Abstract
Introduction
Diarrhoea remains a leading cause of child morbidity and mortality. Systematically collected and analysed data on the aetiology of hospitalised diarrhoea in low-income and middle-income countries are needed to prioritise interventions.
Methods
We established the Global Pediatric Diarrhea Surveillance network, in which children under 5 years hospitalised with diarrhoea were enrolled at 33 sentinel surveillance hospitals in 28 low-income and middle-income countries. Randomly selected stool specimens were tested by quantitative PCR for 16 causes of diarrhoea. We estimated pathogen-specific attributable burdens of diarrhoeal hospitalisations and deaths. We incorporated country-level incidence to estimate the number of pathogen-specific deaths on a global scale.
Results
During 2017–2018, 29 502 diarrhoea hospitalisations were enrolled, of which 5465 were randomly selected and tested. Rotavirus was the leading cause of diarrhoea requiring hospitalisation (attributable fraction (AF) 33.3%; 95% CI 27.7 to 40.3), followed by Shigella (9.7%; 95% CI 7.7 to 11.6), norovirus (6.5%; 95% CI 5.4 to 7.6) and adenovirus 40/41 (5.5%; 95% CI 4.4 to 6.7). Rotavirus was the leading cause of hospitalised diarrhoea in all regions except the Americas, where the leading aetiologies were Shigella (19.2%; 95% CI 11.4 to 28.1) and norovirus (22.2%; 95% CI 17.5 to 27.9) in Central and South America, respectively. The proportion of hospitalisations attributable to rotavirus was approximately 50% lower in sites that had introduced rotavirus vaccine (AF 20.8%; 95% CI 18.0 to 24.1) compared with sites that had not (42.1%; 95% CI 33.2 to 53.4). Globally, we estimated 208 009 annual rotavirus-attributable deaths (95% CI 169 561 to 259 216), 62 853 Shigella-attributable deaths (95% CI 48 656 to 78 805), 36 922 adenovirus 40/41-attributable deaths (95% CI 28 469 to 46 672) and 35 914 norovirus-attributable deaths (95% CI 27 258 to 46 516).
Conclusions
Despite the substantial impact of rotavirus vaccine introduction, rotavirus remained the leading cause of paediatric diarrhoea hospitalisations. Improving the efficacy and coverage of rotavirus vaccination and prioritising interventions against Shigella, norovirus and adenovirus could further reduce diarrhoea morbidity and mortality.
Keywords: public health; epidemiology; infections, diseases, disorders, injuries; PCR; child health
What is already known on this topic
Aetiological studies using quantitative molecular diagnostics have primarily studied children in the community or those presenting to care, of which a minority are hospitalised.
Those studies have suggested that several pathogens other than rotavirus, including Shigella, had a higher or similar burden, especially after rotavirus vaccine introduction.
What this study adds
This study used standardised surveillance, laboratory and analysis protocols to define the aetiology of hospitalised paediatric diarrhoea using quantitative molecular diagnostics from a large, globally representative set of low-income and middle-income countries, including countries and geographies that were not well represented in the published literature.
Despite a substantial impact of rotavirus vaccination, rotavirus remained the leading cause of diarrhoea requiring hospitalisation. Shigella, norovirus and adenovirus 40/41 also had a high burden of disease, with some important geographic heterogeneity.
How this study might affect research, practice or policy
Despite increasing use of rotavirus vaccine globally, countries should expand its use and increase coverage, and strategies to improve rotavirus vaccine efficacy should be pursued.
Interventions targeting Shigella, norovirus and enteric group adenoviruses should be prioritised while recognising that the impacts of such interventions would be expected to vary by region.
Introduction
Despite steady declines in deaths from paediatric diarrhoea over the past three decades, diarrhoea remains a leading cause of death and disease in children less than 5 years of age, causing roughly half a million childhood deaths each year, predominantly in sub-Saharan Africa and south and southeast Asia.1 The introduction of rotavirus vaccines into national immunisation programmes decreased severe rotavirus diarrhoea in children by approximately 40% in low-income and middle-income countries (LMICs) from 2008 to 2016.2 Ongoing monitoring and surveillance of paediatric diarrhoea and its causes are needed to identify diarrhoea prevention and control strategies and guide the prioritisation and development of new vaccines for enteric pathogens.3 4
Diarrhoea requiring hospitalisation represents a consistently severe phenotype of this highly morbid syndrome. This proxy is explicitly used by groups that estimate the global burden of diarrhoeal deaths as diarrhoea severe enough to require hospitalisation should have a similar aetiological profile.1 5 6 Quantitative PCR (qPCR) has emerged as the preferred diagnostic approach for aetiological studies of diarrhoea,7 but most estimates using these diagnostics are from studies of primarily non-hospitalised diarrhoea.8–10 Thus, global estimates of aetiology-specific paediatric diarrhoea hospitalisations and mortality are driven by older studies of hospitalised diarrhoea that used a broad range of non-molecular diagnostics5 11 and studies of non-hospitalised diarrhoea with adjustments to approximate the expected aetiology in hospitalised diarrhoea.1 There is a dearth of globally representative, direct data on the aetiology of diarrhoea in hospitalised children using quantitative molecular diagnostics to inform global burden estimates and to monitor longitudinal trends in diarrhoea aetiology in the era of rotavirus vaccine use, particularly in LMICs where the burden is highest.12–14
To ensure high-quality data on rotavirus from high-burden countries with limited surveillance and laboratory capacity, the WHO has coordinated the Global Rotavirus Surveillance Network (GRSN) since 2008, enrolling children with acute watery diarrhoea and testing for rotavirus at national laboratories and regional reference laboratories (RRLs).15 16 In 2015, a retrospective pilot analysis of a limited number of non-randomly selected diarrhoeal specimens from children hospitalised for acute watery diarrhoea in 16 countries, predominantly from sub-Saharan Africa, trialled the use of a TaqMan Array card (TAC) qPCR assay system to broaden the list of aetiologies monitored in cases of diarrhoea enrolled in GRSN.17 In the current study, we leveraged this existing capacity to create the Global Pediatric Diarrhea Surveillance (GPDS) network, expanding the case definition to all hospitalised diarrhoea and including a globally representative selection of LMICs, with a primary goal of defining and monitoring trends in the causative enteropathogens in all children hospitalised with diarrhoea in these settings.
Methods
Study design and sentinel site selection
We performed a cross-sectional study of children hospitalised with diarrhoea. Sites were chosen from the broader GRSN to be broadly geographically representative. Sites were required to have a history of uninterrupted surveillance, with enrolment of a minimum of 100 cases of hospitalised diarrhoea in children less than 5 years of age per year. The identified surveillance sites expanded their case definition from acute, watery diarrhoea only to prospectively enrol all children admitted for diarrhoea, regardless of duration or the presence of blood.18 19 Thirty-three GRSN sentinel surveillance sites in 28 globally representative countries with a history of uninterrupted surveillance and high-quality data collection participated in prospective, active surveillance of all children less than 5 years of age hospitalised with diarrhoea (online supplemental table 1). The surveillance sites in Côte d’Ivoire, Ethiopia, Mauritius, Moldova and Zimbabwe aggregated patients from two or three surveillance hospitals within the same catchment area. Three GPDS countries had surveillance sites in more than one catchment area: China (three sites), India (three sites) and Zambia (two sites). Each WHO Region had three to six countries participate in GPDS, except the African region, which had 10, and the Eastern Mediterranean region, which was not included. We considered rotavirus vaccines to be introduced at each site if rotavirus vaccination had been added to the immunisation programme at the country or regional level by the end of 2017. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for observational studies is included in the online supplemental materials.
bmjgh-2022-009548supp001.pdf (1.1MB, pdf)
bmjgh-2022-009548supp002.pdf (168.8KB, pdf)
Case definition and enrolment
The inclusion criteria for enrolment in GPDS was any child aged 0–59 months hospitalised with diarrhoea. Diarrhoea was defined as three or more loose stools in a 24-hour period. Children transferred from another hospital or who developed diarrhoea during their hospitalisation were excluded. Caregivers of children enrolled in GPDS were asked about demographic and clinical characteristics of the cases, including reported duration of symptoms and reported or observed presence of blood in stool; medical records were also reviewed. Acute diarrhoea was defined as an episode with a duration prior to enrolment of fewer than 14 days, while longer episodes were considered persistent. Bloody diarrhoea was identified by caregiver report of blood in stool. Dehydration was noted and in a subset of cases, dehydration severity was estimated using the WHO severity scale.20 Deidentified data were used to maintain the anonymity of patients.
