Abstract
In the arginine biosynthetic pathway of the vast majority of prokaryotes, the formation of ornithine is catalyzed by an enzyme transferring the acetyl group of N-α-acetylornithine to glutamate (ornithine acetyltransferase [OATase]) (argJ encoded). Only two exceptions had been reported—the Enterobacteriaceae and Myxococcus xanthus (members of the γ and δ groups of the class Proteobacteria, respectively)—in which ornithine is produced from N-α-acetylornithine by a deacylase, acetylornithinase (AOase) (argE encoded). We have investigated the gene-enzyme relationship in the arginine regulons of two psychrophilic Moritella strains belonging to the Vibrionaceae, a family phylogenetically related to the Enterobacteriaceae. Most of the arg genes were found to be clustered in one continuous sequence divergently transcribed in two wings, argE and argCBFGH(A) [“H(A)” indicates that the argininosuccinase gene consists of a part homologous to known argH sequences and of a 3′ extension able to complement an Escherichia coli mutant deficient in the argA gene, encoding N-α-acetylglutamate synthetase, the first enzyme committed to the pathway]. Phylogenetic evidence suggests that this new clustering pattern arose in an ancestor common to Vibrionaceae and Enterobacteriaceae, where OATase was lost and replaced by a deacylase. The AOase and ornithine carbamoyltransferase of these psychrophilic strains both display distinctly cold-adapted activity profiles, providing the first cold-active examples of such enzymes.
Tracing back the evolution of a metabolic pathway becomes possible when differences in biochemical characters and gene organization can be compared with a phylogenetic progression of the host organisms (23). By applying this rationale to the different branches of the aromatic amino acid biosynthetic pathway, a paradigm was generated to study the molecular evolution of metabolism among Bacteria (1, 8, 23, 24).
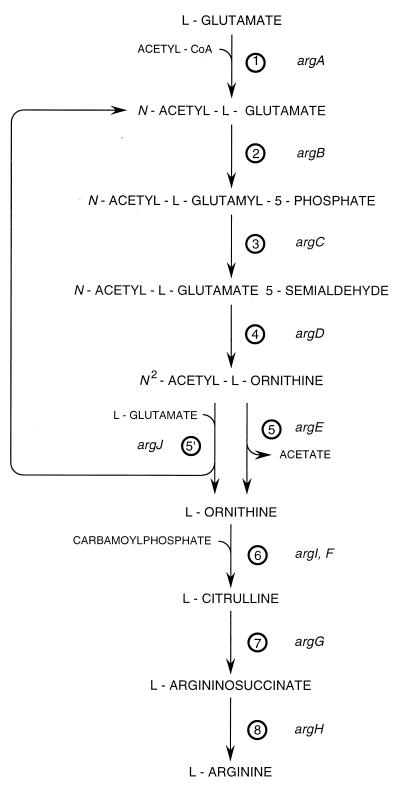
Arginine biosynthesis displays diverse patterns of gene organization (5, 9, 15) and is one of those rare instances where two completely different enzymes may catalyze the formation of a key intermediate, in this case ornithine (Fig. 1). In the linear version of the pathway, characteristic of the Enterobacteriaceae (9), the hydrolysis of N-α-acetylornithine into ornithine and acetate is catalyzed by an acetylornithinase (AOase [EC 3.5.1.16], encoded by argE). In all other prokaryotes, except Myxococcus xanthus (19) and possibly the archaeon Sulfolobus (51), an ornithine acetyltransferase (OATase [EC 2.3.1.35], encoded by argJ) recycles the acetyl group of acetylornithine on glutamate. OATase is also characteristic of fungi and green algae (10).
FIG. 1.
Arginine biosynthesis. 1, N-Acetylglutamate synthase (acetyl coenzyme A [acetyl-CoA]: l-glutamate N-acetyltransferase [EC 2.3.1.1]); 2, N-acetylglutamate 5-phosphotransferase (ATP: N-acetyl-l-glutamate 5-phosphotransferase [EC 2.7.2.8]); 3, N-acetylglutamate 5-semialdehyde dehydrogenase (N-acetyl-l-glutamate-5-semialdehyde: NADP+ oxidoreductase [phosphorylating] [EC 1.2.1.38); 4, N2-acetylornithine 5-aminotransferase (N2-acetyl-l-ornithine:2-oxoglutarate aminotransferase [EC 2.6.1.11]); 5, AOase (N2-acetyl-l-ornithine amidohydrolase [EC 3.5.1.16]); 5′, OATase (N2-acetyl-l-ornithine:l-glutamate N-acetyltransferase [EC 2.3.1.35]); 6, OTCase (carbamoylphosphate:l-ornithine carbamoyltransferase [EC 2.1.3.3]); 7, argininosuccinate synthetase (l-citrulline:l-aspartate ligase [AMP forming] [EC 6.3.4.5]; 8, argininosuccinase (l-argininosuccinate arginine lyase [EC 4.3.2.1]).
Among gram-negative bacteria, the arginine pathway has been thoroughly studied for fluorescent pseudomonads (9, 39) and for Enterobacteriaceae (9, 15), both members of the class Proteobacteria (54, 55), but not for Vibrionaceae, a related family. Early rRNA-DNA homology analyses and comparative studies of the aspartate and aromatic families of amino acid biosynthetic pathways already suggested a common origin for the Vibrionaceae and Enterobacteriaceae (3, 8, 23, 24). In view of this relationship and considering the prevalent position of OATase among prokaryotes, it was of interest to examine the gene-enzyme relationship of the arginine pathway in members of the Vibrionaceae.
