Abstract
Background.
The objective of this work was to evaluate the feasibility of histopathological analysis of tissue extracted on multitined electrodes and assess whether tissue characteristics can be used as biomarkers of oncologic outcomes after lung tumor radiofrequency (RF) ablation.
Methods.
Treatment-related data regarding RF ablation of lung malignancies at our institution was collected using a Health Insurance Portability and Accountability Act-compliant ablation database. Institutional review board waiver was obtained for this study. Immunohistochemical analysis of tissue extracted from the electrodes after lung tumor RF ablation was performed for proliferation (Ki-67) and apoptosis (caspase-3). Patient, tumor demographics, and ablation parameters were recorded. Local tumor progression-free survival (LPFS), disease-specific survival (DSS), and overall survival (OS) were assessed using Kaplan–Meier methodology. Multivariate analysis determined factors affecting these oncological outcomes.
Results.
A total of 47 lung tumors in 42 patients were ablated; 30 specimens were classified as coagulation necrosis (CN) and 17 as Ki-67-positive (+) tumor cells (viable). Tumor sizes were similar in the CN and Ki-67+ groups (P = 0.32). Median LPFS was 10 versus 16 months for Ki-67+ and CN groups, and 1-year LPFS was 34 and 75 %, respectively (P = 0.003). Median OS was 20 and 46 months (P = 0.12), and median DSS was 20 and 68 months (P = 0.01) for the Ki-67 + and CN groups, respectively. Identification of Ki-67+ tumor cells more than tripled the risk of death from cancer [hazard ratio (HR) = 3.65; 95 % confidence interval (95 % CI), 1.34-9.95; P = 0.01] and tripled the risk of local tumor progression (LTP) (HR = 3.01; 95 % CI, 1.39–6.49; P = 0.005).
Conclusions.
Ki-67+ tumor cells on the electrode after pulmonary tumor RF ablation is an independent predictor of LTP, shorter LPFS, and DSS.
Lung cancer is the leading cause of cancer-related death worldwide. Of all cancer patients, 30 % will have lung metastasis, most commonly originating from colorectal cancer (CRC) primaries.1,2 Lobectomy remains the preferred therapy for early-stage primary lung cancer; metastasectomy is used for limited number of small lung metastases.1–4 However, approximately only 20 % patients are surgical candidates at presentation.5 Patients recurring after radiation or pneumonectomy often have limited pulmonary reserve, and ablation may be their only therapeutic option.6 The efficacy of pulmonary tumor radiofrequency (RF) ablation has been demonstrated; however high rates of local tumor progression (LTP) up to 53 % at 5 years remain an important limitation.4,5,7–13 Early identification of patients at risk for LTP may allow for additional treatment and improvement of clinical outcomes.
The feasibility of histopathologic analysis of tissue extracted from RF electrodes after liver tumor ablation is known.14,15 Identification of viable or prolific tumor cells predicted short LTP-free and overall survival after hepatic tumor RF ablation.16–18
Our study used the previously described methodology to evaluate the feasibility of histopathologic analysis of tissue extracted on the electrode and assess whether tissue characteristics can be used as biomarkers of oncologic outcomes after RF ablation of lung malignancies.14,15,17
We hypothesize that when tissue extracted on multitined RF electrodes contains Ki-67+ tumor cells, a marker of cellular proliferation, the probability of incomplete ablation is increased, and a higher rate of LTP, shorter time to LTP, and shorter survivals are expected. To test this hypothesis, we designed a study of histopathological analysis of tissue collected from multitined RF electrodes after lung tumor ablation. Tissue findings were correlated with the radiological and clinical outcomes.
MATERIALS AND METHODS
We identified patients who underwent lung tumor RF ablation from our prospectively maintained, Health Insurance Portability and Accountability Act (HIPAA)-compliant ablation database. Institutional review board waiver was obtained. Pathologic, clinical, and radiological information was collected from the electronic medical records and picture archiving communication systems (PACS).
Inclusion Criteria
Patients with fewer than four lung tumors, each measuring up to 5 cm in largest diameter and with no more than three extrapulmonary lesions deemed stable or responding to chemotherapy by recent computed tomography (CT) scans, were candidates for ablation.
Exclusion Criteria
Patients treated with RF electrodes who had no extracted tissue after ablations were not included in this analysis. Patients with single lung, treated for tumor recurrences after pneumonectomy were also not included in order to prevent confounding the significance of our findings by prior pneumonectomy, a known risk factor of periprocedural morbidity and mortality.6,19
Subjects
Between April 2004 and September 2009, we treated 165 consecutive pulmonary tumors in 141 patients. Of these, 118 tumors were treated with a unipolar, cool-tip device (Valleylab/Covidien, Boulder, CO) with no tissue extracted after ablation and were excluded from this study. All 47 tumors in 42 patients (19 women, 23 men; age range, 19–89 years) that were treated with a multitined RF electrode (RITA XL—AngyoDinamics (n = 30), Latham, NY and Leveen Boston Scientific—Radiotherapeutics (n = 17), Sunnyvale, CA) invariably had tissue extracted on the electrode after ablation and form the study group. Of the 47 lesions, 20 were histopathologically confirmed for their malignant origin; 13 demonstrated primary lung cancer and 7 had various metastatic origins. The rest were diagnosed based on radiologic findings consistent with metastatic disease in the face of previously histologically confirmed malignancy.
