Abstract
The kinetic properties of wild-type and mutant oligopeptide binding proteins of Lactococcus lactis were determined. To observe the properties of the mutant proteins in vivo, the oppA gene was deleted from the chromosome of L. lactis to produce a strain that was totally defective in oligopeptide transport. Amplified expression of the oppA gene resulted in an 8- to 12-fold increase in OppA protein relative to the wild-type level. The amplified expression was paralleled by increased bradykinin binding activity, but had relatively little effect on the overall transport of bradykinin via Opp. Several site-directed mutants were constructed on the basis of a comparison of the primary sequences of OppA from Salmonella enterica serovar Typhimurium and L. lactis, taking into account the known structure of the serovar Typhimurium protein. Putative peptide binding-site residues were mutated. All the mutant OppA proteins exhibited a decreased binding affinity for the high-affinity peptide bradykinin. Except for OppA(D471R), the mutant OppA proteins displayed highly defective bradykinin uptake, whereas the transport of the low-affinity substrate KYGK was barely affected. Cells expressing OppA(D471R) had a similar Km for transport, whereas the Vmax was increased more than twofold as compared to the wild-type protein. The data are discussed in the light of a kinetic model and imply that the rate of transport is determined to a large extent by the donation of the peptide from the OppA protein to the translocator complex.
In bacteria, the binding protein-dependent permeases constitute an important group of transport systems for the uptake of nutrients such as sugars, amino acids, anions, and peptides (1, 8). In gram-negative bacteria, the systems consist of a periplasmic substrate binding protein, a membrane-bound complex formed by two hydrophobic integral membrane proteins (or a single protein with two domains), and two membrane-associated proteins that carry the ATP-binding cassette motif (8). The periplasmic substrate binding protein is usually present in large excess (18), serving to capture the substrate with high affinity and to deliver it to the membrane-bound complex. The substrate binding proteins determine the specificity of the transport systems and therefore the range of molecules that may enter the cell (31).
The oligopeptide transport system (Opp) possesses one of the most versatile binding proteins, since it is able to handle a large variety of peptides present in the medium. Experiments with amino acid auxotrophic strains of Escherichia coli have shown that the Opp system is able to transport peptides from two to five amino acid residues, composed of a large variety of natural and/or modified residues (24). Equilibrium dialysis experiments with OppA of E. coli indicate that the protein has a higher affinity for tri- and tetrapeptides than for di- and pentapeptides (7). The Opp system of Lactococcus lactis is homologous to the Opp systems of enteric bacteria. As for many other binding proteins in gram-positive bacteria, the OppA protein is anchored to the cytoplasmic membrane by a lipid-modified cysteine (6). The Opp system of L. lactis has the capacity to transport peptides from 4 to at least 18 residues (4). Kinetic analysis of binding of the peptides SLSQS, SLSQSKVLP, SLSQSKVLPVPQ, RDMPIQA, and RDMPIQAF to OppA of L. lactis showed a relationship between the peptide dissociation constants (Kd) and the length of the ligand (14), varying from millimolar values for SLSQS to submicromolar values for SLSQSKVLPVPQ.
The crystal structures of the oligopeptide binding protein (OppA) from Salmonella enterica serovar Typhimurium in complex with tripeptides (34), tetrapeptides (33), or dipeptides as well as unliganded binding proteins (31) have been solved, and the residues involved in interactions with the peptides have been identified. The main chain of the peptide is in an extended conformation and forms parallel and antiparallel β-sheet interactions with some residues of OppA. The N terminus of the peptides forms a salt bridge with the side chain of Asp-419. Arg-413 and His-371 each form a salt bridge with the carboxylate groups of the tri- and tetrapeptide ligands, respectively, and Lys-307 has been postulated to form a salt bridge with the C terminus of pentapeptides. In the case of the dipeptide, the C-terminal interaction with OppA is indirect and occurs via a water molecule that interacts with the side chain of Arg-404 and Arg-413. The side chains of the peptides are accommodated in spacious and hydrated pockets, where few direct contacts are made with the protein. Water molecules act as flexible adapters that match the hydrogen-bonding requirements of OppA and the ligand and/or shield charges on the buried ligand (35). The peptides are buried within OppA, according to the Venus flytrap mechanism (19).
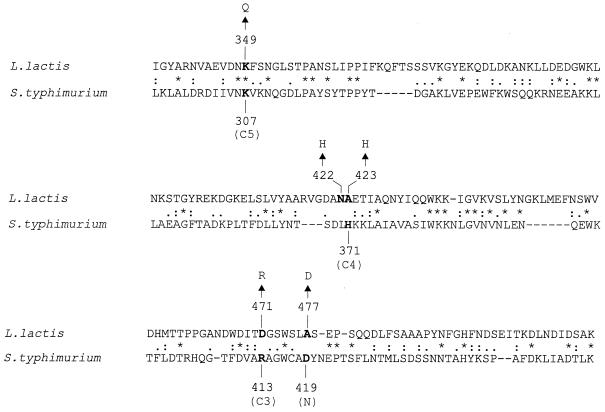
In line with the similar three-dimensional structures of the OppA protein of serovar Typhimurium (OppASt) and the dipeptide binding protein DppA of E. coli and the relatively low degree of identity in primary sequence between these proteins, it seems likely that OppASt and L. lactis (OppALl) also have a similar structural fold (27); the amino acid identity between these proteins is 21 to 22%. Comparison of OppASt and OppALl shows that of the important residues that interact with the peptides in OppASt, only Lys-307 is conserved in OppALl (Fig. 1). On the basis of the structure of OppASt protein, we made amino acid substitutions in OppALl that should be near or at the peptide binding site. The effects of these substitutions on the growth of L. lactis as well as in vivo peptide transport and peptide binding to purified OppA are reported in this paper.
FIG. 1.
Alignment of parts of the OppA proteins from L. lactis and S. enterica serovar Typhymurium. Sequences of the putative peptide binding region were aligned using the CLUSTAL X program. Conserved residues are marked with an asterisk, while similar residues are marked with a single or double dot. N, C3, C4, and C5 correspond to interactions of OppASt with the N terminus of peptides and the C terminus of tri-, tetra-, and pentapeptides, respectively. Characters in boldface represent the identified peptide binding residues in serovar Typhimurium and their putative counterparts in L. lactis. Substitutions made in OppALl are also indicated by arrows.
MATERIALS AND METHODS
Strains, growth conditions, media, and chemicals.