Specimen collection, selection, extraction and qPCR testing
Stool specimen collection was attempted from all enrolled cases. Specimens were stored at a maximum temperature of −20°C and at −80°C when possible. All specimens were tested for rotavirus using a commercial ELISA as previously described.2 To be eligible for random selection for qPCR testing, cases had to have a stool sample collected, and data on the duration of diarrhoea and presence of blood in the stool had to be available. From each surveillance site, 25 cases were randomly selected for qPCR testing for each 3 month quarter starting 1 January 2017 and ending 31 December 2018; the quarters included varied by site (online supplemental table 1). All participating sites contributed data and samples from both 2017 and 2018 except for Bolivia, which was added in 2018. Whole stool aliquots were then shipped with ice packs (or dry ice when possible) to an RRL and stored at −80°C prior to nucleic acid extraction.
All RRLs participated in site assessments and on-site training as well as annual external quality assurance and quality control exercises.19 Total nucleic acid extraction was performed as previously described with the Qiagen QIAamp Fast DNA Stool Mini kit (Qiagen, Hilden, Germany) using a modified protocol involving bead beating, with nucleic acid samples subsequently stored at −80°C prior to testing.17 All samples were spiked with Phocine herpes virus and MS2 phage to be used as external controls for DNA and RNA, respectively, and an extraction blank was included in each extraction batch to monitor for contamination. Sample testing using custom-designed TaqMan Array Cards (Thermo Fisher, Waltham, Massachusetts, USA) was performed as previously described.17 The array card included qPCR assays for 16 enteric pathogens that were associated with diarrhoea in two prior multisite studies that incorporated diarrhoeal cases and non-diarrhoeal controls (online supplemental table 2).9 10
Raw data analysis was carried out at each of the RRLs with fluorescence thresholds standardised for each qPCR assay between all eight RRLs using software appropriate to each laboratory’s instrument. Detections with a quantification cycle (Cq) value less than 35 were considered positive. Negative results were valid only when the corresponding external control was positive, while positive results were valid only when the corresponding extraction blank was negative for the target. We excluded data flagged by the PCR software.
Statistical analysis
Because samples were selected for qPCR testing by stratified random selection for each 3 month quarter, we applied inverse probability of selection weights such that cases selected for testing were representative of all enrolled cases and appropriately captured seasonal variation in diarrhoea aetiology. Specifically, we fit a generalised linear model with selection for qPCR testing as the outcome and site, 3 month quarter and an interaction between site and 3 month quarter as the covariates. Weights were then calculated as the inverse of the model-predicted probability. These weights were applied to all study estimates that used the subset of tested samples, including clinical characteristics, pathogen prevalence, attributable fractions (AFs) and incidence of hospitalised diarrhoea and attributable deaths.
Asymptomatic identification of enteropathogens is common in these settings, especially when using molecular testing methods, and non-diarrhoeal controls were not collected in the present study. Therefore, we attributed diarrhoea to specific enteropathogens based on pathogen quantity using models developed from qPCR reanalyses of two multisite aetiologic studies of diarrhoea that incorporated non-diarrhoeal controls, the GEMS case–control study and the MAL-ED birth cohort study.8–10 Model development for those studies has previously been described in detail.9 10 Briefly, using a conditional logistic regression model for GEMS and a generalised linear mixed-effects model with a random effect for each individual for MAL-ED, a model was fit for each pathogen to describe the association between pathogen quantity and diarrhoea, with a random slope for site to allow for variation in the strength of association between pathogen quantity and diarrhoea. The MAL-ED model was additionally adjusted for sex, age and TAC card batch. Pathogen quantity was defined as the log10 increase in pathogen quantity above the analytical cut-off based on the Cq, namely . A weighted population AF for each pathogen was then calculated for any stratum of j cases as , where wti is the episode-specific inverse probability weight, and ORi is the episode-specific and quantity-specific OR derived from the regression model.21 We propagated uncertainty from the AF estimates using a Monte Carlo approach, which simulated 1000 new OR estimates from a normal distribution with the mean derived from the model coefficients and variance–covariance from the covariance matrix.1 To optimise the alignment between GPDS sites and the sites in which attribution modelling had been previously performed, these 1000 estimates were obtained using site-specific model coefficients from GEMS and MAL-ED with a draw distribution determined by weights that minimised the root mean square error distance between the pathogen density distribution in cases at each GPDS site and the proportionally weighted aggregate distribution across the GEMS and MAL-ED sites. In a separate sensitivity analysis, 1000 AF estimates were obtained evenly from the 15 site-specific model coefficients. Point estimates and 95% CIs were then calculated as the median and 2.5th and 97.5th quantiles, respectively.
To estimate diarrhoea incidence, we used national-level estimates of the number of diarrhoea hospitalisations, deaths and under 5 populations in 2017 from the Global Burden of Disease (GBD) 2019 study. Estimates of the incidence of diarrhoea and diarrhoeal deaths as well as the under 5 population for 2017 were obtained as previously described.22 23 To estimate diarrhoea hospitalisations, we used the incidence of hospital admissions from International Classification of Diseases codes due to diarrhoea from 67 sources and 42 countries and found the ratio of hospital admissions to all diarrhoea episodes. This proportion represented the proportion of diarrhoea that required hospitalisation. We modelled this value using DisMod-MR 2.1, a hierarchical Bayesian meta-regression model1 with the healthcare access and quality index as a predictor.24 We then estimated the proportion of diarrhoea episodes in children under 5 that were hospitalised for each GPDS country and multiplied these by diarrhoea incidence to obtain the incidence of hospitalised diarrhoea. To estimate variance for all of these estimates, 1000 draw values were obtained from the posterior distribution and propagated through the analyses.
To obtain AF or attributable incidence (AI) estimates across multiple countries, country-level estimates were aggregated. First, for countries with more than one surveillance site, AFs were combined as the mean of each of the 1000 estimates, save for estimates stratified by vaccination status, where AF estimates from India were included independently from each site due to state-level rotavirus vaccine introduction. Then, for each country and pathogen, we calculated the number of pathogen-attributable hospitalisations as the product of one randomly sampled draw of the country-level AF and one randomly sampled draw of the number of diarrhoea hospitalisations; this was repeated 10 000 times. Each estimate was summed across all included countries to obtain the total number of attributable episodes for each pathogen and was then either divided by the under 5 population denominator to obtain AI estimates or divided by the total number of diarrhoea hospitalisations to obtain AF estimates. An analogous analysis was conducted to obtain attributable episodes and incidence estimates for diarrhoeal deaths.
Lastly, to extrapolate from GPDS countries to estimate global and regional all-cause and aetiology-specific diarrhoeal deaths, we used the GBD country-level estimates to obtain 1000 estimates of the proportion of diarrhoeal deaths in each WHO region that were from GPDS countries. For the African and South-East Asian Regions, we further stratified these estimates by countries that had and had not introduced rotavirus vaccine, and this stratification was performed at the state level for India due to state-level rotavirus vaccine introduction. We then divided the total number of all-cause and pathogen-attributable deaths in GPDS countries in each regional stratum by this proportion. Because no countries from the Eastern Mediterranean region (EMR) were included in GPDS, regional AF estimates for the African region were applied to the EMR countries located in Africa, and those for the South-East Asian region were applied to the EMR countries located in Asia. These regional estimates were then summed to obtain global estimates. For all analyses, point estimates and 95% CIs were derived from the median and 2.5th and 97.5th quantiles, respectively, of the estimate distributions. All analyses were conducted in R V.4.0.2.