PROCEDURE
All tumors were treated using CT-guidance under general anesthesia. RF ablation was performed using either the Radiotherapeutics Leveen or the RITA Starburst XL ablation electrode depending on tumor size, geometry, location, and operator’s preference. The goal was to create an ablation zone larger than the target tumor with a margin of at least 5 mm circumferentially around the tumor.
In all cases, we applied the manufacturer’s recommended protocol.
Starburst XL Protocol
Deployment of the tines always started at 2 cm, and after target temperature was reached (mean, 105 °C) the tines were expanded to 3, 4, or 5 cm, depending on the desired ablation zone. The ablation protocol was modified from the manufacturer guidelines to start at a lower energy to accommodate ablation of lung tissue. The ablation endpoint for the Starburst electrode is temperature dependent. After deployment of the tines, evidence of tissue temperature exceeding 70 °C at the end of cool-down cycle indicated ablation endpoint. On the contrary, lower temperatures at the end of cool-down cycle indicate incomplete ablation. In such instances we retracted the tines, rotated the device 45°, and redeployed the tines for retreatment according to the manufacturer instructions.
Radiotherapeutics Leveen Protocol
The appropriate size needle was chosen (2–5 cm) and tines expanded to cover the tumor. The energy was very slowly increased to 90–130 W or until roll-off (dramatic rise in impedance indicating no further effective heating of tissue possible). This roll-off is used as an endpoint during a single cycle of use. If roll-off occurred within 5 min, ablation was repeated, starting at 70 % of the energy at which roll-off was achieved. Then the energy was increased again to 90–130 W or until subsequent roll-off.
Overlapping ablations (median 2 per lesion, range 1–5) were performed as needed to cover the lesion and create a margin of at least 5 mm around the target tumor.
Only one of the patients had two nodules that were ablated in the same session. The nodules were ablated using two separate ablation electrodes in order to prevent cross-contamination.
Postablation CT was obtained to demonstrate a ground-glass opacity representing the ablation zone, covering the target tumor with sufficient (5-mm) margins.20–23 Enlarging or symptomatic pneumothoraces were managed with thoracostomy.
HISTOPATHOLOGIC AND IMMUNOHISTOCHEMICAL ANALYSIS
We collected all tissue fragments extracted on the RF electrodes immediately after ablation. All tissue fragments were fixed in formalin, dehydrated, embedded in paraffin, cut into 5-μm thick sections, and stained with hematoxylin and eosin (H–E). The specimens were classified by the study pathologists as thermal artifact/coagulation necrosis (CN) only, tumor cells with thermal injury, and intact tumor cells. Specimens that showed only thermal artifact (CN) did not require further evaluation. Specimens containing tumor cells were further analyzed immunohistochemically for apoptosis with caspase-3 and for cell proliferation with Ki-67.24,25
Immunohistochemical analysis of Apoptosis and Proliferation
Activated caspase-3 cleaves cytoskeletal and nuclear proteins, inducing apoptosis.25 Monoclonal antibodies for caspase-3 were used for immunohistochemical evaluation of cell death. Polyclonal antibodies that detect Ki-67 will be positive in the proliferation phase of the cell cycle and indicate cell viability.26 Specimens positive for Ki-67 and negative for caspase-3 were classified as viable tumor (V). Specimens positive for both markers were also classified as V because even if 1 cell remains able to proliferate (Ki-67+) after ablation, there is a higher risk for LTP. Specimens negative for Ki-67 and positive for caspase-3 were classified as CN.
IMAGING FOLLOW-UP
Contrast-enhanced CT was performed within 4–8 weeks of the procedure to evaluate response to treatment. A ground-glass opacity larger than the target tumor and lack of enhancement were considered evidence of complete ablation and technique effectiveness. Irregular peripheral or nodular enhancement within 1 cm of the ablated area was considered untreated or residual tumor, an indication of incomplete ablation.27,28 Imaging follow-up continued at approximately 3-month intervals for at least 1 year. Evidence of increasing tumor size or of irregular/nodular shape of the postablation zone was considered LTP.28,29 F18-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT) was used to assess for LTP in cases with equivocal CT results.29
DEFINITIONS
Technical success: Complete coverage of the target tumor by the ablation zone, indicated as a ground-glass opacity forming a rim of at least 5 mm all around the tumor in a postablation CT.
Technique effectiveness: Imaging findings of ablation zone covering the target tumor without evidence of residual tumor (i.e., nodular lesional or perilesional contrast enhancement on CT or nodular focal FDG uptake on PET) at 4–8 weeks postablation imaging.
LTP-free survival (LPFS): The time between the initial RF ablation and the first radiological evidence of LTP.
