Abstract
With the increase in world population, the demography of humans is estimated to be exceeded and it has become a major challenge to provide an adequate amount of food, feed, and agricultural products majorly in developing countries. The use of chemical fertilizers causes the plant to grow efficiently and rapidly to meet the food demand. The drawbacks of using a higher quantity of chemical or synthetic fertilizers are environmental pollution, persistent changes in the soil ecology, physiochemical composition, decreasing agricultural productivity and cause several health hazards. Climatic factors are responsible for enhancing abiotic stress on crops, resulting in reduced agricultural productivity. There are various types of abiotic and biotic stress factors like soil salinity, drought, wind, improper temperature, heavy metals, waterlogging, and different weeds and phytopathogens like bacteria, viruses, fungi, and nematodes which attack plants, reducing crop productivity and quality. There is a shift toward the use of biofertilizers due to all these facts, which provide nutrition through natural processes like zinc, potassium and phosphorus solubilization, nitrogen fixation, production of hormones, siderophore, various hydrolytic enzymes and protect the plant from different plant pathogens and stress conditions. They provide the nutrition in adequate amount that is sufficient for healthy crop development to fulfill the demand of the increasing population worldwide, eco-friendly and economically convenient. This review will focus on biofertilizers and their mechanisms of action, role in crop productivity and in biotic/abiotic stress tolerance.
Keywords: abiotic stress, biotic stress, biofertilizers, crop productivity, plant-root interaction
Introduction
The world population will reach 9 billion by 2050 in accordance with Food and Agricultural Organization; as a result, there should be an enhancement in crop yield to meet the food demand. Soil is an important source of food production in human lifespan. In the last decades, due to the increase in agricultural practices such as pesticides and chemical fertilizers it has been degraded at a universal scale and causes lower fertility due to loss in biodiversity, water retention, and disturbance in biogeochemical cycles. Soil health and plant productivity are severely influenced by numerous interactions among plant, soil, and microorganisms (Harman et al., 2020). Soil microbes cooperate with one another and also with plant roots in numerous means providing a wide variety of essential acts which are valuable for sustaining the ecological balance in soil (Kumar et al., 2021c). Plant microbial interactions are positive if they improve plant survival, nutritional status, and crop productivity and they are negative if they reduce plant growth. Soil fertility is inextricably linked to the balance of microorganisms and plants (Vishwakarma et al., 2020). The application of biofertilizers can be a probable approach to improve soil microbial status that stimulates the natural soil microbiota therefore influencing nutrient accessibility and decomposition of organic matter (Chaudhary et al., 2021). It was observed that the supply of biofertilizers in apricot modifies the microbial composition and degradation process which could be efficient in nutrient cycles in soil under field conditions (Agri et al., 2021; Baldi et al., 2021). The capability of biofertilizers to form a high-level microbial diversity in soil may outcome better crop productivity for sustainable agriculture (Agri et al., 2022). Recently, many studies reported the positive impact of beneficial soil microbes on crop productivity, but the role of consortium in agriculture is not entirely unstated. Usage of consortium has positive impact on nutrient uptake efficiency by plants, protection from pathogens, and stress conditions (Aguilar-Paredes et al., 2020). This review provided information on effective approaches such as biofertilizers which help in the restoration of agricultural soil thus improving crop health for sustainable agriculture. This can permit agriculturalists to enhance farming and reach a high standard of soil quality and subsequently lead to raised plant development.
Nutrients are required by every living creature in this world. A total of 17 essential plant nutrients are mandatory for the proper development of plants (Kumar et al., 2021a). These 17 nutrients are divided into three classes based on the amount required such as major nutrients (carbon, hydrogen, oxygen, nitrogen, phosphorus, and potassium), minor nutrients such as sulfur, calcium, and magnesium, and micronutrients (nickel, zinc, molybdenum, manganese, iron, copper, chlorine, and boron). The plant takes up oxygen, hydrogen, and carbon from air and water, but the other nutrients are taken from soils in inorganic forms (Gong et al., 2020). Biofertilizer or biological fertilizer is a material that contains living or dormant microorganisms that colonize the rhizosphere or present inside the plants and directly or indirectly promotes the growth of plants by supplying nutrition (Malusa and Vassilev, 2014; Fasusi et al., 2021). Microorganisms present in soil used as biofertilizers can mobilize the nutrient from soil and convert them into a usable form from unusable form through biological processes like nitrogen fixation, phosphorus solubilization, zinc solubilization, siderophores production, and producing plant growth-promoting substances (Bhattacharjee and Dey, 2014; Mazid and Khan, 2015). Biofertilizers are applied to seed, root, soil, or by the foliar spray to enhance the microbial activity through their multiplication which then mobilizes the nutrients to target plants which remarkably improved the soil fertility and sooner increases the crop health and production (Pandey and Singh, 2012; Ismail et al., 2013).
Biotic stress is responsible to damage plants by pathogenic organisms like bacteria, fungi, viruses, parasites, and insects and by other harmful plants. They lead to declining the crop productivity by causing diseases such as vascular wilts, leaf spots, cankers, nutrient deficiency, systematic damage, chlorosis, stunting and reduce plant vigor, ultimately causing the death of the plants (Iqbal et al., 2021). Plant protects themselves to biotic stress via direct mechanisms like synthesis of secondary metabolite, hormones, cell-wall-degrading enzymes, and antioxidants (Kaur et al., 2022). The indirect mechanisms include the induction of acquired systematic resistance, plant pathogen molecular patterns (PAMPs) which in turn trigger the immunity and plant resistance proteins (Yu et al., 2022). Microorganisms solubilize the phosphorus and zinc, fixing the nitrogen and other macro- and micronutrients which promote the growth of the plants under biotic stress condition by providing nutrition (Singh et al., 2022a). They also enhance the stress resistance in plants by expressing the gene of phytohormones and stress-related metabolite. Some microorganisms also produce the volatile organic compounds (VOCs) such as melatonin to protect the plant from pathogens (Moustafa-Farag et al., 2019). When pathogen attacks, the plant produces various compounds within the tissues that lead to the activation of defense mechanisms inside the plants such as induced systematic resistance, peroxidases, phenylalanine ammonia-lyase, polyphenol oxidase, and hypersensitivity (Kaur et al., 2022).
Climatic change is one of the major factors for enhancing abiotic stress on crops which results in reduced crop productivity (Liu et al., 2017a). Climatic-related abiotic stresses included drought, waterlogging, excessive heat, and soil-related abiotic stresses are fertility, heavy metals, and salinity; all these are responsible for the poor yields of crops around the whole globe (Upadhyay et al., 2019). There is less water available to plants during drought conditions, and biofertilizers have the potential to produce cytokinin, gibberellins, abscisic acid, and IAA, which cause the plant to increase its growth, root length, total surface area, and the formation of root hairs and lateral roots, which increases water absorption from water-deficient soil (Kenneth et al., 2019; Raza et al., 2019). Pollutants released from industry without any further operation if released in the environment then they cause the accumulation of heavy metals such as copper, lead, nickel, zinc, etc., which have detrimental effects on the plants and animals (Popp et al., 2013). These heavy metals are removed from the environment by micro- and macro-nutrient solubilizing and mineralizing microorganisms (Bhojiya et al., 2021). Heat stress causes cellular changes like production of reactive oxygen species, reduction in cell turgidity, reduction in water uptake, reduction in growth of plants, ultimately leading to death of plant by showing initial symptoms like leaf senescence, damages to chloroplast, wilting of plant, and chlorosis (Ahluwalia et al., 2021), whereas low temperature causes the inactivation of protein and reduces the cell membrane fluidity leading to increases in photosynthesis, imbalance of water transport (Odoh et al., 2020). All these temperature-related stresses coped up by plants after the accumulation of the hydrophilic and osmolytes protein. Huang et al. (2015) reported that due to high salt concentration there is increased toxicity to cell due to accumulations of sodium and chloride ions inside the cell which in turn disturb the photosynthetic processes, stomatal opening and closing, shrinkage of cell within pant tissue. Various studies showed that bacteria and arbuscular mycorrhizae fungi help in surviving the plants under salinity stress condition by enhancing the plant growth and development. In this review, we will discuss about the biofertilizers and its mechanism for crop production and biotic/abiotic tolerance for sustainable agriculture.
Biofertilizers
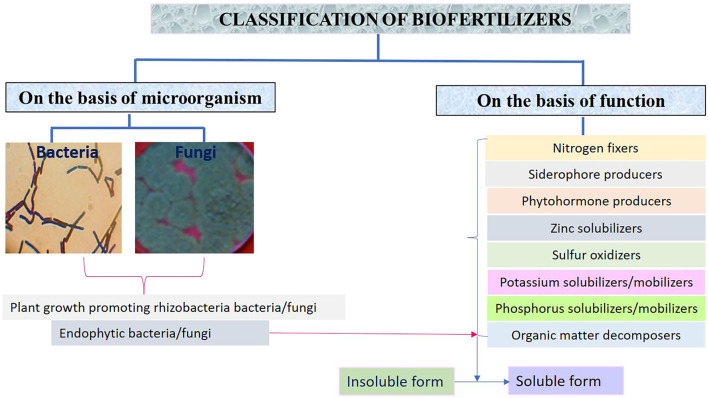
In India, biofertilizer refers to the use of microorganisms to meet nutritional needs, whereas in other countries, the term microbial bioinoculant is used (Mitter et al., 2021). Biofertilizers are bio-based organic fertilizers that either could be from plant or animal sources or from living or dormant microbial cells that have the potential to improve the bioavailability and bioaccessibility of nutrient uptake in plants (Lee et al., 2018; Abbey et al., 2019). Bhardwaj et al. (2014) reported that live microbial mass is a major ingredient of biofertilizers. So biofertilizers are properly defined as “the preparations containing live microbes that help in enhancing soil fertility by fixing atmospheric nitrogen, solubilizing phosphorus or decomposing organic wastes or by elevating plant growth through the production of growth hormones with their biological activities” (Okur, 2018). Biofertilizers are generally applied in solid or dry forms, which are prepared after packing on suitable carriers such as clay minerals, rice bran, peat, lignite, wheat bran, humus, and wood charcoal. Carriers increase the shelf life and enable the easy handling of microbial inoculants (Bhattacharjee and Dey, 2014). The benefits of biofertilizers include low cost, enhanced nutrient availability, improved soil fertility, protect plants from soil-borne pathogens, sustainable agricultural production, enhanced biotic and abiotic stress tolerance, promote phytohormone production, improve soil health, causing less environmental pollution, and its continued use improves the fertility of soil considerably (Chaudhary et al., 2021, 2022a). Based on the source and raw material, global biofertilizer is marketed under two major categories like organic residue-based biofertilizer and microorganisms-based biofertilizer. Green manure, crop residues, treated sewage sludge, and farmyard manure are generally organic-based biofertilizers. While on the contrary, microorganism-based biofertilizers contain beneficial microorganisms like bacteria, fungi, and algae. Directly or indirectly, these biofertilizers mediate the performance of plant growth (Figure 1). Direct mechanisms that act upon plants directly include nitrogen fixation, phosphate solubilization, micronutrient solubilization, and the production of phytohormones (Chaudhary et al., 2021). The indirect mechanism generally protects the plant from the deleterious effect of the pathogens by releasing lytic enzymes, antibiotics, siderophores, and cyanide production (Mahmud et al., 2021).
Figure 1.
Types of biofertilizers on the basis of microorganism and functional characteristics.
Types of biofertilizers and their role in crop production and soil health maintenance
Various types of biofertilizers are classified based on microorganisms such as bacteria and fungi and function of the biofertilizers as shown in Figure 1.
Nitrogen-fixing biofertilizers
Nitrogen is the vital macro-nutrient essential by plants because it improves the growth of the shoot system, helps in reproduction, is a constituent of chlorophyll responsible for the deep green color, and also increases the size of the grains (Sandhu et al., 2021). Although the nitrogen content in the atmosphere is 78% by a mass fraction, dinitrogen contains triple bonds and is an unavailable form of nitrogen present in the air for the plants. Dinitrogen should be first converted into soluble non-toxic form ammonia by the diazotrophs through the biological process of nitrogen fixations (Abbey et al., 2019). This ammonia is then converted to the nitrite and nitrate by the ammonia-oxidizing bacteria and by nitrifying bacteria, respectively (Roy et al., 2020). The unused nitrate is converted to the atmospheric nitrogen in the deeper soil horizons through the process of denitrification which will then escape to the atmosphere as dinitrogen gas. This is the typical path of the nitrogen cycle (Mahanty et al., 2017). Azotobacter and Bacillus sp. are involved in N fixation, growth promotion of maize plants, and forest crops (Etesami et al., 2014; Azeem et al., 2022). Inoculation of Bradyrhizobium japonicum in soybean plants improved plant biomass, nodulation, and N fixation (Htwe et al., 2019). Azotobacter chroococcum improved the plant height and chlorophyll content in maize plants (Jain et al., 2021). Bradyrhizobium sp. showed nitrogen fixation, IAA, and siderophores production and improved the yield of mung bean (Alkurtany et al., 2018). Nitrogen-fixing microbes are considered as symbiotic, free-living, and associative nitrogen-fixing bacteria (Aasfar et al., 2021). Jing et al. (2020) reported that the application of Pseudomonas protegens promoted plant growth in nitrogen-deficient conditions.
Symbiotic nitrogen-fixing microbes
In the process of symbiosis, macro-symbiont is the plant and microsymbionts are the prokaryotic bacteria. Rhizobium and legume symbiosis is one of the most studied mutualistic relationships between plant root nodules and nitrogen-fixing microorganisms. Mutualistic relationships are initiated when the plant began to secrete the flavonoids and iso-flavonoids in its rhizosphere, where it is recognized by Rhizobium (Hawkins and Oresnik, 2022). It started to do infection by differentiating root hairs, developing infection thread up to the root hair cell where infectious thread releases all its bacteria in the cytoplasmic region. Then, bacterial cell are terminally differentiated into the bacteroides, and the further development of bacteroides leads to the formation of symbiosome which is the site of nitrogen fixation (Cissoko et al., 2018; Jimenez-Jimenez et al., 2019; Suzaki et al., 2019). This atmospheric nitrogen fixation inside the nodule is carried out by the nitrogenase enzyme (Brahmaprakash and Sahu, 2012). Examples include Rhizobium associated with leguminous plants, Frankia (actinomycetes) associated with non-leguminous plants (Alnus, Casuarina), Azolla and the blue-green alga Anabaena azollae, and association of cyanobacteria with gymnosperms (Ghodhbane-Gtari et al., 2021). Fixation of N helps to improve the soil fertility and crop productivity. Mondal et al. (2020) reported that Rhizobium meliloti involved in N2 fixation produced chitinase enzyme and improved the yield of peanut plants. The alfalfa-Rhizobium symbiotic system can stimulate plant N fixation, increase phytohormone production, and promote plant growth (Fang et al., 2020).
Free-living nitrogen-fixing bacteria
Mostly Azotobacter is studied because it is a free-living, non-symbiotic, and phototropic bacterium. Azotobacter chroococcum can be used as a biofertilizer because it has the potential to fix 10 mgN/g of carbon source supplied in-vitro (Mukherjee et al., 2022). Plant hormones such as indole acetic acids, gibberellic acids, naphthalene acetic acid, and vitamin B complex are produced by Azotobacter. It inhibits the root pathogens while promoting root growth, helps in mineral uptake, and improves soil fertility (Sumbul et al., 2020). Examples include Azotobacter, Bacillus, Clostridium, and Azospirillum. Application of Bacillus sp. significantly enhanced the growth of Arachis hypogea plant, protects plants from stress, and exhibits the production of ammonia and IAA (Gohil et al., 2022). Azospirillum brasilense reduces N fertilization, improves plant nutrition, and increases plant biomass and wheat grain yield as reported by Galindo et al. (2022).
Associative nitrogen-fixing bacteria
The Spirillum lipoferum was firstly isolated by M.W. Beijerinck in 1925. Spirillum was found associated with the roots of the grain which were also capable of fixing nitrogen (Soumare et al., 2020). Azospirillum is gram-negative, non-nodulating, aerobic-associative nitrogen-fixing bacteria with plants having a C4 dicarboxylic pathway of photosynthesis, such as sugarcane, maize, sorghum, bajra, and cereals like wheat, rice, barley (Yasuda et al., 2022). They also produce cytokinin, gibberellins, and indole acetic acid, which aid in the uptake of N, P, and K and promote the growth of roots. Examples such as Gluconobacter, Acetobacter, Herbaspirillum, and Azoarcus.
