Abstract
Tendinopathy is a term used to describe tendon disorders that are marked by pain and a loss of function. Recent studies demonstrated that inflammation plays an important role throughout the broad spectrum of tendinopathy. Conventional treatments such as steroid injections, analgesics, and physical modalities simply give pain relief and do not alter the disease progression without the tendon regeneration effect. Tenocytes are responsible for maintaining the tendon matrix and understanding how they function is essential to studying new treatments for tendinopathy. Our previous study showed the protective effects of vitamin D (Vit D) on damaged tenocytes. Besides its well-known effects on bone metabolism, the non-classical action of Vit D is the pleiotropic effects on modulating immune function. In the present study, we developed a Vit D delivery system with hyaluronic acid (HA), which is one of the major components of the extracellular matrix that has anti-inflammation and wound-healing properties. A novel Vit D delivery system with cross-linked HA hydrogel (Gel) and Tween 80 (T80), Vit D@Gel/T80, could be a new regeneration technique for the treatment of tendinopathy. Vit D@Gel/T80 reduced TNF-α induced damage to human tenocytes in vitro. In an animal study, the Vit D@Gel/T80 injected group demonstrated tendon restoration features. As a result, this Vit D@Gel/T80 system might be a local injection material in the treatment for tendinopathy.
Keywords: Tendinopathy, tendon regeneration, HA hydrogel, vitamin D, Tween 80
Introduction
Tendinopathy is a widespread musculoskeletal condition associated with overuse and aging. Although its pathogenesis is multi-faceted, inflammation is considered to be the key mechanism of tendinopathy.1,2 Traditional anti-inflammatory treatment such as glucocorticoid injection failed to have long-term benefits, conversely resulting in damage to the tendon structure.3,4 In this context, regenerative therapy based on platelet-rich plasma, growth factors, or stem cells has been tested as alternatives.5,6 Nonetheless, none of the aforementioned techniques have demonstrated repeatable and favorable results in terms of tendon repair.7,8 As a result, clinical solutions for effective regenerative therapy are lacking.
Vitamin D (Vit D) is a pro-hormone that is the essential precursor of the potent steroid calcitriol, classically regulates calcium and phosphate metabolism. 9 Additionally, vitamin D is fat-soluble and plays a crucial role in the development of bone, skeletal muscle, and overall health. Vit D insufficiency leads to low bone mineral density, bone fractures, osteopenia, osteoporosis, and muscle weakness. 10 Moreover, vitamin D exerts anti-inflammatory effects on the immune cell lines thus playing an important role in the pathogenesis of tendinopathy. 11 The physiologically active form of vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] causes down-regulation of proinflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ) and up-regulation of anti-inflammatory cytokines such as interleukin-10 (IL-10) in Mycobacterium tuberculosis-infected human peripheral blood mononuclear cells. 12 Additionally, Vit D regulates reactive oxygen species levels, cyclooxygenase activity, and nuclear factor-kappa B (NF-κB) pathways through its anti-inflammatory effects. 13 In our previous study, Vit D displayed its protective property in damaged tenocytes, suggesting its use as a potential therapeutic agent for tendinopathy. 14 Additionally, Vit D increased proliferation and repaired tendon-related indicators in injured tenocytes through simulating the extracellular signal-regulated kinases (ERK) and p38 pathways. 14 Therefore, Vit D could be a good therapeutic candidate for tendon restoration by effectively modulating the immune response and cell proliferation.
In addition to bioactive molecules, a scaffold is an important component of tissue engineering for tendon regeneration. 15 Many attempts have recently been made in the field of tissue engineering to develop an effective biomaterial for tendon repair. Jiang et al. 16 for example, proposed a 3D printed multilayer scaffold for tendon regeneration that included collagen-fibrin hydrogels and stem cells and showed great potential tendon healing capability. Additionally, Cai et al. 17 investigated a microfiber-nanofiber core-sheath yarns scaffold to see whether it may improve biocompatibility and biomechanical properties. Several studies have used natural biomaterials such as collagen, fibrin, and hyaluronic acid (HA) to mimic the extracellular matrix (ECM) of tendon. 18
ECM-based hydrogels (such as collagen, HA, glycosaminoglycans, fibronectin, and others) are particularly useful as biomaterials, because they can imitate the natural environment. 19 Among them, HA is one of the most commonly used materials for drug delivery. 20 HA, a widely distributed polysaccharide in mammalian connective tissue, is found to be an important molecule of the tendon ECM. 21 For decades, HA hydrogel has been widely used as a biomaterial in arthritis research as a lubricant, 22 and its application has lately been extended to other fields such as tendinopathy. Tendon sheath secretes HA, which promotes gliding motion and reduces tendon adhesion. 23 Besides its lubricating properties, the anti-inflammatory impact has been identified as one of its benefits for tendon pathologies. 24 Hence, HA hydrogel-based drug delivery systems have been widely used in various tissue regeneration procedures due to their excellent features, such as biocompatibility, water absorption, structural rigidity, and drug retentibility.25,26 Niiyama and Kuroyanagi 27 developed vitamin C loaded-HA and collagen scaffold for wound healing. Hsiao et al. 28 suggested that antioxidant-loaded HA hydrogel might be used as a drug carrier system to treat tendinopathy. HA possesses its protective effects in tendinopathy, but it can also be used as a scaffold for another drug.
Controlled release from HA provides advantages regarding safety and efficacy.29,30 We hypothesized that HA would have a synergistic effect with Vit D as an appropriate delivery system for tendon regeneration.
In the current study, we developed a biocompatible, cell-free, non-invasive, injectable, and long-term stable Vit D delivery system. The Vit D@Gel/T80, miscible with cross-linked HA hydrogel (Gel) using surfactants such as Tween 80 (T80), was utilized for tendon restoration. The anti-inflammation, anti-apoptosis, and cell viability properties were investigated using human tenocytes in vitro. In addition, a collagenase-induced animal model was employed to assess the in vivo efficacy of Vit D@Gel/T80 in tendon regeneration.
Materials and methods
Materials
Vitamin D3 (Vit D, Cholecalciferol), Kolliphor®P188 (Poloxamer 188, F-68), Tween®20 (T20), and Tween®80 (T80) were purchased (Sigma-Aldrich, USA). The cross-linked hyaluronic acid (HA, 20 mg/ml) hydrogel with 1,4-butanediol diglycidyl ether (BDDE) was provided (Hyundae Meditech Co., Ltd., Korea).
Methods
Preparation of Vit D@Gel/Surfactant
The Vit D@Gel/T80 was prepared in two steps. In the first step, Vit D (7.7 mg) was dissolved in ethanol (400 μl). This solution (0.8 μl) was sonicated for 30 min in phosphate-buffered saline (PBS, pH 7.4) solution (19.2 μl) containing F-68, T20, or T80 (0.4% and 0.8%, w/v), respectively. In the second step, the water-insoluble Vit D nanoparticle dispersion was stabilized overnight at 4°C. The stabilized dispersion was mixed with HA hydrogel (ratio 1:1).
Dispersion stability test
Turbidity measurements and the size of vitamin D nanoparticles were used to evaluate the stability of the Vit D delivery systems that were cross-linked with HA hydrogel using F-68, T20, or T80 surfactants. The turbidity measurements were performed using a SpectraMax M2 Microplate Reader (Molecular Devices; California, USA). Subsequently, the Vit D nanoparticle dispersion on the 96-well plate was observed at a wavelength of 550 nm. Additionally, the size distributions of Vit D nanoparticles were determined with the Zetasizer instrument (Malvern Instruments Ltd., UK).