We have investigated two psychrophilic and barotolerant strains previously designated Vibrio strains 2674 and 2693 (29, 56) which proved to belong to the genus Moritella. Both were found to possess an AOase and to present an organization of arginine genes which appears ancestral with respect to the different patterns found among Enterobacteriaceae.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains are listed in Table 1. For genetic experiments cells were grown either in liquid broth 869 (14) supplemented with 0.7 g of K2HPO4 and 0.2 g of KH2PO4 per liter or on agar plates supplemented with the same base or with minimal medium 132 (14). For enzyme assays, cells (Escherichia coli or Moritella) were grown in arginine- and uracil-free (AUF) rich synthetic medium (56). For Moritella strains this medium was supplemented with artificial seawater as a minimal base (42). Plasmid vectors pTrc99A and pBK-CMV were from Pharmacia and Stratagene, respectively.
TABLE 1.
Characteristics of strains
| Strain | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Moritella | ||
| Strain 2674 | Psychrophilic member of the Vibrionaceae | This work |
| Strain 2693 | Psychrophilic member of the Vibrionaceae | This work |
| E. coli | ||
| XA4 | argA | S. Baumberga |
| P4XSB171 | argA metB | This laboratory |
| XB25 | argB | S. Baumberga |
| P4XSB53 | argC metB | This laboratory |
| XD1 | argD pro | This laboratory |
| LCB853 | argD pro | This laboratory |
| XS1D2 | argE ppc | S. Baumberga |
| C600 OTC | argF argI | This laboratory |
| 30S0MA5 | argG | W. K. Maasb |
| P4XSB145 | argH metB | This laboratory |
| 145M1 | argH | This laboratory |
From the School of Biology, University of Leeds, Leeds, United Kingdom.
From New York University Medical School, New York, N.Y.
Cloning and sequencing strategies.
Partially digested (with Sau3A) DNA from cells of Moritella strain 2693 grown in Difco Marine Broth was ligated with vector pTrc99A, which had been predigested with BamHI, and was used to transform E. coli C600 OTC argF argI. Colonies appearing at 37°C on 856 plates supplemented with ampicillin (50 μg ml−1) were replicated on minimal medium containing glucose (0.5%), thiamine (1 μg ml−1), and dl-proline (200 μg ml−1) and then incubated at 18°C for 7 days. Several recombinant plasmids were retained for further study and used for the complementation tests whose results are reported in Table 2. Southern blots (48) carried out with pC-11, containing a 3.7-kb insert complementing E. coli argB, -F, and -G mutants, showed that the cloned DNA hybridized to Moritella but not E. coli DNA (data not shown).
TABLE 2.
Complementation patterns of E. coli arg mutants found for different fragments cloned from Moritella strain 2693 DNAa
| Fragment length (kb) | Plasmid designation | Vector used | Mutant(s) complemented |
|---|---|---|---|
| 3.7 | pC-11 | pTrc99A | argB, argF, argG |
| 8.0 | pC-28 | pTrc99A | argE, argC, argB, argF, argG |
| 11.0 | pC-33 | pTrc99A | argC, argB, argF, argG, argH, argA |
| 1.6 | pHBP | pBK-CMV | argH |
| 2.2 | pCMC | pBK-CMV | argH, argA |
Two or three independent clones of each fragment were tested, with identical results. Complemented mutants produced colonies with diameters of 0.6 to 1.0 mm after 3 days, i.e., 1 day later than the E. coli mutant streaked on an appropriate source of arginine (acetylornithine, ornithine, citrulline, or arginine).
DNA from Moritella strain 2674 was used to prepare a λZAP library (Stratagene). Using the insert of pC-11 as a probe, different fragments were identified by plaque hybridization and shown to complement the ornithine carbamoyltransferase (OTCase) deficiency of E. coli C600 argF argI on AUF plates supplemented with 50 μg of uracil ml−1. One of them (pXY-114) was used for the enzyme assays reported in Fig. 5.
FIG. 5.
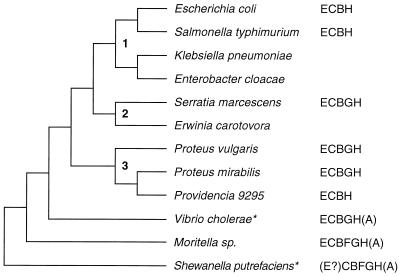
Schematic representation of phylogenetic relationships and patterns of arg genes clustering among Vibrionaceae (Vibrio, Moritella, and Shewanella) and various Enterobacteriaceae. The dendrogram is based on the data reported by Ahmad et al. (1) (a synthetic diagram integrating 16S RNA data and biochemical character states from the aromatic amino acid biosynthetic pathways), by Baumann and Schubert (3), and by Yanagibayashi et al. (57); the numbers refer to the enteroclusters defined in reference 1. ∗, preliminary observations from ongoing sequencing project (see text).
Nucleotide sequences were determined by the method of Sanger et al. (46), using synthetic oligonucleotides as primers. The nucleotide sequence of Moritella strain 2674 DNA was obtained by primer walking from several inserts retrieved by complementation or plaque hybridization.
16S RNA nucleotide sequences.
From DNA extracts obtained from cultures of strains 2674 and 2693, the nucleotide sequence of 16S RNA was determined and interpreted by the identification service of the BCCM/LMG node of the Coordinated Collections of Microorganisms at the University of Ghent, Ghent, Belgium.
Enzyme assays.
