Abstract
Regulatory T cells (Tregs) play an essential role in maintaining immune tolerance and suppressing inflammation. However, Tregs present major hurdle in eliciting potent anti-cancer immune responses. Therefore, curbing the activity of Tregs represents a novel and efficient way towards successful immunotherapy of cancer. Moreover, there is an emerging interest in harnessing Treg-based strategies for augmenting anti-cancer immunity in different types of the disease. This review summarises the crucial mechanisms of Tregs’ mediated suppression of anti-cancer immunity and strategies to suppress or to alter such Tregs to improve the immune response against tumors. Highlighting important clinical studies, the review also describes current Treg-based therapeutic interventions in cancer, and discusses Treg-suppression by molecular targeting, which may emerge as an effective cancer immunotherapy and as an alternative to detrimental chemotherapeutic agents.
Keywords: Anti-cancer immunity, Cancer, CD8+ T cells, CD4+ T cells, Regulatory T cells, Tumor-infiltrating lymphocytes
Highlights
-
•
Tregs are crucial in maintaining immune tolerance and suppressing inflammation.
-
•
Tregs present a major obstacle to eliciting potent anti-tumor immune responses.
-
•
The review summarizes current Treg-based therapeutic interventions in cancer.
-
•
Treg can be an effective cancer immunotherapy target.
Anti-cancer immunity; Cancer, CD8+ T cells; CD4+ T cells; Regulatory T cells; Tumor-infiltrating lymphocytes.
1. Introduction
Despite the immune system possessing the important function of recognising malignant cells, cancers can escape destruction by immune-mediated processes. Major hurdles in mounting an effective anti-tumor immune response include induction of tolerance for tumor-associated antigens, disruption of T cell signalling, and local immune evasion in tumors such as the presence of suppressive regulatory T cells (Tregs). By exerting such mechanisms, tumors can render anti-cancer immunity ineffective.
Treg cells play a key role in maintaining peripheral tolerance in vivo through the active suppression of self-reactive T-cell activation and expansion; thereby help in preventing autoimmune diseases and restraining chronic inflammatory conditions [1]. There are five major classes of Tregs that include, CD4+CD25+FoxP3+ natural Tregs (nTregs; thymus derived), CD4+CD25+FoxP3+ induced Tregs (iTregs), Tr1 type Tregs (IL-10 dependent), Th3 type Tregs (TGF-β dependent, LAP+) and CD8+ Tregs [1]. However, CD4+CD25hiFoxP3+ phenotypic Tregs are the most intriguing. Though FoxP3 serves as specific marker for Tregs, recent evidences suggest that FOXP3+CD4+ T cells are phenotypically and functionally heterogenous and involve both suppressive and non-suppressive T cells [2]. Saito et al. [2] in their report classified FOXP3+CD4+ T cells into three sub-populations on the basis of FOXP3 and CD45RA expression levels which include, FOXP3loCD45RA+ naive Tregs (nTreg), FOXP3hiCD45RA− effector Tregs (eTregs; highly suppressive and functionally stable) and FOXP3loCD45RA− T cells (non-suppressive).
In contrast to autoimmune disease where a reduction in Tregs is observed, increased Treg numbers and activity are found in cancer. Indeed, raised Treg numbers have been correlated with decreased survival rates and poor prognosis in certain cancers [3]. Furthermore, a reduction in Treg cell numbers or inhibiting their suppressive functions can improve the anti-tumor immune response [4]. Mechanistically, Tregs can suppress anti-tumor cytotoxic T cells [5]. Therefore, the balance of Tregs and T effector cells (Teffs) plays crucial role in controlling the malignancy progression, avoiding therapeutic resistance, and improving prognosis. This review discusses the generation of Tregs in Tumor microenvironment (TME), the role of tumor-specific Tregs and the various mechanisms of Treg-mediated suppression of anti-cancer immunity. In addition, the review explores the strategies to suppress or alter Tregs to augment anti-cancer immunity and the development of Treg-based therapeutic intervention in cancer.
2. Regulatory T cells (Tregs) and cancer development
The occurrence of T cells with suppressive function in cancerous lymph node tissues was first shown by Mukherji et al. [6]. Further study reported that these suppressor T cells could be altered using cyclophosphamide which enhanced anti-tumor immunity in cancer patients [7]. Later, Sakaguchi et al. [8], characterised suppressor T cells as CD4+CD25+ natural Treg cells (nTregs) that had both in vivo and in vitro immune suppressive functions. Further, the CD4+CD25+T cells were defined by FOXP3, which is a specific intracellular marker and an indispensable molecule for the growth, development and suppressive capacity of Tregs [9]. The Treg cells maintain immune homeostasis by tolerating self-antigens and suppressing the exaggerated immune responses.
Previously, several studies confirmed the positive association between increased Treg cell number and the occurrence of different types of human cancer (Table 1). The highest frequency of Tregs with in vitro suppressive capacity was reported in cancer patients' peripheral blood, TME, and tumor-draining lymph nodes [10, 11, 12]. Abundant tumor-infiltrating FOXP3+T cells have been correlated with poor prognosis in many cancers including cutaneous T cell lymphoma, hepatocellular carcinoma, cervical, ovarian, and lung cancers [13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33]. Particularly, gastroesophageal cancer's severity was associated with increased Tregs [34] and Treg-cell infiltration was observed in a positive correlation with gastric cancer, gastritis, and peptic ulcers [35, 36, 37]. Moreover, in gastric cancer, the presence of CD4+CD25+ T cells in sentinel lymph nodes was suggestive of metastasis toward the downstream lymph nodes [37]. In particular the study demonstrated that CD4+CD25+ T cells percentage was significantly higher in N1 regional lymph nodes (3.1 ± 0.3%) as compared to mesenteric lymph nodes (1.2 ± 0.3%, P < 0.01) [37]. Additionally, the study reported that CD4+CD25high T-cells were increased in N2 regional lymph nodes and N1 regional lymphnodes adjacent to the tumors indicating for their role in the disease progression [37]. Interestingly, it has been noted that homeostasis between FOXP3+T cells and tumor-infiltrating CD8+T cells can serve as crucial prognostic factor in cervical cancer, hepatic carcinoma, and Hodgkin's lymphoma [28,38, 39, 40]. The CD8+ T cells are crucial lymphocytes that inhibit tumor proliferation and disrupt metastasis through direct recognition and killing the tumor cells. Moreover, a study has shown the prognostic value of tumor-infiltrating regulatory T-cell (Foxp3+)/activated cytotoxic T lymphocyte (granzyme B+) ratio on resected left-sided pancreatic cancer [41].
Table 1.
Increased frequency of regulatory T cells in human cancers.
| Type of cancer | Associated tissue/cells1 | Method of Treg evaluation | Reference |
|---|---|---|---|
| Lung | PBMCs and TILs | Flowcytometry | [11,32] |
| Hodgkin lymphoma | Lymphoma-infiltrating lymphocytes | Flowcytometry | [33] |
| Gastrointestinal | PBMCs and ascites | Flowcytometry | [15] |
| Gastric | PBMCs and TDLN | Flowcytometry and Immunohistochemistry | [16,35, 36, 37] |
| Gastroesophageal | PBMCs and TILs | Flowcytometry | [16,34] |
| Hepatocellular | PBMCs, TILs and ascites | Flowcytometry and Immunohistochemistry | [17,18,31] |
| Melanoma | PBMCs, TILs and TDLN | Flowcytometry | [19,20] |
| Chronic lymphocytic leukaemia | PBMCs | Flowcytometry | [21] |
| Adult T cell leukaemia/lymphoma | Tumor itself has Treg cell phenotype | Immunohistochemistry | [22] |
| Cutaneous T cell lymphoma | Tumor itself has Treg cell phenotype | Flowcytometry | [23] |
| Head and neck | PBMCs and TILs | Flowcytometry | [24,26] |
| Cervical | TDLN | Flowcytometry | [12] |
| Ovarian | Tumor-associated lymphocytes and ascites | Flowcytometry | [11,13] |
| Adenocarcinoma (breast and pancreas) | PBMCs, TILs and TDLN | Flowcytometry and Immunohistochemistry | [10,29] |
| Pancreatic ductal adenocarcinoma | PBMCs, TILs and TDLN | Immunohistochemistry | [30] |
| Colorectal | Established Treg cell line from patient's PBMCs | Flowcytometry | [201] |
| Epithelial Cancers | PBMCs | Flowcytometry | [202] |
| Endometrial | TDLN | Flowcytometry | [12] |
PBMCs, peripheral blood mononuclear cells; TDLN, tumor-draining lymph nodes; TILs, tumor-infiltrating lymphocytes.
Several animal models have also revealed the involvement of Treg cells in cancer development. The first animal model study in Lewis lung tumor mice reported that mice thymocytes accelerated the tumor growth in syngeneic recipients [42]. Interestingly, thymectomised recipients exhibited slower tumor growth and suggested that tumor progression could be due to thymus-derived suppressor T cells. Further studies with lethally irradiated, syngeneic bone marrow-transplanted mice indicated that deficiency of suppressor T cells could lead to increased resistance to tumor challenge [43].
Other studies with animal models have furthered the idea that Tregs can influence tumor development. The suppression of tumor-specific cytotoxic T cell generation was shown by suppressor T cells, in vitro [44]. Moreover, Ly-1+2−CD4+suppressor T cells resulted into loss of the effector Ly-1-2+ CD8+T cells in mice model of methylcholanthrene (Meth A)-induced fibrosarcoma [45]. Finally, in vivo adoptive transfer and CD4-specific monoclonal antibody (mAb) experiments demonstrated that CD4+ suppressor T cells are crucial in cancer development [46]. Overall, several studies in humans and animals have evidenced for the crucial role of Treg cells in both tumor induction and development.
3. Characteristics of tumor-specific regulatory T cells
The tumor microenvironment (TME) is characterised by presence of numerous Tregs that express immune suppressive molecules like cytotoxic T lymphocyte antigen-4 (CTLA-4) and T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT) and few naive Tregs [2]. Initially, a study by Hansen et al. [47] suggested the concept of intra-tumoral Treg cells. The study demonstrated that tumor-derived vascular endothelial growth factor (VEGF) led to Treg cells’ migration in vitro [47] and it is now known that Treg cells are attracted to tumors through VEGF and/or various chemokines (e.g., CXCL12, CCL28, CCL22, CCL17), secreted by tumor cells [48].
Neuropilin 1 (Nrp-1) acts as a receptor for VEGF Soker et al. [49] and Hansen et al. [47] showed that increased expression of Neuropilin 1 (Nrp-1) on FOXP3+ Tregs resulted into migration of Treg cells into tumors, thereby regulating the immunological anti-tumor control [47]. Moreover, expression of VEGF is associated with increased angiogenesis and advanced stage disease in a variety of solid tumor types [50]. Furthermore, Hansen et al. [47] suggested that Nrp-1 is required for T cell migration toward VEGF. In particular, the in vitro study conducted transwell assay with Nrp-1-deficient T cell hybridoma line and Nrp-1–expressing mutant (Nrp-1+) and found that only Nrp-1+ cells migrated toward the VEGF whereas migration of Nrp-1-deficient cells toward the VEGF was completely abolished, indicating that VEGF and Nrp-1 expression are crucial for Treg infiltration into tumors [47]. Interestingly, animal model study demonstrated that knockdown of Nrp-1 expression in Treg cells resulted in delayed tumor formation and progression due to decreased FOXP3+ Tregs infiltration into the tumor site which is mediated by tumor-derived VEGF attraction [47].
