Abstract
Serous endometrial cancer represents a relative rare entity accounting for about 10% of all diagnosed endometrial cancer, but it is responsible for 40% of endometrial cancer-related deaths. Patients with serous endometrial cancer are often diagnosed at earlier disease stage, but remain at higher risk of recurrence and poorer prognosis when compared stage-for-stage with endometrioid subtype endometrial cancer. Serous endometrial cancers are characterized by marked nuclear atypia and abnormal p53 staining in immunohistochemistry. The mainstay of treatment for newly diagnosed serous endometrial cancer includes a multi-modal therapy with surgery, chemotherapy and/or radiotherapy. Unfortunately, despite these efforts, survival outcomes still remain poor. Recently, The Cancer Genome Atlas (TCGA) Research Network classified all endometrial cancer types into four categories, of which, serous endometrial cancer mostly is found within the “copy number high” group. This group is characterized by the increased cell cycle deregulation (e.g., CCNE1, MYC, PPP2R1A, PIKCA, ERBB2 and CDKN2A) and TP53 mutations (90%). To date, the combination of pembrolizumab and lenvatinib is an effective treatment modality in second-line therapy, with a response rate of 50% in advanced/recurrent serous endometrial cancer. Owing to the unfavorable outcomes of serous endometrial cancer, clinical trials are a priority. At present, ongoing studies are testing novel combinations of various targeted and immunotherapeutic agents in newly diagnosed and advanced/recurrent endometrial cancer - an important strategy for serous endometrial cancer, whereby tumors are usually p53+ and pMMR, making response to PD-1 inhibitor monotherapy unlikely. Here, the rare tumor working group (including members from the European Society of Gynecologic Oncology (ESGO), Gynecologic Cancer Intergroup (GCIG), and Japanese Gynecologic Oncology Group (JGOG)), performed a narrative review reporting on the current landscape of serous endometrial cancer and focusing on standard and emerging therapeutic options for patients affected by this difficult disease.
Keywords: Endometrial cancer, Serous Uterine Cancer, Targeted therapy, Immunotherapy
Introduction
Endometrial cancer is one of the most common gynecological malignancies in developed countries, accounting for about 65,000 newly diagnosed cases estimated in the United States in 2020 [1]. A rising incidence of endometrial cancer by more than 20,000 new cases per year has been observed in the last decade [2], likely attributable to the ageing of populations and increased prevalence of obesity in the developed world [3]. Endometrioid carcinoma of the endometrium is the most common histological subtype of endometrial cancer accounting for 85–90% of cases [4] and is generally associated with a lower risk for progression and favorable prognosis, particularly for low-grade disease[4]. The most representative non-endometrioid endometrial carcinoma is uterine serous carcinoma (USC) that accounts for about 10% of all endometrial cancers. Other type non-endometrioid endometrial cancer included carcinosarcoma (2–5%), undifferentiated (5%), clear cell (2–4%), and squamous cell (0.1–0.5%) carcinoma [4]. Table 1 displays the most important differences between endometrioid and non-endometrioid endometrial cancer.
Table 1.
Clinicopathological and molecular features of endometrioid and non-endometrioid endometrial cancer.
| Endometrioid |
Non-endometroid |
|
|---|---|---|
| Frequency | 75–80% | 20–25% |
|
| ||
| Clinical characteristics | ||
| Median age at diagnosis | 50–60 years | 70–80 years |
| Obesity | Present | Absent |
| Hyperlipidemia | Present | Absent |
| Diabetes | Present | Absent |
| Hypertension | Present | Absent |
| Reproductive history | Frequent infertility | Normal |
| Pathological characteristics | ||
| Histology | Endometrioid | Serous, clear cells |
| Surrounding endometrium | Hyperplasia | Atrophy |
| FIGO grade | G1 and G2 | G3 |
| Myometrial invasion | Superficial | Deep |
| Nodal involvement | Infrequent | Frequent |
| Peritoneal involvement | Rare | Frequent |
| Receptor for estrogens/progesterone | + | − |
| Molecular characteristics | ||
| ARID1A | 25–50% | <10% |
| PTEN | 50–80% | <10% |
| KRAS | 20–40% | <10% |
| PIK3CA | 40–50% | 20–40% |
| PPP2R1A | <10% | 10–40% |
| CTNNB1 | 25% | 1% |
| TP53 | 10% | 80–90% |
| HER2 | 1% | 30–40% |
Abbreviations: yrs., years; FIGO, International Federation of Obstetrics and Gynecology.
Despite being the second most common type of endometrial cancer, USC is still considered a relatively rare tumor [5]. Patients with USC are often diagnosed at earlier disease stage compared to endometroid subtype endometrial cancer, but remain at a higher risk for relapse and have an overall worse prognosis when compared stage-for-stage with endometroid subtype endometrial cancer. Importantly, the rising incidence of USC as well as the fact that it is responsible for 40% of the total number of endometrial cancer-related deaths [6], has led to increasing interest in deciphering the biological features of USC [3]. In apparent early-stage disease (even in case of limited myometrial invasion), patients with USC are more likely to be detected with lymph vascular space invasion, nodal involvement, and microscopic diffusion to the peritoneal surfaces, leading to a 2.5-fold increased risk for apparent early-stage USC to be diagnosed with stage III and IV disease, compared to their endometroid-subtype counterparts (46% in USC vs. 20% in endometrioid endometrial cancer)[6]. Tumor biology plays an important role in influencing extra-uterine spread and the poor oncologic outcomes of patients with USC. In order to consolidate knowledge on USC the European Society of Gynecologic Oncology (ESGO), Gynecologic Cancer Intergroup (GCIG), and Japanese Gynecologic Oncology Group (JGOG) collaborate in the rare tumor working group (RTWG) to improve our collective understanding of USC diagnostic features and therapeutic landscape.
Pathological characteristics
USC commonly arises on the surface of endometrial polyps in the background of an atrophic endometrium. USC is microscopically characterized by (1) papillae with or without a fibrovascular core, (2) marked nuclear atypia, (3) slit-like spaces, (4) solid growth, (5) scant cytoplasm (but in few cases, it can be abundant with eosinophilia or clearing), and (6) numerous mitotic figures (in most cases). Additionally, gland-like spaces, cilia, and psammoma bodies might be observed in up to 30–40% of patients [7] (Figure 1). Unlikely serous ovarian cancer, USC should not be graded into low and high-grade serous carcinoma, but are considered high-grade disease by default. To the well-experienced gynecologic-pathologist, making the diagnosis of USC is not challenging, though it is important to note that histopathological characteristics of USC may overlap with those of International Federation of Gynecology and Obstetrics (FIGO) grade 3 endometrioid endometrial cancer, thus making difficult the diagnostic process [7]. These two entities may be distinguished via immohistochemical stains, the most useful of which are p53, p16, DNA mismatch repair proteins (MLH1, MSH2, MSH6 and PMS2), PTEN, PPP2R1A, and ARID1A. USPCs are characterized by the following abnormal stains: (1) p53, (2) p16 (not related to papillomavirus infection), (3) PAX8, (4) AE1 / AE3 and CK7 strong membranous staining. Meanwhile, USPCs are characterized by the negative stains for: (1) CK20, (2) ER/PR (focally positive in up 50% of cases), (3) WT-1 (focally positive in up 30% of cases). Generally, DNA mismatch repair proteins are retained but may show loss of at least one marker in 10% of cases [7, 8]. Interestingly, Ambros et al., reported endometrial intraepithelial carcinoma in 98% of USC lesions, in contrast to only 6% of endometrioid tumors [8].
