Abstract
A 63-year-old man with a history of bipolar and schizoaffective disorder was admitted to the psychiatry unit. His comorbidities included active smoking, hypertension, diabetes, hyperlipidemia, coronary artery disease after coronary artery bypass grafting, and peripheral arterial disease. During the admission, the patient began to complain of right foot pain at rest. Angiography revealed occlusion of a previously placed right superficial femoral artery and popliteal stents, severe common femoral and distal popliteal stenosis with only a patent posterior tibial (PT) artery runoff. Serial venoplasty was performed and revealed an inadequately sized, ipsilateral great saphenous vein, followed by a delayed femoral–PT in situ saphenous vein bypass. Angiography at 32 months demonstrated a patent femoral–PT great saphenous vein bypass.
Keywords: Chronic limb-threatening ischemia, Great saphenous vein, In situ bypass, Peripheral arterial disease, Venoplasty
Case report
A 63-year-old man with a history of bipolar disorder and schizoaffective disorder had been initially admitted to the psychiatry unit because of agitation and substance abuse. His contributory comorbidities included active smoking with a 40 pack-year history, hypertension, non–insulin-dependent diabetes mellitus, hyperlipidemia, coronary artery disease, and peripheral arterial disease. His surgical history was notable for coronary artery bypass grafting with the left lower extremity great saphenous vein (GSV) in 2016 and stenting of the right superficial femoral artery (SFA) and popliteal artery. During the admission, he had begun to complain of increasing right foot pain at rest, which had progressively worsened over several days.
The physical examination revealed a warm, chronically ischemic right limb, no tissue loss, and intact sensory and motor function without signs of acute ischemia. Noninvasive vascular studies revealed a right posterior tibial (PT) ankle brachial index of 0.41 and a dorsalis pedis artery without detectable Doppler signals. The waveforms were low and monophasic throughout. Arterial duplex ultrasound (US) demonstrated an occluded right common femoral artery and SFA, and computed tomography angiography showed discontinuous runoff secondary to an occluded SFA and popliteal stents and diffuse calcification. The catheter-directed angiography findings were remarkable for occluded stents from the right SFA through the adductor hiatus into the popliteal artery with reconstitution at the distal PT artery (Fig 1, A and B). The distal popliteal artery showed severe stenosis into the tibioperoneal trunk, the anterior tibial and peroneal vessels were both occluded at their origins, and the PT artery was patent to the foot. Vein mapping (supine and without a tourniquet) of the right lower extremity (RLE) revealed a GSV with a diameter of <2 mm (range, 0.13-0.09 mm) throughout much of its length. The patient provided written informed consent for the report of his case details and imaging studies.
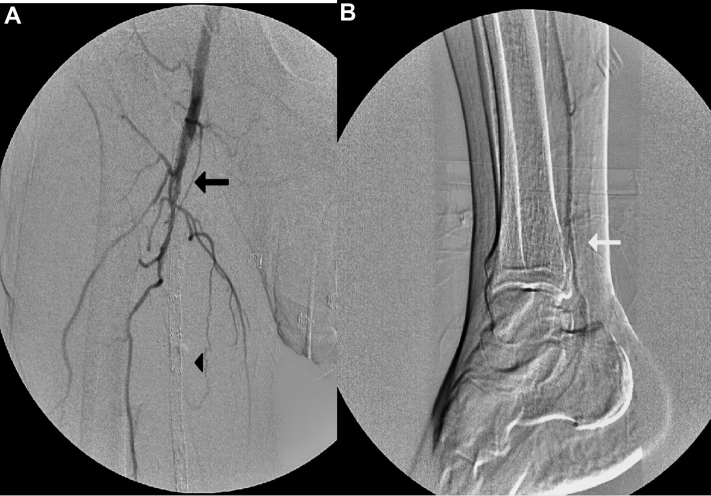
Fig 1.
A, Angiogram of right lower extremity (RLE) revealing an occluded superficial femoral artery (SFA) stent (black arrow and black arrowhead). B, Reconstitution of distal posterior tibial (PT) artery (white arrow).