Phosphorus-solubilizing biofertilizers
Phosphorus is the second macro-nutrient that is responsible for limiting the growth of plants (Bechtaoui et al., 2021). It is an important constituent of organic and nucleic acids and is responsible for the synthesis of ATP and several amino acids. P helps in the nodulation process, amino acid synthesis, and proteins in leguminous plants (Wang et al., 2020). Soluble form of phosphorus is phosphate anion (orthophosphate), and their uptake is facilitated by rhizospheric microbes which help in plant nutrition. There are different microbes which can solubilize the remaining unavailable form of P into available form via organic acid production by bacteria which lowers the pH of the soil, leads to the dissolution of the phosphate compounds, and makes them available for the plant's nutrition (Mahanty et al., 2017). Examples of phosphate-solubilizing bacteria and fungi (PSB and PSF) are Bacillus, Rhizobium, Aerobacter, Burkholderia, Aspergillus, and Penicillium. Inoculation of Alcaligenes sp. improved plant growth parameters via P solubilization and IAA production (Abdallah et al., 2016). Rhizobium leguminosarum and Pseudomonas moraviensis enhanced the yield and growth of wheat plants and showed IAA and solubilization (Igiehon et al., 2019; Fahsi et al., 2021). Application of Arbuscular fungi can make greater availability of P in plants and protects them from stress condition as reported by Nacoon et al. (2020). Bacillus subtilis is also known as PSB which improved safflower growth and protects plants from salinity stress as reported by Zhang et al. (2019). NanoPhos containing phosphate-solubilizing bacteria enhanced the maize production via increasing the soil enzymes and bacteria population under field conditions (Chaudhary et al., 2021).
Phosphorus-mobilizing biofertilizers
They are beneficial bacteria that effectively mobilize the soluble phosphorus and mineralization of the organic phosphorus compound, both are unavailable form of phosphorus. Bacillus, Pseudomonas, and Rhizobium are representative phosphorus-mobilizing microorganisms (PMB) (Kirui et al., 2022). Three different mechanisms have been reported for this process. First, PMB is releasing the phosphatases enzyme. Second, PMB is producing organic acids. The last one added PMB may interact symbiotically with the other fungal mycorrhiza which mobilizes the soluble phosphorus from distant places where plant roots cannot reach by absorbing soluble phosphate by hyphae (Nassal et al., 2018; Etesami et al., 2021). One of the major advantages of Arbuscular mycorrhiza is transporting both inorganic and organic forms of phosphorus to plants. Examples of arbuscular mycorrhiza fungi (AMF) include Acaulospora sp., Glomus sp., Entrophospora, and Paraglomus sp. and ectomycorrhiza include Amanita, Laccaria, and Boletus spp. Fungal endophyte (Serendipita) increased the K content in maize and protects plants from salinity stress (Haro and Benito, 2019).
Potassium-solubilizing biofertilizers
Subsequently, potassium (K) is the third major constituent of the macro-nutrients required by plants. It is mainly intricate in the regulation of stomatal closing and opening, nutrient uptake, protein synthesis improving the quality of products and provides resistance against stress environment (Santosh et al., 2022). K is present in different forms in soil depending upon the type of the soil composition like water-soluble, available form, and non-available form of the K (Basak et al., 2022). K is present in immobilized forms in silicate minerals like illite, orthoclase, biotite, illite, feldspar, etc. K solubilization occurs by both bacteria and fungi, and the major mechanism for solubilization of the unavailable form of K is acidification (means release of organic acids) (Varga et al., 2020). There are mechanisms also for solubilization of the K, namely, siderophores production, exchange reaction, and complexation (Sattar et al., 2019). Examples of potassium-solubilizing bacteria include Bacillus mucilaginous, B. edaphicus, B. circulans, Acidithiobacillus ferrooxidans, Frateuria aurantia, Herbaspirillum spp., and Clostridium spp., and potassium-solubilizing fungi include Aspergillus spp. and some arbuscular mycorrhiza fungi. Bacillus cereus showed K solubilization and improved potato plant health parameters and yield (Ali et al., 2021). Dal et al. (2020) reported that the combination of Rhizophagus irregularis and A. vinelandii improved soil enzyme activities and plant growth of wheat plants via P and K solubilization.
Potassium-mobilizing biofertilizers
The potassium-mobilizing microorganisms (PMMs) effectively release the unavailable potassium through the solubilization process (Patel et al., 2021). PMM is also recognized as potassium-dissolving bacteria or potassium-solubilizing bacteria. Ghaffari et al. (2018) observed that Frateuria and B. megaterium are efficient K-mobilizing bacteria used for crop farming purposes. Azotobacter showed K mobilization and solubilization in wheat plant and improved growth and soil microbial activities as reported by Game et al. (2020). Enterococcus and Pseudomonas aeruginosa also showed P and K solubilization and improved the maize height, yield, and nutrient acquisition (Kumar et al., 2021b). Bacillus aryabhattai showed K solubilization, protects plants from stress, and improves their growth via the expression of K-solubilizing genes (Chen et al., 2022).
Sulfur-solubilizing biofertilizers
Sulfur helps in chlorophyll formation, activation of a certain enzyme, amino acid formation, vitamin formation and promotes nodulation, vital for the development of all plants (Wang et al., 2019). Sulfur solubilizers are also known as sulfur-oxidizing bacteria because they are transforming the most insoluble form of sulfur that is hydrogen sulfide (H2S) into an available form of sulfur known as sulfate (SO4−2), and the reverse of this process is known assimilatory sulfate reduction which is mediated by sulfate-reducing bacteria (Wang et al., 2019). Sulfur transformation in the soil is primarily due to the microbial activity through the processes of mineralization, immobilization, oxidation, and reduction (Malik et al., 2021). Examples of aerobic sulfur-oxidizing bacteria include Bacillus, Beggiatoa, Aquifer, Paracoccus, Sulfolobus, Thiobacillus, Thermithiobacillus, Xanthobacter; phototropic anaerobic sulfur-oxidizing bacteria include Allochromatium, Chlorobium, Rhodobacter, Rhodopseudomonas; non-phototrophic obligate anaerobes include Wolinella succinogenes; and aerobic sulfur-oxidizing archaea include Sulfolobales members (Kusale et al., 2021). Thiobacillus thiooxidans and Bradyrhizobium japonicum are sulfur-oxidizing biofertilizers which showed better effect on cereal crops, medicinal plants, and forage crops (Zhang et al., 2018). Halothiobacillus bacteria tolerated the high salt concentration and improved crop production in saline soils (Boroujeni et al., 2021).
Zinc-solubilizing biofertilizers
Zinc is required during protein synthesis, DNA–protein interaction, growth hormone production, seed development, production of chlorophyll and protects plants from stress conditions (Umair Hassan et al., 2020). Insoluble forms of zinc are mostly ZnO, Zn3(PO4)2, ZnCO3, and metallic Zn. The usable form of zinc by the plant is divalent cations (Ayoub et al., 2022). Zinc-solubilizing fertilizers contain the zinc solubilization bacteria which produce the organic acids to solubilize the insoluble zinc to Zn+2, thereby enhancing zinc uptake in plants (Nitu et al., 2020). Examples of zinc-solubilizing bacteria and fungi are Bacillus subtilis, Pseudomonas striata, Serratia, Burkholderia cenocepacia, Aspergillus niger, A. nomius, and A. oryza which improved the soil enzyme activities and availability of Zn in crop plants (Batool et al., 2021). Leclercia adecarboxylata solubilizes Zn and produced siderophores which enhanced the Zn uptake in the roots of cucumber plants (Kang et al., 2021). Bacillus spp. and Pseudomonas taiwanensis showed a positive impact on the growth and chlorophyll content of maize plants (Chaudhary and Sharma, 2019; Hussain et al., 2020). Inoculation of Trichoderma longibrachiatum and Bacillus megaterium improved the seed germination of soybean plants in the pot experiment (Bakhshandeh et al., 2020). The application of PSB along with fertilizers improved the growth of faba bean in sandy soils (Ding et al., 2021).
Phytohormone-producing biofertilizers
Plant hormone or phytohormone plays a substantial role in plant development, secreted by both plants and microorganisms (Usman et al., 2022). Plant hormone production is an important feature of the beneficial microbes which is producing the indole-3-acetic acids, gibberellins, cytokinin, etc. (Eichmann et al., 2021). Auxin helped in the differentiation and division of plant cells. Cytokinin prevents the premature leaf senescence of plants (Wu et al., 2021a). Abscisic acid is also identified as hormone which is produced by plants during stress conditions. Gibberellins are involved in seed germination, shoot elongation, flowering, and fruiting (Binenbaum et al., 2018). These hormones are generally secreted by microorganisms under environmental stress conditions to protect the plants by modulating the phytohormone level inside the host plants (Lopes et al., 2021). Bacillus thuringiensis has the genes required for IAA production which improved the growth of tomato plants (Batista et al., 2021). B. licheniformis is known for the production of IAA, ABA, and gibberellin which improved the growth of grapevine and protects plants from stress conditions (Salomon et al., 2014).
Siderophores producing biofertilizers
Iron (Fe) is a micronutrient that performs various functions like photosynthesis, respiration, chlorophyll, and many of the enzymatic reactions in plants (Gao et al., 2022). The unavailable form of iron in nature present under aerobic environment predominately is Fe+3 and is more probable form of insoluble oxyhydroxides and hydroxides complex. So, bacteria are producing the low-molecular weight iron-binding protein molecules called siderophores (Lurthy et al., 2020). Siderophores are water-soluble molecules that exist in two forms, namely, extracellular and intracellular. After capturing Fe+3 by siderophore inside bacteria, Fe+3 is reduced to the Fe+2 inside the cytoplasmic membrane which is then transported inside the cytoplasm by gating mechanisms (Gu et al., 2020). This available form of iron is given by the bacteria to the host plant for its development (Mahanty et al., 2017). Plant assimilates the iron with the help of siderophores by releasing the chelating agent via bacteria. Examples include Pseudomonas fluorescens C7, Pseudomonas aeruginosa RSP5, and Pseudomonas aeruginosa RSP8. Application of siderophore-producing Bacillus sp. improves the growth of groundnut (Sarwar et al., 2020). Pseudomonas koreensis inoculation in maize plants inhibited the growth of plant pathogens via the production of siderophore and antioxidant enzymes (Ghazy and El-Nahrawy, 2021).
Organic matter decomposer biofertilizers
Soil organic matter is a mixture of living organisms consisting of bacteria, fungi, and insects, and the non-living part which includes fresh organic residues or waste, the dead and decaying matter of living organisms is generally known as humus (Lou et al., 2022). In organic matter generally, cellulose, lignin, hemicellulose, chitin, and lipids are present which are degraded by microbes such as bacteria, actinomycetes, and fungi. The organic-matter-degrading organisms break down the SOM into simpler or inorganic from which they derive energy and carbon for their growth. Examples of bacteriainclude Bacillus subtilis and Pseudomonas fluorescens and of fungi include ectomycorrhizal fungi. Trichoderma spp. involved in the degradation of litter at a faster rate releases antimicrobial compounds, improves the physicochemical properties of soil, and improves microbial diversity (Baldi et al., 2021). Bacillus subtilis and B. hisashii are involved in lignocellulose biodegradation by secreting the microbial enzymes as reported by Niu and Li (2022).
Endophytic bacteria as biofertilizers
Mutualistic microorganisms that employ the whole or part of their life cycle inside the plant tissues are known as endophytes (Fadiji and Babalola, 2020). Endophytes are of interest because they improve the nutritional requirements of the non-leguminous and leguminous plants by nitrogen fixation, phosphate solubilization, or by siderophores production (Janati et al., 2021). These bacteria have the potential to suppress pathogenic effects by activating the plant defense system (Dicko et al., 2021). Examples of endophytic bacteria include Klebsiella spp., Pseudomonas spp., Serratia spp., Bacillus spp., Burkholderia spp., Citrobacter spp. and endophytic fungi include Colletotrichum, Fusarium, Alternaria, and Aspergillus. Penicillium and Aspergillus isolated from roots of Taxus wallichiana solubilized P and produced phosphatase and phytase enzymes (Adhikari and Pandey, 2019). Kang et al. (2014a) observed that Bacillus megaterium regulates the content of amino acids and carbohydrates to promote the growth of mustard plant. Endophytes isolated from rice such as Bradyrhizobium sp., Paraburkholderia sp., showed acetylene reduction properties and high sugar content contributing to high nitrogen-fixing ability. High content of sugar in different crops such as sweet potato, pineapple, and sugar has known to assist endophytic N-fixing activity among non-leguminous plants (Okamoto et al., 2021).
Plant growth-promoting rhizobacteria
PGPR is used as biofertilizers; it represents the variation of soil bacteria that live in association with the rhizosphere, rhizoplane associated to root surface, and endophytes present inside the intercellular places (Vandana et al., 2021). PGPRs are soil bacteria which increase the growth and enhance the tolerance of plants toward stress conditions (Ghosh et al., 2019). There are diverse mechanisms shown by PGPR which support the plant growth such as N2 fixation, macro- and micronutrient mineralization, secretion of exopolysaccharides, phytohormone production, siderophore, hydrogen cyanide to prevent the growth of phytopathogens, antibiotics, etc. (Gouda et al., 2018; Numan et al., 2018). Rhizobium lupini increased alfalfa growth and enhanced nutrient uptake efficiency (Duan et al., 2022). Application of biofertilizers such as Pseudomonas taiwanensis, Bacillus spp., and Pantoea agglomerans improved the maize growth, yield, and soil health parameters (Khati et al., 2018; Chaudhary et al., 2022b). Application of Bacillus spp. improved the plant/soil health parameters and maize productivity as reported by Chaudhary et al. (2021). Kukreti et al. (2020) reported that Pseudomonas taiwanensis improved maize plant health and soil enzyme activities in the pot experiment.
Role of biofertilizers in biotic stress management
The outbreak of plant diseases in nature necessitates sustainable agriculture with minimum use of agrochemicals. For a long time, the use of chemicals has posed a significant risk to the environment and the agricultural sector (Akanmu et al., 2021). Long-term use of pesticides, on the other hand, harms both plant/soil health and eventually leads to significant crop loss. Thus, effective and eco-friendly phytopathogen control strategies such as biofertilizers are required. The exploitation of potential biofertilizers as endophytes could be useful to improve crop plants from various bacterial and fungal diseases (Collinge et al., 2022). Biological control of plant diseases occurs via destruction of pathogens via beneficial microbes such as Bacillus spp., Pseudomonas spp., Streptomyces, Pantoea spp., and several fungal spp. (Köhl et al., 2019; Chaudhary et al., 2021). Such endosymbiont group of biocontrol agents being friendly, they not only colonize internal plant tissue but also protect host plant throughout its life cycle without causing any apparent damage (Lahlali et al., 2022). The use of Bacillus sp. for crop growth promotion and biocontrol has a long history (Zhu et al., 2021). Bacillus thuringiensis (Bt), a producer of endotoxins that can be used as biopesticide and a source of genes for the creation of transgenic plants that are resistant to insects, is currently the most effective biopesticide on the market (Sujayanand et al., 2021).
Biofertilizers in the form of potential biocontrol agents represent a safe alternative to harmful chemicals like fertilizers, herbicides, pesticides, and insecticides (Hernández-Fernández et al., 2021). Consequently, the use of biofertilizers is receiving special attention for the management of phytopathogens that are comprised of bacteria, fungi, virus, aphids, and nematodes (Table 1). Their ubiquitous nature and the ability to reside within plant tissues make them unique, showing multidimensional interactions within the host plant (Khare et al., 2018). The biodiversity of endophytes is hyperdiverse in almost every other plant species ranging from small non-vascular plants to large conifers like Pinus radiata (Liu et al., 2017b). Some of the known endophytes are Burkholderia, Stenotrophomonas, Rhizobium, Microbacterium, and Bacillus spp. (Kandel et al., 2017).
Table 1.