In terms of cell density, a meta-analysis involving the clinical studies suggested that many solid tumors in melanoma, and cervix, breast, and kidney cancers contain increased frequency of tumor-infiltrating FOXP3+T cells and that these negatively correlate with survival of patients [51]. In addition, analysis of Tregs isolated from human breast cancer lesions demonstrated an increased percentage of Tregs as compared to those isolated from normal breast tissue [52]. Clinical studies suggest that the intra-tumoral Tregs are also highly proliferative and show increased surface expression of T cell antigen CD25, programmed cell death protein 1 (PD-1) and CTLA-4 [53].
With respect to gene expression, de Simone et al. [54] demonstrated that a specific set of transcripts were up-regulated by Tregs in colorectal carcinoma and non-small cell lung cancers as compared to that of Tregs from non-lymphoid tissues and blood. Tumor-infiltrating Tregs exhibited increased levels of Treg cell activation markers including TNF receptor super family member 4 (OX40; TNFRSF4; CD134), T cell immunoglobulin mucin receptor 3 (TIM3), lymphocyte activation gene 3 (LAG3), glucocorticoid-induced TNFR-related protein (GITR), and inducible T cell costimulator (ICOS) [54]. Moreover, single-cell analysis of intra-tumoral Tregs isolated from colorectal carcinoma, breast and gastric cancers and metastases of non-small cell lung cancer (NSCLC) also revealed up-regulation of these signature genes [54].
Further recent research using single-cell RNA sequencing of FOXP3+ Treg cells has revealed up-regulation of approximately400 genes in patients with hepatocellular carcinoma compared to Tregs from normal adjacent tissue [55]. The tumor-specific Treg cell expression signature included genes encoding CTLA-4, GITR, TIGIT, TNFRSF4, ICOS, TNF ligand super family member 9 (4-1BB ligand), T cell activation antigen CD27, IL-1 decoy receptor (IL1R2), chemokine receptor CCR8, Fc receptor-like protein (FCRL3), type-II MAGE family protein (MAGEH1), and transferrin receptor (TFRC). Overall, the gene expression data suggest that intra-tumoral Tregs with the same specificities are involved in different types of cancer [54,55].
In terms of cancer antigen recognition, Wang et al. [56] detected LAGE1-specific CD4+CD25+GITR+ functional Treg cells in cancer patients. Nishikawa et al. [57] showedthe presence NY-ESO-1 specific Treg cells in cancer patients. These Treg cells had to be deleted for inducing NY-ESO-1 specific Th1 cells, indicating that antigen-specific Tregs were required in suppressing the tumor antigen-specific T cells [57]. The NY-ESO-1 (New York esophageal squamous cell carcinoma 1) is a germ-cell derived protein and often expressed by cancer cells [57]. Increased expression of NY-ESO-1 expression have been reported in myeloma, synovial sarcoma, melanoma, neuroblastoma, and bladder, esophageal, hepatocellular, head and neck, ovarian, prostate, breast and non-small cell lung cancers [58]. Moreover, cancer patients exhibited antibody response to NY-ESO-1 and these were correlated with NY-ESO-1 expression in tumor cells and presence of NY-ESO-1 specific CD8+ T cells [59]. Recently, NY-ESO-1 has been suggested as potential target for cancer immunotherapy [58].
It has been observed that both proliferative and dying tumor cells present abundant self-antigens [57], which are preferentially recognised by Tregs [57]. In summary, the aforementioned characteristics of tumor-specific Tregs suggest that these cells play a critical role in suppression of antigen-specific anti-tumor immunity.
4. Generation of regulatory T cells in tumor microenvironment
Tumor microenvironment (TME) is characterised by its acidic, hypoxic, and nutrient poor. However, Treg cells can proliferate here and retain their immunosuppressive function in such harsh conditions [60]. The different mechanisms of metabolic adaptation in TME by Tregs have been reviewed in detail by Koyama et al. [60]. Tregs can utilise tumor metabolites to obtain energy through different mechanisms of fatty acid metabolism [61]. For example, upragulation of fatty acid transporter CD36 and signalling of sterol-regulatory-element-binding protein allow Tregs to survive in fatty acid-rich environment [62,63]. Nevertheless, in Tregs, FOXP3 suppresses glycolysis and stimulate oxidative phosphorylation and NAD+ oxidation thereby allowing Tregs to utilize lactate as energy source [64]. Moreover, in TME, conversion of pyruvate into acetyl-CoA in Tregs leads to tricarboxylic acid (TCA) cycle; thereby rendering Tregs a survival benefit over other T cells [64]. Recently a study suggested that altered sensitivity to PD-1 blokade therapy in gastric cancer is due to RHOA Y42 mutation that results into increased fatty acid synthesis via upregulation of fatty acid synthase; thereby leading to increased numbers of Tregs and less numbers of CD8+ T cells in TME [65].
Koyama et al. [60] also reviewed the mechanisms that lead to Treg abundance in the TME including chemokine and cytokine dependent infiltration and conversion of Tregs. Different chemokines such as CCR4-CCL17/22, CCR5-CCL5, CCR8-CCL1, and CCR10-CCL28 are involved in recruiting Tregs into the TME [60]. For example, in human breast cancer upregulation of CCR8 was shown by tumor-infiltrating Tregs suggesting that CCR8 is involved in Tregs’ recruitment in TME [52,60]. Moreover, generation of Tregs in the TME can occur via hyper-activation of focal adhesion kinase (FAK) in tumor cells. This leads to Treg cell recruitment and CD8+T cell exhaustion by CCL5 regulation [66].
Other mechanisms are also involved in Treg cell induction and accumulation in the TME. For example, an increased frequency of DCs and higher levels of IL-10, transforming growth factor-β (TGF-β) and indoleamine 2, 3-dioxygenase can differentiate Teffs into CD4+CD25+FOXP3+ Tregs. Moreover, an animal model study has suggested that, in tumors, a subset of immature DCs can induce Treg cells’ activation and proliferation in an TGFβ-dependent manner [67]. In addition, the hypoxic environment of the tumor induces CD4+ T cells to express hypoxia-inducible factor-1α (HIF-1α), which associates with HIF-1β in nucleus resulting in FOXP3 as well as TGF-β expression [68,69]. Tumor-derived exosomes can also secrete TGF-β and IL-10 cytokines. Subsequently, the TGF-β binding to T cells causes their differentiation into Tregs through the SMAD signalling pathway [69]. The activation of JAK/STAT5 pathway upon IL-2 binding to the receptor results into increased FOXP3 expression [70]. Hypoxia in the TME also induces PD-L1 expression by maintaining HIF-2α expression, as observed in renal cell carcinoma. PD-1 binding to T cells induces Treg cell differentiation [71]. Finally, it has been suggested that FOXP3 hypo-methylation correlates with Treg cell stability [72].
5. Mechanisms of regulatory T cell mediated suppression of anti-cancer immunity
CD8+ T cells are generated towards different antigens presented on tumor cells. These include neoantigens that are abnormal self-proteins or non-self, oncogenic viral proteins. Tregs exert different immunosuppressive mechanisms against these antigens-specific CD8+ T cells [73]. Animal model studies suggested that Tregs target non-self antigen specific CD8+T cells by increased affinity T cell receptors (TCRs) and regulating co-stimulatory molecules [73]. However, self antigen specific CD8+T cells become anergic by APCs through reduced co-stimulatory signals remaining to be suppressed by Tregs [74].
Interestingly, non-self antigen specific CD8+T cells that target neoantigens show resistance towards suppression by Tregs [74]. Patients harbouring non-self antigen specific CD8+T cells targeting neoantigens did not respond to either single or multiple immune checkpoint inhibitors used in combination [75]. Abundant neoantigens found on cancer cells are susceptible to immune checkpoint inhibitors compared to those having less mutations and these cancer cells remain unresponsive [76]. Such studies suggest that Treg-based immunosuppression prominently occurs in tumor cells that exhibit shared antigens compared to those that express neoantigens.
Treg cells that are involved in the inhibition of an acquired immune response towards tumors resulted into decreased frequency of tumor specific Teffs and B cells [77,78]. These Teff cells, particularly cytotoxic CD8+ Teff cells play crucial role in exerting the anti-tumor effects in TME by secreting cytotoxic cytokines and other lytic molecules (such as granzymes and perforins) and hence, the Treg and Teff cells’ ratio serves as crucial prognostic marker for the clinical outcome in cancer patients [38, 39, 40]. Tregs can also curb the natural killer (NK) cells, dendritic cells (DCs) and macrophages by humoral immune as well as cell-mediated mechanisms [78]. The following sections summarise the different Treg-based suppressive mechanisms of anti-tumor T cells (Figure 1).
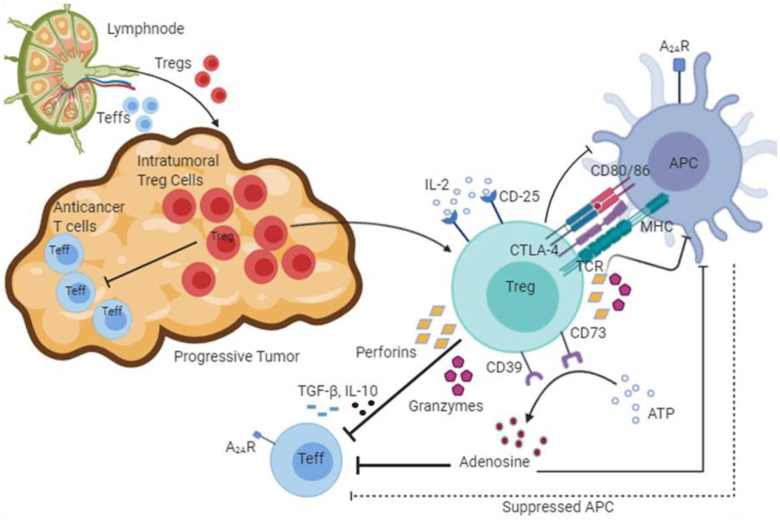
Figure 1.
Mechanisms of regulatory T cells (Tregs) in suppression of anticancer immunity. The interactions of immune cells at the site of a progressive tumor are shown. The Teffs (CD4+ and CD8+ cells) are suppressed by intra-tumoral Tregs using multiple mechanisms that lead to diminished anticancer immunity at the tumor site. Firstly, Tregs secrete Teff-suppressive cytokines including TGF-β, IL-10 and IL-35. In addition, CD25, also known as IL-2 receptor subunit-α (IL-2Rα), is present on Tregs upon which IL-2 binds, thereby depleting the IL-2 cytokine, so it is less available to fight the cancer. Secondly, perforins and granzymes secreted by Tregs lead to the destruction of Teff cells. They also reduce proliferation of APCs, thus decreasing their effectiveness in presenting antigens to Teffs. Thirdly, CD39 and CD73 convert ATP into adenosine. In the tumor microenvironment, this gives a suppressive signal via engagement of adenosine A2A receptor to the anticancer Teff cells as well as to APCs. Finally, CTLA-4 binding to CD80 and CD86 molecules suppresses the activation of Teffs indirectly through the APCs (The figure has been prepared using Biorender;https://biorender.com/).