Fabrication of the Vit D@Gel/T80
To dissolve Vit D completely, T80 was added into a PBS solution (0.4%, w/v). Vit D was first dissolved in ethanol. Subsequently, 20 μl of the HA hydrogel was blended with 20 μl of the abovementioned solution with 1 ml Henke-Ject (HENKE SASS WOLF Co., Germany) in sequence.
Gel swelling test
The HA hydrogels were distributed (40 μl) in a 24-well cell culture insert with 1 ml of PBS solution. The inserts were then removed from the plate, wiped with tissue paper to remove any remaining water, and the remaining masses were weighed immediately.
Rheological analysis
The rheological characteristics of hydrogels were analyzed using a stress-controlled rheometer from Anton-Paar (Graz, Austria), which was employed with a parallel-plate geometry at a diameter of 25 mm. The gap was fixed at 1000 μm. The frequency sweep was carried out with a frequency range of 0.1–10 Hz at 25°C to determine the linear viscoelastic (LVE) zone for the hydrogel.
In vitro Vit D release study
For the innate release test of Vit D, a 40 μl sample was injected in 1 ml of PBS solution containing T20 (0.5%, w/v). At a predetermined time, the released Vit D was extracted from the solution and freeze-dried overnight. Thereafter, the supplement was refilled with 0.5 ml of PBS solution containing T80. Additionally, a minimum of three trials were conducted with different gel formulations.
High-performance liquid chromatography (HPLC) analysis
An HPLC system (Agilent 1100 series, Santa Clara, USA) equipped with a UV detector was used for the chromatographic analysis. The separation was carried out using a Luna 3u c18(2) 100A, LC column measuring 150 × 460 mm, with a 3-micron particle size (Phenomenex Inc.; Torrance, USA). The mobile phase was composed of 70% acetonitrile, 25% methanol, and 5% water with a flow rate of 1 ml/min. A total of 10 μl of each sample was injected into the column to carry out the analysis. The detection wavelength was carried out at 265 nm for 15 min. 31 To stabilize the peaks, the blank samples were measured at least once before starting the measurement. Additionally, the ChemStation (Agilent™) suite was used to conduct the LC analysis.
Cell culture and reagents
Human primary tenocytes were purchased (Cryopreserved Adult Tenocytes, TEN-F, ZenBio Inc., NC, USA) and grown in Dulbecco’s modified Eagle’s minimal essential medium (HyClone Laboratories Inc., UT, USA), supplemented with 10% fetal bovine serum (HyClone; UT, USA) and 1% antibiotic antimycotic solution for less than passage 5 (100X; Gibco, USA). The cells were maintained at 37°C in a controlled humidified air atmosphere supplied with 5% CO2, and the medium was changed every 3 days. Before culturing, the plates were coated with collagen type I (RatCol® Rat Tail Collagen; Advanced BioMatrix; CA, USA). Using filtered 0.1% acetic acid solution, further dilution was carried out to achieve the desired concentration. Additionally, the working concentration was adjusted to 100 μg/ml in a sterile 0.1% acetic acid solution. Subsequently, the cells were cultured in an incubator that was adjusted to 5% CO2 and 37°C in a controlled humidified atmosphere. Every 2 days, the medium was replaced and cultivated until 80% confluence was reached.
Cytotoxicity assays of the Vit D@Gel/T80
In a 24-well plate, human tenocytes were planted at a density of 8000 cells per well. To induce cell damage, the cells were treated with TNF-α (PEPROTECH; NJ, USA) at a concentration of 25 ng/ml. Additionally, to provide indirect diffusion of Vit D, 24-well inserts were used. The HA hydrogel was placed at a quantity of 40 μl/well. Subsequently, the cell viability was measured by a CCK-8 assay. To begin, 50 μl of CCK-8 solution was added to each well and incubated for 1 h at 37°C. The optical density was then measured using a microplate reader at 450 nm. For live-dead staining, cells were treated for 15 min at 37°C in the dark with 2 μM calcein AM and 4 μM EthD-1 (Invitrogen; Thermo Scientific Inc., Waltham, MS, USA) in PBS solution and then observed using a fluorescence microscopy.
Antioxidant effect of the Vit D@Gel/T80
2,2-Diphenyl-1-(2,4,6-trinitrophenyl)-hydrazyl and 2,7-dichlorodihydrofluorescein diacetate (DCF-DA) were purchased (Cayman, MI, USA). Human tenocytes were cultured and treated with an indirect diffusion technique in the same way as the cell cytotoxicity assay conducted earlier. Then, 20 μM DCF-DA was treated for 45 min at 37°C in the dark. Live and DCF-DA positive cells were imaged by fluorescent microscopy (CKX53, Olympus, Tokyo, Japan).
Quantitative real time PCR (qRT-PCR) analysis of human tenocyte and rat tendon tissue
To analyze the effect of the Vit D@Gel/T80 on the proliferation and inflammation of tenocytes, the gene expression levels of relevant proteins were evaluated using qRT-PCR from tenocytes and tendon tissues. The cells under the same experimental conditions as the cytotoxicity test were treated with TNF-α and incubated for 72 h before mRNA was retrieved. For mRNA isolation from whole tissue, Trizol reagent (Invitrogen; Thermo Scientific Inc., Waltham, MS, USA) was used. The cDNA was then synthesized using random hexamer primers and TAKARA RT reagent kit (Perfect Real time; Takara Bio Inc., MI, USA). Supplemental Tables S1 and S2 lists all primer pairs used in qRT-PCR, which was carried out with SYBR Green Master Mix (Applied Biosystems, CA, USA). The expression of target genes was standardized by 18S rRNA as a reference gene.
Immunohistochemistry (ICC) and Western blot analysis
Human tenocytes were fixed for 15 min in a 4% paraformaldehyde/PBS solution, permeabilized for 30 min in 0.3% Triton X-100, and blocked for 1 h in a 5% BSA/PBS solution. The cells were then probed with a Col 1 antibody (Santa Cruz Biotechnology; CA, USA) in a 1% BSA/PBS solution overnight at 4°C. Following three consecutive washes with the PBS solution, the cells were incubated with the secondary antibody (Alexa-Fluor 568 goat antirabbit; Molecular Probes Inc.; OR, USA) in 1% BSA/PBS solution at RT for 1 h in the dark. After three washes with PBS solution, the cells were mounted using the Vectashield mounting medium with DAPI (Vector Laboratories Inc.; CA, USA). The cells were imaged using fluorescent microscopy (Olympus IX 71 microscope, Center Valley, PA).
The cells were washed twice with PBS solution and then lysed for 30 min at 4°C using the RIPA cell lysis buffer. The lysates were then centrifuged for 15 min at 13,000 rpm at 4°C. The protein concentration was subsequently determined using the Pierce™ BCA Protein Assay Kit (Thermo Scientific Inc., Waltham, MS, USA). The protein lysates (15 μg) were loaded and separated on a 10% SDS-PAGE gel before being transferred onto nitrocellulose membranes. After blocking with 5% Difco™ Skim Milk (BD Difco; NJ, USA), the membrane was then incubated overnight at 4°C with primary antibodies such as antiNFκB p65 Antibody (sc-8008, 1/200, Santa Cruz Biotechnology, TX, USA), antiIL-6Rα Antibody (sc-373708, 1/500, Santa Cruz Biotechnology, TX, USA), COX2 Polyclonal Antibody (aa 570–598, 1/200, Cayman Chemical, MI, USA), and antiβ-Actin Antibody (sc-47778, 1/1000, Santa Cruz Biotechnology, TX, USA). The membranes were then washed in TBS-T and labeled with mouse antirabbit IgG HRP (sc-2357, 1/5000, Santa Cruz Biotechnology, TX, USA) and antimouse IgG HRP-linked antibody (7076S, 1/5000, Cell Signaling Technology, Massachusetts, USA). Finally, the immunoreactivity was revealed by the use of the ECL Select™ Luminal Solution (Cytiva; MA, USA).