Disease-specific survival (DSS): The time between the initial RF ablation and patient’s death as a result of his cancer disease.
Overall survival (OS): The time between the initial RF ablation and the patient’s death from any etiology or most recent follow-up.
Coagulation necrosis (CN): Specimens with thermal artifact only and no identifiable viable tumor cells.
Viable tumor (V): Specimens with tumor cells positive to Ki-67.
Statistical Analysis
Tumor size was expressed as the mean ± standard deviation. The t test was used to detect differences in tumor size between the CN and viable tumor groups. Rates of LPFS, DSS, and OS were calculated with the Kaplan–Meier method. A multivariate analysis by Cox regression was used to help assess the factors related to LTP, and an estimating equations approach was used for variance calculations to take into account the fact that some patients had more than one tumor.30
To further assess the value of histologic and immunohistochemical findings as independent predictors of LTP, regardless of tumor size, we further subdivided the CN and viable tumor groups according to tumor size (≤2 and 2–5 cm), generating four categories: category 0 = CN (≤2 cm), category 1 = viable tumor (≤2 cm), category 2 = CN (>2 cm), and category 3 = viable (>2 cm). Corresponding LPFS for each category were calculated by Kaplan–Meier methodology. Cox regression analysis was performed to evaluate differences between groups. All data were analyzed with the statistical software R, version 2.12.2.31
RESULTS
The study included 42 patients with 47 pulmonary tumors ranging in size between 0.5 and 5 cm (mean = 2.1 cm, SD = 1.13). Patient and tumor demographics are summarized in Table 1. One patient died before obtaining postablation imaging, making assessment of technique effectiveness and LTP impossible. This patient was included within survival calculations.
TABLE 1.
Demographics of 42 patients with 47 malignant pulmonary tumors
| Demographics | CN group | Viable group | Total |
|---|---|---|---|
| Mean age (years) | 65.04 (19–85) | 71.53 (52–89) | 67.67 (19–89) |
| Ratio men to women | 16:9 | 7:10 | 23:19 |
| Mean tumor size ± SD (cm) | 2.2 ± 1.2 | 1.8 ± 0.96 | 2.1 ± 1.1 |
| Tumor type | |||
| NSCLC—adenocarcinoma | 9 | 2 | 11 |
| NSCLC—squamous | 3 | 3 | 6 |
| NSCLC—unknown | 3 | – | 3 |
| Colorectal | 6 | 7 | 13 |
| SCLC | 1 | – | 1 |
| Other | 8 | 5 | 14 |
| No. of patients with 2 sitesa | 3/25 (12 %) | 2/17 (12 %) | 5/42 (12 %) |
NSCLC non-small cell lung cancer, SCLC small cell lung cancer, CN coagulation necrosis
2 patients had tumors in both CN and Viable group: for the purpose of patients’ demographics in this table they were classified as Viable group
Technical success was demonstrated in all cases (100 %). Technique effectiveness was documented in 44 of 46 lesions (96 %) available for first post-treatment imaging 4–8 weeks after ablation.
Tissue Examinations
Tissue extracted on the electrode was obtained in all cases, resulting in 47 specimens sent for histopathologic analysis. Of 47 specimens, 18 (38 %) contained tumor cells in between areas of variable degrees of coagulation necrosis. These were further analyzed by immunohistochemistry. In 17 of 18 specimens, tumor cells were positive for Ki-67 and classified as viable tumor (V). Of the 17 (V) specimens, four were also positive for caspase-3. The 18th specimen contained tumor cells negative for Ki-67 and positive for caspase-3 and was classified as CN. There were 29 specimens that had thermal artifact coagulation necrosis only with no identifiable cells. Thus, 30 specimens were classified as CN and 17 as viable tumor cells (V). Demographics and tumor sizes were similar between the CN and V groups (P = 0.32) (Table 1).
LTP and LTP-Free Survival (LPFS)
During median follow-up period of 35 months (range, 12–74 months), LTP occurred in 9 of 17 tumors (53 %) and 10 of 29 tumors (34 %) with available follow-up in the V and CN groups, respectively (one patient in the CN group died prior to follow-up imaging). The 1-, 2-, and 3-year LPFS rates were 34 and 75 %, 14 and 43 %, and 0 and 31 %, in the V and CN group, respectively (P = 0.003) (Fig. 1). In 14 of 19, progression in the same lung, in the ipsilateral lung, and/or distant extrapulmonary progression was also observed.
FIG. 1.