Figure 1:
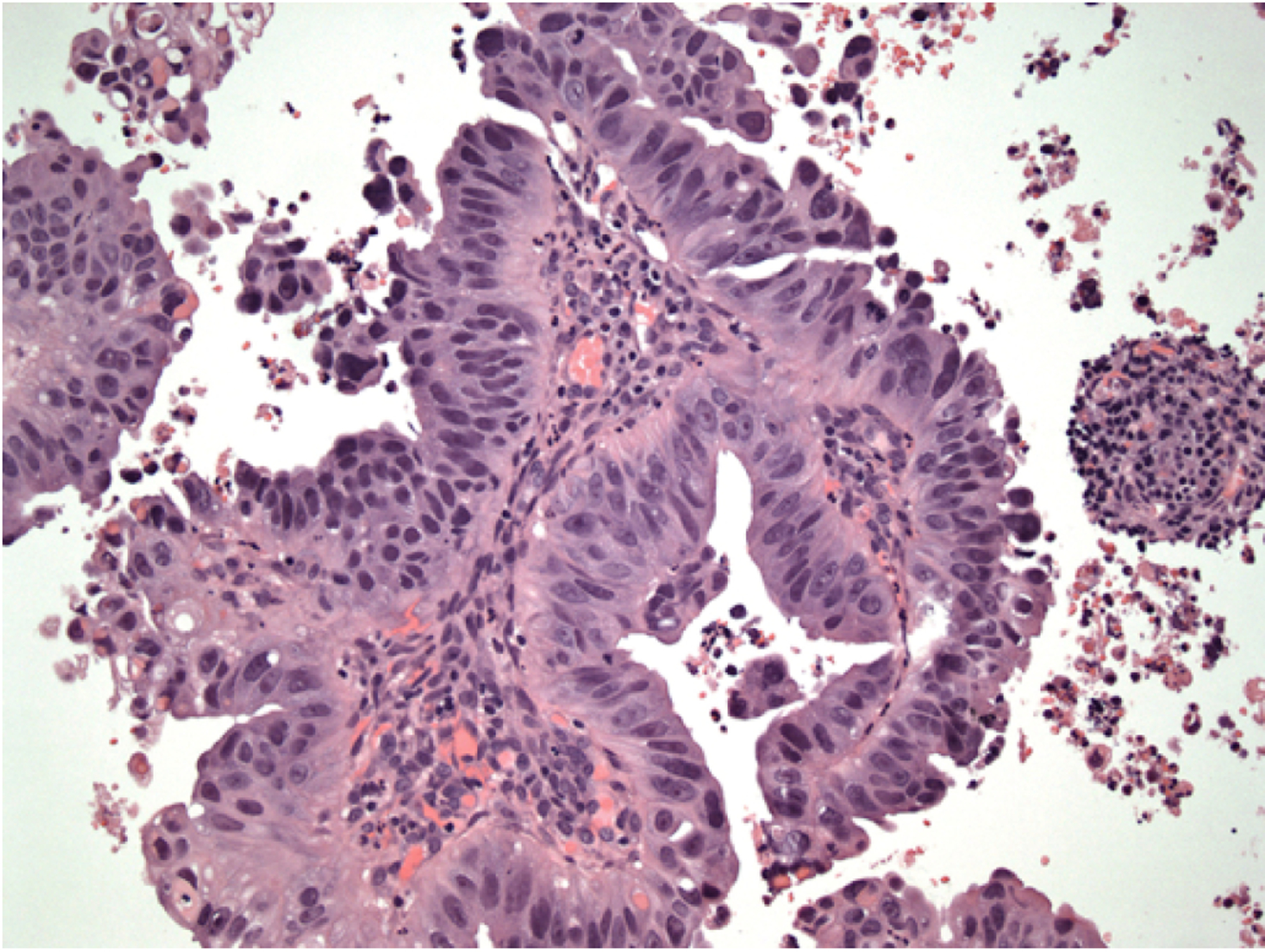
Histological features of a uterine serous papillary carcinoma
Molecular and genomic profile of USC
The Cancer Genome Atlas (TCGA) program was born thanks to the joint effort between the National Cancer Institute and the National Human Genome Research Institute [9]. The TCGA characterized various type of tumor including endometrial cancer. For this project, TCGA evaluated endometrial tumor samples and corresponding germline DNA from 373 patients, including 307 (82.3%) and 66 (17.7%) endometrioid and non-endometrioid cases, respectively. This latter group included 53 (14.2%) USC cases. One of the most important findings reported in the TCGA Research Network was that endometrial cancer shares genomic features with serous ovarian cancer, breast cancer (basal-like subtype), and colorectal cancer. Additionally, the TCGA classified endometrial cancer into four classes: (1) POLE ultra-mutated, (2) microsatellite instability hypermutated, (3) copy-number low, and (4) copy-number high (Table 2) [9]. This latter group included most of USPCs (50 out of 53 (94%)) [9]. Characteristics of USC included focal amplifications of the oncogenes MYC (8q24.12), ERBB2 (17q12), and CCNE1 (19q12) and other SCNAs previously unreported in other types of endometrial cancers (FGFR3 (4p16.3) and SOX17 (8q11.23)). The “copy-number high” group is also characterized by the increased cell cycle dysregulation (e.g., CCNE1, MYC, PPP2R1A, and CDKN2A) and TP53 mutations (90%). The prevalence of microsatellite instability (2–6% vs. 40%), and the PTEN mutations (11% vs. 84%) are lower than in endometrioid tumors. ERBB2 and PIK3CA are focally mutated / altered in 27% and 42% of cases in USC. The LRP1B deletion was also reported in this group [9]. This feature might have direct implication in clinical practice. LRP1B deletion correlates with resistance to liposomal doxorubicin in serous ovarian cancer [10]. Furthermore, Zhao et al., reported the genetic landscape of 57 USC [11]. Copy number variation (CNV) analysis identified frequent amplifications, including the well-known cancer genes PIK3CA (60%) and ERBB2 (encoding HER2/neu; 44%) [11]. ErbB2 overexpression has been previously reported to be associated with cancer cell proliferation, poor survival, and resistance to therapy in multiple human tumors including USC [12]. These particular mutational patterns might be useful in identifying specific molecular targets for USC.
Table 2.
The Cancer Genome Atlas Research Network stratification of endometrial cancer.
| Group | Frequency | Histology | FIGO grade | Mutation rate | Mutated genes |
|---|---|---|---|---|---|
|
| |||||
| POLE ultramutated | 4% of endometrioid tumors | Endometrioid | G1, G2, G3 | 232 × 106 / Mb | POLE (100%) |
| PTEN (>90%) | |||||
| ARID1A (75%) | |||||
| PIK3CA (70%) | |||||
| Microsatellite instability hypermutated | 39% of endometrioid tumors | Endometrioid | G1, G2, G3 | 18 × 106 / Mb | PTEN (85%) |
| PIK3CA (55%) | |||||
| PIK3R1 (40%) | |||||
| ARID1A (35%) | |||||
| Copy number low / microsatellite stable | 49% of endometrioid tumors | Endometrioid (most) | G1, G2 | 2.9 × 106 / Mb | PTEN (75%) |
| PIK3CA (50%) | |||||
| CTNNB1 (50%) | |||||
| ARID1A (40%) | |||||
| Copy number high / “serous” like | 9% of endometrioid tumors: typical for non-endometrioid | Non-endometroid Endometrioid | G3 | 2.3 × 106 / Mb | TP53 (90%) |
| PIK3CA (45%) | |||||
| PPP2R1A (>20%) | |||||
| HER2 (>20%) | |||||
Abbreviations: FIGO, International Federation of Obstetrics and Gynecology; Mb, megabase.