Animal model and surgery procedure
The experimental protocol for the use of animals was approved by the Institutional Animal Care and Use Committee at CHA University (IACUC200107). A total of 40 male Sprague-Dawley rats (8 weeks old; 230–330 g in weight) were used in this study (Supplemental Table S3). Following shaving, a 1 cm of longitudinal skin incision was made in the medial region of the hind limb. Subsequently, collagenase (30 μl, 20 mg/ml) was injected into the aforementioned region. 32 Finally, the tendon skin was sutured by black silk 4–0 (AILEE Co., Korea). After the collagenase-induced tendinopathy procedure, rats were randomly assigned to post-injury groups. Following a period of 2 weeks, the incision was made again, and 40 μl of HA hydrogel was injected into each group using a 26 G needle (KOVAX, Korea). After 2 and 4 weeks post-injection, both Achilles tendons were harvested under general anesthesia. The tendons were then sliced into sections along the sagittal plane.
Histological analysis
The tendon specimens were fixed with 10% formalin solution (Sigma-Aldrich, Germany) for 72 h. Subsequently, the samples were dehydrated in anhydrous ethanol. The samples were then processed and prepared for paraffin tissue slides following the standard procedure. Hematoxylin and eosin (H&E; Abcam, UK) and Masson’s trichrome (MT; VitroVivo Biotech, MD, USA) were used to stain the 5 µm thick slides. Each staining adhered to the established guidelines.
Statistical analysis
All experiments were repeated at least three times. The results are shown as means ± SD. #p < 0.0001, ***p < 0.001, **p < 0.01, and *p < 0.05 indicate statistically significant differences, respectively. Statistically significant differences were evaluated by one-way analysis of variance with post hoc analysis (Tukey method) using GraphPad Prism 7.0 software (GraphPad Software Inc., CA, USA). 33
Results and discussion
Fabrication and characterization of the vitamin D delivery system with the Vit D@Gel
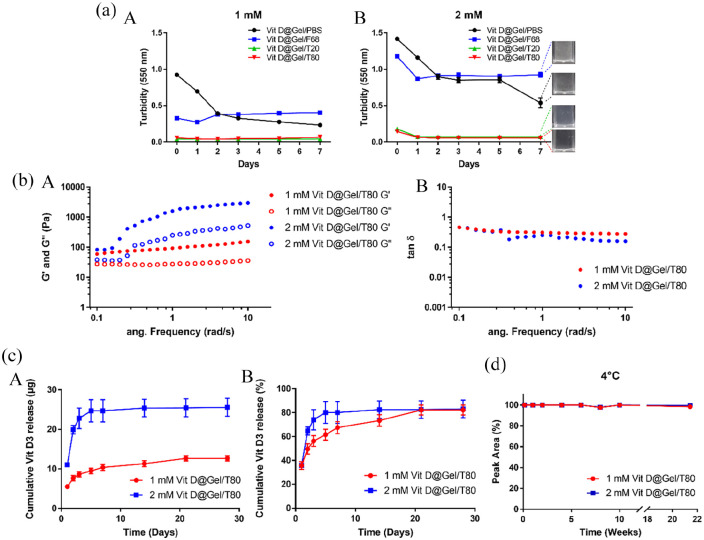
Based on our prior research, Vit D (specifically D3) was chosen as a growth factor to promote tendon regeneration as a drug enriched in HA. 14 Because Vit D is widely recommended for bone health, it could be a safe candidate. Vitamin D consumption (800–1000 IU/day, 1 IU = 0.025 μg) is recommended by the National Osteoporosis Foundation for postmenopausal women and men aged 50 and older. 34 Typical Vit D3 intramuscular injections used 200,000 IU (5 mg) per injection with a 3- or 6-month interval between injections. 35 Thus, based on the existing clinical practice of D3 injections, we hypothesized that 1 and 2 mM Vit D in a single injection would be of the appropriate concentration. However, unlike water-soluble vitamins, because fat-soluble vitamins have low loading efficiency and homogeneity in the hydrogel matrix, large concentrations of Vit D are frequently incompatible with hydrogel. Because of its short half-life and hydrophobicity, Kim et al. 36 suggested using a Vit D delivery method loaded in poly(lactic-co-glycolic) acid nanoparticles on microneedles. But, 82.2% of Vit D released within 48 h in their system. Therefore, in this study, we applied a nanoemulsion-based dispersion method to fabricate the controlled Vit D delivery system in HA hydrogel (Vit D@Gel) for long-term using surfactants such as Poloxamer 188 (F68), Tween®20 (T20), or Tween®80 (T80). 37 Figure 1(a) (A, B) shows that the Vit D@Gel/F68 had a higher turbidity value than the Vit D@Gel/T20 and Vit D@Gel/T80 after 7 days at concentrations of 1 and 2 mM. This result showed that the dispersion stability of Vit D nanoparticles in Vit D@Gel/F68 decreased, and Vit D nanoparticles aggregated. On the other hand, the Vit D@Gel/T20 and the Vit D@Gel/T80 had shown stable dispersion for 7 days.
Figure 1.
Injectable cross-linked HA-based Vit D delivery system. (a) Dispersion of Vit D in HA hydrogel with three different surfactants: Pluronic F-68 (Vit D@Gel/F68), Tween 20 (Vit D@Gel/T20), and Tween 80 (Vit D@Gel/T80). (A, B) Turbidity assay at 550 nm for 7 days. (b) Rheology measurement of the 1 and 2 mM Vit D@Gel/T80. (c) In vitro Vit D release profile of the 1 and 2 mM Vit D@Gel/T80 for 4 weeks. (d) In vitro long-term stability test of the 1 and 2 mM Vit D@Gel/T80 at 4°C for 22 weeks.
HA: hyaluronic acid; Vit D: vitamin D.