Patient and public involvement
Patients and the public were not involved in the surveillance.
Reflexivity statement
To promote equitable authorship of research publications from international partnerships, we have included a reflexivity statement as a online supplemental appendix.
Results
From January 2017 to December 2018, 29 502 children under 5 years of age hospitalised with diarrhoea were enrolled from 33 surveillance sites in 28 countries (online supplemental table 1 and figure 1). Using GBD incidence and population estimates, these 28 countries included 51.4% (299 119/582 295) of all estimated diarrhoeal deaths and 49.9% (337 863 737/677 362 399) of the estimated global under 5 population. Most enrolled children were less than 2 years of age (22716, 77.0%)(table 1). Most cases were accompanied by vomiting and dehydration, and almost all patients received some form of rehydration therapy in the hospital. Of 20 471 cases for which dehydration was present and the severity was estimated, 6681 (32.6%) had severe dehydration. Of 26 728 (90.6%) cases that could be classified by both duration and presence of blood, 24 616 (92.1%) presented with acute watery diarrhoea, 1728 (6.5%) with acute bloody diarrhoea and 384 (1.4%) with watery or bloody persistent diarrhoea.
Table 1.
Demographic and clinical characteristics of patients less than 5 years of age hospitalised with diarrhoea enrolled in Global Pediatric Diarrhea Surveillance, 2017–2018
| Demographic and clinical characteristics | All enrolled cases | qPCR-tested cases (unweighted) | qPCR-tested cases (weighted)* |
| N | 29 502 | 5465 | 29 502 |
| Male sex | 17 171 (58.2) | 3226 (59.0) | 17 217 (58.4) |
| Female sex | 12 331 (41.8) | 2239 (41.0) | 12 285 (41.6) |
| Age (months) | |||
| 0–11 | 12 828 (43.5) | 2433 (44.5) | 12 806 (43.4) |
| 12–23 | 9888 (33.5) | 1764 (32.3) | 9785 (33.2) |
| 24–59 | 6786 (23.0) | 1268 (23.2) | 6911 (23.4) |
| Calendar quarter | |||
| January–March | 6905 (23.4) | 1161 (21.2) | 6905 (23.4) |
| April–June | 7262 (24.6) | 1493 (27.3) | 7262 (24.6) |
| July–September | 8122 (27.5) | 1384 (25.3) | 8122 (27.5) |
| October–December | 7213 (24.4) | 1427 (26.1) | 7213 (24.4) |
| WHO Region | |||
| African | 7880 (26.7) | 1658 (30.3) | 7880 (26.7) |
| Americas | 5414 (18.4) | 1012 (18.5) | 5414 (18.4) |
| European | 9164 (31.1) | 1000 (18.3) | 9164 (31.1) |
| South-East Asian | 3104 (10.5) | 839 (15.4) | 3104 (10.5) |
| Western Pacific | 3940 (13.4) | 956 (17.5) | 3940 (13.4) |
| Acute diarrhoea (<14 days) | 29 055 (98.5) | 5296 (98.5) | 29 097 (98.6) |
| Bloody diarrhoea | 1777 (6.6) | 277 (5.4) | 1614 (5.7) |
| Diarrhoeal duration (days) | 2 (1–4) | 2 (1–4) | 2 (1–4) |
| Vomiting | 20 074 (69.6) | 3606 (68.0) | 20 176 (69.7) |
| Dehydration | 20 478 (75.8) | 3412 (74.0) | 20 227 (74.9) |
| Rehydration therapy given | 26 288 (98.7) | 4818 (98.1) | 26 142 (99.2) |
| In-hospital deaths | 125 (0.5) | 35 (0.7) | 98 (0.4) |
Dichotomous estimates are shown as n (%), and continuous estimates are shown as median (IQR).
*Weighted Ns are rounded to the nearest integer.
Of these 26 728 cases, 25 688 (96.1%) had a stool sample collected and were eligible for random selection, of which 5465 (21.3%) were selected and tested by qPCR (online supplemental table 1). The demographic and clinical characteristics of the qPCR-tested cases were similar to those characteristics for all cases enrolled in GPDS after application of inverse probability of selection weights (table 1). Rotavirus was detected in 6246 of 25 983 children (24.0%) tested by EIA and in 1156 of 5041 children selected for qPCR testing (22.9%), while the weighted proportion in children selected for qPCR testing was 23.9%. Among all cases tested, the most prevalent pathogen detected, regardless of quantity, was rotavirus (weighted prevalence 9896, 33.5%), followed by adenovirus 40/41 (5667, 19.2%), norovirus (5324, 18.0%), Shigella (4224, 14.3%) and sapovirus (2938, 10.0%), with 12 pathogens present in at least 1% of stool samples (online supplemental figure 2).
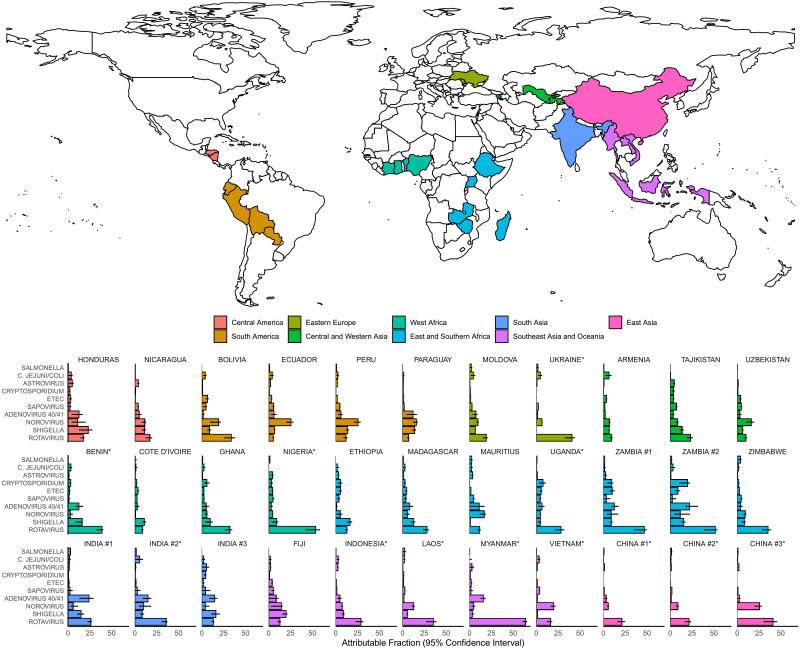
The top aetiologies of diarrhoea were consistent across this wide range of low-income and middle-income countries (figure 1 and online supplemental table 3). The leading aetiology of diarrhoea was rotavirus at 22 of 33 sites (66.7%), norovirus at 6 (18.2%) and Shigella at 5 (15.2%). Four pathogens had an AF of more than 5% at the majority of sites (rotavirus at all 33 sites, norovirus at 27 (81.8%), Shigella at 25 (75.8%) and adenovirus 40/41 at 18 (54.5%)). Enterotoxigenic Escherichia coli (ETEC) and Cryptosporidium had AFs of more than 5% at five sites, and for both pathogens, four of these sites were in East and Southern Africa.
Figure 1.
Pathogen-specific attributable fractions of hospitalised diarrhoea in children less than 5 years of age in 2017–2018 for the 33 surveillance sites from 28 countries in Global Pediatric Diarrhea Surveillance. The map indicates the country locations as well as their geographic groupings and the bar plots are coloured according to those groupings. Attributable fractions are expressed as a percent. *Rotavirus vaccine not introduced by 2017. ETEC, enterotoxigenic E. coli.
