Abstract
We have shown that membrane-associated guanylate kinase with inverted domain structure-1 (MAGI1), a scaffold protein with six PSD95/DiscLarge/ZO-1 (PDZ) domains, is involved in the regulation of endothelial cell (EC) activation and atherogenesis in mice. In addition to causing acute respiratory disease, influenza A virus (IAV) infection plays an important role in atherogenesis and triggers acute coronary syndromes and fatal myocardial infarction. Therefore, the aim of this study is to investigate the function and regulation of MAGI1 in IAV-induced EC activation. Whereas, EC infection by IAV increases MAGI1 expression, MAGI1 depletion suppresses IAV infection, suggesting that the induction of MAGI1 may promote IAV infection. Treatment of ECs with oxidized low-density lipoprotein (OxLDL) increases MAGI1 expression and IAV infection, suggesting that MAGI1 is part of the mechanistic link between serum lipid levels and patient prognosis following IAV infection. Our microarray studies suggest that MAGI1-depleted ECs increase protein expression and signaling networks involve in interferon (IFN) production. Specifically, infection of MAGI1-null ECs with IAV upregulates expression of signal transducer and activator of transcription 1 (STAT1), interferon b1 (IFNb1), myxovirus resistance protein 1 (MX1) and 2′-5′-oligoadenylate synthetase 2 (OAS2), and activate STAT5. By contrast, MAGI1 overexpression inhibits Ifnb1 mRNA and MX1 expression, again supporting the pro-viral response mediated by MAGI1. MAGI1 depletion induces the expression of MX1 and virus suppression. The data suggests that IAV suppression by MAGI1 depletion may, in part, be due to MX1 induction. Lastly, interferon regulatory factor 3 (IRF3) translocates to the nucleus in the absence of IRF3 phosphorylation, and IRF3 SUMOylation is abolished in MAGI1-depleted ECs. The data suggests that MAGI1 inhibits IRF3 activation by maintaining IRF3 SUMOylation. In summary, IAV infection occurs in ECs in a MAGI1 expression-dependent manner by inhibiting anti-viral responses including STATs and IRF3 activation and subsequent MX1 induction, and MAGI1 plays a role in EC activation, and in upregulating a pro-viral response. Therefore, the inhibition of MAGI1 is a potential therapeutic target for IAV-induced cardiovascular disease.
Keywords: IAV, MAGI1, MX1, interferon signaling, IRF3, EC inflammation
Highlights
- IAV infection increases MAGI1 expression.
- MAGI1 depletion upregulates anti-viral response including STATs and IRF3 activation and inhibits EC infection by IAV.
- OxLDL induces MAGI1 expression and amplifies IAV infection in ECs.
- Anti-viral effects of MAGI1 depletion is at least partially depends on MX1 induction.
- De-SUMOylation and the consequent activation of IRF3 are induced by MAGI1 depletion.
- MAGI1 plays a crucial role in both accelerating EC activation and IAV infection.
Introduction
Whereas, three types of influenza viruses (A, B, and C) (1), negative-strand RNA viruses belonging to the Orthomyxoviridae family, infect humans, seasonal flu epidemics are usually caused by influenza A virus (IAV) (2, 3), which targets epithelial cells in the human respiratory tract. The gap between the airspace and the capillary ECs is ~0.5 μm, and alveolar wall cells and capillary ECs are separated only by the basal lamina and so that the endothelium is likely to be also exposed to free virus particles during IAV infection (4). According to some studies, IAV infects ECs in vivo, in association with development of flu symptom (5–8). However, other studies have suggested that ECs indirectly contribute to the pathogenesis of virus infection through control of the local inflammatory milieu in the lungs (9, 10). Indeed, some studies implicate EC function in IAV pathogenesis; i.e., in addition to causing acute respiratory disease IAV may trigger acute coronary syndromes and fatal myocardial infarction (11–15). Also, IAV infection of the vascular wall and/or increased levels of circulating pro-inflammatory cytokines may contribute to atherosclerotic lesion progression (16, 17). However, the molecular pathway by which IAV promotes atherogenesis has not been identified so that these processes may simply share common risk factors (10, 17).
The severity of IAV infection depends on the host inflammatory response. During inflammation, EC activation is a key step. Our previous studies showed that MAGI1 depletion prevents EC activation induced by pro-atherogenic type of flow (18–20). MAGI1, a scaffold protein with six PDZ domains, one guanylate kinase domain, and two WW (rsp5) domains flanked by the first and second PDZ domains, localizes to the tight and adherens junctions (20–22). A genome-wide association study revealed a strong association between MAGI1 locus and various chronic inflammatory diseases including Crohn's disease, in which MAGI1-dependent maintenance of the tight seal of the gastrointestinal tract is compromised (23). Indeed, we also showed that MAGI1 plays a critical role in regulating EC permeability (19).
Although the role of MAGI1 in EC activation has been reported (18–20), its role in IAV-induced EC activation is unknown. The aim of the study was to test the hypothesis that MAGI1 drives EC activation after IAV infection, and that this process promotes IAV-mediated cardiovascular disease.
Experimental procedures
Mice
All mice were fed a normal diet and housed in a room with an ambient temperature of 22°C and a 12-h light/12-h dark cycle in a pathogen-free environment at the Texas A&M Institute of Biosciences and Technology. MAGI1−/− (homozygous knocked out) mice were generated and characterized as we have previously described (20). C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). First, MAGI1−/− mice were crossed with C57BL/6 mice to generate MAGI1−/+ (heterozygous) mice, which were then used to generate MAGI1−/− and the wild type (WT) littermate control mice. All procedures on mice were approved by the Institutional Care and Use Committees of the Texas A&M Institute of Biosciences and Technology (2014-0231, 2017-0154) and The University of Texas MD Anderson Cancer Center (00001652, 00001109).
Cells
HUVECs were isolated through a collagenase digestion of the endothelium of human umbilical cord veins and were grown on culture dishes coated with 0.2% gelatin type A (MP Biomedicals, Solon, OH, USA) in Endothelial Cell Medium (ECM, Catalog #1001, Science Cell, San Diego, CA, USA). HUVEC isolation was approved by the Houston Methodist Research Institute (HMRI) Institutional Review Board (IRB, permit No. Pro00020559). Mouse lung ECs were isolated as we have performed and described previously (17, 22, 23). Mouse lung EC isolation was approved by the HMRI Institutional Animal Care and Use Committee (IACUC, permit No. IS00006725). Briefly, lungs were harvested from 6 to 8 weeks old mice, washed thoroughly in cold PBS, minced finely with scissors, then digested by collagenase. Sheep anti-rat PECAM-1-conjugated Dynabeads (Catalog #11035, Invitrogen, Carlsbad, CA, USA) were prepared and used to isolate mouse lung ECs. Mouse lung ECs were cultured in DMEM (Catalog #SH30243.0, Hyclone, Logan, UT, USA) supplemented with 20% FBS (Catalog #F2442, Sigma-Aldrich, Saint Louis, MO), 1% EC growth supplement (Promo Cell, Heidelberg, Germany), 25 mM HEPES buffer (Catalog #25-060-CI, Corning, Manassas, VA, USA), 1% non-essential amino acid solution (Catalog #25-025-CI, Corning, Manassas, VA, USA), 100 mg/ml heparin (Catalog #67457037399, Mylan Institutional, Rockford, IL, USA) and 1% penicillin/streptomycin solution (Catalog #30-002-CI, Corning, Manassas, VA, USA). HULECs (ATCC, CRL-3244, Manassas, VA, USA) were cultured in MCDB 131 medium (Catalog #10372019, Thermo Fisher Scientific (Waltham, MA, USA) supplemented with Microvascular Endothelial Cell Growth Kit-BBE (Catalog #PCS-110-040, ATCC, Manassas, VA, USA) containing 0.2% bovine brain extract, 5 ng/mL rh EGF, 10 mM L-glutamine, 0.75 Unit/mL heparin sulfate, 1 μg/mL hydrocortisone, 50 μg/mL ascorbic acid and 5% FBS.
Influenza A viral infection
For in vitro influenza A viral infection, as previously described (24), frozen stocks of mouse-adapted influenza A/Hong Kong/8/68 virus (H3N2) were diluted 1:1000 in 1x PBS and viral inocula (multiplicities of infection [MOI] of 0.1) were added to EC monolayer. For heat inactivation: influenza virus stocks were incubated for 30 min at 22.0, 35.0, 38.3, 43.7, 49.6, 55.6, 61.3, 66.7, and 70°C in a T-Gradient thermal cycler (Biometra, Göttingen, Germany) and subsequently stored at −80°C until in vitro infection (24, 25).
Gene expression profiling
Gene expression profiling was performed as we have previously described (20). Briefly, HUVECs were transfected with either control siRNA (siCont) or MAGI1 siRNA (siMAGI1), and total RNA was isolated approximately 48 h post-transfection using RNeasy Plus Micro Kit (Cat# 74034, QIAGEN, Germantown, MD) with DNA digestion. Total RNA was then hybridized using a GeneChip Human Transcriptome Array 2.0 (Affymetrix, Santa Clara, CA). The array data was analyzed using the Transcriptome Analysis Console 3.0 (Affymetrix) followed by data normalization using Tukey's biweight average algorithm. Significance was determined using unpaired ANOVA (P < 0.05) as we have previously described (20). The differential expression data were analyzed using Ingenuity Pathway Analysis (IPA) (application build 377306M [2016-03-16] and content version 27216297 [2016-03-16]; QIAGEN). All molecular interactions and relationships were based on curated findings in the literature stored in the Ingenuity Knowledge Base (QIAGEN).
siRNA-mediated MAGI1 depletion and transient MAGI1 overexpression
The siRNA sequence corresponding to nucleotides 843-857 of human MAGI1 coding sequence (5'-GGACCCUUCUCAGAAGUUCCCUCAA) (20) that specifically targets human MAGI1 for degradation was obtained from Sigma-Aldrich (Burlington, MA, USA). Authenticated siRNA sequence targeting human MX1 was obtained from Santa Cruz Biotechnology (Santa Cruz, Dallas, TX, USA). Non-target siRNA sequence (control) was obtained from Thermo Fisher Scientific (Waltham, MA, USA). siRNA-mediated MAGI1 depletion and transient MAGI1 overexpression were performed as we have previously described (20).