The size distribution of Vit D nanoparticles prepared with different types of surfactants (T20 and T80) and different concentrations of Vit D was shown in Figure S2 (1 and 2 mM). The size of the Vit D nanoparticles varied depending on the surfactant, and T80 produced Vit D nanoparticles that were smaller than T20. This finding is in line with a recent study that used emulsification to create Vit D nano-emulsions. 38 Furthermore, higher Vit D concentration (1 → 2 mM) increased the mean size of Vit D nanoparticles in both surfactants (3.6 → 6.8 nm for T80 and 7.8 → 8.1 nm for T20) although still Vit D nanoparticles for T80 maintained smaller mean size. Consequently, T80 was adopted to preparation of the further Vit D delivery system for tendon repair. The 1 mM Vit D@Gel/T80 was more stable than the 2 mM Vit D@Gel/T80 in Figure 1(b). In both concentrations of Vit D@Gel/T80, the storage modulus (G’) was larger than the loss modulus (G”), indicating solid-like behavior with a three-dimensional (3D) structure following injection. In both 1 and 2 mM Vit D@Gel/T80, the ratio of viscous to elastic response (tan δ) was less than 1, indicating the hydrogel’s elasticity. Additionally, ATR-FTIR spectra for the HA, 100% Gel, 50% Gel/T-PBS, and Vit D@Gel/T80 are shown in Supplemental Figure S3. The characteristic peaks of HA were recorded at 3270 cm−1 (-OH stretching vibration), 2930 cm−1 (stretching vibration of CH2), 1605 and 1406 cm−1 (symmetric and asymmetric stretching vibrations of COO-), and 1028 cm−1 (C–O–C stretching vibration). 39 The characteristic peaks of HA were also observed in spectra of the 100% Gel, 50% Gel/T-PBS, and Vit D@Gel/T80. After Cross-linking or the addition of T-PBS and Vit D did not change the characteristic bands of HA gel. 40 Moreover, the long-term Vit D stability of the drug was analyzed using HPLC for 22 weeks at 4°C and 25°C, respectively (Supplemental Figure S3). Although there was no change in vitamin D peak area or isomerization in Vit D@Gel/T80 at 4°C after 150 days, Vit D@Gel/T80 at 25°C changed to pre-, trans-, and tachysterol type isomers. 41 Thus, our Vit D delivery system (Vit D@Gel/T80) could be employed as an injectable therapeutic or regenerative substance to supplement a surgical implant for tendon rupture healing. 42
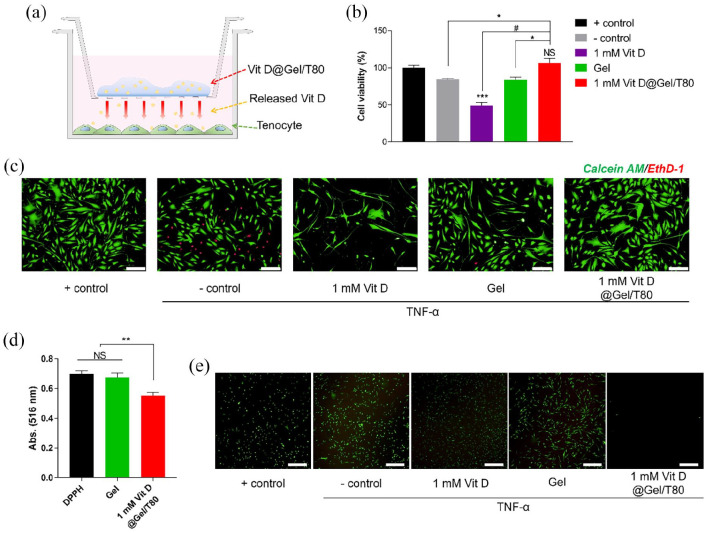
Biocompatibility of the Vit D@Gel/T80 in vitro
To assess in vitro biocompatibility of the Vit D@Gel with human tenocytes and to simulate local injection, the trans-well inserts were used. The trans-well insert device formed an indirect contact environment on cells with the Vit D@Gel/T80 (Figure 2(a)). TNF-α is a cytokine that induces apoptosis and is a key player in the inflammatory cascade. 43 TNF-α, IL-6, and monocyte chemoattractant protein 1 (MCP-1) all increased in plasma in tendinopathy patients. 2 Human tenocytes were treated with TNF-α to induce tendinopathy-like damage. After 72 h of exposure to TNF-α, tenocyte viability was evaluated using CCK-8 (Figure 2(b)). TNF-α treated, TNF-α untreated, Gel only, Vit D (1 mM) only, and 1 mM Vit D@Gel/T80 were the five groups conducted in the study. When compared to the positive control (TNF-α untreated), cell viability fell by roughly 49% in group simply treated with Vit D and 16% with Gel. Interestingly, the 1 mM Vit D treated group without Gel group showed lower viability than the negative control group (TNF-α treated). Previously, our study stated that Vit D improved cell viability in a dose-dependent manner (1, 10, 20, and 40 μM). 14 We decided to use a much higher concentration such as 1 mM of Vit D to maintain its effect for a longer time in our HA hydrogel system. This finding suggests that 1 mM Vit D may be toxic to tenocytes in the absence of a delivery method. On the other hand, when Vit D was encapsulated in Gel, cell viability increased without causing harm to the cells. Because the Gel suppressed the initial burst release of Vit D and allowed it to be delivered continuously. The 1 mM Vit D@Gel/T80 group exhibited similar cell viability with the control group (TNF- α untreated) (p < 0.05). The cell viability test was represented by the live/dead staining (Figure 2(c)). Tenocyte viability was considerably reduced when cells were exposed to a high concentration of Vit D (1 mM) directly. The 1 mM Vit D@Gel/T80 group had the highest ratio of live cells to dead cells of all the groups. As a result, the 1 mM Vit D@Gel/T80 might be an effective way to enhance the proliferation of TNF-α damaged human tenocytes.
Figure 2.
(a) Schematic illustration for cell cytotoxicity test of the Vit D@Gel/T80 using TNF-α damaged human tenocytes. (b) Cell viability test by CCK-8 for 24 h. (c) Live/dead staining images for 24 h. Scale bar indicates 200 µm. (d) 2,2-Diphenyl-1-(2,4,6-trinitrophenyl)-hydrazyl (DPPH) radical scavenging activity and (e) fluorescence microscopy images of H2 DCF-DA-treated tenocytes. All values are expressed as the mean ± SEM. N ⩾ 3, *p < 0.05, **p < 0.01, and #p < 0.0001.
TNF- α: tumor necrosis factor-α.
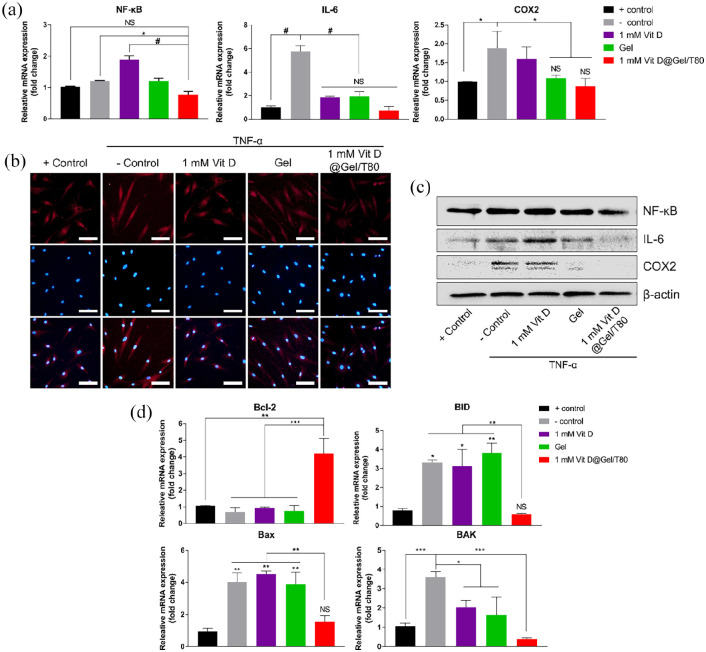
Anti-inflammatory and anti-apoptotic responses of the Vit D@Gel/T80 with TNF-α damaged human tenocyte in vitro
As mentioned previously, Vit D might have a crucial role in the proper activation of the immune system.12,44 We further hypothesized that the secreted Vit D from the Vit D@Gel/T80 would reduce tendon inflammation. Hence, we evaluated the anti-inflammatory and furthermore, anti-apoptotic abilities of the Vit D@Gel/T80 using TNF-α damaged human tenocytes. Quantitative real-time PCR (qRT-PCR) was performed to determine how Vit D functioned on tenocytes at the RNA level. Same as the above-mentioned experiment, TNF-α was treated with all of the groups to induce inflammatory responses. NF-κB is a transcription factor that directs the transcriptional activity of various promoters of proinflammatory cytokines like interleukin 6 (IL-6). 45 Cyclooxygenase-2 (COX2) is expressed by inflammatory cells such as macrophages and is known to be inhibited by Vit D. 13 After being exposed to TNF-α, the gene expressions of NF-κB increased considerably as shown in Figure 3(a). The gene expressions of NF- κB at the 1 mM Vit D@Gel/T80 level, on the other hand, were downregulated to a level similar to that of the control group. The gene expressions of IL-6 and COX2 were lower in the 1 mM Vit D@Gel/T80 group compared to the solely treated 1 mM Vit D group and the negative control (TNF-α treated) group (7.67 and 2.17 times each). This result implied that Vit D treatment with the proper delivery system has an effect on anti-inflammation caused by TNF-α. According to the result of NF-κB, Vit D without any adequate vehicles increased the immune response excessively, and consequently the excessive Vit D administration caused cytotoxicity on tenocytes in the cell viability test. Additionally, an immunocytochemistry (ICC) assay was conducted with an antiNF-κB antibody. Figure 3(b) shows that the number of NF-κB-positive cells notably decreased in the 1 mM Vit D@Gel/T80 group compared to that of the negative control. Similar results were observed in NF-κB, IL-6, and COX2 protein levels by Western blot analysis (Figure 3(c)).