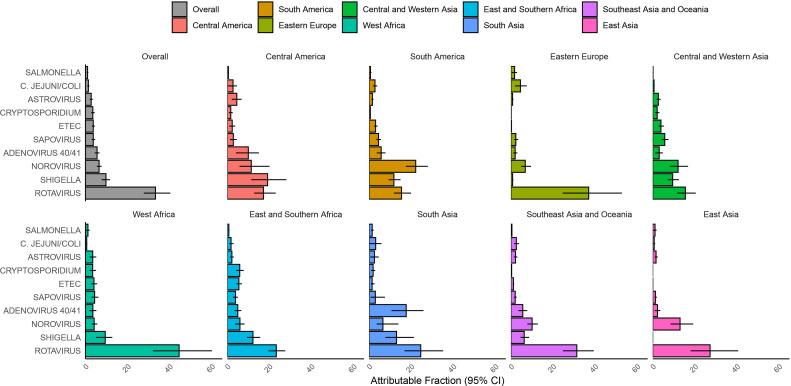
In this network, 72.6% of hospitalised diarrhoea was attributable to one of the pathogens included in this analysis, ranging from 47.3% in East Asia to 79.2% in West Africa and South Asia (figure 2 and online supplemental table 4). Overall, the leading aetiologies of hospitalised diarrhoea were rotavirus (AF 33.3%; 95% CI 27.7 to 40.3), Shigella (9.7%; 95% CI 7.7 to 11.6), norovirus (6.5%; 95% CI 5.4 to 7.6) and adenovirus 40/41 (5.5%; 95% CI 4.4 to 6.7). Rotavirus was the leading aetiology of diarrhoea in all regions, with the exception of the Americas, where all countries included in GPDS had introduced rotavirus vaccine into their national immunisation programme by 2010. In Central America, Shigella was the leading aetiology (AF 19.2%; 95% CI 11.4 to 28.1), while in South America, norovirus was the leading aetiology (22.2%; 95% CI 17.5 to 27.9). Some pathogens were particularly important for certain geographic settings, including adenovirus 40/41 in South and Southeast Asia, Vibrio cholerae in India and ETEC and Cryptosporidium in Africa.
Figure 2.
Pathogen-specific attributable fractions of hospitalised diarrhoea in children less than 5 years of age in 2017–2018 in Global Pediatric Diarrhea Surveillance both overall and by geographic region. Within each grouping and pathogen to attributable fractions were weighted by the site-level attributable incidence of hospitalised diarrhoea. Attributable fractions are expressed as a per cent. ETEC, enterotoxigenic E. coli.
Except for rotavirus and norovirus in the Americas and the Western Pacific, aetiology estimates were stable between 2017 and 2018 (online supplemental figure 3). Rotavirus was the leading aetiology of hospitalised diarrhoea across all age groups (0–11 months: AF 32.5%; 95% CI 26.6 to 39.6; 12–23 months: 33.5%; 95% CI 28.2 to 39.8; 24–59 months: 29.7%; 95% CI 25.1 to 35.1), while Shigella increased substantially in older age groups (0–11 months: AF 5.1%; 95% CI 3.8 to 6.2; 12–23 months: 12.9%; 95% CI 9.9 to 15.7; 24–59 months: 17.9%; 95% CI 14.0 to 21.4) (online supplemental table 5 and figure 4). Rotavirus was the leading aetiology of acute, watery diarrhoea, while Shigella was the leading aetiology of both bloody and persistent diarrhoea (online supplemental figure 5). The aetiological distribution was similar between males and females (online supplemental figure 6).
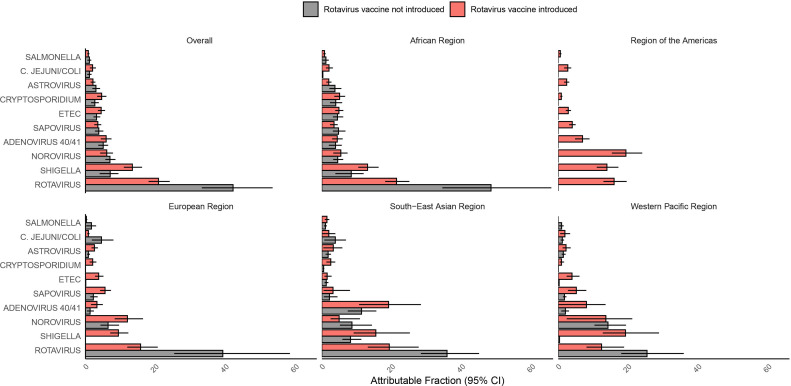
Rotavirus vaccines had been introduced at 21 (63.6%) of the 33 surveillance sites by the end of 2017. We compared the AF of rotavirus at sites where rotavirus vaccine had been introduced to those where it had not been introduced (figure 3 and online supplemental table 6). Overall, the rotavirus AF was slightly more than 50% lower in sites that had introduced rotavirus vaccine (AF 20.8%; 95% CI 18.0 to 24.1) compared with sites that had not (42.1%; 95% CI 33.2 to 53.4). Across the included WHO regions, the rotavirus AF was between 45% and 60% lower in sites that had introduced rotavirus vaccine. However, even in sites that had introduced rotavirus vaccine, rotavirus remained the leading aetiology of hospitalised diarrhoea both overall and in the African, European and South-East Asian regions. In the South-East Asian region, the burden of rotavirus (19.2%; 95% CI 13.1 to 27.6) and adenovirus 40/41 (19.0%; 10.6 to 28.3) was similar in countries that had introduced rotavirus vaccination.
Figure 3.
Pathogen-specific attributable fractions of hospitalised diarrhoea in children less than 5 years of age in 2017–2018 in Global Pediatric Diarrhea Surveillance both overall and by geographic region to by rotavirus vaccination introduction status as of 2017. For each grouping, attributable fractions were weighted by the site-level attributable incidence of hospitalised diarrhoea. Attributable fractions are expressed as a percent. ETEC, enterotoxigenic E. coli.
Comparing the overall AF with the prevalence of each pathogen using Cq cut-offs of 35 (the analytical limit of detection) and 30 (a more conservative cut-off to exclude low-quantity pathogen detections), the same five pathogens had the highest burden using all three metrics, and the overall weighted prevalence using a Cq cut-off of 30 roughly approximated the overall AF (online supplemental figure 7). In comparison with AFs estimated using an even number of draws from each site-specific model (online supplemental figure 8), the overall AF estimate using an optimised draw distribution changed by more than 20% for only two of the top 10 pathogens: norovirus (from 4.9% to 6.5%; +31.2%) and Campylobacter jejuni/coli (from 1.1 to 1.4%; +24.3%).
Using GBD incidence estimates for the GPDS countries, the incidence of all-cause diarrhoea hospitalisations was 19.2 per 1000 child-years (95% CI 17.0 to 21.9) and ranged from 3.9 (95% CI 2.8 to 5.4) in Eastern Europe to 61.3 (95% CI 48.0 to 78.4) in West Africa and 60.2 (95% CI 50.4 to 72.2) in East and Southern Africa. Overall, rotavirus had the highest AI of hospitalised diarrhoea in children (AI 6.4; 95% CI 5.3 to 7.8), followed by Shigella (1.9; 95% CI 1.5 to 2.2), norovirus (1.2; 95% CI 1.0 to 1.5) and adenovirus 40/41 (1.1; 95% CI 0.8 to 1.3) (table 2). The incidence of all-cause diarrhoeal deaths was 88.5 per 100 000 child-years (95% CI 76.2 to 103.0) and ranged from 0.8 (95% CI 0.6 to 1.1) in Eastern Europe to 358.1 (95% Ci 280.0 to 459.2) in West Africa. Rotavirus had the highest incidence of fatal diarrhoea (AI 34.0 per 100 000 child-years; 95% CI 27.1 to 42.2), followed by Shigella (9.6; 95% CI 6.9 to 12.0), norovirus (6.3; 95% CI 4.7 to 7.8) and adenovirus 40/41 (4.9; 95% CI 3.9 to 6.4).