Surgical treatments
In early stage USC, surgery is the mainstay of treatment for patients. Hysterectomy plus bilateral salpingo-oophorectomy allows the removal of the primary tumor and to identify risk factors which may indicate the need for adjuvant treatment. Although no prospective studies investigated the role of the surgical approach specifically in USC, level A evidence from randomized control trials supports the adoption of minimally invasive surgery in apparent early-stage endometrial cancer, regardless of histological types [13]. Open and minimally invasive surgery provides superimposable long-term outcomes, but the latter approach correlates with better short-term perioperative outcomes in comparison to open surgery [13]. Peritoneal staging (with the execution of peritoneal biopsies and omentectomy) is recommended in USC. In apparent early-stage USC, microscopic omental involvement is reported to be between 2 and 17% [14, 15] and omental biopsy/omentectomy should be performed. Current guidelines recommend the adoption of retroperitoneal staging to assess the extent of disease and to provide information for adjuvant treatment decision [16, 17]. Sentinel node (SLN) mapping represents an emerging opportunity for patients affected by USC [18]. Four prospective cohort trials have shown high sensitivity to detect pelvic lymph node metastases and a high negative predictive value by applying a SLN algorithm in high-risk/high-grade endometrial carcinoma in the hands of experienced surgeons [17, 19–22]. The joint European guidelines of ESGO-ESTRO-ESP stated that lymph node staging should be performed in high-intermediate/high-risk disease and consider SLN biopsy an acceptable alternative to systematic lymphadenectomy for lymph node staging in stage I/II endometrial carcinoma, including USC [17]. It is important to point out that there is Level A evidence suggesting that systematic lymphadenectomy does not correlate with a survival benefit in early-stage endometrial carcinoma [17]. It is important to point out that the cumulative prevalence of USC in this trial is less than 3% [17]. The retrospective SEPAL trial reported that patients with intermediate- and high-risk endometrial cancer who had pelvic plus para-aortic lymphadenectomy experienced better overall survival than patients who had pelvic lymphadenectomy alone (HR: 0.53; 95%CI: 0.38–0.76; p<0.001) [23]. However, only 5% of patients included in the SEPAL trial were affected by USC [23].
Accumulating data support the safety and effectiveness of SLN mapping for apparent early-stage USC [24, 25]. The adoption of SLN mapping would be useful in detecting disease harbored in the nodes, reducing post-operative morbidity of lymphadenectomy [25]. In 2018, the National Comprehensive Cancer Network (NCCN) guidelines approved the execution of SLN mapping for staging purposes in high-risk endometrial cancer [16]. Recently, Nasioudis et al., reported patterns of use and outcomes of SLN mapping for patients with high-grade endometrial cancer (including USC) [18]. Evaluating data from the National Cancer Database, the authors observed that from the year 2012 to 2015, a rapid increase in the adoption of sentinel node mapping has occurred. Interestingly, comparing SLN mapping and lymphadenectomy, the prevalence of positive nodes and overall survival was similar [18].
For advanced USC, there are data that suggest that primary cytoreductive surgery may be considered in stage IV disease [26]. In advanced USC, cytoreductive surgery may be of benefit if macroscopic complete resection is feasible with acceptable morbidity. Surgery should be performed in a specialized center. In the case of advanced-stage disease (with peritoneal involvement), systematic lymphadenectomy should be omitted and only bulky nodes resected [16, 17]. Previous studies highlighted that residual disease represents one of the most important prognostic factors, thus supporting the need for extensive surgical cytoreduction in this setting [26]. However, neoadjuvant systemic chemotherapy is also a reasonable option in patients with stage IVB endometrial cancer, as this may reduce the burden of disease and allow easier cytoreduction at the time of interval debulking surgery [26]. Evaluating the data from the National Cancer Database, Tobias et al., described the impact of neoadjuvant chemotherapy on the survival outcomes of stage IV endometrial cancer [27]. Adopting a propensity score algorithm, the authors observed that the use of neoadjuvant chemotherapy displayed a time-varying association with survival outcomes. In the intention-to-treat analysis, neoadjuvant chemotherapy was associated with improved short-term outcomes such as decreased mortality for the first three months. These retrospective data should be interpreted with caution, given possible selection bias for the use of the neoadjuvant chemotherapy approach for patients with a more advanced disease burden. In light of these results, the authors concluded that neoadjuvant chemotherapy might be an option for selected patients with advanced USC [27]. Further prospective studies investigating the role of neoadjuvant chemotherapy in advanced stage USC are certainly warranted.
Adjuvant treatments
To date, various types of adjuvant therapies have been proposed for patients after surgical treatment for USC. Only few trials evaluated the role of adjuvant therapy specifically in patients with USC [17]. According to the 25th FIGO annual report, the 5-year survival rate for surgically staged stage I USC who had no adjuvant therapy was 77% [28]. The addition of radiotherapy improved 5-year outcomes by 8% [28]. However, there is no level A evidence demonstrating an overall survival advantage for the use of radiotherapy in the treatment of endometrial cancer in general, including USC.
However, since most patients with USC experience distant/hematogenous dissemination, several studies have subsequently evaluated the role of systemic treatments (i.e., chemotherapy) [29–32]. In 2010, Hogberg et al., reported on pooled data from two randomized trials (NSGO-EC 9501/EORTC-55991 and MaNGO ILIADE-III) investigating the role of sequential radiotherapy and chemotherapy (either sequence) in high-risk endometrial cancer. Seventy five (14%) of the 534 patients included in the combined studies had serous cancer. The cumulative results of these studies suggested that the addition of adjuvant chemotherapy to radiation improves progression-free survival, but this was not confirmed restricting the analysis on non-endometrioid endometrial cancer (which included USC and clear cells histological subtypes) [29]. The PORTEC-3 trial investigated the role of concurrent and adjuvant chemotherapy added to radiotherapy (two cycles of cisplatin 50mg/m2 during radiotherapy, followed by four cycles of carboplatin AUC5 and paclitaxel 175mg/m2) versus radiotherapy alone in high-risk endometrial cancer patients [30]. Overall, 686 patients were enrolled in the trial, including 105 (15.3%) patients with USC. In 2019, the updated analysis of the PORTEC-3 trial (median follow-up of 72.6 months and 75% of patients with at least 5-year follow-up) reported that concurrent and adjuvant addition of chemotherapy to radiotherapy improves disease-free and overall survival in high-risk endometrial cancer in comparison to pelvic radiotherapy (48.6 Gy in 1.8 fractions) alone [30]. The 5-year disease-free survival was 59.7% and 47.9% after combined chemotherapy & radiotherapy and radiotherapy alone, respectively. The 5-year overall survival was 71.4% and 52.8% after combined chemotherapy & radiotherapy and radiotherapy alone, respectively [30]. These results are confirmed even after the restriction of the analysis on patients with USC [30]. After adjusting for stratification factors, significant improvements in overall survival and failure-free survival were observed for serous cancers treated with chemoradiotherapy versus radiotherapy alone: 5-year overall survival was 71·4% (95% CI 60·1–84·7) with combined chemotherapy & radiotherapy versus 52·8% (40·6–68·6) with radiotherapy alone (HR 0·48 [95% CI 0·24–0·96]; p=0·037), and 5-year failure-free survival was 59·7% (95% CI 45·1–71·6) in the combined treatment arm versus 47·9% (33·9–60·6) with radiotherapy alone (HR 0·42 [95% CI 0·22–0·80]; p=0·008) [30]. Of note, no treatment arm with chemotherapy alone was included in the PORTEC-3 trial, thus the role of radiotherapy in comparison to chemotherapy alone remains unclear. The GOG 258 looked at the role of radiation in locally advanced endometrial cancer [31]. In this latter trial, Matei et al., included 736 patients with stage III-IVA endometrial cancer (any histology) and stage I-II clear cell or USC with positive peritoneal cytology [31]. This trial compared the chemotherapy-radiotherapy schedule used in the PORTEC-3 trial with chemotherapy alone comprising 6 cycles of carboplatin AUC6 and paclitaxel 175mg/m2. USC made up 17.8% (n=131) of the study population. Overall relapse free- survival was not different between the 2 arms; the numbers were too small to assess any difference between the arms in the USC subgroup [31]. The randomized phase III GOG-249 trial evaluated the role of vaginal brachytherapy plus chemotherapy versus radiotherapy alone in high-intermediate and high-risk early-stage endometrial cancer, including 15% of USC. The results of this study suggested that these two methods correlated with similar survival outcomes (overall and recurrence-free), but USC experienced non-significant improved outcomes with the use of vaginal brachytherapy plus chemotherapy in comparison to radiotherapy alone [32]. However, loco-regional recurrences were higher in the chemotherapy-brachytherapy group in comparison to the radiotherapy alone group [28]. Table 3 reports detail of most representative studies investigating phase III trials investigating the role of chemotherapy in high-risk endometrial cancer. Interestingly, optimal sequencing of adjuvant treatment modalities may play a role in survival outcomes [33]. Recent retrospective evidence on stage IIIC endometrial cancer patients has suggested that a strategy employing chemotherapy first, followed by radiotherapy was associated with improved survival compared to concurrent chemo-radiotherapy [33]. Another treatment option for early-stage USC is vaginal brachytherapy. It is widely adopted in Western Countries, especially in stage IA disease [34]. Although no Level A evidence demonstrated the beneficial effects of vaginal brachytherapy vs. observation, the NCCN guidelines support the adoption of vaginal brachytherapy in selected patients with non-invasive disease (confined to the endometrium) [16, 17]. The ESGO/ESTRO/ESP guidelines categories USC without myometrial invasion into the intermediate prognostic risk group. Brachytherapy can be recommended to decrease vaginal recurrence. Omission of adjuvant brachytherapy can be considered, especially for patients aged <60 years [17].
