Abstract
Purpose:
Stereotactic body radiotherapy (SBRT) in the management of adrenal metastases is emerging as a well-tolerated, effective method of treatment for patients with limited metastatic disease. SBRT planning and treatment utilization is widely variable, and publications report heterogeneous radiation dose fractionation schemes and treatment outcomes. The objective of this analysis was to review the current literature on SBRT for adrenal metastases and to develop treatment guidelines and a model for tumor control probability (TCP) of SBRT for adrenal metastases based on these publications.
Methods:
A literature search of all published studies of SBRT for adrenal metastases from 2008–2017 was performed and outcomes in these studies were reviewed. Local control (LC) rates were fit to a statistically significant Poisson model using maximum likelihood estimation techniques.
Results:
One-year LC greater than 95% was achieved at an approximated biological equivalent dose with α/β=10 Gy (BED10) of 116.4 Gy.
Conclusion:
While respecting normal tissue tolerances, tumor doses greater than or equal to a BED10 of 116.4 Gy are recommended to achieve high LC. Further studies following unified reporting standards are needed for more robust prediction.
Keywords: SBRT, tumor control probability, adrenal, metastasis
Summary
Based on the published literature on outcomes of SBRT for adrenal tumors, a model for the tumor control probability (TCP) was developed, and an optimal dose/fractionation schema is recommended to achieve high tumor control while respecting nearby organs at risk.
1. Clinical Significance
The most common tumors involving the adrenal gland are metastases from other primary cancers. An autopsy study of patients with metastatic carcinoma revealed that approximately one-quarter of patients had metastatic involvement of the adrenal gland by the primary cancer, most commonly from lung, gastric, and renal cancer (1). Surgery has historically been the primary treatment modality for patients with adrenal metastases, predominantly in the setting of oligometastatic disease (limited metastases to typically one to five sites/lesions).
Radiotherapy (RT) in the treatment of adrenal metastases has gained traction in recent years, although conventional RT techniques were typically only employed for palliation of pain (2–4). In the modern era, the development and utilization of stereotactic body radiotherapy (SBRT) has expanded the use of RT in treatment of adrenal tumors (5, 6). SBRT delivers a very conformal, high dose of radiation in a reduced fractionation scheme.
In patients who are ineligible for surgery (due to medical co-morbidities, sub-optimal anatomy, local tumor invasion) or patients who refuse surgery, SBRT can be considered a reasonable alternative. With advances in management of tumor motion, imaged-guided treatments, and more sophisticated treatment planning software, SBRT can be delivered safely to intra-abdominal tumors including malignancies of the adrenal gland. No randomized controlled trials have been conducted comparing surgery to SBRT for adrenal malignancies; however, recent studies that report SBRT outcomes are encouraging. Utilization of SBRT has classically been focused on patients with limited disease who are poor surgical candidates in an attempt to increase progression-free survival (PFS) (6).
With the increasing use of SBRT for many sites and the growing interest in using SBRT as an alternative to surgical resection in patients with oligometastatic disease, there has been an attempt to quantify the benefit of this approach and to establish appropriate dose and fractionation schemes. Although SBRT for adrenal metastases is less commonly performed than for liver and lung tumors, there is a move toward using this approach for patients with oligometastatic disease and there have been multiple recent additions to the literature on SBRT for adrenal tumors. The objective of this work was to review the existing publications of primarily single institution, retrospective studies reporting the use of SBRT for adrenal metastases and to model tumor control probability (TCP) with SBRT in this particular clinical scenario. Despite the variation in the available datasets, there appeared to be sufficient discrete data points for outcomes and a range of doses and fractionations to develop a model encompassing these differing dose and fractionation schema to allow for an evaluation of the efficacy of these regimens.
2. Endpoints
Several endpoints have been reported in the literature including overall survival (OS) (median, one- and two-year survival), local control (LC) at similar time points, or PFS. The most commonly reported outcomes were LC and OS (5, 7–16). These were the endpoints selected for analysis and modeling. LC is generally defined as no evidence of locally progressive disease. All the studies reported in Table 1 employed Response Evaluation Criteria in Solid Tumors (RECIST) to evaluate the objective response of solid tumors after treatment (17) except for studies by Torok, Desai, and Chance which stated that radiographic progression on follow-up PET and CT studies was used. For example, Chance, et al, characterized failure by “CT evidence of progressive abnormalities over time that corresponded to PET-avid areas (18).” While RECIST criteria are the most commonly used assessments of response, they may be limited in evaluating response to SBRT where tumors are less likely to change in size, and treatment response can evolve over time with initial increase in size of lesions followed by gradual reduction in tumor size.
Table 1:
Adrenal SBRT studies reviewed in the study. Ones included in the adrenal tumor control probability model are shown in Figure 1.