Cell extracts were obtained by 5-min sonic disruption of 0.9% NaCl-washed mid-exponential-phase cells (either Moritella grown at 6°C or recombinant E. coli grown at 30°C) suspended in 50 mM Tris-HCl buffer (pH 8.0). The extracts were centrifuged for 15 min at 20,000 × g, and the supernatants were used for assays. AOase was assayed as described by Vogel and McLellan (53), OATase was assayed according to the method of Van de Casteele et al. (51), and OTCase (EC 2.1.3.3) was assayed as described by Stalon et al. (49). One enzyme unit is defined as the amount of enzyme converting 1 μmol of substrate to product per h. Protein concentrations were determined by the Lowry method.
Primer extension.
The antisense oligonucleotides 5′ TGCATATAACGTTCCTGT 3′ and 5′ ACTGTCTCGTCGAAACCATGA 3′ (corresponding, respectively, to positions +83 to +66 and +63 to +43 of the argE and argC genes) were used for extension by reverse transcriptase. The protocol was as described by Treizenberg (50). Hybridization was performed at 42°C.
Nucleotide sequence accession numbers.
The sequences of the genomic regions reported have been deposited in the EMBL, GenBank, and DDBJ databases under accession numbers AJ252020 to AJ252023.
RESULTS
Main characteristics of Moritella strains 2674 and 2693.
Both strains were isolated from the upper sediment layer of the deep Atlantic (−2,815 m; 05°37.0N, 19°58.9W) at a temperature of 2°C. By their morphology and other characteristics they had been provisionally assigned to the genus Vibrio (29, 56). Comparative analysis of their 16S rRNA nucleotide sequence brought to light highest similarities with reference organisms of the genus Moritella (from 98.5 to above 99%), the two strains being 98.5% identical with each other.
Growth was observed between 2 and 14°C, a strict psychrophilic profile (34). A minimal doubling time of 6 h was obtained aerobically in Difco Marine Broth 2216 at 6°C. At the same temperature in AUF rich synthetic medium, the doubling time was 8.5 h, with a final density of about 109 cells ml−1.
Isolation of arg genes from Moritella and analysis of their nucleotide sequence.
The argF gene (encoding OTCase) was cloned from both strains by complementation of E. coli C600 OTC argF argI (E. coli K-12 harbors duplicate genes for OTCase synthesis [15, 28]). Plasmid pC-11 (containing 3.7 kb of strain 2693 DNA) also complemented argB and argG mutants of E. coli. Other fragments belonging to the same region complemented argA, argE, or argH E. coli mutants (Table 2). None were found to complement argD mutants.
The nucleotide sequence of the 3.7-kb insert carried by pC-11 and of flanking segments brought to light a series of open reading frames (ORF) homologous to argC, argB, argF, argG, and argH in one orientation and to argE in the reverse orientation (Fig. 2). Left of argE is the beginning of an ORF homologous to the E. coli ppc gene, encoding phosphoenolpyruvate carboxylase. Curiously, the ORF homologous to argH (encoding argininosuccinase) extends beyond the region expected to correspond to the carboxy terminus into a sequence whose product has similarity over 173 amino acids (36% amino acid identity) to a putative acetyltransferase of Aquifex aeolicus (11) and with the part (from residues 294 to 423) of the E. coli N-acetylglutamate synthetase gene corresponding to the carboxy terminus (argA [26% identity]). This composite gene complemented both argH and argA E. coli auxotrophs. When trimmed down to the portion homologous to E. coli argH, the gene complemented only argH mutants. The absence of any stop codon between the sequence homologous to argH and the distal portion of the gene was confirmed by determining the nucleotide sequence of fragments retrieved by PCR from genomic DNA. This gene is provisionally designated argH(A).
FIG. 2.
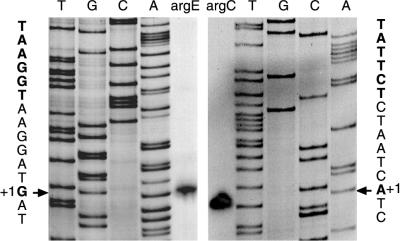
Arginine operons from Moritella strains. The box presents an enlargement of the control region. +1, transcription start site; underlining, putative ARG boxes (operators).
Similarities of derived amino acid sequences for arg genes vary from 88 to 96% for the two Moritella strains. High identities (40 to 70%) were observed with the E. coli homologues of argB, -C, -E, -F, and -H; for argG, however, the level of identity was curiously much lower with homologues from the γ-3 group (28% with E. coli) than from gram-positive bacteria, archaea, eucarya (41 to 44%) and, notably, A. aeolicus (47%), whose genome analysis revealed many genes resembling archaeal genes (11). The presence of argE indicates that Moritella uses the linear pathway for arginine biosynthesis, as confirmed by enzyme assays (see below).
The occurrences of a divergent argEargCBFGH(A) gene cluster and of an extended argH(A) gene are both unprecedented. This pattern is, however, related to those reported for various Enterobacteriaceae (see Discussion).
Putative ribosome binding sites were found at positions compatible with the translation mechanism operating in E. coli. There are 5 nucleotides (nt) between the stop codon of argC and the putative start of argB, 26 between argB and argF, and 13 between argF and argG. It is thus likely that these genes are part of one and the same operon. Between argG and argH there are 110 nt. Several putative −10 elements, but no obvious −35 sequence, were found in this region; little is known of promoter elements in these strains, and the first one to be identified (56) is atypical as regards the −35 region. It is therefore possible that the argH(A) gene is or can be transcribed independently.
Functional analysis of the argE-argC control region.
