Abstract
Hepatic fibrosis (HF) refers to the pathophysiological process of connective tissue dysplasia in the liver caused by various pathogenic factors. Nowadays, HF is becoming a severe threat to the health of human being. However, the drugs available for treating HF are limited. Currently, increasing natural agents derived from traditional Chinese medicines (TCMs) have been found to be beneficial for HF. A systemic literature search was conducted from PubMed, GeenMedical, Sci-Hub, CNKI, Google Scholar and Baidu Scholar, with the keywords of “traditional Chinese medicine,” “herbal medicine,” “natural agents,” “liver diseases,” and “hepatic fibrosis.” So far, more than 76 natural monomers have been isolated and identified from the TCMs with inhibitory effect on HF, including alkaloids, flavones, quinones, terpenoids, saponins, phenylpropanoids, and polysaccharides, etc. The anti-hepatic fibrosis effects of these compounds include hepatoprotection, inhibition of hepatic stellate cells (HSC) activation, regulation of extracellular matrix (ECM) synthesis & secretion, regulation of autophagy, and antioxidant & anti-inflammation, etc. Natural compounds and extracts from TCMs are promising agents for the prevention and treatment of HF, and this review would be of great significance to development of novel drugs for treating HF.
Keywords: natural products, hepatic fibrosis, hepatic stellate cells, extracellular matrix, hepatoprotection, mechanism, traditional Chinese medicines, Fufang-Biejia-Ruangan pill
Introduction
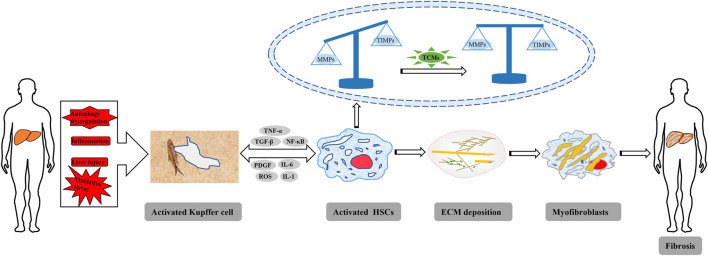
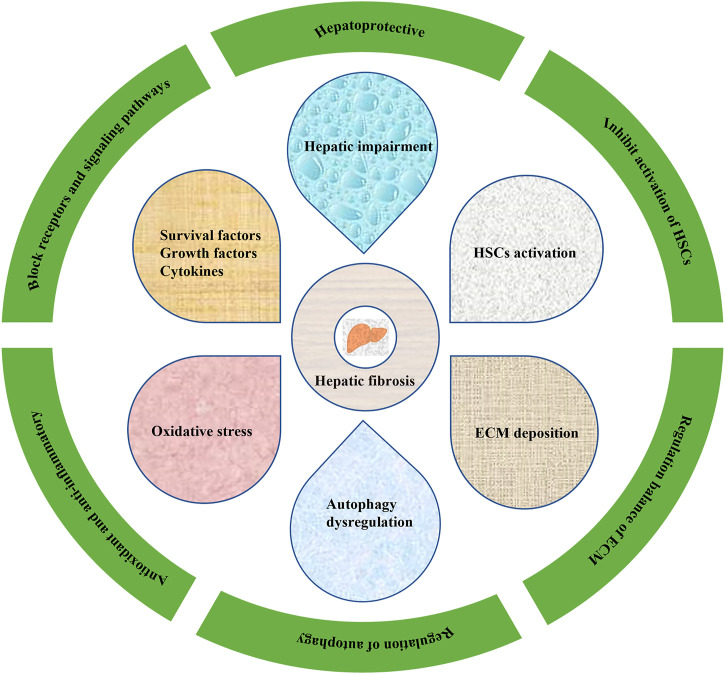
Hepatic fibrosis (HF) refers to abnormal hyperplasia of connective tissue in the liver caused by various pathogenic factors, and is a pathophysiological process of the repair of chronic liver injury. HF is a key step in the development of various chronic liver diseases to cirrhosis (Zhang C. Y. et al., 2016; Parola and Pinzani, 2019), and activation of HSCs is the central link in the development of HF, which is characterized by excessive and abnormal deposition of ECM in the liver (Chen S. R. et al., 2015; Povero et al., 2015) (Figure 1). In fact, HF is reversible (Roeb, 2018), and liver injury commonly has a process of HF in the process of liver repair and healing. However, if the injury factors cannot be removed for a long time, HF will develop into irreversible cirrhosis or even liver cancer (Tao et al., 2020). Liver cancer is one of the most commonly ones with high morbidity and mortality, and the epidemic investigations also reported that liver cancer is one of the currently leading causes of cancer-related death in the world (Mehta et al., 2015; Xiao et al., 2017). According to statistical analyses, approximately one million deaths annually were caused by liver cancer (Rawat et al., 2018; Martínez-Chantar et al., 2020). Therefore, it is important to treat HF before it evolves into cirrhosis or even liver cancer. However, nowadays, no specific therapeutic drugs could be used in clinical for treating HF, and western drugs don’t have good curative effects on HF but often result in some adverse reactions (Daniyal et al., 2019; Shan et al., 2019; Ma et al., 2020). Natural products have a general health promoting effect on the prevention and treatment of different diseases, especially liver ones. Polydatin, an active monomer from the Poygonum cuspidatum Sieb.et Zucc. (Polygonaceae), can protect hepatocytes from oxidative injury and ameliorate liver damage induced by carbon tetrachloride in rats (Peng et al., 2013). Hawthorn herbal preparation from Crataegus oxyacantha (Rosaceae) attenuates carbon tetrachloride-induced hepatic fibrosis in vivo via modulating oxidative stress and inflammation (Hamza et al., 2020). TCMs are derived from animals, plants and mineral agents with certain pharmacological activities, and herbal medicines and their formulas are dominant (Shan et al., 2019). TCMs have attracted much attention due to their unique advantages, such as wide sources, lower prices, few side effects, etc. Previous evidence suggests that many agents derived from TCMs could be beneficial for the treatment or prevention of HF (Shan et al., 2019). So far, more than 76 natural compounds with anti-hepatic fibrosis activities have been isolated and identified from TCMs, including alkaloids, flavones, quinones, terpenoids, saponins, phenylpropanoids, and polysaccharides, etc. The anti-hepatic fibrosis effects of these compounds include hepatoprotection, inhibition of hepatic stellate cells (HSC) activation, regulation of extracellular matrix (ECM) synthesis & secretion, regulation of autophagy, and antioxidant & anti-inflammation, etc. (Figure 2). In the present review, we summarized the potential anti-hepatic fibrosis components from TCMs and their possible molecular mechanisms, which would be of great significance to develop novel drugs against hepatic fibrosis.
FIGURE 1.
Schematic representation of the pathogenesis of HF.
FIGURE 2.
Pharmacological effects of TCMs on HF.
Pathogenesis of hepatic fibrosis
HSCs-mediated hepatic fibrosis
Hepatic stellate cells (HSCs) are a kind of non-parenchymal cells of liver, located in the disse space, accounting for 5–10% of total of hepatocytes, and maintain close interaction with hepatic sinusoidal endothelial cells and hepatocytes (Puche et al., 2013; de Oliveira da Silva et al., 2017). HSCs are the most important source cells of extracellular matrix (ECM) in HF, and activation of HSCs is a key and central step in the development of HF (Cai et al., 2020). In normal liver, HSCs are in a quiescent state without α-smooth muscle actin (α-SMA) secretion, have low proliferative activity and low ability to synthesize collagen, and their main functions are to store and metabolize VitA lipid droplets. When the liver is damaged by inflammation or mechanical stimulation, HSCs could be activated, and their phenotype changes from “quiescent” to “active” (Dewidar et al., 2019).
HSC activation includes two main stages: initiation phase and persistence phase. The priming stage refers to the changes in gene expression in early stage and in cell phenotype caused by stimulating factors such as cytokines. When liver parenchymal cells are injured, cytokines are created by the neighboring hepatocytes, Kupffer cells through paracrine action, such as tumor necrosis factor α (TNF-α), transforming growth factor β (TGF-β), insulin growth factor (IGF), hepatocyte growth factor (HGF), and platelet-derived growth factor (PDGF), etc. (Tsuchida and Friedman, 2017), which can significantly promote the activation, division, proliferation, transformation of HSCs into myofibroblasts (MFBs) to synthesize ECM. Furthermore, activated HSCs can also secrete cytokines such as TNF, TGF and PDGF to sustain the activation, leading to further aggravation of HF. Persistent phase refers to the maintenance of HSC activation due to the role of above factors, increasing the deposition of ECM, resulting in HF. Thus, HSC activation is regulated by both autocrine and paracrine at this stage (Chen et al., 2008; Wallace et al., 2015; de Oliveira da Silva et al., 2017; Tsuchida and Friedman, 2017; Barcena-Varela et al., 2019). Obviously, activation of HSC is the cytological basis of HF.
ECM-mediated hepatic fibrosis
Extracellular matrix (ECM) is mainly composed of collagen, non-collagen glycoproteins (including fibronectin, laminin, etc.), elastin, proteoglycans and aminoglycans, whose intercellular adhesion structure is interstitial connective tissue. The formation of HF is due to the deposition of large amounts of ECM in the liver, and HSC activation is the main cause of ECM production (Seki and Brenner, 2015; Zhao et al., 2019). In normal liver, the synthesis and degradation of ECM mainly composed of collagens IV and VI maintain a dynamic balance, which regulates cell growth and metabolic activities. When the liver is damaged, the synthesis of ECM is greater than degradation. ECM proliferation leads to a large amount of collagen deposition. The original collagens IV and VI are replaced by collagen I, III and fibronectin to promote the formation of fibrosis. On the other hand, ECM stimulates HSC activation and proliferation. Activated HSCs can produce matrix metalloproteinases (MMP) -9 and -13, breaking the balance mechanism between MMPs and tissue inhibitors of metalloproteinases (TIMPs), which leads to partial hydrolysis of ECM deposited in large amount, reducing the occurrence of fibrosis (Nunes de Carvalho et al., 2013; Motawi et al., 2014; Karsdal et al., 2015). MMPs are the main enzymes degrading ECM and can specifically hydrolyze collagen and other matrix proteins, while TIMPs can prevent ECM degradation and promote the formation of fibrosis by inhibiting the activity of MMPs (Pinlaor et al., 2010; Baig et al., 2016). Therefore, regulating the balance of MMPs/TIMPs is essential for the degradation of ECM.
Autophagy-mediated hepatic fibrosis
Autophagy is a process of degrading excess or damaged organelles or other components in the cell with the participation of lysosomes, is essential for cell survival, differentiation, growth, and homeostasis (Tao et al., 2020). Autophagy not only regulates hepatocyte functions but also impacts on non-parenchymal cells, such as endothelial cells, macrophages and hepatic stellate cells (Allaire et al., 2019). Dysregulation of autophagy has been implicated in many liver diseases, and its modulation has also been suggested as a potential new therapeutic strategy for hepatic fibrosis. Studies have shown that autophagy has important regulatory effects on hepatic stellate cells (HSCs) energy metabolism, and then affects the activation state of HSCs (Hou et al., 2022), and further affects the progression of hepatic fibrosis. It is reported that, on the one hand, autophagy has a direct promoting effect on liver fibrosis because it could participate in the digestion of lipid droplets and provide energy for the activation of HSCs; on the other hand, autophagy could inhibit the emergence of hepatic fibrosis through anti-inflammatory effects (Sun et al., 2021). Undoubtedly, autophagy is seen in both physiological and pathological processes of the organism, and whether the role it plays is positive or negative has not been fully elucidated, and research on hepatic fibrosis, in particular, is of concern.
Oxidative stress-mediated hepatic fibrosis
Oxidative stress refers to a state in which reactive oxygen species (ROS) are produced and/or the antioxidant defense function is weakened in the body or cells during the transition, resulting in a serious imbalance between the two and tissue and cell damage (van der Pol et al., 2019). When the liver is injured, firstly, ROS production increases and clearance decreases in the liver tissue, Kupffer cells are activated to release many inflammatory mediators and cytokines (Gong and Yang, 2020). On the other hand, chemokines secreted by Kupffer cells recruit other immune cells to the site of injury to aggravate inflammatory injury, which can directly stimulate HSCs activation and proliferation (Ghatak et al., 2011) through autocrine and paracrine, resulting in a large deposition of ECM, thereby promoting the formation of hepatic fibrosis (Yang J. J. et al., 2013; Richter et al., 2015).
Hepatic fibrosis mediated by cytokines and signaling pathways
TGF-β signaling pathway
TGF-β, an activator of MFBs, is a central regulator of chronic liver disease and potent profibrotic cytokine (Deng et al., 2012; Fabregat et al., 2016). When the liver is damaged, Kupffer cells produce TGF-β in a paracrine manner, causing HSC activation. Activated HSCs can also secrete a large amount of TGF-β, which continues to stimulate the active expression of TGF-β, forming a step-by-step amplification effect (Dooley and ten Dijke, 2012). When activated TGF-β binds to the membrane receptor in HSC, TGF-β receptor activates and phosphorylates R-smad protein that binds to this receptor. Activated R-smad forms heteropolymers with co-Smad protein shared by the cytoplasm and translocated into the nucleus. Smad protein regulates transcription through target gene, thereby triggering the overexpression of fibrotic genes. Many studies have shown that TGF-β1/Smad-2 and Smad-3 are major downstream regulators promoting TGF-β-mediated HF, while Smad-7 is a negative feedback regulator of the TGF-β1 signaling pathway which has a protective effect on TGF-β-mediated HF (Xu et al., 2016; Hu H. H. et al., 2018; Dewidar et al., 2019).
NF-κB signaling pathway
NF-κB is an important nuclear transcription factor in cells (Robinson and Mann, 2010), and a major regulator of inflammatory response and apoptosis (Sun and Karin, 2008). In HF, NF-κB can be activated by LPS, TNF and IL-1β, leading to enhanced TGF-β signaling and HSC activation, while NF-κB secretion of macrophage recruitment chemokines and its anti-apoptotic effect can promote the formation of fibrosis (Elsharkawy and Mann, 2007; Tacke et al., 2009). On the other hand, NF-κB plays an anti-fibrotic role by inhibiting the transcription of COL1a1 gene (Zhang Y. Z. et al., 2019). Therefore, NF-κB may act as both pro-fibrotic and anti-fibrotic effects.
TLR4 signaling pathway
TLR4 is an important regulator of hepatic inflammatory response in chronic injury (Roy et al., 2016). In the liver, HSC has a complete TLR4 signaling pathway. TLR4 can be activated by LPS and HMGB1, and the activated TLR4 could induce downregulation of TGF-β pseudo receptor BAMBI through the downstream NF-κB signaling pathway, leading to increased activity of TGF-β1 (Roh and Seki, 2013; Li Z. et al., 2019; Nakanishi et al., 2019; Dong Z. et al., 2020). Meanwhile, TLR4 activation in HSCs promotes the recruitment of Kupffer cells, which in turn promotes the release of TGF-β, leading to the activation of HSCs and the formation of fibrosis (Pradere et al., 2010; Gao et al., 2011).
Wnt/β-catenin signaling pathway
The Wnt signaling pathway is a signal transduction one that activates downstream channels by binding of ligand protein Wnt and membrane protein receptors, enabling extracellular signals to be transmitted into cells (Thompson and Monga, 2007). In the classical Wnt signaling pathway, its membrane receptor is β-catenin, so it is also called Wnt/β-catenin signaling pathway (Huang et al., 2018). The canonical Wnt signaling pathway is active in HSCs, which promotes the formation of fibrosis by inducing the activation and survival of HSCs, leading to increased expression of type I collagen and α-SMA (Wang J. N. et al., 2018; Nishikawa et al., 2018; Li L. Y. et al., 2019; Rong et al., 2019).
PDGF signaling pathway
Platelet-derived growth factor (PDGF) is the most effective one to stimulate HSC proliferation, differentiation, and migration (Ying et al., 2017). PDGF also promotes collagen production and deposition, and converts HSCs into MFBs. The receptor of PDGF (PDGFR) belongs to the protein tyrosine kinase receptor family and has the function of protein tyrosine kinase, mainly located in vascular endothelial cells, fibroblasts and Kupffer cells (Borkham-Kamphorst and Weiskirchen, 2016). When liver injury occurs, hepatocytes release excessive PDGF, which binds to and activates PDGFR to initiate a variety of protein cascade phosphorylation signal transduction pathways, thereby causing a cellular response. The main downstream signaling pathways of PDGF include: 1) Ras/ERK/MAPK pathway; 2) AKT/mTOR/p27 pathway; 3) JAK/STAT pathway; 4) Ca2+ pathway (Kocabayoglu et al., 2015; Borkham-Kamphorst and Weiskirchen, 2016; Ying et al., 2017). Collectively, PDGF continuously activates and proliferates through autocrine and paracrine mechanisms, which leads to mitosis of HSCs and excessive deposition of ECM, promoting the formation of fibrosis.
Effects of extracts/monomers from TCMs against hepatic fibrosis
Hepatoprotective
Liver injury can be caused by a variety of reasons, including alcohol, drug poisoning, autoimmune overreaction, etc. In the process of liver injury, activated HSC could produce excessive ECM and promote the formation of fibrosis (Hu et al., 2020a). Therefore, search for effective hepatoprotective agents has attracted extensive attention (Li et al., 2020; Wu et al., 2020). It has been reported that some monomers extracted from TCMs have hepatoprotective effects, such as glycyrrhetinic acid, curcumin, emodin, baicalin and some plant polysaccharides (Stanković et al., 2017), which can reduce the occurrence of fibrosis by protecting the liver. Chen et al. (Chen et al., 2013) found that glycyrrhetinic acid (25, 50, 100 mg/kg) might enhance the expression of its target genes and the activity of antioxidant enzymes by activating the nuclear translocation of Nrf2 to protect the liver of mice from oxidative stress. It can be used as a feasible candidate drug to prevent HF. Kong et al. (Kong et al., 2020) found that curcumin (100, 200, 400 mg/kg) could blunt the epithelial-mesenchymal transition (EMT) of hepatocytes to reduce the occurrence of HF.