RNA extracts and qRT-PCR
Studies were performed 48 h after siRNA transfection, as indicated in each corresponding figure. Total RNA was then extracted using the PureLink RNA mini-Kit (Thermo Fisher Scientific; Waltham, MA, USA). cDNAs were synthesized using the iScript cDNA synthesis Kit (Bio-Rad, Hercules, CA 94547, USA). Mixtures for qRT-PCR reactions (10 μL) contained cDNA synthesized from 20 ng of total RNA, 5 μ L of iQ SYBR Green Supermix (Bio-Rad, Hercules, CA 94547, USA), and 0.5 μM each forward and reverse primer. qRT-PCR reactions were carried out at 95°C for 3 min followed by 40 cycles of denaturation at 95°C for 10 s, 60°C for 15 s, and 72°C for 30 s. qRT-PCR data acquisition was carried out using the CFX Connect Real-Time PCR Detection System (Bio-Rad, Hercules, CA 94547, USA). The comparative Ct (2−ΔΔCt) method was used to relatively quantified changes in mRNA expression of samples, in which cycle threshold (Ct) values of target genes were normalized to that of the reference genes (26). All qRT-PCR primers were obtained from Sigma-Aldrich. The sequences of qRT-PCR primers were listed in the Supplementary Table 1.
Protein extracts and immunoblotting
The cells were lysed on ice in RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 0.25% sodium deoxycholate) containing protease and phosphatase inhibitors (Sigma-Roche, Mannheim, Germany) (20). Cell lysates were briefly sonicated and then centrifuged at 16,000 xg for 10 min at 4°C to remove any debris. Cell extracts were mixed with SDS gel loading buffer and loaded on SDS-PAGE gels and separated proteins were transferred to nitrocellulose membranes. The membranes were probed with antibodies that specifically recognize proteins of interest. Some samples were analyzed by using the automated capillary electrophoresis Western analysis (Wes) system (ProteinSimple, San Jose, CA, USA) as we have performed previously (27).
Antibodies and reagents
The following antibodies were purchased: MAGI1 (Catalog #M5691, Sigma-Aldrich, St. Louis, MO, USA), STAT1 (Catalog #ab103813, Abcam, Cambridge, MA, USA), MX1 (Catalog #sc-271024, Santa Cruz Biotechnology, Dallas, TX, USA), phopsho-STAT1 Y701 (Catalog #9167S, Cell Signaling, Danvers, MA, USA), IRF3 (Catalog #ab76409, Abcam, Cambridge, MA, USA), phopsho-IRF3 S396 (Catalog #29047, Cell Signaling, Danvers, MA, USA), GAPDH (Catalog #ab9484, Abcam, Cambridge, MA, USA), b-actin (Catalog #4970, Cell Signaling, Danvers, MA, USA), NP (Catalog #sc-80481, Santa Cruz, Biotechnology, Dallas, TX, USA), and M2 (Catalog #sc32238, Santa Cruz, Biotechnology, Dallas, TX, USA).
OxLDL preparation
LDL at 0.2 mg/mL was incubated with 5 mM CuSO4 at 37°C for 24 h. The oxidation reaction was stopped by the addition of 20 mM EDTA. The preparations were concentrated by cone filtration and followed by dialyzed and sterilized by filtration through a 0.22 mm filter. The extent of oxidation of the LDL preparation was determined by measuring Thiobarbituric acid reactive substance (TBARS, Catalog #10009055; Cayman Chemicals, Ann Arbor, MI, USA). Another control LDL sample was processed in parallel, without CuSO4 addition. The results showed that OxLDL contained 14 nmol/mg protein and LDL contained 1 TBBARS/mg protein.
Human IFN-β in cell culture supernates
ECs were transfected with either siCont or siMAGI1. Approximately after 48 h of transfection, conditioned medium was collected and levels of IFNβ were measured using the human IFN-β Quantikine ELISA kit (Catalog #DIFNB0, R&D Systems, MN, USA).
Immunofluorescent staining
At the end of experiment, the cells were quickly washed twice with warm PBS, fixed with 4% paraformaldehyde in PBS for 15 min, and permeabilized with 0.2% Triton X-100 in PBS for 10 min. The cells were then incubated with a blocking solution (5% goat serum and 0.1% NP-40 in PBS) for 60 min at room temperature and incubated with the primary antibodies (IRF3 or NP) overnight at 4°C. Next, the cells were washed three times with PBS and incubated with fluorescently labeled secondary antibodies for 1 h at room temperature then were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) to identify nuclei at room temperature. Imaging was done using an Olympus FV1200 multiphoton-confocal dual microscope (Olympus, Tokyo, Japan).
Statistical analysis
First, we performed Shapiro-Wilk test for checking the normality of each group, and we performed an ordinary one-way ANOVA followed by Fisher's LSD testing for multiple group comparisons or unpaired student t-test with only the data that passed these normality tests. If the data did not pass the normality test, we performed Brown-Forsythe and Welch ANOVA or unpaired t-test with Welch's correction tests using the Prism software (GraphPad Software). P-values < 0.05 were considered statistically significant.
Data availability
The microarray data and MAGI1 sequence were deposited in the NCBI's Gene Expression Omnibus database (accession GSE95066) and GenBank (accession KY651081), respectively. All other data, analytic methods, and study materials that support the findings of this study are available in the Data supplement or from the corresponding authors upon reasonable request.
Results
IAV infection increases MAGI1 expression and MAGI1 depletion suppresses IAV infection
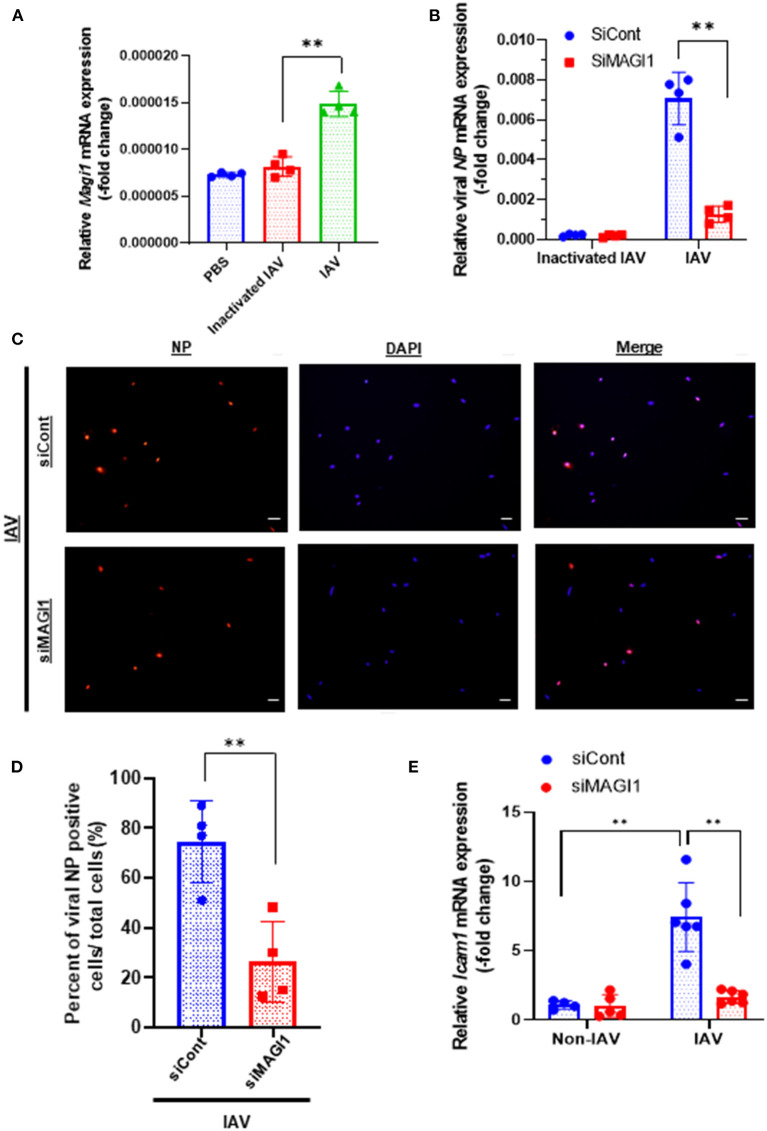
To determine if MAGI1 expression in ECs in response to IAV infection promotes atherogenesis, we infected primary HUVECs with IAV. Given that the umbilical vein carries oxygenated blood, which is completely different from the systemic venous blood, HUVEC is the preferred in vitro cell culture system for determining the molecular mechanism of EC activation-induced atherogenesis (28). HUVECs infected with heat-inactivated virus was used as the mock control. After 24 h of infection, the cells were lysed, total RNA was extracted and analyzed by qRT-PCR to determine relative changes in Magi1 mRNA expression levels (Figure 1A). Neither phosphate-buffered saline (PBS, vehicle control) nor the heat-inactivated virus (mock treatment control) had an effect, whereas the IAV infected cells increased Magi1 mRNA expression level. We next knocked down MAGI1 by transfecting HUVECs with either the small interfering RNA (siRNA) that specifically targets Magi1 mRNA for degradation (siMAGI1) or the siRNA negative control (siCont). After 48 h of siRNA transfection, the cells were infected with either IAV or mock control. After 24 h of virus infection, the cells were lysed, and total RNA was extracted and analyzed by qRT-PCR to determine relative changes in viral nucleoprotein (NP) mRNA expression levels. With the heat-inactivated IAV mock infection, there was no difference between siMAGI1 and siCont samples in the level of viral NP mRNA expression (Figure 1B). Conversely, infecting HUVECs transfected with siMAGI1 with live virus drastically downregulates the viral NP mRNA expression level relative to that of the siCont (Figure 1B). Studies of the cells themselves using immunofluorescence staining for the influenza NP protein showed similar results (Figures 1C,D).
Figure 1.
IAV infection increases MAGI1 expression and MAGI1 depletion suppresses IAV infection and inhibits EC inflammation. (A) HUVECs were infected with IAV, heat-inactivated virus, or treated with PBS for 24 h. Total RNA was extracted and qRT-PCR was performed. Relative changes in Magi1 mRNA expression was calculated using the comparative Ct (2−ΔΔCt) method. Ct values of Magi1 were normalized to that of Gapdh (26). Each group passed the Shapiro-Wilk normality test, then one-way ANOVA followed by Turkey's multiple comparisons test were performed using the Prism software (GraphPad Software). The graph shows mean ± SD (n = 4). **p < 0.01. (B) HUVECs were transfected with either siCont or siMAGI1. After 48 h of transfection, the cells were infected with IAV or heat-inactivated virus for 24 h. Total RNA was extracted then qRT-PCR was performed. Relative changes in the viral NP mRNA expression was calculated using the comparative Ct (2−ΔΔCt) method. Ct values of the viral NP were normalized to that of the 18S ribosomal RNA (26). Each group passed the Shapiro-Wilk normality test, then two-way ANOVA followed by Turkey's multiple comparisons test were performed using the Prism software (GraphPad Software). The graph shows mean ± SD (n = 3). **p < 0.01. (C) HUVECs were transfected with either siCont or siMAGI1. After 48 h of transfection, the cells were infected with IAV and immunofluorescence staining for the viral NP protein (red) and DAPI staining for the nucleus (blue) were performed. Scale bars: 20 μM. (D) We analyzed a total of 30–120 cells per 2–3 fields per dish to determine the percentage of NP positive cells in a blinded manner. Each group passed the Shapiro-Wilk normality test, then unpaired student t-test was performed using the Prism software (GraphPad Software). The graph shows mean ± SD (n = 4). **p < 0.01. (E) HUVECs were transfected with either siCont or siMAGI1. After 48 h of transfection, the cells were infected or not infected with IAV for 24 h. Total RNA was extracted, and qRT-PCR was performed. Relative changes in Icam-1 mRNA expression was calculated using the comparative Ct (2−ΔΔCt) method. Ct values of Icam-1 were normalized to that of gapdh (26). Each group passed the Shapiro-Wilk normality test, then two-way ANOVA followed by Turkey's multiple comparisons test was performed using the Prism software (GraphPad Software). The graph shows mean ± SD (n = 4–6). **p < 0.01.