| Author (citation) | No. of Patients | Median FU (m) [range] | No. of Lesions | Primary Site | Volumes (mm) | Median Total Dose (Gy) [range] | No. of Fractions | Local Control | Overall Survival | Rx type | Target Dose Coverage | GTV size (cc) [range] |
PTV size (cc) [range] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *Ahmed, 2013 (7) | 13 | 12.3 [3.1–18] | 13 | Lung, Kidney, Skin, Other | GTV=CTV PTV=ITV+5 | 45 [33.75–60] | 5 (all) | 12.3mo-100% | 12 mo- 62.9% | NR | NR | NR | 88.4 [37.7–379.6] |
| Barney, 2012 (8) | 6 (subgroup of 50 abdo/pelvic mets:numbers quoted are for entire patient cohort). | 12 [2–28] | 6 | GI, Lung, Kidney, Other | GTV=CTV PTV=ITV+5 | 45 [20–60] | 5 [1–5] | 6 mo- 98%; 12 mo-87% | 6 mo- 90% 12 mo- 62% | 95% IDL | 99% target received at least 90% dose | NR | NR |
| *Casamassima, 2012 (9) | 48 | 16.2 [3–63] | 48 | Lung, Colon, Melanoma, Other | GTV=CTV PTV=ITV+3 | 36 [21.69–54.09] | 3 [1–3] | 12 mo- 90% 24 mo- 90% | 12 mo- 39.7% 24 mo- 14.5% | 70% IDL | NR | 103.4 [6.6–497] | NR |
| *Chawla, 2009 (5) | 30 | 9.8 [3.2–28.3] | 35 | Lung, Liver, Breast, Other | GTV=CTV PTV=CTV+7–10 | 40 [16–50] | 10 [4–16] | 6 mo- 91% 12 mo- 55% 24 mo- 27% | 12 mo- 44% 24 mo- 25% | 100% IDL | 80% to PTV | 18.6 [7.5–156] | NR |
| *Guiou, 2012 (10) | 9 | 7.3 (mean) [0–26] |
10 | Lung | ITV PTV=ITV+ expansion | 25 [20–37.5] | 5 (all) | 12 mo- 44% 24 mo- 44% | 12 mo- 52% 24 mo- 13% | NR | NR | 163 [22.3–599.7] | 306.62 [127.5–953.6] |
| Holy, 2011 (11) | 18 | 12 [2–61] | 18 | Lung | CTV=GTV+2 PTV=CTV+5–10 | 40 [24–40] | 5 [3–6] | Median PFS- 4.2 mo | Median- 21 mo | 100% IDL | ~100% | NR | 176 [20–422] |
| *Katoh, 2008 (12) | 9 | 16 [5–21] | 10 | Lung, Prostate, Kidney, Liver | CTV=GTV+3 PTV=CTV+5 | 48 [30–48] | 8 (all) | 12 mo- 100% | 12 mo- 78% | iso | 80% to PTV | NR | NR |
| Oshiro, 2011 (27) | 19 (11 underwent hypofractionation and 8 underwent conventional fractionation) |
10.1 [0.7–87.8] | 19 | Lung | CTV=GTV PTV=CTV+5–10 | 45 [30–60] | 10 [1–27] | NR | For synchronous mets; 12 mo- 55.6% 24 mo- 33.4% 60 mo- 22.3% Metachronous mets: 12 mo-83.3% 24 mo and 60 mo- 55.6% |
NR | NR | NR | NR |
| *Rudra, 2011 (13) | 10 | 14.9 [5–45.8] | 13 | Lung, Kidney | ITV PTV=ITV+5–10 | 36 [24–50] | 3 [3–10] | 12 mo- 73% | 12 mo- 90% | 80–90% IDL | V95%>95% | NR | NR |
| *Scorsetti, 2012 (14) | 34 | 41 [12–75] | 36 | Lung, Melanoma, Other | CTV=GTV+3 PTV=CTV+5 | 32 [20–45] | 4 [4–18] | 12 mo- 66% 24 mo- 32% | 24 mo- 53% | NR | NR | NR | NR |
| *Torok, 2011 (15) | 7 | 14 [1–60] | 9 | Lung, Liver | GTV=CTV PTV=ITV | 16 (for 5 lesions) [10–22] 27 (for 4 lesions) [24–36] | 16 Gy in 1 fx 27 Gy in 3 fx | 12 mo- 63% | Median- 8 mo | 1fx: 80% 3fx: 94% | 86% | 63.8 [5–123.6] |
NR |
| Desai, 2015 (22) | 14 | NR | 14 | Lung, Renal, Other | NR | 25 [13–30] | 3 [1–5] | NR | Median- 7 mo | NR | NR | NR | NR |
| *Chance, 2016 (18) | 43 | 16 [3–94] |
49 | Lung, Ovary, Esophagus, Other | ITV PTV=ITV+3–5 | 60 [40–70] | 10 [4–15] | 12 mo- 74% 24 mo- 57% |
12 mo- 65% 24mo- 42% |
95% IDL | 97% (median) | 13 [3–90] |
41 [11–262] |
| *Franzese, 2017 (26) | 46 | 7.6 | Lung, Other | CTV PTV = CTV + 5 | 40 (all) | 4 (all) | 12 mo- 66% (±12%) 24 mo 41% (±16%) |
12 mo- 87.6% | V95%>90% (for PTV) | V95%>90% | NR | 63 (mean) (±34) | |
| *Gamsiz, 2015 (16) | 15 | 16 | 17 | Lung | GTV=CTV (DIBH) PTV=CTV+3–5 | 30 (all) | 3 (all) | 16 mo- 86.7% | 16 mo- 33% | 90–100% IDL | NR | 28.4 (mean) [6.6–101.5] | 57.4 (mean) [16.5–143.8] |
| Li, 2013 (23) | 26 | NR | 26 | Various | PGTV=GTV + 3 CTV=GTV +8 PTV=CTV+2–3 | 43 [30–50] | 5 [3–5] | NR | Median- 17 mo | Median- 70% IDL (58–80%) | 95% | NR | NR |
Abbreviations: No. (Number); Gy (Gray); NR (not reported); mo (months); PFS (progression-free survival); fx (fraction); DIBH (deep inspiration breath hold);
studies used in TCP model
Dosimetric data were also reported in the studies and included median total dose, number of fractions, target dose coverage, and target volume size. For patients whose outcomes were reported at 1 year, data were recorded, and the equivalent dose in 2 Gy fractions (α/β=10 Gy) (EQD210) was calculated and used for analysis.
3. Challenges Defining and Segmenting Volumes
The delineation of target volumes when utilizing SBRT can be complicated and necessitates multiple imaging modalities and optimal CT simulation with intravenous contrast if possible and immobilization of the patient. If diagnostic imaging studies including PET/CT, CT, and MRI are available they can be fused with the CT scan obtained during simulation of the patient and reviewed by the treating physician prior to contouring. The gross tumor volume (GTV) is defined by the axial images from the simulation and diagnostic images. Although the studies in Table 1 primarily used CT for tumor delineation , 5 of them (5, 7, 8, 13, 18) also fused PET and/or MR scans from staging imaging to aid this task. As evidenced in the reported retrospective data (Table 1), frequently no expansion for microscopic disease is applied to the GTV; in such cases, the GTV and clinical target volume (CTV) are identical (8, 15). It is common in the practice of SBRT to omit a CTV expansion as microscopic disease is not the intended target. Given high dose and planning techniques with SBRT, tighter margins are typically employed. Nonetheless, contouring adrenal tumor volumes remains challenging and a source of uncertainty despite multiple diagnostic imaging modalities available, thus the input of a diagnostic radiologist may be helpful to accurately delineate the GTV.