Inhibiting activation and promoting apoptosis in HSC
Activation of HSCs is a major event in the pathogenesis of HF (Jin et al., 2016), and inhibiting activation and proliferation of HSC is a possible step in the intervention of HF. Following observing the effect of conophylline (0.9 μg/g) on the expression of α-SMA and collagen I in rat HSCs. Kubo et al. (2014) found that conophylline reduced the expression of α-SMA and collagen-1, thereby inhibiting HSC activation. Ligustrazine (50, 100, 200 mg/kg) inhibited the production, migration, metabolism, and contraction of angiogenic factors in HSCs (Zhang et al., 2018). Artesunate (50, 100, 200 mg/kg) significantly induced apoptosis of activated HSCs in CCl4-induced mouse hepatic fibrosis (Kong et al., 2019). Consistent with the experimental results in vivo, artesunate treatment markedly repressed HSC activity, raised cell mortality, increased lipid peroxides and reduced antioxidant capacity in vitro.
Regulation synthesis, secretion and degradation of ECM
Overaccumulation of ECM is the initial stage of HF, and HSC activation is a central event in the pathogenesis of HF (Xie et al., 2021). Therefore, regulation of the synthesis and degradation of ECM is of great significance for the treatment of HF. Oroxylin A (20, 30, 40 mg/kg) could significantly inhibit the deposition of ECM in CCl4-induced mouse HF model, suggesting the possibility of oroxylin A for the treatment of HF (Chen et al., 2018). In the experiment of CCl4-induced hepatic fibrosis in rats, tanshinone IIA (2.5, 5.0, 10 mg/kg) significantly improved liver function, reduced liver injury, decreased ECM accumulation, inhibited HSC proliferation and activation, thus improving fibrosis (Shi et al., 2020).
Regulation of autophagy
Numerous studies suggest a key role of autophagy dysbiosis in the progression of hepatic fibrosis. MFBs are considered to be the main products of HSCs, and their massive production and activation are key to the development of hepatic fibrosis (Kisseleva and Brenner, 2021). More recently, in a model of hepatic fibrosis in rats injected intraperitoneally with CCl4, Kong et al. recently provided evidence that curcumin (100, 200, 400 mg/kg) inhibited TGF-β/Smad signaling transmission by activating autophagy, thereby inhibiting EMT, further inhibiting the production of MFBs to protect against hepatic fibrosis (Kong et al., 2020). In addition, in vitro, curcumin (10, 20, 30 μM/L) suppressed levels of ROS and oxidative stress in hepatocytes, and modulated upstream signaling pathways of autophagy AMPK and PI3K/AKT/mTOR, leading to an increase of the autophagic flow in hepatocytes (Kong et al., 2020). Furthermore, studies have also revealed the role of autophagy in CCl4-induced hepatic fibrosis, and to further examine the molecular mechanisms after treatment oroxylin A, at the same time leading to a decrease in the levels of gene expression of the main actors of liver damage, such as ALP, AST, ALT, TGF-β1, TNF-α, α-SMA, and the expression of autophagy makers, such as LC3-B, Atg3, Atg4, Atg5, Atg6, Atg7, Atg9, Atg12, Atg14 Beclin1, and p62, etc. was significantly up-regulated, consequently, oroxylin A is required to activate autophagy to attenuate hepatic fibrosis and HSCs activation (Chen et al., 2018). Hence, based on these studies, we reasonably point to the possibility of using natural drugs to treat hepatic fibrosis by regulating autophagy.
Antioxidant and anti-inflammatory effects
Oxidative stress can create a vicious circle of fibrous deposition, and persistent inflammation is an initiation and maintenance factor of HF (Ruart et al., 2019; Bai et al., 2020). Therefore, antioxidation and inhibition of inflammation can effectively improve HF. The natural compouds exerting antifibrosis effect through antioxidation and anti-inflammation mainly include acanthopanax senticosus alkaloid A, berberine, betaine, astragaloside IV, breviscapine, rhein, oxymatrine, cucurbitacin B, and so on. For example, berberine (50 mg/kg) has antioxidant and anti-inflammatory activities and is a potential drug for the treatment of HF (Eissa et al., 2018). Astragaloside IV (50 mg/kg) inhibited the activation of HSC by inhibiting oxidative stress and p38 MAPK pathway, thus achieving the anti-hepatic fibrosis effect (Li et al., 2013). Breviscapine (15, 30 mg/kg) attenuated CCl4-induced liver injury in mice by inhibiting inflammatory apoptotic response and ROS production (Liu Y. et al., 2018). In addition, nonalcoholic steatohepatitis (NASH) is characterized by histological lobular inflammation and hepatocyte ballooning, the active form of non-alcoholic fatty liver disease NAFLD, and is associated with disease progression, development of fibrosis, eventually, cirrhosis and hepatocellular carcinoma (Castera et al., 2019). A mouse model of NASH was established by feeding a high-fat/high-cholesterol diet, for 16 weeks, and breviscapine (15 and 30 mg/kg) was administered daily by oral gavage after 8 weeks of treatment, significantly reduced lipid accumulation, inflammatory cell infiltration, liver injury, and fibrosis in mice. Further RNA-sequencing and multiomics analyses indicated that breviscapine inhibited TGF-β-activated kinase 1 (TAK1) -dependent signaling to alleviate NASH (Lan et al., 2022). Moreover, in vitro and in vivo results of the experiment showed that gastrodin (25, 50, 100, 200 μg/ml; 10, 20, 50 mg/kg) activated the AMPK/Nrf2 pathway, ameliorated hepatic oxidative stress/proinflammatory response and significantly improved metabolic disorders in NAFLD (Qu et al., 2016). These findings will provide scientific basis for the clinical application of natural botanical compounds to treat HF in the future.
Blockade of receptors and signaling pathways
TGF-β, PDGF and PPARγ are important fibrogenic factors, and blocking the binding of these cytokines to HSC membrane receptors or inactivating their signal transduction pathways can all achieve anti-fibrotic effects (Zhang et al., 2013; Borkham-Kamphorst and Weiskirchen, 2016; Frangogiannis, 2020; Sharma et al., 2020). For example, caffeine (37.5 mg/kg) inhibited the formation of HF by inhibiting the expression of TGF-β-stimulated connective tissue growth factor (CTGF) in hepatocytes through PPARγ and Smad2/3 pathways (Gressner et al., 2008). A rat model of liver injury was established and treated with different doses of baicalin (25, 50, 100 mg/kg). The results showed that baicalin reversed the biochemical indicators of liver injury, inhibited the upregulation of TGF-β1 expression, and enhanced the expression of PPARγ (Qiao et al., 2011).
Traditional applications of TCMs against hepatic fibrosis
In traditional Chinese medicine system, the HF belongs to the scopes of “hypochondriac pain,” “jaundice,” “liver impediment” and “tympanites” and commonly induced by “deficiency of Qi and blood stasis,” “liver Qi stagnation,” “moist-heat collecting in the splenetic and stomach systems” and “damp abundance due to splenic asthenia,” etc. (Zhang X. Y. et al., 2019; Xu, 2020). In China, TCMs have been comprehensively used for treating HF for thousands of years based on the functions of “promoting blood circulation and removing blood stasis,” “tonifying Qi,” “soothing liver and strengthening spleen” and “clearing away heat and toxic material,” etc. (Duan et al., 2020; Xu, 2020). The commonly used TCMs include the Danshen (roots of Salvia miltiorrhiza Bge., Lamiaceae), Gancao (roots of Glycyrrhiza uralensis Fisch., Leguminosae), Banxia (roots of Pinellia ternata (Thunb.) Breit., Araceae), Renshen (roots of Panax ginseng C.A.Mey., Araliaceae), Huangqin (roots of Scutellaria baicalensis Georgi, Lamiaceae), Shengjiang (roots of Zingiber officinale Rosc., Zingiberaceae), Baizhu (roots of Atractylodes macrocephala Koidz., Asteraceae), and Yujin (roots of Curcuma wenyujin Y.H. Chen et C. Ling, Zingiberaceae), etc. (Duan and Wang., 2020). TCMs are often applied in clinical to treat diseases in the form of formula, and importantly, TCM formula is also recognized as one of promising therapies against HF due to its feature of “multiple components, multiple targets and multiple pathways” (Li, 2020; Sun, 2020).
The Fufang-Biejia-Ruangan pill (FBRP) is the first clinically approved classic Chinese herbal formula against fibrosis in China (Zhang et al., 2020). In CCl4-induced hepatic fibrosis rat model, daily orally administration with FBRP, at doses of 0.55 g/kg, for 8 weeks, decreased the serum levels of ALT, AST, PCIII, HA, LN and IV-C, alleviated the level of infiltration of leukocytes, necrosis, bile duct proliferationa, and collagen deposition as well as downregulated reduced the protein expression of TGF-β1 and Smad3 in the rat liver (Yang F. R. et al., 2013). Further in vitro investigations demonstrated that FBRP significantly suppressed HSC-LX-2 cell proliferation and reduced hydroxyproline content (Yang F. R. et al., 2013). These results indicated that FBRP shows significant anti-hepatic fibrosis effects via inhibition of TGF-β/Smad signaling pathway may be an underlying mechanism. Moreover, in vivo model of diethylnitrosamine (DEN) induced hepatocellular carcinoma (HCC) in rats, daily orally administrated with FBRP at doses of 1 g/kg, for 18 weeks, effectively reduced the serum levels of ALT, AST, ALP, TP, HA and alpha fetoprotein (AFP), significantly suppressed the liver tissues expression of p-PI3K, p-AKT, p-IKkB, and p-NF-κB, as well as the ratios of p-IKkB/total IKkB and p-NF-κB/total NF-κB, suggested FBRP may be a promising candidate drug for reduction of fibrogenesis and prevention of HCC via blocking PI3K/AKT/NF-κB activation (Zhang et al., 2020). Meanwhile, in vitro, Huh7 cells were treated with 5.0 and 10.0 mg/ml FBRP for 24 h, suppressed HCC cell proliferation and induced cell cycle arrest, obviously downregulated the expression of related factors in the PI3K/AKT/NF-κB signaling pathway (Zhang et al., 2020). Notably, network-based pharmacological strategies, Zhang et al. (2020) also provided a reasonable data for unveiling that FBRP may block PI3K/AKT/NF-κB activation, further highlighting the multi-target anti-HCC. Similarly, in vivo, orally administrated with FBRP, at doses of 0.625 g/kg, once a day during the 7 weeks, detection of biochemical indicators, fibrosis-related index and the MAPK pathway involved factors at the end of experiment. The results showed that FBRP dramatically the serum level of ALT, AST, HA, LN, IV-C and PCIII, significantly reduced the levels of α-SMA, TNF-α, IL-13, p-p38, p-ERK both in serum and liver tissues in rats (Cai et al., 2010).
Besides, in vivo model of CCl4-induced hepatic fibrosis in rats, daily orally administration with FZHY formula, at doses of 4.6 g/kg, for 9 weeks, alleviated the hepatic lobular distortion and collagen fiber accumulation, improved the hepatocellular steatosis and ballooning. Moreover, FZHY dramatically elevated the expression of Hexokinase 2 (Hk2) and glutamine synthetase (Gs), regulated the both glucose metabolism and amino acid metabolism as well as reduced dehydrogenase 1 (Adh1), acetyl-CoA synthetase 2 (Acss2) and glutamic-pyruvictransaminase (Gpt) (Hu et al., 2019), suggested FZHY has good anti-hepatic fibrosis effect via altering the metabolic pathways. Fu et al. (2021) found a novel Chinese medicine, namely JY5 formula, composed of salvianolic acid B, schisantherin A and amygdalin, the main active ingredients of FZHY, and evaluated its anti-hepatic fibrosis activity both in vitro and in vivo. In the CCl4-and BDL-induced hepatic fibrosis rat experiment, after treatment with JY5 (salvianolic acid B: 16 mg/kg, schisantherin A: 2 mg/kg, amygdalin: 0.5 mg/kg), significantly alleviated hepatic injury and collagen deposition, the expressions of Jagged1, Notch2, Notch3, Notch4 and recombination signal binding protein-κB (RBP-κB) were significantly decreased. Meanwhile, consistent results were obtained in CCl4-induced hepatic fibrosis in mice, after JY5 (salvianolic acid B, 16 mg/kg amygdalin, 0.5 mg/kg schisantherin A, 2 mg/kg) treatment (Fu et al., 2021). These results suggest that JY5 could significantly inhibit the activation of Notch signaling pathway in CCl4-and BDL-induced liver fibrosis. Moreover, in vitro results showed, compared to the TGF-β1-induced activation of hepatic stellate cell line (LX-2) group, after treatment with JY5, at concentrations of 6.587, 13.174 and 26.348 μg/ml, the mRNA levels of α-SMA, Col-I, Jagged1, Notch2, Notch3 and RBP-кB were significantly reduced, the expression above these proteins were significantly reduced in the JY5-treated groups, and the high-dose JY5 group more significantly (Fu et al., 2021). The commonly clinically used TCM formulas against HF were displayed in Table 1.
TABLE 1.
Traditional Chinese medicine formulas with anti-hepatic fibrosis effect.