Figure 3.
In vitro biological properties of the Vit D@Gel/T80 in TNF-α damaged human tenocytes. (a) The mRNA expression levels of inflammation-related gene (NF-κB, IL-6, and COX2) by qRT-PCR. (b) Immunocytochemistry labeled with antiNF-κB antibody. Scale bar indicates 100 µm. (c) The expression levels of inflammation-related proteins (NF-κB, IL-6, and COX2) using Western blot analysis. (d) The mRNA expression levels of an apoptosis-related gene (Bcl-2, BID, Bax, and BAK) by qRT-PCR. *p < 0.05, **p < 0.01, ***p < 0.001, and #p < 0.0001 indicate statistically significant differences, respectively.
NF-κB: nuclear factor-kappa-light-chain-enhancer of activated B cells; IL: interleukin; COX2: cyclooxygenase 2; Bcl-2: B-cell lymphoma 2; BID: BH3-interacting domain death agonist; Bax: Bcl-2 associated X; BAK: Bcl-2 homologous antagonist killer.
Only the 1 mM Vit D@Gel/T80 group showed a significant increase (6.10 times) in the expression of the B-cell lymphoma 2 (BCL-2) gene, which inhibits cell destruction as an anti-apoptotic molecule (Figure 3(d)). The pro-apoptotic genes, such as BH3 interacting domain death agonist (BID), Bcl-2-associated X protein (BAX), and Bcl-2 homologous killer (BAK) were significantly down-expressed in the 1 mM Vit D@Gel/T80 group compared to negative control (5.69, 2.57, and 9.14 times each). As a result of Vit D’s activity in modulating the ERK and p38 pathways, 14 the 1 mM Vit D@Gel/T80 possesses excellent anti-inflammation and anti-apoptosis properties for tendon regeneration.
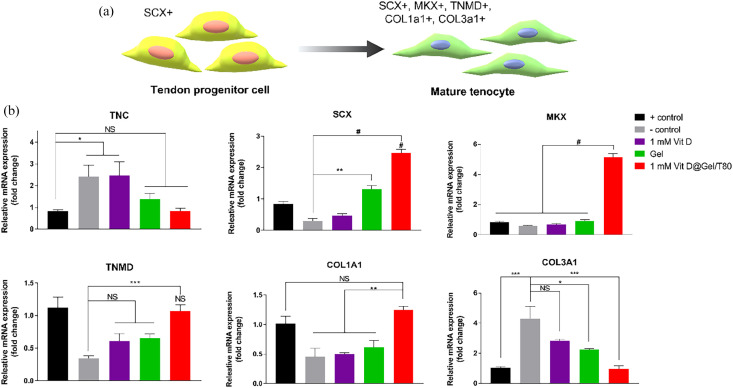
Tendon repairing ability of the Vit D@Gel/T80 in vitro
As shown in Figure 4(a), the tendon progenitor cells expressed scleraxis (SCX) and mohawk (MKX). Tenomodulin (TNMD) and collagen types 1 and 3 are all expressed once the cell has matured into mature tenocytes. 46 The RT-PCR was analyzed to confirm the ability of Vit D and Gel to differentiate. Tenascin C (TNC) was formerly thought to be overexpressed in inflammatory or infectious conditions. 47 As seen in Figure 4(b), mRNA expression level of TNC was up-regulated in the presence of TNF-α. The groups treated with Gel and 1 mM Vit D@Gel/T80 effectively reduced TNC expression to a level similar to that of the native group. Although the inflammatory response is known that suppress the differentiation of tenocyte progenitor cells, the 1 mM Vit D@Gel/T80 could restore damaged tenocyte progenitor cell through attenuating the inflammation response. The 1 mM Vit D@Gel/T80 can develop progenitor cells into tenocytes by significantly increasing the expression of tenocyte-related markers (SCX and MKX). Ultimately, when compared to the control group, the expression of TNMD, a mature tendon marker, was recovered identically.
Figure 4.
(a) Schematic illustration of tendon-related marker expressions during tendon development for regenerative capacity analysis of the Vit D@Gel/T80 using TNF-α damaged human tenocyte in vitro. (b) The mRNA expression levels of the tendon-related gene in TNF-α damaged human tenocytes by qRT-PCR. *p < 0.05, **p < 0.01, ***p < 0.001, and #p < 0.0001 indicate statistically significant differences, respectively.
TNC: tenascin C; SCX: scleraxis; MKX: mohawk; TNMD: tenomodulin; COL1A1: collagen type 1 alpha 1; COL3A1: collagen type 3 alpha 1.
Because of TNF-α damage, the expression of collagen type 1 alpha 1 (COL1A1) mRNA expressions in the negative control, 1 mM Vit D only, and Gel substantially decreased. However, COL1A1 expression was up-regulated in the 1 mM Vit D@Gel/T80. Finally, the negative control group showed aberrant collagen type 3 alpha 1 (COL3A1) expression. The expression of COL3A1 in the 1 mM Vit D, Gel, and 1 mM Vit D@Gel/T80 groups, on the other hand, decreased gradually. The level of COL3A1 expression was investigated negligible difference between healthy tenocytes and the 1 mM Vit D@Gel/T80. SCX and MKX are tendon-ligament-specific markers that are expressed in the early stages of tendon formation. SCX-expressing early tendon progenitor cells, in particular, are responsible for tendon tissue development and muscle attachment. MKX and TNMD are well-known tendon-specific markers that belong to the type II transmembrane glycoproteins family and are expressed during tendon development. Tendon is also a collagen-rich tissue that resists tensile forces. Collagen type 1 accounts for about 95% of the collagen in the body. Collagen type 1 is considered one of the key indications of tendon regeneration. The second most frequent kind of collagen found in a tendon is collagen type 3. However, at the tendon rupture site, there is excessive collagen type 3 expression. In the same context, Liu et al. 48 reported the use of nitric oxide-releasing exosome-loaded microneedles to treat Achilles tendinopathy, which improved collagen type 1 expression while reducing collagen type 3 expression in vivo. Consequently, the 1 mM Vit D@Gel/T80 showed excellent tenocyte regeneration in vitro.