All strains and plasmids are listed in Table 1. E. coli BZ234 was grown at 37°C with vigorous aeration in Luria broth (29), supplemented with 500 μg of erythromycin per ml when carrying plasmids pAMP21 or pAMP31. L. lactis strains were grown in M17 broth (Difco Laboratories, East Molesey, United Kingdom) at 30°C as stand cultures or on M17 broth solidified with 1.5% agar (36) supplemented with 0.5% (wt/vol) glucose and 5 μg of erythromycin per ml, if required. For purification purposes, the L. lactis strains were grown in fed batch in 10-liter fermentors with pH control (ADI 1065 fermentor; Applikon Dependable Instruments, B. V., Schiedam, The Netherlands). The pH value was kept constant at 6.5 by the addition of 1 M KOH. Complementation studies were performed on plates or liquid cultures of chemically defined medium (CDM) (25) lacking leucine and containing a tetra- or pentapeptide (400 μM, final concentration) as the sole source of leucine. All peptides used were from Bachem Feinchemikalien AG (Bubendorf, Switzerland); Na125I (2,145 Ci/mmol) and [3,4(n)-3H]-bradykinin (71 Ci/mmol) were obtained from Amersham (Buckinghamshire, United Kingdom); Ni-nitrilotriacetic acid resin was from Qiagen, Inc.; n-dodecyl-β-d-maltoside (DDM) was from Sigma (St. Louis, Mo.). All other chemicals were of reagent grade and were obtained from commercial sources.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| L. lactis | ||
| MG1363 | Plasmid-free derivative of NCD0712; Lac− Prot− | 5 |
| IM15 | MG1363 ΔpepX ΔpepT ΔpepC ΔpepN | 20 |
| AMP15 | MG1363 ΔoppA | This work |
| AMP2 | IM15 ΔoppA | This work |
| E. coli | ||
| BZ234 | C600 derivative; Ems | |
| Plasmids | ||
| pVS8 | Cmr; pSH71 replicon; opp operon of L. lactis | 37 |
| pORI280 | Emr; lacZ+; deletion derivative of pWV01 lacking repA | 16 |
| pAP2 | pORI280 containing the 5′ and 3′ flanking regions of oppA | This work |
| pSKII(+) | Carbr; high-copy-number expression vector | Stratagene |
| pSKE8His | pSKII(+)-derivative carrying lacS with NcoI site on the initiation codon and a C-terminal His tag | 10 |
| pGK13 | Emr Cmr; pWV01 replicon; E. coli-L. lactis shuttle vector | 11 |
| pGKGS8 | pGK13 derivative carrying lacS as a 3,784-bp EcoRI-DraI fragment from pSKE8His ligated into the EcoRI-EcoRV sites | 10 |
| pMG36e | P32 promoter of L. lactis subsp. cremoris Wg2; Emr | 38 |
| pAMP21 | pGK13 derivative with oppA as a 1,736-bp NcoI-BamHI fragment | This work |
| pAMP31 | pGK13 derivative with oppA as a 1,802-bp NcoI-BamHI fragment | This work |
| pAMP31(K349Q) | pAMP31 with Lys-349 of OppA-His6 replaced by Gln | This work |
| pAMP31(N422H) | pAMP31 with Asn-422 of OppA-His6 replaced by His | This work |
| pAMP31(A423H) | pAMP31 with Ala-423 of OppA-His6 replaced by His | This work |
| pAMP31(D471R) | pAMP31 with Asp-471 of OppA-His6 replaced by Arg | This work |
| pAMP31(A477D) | pAMP31 with Ala-477 of OppA-His6 replaced by Asp | This work |
General DNA techniques.
Plasmid and chromosomal DNA were isolated by the alkaline lysis method as previously described (29). PCR was performed with VENT DNA polymerase (New England Biolabs). After 30 cycles of amplification, the PCR products were purified using the QIAquick spin PCR purification kit (Qiagen). DNA modification enzymes were obtained from Boehringer GmbH (Mannheim, Germany). Digestions were carried out according to the manufacturer's recommendations. Ligation of DNA fragments was performed as described previously (29). L. lactis was transformed by electroporation as described (9). DNA was sequenced by the dideoxy-chain termination method (30) using T7 DNA polymerase.
Construction of oppA deletion mutants.
The oppA gene was deleted from the chromosome of L. lactis MG1363 and IM15 via homologous recombination (16). An integration plasmid, pAP2, which contains the 5′ and 3′ flanking sequences of oppA was constructed for this purpose. Both flanking regions were amplified by PCR using pVS8 (37) as template and the primers FBB plus RCP (5′ region) and FOP plus ROS (3′ region). Primers are listed in Table 2. The PCR products corresponding to the 5′ (1,086 bp) and 3′ (1,096 bp) flanking regions were restricted with BamHI plus PstI and PstI plus SphI, respectively, and ligated into the multiple cloning site of pORI280 (15). L. lactis MG1363 and IM15 were transformed with pAP2, and transformants were selected on CDM plates, supplemented with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) plus erythromycin. Blue colonies arose from the integration of the plasmid at one of the two loci. Subsequently, the recombinant strains were grown on CDM liquid medium without erythromycin for about 100 generations to allow for another recombination event. A number of white colonies were selected on CDM plates supplemented with X-Gal and further analyzed by PCR and Western analysis.
TABLE 2.
Oligonucleotides used for cloning and mutagenesisa
| Primer | Oligonucleotide | Characteristic |
|---|---|---|
| FBB | 5′-CGCGGATCCGAGCCCTCCTGCCGGAT-3′ | BamHI |
| RCP | 5′-AAAACTGCAGGTTCATCTTAGTTTCTCC-3′ | PstI |
| FOP | 5′-CAGAAATTCCTGCAGACAAACC-3′ | PstI |
| ROS | 5′-TCACATGCATGCGGGAAACCAATGAAAGG-3′ | SphI |
| FSA | 5′-CAAGCCATGGCCAAATTAAAAGTAACTTTA-3′ | NcoI |
| FA | 5′-CAAGCCATGGGGTTCTAATCAAAGCTCA-3′ | NcoI |
| RAB | 5′-CGGTGGATCCTTTGGTGGCCAACT-3′ | BamHI |
| FP32S | 5′-GGAAGGCCTAATTCGGTCCTCGGG-3′ | StuI |
| RP32 | 5′-CGATTGCCATGGCAAAATTCCTCCGA-3′ | NcoI |
| XbaF | 5′-GTTGCTCTAGATAAAGAGTC-3′ | XbaI |
| K349QF | 5′-GTGGATAATCAATTCTCAAACG-3′ | K349Q |
| K349QR | 5′-CCGTTTGAGAATTGTTATCCAC-3′ | K349Q |
| N422HF | 5′-GGTGATGCACATGCTGAAACC-3′ | N422H |
| N422HR | 5′-GGTTTCAGCATGTGCATCACC-3′ | N422H |
| A423HF | 5′-GATGCAAACCATGAAACCATTGC-3′ | A423H |
| A423HR | 5′-GCAATGGTTTCATGGTTTGCATC-3′ | A423H |
| D471RF | 5′-GGATATCACTCGTGGTTCTTGG-3′ | D471R |
| D471RR | 5′-CCAAGAACCACGAGTGATATCC-3′ | D471R |
| A477DF | 5′-GGTCATTGGATTCTGAACC-3′ | A477D |
| A477DR | 5′-GGTTCAGAATCAATGACC-3′ | A477D |
Nucleotides in boldface type correspond to restriction site; nucleotide changes that give rise to an amino acid substitution are underlined.
oppA expression vector.