An important component of SBRT is the consideration and management of motion typically driven by respiration. Adrenal tumor motion is marked in the cranio-caudal dimension, ranging from 5–12mm in one report (19), but less so in the left-to-right or antero-posterior dimensions (20, 21). In the reported series in Tables 1 and 2, motion was accounted for in a myriad of ways including the use of four-dimensional CT (4DCT), deep inspiration breath hold (DIBH), and abdominal compression. In most cases an internal target volume (ITV) was contoured based on the 4DCT. Lastly, a planning target volume (PTV) was created by adding an approximately 3–5 mm margin to the CTV or ITV in most cases. This volume accounts for daily setup error, breathing pattern variations, and motion of the target (if an ITV had not previously been defined).
Table 2:
Summary of motion management and image guidance utilized in Adrenal SBRT studies at the time of simulation, planning, and treatment.
| Author (citation) | Motion Management: Simulation and Planning | Motion management: Image Guidance for Treatment |
|---|---|---|
| Ahmed, 2013 (7) | 4DCT/ITV | ^Daily CB (2nd confirming CB for shifts> 3 mm) |
| Barney, 2012 (8) | Compression for diaphragm motion ≥ 5 mm + 4DCT to construct ITV | ^Daily CB (2nd confirming CB for shifts > 3 mm) |
| Casamassima, 2012 (9) | ITV from free-breathing, end inhale and end exhale CT scans at simulation | Daily CB (matched on bone) |
| Chawla, 2009 (5) | End Exhale Breath-hold | NR |
| Guiou, 2012 (10) | Compression if tolerated (5/9 pts); 4DCT for ITV | Daily CB |
| Holy, 2011 (11) | Compression (14/18 pts): SI margins from fluoroscopy of diaphragm | Daily orthogonal radiographs compared to DRR |
| Katoh, 2008 (12) | One implanted fiducial; Planning CT at end-exhale. | RTRT fluoroscopic tracking system, tracking fiducial |
| Oshiro, 2011 (27) | End exhale gating (9/11 pts) | Gating with modified Microtron |
| Rudra, 2011 (13) | Simulation with free-breathing, 4DCT and end exhale scans; plan for ITV (‘not significant tumor motion’) or end exhale | Daily orthogonal radiographs (match on bone) followed by ungated CB to verify PTV margin |
| Scorsetti, 2012 (14) | Population based | Orthogonal KV radiographs (2D-2D) |
| Torok, 2011 (15) | 4DCT for the 2/7 gating patients | 5/7 pts CK (Synchrony system); 2/7 pts Trilogy with gating |
| Desai, 2015 (22) | (single fiducial placed; free-breathing CT; margins NR | CK (Synchrony system) |
| Chance, 2016 (18) | 4DCT; ITV for 86% of pts, voluntary deep-inhale breath-hold for tumor motion> 1 cm (14% pts) | Daily CB or CT on Rails |
| Franzese, 2017 (26) | Abdominal compression and 4DCT for construction of ITV (union of CTVs on all phases) | Daily CB compared to simulation scan |
| Gamsiz, 2015 (16) | Moderate deep inhale using ABC™ device | Daily CB |
| Li, 2013 (23) | ≥ 3 fiducials placed; generic margins | CK |
Abbreviations: ITV (internal target volume); CB (cone beam); NR (not reported); DRR (digitally reconstructed radiograph); KV (kilovoltage); CK (CyberKnife);RTRT (Real-time Tumor-tracking radiotherapy (40)
matching structure not stated
In the literature, a large range of PTV sizes (see Table 1) is reported; median reported PTVs in these studies ranged from 41 cc to 306.6 cc. The size of the target volume and presence of nearby organs at risk pose limitations to the total dose and number of fractions that can be safely delivered to these mobile, intra-abdominal tumors.
Fiducials are commonly used to improve target localization when delivering SBRT. However, in this report, a minority of the published experiences utilized fiducial markers for adrenal tumors (12, 22, 23). This is likely due to the relative ease in identifying an adrenal mass with the difference in Hounsfield Units of surrounding fat and other nearby organs on cone-beam CT (CBCT), which was used for image guidance in many of the reviewed studies. Fiducials remain an option for optimizing treatment delivery, though are not essential per the summarized reports here and remain institutionally dependent. Further discussion on this topic is included in the American Society for Radiation Oncology (ASTRO) quality and safety considerations in SBRT (24) and in the report of AAPM Task Group 101 on stereotactic body radiation therapy (25).
4. Review of Outcomes
A literature search using Pub Med was undertaken to identify publications capturing patients treated with SBRT for adrenal malignancies with available outcomes. Specifically, the search criteria included keyword in the Title/Abstract of “stereotactic” or “radiotherapy” and “adrenal” published from January 01, 2007 through March 01, 2017. Results were limited to those in the English language. This yielded 250 relevant results. These results were manually reviewed. Studies were excluded if not pertaining to adrenal SBRT. Those that were reviews only were excluded. Studies with available outcomes (LC or OS) were included. One study, Guiou et al, was not available in the Pub Med database. However, this was later included when referenced in another included source.