Because MAGI1 induces EC activation (20), we investigated whether IAV activated ECs through upregulation of MAGI1 by determining relative changes in mRNA expression level of intercellular adhesion molecule 1 (ICAM-1), a marker of EC activation, using qRT-PCR in MAGI1-depleted HUVECs with or without virus infection. IAV infection increased Icam-1 mRNA expression level and knocking down MAGI1 prevented this virus induced upregulation of Icam-1 expression (Figure 1E). These results indicate that MAGI1 is essential to IAV infection, and in addition, mediates the activation of ECs by the virus. Interestingly, 24 h after virus infection, vascular cell adhesion molecule 1 (Vcam-1) mRNA expression was downregulated (Supplementary Figure 2).
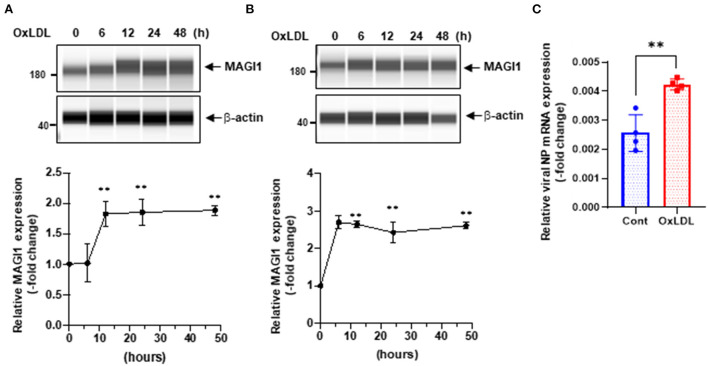
We speculated that other pathological conditions in ECs also upregulate MAGI1 expression. IAV induces acute respiratory disease and the association between serum lipid levels and prognosis after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza virus has been reported (29–34). Therefore, we investigated the role of MAGI1 in HUVECs and in human lung endothelial cells (HULECs) and found that OxLDL increased MAGI1 expression in both HUVECs and HULECs (Figures 2A,B). Furthermore, we also found that the pre-treatment of EC with OxLDL increased IAV infection (Figure 2C). Taken together, these data suggest a possible role for MAGI1 in IAV-associated cardiovascular and acute respiratory disease in dyslipidemia patients through driving virus infection.
Figure 2.
OxLDL increases MAGI1 expression and enhances IAV infection in ECs. (A,B) HUVECs (A) and HULECs (B) were pre-treated with OxLDL (10 μg/mL) for the indicated times. Upper: relative changes in MAGI1 protein expression were analyzed using the automated capillary electrophoresis Western analysis (Wes) system (ProteinSimple, San Jose, CA, USA) (27). Lower (densitometric quantification): relative changes in MAGI1 protein expression after OxLDL treatment. Fold increases are shown after normalization with β-actin at each time point. Quantification was performed using Image J. Each group passed the Shapiro-Wilk normality test, then one-way ANOVA followed by Turkey's multiple comparisons test was performed using the Prism software (GraphPad Software). The graph shows mean ± SD (n = 3). **P < 0.01. (C) HUVECs were pre-treated with OxLDL (10 μg/mL) for 24 h then infected with IAV for another 24 h. Total RNA was extracted then qRT-PCR was performed. Relative changes in the viral NP mRNA expression were calculated using the comparative Ct (2−ΔΔCt) method. Ct values of the viral NP were normalized to that of the 18S ribosomal RNA (26). Each group passed the Shapiro-Wilk normality test, then an unpaired student t-test was performed using the Prism software (GraphPad Software). The graph shows mean ± SD (n = 4). **p < 0.01.
MAGI1 depletion upregulates IFN signaling
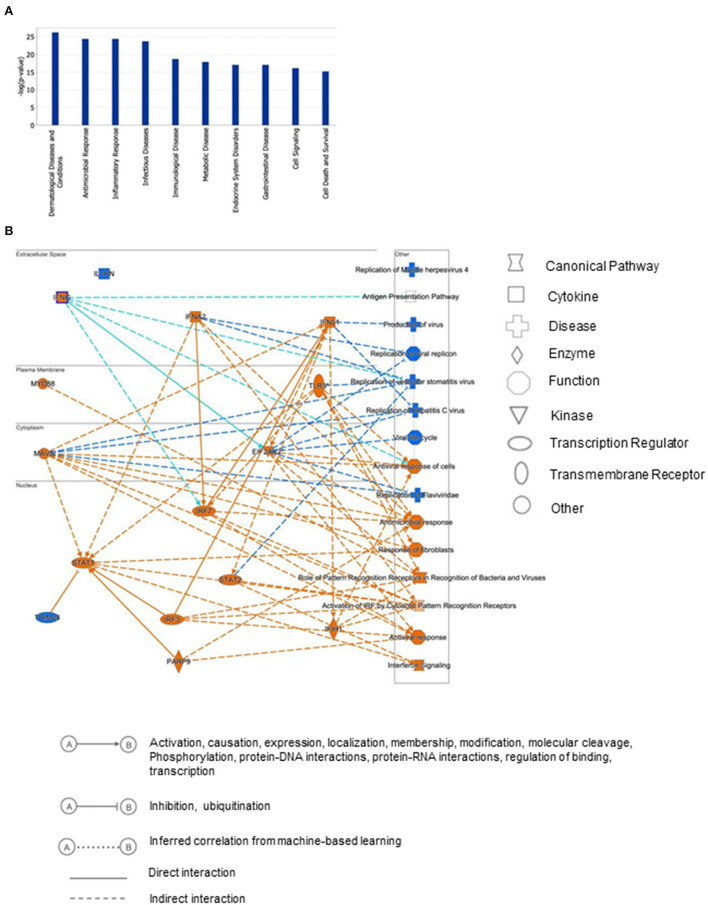
To gain insights into the biological relevance of the reduced MAGI1 expression in ECs, we transfected HUVECs with either siCont or siMAGI1. Approximately 48 h after transfection, the cells were lysed, total RNA were extracted and transcriptionally profiled. A set of genes in the siMAGI1-treated ECs with Significant Differential Gene Expression (absolute fold-change >2, p-value < 0.05) was submitted to IPA (QIAGEN, Redwood City, CA) for core analysis. We identified 252 genes that were differentially expressed in siMAGI1-transfected ECs compared to that in siCont-transfected ECs. Using Fisher's Exact Test enrichment analysis for curated gene sets categorized by biological function and disease, we find that gene sets categorized as Dermatological-Disease and Conditions, Antimicrobial Response, Inflammatory Response, and Infectious Disease are the top four most significant groups (-log[P] >20, Figure 3A). The gene enrichment calculations and predicted biological gene effects, based upon directional expression changes and literature-substantiated causal relationships, indicated that the gene expression pattern will reduce viral infection in siMAGI1-transfected ECs compared to that in siCont-transfected ECs and that 45 of the 82 genes had the measurement direction consistent with decreased viral infection (z-score, −2.7; P = 5.6E-23) (Supplementary Table 2). The high z-score predicts that MAGI1 depletion will down-regulate the expression of genes belonging to the viral infection category.
Figure 3.
Differential gene expression profiles between siMAGI1 vs. siCont-treated ECs. (A) Statistical significance of the IPA-determined biological function and disease gene enrichment in siMAGI1-treated ECs calculated by the right-tailed Fisher exact test and presented as –log(P) value. A larger value on the y-axis represents greater significance. A total of 252 genes were differentially expressed in siMAGI1-transfected ECs compared to that in siCont-transfected ECs (P < 0.05; absolute fold change >2). The p-value, calculated by the Fischer's exact test, reflects the likelihood that the association among a set of genes in the data set and a related biological function is significant. (B) Graphical summary of IPA results showing significant predictor genes that are related to antiviral response.
Figure 3B is the graphical summary constructed by the algorithm based on machine learning techniques to prioritize and connect entities identified by IPA. It shows the relationship which may not yet be connected by findings in the QIAGEN Knowledge Graph and suggests that MAGI1 depletion induces strong antiviral responses and IFN signaling by increasing Ifih1 (interferon-induced helicase C domain-containing protein 1), Irf (interferon regulatory factor) 3/7, Parp9 (poly(ADP-ribose) polymerase family member 9), Stat (signal transducer and activator of transcription) 1/2, Mavs (mitochondrial antiviral signaling protein), Eif2ak2 (eukaryotic translation initiation factor 2 alpha kinase), and Ifng (interferon gamma).