The oppA gene was obtained by PCR using pVS8 as template and the primers FA and RAB (oppA without the nucleotide sequence coding for the signal sequence; oppAΔss) or FSA and RAB (oppA including the nucleotide sequence for the signal sequence). In both cases, a unique NcoI site was engineered at the translation initiation site. Both PCR products were digested with NcoI plus BamHI, ligated into the vector pGKHis (10), which places the gene fragments in frame with a sequence specifying a 6-His tag at the C terminus of the protein. The StuI-NcoI (2,783 bp) fragment containing the cat and galM genes and the lacS promoter region was replaced by a PCR product that specifies the P32 promoter of L. lactis subsp. cremoris Wg2 (41). The P32 PCR product (191 bp) was obtained using pMG36e (38) as template and the primers FP32S and RP32. The resulting plasmids were named pAMP21 (OppA without signal sequence) and pAMP31 (OppA with signal sequence).
Immunogold labelling.
Immunogold labelling of ultrathin sections of L. lactis IM15, AMP2/pAMP21, and AMP2/pAMP31 with polyclonal antibodies raised against OppA (20) (1:2,000 serum dilution) was performed as previously described (32). Samples were analyzed with a Philips CM 10 transmission electron microscope.
Sequence alignment.
A multiple alignment of the OppA protein from serovar Typhimurium, DppA protein from E. coli (22), and OppA from L. lactis was generated using the CLUSTAL X program. A gap penalty of 30 and an extension gap penalty of 0.05 were used. The alignment was then manually modified to prevent gaps in the sequences that aligned with the known secondary structure elements of OppASt and DppA.
Construction of mutants of OppA.
Oligonucleotide-directed site-specific mutagenesis was used to generate single mutations in OppA. The mutants were constructed by a two-step PCR method. The synthetic mutagenic primers used are listed in Table 2. The oligonucleotides XbaF plus XnYR (X, amino acid residue present in OppA; n, position in the mature OppA; Y, mutated residue; and R, reverse primer) and XnYF (F, forward primer) plus RAB were used as primers in the first PCR step with plasmid pAMP31 as template. Subsequently, both PCR products were purified together and used as template for the second PCR step with the oligonucleotides XbaF plus RAB. The resulting 1,483-bp fragments were digested with XbaI plus BamHI and exchanged for the equivalent fragment of pAMP31. All 1,465-bp XbaI-BamHI fragments were checked by nucleotide sequencing.
Western analyses.
L. lactis cells were harvested at the end of the exponential phase of growth, washed once with water, and resuspended in water to A660 of approximately 10. The cells were sonicated for nine cycles of 5 s at an amplitude of 4 μm with 15 s cooling, on ice, using an MSE Soniprep 150-probe sonicator (Crawley, United Kingdom). Subsequently, sample buffer was added and the lysates were boiled for 5 min. Cell debris was removed by centrifugation (12,000 × g; 3 min). Samples (20 μg/lane) were subjected to sodium dodecyl sulfate (SDS)–10% polyacrylamide gel electrophoresis, and the proteins were transferred to polyvinylidene difluoride sheets (Millipore) by semidry electroblotting (13). OppA was detected with polyclonal anti-OppA antibodies (1:20,000 serum dilution) using the Western-Light chemiluminescence kit with CSPD as substrate (Tropix, Inc.).
Iodination of the tetrapeptide KYGK.
The tetrapeptide KYGK was iodinated at the tyrosine residue with the iodinating reagent 1,3,4,6-tetrachloro-3α, 6α-diphenylglycouril (Pierce Chemical Co., Rockford, Ill.) plus 200 μCi of Na125I (2,145 mCi/μmol; Amersham) as previously described (4).
Transport assays.
Cells grown to an optical density at 600 nm of 1.0 were harvested by centrifugation, washed twice, and resuspended in buffer A (100 mM potassium phosphate [pH 6.5], 5 mM magnesium sulphate). A total of 50 μl of the cell suspension (≈1.2 mg of protein/ml for KYGK uptake; ≈0.17 mg of protein/ml for bradykinin uptake) was added to 200 μl of buffer A supplemented with glucose (25 mM final concentration). Cells were incubated for 3 min at 30°C (in assays with KYGK as substrate) or 10°C (bradykinin as substrate), after which the transport reaction was initiated by the addition of 5.85 μM 125I-KYGK or 0.7 μM bradykinin (3H-RPPGFSPFR diluted with RPPGFSPFR), unless specified otherwise. At given time points, 50-μl samples were withdrawn and diluted with 2 ml of ice-cold 0.1 M LiCl. The samples were rapidly filtered through 0.45-μm-pore-size cellulose-acetate filters (Schleicher & Schuell GmbH, Dassel, Germany) and washed with 2 ml of ice-cold 0.1 M LiCl. The radioactivity of the filter was determined by liquid scintillation. To estimate the binding, the same procedure was followed except that the cells were incubated in buffer A without glucose for 6 min, and the amount obtained for strain AMP2 was subtracted in all cases. To determine the kinetic constants for bradykinin uptake, the amounts of bradykinin were varied from 0 to 5 μM. The uptake rate for each concentration was calculated by linear regression from the intracellular peptide concentration at different time points up to 90 s. The uptake rate as a function of the substrate concentration was fitted to the Michaelis-Menten equation.
Purification of OppA-His6.
Membrane-bound OppA-His6 was purified from inside-out membrane vesicles of L. lactis. The membrane vesicles were isolated as previously described (26) and solubilized at 5 mg of protein/ml in buffer B (50 mM potassium phosphate, 100 mM KCl, 10% glycerol [pH 7.6]) plus 0.2% (wt/vol) DDM. The mixture was incubated on ice for 30 min, and the insoluble material was removed by centrifugation (280,000 × g; 15 min). The solubilized membrane proteins were mixed with Ni-nitriloacetic acid resin previously equilibrated with buffer B. The mixture was incubated for 1 h at 4°C under continuous shaking and subsequently poured into a Bio-spin column (Bio-Rad). The column was washed with 20 column volumes of buffer B, pH 6.5, plus 0.05% DDM supplemented with 15 mM imidazole. The protein was eluted with buffer B plus 0.05% DDM containing 500 mM imidazole. A desalting step on a PD10 column (Bio-Rad) was performed in order to remove the imidazole. All handlings were performed at 4°C. The endogenous ligand copurified with OppA was removed by controlled denaturation-renaturation with 2 M guanidinium-HCl as described (14), except that 0.05% DDM was present in all solutions.
NCE.
Samples (each, 1 μg of protein) were prepared by incubating OppA-His6 with an equimolar amount of trypsin for 1 h at 30°C. The reaction was stopped by adding a 10-fold excess of trypsin inhibitor. When appropriate, peptide was added at a final concentration of 1 mM, and the mixture was incubated for 5 min at room temperature. Native cationic gel electrophoresis (NCE) was performed according to the method of Reisfield et al. (28) with some modifications (13).
Intrinsic protein fluorescence.
Peptide binding to OppA-His6 was observed as changes in intrinsic protein fluorescence, as previously described (14), except that 0.05% DDM was present in the buffer solution. All measurements were done in an Aminco 4800 spectrofluorimeter. The effect of peptide addition on fluorescence was measured at 15°C by exciting OppA (0.6 μM) at 280 nm with a slit width of 2 nm and measuring the emission at 315 nm with a slit width of 8 nm. Data analyses were performed as previously described (14).