| TCM formula | Compositions | Mechanisms | Refs |
|---|---|---|---|
| Fufang-Biejia-Ruangan pill | Carapace of Trionyx sinensis Wiegmann; Roots of Paeonia lactiflora Pall.; Cordyceps sinensis (BerK.) Sacc.; Roots of Panax notoginseng (Burk.) F.H.Chen; Hominis placenta; Fruits of Forsythia suspensa (Thunb.) Vahl; Roots of Angelica sinensis (Oliv.) Diels; Roots of Curcuma phaeocaulis Val.; Roots of Codonopsis pilosula (Franch.) Nannf.; Roots of Astragalus membranaceus (Fisch.) Bge.; Roots of Isatis indigotica Fort. | Inhibiting activation of TGF-β/Smad signaling; Inhibiting activation of PI3K/AKT/NF-κB signaling; Downregulating MAPK pathway | Cai et al. (2010), Feng-Rui Yang et al. (2013), Zhang et al. (2020) |
| Fuzheng Huayu formula | Roots of Salvia miltiorrhiza Bge.; Seeds of Prunus persica (L.) Batsch; Whole herb of Gynostemma pentaphyllum (Thunb.) Makino; Fruits of Schisandra chinensis (Turcz.) Baill., Cordyceps sinensis (BerK.) Sacc. | Altering the metabolic pathways and regulating gene expression of involved metabolic enzymes; Inhibiting the Notch signaling pathway | Hu et al. (2019), Fu et al. (2021) |
| Yinchenhao decoction | Whole herb of Artemisia capillaris Thunb.; Fruits of Gardenia jasminoides Ellis; Roots of Rheum palmatum L. | Regulating bile acid metabolism and TGF-β/Smad/ERK signaling pathway | Cai et al. (2018) |
| PienTze Huang | Gallstone of Bos taurus domesticus Gmelin Cow bezoar; Secretion of Moschus berezovskii Flerov; Roots of Panax notoginseng (Burk.) F.H.Chen; Gallbladder of Zaocys dhumnades Contor | Suppressing NF-κB pathway and promoting HSC apoptosis; Enhancing the immune process | Zheng et al. (2019), Zhu et al. (2021) |
| Ganshuang granule | Roots of Codonopsis pilosula (Franch.)Nannf.; Roots of Bupleurum chinense DC.; Roots of Paeonia lactiflora Pall.; Roots of Angelica sinensis (Oliv.) Diels; Sclerotium of Poria cocos (Schw.) Wolf; Roots of Atractylodes macrocephala Koidz.; Whole herb of Taraxacum mongolicum Hand. Mazz.; Roots of Polygonum cuspidatum Sieb.et Zucc.; Ear of Prunella vulgaris L.; Roots of Salvia miltiorrhiza Bge.; Seeds of Prunus persica (L.) Batsch; Carapace of Trionyx sinensis Wiegmann; Immature fruit of Citrus aurantium L. | Inhibiting HSCs activation | Shi et al. (2017) |
| Herbal compound 861 | Roots of Salvia miltiorrhiza Bge; Roots of Astragalus membranaceus (Fisch.) Bge.; Roots of Bupleurum chinense DC.; Caulis of Spatholobus suberectus Dunn; Roots of Ligusticum chuanxiong Hort.; Roots of Cyperus rotundus L.; Roots of Paeonia lactiflora Pall.; Peel of Citrus reticulata Blanco; Roots of Angelica sinensis (Oliv.) Diels; Flower of Carthamus tinctorius L. | Increasing SnoN protein expression and inhibiting the TGF-β1/Smad signaling pathway | Chi et al. (2018) |
| Xiaochaihu decoction | Roots of Bupleurum chinense DC.; Roots of Scutellaria baicalensis Georgi; Roots of Codonopsis pilosula (Franch.) Nannf.; Roots of Pinellia ternata (Thunb.) Breit.; Roots of Glycyrrhiza uralensis Fisch.; Roots of Zingiber officinale Rosc.; Fruits of Ziziphus jujube Mill.; Roots of Panax ginseng C.A.Mey. | Up-regulating Nrf2 pathway against oxidative stress and inhibiting activated HSCs | Li et al. (2017) |
| Liuwei wuling tablets | Fruits of Schisandra chinensis (Turcz.) Baill; Fruits of Ligustrum lucidum Ait.; Fruits of Forsythia suspensa (Thunb.) Vahl; Roots of Curcuma wenyujin Y.H. Chen et C. Ling; Roots of Curcuma aeruginosa Roxb.; Ganoderma lucidum (Leyss.ex Fr.) Karst. | Modulating TGF-β/Smad and NF-κB signaling pathways | Huimin Liu et al. (2018) |
| Anluo huaxian pill | Roots of Rehmannia glutinosa Libosch.; Roots of Panax notoginseng (Burk.) F.H.Chen; Whitmania pigra Whitman; Pheretima aspergillum (E.Perrier); Bombyx mori Linnaeus; Roots of Atractylodes macrocephala Koidz.; Roots of Curcuma wenyujin Y.H. Chen et C. Ling; Gallstone of Bos taurus domesticus Gmelin Cow bezoar; Shell of Arca subcrenata Lischke;Root and bark of Paeonia suffruticosa Andr.; Roots of Rheum palmatum L.; Fruits of Hordeum vulgare L.; Gizzard of Gallus gallus domesticus Brisson; Shell of Bubalus bubalis Linnaeus | Improving liver function, inhibiting the activation of hepatic stellate cells, enhancing the expression of MMP-13, and inhibiting the expression of MMP-2 and TIMP-1/2 | Wang L. et al. (2019) |
| Huang Qi decoction | Roots of Astragalus membranaceus (Fisch.) Bge.; Roots of Glycyrrhiza uralensis Fisch. | Inhibiting Notch signaling activation | Zhang et al. (2017) |
| Qinggan Huoxue decoction | Roots of Bupleurum chinense DC.; Roots of Scutellaria baicalensis Georgi; Roots of Salvia miltiorrhiza Bge.; Carapace of Trionyx sinensis Wiegmann; Roots of Pueraria lobata (Willd.) Ohwi | Inhibiting TGF-β1/Smad1 signaling pathway | Wu et al. (2016) |
| Gan-fu-kang | Fruits of Schisandra chinensis (Turcz.) Baill; Roots of Pseudostellaria heterophylla (Miq.) Pax ex Pax et Hoffm.; Whole herb of Hedyotis diffusa Willd. | Downregulating Wnt/Ca2+ signaling | Jia et al. (2016) |
| Taohong Siwu decoction | Roots of Rehmannia glutinosa Libosch.; Roots of Paeonia lactiflora Pall.; Roots of Angelica sinensis (Oliv.) Diels; Roots of Ligusticum chuanxiong Hort.; Seeds of Prunus persica (L.) Batsch; Flos of Carthamus tinctorius L. | Reducing inflammatory reaction and VEGF expression and downstream signaling transduction | Xi et al. (2016) |
| Xia-yu-xue decoction | Roots of Rheum palmatum L.; Seeds of Prunus persica (L.) Batsch; Eupolyphaga sinensis Walker | Inhibiting hepatic stellate cell activation by targeting NF-κB and TGF-β1 signaling pathways | Liu et al. (2015) |
| Ger-Gen-Chyn-Lian decoction | Roots of Pueraria lobata (Willd.) Ohwi; Roots of Scutellaria baicalensis Georgi; Roots of Coptis chinensis Franch.; Roots of Glycyrrhiza uralensis Fisch. | Inhibiting hypoxia-inducible factor-1α-mediated pathway | Chang et al. (2019) |
| Dahuang zhechong pill | Roots of Rheum palmatum L.; Roots of Scutellaria baicalensis Georgi; Roots of Glycyrrhiza uralensis Fisch.; Seeds of Prunus persica (L.) Batsch; Seeds of Prunus armeniaca L.; Roots of Paeonia lactiflora Pall.; Roots of Rehmannia glutinosa Libosch.; Whitmania pigra Whitman; Eupolyphaga sinensis Walker; Toxicodendri Resina; Tabanus bivittatus Matsumura; Holotrichia diomphalia Bates | Decreasing the secretion of TNF-α and IL-13 through downregulating p38 and ERK phosphorylation | Cai et al. (2010) |
| Compound kushen injection | The roots of Kushen and Baituling with several identified bioactive alkaloids, including oxymatrine, matrine, oxysophocarpine, and sophocarpine | Rebalancing TGF-β/Smad7 signaling in HSCs | Yang et al. (2021) |
| Yiguanjian decoction | Radix rehmanniae (Rehmannia glutinosa (Gaetn.) Libosch. ex Fisch. et Mey.), Radix glehniae (Glehnia littoralis Fr. Schmidt ex Miq.), Radix ophiopogonis (Ophiopogon japonicus (Thunb.) Ker-Gawl.), Fructus lycii (Lycium barbarum L.), Radix Angelicae sinensis (Angelica sinensis (Oliv.) Diels), and Fructus toosendan (Melia toosendan Sieb. et Zucc.) | Inhibition of M1 polarization of macrophages | Xu et al. (2021) |
| Kangxian ruangan capsule | Artemisia capillaris (20 g), Astragalus membranaceus (10 g), Turtle shell (10 g); minister drug: Coix seed (20 g), Salvia miltiorrhiza (30 g), Angelica sinensis (10 g), Curcuma zedoaria (10 g), Ground beetle (10 g); assistant drug: Panax notoginseng (6 g), peach seed (10 g), Parched pangolin scales (6 g), ambassador drug: Rhizoma atractylodis macrocephalae (10 g) | Reducing HSC activation by suppressing the TGF-β1 and TLR4 signaling pathways | Liu et al. (2021) |
| Yinchen Wuling powder | artemisia Capillaris Herba, Polyporus Umbellatus, Alismatis Rhizoma, Atractylodes Macrocephalae Rhizoma stir-fried with wheat bran, Poria and Cinnamomi Ramulus | Regulating the imbalance of gut microbiota | Zhang et al. (2021) |
| Xueshisanjia no-decoction granule prescription | Turtle shell 15 g, pangolin 3 g, batryticated silkworm 10 g, ground beetle 10 g, peach kernel 10 g, bupleurum 5 g (total 53 g) | Promoting the miR-29b-3p/VEGFA axis | Zhang et al. (2022) |
| Si-Ni-San | Roots of Bupleurum scorzonerifolium Willd.; Roots of Paeonia lactiflora Pall.; Citrus aurantium L.; Glycyrrhiza uralensis Fisch. | Alleviating inflammation, ECM accumulation, aberrant angiogenesis and apoptosis of hepatic parenchymal cells, inhibiting activation of HSCs | Siliang Wang et al. (2021) |
TCMs treating hepatic fibrosis
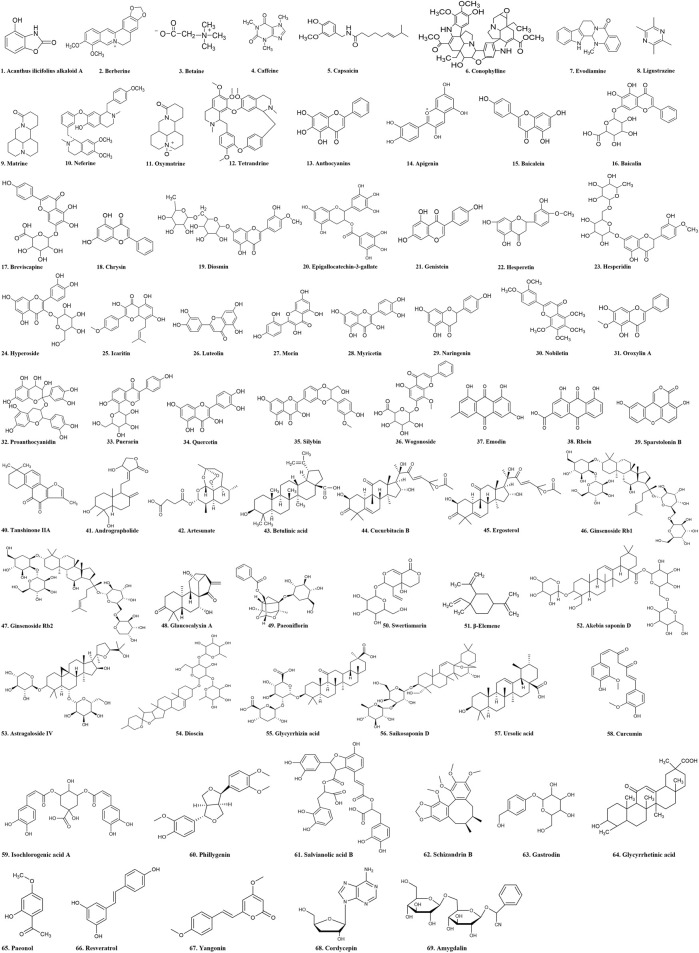
Nowadays, HF becomes a major public health problem with severe consequence (Berumen et al., 2021). Finding and discovering antifibrotic drugs from TCMs has become a hot research field. TCMs have unique advantages and great application prospects in anti-hepatic fibrosis due to their diverse structures, low toxicity, and wide sources (Daniyal et al., 2019; Hamza et al., 2020). Studies have shown that natural products derived from TCMs, such as alkaloids, flavones, quinones, terpenoids, saponins, phenylpropanols and polysaccharides, have promising anti-fibrotic activities (Shan et al., 2019). In addition, some TCMs and their formulas would be also beneficial for treating HF. The anti-fibrotic effects of these natural products were summarized in the following section according to the classification of chemical structure (Figure 3).
FIGURE 3.
Chemical structural formula of natural products.
Alkaloids
Alkaloids is a class of basic organic compounds containing nitrogen that mainly exist in plants or herbs. Most of them have complex ring structures, conjugation, and optical rotation. They have alkali like properties and significant biological activities, which are an important class of effective components in TCMs (Mondal et al., 2019). For example, acanthus ilicifolius alkaloid A, berberine and betaine are effective antioxidants with strong free radical scavenging effects, which can reduce the bio-markers’ levels of HF, so significantly improve liver functions (Wai et al., 2015; Eissa et al., 2018; Shedid et al., 2019). In addition, caffeine, capsaicin, evodiamine, matrine, neferine, oxymatrix and tetrandrine exert anti-fibrotic effects through regulating the TGF-β signal transduction pathway (Hsu et al., 2007; Gressner et al., 2008; Yu et al., 2014; Chen M. S. et al., 2015; Zhao et al., 2016; Choi et al., 2017; Yang et al., 2018). Besides, conophylline and ligustrazine can inhibit the activation of HSCs, and reduce the deposition of ECM (Kubo et al., 2014; Zhang et al., 2018). Table 2 summarized the anti-fibrotic effects of alkaloids found in TCMs against HF.
TABLE 2.
Alkaloids with anti-hepatic fibrosis effect.
| No. | Compounds | Source | Molecular formula | Mechanisms | Refs |
|---|---|---|---|---|---|
| 1 | Acanthus ilicifolius alkaloid A | Acanthus ilicifolius L. | C7H5NO3 | Inhibiting inflammatory response; | Wai et al. (2015) |
| Reducing lipid peroxidation and oxidative stress | |||||
| 2 | Berberine | Coptis chinensis Franch. | C20H18NO4 | Antioxidant and anti-inflammatory responses | Eissa et al. (2018) |
| 3 | Betaine | Beta vulgaris L. | C5H11NO2 | Reducing oxidative stress response | Shedid et al. (2019) |
| 4 | Caffeine | Coffea arabica L. | C8H10N4O2 | Inhibiting TGF-β-induced CTGF expression by inhibiting Smad2/3 phosphorylation and up-regulating PPARγ receptor | Gressner et al. (2008) |
| 5 | Capsaicin | Capsicum annuum L. | C18H27NO3 | Inhibiting TGF-β1/Smad pathway | Choi et al. (2017) |
| 6 | Conophylline | Catharanthus roseusvar. albus Lochnera rosea var. flava | C44H50N4O10 | Inhibiting HSCs activation and induction of apoptosis | Kubo et al. (2014) |
| 7 | Evodiamine | Euodia rutaecarpa (Juss.) Benth | C19H17N3O | Inhibiting TGF-β1 and p-Smad2/3 signaling pathway expression | Yang et al. (2018) |
| 8 | Ligustrazine | Ligusticum chuanxiong hort. | C8H12N2 | Inhibiting HSCs activation by interfering with PDGF-βR | Zhang et al. (2018) |
| 9 | Matrine | Sophora flavescens Alt. | C15H24N2O | Inhibiting TGF-β1 expression, enhance the activity of hepatocyte growth factor | Yu et al. (2014) |
| 10 | Neferine | Nelumbo nucifera Gaertn | C38H44N2O6 | Decreasing expression of TGF-β1 in the liver | Mo-Si Chen et al. (2015) |
| 11 | Oxymatrine | Sophora flavescens Alt. | C15H24N2O2 | Regulating TLR4-dependent inflammation and TGF-β1 signaling pathway | Zhao et al. (2016) |
| 12 | Tetrandrine | Stephania tetrandra S. Moore | C38H42N2O6 | Inhibiting NF-κB transcriptional activity induced by TNF-α; Inhibiting TGF-β1-induced α-SMA secretion and collagen deposition in HSC-T6 cells | Hsu et al. (2007) |
Flavonoids
Flavonoids is a class of compounds with 2-phenyl chromogenic ketone structure as the basic parent core, which widely exists in berries and vegetables in nature. Flavonoids have notable antioxidant, anti-inflammatory, and anticancer activities, and can be used to treat a variety of liver diseases (Akhlaghi, 2016). For example, anthocyanins can significantly improve the severity of liver injury and fibrosis by reducing oxidative stress and inflammation (Sun et al., 2018). Epigallocatechin-3-gallate has anti-hepatic fibrosis effect in vitro by inhibiting PI3K/Akt/Smad pathway (Yu et al., 2015). Breviscapine, nobiletin, proanthocyanidin, silybin and wogonoside have anti-inflammatory and antioxidant activities, and these bioactive flavonoids can be used as promising agents for the treatment of HF and related liver diseases (Trappoliere et al., 2009; Li et al., 2012; Wang Q. et al., 2015; Liu Y. et al., 2018; Wu et al., 2020). Previous investigations showed that apigenin could improve CCl4-induced HF by mediating VEGF-mediated FAK phosphorylation through multiple pathways (Qiao et al., 2020). Baicalein, chrysin, icaritin and myricetin can prevent or reverse the progression of HF by inhibiting the activation of HSCs and inducing apoptosis of activated HSCs (Sun et al., 2010; Li et al., 2011; Balta et al., 2015; Geng et al., 2017). Besides, baicalin and diosmin exert anti-fibrotic effects by stimulating the expression of PPAR-γ (Qiao et al., 2011; Hasan et al., 2017). Genistein, hesperetin and luteolin can block the development of HF by decreasing TGF-β and activating TGF-β/Smad signaling (Li et al., 2015; Ganai and Husain, 2017; Kong et al., 2018). Moreover, hesperidin, naringenin, puerarin and quercetin inhibit liver tissue injury, necrosis, and fibrosis by reducing the activity of cytokines and biological receptors such as NF-κB (Pérez-Vargas et al., 2014; Wang et al., 2016; Wang R. et al., 2017; Hernández-Aquino et al., 2017). In addition, hyperoside and morin improve liver function and reduce the occurrence of HF by enhancing the expression of liver Nrf2 (Sang et al., 2017; Zou et al., 2017). Furthermore, oroxylin A can dose-dependently reduce biomarkers of liver injury and HSC activation by activating autophagy (Chen et al., 2018). Table 3 summarized the anti-fibrotic effects of flavonoids from TCMs against HF.
TABLE 3.
Flavonoids with anti-hepatic fibrosis effect.
| No. | Compounds | Source | Molecular formula | Mechanisms | Refs |
|---|---|---|---|---|---|
| 13 | Anthocyanins | Vaccinium corymbosum L. | C15H11O6 | Regulating oxidative stress, inflammation and activation of HSCs | Sun et al. (2018) |
| 14 | Apigenin | Apium graveolens L. | C15H10O5 | VEGF-mediated FAK phosphorylation through multiple pathways | Qiao et al. (2020) |
| 15 | Baicalein | Scutellaria baicalensis Georgi | C15H10O5 | Downregulating PDGF-β receptor; inhibiting the activation and proliferation of HSC | Sun et al. (2010) |
| 16 | Baicalin | Scutellaria baicalensis Georgi | C21H18O11 | Activating PPARγ and inhibiting TGF-β1 | Qiao et al. (2011) |
| 17 | Breviscapine | Erigeron breviscapus (Vant.) Hand. -Mazz. | C21H18O12 | Inhibiting inflammation and apoptosis | Yu Liu et al. (2018), Lan et al. (2022) |
| 18 | Chrysin | Oroxylum indicum (Linn.) Kurz | C15H10O4 | Inhibiting HSCs activation and proliferation through TGF-β1/Smad pathway | Balta et al. (2015) |
| 19 | Diosmin | Citrus aurantium L. | C28H32O15 | Boosting PPAR-γ expression and hampering miR-17-5p-activated canonical Wnt-β-catenin signaling | Hasan et al. (2017) |
| 20 | Epigallocatechin-3-gallate | Acacia catechu (L.f.) Willci. | C22H18O11 | Inhibiting PI3K/Akt/Smad pathway | Yu et al. (2015) |
| 21 | Genistein | Genista tinctoria Linn. | C15H10O5 | Blocking the TGF-β/Smad signaling pathway | Ganai and Husain, (2017) |
| 22 | Hesperetin | Citrus reticulata L. | C16H14O6 | Inhibiting TGF-β1/Smad pathway | Kong et al. (2018) |
| 23 | Hesperidin | Citrus reticulata L. | C28H34O15 | Decreasing the expression of NF-κB, TGF-β and connective tissue growth factor | Pérez-Vargas et al. (2014) |
| 24 | Hyperoside | Hypericum perforatum L. | C21H20O12 | Activating Nrf2 nuclear translocation | Zou et al. (2017) |
| 25 | Icaritin | Epimedium brevicornu Maxim. | C21H20O6 | Inducing activated HSCs death | Li et al. (2011) |
| 26 | Luteolin | Reseda odorata L. | C15H10O6 | Inhibiting AKT/mTOR/p70S6K and TGF-β/Smad signaling pathway | Li et al. (2015) |
| 27 | Morin | Cudrania cochin chinensis (Lour.) Kudo et Masam. | C15H10O7 | Enhancing hepatic Nrf2 expression | Sang et al. (2017) |
| 28 | Myricetin | Myrica rubra (Lour.) S. et Zucc. | C15H10O8 | Inhibiting HSCs activation | Geng et al. (2017) |
| 29 | Naringenin | Anacardium occidentalie Linn. | C15H12O5 | Inhibiting NF-κB, TGF-β-Smad3 and JNK-Smad3 signaling pathways | Hernández-Aquino et al. (2017) |
| 30 | Nobiletin | Citrus nobilis Lour. | C21H22O8 | Reducing inflammation | Hernández-Aquino et al. (2017) |
| 31 | Oroxylin A | Oroxylum indicum (Linn.) Kurz. | C16H12O5 | Activating autophagy | Chen et al. (2018) |
| 32 | Proanthocyanidin | Vitis vinifera L. | C30H26O13 | Anti-lipid peroxidation | Li et al. (2012) |
| 33 | Puerarin | Pueraria montanavar. lobata (Willd.) Sanjappa & Pradeep | C21H20O10 | Inhibiting PARP-1 and subsequent attenuating NF-κB, ROS production and mitochondrial dysfunction | Wang et al. (2016) |
| 34 | Quercetin | Sophora flavescens Ait. | C15H10O7 | Reducing inflammation; Regulating NF-κB and p38 MAPK pathway | Rong Wang et al. (2017) |
| 35 | Silybin | Silybum marianum (L.) Gaertn. | C25H22O10 | Anti-inflammatory and antioxidant activity | Trappoliere et al. (2009) |
| 36 | Wogonoside | Scutellaria baicalensis Georgi. | C22H20O11 | Reducing inflammation | Qichao Wang et al. (2015) |
Quinones
Quinones is a kind of chemical constituents with quinone structure, which are mainly divided into four types: benzoquinone, naphthoquinone, phenanthrenequinone and anthraquinone. Quinones are widely distributed in plants and have various biological activities. Studies have shown that quinones have anti-inflammatory, anticancer, anti-viral and hepatoprotective activities, which lay a foundation for the treatment of HF (Dong X. et al., 2020). For example, rhein can significantly reduce alanine aminotransferase (ALT) activity in liver tissue of CCl4/ethanol-injured rats, improve the histological changes of HF, and decrease the expression of α-SMA and TGF-β1 in liver tissue. Therefore, rhein has a protective effect on CCl4/ethanol-induced liver injury in rats, inhibiting HF. Its mechanism of action may be related to antioxidant and anti-inflammatory effects, but can also inhibit TGF-β1 to stop HSC activation (Guo et al., 2002). Emodin is an active ingredient isolated from rhubarb, which has antimicrobial, immunosuppressive and anti-inflammatory effects. CCl4 was used to establish an experimental HF model in rats, and emodin was administered for treatment. The results suggested that Emodin might play an anti-fibrotic role by inhibiting TGF-β1 signal transduction to prevent HSC activation (Dong et al., 2009; Liu F. et al., 2018). Studies have found that tanshinone IIA can significantly improve liver function, alleviate liver injury, reduce ECM accumulation, inhibit HSC proliferation and activation, thereby playing an anti-fibrotic role. Tanshinone IIA may alleviate HF through multi-target and multi-signal pathways (Liu and Huang, 2014; Shi et al., 2020). In addition, sparstolonin B can play an anti-hepatic fibrosis role by inhibiting the TLR4 signal transduction pathway (Dattaroy et al., 2018). Table 4 summarized the anti-fibrotic effects of quinones from TCMs against HF.