Table 3.
Phase III prospective randomized trials investigating the role of chemotherapy in high-risk endometrial cancer.
| Trail | Study population | Uterine serous carcinoma (USPC) | Progression-free survival (PFS) | Overall survival (OS) |
|---|---|---|---|---|
|
| ||||
| NSGO-EC 9501/EORTC-55991 [25] | 191 RT | 40 (21%) | Better outcomes for RT + CT (36% improvement in PFS) | Not statistically significant |
| 187 RT + CT | 32 (18%) | |||
| MaNGO ILIADE-III* [25] | 76 RT | 0 | Not statistically significant | Not statistically significant |
| 80 CT + RT | 1 (1.3%) | |||
| PORTEC-3 [26] | 330 RT | 52 (16%) | Better outcomes for CTRT+CT (also in USPC) | Better outcomes for CTRT+CT (also in USPC) |
| 330 CTRT + CT | 53 (16%) | |||
| GOG-258 [27] | 370 CTRT + CT | 66 (18%) | Not statistically significant | No mature data |
| 366 CT | 65 (18%) | |||
| GOG-249 [28] | 301 RT | 46 (15%) | Not statistically significant | Not statistically significant |
| 300 VB + CT | 42 (14%) | |||
Abbreviations: RT, radiotherapy; CT, chemotherapy; CTRT, Chemoradiation; VB, vaginal bradwtherapy; USPC, uterine serous papillary cancer; PFS, progression-free survival; OS, overall survival;
Serous histology was an exclusion criteria. This table reports most representative phase Ill prospective trials evaluating the role of chemotherapy in high-risk endometrial cancer.
There is controversy as to whether patients with all stages and substages of USC should be offered adjuvant treatment. Because of the small number of patients included in the randomized trial data, the evidence for patients with this subgroup is largely limited to database analysis, so is prone to bias. Data from two large database analyses showed overall survival benefit for chemotherapy in patients with stage IA USC [35, 36]. In the study by Nasioudis et al, benefit was even demonstrated in patients with stage IA USC without myometrial invasion [36]. Given this is retrospective data however, this information should form part of a full discussion around benefit and risk of treatment, rather than be uniformly recommended.
Recently, a randomized phase II study of HER2-overexpressing USC, combining trastuzumab with carboplatin/ paclitaxel showed a progression-free survival benefit when compared to chemotherapy alone in advanced-stage disease (median 17.9 months vs 9.3 months, p = 0.013, HR = 0.40) and the recurrent setting (9.2 months vs 6.0 months, p = 0.003, HR = 0.14) [37]. The benefit was most pronounced in those treated upfront. These findings led to the incorporation of trastuzumab into the National Comprehensive Cancer Network (NCCN) guidelines [38] and the recommendation that carboplatin/paclitaxel/trastuzumab should be considered the preferred regimen for HER2 positive advanced or platinum-sensitive recurrent USC. Additionally, an updated trial survival analysis demonstrated that overall survival was also significantly higher in the trastuzumab compared with the control arm, with medians of 29.6 months versus 24.4 months (HR = 0.58; 90% CI, 0.34–0.99; p = 0.046) [39]. Again, the benefit was most notable in those with stage III to IV disease, with survival median not reached in the trastuzumab-containing arm versus 24.4 months in the control arm (HR = 0.49; 90% CI, 0.25–0.97; p=0.041) [39].
Considering the available evidence, patients with USC should receive adjuvant chemotherapy in order to reduce the risk of developing distant recurrence. No clear recommendation for adjuvant chemotherapy in stage IA USC without myometrial invasion exists. In HER2+ patients affected by stage III and IV disease, trastuzumab can be added (8mg/kg for the first dose and 6mg/kg in subsequent cycles). Adjuvant radiotherapy can be considered to reduce local and loco-regional control (especially in patients with nodal involvement). Adjuvant radiotherapy recommendations strongly depend on stage/risk group of disease and knowledge on lymph node status Further evidence focusing on USC is necessary to better understand the better adjuvant modality. Innovative treatment modalities are needed to improve the outcomes of those patients.
Post-treatment Surveillance and Follow-up
The main aim of the follow-up is the early detection of recurrence or disease progression. The majority of recurrences occur up to two years after the end of the primary treatment. In general, clinical trials have a follow-up with a pelvic exam every three months for two years (or every three months in the first year and every four months in the second year), every 6 months up to five years, then annually [36]. Imaging assessments are reserved for patients with symptoms or abnormalities on physical exam (E.g., pelvic or vaginal mass, lymph nodes enlargement, new pain). Computed tomography of the abdomen, pelvis, or chest is the most used method [40]. PET/CT or MRI can be considered for selected patients based on clinical findings. The use of serum CA 125 in the follow-up of patients with USC is controversial. Data from retrospective studies show some utility of serial CA 125 in anticipating recurrence [41]; however, there are no prospective data supporting the role of assessing CA125 levels.
Treatments for recurrent / progressive disease
The treatment of recurrent disease depends on various features including the patients’ demographic characteristics, comorbidity, performance status, response to previous treatments, and location of metastatic sites. The multi-disciplinary tumor board should be consulted regarding multi-modal therapy for recurrent disease, including surgical, radiotherapy, and chemotherapeutic inputs. In oligometastatic disease, surgery or radiotherapy, generally followed by chemotherapy, might be considered in selected cases. However, owing to the high prevalence of hematogenous dissemination most patients with recurrent/progressive USC are submitted to systemic treatments including chemotherapy and/or immunotherapy.
Immunotherapy has gained popularity for the treatments of various solid tumors characterized by microsatellite instability, including MSI-high endometrial cancer [42, 43]. However, as virtually all of USC are p53+ and pMMR/MSS, anti-PD-1 monotherapy is usually ineffective in these tumors [40]. Makker et al., has reported data on the combination of pembrolizumab and lenvatinib in recurrent or metastatic (>1 line) endometrial cancer [42, 43]. The objective response (complete response + partial response) rate was 63.6% in MSI-H/dMMR patients (n=11) and 38.3% in MSS/dMMR (n=94). This latter group experienced a duration of response >6 months in 25 cases (69%). This study highlighted an unprecedented response rate in USC of around 50% [43]. In October 2019, the Food and Drug Administration (FDA) approved the combination of pembrolizumab and lenvatinib also for microsatellite-stable endometrial cancer (including also USC), based on the results of this phase II single-arm trial [44]. Confirmatory phase III studies are ongoing and will be presented at the next meeting of the Society of Gynecologic Oncology (SGO). To date, owing to poor patient outcomes, patients’ enrollment into clinical trials should be made a priority.
Ongoing trials and future directions
Several ongoing trials are testing novel therapies for patients with advanced/recurrent endometrial cancer, including USPC [45] (Table 4). The majority of these studies are designed to investigate the role of novel strategies in advanced/recurrent endometrial cancer, but are not powered to assess their specific benefit in USC. The immunotherapeutic agents tested in advanced/recurrent endometrial cancer included: pembrolizumab, durvalumab, atezolizumab, nivolumab (with or without ipilimumab). Several studies are testing various immunotherapeutic agents in high risk endometrial cancer [45]. The RUBY/ENGOT-en6 phase III trials investigating the role of adding dostarlimab (TSR-042) (a humanized monoclonal PD-1 antibody) to platinum-based chemotherapy [45]. Other trials evaluating the role of immunotherapy in endometrial cancer alone or in combination include the AtTEnd/ENGOT-en7 and the LEAP/ENGOT-en9 trials [45].