5.1. IL-2 and IL-2 receptor
The Tregs deplete IL-2 in their surroundings through constitutive CD-25 (IL-2 receptor subunit-α) expression. This results into less availability of the cytokine to activate Teff cells. Indeed, reduction of IL-2 production due to Treg cell activity suppresses proliferation of CD4+ and CD8+T cells [77,79,80]. It has also been shown that the in vitro addition of exogenous IL-2 nullifies the suppressive function of Tregs [77]. Thus, both IL-2 receptor and IL-2 serve as potential targets for regulating Treg cells’ survival and suppressive capacity [81].
5.2. Cytotoxic T lymphocyte antigen-4
The cytotoxic T lymphocyte antigen-4 (CTLA-4) is expressed constitutively on Tregs. Binding of CTLA-4 to co-stimulatory molecules such as CD80 and CD86 on antigen-presenting cells (APCs) results in the suppression of APCs and reduces their capacity to activate Teff cells [82]. Due to higher affinity ofCTLA-4 binding to CD80 and CD86 as compared to CD28; CTLA-4 competes with CD28 and causes CD80 and CD86 sequestration from APCs to Tregs by a process known as ‘trogocytosis’ [82]. Due to the deficiency of the co-stimulatory molecules, high-affinity TCRs containing antigen-specific Teffs undergo apoptosis, intermediate-affinity TCRs containing Teffs become anergic, and low-affinity TCRs containing Teffs remain dormant. This finally leads to the disruption of Teff cell priming and/or their activation [82]. It has been shown that Treg cells can down regulate MHC class II molecules, CD80 and CD86 expression on DCs and thereby affect the function of APCs [83]. Moreover, CTLA-4-deficient mice exhibited altered Treg-mediated immunosuppression [84]. Finally, CTLA-4 mutations can cause autoimmune manifestations where patients exhibit impaired Treg immunosuppressive capacity [85].
5.3. Indoleamine 2,3-dioxygenase
Indoleamine 2,3-dioxygenase (IDO) enzyme is involved in degradation of essential amino acids such as tryptophan resulting in T cell exhaustion in the TME. IDO is expressed in APCs upon binding of CTLA-4 on Tregs to B7 ligand and leads to Teff cells' suppression and Treg cells' expansion [86]. In TME, the catabolism of tryptophan through IDO results into synthesis of kynurenine, which suppresses anti-cancer immunity. Moreover, IDO expression in the TME is directly proportional to the number of intratumoral Tregs [87]. The conversion of Tconv cells to Tregs can occur through IDO-expressing myeloid cells via aryl hydrocarbon receptor (AHR) [88]. The binding of kynurenine to AHR results into increased Treg cells’ proliferation and immunosuppressive activity [89]. It has been suggested that administration of an IDO inhibitor can improve the efficacy of therapeutic vaccination of cancer patients [90].
5.4. Immuno-suppressive cytokines
By secreting suppressor cytokines such as TGF-β, IL-10 and IL-35, Treg cells suppress the activity of APCs and Teff cells leading to their cell cycle arrest. These cytokines also lead to Tregs' mediated secretion of granzymes and perforins that kill Teff cells directly [91,92]. The IL-10 treatment to immature DCs resulted into decreased stimulation of CD4+T cell responses [93] and IL-35 leads to inhibition of T cell proliferation and function. Moreover, it has been demonstrated that both IL-35 specific antibody and Treg cell restricted deletion of IL-35 production resulted into suppressed tumor in murine cancer model [94]. In addition, Treg cells can adversely affect B cell proliferation, both in T cell-dependent and T cell-independent manner [95,96]. Treg cells can also destroy B cells containing cognate antigen and MHC class II molecules [97]. It has been shown that Treg cells promote monocyte apoptosis via Fas/Fas ligand pathway [98]. Moreover, in vitro co-culture system of Treg cells and macrophages demonstrated decrease in HLA-DR expression on macrophages and reduction of pro-inflammatory cytokines' production [99]. Furthermore, intra-tumoral Treg cells from mice have been shown to alter the DC co-stimulatory molecules’ (CD80, CD86, and CD40) expression, to suppress DC mediated IL-12 and TNF-α cytokine production and inhibit their ability to induce T cell activation [100]. The study suggested that the intra-tumoral Treg cells mediated suppression of DCs might involved the IL-10 and TGF-β cytokines [100].
5.5. Inhibition of cytokine secretion by T helper (Th) cells
Treg cells inhibit both Th1 and Th2 cytokines' secretion [101]as well as Th1 and Th17 cells' proliferation [102]. Indeed, removal of Treg cells results in activation of autoantigen-specific CD4+T cells which secrete large amounts of IL-2, thereby stimulating the tumor-specific CD8+T cells involved in rejection of multiple tumors. The IL-2 secretion by CD4+T cells, in absence of Treg cells also contributes to anti-melanoma immunity in mice [80]. In addition, CD4+T cells’ ability to secrete IFN-γ is enhanced by removing CD25+T cells and thus IFN-γ induced macrophages kill the tumor cells [103].
5.6. Adenosine A2A receptor
The endogenous purine nucleoside adenosine is an important anti-inflammatory mediator responsible for controlling the CD4+T cell response [104]. When ATP is converted into adenosine, it prevents the T cell activation [104,105]. In the TME, apoptotic Tregs convert large amounts of ATP into adenosine through the adenosine A2A receptor pathway involving CD73 and CD39, which results into suppression of local immune cells including Teffs and APCs [104,105].
In particular, it has been suggested that hypoxic stress in TME leads to ATP release into extracellular space by exocytosis or pannexin-1 channels [106]. Following this, major nucleotide catabolising enzymes such as CD39 and CD73 convert ATP into AMP and then to hypoxia-inducible factor (HIF)-dependent adenosine [106]. Adenosine leads to tumor-mediated immune suppression through autocrine and/or paracrine fashion. The accumulation of adenosine in the extracellular space is supported by HIF-mediated inhibition of the nucleoside transporter ENT-1 which impedes adenosine transport into the cell and removes it from the extracellular space [106]. Adenosine can sabotage not only spontaneous anti-tumor immune responses, but also anti-tumor immune functions artificially introduced with therapeutic intention, such as radiotherapy [106].
5.7. FOXP3 expression
Forkhead Box P3 (FOXP3) is a Treg cell-defining molecule that can suppress IL-2gene transcription and up-regulate IL-2RA (CD25) and CTLA-4 transcription [107]. Nevertheless, FOXP3 itself is not the effector of immune-suppression, and it induces CTLA-4 expression on Tregs for immune-suppression activity, as mentioned in section 5.2. In addition, FOXP3 expression in Treg cells renders them hypo-responsive to TCR stimulation [9]. This is achieved via FOXP3 mediated suppression of Zeta-chain-associated protein kinase-70(ZAP-70; a TCR signalling molecule), which allows Tregs to escape activation-induced cell death upon antigen exposure [72]. Attenuation of TCR-signalling through suppressing ZAP-70 also contributes to allowing Treg-specific functions to be optimised [108].
5.8. Prevention of immune trafficking
Tumor-infiltrating Tregs exert discrete pattern of chemokine receptor expression as compared to the peripheral Tregs. In the TME, CCR4-CCL22 pathway leads to recruitment of intra-tumoral Tregs in multiple type of tumors [109]. Treg cells have been shown to alter the immune trafficking that is required for the tumor immunity generation. Tregs inhibited the infiltration of CD8+T cells into tumors (in vivo), and reduced occurrence of CD8+T cells in effector target conglomerates (in vitro) [110,111]. Additionally, Treg cells are also involved in reducing the retention and infiltration of monocytes, NK cells, neutrophils and eosinophils in lamina propria of mice infected with Helicobacter hepaticus [112].
5.9. Inhibition of CD8+ T, NK and NKT cells’ cytolytic activity by regulatory T cells
Treg cells can suppress anti-tumor immunity by inhibiting the cytolytic activity of cytotoxic CD8+T, NK and NKT cells [113,114]. For example, Treg cells reduced NK cell cytolytic activity towards Meth A-induced tumors, YAC1 tumor cells and allogeneic cells [79,111]. Tregs can also cause direct cytolysis of CD8+T and NK cells by granzyme B and perforins, which are expressed by 5–30% of intra-tumoral Treg cells [115]. In addition, activated Treg cells express surface molecule such as galectin-1 which binds to its receptors present on Teffs and results into cell cycle arrest [116].
5.10. Immune checkpoint molecules
Treg cells can disrupt Teff cell function through various mechanisms such asMHC class II- LAG-3 interaction, ICOS-ligand mediated T cell activation, and PD-1 & PDL-1 interaction [117]. PD-1 is an important immune checkpoint and targeting this molecule in different cancer types results into anti-cancer response and improved prognosis [117]. The binding of PD-1 to Teff cells resulted into enhanced activation and proliferation of Teff cells in tumor sites [118]. However, the effect of PD-1 on Treg cells is not clear but evidence suggest that it enhances the FOXP3 expression and thereby increases stability of Treg cells [119]. Recent studies suggest that the PD-1 expression is essential for maintaining the balance between effector and Treg cells and is useful for predicting the clinical efficacy of PD-1 blockade therapies [120,121] In particular, Kumagai et al. [120] reported that in TME, relative frequency of PD-1+CD8+ T cells and PD-1+ Treg cells can predict the clinical efficacy of PD-1 blockade therapy and it is more efficient than other available predictors such as PD-L1 [120]. Moreover, in the successive study, the research group suggested that PD-1 expression in Treg cells is induced by lactic acid in a highly glycolytic TME [121]. It has been suggested that in TME, low glucose environment promotes Tregs to consume lactic acid through monocarboxylate transporter 1 (MCT1) which leads to nuclear translocation of NFAT, resulting into increased PD-1 expression [121]. Similarly, PD-L1 has been suggested to stimulate differentiation and function of iTregs by increasing the FOXP3 expression [122]. Nevertheless, PD-1lowCD4+ Tregs efficiently induced B cell apoptosis and inhibited CD4+T cells as compared to PD-1hiCD4+ Tregs [123]. These studies suggest that PD-1/PD-L1 signalling pathway is crucial in regulating the development and function of Treg cell.
Tregs can also express increased levels of TIM-3, TGIT, LAG-3 and VISTA (V domain containing Ig suppressor of T cell activation) in the TME [124]. Expression of these immune checkpoint molecules leads to Teff cell suppression. In particular, LAG-3 expression on Tregs results in suppression of antigen specific CD4+Teff cells and their cytokine secretion [125]. Additionally, binding of LAG-3 to galectin-3 on tumor cells results into the abolition of CD8+T cells’ anti-cancer responses [126]. Moreover, increased frequencies of LAG-3+ Treg cells were found in NSCLC and head & neck squamous cell carcinoma patients [127,128]. The LAG-3+ Treg cells isolated from CRC and melanoma patients demonstrated increased suppressive capacity [125]. Additionally, LAG-3-deficient Treg cells showed reduced suppressive capacity with decreased immune suppressive cytokines such as TGF-β and IL-10 in mouse tumor models [129].