TABLE 4.
Quinones with anti-hepatic fibrosis effect.
| No. | Compounds | Source | Molecular formula | Mechanisms | Refs |
|---|---|---|---|---|---|
| 37 | Emodin | Rheum palmatum L. | C15H10O5 | Inhibiting HSC activation by mediating TGF-β/Smads signaling pathway | Dong et al. (2009), Feng Liu et al. (2018) |
| 38 | Rhein | Rheum palmatum L. | C15H8O6 | Antioxidant and anti-inflammatory responses. | Guo et al. (2002) |
| 39 | Sparstolonin B | Sparganium stoloniferum (Graebn.), Buch-Ham. ex Juz. | C15H8O5 | Inhibiting TLR4 signaling transduction pathway | Dattaroy et al. (2018) |
| 40 | Tanshinone IIA | Salvia miltiorrhiza Bge. | C19H18O3 | Inhibiting HSC activation and proliferation | Liu and Huang, (2014), Stanković et al. (2017) |
Terpenoids
Terpenoids and their derivatives are the compounds with isoprene units as their basic structures widely existed in nature. Terpenoids have antioxidant, metabolic, immunomodulatory, and anti-inflammatory activities (Sánchez-Crisóstomo et al., 2019), and have promising potentials for the treatment of some hepatic chronic diseases. For example, cucurbitacin B attenuates HF by inhibiting oxidative stress, inflammation, and STAT3 signaling pathways (Sallam et al., 2018). β-Elemene could reduce plasma endotoxin level, serum tumor necrosis factor-alpha level and CD14 mRNA expression in liver of rats with HF rats by CCl4 treatment (Liu et al., 2011). In addition, andrographolide, ginsenoside Rb1 and paeoniflorin could inhibit HF by regulating the TGF-β1/Smads signaling pathway (Lo et al., 2011; Hu Z. et al., 2018; Lin et al., 2018), and artesunate, ergosterol, ginsenoside Rb2, glaucocalyxin A and swertiamarin can exert anti-fibrotic effects by inhibiting the activation of HSCs (Park et al., 2006; Tai et al., 2016a; Li S. et al., 2016; Dong et al., 2018; Kong et al., 2019). Betulinic acid exerts significant anti-fibrotic effects on rats treated with intraperitoneal injection of thioacetamide (TAA) by regulating TLR4/MyD88/NF-κB signaling pathway (Wan et al., 2012). Table 5 summarized the anti-fibrotic effects of terpenoids found in TCMs against HF.
TABLE 5.
Terpenoids with anti-hepatic fibrosis effects.
| No. | Compounds | Source | Molecular formula | Mechanisms | Refs |
|---|---|---|---|---|---|
| 41 | Andrographolide | Andrographis paniculate (Burm. f.) Nees | C20H30O5 | Inhibiting the TGF-β1/Smad2 and TLR4/NF-κB p50 pathways | Lin et al. (2018) |
| 42 | Artesunate | Artemisia annua Linn. | C19H28O8 | Inhibiting activation and proliferation of HSC; Inhibiting LPS/TLR4/NF-κB signaling pathway | Kong et al. (2019) |
| 43 | Betulinic acid | Betula platyphylla Suk. | C30H48O3 | Inhibiting NF-κB signaling pathway | Wan et al. (2012) |
| 44 | Cucurbitacin B | Cucumis melo L. | C32H46O8 | Inhibiting oxidative stress, inflammation and STAT3 signaling | Sallam et al. (2018) |
| 45 | Ergosterol | Ganoderma lucidum (Leyss. ex Fr.) Karst | C28H44O | Inhibiting HSCs activation | Tai et al. (2016a) |
| 46 | Ginsenoside Rb1 | Panax ginseng C.A. Mey | C54H92O23 | Regulating the expression of collagen, TGF-β1, MMP-2 and TIMP-1 | Lo et al. (2011) |
| 47 | Ginsenoside Rb2 | Panax ginseng C.A. Mey | C53H90O22 | Inducing apoptosis in HSC via caspase-3 activation | Park et al. (2006) |
| 48 | Glaucocalyxin A | Rabdosia japonica (Burm.f.) Hara var.glaucocalyx (Maxim.) Hara | C20H28O4 | Attenuating the activation of HSC through the TGF-β1/Smad signaling pathway | Dong et al. (2018) |
| 49 | Paeoniflorin | Paeonia lactiflora Pall. | C23H28O11 | Inhibiting TGF-β1/Smads signaling pathway | Zongtao Hu et al. (2018) |
| 50 | Swertiamarin | Gentianaceae Swertia L. | C16H22O10 | Inhibiting HSC activation by regulating the RAS | Shu Li et al. (2016) |
| 51 | β-Elemene | Curcuma Wenyujin Y.H.Chen et C.Ling | C15H24 | Regulating endotoxin signaling transduction pathway | Liu et al. (2011) |
Saponins
Saponins, a class of glycosides whose aglycones are triterpenoids or spirosterols, are complex compounds in the structure, which widely exist in plants and have a wide variety of species. Saponins have antioxidant, anticancer and multi-target activities, which play an important regulatory role in the treatment of HF (Li X. et al., 2018). For example, akebia saponin D and saikosapoin D can reduce HF through anti-inflammatory effects (Dang et al., 2007; Gong et al., 2019), and astragaloside IV, glycyrrhizin acid and ursolic acid could alleviate HF by inhibiting HSC activation and inducing apoptosis of activated HSCs (Wang et al., 2011; Li et al., 2013; Liang et al., 2015). In addition, dioscin can specifically inhibit collagen synthesis by regulating the PI3K/Akt pathway, and can be used as an effective anti-fibrotic drug (Xu et al., 2017). Table 6 summarized the anti-fibrotic effects of saponins found in TCMs against HF.
TABLE 6.
Saponins with anti-hepatic fibrosis effect.
| No. | Compounds | Source | Molecular formula | Mechanisms | Refs |
|---|---|---|---|---|---|
| 52 | Akebia saponin D | Dipsacus asperoides C.Y. Cheng et T.M.Ai. | C47H76O18 | Anti-inflammatory effect | Gong et al. (2019) |
| 53 | Astragaloside IV | Astragalus membranaceus (Fisch.) Bungede | C41H68O14 | Inhibiting HSC activation by inhibiting oxidative stress and p38 MAPK signaling pathway | Li et al. (2013) |
| 54 | Dioscin | Dioscorea japonica Thunb. | C45H72O16 | Inhibiting collagen synthesis by regulating PI3K/Akt pathway | Xu et al. (2017) |
| 55 | Glycyrrhizin acid | Glycyrrhize glabra L. | C42H62O16 | Inhibiting hepatocyte apoptosis and activation of HSCs | Liang et al. (2015) |
| 56 | Saikosaponin D | Bupleurum chinense DC. | C42H68O13 | Reducing inflammation and hepatocyte injury | Dang et al. (2007) |
| 57 | Ursolic acid | Prunella vulgaris L. | C30H48O3 | Inducing apoptosis of activated HSCs | Wang et al. (2011) |
Phenylpropanoids
Phenylpropanoids is a kind of natural compounds composed of benzene ring and three straight chain carbon, and have anti-tumor, antioxidant and hepatoprotective effects (Teponno et al., 2016). For example, curcumin can significantly improve the severity of liver injury and fibrosis by reducing oxidative stress and inflammation (Kong et al., 2020). Experimental studies have found that the anti-fibrotic mechanism of salvianolic acid B may be through inhibiting HSC proliferation (Liu et al., 2002). In addition, isochlorogenic acid A could delay the progression of HF by regulating HMGB1/TLR4/NF-κB signaling pathway (Liu et al., 2020). Schizandrin B plays an anti-fibrotic role by regulating Nrf2-ARE and TGF-β/Smad signaling pathways (Chen et al., 2017). Phillygenin is a phenylpropanoid compound isolated from Forsythia and has good anti-inflammatory effects. Phillygenin inhibits LPS induced proinflammatory response and Lx2 cell activation through TLR4/MyD88/NF-κB signaling pathway, thereby inhibiting HF (Hu et al., 2020b). Table 7 summarized the anti-fibrotic effects of phenylpropanoids found in TCMs against HF.
TABLE 7.
Phenylpropanoids with anti-hepatic fibrosis effect.
| No. | Compounds | Source | Molecular formula | Mechanisms | Refs |
|---|---|---|---|---|---|
| 58 | Curcumin | Curcuma longa L. | C21H20O6 | Regulating oxidative stress and autophagy, passivation of hepatocyte epithelial-mesenchymal transformation | Kong et al. (2020) |
| 59 | Isochlorogenic acid A | Lonicera japonica Thunb. | C25H24O12 | Inhibiting HMGB1/TLR4/NF-κB signaling pathways | Liu et al. (2020) |
| 60 | Phillygenin | Forsythia suspensa (Thunb.) Vahl | C21H24O6 | Regulating TLR4/MyD88/NF-κB signaling pathways | Hu et al. (2020b) |
| 61 | Salvianolic acid B | Salvia miltiorrhiza Bge. | C36H30O16 | Inhibiting HSC proliferation and collagen synthesis, Decreasing TGF-β1 autocrine and MAPK activity | Liu et al. (2002) |
| 62 | Schizandrin B | Schisandra chinensis (turcz.) baill. | C23H28O6 | Regulating Nrf2-ARE and TGF-β/Smad signaling pathways | Chen et al. (2017) |
Polysaccharides
Polysaccharides are polysaccharide macromolecular carbohydrates consisting of glycosidic bonded sugar chains with at least 10 monosaccharides. Polysaccharides are widely distributed in nature and have the characteristics of multi-component, multi-target, high efficiency, and low toxicity (Qu et al., 2020). In recent years, increasing investigations reported that polysaccharides possess promising hepatoprotective effects against HF. Table 8 summarized the anti-fibrotic effects of polysaccharides found in TCMs against HF.
TABLE 8.
Polysaccharides with anti-hepatic fibrosis effect.
| Polysaccharides | Source | Mechanisms | Refs |
|---|---|---|---|
| Astragalus polysaccharides | Astragalus membranaceus (Fisch.) Bge. | Inhibiting LX-2 cell proliferation | Zheng et al. (2014) |
| Cordyceps polysaccharides | Cordyceps sinensis (BerK.) Sacc. | Reducing ROS production | Liu et al. (2013) |
| Dicliptera chinensis polysaccharides | Dicliptera chinensis (L.) Juss. | Reducing inflammation | Kefeng Zhang et al. (2016) |
| Fucoidan | Fucus vesiculosus | Inhibiting TGF-β1/Smads signaling pathway | Jingjing Li et al. (2016) |
| Garlic polysaccharide | Allium sativum L. | Inhibiting intestinal flora imbalance | Yuchuan Wang et al. (2018) |
| Lycium barbarum polysaccharides | Lyciumbarbarum L. | Inhibiting TLRs/NF-κB signaling pathway expression | Gan et al. (2018) |
| Polysaccharide from Amusium Pleuronect | Amusium Pleuronectes pleuronectes Linnaeus | Hepatoprotective effect | Tang et al. (2018) |
Other natural compounds
Many other compounds extracted from TCMs have therapeutic effects on HF through complex mechanisms. Polyphenols have been reported to have good effects in alleviating oxidative stress, lipid metabolism and inflammation (Luk et al., 2007; Li S. et al., 2018). For example, gastrodin can significantly improve the severity of liver injury and fibrosis by reducing oxidative stress and inflammation (Zhao et al., 2014; Zhao et al., 2015). Glycyrrhetinic acid protects the liver by activating the nuclear translocation of Nrf2 and increasing the activity of antioxidant enzymes. Therefore, glycyrrhetinic acid may be an effective hepatoprotective drug for the treatment of HF (Chen et al., 2013; Lam et al., 2016). Experimental studies have found that the anti-fibrotic mechanism of paeonol may be through inhibiting HSC proliferation (Kong et al., 2013; Tai et al., 2016b). Resveratrol delays the progression of HF by reprogramming macrophage phenotype from LPS to IL-4 by upregulating endogenous IL-10 (Hsu et al., 2019; Yu et al., 2019). The anti-fibrotic mechanism of yangonin may be that it reduces collagen content by regulating HF-related genes Col1-α-1 and TIMP-1 (Cao et al., 2018; Wang X. et al., 2019). In addition, Lan et al. (2021) established the fibrosis model in mice induced by 16 weeks of high-fat/high-cholesterol diet administration. They found that cordycepin (100 and 200 mg/kg) treatment significantly inhibited the expression of fibrogenic genes such as ColIa1, ColIIIa1, α-SMA, and decreased the serum levels of ALT and aspartate aminotransferase (AST). It is revealed that cordycepin plays an important protective role in hepatic fibrosis mice. Furthermore, Wang R. et al. (2021) demonstrated the suppressive effects of amygdalin in TGF-β1-induced HSC activation in vitro and found that amygdalin (3 mg/kg) could reduce the levels of ALT and AST in serum, decrease the levels of α-SMA, collagen I, and TIMP-1 in hepatic tissue samples, improve focal necrosis and collagen fiber accumulation in CCl4-induced hepatic fibrosis rats. Table 9 summarized the anti-fibrotic effects of other compounds found in TCMs against HF.
TABLE 9.
Other natural compounds with anti-hepatic fibrosis effect.
| No. | Compounds | Source | Molecular formula | Mechanisms | Refs |
|---|---|---|---|---|---|
| 63 | Gastrodin | Gastrodiaelataf glauca | C13H18O7 | Reducing oxidative stress and inflammation | Zhao et al. (2014), Qu et al. (2016) |
| 64 | Glycyrrhetinic acid | Glycyrrhize glabra L. | C30H46O4 | Hepatoprotective effect | Lam et al. (2016) |
| 65 | Paeonol | Paeonia moutan Sim. | C9H10O3 | Inhibiting HSC production and NF-κB pathway | Tai et al. (2016b) |
| 66 | Resveratrol | Veratrum nigrum L. | C14H12O3 | Up-regulating endogenous IL-10 to reprogramme macrophages phenotype from LPS to IL-4 | Hsu et al. (2019) |
| 67 | Yangonin | Piper methysticum Forst. | C15H14O4 | Activating farnesoid X receptor | Cao et al. (2018) |
| 68 | Cordycepin | Cordyceps sinensis (Berk.) Sacc. | C10H13N5O3 | Activating AMPK signaling pathway | Lan et al. (2021) |
| 69 | Amygdalin | Prunus armeniaca L.var.ansu Maxim | C20H27NO11 | Inhibiting HSC proliferation and activation | Ruoyu Wang et al. (2021) |
Other extracts from TCMs
Besides, some TCM extracts are also beneficial for the treatment of HF via protecting hepatocytes and inhibiting hepatitis and fibrosis (Wang Y. et al., 2015; Wang Y. Y. et al., 2017; Miao et al., 2019). Table 10 summarized the anti-fibrotic effects of these extracts.
TABLE 10.