Captured in Table 1 are important clinical and treatment parameters. The studies themselves were fairly small. The number of treated lesions ranged from 6–49 total tumors (6–48 patients) per study. Multiple primary sites were included with different histologies. As aforementioned, publications often included information regarding PTV and GTV with ranges annotated for each. The median total physical dose was recorded, with a median between all studies of 40 Gy (mean, 37.2 Gy). The range and median number of fractions for each publication varied both within studies and across studies; a median of 5 fractions (range, 1–27) was delivered across all studies. Prescription dose was intended to cover the PTV with the 70–100% isodose line (IDL) in most studies (when it was reported). Motion management methods were described in all the publications (Table 2). If treatment was delivered on a conventional linac, most studies used an internal target volume (ITV) based on imaging multiple breathing phases at simulation, sometimes supplemented with compression to suppress large breathing motion (7–11, 13, 18, 26). Three studies used breath-hold or respiratory gated treatment based on a 4DCT (5, 16, 27). One study did not use patient-specific GTV-to-PTV margins (14). Immobilization was often provided by thermoplastic chest masks and/or abdominal compression, and image guidance (Table 2) was employed. In 9 studies, image guidance was done by comparing cone beam CT imaging at treatment setup with the simulation scans; one also used CT-on-rails (18). Three studies (15, 22, 23) used CyberKnife, one(12) used a dedicated tracking system (RTRT), two (11, 14) matched bony landmarks in on-treatment radiographs with simulation DRRs and two did not describe image guidance. Some studies utilized implanted gold fiducials [length 5mm, diameter 0.8mm in one experience (19)] to track and target adrenal tumors for SBRT. Both the ASTRO guidelines on safety of SBRT and the report of AAPM’s Task Group 101 recommend a comprehensive image guidance and motion management strategy for safe delivery of SBRT (24, 25). Of the studies used for modeling, response was assessed by the RECIST criteria with one exception (18) that used CT and PET responses. With the exception of the smallest study (15), the Kaplan-Meier method was used to assess LC and OS.
While the studies summarized in Table 1 reveal promising survival and LC rates, there is high variability in LC ranging from 44–100% at 1 year (5, 7, 10, 13). The extent to which this is due to patient selection versus dosimetric and technical factors is unclear. For example, in a study reported by Oshiro et al., patients who were deemed ineligible for adrenalectomy if they had advanced disease, intercurrent disease, or small cell histology were treated with SBRT. Median dose was 45 Gy (range 30–60 Gy) in median 10 fractions (1–27). The 2-year and 5-year OS rates were 83% and 56%, respectively for the 6 patients with metachronous adrenal metastases (27). This cohort fared significantly better than the 13 patients with synchronous and coexisting metastases (0% at 2 and 5 years) but they also had more aggressive dose schedules (median BED10 of 81.3 Gy vs 50 Gy). Nonetheless, the outcomes for the metachronous adrenal metastases cohort are similar to the outcomes for patients undergoing surgery for adrenal metastases with one surgical study reporting 1- and 2-year survival rates of 80% and 52% (28). A more recent SBRT series published by Franzese et al, notes OS of 88% at 1 year in a 46-patient cohort receiving 40 Gy in 4 daily fractions (prescription BED10 of 80 Gy) (26). They did not find an OS difference between patients with a solitary adrenal metastasis and ‘oligometastatic’ patients (≤ 5 metastases). In a retrospective review of 14 adrenal tumors treated with CyberKnife, Desai et al found BED10 to be the most significant correlate to LC (22). In this publication, which does not specify the prescription isodose line, local failures correlated with a mean BED10 of 76 Gy while durable LC was ascertained at a mean BED10 greater than 96.76 Gy. Other trials, typically in the setting of early stage NSCLC, have supported the finding of increased LC with BED10 greater than or equal to 100 Gy with SBRT (29, 30).
5. Factors Affecting Outcome
Many factors that have the potential to influence outcomes in this population of patients and the data are reported in Table 1. As described above, there is considerable heterogeneity in the datasets regarding target delineation, dose prescription (dose per fraction, prescription isodose, number of fractions), primary histology, types of outcomes analysis and treatment intent (palliative or definitive). It is also important to realize that target coverage for each patient depends on many factors including treatment planning and delivery technique, respiratory motion control, proximity to critical structures, and previous treatments. The planning target volume (PTV) definition differed from study to study; the PTV had a margin of as little as zero (PTV = GTV) (15) or as large as 10 mm expansion from CTV or ITV (11, 13, 27). Given variability in the available data, we are unable to establish a clear correlation between margin size and LC. Margin adequacy could impact tumor control and a standardized margin would be desirable for future studies. Finally, all of these studies had small patient numbers with competing risks of death from other sites of metastatic disease, thus impacting the reliability of the local control data. Despite these caveats, there was a clear dose response in terms of LC and OS in patients with adrenal metastases treated with SBRT.
6. Mathematical/Biological Models
Analyzing the publications in Table 1, we found no correlation of PTV margin with LC. Prescription isodose lines (IDL) varied from 70% to 100%; these did not seem to correlate with the observed LC rate based on the crude observation. We did not perform statistical analysis of the IDL effect due to very limited data points available.
The 1-year LC rates of the suitable studies in Table 1 were fit to a statistically significant Poisson model, shown in Equation 1 (31), using maximum likelihood estimation techniques. The 95% confidence intervals of the parameters were estimated using profile likelihood. The Poisson model for TCP is among the most commonly used in the literature due to its radiobiological relevance. To account for the variety of fractionations, doses were converted into the equivalent dose in 2 Gy fractions with α/β=10 (EQD210) (32).
| Equation 1 |
where TCP is 50% at EQD250,10 and γ50 is the normalized slope at 50% TCP.
Because detailed DVH data were not available, we used the median prescription dose, isodose line, and number of fractions from each publication to estimate the median EQD210 encompassing the tumor; the dose was adjusted accordingly, if the prescription IDL was known. The fit to these data was statistically significant using chi-squared test (χ2 = 57.5, χ < 0.0001) (the p value rejects the null hypothesis that the fit is has a zero slope (horizontal line)).
Figure 1 shows the model fit (solid curve), the 95% confidence intervals (dotted), and the data points. The circle size is proportional to the number of patients in the study and the error bars are calculated using Agresti–Coull approximation for binomial proportions (33). It is noted that LC at 95% is achieved with an approximate BED10 of 116.4 Gy (EQD210 ~ 97 Gy). In addition, a dose-response model using equation 1 for the published OS rates at year 1 is presented (Figure 2). This fit is also statistically significant (χ2is 44.7, p <0.001), though we note that its confidence intervals are quite wide compared to LC, given that many factors other than dose may also contribute to OS.