In vivo tendon restoration effect of the Vit D@Gel/T80
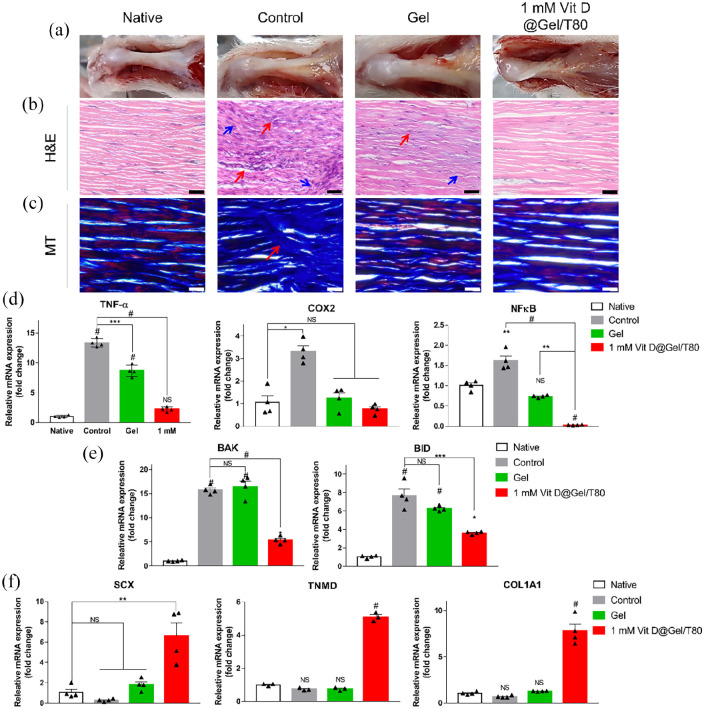
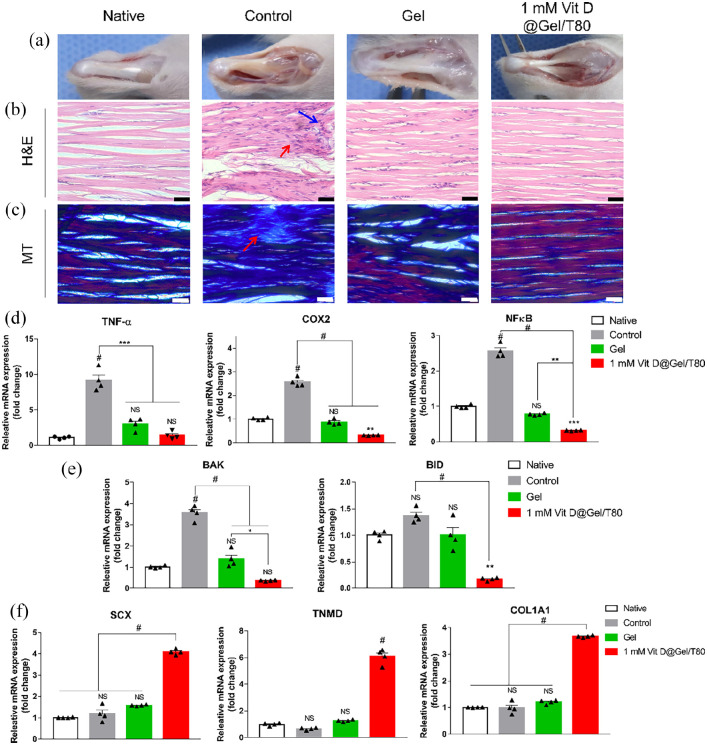
To investigate tendon regeneration ability in vivo, we investigated the hydrogel and 1 mM Vit D@Gel/T80 using a collagenase-induced animal tendinopathy model. Two weeks after collagenase injection into the Achilles tendons, 1 mM Vit D@Gel/T80 was injected into the lesion site. The gross images of each group were examined at 2 and 4 weeks following injection in Figures 5(a) and 6(a). The negative control has an opaque yellow tissue on the surface. At 4 weeks following injection, the regenerated tendon in the 1 mM Vit D@Gel/T80 restored its original clear glossy appearance, which was similar to that of a native tendon (Figure 5(a)). Histological analyses utilizing H&E and MT stainings were used to demonstrate the tissue regeneration impact of the 1 mM Vit D@Gel/T80 (Figure 5(b) and (c)). Tendon tissue in the control group was observed abnormal fiber structure and arrangement based on H&E and MT stainings (red arrow). The Gel group’s fiber arrangement was restored 2 weeks after injection, as seen in Figure 6(b) and (c). The morphology and positioning of nuclei are also crucial histology indicators of tendon health. Round nuclei were found in the control and gel groups (blue arrow); however, the 1 mM Vit D@Gel/T80 group had the most similar nuclei appearance to the native group, with a flat shape.
Figure 5.
In vivo evaluation using collagenase-induced rat tendinopathy model in 2 weeks after injection. (a) Gross images of the injected site. (b) H&E staining images of the tendon (top) and muscle (below). (c) MT staining images of the tendon. Scale bar indicates 40 µm. The mRNA expression levels of (d) inflammation-, (e) apoptosis-, and (f) tendon-related genes in rat tendon. *p < 0.05, **p < 0.01, ***p < 0.001, and #p < 0.0001 indicate statistically significant differences, respectively.
H&E: Hematoxylin and eosin; MT: Masson’s trichrome.
Figure 6.
In vivo evaluation using collagenase-induced rat tendinopathy model in 4 weeks after injection. (a) Gross images of the injected site. (b) H&E staining images of the tendon (top) and muscle (below). (c) MT staining images of the tendon. Scale bar indicates 40 µm. The mRNA expression levels of (d) inflammation-, (e) apoptosis-, and (f) tendon-related genes in rat tendon. *p < 0.05, **p < 0.01, ***p < 0.001, and #p < 0.0001 indicate statistically significant differences, respectively.
In addition, the mRNA expressions were examined to further support the histological results and to confirm the internal tendon tissue restoration. Inflammation-related genes (TNF-α, COX2, and NF-κB) had their mRNA expressions downregulated in the 1 mM Vit D@Gel/T80, which was significantly lower than the control and decreased to a level comparable to those of native cells (Figures 5(d) and 6(d)). And apoptosis-related gene expressions (BAK and BID) were likewise significantly lower than in that of the control group (p < 0.0001). Finally, at 2 and 4 weeks after injection, mRNA expressions of tendon-related genes (SCX, TNMD, and COL1A1) increased dramatically in the 1 mM Vit D@Gel/T80 group. According to in vivo tests, 1 mM Vit D@Gel/T80 will cure tendon dysfunction while also having anti-inflammatory and anti-apoptotic properties.
Tendon tissue restoration effect of the Vit D@Gel/T80
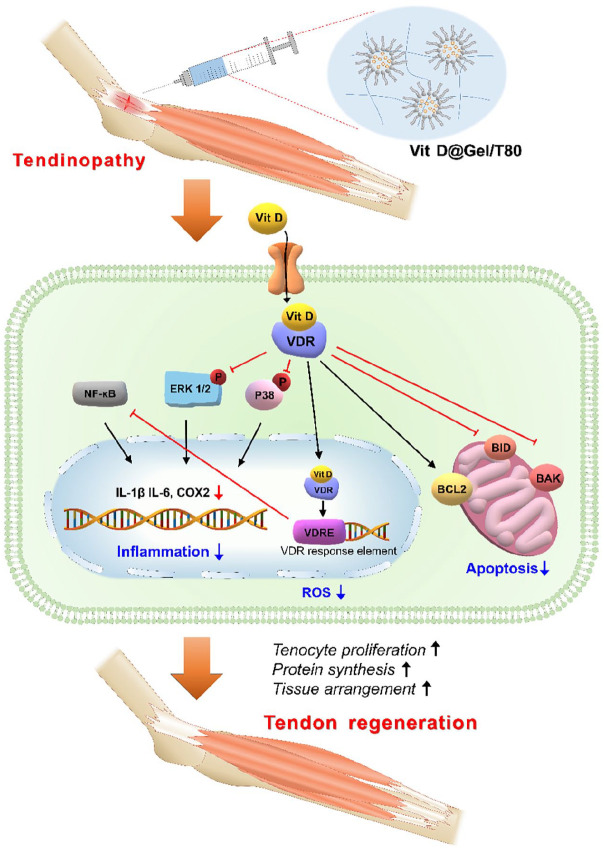
Reactive oxygen species (ROS) regulate cell growth and differentiation in a steady state. However, abnormal ROS accumulation can cause oxidative stress, accelerating the development of various diseases including tendinopathy. 49 Excessive ROS has been shown to influence the proliferation and migration of tendon-derived stem cells (TDSCs) leading to the initiation of apoptosis in vitro. 50 Hypoxia is a critical regulator of tendinopathy, resulting in high levels of ROS in mitochondria, dimerization of NF-κB, and mitophagy. 51 Vit D is one of the well-known antioxidants found in nature via ROS scavenging. Therefore, to reduce oxidative stress in the tendon, the usage of Vit D would be a great approach for effective tendinopathy treatment in the clinic. In this study, the ROS scavenging ability of the 1 mM Vit D@Gel/T80 was confirmed (Figure 2(d) and (e)), as was the reduction in proinflammatory cytokine expression at the cellular level (Figure 3(a)–(c)). In addition, the effects of Vit D on tendon repair via the ERK and p38 pathways as well as antioxidant activities were investigated. 14 The ERK and p38 are the most well-studied mitogen-activated protein kinase (MAPK) pathways that govern cell proliferation in general. 52 Furthermore, these signaling pathways also relay physiological responses in mammalian cells, such as cell differentiation, inflammatory response, and apoptosis. Numerous studies have established Vit D as an antioxidant. The 1 mM Vit D@Gel/T80 decreased the expression of apoptosis-related markers in tenocytes (Figure 3(d)) and eventually increased the expression of tendon-related markers in damaged tendons (Figure 4). Taken together, it appears that 1 mM Vit D@Gel/T80 has multiple functions, including reducing tenocyte apoptosis, enhancing cell proliferation, protein synthesis, and tissue organization in the injured tendon by modulating the ERK and p38 pathways (Figure 7).