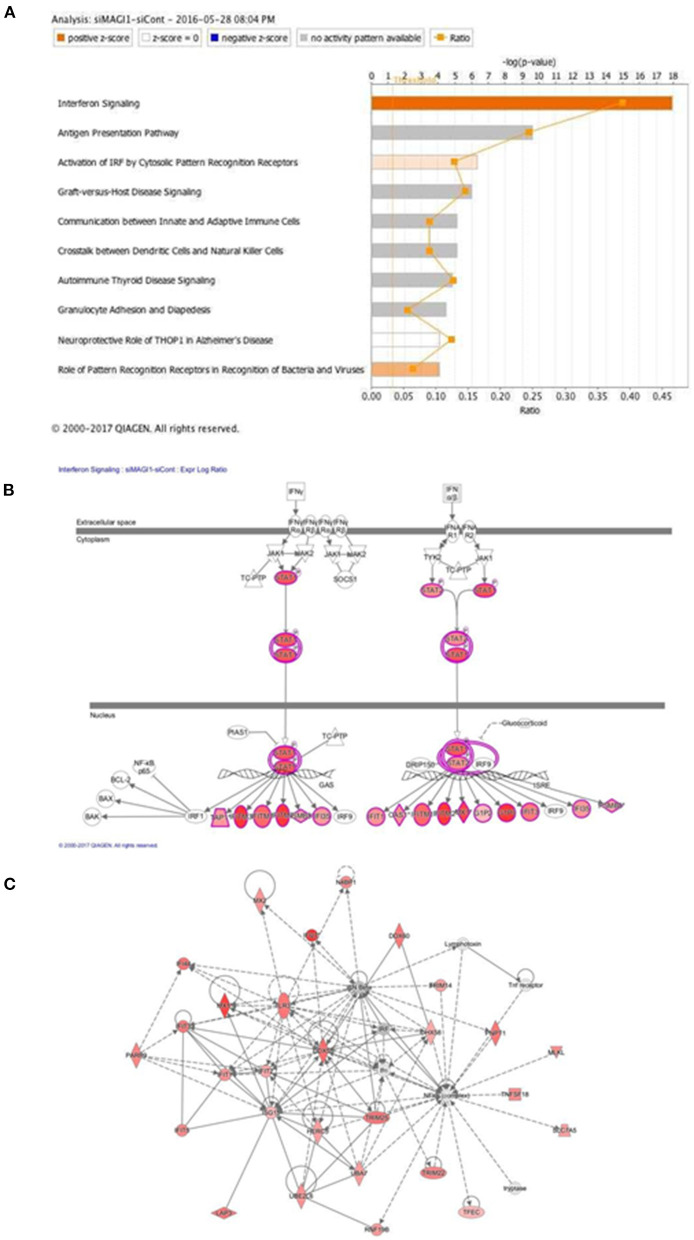
The predicted biological gene expression affected by MAGI1 depletion is shown as heat maps (Supplementary Figure 1). The results show the unique and strong downregulation of infectious disease category (Supplementary Figure 1) including viral infection (z-score, −2.7; P = 5.6E-23, Supplementary Table 2), replication of virus (z-score, −4.1; P = 1.97E-24, Supplementary Table 3), infection of mammalia (z-score, −3.6; P = 9.1E-17, Supplementary Table 4), and production of virus (z-score, −3.1; P = 3.1E-07, Supplementary Table 5; Supplementary Figure 1B). We also find that IFN signaling is the top-ranked enriched IPA canonical pathway, which comprises of 36 genes, 14 of which are expressed at higher levels and thereby supporting the prediction that this pathway activation is increased in siMAGI1-transfected ECs compared to that in siCont-transfected ECs (z-score, 3.7; P = 1.1E-18) (Figures 4A,B; Supplementary Table 6). Following the IPA generated network, which optimizes the interconnectivity of differentially expressed genes under the constraint of the maximal network size, we carried out Fisher's Exact Test gene set function and disease enrichment analysis to predict biological functions that are expected to be affected by a given interaction network. The gene set function and disease enrichment of the top ranked interaction networks, based on the number of interconnected differentially expressed genes, were Antimicrobial Response, Inflammatory Response, and Infectious Diseases (differentially expressed genes, 28) (Figure 4C; Supplementary Table 7). As demonstrated in our network analysis (Figure 4C), although the expression of antiviral response-related genes, including Mx1, Mx2, and Ddx58, were upregulated in MAGI1-depleted ECs, NF-κB expression did not exceed the gene expression significance threshold.
Figure 4.
IFN signaling is the top-rank enriched IPA canonical pathway. (A) IPA-based identification of canonical pathways (–log[P] >5) associated with differentially expressed genes in HUVECs transfected with siMAGI1 vs. siCont. The bar chart indicates the –log(P) value of the significance of enrichment of each category. (B) Induction of IFN signaling in MAGI1-depleted ECs. Pathway models of IFN signaling in ECs based on data in the Ingenuity Knowledge Base are shown. Expression of genes in red is higher in siMAGI1-treated ECs compared to that in siCont-treated ECs. (C) Gene enrichment analyses (right-tailed Fisher exact test) according to the IPA downstream effects analysis identified Antimicrobial Response, Inflammatory Response, and Infectious Diseases as the highest scoring IPA interaction networks. Green and red symbols denote genes with lower and higher expression, respectively, in siMAGI1-treated ECs compared to that in siCont-treated ECs. The arrows with solid lines indicate direct (usually physical) interactions between two molecules in the direction of the arrow, Whereas, arrows with dashed lines denote indirect interactions (e.g., molecule/gene X affects molecule/gene Y). The abbreviations shown are defined in Supplementary Table 6.
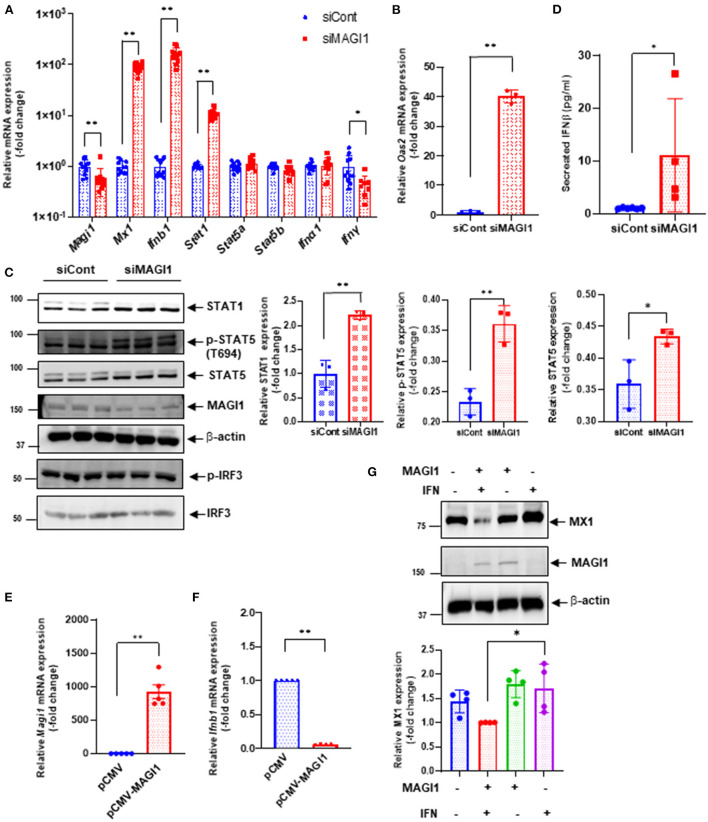
To verify key microarray results, we performed qRT-PCR using HUVECs transfected with siCont or siMAGI1 and evaluated relative changes in mRNA expression level of Magi1, Mx1, Ifnb1, Ifna1, Ifng, Stat1, Stat5a, and Stat5b. Knocking down MAGI1 increases mRNA expression level of Ifnb1, Mx1 and Stat1, but decreases mRNA expression level of Ifng. MAGI1 depletion did not alter mRNA levels of Ifna1, Stat5a, and Stat5b (Figure 5A). To determine the anti-viral effects by MAGI1 depletion, we also evaluated Oas2, another anti-viral molecule that can cause viral RNA degradation and inhibition of viral replication (35) and find that depletion of MAGI1 by siRNA upregulated Oas2 mRNA expression (Figure 5B). Although the goal of this study is to investigate the role of MAGI1 in IAV-associated cardiovascular diseases, we also treated HULECs with siCont or siMAGI1 and quantified Ifnb1 mRNA expression and observed a clear increase in Ifnb1 mRNA expression (Supplementary Figures 2B,C), indicating that the yin-yang form of correlation between Magi1 and Ifnb1 expressions is not specific to HUVECs.
Figure 5.
MAGI1 suppresses IFN signaling in ECs. (A) HUVECs were transfected with either siCont or siMAGI1. After 48 h of transfection, total RNA was extracted then qRT-PCR was performed. Relative changes in Magi1, Mx1, Ifnb1, Ifna1, IfngI, Stat1, Stat5a, and Stat5b expression were calculated using the comparative Ct (2−ΔΔCt) method. Ct values of each target gene were normalized to that of the Gapdh (26). Each group passed the Shapiro-Wilk normality test, then an unpaired student t-test was performed using the Prism software (GraphPad Software). The graph shows mean ± SD (n = 11). **p < 0.01, *p < 0.05. (B) HUVECs were transfected with either siCont or siMAGI1. After 48 h of transfection, total RNA was extracted then qRT-PCR was performed. Relative changes in Oas2 mRNA expression were calculated using the comparative Ct (2−ΔΔCt) method. Ct values of Oas2 were normalized to that of the Gapdh (26). Each group passed the Shapiro-Wilk normality test, then an unpaired student t-test was performed using the Prism software (GraphPad Software). The graph shows mean ± SD (n = 3). **p < 0.01. (C) HUVECs were transfected with either siCont or siMAGI1. After 48 h of transfection, cell lysates were analyzed by immunoblotting with each specific antibody as indicated. The graphs represent densitometry data from 3 independent gels, one of which is shown in the left panel. Each group passed the Shapiro-Wilk normality test, then an unpaired student t-test was performed using the Prism software (GraphPad Software). The graph shows mean ± SD, n = 3, **P < 0.01, and * P < 0.05. Uncropped figures were provided in the supplements. (D) HUVECs were transfected with either siCont or siMAGI1. After 48 h of transfection, conditioned medium was collected and levels of IFNβ were measured. Each group passed the Shapiro-Wilk normality test, then an unpaired student t-test was performed using the Prism software (GraphPad Software). The graph shows mean ± SD, n = 4–6, *P < 0.05. (E,F) HUVECs were transfected with either the pCMV-MAGI1 or the pCMV-2B backbone construct (control) (20). After 24 h of transfection, total RNA was extracted then qRT-PCR was performed. Relative changes in Magi1 (E) and Ifnb1 (F) mRNA expression were calculated using the comparative Ct (2−ΔΔCt) method. Ct values of each target gene were normalized to that of the gapdh (26). At least, one of the groups did not pass the Shapiro-Wilk normality test, we performed an unpaired t-test with Welch's correction using the Prism software (GraphPad Software). The graph shows mean ± SD, n = 5, **P < 0.01. (G) HUVECs were transfected with either the pCMV-MAGI1 or the pCMV-2B backbone construct (control) (20). After 24 h of transfection, the cells were treated with IFN (200 U/mL) for 6 h, and immunoblotting was performed with each specific antibodies as indicated. The graph (lower) represents densitometry data from 4 independent gels, one of which is shown in the top panel. Each group passed the Shapiro-Wilk normality test, then one-way ANOVA followed by Turkey's multiple comparisons test was performed using the Prism software (GraphPad Software). The graph shows mean ± SD (n = 4). *p < 0.05.