The presence of DNA transcription signals in the 242-nt region (243-nt region in strain 2693) separating the putative translation start codons of argE and argC was established by primer extension. For argE, a predominant start was identified at a G residue preceded by putative −10 and −35 elements: TAAGGT (or TAAAGT in strain 2 693) and TTCATT, respectively. For argC, transcription was found to start at an A residue in front of the sequences TATTCT and TTGCAT (Fig. 2 and 3). The two promoters face each other as in E. coli (12, 15, 21). In Moritella, however, there is no overlap between the transcripts, whereas in E. coli the overlap is 13 nt long (15). Most of the 242-nt segment is in front of argC; such a long and presumably untranslated sequence is also present in E. coli. Putative operator sequences (Fig. 2) were identified by their similarity to E. coli ARG boxes, i.e., the 18-bp and partly symmetric elements interacting with the E. coli ArgR repressor (15, 30). A close match to the E. coli consensus overlaps the putative argE −35 element. The argC promoter region contains two ARG boxes separated by 3 bp (i.e., the arrangement found in most E. coli arg operators), but the almost completely conserved C residue at position 15 in the right-hand half of the box is either shifted forward by one nucleotide or absent. Since repression was observed in vivo and since there is a gene highly similar to E. coli argR in Moritella (see below), it is likely that at least some of these sequences have a regulatory function.
FIG. 3.
Identification of transcription start sites for the arginine operon by primer extension in strains 2674 and 2693 using RNA extracted from cells grown in AUF medium. No bands were observed in the control without RNA. For argC the sequence shown was identical in both strains. For argE the results shown are from strain 2674; the transcription start in strain 2693 was found to be the same.
Enzyme assays and repression in the native context.
In extracts of cells grown in AUF medium, an AOase activity of 0.25 U/mg of protein could be detected but no OATase activity was detected; i.e., the level was <0.001 U/mg of protein. Thus, OATase activity was less than 0.05, 0.3, and 2% of the activities measured in extracts of Pseudomonas aeruginosa (33), Bacillus stearothermophilus, and Bacillus subtilis (43), respectively. Extracts of Moritella grown at 6°C in AUF medium displayed enough OTCase activity to determine a repression ratio with some accuracy. In an assay conducted at 15°C, OTCase in extracts of cells of strains 2674 and 2693 was found to have specific activities of 199 and 60 U/mg of protein, respectively, in the absence of arginine and 7 U/mg of protein (for both strains) in the presence of arginine. Thus, the synthesis of this enzyme was repressed by arginine to a considerable extent, as in E. coli.
The OTCase assays and the presence of putative ARG boxes in the control region of the operon suggest that the arg genes of Moritella are regulated by a repressor homologous to E. coli ArgR. We were indeed able to retrieve, by PCR and colony hybridization, from the DNA of strain 2674 a fragment harboring an ORF having 70% codon identity with the E. coli argR gene (Xu Ying, unpublished data).
Enzyme assays in recombinant E. coli cells.
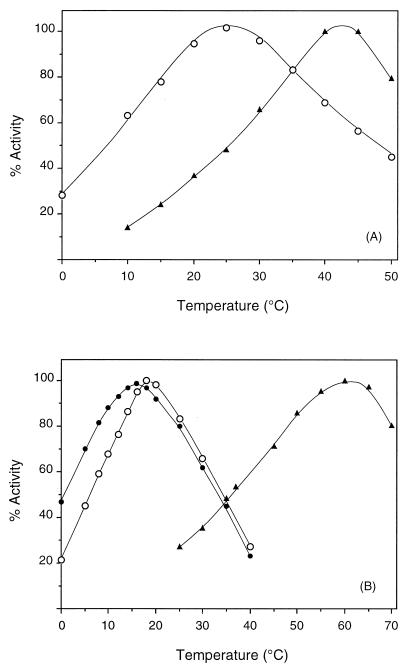
Both AOase and OTCase displayed a distinctly psychrophilic profile, with apparent temperature optima lower than those of their E. coli homologues (Fig. 4). Considerable activity was still observed in the actual temperature range of the organisms (from 0 to 14°C). The lower performance of strain 2674 OTCase at a low temperature appears to be compensated for by a higher specific activity (see above).
FIG. 4.
Comparison of the temperature dependence of AOase and OTCase activities from E. coli and Moritella. (A) Effect of temperature on AOase activity in cell extracts of E. coli (▴) and of E. coli transformed with plasmid pC-28 harboring argE from strain 2693 (○). (B) Effect of temperature on OTCase activity in cell extracts of E. coli (▴) and of E. coli transformed with plasmid pC-11 harboring argF from strain 2693 (●) and plasmid pXY-114 harboring argF from strain 2674 (○). The assays were performed at various temperatures under the conditions described in Materials and Methods. Data are means of at least duplicate measurements; the standard error of the mean was less than 10%.
DISCUSSION
A major objective of this work was to determine whether the genetic organization of arginine biosynthetic genes and the mode of ornithine synthesis in Moritella strains 2674 and 2693 could shed some light on the evolution of the arginine pathway within the γ-3 group of the class Proteobacteria. The data improve our understanding of this process and disclose a new, peculiar type of bifunctional argininosuccinase.
To synthesize ornithine Moritella uses an AOase instead of an OATase, and most of its arg genes are clustered in one continuous sequence divergently transcribed in two wings: argE and argCBFGH(A). This new pattern is related to the argECBH unit of divergent transcription characterized in E. coli (12, 21), the similar cluster found in Salmonella enterica serovar Typhimurium (45) and Providencia strain 9295 (41), and the argECBGH cluster of different Proteus species (41) and Serratia marcescens (32). Other similarities between Moritella and Enterobacteriaceae are the presence of a homologous arginine repressor and the close linkage between ppc and argE.