Figure 7.
Schematic illustration of the potential tendon regeneration effect of the 1 mM Vit D@Gel/T80.
There are some limitations in this study. First, collagenase-induced tendinopathy model does not sufficiently replicate degenerative tendon pathologies in human.53,54 In addition, inconsistent and variable degree of tendon injury by collagenase injection were reported. 55 Second, our histologic data are not completely quantitative. Finally, we just combined Vit D and hydrogel using a specific surfactant. To develop more effective composition of Vit D delivery system, nanofiber hydrogels could be a superior scaffold for sustained Vit D delivery. 29
Conclusions
Our 1 mM Vit D@Gel/T80, a novel Vit D delivery hydrogel system with cross-linked HA and surfactant T80, showed its capability of repairing tendinopathy based on tendon restoration properties in vitro and in vivo. Vit D in the 1 mM Vit D@Gel/T80 formulation, in particular, had a tendon regenerating impact in vivo, with biological properties such as anti-apoptosis, tenocyte proliferation, tendon-related protein production, and tissue alignment. According to these findings and earlier research, Vit D downregulated NF-κB expression and inhibited phosphorylation of ERK and p38 pathway, which could reduce proinflammatory cytokines. Overall, the Vit D@Gel/T80 considerably aided the tendon regenerating process. This approach could be a candidate for tendon treatment. For more advanced Vit D delivery system, the development of combining hydrophobic Vit D into hydrogel and sophisticated scaffold such as nanofiber hydrogels could be explored in the future.
Supplemental Material
Supplemental material, sj-docx-1-tej-10.1177_20417314221122089 for Controlled vitamin D delivery with injectable hyaluronic acid-based hydrogel for restoration of tendinopathy by Da-Seul Kim, Jun Hyuk Kim, Seung-Woon Baek, Jun-Kyu Lee, So-Yeon Park, Bogyu Choi, Tae-Hyung Kim, Kyunghoon Min and Dong Keun Han in Journal of Tissue Engineering
Footnotes
Author contributions: D. K. Han and K. Min conceived and supervised the project. D. -S. Kim, J. H. Kim, and B. Choi performed the experiments and analyzed the data. S. -W. Baek optimized the condition of Vit D dispersion. The first draft of manuscript was written by D. -S. Kim, J. H. Kim, J. -K. Lee, and T. -H. Kim. All authors have given approval to the final version of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Basic Science Research Program (2020R1A2B5B03002344 and 2020R1F1A1048532) and Bio & Medical Technology Development Program (2018M3A9E2024579) through the National Research Foundation of Korea funded by the Ministry of Science and ICT (MSIT) and the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (202011A05-05), Republic of Korea.
ORCID iDs: Da-Seul Kim  https://orcid.org/0000-0002-6281-0434
https://orcid.org/0000-0002-6281-0434
Kyunghoon Min  https://orcid.org/0000-0003-3357-9795
https://orcid.org/0000-0003-3357-9795
Dong Keun Han  https://orcid.org/0000-0003-4641-7883
https://orcid.org/0000-0003-4641-7883
Supplemental material: Supplemental material for this article is available online.
References
- 1. Abate M, Silbernagel KG, Siljeholm C, et al. Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res Ther 2009; 11: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Del Buono A, Battery L, Denaro V, et al. Tendinopathy and inflammation: some truths. Int J Immunopathol Pharmacol 2011; 24: 45–50. [DOI] [PubMed] [Google Scholar]
- 3. Andres BM, Murrell GAC. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res 2008; 466: 1539–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet 2010; 376: 1751–1767. [DOI] [PubMed] [Google Scholar]
- 5. Zhang J, Nie D, Williamson K, et al. Selectively activated PRP exerts differential effects on tendon stem/progenitor cells and tendon healing. J Tissue Eng 2019; 10: 2041731418820034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou H, Lu H. Advances in the development of anti-adhesive biomaterials for tendon repair treatment. Tissue Eng Regen Med 2021; 18(1): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Janvier AJ, Canty-Laird E, Henstock JR. A universal multi-platform 3D printed bioreactor chamber for tendon tissue engineering. J Tissue Eng 2020; 11: 2041731420942462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Z, Liu H, Luo W, et al. Regeneration of skeletal system with genipin crosslinked biomaterials. J Tissue Eng 2020; 11: 2041731420974861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeon S-M, Shin E-A. Exploring vitamin D metabolism and function in cancer. Exp Mol Med 2018; 50: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wintermeyer E, Ihle C, Ehnert S, et al. Crucial role of vitamin D in the musculoskeletal system. Nutrients 2016; 8: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Millar NL, Murrell GA, McInnes IB. Inflammatory mechanisms in tendinopathy - towards translation. Nat Rev Rheumatol 2017; 13: 110–122. [DOI] [PubMed] [Google Scholar]
- 12. Khoo A-L, Chai LY, Koenen HJ, et al. Vitamin D3 down-regulates proinflammatory cytokine response to Mycobacterium tuberculosis through pattern recognition receptors while inducing protective cathelicidin production. Cytokine 2011; 55: 294–300. [DOI] [PubMed] [Google Scholar]
- 13. Chen J, Tang Z, Slominski AT, et al. Vitamin D and its analogs as anticancer and anti-inflammatory agents. Eur J Med Chem 2020; 207: 112738. [DOI] [PubMed] [Google Scholar]
- 14. Min K, Lee JM, Kim MJ, et al. Restoration of cellular proliferation and characteristics of human tenocytes by vitamin D. J Orthop Res 2019; 37: 2241–2248. [DOI] [PubMed] [Google Scholar]
- 15. Ruiz-Alonso S, Lafuente-Merchan M, Ciriza J, et al. Tendon tissue engineering: cells, growth factors, scaffolds and production techniques. J Control Release 2021; 333: 448–486. [DOI] [PubMed] [Google Scholar]
- 16. Jiang X, Wu S, Kuss M, et al. 3D printing of multilayered scaffolds for rotator cuff tendon regeneration. Bioact Mater 2020; 5: 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cai J, Xie X, Li D, et al. A novel knitted scaffold made of microfiber/nanofiber core-sheath yarns for tendon tissue engineering. Biomater Sci 2020; 8: 4413–4425. [DOI] [PubMed] [Google Scholar]
- 18. Xu Y, Yin H, Chu J, et al. An anisotropic nanocomposite hydrogel guides aligned orientation and enhances tenogenesis of human tendon stem/progenitor cells. Biomater Sci 2021; 9: 1237–1245. [DOI] [PubMed] [Google Scholar]
- 19. Park H, Choi B, Hu J, et al. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater 2013; 9: 4779–4786. [DOI] [PubMed] [Google Scholar]