Miscellaneous.
Protein content was determined according to Lowry et al. (17) with bovine serum albumin as standard. The concentration and stability of purified OppA proteins were evaluated by measuring the absorption spectrum between 240 and 340 nm. The extinction coefficient of OppA was calculated as previously described (24), obtaining a value of 1.605 (mg/ml)−1 · cm−1.
RESULTS
Analysis of oppA deletion mutants of L. lactis.
To study the properties of wild-type and mutant alleles of OppA in vivo, the oppA gene was deleted from the chromosome of strains MG1363 and IM15 by a crossover in each of the flanking regions with the integration plasmid pAP2. This procedure allows the complete deletion of oppA, leaving intact the other genes of the opp operon. Some putative mutants were analyzed by PCR, and the absence of the OppA protein was confirmed by immunoblotting. One mutant of each parent strain was chosen for further studies and named L. lactis AMP15(MG1363 ΔoppA) and L. lactis AMP2(IM15 ΔoppA) (Fig. 2).
FIG. 2.
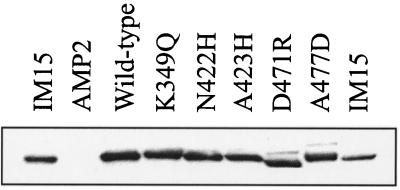
Immunoblot of wild-type and mutant OppA proteins. Total cell lysates were resolved by SDS–10% polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and probed with antibodies raised against OppA. Lysates of IM15, AMP2, AMP2/pAMP31, AMP2/pAMP31(K349Q), AMP2/pAMP31(N422H), AMP2/pAMP31(A423H), AMP2/pAMP31(D471R), AMP2/pAMP31(A477D), and IM15 are indicated; 20 μg of protein was present in each lane.
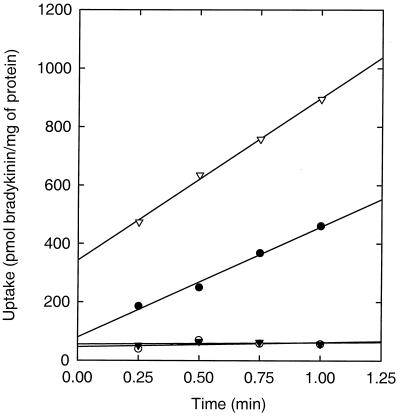
Lactic acid bacteria are multiple amino acid auxotrophs (3), and their nitrogen requirements are met by taking up free amino acids or peptides from the medium. Since it has been proved that the Opp system is essential for the uptake of peptides longer than three residues (12), the deletion of the oppA gene should result in a strain unable to grow on peptides as the source of one of these essential amino acids. Indeed, L. lactis AMP15 was unable to grow on CDM liquid medium with one of the tetra- or pentapeptides GLGL, LWMR, SLSQS, and YGGFL as the sole source of leucine, whereas the strain grew normally on CDM containing l-leucine. L. lactis IM15 is impaired in the degradation of peptides due to the deletion of four peptidases, but it is still able to use leu-enkephalin (YGGFL) as a source of leucine. As anticipated, L. lactis AMP2 was unable to grow on CDM plates containing 100 μM of leu-enkephalin as a sole source of leucine, whereas the strain grew normally on CDM plates containing l-leucine. To show directly that L. lactis AMP2 is defective in oligopeptide uptake, we monitored the uptake of 3H-bradykinin. Transport of bradykinin was completely abolished in L. lactis AMP2, whereas the uptake rate in the parent strain (IM15) was about 400 pmol · min−1 · mg of protein−1 (Fig. 3).
FIG. 3.
Bradykinin uptake by L. lactis IM15 (●), AMP2 (○), AMP2/pAMP21 (▾), and AMP2/pAMP31 (▿). The cells were energized for 3 min prior to the addition of bradykinin (0.7 μM, final concentration) at time zero. The intercept with the y axis reflects the amount of bradykinin binding to the cells.
L. lactis AMP2 was tested as host for the expression of OppA with the plasmid pAMP21 or pAMP31. Both plasmids contain the oppA gene under the control of the P32 promoter, and the genes are fused to a sequence that specifies a 6-His tag. Unlike in pAMP31, the oppA gene in pAMP21 lacks the signal sequence. The protein specified by oppAΔss is referred to as OppA*. As anticipated, pAMP21(oppAΔss) was unable to restore the ability of L. lactis AMP2 to utilize leu-enkephalin as a source of leucine (data not shown), and the transport of bradykinin was negligible (Fig. 3). L. lactis AMP2/pAMP31(oppA) was able to use leu-enkephalin as a source of leucine and transported bradykinin with an uptake rate of about 550 pmol · min−1 · mg of protein−1 (Fig. 3).
Overall, the results demonstrate that the oppA gene has been deleted from the chromosome of L. lactis MG1363 and IM15 and that OppA is the only binding protein that allows the organism to transport the tested oligopeptides. Complementation occurs with the oppA gene in trans.
Overexpression and localization of OppA.
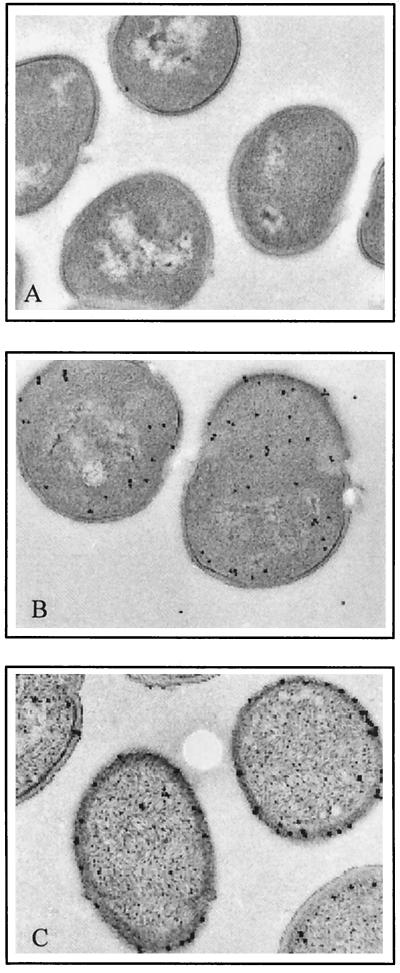
The amount of OppA present in AMP2/pAMP31 was approximately eight times higher than the level present in the parent strain (data not shown). Electron microscopy studies showed that all OppA produced by AMP2/pAMP31 was localized at the surface of the cell (Fig. 4). As was anticipated, OppA* was found in the cytoplasm of strain AMP2/pAMP21, due to the lack of a signal sequence. The observation that the rate of bradykinin uptake by AMP2/pAMP31 is at best 40% higher than that of IM15, whereas the expression level of OppA increased eightfold, indicates that transport of this peptide is not to a large extent rate determined by OppA activity.
FIG. 4.