We also confirmed key microarray results using immunoblotting to detect protein expression level. STAT1 protein expression was augmented by MAGI1 depletion (Figure 5C). Total STAT5 activity (p-STAT5 T694 represents total STAT5 activity including total STAT5 expression) was increased by siMAGI1 transfection (Figure 5C). We have also shown that the level of secreted IFNb in conditioned medium from siMAGI1-transfected ECs was increased compared to that from siCont-transfected ECs (Figure 5D). These data suggest that MAGI1 depletion differentially affects IFN signaling both in mRNA and protein levels.
To clarify the role of MAGI1 in IFN signaling, we performed two types of overexpression studies. First, we overexpressed MAGI1 and measured the relative changes in Ifnb1 mRNA expressions. In ECs overexpressing MAGI1, Ifnb1 mRNA expression levels were decreased (Figures 5E,F). Next, we determined if MAGI1 overexpression altered MX1 expression and observed little change (data not shown). Therefore, we stimulated ECs overexpressing MAGI1 with a low dose of IFN (200 U/mL), and observed a decreased MX1 expression (Figure 5G). It is unclear why Ifnb1 mRNA and MX1 protein expression respond differently to MAGI1 overexpression; however, it is possible that IFN-mediated signaling such as p90RSK activation may potentiate the inhibitory effects of MAGI1 on MX1 expression through promoting MAGI1 post-translational modification (20). These data support a role for MAGI1 in promoting viral infection in ECs.
Knocking down MAGI1 suppresses IAV infection partially through increasing MX1 expression
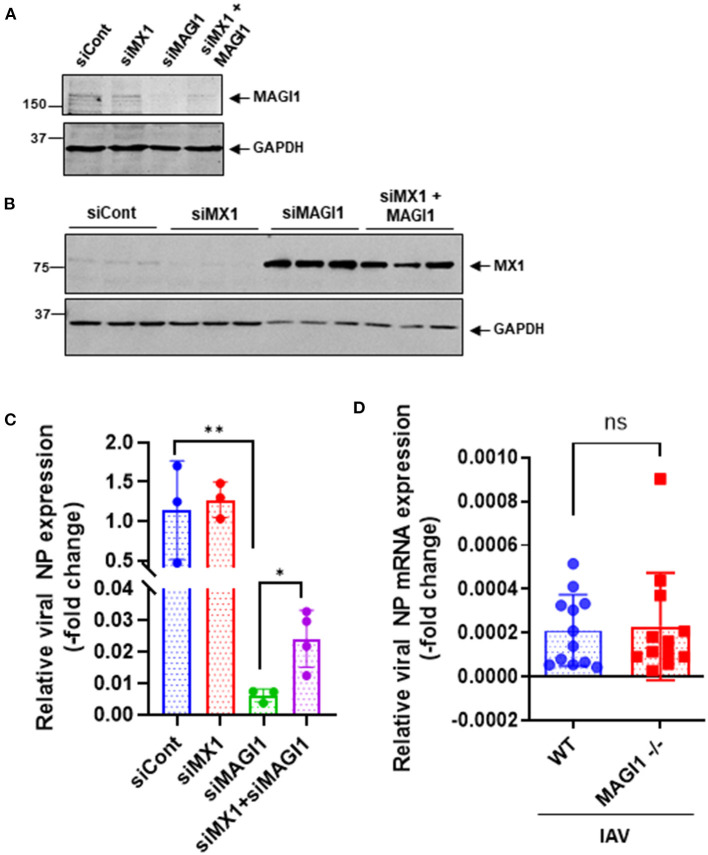
Since MX1 exerts broad antiviral activity against several strains of viruses including IAV (36–38), we tested whether the MAGI1 depletion-induced virus suppression could be due to increased MX1 expression. HUVECs were transfected with siCont, siMX1, siMAGI1, or siMX1+siMAGI1 and then infected with IAV. We extracted total RNA from these cells and performed qRT-PCR to detect relative changes in viral NP mRNA expressions. Cell lysates were also immunoblotted for MX1 protein expression. Knocking down MAGI1 increased MX1 expression. However, depletion of MX1 exerted no effect on MAGI1 expression (Figures 6A,B). Since we also noted the reduced MX1 expression by MAGI1 overexpression only occurs under IFN stimulation (Figure 5G), these data have suggested that MAGI1 is an upstream signaling event for regulating MX1 expression in ECs. The depletion of MAGI1 by siMAGI1 increased MX1 expression (Figure 6B), and the partial significant inhibition of MX1 expression after siMX1 transfection was due to the incomplete transfection efficiency by siMX1. We also observed that siMX1 reversed the anti-viral effects induced by siMAGI1, suggesting that siMAGI1-mediated induction of MX1 plays a meaningful role in siMAGI1-mediated anti-viral response. However, siMAGI1 leads to the inhibition of influenza infection (Figure 6C), which was in part due to the induction of MX1. In fact, in addition to MX1, we also found that the depletion of MAGI1 by siMAGI1 upregulated Oas2 mRNA expression (Figure 5B). Furthermore, we also found the upregulation of Ifnb1 (Figures 5A,B) and Stat1 (Figures 5A,C) expression, STAT5 activation (Figure 5C), and IRF3 nuclear translocation (Figure 7B) which played a significant role in developing anti-viral response (Figure 7E) (39–42). Therefore, siMAGI1 can induce anti-viral effects not only through MX1 but also by induction of other anti-viral molecules (Figure 7E).
Figure 6.
MX1 depletion reverses MAGI1 depletion-mediated suppression of IAV infection. (A,B) HUVECs were transfected with either siCont, siMX1, siMAGI1, or siMX1+siMAGI1. After 48 h of transfection, cell lysates were analyzed by immunoblotting with each specific antibody as indicated. Gels are representative of 3 independent experiments. (C) HUVECs were transfected with either siCont, siMX1, siMAGI1, or siMX1+siMAGI1. After 48 h of transfection, the cells were infected with IAV for 24 h. Total RNA was extracted then qRT-PCR was performed. Relative changes in the viral NP mRNA expression were calculated using the comparative Ct (2−ΔΔCt) method. Ct values of the viral NP were normalized to that of the 18S ribosomal RNA (26). Each group passed the Shapiro-Wilk normality test, then one-way ANOVA followed by Turkey's multiple comparisons test was performed using the Prism software (GraphPad Software). The graph shows mean ± SD (n = 3-4). **p < 0.01, *p < 0.05. (D) Mouse lung ECs were isolated from Magi1−/− and littermate wild type (WT) control mice and cultured. The cells were infected with IAV for 24h. Total RNA was extracted then qRT-PCR was performed. Relative changes in the viral NP mRNA expression were calculated using the comparative Ct (2−ΔΔCt) method. Ct values of the viral NP were normalized to that of the 18S ribosomal RNA (26). Each group passed the Shapiro-Wilk normality test, then an unpaired student t-test was performed using the Prism software (GraphPad Software). The graph shows mean ± SD, n =11. NS: not significant.
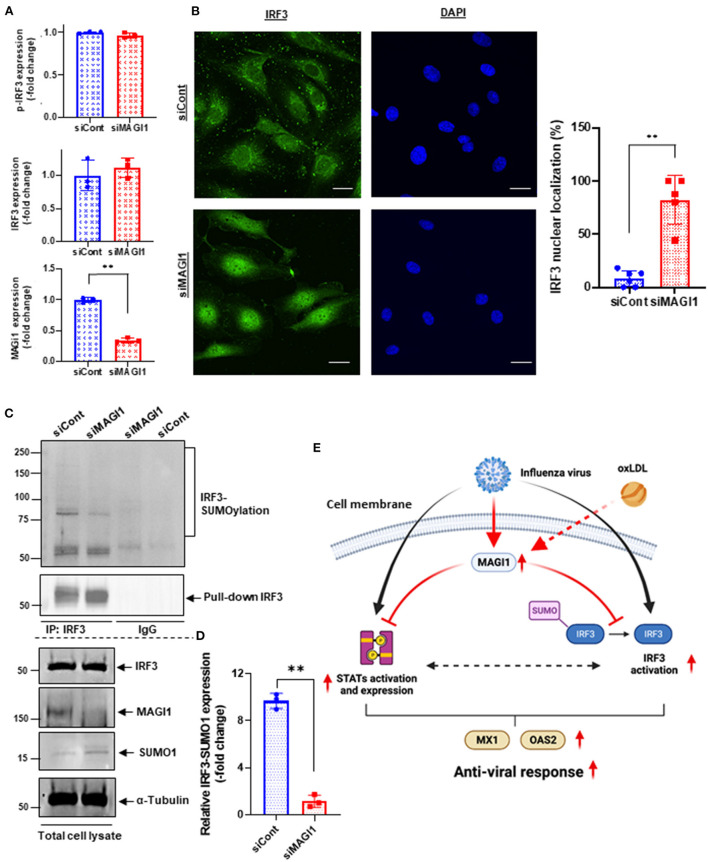
Figure 7.
MAGI1 depletion promotes IRF3 nuclear translocation and de-SUMOylation without affecting IRF3 S396 phosphorylation. (A) HUVECs were transfected with either siCont or siMAGI1. After 48 h of transfection, cell lysates were analyzed by immunoblotting with each specific antibody as indicated. The graphs represent densitometry data from 3 independent gels, one of which is shown in Figure 5C. Each group passed the Shapiro-Wilk normality test, then an unpaired student t-test was performed using the Prism software (GraphPad Software). The graphs show mean ± SD, n = 3, **P < 0.01. (B) HUVECs were transfected with either siCont or siMAGI1. After 48 h of transfection, immunofluorescence staining for IRF3 protein (green) and DAPI staining for the nuclei (blue) were performed. Scale bars: 20 μM. Robust IRF3 nuclear translocation noted in siMAGI1-transfected cells. The percentages of cells with nuclear staining were quantified by counting the cells with the mean intensity of IRF3 staining in the nucleus ≥ mean intensity of IRF3 cytoplasmic staining by each dish (n = 5–6) in a blinded manner. (C) HUVECs were transfected with either siCont or siMAGI1. After 48 h of transfection, cell lysates were immuno-precipitated IRF3 with the antibody that specifically recognizes this protein then immunoblotting with the antibody that recognizes SUMO1 to detect IRF3 SUMOylation (20). IgG antibody was used as a negative control. Total cell lysates were immunoblotted with each specific antibody as indicated. (D) The graphs represent densitometry data from 3 independent gels, one of which is shown in shown in (C). Median intensities of IRF3 SUMOylation were calculated after subtracting the background, which is median intensities of IgG control. Each group passed the Shapiro-Wilk normality test, then an unpaired student t-test with Welch's correction was performed using the Prism software (GraphPad Software). The graph shows mean ± SD, n = 3, **P < 0.01. (E) The scheme depicts the relationship between MAGI1 and IFN signaling. IAV infection induces anti-viral responses including STATs and IRF3 activation and subsequent MX1 and OAS2 induction. For efficient infection of IAV to ECs, IAV induces MAGI1 expression, resulting in inhibition of anti-viral responses by inhibiting STATs and IRF3 activation. Especially, MAGI1 inhibits IRF3 de-SUMOylation and subsequent IRF3 activation. Importantly, OxLDL also upregulates MAGI1 expression and promotes IAV infection, which may explain the relationship between hypercholesterolemia and IAV infection in patients. The scheme was generated using BioRender.