Other extracts from TCM with anti-hepatic fibrosis effect.
| Extracts | Source | Mechanisms | Refs |
|---|---|---|---|
| Water extracts from Solanum nigrum | Solanum nigrum L. | Downregulating HSCs activation by inhibiting advanced glycation end products-associated signaling pathways involved in anti-glycation and Nrf2 activity | Sha Li et al. (2018) |
| Graptopetalum paraguayense | Graptopetalum paraguayense E. Walther | Regulating HSC activation by the Inhibiting the TGF-β pathway | Zhao et al. (2015) |
| Semen brassicae | Brassica campeatris L. | Regulating TGF-β1/Smad, NF-κB, and AKT signaling pathways and the reduction of extracellular matrix deposition | Chen et al. (2013) |
| Water extract of Lonicerae japonicae | Lonicera japonica Thunb. | Inhibiting HSC activation, reducing hepatic ECM deposition, ameliorating liver oxidative stress injury and reversing EMT | Kong et al. (2013) |
| Ginkgo biloba extract | Ginkgo biloba L. | Regulating p38, MAPK and NF-κB/IκBα signaling to inhibit HSC activation and disrupting Bcl-2/Bax signaling to inhibit hepatocyte apoptosis | Yu et al. (2019) |
| Glechoma hederacea extracts | Glechoma longituba (Nakai) Kupr. | Downregulating NF-κB, AP-1 and TGF-β/Smad signals by interfering with HMGB1/TLR4 axis | Xiaohui Wang et al. (2019) |
| Germacrone | Zedoary turmeric oil | Inhibiting the activation and survival of HSCs | Li et al. (2021) |
| Total C-21 steroidal glycosides from baishouwu | Roots of Cynanchum auriculatum Royle ex Wight | Regulating IL-1β/MyD88 inflammation signaling | Qin et al. (2021) |
| Red raspberry extract | Rubus idaeus L. | Inducing apoptosis and trans differentiation of activated HSCs | Wu et al. (2021) |
Clinical trials
To date, there are several clinical trials on the anti-hepatic fibrosis effect of TCMs. Chronic hepatitis B (CHB) can progress to liver fibrosis, leading to cirrhosis with liver-related disease, and ultimately markedly increasing the risk of hepatocellular carcinoma (HCC) (Inoue and Tanaka, 2020). At present, a randomized, double-blind, and placebo-controlled clinical trial was carried out to estimate the antifibrotic activities of Anluo huaxian pill (AHP) on chronic hepatitis B (CHB) patients with advanced fibrosis or cirrhosis. The results found that the rate of histological improvement in patients with liver fibrosis was significantly higher in the AHP group than in the placebo group, the level of liver stiffness measurement (LSM) decreased dramatically from baseline in the AHP group but not in the placebo group, after orally administration with AHP at a dose of 6 g, twice daily for 48 weeks (Xiao et al., 2022), which indicated that AHP could improve liver fibrosis and may be useful as a functional food medicine. Besides, cirrhosis developed in one patient in the placebo group but in none of the patients in the AHP group, and no serious side effects occurred in patients treated with AHP (Xiao et al., 2022). Moreover, a multicenter, randomized, double-blind, placebo-controlled trial, patients with CHB were randomly assigned to receive entecavir (ETV) (0.5 mg/day)+FBRP (2 g three times/day), after 72 weeks of treatment, the rate of regression of fibrosis/cirrhosis was significantly higher in the ETV + BR group than ETV monotherapy in overall patients with CHB and those with liver cirrhosis at baseline, and this combination therapy also showed an excellent safety profile (Rong et al., 2022). To evaluate the efficacy and safety of Chunggan extract (CGX), Joung et al. (2020) conducted a randomized controlled clinical trial of CGX in patients with liver fibrosis diagnosed by Fibroscan. Orally administration with CGX (1 g or 2 g, twice daily) to the subjects for 24 weeks could dramatically decreased the LSM scores compared to the placebo group, and no notable adverse events were present, which firstly demonstrated that the potent pharmacological effects of CGX in restoring early liver fibrosis in patients with chronic liver disease. Based on a collective research report, luteolin, quercetin, ursolic acid and salvianic acid A are the main potential active components of FZHY formula to exert anti-inflammatory and anti-hepatic fibrosis pharmacological effects (Xin et al., 2022). Furthermore, the active components of FBRP to prevent HF mainly include total flavonoids from Astragalus membranaceus, salvianic acid A, notoginseng saponins, cordyceps polysaccharides (Yao et al., 2022). More notably, among these biological compounds from TCMs similarly positively affect fibrosis, as reported in the previous section.
At least, these established evidence indicated that TCMs might be considered as a viable, efficacious supplement for hepatic fibrosis, and can be used in daily life to prevent associated with liver disease. However, there is insufficient evidence to support the optimal dose of TCMs and what kind of hepatic fibrosis it has effect on deserve further research. Besides, the underlying mechanisms for the benefit of these TCMs therapy is unclear, and the specific medicinal components of TCMs responsible for the antifibrotic effects remain unknown. Therefore, it is necessary to investigate the effects of TCMs blockade and reversal of hepatic fibrosis in a larger, more rigorous methodological design and more diverse samples.
Conclusion and prospect
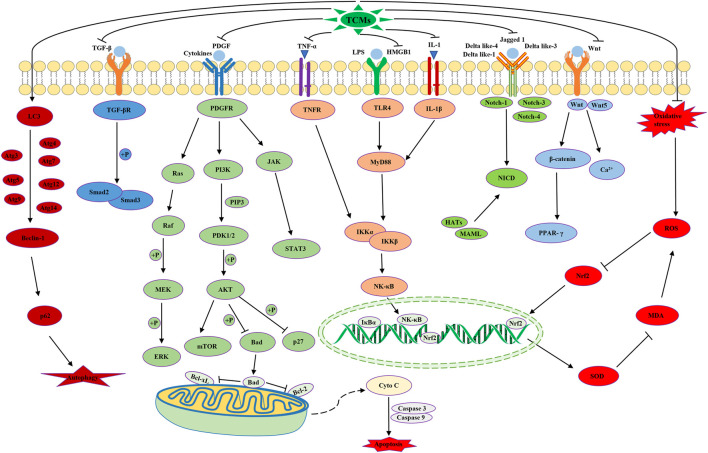
Hepatic fibrosis (HF) is a complex pathological process related to multiple factors, targets, and pathways, which causes serious harm to human health. In recent years, many TCMs and their active ingredients have been found to be effective in treating HF via multiple mechanisms (Figure 4). However, most of them remain at the stage of in vitro and in vivo experimental studies, which require more detailed preclinical and clinical studies.
FIGURE 4.
Schematic representation of the possible mechanism of TCMs on HF.
First, clinical studies on anti-hepatic fibrosis ingredients derived from TCMs are lacking, the assessment of pre-clinical safety is rarely documented, and the possible side effects and toxicity are still uncovered. Therefore, more work should be devoted to clinical data mining and experimental research in the future. Second, there is currently insufficient evidence to explain the specific molecular mechanisms of these anti-fibrotic components. Therefore, the pharmacokinetics and pharmacological properties of these natural anti-hepatic fibrosis products from TCMs are needed to be further studied. Third, the current research on anti-hepatic fibrosis drugs mainly focuses on plant medicines, while the research on animal and mineral drugs is less. Therefore, future research on anti-hepatic fibrosis drugs can also involve more animal or mineral drugs.
In conclusion, TCMs have more advantages in the treatment of HF due to its unique theoretical system and long-term practice clinically. This review systematically summarized the potential anti-hepatic fibrosis components from TCMs and their related molecular mechanisms, which would be helpful for further investigations on TCMs derived remedies for HF. In addition, it is hoped that this review will inspire more scholars to make more contributions to the study of TCMs in the treatment of HF.
Author contributions
HZ, WP, and LS designed this manuscript; W-QL, W-HL, JL, S-QZ, DQ and LZ wrote the manuscript; HZ and WP revised the manuscript. All authors collaborated in the writing of the present manuscript and approved its submission.
Funding
This work was supported by funds from the National Natural Science Foundation of China (No. 82174023) and National Key R&D Program for key research project of modernization of traditional Chinese medicine (No. 2019YFC1711602).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Akhlaghi M. (2016). Non-alcoholic fatty liver disease: Beneficial effects of flavonoids. Phytother. Res. 30 (10), 1559–1571. 10.1002/ptr.5667 [DOI] [PubMed] [Google Scholar]
- Allaire M., Rautou P. E., Codogno P., Lotersztajn S. (2019). Autophagy in liver diseases: Time for translation? J. Hepatol. 70 (5), 985–998. 10.1016/j.jhep.2019.01.026 [DOI] [PubMed] [Google Scholar]
- Bai X., Su G., Zhai S. (2020). Recent advances in nanomedicine for the diagnosis and therapy of liver fibrosis. Nanomater. (Basel) 10 (10), E1945. 10.3390/nano10101945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig M. S., Yaqoob U., Cao S., Saqib U., Shah V. H. (2016). Non-canonical role of matrix metalloprotease (MMP) in activation and migration of hepatic stellate cells (HSCs). Life Sci. 155, 155–160. 10.1016/j.lfs.2016.04.031 [DOI] [PubMed] [Google Scholar]
- Balta C., Herman H., Boldura O. M., Gasca I., Rosu M., Ardelean A., et al. (2015). Chrysin attenuates liver fibrosis and hepatic stellate cell activation through TGF-β/Smad signaling pathway. Chem. Biol. Interact. 240, 94–101. 10.1016/j.cbi.2015.08.013 [DOI] [PubMed] [Google Scholar]
- Barcena-Varela M., Colyn L., Fernandez-Barrena M. G. (2019). Epigenetic mechanisms in hepatic stellate cell activation during liver fibrosis and carcinogenesis. Int. J. Mol. Sci. 20 (10), E2507. 10.3390/ijms20102507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berumen J., Baglieri J., Kisseleva T., Mekeel K. (2021). Liver fibrosis: Pathophysiology and clinical implications. WIREs Mech. Dis. 13 (1), e1499. 10.1002/wsbm.1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkham-Kamphorst E., Weiskirchen R. (2016). The PDGF system and its antagonists in liver fibrosis. Cytokine Growth Factor Rev. 28, 53–61. 10.1016/j.cytogfr.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Cai F. F., Wu R., Song Y. N., Xiong A. Z., Chen X. L., Yang M. D., et al. (2018). Yinchenhao decoction alleviates liver fibrosis by regulating bile acid metabolism and TGF-β/smad/ERK signalling pathway. Sci. Rep. 8 (1), 15367. 10.1038/s41598-018-33669-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H. B., Sun X. G., Liu Z. F., Liu Y. W., Tang J., Liu Q., et al. (2010). Effects of dahuangzhechong pills on cytokines and mitogen activated protein kinase activation in rats with hepatic fibrosis. J. Ethnopharmacol. 132 (1), 157–164. 10.1016/j.jep.2010.08.019 [DOI] [PubMed] [Google Scholar]
- Cai X., Wang J., Wang J., Zhou Q., Yang B., He Q., et al. (2020). Intercellular crosstalk of hepatic stellate cells in liver fibrosis: New insights into therapy. Pharmacol. Res. 155, 104720. 10.1016/j.phrs.2020.104720 [DOI] [PubMed] [Google Scholar]
- Cao S., Zheng B., Chen T., Chang X., Yin B., Huang Z., et al. (2018). Semen Brassicae ameliorates hepatic fibrosis by regulating transforming growth factor-β1/Smad, nuclear factor-κB, and AKT signaling pathways in rats. Drug Des. devel. Ther. 12, 1205–1213. 10.2147/dddt.S155053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castera L., Friedrich-Rust M., Loomba R. (2019). Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 156 (5), 1264–1281. e1264. 10.1053/j.gastro.2018.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z. Y., Chen C. C., Liu H. M., Yeh Y. C., Lin T. Y., Lee T. Y., et al. (2019). Positive effects of ger-gen-chyn-lian-tang on cholestatic liver fibrosis in bile duct ligation-challenged mice. Int. J. Mol. Sci. 20 (17), E4181. 10.3390/ijms20174181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. S., Zhang J. H., Wang J. L., Gao L., Chen X. X., Xiao J. H. (2015). Anti-fibrotic effects of neferine on carbon tetrachloride-induced hepatic fibrosis in mice. Am. J. Chin. Med. 43 (2), 231–240. 10.1142/s0192415x15500159 [DOI] [PubMed] [Google Scholar]
- Chen Q., Zhang H., Cao Y., Li Y., Sun S., Zhang J., et al. (2017). Schisandrin B attenuates CCl(4)-induced liver fibrosis in rats by regulation of Nrf2-ARE and TGF-β/Smad signaling pathways. Drug Des. devel. Ther. 11, 2179–2191. 10.2147/dddt.S137507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. R., Chen X. P., Lu J. J., Wang Y., Wang Y. T. (2015). Potent natural products and herbal medicines for treating liver fibrosis. Chin. Med. 10, 7. 10.1186/s13020-015-0036-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. W., Zhang X. R., Wang C. Z., Chen W. Z., Xie W. F., Chen Y. X. (2008). RNA interference targeting the platelet-derived growth factor receptor beta subunit ameliorates experimental hepatic fibrosis in rats. Liver Int. 28 (10), 1446–1457. 10.1111/j.1478-3231.2008.01759.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zou L., Li L., Wu T. (2013). The protective effect of glycyrrhetinic acid on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. PLoS One 8 (1), e53662. 10.1371/journal.pone.0053662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Zhang Z., Yao Z., Wang L., Zhang F., Shao J., et al. (2018). Activation of autophagy is required for Oroxylin A to alleviate carbon tetrachloride-induced liver fibrosis and hepatic stellate cell activation. Int. Immunopharmacol. 56, 148–155. 10.1016/j.intimp.2018.01.029 [DOI] [PubMed] [Google Scholar]
- Chi C., Liu X. Y., Hou F., Yu X. Z., Li C. Y., Cui L. J., et al. (2018). Herbal compound 861 prevents hepatic fibrosis by inhibiting the TGF-β1/Smad/SnoN pathway in bile duct-ligated rats. BMC Complement. Altern. Med. 18 (1), 52. 10.1186/s12906-018-2119-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. H., Jin S. W., Choi C. Y., Kim H. G., Lee G. H., Kim Y. A., et al. (2017). Capsaicin inhibits dimethylnitrosamine-induced hepatic fibrosis by inhibiting the TGF-β1/smad pathway via peroxisome proliferator-activated receptor gamma activation. J. Agric. Food Chem. 65 (2), 317–326. 10.1021/acs.jafc.6b04805 [DOI] [PubMed] [Google Scholar]
- Dang S. S., Wang B. F., Cheng Y. A., Song P., Liu Z. G., Li Z. F. (2007). Inhibitory effects of saikosaponin-d on CCl4-induced hepatic fibrogenesis in rats. World J. Gastroenterol. 13 (4), 557–563. 10.3748/wjg.v13.i4.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniyal M., Akram M., Zainab R., Munir N., Sharif A., Shah S. M. A., et al. (2019). Prevalence and current therapy in chronic liver disorders. Inflammopharmacology 27 (2), 213–231. 10.1007/s10787-019-00562-z [DOI] [PubMed] [Google Scholar]
- Dattaroy D., Seth R. K., Sarkar S., Kimono D., Albadrani M., Chandrashekaran V., et al. (2018). Sparstolonin B (SsnB) attenuates liver fibrosis via a parallel conjugate pathway involving P53-P21 axis, TGF-beta signaling and focal adhesion that is TLR4 dependent. Eur. J. Pharmacol. 841, 33–48. 10.1016/j.ejphar.2018.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira da Silva B., Ramos L. F., Moraes K. C. M. (2017). Molecular interplays in hepatic stellate cells: Apoptosis, senescence, and phenotype reversion as cellular connections that modulate liver fibrosis. Cell. Biol. Int. 41 (9), 946–959. 10.1002/cbin.10790 [DOI] [PubMed] [Google Scholar]
- Deng Y. L., Xiong X. Z., Cheng N. S. (2012). Organ fibrosis inhibited by blocking transforming growth factor-β signaling via peroxisome proliferator-activated receptor γ agonists. Hepatobiliary Pancreat. Dis. Int. 11 (5), 467–478. 10.1016/s1499-3872(12)60210-0 [DOI] [PubMed] [Google Scholar]
- Dewidar B., Meyer C., Dooley S., Meindl-Beinker A. N. (2019). TGF-Β in hepatic stellate cell activation and liver fibrogenesis-updated 2019. Cells 8 (11), E1419. 10.3390/cells8111419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M. X., Jia Y., Zhang Y. B., Li C. C., Geng Y. T., Zhou L., et al. (2009). Emodin protects rat liver from CCl(4)-induced fibrogenesis via inhibition of hepatic stellate cells activation. World J. Gastroenterol. 15 (38), 4753–4762. 10.3748/wjg.15.4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Zeng Y., Liu Y., You L., Yin X., Fu J., et al. (2020). Aloe-emodin: A review of its pharmacology, toxicity, and pharmacokinetics. Phytother. Res. 34 (2), 270–281. 10.1002/ptr.6532 [DOI] [PubMed] [Google Scholar]
- Dong Z., Gao Q., Guo H. (2018). Glaucocalyxin A attenuates the activation of hepatic stellate cells through the TGF-β1/smad signaling pathway. DNA Cell. Biol. 37 (3), 227–232. 10.1089/dna.2017.3992 [DOI] [PubMed] [Google Scholar]