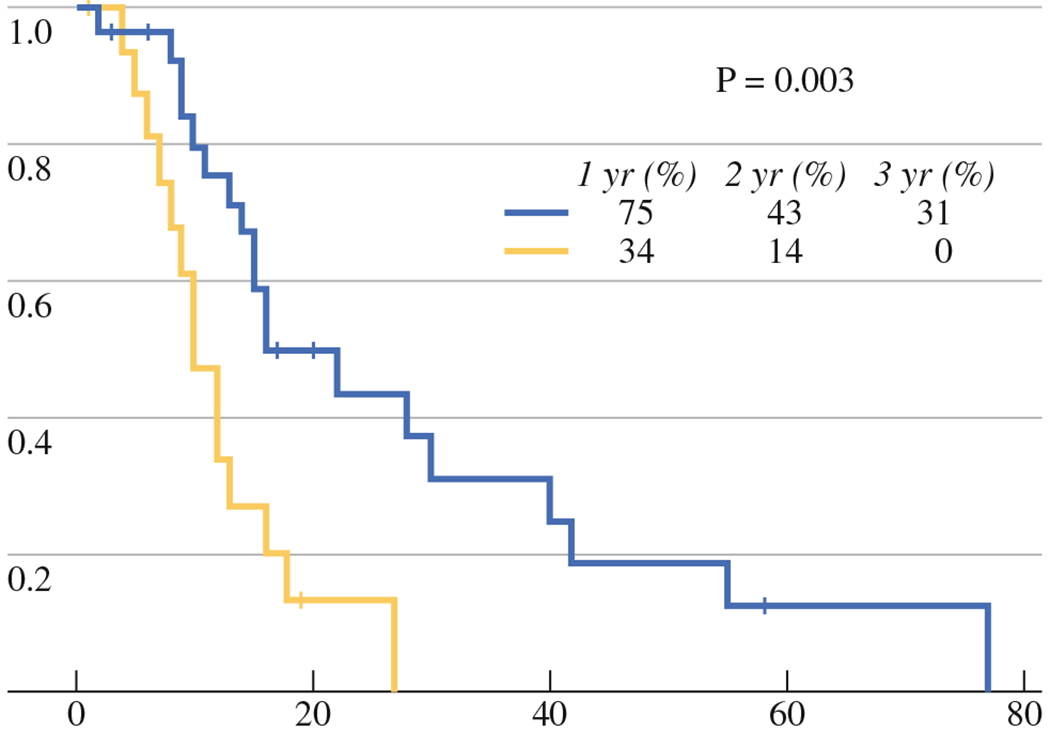
LPFS for viable (V) (yellow line) and CN groups (blue line)
Patient Survival
Of the 42 patients, 26 (62 %) were in the CN group and 16 (38 %) in the V group. Also, 25 patients died: 14 in the CN and 11 in the V group. The median OS was 46 and 20 months, and the 1-, 2-, and 3- year OS rates were 96, 69, and 65 %, and 94, 47, and 34 % for the CN and V groups, respectively (P = 0.12).
In the CN group, five patients died from causes other than their cancer. Resulting median DSS was 68 and 20 months, and the 1-, 2- and 3-year DSS rates were 87, 78, and 73 % and 94, 40, and 33 for the CN and V group (P = 0.007), respectively (Fig. 2).
FIG. 2.
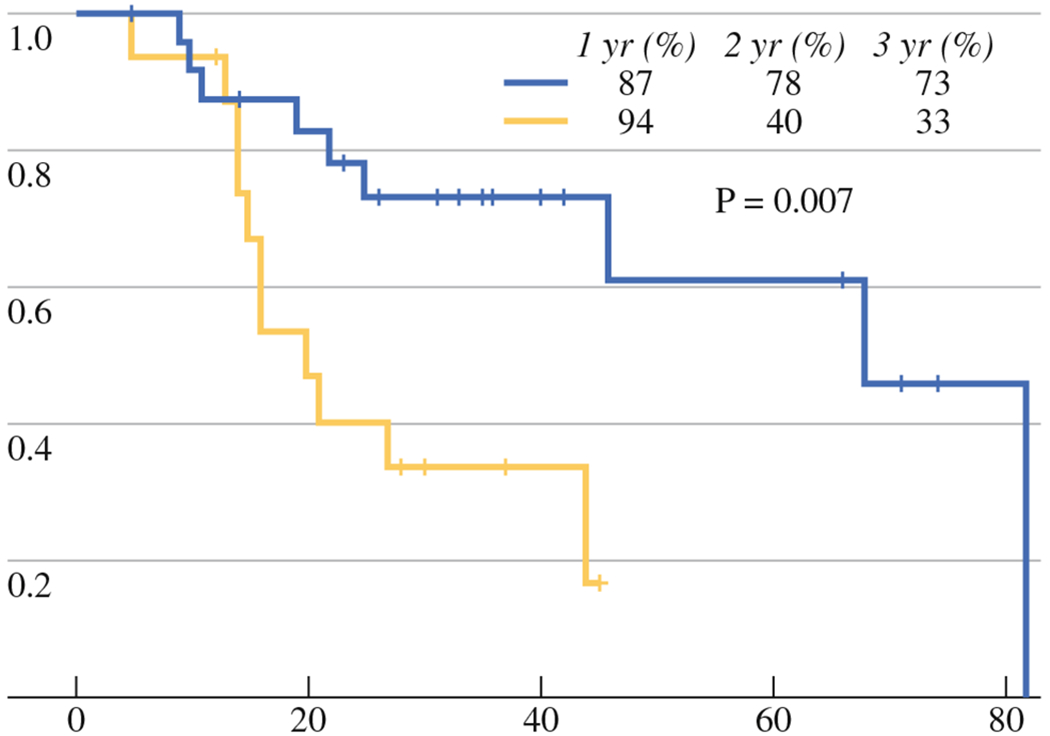
DSS for V (yellow line) and CN (blue line) groups
Multivariate Analysis
Primary tumor pathology (primary lung cancer vs. metastatic lung cancer) was not an independent factor for OS (hazard ratio [HR], 1.18; 95 % CI, 0.53–2.64; P = 0.69), DSS (HR, 0.89; 95 % CI, 0.35–2.17; P = 0.76), and PFS (HR, 1.03; 95 % CI, 0.5–2.11; P = 0.93) at univariate analysis. Therefore, tumor pathology was not included in the multivariate analysis.