Role of biofertilizers in biotic stress tolerance.
| Biofertilizers | Host plant | Pathogen | Response | References |
|---|---|---|---|---|
| Bacillus subtilis | Atractylodes macrocephala | Ceratobasidium sp. | Inhibit growth of pathogen and promote plant growth | You et al., 2018 |
| Bacillus cereus | Arabidopsis | Botrytis cinerea | Regulates signaling pathway such as JA and MAPK | Nie et al., 2019 |
| Bacillus velezensis | Arabidopsis | Myzus persicae | Protects host plant from pathogen via systemic resistance response | Rashid et al., 2017 |
| Bacillus safensis | Vaccinium | Botrytis cinerea | Enhanced the chitinase, hydrolytic, protease production and protects plants from pathogen | Hassan et al., 2021 |
| Pseudomonas aeruginosa | Cruciferous vegetables | Xanthomonas campestris | Protects plants from pathogen via chitinase production | Mishra and Arora, 2012 |
| Gluconacetobacter diazotrophicus | Arabidopsis thaliana | Ralstonia solanacearum | Protects from pathogen and activates defense response in plants | Rodriguez et al., 2019 |
| Streptomyces spp. | Oryzae sativa | Xanthomonas oryzae | Provides immunity to plants and protect from disease via increasing antioxidant enzymes | Hata et al., 2021 |
| Paenibacillus polymyxa | Brassica napus | Verticillium spp. | Increased production of volatile fatty acids and antibiotics | Rybakova et al., 2017 |
| Bacillus subtilis | Solanum lycopersicum | Fusarium oxysporum | Increased expression of auxin-related genes and improved plant growth | Samaras et al., 2021 |
| Trichoderma koningii | Nicotiana tabacum | Tobacco Mosaic Virus | Enhanced proline content and pathogen-related enzymes and inhibit the growth of pathogens | Taha et al., 2021 |
| Aureobasidium pullulans | Olive trees | Colletotrichum acutatum | Increased production of volatile fatty acids and improves seed germination | Sdiri et al., 2022 |
| Trichoderma harzianum | Zea mays | Curvularia lunata | Provides protection to plants from pathogen via JA signaling and platelet-activating factor | Yu et al., 2015 |
| Bacillus amyloliquefaciens | Solanum lycopersicum | Viruses | Induced SA and JA signaling and protects plants from disease | Beris et al., 2018 |
| Bacillus endophyticus, Pseudomonas aeruginosa | Solanum lycopersicum | Spodoptera litura | Increased secondary metabolite, phytohormone production and improved plant growth | Kousar et al., 2020 |
| Bacillus subtilis | Solanum lycopersicum | Fusarium oxysporum | Increased plant growth and suppress the growth of pathogens | Sundaramoorthy and Balabaskar, 2013 |
| Bacillus sp. | Common bean | Rhizoctonia solani | Inhibit growth of pathogens via production of cyanogens and lytic enzymes | Kumar et al., 2012 |
| Pseudomonas spp. | Gossypium | Fusarium spp. | Inhibit pathogens via production of HCN and enzymes | Zain et al., 2019 |
| Bacillus halotolerans, Agrobacterium fabrum | Common bean | Alternaria sp. | Improved plant growth and increased chitinase, siderophore, and IAA production | Sendi et al., 2020 |
| P.putida | Solanum tuberosum | Phytophthora infestans | Increased production of HCN against pathogens | Anand et al., 2020 |
| Aureobasidium pullulans | Crops | Botrytis cinerea, Alternaria alternata | Increased production of volatile organic acids and inhibit pathogen growth | Don et al., 2020 |
There are several enzymes which protect the plants from stress conditions such as antioxidant enzymes like peroxidase (POD), polyphenol oxidase, phenylalanine ammonia-lyase (PAL), lipoxygenase, and chitinase (Cataldo et al., 2022). Lipoxygenase enzymes have its place to non-heme iron comprising dioxygenases which contribute to stress response via lipid oxidation. Also, it is found to act as signals for communication with the plant host, with associated endophytes and pathogens (Singh et al., 2022b). In response to pathogen attack, endophytes boost plant immunity by priming induced systemic resistance (ISR) and systemic acquired resistance (SAR) via several phytohormones (Romera et al., 2019; Oukala et al., 2021). Pathogenesis-related proteins with antimicrobial properties are produced and accumulated by several endophytes symbiotically living with their host plants. Many endosymbionts have the capability to complement the inefficient antioxidative system of plants by different mechanisms (Shukla et al., 2022). In some strains, production of lipopeptides, surfactin, plipastatin, and mycosubtilin differentially activated the plant innate immune response (Kumar et al., 2021c). Production of surfactin may have an important role in the suppression of Fusarium infestation on germinating seeds (Eid et al., 2021). Bacillus strains inhibited the verticillium wilt caused due to Verticillium dahliae by the production of secondary metabolites such as surfactin, fengycin, and bacillibactin, as well as expressing defense-related genes such as SOD and PAL (Hasan et al., 2020). Bacillus atrophaeus inhibits Meloidogyne incognita growth by producing volatile dimethyl disulfide and antioxidant enzymes (Ayaz et al., 2021). According to Nie et al. (2019), Bacillus cereus inhibits the growth of Pseudomonas syringae by producing antioxidant enzymes. Pseudomonas fluorescent controls the iron uptake genes and protects plants from phytopathogens as reported by Desrut et al. (2020). Acrophialophora jodhpurensis defends tomato plants from Rhizoctonia solani which causes crown root disease via the production of peroxidase enzyme, chitinase, and phenylalanine (Daroodi and Taheri, 2021). Plants are protected from Botrytis cinerea by Trichoderma atroviride via the production of glutamate and glyoxylate aminotransferase (González-López et al., 2021).
Secondary metabolites play the foremost role in defense mechanism toward pathogens, pests, and herbivores. Many plants microbiome especially endosymbionts regulate defense mechanisms through secreting various metabolites (Divekar et al., 2022). Secondary plant metabolites belonging to the family of steroids, alkaloids, phenolics, flavonoids, and terpenoids function in innate immunity and defense response signaling (Pang et al., 2021). Volatile compounds from endophytes modulate plant microbiome and possess antimicrobial properties. A variety of fungi, including Ascomycetes and Deuteromycetes, are inhibited by a mixture of VOCs produced by the fungal endophyte Phomopsis sp. (Hummadi et al., 2022). Three VOCs, including caryophyllene, 2-methoxy-4-vinylphenol, and 3,4-dimethoxystyrol, produced by endophytic fungi Sarocladium bravhiariae HND5 have been found to have antifungal activity against Fusarium oxysporum (Yang et al., 2021). Alkaloid produced by Epichloe sp. in a variety of grass species is one of the well-known secondary metabolites produced by endophytic fungi. Hennessy et al. (2022) reported Epichloe festucae colonized agricultural forage grasses and offered the plant defense against herbivorous insects. Streptomyces hydrogenans metabolites can be used as safe biocontrol agents against Meloidogyne incognita and plant growth promoters for Solanum lycopersicum (Sharma et al., 2020). Bacillus velezensis is a potential pesticide due to its strong biocontrol activity and ability to strengthen host defense against Magnaporthe oryzae fungi, which cause rice blast disease in plants (Chen et al., 2021). An isolate of Trichoderma asperellum increased the resistance in tomato seedling to the disease A. alternata leaf spot (Yu et al., 2021). Trichoderma asperellum also produces mycolytic enzymes such as chitinase and 1,3, glucanase which may be capable of destroying phytopathogens cell walls (Win et al., 2021). Trichoderma spp. also have biocontrol potential against V. dahliae, which causes olive tree wilting, and inhibit the pathogenic fungus mycelial growth (Reghmit et al., 2021). Trichoderma sp. has been shown successfully to suppress Sclerospora graminicola, the cause of pearl millet downy mildew disease, and develop systemic resistance (Nandini et al., 2021).
Some endophytes can also regulate stress management through SAR mediated by salicylic acid. SAR offers long-lasting stress management and broad-spectrum effectiveness against a variety of pathogens (Xia et al., 2022). It frequently involves the accumulation of chitinase and pathogenesis-related proteins (PR). In a study by Samain et al. (2019), Paenibacillus strain (PB2) used to control Mycosphaerella graminicola induced pathogenesis-related proteins (PR1) which is considered as marker of SAR. Application of Bacillus aryabhattai activated a durable defense response against pathogens facilitated through salicylic acid/ethylene pathways (Portieles et al., 2021). Trichoderma harzianum helps to improve plant immediate resistance against Nezara viridula feeding invasion via enhancing JA marker gene transcript levels (Alınç et al., 2021). Bacillus subtilis and Pseudomonas fluorescens mediated systemic alleviated the biotic stress in Solanum lycopersicum against Sclerotium rolfsii. Heat-killed endophytic strain B. aryabhattai (HKEB) induced defense-related genes protein (PR1) and phytoalexin-deficient 3 in A. thaliana. PR1 gene expression was found to be 20-fold higher in treated plants than control, and other genes found in the study were associated with jasmonic and salicylic acid pathways (Portieles et al., 2021). Endophytes exhibit different gene upregulation and a distinct signaling pathway in response to distinct colonization strategies (Morelli et al., 2020). Trichoderma spp. demonstrated antagonistic activity against phytopathogens such as B. cinerea, Fusarium solani, and Rhizoctonia solani used as biocontrol agent in greenhouse experiment (Sánchez-Montesinos et al., 2021).
Role of biofertilizers in abiotic stress management
Climate change is one of the major reasons for the increasing abiotic stresses on the crops, which results in reducing the world's agriculture productivity. Abiotic stresses like drought, salinity, waterlogging, and excessive heat are responsible for the poor yield of crops (He et al., 2018). In recent years, the abiotic stress has increased so fast, because of the fluctuation of climates or climate change, and it has caused an unusual rise in the weather conditions and incidents, which is responsible for the substantial losses of crops around the globe. These abiotic stresses induce several physiological, biochemical, and morphological changes in plant that finally affect the economic yield of crop plants, and it was reported that the yield loss from abiotic stress is about 51–82%, which if continues will affect the goal of sustainable food production (dos Santos et al., 2022).
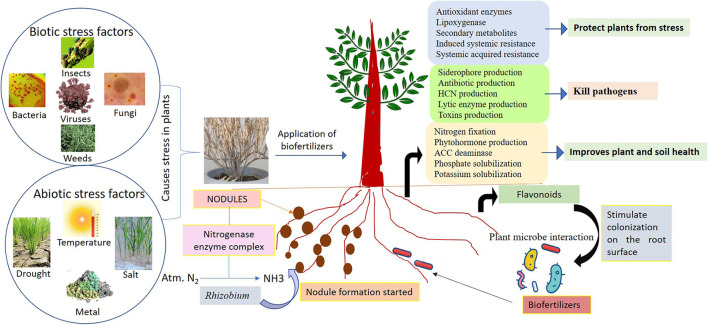
The use of beneficial microbes such as endophytes capable of producing growth hormones, like IAA, ACC deaminase, augmented the K uptake in plant tissues but decreased the ethylene level which helps in tolerance of stress in diverse plants (Figure 2). Biofertilizers as endophytes are found to have diverse associations with its host plant such as symbiotic, parasitic, and mutualistic and colonize plant tissues without causing any disease, thus benefiting for plants (Chaudhary et al., 2022c). Endophytes may benefit from mutualistic associations as they obtain nutrients from the hosts, and they spread by host seed transmission. They are also able to enhance the nutrients uptake like nitrogen, magnesium, zinc, and phosphorus from soil and provide to the host plant for better growth and survival (Bamisile et al., 2018). It is well-identified that plant biofertilizers play a significant role in supporting the growth of crops under different abiotic stresses (Table 2). Actinobacteria are well-known for plant growth via metabolite production and antibiotics under stress conditions (Yadav et al., 2018). Abd El-Daim et al. (2014) observed that the application of Pseudomonas sp. improved plant growth under heat stress via HSPs and ROS reduction. Paenibacillus sp. improved Phaseolus vulgaris growth via facilitating the siderophore, IAA and HCN production under salinity stress conditions (Gupta and Pandey, 2019). Trichoderma harzianum inoculation in rice plants improved root growth and protects from drought stress as reported by Shukla et al. (2012). Mukhtar et al. (2020a) reported that Bacillus cereus enhanced the production of ACC deaminase and exopolysaccharide which protects Solanum lycopersicum plants from heat stress.
Figure 2.
Role of biofertilizers for maintenance of crop productivity and soil health.
Table 2.
Role of biofertilizers in abiotic stress tolerance.
| Biofertilizers | Host plant | Stress | Response | References |
|---|---|---|---|---|
| Bacillus aryabhattai | Oryza sativa | Salinity, heavy metals | Improved salt tolerance ability via exopolysaccharide production | Sultana et al., 2020 |
| Bacillus amyloliquefaciens | Arabidopsis | Salt | Improved plant growth via regulation of JA pathway and antioxidant enzymes | Liu et al., 2020 |
| Bacillus licheniformis | Chrysanthemum | Salt | Improved salt tolerance ability in stressed plants via production of antioxidant enzymes and Fe attainment | Zhou et al., 2017 |
| Bacillus HL3RS14 | Zea mays | Salt | Increased weight of roots and shoots via production of IAA, proline, and glycine betaine | Mukhtar et al., 2020b |
| Bacillus subtilis, Pseudomonas sp. | Solanum melongena | Salt | Increase chlorophyll content and protects plants from stress | Mokabel et al., 2022 |
| Bacillus sp. | Pisum sativum | Salt | Improved plant growth and photosynthesis via antioxidant enzyme and AAC and siderophore production | Gupta et al., 2021 |
| Gluconacetobacter diazotrophicus | Zea mays | Drought and nitrogen | Increase plant biomass and chlorophyll content | Tufail et al., 2021 |
| Pseudomonas pseudoalcaligenes | Glycine max | Salt | Improved plant health parameters via production antioxidant enzyme, proline contents in shoots and roots | Yasmin et al., 2020 |
| Alternaria alternata | Triticum aestivum | Drought | Improved photosynthesis via increasing antioxidant enzymes | Qiang et al., 2019 |
| Aspergillus flavus | Glycine max | Salt | Increased antioxidant enzyme activity and chlorophyll content | Asaf et al., 2018 |
| Aspergillus violaceofucus | Helianthus annuus | Heat | Improved plant height, biomass, and chlorophyll content | Ismail et al., 2020 |
| Funneliformis mosseae | Trifoliate orange | Drought | Improved phenols, terpenoids and soil protein and enzyme activities | Cheng et al., 2021 |
| Glomus lomus | Date palm | Salt | Improved shoot weight and growth | Meddich et al., 2018 |
| Piriformospora indica | Triticum aestivum | Nutrient | Improved Zn uptake and root and shoot biomass | Abadi et al., 2021 |
| Piriformospora indica | Arabidopsis | Cold | Increased proline content and cold stress tolerance genes | Jiang et al., 2020 |
| Trichoderma atroviridae | Arabidopsis | Cold | Improved auxin production and cold-related gene expression | González-Pérez et al., 2018 |
| Rhizophagus intraradices | C3 plants | Salt | Improved chlorophyll content in plants | Chandrasekaran et al., 2019 |
| AMF and Bradyrhizobium japonicum | Glycine max | Drought | Increased yield and protects from stress via upregulation of CAT and POD activity | Sheteiwy et al., 2021 |
|
AMF and Rhizobium spp. Serratia marcescens |
Glycine max Lactuca sativa | Drought Salinity |
Improved plant health and microbial diversity in soil Triggered CAT, proline, and IAA production | Igiehon et al., 2021 Fortt et al., 2022 |
Drought stress
Drought stress is one of the main abiotic stresses which causes water scarcity to meet the plant necessity and causes economic fatalities in agriculture production. The normal progress of plants is hindered due to decrease in water shortage in their cells. Drought stress decreased the rate of photosynthesis, germination in plants, and loss in crop productivity (Lata et al., 2018). Inoculation of beneficial biofertilizers (rhizospheric and endophytic microbes) improved plant growth and development via different direct/indirect mechanisms under stress situations. Stress can be overcome via using biofertilizers which produced growth hormones such as IAA and cytokinins and improved plant development (Fasusi et al., 2021). Inoculation of Pseudomonas putida boosted the flavonoids, salicylic, and abscisic acid production which protects soybean plants from drought stress (Kang et al., 2014b). Inoculation of Pseudomonas spp. protects maize plants and improved biomass and sugar content in treated plants from drought stress via upregulation of dehydrin proteins and proline content (Sandhya et al., 2010). Khan et al. (2018) found that Bacillus thuringiensis improved chickpea growth under drought conditions via production of volatile organic compounds. Application of Microbacterium sp. improved maize plant growth, root length, photosynthetic rate, and yield under drought stress (Romera et al., 2019). Usage of Phoma improved the drought tolerance in Pinus tabulaeformis plants and increased seedling growth by improving the mechanism of water uptake, proline, and SOD (Zhou et al., 2021). Sheteiwy et al. (2021) reported that Bradyrhizobium japonicum and AMF improved the yield of soybean bacterial count and enzyme activities of soil via improving the nutrient accessibility in soil under drought stress. AMF and Rhizobium inoculation improved the Glycyrrhiza plant growth and phosphorus content in roots in drought stress (Hao et al., 2019). Combined inoculation of arbuscular fungi and bioinoculants improved plant biomass and chlorophyll content in date palm (Phoenix dactylifera) under water-deficit conditions via enhanced antioxidant enzyme activities, soluble sugars, and proteins (Anli et al., 2020). Inoculation of Glomus mosseae and Bacillus amyloliquefaciens in Phaseolus vulgaris significantly improved the photosynthetic rate and yield under water stress conditions (Salem and Al-Amri, 2021).