In Fig. 5 we have combined these data in a schematic and qualitative dendrogram with concurrent phylogenetic information gained by rRNA-DNA homology studies (3), 16S RNA analysis (1, 57), and character state analysis of aromatic amino acid biosynthesis (1). The data suggest that higher degrees of clustering of arg biosynthetic genes correspond to ancestral states in the evolution of this pathway and that the pattern found in Moritella originates from an ancestor common to Vibrionaceae and Enterobacteriaceae.
Preliminary and still-incomplete data obtained from the Institute of Genomics Research (http://www.tigr.org) and also reported in Fig. 5 support this view: in Vibrio cholerae, which appears more closely related to E. coli than Moritella (57), there is a cluster comprising at least the argE, -C, -B, -G, and -H genes. Interestingly, for Shewanella putrefaciens, which is closely related to Moritella, the same source of information mentions an argCBFGH cluster, with the gene responsible for acetylornithine deacylation remaining as yet undefined.
The observation that the argH gene is extended by a stretch of 173 codons able to complement argA mutants emerges as unprecedented in an already wide variety of organisms comprising Eucarya, Archaea, and Bacteria, among which are several Proteobacteria (E. coli, Haemophilus influenzae, Campylobacter jejuni, and Zymomonas mobilis [http://www.ncbi.nlm.nih.gov]). However, preliminary data from the Institute of Genetic Research (see above) suggest that a similar situation may prevail in V. cholerae and S. putrefaciens as well. The ability of this new gene to compensate for a defect in enzymatic acetylation of glutamate is intriguing from both the functional and evolutionary points of view. It should be noted that the origin of glutamate acetylation is far from clear and that it is not impossible that this metabolic function is accomplished in different organisms by unrelated proteins (7, 44). This novel type of argininosuccinase is currently under investigation.
Enterobacterial AOase is thus not anymore an isolated singularity among organisms of the γ group of the class Proteobacteria. The other bacteria screened by genomic analysis and/or analyzed at the enzymatic level belong to 6 of the 11 major subdivisions of their domain, not counting the chlamydiae (Table 3). They all produce an OATase except M. xanthus, a δ-group proteobacterium (19). Putative OATase genes were also reported for several archaea (6, 27, 47). It therefore appears likely that the last common ancestor of the three domains relied on an OATase rather than an AOase.
TABLE 3.
Distribution of OATase and AOase in the major lineages of Bacteria according to 16S RNA-based phylogeny (54)
| Enzyme | Group(s) | Subgroup | Genus |
|---|---|---|---|
| OATase | Hydrogenobacteria | Aquifex (11) | |
| Thermotogales | Thermotoga (35) | ||
| Deinococcus group | Thermus (2) | ||
| Cyanobacteria | Synechocystis (25) | ||
| Gram positives | Streptomyces (20) | ||
| Lactobacillus (5) | |||
| Bacillus (43) | |||
| Corynebacterium (44) | |||
| Proteobacteria | β | Neisseria (31, 40) | |
| γ | Pseudomonas (17, 18) | ||
| AOase | γ | Escherichia (15) | |
| γ | Salmonella (45) | ||
| γ | Serratia (32) | ||
| γ | Providencia (41) | ||
| γ | Proteus (41) | ||
| γ | Moritella (this work) | ||
| δ | Myxococcus (19) | ||
| NRa | Planctomyces, spirochetes, green sulfur bacteria, Cytophagas |
NR, not reported.
Is it possible to infer from available data how ornithine synthesis and the organization of the arginine regulon have evolved among Proteobacteria and in other branches of the Bacteria? As regards ornithine synthesis among γ-3 Proteobacteria, the evidence thus distinguishes Vibrionaceae and enteric bacteria (with an AOase) from P. aeruginosa and closely related species, which have an OATase. On the 16S RNA phylogenetic tree (38), P. aeruginosa branches off at a lower level than H. influenzae (which has no pathway for de novo ornithine synthesis), Vibrio parahaemolyticus, Proteus vulgaris, Erwinia carotovora, and E. coli. OATase may thus have been lost in an organism located near the bifurcation between P. aeruginosa and the branch containing the latter organisms; ornithine synthesis would have been maintained by recruiting (22) internally or acquiring horizontally a deacylase able to split off the acetyl group of N-acetylornithine. AOase itself acts on a variety of N-acetylated compounds (53) and is homologous to other deacylases (4). Since γ- and β-group Proteobacteria appear to have diverged relatively late (38), the presence of an OATase in Neisseria gonorrhoeae (belonging to the β group) suggests that it was maintained in Proteobacteria from their origin as far as the splitting between the γ and β groups. The α branch is not yet documented, and among the earlier-branching ɛ and δ branches the only case known is M. xanthus, which uses an AOase (19); this suggests that the bacterial ancestral OATase gene was lost in this branch as well, much earlier than in the γ group. Further analysis of various Proteobacteria could disclose the exact path followed by the evolution of ornithine synthesis. In particular, it would be interesting to characterize arginine genes in Leucothrix mucor. Indeed, both Vibrionaceae and enteric bacteria possess a class B aspartate carbamoyltransferase, i.e., a dodecameric enzyme constituted by six catalytic and six regulatory subunits, which was detected in Vibrio natriegens (26) and in the two strains analyzed in this work (56). By contrast, fluorescent pseudomonads and Acinetobacter calcoaceticus have a class A ATCase, which has a quite different architecture. As pointed out by Kenny et al. (26), L. mucor may be located near the bifurcation dividing species possessing class A and class B ATCases within the γ group.