The Mx1 gene in C57BL/6 mice contains a large deletion, which creates the MX1 null phenotype, a condition that increases susceptibility of this mouse strain to IAV (43, 44). We took advantage of this fact (i.e., Mx1 null condition in C57BL/6 mice) to further determine if MAGI1 depletion in mice lacking MX1 still inhibits IAV infection. Mouse ECs were isolated from the lungs of Magi1−/− and WT littermate control mice and cultured to confluence on gelatin-coated plates. The cells were then infected with virus for 24 h. Total mRNA was extracted from the infected cells, and relative changes in viral NP mRNA expression levels were determined by qRT-PCR (Figure 6D). No difference in NP mRNA expression was found between WT and Magi−/− mouse ECs. Whereas, we showed that NP mRNA burden is correlated with viral burden and infectivity (24), these results indicate that MX1 promotes the inhibition of virus replication caused by MAGI1 depletion.
Knocking down MAGI1 promotes IRF3 nuclear translocation and de-SUMOylation without affecting IRF3 phosphorylation
MAGI1 depletion in human lung epithelial cells increases phosphorylation of IRF3, an upstream transcriptional regulator of IFN genes, at S396, which triggers IRF3 translocation into the nucleus and activation IFN-β signaling (45, 46). Following activation in the cytoplasm, IRF3 translocate into the nucleus and promotes the transcription of IFN-α and -β and IFN-stimulated genes by binding to the interferon-stimulated response element (ISRE) (47, 48). However, it is still not clear if ECs respond in the same manner. To address this question, we treated HUVECs with either siMAGI1 or siCont and detected IRF3 S396 phosphorylation and nuclear translocation. According to immunoblot analysis, similar levels of IRF3 phosphorylation at S396 was observed in control and MAGI1-depleted cells (Figures 5C, 7A,C). By contrast, IRF3 nuclear translocation was clearly observed in MAGI1-depleted HUVECs while the cytoplasmic staining was prevalent in cells treated with siCont (Figure 7B), suggesting that IRF3 nuclear translocation is involved in siMAGI1-mediated upregulation of IFN expression. Kubota et al. showed that virus infection enhances IRF3 SUMOylation, thereby attenuating IFN production (49). This finding has led us to investigate the potential role of MAGI1 on IRF3 SUMOylation. After 48 h of either siMAGI1 or siCont transfection, we lysed the cells and immuno-precipitated IRF3 with the antibody that specifically recognizes this protein. IgG antibody was used as a negative control. We then utilized immunoblotting to assess SUMOylated IRF3 using the antibody that recognizes only SUMO1. We found a significant decrease in IRF3 SUMOylation by MAGI1 depletion (Figures 7C,D), suggesting the upregulation of IRF3 transcriptional activity as described previously (49). These data suggest that MAGI1 depletion increased IRF3 transcriptional activity via downregulated IRF3 SUMOylation and promoted IRF3 nuclear translocation, which may be independent of IRF3 phosphorylation (Figure 7E).
Discussion
In this study, we find that IAV infection and OxLDL pre-treatment upregulate MAGI1 expression in ECs. Influenza replication is increased by OxLDL pre-treatment, Whereas, MAGI1 depletion decreased virus replication, suggesting the crucial role of IAV-induced MAGI1 expression in promoting IAV infection in hypercholesterolemia patients. In MAGI1-depleted ECs, we also noted heightened anti-viral events such as upregulation of IFN signaling and response-related gene expression including MX1 and OAS2. Lastly, we find that MAGI1 depletion inhibits IRF3 SUMOylation, which subsequently induces IRF3 activation. Our results suggest that IAV and/or OxLDL-induced MAGI1 expression accelerates virus infection in ECs and that MAGI1 depletion effectively inhibits this process. The data collectively provides insights into the role of MAGI1 in influenza infection as well as associated cardiovascular disease observed in dyslipidemia patients (11–15).
This study is the first to show that IAV infection increases MAGI1 expression in cultured ECs and that MAGI1 depletion suppresses IAV replication by increasing MX1 expression, a process that appears to induce IFN production. Indeed, others have shown that increased MX1 expression induces IFN-β production (46). The consensus PDZ-binding motif ESEV (glu-ser-glu-val) (PBM) in NS1 protein of IAV binds the PDZ domain of various proteins. MAGI1 has six PDZ domains, thus, it is possible that the binding between the virus NS1 PBM and MAGI1 plays a major role in viral infection and replication (48, 50). Since we find that virus infection upregulated MAGI1 expression, this increased MAGI1 expression provides a favorable environment for virus to replicate and thereby forming a positive feedback loop. Thus, MAGI1 depletion should break this cycle and suppress virus infection and replication. Our results have supported this model. In this study, we have also shown that knocking down MX1 impairs MAGI1 depletion-mediated IAV suppression. Moreover, the impaired virus infection caused by MAGI1 depletion was not detected in cultured lung ECs derived from C57BL/6 mice that are known to carry a truncated Mx1 gene, which results in the MX1 null phenotype. The anti-viral effect of MX1 in vivo was clearly demonstrated using mouse models with and without MX1 expression (51). Together, these results suggest that MAGI1 depletion mediates the suppression of IAV infection, at least in part, by increasing MX1 expression. We also find upregulation of a broad range of IFN signaling network-related molecules (Figure 4) including the increase of STAT1 and Oas2 expression and STAT5 activity (Figure 5), revealing how the depletion of MAGI1 can induce a strong anti-viral response (Figure 7E).
IRF3, a key transcriptional regulator of type I interferon, regulates the transcription of IFN-β and IFN-stimulated genes by binding the ISRE in their promoters. Upon viral infection, IRF3 in the cytoplasm of the infected cells is phosphorylated by the kinases TBK1 and IKKε, and undergoes a conformational change and homo-dimerization, which permits its translocation to the nucleus where it binds the ISRE of target genes (46, 47). Knocking down MAGI1 specifically in lung epithelial cells causes IRF3 S396 phosphorylation and increased IFN production (46). Interestingly, although we found a clear IRF3 nuclear translocation after MAGI1 depletion, we did not detect significant increases of IRF3 phosphorylation under the same condition (Figures 7A,B). This led us to investigate other types of IRF3 post-translational modifications, and we found a significant decrease of IRF3 SUMOylation induced by MAGI1 depletion (Figures 7C,D). Since the activation of IRF3 by attenuating IRF3 SUMOylation has been reported (49), the decrease of IRF3 SUMOylation by siMAGI1 transfection can induce IRF3 nuclear translocation and transcriptional activity. Of note, we have reported that the similar effect of p53 SUMOylation in ECs cultured under disturbed flow leading to p53 nuclear export (52). Therefore, both p53 and IRF3 SUMOylation may be important to retrain these proteins in the cytoplasm, and after losing this modification, p53 and IRF3 translocate to the nucleus. Furthermore, we have previously reported that de-SUMOylation enzyme of sentrin/SUMO-specific protease 2 (SENP2) regulates MAGI1 SUMOylation and de-SUMOylation of MAGI1 induced by SENP2 promotes MAGI1 nuclear translocation and subsequent EC activation (20). Therefore, MAGI1 may inhibit IRF3 SUMOylation by modulating SENP2 function. Future studies will be necessary to clarify these issues.
EC activation is a major cause of cardiovascular disease. Our study revealed upregulation of ICAM-1 expression in IAV-infected ECs, indicating that virus infection activates ECs, and increasing the risk of cardiovascular dysfunction in influenza patients. In our recent study, we have shown that MAGI1 plays a major role in atherogenesis by inducing pro-inflammatory signaling in ECs (20). The role of MAGI1 in EC activation was confirmed by knock down studies, in which the virus induced ICAM-1 elevation was strongly inhibited. Together, these results show that MAGI1 could be the molecular link between IAV infection and cardiovascular disease. Furthermore, our current study shows that MAGI1 expression is upregulated under a pro-atherogenic, physiological perturbation i.e., OxLDL treatment (Figures 2A,B). More importantly, the pre-treatment of OxLDL upregulates virus infection in ECs (Figure 7C), suggesting that the induction of MAGI1 by OxLDL increases EC activation and accelerates virus infection by inhibiting IAV-mediated anti-viral responses (Figure 7E). It has been reported that IL-1β stimulation induces VCAM-1 expression rapidly and temporally although sustaining ICAM-1 expression over 24–72 h (53). Therefore, the inhibition of VCAM-1 expression in our study may be due to a counter-response to the initial increase of VCAM-1 expression induced by influenza A.
In addition to MAGI1, which has six PDZ domains, there are other PDZ domain-containing proteins—Dlg1, MAGI2, MAGI3, Scribble, Lin7C, PDLIM2 and PSD-95 - are potential targets of the NS1 protein of IAV that has the consensus PDZ-binding motif, ESEV (glu-ser-glu-val) (PBM). Indeed, PBM association with Scribble and Dlg1 promotes virus infection (48). Our study shows that MAGI1 also promotes virus infection, presumably via its interaction with PBM. Since MAGI1 depletion robustly inhibits IAV infection, MAGI1's role in supporting virus infection is larger than that of Scribble and Dlg1.
In conclusion, we have found that MAGI1 is involved in IAV infection of ECs. Previously, we have reported the crucial role of MAGI1 in EC activation and subsequent atherogenesis (20). In the current report, we found that IAV infection increases MAGI1 expression and enhances virus infection by inhibiting various anti-viral responses including STATs and IRF3 activation and induction of MX1 and OAS2 (Figure 7E). We also find that MAGI1 inhibits IRF3 de-SUMOylation, resulting in inhibiting IRF3 activation. These data suggest the crucial role of MAGI1 in inhibiting IAV-mediated anti-viral responses, and the induction of MAGI1 by OxLDL and IAV infection itself can accelerate further virus infection and promote severe EC activation (Figure 7E). Thus, MAGI1 as a promotor of both EC activation and virus infection, is a potential therapeutic target for influenza virus infection.
Data availability statement
The datasets presented in this study can be found in online repositories. The microarray data and MAGI1 sequence were deposited in the NCBI's Gene Expression Omnibus database (accession GSE95066) and GenBank (accession KY651081), respectively.
Ethics statement
The animal study was reviewed and approved by the Institutional Care and Use Committees of the Texas A&M Institute of Biosciences and Technology and The University of Texas MD Anderson Cancer Center.