Table 4.
Ongoing trials on high-risk endometrial cancer and uterine serous papillary carcinoma.
| Agents | Type of study | Mecchanism of Acation | Partecipants | Primary endopoint | Estimated completion date |
|---|---|---|---|---|---|
|
| |||||
| Atezolizumab (NCT01375842) | Phase III | lummotherapic agent (anti PD-1) | 550 pts. with advanced or recurrent EC, including also USPC | Overall survival Progression-free survival | July 2022 |
| Avelumab | Phase II | lummotherapic agent (anti PD-1) | 120 pts. with advanced or recurrent EC, including also USPC | Progression-free survival | December 2023 |
| AZD1775 | Phase II | blocks the activity of Wee1 | 80 pts. with USPC | Objective response rate Progression-free survival | June 2023 |
| Copanlisib | Phase II | PIK inhibitor | 11 pts. with endometrial cancer (with PIK3CA) | Objective response rate | June 2020 |
| Dostarlimab (TSR-042) | Phase III | lummotherapic agent (anti PD-1) | 470 pts. with advanced or recurrent EC, including also USPC | Progression-free survival | February 2026 |
| Durvalumab, Lenvatinib | Phase II | lummotherapic agent (anti PD-1), TKI | 20 pts. with advanced or recurrent EC, including also USPC | Objective response rate Progression-free survival | May 2021 |
| IMGN853, Pembrolizumab (MK-3475) | Phase II | Antibody-drug conjugate lummotherapic agent (anti PD-1) | 35 pts. with USPC (micro satellite stable) | Objective response rate Progression-free survival | October 2023 |
| Niraparib | Phase II | PARP inhibitors (maintenance after platinum-based chemotherapy) | 45 pts. with advanced or platinum sensitive recurrent USPC | Progression-free survival | July 2025 |
| Nivolumab BMS-986205 | Phase II | Anti PDL-1, IDO- inhibitor | 45 pts. with advanced or platinum sensitive recurrent EC, including also USPC | Overall response rate | September 2022 |
| ONC201 | Phase II | ONC201 targets the G protein-coupled receptor DRD2 | 36 pts. with advanced or recurrent, EC including also USPC | Objective response rate Progression-free survival | October 2022 |
| Pembrolizumab (MK-3475) | Phase II | lummotherapic agent (anti PD-1) | 20 pts. with clinical stage 1, grade 3 EC, encompassing endometrioid, serous and clear cell histologies | Change in the number of Tumor Infiltrating Lymphocytes | May 2022 |
| Pembrolizumab (MK-3475) | Phase III | Anti PDL-1 (added to platinum-based chemotherapy) | 810 pts. with advanced or recurrent EC, including also USPC | Progression-free survival | June 2023 |
| Selinexor | Phase III | XPO1 inhibitors (maintenance after platinum-based chemotherapy) | 248 pts. with advanced or recurrent EC, including also USPC | Progression-free survival | March 2023 |
| Trastuzumab | Phase II | Anti HER-2 | 61 pts. with advanced or recurrent USPC | Progression-free survival | December 2021 |
| VSV-hIFNbeta-NIS, with/without Ruxolitinib | Phase I | Oncolytic vesicular stomatitis virus-human interferon beta-sodium iodide symporter | 77 pts. with advanced or recurrent EC, including also USPC | Maximum tolerated dose of VSV-hIFNbeta-NIS | June 2021 |
Abbreviations: EC, endometrial cancer; USPC, uterine serous papillary carcinoma; pts., patients; PD-1, Programmed cell death protein 1; TKI, Tyrosine Kinase Inhibitors; PARP, Poly (ADP-ribose) polymerase; PIK3, phosphatidylinositol-4,5-bisphosphate 3-kinase; IDO, Indoleamine 2,3-dioxygenase; XPO1, Exportin 1.
Further trials are investigating other targeted agents include HER2 targeting medications (e.g., trastuzumab, SYD985), multi-kinase TKI (e.g., lenvatinib, TKI258), PIK3CA inhibitors (e.g., copanlisib, XL147 (SAR245408)), and PARP inhibitors [45]. Targeting of HER2 with trastuzumab has shown promising results [37, 38]. For these reasons, HER2 represents an attractive therapeutic target in USC. Antibody-drug conjugates (ADC) or small molecule inhibitors have been extensively evaluated in preclinical and clinical studies [46]. SYD985 (Synthon Biopharmaceuticals) is a novel HER2-targeting ADC composed of trastuzumab linked to duocarmycin, a highly potent DNA-alkylating agent [46]. In preclinical experiments against T-DM1, SYD985 demonstrated significantly higher activity against primary USC cell lines with strong (3+) as well as low to moderate (1+/2+) HER2 expression. SYD985 was 10- to 70-fold more effective than T-DM1 in comparative experiments and, unlike T-DM1, it was active against USC demonstrating heterogeneous HER2 expression [47]. In a Phase I trial of HER2-expressing cancers, responses were observed in 5 of 13 (39%) endometrial carcinoma patients [43]. A Phase II trial evaluating the effectiveness of SYD985 in recurrent endometrial carcinoma is ongoing (NCT04205630) [45]. Afatinib and neratinib, irreversible small molecule inhibitors of EGFR, HER2 and HER4 that are FDA-approved for the treatment of EGFR-positive squamous non-small cell lung cancer and HER2+ breast cancer, have demonstrated significant in vitro and in vivo activity against primary HER2-amplified cell lines and xenografts [48] and are currently being studied in a phase II trial in HER2-positive USC (NCT02491099) [45]. Dual anti-HER2 inhibition is considered established therapy in locally advanced and advanced breast cancer, and is another area of active exploration in Her2-expressing endometrial cancer. The combination of trastuzumab with pertuzumab (a humanized HER2 monoclonal antibody that prevents receptor dimerization), has shown antitumor activity in USC cell lines [49]. Combination treatment was observed to significantly increase antibody-dependent cytotoxicity, even in low HER2-expressing cells [49].
Since it has been reported that a large number of USC shows alterations in PI3K pathway-related genes [50], several PI3K/AKT/mTOR inhibitors have recently been tested against primary USC cell lines and xenografts [51]. Preclinical studies of AZD8055 (mTORC 1/2 inhibitor), GDC-0980 (inhibitor of class one PI3K and mTORC 1/2), and GDC-0032 (taselisib, PIK3CA inhibitor) have shown promising results [45]. Furthermore, preclinical data combining the PIK3CA inhibitor taselisib and the pan-Her inhibitor neratinib, found the combination to be highly synergistic and well-tolerated in vivo (in animal models) [45]. The combination prevented the development of resistance in preclinical USC models, and led to substantial tumor regression in large USC xenografts that were previously resistant to single-agent PIK3CA or pan-Her inhibition [52]. These preclinical results are concordant with recent clinical data in a variety of human tumors, suggesting that combination regimens using highly targeted drugs may improve responses and clinical benefit. Taken together, these data support the view that future trials with C-ERB/PIK3CA/AKT/mTOR inhibitors may require the use of synergistic combinations to induce more durable clinical responses in USC.