Regarding the organization of the arginine regulon in Proteobacteria, we observe on the one hand complete scattering in the fluorescent pseudomonads and Neisseria (from the γ-3 and β groups, respectively) (17, 31, 40) and on the other hand the argECBFGH, ECBGH, and ECBH gene patterns found in Vibrionaceae and Enterobacteriaceae, both from the γ-3 group. In the latter organisms the very presence of an AOase is correlated with integration of argE in a unit of divergent transcription. In M. xanthus, however, argE is located between two unrelated genes (19). In other branches of the bacterial domain we observe other interrelated modes of clustering, such as argCJ in Thermus (2), argCJBD or argCJBDF in several gram-positive organisms (5) (Table 3), and argGHCJBD in Thermotoga maritima (35) and Thermotoga neapolitana (V. Sakanyan, personal communication), which both belong to a primeval line of descent on the 16S RNA tree (38). However, in Aquifex (11), another ancient branch by the 16S RNA criterion, and in Synechocystis (25), a cyanobacterium, the arginine genes are scattered. It thus seems that extensive reorganization of arg genes has accompanied the emergence of major subdivisions of the Bacteria, and, within the Proteobacteria themselves, of their main ramifications. Within the γ group, one important event was the recruitment of the ancestor of AOase. As regards the mechanism controlling the expression of arginine genes, another major event occurred in the branch leading to P. aeruginosa; indeed, this organism uses an argR gene which is not homologous to its functional equivalent in Enterobacteriaceae, gram-positive bacteria, and Moritella (15, 30, 37, 39; also this paper). This may be related to the integration of arginine biosynthesis in the complex nitrogen metabolism of this organism.
In keeping with the strict psychrophily of both Moritella strains, AOase and OTCase present clear-cut cold-adapted temperature activity profiles, with relatively high activities at 0°C. With respect to other cold-active enzymes, many of which were characterized from psychrotolerant rather than strictly psychrophilic hosts (13, 16), the OTCase from Moritella strain 2693 has a comparatively low apparent temperature optimum (17°C). It will therefore be interesting to compare this enzyme with its mesophilic and thermophilic homologues (52). The disclosure of an arginine repressor from a strict psychrophile also calls for structural comparisons between this protein and its homologues operating in the mesophilic and thermophilic ranges (36).
ACKNOWLEDGMENTS
This work was supported by the Belgian Foundation for Joint and Fundamental Research (FKFO), by the Flanders Actionprogramme Biotechnology, by the EC-sponsored programmes Coldzyme and Eurocold, and by grants from the Research Council of the Free University of Brussels (VUB).
REFERENCES
- 1.Ahmad S, Weisburg W G, Jensen R A. Evolution of aromatic amino acid biosynthesis and application to the fine-tuned phylogenetic positioning of enteric bacteria. J Bacteriol. 1990;172:1051–1061. doi: 10.1128/jb.172.2.1051-1061.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baetens M, Legrain C, Boyen A, Glansdorff N. Genes and enzymes of the acetyl cycle of arginine biosynthesis in the extreme thermophilic bacterium Thermus thermophilus HB27. Microbiology. 1998;144:479–492. doi: 10.1099/00221287-144-2-479. [DOI] [PubMed] [Google Scholar]
- 3.Baumann P, Schubert R H W. Vibrionaceae Veron 1965, 5245AL. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams & Wilkins; 1984. pp. 516–517. [Google Scholar]
- 4.Boyen A, Charlier D, Charlier J, Sakanyan V, Mett I, Glansdorff N. Acetylornithine deacetylase, succinyldiaminopimelate desuccinylase and carboxypeptidase G2 are evolutionarily related. Gene. 1992;116:1–6. doi: 10.1016/0378-1119(92)90621-u. [DOI] [PubMed] [Google Scholar]
- 5.Bringel F, Frey L, Boivin S, Hubert J-C. Arginine biosynthesis and regulation in Lactobacillus plantarum: the carA gene and the argCJBDF cluster are divergently transcribed. J Bacteriol. 1997;179:2697–2706. doi: 10.1128/jb.179.8.2697-2706.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bult C J, White O, Olsen G J, Zhou L, Fleischmann R D, et al. Complete genome sequence of the methanogenic archaeon Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 7.Crabeel M, Abadjieva A, Hilven P, Desimpelaere J, Soetens O. Characterisation of the Saccharomyces cerevisiae ARG7 gene encoding ornithine acetyltransferase, an enzyme also endowed with acetylglutamate synthase activity. Eur J Biochem. 1997;250:232–241. doi: 10.1111/j.1432-1033.1997.0232a.x. [DOI] [PubMed] [Google Scholar]
- 8.Crawford I P. Evolution of a biosynthetic pathway: the tryptophan paradigm. Annu Rev Microbiol. 1989;43:567–600. doi: 10.1146/annurev.mi.43.100189.003031. [DOI] [PubMed] [Google Scholar]