Table 2.
Pathogen-specific attributable incidence with 95% CIs of diarrhoea hospitalisations and deaths in 2017–2018 both globally and by geographic grouping in children less than 5 years of age in Global Pediatric Diarrhea Surveillance countries
| All cause | Rotavirus | Shigella | Norovirus | Adenovirus 40/41 | Sapovirus | ETEC | Cryptosporidium | Astrovirus | C. jejuni/C. coli | Salmonella | |
| Hospitalisations* | 19.2 (17.0 to 21.9) | 6.4 (5.3 to 7.8) | 1.9 (1.5 to 2.2) | 1.2 (1.0 to 1.5) | 1.1 (0.8 to 1.3) | 0.7 (0.6 to 0.9) | 0.7 (0.6 to 0.9) | 0.7 (0.5 to 0.8) | 0.5 (0.4 to 0.7) | 0.3 (0.2 to 0.4) | 0.2 (0.1 to 0.3) |
| Central America | 17.2 (13.2 to 22.3) | 3.0 (2.2 to 4.0) | 3.3 (1.9 to 4.8) | 2.0 (1.0 to 3.5) | 1.7 (0.7 to 2.6) | 0.5 (0.2 to 0.8) | 0.4 (0.2 to 0.6) | 0.3 (0.1 to 0.5) | 0.8 (0.4 to 1.2) | 0.5 (0.0 to 0.8) | 0.1 (0.0 to 0.1) |
| South America | 10.8 (8.9 to 13.1) | 1.7 (1.3 to 2.2) | 1.3 (1.0 to 1.6) | 2.4 (1.9 to 3.0) | 0.6 (0.4 to 0.8) | 0.5 (0.4 to 0.6) | 0.3 (0.2 to 0.4) | 0.1 (0.0 to 0.1) | 0.2 (0.1 to 0.2) | 0.3 (0.2 to 0.4) | 0.1 (0.0 to 0.1) |
| Eastern Europe | 3.9 (2.8 to 5.4) | 1.4 (1.0 to 2.1) | 0.0 (0.0 to 0.0) | 0.3 (0.2 to 0.4) | 0.1 (0.0 to 0.1) | 0.1 (0.1 to 0.1) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) | 0.2 (0.1 to 0.3) | 0.1 (0.0 to 0.1) |
| Central and Western Asia |
4.4 (3.4 to 5.6) | 0.7 (0.5 to 0.9) | 0.4 (0.3 to 0.5) | 0.5 (0.4 to 0.7) | 0.1 (0.1 to 0.2) | 0.3 (0.2 to 0.3) | 0.2 (0.1 to 0.2) | 0.1 (0.1 to 0.1) | 0.1 (0.1 to 0.2) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) |
| West Africa | 61.3 (48.0 to 78.4) | 27.5 (19.7 to 36.9) | 5.8 (3.1 to 7.8) | 2.6 (1.8 to 3.4) | 2.2 (1.1 to 3.2) | 2.7 (1.8 to 3.8) | 2.5 (1.7 to 3.4) | 2.1 (1.2 to 3.1) | 2.1 (1.2 to 3.2) | 0.4 (0.2 to 0.6) | 0.7 (0.2 to 1.2) |
| East and Southern Africa |
60.2 (50.4 to 72.2) | 14.1 (11.8 to 16.6) | 7.4 (5.8 to 9.4) | 3.6 (2.2 to 5.0) | 3.0 (2.0 to 4.1) | 2.4 (1.6 to 3.1) | 3.3 (2.6 to 4.2) | 3.6 (2.5 to 4.7) | 1.3 (0.8 to 1.8) | 1.1 (0.5 to 1.8) | 0.4 (0.3 to 0.6) |
| South Asia | 4.9 (3.4 to 7.1) | 1.2 (0.8 to 1.8) | 0.7 (0.4 to 1.1) | 0.3 (0.2 to 0.7) | 0.9 (0.5 to 1.3) | 0.2 (0.0 to 0.4) | 0.1 (0.0 to 0.1) | 0.1 (0.0 to 0.1) | 0.1 (0.0 to 0.2) | 0.2 (0.0 to 0.3) | 0.1 (0.0 to 0.1) |
| Southeast Asia and Oceania | 14.3 (11.5 to 17.8) | 4.5 (3.6 to 5.7) | 0.9 (0.7 to 1.2) | 1.5 (1.1 to 1.8) | 0.8 (0.5 to 1.1) | 0.3 (0.2 to 0.4) | 0.2 (0.1 to 0.2) | 0.0 (0.0 to 0.1) | 0.3 (0.2 to 0.4) | 0.4 (0.2 to 0.5) | 0.1 (0.0 to 0.1) |
| East Asia | 6.2 (4.2 to 9.1) | 1.7 (1.1 to 2.5) | 0.0 (0.0 to 0.0) | 0.8 (0.5 to 1.2) | 0.1 (0.0 to 0.2) | 0.1 (0.1 to 0.1) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) | 0.1 (0.1 to 0.2) | 0.0 (0.0 to 0.1) | 0.1 (0.0 to 0.1) |
| Deaths† | 88.5 (76.2 to 103.0) | 34.0 (27.1 to 42.2) | 9.6 (6.9 to 12.0) | 6.3 (4.7 to 7.8) | 4.9 (3.9 to 6.4) | 3.6 (2.6 to 4.9) | 3.3 (2.6 to 4.1) | 3.0 (2.0 to 3.9) | 2.9 (2.0 to 3.8) | 1.2 (0.4 to 1.8) | 1.0 (0.5 to 1.4) |
| Central America | 33.0 (22.2 to 47.7) | 5.7 (3.7 to 8.5) | 6.9 (3.6 to 11.5) | 3.7 (1.3 to 6.0) | 3.9 (1.6 to 7.8) | 0.9 (0.3 to 1.6) | 0.9 (0.3 to 1.6) | 1.5 (0.6 to 2.6) | 0.6 (0.3 to 1.1) | 1.0 (0.0 to 1.9) | 0.2 (0.1 to 0.3) |
| South America | 11.6 (7.6 to 17.1) | 2.1 (1.2 to 3.7) | 1.3 (0.8 to 1.9) | 0.6 (0.3 to 0.9) | 2.6 (1.7 to 3.8) | 0.5 (0.3 to 0.8) | 0.4 (0.2 to 0.8) | 0.2 (0.1 to 0.3) | 0.1 (0.0 to 0.1) | 0.4 (0.2 to 0.6) | 0.1 (0.0 to 0.1) |
| Eastern Europe | 0.8 (0.6 to 1.1) | 0.3 (0.2 to 0.4) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) | 0.1 (0.0 to 0.1) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.1) | 0.0 (0.0 to 0.0) |
| Central and Western Asia | 16.8 (11.0 to 25.9) | 3.5 (2.1 to 5.7) | 2.1 (1.3 to 3.4) | 0.6 (0.2 to 1.0) | 1.6 (1.0 to 2.4) | 1.1 (0.7 to 1.8) | 0.7 (0.4 to 1.1) | 0.7 (0.4 to 1.1) | 0.5 (0.2 to 1.0) | 0.1 (0.0 to 0.1) | 0.0 (0.0 to 0.0) |
| West Africa | 358.1 (280.0 to 459.2) | 182.0 (131.7 to 241.6) | 33.2 (14.3 to 45.7) | 12.1 (4.8 to 18.5) | 16.0 (11.5 to 21.4) | 17.3 (11.4 to 24.1) | 15.4 (10.4 to 21.0) | 14.7 (8.3 to 21.3) | 12.1 (5.8 to 18.4) | 1.1 (0.6 to 1.7) | 4.5 (0.6 to 7.6) |
| East and Southern Africa | 161.2 (128.3 to 210.4) | 34.8 (27.6 to 43.8) | 21.9 (16.3 to 30.4) | 7.7 (4.5 to 10.9) | 9.4 (4.9 to 13.9) | 6.1 (3.7 to 8.7) | 9.1 (6.8 to 12.4) | 3.9 (2.4 to 6.0) | 9.4 (6.1 to 13.3) | 3.2 (1.4 to 5.6) | 0.8 (0.4 to 1.2) |
| South Asia | 52.8 (39.5 to 69.0) | 13.1 (9.7 to 17.5) | 7.0 (4.4 to 10.7) | 9.5 (5.9 to 12.8) | 3.5 (2.0 to 7.3) | 1.6 (0.4 to 3.9) | 0.8 (0.4 to 1.3) | 1.4 (0.3 to 2.3) | 1.0 (0.5 to 1.4) | 1.7 (0.0 to 3.0) | 0.8 (0.5 to 1.1) |
| Southeast Asia and Oceania | 38.8 (30.3 to 49.0) | 13.3 (10.1 to 17.3) | 3.1 (2.2 to 4.2) | 2.5 (1.4 to 3.6) | 2.9 (2.1 to 3.7) | 0.6 (0.4 to 0.8) | 0.4 (0.3 to 0.6) | 1.0 (0.6 to 1.4) | 0.1 (0.1 to 0.2) | 1.0 (0.4 to 1.6) | 0.2 (0.1 to 0.4) |
| East Asia | 2.3 (1.9 to 2.8) | 0.6 (0.5 to 0.8) | 0.0 (0.0 to 0.0) | 0.1 (0.0 to 0.1) | 0.3 (0.2 to 0.4) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) |
*Per 1000 child-years.