In an effort to improve the caliber of the vein, we performed RLE venography (Fig 2), followed by balloon venoplasty of the entire right GSV using a 3-mm × 100-mm balloon, followed by a 4-mm × 100-mm balloon. A 4F, followed by a 5F, sheath was placed in the GSV at the ankle, and an angled Glidewire was maneuvered through the GSV into the saphenofemoral junction under US guidance. Postoperatively, a low-dose heparin infusion was started through a 4F microsheath left in the distal GSV and continued for 48 hours postoperatively. Duplex US scanning after venography of the RLE showed some recoil of the GSV, with a diameter of ≤3 mm. Subsequent venoplasty was delayed for 10 days secondary to a urinary tract infection (UTI). However, after resolution of the UTI, the patient was taken to the operating room for repeat balloon venoplasty and bypass. This dilation was successful, resulting in a right GSV with a diameter of ≤4 mm (range, 3-4 mm) on US. The vein appeared normal, with few signs of inflammation or damage. Because the stents were encroaching on the profunda femoral artery, the proximal SFA stents were explanted to facilitate performance of the proximal anastomosis of the planned bypass. Arteriotomy was performed in the tibioperoneal trunk and carried onto the PT artery with endarterectomy to remove plaque at the tibioperoneal trunk bifurcation and improve peroneal flow. Finally, femoral to PT artery bypass was performed, using the previously dilated in situ GSV (Fig 3). The hood of the vein was then sewn in at the common femoral artery bifurcation, and the distal anastomosis was sewn in at the tibioperoneal trunk and proximal PT arteriotomy site. An endovascular valvulotome (LeMaitre, Burlington, MA) was used to disrupt the valves before completion. Postoperatively, the examination was notable for a PT artery with biphasic Doppler signals, anterior tibial artery monophasic Doppler signals, and capillary refill of 1 to 2 seconds. The patient was discharged without ischemic rest pain with a prescription for aspirin and clopidogrel.
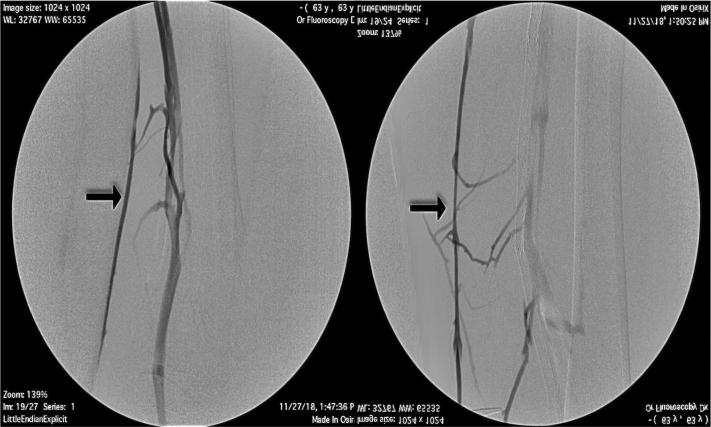
Fig 2.
Preoperative venogram of right lower extremity (RLE) great saphenous vein (GSV) showing a small caliber vein.
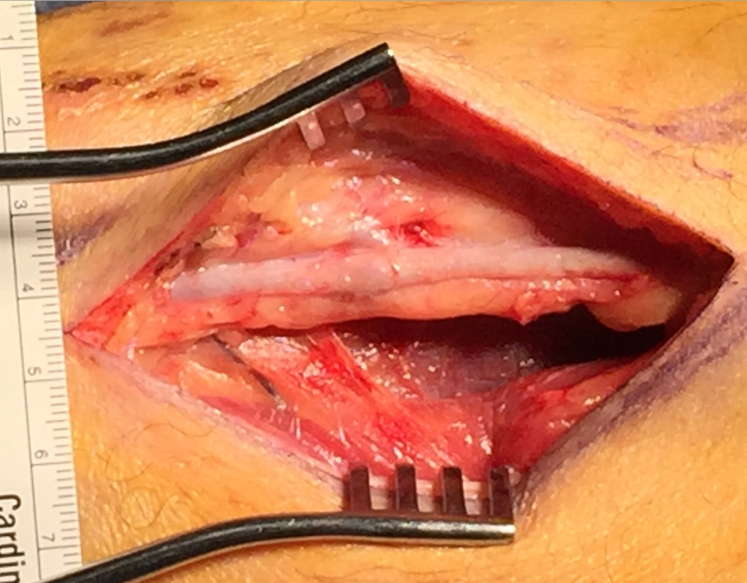
Fig 3.