- Dong Z., Zhuang Q., Ning M., Wu S., Lu L., Wan X. (2020). Palmitic acid stimulates NLRP3 inflammasome activation through TLR4-NF-κB signal pathway in hepatic stellate cells. Ann. Transl. Med. 8 (5), 168. 10.21037/atm.2020.02.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley S., ten Dijke P. (2012). TGF-β in progression of liver disease. Cell. Tissue Res. 347 (1), 245–256. 10.1007/s00441-011-1246-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan G. J., Jiang R. Y., Wang Z. C. (2020). Research progress on the mechanism of anti-hepatic fibrosis of traditional Chinese medicine. Guid. J. TCM Pharm. 26 (05), 113–117. 10.13862/j.cnki.cn43-1446/r.2020.05.029 [DOI] [Google Scholar]
- Duan G. J., Wang Z. C. (2020). Prescription law of traditional Chinese medicine in the treatment of liver fibrosis. Acta Chin. Med. 35 (04), 894–900. 10.16368/j.issn.1674-8999.2020.04.199 [DOI] [Google Scholar]
- Eissa L. A., Kenawy H. I., El-Karef A., Elsherbiny N. M., El-Mihi K. A. (2018). Antioxidant and anti-inflammatory activities of berberine attenuate hepatic fibrosis induced by thioacetamide injection in rats. Chem. Biol. Interact. 294, 91–100. 10.1016/j.cbi.2018.08.016 [DOI] [PubMed] [Google Scholar]
- Elsharkawy A. M., Mann D. A. (2007). Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis. Hepatology 46 (2), 590–597. 10.1002/hep.21802 [DOI] [PubMed] [Google Scholar]
- Fabregat I., Moreno-Càceres J., Sánchez A., Dooley S., Dewidar B., Giannelli G., et al. (2016). TGF-β signalling and liver disease. Febs J. 283 (12), 2219–2232. 10.1111/febs.13665 [DOI] [PubMed] [Google Scholar]
- Frangogiannis N. (2020). Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 217 (3), e20190103. 10.1084/jem.20190103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Xiao Z., Tian X., Liu W., Xu Z., Yang T., et al. (2021). The novel Chinese medicine JY5 formula alleviates hepatic fibrosis by inhibiting the Notch signaling pathway. Front. Pharmacol. 12, 671152. 10.3389/fphar.2021.671152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan F., Liu Q., Liu Y., Huang D., Pan C., Song S., et al. (2018). Lycium barbarum polysaccharides improve CCl(4)-induced liver fibrosis, inflammatory response and TLRs/NF-kB signaling pathway expression in wistar rats. Life Sci. 192, 205–212. 10.1016/j.lfs.2017.11.047 [DOI] [PubMed] [Google Scholar]
- Ganai A. A., Husain M. (2017). Genistein attenuates D-GalN induced liver fibrosis/chronic liver damage in rats by blocking the TGF-β/Smad signaling pathways. Chem. Biol. Interact. 261, 80–85. 10.1016/j.cbi.2016.11.022 [DOI] [PubMed] [Google Scholar]
- Gao B., Seki E., Brenner D. A., Friedman S., Cohen J. I., Nagy L., et al. (2011). Innate immunity in alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 300 (4), G516–G525. 10.1152/ajpgi.00537.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Sun Q., Li W., Lu Z. M., Xu H. Y., Shi J. S., et al. (2017). The common dietary flavonoid myricetin attenuates liver fibrosis in carbon tetrachloride treated mice. Mol. Nutr. Food Res. 61 (4), 1600392. 10.1002/mnfr.201600392 [DOI] [PubMed] [Google Scholar]
- Ghatak S., Biswas A., Dhali G. K., Chowdhury A., Boyer J. L., Santra A. (2011). Oxidative stress and hepatic stellate cell activation are key events in arsenic induced liver fibrosis in mice. Toxicol. Appl. Pharmacol. 251 (1), 59–69. 10.1016/j.taap.2010.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L. L., Yang S., Liu H., Zhang W., Ren L. L., Han F. F., et al. (2019). Anti-nociceptive and anti-inflammatory potentials of Akebia saponin D. Eur. J. Pharmacol. 845, 85–90. 10.1016/j.ejphar.2018.11.038 [DOI] [PubMed] [Google Scholar]
- Gong Y., Yang Y. (2020). Activation of nrf2/AREs-mediated antioxidant signalling, and suppression of profibrotic TGF-β1/smad3 pathway: A promising therapeutic strategy for hepatic fibrosis - a review. Life Sci. 256, 117909. 10.1016/j.lfs.2020.117909 [DOI] [PubMed] [Google Scholar]
- Gressner O. A., Lahme B., Rehbein K., Siluschek M., Weiskirchen R., Gressner A. M. (2008). Pharmacological application of caffeine inhibits TGF-beta-stimulated connective tissue growth factor expression in hepatocytes via PPARgamma and SMAD2/3-dependent pathways. J. Hepatol. 49 (5), 758–767. 10.1016/j.jhep.2008.03.029 [DOI] [PubMed] [Google Scholar]
- Guo M. Z., Li X. S., Xu H. R., Mei Z. C., Shen W., Ye X. F. (2002). Rhein inhibits liver fibrosis induced by carbon tetrachloride in rats. Acta Pharmacol. Sin. 23 (8), 739–744. [PubMed] [Google Scholar]
- Hamza A. A., Lashin F. M., Gamel M., Hassanin S. O., Abdalla Y., Amin A. (2020). Hawthorn herbal preparation from Crataegus oxyacantha attenuates in vivo carbon tetrachloride -induced hepatic fibrosis via modulating oxidative stress and inflammation. Antioxidants (Basel) 9 (12), E1173. 10.3390/antiox9121173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan H. F., Abdel-Rafei M. K., Galal S. M. (2017). Diosmin attenuates radiation-induced hepatic fibrosis by boosting PPAR-γ expression and hampering miR-17-5p-activated canonical Wnt-β-catenin signaling. Biochem. Cell. Biol. 95 (3), 400–414. 10.1139/bcb-2016-0142 [DOI] [PubMed] [Google Scholar]
- Hernández-Aquino E., Zarco N., Casas-Grajales S., Ramos-Tovar E., Flores-Beltrán R. E., Arauz J., et al. (2017). Naringenin prevents experimental liver fibrosis by blocking TGFβ-Smad3 and JNK-Smad3 pathways. World J. Gastroenterol. 23 (24), 4354–4368. 10.3748/wjg.v23.i24.4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L. S., Zhang Y. W., Li H., Wang W., Huan M. L., Zhou S. Y., et al. (2022). The regulatory role and mechanism of autophagy in energy metabolism-related hepatic fibrosis. Pharmacol. Ther. 234, 108117. 10.1016/j.pharmthera.2022.108117 [DOI] [PubMed] [Google Scholar]
- Hsu W. H., Liao S. C., Chyan Y. J., Huang K. W., Hsu S. L., Chen Y. C., et al. (2019). Graptopetalum paraguayense inhibits liver fibrosis by blocking TGF-β signaling in vivo and in vitro . Int. J. Mol. Sci. 20 (10), E2592. 10.3390/ijms20102592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y. C., Chiu Y. T., Cheng C. C., Wu C. F., Lin Y. L., Huang Y. T. (2007). Antifibrotic effects of tetrandrine on hepatic stellate cells and rats with liver fibrosis. J. Gastroenterol. Hepatol. 22 (1), 99–111. 10.1111/j.1440-1746.2006.04361.x [DOI] [PubMed] [Google Scholar]
- Hu H. H., Chen D. Q., Wang Y. N., Feng Y. L., Cao G., Vaziri N. D., et al. (2018). New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 292, 76–83. 10.1016/j.cbi.2018.07.008 [DOI] [PubMed] [Google Scholar]
- Hu N., Liu J., Xue X., Li Y. (2020a). The effect of emodin on liver disease -- comprehensive advances in molecular mechanisms. Eur. J. Pharmacol. 882, 173269. 10.1016/j.ejphar.2020.173269 [DOI] [PubMed] [Google Scholar]
- Hu N., Wang C., Dai X., Zhou M., Gong L., Yu L., et al. (2020b). Phillygenin inhibits LPS-induced activation and inflammation of LX2 cells by TLR4/MyD88/NF-κB signaling pathway. J. Ethnopharmacol. 248, 112361. 10.1016/j.jep.2019.112361 [DOI] [PubMed] [Google Scholar]
- Hu X. Q., Song Y. N., Wu R., Cai F. F., Zhang Y., Peng J. H., et al. (2019). Metabolic mechanisms of Fuzheng-Huayu formula against liver fibrosis in rats. J. Ethnopharmacol. 238, 111888. 10.1016/j.jep.2019.111888 [DOI] [PubMed] [Google Scholar]
- Hu Z., Qin F., Gao S., Zhen Y., Huang D., Dong L. (2018). Paeoniflorin exerts protective effect on radiation-induced hepatic fibrosis in rats via TGF-β1/Smads signaling pathway. Am. J. Transl. Res. 10 (3), 1012–1021. [PMC free article] [PubMed] [Google Scholar]
- Huang G. R., Wei S. J., Huang Y. Q., Xing W., Wang L. Y., Liang L. L. (2018). Mechanism of combined use of vitamin D and puerarin in anti-hepatic fibrosis by regulating the Wnt/β-catenin signalling pathway. World J. Gastroenterol. 24 (36), 4178–4185. 10.3748/wjg.v24.i36.4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Tanaka Y. (2020). Novel biomarkers for the management of chronic Hepatitis B. Clin. Mol. Hepatol. 26 (3), 261–279. 10.3350/cmh.2020.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., Yuan L., Tingting X. U., Hanshu L. I., Yang G., Jiang M., et al. (2016). Herbal medicine Gan-fu-kang downregulates Wnt/Ca2+ signaling to attenuate liver fibrogenesis in vitro and in vivo . Mol. Med. Rep. 13 (6), 4705–4714. 10.3892/mmr.2016.5148 [DOI] [PubMed] [Google Scholar]
- Jin H., Lian N., Zhang F., Chen L., Chen Q., Lu C., et al. (2016). Activation of PPARγ/P53 signaling is required for curcumin to induce hepatic stellate cell senescence. Cell. Death Dis. 7 (4), e2189. 10.1038/cddis.2016.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung J. Y., Kim H. G., Lee J. S., Cho J. H., Ahn Y. C., Lee D. S., et al. (2020). Anti-hepatofibrotic effects of CGX, a standardized herbal formula: A multicenter randomized clinical trial. Biomed. Pharmacother. 126, 110105. 10.1016/j.biopha.2020.110105 [DOI] [PubMed] [Google Scholar]
- Karsdal M. A., Manon-Jensen T., Genovese F., Kristensen J. H., Nielsen M. J., Sand J. M., et al. (2015). Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 308 (10), G807–G830. 10.1152/ajpgi.00447.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T., Brenner D. (2021). Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 18 (3), 151–166. 10.1038/s41575-020-00372-7 [DOI] [PubMed] [Google Scholar]
- Kocabayoglu P., Lade A., Lee Y. A., Dragomir A. C., Sun X., Fiel M. I., et al. (2015). β-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J. Hepatol. 63 (1), 141–147. 10.1016/j.jhep.2015.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D., Zhang F., Wei D., Zhu X., Zhang X., Chen L., et al. (2013). Paeonol inhibits hepatic fibrogenesis via disrupting nuclear factor-κB pathway in activated stellate cells: In vivo and in vitro studies. J. Gastroenterol. Hepatol. 28 (7), 1223–1233. 10.1111/jgh.12147 [DOI] [PubMed] [Google Scholar]
- Kong D., Zhang Z., Chen L., Huang W., Zhang F., Wang L., et al. (2020). Curcumin blunts epithelial-mesenchymal transition of hepatocytes to alleviate hepatic fibrosis through regulating oxidative stress and autophagy. Redox Biol. 36, 101600. 10.1016/j.redox.2020.101600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong R., Wang N., Luo H., Lu J. (2018). Hesperetin mitigates bile duct ligation-induced liver fibrosis by inhibiting extracellular matrix and cell apoptosis via the TGF-β1/smad pathway. Curr. Mol. Med. 18 (1), 15–24. 10.2174/1566524018666180608084947 [DOI] [PubMed] [Google Scholar]
- Kong Z., Liu R., Cheng Y. (2019). Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway. Biomed. Pharmacother. 109, 2043–2053. 10.1016/j.biopha.2018.11.030 [DOI] [PubMed] [Google Scholar]
- Kubo N., Saito R., Hamano K., Nagasawa M., Aoki F., Takei I., et al. (2014). Conophylline suppresses hepatic stellate cells and attenuates thioacetamide-induced liver fibrosis in rats. Liver Int. 34 (7), 1057–1067. 10.1111/liv.12328 [DOI] [PubMed] [Google Scholar]
- Lam P., Cheung F., Tan H. Y., Wang N., Yuen M. F., Feng Y. (2016). Hepatoprotective effects of Chinese medicinal herbs: A focus on anti-inflammatory and anti-oxidative activities. Int. J. Mol. Sci. 17 (4), 465. 10.3390/ijms17040465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan T., Jiang S., Zhang J., Weng Q., Yu Y., Li H., et al. (2022). Breviscapine alleviates NASH by inhibiting TGF-β-activated kinase 1-dependent signaling. Hepatology 76 (1), 155–171. 10.1002/hep.32221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan T., Yu Y., Zhang J., Li H., Weng Q., Jiang S., et al. (2021). Cordycepin ameliorates nonalcoholic steatohepatitis by activation of the AMP-activated protein kinase signaling pathway. Hepatology 74 (2), 686–703. 10.1002/hep.31749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. (2020). Advances in anti hepatic fibrotic therapy with Traditional Chinese Medicine herbal formula. J. Ethnopharmacol. 251, 112442. 10.1016/j.jep.2019.112442 [DOI] [PubMed] [Google Scholar]
- Li J., Chen K., Li S., Feng J., Liu T., Wang F., et al. (2016). Protective effect of fucoidan from Fucus vesiculosus on liver fibrosis via the TGF-β1/Smad pathway-mediated inhibition of extracellular matrix and autophagy. Drug Des. devel. Ther. 10, 619–630. 10.2147/dddt.S98740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Hu R., Xu S., Li Y., Qin Y., Wu Q., et al. (2017). Xiaochaihutang attenuates liver fibrosis by activation of Nrf2 pathway in rats. Biomed. Pharmacother. 96, 847–853. 10.1016/j.biopha.2017.10.065 [DOI] [PubMed] [Google Scholar]
- Li J., Li J., Li S., He B., Mi Y., Cao H., et al. (2012). Ameliorative effect of grape seed proanthocyanidin extract on thioacetamide-induced mouse hepatic fibrosis. Toxicol. Lett. 213 (3), 353–360. 10.1016/j.toxlet.2012.07.019 [DOI] [PubMed] [Google Scholar]
- Li J., Li X., Xu W., Wang S., Hu Z., Zhang Q., et al. (2015). Antifibrotic effects of luteolin on hepatic stellate cells and liver fibrosis by targeting AKT/mTOR/p70S6K and TGFβ/Smad signalling pathways. Liver Int. 35 (4), 1222–1233. 10.1111/liv.12638 [DOI] [PubMed] [Google Scholar]
- Li J., Liu P., Zhang R., Cao L., Qian H., Liao J., et al. (2011). Icaritin induces cell death in activated hepatic stellate cells through mitochondrial activated apoptosis and ameliorates the development of liver fibrosis in rats. J. Ethnopharmacol. 137 (1), 714–723. 10.1016/j.jep.2011.06.030 [DOI] [PubMed] [Google Scholar]
- Li L. Y., Yang C. C., Yang J. F., Li H. D., Zhang B. Y., Zhou H., et al. (2019). ZEB1 regulates the activation of hepatic stellate cells through Wnt/β-catenin signaling pathway. Eur. J. Pharmacol. 865, 172787. 10.1016/j.ejphar.2019.172787 [DOI] [PubMed] [Google Scholar]
- Li S., Tan H. Y., Wang N., Cheung F., Hong M., Feng Y. (2018). The potential and action mechanism of polyphenols in the treatment of liver diseases. Oxid. Med. Cell. Longev. 2018, 8394818. 10.1155/2018/8394818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Wang Q., Tao Y., Liu C. (2016). Swertiamarin attenuates experimental rat hepatic fibrosis by suppressing angiotensin II-angiotensin type 1 receptor-extracellular signal-regulated kinase signaling. J. Pharmacol. Exp. Ther. 359 (2), 247–255. 10.1124/jpet.116.234179 [DOI] [PubMed] [Google Scholar]
- Li X., Ge J., Zheng Q., Zhang J., Sun R., Liu R. (2020). Evodiamine and rutaecarpine from Tetradium ruticarpum in the treatment of liver diseases. Phytomedicine 68, 153180. 10.1016/j.phymed.2020.153180 [DOI] [PubMed] [Google Scholar]
- Li X., Li X., Huang N., Liu R., Sun R. (2018). A comprehensive review and perspectives on pharmacology and toxicology of saikosaponins. Phytomedicine 50, 73–87. 10.1016/j.phymed.2018.09.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang X., Han C., Wang X., Xing G., Zhou L., et al. (2013). Astragaloside IV suppresses collagen production of activated hepatic stellate cells via oxidative stress-mediated p38 MAPK pathway. Free Radic. Biol. Med. 60, 168–176. 10.1016/j.freeradbiomed.2013.02.027 [DOI] [PubMed] [Google Scholar]
- Li Z., Chen D., Jia Y., Feng Y., Wang C., Tong Y., et al. (2019). Methane-rich saline counteracts cholestasis-induced liver damage via regulating the TLR4/NF-κB/NLRP3 inflammasome pathway. Oxid. Med. Cell. Longev. 2019, 6565283. 10.1155/2019/6565283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wang Z., Dong F., Shi W., Dai W., Zhao J., et al. (2021). Germacrone attenuates hepatic stellate cells activation and liver fibrosis via regulating multiple signaling pathways. Front. Pharmacol. 12, 745561. 10.3389/fphar.2021.745561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B., Guo X. L., Jin J., Ma Y. C., Feng Z. Q. (2015). Glycyrrhizic acid inhibits apoptosis and fibrosis in carbon-tetrachloride-induced rat liver injury. World J. Gastroenterol. 21 (17), 5271–5280. 10.3748/wjg.v21.i17.5271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Li R., Cai M., Huang J., Huang W., Guo Y., et al. (2018). Andrographolide ameliorates liver fibrosis in mice: Involvement of TLR4/NF-κB and TGF-β1/smad2 signaling pathways. Oxid. Med. Cell. Longev. 2018, 7808656. 10.1155/2018/7808656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Yuan X., Tao L., Cheng Z., Dai X., Sheng X., et al. (2015). Xia-yu-xue decoction (XYXD) reduces carbon tetrachloride (CCl4)-induced liver fibrosis through inhibition hepatic stellate cell activation by targeting NF-κB and TGF-β1 signaling pathways. BMC Complement. Altern. Med. 15, 201. 10.1186/s12906-015-0733-1 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liu F., Zhang J., Qian J., Wu G., Ma Z. (2018). Emodin alleviates CCl4-induced liver fibrosis by suppressing epithelial-mesenchymal transition and transforming growth factor-β1 in rats. Mol. Med. Rep. 18 (3), 3262–3270. 10.3892/mmr.2018.9324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Dong F., Li G., Niu M., Zhang C., Han Y., et al. (2018). Liuweiwuling tablets attenuate BDL-induced hepatic fibrosis via modulation of TGF-β/Smad and NF-κB signaling pathways. J. Ethnopharmacol. 210, 232–241. 10.1016/j.jep.2017.08.029 [DOI] [PubMed] [Google Scholar]