Increased TIM-3+Tregs were found in tumor tissues of several cancers [130]. They exhibit increased suppressive capacity by elevated production of IL-10, granzymes, and perforin [131]. Furthermore, an increased CD8+ T cell:Treg ratio with suppressed tumor growth was observed after treatment of anti-PD-L1 and anti-TIM-3 mAbs [132]. Another immune checkpoint molecule TIGIT is expressed on Tregs, CD4+ and CD8+ T cells. The binding of TIGIT to CD155 and CD112 on DCs leads to suppression of Teffs’ activation [133]. TIGIT+ Tregs showed an increased suppressive capacity with increased IL-10 production in the TME [134]. VISTA is highly expressed on Tregs in the TME and its binding to VSIG-3 (V-set and immunoglobulin domain containing 3) results into suppression of proliferation of Teffs [124,135]. Moreover, VISTA expression was positively correlated with poor survival rates in oral squamous cell carcinoma patients [136].
6. Regulatory T cell-based therapeutic interventions for cancer
6.1. Immune checkpoint inhibitors for targeting effector regulatory T cells
Given the fact that Tregs are well equipped with increased CTLA-4 and PD-1, as well as other immune-modulatory receptors such as OX40, 4-1BB and ICOS in TME; the role of therapeutic antibodies targeting these molecules is a possible way to selectively deplete the activity of intra-tumoral Treg cells. Such antibodies are referred to as immune checkpoint blockade antibodies because they block the binding of PD-1 or CTLA-4 to their respective ligands, thus releasing tumor-specific T cells from signals that control their activities [137].
6.1.1. Anti-PD-1/anti-PD-L1 mAbs
Anti-PD-1 and anti-PD-L1 mAbs can lead to an increased ratio of Teffs: Tregs in TME and can activate Teffs to raise IFN-γ and IL-2 production [138]. The anti-PD-1 mAbs approved for the cancer treatment include nivolumab and pembrolizumab, whereas anti-PD-L1 mAbs include avelumab, durvalumab and atezolizumab [139]. In melanoma patients, blockade of PD-1 by pembrolizumab resulted into increased CD8+T cells’ activation and proliferation along with decreased Tregs [135]. However, in certain cancers, such as that of the colon and metastatic renal cell carcinoma, the anti-PD-1 mAb did not suppress Treg cells although it did lead to an increase in Teffs and IFN-γ levels in the TME [140,141].
The PD-1 or PD-L1 blockade can also target B cells, NK cells and DCs and thus induces T cell independent anti-cancer responses [138]. Of note, in breast cancer, PD-1 or PD-L1 blockade led to B cells’ proliferation and activation and reduced the suppressive capacity of regulatory B cells; thereby enhanced the anti-cancer immunity [142,143]. Moreover, PD-1/PD-L1 blockade was demonstrated to prevent interaction of PD-1+NK cells and PD-L1+DCs; thereby restored the cytotoxic action of NK cells and led to therapeutic benefits in multiple myeloma [144].
6.1.2. Anti-CTLA-4 mAbs
Ipilimumab, an anti-CTLA-4 mAb acts as immune checkpoint inhibitor and was approved for treatment of cancer [139]. In TME, anti-CTLA-4 mAbs deplete the cells expressing CTLA-4 by binding to the target cells and activating the antibody dependent cell mediated cytotoxicity (ADCC) [145]. This was shown in mice with melanoma where treatment reduced intra-tumoral Treg cells without affecting intra-tumoral Tconv cells or Tregs outside of the tumor [146]. In this case, activity of the anti-CTLA-4 mAbs depended upon Fc receptors causing Treg cell depletion via ADCC. Similarly, another study also reported that anti-CTLA-4 mAb efficacy was correlated with the ability of the antibody's Fc region to bind and activate Fc receptors [147].
6.1.3. Anti-CD-25 mAbs
CD25 can be a suitable target to deplete the intratumoral Treg cells, as these cells express increased levels of CD25. One such strategy is used by denileukindiftitox. In cutaneous T cell lymphoma the denileukindiftitox has been shown to bind and kill CD-25 expressing cells; however, its efficacy was less in melanoma patients [148]. Moreover, other mAbs of CD-25 such as RG6292 and ADCT-301 have been developed for depleting the Tregs in human cancers including lymphoma and are under clinical trials (NCT04158583; NCT03621982 respectively) [149,150].
6.1.4. Anti-GITR and OX40 mAbs
Therapeutic antibodies that target T cell expressed co-receptors GITR and OX40 are undergoing clinical trials for cancer treatment [145]. The suppressive function of Tregs mediated was altered when an agonistic anti-GITR mAb was administered in mice, resulting in increased activity of Tconv cells [151]. The anti-GITR mAb administered to tumor-bearing mice induced anti-tumor activity along with increased tumor-destructive CD4+ and CD8+ T cells [152]. Moreover, in mice anti-GITR mAb demonstrated efficient tumor killing by selectively decreasing Tregs [153]. The GITR agonistic mAb such as MK-4166135 (NCT02132754) is undergoing clinical trials for determining its efficacy against human cancers including melanoma and other solid tumors [154].
Similarly, OX40 agonistic mAb such as MEDI6469134 (NCT02274155) is under clinical trials in head and neck squamous cell carcinoma patients [155]. An initial study in mice showed reduction of Tregs numbers in blood and secondary lymphoid organs, but it did not deplete intra-tumoral Treg cells [156]. However, a clonal variant of the mAb did induce effective depletion of intra-tumoral Tregs and also improved the anti-tumor immunity, possibly by engaging with Fc receptors [156]. Thus, the antibody's Fc region design is a major determinant of biological activity as well as in vivo efficacy. Although, animal studies have shown the rejection of tumors following anti-OX40 mAb treatment, the efficacy of such antibodies remains indeterminate in cancer patients.
6.1.5. Anti-TIGIT mAbs
After studies in animal models, TIGIT could be a potential target for Treg suppression for enhancing anti-cancer immunity. In a glioma mouse model, TIGIT was blocked through an anti-TIGIT mAb that resulted in an increased Teff:Treg cell ratio and improved the survival rate of tumor-bearing mice [157]. In another study, the TIGIT blockade, along with an anti-PD-1 mAb, showed enhanced survival rate of tumor-bearing mice [158].
6.1.6. Anti-VISTA mAbs
In a mouse tumor model, anti-VISTA mAbs resulted in induction of anti-tumor immunity by activating Teffs and reducing Treg cell numbers [159]. In addition, combination of anti-VISTA and anti-CTLA-4 or anti-VISTA and anti-PD-1 mAbs resulted into increased Teff:Treg cell ratio in mouse model of head and neck squamous cell carcinoma [160]. Moreover, VISTA and PD-L1 co-blockade led to effective tumor regression with increased survival rate in mouse model of colon cancer and melanoma [161]. Interestingly for humans, ipilimumab treatment of melanoma and prostate cancer resulted in increased frequency of VISTA+ macrophages and VISTA+ tumor-infiltrating lymphocytes, suggesting that up-regulation of VISTA can be a compensatory resistance mechanism [162].
6.1.7. Anti-CCR4 and anti-CCR8 mAbs
In tumor tissues, depletion of effector Tregs (eTregs) shifts the homeostasis from immune suppression to immune activation against tumor cells. The CCR4 receptor is exclusively expressed on eTregs [163]. CCR4 receptor ligands such as CCL17 and CCL22 are produced by tumor cells or macrophages and are responsible for eTreg cell migration and infiltration of tumor tissues [13,164]. In the in vivo study, reducing the activity of eTregs by anti-CCR4 antibody was found to be effective in enhancing tumor antigen specific CD8+ and CD4+ T cells activity [163]. The FDA approved anti-CCR4 antibody Mogamulizumab (KW-0761) has been shown to decrease the CCR4+ Tregs in solid tumors [165]. Moreover, in a clinical trial the combination of mogamulizumab and nivolumab (anti-PD-1 antibody) resulted into significant depletion of Tregs [166]. In addition, a phase I/II clinical trial is involving FLX475 (NCT03674567; a CCR4 antagonist) and pembrolizumab for treating advanced cancers (NCT03674567) [60]. Moreover, another phase-II clinical trial study demonstrated that anti-CCR4 mAb effectively treated the T cell leukaemia/lymphoma by inducing ADCC [167].
Furthermore, in TME the exclusive upregulation of CCR8 in Treg cells renders it as potential therapeutic target for suppressing the Treg cells’ recruitment in TME, without inducing systemic autoimmunity [52,60]. In preclinical studies, the anti-CCR8 antibodies with Fc-dependent ADCC have been shown to specifically deplete intratumoral Tregs resulting into induction of anti-cancer immunity [168,169].
6.1.8. Anti-FR4 mAbs
Folate receptor 4 (FR4) is expressed at increased level by rodent Tregs and it is required for folate influx. The folate is required by naive T cells for nucleic acid and protein synthesis and upon TCR stimulation, FR4 expression is up-regulated many folds in FOXP3+ Tregs [170]. Therefore, FR4 could be a suitable target and an anti-FR4 mAb-based therapy can deplete Tregs and enhance anti-cancer immunity. However, folate is essential for other cells for their proliferation and growth, which may preclude anti-FR4 mAbs as successful cancer treatments.
6.1.9. Anti-CD73 and anti-CD39 mAbs
As mentioned above (section 5.3), in the TME, Tregs convert large amounts of ATP into adenosine through the adenosine A2A receptor (A2AR) pathway involving CD73 and CD39, and results into suppression of Teffs and APCs. Hence, mAbs that target CD73 and CD39 are of therapeutic value in inducing anti-cancer immunity. Such mAbs include TTX-30, MEDI9447, and BMS-986179 and are currently under clinical trials (NCT03884556, NCT03742102, and NCT02754141 respectively) [60]. These anti-CD73 and anti-CD39 mAbs by blocking the ectonucleotidase activity induce anti-cancer immunity. Moreover, the anti-cancer immunity can be augmented by targeting the A2AR through receptor antibodies such as CPI-444, AZD4635, and PBF-509 which prevent the adenosine-dependent T cell suppression. This A2AR receptor antibody based strategy is under clinical trials (NCT02655822, NCT04089553, and NCT02403193) [60].
6.2. Anti-regulatory T cell vaccines
Since, FOXP3 is an intracellular nuclear product; anti-FOXP3 mAbs are ineffective at suppressing the activity of Treg cells. As an alternative, vaccine-based immunotherapy offers an approach to specifically eliminate Treg cells and so induce Teff cell-based anti-cancer immunity. Recently, anti-regulatory T cell vaccines that target the FOXP3 molecule of Treg cells have been investigated [171]. Previously, DCs pulsed with FOXP3 mRNA results into significant Teff cell response and depleted the FOXP3+ Tregs [172]. Klages et al. [173] showed that the selective depletion of FOXP3+ Tregs improved effective vaccination against established melanoma in mice. Another murine melanoma study demonstrated that the silencing of FOXP3 delayed tumor growth and altered the tumor immunosuppressive environment through decreasing IL-10, IL-2, and TGF-β production [174]. An improved anti-FOXP3+Treg vaccine has been proposed by Mousavi-Niri et al. [175]. They demonstrated that mice vaccinated with a FOXP3-Fc fusion DNA vaccine resulted in enhanced cytotoxic T cell response against FOXP+ Tregs. Overall, the studies mentioned suggest that anti-Treg cell FOXP3-based vaccines can be an effective immunotherapeutic approach for cancers [175]. Additionally, an antisense oligonucleotide (AZD8701) for FOXP3 has recently been developed to deplete the Treg cells and its immunosuppressive molecules in humanized mouse model and it is under clinical trial (NCT04504669) [60].