Author contributions
YinW performed experiments, analyzed data, and drafted manuscript. J-iA planned and oversaw the project, funded research, performed data analysis, and wrote the manuscript. KC, YongW, HV, LR, and FG performed experiments. MI, VS, MN, KK, L-LL, and TT supported the experiments and interpretation of the data. EO-D, JC, SK, and KF contributed to the interpretation of the data and edited the manuscript. SE and N-TL planned and generated the study design, obtained funding, interpreted data, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was partially supported by funding's from the National Institutes of Health (NIH) to J-iA, N-TL, and JC (HL149303), J-iA (AI156921), JC (HL148338 and HL157790), N-TL (HL-134740), and SE (HL144805).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
All authors would like to thank Dr. Henry J. Pownall (Houston Methodist Research Institute) for a critical reading of this manuscript.
Glossary
Abbreviations
- DAPI, 4′
6-diamidino-2-phenylindole
- ECs
endothelial cells
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HULECs
human lung microvascular ECs
- HUVECs
human umbilical vein ECs
- IAV
influenza A virus
- ICAM-1
intercellular adhesion molecule 1
- IFN
interferon
- IFIH1
interferon-induced helicase C domain-containing protein 1
- IPA
ingenuity pathway analysis
- IRF3
interferon regulatory factor 3
- ISG
IFN-stimulated genes
- ISRE
interferon-stimulated response element
- M2
matrix protein 2
- MAGI1
membrane-associated guanylate kinase with inverted domain structure-1
- MAVS
mitochondrial antiviral signaling protein
- MOI
multiplicities of infection
- MX1
myxovirus resistance protein 1
- OAS2
2′-5′-oligoadenylate synthetase 2
- PARP9
poly(ADP-ribose) polymerase family member 9
- PBM
PDZ binding motif
- PBS
phosphate buffered saline
- NP
nucleoprotein
- OxLDL
oxidized low-density lipoprotein
- PDZ
PSD95/DiscLarge/ZO-1
- PTEN
phosphatase and tension homolog
- qRT-PCR
quantitative reverse-transcriptase polymerase chain reaction
- RIPA
radioimmunoprecipitation assay buffer
- SDS
sodium dodecyl sulfate
- SDS-PAGE
SDS polyacrylamide gel electrophoresis
- STAT1/2
signal transducer and activator of transcription
- siRNA
small interfering RNA
- VCAM-1
vascular cell adhesion molecule 1.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.791143/full#supplementary-material
Heat maps based on Ingenuity Downstream Effects Analysis (Diseases and Functions analysis). The maps show the functional hierarchy for differential gene expression in siMAGI1-transfected HUVECs with the significance and predicted activity state of gene sets identified by the Ingenuity Knowledge Base. The color-coded scales above both heat maps reflect the direction of change for each function based on activation z-scores (orange, upregulation; blue, downregulation; white, z-score of 0; gray, not available). (A) The level 1 squares in this heat map (high-level functional categories) indicate the functions predicted to increase or decrease with MAGI1 depletion. The size of each box reflects the P-value (the larger the box, the smaller the P-value). The P-values for the Infectious Disease categories are within the six highest category. The z-score for the Infectious Disease category is predicted to decrease with MAGI1 depletion. (B) The hierarchical heat map of the level 1 category Infectious Disease.
Magi1, Vcam1, and Ifnβ1 mRNA expression. (A) HUVECs were transfected with either siCont or siMAGI1. Approximately after 48 h of transfection, the cells were infected or not infected with IAV for 24 h. Total RNA was extracted then qRT-PCR was performed. Relative changes in Vcam1 mRNA expression were calculated using the comparative Ct (2−ΔΔCt) method. Ct values of Vcam1 mRNA expression were normalized to that of Gapdh (26). Each group passed the Shapiro-Wilk normality test, then two-way ANOVA followed by Turkey's multiple comparisons test was performed using the Prism software (GraphPad Software). The graph shows mean ± SD (n = 3). **p < 0.01. (B,C) HULECs were transfected with either siCont or siMAGI1. Approximately after 48 h of transfection, total RNA was extracted then qRT-PCR was performed. Relative changes in Magi1 (B) and Ifnb1 (C) mRNA expression were calculated using the comparative Ct (2−ΔΔCt) method. Ct values of each target gene were normalized to that of β-actin (26). At least, one of the groups did not pass the Shapiro-Wilk normality test, we performed an unpaired t-test with Welch's correction using the Prism software (GraphPad Software). The graph shows mean ± SD, n = 3, **P < 0.01.
Oligonucleotide sequences.
Viral infection.
Replication of virus.
Infection of mammalia.
Production of virus.
Interferon signaling.
Antimicrobial response, inflammatory response, and infectious diseases.
References
- 1.ReferencesDou D, Revol R, Ostbye H, Wang H, Daniels R. Influenza A virus cell entry, replication, virion assembly and movement. Front Immunol. (2018) 9:1581. 10.3389/fimmu.2018.01581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisfeld AJ, Neumann G, Kawaoka Y. Influenza A virus isolation, culture and identification. Nat Protoc. (2014) 9:2663–81. 10.1038/nprot.2014.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flerlage T, Boyd DF, Meliopoulos V, Thomas PG, Schultz-Cherry S. Influenza virus and SARS-CoV-2: pathogenesis and host responses in the respiratory tract. Nat Rev Microbiol. (2021) 19:425–41. 10.1038/s41579-021-00542-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piantadosi CA, Schwartz DA. The acute respiratory distress syndrome. Ann Intern Med. (2004) 141:460–70. 10.7326/0003-4819-141-6-200409210-00012 [DOI] [PubMed] [Google Scholar]
- 5.Sugiyama MG, Gamage A, Zyla R, Armstrong SM, Advani S, Advani A, et al. Influenza virus infection induces platelet-endothelial adhesion which contributes to lung injury. J Virol. (2016) 90:1812–23. 10.1128/JVI.02599-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiyoshi M, Indalao IL, Yano M, Yamane K, Takahashi E, Kido H, et al. Influenza A virus infection of vascular endothelial cells induces GSK-3beta-mediated beta-catenin degradation in adherens junctions, with a resultant increase in membrane permeability. Arch Virol. (2015) 160:225–34. 10.1007/s00705-014-2270-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong SM, Wang C, Tigdi J, Si X, Dumpit C, Charles S, et al. Influenza infects lung microvascular endothelium leading to microvascular leak: role of apoptosis and claudin-5. PLoS ONE. (2012) 7:e47323. 10.1371/journal.pone.0047323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Short KR, Kroeze E, Fouchier RM, Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis. (2014) 14, 57–69. 10.1016/S1473-3099(13)70286-X [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Tang H. Aberrant coagulation causes a hyper-inflammatory response in severe influenza pneumonia. Cell Mol Immunol. (2016) 13:432–42. 10.1038/cmi.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fong IW. New perspectives of infections in cardiovascular disease. Curr Cardiol Rev. (2009) 5:87–104. 10.2174/157340309788166679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebsur S, Vakil E, Oetgen WJ, Kumar PN, Lazarous DF. Influenza and coronary artery disease: exploring a clinical association with myocardial infarction and analyzing the utility of vaccination in prevention of myocardial infarction. Rev Cardiovasc Med. (2014) 15:168–75. 10.3909/ricm0692 [DOI] [PubMed] [Google Scholar]
- 12.Ludwig A, Lucero-Obusan C, Schirmer P, Winston C, Holodniy M. Acute cardiac injury events < /=30 days after laboratory-confirmed influenza virus infection among U.S. veterans, 2010-2012. BMC Cardiovasc Disord. (2015) 15:109. 10.1186/s12872-015-0095-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vejpongsa P, Kitkungvan D, Madjid M, Charitakis K, Anderson HV, Arain S, et al. Outcomes of acute myocardial infarction in patients with influenza and other viral respiratory infections. Am J Med. (2019) 132:1173–81. 10.1016/j.amjmed.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 14.Kytomaa S, Hegde S, Claggett B, Udell JA, Rosamond W, Temte J, et al. Association of influenza-like illness activity with hospitalizations for heart failure: the atherosclerosis risk in communities study. JAMA Cardiol. (2019) 4:363–9. 10.1001/jamacardio.2019.0549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haidari M, Wyde PR, Litovsky S, Vela D, Ali M, Casscells SW, et al. Influenza virus directly infects, inflames, and resides in the arteries of atherosclerotic and normal mice. Atherosclerosis. (2010) 208:90–6. 10.1016/j.atherosclerosis.2009.07.028 [DOI] [PubMed] [Google Scholar]
- 16.Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. (2011) 146:980–91. 10.1016/j.cell.2011.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bazaz R, Marriott HM, Francis SE, Dockrell DH. Mechanistic links between acute respiratory tract infections and acute coronary syndromes. J Infect. (2013) 66:1–17. 10.1016/j.jinf.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 18.Abe RJ, Savage H, Imanishi M, Banerjee P, Kotla S, Paez-Mayorga J, et al. Corrigendum: p90RSK-MAGI1 module controls endothelial permeability by post-translational modifications of magi1 and hippo pathway. Front Cardiovasc Med 8:663486 (2021). 10.3389/fcvm.2021.663486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe RJ, Savage H, Imanishi M, Banerjee P, Kotla S, Paez-Mayorga J, et al. 90RS.K-MAGI1 module controls endothelial permeability by post-translational modifications of MAGI1 and hippo pathway. Front Cardiovasc Med. (2020) 7:542485. 10.3389/fcvm.2020.542485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe JI, Ko KA, Kotla S, Wang Y, Paez-Mayorga J, Shin IJ, et al. MAGI1 as a link between endothelial activation and ER stress drives atherosclerosis. JCI Insight. (2019) 4:e125570. 10.1172/jci.insight.125570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobrosotskaya I, Guy RK, James GL. MAGI-1, a membrane-associated guanylate kinase with a unique arrangement of protein-protein interaction domains. J Biol Chem. (1997) 272:31589–97. 10.1074/jbc.272.50.31589 [DOI] [PubMed] [Google Scholar]
- 22.Hirabayashi S, Tajima M, Yao I, Nishimura W, Mori H, Hata Y, et al. JAM4, a junctional cell adhesion molecule interacting with a tight junction protein, MAGI-1. Mol Cell Biol. (2003) 23:4267–82. 10.1128/MCB.23.12.4267-4282.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alonso A, Domenech E, Julia A, Panes J, Garcia-Sanchez V, Mateu PN, et al. Identification of risk loci for Crohn's disease phenotypes using a genome-wide association study. Gastroenterology. (2015) 148:794–805. 10.1053/j.gastro.2014.12.030 [DOI] [PubMed] [Google Scholar]