- Liu J., Zhang Z., Gao J., Xie J., Yang L., Hu S. (2011). Downregulation effects of beta-elemene on the levels of plasma endotoxin, serum TNF-alpha, and hepatic CD14 expression in rats with liver fibrosis. Front. Med. 5 (1), 101–105. 10.1007/s11684-011-0111-4 [DOI] [PubMed] [Google Scholar]
- Liu L., Zhou Y., Dai D., Xia H., Zhao K., Zhang J. (2021). Antifibrotic effects of kangxian ruangan capsule on rats with nonalcoholic fatty liver fibrosis and hepatic stellate cells through regulation of TGF-β and TLR4 signaling pathways. Evid. Based. Complement. Altern. Med. 2021, 5649575. 10.1155/2021/5649575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Liu C. H., Wang H. N., Hu Y. Y., Liu C. (2002). Effect of salvianolic acid B on collagen production and mitogen-activated protein kinase activity in rat hepatic stellate cells. Acta Pharmacol. Sin. 23 (8), 733–738. [PubMed] [Google Scholar]
- Liu X., Huang K., Zhang R. J., Mei D., Zhang B. (2020). Isochlorogenic acid A attenuates the progression of liver fibrosis through regulating HMGB1/TLR4/NF-κB signaling pathway. Front. Pharmacol. 11, 582. 10.3389/fphar.2020.00582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., E Q., Zuo J., Tao Y., Liu W. (2013). Protective effect of Cordyceps polysaccharide on hydrogen peroxide-induced mitochondrial dysfunction in HL-7702 cells. Mol. Med. Rep. 7 (3), 747–754. 10.3892/mmr.2012.1248 [DOI] [PubMed] [Google Scholar]
- Liu Y., Wen P. H., Zhang X. X., Dai Y., He Q. (2018). Breviscapine ameliorates CCl4-induced liver injury in mice through inhibiting inflammatory apoptotic response and ROS generation. Int. J. Mol. Med. 42 (2), 755–768. 10.3892/ijmm.2018.3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. W., Huang Y. T. (2014). Inhibitory effect of tanshinone IIA on rat hepatic stellate cells. PLoS One 9 (7), e103229. 10.1371/journal.pone.0103229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y. T., Tsai Y. H., Wu S. J., Chen J. R., Chao J. C. (2011). Ginsenoside Rb1 inhibits cell activation and liver fibrosis in rat hepatic stellate cells. J. Med. Food 14 (10), 1135–1143. 10.1089/jmf.2010.1485 [DOI] [PubMed] [Google Scholar]
- Luk J. M., Wang X., Liu P., Wong K. F., Chan K. L., Tong Y., et al. (2007). Traditional Chinese herbal medicines for treatment of liver fibrosis and cancer: From laboratory discovery to clinical evaluation. Liver Int. 27 (7), 879–890. 10.1111/j.1478-3231.2007.01527.x [DOI] [PubMed] [Google Scholar]
- Ma X., Jiang Y., Wen J., Zhao Y., Zeng J., Guo Y. (2020). A comprehensive review of natural products to fight liver fibrosis: Alkaloids, terpenoids, glycosides, coumarins and other compounds. Eur. J. Pharmacol. 888, 173578. 10.1016/j.ejphar.2020.173578 [DOI] [PubMed] [Google Scholar]
- Martínez-Chantar M. L., Avila M. A., Lu S. C. (2020). Hepatocellular carcinoma: Updates in pathogenesis, detection and treatment. Cancers (Basel) 12 (10), E2729. 10.3390/cancers12102729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A., Herrera H., Block T. (2015). Glycosylation and liver cancer. Adv. Cancer Res. 126, 257–279. 10.1016/bs.acr.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H., Zhang Y., Huang Z., Lu B., Ji L. (2019). Lonicera japonica attenuates carbon tetrachloride-induced liver fibrosis in mice: Molecular mechanisms of action. Am. J. Chin. Med. 47 (2), 351–367. 10.1142/s0192415x19500174 [DOI] [PubMed] [Google Scholar]
- Mondal A., Gandhi A., Fimognari C., Atanasov A. G., Bishayee A. (2019). Alkaloids for cancer prevention and therapy: Current progress and future perspectives. Eur. J. Pharmacol. 858, 172472. 10.1016/j.ejphar.2019.172472 [DOI] [PubMed] [Google Scholar]
- Motawi T. M., Atta H. M., Sadik N. A., Azzam M. (2014). The therapeutic effects of bone marrow-derived mesenchymal stem cells and simvastatin in a rat model of liver fibrosis. Cell. biochem. Biophys. 68 (1), 111–125. 10.1007/s12013-013-9698-1 [DOI] [PubMed] [Google Scholar]
- Nakanishi K., Kaji K., Kitade M., Kubo T., Furukawa M., Saikawa S., et al. (2019). Exogenous administration of low-dose lipopolysaccharide potentiates liver fibrosis in a choline-deficient l-amino-acid-defined diet-induced murine steatohepatitis model. Int. J. Mol. Sci. 20 (11), E2724. 10.3390/ijms20112724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K., Osawa Y., Kimura K. (2018). Wnt/β-Catenin signaling as a potential target for the treatment of liver cirrhosis using antifibrotic drugs. Int. J. Mol. Sci. 19 (10), E3103. 10.3390/ijms19103103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes de Carvalho S., Helal-Neto E., de Andrade D. C., Costa Cortez E. A., Thole A. A., Barja-Fidalgo C., et al. (2013). Bone marrow mononuclear cell transplantation increases metalloproteinase-9 and 13 and decreases tissue inhibitors of metalloproteinase-1 and 2 expression in the liver of cholestatic rats. Cells Tissues Organs 198 (2), 139–148. 10.1159/000353215 [DOI] [PubMed] [Google Scholar]
- Park E. J., Zhao Y. Z., Kim J., Sohn D. H. (2006). A ginsenoside metabolite, 20-O-beta-D-glucopyranosyl-20(S)-protopanaxadiol, triggers apoptosis in activated rat hepatic stellate cells via caspase-3 activation. Planta Med. 72 (13), 1250–1253. 10.1055/s-2006-947223 [DOI] [PubMed] [Google Scholar]
- Parola M., Pinzani M. (2019). Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Asp. Med. 65, 37–55. 10.1016/j.mam.2018.09.002 [DOI] [PubMed] [Google Scholar]
- Peng W., Qin R., Li X., Zhou H. (2013). Botany, phytochemistry, pharmacology, and potential application of polygonum cuspidatum Sieb.et Zucc.: A review. J. Ethnopharmacol. 148 (3), 729–745. 10.1016/j.jep.2013.05.007 [DOI] [PubMed] [Google Scholar]
- Pérez-Vargas J. E., Zarco N., Shibayama M., Segovia J., Tsutsumi V., Muriel P. (2014). Hesperidin prevents liver fibrosis in rats by decreasing the expression of nuclear factor-κB, transforming growth factor-β and connective tissue growth factor. Pharmacology 94 (1-2), 80–89. 10.1159/000366206 [DOI] [PubMed] [Google Scholar]
- Pinlaor S., Prakobwong S., Hiraku Y., Pinlaor P., Laothong U., Yongvanit P. (2010). Reduction of periductal fibrosis in liver fluke-infected hamsters after long-term curcumin treatment. Eur. J. Pharmacol. 638 (1-3), 134–141. 10.1016/j.ejphar.2010.04.018 [DOI] [PubMed] [Google Scholar]
- Povero D., Panera N., Eguchi A., Johnson C. D., Papouchado B. G., de Araujo Horcel L., et al. (2015). Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-γ. Cell. Mol. Gastroenterol. Hepatol. 1 (6), 646–663. e644. 10.1016/j.jcmgh.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradere J. P., Troeger J. S., Dapito D. H., Mencin A. A., Schwabe R. F. (2010). Toll-like receptor 4 and hepatic fibrogenesis. Semin. Liver Dis. 30 (3), 232–244. 10.1055/s-0030-1255353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puche J. E., Saiman Y., Friedman S. L. (2013). Hepatic stellate cells and liver fibrosis. Compr. Physiol. 3 (4), 1473–1492. 10.1002/cphy.c120035 [DOI] [PubMed] [Google Scholar]
- Qiao H., Han H., Hong D., Ren Z., Chen Y., Zhou C. (2011). Protective effects of baicalin on carbon tetrachloride induced liver injury by activating PPARγ and inhibiting TGFβ1. Pharm. Biol. 49 (1), 38–45. 10.3109/13880209.2010.493179 [DOI] [PubMed] [Google Scholar]
- Qiao M., Yang J., Zhu Y., Zhao Y., Hu J. (2020). Transcriptomics and proteomics analysis of system-level mechanisms in the liver of apigenin-treated fibrotic rats. Life Sci. 248, 117475. 10.1016/j.lfs.2020.117475 [DOI] [PubMed] [Google Scholar]
- Qin T., Wang M., Zhang T., Wang Y., Zhang Y., Hasnat M., et al. (2021). Total C-21 steroidal glycosides from baishouwu ameliorate hepatic and renal fibrosis by regulating IL-1β/MyD88 inflammation signaling. Front. Pharmacol. 12, 775730. 10.3389/fphar.2021.775730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J., Huang P., Zhang L., Qiu Y., Qi H., Leng A., et al. (2020). Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol. 161, 24–34. 10.1016/j.ijbiomac.2020.05.196 [DOI] [PubMed] [Google Scholar]
- Qu L. L., Yu B., Li Z., Jiang W. X., Jiang J. D., Kong W. J. (2016). Gastrodin ameliorates oxidative stress and proinflammatory response in nonalcoholic fatty liver disease through the AMPK/Nrf2 pathway. Phytother. Res. 30 (3), 402–411. 10.1002/ptr.5541 [DOI] [PubMed] [Google Scholar]
- Rawat D., Shrivastava S., Naik R. A., Chhonker S. K., Mehrotra A., Koiri R. K. (2018). An overview of natural plant products in the treatment of hepatocellular carcinoma. Anticancer. Agents Med. Chem. 18 (13), 1838–1859. 10.2174/1871520618666180604085612 [DOI] [PubMed] [Google Scholar]
- Richter K., Konzack A., Pihlajaniemi T., Heljasvaara R., Kietzmann T. (2015). Redox-fibrosis: Impact of TGFβ1 on ROS generators, mediators and functional consequences. Redox Biol. 6, 344–352. 10.1016/j.redox.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. M., Mann D. A. (2010). Role of nuclear factor kappaB in liver health and disease. Clin. Sci. 118 (12), 691–705. 10.1042/cs20090549 [DOI] [PubMed] [Google Scholar]
- Roeb E. (2018). Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biol. 68-69, 463–473. 10.1016/j.matbio.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Roh Y. S., Seki E. (2013). Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J. Gastroenterol. Hepatol. 28 (0 1), 38–42. 10.1111/jgh.12019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong G., Chen Y., Yu Z., Li Q., Bi J., Tan L., et al. (2022). Synergistic effect of biejia-ruangan on fibrosis regression in patients with chronic hepatitis B treated with entecavir: A multicenter, randomized, double-blind, placebo-controlled trial. J. Infect. Dis. 225 (6), 1091–1099. 10.1093/infdis/jiaa266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong X., Liu J., Yao X., Jiang T., Wang Y., Xie F. (2019). Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell. Res. Ther. 10 (1), 98. 10.1186/s13287-019-1204-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Srivastava M., Saqib U., Liu D., Faisal S. M., Sugathan S., et al. (2016). Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int. Immunopharmacol. 40, 79–89. 10.1016/j.intimp.2016.08.026 [DOI] [PubMed] [Google Scholar]
- Ruart M., Chavarria L., Campreciós G., Suárez-Herrera N., Montironi C., Guixé-Muntet S., et al. (2019). Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury. J. Hepatol. 70 (3), 458–469. 10.1016/j.jhep.2018.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam A. M., Esmat A., Abdel-Naim A. B. (2018). Cucurbitacin-B attenuates CCl(4) -induced hepatic fibrosis in mice through inhibition of STAT-3. Chem. Biol. Drug Des. 91 (4), 933–941. 10.1111/cbdd.13160 [DOI] [PubMed] [Google Scholar]
- Sánchez-Crisóstomo I., Fernández-Martínez E., Cariño-Cortés R., Betanzos-Cabrera G., Bobadilla-Lugo R. A. (2019). Phytosterols and triterpenoids for prevention and treatment of metabolic-related liver diseases and hepatocellular carcinoma. Curr. Pharm. Biotechnol. 20 (3), 197–214. 10.2174/1389201020666190219122357 [DOI] [PubMed] [Google Scholar]
- Sang L., Wang X. M., Xu D. Y., Sang L. X., Han Y., Jiang L. Y. (2017). Morin enhances hepatic Nrf2 expression in a liver fibrosis rat model. World J. Gastroenterol. 23 (47), 8334–8344. 10.3748/wjg.v23.i47.8334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E., Brenner D. A. (2015). Recent advancement of molecular mechanisms of liver fibrosis. J. Hepatobiliary. Pancreat. Sci. 22 (7), 512–518. 10.1002/jhbp.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L., Liu Z., Ci L., Shuai C., Lv X., Li J. (2019). Research progress on the anti-hepatic fibrosis action and mechanism of natural products. Int. Immunopharmacol. 75, 105765. 10.1016/j.intimp.2019.105765 [DOI] [PubMed] [Google Scholar]
- Sharma A., Verma A. K., Kofron M., Kudira R., Miethke A., Wu T., et al. (2020). Lipopolysaccharide reverses hepatic stellate cell activation through modulation of cMyb, small mothers against decapentaplegic, and CCAAT/Enhancer-Binding protein C/EBP transcription factors. Hepatology 72 (5), 1800–1818. 10.1002/hep.31188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedid S. M., Abdel-Magied N., Saada H. N. (2019). Role of betaine in liver injury induced by the exposure to ionizing radiation. Environ. Toxicol. 34 (2), 123–130. 10.1002/tox.22664 [DOI] [PubMed] [Google Scholar]
- Shi H., Shi H., Ren F., Chen D., Chen Y., Duan Z. (2017). Naringin in Ganshuang Granule suppresses activation of hepatic stellate cells for anti-fibrosis effect by inhibition of mammalian target of rapamycin. J. Cell. Mol. Med. 21 (3), 500–509. 10.1111/jcmm.12994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M. J., Yan X. L., Dong B. S., Yang W. N., Su S. B., Zhang H. (2020). A network pharmacology approach to investigating the mechanism of Tanshinone IIA for the treatment of liver fibrosis. J. Ethnopharmacol. 253, 112689. 10.1016/j.jep.2020.112689 [DOI] [PubMed] [Google Scholar]
- Stanković V., Mihailović V., Mitrović S., Jurišić V. (2017). Protective and therapeutic possibility of medical herbs for liver cirrhosis. Rom. J. Morphol. Embryol. 58 (3), 723–729. [PubMed] [Google Scholar]
- Sun B., Karin M. (2008). NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene 27 (48), 6228–6244. 10.1038/onc.2008.300 [DOI] [PubMed] [Google Scholar]
- Sun H., Che Q. M., Zhao X., Pu X. P. (2010). Antifibrotic effects of chronic baicalein administration in a CCl4 liver fibrosis model in rats. Eur. J. Pharmacol. 631 (1-3), 53–60. 10.1016/j.ejphar.2010.01.002 [DOI] [PubMed] [Google Scholar]
- Sun J., Wu Y., Long C., He P., Gu J., Yang L., et al. (2018). Anthocyanins isolated from blueberry ameliorates CCl(4) induced liver fibrosis by modulation of oxidative stress, inflammation and stellate cell activation in mice. Food Chem. Toxicol. 120, 491–499. 10.1016/j.fct.2018.07.048 [DOI] [PubMed] [Google Scholar]
- Sun M., Tan L., Hu M. (2021). The role of autophagy in hepatic fibrosis. Am. J. Transl. Res. 13 (6), 5747–5757. [PMC free article] [PubMed] [Google Scholar]
- Sun X. (2020). Research progress of traditional Chinese medicine in the treatment of liver fibrosis. Jiangsu Sci Technol. Inf. 37 (13), 35–38. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=KJXY202013009&DbName=CJFQ2020 [Google Scholar]
- Tacke F., Luedde T., Trautwein C. (2009). Inflammatory pathways in liver homeostasis and liver injury. Clin. Rev. Allergy Immunol. 36 (1), 4–12. 10.1007/s12016-008-8091-0 [DOI] [PubMed] [Google Scholar]
- Tai C. J., Choong C. Y., Lin Y. C., Shi Y. C., Tai C. J. (2016a). The anti-hepatic fibrosis activity of ergosterol depended on upregulation of PPARgamma in HSC-T6 cells. Food Funct. 7 (4), 1915–1923. 10.1039/c6fo00117c [DOI] [PubMed] [Google Scholar]
- Tai C. J., Choong C. Y., Shi Y. C., Lin Y. C., Wang C. W., Lee B. H., et al. (2016b). Solanum nigrum protects against hepatic fibrosis via suppression of hyperglycemia in high-fat/ethanol diet-induced rats. Molecules 21 (3), 269. 10.3390/molecules21030269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Hu W., Lv Y., Zhang W., Sun T., Jiang Y., et al. (2018). A polysaccharide from amusium Pleuronectes combined with praziquantel treatment ameliorates hepatic fibrosis in schistosoma japonicum-infected mice. Med. Sci. Monit. 24, 1597–1603. 10.12659/msm.909320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Wang N., Qiu T., Sun X. (2020). The role of autophagy and NLRP3 inflammasome in liver fibrosis. Biomed. Res. Int. 2020, 7269150. 10.1155/2020/7269150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teponno R. B., Kusari S., Spiteller M. (2016). Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 33 (9), 1044–1092. 10.1039/c6np00021e [DOI] [PubMed] [Google Scholar]