6.3. Other regulatory T cell-depletion strategies in tumor tissues
Recently, Verma et al. [176] reviewed Treg cell-based therapeutic approaches for cancer and highlighted that DNA-targeting drugs and drugs that target Treg cell biosynthetic pathways associate with significant anti-cancer activity. The next sections summarise the use of such chemotherapeutic agents and other mechanisms to deplete Treg cells in the TME.
6.3.1. Cyclophosphamide
The depletion of Tregs can be achieved through small molecular drugs with their specific features. Cyclophosphamide (alkylating agent) is one such drug which act by interfering with replication of DNA and killing hyper-proliferating cells. Studies have suggested that lower doses of cyclophosphamide can specifically deplete highly proliferating Tregs in tumor tissues and improve the anti-tumor immunity in rodents and humans [177,178]. Moreover, a study demonstrated that cyclophosphamide can enhance radio-sensitisation by the 2-deoxy-D-glucose (2-DG; a glycolytic inhibitor) and results into depletion of iTregs [179]. Furthermore, treatment of metastatic carcinoma with cyclophosphamide and intra-tumor BCG vaccine immunotherapy led to decreased Treg and an increase CD8+ T cell infiltration [180]. Such studies suggest that cyclophosphamide inhibits Treg cell function and thereby enhances anti-cancer immunity.
6.3.2. Mitoxantrone
Mitoxantrone is a anthracenedione, which upon binding to deoxyribose sugars causes DNA strand breakage [181]. Mitoxantrone treatment of breast cancer resulted into depletion of B cells and Treg cells; however, the CD4+:CD8+ ratio was not changed significantly [182]. Alternatively, mitoxantrone has been suggested to induce anti-cancer immunity by increasing calreticulin expression in cancer cells. Such an effect promotes the phagocytosis of tumor cells by DCs [183]. In particular, the study demonstrated that anthracyclins (such as mitoxantrone) treatment to tumor cells induce pre-apoptotic translocation of calreticulin to the cell surface and calreticulin blockade or knockdown resulted into suppression of phagocytosis of anthracyclin-treated tumor cells by Dcs in mice [183]. It has been suggested that the translocation of molecules such as phosphatidylserine and calreticulin, from inside the cell to the surface induce the phagocytosis mediated removal of apoptotic and tumor cells [184]. Though, mitoxantrone treatment seems to be an effective treatment; but the effects of mitoxantrone may be non-specific, so further animal model studies are needed to analyse its use in cancer immunotherapy [176].
6.3.3. 2-Deoxy-D-glucose (2-DG)
2-DG is a glycolytic inhibitor and exerts chemo- and/or radio-sensitising effects on cancer cells [185]. These effects include impaired anti-oxidant defence, ATP crunch, increased unfolded protein response (UPR), impaired regulation of cell cycle, impaired influx of calcium, inhibition of DNA repair and apotosis [185]. Glycolysis regulates the activity of Tregs by modulating FOXP3 expression and treatment with 2-DG has been shown to decrease FOXP3 expression [186]. In the presence of glycolysis, optimal TCR-stimulation of CD4+T cells leads to IL-2 secretion and limited number of Treg cells are generated [187]. However, in sub-optimal TCR stimulation, tumor cells can be directly sensitised by 2-DG through impaired energy-dependent repair and recovery processes, leading to a decrease in Tregs [188]. The 2-DG treatment along with radiation in a murine tumor model resulted in regression of tumors and a better survival rate of the animals [189]. Clinical trial studies suggested that 2-DG along with hypofractionated radiotherapy results in improved quality of life and increased survival rate in cerebral glioma patients [185,190,191].
6.3.4. Indoleamine 2,3-dioxygenase (IDO) inhibitors
As mentioned earlier (section), IDO is constitutively expressed on intratumoral APCs and DCs, and is involved in Teff cells' suppression and Treg cells’ expansion [86]. Therefore IDO is a potential target to suppress the Tregs and inducing the anti-cancer immunity. IDO inhibitors such as Epacadostat, NLG802, GDC-0919 are currently under clinical trials for soildtumors (NCT01685255, NCT03164603, NCT02048709 respectively) [60]. Nevertheless, intratumoral IDO-expressing cells operate AHR pathway, targeting the IDO- Kynurenine-AHR axis has been suggested as efficient strategy to induce anti-cancer immunity by depleting Treg cells [60,192].
6.3.5. Attenuation of tumor-derived exosomes
Exosomes are eukaryotic cells derived extracellular vesicles that include immune network components that facilitate communication among various immune cells [193]. Exosomes within the TME contribute to enhance Treg cell function by TGF-β1 (surface bound form), which induces expression of FOXP3 and enhances suppressive capacity of Treg cells in tumors [2,194]. Therefore, the inhibition and/or control of exosomes in the TME may be an efficient immunotherapy for management of cancers. Since tumor derived exosomes have crucial role in DCs associated with anti-cancer immunity; an in vitro study demonstrated that tumor derived exosomes decrease the expression of PD-L1 on DCs, thereby causing downregulation of Tregs [62]. Moreover, study in mice model of hepatocellular carcinoma demonstrated that exosomes derived from α-fetoprotein (AFP) expressing DCs induced the production of IFN-γ expressing CD8+ T cells with increased IFN-γ and IL-2 and reduced CD25+FOXP3+Tregs, TGF-β and IL-10 [195]. Recently, the use of exosome-vaccination for immunotherapy has been suggested to suppress Tregs and inducing the anti-cancer immunity in TME. Studies have suggested that vaccination within tumor associated exosomes-exosome loaded T cells can counteract the CD4+CD25+ Treg cell-mediated immunosuppression and induce long term anti-cancer immunity against B16 melanoma [196,197]. However, further animal model studies and clinical trials are needed to verify the exosome based Treg suppression modality for cancer immunotherapy.
6.3.6. Targeting T cell receptor (TCR) signalling molecules
Treg cells can be controlled through targeting of TCR signalling molecules that are expressed differentially in Tregs. ZAP-70 is one such molecule [70,198]. In addition, a mouse study showed that anti-tumor activity was enhanced by mutated phosphatidylinositol-3-kinase (PI3K) or by conditional PI3K-knockout in Tregs [199]. It has been suggested that Tregs can be selectively depleted by PI3Kδ isoform-specific PI3K inhibitor which results into tumor suppression and prevention of metastasis [200]. In addition, combined treatment of PI3Kδ inhibitor (INCB050465) and anti-PD-1 mAb (pembrolizumab) is under clinical trial (NCT02646748).
7. Future perspectives
The role of Treg cells in cancer has emerged as an interesting research field with respect to pathogenesis and therapeutic aspects. Previous studies have explained the pathogenic role of Tregs in tumor development and metastasis. Studies with animal models have suggested that Tregs depletion in TME can result into enhancement of anti-tumor activity. However, a clear understanding of basic Treg cell biology and its various suppressive mechanisms in the TME is required, since Tregs might act differently in tumors of different aetiology and stage of progression.
With respect to therapy, novel strategies for the selective targeting of Tregs residing in the TME are required. These may include targeting of Treg cell-recruiting molecules as well as Treg cell effector and regulatory molecules. Such strategies need to overcome the downsides of existing Treg cell-based therapies that have normal tissue toxicity due to targeting of proteins or receptors shared with other effector T cells. Also, the depletion of Treg cell numbers can result into autoimmunity generation and hence, further investigations are needed while developing Treg-based therapeutics. Despite the drawbacks, immune-targeted therapies which selectively reduce Treg cell function and/or Treg cell frequency, especially for cancers harbouring predicted neoantigens and low number of somatic mutations would enhance the therapeutic efficacy of anti-cancer armamentarium.
8. Conclusions
Taken together, growing evidence suggests that Treg cells have a crucial pathogenic role in cancers and are suitable targets for the regression of tumors and they may also be helpful in cancer prognosis. Indeed, the depletion or elimination of Treg cells via targeted therapy results in enhanced anti-cancer effects and therapeutic outcomes with better efficacy and increasing survival rates. Interestingly, the combined inhibition of TIM3 and LAG3 or TIGIT and VISTA has been suggested for improved targeting of Treg cell function and to overcome resistance. However, more animal model studies and clinical trials are warranted for new anti-cancer Treg cell-targeted therapies to be developed and current ones improved. For example, the anti-Treg cell/FOXP3-based vaccine immunotherapy is an efficient approach for depleting the Treg cells in the TME, but such strategies need additional clinical trials with different cancers.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Dr Mitesh Dwivedi was supported by Science and Engineering Research Board, Department of Science and Technology (SERB-DST), New Delhi [CRG/2021/002419].
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgments
We thank the Science and Engineering Research Board, Department of Science and Technology (SERB-DST), New Delhi, India, for providing the research grant to Dr Mitesh Dwivedi (CRG/2021/002419). We are grateful to Uka Tarsadia University, Tarsadi, Gujarat, India, for providing the facilities needed for the preparation of this article.