Multivariate analysis assessed the effect of tumor size and tissue findings on LPFS. The CN and V groups were stratified by tumor size, into four categories described previously. Comparisons of calculated Kaplan–Meier LPFS between these categories (Fig. 3) show that V is an independent predictor of LTP and LPFS, regardless of tumor size. Specifically, identification of Ki-67+ tumor cells (V) extracted on RF electrodes after ablation correlates with shorter LPFS, regardless of tumor size (Fig. 3). For large tumors (2–5 cm), median LPFS was 9 versus 22 months for the V and CN group, respectively (P = 0.04). For tumors ≤2 cm, median LPFS was 12 versus 22 months in V and CN groups, respectively (P = 0.003). This indicates that the identification of Ki-67+ tumor cells is an independent strong predictor of LTP (HR, 3.01; 95 % CI, 1.39–6.49; P = 0.005) (Table 2) and short LPFS. In addition, the presence of Ki-67+ tumor cells significantly increased the risk of death from cancer (HR, 3.64; 95 % CI, 1.34–6.49; P = 0.011) (Table 2). For patients with tumors ≤2 cm, Ki-67+ tumor cells increased the risk of LTP (HR, 2.83; 95 % CI, 1.04–7.69; P = 0.041). Similarly, for patients with tumors >2 cm, presence of Ki67+ tumor cells increased the risk of LTP (HR, 3.45; 95 % CI, 0.99–12.2; P = 0.054) (Table 2).
FIG. 3.
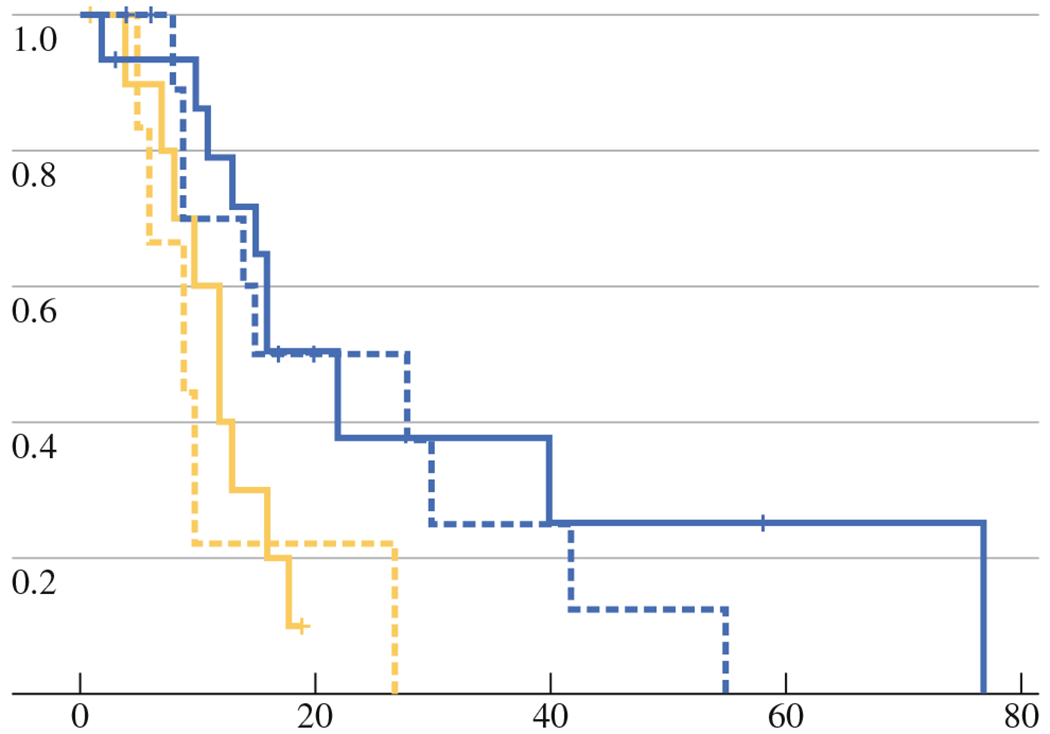
LPFS for V (yellow line) and CN groups (blue line) stratified by size (≤2 cm solid; >2 cm dotted)
TABLE 2.
