Abstract
Purpose:
Numerous dose and fractionation schedules have been used to treat medically inoperable stage I Non-small cell lung cancer (NSCLC) with stereotactic body radiation therapy (SBRT) or stereotactic ablative radiotherapy (SABR). We evaluated published experiences with SBRT to determine local control (LC) rates as a function of SBRT dose.
Methods:
One hundred sixty published articles reporting LC rates following SBRT for stage I NSCLC were identified. Quality of the series was assessed by evaluating the number of patients in the study, homogeneity of the dose regimen, length of follow-up time, and reporting of LC. Clinical data including 1, 2, 3, and 5 year tumor control probabilities for T1, T2, and combined T1 and T2 stage as a function of the biological effective dose were fitted to the linear quadratic (LQ), Universal survival curve (USC), and regrowth models.
Results:
Forty-six studies met inclusion criteria. As measured by the goodness of fit χ2/ndf, with ndf as the number of degrees of freedom, none of the models were ideal fits for the data. Of the three models, the regrowth model provides the best fit to the clinical data. For the regrowth model, the fitting yielded an α/β ratio of approximately 25 Gy for T1 tumors, 19 Gy for T2 tumors, and 21 Gy for T1 and T2 combined. In order to achieve the maximal LC rate, the predicted physical dose schemes when prescribed at the periphery of the planning target volume (PTV) are 43 +/− 1 Gy in 3 fractions, 47 +/− 1 Gy in 4 fractions, and 50 +/− 1 Gy in 5 fractions for combined T1 and T2 tumors.
Conclusion:
Early stage NSCLC is radioresponsive when treated with SBRT/SABR. A steep dose-response relationship exists with high rates of durable LC when physical doses of 43–50 Gy are delivered in 3–5 fractions.
Keywords: Stage I NSCLC, SBRT, TCP Models
Summary
Stereotactic body radiation therapy (SBRT) or stereotactic ablative radiotherapy (SABR) is an effective treatment for medically inoperable early stage NSCLC. The authors quantitatively evaluated published experience with thoracic SBRT for early NSCLC and modeled local control rates as a function of SBRT dose. Early stage NSCLC is radioresponsive when treated with SBRT/SABR. A steep dose-response relationship exists with high rates of durable LC when physical doses of 43–50 Gy are delivered in 3–5 fractions.
1. CLINICAL SIGNIFICANCE
Non-small cell lung cancer (NSCLC) is the 2nd most common cancer overall (228,190 cases per year in the US), and the leading cause of cancer-related death (159,480 deaths) in both men and women [77]. Stage I disease represents approximately a quarter of the patients diagnosed with NSCLC and accounts for the most curable cohort of the population (SEER 18 2004–2010; AJCC Cancer Staging Manual, 7th edition). The standard treatment for medically operable stage I NSCLC has historically been an anatomical resection with lobectomy as well as hilar and mediastinal lymph node dissection [1,16,22,63]. However, the majority of patients with NSCLC have a history of chronic tobacco use and a median age of diagnosis of 65–74 years [SEER 18 2004–2010; AJCC Cancer Staging Manual, 7th edition], and often have cardiopulmonary comorbidities (e.g. cardiac and pulmonary) that make them at high-risk for resection. Some patients are deemed to be medically inoperable [15,82]. The increasing use of screening for lung cancer, based on the National Lung Screening Trial may increase the number of patients with early stage NSCLC appropriate for non-surgical treatments[57].
The historical standard therapy for patients with unresectable early stage NSCLC was conventionally fractionated radiation therapy; e.g. 2–3 Gy per fraction to a dose of ≈ 54–60 Gy. However, the reported long-term local control (≈ 30–70%) and overall survival (≈ 15–30%) rates with this approach are suboptimal[7,28,67]. Advances in imaging, radiation treatment planning, and delivery (e.g. with image-guidance and/or motion management) enable the delivery of “ablative doses” of radiation (e.g. 18–20 Gy times three fractions) to very small targets (often termed SBRT/SABR) that appears to yield better outcomes for early stage NSCLC[50,64,89,90,92,99].