Immunogold labeling of ultrathin sections of L. lactis cells expressing wild-type OppA (A and C) or OppA* (lacking signal sequence) (B). The proteins were detected with polyclonal antibodies raised against OppA. Panel A, IM15 cells; panel B, AMP2/pAMP21 cells; and panel C, AMP2/pAMP31 cells.
Expression of site-specific mutant OppA proteins.
The tertiary structure of OppA of serovar Typhimurium has been elucidated, and the specific residues that may interact with the termini of different peptides have been identified (33). OppA of serovar Typhimurium (OppASt) and OppA of L. lactis (OppALl) are homologous, but the identity between the two proteins is only 21 to 22%. A comparison of the primary sequence of both proteins (Fig. 1) shows that only Lys-307 in OppASt, which interacts with the carboxy terminus of the pentapeptides, is conserved in OppALl (Lys-349). The identification of the other residues that, on the basis of the OppASt structure, could interact with the termini of the peptides is more ambiguous. The residues equivalent to Asp-419 (N terminus of peptides), Arg-413 (C terminus of tripeptides), and His-371 (C terminus of tetrapeptides) in OppALl could be Ala-477, Asp-471, and Asn-422 or Ala-423. To establish the possible role of these residues in peptide binding and transport, substitutions were made on the basis of the structure of OppASt, yielding the following OppALl mutants: K349Q, A477D, D471R, N422H, and A423H.
The plasmids bearing the mutant genes were transformed to strain AMP2. Expression of these mutant OppA proteins was tested by Western analysis in whole cells and in membrane vesicles. In all cases, OppA was present in the membrane fraction and the mutant proteins were produced in amounts comparable to that of the wild-type protein expressed from plasmid pAMP31 (Fig. 2). Mutant D471R had an altered electrophoretic mobility as compared to wild-type OppA, which disappeared in the presence of 6 M urea (data not shown).
In vivo function of mutant OppA proteins.
To determine if these mutant proteins were able to complement the deletion mutants, L. lactis AMP15 and AMP2 were transformed with one of the following plasmids; pAM31(K349Q), pAMP31(N422H), pAMP31(A423H), pAMP31(D471R), or pAMP31(A477D). Transformants were tested for their ability to use oligopeptides (GLGL, LWMR, SLSQS, and YGGFL) as the sole source of leucine. All mutant OppA proteins sustained growth on these tetra- or pentapeptides as the sole source of leucine and had growth rates similar to that of the wild-type protein (data not shown).
Binding of bradykinin to cells expressing wild-type or mutant OppA proteins.
The data presented in Fig. 3 show that the increased amount of OppA present in strain AMP2/pAMP31 resulted in a slightly increased uptake rate but a highly increased binding (340 pmol · min−1 · mg of protein−1 when the uptake curve is extrapolated to time zero). To evaluate the binding of bradykinin quantitatively, the cells were incubated in buffer A without glucose for 6 min, and the amount of bound bradykinin was determined. Under these conditions, the cells did not accumulate the substrate and since the binding of bradykinin to OppA appeared to be tight, it could be quantified by the filtration assay. L. lactis AMP2/pAMP31 bound approximately seven times more bradykinin than IM15, whereas binding to the OppA mutants K349Q, A423H, D471R, and A477D was similar to that of IM15, at a bradykinin concentration of 0.7 μM (Table 3). The N422H mutant displayed intermediate binding. Since the expression levels of these mutant proteins were similar to that of wild-type OppA and functional complementation was observed in growth experiments, the data are consistent with a reduced affinity for bradykinin (see below) but, at this point, it cannot be ruled out that part of the mutant proteins is inactive.
TABLE 3.
Binding of bradykinin (RPPGFSPFR) and uptake of peptides by L. lactis OppA mutants
| Strain | 3H-RPPGFSPFR bounda | 125I-KYGK uptake rateb | 3H-RPPGFSPFR Uptake rateb |
|---|---|---|---|
| AMP2 | 0a | <0.2 | 15 ± 10 |
| IM15 | 39 ± 5 | ND | 400 ± 40 |
| AMP2/pAMP31(WT) | 286 ± 20 | 5 ± 1 | 550 ± 40 |
| AMP2/pAMP31(K349Q) | 56 ± 6 | 4 ± 1 | 33 ± 10 |
| AMP2/pAMP31(N422H) | 106 ± 9 | ND | 39 ± 11 |
| AMP2/pAMP31(A423H) | 32 ± 4 | 3 ± 1 | ND |
| AMP2/pAMP31(D471R) | 51 ± 4 | 28 ± 3 | 1,600 ± 50 |
| AMP2/pAMP31(A477D) | 30 ± 3 | 6 ± 2 | 53 ± 10 |
The amount of bradykinin bound to AMP2 (40 ± 4) was subtracted in all cases. Bradykinin (3H-RPPGFSPFR), (0.7 μM) final concentration.
Uptake rates are in picomoles of peptide per milligram of protein per minute. Rates are shown as means ± standard errors. ND, not determined.
Transport of peptides by cells expressing wild-type or mutant OppA proteins.
KYGK and bradykinin are low- and high-affinity substrates, respectively, of the Opp system of L. lactis (4, 14). Moreover, KYGK was used as substrate because it is not degraded by strains IM15 and AMP2 due to their multiple peptidase deficiencies. The rates of uptake of 125I-KYGK and 3H-bradykinin are shown in Table 3. Each of the mutant OppA proteins restored the uptake of KYGK and bradykinin in the AMP2 background, albeit to different levels. With the exception of OppA(D471R), the rates of KYGK uptake were comparable to that of the system with the wild-type OppA protein (Table 3). The rates of bradykinin uptake by strains expressing OppA proteins K349Q, N422H, and A477D were significantly lower than that of the wild type. The rate of uptake by strain expressing OppA(D471R) was much higher for both peptides (fivefold for KYGK and threefold for bradykinin).
Strain AMP2/pAMP31(D471R) was studied further because of its exciting properties, that is an apparent decreased binding affinity for bradykinin and an increased transport activity. To establish whether these properties are also manifested in the kinetic parameters of transport (Km and Vmax), we determined the uptake rate as a function of the bradykinin concentration and compared the obtained Km and Vmax values to the data obtained for strain AMP2/pAMP31 (Table 4). Strain AMP2/pAMP31(A477D) was also characterized as an example of a system that displayed reduced binding and transport activity. The differences in transport rate between the wild type and mutants were mainly at the level of Vmax, but a small but significant change in Km was also observed for OppA(A477D). Thus, the apparent decrease in binding affinity for bradykinin by OppA(D471R) is accompanied by a higher Vmax for uptake (see Discussion for interpretation).
TABLE 4.
Kinetic parameters for bradykinin uptake in L. lactis cells
| Strain | Vmaxa | Km (μM) |
|---|---|---|
| AMP2/pAMP31(WT) | 1,095 ± 106 | 0.27 ± 0.03 |
| AMP2/pAMP31(D471R) | 2,407 ± 228 | 0.24 ± 0.04 |
| AMP2/pAMP31(A477D) | 111 ± 48 | 0.54 ± 0.06 |
Vmax values are in picomoles per milligram of protein per minute. Rates are shown as means ± standard errors.