Figure 1:
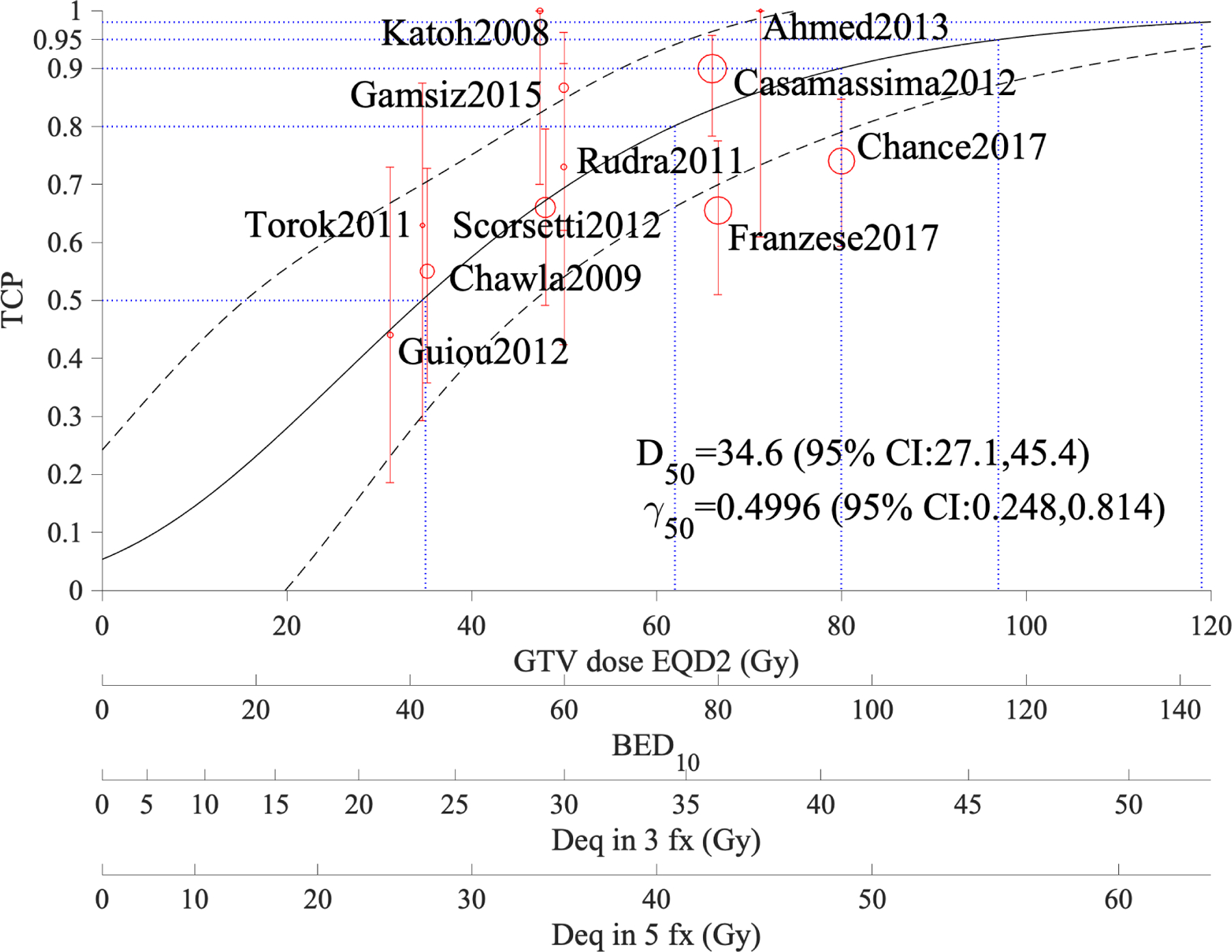
Tumor control probability of adrenal metastases treated with SBRT based on one-year local control; α/β=10 Gy in this model. The figure shows TCP estimates at 50%, 80%, 90%, and 95% (blue dotted horizontal lines). The black dashed curves show the 95% confidence band and the GTV coverage dose scales are for EQD210, BED10, and equivalent doses in 3 and 5 fractions.
Figure 2:
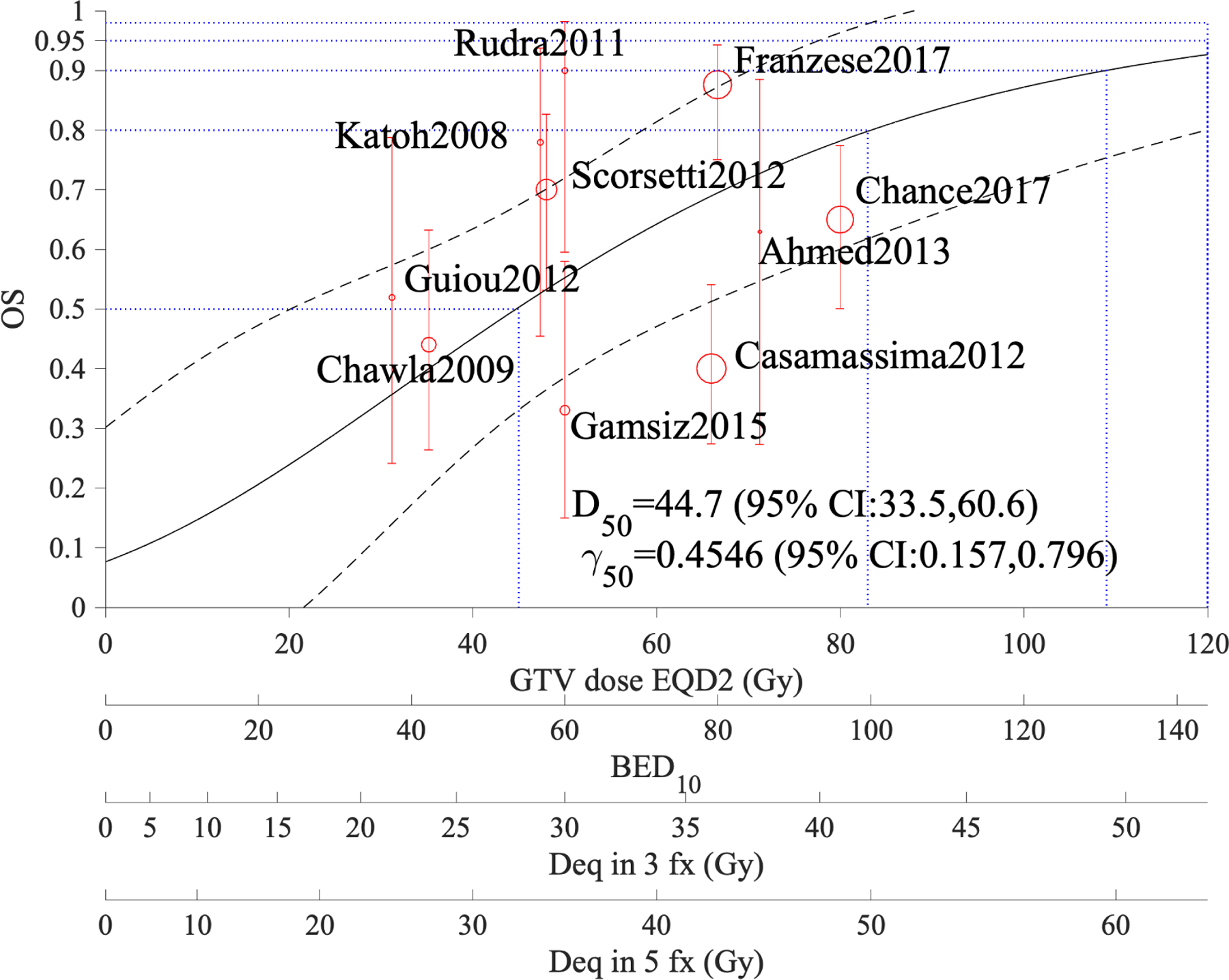
Overall survival of patients with adrenal metastases treated with SBRT based on one-year estimates; α/β=10 Gy in this model. The figure shows OS estimates at 50%, 80%, 90%, and 95% (blue dotted horizontal lines). The black dashed curves show the 95% confidence band and the GTV coverage dose scales are for EQD210, BED10, and equivalent doses in 3 and 5 fractions.
Three additional studies have been published during the write up of this work which were not included in the present model as the manuscripts were not available at the time of this analysis. One recent study (31 patients, 33 lesions) fit this model very well. It treated to a median BED10 of 112.5 Gy with 96.5% LC at 1 year (34). The two additional publications showed a high LC at 1 year with 30 patients and a BED10 of 85.5 Gy (35) and one with 35 patients and median BED10 of 72 Gy (36). The one-year LC for all three studies fell within the 95% CI of the model. The LC dropped off in the two studies with the lower BED10 at two and three years suggesting that the durability of LC may be impacted by dose (35, 36). Most recently, a meta-analysis of outcomes for SBRT of adrenal metastases is in press(37). The authors extracted data from 39 of an initially identified 569 published between 2009 and September 2019. They also report that LC is strongly correlated with BED10. Their analysis, based on 22 studies, predicts LC at 1 year of 70.5% for BED10 of 60 Gy, 84.8% at BED10 of 80 Gy and 92.9% for BED10 of 100 Gy, which is well within the CI of our model. They also found BED10 to be significantly correlated with 2 year LC (19 studies) and 2 year OS, though not with 1 year OS.
7. Special Situations
A recently published review of the literature compared surgical adrenalectomy, SBRT, and percutaneous catheter ablation (PCA) (38). Reported OS and LC were greater in patients undergoing surgery. However, the manuscript aptly reports significant selection biases amongst patients. Tumor histology, performance status, extent of uncontrolled extra-adrenal disease, and co-morbidities were among some of the biases noted. For