References
- 1.Dwivedi M., Kumar P., Laddha N.C., Kemp E.H. Induction of regulatory T cells: a role for probiotics and prebiotics to suppress autoimmunity. Autoimmun. Rev. 2016;15:379–392. doi: 10.1016/j.autrev.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Saito T., Nishikawa H., Wada H., et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. 2016;22:679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi Y., Nishikawa H. Roles of regulatory T cells in cancer immunity. Int. Immunol. 2016;28:401–409. doi: 10.1093/intimm/dxw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plitas G., Rudensky A.Y. Regulatory T cells in cancer. Annu. Rev. Cancer Biol. 2020;4:459–477. [Google Scholar]
- 5.Ohue Y., Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 2019;110:2080–2089. doi: 10.1111/cas.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherji B., Wilhelm S.A., Guha A., Ergin M.T. Regulation of cellular immune response against autologous human melanoma. I. Evidence for cell-mediated suppression of in vitro cytotoxic immune response. J. Immunol. 1986;136:1888–1892. [PubMed] [Google Scholar]
- 7.Berd D., Mastrangelo M.J. Active immunotherapy of human melanoma exploiting the immunopotentiating effects of cyclophosphamide. Cancer Invest. 1988;6:337–349. doi: 10.3109/07357908809080657. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S., Sakaguchi N., Asano M., et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 9.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 10.Liyanage U.K., Moore T.T., Joo H.-G., et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 11.Woo E.Y., Chu C.S., Goletz T.J., et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 12.Fattorossi A., Battaglia A., Ferrandina G., et al. Lymphocyte composition of tumor draining lymph nodes from cervical and endometrial cancer patients. Gynecol. Oncol. 2004;92:106–115. doi: 10.1016/j.ygyno.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Curiel T.J., Coukos G., Zou L., et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 14.Wolf D., Wolf A.M., Rumpold H., et al. The expression of the regulatory T cell–specific forkhead Box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin. Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 15.Sasada T., Kimura M., Yoshida Y., et al. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 16.Ichihara F., Kono K., Takahashi A., et al. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003;9:4404–4408. [PubMed] [Google Scholar]
- 17.Ormandy L.A., Hillemann T., Wedemeyer H., et al. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 18.Unitt E., Rushbrook S.M., Marshall A., et al. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41:722–730. doi: 10.1002/hep.20644. [DOI] [PubMed] [Google Scholar]
- 19.Gray C.P., Arosio P., Hersey P. Association of increased levels of heavy-chain ferritin with increased CD4+ CD25+ regulatory T-cell levels in patients with melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003;9:2551–2559. [PubMed] [Google Scholar]
- 20.Viguier M., Lemaître F., Verola O., et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J. Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 21.Beyer M., Kochanek M., Darabi K., et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 22.Karube K., Ohshima K., Tsuchiya T., et al. Expression of FoxP3, a key molecule in CD4CD25 regulatory T cells, in adult T-cell leukaemia/lymphoma cells. Br. J. Haematol. 2004;126:81–84. doi: 10.1111/j.1365-2141.2004.04999.x. [DOI] [PubMed] [Google Scholar]
- 23.Berger C.L., Tigelaar R., Cohen J., et al. Cutaneous T-cell lymphoma: malignant proliferation of T-regulatory cells. Blood. 2005;105:1640–1647. doi: 10.1182/blood-2004-06-2181. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer C., Kim G.G., Albers A., et al. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br. J. Cancer. 2005;92:913–920. doi: 10.1038/sj.bjc.6602407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gjerdrum L.M., Woetmann A., Odum N., et al. FOXP3+ regulatory T cells in cutaneous T-cell lymphomas: association with disease stage and survival. Leukemia. 2007;21:2512–2518. doi: 10.1038/sj.leu.2404913. [DOI] [PubMed] [Google Scholar]
- 26.Sakakura K., Chikamatsu K., Takahashi K., et al. Maturation of circulating dendritic cells and imbalance of T-cell subsets in patients with squamous cell carcinoma of the head and neck. Cancer Immunol. Immunother. 2006;55:151–159. doi: 10.1007/s00262-005-0697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen R.P., Campa M.J., Sperlazza J., et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 28.Jordanova E., Gorter A., Ayachi O., et al. HLA Class I, MICA and CD8+/regulatory T-cell ratio: which parameter determines survival of cervical cancer patients. Clin. Cancer Res. 2008;14:2028–2035. doi: 10.1158/1078-0432.CCR-07-4554. [DOI] [PubMed] [Google Scholar]
- 29.Bates G.J., Fox S.B., Han C., et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 30.Hiraoka N., Onozato K., Kosuge T., Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 31.Fu J., Xu D., Liu Z., et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 32.Woo E.Y., Yeh H., Chu C.S., et al. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J. Immunol. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 33.Marshall N.A., Christie L.E., Munro L.R., et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103:1755–1762. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 34.Kono K., Kawaida H., Takahashi A., et al. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol. Immunother. 2006;55:1064–1071. doi: 10.1007/s00262-005-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mesali H., Ajami A., Hussein-Nattaj H., et al. Regulatory T cells and myeloid-derived suppressor cells in patients with peptic ulcer and gastric cancer. Iran J. Immunol. 2016;13:167–177. [PubMed] [Google Scholar]
- 36.Cheng H.-H., Tseng G.-Y., Yang H.-B., et al. Increased numbers of Foxp3-positive regulatory T cells in gastritis, peptic ulcer and gastric adenocarcinoma. World J. Gastroenterol. 2012;18:34–43. doi: 10.3748/wjg.v18.i1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawaida H., Kono K., Takahashi A., et al. Distribution of CD4 (+) CD25 high regulatory T-cells in tumor-draining lymph nodes in patients with gastric cancer. J. Surg. Res. 2005;125:151–157. doi: 10.1016/j.jss.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Alvaro T., Lejeune M., Salvadó M.T., et al. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005;11:1467–1473. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 39.Gao Q., Qiu S.-J., Fan J., et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi N., Hiraoka N., Yamagami W., et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 41.Hwang H.K., Kim H Il, Kim S.H., et al. Prognostic impact of the tumor-infiltrating regulatory T-cell (Foxp3 +)/activated cytotoxic T lymphocyte (granzyme B +) ratio on resected left-sided pancreatic cancer. Oncol. Lett. 2016;12:4477–4484. doi: 10.3892/ol.2016.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umiel T., Trainin N. Immunological enhancement of tumor growth by syngeneic thymus-derived lymphocytes. Transplantation. 1974;18:244–250. doi: 10.1097/00007890-197409000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Rotter V., Trainin N. Elimination of suppressor T-cells in mice undergoing a graft-versus-host reaction expressed by increased response to polyvinylpyrrolidone. Cell Immunol. 1975;18:199–209. doi: 10.1016/0008-8749(75)90048-9. [DOI] [PubMed] [Google Scholar]
- 44.Bear H.D. Tumor-specific suppressor T-cells which inhibit the in vitro generation of cytolytic T-cells from immune and early tumor-bearing host spleens. Cancer Res. 1986;46:1805. –1812. [PubMed] [Google Scholar]
- 45.North R.J., Bursuker I. Generation and decay of the immune response to a progressive fibrosarcoma. I. Ly-1+2- suppressor T cells down-regulate the generation of Ly-1-2+ effector T cells. J. Exp. Med. 1984;159:1295–1311. doi: 10.1084/jem.159.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Awwad M., North R.J. Cyclophosphamide (Cy)-facilitated adoptive immunotherapy of a Cy-resistant tumour. Evidence that Cy permits the expression of adoptive T-cell mediated immunity by removing suppressor T cells rather than by reducing tumour burden. Immunology. 1988;65:87–92. [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen W., Hutzler M., Abel S., et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J. Exp. Med. 2012;209:2001–2016. doi: 10.1084/jem.20111497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Facciabene A., Peng X., Hagemann I.S., et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 49.Soker S., Takashima S., Miao H.Q., et al. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 50.Price D.J., Miralem T., Jiang S., et al. Role of vascular endothelial growth factor in the stimulation of cellular invasion and signaling of breast cancer cells. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 2001;12:129–135. [PubMed] [Google Scholar]
- 51.Shang B., Liu Y., Jiang S., Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci. Rep. 2015;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plitas G., Konopacki C., Wu K., et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity. 2016;45:1122–1134. doi: 10.1016/j.immuni.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen B.-J., Zhao J.-W., Zhang D.-H., et al. Immunotherapy of cancer by targeting regulatory T cells. Int. Immunopharm. 2022;104:108469. doi: 10.1016/j.intimp.2021.108469. [DOI] [PubMed] [Google Scholar]
- 54.De Simone M., Arrigoni A., Rossetti G., et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity. 2016;45:1135–1147. doi: 10.1016/j.immuni.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng J.H., Nguyen V.H., Jiang S.-N., et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aak9537. [DOI] [PubMed] [Google Scholar]
- 56.Wang H.Y., Lee D.A., Peng G., et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 57.Nishikawa H., Jäger E., Ritter G., et al. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005;106:1008–1011. doi: 10.1182/blood-2005-02-0607. [DOI] [PubMed] [Google Scholar]
- 58.Thomas R., Al-Khadairi G., Roelands J., et al. NY-ESO-1 based immunotherapy of cancer: current perspectives. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jäger E., Nagata Y., Gnjatic S., et al. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishikawa H., Koyama S. Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2021-002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Locasale J.W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H., Franco F., Tsui Y.C., et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat. Immunol. 2020;21:298–308. doi: 10.1038/s41590-019-0589-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim S.A., Wei J., Nguyen T.L.M., et al. Lipid signalling enforces functional specialization of T reg cells in tumours. Nature. 2021;591:306–311. doi: 10.1038/s41586-021-03235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Angelin A., Gil-de-Gómez L., Dahiya S., et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017;25:1282–1293. doi: 10.1016/j.cmet.2016.12.018. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumagai S., Togashi Y., Sakai C., et al. An oncogenic alteration creates a microenvironment that promotes tumor progression by conferring a metabolic advantage to regulatory T cells. Immunity. 2020;53:187–203. doi: 10.1016/j.immuni.2020.06.016. e8. [DOI] [PubMed] [Google Scholar]
- 66.Serrels A., Lund T., Serrels B., et al. Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity. Cell. 2015;163:160–173. doi: 10.1016/j.cell.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghiringhelli F., Puig P.E., Roux S., et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu J., Cui H., Zhu Z., et al. Effect of HIF1α on Foxp3 expression in CD4+ CD25- T lymphocytes. Microbiol. Immunol. 2014;58:409–415. doi: 10.1111/1348-0421.12168. [DOI] [PubMed] [Google Scholar]
- 69.Deng B., Zhu J.-M., Wang Y., et al. Intratumor hypoxia promotes immune tolerance by inducing regulatory T cells via TGF-β1 in gastric cancer. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zorn E., Nelson E.A., Mohseni M., et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruf M., Moch H., Schraml P. PD-L1 expression is regulated by hypoxia inducible factor in clear cell renal cell carcinoma. Int. J. Cancer. 2016;139:396–403. doi: 10.1002/ijc.30077. [DOI] [PubMed] [Google Scholar]
- 72.Ohkura N., Hamaguchi M., Morikawa H., et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 73.Pace L., Tempez A., Arnold-Schrauf C., et al. Regulatory T cells increase the avidity of primary CD8+ T cell responses and promote memory. Science. 2012;338:532–536. doi: 10.1126/science.1227049. [DOI] [PubMed] [Google Scholar]