Trop-2 is a transmembrane glycoprotein that is upregulated in all cancer types, including gynecological cancers [53]. It has been shown that Trop-2 expression was detected in 95.1% of USC samples [53]. Thus, researchers evaluated the role of sacituzumab govitecan in Trop-2 overexpressing USC cell lines [54]. Sacituzumab govitecan was highly active against USPC overexpressing Trop-2 in vitro and in vivo. Moreover, sacituzumab govitecan showed promising responses against multiple chemotherapy-resistant human tumors [54]. Due to the high molecular similarities with high-grade serous carcinoma of the ovary such as mutations affecting DNA repair pathways, the potential role for PARP inhibition (PARPi) in USC has been investigated [55, 56]. An ongoing phase II trial (NCT 04080284) is investigating the activity of maintenance niraparib in stage III-IV or platinum-sensitive recurrent USC [45]. Similarly, another ongoing randomized phase II trial (UTOLA, NCT03745950) is investigating the activity of maintenance olaparib in Stage III-IV or platinum-sensitive recurrent endometrial carcinoma including USC [45]. A multi-arm phase II trial (NRG-GY012, NCT03660826) is also examining several olaparib-based combinations in unselected uterine cancer subtypes [45]. Adding immune checkpoint inhibition to PARP inhibitors seeks to exploit the immunomodulatory effects of PARP inhibitors and it is under evaluation in several studies in recurrent endometrial cancer (olaparib/durvalumab NCT03951415; rucaparib/nivolumab NCT03572478; niraparib/dostarlimab NCT03016338) [45]. In particular, the DUO-E (NCT03951415) study is a randomized trial investigating the effect of durvalumab (with or without olaparib) as maintenance after first line treatment of advanced/recurrent endometrial cancer. This study will aim to include approximately 700 patients randomized in three different arms: (1) platinum-based chemotherapy and durvalumab placebo followed by maintenance durvalumab placebo and olaparib placebo; (2) platinum-based chemotherapy and durvalumab followed by maintenance durvalumab and olaparib placebo; and (3) platinum-based chemotherapy and durvalumab followed by maintenance durvalumab and olaparib [45].
At present, despite the growing number of studies investigating the role of emerging therapies in endometrial cancer, only a few trials are investigating the safety and effectiveness of those novel medications specifically for USC. Further studies stratifying patients by molecular classification and/or aiming to assess the role of new targeted agents and immune checkpoint inhibitors (combinations) specifically in USC are warranted.
Conclusions
In this narrative review, we have summarized the current evidence and further prospective on the management of USC. USC represents a relatively rare entity, characterized by aggressive behavior. The optimal therapeutic strategy is not fully understood. Given the available evidence, the authors of this review are in favor of a multimodal approach to USC treatment, consisting of surgery, chemotherapy, and radiotherapy. Given the prevalence for Her2 over expression in USC, early testing for HER2 should be considered for advanced disease, with the addition of trastuzumab to the therapeutic armamentarium for patients who harbor this biomarker. While the combination of pembrolizumab and lenvatinib is considered an important treatment modality for second-line treatment of advanced/recurrent endometrial cancer, with an objective response rate of 50%. New targeted agents and immune checkpoint inhibitors are being tested in this setting. Further prospective studies and collaborative trials focusing on USC and p53 mutated endometrial cancer supported by GCIG and ENGOT network are necessary to improve knowledge and treatment-related outcomes of those
Highlights:
Uterine serous carcinoma (USC) is a rare and aggressive variant of endometrial cancer
USCs are generally mismatch repair proficient and have extensive copy number alterations
Multi-modal treatment including surgery, chemotherapy and/or radiotherapy should be considered in the majority patient with USC
Novel combinations of immune checkpoint inhibitors with targeted therapies are under evaluation
USCs are generally mismatch repair proficient and have extensive copy number alterations
Multi-modal treatment including surgery, chemotherapy and/or radiotherapy should be considered in the majority patient with USC
Novel combinations of immune checkpoint inhibitors with targeted therapies are under evaluation
Legend:
- CA
consulting/advisory relationship
- SH
speaker honoraria
- H
honoraria
- RF
research funding
- OI
ownership interest
- IP
intellectual propriety/patent holder
- SAB
scientific advisory board
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
Giorgio Bogani: Novartis AG Pharma (C/A, H), Janssen (H), Italian Ministry of Health (RG)
Nicole Concin: AstraZeneca (C/A, SH), Seattle Genetics (C/A, SH), MSD (SAB), Mersana (C/A, SH), eTheRNA immunotherapies NV (C/A, SH), Roche (travel expenses), Genmab (travel expenses), Amgen (travel expenses)
Isabelle Ray-Coquard: AstraZeneca (C/A, H), Clovis (C/A, H), Genmab (C/A), MSD (C/A) PharmaMar (C/A, H) Pfizer (C/A), Roche (C/A) and Tesaro (C/A, H);
Natalie YL Ngoi: Thermofisher (H)
Domenica Lorusso: Clovis Oncology (C/A), Amgen (C/A), AstraZeneca (C/A, H), ImmunoGen (C/A), Genmab (C/A), Merck (C/A), PharmaMar (C/A, H), Roche (C/A, H), Takeda (C/A), and Tesaro (C/A, H);
Diane Provencher: AstraZeneca (CA, SH, SAB), GSK (CA, SH, SAB)
Hannelore Denys: Roche (CA, SH, SAB), Pfizer (CA, SH, SAB), AstraZeneca (SH, SAB), Lily (SAB), GSK (SAB), Novartis (SH), Pharmamar (SH)
Yakir Segev: AstraZeneca (CA), GSK (CA)
Pauline Wimberger: Amgen (CA, SH, RF), AstraZeneca (CA, SH), MSD (CA, SH), Novartis (CA, SH, RF), Pfizer (CA, SH), Lilly (CA, SH), Roche (CA, SH, RF), TEVA (CA, SH), Eisai (CA, SH), Clovis (CA, SH), GSK (CA, SH, RF).
Hannelore Denys: Roche (CA, SH, SAB), Pfizer (CA, SH, SAB), AstraZeneca (SH, SAB), Lily (SAB), GSK (SAB), Novartis (SH), Pharmamar (SH)
Takayuki Enomoto: Takeda (SH), Astra Zeneca (SH), Eisai (SH), Chugai Pharma (SH, RF), MSD (SH), Mochida (SH)
Kazuhiro Takehara: Takeda (SH), Astra Zeneca (SH), Eisai (SH), Chugai Pharma (SH, RF), MSD (SH), Mochida (SH)
Bradley J Monk: AstraZeneca (SH, SAB), GSK (SH, SAB), Incyte (SAB), Merck (SH, SAB), Roche/Genentech (SH, SAB), Eisai (SAB), GOG-Foundation (E), US Oncology (E)
The other authors indicated no financial relationship
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020. Jan;70(1):7–30. Doi: 10.3322/caac.21590. Epub 2020 Jan 8. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010. Sep-Oct;60(5):277–300. Doi: 10.3322/caac.20073. Epub 2010 Jul 7. Erratum in: CA Cancer J Clin. 2011 Mar-Apr;61(2):133–4. [DOI] [PubMed] [Google Scholar]
- 3.Simon MS, Hastert TA, Barac A, Banack HR, Caan BJ, Chlebowski RT, et al. Cardiometabolic risk factors and survival after cancer in the Women’s Health Initiative. Cancer 2020. Nov 5. Doi: 10.1002/cncr.33295. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 4.Lu KH, Broaddus RR. Endometrial Cancer. N Engl J Med 2020. Nov 19;383(21):2053–2064. Doi: 10.1056/NEJMra1514010. [DOI] [PubMed] [Google Scholar]