example, patients in the SBRT group tended to more often have lung primary histology as compared to the surgery or PCA groups (68% vs 33% and 27%, respectively). The authors assert that patients with lung primary metastases have an overall poorer prognosis than other malignancies. Another observation made was that patients offered surgery tended to have isolated adrenal disease (75% vs 48% in the surgical and SBRT cohorts, respectively). Given these heterogeneities in patient cohorts, it remains unclear whether adrenalectomy, SBRT, or other local ablative treatment options offer the most effective approach for adrenal metastases. When considering patients for localized therapy and critically evaluating the literature, these selection biases should be considered. Finally, all but one study that we reviewed included only patients who had not received prior radiation to the adrenal gland. Chawla et al included two patients who underwent re-irradiation and were both still alive at the time of publication with LC of 16 and 5 months (5). However, given the low number of patients represented, our results may not be applicable to patients undergoing re-irradiation to the adrenal disease.
8. Recommended Dose/Volume Objectives
As discussed, there were a range of doses reported in the studies analyzed in this model. The median dose prescribed was 40 Gy, and the median fraction number was 5. In Table 1, the results of the largest retrospective institutional analysis by Casamassima et al are presented (9). In this report, patients received a BED10 of just over 139 Gy to isocenter (100% isodose) with an actuarial LC rate of 90% at 1- and 2-years. This corresponded to a dose of 36 Gy prescribed to the 70% IDL over 3 fractions, and it yielded acceptable toxicities with no acute gastrointestinal, hepatic, or renal issues reported. Within the PTV, the BED10 varies between 79.2 to 139 Gy (EQD210 between 66–116.3 Gy).
The LC model of Figure 1, which incorporates the reviewed studies with dosimetric and LC information, suggests that at 1 year LC over 95% is achieved at an approximate BED10 of 116.4 Gy (EQD210 ~ 97 Gy). Clinical examples of fractionation schema providing this BED10 include (though are not limited to) 45 Gy delivered in 3 fractions (BED10 112.5 Gy) or 55 Gy in 5 fractions (BED10 115.5 Gy). Providing normal tissue tolerances are respected, tumor doses at or above this range are a desirable goal. However, larger studies with more consistent dosimetric reporting and longer follow-up are needed for firm recommendations.