Intraoperative distal great saphenous vein (GSV) after balloon angioplasty.
At the 7-month follow-up appointment, the patient continued to be free of rest pain and claudication and was successfully ambulating without assistance. Noninvasive imaging studies showed an ankle brachial index of 0.72, and duplex US revealed a patent bypass graft. The patient was subsequently lost to follow-up but had presented again at 30 months to an outside hospital with right toe pain. Angiography of the RLE revealed a patent right femoral to PT GSV bypass with mild mid-graft valve stenosis, which was treated with a stent, and stenosis in the PT distal to the bypass, for which angioplasty and atherectomy were performed. At 32 months, the patient had developed right third toe osteomyelitis and gangrene secondary to an injury. He underwent balloon angioplasty to treat the in-stent stenosis and, 2 days later, amputation of the right third toe. He was discharged with a prescription for daily aspirin and 2.5 mg of rivaroxaban.
Discussion
When endovascular revascularization strategies have been exhausted for the treatment of chronic limb-threatening ischemia, one option remaining is surgery. However, unsuitable veins often preclude their use and only synthetic grafts remain. The patency rates of synthetic grafts compared with autologous vein conduits, particularly in the setting of below-the-knee bypass, have improved, especially with heparin bonding, but have remained at 52% to 59.6% at 5 years.1,2 However, given that autologous conduits in below-the-knee peripheral bypass surgery have demonstrated a 68.9% 5-year patency, venous conduits are generally preferred compared with prosthetic bypass grafts.3
In an effort to extend and improve the quality of life in our highly ambulatory patient, we opted to use his marginally sized GSV by performing balloon dilation of the vein before an in situ bypass. Such balloon dilation is rooted in the practice of primary balloon angioplasty in small caliber veins before arteriovenous fistula creation with subsequent balloon-assisted maturation, yielding 85% arteriovenous fistula maturation rates as reported in some case series.4 Autologous veins are preferred in peripheral bypass surgery even for above-the-knee revascularization, with a single segment GSV remaining the optimal choice for below-the-knee targets.5, 6, 7, 8 However, veins will often not be functional secondary to quality (wall thickness, sclerotic/postphlebitic changes) and luminal diameter, with the most important, the luminal size and length necessary for revascularization. Together with adequate inflow and outflow, the luminal diameter is considered essential for vein graft patency, with a diameter >3 mm correlating with improved crural bypass patency. The 3-year patency for a minimum internal diameter of >3.0 mm was 66% ± 12% compared with 27% ± 12% with a diameter of <3.0 mm.9
Numerous important questions remain regarding which conduit will provide lasting patency when single segment GSV is unusable. Alternatives include prosthetic heparin-coated polytetrafluoroethylene (PTFE) or noncoated PTFE, with recent literature revealing the superiority of heparin-coated PTFE.10,11 Nonprosthetic options, including cryopreserved veins and alternate autogenous veins such as upper extremity veins, have demonstrated disappointing patency.12 However, when evaluating the 5-year overall limb salvage rate, autologous veins (90.0%) were far superior to heparin-coated PTFE (62.9%).13 Therefore, in our ambulatory patient, our goal in treating his chronic limb-threatening ischemia was achieved by using his inadequately sized autogenous vein after serial balloon dilations.
The present report had some limitations. The patient had undergone only one additional vein dilation other than that performed during the bypass. His extended stay was related to a UTI and not because of the bypass procedure. In the future, the first dilation could have been performed during his initial angiogram. Alternatively, it is possible that additional dilation had been unnecessary, which would have put this procedure on par, cost wise, with prosthetic bypass or arm vein harvest bypass. Additionally, the remnants of the GSV were not sent for pathologic analysis. Thus, although similar to balloon-assisted maturation for hemodialysis, it would be interesting to compare.