Peptide binding studied by NCE.
It has been shown that OppA* (expressed from plasmid pAMP21) exhibits a shift in mobility in the presence of oligopeptides (14). The method provides direct proof for the ability of the protein to bind peptide, and it yields semiquantitative information about the dissociation constants. It was used to study peptide binding to OppA(WT), OppA(D471R), and OppA(A477D). Each of the proteins was purified, the endogenous ligand was removed by guanidinium-HCl treatment, and the amino-terminal lipid anchor-signal sequence was removed by trypsin treatment. The latter step was performed because the lipid anchor prevented entry of the protein into the polyacrylamide gel. NCE of trypsin-treated OppA(WT) and OppA(A477D) yielded two species that correspond to the open unliganded and the closed liganded forms of OppA (14). Upon addition of bradykinin or SLSQSKVLPVPQ, the fast-migrating form became predominant. In the case of OppA(D471R), only one form was observed before and after incubation with peptides (data not shown). Due to the altered electrophoretic mobility of OppA(D471R) even in the presence of SDS, it was not possible to conclude if this unique form corresponds to the open or closed conformation.
Peptide binding studied by intrinsic protein fluorescence.
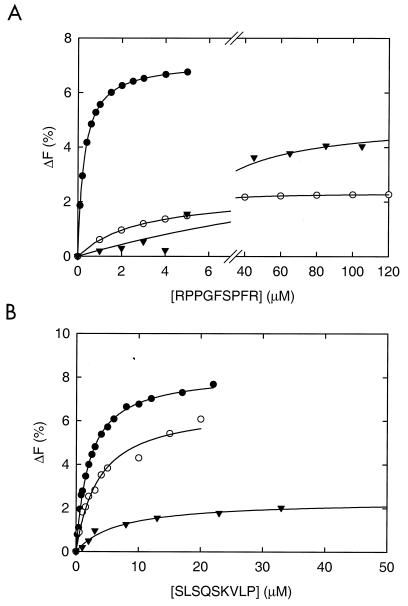
To study the binding of peptides in a more quantitative manner, purified and guanidinium-HCl-treated OppA(WT), OppA(D471R), and OppA(A477D) were used in intrinsic protein fluorescence assays. The emission spectrum of OppA showed a maximum at 332 nm. Upon binding of peptides, a blue shift of approximately 2 nm was observed. Binding of bradykinin or SLSQSKVLP resulted in an increase in fluorescence below 340 nm and in a decrease above 340 nm (data not shown). The increase in fluorescence at 315 nm was concentration dependent and could be used to determine the kinetic parameters for peptide binding. Binding of bradykinin and SLSQSKVLP to OppA(WT), OppA(D471R), and OppA(A477D) yielded a dependence on the peptide concentration that could be equated with ΔF = (Fmax*[S])/(Kd+[S]), where ΔF is the observed fluorescence change, Fmax is the maximal change in fluorescence, Kd is the dissociation constant, and [S] is the peptide concentration (Fig. 5). The dissociation constants determined for bradykinin were 0.29 μM for OppA(WT), 2.83 μM for OppA(D471R), and 20.9 μM for OppA(A477D). The Kd values of the three proteins were of the same order of magnitude when the nonapeptide SLSQSKVLP was used as test substrate. These results indicate that the mutations affect the affinity of OppA for bradykinin but not the affinity for peptides in general.
FIG. 5.
Concentration dependence of the fluorescence increase at 315 nm induced by peptides binding to OppA(WT) (●), OppA(D471R) (○), and OppA(A477D) (▾) in the presence of bradykinin (RPPGFSPFR) (A) and SLSQSKVLP (B). The concentration of all OppA proteins in this experiment was 0.6 μM. The solid line through the data points represents the best fit. The Kd values obtained for OppA(WT) were 0.29 ± 0.02 for bradykinin and 2.07 ± 0.07 for SLSQSKVLP. The Kd values for OppA(D471R) were 2.83 ± 0.09 for bradykinin and 2.26 ± 0.05 for SLSQSKVLP. The Kd values for OppA(A477D) were 20.86 ± 0.56 for bradykinin and 6.22 ± 0.13 for SLSQSKVLP.
DISCUSSION
In this paper, we show that deletion of the oppA gene from the chromosome rendered L. lactis MG1363 and IM15 inactive in the uptake of oligopeptides. These strains could be complemented with the oppA gene in trans. The presence of a His tag at the C terminus did not affect the functionality of OppA; all of the overexpressed protein was directed to the cell surface as shown by the electron microscopy studies. The increase in the expression of OppA resulted in a highly increased peptide binding capacity, whereas the uptake rate was only marginally affected. These initial studies have set the stage for the in vivo and in vitro analyses of mutations in the peptide binding protein of the Opp system of L. lactis.
Several site-directed mutants of OppA were constructed on the basis of a comparison between the primary sequence of OppA from serovar. Typhimurium and L. lactis, taking advantage of the three-dimensional structure of OppASt. The expression of all of these mutant OppA proteins restored the transport of peptides as well as the growth of ΔoppA mutants of MG1363 and IM15 on peptides as a source of essential amino acids. Mutant D471R displayed a five- and threefold-higher uptake rate for KYGK and bradykinin (RPPGFSPFR), respectively. The rate of transport of KYGK was not significantly affected in the other mutants, whereas that of bradykinin was approximately 10-fold lower. The apparent increase in uptake rate in OppA(D471R) and the decrease in OppA(A477D) correspond to a change in Vmax rather than to large alterations in the affinity constants for uptake. Studies of peptide binding to wild-type and mutant OppA proteins showed that the Kd values for bradykinin binding to OppA(D471R) and OppA(A477R) increased by 1 and 2 orders of magnitude, respectively, as compared to OppA(WT). The same proteins exhibited wild-type binding kinetics for the other nonapeptide tested (SLSQSKVLP). The consequences of these differences in Kd and Km values and their dependence on the actual peptide used are discussed below.
Since the Kd for bradykinin binding to OppA(D471R) and OppA(A477R) was greatly increased, it was not possible to determine the binding stoichiometry for these mutants. To determine the actual number of binding sites, one needs a high-affinity ligand such that Kd << [OppA] under the experimental conditions (see our previous analysis in reference 14). In our opinion, the diminished amount of bradykinin binding is consistent with the increase in Kd and does not involve a decrease in the binding stoichiometry as a result of a fraction of inactive protein. This notion is supported by the observation that the kinetics of SLSQSKVLP binding to OppA(D471R) is very similar to that of the wild-type protein.
Comparison of the specificities of OppALl and OppASt in relation to the structure.
All the mutations introduced into OppALl seem to affect the specificity of the protein for peptides. The positions were selected for mutagenesis studies on the basis of their proposed interactions with the tri-, tetra-, or pentapeptides in OppASt. The selected residues in OppALl clearly have a more global effect on the interactions with the peptides, as pronounced differences in transport and binding activities were observed when the nonameric peptide bradykinin was used as test substrate. Since the transport of peptides by Opp is rate determined by the kinetics of bradykinin binding to only a small extent, changes in this parameter may not be observed in the overall transport reaction. The same may apply for other peptides, and it would require a full analysis of both peptide binding and transport. Unfortunately, the availability of radiolabelled oligopeptides for transport studies is limited, whereas the dissociation constants of small peptides (five or fewer residues) are too high (in the millimolar range) to be analyzed by NCE or intrinsic fluorescence. As a consequence, we cannot rule out the possibility that some of the mutants have an altered Kd for tripeptides (D471R) or tetrapeptides (N422H or A423H) specifically.