A current ongoing clinical trial, NRG-BR001, may provide further information on TCP for adrenal metastases. This is a phase 1 study of SBRT for the treatment of oligometastases arising from the breast, lung or prostate to various sites (39), prescribes 45 Gy delivered in 3 fractions (acceptable variation of 42.5–45Gy) with a plan to decrease dose to 42 Gy in 3 fractions should dose limiting toxicities be reported. The protocol defines prescription dose as the dose covering 95% of the PTV with the 80–90% IDL generally used, but a range of 60–90% is acceptable. In this protocol, hot spots are to be restricted to within the target unless the target is overlapping with an organ at risk. OAR constraints are provided in the protocol. Normalization is employed such that 100% prescription dose corresponds to the maximum dose in the PTV. Of note, our suggested dose-volume objectives are based on studies which all included respiratory motion management (Table 2). Many techniques are available, as described in the references, and clinicians can adopt motion management techniques in accordance with their available resources.
9. Future Studies
Prospective studies comparing the efficacy and toxicity of adrenal SBRT to adrenal metastectomy could provide essential data to assist in selecting the optimal modality based on individual patient and disease characteristics. Studies should clearly describe the patient characteristics (number of metastases, other sites of disease, performance status) and treatment details (for radiation therapy, motion management and prescription methods are key) and provide data by which future analyses can more confidently determine the optimal dose and fractionation of SBRT. The results of the aforementioned NRG trial will also help elucidate LC rates and outcomes for patients with oligometastatic disease (39). This study includes various histologies which would be representative of typical adrenal metastases. The overall disease response to SBRT in this study will provide prospective data to assist in establishing more standard fractionation patterns for adrenal metastases.
10. Reporting Standards for Outcomes
In an effort to standardize reporting of outcomes for adrenal SBRT, we propose recording and publishing the following parameters, making use of electronic supplements if necessary:
Primary tumor histology
Volume of GTV (or ITV) and PTV
GTV (or ITV) to PTV margin including methods used to mitigate target motion and daily set-up verification measures
- In addition to summary dose and outcome statistics, at least have electronically available
- Prescription dose and IDL and fractionation for each individual patient together with patients’ failure and survival endpoints reported in published data (use of supplementary data if necessary).
- Dose to organs at risk and reported toxicities
Criteria used to determine LC (e.g. RECIST- Response Evaluation Criteria in Solid Tumors used to evaluated the objective response of solid tumors after treatment (17))
Outcomes to consider include but are not limited to cancer-specific survival, LC, time to local progression, time to distant progression
Follow-up protocol (frequency, imaging and other methods)
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
IEN has grants from NIH, Endectra LLC, Resero AI LLC, outside the submitted work. JG reports grants from Accuray, Novocure, and patent DVH Evaluator issued, outside the submitted work. KG reports work with RenovoRX, outside the submitted work.
References:
- 1.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 1950;3(1):74–85. [DOI] [PubMed] [Google Scholar]
- 2.Soffen EM, Solin LJ, Rubenstein JH, Hanks GE. Palliative radiotherapy for symptomatic adrenal metastases. Cancer 1990;65(6):1318–20. [DOI] [PubMed] [Google Scholar]
- 3.Short S, Chaturvedi A, Leslie MD. Palliation of symptomatic adrenal gland metastases by radiotherapy. Clin Oncol (R Coll Radiol) 1996;8(6):387–9. [DOI] [PubMed] [Google Scholar]
- 4.Zeng ZC, Tang ZY, Fan J, Zhou J, Qin LX, Ye SL, et al. Radiation therapy for adrenal gland metastases from hepatocellular carcinoma. Jpn J Clin Oncol 2005;35(2):61–7. [DOI] [PubMed] [Google Scholar]
- 5.Chawla S, Chen Y, Katz AW, Muhs AG, Philip A, Okunieff P, et al. Stereotactic body radiotherapy for treatment of adrenal metastases. Int J Radiat Oncol Biol Phys 2009;75(1):71–5. [DOI] [PubMed] [Google Scholar]
- 6.Ippolito E, D’Angelillo RM, Fiore M, Molfese E, Trodella L, Ramella S. SBRT: A viable option for treating adrenal gland metastases. Rep Pract Oncol Radiother 2015;20(6):484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed KA, Barney BM, Macdonald OK, Miller RC, Garces YI, Laack NN, et al. Stereotactic body radiotherapy in the treatment of adrenal metastases. Am J Clin Oncol 2013;36(5):509–13. [DOI] [PubMed] [Google Scholar]
- 8.Barney BM, Olivier KR, Macdonald OK, Fong de Los Santos LE, Miller RC, Haddock MG. Clinical outcomes and dosimetric considerations using stereotactic body radiotherapy for abdominopelvic tumors. Am J Clin Oncol 2012;35(6):537–42. [DOI] [PubMed] [Google Scholar]
- 9.Casamassima F, Livi L, Masciullo S, Menichelli C, Masi L, Meattini I, et al. Stereotactic radiotherapy for adrenal gland metastases: university of Florence experience. Int J Radiat Oncol Biol Phys 2012;82(2):919–23. [DOI] [PubMed] [Google Scholar]
- 10.Guiou MM NA; Kim EY; Williams T; Lo SS. Stereotactic body radiotherapy for adrenal metastases from lung cancer. J Radiat Oncol 2012:155–63. [Google Scholar]
- 11.Holy R, Piroth M, Pinkawa M, Eble MJ. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol 2011;187(4):245–51. [DOI] [PubMed] [Google Scholar]
- 12.Katoh N, Onimaru R, Sakuhara Y, Abo D, Shimizu S, Taguchi H, et al. Real-time tumor-tracking radiotherapy for adrenal tumors. Radiother Oncol 2008;87(3):418–24. [DOI] [PubMed] [Google Scholar]