Conclusions
We have described the case of a patient who had undergone in situ GSV bypass via serial dilation of a previously unsuitable saphenous vein. Through this technique, we successfully avoided the need to use a prosthetic conduit. This technique could provide new insights into autologous options for patients with a seemingly inadequate vein conduit.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Gessaroli M., Tarantini S., Leone M., Fabbri E., Panzini I. A comparison of femorocrural bypasses performed with modified heparin-bonded expanded polytetrafluorethylene grafts and those with great saphenous vein grafts to treat critical limb ischemia. Ann Vasc Surg. 2015;29:1255–1264. doi: 10.1016/j.avsg.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 2.Samson R.H., Morales R., Showalter D.P., Lepore M.R., Nair D.G. Heparin-bonded expanded polytetrafluoroethylene femoropopliteal bypass grafts outperform expanded polytetrafluoroethylene grafts without heparin in a long-term comparison. J Vasc Surg. 2016;64:638–647. doi: 10.1016/j.jvs.2016.03.414. [DOI] [PubMed] [Google Scholar]
- 3.Pereira C.E., Albers M., Romiti M., Brochado-Neto F.C., Pereira C.A. Meta-analysis of femoropopliteal bypass grafts for lower extremity arterial insufficiency. J Vasc Surg. 2006;44:510–517. doi: 10.1016/j.jvs.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 4.De Marco Garcia L.P., Davila-Santini L.R., Feng Q., Calderin J., Krishnasastry K.V., Panetta T.F. Primary balloon angioplasty plus balloon angioplasty maturation to upgrade small-caliber veins (<3 mm) for arteriovenous fistulas. J Vasc Surg. 2010;52:139–144. doi: 10.1016/j.jvs.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Klinkert P., Schepers A., Burger D.H., van Bockel J.H., Breslau P.J. Vein versus polytetrafluoroethylene in above-knee femoropopliteal bypass grafting: five-year results of a randomized controlled trial. J Vasc Surg. 2003;37:149–155. doi: 10.1067/mva.2002.86. [DOI] [PubMed] [Google Scholar]
- 6.Johnson W.C., Lee K.K. A comparative evaluation of polytetrafluoroethylene, umbilical vein, and saphenous vein bypass grafts for femoral-popliteal above-knee revascularization: a prospective randomized Department of Veterans Affairs cooperative study. J Vasc Surg. 2000;32:268–277. doi: 10.1067/mva.2000.106944. [DOI] [PubMed] [Google Scholar]
- 7.Ambler G.K., Twine C.P. Graft type for femoro-popliteal bypass surgery. Cochrane Database Syst Rev. 2018;2:CD001487. doi: 10.1002/14651858.CD001487.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah D.M., Darling R.C., III, Chang B.B., Fitzgerald K.M., Paty P.S., Leather R.P. Long-term results of in situ saphenous vein bypass: analysis of 2058 cases. Ann Surg. 1995;222:438–446. doi: 10.1097/00000658-199510000-00003. discussion: 446, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishii Y., Gossage J.A., Dourado R., Sabharwal T., Burnand K.G. Minimum internal diameter of the greater saphenous vein is an important determinant of successful femorodistal bypass grafting that is independent of the quality of the runoff. Vascular. 2004;12:225–232. doi: 10.1258/rsmvasc.12.4.225. [DOI] [PubMed] [Google Scholar]
- 10.Dorigo W., Pulli R., Castelli P., Dorrucci V., Ferilli F., De Blasis G., et al. A multicenter comparison between autologous saphenous vein and heparin-bonded expanded polytetrafluoroethylene (ePTFE) graft in the treatment of critical limb ischemia in diabetics. J Vasc Surg. 2011;54:1332–1338. doi: 10.1016/j.jvs.2011.05.046. [DOI] [PubMed] [Google Scholar]
- 11.Neville R.F., Capone A., Amdur R., Lidsky M., Babrowicz J., Sidawy A.N. A comparison of tibial artery bypass performed with heparin-bonded expanded polytetrafluoroethylene and great saphenous vein to treat critical limb ischemia. J Vasc Surg. 2012;56:1008–1014. doi: 10.1016/j.jvs.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Avgerinos E.D., Sachdev U., Naddaf A., Doucet D.R., Mohapatra A., Leers S.A., et al. Autologous alternative veins may not provide better outcomes than prosthetic conduits for below-knee bypass when great saphenous vein is unavailable. J Vasc Surg. 2015;62:385–391. doi: 10.1016/j.jvs.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Uhl C., Grosch C., Hock C., Topel I., Steinbauer M. Comparison of long-term outcomes of heparin bonded polytetrafluoroethylene and autologous vein below knee femoropopliteal bypasses in patients with critical limb ischemia. Eur J Vasc Endovasc Surg. 2017;54:203–211. doi: 10.1016/j.ejvs.2017.05.001. [DOI] [PubMed] [Google Scholar]