†Per 100 000 child-years.
ETEC, enterotoxigenic E. coli.
Using the aetiology distribution as a proxy for the aetiology of diarrhoeal deaths, we then extrapolated to estimate annual diarrhoeal deaths for WHO regions and globally (table 3). The top causes of diarrhoeal deaths were rotavirus (208 009 annual deaths; 95% CI 169 561 to 2 59 216), Shigella (62 853; 95% CI 48 656 to 78805), adenovirus 40/41 (36 922; 95% CI 28 469 to 46 672) and norovirus (35 914; 95% CI 27 258 to 46 516). Sapovirus, ETEC, Cryptosporidium, astrovirus, Campylobacter jejuni/coli and Salmonella were also estimated to be the cause of more than 5000 deaths per year.
Table 3.
Estimated all-cause and pathogen-attributable diarrhoeal deaths in 2017–2018 with 95% CIs both globally and by WHO region in children less than 5 years of age
| Global | African region | Region of the Americas | Eastern Mediterranean region* | European region | South-East Asian region | Western Pacific region | |
| All cause | 582 295 (493 241 to 683 788) | 396 459 (321 310 to 482 064) | 10 483 (7385 to 14 682) | 79 661 (56 853 to 108 689) | 1623 (1069 to 2597) | 84 565 (70 943 to 101 038) | 8175 (6057 to 10 868) |
| Rotavirus | 208 009 (169 561 to 259 216) | 148 931 (115 068 to 191 171) | 1857 (1221 to 2898) | 28 343 (20 445 to 39 430) | 342 (207 to 560) | 25 829 (20 780 to 31 466) | 2283 (1590 to 3307) |
| Shigella | 62 853 (48 656 to 78 805) | 43 947 (30 852 to 57 086) | 1570 (995 to 2376) | 7837 (5221 to 11 774) | 193 (116 to 319) | 9164 (6608 to 11 997) | 106 (54 to 211) |
| Adenovirus 40/41 | 36 922 (28 469 to 46 672) | 15 117 (9339 to 20 597) | 765 (439 to 1166) | 8182 (5333 to 12 275) | 54 (17 to 97) | 12 701 (9130 to 16 202) | 175 (100 to 250) |
| Norovirus | 35 914 (27 258 to 46 516) | 19 562 (13 936 to 26 002) | 1843 (1201 to 2883) | 5881 (3267 to 9851) | 156 (96 to 243) | 6960 (3958 to 11 553) | 1094 (816 to 1475) |
| Sapovirus | 22 704 (16 452 to 29 354) | 17 060 (12 249 to 22 275) | 396 (245 to 605) | 2539 (1176 to 4507) | 108 (65 to 185) | 2302 (578 to 4550) | 143 (103 to 208) |
| ETEC | 22 530 (17 762 to 28 869) | 18 879 (14 817 to 24 304) | 338 (205 to 559) | 1988 (1224 to 2971) | 63 (37 to 109) | 1158 (726 to 1700) | 28 (17 to 52) |
| Cryptosporidium | 19 905 (14 364 to 26 984) | 17 121 (11 950 to 23 540) | 116 (62 to 194) | 1553 (949 to 2527) | 51 (22 to 95) | 984 (664 to 1278) | 22 (8 to 55) |
| Astrovirus | 17 213 (12 095 to 22 573) | 13 208 (8547 to 18 064) | 289 (164 to 460) | 1832 (1077 to 2773) | 63 (32 to 110) | 1670 (862 to 2477) | 110 (80 to 151) |
| C. jejuni/C. coli | 9741 (4023 to 15 478) | 4130 (2144 to 6778) | 321 (150 to 503) | 2263 (372 to 4468) | 9 (5 to 16) | 3032 (291 to 5388) | 92 (53 to 153) |
| Salmonella | 6021 (3391 to 8442) | 3688 (1188 to 5794) | 55 (29 to 97) | 965 (614 to 1404) | 1 (0 to 3) | 1160 (788 to 1450) | 104 (44 to 175) |
*Aetiology-specific diarrhoeal deaths for countries in the Eastern Mediterranean region were estimated using attributable fractions from the African region for countries that are physically in Africa and from the South-East Asian region for countries that are physically in Asia.
ETEC, enterotoxigenic E. coli.
Discussion
By incorporating quantitative molecular diagnostics and pathogen attribution modelling into a global network with standardised surveillance, sample collection and testing, this study provides direct estimates of the aetiology of diarrhoea requiring hospitalisation in children under 5 years of age in LMICs in 2017–2018. Despite the substantial and consistent impact of rotavirus vaccine introduction across geographies, rotavirus remained the leading cause of diarrhoea hospitalisations. We estimated that rotavirus was still responsible for one in three diarrhoeal hospitalisations in these populations. By focusing on this most severe subset of diarrhoea, we additionally identified Shigella, adenovirus 40/41 and norovirus as pathogens with a high burden of disease and global relevance. While these four pathogens had a consistently high attributable burden of diarrhoea, a wide range of additional pathogens accounted for the remaining pathogen-attributable hospitalisations with significant geographic variation. The estimates were robust to several assumptions around the diagnostic and analytical approaches and due to the inclusion of a breadth of LMICs representing about half of the total global disease burden, the generalisability of the aggregated results to all LMICs should be high.