In most of the reports using this approach, typical patient selection criteria include co-morbid conditions that preclude a safe oncologic resection, such as poor pulmonary function tests (FEV1 < 1.2 liters, and DLCO < 50%) [30,49,90]. Most patients are staged with a whole body Computed tomography (CT),and/or Positron emission tomography-computed tomography (PET-CT) scan. Patients suspected of having lymph node involvement (interlobar, hilar, or mediastinal) are not candidates for SBRT. More often than not, pathological staging of the mediastinal nodes is not done due to the risk of invasive procedures in this patient population[17,72]. However, tissue diagnosis of the NSCLC subtype using CT-guided or endobronchial ultrasound-guided (EBUS-guided) needle biopsy is recommended [17,78]. Of note, EBUS directed biopsy is usually only appropriate for centrally located primary tumors. Ideally, primary tumor size is restricted to ≤ 5 cm (e.g. T1a – T2a), and thus the optimal patients for SBRT include clinically staged IA and IB medically inoperable NSCLC. Of note, recent ASTRO guidelines conditionally recommend SBRT for tumors larger than five cm that are not suitable for surgical resection with appropriate counseling of patients regarding higher risk of locoregional and distant failures[100]. Nevertheless, the majority of patients in the available literature were treated for lesions ≤ 5 cm and in non-central locations as tumors in central locations have less favorable outcomes with SBRT (see section 7, “Special Situations).
2. ENDPOINTS
The primary endpoint reported in the literature was local tumor control (LC) at the primary site of SBRT. When reported, the actuarial rates of local control, defined as no local progression at the primary tumor site as assessed by CT or PET-CT imaging, at 1, 2, 3, and 5 years were recorded; however the majority of the studies only reported outcomes up to 3 years. Overall survival data is often reported in the literature and was collected in this review. However, this data was not used in the final analysis and modeling due to the lack of consistent reporting of this endpoint in the reviewed literature. Distant failure was not recorded in our review due to minimal observed correlations to models assessing local tumor control probabilities (TCP).
Comparisons with surgical series are challenging since most surgical series define local failure to include failure within the lobe of the lung (in cases of sublobar resection) as well as locoregional or regional failures (failures within hilar and mediastinal lymph nodes). These metrics are not routinely reported in the SBRT literature. Further, patients undergoing SBRT for early stage NSCLC generally have greater competing risks for death from causes other than their lung cancer compared to patients undergoing surgery, as the latter have fewer competing comorbidities[46,107]. Thus, it is possible that reported actuarial local control rates at 1–3 years after SBRT over-estimate the true LC. Since patients dying of intercurrent deaths (death not due to lung cancer during the follow-up period for lung cancer after treatment) are censored, perhaps leaving an “enriched healthier” subset of evaluable patients while those that died of intercurrent illnesses may have had occult local progression prior to death. Indeed, most matched-pair comparisons between SBRT versus surgery report an inferior overall survival with SBRT despite comparable cause-specific survival[66,76].
In most SBRT series, LC was assessed using CT and PET-CT based imaging and applying the Response Evaluation Criteria in Solid Tumors (RECIST) and/or changes in PET-FDG activity. Some studies reported pathological confirmation of tumor recurrence in a subset of the patients. Nevertheless, given that the majority of the recurrences were assessed radiographically, there is certainly some uncertainty in the reported LC rates. SBRT can cause scarring/inflammatory changes that result in tissue distortion making radiographic interpretations difficult[36,37,42]. These changes can mask, and thus delay, the diagnosis of tumor recurrence. Similarly, local inflammation soon (within 1 year) after SBRT often causes an increase in FDG uptake which can make response assessment unreliable and can lead to false positives[79,108]. Thus, data from studies with longer follow-up are likely more accurate in their assessment of LC.
3. CHALLENGES DEFINING AND SEGMENTING ANATOMIC VOLUMES
Respiration-induced tumor motion is a challenge for target definition. Older series often used breath hold (both deep-exhalation and deep-inhalation) CT’s or used fluoroscopy as a surrogate to define an extreme borders of the target’s motion envelope. Most of the modern studies use 4-dimensional CT (4D-CT) scans to define the target volume, where the images acquired in the same respiratory phase or amplitude are grouped together to reconstruct multiple 3DCTs. The amplitude-based 4DCT reconstruction is preferred because it generates less image motion artifacts. A separate free breathing scan, with or without contrast, is often also obtained. Intravenous (IV) contrast may be useful in settings where the target lesion abuts a large vessel and/or the mediastinal structures[23,34,105].
Some studies have used methods to control the amplitude of tidal volume and thus tumor excursion by simulating the patient with a 4D-CT or fluoroscopy while also using abdominal compression devices[8]. The degree of abdominal compression can be determined by using fluoroscopy and/or imaging implanted radio-opaque fiducial markers within or near the tumor such that the excursion of the marker and tumor is within an acceptable range[29,54]. It is of note that the use of implanted fiducial markers is optional for all respiratory management tools during CT simulation. These motion management strategies at the time of CT simulation are also used to characterize respiratory motion of organs at risk (OARs). Fusion of a PET and a CT scan can help define the tumor borders and is especially helpful when the tumor is adjacent to lung atelectasis, the mediastinum, diaphragm, stomach, liver, etc.[40,68]. Ideally, the PET-CT fusion should be performed in the same respiratory phase (amplitude).