- 5.Mendivil A, Schuler KM, Gehrig PA. Non-endometrioid adenocarcinoma of the uterine corpus: a review of selected histological subtypes. Cancer Control 2009;16(1):46–52. Doi: 10.1177/107327480901600107 [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Yu M, Yang JX, Cao DY, Shen K, Lang JH. Clinicopathological and survival analysis of uterine papillary serous carcinoma: a single institutional review of 106 cases. Cancer Manag Res 2018. Oct 25;10:4915–4928. Doi: 10.2147/CMAR.S179566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soslow RA, Tornos C, Park KJ, Malpica A, Matias-Guiu X, Oliva E, et al. Endometrial Carcinoma Diagnosis: Use of FIGO Grading and Genomic Subcategories in Clinical Practice: Recommendations of the International Society of Gynecological Pathologists. Int J Gynecol Pathol 2019. Jan;38 Suppl 1:S64–S74. Doi: 10.1097/PGP.0000000000000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambros RA, Sherman ME, Zahn CM, Bitterman P, Kurman RJ. Endometrial intraepithelial carcinoma: a distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Pathol 1995. Nov;26(11):1260–7. doi: 10.1016/0046-8177(95)90203-1. [DOI] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research Network, Kandoth C, Schultz N, Cherniack AD, R Akbani, Y Liu, et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013. May 2;497(7447):67–73. Doi: 10.1038/nature12113. Erratum in: Nature. 2013 Aug 8;500(7461):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowin PA, George J, Fereday S, Loehrer E, Van Loo P, Cullinane C, et al. LRP1B deletion in high-grade serous ovarian cancers is associated with acquired chemotherapy resistance to liposomal doxorubicin. Cancer Res 2012. Aug 15;72(16):4060–73. Doi: 10.1158/0008-5472.CAN-12-0203. [DOI] [PubMed] [Google Scholar]
- 11.Zhao S, Choi M, Overton JD, Bellone S, Roque DM, Cocco E, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci U S A 2013. Feb 19;110(8):2916–21. Doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Sahwi KS, Schwartz PE, Santin AD. Development of targeted therapy in uterine serous carcinoma, a biologically aggressive variant of endometrial cancer. Expert Rev Anticancer Ther 2012. Jan;12(1):41–9. Doi: 10.1586/era.11.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol 2012. Mar 1;30(7):695–700. Doi: 10.1200/JCO.2011.38.8645. Epub 2012 Jan 30. Erratum in: J Clin Oncol. 2012 May 1;30(13):1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaban A, Topuz S, Erdem B, Sozen H, Numanoğlu C, Salihoğlu Y. Is Omentectomy Necessary for Non-Endometrioid Endometrial Cancer. Gynecol Obstet Invest 2018;83(5):482–486. doi: 10.1159/000480237. Epub 2017 Aug 26. [DOI] [PubMed] [Google Scholar]
- 15.Bayrak M, Yılmaz A, Yılmaz F, İlhan O, Oz Atalay F, Ozan H. Omental Micrometastasis in Endometrial Cancer. Oncol Res Treat 2019;42(9):466–469. Doi: 10.1159/000501727. Epub 2019 Jul 24. [DOI] [PubMed] [Google Scholar]
- 16.Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018. Feb;16(2):170–199. Doi: 10.6004/jnccn.2018.0006. [DOI] [PubMed] [Google Scholar]
- 17.Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer 2021. Jan;31(1):12–39. doi: 10.1136/ijgc-2020-002230. Epub 2020 Dec 18. [DOI] [PubMed] [Google Scholar]
- 18.Nasioudis D, Albright BB, Roy A, Ko EM, Giuntoli RL 2nd, Haggerty AF, et al. Patterns of use and outcomes of sentinel lymph node mapping for patients with high-grade endometrial cancer. Gynecol Oncol 2020. Dec;159(3):732–736. Doi: 10.1016/j.ygyno.2020.09.023. Epub 2020 Sep 29. [DOI] [PubMed] [Google Scholar]
- 19.Cusimano MC, Vicus D, Pulman K, Maganti M, Bernardini MQ, Bouchard-Fortier G, Laframboise S, May T, Hogen LF, Covens AL, Gien LT, Kupets R, Rouzbahman M, Clarke BA, Mirkovic J, Cesari M, Turashvili G, Zia A, Ene GEV, Ferguson SE. Assessment of Sentinel Lymph Node Biopsy vs Lymphadenectomy for Intermediate- and High-Grade Endometrial Cancer Staging. JAMA Surg 2021. Feb 1;156(2):157–164. doi: 10.1001/jamasurg.2020.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persson J, Salehi S, Bollino M, Lönnerfors C, Falconer H, Geppert B. Pelvic Sentinel lymph node detection in High-Risk Endometrial Cancer (SHREC-trial)-the final step towards a paradigm shift in surgical staging. Eur J Cancer 2019. Jul;116:77–85. doi: 10.1016/j.ejca.2019.04.025. Epub 2019 Jun 7. [DOI] [PubMed] [Google Scholar]
- 21.Soliman PT, Westin SN, Dioun S, Sun CC, Euscher E, Munsell MF, Fleming ND, Levenback C, Frumovitz M, Ramirez PT, Lu KH. A prospective validation study of sentinel lymph node mapping for high-risk endometrial cancer. Gynecol Oncol 2017. Aug;146(2):234–239. doi: 10.1016/j.ygyno.2017.05.016. Epub 2017 May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi EC, Kowalski LD, Scalici J, Cantrell L, Schuler K, Hanna RK, Method M, Ade M, Ivanova A, Boggess JF. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol 2017. Mar;18(3):384–392. doi: 10.1016/S1470-2045(17)30068-2. Epub 2017 Feb 1. [DOI] [PubMed] [Google Scholar]
- 23.Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet 2010. Apr 3;375(9721):1165–72. Doi: 10.1016/S0140-6736(09)62002-X. Epub 2010 Feb 24. Erratum in: Lancet. 2010 Aug 21;376(9741):594. [DOI] [PubMed] [Google Scholar]
- 24.Bogani G, Papadia A, Buda A, Casarin J, Di Donato V, Gasparri ML, et al. Sentinel node mapping vs. sentinel node mapping plus back-up lymphadenectomy in high-risk endometrial cancer patients: Results from a multi-institutional study. Gynecol Oncol 2021. Jan 20:S0090–8258(21)00055-X. doi: 10.1016/j.ygyno.2021.01.008. Epub ahead of print. [DOI] [PubMed]
- 25.Bogani G, Murgia F, Ditto A, Raspagliesi F. Sentinel node mapping vs. lymphadenectomy in endometrial cancer: A systematic review and meta-analysis. Gynecol Oncol 2019. Jun;153(3):676–683. Doi: 10.1016/j.ygyno.2019.03.254. Epub 2019 Apr 2. [DOI] [PubMed] [Google Scholar]
- 26.Bogani G, Ditto A, Leone Roberti Maggiore U, Scaffa C, Mosca L, Chiappa V, et al. Neoadjuvant chemotherapy followed by interval debulking surgery for unresectable stage IVB Serous endometrial cancer. Tumori 2019. Feb;105(1):92–97. Doi: 10.1177/0300891618784785. Epub 2018 Jul 9. [DOI] [PubMed] [Google Scholar]
- 27.Tobias CJ, Chen L, Melamed A, St Clair C, Khoury-Collado F, Tergas AI, et al. Association of Neoadjuvant Chemotherapy With Overall Survival in Women With Metastatic Endometrial Cancer. JAMA Netw Open 2020. Dec 1;3(12):e2028612. Doi: 10.1001/jamanetworkopen.2020.28612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Creasman WT, Kohler MF, Odicino F, Maisonneuve P, Boyle P. Prognosis of papillary serous, clear cell, and grade 3 stage I carcinoma of the endometrium. Gynecol Oncol 2004. Dec;95(3):593–6. Doi: 10.1016/j.ygyno.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Hogberg T, Signorelli M, de Oliveira CF, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer—results from two randomised studies. Eur J Cancer 2010. Sep;46(13):2422–31. Doi: 10.1016/j.ejca.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol 2019. Sep;20(9):1273–1285. Doi: 10.1016/S1470-2045(19)30395-X. Epub 2019 Jul 22. Erratum in: Lancet Oncol. 2019 Sep;20(9):e468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matei D, Filiaci V, Randall ME, Mutch D, Steinhoff MM, DiSilvestro PA, et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. N Engl J Med 2019. Jun 13;380(24):2317–2326. Doi: 10.1056/NEJMoa1813181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tatebe K, Hasan Y, Son CH. Adjuvant vaginal brachytherapy and chemotherapy versus pelvic radiotherapy in early-stage endometrial cancer: Outcomes by risk factors. Gynecol Oncol 2019. Dec;155(3):429–435. Doi: 10.1016/j.ygyno.2019.09.028. Epub 2019 Oct 11. [DOI] [PubMed] [Google Scholar]