Salinity stress
Accumulation of salt in agricultural soil will have a negative impact on plants including its physiological, morphological, and molecular aspects. This affects plants via creating osmotic stress, ion toxicity and reducing the photosynthesis, CO2 fixation, and transpiration rate in plants. Availability of nutrients and microbial diversity are also affected due to the salinity stress (Luo et al., 2021). Usage of bioinoculants is enormously supportive in countering the lethal properties of soil salinity via improving the soil physicochemical properties and thus improved crop production (Jiménez-Mejía et al., 2022). Interaction between microbes and plants can overcome stress problem. Gond et al. (2015) reported that inoculation of Pantoea agglomerans in tropical corn under salt stress (0–100 mM) improves tolerance and growth of plants due to the upregulation of aquaporins. Bacillus megaterium also regulates the aquaporin genes during salt stress in maize plants and improved root growth and leaf water content (Marulanda et al., 2010). Waqas et al. (2012) reported that Penicillium and Phoma glomerata improved the rice plant growth under salinity stress via increased production of CAT, POD, and IAA. Checchio et al. (2021) observed that Azospirillum brasilense improved resistance in corn plants via enhancing the production of antioxidant enzymes and glycine betaine. Application of Pseudomonas sp. improves Arabidopsis thaliana germination and growth via upregulation of lipoxygenase genes which are involved in tolerance mechanism via jasmonic pathway (Chu et al., 2019). The Arthrobacter nitroguajacolicus improved wheat growth under salt stress via upregulation of IAA, ACC, flavonoid, stilbenoid, terpenoids, and cytochrome P450 genes (Safdarian et al., 2019). Inoculation of Planococcus rifietoensis protects Cicer arietinum plants from salt stress (200 mM) via EPS and biofilm production (Qurashi and Sabri, 2012). Gupta and Pandey (2019) observed that inoculation of Paenibacillus sp. protects and improved Phaseolus vulgaris plant growth under salinity stress via the production of IAA and ACC deaminase. Meena et al. (2020) reported that Nocardioides sp. improved seedling growth of Triticum aestivum under salt stress (0–100 mM) via increasing the CAT and POD genes. Inoculation of Penicillium and Ampelomyces spp. improved drought and salinity stress tolerance in tomato plants via the production of osmolytes, stress-responsive genes, and antioxidant enzymes (Morsy et al., 2020). Inoculation of Piriformospora indica highly enhanced plant development and attenuated NaCl-induced lipid peroxidation which helps to build tolerance during salinity stress (Ghaffari et al., 2018). Studies show that the inoculation of Trichoderma longibrachiatum T6 in wheat increased the levels of antioxidant enzymes (SOD, POD, and CAT) which helped to improve the stress tolerance in plants during salt stress (Zhang et al., 2016). Agrobacterium and Raoultella showed production of IAA, HCN, and ACC under salt stress and improved growth of Tetragonia tetragonioides plants (Egamberdieva et al., 2022). Fortt et al. (2022) reported that the application of PGPR improved the growth of lettuce under salt stress via the production of IAA and antioxidant enzymes which provide protection to plants.
Temperature stress
Global warming is a serious risk to all living creatures and is becoming a worldwide concern. Temperature stress such as heat and cold greatly limits the growth and development of plants (Yadav et al., 2018). Heat stress causes modification in homeostasis, degradation of proteins, which have lethal effects on physiology of plants as it delays the seed germination, damages to seeds and affects agricultural production (Imran et al., 2021). Cold stress causes dehydration due to ice formation which is responsible for protein denaturation. It also causes plant leaves lesions, yellowing of leaves, and rotting. It also affects the seed germination and yield of crops (Wu et al., 2021b). The application of several microbes alleviated the damaging effects of heat stress in various plants such as wheat, tomato, and sorghum via producing phytohormones, biofilm formation, and enhancing heat shock proteins (Issa et al., 2018; Sarkar et al., 2018). Bacillus cereus inoculation in tomato plants increased the production of HSPs, IAA, essential amino acids, and organic acids and protects plants from stress conditions (Khan et al., 2020). Inoculation of Azospirillum and B. amyloliquefaciens improved the heat tolerance via reducing oxidative damage in wheat seedling (Abd El-Daim et al., 2014). Duc et al. (2018) reported that Glomus sp. tolerates heat stress and protects tomato plants via scavenging ROS generation. Bacillus velezensis improved wheat plant survival under cold stress via increase in cold stress-related proteins as reported by Abd El-Daim et al. (2019). Zulfikar et al. (2011) reported that Pseudomonas putida also improved the growth of wheat plants under heat stress via enhanced production of proline, sugars, and antioxidant enzymes. R. irregularis and F. mosseae increased plant height, transpiration rate in maize, and nutrient composition in roots of triticum aestivum during heat stress (Cabral et al., 2016). Paraburkholderia phytofirmans having ACC deaminase-producing efficiency helps in normal growth of tomato plants under heat stress as reported by Esmaeel et al. (2018). Bacterial inoculants such as Rhodococcus and Burkholderia protect the medicinal plant Atractylodes lancea from heat stress and improved their growth via enriched root-associated microbes (Wang et al., 2022a).
Heavy metal stress
Extreme usage of inorganic chemical fertilizers in agriculture system causes the accumulation of toxic metals such as nickel, manganese, cadmium, iron, and zinc in soil (Ghori et al., 2019). These metals are beneficial for plants at low level, but if their concentration increases cause stress via decrease in plant growth due to the decrease in photosynthesis, deprived nutrients, membrane integrity, and enzyme activities. It causes oxidative stress via ROS and H2O2 generation and reduces plant growth and crop productivity (Ahmad et al., 2019; Gong et al., 2020). ROS generation occurs both under favorable and unfavorable circumstances, and it has a negative impact on vital macromolecules (Köhl et al., 2019). Rhizobium inoculation at nickel-contaminated site improves the chlorophyll content and increased lentil plant growth (Wani and Khan, 2013). Bradyrhizobium increased IAA production and siderophore production and improved the shoot weight of Lolium multiflorum at cadmium-contaminated site (Guo and Chi, 2014). Candida parapsilosis and B. cereus protect Trifolium repens plants from heavy metal stress conditions as reported by Azcón et al. (2010). Toxicity of arsenic in Brassica juncea is reduced by Staphylococcus arlettae via enhanced production of dehydrogenase and phosphatase enzyme in soil (Srivastava et al., 2013). Inoculation of Talaromyces pinophilus in Triticum aestivum plants stimulates plant growth via the production of gibberellic acid under heavy metal stress (El-Shahir et al., 2021). Paredes-Páliz et al. (2018) reported that inoculation of metal-resistant bacteria such as B. aryabhattai and Pantoea agglomerans brings production of phenylalanine ammonia-lyase enzyme and SOD which protects plants from metal stress. The addition of bioinoculants like P. aeruginosa and Burkholderia gladioli reduced Cd toxicity in Solanum lycopersicum by producing phenols, organic acids, and osmoprotectants (Khanna et al., 2019). Application of Serratia marcescens and E. bugandensis improved spinach (Ipomoea aquatica) growth via the production of polyamine under Pb and Cd toxicity (Wang et al., 2022b). Citrobacter and Enterobacter cloacae mitigate the Cd and Pb toxicity, improve the wheat plant health parameters, and protect from stress via the generation of antioxidant enzymes (Ajmal et al., 2022). Oubohssaine et al. (2022) reported that Pseudarthrobacter oxydans improved Sulla spinosissima growth and can be used as biofertilizer at heavy metal-contaminated sites. Cadmium tolerance bacteria such as Curtobacterium ocenosedimentum having P-solubilizing, IAA, and siderophore-producing possessions improved chili growth and increased shoot/root length (Patel et al., 2022). Inoculation of Pseudoarthrobacter and Vibrio neocaledonicus improved the Salicornia ramosissima growth at As- and Cu-polluted sites (Mesa-Marín et al., 2020). Rhizobium inoculation can promote soil nutrient cycling by increasing enzyme activity in metal-contaminated soil, thereby providing more N and P for microbial activity and growth of plants (Ma et al., 2021; Duan et al., 2022). Heavy metal toxicity is a growing problem in the world; therefore, finding appropriate microbes proficient to depollution of the metals can benefit to improve the crop efficiency. Application of biofertilizers for sustainable food crop production and boosting various stress tolerance of plants are gaining popularity. Still, further studies are crucial to unravel the potential role of biofertilizers in responding to the impact of different stresses at molecular level.
Conclusion
Agriculture systems have to face the task of food production, stress management, and dependency on agrochemicals. The presence of pest and pathogen in crops causes decrease in crop yield and heavy crop losses every year. The occurrence of abiotic stresses due to the change in climatic conditions leads to difficult challenge to crop production worldwide. Different effective approaches should be employed to reduce crop output loss and control diseases. Hence, the necessity to implement the eco-friendly approaches such as biofertilizers is of great importance for sustainable agriculture. The application of biofertilizers not only improves plant heath parameters but also enhances the crop productivity, soil health and protects from stress environment. More research has been focused on physiological and molecular aspects under different conditions with different crops using biofertilizers under field conditions.
Author contributions
PC: conceptualization and wrote the manuscript. SS, AC, AS, and GK: editing the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to acknowledge the Microbiology Department, Govind Ballabh Pant University of Agriculture and Technology.
References
- Aasfar A., Bargaz A., Yaakoubi K., Hilali A., Bennis I., Zeroual Y., Kadmiri M. I. (2021). Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Front. Microbiol. 12, 628379. 10.3389/fmicb.2021.628379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abadi V. A. J. M., Sepehri M., Khatabi B., Rezaei M. (2021). Alleviation of zinc deficiency in wheat inoculated with root endophytic fungus Piriformospora indica and Rhizobacterium, Pseudomonas putida. Rhizosphere 17:100311. 10.1016/j.rhisph.2021.100311 [DOI] [Google Scholar]
- Abbey L., Abbey J., Leke-Aladekoba A., Iheshiulo E. M. A., Ijenyo M. (2019). Biopesticides and biofertilizers: types, production, benefits, and utilization. Byprod. Agri. Fisher. 2019, 479–500. 10.1002/9781119383956.ch20 [DOI] [Google Scholar]
- Abd El-Daim I., Bejai S., Meijer J. (2014). Improved heat stress tolerance of wheat seedlings by bacterial seed treatment. Plant Soil. 379, 337–350. 10.1007/s11104-014-2063-3 [DOI] [Google Scholar]
- Abd El-Daim I. A., Bejai S., Meijer J. (2019). Bacillus velezensis 5113 induced metabolic and molecular reprogramming during abiotic stress tolerance in wheat. Sci. Rep. 9:16282. 10.1038/s41598-019-52567-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah R. A. B., Trabelsi B. M., Nefzi A., Khiareddine H. J., Remadi M. D. (2016). Isolation of endophytic bacteria from Withania somnifera and assessment of their ability to suppress Fusarium wilt disease in tomato and to promote plant growth. J. Plant Pathol. Microbiol. 7:352. 10.4172/2157-7471.1000352 [DOI] [Google Scholar]
- Adhikari P., Pandey A. (2019). Phosphate solubilization potential of endophytic fungi isolated from Taxus wallichiana Zucc. roots. Rhizosphere 9, 2–9. 10.1016/j.rhisph.2018.11.00232738763 [DOI] [Google Scholar]
- Agri U., Chaudhary P., Sharma A. (2021). In vitro compatibility evaluation of agriusable nanochitosan on beneficial plant growth-promoting rhizobacteria and maize plant. Natl. Acad. Sci. Lett. 44, 555–559. 10.1007/s40009-021-01047-w [DOI] [Google Scholar]
- Agri U., Chaudhary P., Sharma A., Kukreti B. (2022). Physiological response of maize plants and its rhizospheric microbiome under the influence of potential bioinoculants and nanochitosan. Plant Soil 474, 451–468. 10.1007/s11104-022-05351-2 [DOI] [Google Scholar]
- Aguilar-Paredes A., Valdés G., Nuti M. (2020). Agronomy ecosystem functions of microbial consortia in sustainable agriculture. Agronomy. 10. 10.3390/agronomy10121902 [DOI] [Google Scholar]
- Ahluwalia O., Singh P. C., Bhatia R. (2021). A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Res. Environ. Sustain. 2021:100032. 10.1016/j.resenv.2021.100032 [DOI] [Google Scholar]
- Ahmad P., Tripathi D. K., Deshmukh R., Pratap S. V., Corpas F. J. (2019). Revisiting the role of ROS and RNS in plants under changing environment. Environ. Experi. Botany 161, 1–398. 10.1016/j.envexpbot.2019.02.017 [DOI] [Google Scholar]
- Ajmal A. W., Yasmin H., Hassan M. N., Khan N., Jan B. L., Mumtaz S. (2022). Heavy metal-resistant plant growth-promoting Citrobacter werkmanii strain WWN1 and Enterobacter cloacae strain JWM6 enhance wheat (Triticum aestivum L.) growth by modulating physiological attributes and some key antioxidants under multi-metal stress. Front. Microbiol. 6, 815704. 10.3389/fmicb.2022.815704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanmu A. O., Babalola O. O., Venturi V., Ayilara M. S., Adeleke B. S., Amoo A. E., et al. (2021). Plant disease management: leveraging on the plant-microbe-soil interface in the biorational use of organic amendments. Front. Plant Sci. 12, 700507. 10.3389/fpls.2021.700507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A. A., Awad M. Y. M., Hegab S. A., Abd El Gawad A. M., Eissa M. A. (2021). Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J. Plant. Nutr. 44, 411–420. 10.1080/01904167.2020.1822399 [DOI] [Google Scholar]
- Alınç T., Cusumano A., Peri E., Torta L., Colazza S. (2021). Trichoderma harzianum strain T22 modulates direct defense of tomato plants in response to Nezara viridula feeding activity. J Chem Ecol. 47, 455–462. 10.1007/s10886-021-01260-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkurtany A., Ali S., Mahdi W. (2018). The efficiency of prepared biofertilizer from local isolate of Bradyrhizobium sp. on growth and yield of mungbean plant. Iraqi J. Agric. Sci. 49, 722–730. [Google Scholar]
- Anand A., Chinchilla D., Tan C., Mène-Saffrané L., L'Haridon F., Weisskopf L. (2020). Contribution of hydrogen cyanide to the antagonistic activity of Pseudomonas strains against Phytophthora infestans. Microorganisms 8:1144. 10.3390/microorganisms8081144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anli M., Baslam M., Tahiri A., Raklami A., Symanczik S., Boutasknit A., et al. (2020). Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front. Plant Sci. 11, 516818. 10.3389/fpls.2020.516818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaf S., Hamayun M., Khan A. L., Waqas M., Khan M. A., Jan R., et al. (2018). Salt tolerance of Glycine max. L induced by endophytic fungus Aspergillus flavus CSH1, via regulating its endogenous hormones and antioxidative system. Plant Physiol. Biochem. 128, 13–23. 10.1016/j.plaphy.2018.05.007 [DOI] [PubMed] [Google Scholar]
- Ayaz M., Ali Q., Farzand A., Khan A., Ling H., Gao X. (2021). Nematicidal volatiles from Bacillus atrophaeus GBSC56 promote growth and stimulate induced systemic resistance in tomato against Meloidogyne incognita. Int. J. Mol. Sci. 22:5049. 10.3390/ijms22095049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub I., Kumar V., Abolhassani R., Sehgal R., Sharma V., Sehgal R., et al. (2022). Advances in ZnO: Manipulation of defects for enhancing their technological potentials. Nanotechnol. Rev. 11, 575–619. 10.1515/ntrev-2022-0035 [DOI] [Google Scholar]