- 20. Lee BM, Park SJ, Noh I, et al. The effects of the molecular weights of hyaluronic acid on the immune responses. Biomater Res 2021; 25: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abate M, Schiavone C, Salini V. The use of hyaluronic acid after tendon surgery and in tendinopathies. Biomed Res Int 2014; 2014: 783632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han Y, Yang J, Zhao W, et al. Biomimetic injectable hydrogel microspheres with enhanced lubrication and controllable drug release for the treatment of osteoarthritis. Bioact Mater 2021; 6: 3596–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao C, Sun YL, Amadio PC, et al. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic acid. An in vivo canine model. J Bone Joint Surg Am 2006; 88: 2181–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaux JF, Samson A, Crielaard JM. Hyaluronic acid and tendon lesions. Muscles Ligaments Tendons J 2015; 5: 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Birajdar MS, Joo H, Koh W-G, et al. Natural bio-based monomers for biomedical applications: a review. Biomater Res 2021; 25: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prager J, Adams CF, Delaney AM, et al. Stiffness-matched biomaterial implants for cell delivery: clinical, intraoperative ultrasound elastography provides a ‘target’ stiffness for hydrogel synthesis in spinal cord injury. J Tissue Eng 2020; 11: 2041731420934806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niiyama H, Kuroyanagi Y. Development of novel wound dressing composed of hyaluronic acid and collagen sponge containing epidermal growth factor and vitamin C derivative. J Artif Organs 2014; 17: 81–87. [DOI] [PubMed] [Google Scholar]
- 28. Hsiao M-Y, Lin A-C, Liao W-H, et al. Drug-loaded hyaluronic acid hydrogel as a sustained-release regimen with dual effects in early intervention of tendinopathy. Sci Rep 2019; 9: 4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bayer IS. Hyaluronic acid and controlled release: a review. Molecules 2020; 25: 2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bayer IS. A review of sustained drug release studies from nanofiber hydrogels. Biomedicines 2021; 9: 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Galunska B, Gerova D, Boncheva M, et al. HPLC method for measuring the circulating levels of 25-hydroxy vitamin D: validation and comparison with ID LC/MS/MS and immunoassay. Integr Food Nutr Metab 2014; 1: 119–123. [Google Scholar]
- 32. Hast MW, Zuskov A, Soslowsky LJ. The role of animal models in tendon research. Bone Jt Res 2014; 3: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim D-S, Lee J-K, Kim JH, et al. Advanced PLGA hybrid scaffold with a bioactive PDRN/BMP2 nanocomplex for angiogenesis and bone regeneration using human fetal MSCs. Sci Adv 2021; 7: eabj1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee JH. Pharmacologic supplementation of vitamin D. J Korean Med Assoc 2017; 60: 330–335. [Google Scholar]
- 35. Choi HS, Chung Y-S, Choi YJ, et al. Efficacy and safety of vitamin D3 B.O.N intramuscular injection in Korean adults with vitamin D deficiency. Osteoporos Sarcopenia 2016; 2: 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim H-G, Gater DL, Kim Y-C. Development of transdermal vitamin D3 (VD3) delivery system using combinations of PLGA nanoparticles and microneedles. Drug Deliv Transl Res 2018; 8: 281–290. [DOI] [PubMed] [Google Scholar]
- 37. Larrañeta E, Stewart S, Ervine M, et al. Hydrogels for hydrophobic drug delivery. Classification, synthesis and applications. J Funct Biomater 2018; 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guttoff M, Saberi AH, McClements DJ. Formation of vitamin D nanoemulsion-based delivery systems by spontaneous emulsification: factors affecting particle size and stability. Food Chem 2015; 171: 117–122. [DOI] [PubMed] [Google Scholar]
- 39. Park HS, Lee SY, Yoon H, et al. Biological evaluation of micro-patterned hyaluronic acid hydrogel for bone tissue engineering. Pure Appl Chem 2014; 86: 1911–1922. [Google Scholar]
- 40. Heidari A. Measurement the amount of vitamin D2 (ergocalciferol), vitamin D3 (cholecalciferol) and absorbable calcium (Ca2+), iron (II)(Fe2+), magnesium (Mg2+), phosphate (PO4–) and zinc (Zn2+) in apricot using high–performance liquid chromatography (HPLC) and spectroscopic techniques. J Biom Biostat 2016; 7: 292. [Google Scholar]
- 41. Mahmoodani F, Perera CO, Fedrizzi B, et al. Degradation studies of cholecalciferol (vitamin D3) using HPLC-DAD, UHPLC-MS/MS and chemical derivatization. Food Chem 2017; 219: 373–381. [DOI] [PubMed] [Google Scholar]
- 42. Walden G, Liao X, Donell S, et al. A clinical, biological, and biomaterials perspective into tendon injuries and regeneration. Tissue Eng Part B Rev 2017; 23: 44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol 2004; 14: 433–439. [DOI] [PubMed] [Google Scholar]
- 44. Tagliaferri S, Porri D, De Giuseppe R, et al. The controversial role of vitamin D as an antioxidant: results from randomised controlled trials. Nutr Res Rev 2019; 32: 99–105. [DOI] [PubMed] [Google Scholar]
- 45. Kim D-H, Meza CA, Clarke H, et al. Vitamin D and endothelial function. Nutrients 2020; 12: 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Im GI, Kim T-K. Stem cells for the regeneration of tendon and ligament: a perspective. Int J Stem Cells 2020; 13: 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jelinsky SA, Rodeo SA, Li J, et al. Regulation of gene expression in human tendinopathy. BMC Musculoskelet Disord 2011; 12(1): 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu A, Wang Q, Zhao Z, et al. Nitric oxide nanomotor driving exosomes-loaded microneedles for Achilles tendinopathy healing. ACS Nano 2021; 15: 13339–13350. [DOI] [PubMed] [Google Scholar]
- 49. Wu H, Li F, Wang S, et al. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018; 151: 66–77. [DOI] [PubMed] [Google Scholar]
- 50. Li K, Deng Y, Deng G, et al. High cholesterol induces apoptosis and autophagy through the ROS-activated AKT/FOXO1 pathway in tendon-derived stem cells. Stem Cell Res Ther 2020; 11: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee JM, Hwang JW, Kim MJ, et al. Mitochondrial transplantation modulates inflammation and apoptosis, alleviating tendinopathy both in vivo and in vitro. Antioxidants 2021; 10: 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 2002; 12: 9–18. [DOI] [PubMed] [Google Scholar]
- 53. de Cesar Netto C, Godoy-Santos AL, Augusto Pontin P, et al. Novel animal model for Achilles tendinopathy: controlled experimental study of serial injections of collagenase in rabbits. PLoS One 2018; 13: e0192769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Warden SJ. Animal models for the study of tendinopathy. Br J Sports Med 2007; 41: 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lake SP, Ansorge HL, Soslowsky LJ. Animal models of tendinopathy. Disabil Rehabil 2008; 30: 1530–1541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tej-10.1177_20417314221122089 for Controlled vitamin D delivery with injectable hyaluronic acid-based hydrogel for restoration of tendinopathy by Da-Seul Kim, Jun Hyuk Kim, Seung-Woon Baek, Jun-Kyu Lee, So-Yeon Park, Bogyu Choi, Tae-Hyung Kim, Kyunghoon Min and Dong Keun Han in Journal of Tissue Engineering