During treatment planning, the contrast enhanced CT as well as the 4D-CT can be used to segment an internal target volume (ITV) using a Boolean operation to account for the motion envelope [41,105]. Various methods including using only the end-expiratory and end-inspiratory phases, or segmenting the tumor in all respiratory phases and using a Boolean operations to combine the contours, as well as generating maximal intensity projection (MIP) images and segmenting the target, have been used to defined the ITV. In addition, based on the ICRU 62 definition, CTV=GTV with no margin[20,53]. Further, due to the high doses per fraction, the doses to the ‘non-target’ tissues immediately adjacent to the PTV receive relatively-high, and likely ‘therapeutic dose’ for potential microscopic disease. Thus, the favorable outcomes reported without a formal CTV expansion should not be taken as proof that there is no microscopic spread. Typical expansions from ITV to PTV are in the range of 3–8 mm in the axial dimensions, and 5–10 mm in the cranio-caudal dimensions with or without respiratory gating or tracking. If respiratory gating is utilized, then the ITV is defined based on the phases selected for treatment. Commonly, near end-expiratory phases (gating phase 30%–70%) are used due to maximal tumor stability and minimal tumor motion in these phases. Alternatively, some studies choose near-inspiratory phases (gating phase 90%–10% or inspiration breath-hold) as the total lung volume is larger, and the percent of lung irradiated to any given dose is likely lowest, in these phases.
Various techniques to control, monitor and/or limit respiratory motion can be used including passive breath-hold with visual or audio feedback to the patient, active breath control (where air movement is restricted by a device), tracking of external (e.g. surface markers) or internal (e.g. implanted markers, diaphragm, or the tumor itself), and abdominal compression[58,80]. Each of these approaches has its own benefits and limitations. Typically, gating and tracking to improve normal tissue sparing are most useful for tumors with relatively-large respiratory excursions[93,95], but these approaches typically increase treatment times. Tumors in the middle and lower lobes are generally more mobile than those in the upper lobes. Caveats in characterizing tumor and organ motion using a 4D-CT include artifacts induced due to patients’ irregular breathing patterns and reproducibility of the breathing patterns at CT simulation compared to treatment days.
Adjusting the window and level on the CT scan will impact target definition[45]. Typically, a ‘lung window’ is best to define a parenchymal tumor as irregular spiculations can be better appreciated. When a tumor abuts another organ composed mostly of soft-tissue (heart, mediastinum, diaphragm/liver, and chest wall), assessing the boundaries of the target at the interface is best done using a mediastinal or soft tissue windows[83].
4. REVIEW OF OUTCOMES DATA
A keyword search for ‘SBRT and lung’ and ‘stereotactic ablative radiotherapy (SABR) and lung’ using PubMed identified 160 studies reporting clinical outcomes from thoracic SBRT for early stage NSCLC published through May 2014. These studies were systematically reviewed. Articles relating to the treatment of oligometastatic disease to the lung were specifically excluded. Each publication was assessed to determine whether the data was collected prospectively or retrospectively, the number of patients, homogeneity of dose prescriptions, the length of follow-up, and whether local control was reported. Studies with fewer than 10 patients, or tumor stage higher than T2 were excluded. Three of the included studies that met the above publication selection criteria had doses in the 3–4 Gy per fraction range (211 patients). Due to the paucity of data in the intermediate dose per fraction range, these studies were included in order to improve model fitting in the shoulder region. The rest of the included studies had dose per fraction ≥ 6 Gy per fraction (3268 patients). Based on this metric for “quality”, 46 studies[3,4,6,9–14,18,19,21,24,26,27,31–33,38,39,44,47,48,52,55,56,59–61,69–71,73,75,81,84,87,88,90,94,97,98,101,103,106,109] were identified and included for data collection and modeling of outcomes. Of note a retrospective series was included if it met all the other criteria for “quality” listed above, as long as it had included ≥ 10 patients (see supplementary material for all included studies and input from each study).
Extracting/comparing dose information:
The reported dose was prescribed to the isocenter in 17 (36%) studies, or to an isodose surface that covered a certain percent of the PTV in 30 (64%) studies. To facilitate the pooling of data from multiple studies for analysis, in the latter studies, reported doses were converted to presumed isocenter doses by the formula: Reported Dose/Reported percent covering isodose. In the 10 cases where percent isodose was not given, authors were contacted directly for this information (3 cases), or 80% isodose coverage was assumed (7 cases). Thus, from each study, we extracted an estimate of the isocenter dose, as well as the dose per fraction, the number of fractions, and an estimate of the total elapsed days of treatment.