- 24.Kirkpatrick CT, Wang Y, Leiva Juarez MM, Shivshankar P, Pantaleon Garcia J, Plumer AK, et al. Inducible lung epithelial resistance requires multisource reactive oxygen species generation to protect against viral infections. MBio. (2018) 9:e00696–18. 10.1128/mBio.00696-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonges M, Liu WM, Van Der Vries E, Jacobi R, Pronk I, Boog C, et al. Influenza virus inactivation for studies of antigenicity and phenotypic neuraminidase inhibitor resistance profiling. J Clin Microbiol. (2010) 48:928–40. 10.1128/JCM.02045-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques. (2005) 39:75–85. 10.2144/05391RV01 [DOI] [PubMed] [Google Scholar]
- 27.Paez-Mayorga J, Chen AL, Kotla S, Tao Y, Abe RJ, He ED, et al. Ponatinib activates an inflammatory response in endothelial cells via ERK5 SUMOylation. Front Cardiovasc Med. (2018) 5:125. 10.3389/fcvm.2018.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onat D, Brillon D, Colombo PC, Schmidt AM. Human vascular endothelial cells: a model system for studying vascular inflammation in diabetes and atherosclerosis. Curr Diab Rep. (2011) 11:193–202. 10.1007/s11892-011-0182-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aparisi A, Iglesias-Echeverria C, Ybarra-Falcon C, Cusacovich I, Uribarri A, Garcia-Gomez M, et al. Low-density lipoprotein cholesterol levels are associated with poor clinical outcomes in COVID-19. Nutr Metab Cardiovasc Dis. (2021) 31:2619–27. 10.1016/j.numecd.2021.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen M, Bourredjem A, Piroth L, Bouhemad B, Jalil A, Pallot G, et al. High plasma concentration of non-esterified polyunsaturated fatty acids is a specific feature of severe COVID-19 pneumonia. Sci Rep. (2021) 11:10824. 10.1038/s41598-021-90362-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masana L, Correig E, Ibarretxe D, Anoro E, Arroyo JA, Jerico C, et al. Low HDL and high triglycerides predict COVID-19 severity. Sci Rep. (2021) 11:7217. 10.1038/s41598-021-86747-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Kerkhove MD, Vandemaele KA, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. (2011) 8:e1001053. 10.1371/journal.pmed.1001053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson M. R, Geleris J, Anderson D. R, Zucker J, Nobel Y. R, Freedberg D., Ferrante A., Anderson M. R, et al. (2020). Body mass index and risk for intubation or death in SARS-CoV-2 infection : a retrospective cohort study. Ann Intern Med 173, 782–790. 10.7326/M20-3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tartof SY, Qian L, Hong V, Wei R, Nadjafi RF, Fischer H, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. (2020) 173:773–81. 10.7326/M20-3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahony R, Gargan S, Roberts KL, Bourke N, Keating SE, Bowie AG, et al. A novel anti-viral role for STAT3 in IFN-alpha signalling responses. Cell Mol Life Sci. (2017) 74:1755–64. 10.1007/s00018-016-2435-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collet B, Boudinot P, Benmansour A, Secombes CJ. An Mx1 promoter-reporter system to study interferon pathways in rainbow trout. Dev Comp Immunol. (2004) 28:793–801. 10.1016/j.dci.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 37.Verhelst J, Parthoens E, Schepens B, Fiers W, Saelens X. Interferon-inducible protein Mx1 inhibits influenza virus by interfering with functional viral ribonucleoprotein complex assembly. J Virol. (2012) 86:13445–55. 10.1128/JVI.01682-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wisskirchen C, Ludersdorfer TH, Muller DA, Moritz E, Pavlovic J. Interferon-induced antiviral protein MxA interacts with the cellular RNA helicases UAP56 and URH49. J Biol Chem. (2011) 286:34743–51. 10.1074/jbc.M111.251843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baturcam E, Vollmer S, Schluter H, Maciewicz RA, Kurian N, Vaarala O, et al. MEK inhibition drives anti-viral defence in RV but not RSV challenged human airway epithelial cells through AKT/p70S6K/4E-BP1 signalling. Cell Commun Signal. (2019) 17:78. 10.1186/s12964-019-0378-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raftery N, Stevenson NJ. Advances in anti-viral immune defence: revealing the importance of the IFN JAK/STAT pathway. Cell Mol Life Sci. (2017) 74:2525–35. 10.1007/s00018-017-2520-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Islam S, Espitia CM, Persky DO, Carew JS, Nawrocki ST. Targeting JAK/STAT signaling antagonizes resistance to oncolytic reovirus therapy driven by prior infection with HTLV-1 in models of T-cell lymphoma. Viruses. (2021) 13. 10.3390/v13071406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry AK, Chen G, Zheng D, Tang H, Cheng G. The host type I interferon response to viral and bacterial infections. Cell Res. (2005) 15:407–22. 10.1038/sj.cr.7290309 [DOI] [PubMed] [Google Scholar]
- 43.Verhelst J, Spitaels J, Nurnberger C, De Vlieger D, Ysenbaert T, Staeheli W, et al. Functional comparison of Mx1 from two different mouse species reveals the involvement of loop L4 in the antiviral activity against influenza A viruses. J Virol. (2015) 89:10879–90. 10.1128/JVI.01744-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staeheli P, Grob R, Meier E, Sutcliffe JG, Haller O. Influenza virus-susceptible mice carry Mx genes with a large deletion or a nonsense mutation. Mol Cell Biol. (1988) 8:4518–23. 10.1128/mcb.8.10.4518-4523.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al Hamrashdi M, Brady G. Regulation of IRF3 activation in human antiviral signaling pathways. Biochem Pharmacol. (2022) 200:115026. 10.1016/j.bcp.2022.115026 [DOI] [PubMed] [Google Scholar]
- 46.Kumar M, Liu H, Rice AP. Regulation of interferon-beta by MAGI-1 and its interaction with influenza A virus NS1 protein with ESEV PBM. PLoS ONE. (2012) 7:e41251. 10.1371/journal.pone.0041251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bakshi S, Taylor J, Strickson S, Mccartney T, Cohen P. Identification of TBK1 complexes required for the phosphorylation of IRF3 and the production of interferon beta. Biochem J. (2017) 474:1163–74. 10.1042/BCJ20160992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fitzgerald KA, Mcwhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. (2003) 4:491–6. 10.1038/ni921 [DOI] [PubMed] [Google Scholar]
- 49.Kubota T, Matsuoka M, Chang TH, Tailor P, Sasaki T, Tashiro M, et al. Virus infection triggers SUMOylation of IRF3 and IRF7, leading to the negative regulation of type I interferon gene expression. J Biol Chem. (2008) 283:25660–70. 10.1074/jbc.M804479200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonzalez-Mariscal L, Dominguez-Calderon A, Raya-Sandino A, Ortega-Olvera JM, Vargas-Sierra O, Martinez-Revollar G, et al. Tight junctions and the regulation of gene expression. Semin Cell Dev Biol. (2014) 36:213–23. 10.1016/j.semcdb.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 51.Moritoh K, Yamauchi H, Asano A, Yoshii K, Kariwa H, Takashima I, et al. Generation of congenic mouse strains by introducing the virus-resistant genes, Mx1 and Oas1b, of feral mouse-derived inbred strain MSM/Ms into the common strain C57BL/6J. Jpn J Vet Res. (2009) 57:89–99. 10.14943/jjvr.57.2.89 [DOI] [PubMed] [Google Scholar]
- 52.Heo KS, Lee H, Nigro P, Thomas T, Le NT, Chang E, et al. PKCzeta mediates disturbed flow-induced endothelial apoptosis via p53 SUMOylation. J Cell Biol. (2011) 193:867–84. 10.1083/jcb.201010051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scholz D, Devaux B, Hirche A, Potzsch B, Kropp B, Schaper W, et al. Expression of adhesion molecules is specific and time-dependent in cytokine-stimulated endothelial cells in culture. Cell Tissue Res. (1996) 284:415–23. 10.1007/s004410050602 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Heat maps based on Ingenuity Downstream Effects Analysis (Diseases and Functions analysis). The maps show the functional hierarchy for differential gene expression in siMAGI1-transfected HUVECs with the significance and predicted activity state of gene sets identified by the Ingenuity Knowledge Base. The color-coded scales above both heat maps reflect the direction of change for each function based on activation z-scores (orange, upregulation; blue, downregulation; white, z-score of 0; gray, not available). (A) The level 1 squares in this heat map (high-level functional categories) indicate the functions predicted to increase or decrease with MAGI1 depletion. The size of each box reflects the P-value (the larger the box, the smaller the P-value). The P-values for the Infectious Disease categories are within the six highest category. The z-score for the Infectious Disease category is predicted to decrease with MAGI1 depletion. (B) The hierarchical heat map of the level 1 category Infectious Disease.
Magi1, Vcam1, and Ifnβ1 mRNA expression. (A) HUVECs were transfected with either siCont or siMAGI1. Approximately after 48 h of transfection, the cells were infected or not infected with IAV for 24 h. Total RNA was extracted then qRT-PCR was performed. Relative changes in Vcam1 mRNA expression were calculated using the comparative Ct (2−ΔΔCt) method. Ct values of Vcam1 mRNA expression were normalized to that of Gapdh (26). Each group passed the Shapiro-Wilk normality test, then two-way ANOVA followed by Turkey's multiple comparisons test was performed using the Prism software (GraphPad Software). The graph shows mean ± SD (n = 3). **p < 0.01. (B,C) HULECs were transfected with either siCont or siMAGI1. Approximately after 48 h of transfection, total RNA was extracted then qRT-PCR was performed. Relative changes in Magi1 (B) and Ifnb1 (C) mRNA expression were calculated using the comparative Ct (2−ΔΔCt) method. Ct values of each target gene were normalized to that of β-actin (26). At least, one of the groups did not pass the Shapiro-Wilk normality test, we performed an unpaired t-test with Welch's correction using the Prism software (GraphPad Software). The graph shows mean ± SD, n = 3, **P < 0.01.
Oligonucleotide sequences.
Viral infection.
Replication of virus.
Infection of mammalia.
Production of virus.
Interferon signaling.
Antimicrobial response, inflammatory response, and infectious diseases.
Data Availability Statement
The microarray data and MAGI1 sequence were deposited in the NCBI's Gene Expression Omnibus database (accession GSE95066) and GenBank (accession KY651081), respectively. All other data, analytic methods, and study materials that support the findings of this study are available in the Data supplement or from the corresponding authors upon reasonable request.
The datasets presented in this study can be found in online repositories. The microarray data and MAGI1 sequence were deposited in the NCBI's Gene Expression Omnibus database (accession GSE95066) and GenBank (accession KY651081), respectively.