In our opinion, however, the fact that these residues are not conserved may reflect the differences in function of both OppA proteins; that is, OppALl serves to accumulate rather long peptides (>5 residues) (4), whereas the optimal activity of OppASt is for tri- and tetrapeptides (7). Part of the binding affinity of OppASt for tri- and tetrapeptides will be obtained from the interactions of the carboxyl-terminal ends of these peptides with the corresponding residues in the protein. The dissociation constants of OppALl for tri- and tetrapeptides are much higher than those of OppASt, most likely because the interactions with the termini of the peptides are absent. In this regard, it is worth emphasizing that, despite the high dissociation constants of OppALl for tri- and tetrapeptides, all the peptides tested thus far are taken up by Opp of L. lactis (4); the capacity of OppLl to transport tripeptides is more ambiguous.
A moderate decrease in binding affinity results in an higher uptake rate.
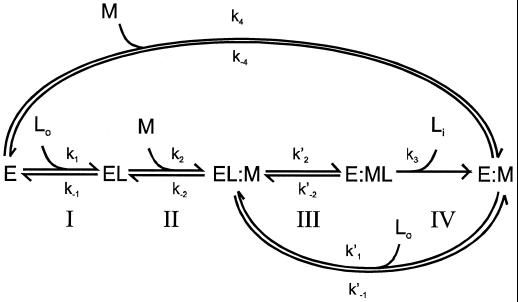
The Kd obtained for bradykinin for OppA(D471R) is about 10-fold higher than that of OppA(WT). This difference is in agreement with the observed lower-binding activity in cells expressing OppA(D471R). Due to its very fast association, it was not possible to determine the association (k1) and dissociation (k−1) rates for bradykinin by stopped-flow fluorescence measurements. Nevertheless, we speculate that the increased Kd of OppA(D471R) for bradykinin is caused by an increased dissociation rate constant (k−1). This suggestion follows from the observation that the large variation in Kd of OppA* for a range of peptides relates to differences in k−1 (14). Site-directed mutagenesis studies of the arabinose-binding protein of E. coli (40, 41) also showed that variations in Kd relate to an altered k−1 rather than to a change in the association constant (k1). This implies that bradykinin gains access to the active sites of OppA(WT) and OppA(D471R) equally well but that the dissociation rates from these binding proteins are different. The consequences of this suggestion on the overall transport by Opp can be analyzed from a previously published scheme (14). According to this model (Fig. 6) transport takes place in four steps: I, binding of the ligand to the binding protein; II, docking of the liganded binding protein to the membrane complex; III, donation of the ligand to the membrane complex; and IV, translocation of the substrate across the membrane. It has been proposed that the donation of the ligand from the binding protein to the membrane-bound complex determines the rate of the whole transport process (14, 21), which corresponds to step III of the scheme. In this case, the rate of transport can be described by the following equation:
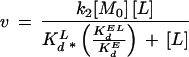 |
in which K′2 is the rate constant of this donation step, [Mo] is the total concentration of membrane-bound complex, [L] is the concentration of the ligand, Kdl corresponds to the equilibrium constant for binding of the ligand to the binding protein (k−1/k1), KdEL is the equilibrium constant for binding of the unliganded binding protein to the membrane complex. If we assume that k−1 and k′2 are related, that is, that the rate of dissociation of the peptide from OppA is the same for free (EL) and membrane-docked (EL:M) binding protein, then the rate of transport will increase in proportion to k′2. In other words, the increase in Vmax for bradykinin uptake in OppA(D471R) reflects an enhanced donation of the peptide from the binding protein to the membrane complex.
FIG. 6.
In this scheme, E and EL represent the unliganded and liganded binding protein, respectively; Lo and Li are the external and internalized ligand, respectively; and M refers to the membrane-bound complex.
A large decrease in binding affinity results in a lower uptake rate.
In cells expressing OppA(A477D), the lower Vmax value for bradykinin uptake parallels a dramatic decrease in the binding affinity of the OppA(A477D) protein for bradykinin. If we assume that the increased Kd is a consequence of a higher value for k−1, and thus k′2, then following the same line of reasoning as in OppA(D471R), one would also expect an increased rate of uptake for OppA(A477D). However, if the Kd becomes too low, the equilibrium between liganded and unliganded OppA will be towards unliganded binding protein and step I in the scheme may become rate determining. In this regard, it is worth noting that unliganded and liganded binding proteins are believed to have a similar affinity for the membrane complex in the case of the histidine system (2).
ACKNOWLEDGMENTS
This work was supported by grants from the Spanish Ministerio de Educacion y Cultura (FP 95 27509037) and the E.U. agriculture and fisheries program (FAIR-CT96-5030). Additional support was from the E.U. biotechnology program (BIO4-CT96-0016).
We thank the following persons for assistance: Klaas Sjollema (electron microscopy), André Boorsma (DNA sequencing), and Bert Klunder (large scale fermentations). We also thank Karel van Wely for helpful suggestions and fruitful discussions.