- 33.Latham AH, Chen L, Hou JY, Tergas AI, Khoury-Collado F, St Clair CM, et al. Sequencing of therapy in women with stage III endometrial carcinoma receiving adjuvant combination chemotherapy and radiation. Gynecol Oncol 2019. Oct;155(1):13–20. Doi: 10.1016/j.ygyno.2019.07.021. Epub 2019 Aug 6. [DOI] [PubMed] [Google Scholar]
- 34.Lancellotta V, De Felice F, Vicenzi L, Antonacci A, Cerboneschi V, Costantini S, et al. The role of vaginal brachytherapy in stage I endometrial serous cancer: a systematic review. J Contemp Brachytherapy 2020. Feb;12(1):61–66. Doi: 10.5114/jcb.2020.92698. Epub 2020 Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu XM, Velker VM, Leung E, Kwon JS, Elshaikh MA, Kong I, et al. The role of adjuvant therapy in stage IA serous and clear cell uterine cancer: A multi-institutional pooled analysis. Gynecol Oncol 2018. May;149(2):283–290. doi: 10.1016/j.ygyno.2018.03.002. Epub 2018 Mar 12. [DOI] [PubMed] [Google Scholar]
- 36.Nasioudis D, Roy AG, Ko EM, Cory L, Giuntoli Ii RL, Haggerty AF, et al. Adjuvant treatment for patients with FIGO stage I uterine serous carcinoma confined to the endometrium. Int J Gynecol Cancer 2020. Aug;30(8):1089–1094. doi: 10.1136/ijgc-2020-001379. Epub 2020 Jul 15. [DOI] [PubMed] [Google Scholar]
- 37.Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/neu. J Clin Oncol 2018. Jul 10;36(20):2044–2051. Doi: 10.1200/JCO.2017.76.5966. Epub 2018 Mar 27. [DOI] [PubMed] [Google Scholar]
- 38.Pelligra S, Buza N, Hui P, Bellone S, Zeybek B, Ratner E, et al. Selection of HER2/NEU negative tumor cells as a mechanism of resistance to trastuzumab in uterine serous carcinoma. Gynecol Oncol Rep 2020. Feb 25;32:100554. Doi: 10.1016/j.gore.2020.100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Compared with Carboplatin-Paclitaxel-Trastuzumab in Advanced (Stage III-IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2/Neu (NCT01367002): Updated Overall Survival Analysis. Clin Cancer Res 2020. Aug 1;26(15):3928–3935. Doi: 10.1158/1078-0432.CCR-20-0953. Epub 2020 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salani R, Khanna N, Frimer M, Bristow RE, Chen LM. An update on post-treatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncology (SGO) recommendations. Gynecol Oncol 2017. Jul;146(1):3–10. doi: 10.1016/j.ygyno.2017.03.022. Epub 2017 Mar 31. [DOI] [PubMed] [Google Scholar]
- 41.Frimer M, Hou JY, McAndrew TC, Goldberg GL, Shahabi S. The clinical relevance of rising CA-125 levels within the normal range in patients with uterine papillary serous cancer. Reprod Sci 2013. Apr;20(4):449–55. doi: 10.1177/1933719112459218. Epub 2012 Sep 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makker V, Taylor MH, Aghajanian C, Oaknin A, Mier J, Cohn AL, et al. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J Clin Oncol 2020. Sep 10;38(26):2981–2992. Doi: 10.1200/JCO.19.02627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makker V, Rasco D, Vogelzang NJ, Brose MS, Cohn AL, Mier J, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 2019. May;20(5):711–718. Doi: 10.1016/S1470-2045(19)30020-8. Epub 2019 Mar 25. [DOI] [PubMed] [Google Scholar]
- 44.Arora S, Balasubramaniam S, Zhang W, Zhang L, Sridhara R, Spillman D, et al. FDA Approval Summary: Pembrolizumab plus Lenvatinib for Endometrial Carcinoma, a Collaborative International Review under Project Orbis. Clin Cancer Res 2020. Oct 1;26(19):5062–5067. Doi: 10.1158/1078-0432.CCR-19-3979. Epub 2020 Apr 15. [DOI] [PubMed] [Google Scholar]
- 45.NIH US National Library of Medicine. ClinicalTrial.gov. Avaibale at: https://www.clinicaltrials.gov/ct2/results?cond=Endometrial+Cancer&term=serous&cntry=&state= &city=&dist=. Last accessed December 31, 2020.
- 46.Sheng X, Yan X, Wang L, Shi Y, Yao X, Luo H, et al. Open-label, Multicenter, Phase II Study of RC48-ADC, a HER2-Targeting Antibody-Drug Conjugate, in Patients with Locally Advanced or Metastatic Urothelial Carcinoma. Clin Cancer Res 2020. Oct 27. Doi: 10.1158/1078-0432.CCR-20-2488. Epub ahead of print. [DOI] [PubMed]
- 47.Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V, Macpherson IR, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol 2019. Aug;20(8):1124–1135. Doi: 10.1016/S1470-2045(19)30328-6. Epub 2019 Jun 27. [DOI] [PubMed] [Google Scholar]
- 48.Ivanova E, Kuraguchi M, Xu M, Portell AJ, Taus L, Diala I, et al. Use of ExVivo Patient-Derived Tumor Organotypic Spheroids to Identify Combination Therapies for HER2 Mutant Non-Small Cell Lung Cancer. Clin Cancer Res 2020. May 15;26(10):2393–2403. Doi: 10.1158/1078-0432.CCR-19-1844. Epub 2020 Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-Sahwi K, Bellone S, Cocco E, Cargnelutti M, Casagrande F, Bellone M, et al. In vitro activity of pertuzumab in combination with trastuzumab in uterine serous papillary adenocarcinoma. Br J Cancer 2010. Jan 5;102(1):134–43. Doi: 10.1038/sj.bjc.6605448. Epub 2009 Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santin AD, Filiaci V, Bellone S, Ratner ES, Mathews CA, Cantuaria G, et al. Phase II evaluation of copanlisib, a selective inhibitor of Pi3kca, in patients with persistent or recurrent endometrial carcinoma harboring PIK3CA hotspot mutations: An NRG Oncology study (NRG-GY008). Gynecol Oncol Rep 2020. Jan 2;31:100532. Doi: 10.1016/j.gore.2019.100532. Erratum in: Gynecol Oncol Rep. 2020 May 28;33:100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonazzoli E, Cocco E, Lopez S, Bellone S, Zammataro L, Bianchi A, et al. PI3K oncogenic mutations mediate resistance to afatinib in HER2/neu overexpressing gynecological cancers. Gynecol Oncol 2019. Apr;153(1):158–164. Doi: 10.1016/j.ygyno.2019.01.002. Epub 2019 Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez S, Cocco E, Black J, Bellone S, Bonazzoli E, Predolini F, et al. Dual HER2/PIK3CA Targeting Overcomes Single-Agent Acquired Resistance in HER2-Amplified Uterine Serous Carcinoma Cell Lines In Vitro and In Vivo. Mol Cancer Ther 2015. Nov;14(11):2519–26. Doi: 10.1158/1535-7163.MCT-15-0383. Epub 2015 Sep 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaman GJR, de Roos JADM, Libouban MAA, Prinsen MBW, de Man J, Buijsman RC, et al. TTK Inhibitors as a Targeted Therapy for CTNNB1 (β-catenin) Mutant Cancers. Mol Cancer Ther 2017. Nov;16(11):2609–2617. Doi: 10.1158/1535-7163.MCT-17-0342. Epub 2017 Jul 27. [DOI] [PubMed] [Google Scholar]
- 54.Webster EM, Zeybek B, Tymon-Rosario J, Santin AD. Sacituzumab govitecan: a promising antibody-drug conjugate for the treatment of poorly differentiated endometrial cancer. Oncoscience 2020. Jun 8;7(9–10):68–69. Doi: 10.18632/oncoscience.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romero I, Rubio MJ, Medina M, Matias-Guiu X, Santacana M, Schoenenberger JA, et al. An olaparib window-of-opportunity trial in patients with early-stage endometrial carcinoma: POLEN study. Gynecol Oncol 2020. Dec;159(3):721–731. Doi: 10.1016/j.ygyno.2020.09.013. Epub 2020 Sep 26. [DOI] [PubMed] [Google Scholar]
- 56.Post CCB, Westermann AM, Bosse T, Creutzberg CL, Kroep JR. PARP and PD-1/PD-L1 checkpoint inhibition in recurrent or metastatic endometrial cancer. Crit Rev Oncol Hematol 2020. Aug;152:102973. Doi: 10.1016/j.critrevonc.2020.102973. Epub 2020 May 18. [DOI] [PubMed] [Google Scholar]