- Thompson M. D., Monga S. P. (2007). WNT/beta-catenin signaling in liver health and disease. Hepatology 45 (5), 1298–1305. 10.1002/hep.21651 [DOI] [PubMed] [Google Scholar]
- Trappoliere M., Caligiuri A., Schmid M., Bertolani C., Failli P., Vizzutti F., et al. (2009). Silybin, a component of sylimarin, exerts anti-inflammatory and anti-fibrogenic effects on human hepatic stellate cells. J. Hepatol. 50 (6), 1102–1111. 10.1016/j.jhep.2009.02.023 [DOI] [PubMed] [Google Scholar]
- Tsuchida T., Friedman S. L. (2017). Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 14 (7), 397–411. 10.1038/nrgastro.2017.38 [DOI] [PubMed] [Google Scholar]
- van der Pol A., van Gilst W. H., Voors A. A., van der Meer P. (2019). Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 21 (4), 425–435. 10.1002/ejhf.1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai K. K., Liang Y., Zhou L., Cai L., Liang C., Liu L., et al. (2015). The protective effects of Acanthus ilicifolius alkaloid A and its derivatives on pro- and anti-inflammatory cytokines in rats with hepatic fibrosis. Biotechnol. Appl. Biochem. 62 (4), 537–546. 10.1002/bab.1292 [DOI] [PubMed] [Google Scholar]
- Wallace M. C., Friedman S. L., Mann D. A. (2015). Emerging and disease-specific mechanisms of hepatic stellate cell activation. Semin. Liver Dis. 35 (2), 107–118. 10.1055/s-0035-1550060 [DOI] [PubMed] [Google Scholar]
- Wan Y., Wu Y. L., Lian L. H., Xie W. X., Li X., Ouyang B. Q., et al. (2012). The anti-fibrotic effect of betulinic acid is mediated through the inhibition of NF-κB nuclear protein translocation. Chem. Biol. Interact. 195 (3), 215–223. 10.1016/j.cbi.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Wang J. N., Li L., Li L. Y., Yan Q., Li J., Xu T. (2018). Emerging role and therapeutic implication of Wnt signaling pathways in liver fibrosis. Gene 674, 57–69. 10.1016/j.gene.2018.06.053 [DOI] [PubMed] [Google Scholar]
- Wang L., Lu W., Gao Y. H., Cao X., Pei F., Liu X. E., et al. (2019). Effect of Anluohuaxianwan on the expression of matrix metalloproteinases and their inhibitors in rat liver with fibrosis. Zhonghua Gan Zang Bing Za Zhi 27 (4), 267–273. 10.3760/cma.j.issn.1007-3418.2019.04.006 [DOI] [PubMed] [Google Scholar]
- Wang Q., Wen R., Lin Q., Wang N., Lu P., Zhu X. (2015). Wogonoside shows antifibrotic effects in an experimental regression model of hepatic fibrosis. Dig. Dis. Sci. 60 (11), 3329–3339. 10.1007/s10620-015-3751-4 [DOI] [PubMed] [Google Scholar]
- Wang R., Zhang D., Tang D., Sun K., Peng J., Zhu W., et al. (2021). Amygdalin inhibits TGFβ1-induced activation of hepatic stellate cells (HSCs) in vitro and CCl(4)-induced hepatic fibrosis in rats in vivo . Int. Immunopharmacol. 90, 107151. 10.1016/j.intimp.2020.107151 [DOI] [PubMed] [Google Scholar]
- Wang R., Zhang H., Wang Y., Song F., Yuan Y. (2017). Inhibitory effects of quercetin on the progression of liver fibrosis through the regulation of NF-кB/IкBα, p38 MAPK, and Bcl-2/Bax signaling. Int. Immunopharmacol. 47, 126–133. 10.1016/j.intimp.2017.03.029 [DOI] [PubMed] [Google Scholar]
- Wang S., Shi X. L., Feng M., Wang X., Zhang Z. H., Zhao X., et al. (2016). Puerarin protects against CCl4-induced liver fibrosis in mice: Possible role of PARP-1 inhibition. Int. Immunopharmacol. 38, 238–245. 10.1016/j.intimp.2016.06.008 [DOI] [PubMed] [Google Scholar]
- Wang S., Tang C., Zhao H., Shen P., Lin C., Zhu Y., et al. (2021). Network pharmacological analysis and experimental validation of the mechanisms of action of Si-Ni-san against liver fibrosis. Front. Pharmacol. 12, 656115. 10.3389/fphar.2021.656115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Fu T., Wang J., Wang C., Liu K., Wu J., et al. (2019). Hepatoprotection of yangonin against hepatic fibrosis in mice via farnesoid X receptor activation. Int. Immunopharmacol. 75, 105833. 10.1016/j.intimp.2019.105833 [DOI] [PubMed] [Google Scholar]
- Wang X., Ikejima K., Kon K., Arai K., Aoyama T., Okumura K., et al. (2011). Ursolic acid ameliorates hepatic fibrosis in the rat by specific induction of apoptosis in hepatic stellate cells. J. Hepatol. 55 (2), 379–387. 10.1016/j.jhep.2010.10.040 [DOI] [PubMed] [Google Scholar]
- Wang Y., Guan M., Zhao X., Li X. (2018). Effects of garlic polysaccharide on alcoholic liver fibrosis and intestinal microflora in mice. Pharm. Biol. 56 (1), 325–332. 10.1080/13880209.2018.1479868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang R., Wang Y., Peng R., Wu Y., Yuan Y. (2015). Ginkgo biloba extract mitigates liver fibrosis and apoptosis by regulating p38 MAPK, NF-κB/IκBα, and Bcl-2/Bax signaling. Drug Des. devel. Ther. 9, 6303–6317. 10.2147/dddt.S93732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Y., Lin S. Y., Chen W. Y., Liao S. L., Wu C. C., Pan P. H., et al. (2017). Glechoma hederacea extracts attenuate cholestatic liver injury in a bile duct-ligated rat model. J. Ethnopharmacol. 204, 58–66. 10.1016/j.jep.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Wu J., Huang G., Li Y., Li X. (2020). Flavonoids from aurantii fructus immaturus and aurantii fructus: Promising phytomedicines for the treatment of liver diseases. Chin. Med. 15, 89. 10.1186/s13020-020-00371-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Chen J. M., Xiao T. G., Shu X. B., Xu H. C., Yang L. L., et al. (2016). Qinggan Huoxue Recipe suppresses epithelial-to-mesenchymal transition in alcoholic liver fibrosis through TGF-β1/Smad signaling pathway. World J. Gastroenterol. 22 (19), 4695–4706. 10.3748/wjg.v22.i19.4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. H., Wang P. W., Lin T. Y., Yang P. M., Li W. T., Yeh C. T., et al. (2021). Antioxidant properties of red raspberry extract alleviate hepatic fibrosis via inducing apoptosis and transdifferentiation of activated hepatic stellate cells. Biomed. Pharmacother. 144, 112284. 10.1016/j.biopha.2021.112284 [DOI] [PubMed] [Google Scholar]
- Xi S., Shi M., Jiang X., Minuk G. Y., Cheng Y., Peng Y., et al. (2016). The effects of Tao-Hong-Si-Wu on hepatic necroinflammatory activity and fibrosis in a murine model of chronic liver disease. J. Ethnopharmacol. 180, 28–36. 10.1016/j.jep.2016.01.030 [DOI] [PubMed] [Google Scholar]
- Xiao H. M., Shi M. J., Jiang J. M., Cai G. S., Xie Y. B., Tian G. J., et al. (2022). Efficacy and safety of AnluoHuaxian pills on chronic Hepatitis B with normal or minimally elevated alanine transaminase and early liver fibrosis: A randomized controlled trial. J. Ethnopharmacol. 293, 115210. 10.1016/j.jep.2022.115210 [DOI] [PubMed] [Google Scholar]
- Xiao J., Lv Y., Jin F., Liu Y., Ma Y., Xiong Y., et al. (2017). LncRNA HANR promotes tumorigenesis and increase of chemoresistance in hepatocellular carcinoma. Cell. Physiol. biochem. 43 (5), 1926–1938. 10.1159/000484116 [DOI] [PubMed] [Google Scholar]
- Xie X., Dou C. Y., Zhou Y., Zhou Q., Tang H. B. (2021). MicroRNA-503 targets mothers against decapentaplegic homolog 7 enhancing hepatic stellate cell activation and hepatic fibrosis. Dig. Dis. Sci. 66 (6), 1928–1939. 10.1007/s10620-020-06460-7 [DOI] [PubMed] [Google Scholar]
- Xin L., Hu X. D., Tao Y. Y., Peng Y., Liu J. H. (2022). Action mechanism and active components of Fuzheng Huayu Recipe against liver fibrosis via regulating macrophage with network pharmacology and transcriptomics methods. China J. Chin. Materia Medica 47 (11), 3029–3037. 10.19540/j.cnki.cjcmm.20211214.401 [DOI] [PubMed] [Google Scholar]
- Xu F., Liu C., Zhou D., Zhang L. (2016). TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J. Histochem. Cytochem. 64 (3), 157–167. 10.1369/0022155415627681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L. M. (2020). Unscrambling of guideline on the diagnosis and treatment of hepatic fibrosis with integrated traditional Chinese and Western medicine (2019 Edition). Sh. J. TCM 54 (03), 29–31+52. 10.16305/j.1007-1334.2020.03.008 [DOI] [PubMed] [Google Scholar]
- Xu L., Yin L., Tao X., Qi Y., Han X., Xu Y., et al. (2017). Dioscin, a potent ITGA5 inhibitor, reduces the synthesis of collagen against liver fibrosis: Insights from SILAC-based proteomics analysis. Food Chem. Toxicol. 107, 318–328. 10.1016/j.fct.2017.07.014 [DOI] [PubMed] [Google Scholar]
- Xu Y., Xu W., Liu W., Chen G., Jiang S., Chen J., et al. (2021). Yiguanjian decoction inhibits macrophage M1 polarization and attenuates hepatic fibrosis induced by CCl(4)/2-AAF. Pharm. Biol. 59 (1), 1150–1160. 10.1080/13880209.2021.1961820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Li L., Qian S., Liu L. (2018). Evodiamine ameliorates liver fibrosis in rats via TGF-β1/Smad signaling pathway. J. Nat. Med. 72 (1), 145–154. 10.1007/s11418-017-1122-5 [DOI] [PubMed] [Google Scholar]
- Yang F. R., Fang B. W., Lou J. S. (2013). Effects of Fufang biejia ruangan pills on hepatic fibrosis in vivo and in vitro . World J. Gastroenterol. 19 (32), 5326–5333. 10.3748/wjg.v19.i32.5326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. J., Tao H., Huang C., Li J. (2013). Nuclear erythroid 2-related factor 2: A novel potential therapeutic target for liver fibrosis. Food Chem. Toxicol. 59, 421–427. 10.1016/j.fct.2013.06.018 [DOI] [PubMed] [Google Scholar]
- Yang Y., Sun M., Li W., Liu C., Jiang Z., Gu P., et al. (2021). Rebalancing TGF-β/Smad7 signaling via Compound kushen injection in hepatic stellate cells protects against liver fibrosis and hepatocarcinogenesis. Clin. Transl. Med. 11 (7), e410. 10.1002/ctm2.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H. J., Chen H. X., Li H. Y. (2022). Research progress of Compound Biejia Ruangan Tablet in the prevention and treatment of liver fibrosis. Chin. Med. J. Metallurgical Industry 39 (03), 261–263. 10.13586/j.cnki.yjyx1984.2022.03.104 [DOI] [Google Scholar]
- Ying H. Z., Chen Q., Zhang W. Y., Zhang H. H., Ma Y., Zhang S. Z., et al. (2017). PDGF signaling pathway in hepatic fibrosis pathogenesis and therapeutics (Review). Mol. Med. Rep. 16 (6), 7879–7889. 10.3892/mmr.2017.7641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Qin S. Y., Hu B. L., Qin Q. Y., Jiang H. X., Luo W. (2019). Resveratrol improves CCL4-induced liver fibrosis in mouse by upregulating endogenous IL-10 to reprogramme macrophages phenotype from M(LPS) to M(IL-4). Biomed. Pharmacother. 117, 109110. 10.1016/j.biopha.2019.109110 [DOI] [PubMed] [Google Scholar]
- Yu D. K., Zhang C. X., Zhao S. S., Zhang S. H., Zhang H., Cai S. Y., et al. (2015). The anti-fibrotic effects of epigallocatechin-3-gallate in bile duct-ligated cholestatic rats and human hepatic stellate LX-2 cells are mediated by the PI3K/Akt/Smad pathway. Acta Pharmacol. Sin. 36 (4), 473–482. 10.1038/aps.2014.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. L., Li J. H., Chengz R. G., Ma Y. M., Wang X. J., Liu J. C. (2014). Effect of matrine on transforming growth factor β1 and hepatocyte growth factor in rat liver fibrosis model. Asian pac. J. Trop. Med. 7 (5), 390–393. 10.1016/s1995-7645(14)60062-6 [DOI] [PubMed] [Google Scholar]
- Zhang C. Y., Yuan W. G., He P., Lei J. H., Wang C. X. (2016). Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 22 (48), 10512–10522. 10.3748/wjg.v22.i48.10512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Kong D., Lu Y., Zheng S. (2013). Peroxisome proliferator-activated receptor-γ as a therapeutic target for hepatic fibrosis: From bench to bedside. Cell. Mol. Life Sci. 70 (2), 259–276. 10.1007/s00018-012-1046-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Lu S., He J., Jin H., Wang F., Wu L., et al. (2018). Ligand activation of PPARγ by ligustrazine suppresses pericyte functions of hepatic stellate cells via SMRT-mediated transrepression of HIF-1α. Theranostics 8 (3), 610–626. 10.7150/thno.22237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Gao Y., Zhong M., Xu Y., Li J., Chen Y., et al. (2016). Hepatoprotective effects of Dicliptera chinensis polysaccharides on dimethylnitrosamine-induced hepatic fibrosis rats and its underlying mechanism. J. Ethnopharmacol. 179, 38–44. 10.1016/j.jep.2015.12.053 [DOI] [PubMed] [Google Scholar]
- Zhang T., Yang Y., Wang B., Wang L., Wang D., Cao N., et al. (2022). XSSJS inhibits hepatic fibrosis by promoting the miR-29b-3p/VEGFA axis in vitro and in vivo . Biosci. Rep. 42 (2), BSR20212241. 10.1042/bsr20212241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Xu Y., Chen J. M., Liu C., Du G. L., Zhang H., et al. (2017). Huang Qi decoction prevents BDL-induced liver fibrosis through inhibition of Notch signaling activation. Am. J. Chin. Med. 45 (1), 85–104. 10.1142/s0192415x17500070 [DOI] [PubMed] [Google Scholar]
- Zhang X. Y., Li M. M., Wang J., Wu X., Zhang G. L. (2019). Research progress of traditional Chinese medicine on signal pathways related to the treatment of liver fibrosis. J. Shanxi Univ. Chin. Med. 20 (06), 465–468. 10.19763/j.cnki.2096-7403.2019.06.25 [DOI] [Google Scholar]
- Zhang Y., Mao X., Chen W., Guo X., Yu L., Jiang F., et al. (2020). A discovery of clinically approved formula FBRP for repositioning to treat HCC by inhibiting PI3K/AKT/NF-κB activation. Mol. Ther. Nucleic Acids 19, 890–904. 10.1016/j.omtn.2019.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhao M., Jiang X., Qiao Q., Liu T., Zhao C., et al. (2021). Comprehensive analysis of fecal microbiome and metabolomics in hepatic fibrosis rats reveal hepatoprotective effects of yinchen wuling powder from the host-microbial metabolic Axis. Front. Pharmacol. 12, 713197. 10.3389/fphar.2021.713197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Z., Yao J. N., Zhang L. F., Wang C. F., Zhang X. X., Gao B. (2019). Effect of NLRC5 on activation and reversion of hepatic stellate cells by regulating the nuclear factor-κB signaling pathway. World J. Gastroenterol. 25 (24), 3044–3055. 10.3748/wjg.v25.i24.3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C. Q., Zhou Y., Ping J., Xu L. M. (2014). Traditional Chinese medicine for treatment of liver diseases: Progress, challenges and opportunities. J. Integr. Med. 12 (5), 401–408. 10.1016/s2095-4964(14)60039-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. W., Zhang Z. F., Chai X., Li G. Q., Cui H. R., Wang H. B., et al. (2016). Oxymatrine attenuates CCl4-induced hepatic fibrosis via modulation of TLR4-dependent inflammatory and TGF-β1 signaling pathways. Int. Immunopharmacol. 36, 249–255. 10.1016/j.intimp.2016.04.040 [DOI] [PubMed] [Google Scholar]
- Zhao S., Li N., Zhen Y., Ge M., Li Y., Yu B., et al. (2015). Protective effect of gastrodin on bile duct ligation-induced hepatic fibrosis in rats. Food Chem. Toxicol. 86, 202–207. 10.1016/j.fct.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Zhao Z., Lin C. Y., Cheng K. (2019). siRNA- and miRNA-based therapeutics for liver fibrosis. Transl. Res. 214, 17–29. 10.1016/j.trsl.2019.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Wang X., Zhang Y., Chen L., Hua L., Xu W. (2019). Pien-Tze-Huang ameliorates hepatic fibrosis via suppressing NF-κB pathway and promoting HSC apoptosis. J. Ethnopharmacol. 244, 111856. 10.1016/j.jep.2019.111856 [DOI] [PubMed] [Google Scholar]
- Zheng J., Ma L. T., Ren Q. Y., Li L., Zhang Y., Shi H. J., et al. (2014). The influence of astragalus polysaccharide and β-elemene on LX-2 cell growth, apoptosis and activation. BMC Gastroenterol. 14, 224. 10.1186/s12876-014-0224-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Zhang D., Wang T., Chen Z., Chen L., Wu H., et al. (2021). Target identification of hepatic fibrosis using Pien Tze Huang based on mRNA and lncRNA. Sci. Rep. 11 (1), 16980. 10.1038/s41598-021-96459-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Chen S., Li L., Wu T. (2017). The protective effect of hyperoside on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. Exp. Toxicol. Pathol. 69 (7), 451–460. 10.1016/j.etp.2017.04.001 [DOI] [PubMed] [Google Scholar]