- 74.Maeda Y., Nishikawa H., Sugiyama D., et al. Detection of self-reactive CD8+ T cells with an anergic phenotype in healthy individuals. Science. 2014;346:1536–1540. doi: 10.1126/science.aaa1292. [DOI] [PubMed] [Google Scholar]
- 75.Hellmann M.D., Ciuleanu T.-E., Pluzanski A., et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rizvi N.A., Hellmann M.D., Snyder A., et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thornton A.M., Shevach E.M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sakaguchi S., Yamaguchi T., Nomura T., Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 79.Nishikawa H., Kato T., Tawara I., et al. Accelerated chemically induced tumor development mediated by CD4+CD25+ regulatory T cells in wild-type hosts. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9253–9257. doi: 10.1073/pnas.0503852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Antony P.A., Restifo N.P. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J. Immunother. 2005;28:120–128. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boyman O., Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 82.Tekguc M., Wing J.B., Osaki M., et al. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2023739118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cederbom L., Hall H., Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur. J. Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 84.Wing K., Onishi Y., Prieto-Martin P., et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 85.Kuehn H.S., Ouyang W., Lo B., et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623–1627. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Munn D.H., Sharma M.D., Mellor A.L. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J. Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 87.Godin-Ethier J., Hanafi L.A., Piccirillo C.A., Lapointe R. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin. Cancer Res. 2011;17:6985–6991. doi: 10.1158/1078-0432.CCR-11-1331. [DOI] [PubMed] [Google Scholar]
- 88.Curti A., Pandolfi S., Valzasina B., et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cells. Blood. 2007;109:2871–2877. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 89.Mezrich J.D., Fechner J.H., Zhang X., et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Uyttenhove C., Pilotte L., Théate I., et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 91.Jarnicki A.G., Lysaght J., Todryk S., Mills K.H.G. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J. Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 92.Collison L.W., Workman C.J., Kuo T.T., et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 93.Steinbrink K., Wölfl M., Jonuleit H., et al. Induction of tolerance by IL-10-treated dendritic cells. J. Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 94.Turnis M.E., Sawant D.V., Szymczak-Workman A.L., et al. Interleukin-35 limits anti-tumor immunity. Immunity. 2016;44:316–329. doi: 10.1016/j.immuni.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lim H.W., Hillsamer P., Kim C.H. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J. Clin. Invest. 2004;114:1640–1649. doi: 10.1172/JCI22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seo S., Fields M.L., Buckler J.L., et al. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16:535–546. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 97.Janssens W., Carlier V., Wu B., et al. CD4+CD25+ T cells lyse antigen-presenting B cells by Fas-Fas ligand interaction in an epitope-specific manner. J. Immunol. 2003;171:4604–4612. doi: 10.4049/jimmunol.171.9.4604. [DOI] [PubMed] [Google Scholar]
- 98.Venet F., Pachot A., Debard A.-L., et al. Human CD4+CD25+ regulatory T lymphocytes inhibit lipopolysaccharide-induced monocyte survival through a Fas/Fas ligand-dependent mechanism. J. Immunol. 2006;177:6540–6547. doi: 10.4049/jimmunol.177.9.6540. [DOI] [PubMed] [Google Scholar]
- 99.Tiemessen M.M., Jagger A.L., Evans H.G., et al. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc. Natl. Acad. Sci. U. S. A. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Larmonier N., Marron M., Zeng Y., et al. Tumor-derived CD4+CD25+ regulatory T cell suppression of dendritic cell function involves TGF-β and IL-10. Cancer Immunol. Immunother. 2007;56:48–59. doi: 10.1007/s00262-006-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu D., Liu H., Komai-Koma M., et al. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J. Immunol. 2003;170:394–399. doi: 10.4049/jimmunol.170.1.394. [DOI] [PubMed] [Google Scholar]
- 102.Basdeo S.A., Cluxton D., Sulaimani J., et al. Ex-Th17 (nonclassical Th1) cells are functionally distinct from classical Th1 and Th17 cells and are not constrained by regulatory T cells. J. Immunol. 2017;198:2249–2259. doi: 10.4049/jimmunol.1600737. [DOI] [PubMed] [Google Scholar]
- 103.Ghiringhelli F., Larmonier N., Schmitt E., et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 104.Deaglio S., Dwyer K.M., Gao W., et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wilson J.M., Ross W.G., Agbai O.N., et al. The A2B adenosine receptor impairs the maturation and immunogenicity of dendritic cells. J. Immunol. 2009;182:4616–4623. doi: 10.4049/jimmunol.0801279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vaupel P., Multhoff G. Adenosine can thwart antitumor immune responses elicited by radiotherapy : therapeutic strategies alleviating protumor ADO activities. Strahlenther. Onkol. 2016;192:279–287. doi: 10.1007/s00066-016-0948-1. [DOI] [PubMed] [Google Scholar]
- 107.Birzele F., Fauti T., Stahl H., et al. Next-generation insights into regulatory T cells: expression profiling and FoxP3 occupancy in Human. Nucl. Acid Res. 2011;39:7946–7960. doi: 10.1093/nar/gkr444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morikawa H., Sakaguchi S. Genetic and epigenetic basis of Treg cell development and function: from a FoxP3-centered view to an epigenome-defined view of natural Treg cells. Immunol. Rev. 2014;259:192–205. doi: 10.1111/imr.12174. [DOI] [PubMed] [Google Scholar]
- 109.Liu C., Workman C.J., Vignali D.A.A. Targeting regulatory T cells in tumors. FEBS J. 2016;283:2731–2748. doi: 10.1111/febs.13656. [DOI] [PubMed] [Google Scholar]
- 110.Yu P., Lee Y., Liu W., et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J. Exp. Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trzonkowski P., Szmit E., Myśliwska J., et al. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin. Immunol. 2004;112:258–267. doi: 10.1016/j.clim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 112.Erdman S.E., Poutahidis T., Tomczak M., et al. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am. J. Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kursar M., Bonhagen K., Fensterle J., et al. Regulatory CD4+CD25+ T cells restrict memory CD8+ T cell responses. J. Exp. Med. 2002;196:1585–1592. doi: 10.1084/jem.20011347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Azuma T., Takahashi T., Kunisato A., et al. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003;63:4516–4520. [PubMed] [Google Scholar]
- 115.Cao X., Cai S.F., Fehniger T.A., et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 116.Shevach E.M. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 117.Saleh R., Elkord E. Treg-mediated acquired resistance to immune checkpoint inhibitors. Cancer Lett. 2019;457:168–179. doi: 10.1016/j.canlet.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 118.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Ann. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nair V.S., Elkord E. Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells. Immunol. Cell Biol. 2018;96:21–33. doi: 10.1111/imcb.1003. [DOI] [PubMed] [Google Scholar]
- 120.Kumagai S., Togashi Y., Kamada T., et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 2020;21:1346–1358. doi: 10.1038/s41590-020-0769-3. [DOI] [PubMed] [Google Scholar]
- 121.Kumagai S., Koyama S., Itahashi K., et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. 2022;40:201–218. doi: 10.1016/j.ccell.2022.01.001. e9. [DOI] [PubMed] [Google Scholar]
- 122.Francisco L.M., Salinas V.H., Brown K.E., et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wong M., La Cava A., Hahn B.H. Blockade of programmed death-1 in young (New Zealand Black x New Zealand White)F1 mice promotes the suppressive capacity of CD4+ regulatory T cells protecting from lupus-like disease. J. Immunol. 2013;190:5402–5410. doi: 10.4049/jimmunol.1202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burugu S., Dancsok A.R., Nielsen T.O. Emerging targets in cancer immunotherapy. Semin. Cancer Biol. 2018;52:39–52. doi: 10.1016/j.semcancer.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 125.Camisaschi C., Casati C., Rini F., et al. LAG-3 expression defines a subset of CD4(+)CD25(high)Foxp3(+) regulatory T cells that are expanded at tumor sites. J. Immunol. 2010;184:6545–6551. doi: 10.4049/jimmunol.0903879. [DOI] [PubMed] [Google Scholar]
- 126.Kouo T., Huang L., Pucsek A.B., et al. Galectin-3 shapes antitumor immune responses by suppressing CD8+ T cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol. Res. 2015;3:412–423. doi: 10.1158/2326-6066.CIR-14-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wei S.H., Chen Y.P., Chen M.J. Selecting probiotics with the abilities of enhancing GLP-1 to mitigate the progression of type 1 diabetes in vitro and in vivo. J. Funct. Foods. 2015;18:473–486. [Google Scholar]
- 128.Deng W.-W., Mao L., Yu G.-T., et al. LAG-3 confers poor prognosis and its blockade reshapes antitumor response in head and neck squamous cell carcinoma. Oncoimmunology. 2016;5:e1239005. doi: 10.1080/2162402X.2016.1239005. e1239005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Okamura T., Fujio K., Sumitomo S., Yamamoto K. Roles of LAG3 and EGR2 in regulatory T cells. Ann. Rheum. Dis. 2012;71(Suppl 2):i96–100. doi: 10.1136/annrheumdis-2011-200588. [DOI] [PubMed] [Google Scholar]
- 130.Yan J., Zhang Y., Zhang J.-P., et al. Tim-3 expression defines regulatory T cells in human tumors. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sakuishi K., Ngiow S.F., Sullivan J.M., et al. TIM3(+)FOXP3(+) regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology. 2013;2 doi: 10.4161/onci.23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oweida A., Hararah M.K., Phan A., et al. Resistance to radiotherapy and PD-L1 blockade is mediated by TIM-3 upregulation and regulatory T-cell infiltration. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018;24:5368–5380. doi: 10.1158/1078-0432.CCR-18-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu X., Harden K., Gonzalez L.C., et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 134.Kurtulus S., Sakuishi K., Ngiow S.-F., et al. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Invest. 2015;125:4053–4062. doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Wang W., Lau R., Yu D., et al. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int. Immunol. 2009;21:1065–1077. doi: 10.1093/intimm/dxp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu L., Deng W.-W., Huang C.-F., et al. Expression of VISTA correlated with immunosuppression and synergized with CD8 to predict survival in human oral squamous cell carcinoma. Cancer Immunol. Immunother. 2017;66:627–636. doi: 10.1007/s00262-017-1968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Korman A.J., Peggs K.S., Allison J.P. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O’Donnell J.S., Long G.V., Scolyer R.A., et al. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat. Rev. 2017;52:71–81. doi: 10.1016/j.ctrv.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 139.Davies M., Duffield E.A. Safety of checkpoint inhibitors for cancer treatment: strategies for patient monitoring and management of immune-mediated adverse events. ImmunoTargets Ther. 2017;6:51–71. doi: 10.2147/ITT.S141577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Selby M.J., Engelhardt J.J., Johnston R.J., et al. Preclinical development of ipilimumab and nivolumab combination immunotherapy: mouse tumor models, in vitro functional studies, and cynomolgus macaque toxicology. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Choueiri T.K., Fishman M.N., Escudier B., et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin. Cancer Res. 2016;22:5461–5471. doi: 10.1158/1078-0432.CCR-15-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thibult M.-L., Mamessier E., Gertner-Dardenne J., et al. PD-1 is a novel regulator of human B-cell activation. Int. Immunol. 2013;25:129–137. doi: 10.1093/intimm/dxs098. [DOI] [PubMed] [Google Scholar]