Multivariate analysis of survival times using size and viability for independent variables
| OS (months) |
DSS (months) |
LPFS (months) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viable (median) | CN (median) | HR | 95 % CI | P value | Viable (median) | CN (median) | HR | 95 % CI | P value | Viable (median) | CN (median) | HR | 95 % CI | P value | |
| All patients | 20 | 46 | 1.94 | 0.83–4.5 | 0.125 | 20 | 68 | 3.64 | 1.34–6.49 | 0.011 | 10 | 16 | 3.01 | 1.39–6.49 | 0.005 |
| Size | |||||||||||||||
| ≤2 cm | 21 | 46 | 2.56 | 0.72–9.15 | 0.148 | 21 | 82 | 3.44 | 0.85–13.9 | 0.083 | 12 | 22 | 2.83 | 1.04–7.69 | 0.041 |
| > 2 cm | 18 | 25 | 1.51 | 0.48–4.81 | 0.481 | 18 | 68 | 3.78 | 0.89–15.9 | 0.071 | 9 | 22 | 3.45 | 0.99–12.2 | 0.054 |
DISCUSSION
Ki-67, a cell-proliferation marker, has prognostic value in malignancies.26 Ki-67 is a nuclear component expressed in viable and proliferating cells during all but the G0 phase of cell cycle, and it can be evaluated immunohistochemically.32,33
This study demonstrates a strong correlation between the presence of Ki-67+ tumor (viable) cells (V) on ablated tissue and higher incidence of LTP with a shorter LPFS (P = 0.003). Multivariate analysis demonstrated that V on tissue extracted on the electrode is an independent biomarker of short LPFS after pulmonary tumor RF ablation. The presence of V on the electrode increased the chance of LTP in both the large (P = 0.04) and small (P = 0.03) tumor size groups. In fact, the presence of Ki-67+ tumor cells triples the risk for LTP for tumors under 2 cm (HR, 2.83; 95 % CI, 1.04–7.69, P = 0.041) and more than triples the risk for larger tumors (HR, 3.45; 95 % CI, 0.99–12.2; P = 0.054). This association was significant and is consistent with prior reports evaluating the same marker after liver tumor ablation.17 The difference between the median OS of V and CN groups (20 vs. 46 months) groups was not significant (P = 0.12); however, disease-specific survival (DSS) was significantly shorter in the V group (20 vs. 68 months, P = 0.01).
This study adds to the existing body of work showing that histopathologic examination of tissue after ablation can be used as a prognostic indicator of oncologic outcomes. Prior studies have reported on tissue changes after pulmonary ablation; however, to our knowledge this is the first study assessing tissue extracted on the RF electrode and its use as a biomarker of oncologic outcomes after pulmonary tumor ablation.34–36
Prior publications showed that Ki-67 within neoplastic tissue is indicative of tumor ability to proliferate.37 The expression of Ki-67 stops when treatment halts DNA synthesis by cell damage, but reappears when the insult is removed and the cell starts to proliferate.37 Similarly, the presence of Ki-67+ tumor cells strongly correlated with shorter LPFS and DSS in this cohort. This strong correlation suggests that tissue sampling after ablation could identify patients at risk for LTP.
Unlike surgery where the resected tumor is evaluated by pathology to confirm complete resection with clear margins, no specimen is routinely evaluated after image-guided ablation. This lack of pathological confirmation as a clinical endpoint during treatment may explain the relatively high rates of LTP after image-guided ablations. Our aim was to use histopathological and immunohistochemical analysis on tissue extracted on the RF electrode and to evaluate whether the tissue findings can be used as biomarkers of oncologic outcomes after pulmonary tumor RF ablation. There were several limitations to our study. The most apparent and statistically relevant is the small number of patients (n = 42), limiting conclusions about OS. It is conceivable that the enrollment of more patients and longer follow-up could make OS differences significant. A disadvantage of the examination of tissue adherent to the electrode is the limited sampling of the tumor. LTP was observed in significant number of tumors in the CN group. One explanation for the latter is the fact that our examination did not sample the entire area of the treated tumor, but only tissue extracted on the RF electrode. It is likely that in the CN group some tumors still contained viable tumor cells in areas not represented by the tissue adherent on the RF electrode. Another limitation is the fact that histochemical analysis cannot be performed during or immediately after ablation; thus it cannot be applied to modify treatment that could improve clinical outcomes. Finally, tissue is only extracted on older types of multitined RF electrodes. Such tissue is typically not present in unipolar cool-tip RF electrodes or other ablation needles such as microwave, laser, cryoablation, or irreversible electroporation. However, our preliminary findings support the design of new studies where tissue sampling after ablation may be used to detect residual viable tumor cells in the ablation zone. Such studies, as well as the development of specific stains that can evaluate tumor apoptosis and viability, are very much needed for the immediate assessment of tumor ablation effectiveness, in real time. The development, application, and reproducibility of tissue examinations as a tool for the immediate assessment of tumor ablation effectiveness will establish the value of tissue sampling that will then become standard clinical practice in the field of ablation.