- Azcón R., Perálvarez M. C., Roldán A., Barea J. (2010). Arbuscular mycorrhizal fungi, Bacillus cereus, and Candida parapsilosis from a multicontaminated soil alleviate metal toxicity in plants. Microb. Ecol. 59, 668–677. 10.1007/s00248-009-9618-5 [DOI] [PubMed] [Google Scholar]
- Azeem M., Haider M. Z., Javed S., Saleem M. H., Alatawi A. (2022). Drought stress amelioration in maize (Zea mays L.) by inoculation of Bacillus spp. Strains under sterile soil conditions. Agriculture 12:50. 10.3390/agriculture12010050 [DOI] [Google Scholar]
- Bakhshandeh E., Gholamhosseini M., Yaghoubian Y., Pirdashti H. (2020). Plant growth promoting microorganisms can improve germination, seedling growth and potassium uptake of soybean under drought and salt stress. Plant Growth Regul. 90, 123–136. 10.1007/s10725-019-00556-5 [DOI] [Google Scholar]
- Baldi E., Gioacchini P., Montecchio D., Mocali S., Antonielli L., Masoero G., et al. (2021). Effect of biofertilizers application on soil biodiversity and litter degradation in a commercial apricot orchard. Agronomy 11:1116. 10.3390/agronomy11061116 [DOI] [Google Scholar]
- Bamisile B. S., Dash C. K., Akutse K. S., Keppanan R., Wang L. (2018). Fungal endophytes: beyond herbivore management. Front. Microbiol. 9, 544. 10.3389/fmicb.2018.00544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak B. B., Maity A., Ray P., Biswas D. R., Roy S. (2022). Potassium supply in agriculture through biological potassium fertilizer: a promising and sustainable option for developing countries. Arch. Agronomy Soil Sci. 68, 101–114. 10.1080/03650340.2020.1821191 [DOI] [Google Scholar]
- Batista B. D., Dourado M. N., Figueredo E. F. (2021). The auxin-producing Bacillus thuringiensis RZ2MS9 promotes the growth and modifies the root architecture of tomato (Solanum lycopersicum cv. Micro-Tom). Arch. Microbiol. 203, 3869–3882. 10.1007/s00203-021-02361-z [DOI] [PubMed] [Google Scholar]
- Batool S., Asghar H. N., Shehzad M. A., Yasin S., Sohaib M., Nawaz F., et al. (2021). Zinc-solubilizing bacteria-mediated enzymatic and physiological regulations confer zinc biofortification in chickpea (Cicer arietinum L.). J. Soil Sci. Plant Nutr. 21, 2456–2471. 10.1007/s42729-021-00537-6 [DOI] [Google Scholar]
- Bechtaoui N., Rabiu M. K., Raklami A., Oufdou K., Hafidi M., Jemo M. (2021). Phosphate-dependent regulation of growth and stresses management in plants. Front. Plant Sci. 12, 679916. 10.3389/fpls.2021.679916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beris D., Theologidis I., Skandalis N. (2018). Bacillus amyloliquefaciens strain MBI600 induces salicylic acid dependent resistance in tomato plants against Tomato spotted wilt virus and Potato virus Y. Sci. Rep. 8:10320. 10.1038/s41598-018-28677-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj D., Ansari M. W., Sahoo R. K., Tuteja N. (2014). Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 13, 1–10. 10.1186/1475-2859-13-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee R., Dey U. (2014). Biofertilizer, a way towards organic agriculture: A review. Afr. J. Microbiol. Res. 8, 2332–2343. 10.5897/AJMR2013.637434480627 [DOI] [Google Scholar]
- Bhojiya A. A., Joshi H., Upadhyay S. K., Srivastava A. K., Pathak V. V., Pandey V. C., et al. (2021). Screening and optimization of zinc removal potential in Pseudomonas aeruginosa-HMR1 and its plant growth-promoting attributes. Bull. Environ. Contamination Toxicol. 2021, 1–10. 10.1007/s00128-021-03232-5 [DOI] [PubMed] [Google Scholar]
- Binenbaum J., Weinstain R., Shani E. (2018). Gibberellin localization and transport in plants. Trends Plant Sci. 23:5. 10.1016/j.tplants.2018.02.005 [DOI] [PubMed] [Google Scholar]
- Boroujeni S. M., Kalbasi M., Asgharzadeh A., Baharlouei J. (2021). Evaluating the potential of Halothiobacillus bacteria for sulfur oxidation and biomass production under saline soil. Geomicrobiol. J. 38, 57–65. 10.1080/01490451.2020.1809571 [DOI] [Google Scholar]
- Brahmaprakash G. P., Sahu P. K. (2012). Biofertilizers for sustainability. J. Indian Institute Sci. 92, 37–62. [Google Scholar]
- Cabral M., Cheng X., Singh S., Ivessa A. S. (2016). Absence of non-histone protein complexes at natural chromosomal pause sites results in reduced replication pausing in aging yeast cells. Cell Rep. 8, 1747–1754. 10.1016/j.celrep.2016.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo E., Fucile M., Mattii G. B. (2022). Biostimulants in viticulture: a sustainable approach against biotic and abiotic stresses. Plants 11:162. 10.3390/plants11020162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran M., Chanratana M., Kim K., Seshadri S., Sa T. (2019). Impact of arbuscular mycorrhizal fungi on photosynthesis, water status, and gas exchange of plants under salt stress–a meta-analysis. Front. Plant Sci. 10, 457. 10.3389/fpls.2019.00457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary A., Chaudhary P., Upadhyay A., Kumar A., Singh A. (2022a). Effect of gypsum on plant growth promoting rhizobacteria. Environ. Ecol. 39, 1248–1256.31124074 [Google Scholar]
- Chaudhary A., Parveen H., Chaudhary P., Khatoon H., Bhatt P. (2021). “Rhizospheric microbes and their mechanism,” in Microbial Technology for Sustainable Environment, eds P. Bhatt, S. Gangola, D. Udayanga, and G. Kumar (Singapore: Springer; ). 10.1007/978-981-16-3840-4_6 [DOI] [Google Scholar]
- Chaudhary P., Chaudhary A., Agri U., Khatoon H., Singh A. (2022b). “Recent trends and advancements for agro-environmental sustainability at higher altitudes,” In: Survival Strategies in Cold-adapted Microorganisms, eds R. Goel, R. Soni, D. C. Suyal, and M. Khan M. (Singapore: Springer; ). 10.1007/978-981-16-2625-8_19 [DOI] [Google Scholar]
- Chaudhary P., Chaudhary A., Bhatt P., Kumar G., Khatoon H., Rani A., et al. (2022c). Assessment of soil health indicators under the influence of nanocompounds and Bacillus spp. in field condition. Front. Environ. Sci. 9, 769871. 10.3389/fenvs.2021.769871 [DOI] [Google Scholar]
- Chaudhary P., Sharma A. (2019). Response of nanogypsum on the performance of plant growth promotory bacteria recovered from nanocompound infested agriculture field. Environ. Ecol. 37, 363–372. [Google Scholar]
- Checchio M. V., de Cássia Alves R., de Oliveira K. R., Moro G. V., Santos D. M. M. D., Gratão P. L. (2021). Enhancement of salt tolerance in corn using Azospirillum brasilense: An approach on antioxidant system. J. Plant Res. 24:34302571. 10.1007/s10265-021-01332-1 [DOI] [PubMed] [Google Scholar]
- Chen Y., Yang H., Shen Z., Ye J. (2022). Whole-Genome Sequencing and Potassium-Solubilizing Mechanism of Bacillus aryabhattai SK1-7. Front. Microbiol. 12, 722379. 10.3389/fmicb.2021.722379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Zhao L., Dong Y., Chen W., Li C., Gao X., et al. (2021). The antagonistic mechanism of Bacillus velezensis ZW10 against rice blast disease: Evaluation of ZW10 as a potential biopesticide. PLoS ONE 16, e0256807. 10.1371/journal.pone.0256807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X. F., Wu H. H., Zou Y. N., Wu Q. S., Kuča K. (2021). Mycorrhizal response strategies of trifoliate orange under well-watered, salt stress, and waterlogging stress by regulating leaf aquaporin expression. Plant Physiol. Biochem. 162, 27–35. 10.1016/j.plaphy.2021.02.026 [DOI] [PubMed] [Google Scholar]
- Chu T. N., Tran B., Van Bui L., Hoang M. (2019). Plant growth-promoting rhizobacterium Pseudomonas PS01 induces salt tolerance in Arabidopsis thaliana. BMC Res. Notes 12:11. 10.1186/s13104-019-4046-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cissoko M., Hocher V., Gherbi H., Gully D., Carré-Mlouka A., Sane S., et al. (2018). Actinorhizal signaling molecules: Frankia root hair deforming factor shares properties with NIN inducing factor. Front. Plant Sci. 18:1494. 10.3389/fpls.2018.01494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge D. B., Jensen D. F., Rabiey M., Sarrocco S., Shaw M. W., Shaw R. H. (2022). Biological control of plant diseases – What has been achieved and what is the direction? Plant Pathol. 71, 1024–1047. 10.1111/ppa.13555 [DOI] [Google Scholar]
- Dal C.ortivo C., Ferrari M., Visioli G., Lauro M., Fornasier F., Barion G., et al. (2020). Effects of seed-applied biofertilizers on rhizosphere biodiversity and growth of common wheat (Triticum aestivum L.) in the field. Front. Plant Sci. 11, 72. 10.3389/fpls.2020.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daroodi Z., Taheri P. S. (2021). Direct antagonistic activity and tomato resistance induction of the endophytic fungus Acrophialophora jodhpurensis against Rhizoctonia solani. Biol. Control. 160:104696. 10.1016/j.biocontrol.2021.104696 [DOI] [Google Scholar]
- Desrut A., Moumen B., Thibault F., Le Hir R., Coutos-Thévenot P., Vriet C. (2020). Beneficial rhizobacteria Pseudomonas simiae WCS417 induce major transcriptional changes in plant sugar transport. J. Exp. Bot. 71, 7301–7315. 10.1093/jxb/eraa396 [DOI] [PubMed] [Google Scholar]
- Dicko A. H., Nantoume D., Ouattara D., Kassogue A., Fane R., Malle I., et al. (2021). Screening of rice endophytic natives biofertilizers with plant growth-promoting characteristics. Afr. J. Agri. Res. 17, 1336–1342. 10.5897/AJAR2020.15191 [DOI] [Google Scholar]
- Ding Z., Ali E. F., Almaroai Y. A., Eissa M. A., Abeed A. H. A. (2021). Effect of potassium solubilizing bacteria and humic acid on faba bean (Vicia faba L.) plants grown on sandy loam soils. J. Soil Sci. Plant Nutr. 21, 791–800. 10.1007/s42729-020-00401-z [DOI] [Google Scholar]
- Divekar P. A., Narayana S., Divekar B. A., Kumar R., Gadratagi B. G., Ray A., et al. (2022). Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 23:2690. 10.3390/ijms23052690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don Y., Schmidtke S. M., Gambetta L. M. (2020). Aureobasidium pullulans volatilome identified by a novel, quantitative approach employing SPME-GC-MS, suppressed Botrytis cinerea and Alternaria alternata in vitro. Sci. Rep. 10:4498. 10.1038/s41598-020-61471-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos T. B, Ribas A. F., de Souza S. G. H., Budzinski I. G. F., Domingues D.S. (2022). Physiological responses to drought, salinity, and heat stress in plants: a review. Stresses 2, 113–135. 10.3390/stresses2010009 [DOI] [Google Scholar]
- Duan C., Mei Y., Wang Q., Wang Y., Li Q., Hong M., et al. (2022). Rhizobium inoculation enhances the resistance of alfalfa and microbial characteristics in copper-contaminated soil. Front. Microbiol. 12, 781831. 10.3389/fmicb.2021.781831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc N. H., Csintalan Z., Posta K. (2018). Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 132, 297–307. 10.1016/j.plaphy.2018.09.011 [DOI] [PubMed] [Google Scholar]
- Egamberdieva D., Alimov J., Shurigin V., Alaylar B., Wirth S., Bellingrath-Kimura S. D. (2022). Diversity and plant growth-promoting ability of endophytic, halotolerant bacteria associated with Tetragonia tetragonioides (Pall.) kuntze. Plants. 11:49. 10.3390/plants11010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann R., Richards L., Schafer P. (2021). Hormones as go-betweens in plant microbiome assembly. Plant J. 105, 518–541. 10.1111/tpj.15135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid A. M., Fouda A., Abdel-Rahman M. A., Salem S. S., Elsaied A., Oelmüller R., et al. (2021). Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: an overview. Plants 10:935. 10.3390/plants10050935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shahir A. A., Noha A., El,-T, Omar M. A., Arafat A. H., Abdel L., et al. (2021). The effect of endophytic Talaromyces pinophilus on growth, absorption and accumulation of heavy metals of Triticum aestivum grown on sandy soil amended by sewage sludge. Plants. 10:2659. 10.3390/plants10122659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeel Q., Miotto L., Rondeau M., Leclère V., Clément C., Jacquard C. (2018). Paraburkholderia phytofirmans PsJN-plants interaction: from perception to the induced mechanisms. Front. Microbiol. 9, 2093. 10.3389/fmicb.2018.02093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etesami H., Jeong B. R., Glick B. R. (2021). Contribution of arbuscular mycorrhizal fungi, phosphate–solubilizing bacteria, and silicon to p uptake by plant. Front. Plant Sci. 12, 699618. 10.3389/fpls.2021.699618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etesami H., Mirseyed Hosseini H., Alikhani H. A. (2014). Bacterial biosynthesis of 1-aminocyclopropane-1-caboxylate (ACC) deaminase, a useful trait to elongation and endophytic colonization of the roots of rice under constant flooded conditions. Physiol. Mol. Biol. Plants. 20, 425–434. 10.1007/s12298-014-0251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadiji A. E., Babalola O. O. (2020). Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 8:467. 10.3389/fbioe.2020.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahsi N., Mahdi I., Mesfioui A., Biskri L., Allaoui A. (2021). Plant growth-promoting rhizobacteria isolated from the Jujube (Ziziphus lotus) plant enhance wheat growth, Zn uptake and heavy metal tolerance. Agriculture. 11:316. 10.3390/agriculture11040316 [DOI] [Google Scholar]
- Fang L., Ju W., Yang C., Jin X., Zhang C. (2020). Exogenous application of signaling molecules to enhance the resistance of legume-rhizobium symbiosis in Pb/Cd-contaminated soils. Environ. Pollut. 365:114744. 10.1016/j.envpol.2020.114744 [DOI] [PubMed] [Google Scholar]
- Fasusi O. A., Cruz C., Babalola O. O. (2021). Agricultural sustainability: microbial biofertilizers in rhizosphere management. Agriculture 11:163. 10.3390/agriculture11020163 [DOI] [Google Scholar]
- Fortt J., González M., Morales P., Araya N., Remonsellez F., Coba de la Peña T., et al. (2022). Bacterial modulation of the plant ethylene signaling pathway improves tolerance to salt stress in lettuce (Lactuca sativa L.). Front. Sustain. Food Syst. 6:768250. 10.3389/fsufs.2022.768250 [DOI] [Google Scholar]
- Galindo F. S., Pagliari P. H., Fernandes G. C., Rodrigues W. L., Boleta E. H. M., Jalal A., et al. (2022). Improving sustainable field-grown wheat production with Azospirillum brasilense under tropical conditions: a potential tool for improving nitrogen management. Front. Environ. Sci. 10, 821628. 10.3389/fenvs.2022.821628 [DOI] [Google Scholar]
- Game B. C., Ilhe B. M., Pawar V. S., Khandagale P. P. (2020). Effect of Azotobacter, phosphate solubilizing bacteria and potash mobilizing bacteria inoculants on productivity of wheat (Triticum aestivum L.). Intern. J. Curr. Microbiol. Appl. Sci. 9, 2800–2807. 10.20546/ijcmas.2020.903.322 [DOI] [Google Scholar]
- Gao D., Ran C., Zhang Y., Wang X., Lu S., Geng Y., et al. (2022). Effect of different concentrations of foliar iron fertilizer on chlorophyll fluorescence characteristics of iron-deficient rice seedlings under saline sodic conditions. Plant Physiol. Biochem. 185, 112–122. 10.1016/j.plaphy.2022.05.021 [DOI] [PubMed] [Google Scholar]
- Ghaffari H., Gholizadeh A., Biabani A., Fallah A., Mohammadian M. (2018). Plant growth promoting rhizobacteria (PGPR) application with different nitrogen fertilizer levels in rice (Oryza sativa L.). Pertanika J. Trop. Agric. Sci. 41, 715–728. [Google Scholar]
- Ghazy N., El-Nahrawy S. (2021). Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant. Arch. Microbiol. 203, 1195–1209. 10.1007/s00203-020-02113-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghodhbane-Gtari F., D'Angelo T., Gueddou A., Ghazouani S., Gtari M., Tisa L. S. (2021). Alone yet not alone: frankia lives under the same roof with other bacteria in actinorhizal nodules. Front. Microbiol. 12, 749760. 10.3389/fmicb.2021.749760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghori N. H., Ghori T., Hayat M. Q., Imadi S. R., Gul A., Altay V., et al. (2019). Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 16, 1807–1828. 10.1007/s13762-019-02215-835205031 [DOI] [Google Scholar]