- 143.Guan H., Wan Y., Lan J., et al. PD-L1 is a critical mediator of regulatory B cells and T cells in invasive breast cancer. Sci. Rep. 2016;6 doi: 10.1038/srep35651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ray A., Das D.S., Song Y., et al. Targeting PD1-PDL1 immune checkpoint in plasmacytoid dendritic cell interactions with T cells, natural killer cells and multiple myeloma cells. Leukemia. 2015;29:1441–1444. doi: 10.1038/leu.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Furness A.J.S., Vargas F.A., Peggs K.S., Quezada S.A. Impact of tumour microenvironment and Fc receptors on the activity of immunomodulatory antibodies. Trends Immunol. 2014;35:290–298. doi: 10.1016/j.it.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 146.Simpson T.R., Li F., Montalvo-Ortiz W., et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Selby M.J., Engelhardt J.J., Quigley M., et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 148.Luke J.J., Zha Y., Matijevich K., Gajewski T.F. Single dose denileukin diftitox does not enhance vaccine-induced T cell responses or effectively deplete Tregs in advanced melanoma: immune monitoring and clinical results of a randomized phase II trial. J. Immunother. Cancer. 2016;4 doi: 10.1186/s40425-016-0140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Solomon I., Amann M., Goubier A., et al. CD25-T reg-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity. Nat. Cancer. 2020;1:1153–1166. doi: 10.1038/s43018-020-00133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Flynn M.J., Zammarchi F., Tyrer P.C., et al. ADCT-301, a pyrrolobenzodiazepine (PBD) dimer-containing antibody-drug conjugate (ADC) targeting CD25-expressing hematological malignancies. Mol. Cancer Ther. 2016;15:2709–2721. doi: 10.1158/1535-7163.MCT-16-0233. [DOI] [PubMed] [Google Scholar]
- 151.Stephens G.L., McHugh R.S., Whitters M.J., et al. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J. Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 152.Ko K., Yamazaki S., Nakamura K., et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J. Exp. Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bulliard Y., Jolicoeur R., Windman M., et al. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J. Exp. Med. 2013;210:1685–1693. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sukumar S., Wilson D.C., Yu Y., et al. Characterization of MK-4166, a clinical agonistic antibody that targets human GITR and inhibits the generation and suppressive effects of T regulatory cells. Cancer Res. 2017;77:4378–4388. doi: 10.1158/0008-5472.CAN-16-1439. [DOI] [PubMed] [Google Scholar]
- 155.Duhen R., Ballesteros-Merino C., Frye A.K., et al. Neoadjuvant anti-OX40 (MEDI6469) therapy in patients with head and neck squamous cell carcinoma activates and expands antigen-specific tumor-infiltrating T cells. Nat. Commun. 2021;12:1047. doi: 10.1038/s41467-021-21383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Arce Vargas F., Furness A.J.S., Solomon I., et al. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity. 2017;46:577–586. doi: 10.1016/j.immuni.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hung A.L., Maxwell R., Theodros D., et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology. 2018;7 doi: 10.1080/2162402X.2018.1466769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang Z., Liu L., Tang H., et al. Immunosuppressive effect of the gut microbiome altered by high-dose tacrolimus in mice. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2018;18:1646–1656. doi: 10.1111/ajt.14661. [DOI] [PubMed] [Google Scholar]
- 159.Le Mercier I., Chen W., Lines J.L., et al. VISTA regulates the development of protective antitumor immunity. Cancer Res. 2014;74:1933–1944. doi: 10.1158/0008-5472.CAN-13-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kondo Y., Ohno T., Nishii N., et al. Differential contribution of three immune checkpoint (VISTA, CTLA-4, PD-1) pathways to antitumor responses against squamous cell carcinoma. Oral Oncol. 2016;57:54–60. doi: 10.1016/j.oraloncology.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 161.Liu J., Yuan Y., Chen W., et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc. Natl. Acad. Sci. U. S. A. 2015;112:6682–6687. doi: 10.1073/pnas.1420370112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gao J., Ward J.F., Pettaway C.A., et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 2017;23:551–555. doi: 10.1038/nm.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sugiyama D., Nishikawa H., Maeda Y., et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl. Acad. Sci. U. S. A. 2013;110:17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Faget J., Biota C., Bachelot T., et al. Early detection of tumor cells by innate immune cells leads to T(reg) recruitment through CCL22 production by tumor cells. Cancer Res. 2011;71:6143–6152. doi: 10.1158/0008-5472.CAN-11-0573. [DOI] [PubMed] [Google Scholar]
- 165.Ni X., Jorgensen J.L., Goswami M., et al. Reduction of regulatory T cells by Mogamulizumab, a defucosylated anti-CC chemokine receptor 4 antibody, in patients with aggressive/refractory mycosis fungoides and Sézary syndrome. Clin. Cancer Res. 2015;21:274–285. doi: 10.1158/1078-0432.CCR-14-0830. [DOI] [PubMed] [Google Scholar]
- 166.Doi T., Muro K., Ishii H., et al. A phase I study of the anti-CC chemokine receptor 4 antibody, mogamulizumab, in combination with nivolumab in patients with advanced or metastatic solid tumors. Clin. Cancer Res. 2019;25:6614–6622. doi: 10.1158/1078-0432.CCR-19-1090. [DOI] [PubMed] [Google Scholar]
- 167.Ishida T., Joh T., Uike N., et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:837–842. doi: 10.1200/JCO.2011.37.3472. [DOI] [PubMed] [Google Scholar]
- 168.Van Damme H., Dombrecht B., Kiss M., et al. Therapeutic depletion of CCR8 + tumor-infiltrating regulatory T cells elicits antitumor immunity and synergizes with anti-PD-1 therapy. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2020-001749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Campbell J.R., McDonald B.R., Mesko P.B., et al. Fc-optimized anti-CCR8 antibody depletes regulatory T cells in human tumor models. Cancer Res. 2021;81:2983–2994. doi: 10.1158/0008-5472.CAN-20-3585. [DOI] [PubMed] [Google Scholar]
- 170.Yamaguchi T., Hirota K., Nagahama K., et al. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007;27:145–159. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 171.Mousavi-Niri N., Naseroleslami M., Hadjati J. Anti-regulatory T cell vaccines in immunotherapy: focusing on FoxP3 as target. Hum. Vacc. Immunother. 2019;15:620–624. doi: 10.1080/21645515.2018.1545625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nair S., Boczkowski D., Fassnacht M., et al. Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res. 2007;67:371–380. doi: 10.1158/0008-5472.CAN-06-2903. [DOI] [PubMed] [Google Scholar]
- 173.Klages K., Mayer C.T., Lahl K., et al. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70:7788–7799. doi: 10.1158/0008-5472.CAN-10-1736. [DOI] [PubMed] [Google Scholar]
- 174.Franco-Molina M.A., Miranda-Hernández D.F., Mendoza-Gamboa E., et al. Silencing of Foxp3 delays the growth of murine melanomas and modifies the tumor immunosuppressive environment. Onco. Targets Ther. 2016;9:243–253. doi: 10.2147/OTT.S90476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mousavi Niri N., Memarnejadian A., Pilehvar-Soltanahmadi Y., et al. Improved anti-treg vaccination targeting Foxp3 efficiently decreases regulatory T cells in mice. J. Immunother. 2016;39:269–275. doi: 10.1097/CJI.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 176.Verma A., Mathur R., Farooque A., et al. T-regulatory cells in tumor progression and therapy. Cancer Manag. Res. 2019;11:10731–10747. doi: 10.2147/CMAR.S228887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Motoyoshi Y., Kaminoda K., Saitoh O., et al. Different mechanisms for anti-tumor effects of low- and high-dose cyclophosphamide. Oncol. Rep. 2006;16:141–146. [PubMed] [Google Scholar]
- 178.Ghiringhelli F., Menard C., Puig P.E., et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.A F, N S, A V Immuno-modulation linked to the depletion of T regulatory cells contributes to the radiosensitization of tumors by the glycolytic inhibitor 2-Deoxy-DGlucose. J. Cancer Res. Ther. 2012;8:502. [Google Scholar]
- 180.Audia S., Nicolas A., Cathelin D., et al. Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clin. Exp. Immunol. 2007;150:523–530. doi: 10.1111/j.1365-2249.2007.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nizar S., Copier J., Meyer B., et al. T-regulatory cell modulation: the future of cancer immunotherapy? Br. J. Cancer. 2009;100:1697–1703. doi: 10.1038/sj.bjc.6605040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Correale P., Cusi M.G., Tsang K.Y., et al. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005;23:8950–8958. doi: 10.1200/JCO.2005.12.147. [DOI] [PubMed] [Google Scholar]
- 183.Obeid M., Tesniere A., Ghiringhelli F., et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 184.Gardai S.J., McPhillips K.A., Frasch S.C., et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 185.Dwarakanath B., Jain V. Targeting glucose metabolism with 2-deoxy-D-glucose for improving cancer therapy. Future Oncol. 2009;5:581–585. doi: 10.2217/fon.09.44. [DOI] [PubMed] [Google Scholar]
- 186.De Rosa V., Galgani M., Porcellini A., et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat. Immunol. 2015;16:1174–1184. doi: 10.1038/ni.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Farooque A., Afrin F., Adhikari J.S., Dwarakanath B.S. Protection of normal cells and tissues during radio- and chemosensitization of tumors by 2-deoxy-D-glucose. J. Cancer Res. Ther. 2009;5(Suppl 1):S32–S35. doi: 10.4103/0973-1482.55138. [DOI] [PubMed] [Google Scholar]
- 188.Dwarakanath B.S. Cytotoxicity, radiosensitization, and chemosensitization of tumor cells by 2-deoxy-D-glucose in vitro. J. Cancer Res. Ther. 2009;5(Suppl 1):S27–31. doi: 10.4103/0973-1482.55137. [DOI] [PubMed] [Google Scholar]
- 189.Gupta S., Farooque A., Adhikari J.S., et al. Enhancement of radiation and chemotherapeutic drug responses by 2-deoxy-D-glucose in animal tumors. J. Cancer Res. Ther. 2009;5(Suppl 1):S16–20. doi: 10.4103/0973-1482.55135. [DOI] [PubMed] [Google Scholar]
- 190.Mohanti B.K., Rath G.K., Anantha N., et al. Improving cancer radiotherapy with 2-deoxy-D-glucose: phase I/II clinical trials on human cerebral gliomas. Int. J. Radiat. Oncol. Biol. Phys. 1996;35:103–111. doi: 10.1016/s0360-3016(96)85017-6. [DOI] [PubMed] [Google Scholar]
- 191.Singh D., Banerji A.K., Dwarakanath B.S., et al. Optimizing cancer radiotherapy with 2-deoxy-d-glucose dose escalation studies in patients with glioblastoma multiforme. Strahlenther. Onkol. 2005;181:507–514. doi: 10.1007/s00066-005-1320-z. [DOI] [PubMed] [Google Scholar]
- 192.Campesato L.F., Budhu S., Tchaicha J., et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat. Commun. 2020;11:1–11. doi: 10.1038/s41467-020-17750-z. 2020 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mignot G., Roux S., Thery C., et al. Prospects for exosomes in immunotherapy of cancer. J. Cell. Mol. Med. 2006;10:376–388. doi: 10.1111/j.1582-4934.2006.tb00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wada J., Onishi H., Suzuki H., et al. Surface-bound TGF-beta1 on effusion-derived exosomes participates in maintenance of number and suppressive function of regulatory T-cells in malignant effusions. Anticancer Res. 2010;30:3747–3757. [PubMed] [Google Scholar]
- 195.Lu Z., Zuo B., Jing R., et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J. Hepatol. 2017;67:739–748. doi: 10.1016/j.jhep.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 196.Hao S., Liu Y., Yuan J., et al. Novel exosome-targeted CD4+ T cell vaccine counteracting CD4+ 25+ regulatory T cell-mediated immune suppression and stimulating efficient central memory CD8+ CTL responses. J. Immunol. 2007;179:2731. doi: 10.4049/jimmunol.179.5.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xie Y., Wang L., Freywald A., et al. A novel T cell-based vaccine capable of stimulating long-term functional CTL memory against B16 melanoma via CD40L signaling. Cell Mol. Immunol. 2013;10:72. doi: 10.1038/cmi.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sakaguchi S., Tanaka S., Tanaka A., et al. Thymus, innate immunity and autoimmune arthritis: interplay of gene and environment. FEBS Lett. 2011;585:3633–3639. doi: 10.1016/j.febslet.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 199.Ali K., Soond D.R., Pineiro R., et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510:407–411. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ahmad S., Abu-Eid R., Shrimali R., et al. Differential PI3Kδ signaling in CD4(+) T-cell subsets enables selective targeting of T regulatory cells to enhance cancer immunotherapy. Cancer Res. 2017;77:1892–1904. doi: 10.1158/0008-5472.CAN-16-1839. [DOI] [PubMed] [Google Scholar]
- 201.Somasundaram R., Jacob L., Swoboda R., et al. Inhibition of cytolytic T lymphocyte proliferation by autologous CD4+/CD25+ regulatory T cells in a colorectal carcinoma patient is mediated by transforming growth factor-beta. Cancer Res. 2002;62:5267–5272. [PubMed] [Google Scholar]
- 202.Wolf A.M., Wolf D., Steurer M., et al. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.