High rates of LTP remain 1 of the limitations of the ablation technique. In our group, in 14 of 19 cases (75 %) that progressed locally, there was also progression in the same lung, progression in the ipsilateral lung, and/or distant extrapulmonary progression. This may indicate aggressive tumor biology. Additionally, most of the ablations were performed with intention to create a 5-mm ablation margin. That might have contributed to the high local recurrence rate. In view of recent knowledge, we currently strive and recommend the creation of margin larger than 5 mm, ideally 10 mm all around the target tumor, whenever safe and feasible.
In conclusion, histopathologic and immunohistochemical analysis of tissue extracted on the RF electrode is feasible after pulmonary tumor ablation. The identification of Ki-67+ tumor cells on the RF electrode is an independent predictor of oncologic outcomes, specifically LPFS and DSS. This strong correlation is promising and may lead to the use of prognostic biomarkers of oncologic outcomes after or during pulmonary tumor ablation. The development of such biomarkers may allow for additional treatment in the high-risk group, improving clinical outcomes after lung tumor ablation or locoregional therapies in general.
REFERENCES
- 1.Lencioni R, Crocetti L, Cioni R, Suh R, Glenn D, Regge D, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol. 2008;9:621–8. [DOI] [PubMed] [Google Scholar]
- 2.Pua BB, Solomon SB. Radiofrequency ablation of primary and metastatic lung cancers. Semin Ultrasound CT MR. 2009;30:113–24. [DOI] [PubMed] [Google Scholar]
- 3.Crocetti L, Lencioni R. Radiofrequency ablation of pulmonary tumors. Eur J Radiol. 2010;75:23–7. [DOI] [PubMed] [Google Scholar]
- 4.Thanos L, Mylona S, Ptohis N, Tsiouris S, Sotiropoulou E, Pomoni A, et al. Percutaneous radiofrequency thermal ablation in the management of lung tumors: presentation of clinical experience on a series of 35 patients. Diagn Interv Radiol. 2009;15: 290–6. [DOI] [PubMed] [Google Scholar]
- 5.Simon CJ, Dupuy DE, DiPetrillo TA, Safran HP, Grieco CA, Ng T, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–75. [DOI] [PubMed] [Google Scholar]
- 6.Hess A, Palussiere J, Goyers JF, Guth A, Auperin A, de Baere T. Pulmonary radiofrequency ablation in patients with a single lung: feasibility, efficacy, and tolerance. Radiology. 2011;258:635–42. [DOI] [PubMed] [Google Scholar]
- 7.Dupuy DE, Zagoria RJ, Akerley W, Mayo-Smith WW, Kavanagh PV, Safran H. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174:57–9. [DOI] [PubMed] [Google Scholar]
- 8.Yasui K, Kanazawa S, Sano Y, Fujiwara T, Kagawa S, Mimura H, et al. Thoracic tumors treated with CT-guided radiofrequency ablation: initial experience. Radiology. 2004;231:850–7. [DOI] [PubMed] [Google Scholar]
- 9.Akeboshi M, Yamakado K, Nakatsuka A, Hataji O, Taguchi O, Takao M, et al. Percutaneous radiofrequency ablation of lung neoplasms: initial therapeutic response. J Vasc Interv Radiol. 2004;15:463–70. [DOI] [PubMed] [Google Scholar]
- 10.Sonntag PD, Hinshaw JL, Lubner MG, Brace CL, Lee FT Jr. Thermal ablation of lung tumors. Surg Oncol Clin N Am. 2011;20:369–87, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamagami T, Kato T, Hirota T, Yoshimatsu R, Matsumoto T, Shimada J, et al. Risk factors for occurrence of local tumor progression after percutaneous radiofrequency ablation for lung neoplasms. Diagn Interv Radiol. 2007;13:199–203. [PubMed] [Google Scholar]
- 12.de Baere T, Palussiere J, Auperin A, Hakime A, Abdel-Rehim M, Kind M, et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology. 2006;240:587–96. [DOI] [PubMed] [Google Scholar]
- 13.Hiraki T, Sakurai J, Tsuda T, Gobara H, Sano Y, Mukai T, et al. Risk factors for local progression after percutaneous radiofrequency ablation of lung tumors: evaluation based on a preliminary review of 342 tumors. Cancer. 2006;107:2873–80. [DOI] [PubMed] [Google Scholar]
- 14.Sofocleous CT, Klein KM, Hubbi B, Brown KT, Weiss SH, Kannarkat G, et al. Histopathologic evaluation of tissue extracted on the radiofrequency probe after ablation of liver tumors: preliminary findings. AJR Am J Roentgenol. 2004;183:209–13. [DOI] [PubMed] [Google Scholar]
- 15.Snoeren N, Jansen MC, Rijken AM, van Hillegersberg R, Slooter G, Klaase J, et al. Assessment of viable tumour tissue attached to needle applicators after local ablation of liver tumours. Dig Surg. 2009;26:56–62. [DOI] [PubMed] [Google Scholar]