- 9.Cunin R, Glansdorff N, Piérard A, Stalon V. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev. 1986;50:314–352. doi: 10.1128/mr.50.3.314-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis R. Compartmental and regulatory mechanisms in the arginine pathways of Neurospora crassa and Saccharomyces cerevisiae. Microbiol Rev. 1986;50:280–313. doi: 10.1128/mr.50.3.280-313.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deckert G, Warren P V, Gaasterland T, Young W G, Lenox A L, et al. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature. 1998;392:353–358. doi: 10.1038/32831. [DOI] [PubMed] [Google Scholar]
- 12.Elseviers D, Cunin R, Glansdorff N, Baumberg S, Ashcroft E. Control regions within the argECBH cluster of Escherichia coli K12. Mol Gen Genet. 1972;117:349–366. doi: 10.1007/BF00333028. [DOI] [PubMed] [Google Scholar]
- 13.Gerday C, Aittaleb M, Arpigny J L, Baise E, Chessa J P, François J M, Garsone G, Petrescu I, Feller G. Cold enzymes: a hot topic. In: Margesin R, Schinner F, editors. Cold-adapted organisms. Ecology, physiology, enzymology and molecular biology. Heidelberg, Germany: Springer; 1999. pp. 257–275. [Google Scholar]
- 14.Glansdorff N. Topography of co-transducible arginine mutations in E. coli K12. Genetics. 1965;51:167–179. doi: 10.1093/genetics/51.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glansdorff N. Biosynthesis of arginine and polyamines. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 408–433. [Google Scholar]
- 16.Gounot A M, Russell N J. Physiology of cold-adapted microorganisms. In: Margesin R, Schinner F, editors. Cold-adapted organisms. Ecology, physiology, enzymology and molecular biology. Heidelberg, Germany: Springer; 1999. pp. 33–55. [Google Scholar]
- 17.Haas D, Holloway B W. The genetic organisation of arginine biosynthesis in Pseudomonas aeruginosa. Mol Gen Genet. 1977;154:7–22. doi: 10.1007/BF00265571. [DOI] [PubMed] [Google Scholar]
- 18.Haas D, Kurer V, Leisinger T. N-Acetylglutamate synthetase of Pseudomonas aeruginosa. An assay in vitro and feedback inhibition by arginine. Eur J Biochem. 1972;31:290–295. doi: 10.1111/j.1432-1033.1972.tb02531.x. [DOI] [PubMed] [Google Scholar]
- 19.Harris B Z, Singer M. Identification and characterization of the Myxococcus xanthus argE gene. J Bacteriol. 1998;180:6412–6414. doi: 10.1128/jb.180.23.6412-6414.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hindle Z, Callis R, Dowden S, Rudd B A M, Baumberg S. Cloning and expression in Escherichia coli of Streptomyces coelicolor A3 (2) argCBJ gene cluster. Microbiology. 1994;140:311–320. doi: 10.1099/13500872-140-2-311. [DOI] [PubMed] [Google Scholar]
- 21.Jacoby G A. Control of the argECBH cluster in Escherichia coli. Mol Gen Genet. 1972;177:337–348. doi: 10.1007/BF00333027. [DOI] [PubMed] [Google Scholar]
- 22.Jensen R A. Enzyme recruitment in evolution of new functions. Annu Rev Microbiol. 1976;30:409–425. doi: 10.1146/annurev.mi.30.100176.002205. [DOI] [PubMed] [Google Scholar]
- 23.Jensen R A. Biochemical pathways can be traced backwards through evolutionary time. Mol Biol Evol. 1985;2:92–108. doi: 10.1093/oxfordjournals.molbev.a040338. [DOI] [PubMed] [Google Scholar]
- 24.Jensen R A. An emerging outline of the evolutionary history of aromatic amino acid biosynthesis. In: Mortlock R P, editor. The evolution of metabolic function. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 205–236. [Google Scholar]
- 25.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 26.Kenny M J, McPhail D, Shepherson M. A reappraisal of the diversity and class distribution of aspartate transcarbamoylases in Gram-negative bacteria. Microbiology. 1996;142:1873–1879. doi: 10.1099/13500872-142-7-1873. [DOI] [PubMed] [Google Scholar]
- 27.Klenk H P, Clayton R A, Tomb J F, White O, Nelson K E, et al. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature. 1997;390:364–370. doi: 10.1038/37052. [DOI] [PubMed] [Google Scholar]
- 28.Labédan B, Boyen A, Baetens M, Charlier D, Chen P, et al. The evolutionary history of carbamoyltransferases: a complex set of paralogous genes was already present in the last universal common ancestor. J Mol Evol. 1999;49:461–473. doi: 10.1007/pl00006569. [DOI] [PubMed] [Google Scholar]
- 29.Liang Z. Physiology and molecular biology of enzymatic carbamoylation in marine psychrophilic bacteria. Ph.D. thesis. Brussels, Belgium: Vrije Universiteit Brussel; 1997. [Google Scholar]
- 30.Maas W K. The arginine repressor of Escherichia coli. Microbiol Rev. 1994;58:631–640. doi: 10.1128/mr.58.4.631-640.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin P R, Mulks M H. Sequence analysis and complementation studies of the argJ gene encoding ornithine acetyltransferase from Neisseria gonorrhoeae. J Bacteriol. 1992;174:2694–2701. doi: 10.1128/jb.174.8.2694-2701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto H, Hosogaya S, Suzuki K, Tazaki T. Arginine gene cluster of Serratia marcescens. Jpn J Microbiol. 1975;19:35–44. doi: 10.1111/j.1348-0421.1975.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 33.Mergeay M, Boyen A, Legrain C, Glansdorff N. Expression of Escherichia coli K-12 arginine genes in Pseudomonas fluorescens. J Bacteriol. 1978;136:1187–1188. doi: 10.1128/jb.136.3.1187-1188.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morita R Y. Psychrophilic bacteria. Bacteriol Rev. 1975;39:144–167. doi: 10.1128/br.39.2.144-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson K E, Clayton R A, Gill S R, et al. Evidence for lateral gene transfer between Archaea and Bacteria from genome sequence of Thermotoga maritima. Nature. 1999;399:323–329. doi: 10.1038/20601. [DOI] [PubMed] [Google Scholar]