- Ghosh D., Gupta A., Mohapatra S. (2019). A comparative analysis of exopolysaccharide and phytohormone secretions by four drought-tolerant rhizobacterial strains and their impact on osmotic-stress mitigation in Arabidopsis thaliana. World J. Microbiol. Biotechnol. 35, 1–15. 10.1007/s11274-019-2659-0 [DOI] [PubMed] [Google Scholar]
- Gohil R. B., Raval V. H., Panchal R. R., Rajput K. N. (2022). Plant growth-promoting activity of Bacillus sp. PG-8 isolated from fermented panchagavya and its effect on the growth of arachis hypogea. Front. Agron. 4, 805454. 10.3389/fagro.2022.805454 [DOI] [Google Scholar]
- Gond S. K., Torres M. S., Bergen M. S., Helsel Z., White J. F. (2015). Induction of salt tolerance and up-regulation of aquaporin genes in tropical corn by rhizobacterium Pantoea agglomerans. Lett. Appl. Microbiol. 60, 392–399. 10.1111/lam.12385 [DOI] [PubMed] [Google Scholar]
- Gong Z., Xiong L., Shi H., Yang S., Herrera-Estrella L. R., Xu G., et al. (2020). Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 63, 635–674. 10.1007/s11427-020-1683-x [DOI] [PubMed] [Google Scholar]
- González-López M. D. C., Jijón-Moreno S., Dautt-Castro M., Ovando-Vázquez C., Ziv T., Horwitz B. A., et al. (2021). Secretome analysis of Arabidopsis-Trichoderma atroviride interaction unveils new roles for the plant glutamate:glyoxylate aminotransferase GGAT1 in plant growth induced by the fungus and resistance against botrytis cinerea. Int. J. Mol. Sci. 22:6804. 10.3390/ijms22136804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Pérez E., Ortega-Amaro M. A., Salazar-Badillo F. B., Bautista E., Douterlungne D., Jiménez-Bremont J. F. (2018). The Arabidopsis-Trichoderma interaction reveals that the fungal growth medium is an important factor in plant growth induction. Sci. Rep. 8, 1–14. 10.1038/s41598-018-34500-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouda S., Kerry R. G., Das G. (2018). Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 206, 131–140. 10.1016/j.micres.2017.08.016 [DOI] [PubMed] [Google Scholar]
- Gu S., Wei Z., Shao Z., Friman V. P., Cao K., Yang T. (2020). Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat. Microbiol. 5, 1002–1010. 10.1038/s41564-020-0719-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Chi J. (2014). Effect of Cd-tolerant plant growth promoting Rhizobium on plant growth and Cd uptake by Lolium multiflorum Lam. and Glycine max (L.) Merr. in Cd-contaminated soil. Plant Soil. 375, 205–214. 10.1007/s11104-013-1952-1 [DOI] [Google Scholar]
- Gupta A., Rai S., Bano A., Khanam A., Sharma S., Pathak N. (2021). Comparative evaluation of different salt-tolerant plant growth-promoting bacterial isolates in mitigating the induced adverse effect of salinity in pisum sativum. Biointerface Res. Appl. Chem. 11, 13141–13154. 10.33263/BRIAC115.1314113154 [DOI] [Google Scholar]
- Gupta S., Pandey S. (2019). ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front. Microbiol. 10, 1506. 10.3389/fmicb.2019.01506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z., Xie W., Jiang X., Wu Z., Zhang X., Chen B. (2019). Arbuscular mycorrhizal fungus improves Rhizobium–Glycyrrhiza seedling symbiosis under drought stress. Agronomy. 9:572. 10.3390/agronomy910057228424720 [DOI] [Google Scholar]
- Harman G., Khadka R., Doni F., Uphoff N. (2020). Benefits to plant health and productivity from enhancing plant microbial symbionts. Front. Plant Sci. 11, 610065. 10.3389/fpls.2020.610065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro R., Benito B. (2019). The role of soil fungi in K+ plant nutrition. Int. J. Mol. Sci. 20:3169. 10.3390/ijms20133169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan N., Farzand A., Heng Z., Khan I. U., Moosa A., Zubair M., et al. (2020). Antagonistic potential of novel endophytic Bacillus strains and mediation of plant defense against Verticillium wilt in upland cotton. Plants 9:1438. 10.3390/plants9111438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan E. A., Yasser S., Mostafa A., Mohamed H., Nivien A. N. (2021). Biosafe management of Botrytis grey mold of strawberry fruit by novel bioagents. Plants 12:2737. 10.3390/plants10122737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata E. M., Yusof M. T., Zulperi D. (2021). Induction of systemic resistance against bacterial leaf streak disease and growth promotion in rice plant by Streptomyces shenzhenesis TKSC3 and Streptomyces sp. SS8. Plant Pathol. J. 37, 173–181. 10.5423/PPJ.OA.05.2020.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J. P., Oresnik I. J. (2022). The Rhizobium-legume symbiosis: co-opting successful stress management. Front. Plant Sci. 3, 796045. 10.3389/fpls.2021.796045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., He C.-Q., Ding N.-Z. (2018). Abiotic stresses: general defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 9, 1771. 10.3389/fpls.2018.01771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy L. M., Popay A. J., Glare T. R. (2022). Olfactory responses of Argentine stem weevil to herbivory and endophyte-colonisation in perennial ryegrass. J. Pest Sci. 95, 263–277. 10.1007/s10340-021-01375-2 [DOI] [Google Scholar]
- Hernández-Fernández M., Cordero-Bueso G., Ruiz-Muñoz M., Cantoral J. M. (2021). Culturable yeasts as biofertilizers and biopesticides for a sustainable agriculture: a comprehensive review. Plants (Basel) 21:822. 10.3390/plants10050822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htwe A. Z., Moh S. M., Moe K., Yamakawa T. (2019). Biofertilizer production for agronomic application and evaluation of its symbiotic effectiveness in soybeans. Agronomy 9:162. 10.3390/agronomy9040162 [DOI] [Google Scholar]
- Huang C. J., Wei G., Jie Y. C., Xu J. J., Zhao S. Y., Wang L. C., et al. (2015). Responses of gas exchange, chlorophyll synthesis and ROS-scavenging systems to salinity stress in two ramie (Boehmeria nivea L.) cultivars. Photosynthetica 53, 455–463. 10.1007/s11099-015-0127-0 [DOI] [Google Scholar]
- Hummadi E. H., Cetin Y., Demirbek M., Kardar N. M., Khan S., Coates C. J., et al. (2022). Antimicrobial volatiles of the insect pathogen Metarhizium brunneum. J. Fungi 22:326. 10.3390/jof8040326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A., Zahir Z. A., Ditta A., Tahir M. U., Ahmad M., Mumtaz M. Z., et al. (2020). Production and implication of bio-activated organic fertilizer enriched with zinc-solubilizing bacteria to boost up maize (Zea mays L.) production and biofortification under two cropping seasons. Agronomy 10:39. 10.3390/agronomy10010039 [DOI] [Google Scholar]
- Igiehon N. O., Babalola O. O., Aremu B. R. (2019). Genomic insights into plant growth promoting rhizobia capable of enhancing soybean germination under drought stress. BMC Microbiol. 19:159. 10.1186/s12866-019-1536-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igiehon N. O., Babalola O. O., Cheseto X., Torto B. (2021). Effects of rhizobia and arbuscular mycorrhizal fungi on yield, size distribution and fatty acid of soybean seeds grown under drought stress. Microbiol. Res. 242:126640. 10.1016/j.micres.2020.126640 [DOI] [PubMed] [Google Scholar]
- Imran Q. M., Falak N., Hussain A., Mun B.-G., Yun B.-W. (2021). Abiotic stress in plants; stress perception to molecular response and role of biotechnological tools in stress resistance. Agronomy 11:1579. 10.3390/agronomy11081579 [DOI] [Google Scholar]
- Iqbal Z., Iqbal M. S., Hashem A., Abd_Allah E. F., Ansari M. I. (2021). Plant defense responses to biotic stress and its interplay with fluctuating dark/light conditions. Front. Plant Sci. 12, 631810. 10.3389/fpls.2021.631810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail A. H., Mehmood A., Qadir M., Husna A. I., Hamayun M., Khan N. (2020). Thermal stress alleviating potential of endophytic fungus Rhizopus oryzae inoculated to sunflower (Helianthus annuus L.) and soybean (Glycine max L.). Pak. J. Bot. 52, 1857–1865. 10.30848/PJB2020-5(10) [DOI] [Google Scholar]
- Ismail E. G., Mohamed W. W., Khattab S., Sherif F. E. (2013). Effect of manure and bio-fertilizers on growth, yield, silymarin content, and protein expression profile of Silybum marianum. Int. J. Med. Arom. Plants. 3, 430–438. [Google Scholar]
- Issa A. Esmaeel Q. Sanchez L. Courteaux B. Guise and, J.-F. Gibon Y. (2018). Impacts of Paraburkholderia phytofirmans strain PsJN on tomato (Lycopersicon esculentum L.) under high temperature. Front. Plant Sci. 9, 1397. 10.3389/fpls.2018.01397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain D., Sharma J., Kaur G., Bhojiya A. A., Chauhan S., Sharma V., et al. (2021). Phenetic and molecular diversity of nitrogen-fixing plant growth-promoting Azotobacter isolated from semiarid regions of India. Hindawi BioMed. Res. Intern. 2021:6686283. 10.1155/2021/6686283 [DOI] [Google Scholar]
- Janati W., Benmrid B., Elhaissoufi W., Zeroual Y., Nasielski J., Bargaz A. (2021). Will phosphate bio-solubilization stimulate biological nitrogen fixation in grain legumes? Front. Agron. 3:637196. 10.3389/fagro.2021.637196 [DOI] [Google Scholar]
- Jiang W., Pan R., Wu C., Xu L., Abdelaziz M. E., Oelmüller R., et al. (2020). Piriformospora indica enhances freezing tolerance and post-thaw recovery in Arabidopsis by stimulating the expression of CBF genes. Plant Signal. Behav. 15:1745472. 10.1080/15592324.2020.1745472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Jimenez S., Santana O., Lara-Rojas F., Arthikala M. K., Armada E., Hashimoto K., et al. (2019). Differential tetraspanin genes expression and subcellular localization during mutualistic interactions in Phaseolus vulgaris. PLoS ONE 14, e0219765. 10.1371/journal.pone.0219765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Mejía R., Medina-Estrada R. I., Carballar-Hernández S., Orozco-Mosqueda M., d,.C, Santoyo G., et al. (2022). Teamwork to survive in hostile soils: use of plant growth-promoting bacteria to ameliorate soil salinity stress in crops. Microorganisms 10:150. 10.3390/microorganisms10010150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X., Cui Q., Li X., Yin J., Ravichandran V., Pan D., et al. (2020). Engineering Pseudomonas protegens Pf-5 to improve its antifungal activity and nitrogen fixation. Microb. Biotechnol. 13, 118–133. 10.1111/1751-7915.13335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel S. L., Joubert P. M., Doty S. L. (2017). Bacterial endophyte colonization and distribution within plants. Microorganisms 5:77. 10.3390/microorganisms5040077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.-M., Radhakrishnan R., Khan A. L., Kim M.-J., Park J.-M., Kim B.-R. (2014b). Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 84, 115–124. 10.1016/j.plaphy.2014.09.001 [DOI] [PubMed] [Google Scholar]
- Kang S.-M., Shahzad R., Khan M. A., Hasnain Z., Lee K.-E., Park H.-S., et al. (2021). Ameliorative effect of indole-3-acetic acid- and siderophore-producing Leclercia adecarboxylata MO1 on cucumber plants under zinc stress. J. Plant Inter. 16, 30–41. 10.1080/17429145.2020.1864039 [DOI] [Google Scholar]
- Kang S. M., Radhakrishnan R., You Y. H., Joo G. J., Lee I. J., Lee K. E., et al. (2014a). Phosphate solubilizing Bacillus megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth. Ind. J. Microbiol. 54, 427–433. 10.1007/s12088-014-0476-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S., Samota M. K., Choudhary M., Choudhary M., Pandey A. K., Sharma A., et al. (2022). How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants. 28, 485–504. 10.1007/s12298-022-01146-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenneth O. C., Nwadibe E. C., Kalu A. U., Unah U. V. (2019). Plant growth promoting rhizobacteria (PGPR): a novel agent for sustainable food production. Am. J. Agric. Biol. Sci. 14, 35–54. 10.3844/ajabssp.2019.35.54 [DOI] [Google Scholar]
- Khan M. A., Asaf S., Khan A. L., Jan R., Kang S.-M., Kim K.-M. (2020). Extending thermotolerance to tomato seedlings by inoculation with SA1 isolate of Bacillus cereus and comparison with exogenous humic acid application. PLoS ONE 15, e0232228. 10.1371/journal.pone.0232228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N., Bano A., Zandi P. (2018). Effects of exogenously applied plant growth regulators in combination with PGPR on the physiology and root growth of chickpea (Cicer arietinum) and their role in drought tolerance. J. Plant Interact. 13, 239–247. 10.1080/17429145.2018.1471527 [DOI] [Google Scholar]
- Khanna K., Jamwal V. L., Sharma A., Gandhi S. G., Ohri P., Bhardwaj R., et al. (2019). Supplementation with plant growth promoting rhizobacteria (PGPR) alleviates cadmium toxicity in Solanum lycopersicum by modulating the expression of secondary metabolites. Chemosphere. 230, 628–639. 10.1016/j.chemosphere.2019.05.072 [DOI] [PubMed] [Google Scholar]
- Khare E., Mishra J., Arora N. K. (2018). Multifaceted interactions between endophytes and plant: developments and prospects. Front. Microbiol. 9, 2732. 10.3389/fmicb.2018.02732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khati P., Parul, Bhatt P., Nisha, Kumar R., Sharma A. (2018). Effect of nanozeolite and plant growth promoting rhizobacteria on maize. 3Biotech 8:141. 10.1007/s13205-018-1142-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirui C. K., Njeru E. M., Runo S. (2022). Diversity and phosphate solubilization efficiency of phosphate solubilizing bacteria isolated from semi-arid agroecosystems of Eastern Kenya. Microbiol. Insights 17:15. 10.1177/11786361221088991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhl J., Kolnaar R., Ravensberg W. J. (2019). Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Front. Plant Sci. 10, 845. 10.3389/fpls.2019.00845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousar B., Bano A., Khan N. (2020). PGPR Modulation of secondary metabolites in tomato infested with Spodoptera litura. Agronomy 10:778. 10.3390/agronomy10060778 [DOI] [Google Scholar]
- Kukreti B., Sharma A., Chaudhary P., Agri U., Maithani D. (2020). Influence of nanosilicon dioxide along with bioinoculants on Zea mays and its rhizospheric soil. 3Biotech 10:345. 10.1007/s13205-020-02329-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Maurya B. M., Raghuwanshi R. (2021a). The microbial consortium of indigenous rhizobacteria improving plant health, yield and nutrient content in wheat (Triticum aestivum). J. Plant Nutr. 44, 1942–1956. 10.1080/01904167.2021.1884706 [DOI] [Google Scholar]
- Kumar A., Singh S. K., Kant C., Verma H., Kumar D., Singh P. P., et al. (2021b). Microbial biosurfactant: a new frontier for sustainable agriculture and pharmaceutical industries. Antioxidants 10:1472. 10.3390/antiox10091472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Dubey R. C., Maheshwari D. K. (2012). Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiological Res. 16, 493–499. 10.1016/j.micres.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Kumar S., Kumar S., Mohapatra T. (2021c). Interaction between macro- and micro-nutrients in plants. Front. Plant Sci. 12, 665583. 10.3389/fpls.2021.665583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusale S. P., Attar Y. C., Sayyed R. Z., Malek R. A., Ilyas N., Suriani N. L., et al. (2021). Production of plant beneficial and antioxidants metabolites by Klebsiella variicola under salinity stress. Molecules 26:1894. 10.3390/molecules26071894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlali R., Ezrari S., Radouane N., Kenfaoui J., Esmaeel Q., El Hamss H., et al. (2022). Biological control of plant pathogens: a global perspective. Microorganisms 10:596. 10.3390/microorganisms10030596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lata R., Chowdhury S., Gond S. K., White J.r, J.F. (2018). Induction of abiotic stress tolerance in plants by endophytic microbes. Lett. Appl. Microbiol. 66, 268–276. 10.1111/lam.12855 [DOI] [PubMed] [Google Scholar]