REFERENCES
- 1.Ames G F-L. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- 2.Ames G F-L, Liu C E, Joshi A K, Nikaido K. Liganded and unliganded receptors interact with equal affinity with the membrane complex of periplasmic permeases, a subfamily of traffic ATPases. J Biol Chem. 1996;271:14264–14270. doi: 10.1074/jbc.271.24.14264. [DOI] [PubMed] [Google Scholar]
- 3.Chopin A. Organization and regulation of genes for amino acid biosynthesis in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:21–38. doi: 10.1111/j.1574-6976.1993.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 4.Detmers F J M, Kunji E R S, Lanfermeijer F C, Poolman B, Konings W N. Kinetics and specificity of peptide uptake by the oligopeptide transport system of Lactococcus lactis. Biochemistry. 1998;37:16671–16679. doi: 10.1021/bi981712t. [DOI] [PubMed] [Google Scholar]
- 5.Gasson M J. Plasmid complements of Streptococcus lactis NCDO712 and other lactic streptococci after protoplast induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilson E, Alloing G, Schmidt T, Claverys J P, Dudler R, Hofnung M. Evidence for high affinity binding-protein dependent transport systems in gram-positive bacteria and in Mycoplasma. EMBO J. 1988;7:3971–3974. doi: 10.1002/j.1460-2075.1988.tb03284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyer C A, Morgan D G, Staros J V. Binding specificity of the periplasmic oligopeptide-binding protein from Escherichia coli. J Bacteriol. 1986;168:775–779. doi: 10.1128/jb.168.2.775-779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 9.Holo H, Nes I F. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knol J, Veenhoff L, Liang W J, Henderson P J F, Leblanc G, Poolman B. Unidirectional reconstitution into detergent-destabilized liposomes of the purified lactose transport system of Streptococcus thermophilus. J Biol Chem. 1996;271:15358–15366. doi: 10.1074/jbc.271.26.15358. [DOI] [PubMed] [Google Scholar]
- 11.Kok J, van der Vossen J M B M, Venema G. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl Environ Microbiol. 1984;48:726–731. doi: 10.1128/aem.48.4.726-731.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunji E R S, Fang G, Jeronimus-Stratingh C M, Bruins A P, Poolman B, Konings W N. Reconstruction of the proteolytic pathway for use of β-casein by L. lactis. Mol Microbiol. 1998;27:1107–1118. doi: 10.1046/j.1365-2958.1998.00769.x. [DOI] [PubMed] [Google Scholar]
- 13.Kyhse-Anderson J. Electroblotting of multiple gels: a simple apparatus without buffer for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984;10:203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- 14.Lanfermeijer F C, Picon A, Konings W N, Poolman B. Kinetics and consequences of binding of nona- and dodecapeptides to the oligopeptide binding protein (OppA) of Lactococcus lactis. Biochemistry. 1999;38:14440–14450. doi: 10.1021/bi9914715. [DOI] [PubMed] [Google Scholar]
- 15.Leenhouts K J, Kok J, Venema G. Lactococcal plasmid pWV01 as an integration vector for lactococci. Appl Environ Microbiol. 1991;57:2562–2567. doi: 10.1128/aem.57.9.2562-2567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leenhouts K J, Venema G. Molecular cloning and expression in Lactococcus. Med. Fac. Landbouww. Univ. Genet. 1992;57:2031–2043. [Google Scholar]
- 17.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Manson M D, Boos W, Bassford P J, Rasmussen B A. Dependence of maltose transport and chemotaxis on the amount of maltose-binding protein. J Biol Chem. 1985;260:9727–9733. [PubMed] [Google Scholar]
- 19.Mao B, Pear M R, McCammon J A, Quiocho F A. Hinge-bending in l-arabinose-binding protein. The “Venus flytrap” model. J Biol Chem. 1982;257:1131–1133. [PubMed] [Google Scholar]
- 20.Mierau I, Kunji E R S, Leenhouts K J, Hellendoorn M A, Haandrikman A J, Poolman B, Konings W N, Venema G, Kok J. Multiple-peptidase mutants of Lactococcus lactis are severely impaired in their ability to grow in milk. J Bacteriol. 1996;178:2794–2803. doi: 10.1128/jb.178.10.2794-2803.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller D M, Olson J S, Pflugrath J W, Quiocho F A. Rates of ligand binding to periplasmic proteins involved in bacterial transport and chemotaxis. J Biol Chem. 1983;258:13665–13672. [PubMed] [Google Scholar]
- 22.Nickitenco A V, Trakhanov S, Quiocho F A. 2 Å resolution structure of DppA, a periplasmic dipeptide transport/chemosensory receptor. Biochemistry. 1995;34:16585–16595. doi: 10.1021/bi00051a006. [DOI] [PubMed] [Google Scholar]
- 23.Pace C N, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payne J W, Smith M W. Peptide transport by microorganisms. Adv Microb Physiol. 1994;36:1–80. doi: 10.1016/s0065-2911(08)60176-9. [DOI] [PubMed] [Google Scholar]
- 25.Poolman B, Konings W N. Growth of Streptococcus cremoris in relation to amino acid transport. J Bacteriol. 1988;170:700–707. doi: 10.1128/jb.170.2.700-707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putman M, van Veen H W, Poolman B, Konings W N. Restrictive use of detergents in the functional reconstitution of the secondary multidrug transporter LmrP. Biochemistry. 1999;38:1002–1008. doi: 10.1021/bi981863w. [DOI] [PubMed] [Google Scholar]
- 27.Quiocho F A, Ledvina P S. Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis. Variation of common themes. Mol Microbiol. 1996;20:17–25. doi: 10.1111/j.1365-2958.1996.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 28.Reisfield R A, Lewis U J, Williams D E. Disk electrophoresis of basic proteins on polyacrylamide gels. Nature. 1962;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 30.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sleigh S H, Tame J R H, Dodson E J, Wilkinson A J. Peptide binding in OppA, the crystal structures of the periplasmic oligopeptide binding protein in the unliganded form and in complex with lysyllysine. Biochemistry. 1997;36:9747–9758. doi: 10.1021/bi970457u. [DOI] [PubMed] [Google Scholar]
- 32.Slot J W, Geuze H J. Gold markers for single and double immunolabelling of ultrathin cryosections. In: Polak J M, Varndell I M, editors. Immunolabelling for electron microscopy. New York, N.Y: Elsevier Science Publishing; 1984. pp. 129–142. [Google Scholar]
- 33.Tame J R H, Dodson E J, Murshudov G N, Higgins C F, Wilkinson A J. The crystal structures of the oligopeptide-binding protein OppA complexed with tripeptide and tetrapeptide ligands. Structure. 1995;3:1395–1406. doi: 10.1016/s0969-2126(01)00276-3. [DOI] [PubMed] [Google Scholar]
- 34.Tame J R H, Murshudov G N, Dodson E J, Neil T K, Dodson G G, Higgins C F, Wilkinson A J. The structural basis of sequence-independent peptide binding by OppA protein. Science. 1994;264:1578–1581. doi: 10.1126/science.8202710. [DOI] [PubMed] [Google Scholar]
- 35.Tame J R H, Sleigh S H, Wilkinson A J, Landbury J E. The role of water in sequence-independent ligand binding by an oligopeptide transporter protein. Nat Struct Biol. 1996;3:998–1001. doi: 10.1038/nsb1296-998. [DOI] [PubMed] [Google Scholar]
- 36.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tynkkynen S, Buist G, Kunji E, Kok J, Poolman B, Venema G, Haandrikman A. Genetic and biochemical characterization of the oligopeptide transport system of Lactococcus lactis. J Bacteriol. 1993;175:7523–7532. doi: 10.1128/jb.175.23.7523-7532.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van de Guchte M, van der Vossen J M B M, Kok J, Venema G. Construction of a lactococcal expression vector: expression of hen egg white lysozyme in Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1989;55:224–228. doi: 10.1128/aem.55.1.224-228.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Vossen J M B M, van der Lelie D, Venema G. Isolation and characterization of Streptococcus cremoris Wg2-specific promoters. Appl Environ Microbiol. 1987;53:2452–2457. doi: 10.1128/aem.53.10.2452-2457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vermersch P S, Lemon D D, Tesmer J J G, Quiocho F A. Sugar-binding and crystallographic studies of an arabinose-binding protein mutant (Met108Leu) that exhibits enhanced affinity and altered specificity. Biochemistry. 1991;30:6861–6866. [PubMed] [Google Scholar]
- 41.Vermersch P S, Tesmer J J G, Lemon D D, Quiocho F A. A Pro to Gly mutation in the hinge of the arabinose-binding protein enhances binding and alters specificity. J Biol Chem. 1990;265:16592–16603. [PubMed] [Google Scholar]