- 16.Snoeren N, Huiskens J, Rijken AM, van Hillegersberg R, van Erkel AR, Slooter GD, et al. Viable tumor tissue adherent to needle applicators after local ablation: a risk factor for local tumor progression. Ann Surg Oncol. 2011;18:3702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sofocleous CT, Nascimento RG, Petrovic LM, Klimstra DS, Gonen M, Brown KT, et al. Histopathologic and immunohistochemical features of tissue adherent to multitined electrodes after RF ablation of liver malignancies can help predict local tumor progression: initial results. Radiology. 2008;249:364–74. [DOI] [PubMed] [Google Scholar]
- 18.Sofocleous CT, Garg S, Petrovic LM, Gonen M, Petre EN, Klimstra DS, et al. Ki-67 is a prognostic biomarker of survival after radiofrequency ablation of liver malignancies. Ann Surg Oncol. 2012;19:4262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sofocleous CT, May B, Petre EN, Gonen M, Thornton RH, Alago W, et al. Pulmonary thermal ablation in patients with prior pneumonectomy. AJR Am J Roentgenol. 2011;196:W606–12. [DOI] [PubMed] [Google Scholar]
- 20.Sofocleous CT, Sideras P, Petre EN, Solomon SB. Ablation for the management of pulmonary malignancies. AJR Am J Roentgenol. 2011;197:W581–9. [DOI] [PubMed] [Google Scholar]
- 21.Jin GY, Lee JM, Lee YC, Han YM, Lim YS. Primary and secondary lung malignancies treated with percutaneous radiofrequency ablation: evaluation with follow-up helical CT. AJR Am J Roentgenol. 2004;183:1013–20. [DOI] [PubMed] [Google Scholar]
- 22.Gillams A Lung tumour ablation—where are we now? Cancer Imaging. 2008;8:116–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose SC, Dupuy DE, Gervais DA, Millward SF, Brown DB, Cardella JF, et al. Research reporting standards for percutaneous thermal ablation of lung neoplasms. J Vasc Interv Radiol. 2009; 20:S474–85. [DOI] [PubMed] [Google Scholar]
- 24.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–90. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Ramnath N, Moysich KB, Asch HL, Swede H, Alrawi SJ, et al. Prognostic significance of MCM2, Ki-67 and gelsolin in non-small cell lung cancer. BMC Cancer. 2006;6:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King KL, Hwang JJ, Chau GY, Tsay SH, Chi CW, Lee TG, et al. Ki-67 expression as a prognostic marker in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 1998;13:273–9. [DOI] [PubMed] [Google Scholar]
- 27.Smith S, Gillams A. Imaging appearances following thermal ablation. Clin Radiol. 2008;63:1–11. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S377–90. [DOI] [PubMed] [Google Scholar]
- 29.Kanzaki R, Higashiyama M, Maeda J, Okami J, Hosoki T, Hasegawa Y, et al. Clinical value of F18-fluorodeoxyglucose positron emission tomography-computed tomography in patients with non-small cell lung cancer after potentially curative surgery: experience with 241 patients. Interact Cardiovasc Thorac Surg. 2010;10:1009–14. [DOI] [PubMed] [Google Scholar]
- 30.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84:1065–73. [Google Scholar]
- 31.R: A language and environment for statistical computing. R Development Core Team; R Foundation for Statistical Computing. Vienna; 2011. [Google Scholar]
- 32.Vilar E, Salazar R, Perez-Garcia J, Cortes J, Oberg K, Tabernero J. Chemotherapy and role of the proliferation marker Ki-67 in digestive neuroendocrine tumors. Endocr Relat Cancer. 2007;14:221–32. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Cebrian JM, Nevado Santos M, Vorwald Kuborn P, Pardo de Lama M, Martín-Cavanna J, Pacheco Martínez P, et al. Can the clinical outcome in stage II colon carcinomas be predicted by determination of molecular marker expression? Clin Transl Oncol. 2007;9:663–70. [DOI] [PubMed] [Google Scholar]
- 34.Clasen S, Krober SM, Kosan B, Aebert H, Fend F, Bomches A, et al. Pathomorphologic evaluation of pulmonary radiofrequency ablation: proof of cell death is characterized by DNA fragmentation and apoptotic bodies. Cancer. 2008;113:3121–9. [DOI] [PubMed] [Google Scholar]
- 35.Jaskolka JD, Kachura JR, Hwang DM, Tsao MS, Waddell TK, Asch MR, et al. Pathologic assessment of radiofrequency ablation of pulmonary metastases. J Vasc Interv Radiol. 2010;21:1689–96. [DOI] [PubMed] [Google Scholar]
- 36.Schneider T, Reuss D, Warth A, Schnabel PA, von Deimling A, Herth FJ, et al. The efficacy of bipolar and multipolar radiofrequency ablation of lung neoplasms—results of an ablate and resect study. Eur J Cardiothorac Surg. 2011;39:968–73. [DOI] [PubMed] [Google Scholar]
- 37.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–22. [DOI] [PubMed] [Google Scholar]