- Lee L. H., Wu T. Y., Shak K. P. Y., Lim S. L., Ng K. Y., Nguyen M. N., et al. (2018). Sustainable approach to biotransform industrial sludge into organic fertilizer via vermicomposting: A mini-review. J. Chem. Technol. Biotechnol. 93, 925–935. 10.1002/jctb.5490 [DOI] [Google Scholar]
- Liu H., Carvalhais L. C., Crawford M., Singh E., Dennis P. G., Pieterse C. M., et al. (2017a). Inner plant values: diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 8, 2552. 10.3389/fmicb.2017.02552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Tian Y., Jia M., Lu X., Yue L., Zhao X., et al. (2020). Induction of salt tolerance in Arabidopsis thaliana by volatiles from Bacillus amyloliquefaciens FZB42 via the jasmonic acid signaling patway. Front. Microbiol. 11, 562934. 10.3389/fmicb.2020.562934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Rong Q., Zhou W., Liang G. (2017b). Effects of inorganic and organic amendment on soil chemical properties, enzyme activities, microbial community and soil quality in yellow clayey soil. PLoS ONE 12, e0172767. 10.1371/journal.pone.0172767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes M. J. S., Dias-Filho M. B., Gurgel E. S. C. (2021). Successful plant growth-promoting microbes: inoculation methods and abiotic factors. Front. Sustain. Food Syst. 5, 606454. 10.3389/fsufs.2021.606454 [DOI] [Google Scholar]
- Lou X., Zhao J., Lou X., Xia X., Feng Y., Li H. (2022). The biodegradation of soil organic matter in soil-dwelling humivorous fauna. Front. Bioeng. Biotechnol. 9:808075. 10.3389/fbioe.2021.808075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Zhang Z., Hou Y., Diao F., Hao B., Bao Z., et al. (2021). Exploring microbial resource of different rhizocompartments of dominant plants along the salinity gradient around the hypersaline lake Ejinur. Front. Microbiol. 12, 698479. 10.3389/fmicb.2021.698479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurthy T., Cantat C., Jeudy C., Declerck P., Gallardo K., Barraud C., et al. (2020). Impact of bacterial siderophores on iron status and ionome in pea. Front. Plant Sci. 11, 730. 10.3389/fpls.2020.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Liu Y., Shen W., Kuzyakov Y. (2021). Phosphatase activity and acidification in lupine and maize rhizosphere depend on phosphorus availability and root properties: coupling zymography with planar optodes. Agric. Ecosyst. Environ. Appl. Soil Ecol. 167:104029. 10.1016/j.apsoil.2021.104029 [DOI] [Google Scholar]
- Mahanty T., Bhattacharjee S., Goswami M., Bhattacharyya P., Das B., Ghosh A., et al. (2017). Biofertilizers: a potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 24, 3315–3335. 10.1007/s11356-016-8104-0 [DOI] [PubMed] [Google Scholar]
- Mahmud A. A., Upadhyay S. K., Srivastava A. K., Bhojiya A. A. (2021). Biofertilizers: A Nexus between soil fertility and crop productivity under abiotic stress. Curr. Res. Environ. Sustain. 3:100063. 10.1016/j.crsust.2021.100063 [DOI] [Google Scholar]
- Malik K. M., Khan K. S., Billah M., Akhtar M. S., Rukh S., Alam S., et al. (2021). Organic amendments and elemental sulfur stimulate microbial biomass and sulfur oxidation in alkaline subtropical soils. Agronomy 11:2514. 10.3390/agronomy11122514 [DOI] [Google Scholar]
- Malusa E., Vassilev N. (2014). A contribution to set a legal framework for biofertilisers. Appl. Microbiol. Biotechnol. 98, 6599–6607. 10.1007/s00253-014-5828-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marulanda A., Azcón R., Chaumont F., Ruiz-Lozano J. M., Aroca R. (2010). Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 232, 533–543. 10.1007/s00425-010-1196-8 [DOI] [PubMed] [Google Scholar]
- Mazid M., Khan T. A. (2015). Future of bio-fertilizers in Indian agriculture: an overview. Int. J. Agri. Food Res. 3:132. 10.24102/ijafr.v3i3.132 [DOI] [Google Scholar]
- Meddich A., Ait M. M., Bourzik W., Mitsui T., Baslam M., Hafidi M. (2018). Optimizing Growth and Tolerance of Date Palm (Phoenix dactylifera L.) to Drought, Salinity, and Vascular Fusarium-Induced Wilt (Fusarium oxysporum) by Application of Arbuscular Mycorrhizal Fungi (AMF). Cham: Springer. 10.1007/978-3-319-75910-4_9 [DOI] [Google Scholar]
- Meena K. K., Bitla U. M., Sorty A. M., Singh D. P., Gupta V. K., Wakchaure G. C., et al. (2020). Mitigation of salinity stress in wheat seedlings due to the application of phytohormone-rich culture filtrate extract of methylotrophic Actinobacterium Nocardioides sp. NIMMe6. Front. Microbiol. 11, 2091. 10.3389/fmicb.2020.02091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa-Marín J., Pérez-Romero J. A., Redondo-Gómez S., Pajuelo E., Rodríguez-Llorente I. D., Mateos-Naranjo E. (2020). Impact of plant growth promoting bacteria on Salicornia ramosissima ecophysiology and heavy metal phytoremediation capacity in estuarine soils. Front. Microbiol. 11, 553018. 10.3389/fmicb.2020.553018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S., Arora N. K. (2012). Evaluation of rhizospheric Pseudomonas and Bacillus as biocontrol tool for Xanthomonas campestris pv campestris. World J. Microbiol. Biotechnol. 28, 693–702. 10.1007/s11274-011-0865-5 [DOI] [PubMed] [Google Scholar]
- Mitter E. K., Tosi M., Obregón D., Dunfield K. E., Germida J. J. (2021). Rethinking crop nutrition in times of modern microbiology: innovative biofertilizer technologies. Front. Sustain. Food Syst. 5, 606815. 10.3389/fsufs.2021.606815 [DOI] [Google Scholar]
- Mokabel S., Olama Z., Ali S., El-Dakak R. (2022). The role of plant growth promoting rhizosphere microbiome as alternative biofertilizer in boosting Solanum melongena L. Adaptation to salinity stress. Plants 11:659. 10.3390/plants11050659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal M., Skalicky M., Garai S., Hossain A., Sarkar S. H., et al. (2020). Supplementing nitrogen in combination with Rhizobium inoculation and soil mulch in peanut (Arachis hypogaea L.) production system: Part II. Effect on phenology, growth, yield attributes, pod quality, profitability and nitrogen use efficiency. Agronomy 10:1513. 10.3390/agronomy10101513 [DOI] [Google Scholar]
- Morelli M., Bahar O., Papadopoulou K. K., Hopkins D. L., Obradovi,ć A. (2020). Role of endophytes in plant health and defense against pathogens. Front. Plant Sci. 11, 1312. 10.3389/fpls.2020.01312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsy M., Blake C., Hayden A.-M. (2020). Fungal endophytes promote tomato growth and enhance drought and salt tolerance. Plants 9:7877. 10.3390/plants9070877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa-Farag M., Almoneafy A., Mahmoud A., Elkelish A., Arnao M. B., Li L., et al. (2019). Melatonin and its protective role against biotic stress impacts on plants. Biomolecules 10:54. 10.3390/biom10010054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Gaurav A. K., Singh S., Yadav S., Bhowmick S., Abeysinghe S., et al. (2022). The bioactive potential of phytohormones: A review. Biotechnol. Rep. 8:e00748. 10.1016/j.btre.2022.e00748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar S., Zareen M., Khaliq Z., Mehnaz S., Malik K. A. (2020a). Phylogenetic analysis of halophyte-associated rhizobacteria and effect of halotolerant and halophilic phosphate-solubilizing biofertilizers on maize growth under salinity stress conditions. J. Appl. Microbiol. 128, 556–573. 10.1111/jam.14497 [DOI] [PubMed] [Google Scholar]
- Mukhtar T., Shafiqur R., Smith D., Sultan T., Seleiman M. F., Alsadon A. A., et al. (2020b). Mitigation of heat stress in Solanum lycopersicum L. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: Effects on biochemical profiling. Sustainability 12:2159. 10.3390/su12062159 [DOI] [Google Scholar]
- Nacoon S., Jogloy S., Riddech N., Mongkolthanaruk W., Kuyper T., Boonlue S. (2020). Interaction between phosphate solubilizing bacteria and arbuscular mycorrhizal fungi on growth promotion and tuber inulin content of Helianthus tuberosus L. Sci. Rep. 10:4916. 10.1038/s41598-020-61846-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandini B., Puttaswamy H., Saini R. K., Prakash H. S., Geetha N. (2021). Trichovariability in rhizosphere soil samples and their biocontrol potential against downy mildew pathogen in pearl millet. Sci. Rep. 11:9517. 10.1038/s41598-021-89061-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassal D., Spohn M., Eltlbany N., Jacquiod S., Smalla K., Marhan S., et al. (2018). Effects of phosphorus-mobilizing bacteria on tomato growth and soil microbial activity. Plant Soil. 427, 17–37. 10.1007/s11104-017-3528-y [DOI] [Google Scholar]
- Nie P., Chen C., Yin Q., Jiang C., Guo J., Zhao H., et al. (2019). Function of miR825 and miR825* as negative regulators in Bacillus cereus AR156-elicited systemic resistance to Botrytis cinerea in Arabidopsis thaliana. Int. J. Mol. Sci. 20:5032. 10.3390/ijms20205032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitu R., Rajinder K., Sukhminderjit K. (2020). Zinc solubilizing bacteria to augment soil fertility—A comprehensive review. Int. J. Agri. Sci. Vet. Med. 8, 38–44. [Google Scholar]
- Niu J., Li X. (2022). Effects of microbial inoculation with different indigenous bacillus species on physicochemical characteristics and bacterial succession during short-term composting. Fermentation 8:152. 10.3390/fermentation8040152 [DOI] [Google Scholar]
- Numan M., Bashir S., Khan Y. (2018). Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: a review. Microbiol Res. 209, 21–32. 10.1016/j.micres.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Odoh C. K., Sam K., Zabbey N., Eze C. N., Nwankwegu A. S., Laku C., et al. (2020). Microbial Consortium as Biofertilizers for Crops Growing Under the Extreme Habitats. Plant Microbiomes for Sustainable Agriculture. Cham: Springer. 10.1007/978-3-030-38453-1_13 [DOI] [Google Scholar]
- Okamoto T., Shinjo R., Nishihara A., Uesaka K., Tanaka A., Sugiura D., et al. (2021). Genotypic variation of endophytic nitrogen-fixing activity and bacterial flora in rice stem based on sugar content. Front. Plant Sci. 2021, 1610. 10.3389/fpls.2021.719259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okur N. (2018). A review-bio-fertilizers-power of beneficial microorganisms in soils. Biomed. J. Sci. Tech. Res. 4, 4028–4029. 10.26717/BJSTR.2018.04.0001076 [DOI] [Google Scholar]
- Oubohssaine M., Sbabou L., Aurag J. (2022). Native heavy metal-tolerant plant growth promoting rhizobacteria improves Sulla spinosissima (L.) growth in post-mining contaminated soils. Microorganisms 10:838. 10.3390/microorganisms10050838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oukala N., Aissat K., Pastor V. (2021). Bacterial endophytes: The hidden actor in plant immune responses against biotic stress. Plants 10:1012. 10.3390/plants10051012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey J., Singh A. (2012). Opportunities and constraints in organic farming: an Indian perspective. J. Sci. Res. 56, 47–72. [Google Scholar]
- Pang Z., Chen J., Wang T., Gao C., Li Z., Guo L., et al. (2021). Linking plant secondary metabolites and plant microbiomes: a review. Front. Plant Sci. 12, 621276. 10.3389/fpls.2021.621276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes-Páliz K., Rodríguez-Vázquez R., Duarte B., Caviedes M. A., Mateos-Naranjo E., Redondo-Gómez S., et al. (2018). Investigating the mechanisms underlying phytoprotection by plant growth-promoting Rhizobacteria in Spartina densiflora under Metal Stress. Plant Biol. 20, 497–506 10.1111/plb.12693 [DOI] [PubMed] [Google Scholar]
- Patel M., Patel K., Al-Keridis L. A., Alshammari N., Badraoui R., Elasbali A. M., et al. (2022). Cadmium-tolerant plant growth-promoting bacteria Curtobacterium oceanosedimentum improves growth attributes and strengthens antioxidant system in chili (Capsicum frutescens). Sustainability 14:4335. 10.3390/su14074335 [DOI] [Google Scholar]
- Patel S. H., Viradiya M. B., Prajapati B. J. (2021). Effect of potassium and potassium mobilizing bacteria (KMB) with and without FYM on yield of wheat (Triticum aestivum L.). Pharmac. Phytochem. 10, 1615–1620. [Google Scholar]
- Popp J., Pet,o K., Nagy J. (2013). Pesticide productivity and food security. A review. Agronomy Sustain. Dev. 33, 243–255. 10.1007/s13593-012-0105-x [DOI] [Google Scholar]
- Portieles R., Xu H., Yue Q., Zhao L., Zhang D., Du L., et al. (2021). Heat-killed endophytic bacterium induces robust plant defense responses against important pathogens. Sci. Rep. 11:12182. 10.1038/s41598-021-91837-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang X., Ding J., Lin W., Li Q., Xu C., Zheng Q., et al. (2019). Alleviation of the detrimental effect of water deficit on wheat (Triticum aestivum L.) growth by an indole acetic acid-producing endophytic fungus. Plant Soil. 439, 373–391. 10.1007/s11104-019-04028-7 [DOI] [Google Scholar]
- Qurashi A. W., Sabri A. N. (2012). Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 43, 1183–1191. 10.1590/S1517-83822012000300046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid M. H. O., Khan A., Hossain M. T., Chung Y. R. (2017). Induction of systemic resistance against aphids by endophytic Bacillus velezensis YC7010 via expressing PHYTOALEXIN DEFICIENT4 in Arabidopsis. Front. Plant Sci. 8, 211. 10.3389/fpls.2017.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A., Razzaq A., Mehmood S. S., Zou X., Zhang X., Lv Y., et al. (2019). Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 8, 34. 10.3390/plants8020034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reghmit A., Benzina-tihar F., Escudero F. J. L., Halouane-Sahir F., Oukali Z., Bensmail S., et al. (2021). Trichoderma spp. isolates from the rhizosphere of healthy olive trees in northern Algeria and their biocontrol potentials against the olive wilt pathogen, Verticillium dahliae. Org. Agric. 11, 639–657. 10.1007/s13165-021-00371-1 [DOI] [Google Scholar]
- Rodriguez M. V., Tano J., Ansaldi N., Carrau A., Srebot M. S., Ferreira V., et al. (2019). Anatomical and biochemical changes induced by Gluconacetobacter diazotrophicus stand up for Arabidopsis thaliana seedlings from Ralstonia solanacearum infection. Front. Plant Sci. 10, 1618. 10.3389/fpls.2019.01618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera F. J., García M. J., Lucena C., Martínez-Medina A., Aparicio M. A., Ramos J., et al. (2019). Induced systemic resistance (ISR) and Fe deficiency responses in dicot plants. Front. Plant Sci. 10, 287. 10.3389/fpls.2019.00287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Liu W., Nandety R. S., Crook A., Mysore K. S., Pislariu C. I., et al. (2020). Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell. 32, 15–41. 10.1105/tpc.19.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakova D., Rack-Wetzlinger U., Cernava T., Schaefer A., Schmuck M., Berg G. (2017). Aerial warfare: A volatile dialogue between the plant pathogen Verticillium longisporum and its antagonist Paenibacillus polymyxa. Front. Plant Sci. 8, 1294. 10.3389/fpls.2017.01294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdarian M., Askari H., Shariati J. V., Nematzadeh G. (2019). Transcriptional responses of wheat roots inoculated with Arthrobacter nitroguajacolicus to salt stress. Sci. Rep. 9:1792. 10.1038/s41598-018-38398-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem M., Al-Amri (2021). Application of bio-fertilizers for enhancing growth and yield of common bean plants grown under water stress conditions. Saudi J. Biol. Sci. 28, 3901–3908. 10.1016/j.sjbs.2021.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon M. V., Bottini R., de Souza Filho G. A., Cohen A. C., Moreno D., Gil M. (2014). Bacteria isolated from roots and rhizosphere of Vitis vinifera retard water losses, abscisic acid accumulation and synthesis of defence-related terpenes in in vitro cultured grapevine. Physiol. Plant. 151, 359–374. 10.1111/ppl.12117 [DOI] [PubMed] [Google Scholar]