- 36.Ni J P, Sakanyan V, Charlier D, Glansdorff N, Van Duyne G. Structure of the arginine repressor from Bacillus stearothermophilus. Nat Struct Biol. 1999;6:427–432. doi: 10.1038/8229. [DOI] [PubMed] [Google Scholar]
- 37.North A K, Smith M C M, Baumberg S. Nucleotide sequence of a Bacillus subtilis arginine regulatory gene and homology of its product to the Escherichia coli arginine repressor. Gene. 1989;80:29–38. doi: 10.1016/0378-1119(89)90247-3. [DOI] [PubMed] [Google Scholar]
- 38.Olsen G J, Woese C R, Overbeek R. The winds of (evolutionary) change: breathing new life into microbiology. J Bacteriol. 1994;176:1–6. doi: 10.1128/jb.176.1.1-6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park S M, Lu C D, Abdelal A T. Cloning and characterization of argR, a gene that participates in regulation of arginine biosynthesis and catabolism in Pseudomonas aeruginosa PAO1. J Bacteriol. 1997;179:5300–5308. doi: 10.1128/jb.179.17.5300-5308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picard F J, Dillon J R. Cloning and organization of seven arginine biosynthesis genes from Neisseria gonorrhoeae. J Bacteriol. 1989;171:1644–1651. doi: 10.1128/jb.171.3.1644-1651.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prozesky O W, Grabow W O K, van der Merve S, Coetzee J M. Arginine gene clusters in the Proteus-Providence group. J Gen Microbiol. 1973;77:327–240. doi: 10.1099/00221287-77-1-237. [DOI] [PubMed] [Google Scholar]
- 42.Rüger H J. Substrate-dependent cold adaptations in some deep-sea sediment bacteria. Syst Appl Microbiol. 1988;11:90–93. [Google Scholar]
- 43.Sakanyan V, Kochikyan A, Mett I, Legrain C, Charlier D, Piérard A, Glansdorff N. A re-examination of the pathway for ornithine biosynthesis in a thermophilic and two mesophilic Bacillus species. J Gen Microbiol. 1992;138:125–130. [Google Scholar]
- 44.Sakanyan V, Petrosyan P, Lecocq M, Boyen A, Legrain C, Demarez M, Hallet J N, Glansdorff N. Genes and enzymes of the acetyl cycle of arginine biosynthesis in Corynebacterium glutamicum: enzyme evolution in the early steps of the arginine pathway. Microbiology. 1996;142:99–108. doi: 10.1099/13500872-142-1-99. [DOI] [PubMed] [Google Scholar]
- 45.Sanderson K E, Roth J R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988;52:485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith D R, Doucette-Stamm L A, Deloughery C, Lee H, Dubois J, et al. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genomics. J Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 49.Stalon V, Ramos F, Piérard A, Wiame J M. Regulation of the catabolic ornithine carbamoyltransferase of Pseudomonas fluorescens. A comparison with the anabolic transferase and with a mutationally modified catabolic transferase. Eur J Biochem. 1972;29:25–35. doi: 10.1111/j.1432-1033.1972.tb01953.x. [DOI] [PubMed] [Google Scholar]
- 50.Treizenberg S J. Primer extension. In: Ausubel F M, et al., editors. Current protocols in molecular biology. I. New York, N.Y: Wiley; 1995. pp. 4.8.1–4.8.5. [Google Scholar]
- 51.Van de Casteele M, Demarez M, Legrain C, Glansdorff N, Piérard A. Pathways of arginine biosynthesis in extreme thermophilic archaeo- and eubacteria. J Gen Microbiol. 1990;136:1177–1183. [Google Scholar]
- 52.Villeret V, Clantin B, Tricot C, Legrain C, Roovers M, Stalon V, Glansdorff N, Van Beeumen J. The crystal structure of Pyrococcus furiosus ornithine carbamoyltransferase reveals a key role for oligomerization in enzyme stability at extremely high temperatures. Proc Natl Acad Sci USA. 1998;95:2801–2806. doi: 10.1073/pnas.95.6.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vogel H J, McLellan W L. Acetylornithinase (Escherichia coli) Methods Enzymol. 1970;17A:265–269. [Google Scholar]
- 54.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woese C R, Weisberg W G, Hahn C M, Paster B J, Zablen L B, Lewis B J, Macke T J, Ludwig W, Stackebrandt E. The phylogeny of purple bacteria: the γ subdivision. Syst Appl Microbiol. 1985;6:25–33. [Google Scholar]
- 56.Xu Y, Zhang Y F, Liang Z, Van de Casteele M, Legrain C, Glansdorff N. Aspartate carbamoyltransferase from a psychrophilic deep-sea bacterium, Vibrio strain 2693: properties of the enzyme, genetic organisation and synthesis in Escherichia coli. Microbiology. 1998;144:1435–1441. doi: 10.1099/00221287-144-5-1435. [DOI] [PubMed] [Google Scholar]
- 57.Yanagibayashi M, Nogi Y, Li L, Kato C. Changes in the microbial community in Japan Trench sediment from a depth of 6292 m during cultivation without decompression. FEMS Microbiol Lett. 1999;170:271–279. doi: 10.1111/j.1574-6968.1999.tb13384.x. [DOI] [PubMed] [Google Scholar]