- 13.Rudra S, Malik R, Ranck MC, Farrey K, Golden DW, Hasselle MD, et al. Stereotactic body radiation therapy for curative treatment of adrenal metastases. Technol Cancer Res Treat 2013;12(3):217–24. [DOI] [PubMed] [Google Scholar]
- 14.Scorsetti M, Alongi F, Filippi AR, Pentimalli S, Navarria P, Clerici E, et al. Long-term local control achieved after hypofractionated stereotactic body radiotherapy for adrenal gland metastases: a retrospective analysis of 34 patients. Acta Oncol 2012;51(5):618–23. [DOI] [PubMed] [Google Scholar]
- 15.Torok J, Wegner RE, Burton SA, Heron DE. Stereotactic body radiation therapy for adrenal metastases: a retrospective review of a noninvasive therapeutic strategy. Future Oncol 2011;7(1):145–51. [DOI] [PubMed] [Google Scholar]
- 16.Gamsiz H, Beyzadeoglu M, Sager O, Demiral S, Dincoglan F, Uysal B, et al. Evaluation of stereotactic body radiation therapy in the management of adrenal metastases from non-small cell lung cancer. Tumori 2015;101(1):98–103. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 18.Chance WW, Nguyen QN, Mehran R, Welsh JW, Gomez DR, Balter P, et al. Stereotactic ablative radiotherapy for adrenal gland metastases: Factors influencing outcomes, patterns of failure, and dosimetric thresholds for toxicity. Pract Radiat Oncol 2017;7(3):e195–e203. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Li F, Dong Y, Song Y, Yuan Z. Clinical study on the influence of motion and other factors on stereotactic radiotherapy in the treatment of adrenal gland tumor. Onco Targets Ther 2016;9:4295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies SC, Hill AL, Holmes RB, Halliwell M, Jackson PC. Ultrasound quantitation of respiratory organ motion in the upper abdomen. Br J Radiol 1994;67(803):1096–102. [DOI] [PubMed] [Google Scholar]
- 21.Korin HW, Ehman RL, Riederer SJ, Felmlee JP, Grimm RC. Respiratory kinematics of the upper abdominal organs: a quantitative study. Magn Reson Med 1992;23(1):172–8. [DOI] [PubMed] [Google Scholar]
- 22.Desai A, Rai H, Haas J, Witten M, Blacksburg S, Schneider JG. A Retrospective Review of CyberKnife Stereotactic Body Radiotherapy for Adrenal Tumors (Primary and Metastatic): Winthrop University Hospital Experience. Front Oncol 2015;5:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Shi Z, Wang Z, Liu Z, Wu X, Shen Z, et al. Treating adrenal tumors in 26 patients with CyberKnife: a mono-institutional experience. PLoS One 2013;8(11):e80654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solberg TD, Balter JM, Benedict SH, Fraass BA, Kavanagh B, Miyamoto C, et al. Quality and safety considerations in stereotactic radiosurgery and stereotactic body radiation therapy: Executive summary. Pract Radiat Oncol 2012;2(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 2010;37(8):4078–101. [DOI] [PubMed] [Google Scholar]
- 26.Franzese C, Franceschini D, Cozzi L, D’Agostino G, Comito T, De Rose F, et al. Minimally Invasive Stereotactical Radio-ablation of Adrenal Metastases as an Alternative to Surgery. Cancer Res Treat 2017;49(1):20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oshiro Y, Takeda Y, Hirano S, Ito H, Aruga T. Role of radiotherapy for local control of asymptomatic adrenal metastasis from lung cancer. Am J Clin Oncol 2011;34(3):249–53. [DOI] [PubMed] [Google Scholar]
- 28.Tanvetyanon T, Robinson LA, Schell MJ, Strong VE, Kapoor R, Coit DG, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: a systematic review and pooled analysis. J Clin Oncol 2008;26(7):1142–7. [DOI] [PubMed] [Google Scholar]
- 29.Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81(5):1352–8. [DOI] [PubMed] [Google Scholar]
- 30.Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2(7 Suppl 3):S94–100. [DOI] [PubMed] [Google Scholar]
- 31.Kallman P, Agren A, Brahme A. Tumour and normal tissue responses to fractionated non-uniform dose delivery. Int J Radiat Biol 1992;62(2):249–62. [DOI] [PubMed] [Google Scholar]
- 32.Bentzen SM, Dorr W, Gahbauer R, Howell RW, Joiner MC, Jones B, et al. Bioeffect modeling and equieffective dose concepts in radiation oncology--terminology, quantities and units. Radiother Oncol 2012;105(2):266–8. [DOI] [PubMed] [Google Scholar]
- 33.Agresti AaC B. Approximate Is Better than ‘Exact’ for Interval Estimation of Binomial Proportions. The American Statistician 1998;52(2):119–26. [Google Scholar]
- 34.Scouarnec C, Pasquier D, Luu J, le Tinier F, Lebellec L, Rault E, et al. Usefulness of Stereotactic Body Radiation Therapy for Treatment of Adrenal Gland Metastases. Front Oncol 2019;9:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao X, Zhu X, Fei J, Ren H, Cao Y, Ju X, et al. Short-term outcomes and clinical efficacy of stereotactic body radiation therapy (SBRT) in treatment of adrenal gland metastases from lung cancer. Radiat Oncol 2018;13(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toesca DAS, Koong AJ, von Eyben R, Koong AC, Chang DT. Stereotactic body radiation therapy for adrenal gland metastases: Outcomes and toxicity. Adv Radiat Oncol 2018;3(4):621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen WC, Baal JD, Baal U, Pai J, Gottschalk A, Boreta L, et al. Stereotactic body radiotherapy of adrenal metastases: a pooled meta-analysis and systematic review of 39 studies with 1006 patients. Int J Radiat Oncol Biol Phys 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunjur A, Duong C, Ball D, Siva S. Surgical and ablative therapies for the management of adrenal ‘oligometastases’ - A systematic review. Cancer Treat Rev 2014;40(7):838–46. [DOI] [PubMed] [Google Scholar]
- 39.Oncology N. NRG-BR001: A Phase I Study of Stereotactic Body Radiotherapy (SBRT) for the Treatment of Multiple Metastases [Google Scholar]
- 40.Shirato H, Shimizu S, Shimizu T, Nishioka T, Miyasaka K. Real-time tumour-tracking radiotherapy. Lancet 1999;353(9161):1331–2. [DOI] [PubMed] [Google Scholar]