- Samain E., Aussenac T., Selim S. (2019). The effect of plant genotype, growth stage, and Mycosphaerella graminicola strains on the efficiency and durability of wheat-induced resistance by Paenibacillus sp. Strain B2. Front. Plant Sci. 10, 587. 10.3389/fpls.2019.00587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaras A., Roumeliotis E., Ntasiou P., Karaoglanidis G. (2021). Bacillus subtilis MBI600 promotes growth of tomato plants and induces systemic resistance contributing to the control of soilborne pathogens. Plants 10:1113. 10.3390/plants10061113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Montesinos B., Santos M., Moreno-Gavíra A., Marín-Rodulfo T., Gea F. J., Diánez F. (2021). Biological control of fungal diseases by Trichoderma aggressivum f. europaeum and its compatibility with fungicides. J. Fungi. 24:7. 10.3390/jof7080598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu N., Sethi M., Kumar A., Dang D., Singh J., Chhuneja P. (2021). Biochemical and genetic approaches improving nitrogen use efficiency in cereal crops: a review. Front. Plant Sci. 12, 657629. 10.3389/fpls.2021.657629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhya V., Ali S. Z., Grover M., Reddy G., Venkateswarlu B. (2010). Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 62, 21–30. 10.1007/s10725-010-9479-4 [DOI] [Google Scholar]
- Santosh S., Velmourougane K., Idapuganti R. G., Manikandan A., Blaise D. (2022). Potassium solubilizing potential of native bacterial isolates from cotton rhizosphere of rainfed vertisols. Natl. Acad. Sci. Lett. 2022, 1–4. 10.1007/s40009-022-01113-x [DOI] [Google Scholar]
- Sarkar J., Chakraborty B., Chakraborty U. (2018). Plant growth promoting rhizobacteria protect wheat plants against temperature stress through antioxidant signalling and reducing chloroplast and membrane injury. J. Plant Growth Regul. 37, 1396–1412. 10.1007/s00344-018-9789-8 [DOI] [Google Scholar]
- Sarwar S., Khaliq A., Yousra M., Sultan T., Ahmad N., Khan M. Z. (2020). Screening of siderophore-producing PGPRs isolated from groundnut (Arachis hypogaea L.) rhizosphere and their influence on iron release in soil. Commun. Soil Sci. Plant Anal. 51, 1680–1692. 10.1080/00103624.2020.1791159 [DOI] [Google Scholar]
- Sattar A., Naveed M., Ali M., Zahir Z. A., Nadeem S. M., Yaseen M., et al. (2019). Perspectives of potassium solubilizing microbes in sustainable food production system: A review. Agric,. Ecosyst. Environ,. Appl. Soil Ecol. 133, 146–159. 10.1016/j.apsoil.2018.09.012 [DOI] [Google Scholar]
- Sdiri Y., Lopes T., Rodrigues N., Silva K., Rodrigues I., Pereira J. A., et al. (2022). Biocontrol ability and production of volatile organic compounds as a potential mechanism of action of olive endophytes against Colletotrichum acutatum. Microorganisms 10:571. 10.3390/microorganisms10030571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendi Y., Pfeiffer T., Koch E. (2020). Potential of common bean (Phaseolus vulgaris L.) root microbiome in the biocontrol of root rot disease and traits of performance. J. Plant Dis. Prot. 127, 453–462 10.1007/s41348-020-00338-6 [DOI] [Google Scholar]
- Sharma N., Khanna K., Manhas R. K., Bhardwaj R., Ohri P., Alkahtani J., et al. (2020). Insights into the role of Streptomyces hydrogenans as the plant growth promoter, photosynthetic pigment enhancer and biocontrol agent against Meloidogyne incognita in Solanum lycopersicum Seedlings. Plants 9:1109. 10.3390/plants9091109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheteiwy M. S. Abd Elgawad H. Xiong Y.-C. Macovei A. Brestic M. Skalicky M. (2021) Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress improve seed yield quality of soybean plant. Physiologia Plant. 1–17. 10.1111/ppl.13454 doi: 10.1111/ppl.13454. [DOI] [PubMed] [Google Scholar]
- Shukla P. S., Agarwal P. K., Jha B. I. (2012). Improved salinity tolerance of Arachis hypogaea (L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria. J. Plant Growth Regul. 31, 195–206. 10.1007/s00344-011-9231-y [DOI] [Google Scholar]
- Shukla V., Kumar S., Tripathi Y. N., Upadhyay R. S. (2022). Bacillus subtilis- and Pseudomonas fluorescens-mediated systemic resistance in tomato against sclerotium rolfsii and study of physio-chemical alterations. Front. Fungal Biol. 3:851002. 10.3389/ffunb.2022.851002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Arif Y., Miszczuk E., Bajguz A., Hayat S. (2022a). Specific roles of lipoxygenases in development and responses to stress in plants. Plants 11:979. 10.3390/plants11070979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. K., Wu X., Shao C., Zhang H. (2022b). Microbial enhancement of plant nutrient acquisition. Stress Biol. 2:3. 10.1007/s44154-021-00027-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumare A., Diedhiou A. G., Thuita M., Hafidi M., Ouhdouch Y., Gopalakrishnan S., et al. (2020). Exploiting biological nitrogen fixation: a route towards a sustainable agriculture. Plants 9:1011. 10.3390/plants9081011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Verma P. C., Chaudhry V., Singh N., Abhilash P. C., Kumar K. V., et al. (2013). Influence of inoculation of arsenic-resistant Staphylococcus arlettae on growth and arsenic uptake in Brassica juncea (L.) Czern. Var. R-46. J. Hazard Mater. 15, 1039–1047. 10.1016/j.jhazmat.2012.08.019 [DOI] [PubMed] [Google Scholar]
- Sujayanand G. K., Akram M., Konda A., Nigam A., Bhat S., Dubey J., et al. (2021). Distribution and toxicity of Bacillus thuringiensis (Berliner) strains from different crop rhizosphere in Indo-Gangetic plains against polyphagous lepidopteran pests. Int. J. Trop. Insect Sci. 41, 2713–2731. 10.1007/s42690-021-00451-5 [DOI] [Google Scholar]
- Sultana S., Paul S. C., Parveen S., Alam S., Rahman N., Jannat B., et al. (2020). Isolation and identification of salt-tolerant plant-growth-promoting rhizobacteria and their application for rice cultivation under salt stress. Can. J. Microbiol. 66, 144–160. 10.1139/cjm-2019-0323 [DOI] [PubMed] [Google Scholar]
- Sumbul A., Ansari R. A., Rizvi R., Mahmood I. (2020). Azotobacter: A potential bio-fertilizer for soil and plant health management. Saudi J. Biol. Sci. 27, 3634–3640. 10.1016/j.sjbs.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaramoorthy S., Balabaskar P. (2013). Evaluation of combined efficacy of Pseudomonas fluorescens and Bacillus subtilis in managing tomato wilt caused by Fusarium oxysporum f. sp. lycopersici (FOL). Plant Pathol. J. 12, 154–161. 10.3923/ppj.2013.154.161 [DOI] [Google Scholar]
- Suzaki T., Takeda N., Nishida H., Hoshino M., Ito M., Misawa F., et al. (2019). Lack of symbiont accommodation controls intracellular symbiont accommodation in root nodule and arbuscular mycorrhizal symbiosis in Lotus japonicus. PLoS Genet. 15, e1007865. 10.1371/journal.pgen.1007865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha R. S., Seleiman M. F., Shami A., Alhammad B. A., Mahdi A. H. A. (2021). Integrated application of selenium and silicon enhances growth and anatomical structure, antioxidant defense system and yield of wheat grown in salt-stressed soil. Plants. 10, 1040. 10.3390/plants10061040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufail M. A., Touceda-González M., Pertot I., Ehlers R. U. (2021). Gluconacetobacter diazotrophicus Pal5 enhances plant robustness status under the combination of moderate drought and low nitrogen stress in Zea mays L. Microorganisms 9:870. 10.3390/microorganisms9040870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umair Hassan M., Aamer M., Umer Chattha M., Haiying T., Shahzad B., Barbanti L., et al. (2020). The critical role of zinc in plants facing the drought stress. Agriculture 10:396. 10.3390/agriculture10090396 [DOI] [Google Scholar]
- Upadhyay S. K., Saxena A. K., Singh J. S., Singh D. P. (2019). Impact of native ST-PGPR (Bacillus pumilus; EU927414) on PGP traits, antioxidants activities, wheat plant growth and yield under salinity. Clim. Change Environ. Sustain. 7, 157–168. 10.5958/2320-642X.2019.00021.8 [DOI] [Google Scholar]
- Usman M., Balsalobre-Lorente D., Jahanger A., Ahmad P. (2022). Pollution concern during globalization mode in financially resource-rich countries: Do financial development, natural resources, and renewable energy consumption matter? Renewable Ener. 183, 90–102. 10.1016/j.renene.2021.10.067 [DOI] [Google Scholar]
- Vandana U. K., Rajkumari J., Singha L. P., Satish L., Alavilli H., Sudheer P. D. V. N., et al. (2021). The endophytic microbiome as a hotspot of synergistic interactions, with prospects of plant growth promotion. Biology 10:101. 10.3390/biology10020101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga T., Hixson K. K., Ahkami A. H., Sher A. W., Barnes M. E., Chu R. K., et al. (2020). Endophyte-promoted phosphorus solubilization in populus. Front. Plant Sci. 11, 567918. 10.3389/fpls.2020.567918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwakarma K., Kumar N., Shandilya C., Mohapatra S., Bhayana S., Varma A. (2020). Revisiting plant–microbe interactions and microbial consortia application for enhancing sustainable agriculture: a review. Front. Microbiol. 11, 560406. 10.3389/fmicb.2020.560406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Wang Y., Jiang D., Xiang Z., Wang S., Kang C., et al. (2022a). Soil microbe inoculation alters the bacterial communities and promotes root growth of Atractylodes lancea under heat stress. Plant Soil. 10.1007/s11104-022-05369-6 [DOI] [Google Scholar]
- Wang R., Lin J.-Q., Liu X.-M., Pang X., Zhang C.-J., Yang C.-L., et al. (2019). Sulfur oxidation in the acidophilic autotrophic Acidithiobacillus spp. Front. Microbiol. 9, 3290. 10.3389/fmicb.2018.03290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Cai D., Ji M., Chen Z., Yao L., Han H. (2022b). Isolation of heavy metal-immobilizing and plant growth-promoting bacteria and their potential in reducing Cd and Pb uptake in water spinach. Sci. Total Environ. 819:153242. 10.1016/j.scitotenv.2022.153242 [DOI] [PubMed] [Google Scholar]
- Wang Y., Yang Z., Kong Y., Li X., Li W., Du H., et al. (2020). GmPAP12 is required for nodule development and nitrogen fixation under phosphorus starvation in soybean. Front. Plant Sci. 11, 450. 10.3389/fpls.2020.00450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani P. A., Khan M. S. (2013). Nickel detoxification and plant growth promotion by multi metal resistant plant growth promoting Rhizobium species RL9. Bull. Environ. Contam. Toxicol. 91, 117–124. 10.1007/s00128-013-1002-y [DOI] [PubMed] [Google Scholar]
- Waqas M., Khan A. L., Kamran M., Hamayun M., Kang S. M., Kim Y. H., et al. (2012). Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 17, 10754–10773. 10.3390/molecules170910754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win T. T., Bo B., Malec P., Khan S., Fu P. (2021). Newly isolated strain of Trichoderma asperellum from disease suppressive soil is a potential bio-control agent to suppress Fusarium soil borne fungal phytopathogens. J. Plant Pathol. 103, 549–561. 10.1007/s42161-021-00780-x [DOI] [Google Scholar]
- Wu W., Du K., Kang X., Wei H. (2021a). The diverse roles of cytokinins in regulating leaf development. Hortic Res. 8:118. 10.1038/s41438-021-00558-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Huang W., Tian Q., Liu J., Xia X., Yang X., et al. (2021b). Comparative transcriptomic analysis reveals the cold acclimation during chilling stress in sensitive and resistant passion fruit (Passiflora edulis) cultivars. Peer J. 9:e10977. 10.7717/peerj.10977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Liu J., Chen C., Mo X., Tan Q., He Y., et al. (2022). The multifunctions and future prospects of endophytes and their metabolites in plant disease management. Microorganisms 10:1072. 10.3390/microorganisms10051072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A. N., Verma P., Kumar S., Kumar V., Kumar M., Kumari Sugitha T. C. (2018). Actinobacteria From Rhizosphere in New and Future Developments in Microbial Biotechnology and Bioengineering. 10.1016/B978-0-444-63994-3.00002-3 [DOI] [Google Scholar]
- Yang Y., Chen Y., Cai J., Liu X., Huang G. (2021). Antifungal activity of volatile compounds generated by endophytic fungis Sarocladium brachiariae HND5 against Fusarium oxysporum f. sp. cubense. PLoS ONE 16, e0260747. 10.1371/journal.pone.0260747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmin H., Naeem S., Bakhtawar M., Jabeen Z., Nosheen A., Naz R. (2020). Halotolerant rhizobacteria Pseudomonas pseudoalcaligenes and Bacillus subtilis mediate systemic tolerance in hydroponically grown soybean (Glycine max L.) against salinity stress. PLoS ONE. 15, e0231348. 10.1371/journal.pone.0231348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M., Dastogeer K. M. G., Sarkodee-Addo E., Tokiwa C., Isawa T., Shinozaki S., et al. (2022). Impact of Azospirillum sp. B510 on the rhizosphere microbiome of rice under field conditions. Agronomy 12:1367. 10.3390/agronomy12061367 [DOI] [Google Scholar]
- You J. M., Xiong K., Mu S., Guo J., Guo X. L., Duan Y. Y., et al. (2018). Identification of endophytic bacteria Bzjn1 and research on biological control of root rot of Atractylodes Macrocephala. Zhongguo Zhong Yao Za Zhi. 3, 478–483. 10.19540/j.cnki.cjcmm.20180105.008 [DOI] [PubMed] [Google Scholar]
- Yu C., Fan L., Gao J., Wang M., Wu Q., Tang J., et al. (2015). The platelet-activating factor acetyl hydrolase gene derived from Trichoderma harzianum induces maize resistance to Curvularia lunata through the jasmonic acid signaling pathway. J. Environ. Sci. Health B. 50, 708–717. 10.1080/03601234.2015.1048104 [DOI] [PubMed] [Google Scholar]
- Yu Y., Gui Y., Li Z., Jiang C., Guo J., Niu D. (2022). Induced systemic resistance for improving plant immunity by beneficial microbes. Plants 11:386. 10.3390/plants11030386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Wang Z., Zhang Y., Wang Y., Liu Z. (2021). Biocontrol and growth-promoting effect of Trichoderma asperellum TaspHu1 isolate from Juglans mandshurica rhizosphere soil. Microbiol. Res. 242:e126596. 10.1016/j.micres.2020.126596 [DOI] [PubMed] [Google Scholar]
- Zain M., Yasmin S., Hafeez F. Y. (2019). Isolation and characterization of plant growth promoting antagonistic bacteria from cotton and sugarcane plants for suppression of phytopathogenic Fusarium species. Iran. J. Biotechnol. 17:e1974. 10.21859/ijb.1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Xu M., Liu Y., Zhang F., Hodge A., Feng G. (2016). Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium. New Phytol. 210, 1022–1032. 10.1111/nph.13838 [DOI] [PubMed] [Google Scholar]
- Zhang T., Hu F., Ma L. (2019). Phosphate-solubilizing bacteria from safflower rhizosphere and their effect on seedling growth. Open. Life Sci. 14, 246–254. 10.1515/biol-2019-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Liu Z., Wei G., Yang F., Liu X. (2018). In silico genome-wide analysis reveals the potential links between core genome of Acidithiobacillus thiooxidans and its autotrophic lifestyle. Front. Microbiol. 9, 1255. 10.3389/fmicb.2018.01255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Zhu L., Xie Y., Li F., Xiao X., Ma Z., et al. (2017). Bacillus licheniformis SA03 confers increased saline–alkaline tolerance in Chrysanthemum plants by induction of abscisic acid accumulation. Front. Plant Sci. 8, 1143. 10.3389/fpls.2017.01143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. R., Dai L., Xu G. F., Wang H. S. (2021). A strain of Phoma species improves drought tolerance of Pinus tabulaeformis. Sci. Rep. 11:7637. 10.1038/s41598-021-87105-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Wang Y., Lu J., Liu S., Min Y., Liu X., et al. (2021). Complete genome sequence of Bacillus badius NBPM-293, a plant growth-promoting strain isolated from rhizosphere soil. Am. Soc. Microbiol. 10, e00977–e00921. 10.1128/MRA.00977-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulfikar S. A., Sandhya V., Grover M., Linga V. R., Bandi V. (2011). Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress, J. Plant Inter. 6, 239–246. 10.1080/17429145.2010.545147 [DOI] [Google Scholar]