The impact of rotavirus vaccine introduction seen in this analysis was consistent with the 40% decline in rotavirus prevalence after vaccine introduction in the broader GRSN.2 However, rotavirus remained the leading cause of severe paediatric diarrhoea in LMICs outside of the Americas. The rotavirus AF remained high in some sites that had introduced rotavirus vaccine, a finding that might be explained by a number of factors including poor vaccine coverage, a high baseline burden of disease or a low prevalence of other pathogens. Individual vaccine status for patients enrolled was not available, nor was vaccine coverage in individual site catchment areas, thus we could not directly evaluate the impact of vaccine coverage. These findings support the need to continue to extend rotavirus vaccine introduction and coverage. WHO recommends that every country include rotavirus vaccine in their infant immunisation programmes,25 but fewer than half of infants born in 2018 globally were immunised against rotavirus.26 Efforts to improve the performance of rotavirus vaccines, which currently have 50–60% efficacy in low-income settings, are also critically important.27
This network, which added molecular diagnostic testing to a subset of sites from a broader existing surveillance system for rotavirus, provides a broader context for the relatively importance of additional enteric vaccines as well as a platform for evaluating vaccine impact after introduction.4 While vaccines against cholera are available, WHO recommends use of cholera vaccines only for specific endemic settings and during outbreaks,28 the burden of which cannot be adequately evaluated via sentinel surveillance. Vaccines against Shigella and norovirus are in development, and our findings support their broad potential impact on childhood morbidity and mortality in these settings.29 30 Finally, these data suggest the need for options to control and reduce the burden of enteric adenoviruses, which were also found to be common across many regions.31
Using diarrhoea hospitalisations as a proxy for diarrhoea mortality, our estimates of pathogen-specific diarrhoeal deaths showed some similarities as well as some important differences compared with the most recent modelled estimates.1 Similar to these estimates, we found rotavirus and Shigella to be the leading causes of diarrhoeal deaths, but we identified a substantially higher burden of norovirus and a lower burden of Cryptosporidium, Campylobacter and Salmonella. Norovirus is widely estimated to be the leading aetiology of hospitalised paediatric diarrhoea in high-income countries, but prior estimates of norovirus burden have been lower from LMICs.32 The low burden of Cryptosporidium was unexpected and may reflect that our surveillance sites miss areas of significant endemicity for this pathogen or that prior estimates extrapolated inappropriately from such areas. In our earlier pilot study,17 we found that Cryptosporidium was the second most common aetiology of diarrhoea in African countries, in contrast to being the fifth most common in this study. Seven of the 12 African countries included in the pilot were not included in this study because they did not meet the site inclusion criteria, and this study included an additional five countries from this region. In addition, the pilot study was from 2013 to 2014, 3–4 years prior to this study, and HIV infections in children have declined in Africa during this period,33 which could also contribute to the relatively low burden of Cryptosporidium found in the present study. Campylobacter and Salmonella may be relatively less likely to cause severe acute dehydration,10 and it is possible that the extrapolation of pathogen prevalence data from studies of non-hospitalised diarrhoea, for example, in studies such as GBD, may lead to higher estimates for these pathogens. This study makes a substantial contribution of data for reconciling these differences and improving model-based estimates. These findings are also mostly consistent with two multisite paediatric diarrhoea aetiology studies, GEMS and MAL-ED, which were performed across 15 sites in Asia, Africa and South America from 2007 to 2012 using the same diagnostic platform and provided the data for the attribution modelling used in this analysis.8–10 In those studies, the burden of norovirus was variable, and ETEC and Cryptosporidium were more consistently identified as important pathogens than in the present study. Approximately 20% of subjects in GEMS were hospitalised, while MAL-ED was a community-based cohort study in which hospitalisation for diarrhoea was rare.
We were able to identify an aetiology in approximately three-quarters of cases, which was within the bounds of the proportion of cases without an identified aetiology in multisite studies of community (MAL-ED: 65%)9 and moderate-to-severe diarrhoea (GEMS: 89%)8 using the same diagnostic platform. Relatively high-income sites included in GPDS, including in East Asia and Eastern Europe, where the pretest probability for infectious diarrhoea is likely lower, had less cumulative attribution to the included pathogens. This study did not enrol concurrent controls but rather used attribution models developed in these previous studies.8 9 These studies did not include countries from Europe and few from the Americas and used different case definitions than GPDS. Because these regions are expected to have less subclinical carriage of enteropathogens, the attribution models may have been overly conservative in assigning aetiology in these sites. The use of optimised draw distributions from the attribution models based on pathogen density in cases was designed to close this gap in settings with lower pathogen carriage. This approach increased attribution to norovirus and Campylobacter in particular, pathogens with particularly a high subclinical prevalence in highly endemic settings. Additionally, the overall weighted prevalence using a conservative Cq cut-off approximated the weighted AF estimate for the top pathogens, suggesting that our estimate of the aetiology hierarchy was robust to these assumptions around pathogen attribution.
Several other limitations are important to note. First, GPDS sentinel surveillance hospitals are predominantly but not exclusively referral hospitals in urban centres and thus may not be representative of all hospitalised diarrhoea in each country. Second, these sites were initially chosen for inclusion in the Global Rotavirus Surveillance Network. While we have expanded the case definition to all hospitalised diarrhoea, there may be some bias towards sites with high rotavirus incidence due to the initial site selection. Additionally, hospitalisation may not be a consistent indicator of disease severity across diverse settings. Third, while underlying malnutrition is a major risk factor for diarrhoea hospitalisation and death,34 35 nutritional status indicators were not collected from enrolled children, and thus we could not explore the impact of nutritional status on aetiology. Third, no countries from the Eastern Mediterranean Region were included and thus death estimates for this region required an assumption that diarrhoea aetiology from geographically proximate sites was representative. Fourth, because these sites do not have defined catchment areas, our extrapolation to incidence of diarrhoea hospitalisations and deaths relied on GBD model estimates. Finally, as healthcare access continues to improve, it is possible that the proxy of diarrhoea requiring hospitalisation will become less applicable for understanding the aetiology of fatal diarrhoea.
Conclusion
By leveraging existing sentinel surveillance in a globally representative range of LMICs and applying the best-available diagnostic and analytical methods, we provide a novel picture of the aetiology of hospitalised diarrhoea in children under 5 and substantially increase the available data for estimating aetiology-specific diarrhoeal deaths. These data identify a significant residual burden of rotavirus diarrhoea and associated deaths and identify Shigella, norovirus and adenovirus 40/41 as additional pathogens of global importance.
Acknowledgments
We would like to thank the sentinel surveillance hospitals and staff and the country Ministries of Health for supporting and maintaining surveillance at the country level.
Footnotes
Handling editor: Seye Abimbola
Twitter: @japlattsmills
ALC and JAP-M contributed equally.
Contributors: ALC, JP-M, ERH, UDP and FS conceived of and designed the study. JL and ERH developed the laboratory assays. ALC, FS, TN, SA, JaM, GW, GR-B, LdO, CO, DD, DV, SS, EN, MS, VG, BN and JL directed the surveillance activities and data collection. FS, DJO and TN were responsible for laboratory training and quality assurance and oversaw the laboratory analyses. GA, FD, MS, NM, JM, TF, IM, JPGL, ME, MD, ES, GS, DA, SG, IP, GK, ST, JB and NL oversaw or performed the laboratory analyses. HHK and MD prepared and provided demographic and disease incidence data. JP-M and TM conducted the data analysis with assistance from JET, SA, ALC, TN and ETRM. ALC and JP-M codrafted the manuscript. JP-M acts as the guarantor for this manuscript.
Funding: The study was funded by the Bill & Melinda Gates Foundation and Gavi, the Vaccine Alliance.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention. The funder had no role in data collection, analysis, interpretation, manuscript writing or the decision to submit the manuscript for publication. All authors had full access to the study data, reviewed the final manuscript and agreed with submission for publication.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: The reflexivity statement for this paper is linked as an online supplemental file 2.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The deidentified participant-level data used in these analyses will be made available on request to qualified researchers after publication of this manuscript, after approval of a proposal submitted to the corresponding author and signing of a data access agreement.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This activity was considered to be public health surveillance and thus exempt from ethical review. As previously mentioned, this work was considered to be public health surveillance and thus individual informed consent was not performed.
References
- 1.GBD 2016 Diarrhoeal Disease Collaborators . Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis 2018;18:1211–28. 10.1016/S1473-3099(18)30362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aliabadi N, Antoni S, Mwenda JM, et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008-16: findings from the global rotavirus surveillance network. Lancet Glob Health 2019;7:e893–903. 10.1016/S2214-109X(19)30207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Global strategy for comprehensive vaccine-preventable disease (VPD) surveillance. Available: https://www.who.int/publications/m/item/global-strategy-for-comprehensive-vaccine-preventable-disease-(vpd)-surveillance [Accessed 12 Apr 2022].
- 4.Cohen AL, Aliabadi N, Serhan F, et al. Using surveillance and economic data to make informed decisions about rotavirus vaccine introduction. Vaccine 2018;36:7755–8. 10.1016/j.vaccine.2018.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanata CF, Fischer-Walker CL, Olascoaga AC, et al. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One 2013;8:e72788. 10.1371/journal.pone.0072788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butkeviciute E, Prudden HJ, Jit M, et al. Global diarrhoea-associated mortality estimates and models in children: recommendations for dataset and study selection. Vaccine 2021;39:4391–8. 10.1016/j.vaccine.2021.05.086 [DOI] [PubMed] [Google Scholar]
- 7.Prudden HJ, Hasso-Agopsowicz M, Black RE, et al. Meeting report: who workshop on modelling global mortality and aetiology estimates of enteric pathogens in children under five. Cape Town, 28-29th November 2018. Vaccine 2020;38:4792–800. 10.1016/j.vaccine.2020.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013;382:209–22. 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Platts-Mills JA, Juma J, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 2016;388:1291–301. 10.1016/S0140-6736(16)31529-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platts-Mills JA, Liu J, Rogawski ET, et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health 2018;6:e1309–18. 10.1016/S2214-109X(18)30349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker CLF, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013;381:1405–16. 10.1016/S0140-6736(13)60222-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taniuchi M, Islam K, Sayeed MA, et al. Etiology of diarrhea requiring hospitalization in Bangladesh by quantitative polymerase chain reaction, 2014-2018. Clin Infect Dis 2021;73:e2493–9. 10.1093/cid/ciaa840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okada K, Wongboot W, Kamjumphol W, et al. Etiologic features of diarrheagenic microbes in stool specimens from patients with acute diarrhea in Thailand. Sci Rep 2020;10:4009. 10.1038/s41598-020-60711-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platts-Mills JA, Amour C, Gratz J, et al. Impact of rotavirus vaccine introduction and postintroduction etiology of diarrhea requiring hospital admission in Haydom, Tanzania, a rural African setting. Clin Infect Dis 2017;65:1144–51. 10.1093/cid/cix494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agócs MM, Serhan F, Yen C, et al. WHO global rotavirus surveillance network: a strategic review of the first 5 years, 2008-2012. MMWR Morb Mortal Wkly Rep 2014;63:634–7. [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . WHO global invasive bacterial vaccine-preventable disease and rotavirus and pediatric diarrhea surveillance networks Bulletin, February 2020. Available: https://mailchi.mp/d61958d8bbd1/who-ib-vpd-and-rotavirus-surveillance-bulletin-june-4779233?e [Accessed 12 Apr 2022].
- 17.Operario DJ, Platts-Mills JA, Nadan S, et al. Etiology of severe acute watery diarrhea in children in the global rotavirus surveillance network using quantitative polymerase chain reaction. J Infect Dis 2017;216:220–7. 10.1093/infdis/jix294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray J, Soenarto SY, Mulyani NS, et al. Multicountry Analysis of Spectrum of Clinical Manifestations of Children <5 Years of Age Hospitalized with Diarrhea. Emerg Infect Dis 2019;25:2253–6. 10.3201/eid2512.180712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . Meeting report: global rotavirus and pediatric diarrheal surveillance network meeting, 19-20 November 2019. Available: https://cdn.who.int/media/docs/default-source/immunization/vpd_surveillance/lab_networks/rota/global-rv-ibd-meeting-report-2019.pdf?sfvrsn=ce54740c_2 [Accessed 12 April 2022].
- 20.World Health Organization . The treatment of diarrhoea: a manual for physicians and other senior health workers, 4th revision, Geneva, Switzerland: WHO Press, 2005. Available: https://www.who.int/publications/i/item/9241593180 [Accessed 12 Apr 2022].
- 21.Heeringa SG, Berglund PA, West BT, et al. Attributable fraction estimation from complex sample survey data. Ann Epidemiol 2015;25:174–8. 10.1016/j.annepidem.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 22.GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GBD 2019 Demographics Collaborators . Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: a comprehensive demographic analysis for the global burden of disease study 2019. Lancet 2020;396:1160–203. 10.1016/S0140-6736(20)30977-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.GBD 2016 Healthcare Access and Quality Collaborators . Measuring performance on the healthcare access and quality index for 195 countries and territories and selected subnational locations: a systematic analysis from the global burden of disease study 2016. Lancet 2018;391:2236–71. 10.1016/S0140-6736(18)30994-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization . Rotavirus vaccines. WHO position paper – January 2013. Wkly Epidemiol Rec 2013;88:49–64. [PubMed] [Google Scholar]
- 26.World Health Organization/UNICEF . WHO/UNICEF coverage estimates. Available: https://www.who.int/teams/immunization-vaccines-and-biologicals/immunization-analysis-and-insights/global-monitoring/immunization-coverage/who-unicef-estimates-of-national-immunization-coverage [Accessed 12 Apr 2022].
- 27.Lee B. Update on rotavirus vaccine underperformance in low- to middle-income countries and next-generation vaccines. Hum Vaccin Immunother 2021;17:1787–802. 10.1080/21645515.2020.1844525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cholera vaccines: WHO position paper – August 2017. Wkly Epidemiol Rec 2017;92:477–98. [PubMed] [Google Scholar]
- 29.Mani S, Wierzba T, Walker RI. Status of vaccine research and development for Shigella. Vaccine 2016;34:2887–94. 10.1016/j.vaccine.2016.02.075 [DOI] [PubMed] [Google Scholar]
- 30.Mattison CP, Cardemil CV, Hall AJ. Progress on norovirus vaccine research: public health considerations and future directions. Expert Rev Vaccines 2018;17:773–84. 10.1080/14760584.2018.1510327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee B, Damon CF, Platts-Mills JA. Pediatric acute gastroenteritis associated with adenovirus 40/41 in low-income and middle-income countries. Curr Opin Infect Dis 2020;33:398–403. 10.1097/QCO.0000000000000663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopman BA, Steele D, Kirkwood CD, et al. The vast and varied global burden of norovirus: prospects for prevention and control. PLoS Med 2016;13:e1001999. 10.1371/journal.pmed.1001999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joint United Nations Programme on HIV/AIDS (UNAIDS) . Progress towards the start free, stay free, AIDS free targets: 2020 report. Available: https://www.unaids.org/sites/default/files/media_asset/start-free-stay-free-aids-free-2020-progress-report_en.pdf [Accessed 12 Apr 2022].
- 34.Irena AH, Mwambazi M, Mulenga V. Diarrhea is a major killer of children with severe acute malnutrition admitted to inpatient set-up in Lusaka, Zambia. Nutr J 2011;10:110. 10.1186/1475-2891-10-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acácio S, Mandomando I, Nhampossa T, et al. Risk factors for death among children 0-59 months of age with moderate-to-severe diarrhea in Manhiça district, southern Mozambique. BMC Infect Dis 2019;19:322. 10.1186/s12879-019-3948-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2022-009548supp001.pdf (1.1MB, pdf)
bmjgh-2022-009548supp002.pdf (168.8KB, pdf)
Data Availability Statement
Data are available on reasonable request. The deidentified participant-level data used in these analyses will be made available on request to qualified researchers after publication of this manuscript, after approval of a proposal submitted to the corresponding author and signing of a data access agreement.