In all analyzed studies, treatment plans were based on multiple non-coplanar or coplanar fields delivered with conformal fields or with dynamic conformal arcs or volumetric modulated arc therapy, or with multi-field intensity modulated radiation therapy.
Notably, a variety of tissue inhomogeneity algorithms were used, which confounds the estimation of the dose at the isocenter. Among the various sources of uncertainty, these algorithms may be inaccurate in estimating the PTV dose and distort the dose distribution in a patient-specific manner. Although most protocols recommend prescriptions to the PTV margin[5,91], the dose calculation introduces more uncertainty in this region than at the isocenter[35,48,102,104] or at a suitable calculation point within the tumor for IMRT. In particular, the dosimetric uncertainty at lung-tissue interfaces[35], at the periphery of a PTV[48], and at shallow depths near the skin or surface of a lung tumor[102,104] can be large, up to 120%[102,104], with the less accurate dose calculation methods. In contrast, even for small fields in a heterogeneous environment, dose measurements and Monte Carlo calculations can match within 3%[104] at the isocenter. To resolve the dilemma, the HyTEC dose-response model was constructed using isocenter dose, and for clinical conclusions based on the analysis (Section 8) the results were converted to a PTV margin equivalent using a generic 80% isodose line; the interested reader can convert the results using an applicable isodose line for individual situations.
Most of the studies used linear accelerator-based radiosurgical delivery systems including Cyberknife (Accuray, Sunnyvale, CA), and other more traditional linear accelerator based delivery systems (Truebeam, Triology, Novalis, Artiste, etc.). A few studies utilized either helical tomotherapy or proton beam therapy. Several conventional linac studies noted that only a limited number of non-coplanar beams could be used due to issues relating to patient or couch collision.
Immobilization devices included stereotactic body frames, customized cradles, and evacuated vacuum cushions; a few studies used no special immobilization. Respiratory motion management strategies varied and included free-breathing techniques with a 4D CT from simulation defining an ITV with or without tracking of implanted metallic fiducials, abdominal compression with fluoroscopic assessment of diaphragmatic motion as surrogate for tumor motion, and no (or unreported) motion management. There were too many permutations related to patient set-up, immobilization, respiratory motion management strategies, and target definition to conduct a meaningful analysis relating outcomes to any of these variables. Thus, data were pooled from studies independent of how these issues were addressed, which leads to further uncertainty. Similarly, there was not enough specificity in the available reports to consider factors such as histology, absolute tumor volume (although T1 vs. T2 tumors were evaluated as variables for the regrowth model), total treatment duration, minimum PTV dose, dose heterogeneity within the tumor, fraction number, tumor location, and irradiation technique (e.g. margin, immobilization, set-up considerations, delivery method, etc.). Additional factors that were not addressed that may affect outcome include molecular mutation status, invasive versus in-situ disease, and staging work-up requirements (invasive or non-invasive mediastinal nodal staging).
Extracting/comparing outcome information:
From each study, the reported local control rates at 1, 2, 3, and 5 years were extracted (as able). For the studies where results were presented in Kaplan-Meier/actuarial figures, the corresponding data were extracted from the figures.
Due to the heterogeneity of data reporting as well as insufficient reporting of outcomes other than LC (i.e. overall survival), outcome modeling was limited to the effect of tumor size (T1 vs. T2 disease for regrowth model), total dose prescribed to the isocenter, and number of fractions on LC (Table 2).
Table 2:
Required physical doses (Gy) at isocenter and covering PTV with the 80% isodose line to reach the maximum TCP, calculated from the three models with the parameters determined in Section 6.
| Isocenter Dose (Gy) | 3 fractions | 4 fractions | 5 fractions | |
|
| ||||
| Regrowth | T1 | 52±1 | 57±1 | 60±1 |
| T2 | 56±1 | 62±1 | 66±1 | |
| T1+T2 | 54±1 | 59±1 | 63±1 | |
|
|
||||
| LQ | T1+T2 | 55±1 | 59±1 | 63±1 |
|
|
||||
| USC | T1+T2 | 55±1 | 59±1 | 63±1 |
|
| ||||
| PTV Dose (Gy) | 3 fractions | 4 fractions | 5 fractions | |
|
| ||||
| Regrowth | T1 | 42±1 | 46±1 | 48±1 |
| T2 | 45±1 | 50±1 | 53±1 | |
| T1+T2 | 43±1 | 47±1 | 50±1 | |
|
|
||||
| LQ | T1+ T2 | 44±1 | 47±1 | 50±1 |
|
|
||||
| USC | T1+T2 | 44±1 | 47±1 | 50±1 |
Pooled crude data:
Figure 1 presents TCP of 3-, 4-, and 5-fraction SBRT for combined T1- and T2-stage NSCLC as a function of estimated physical isocenter dose.
Fig.1.
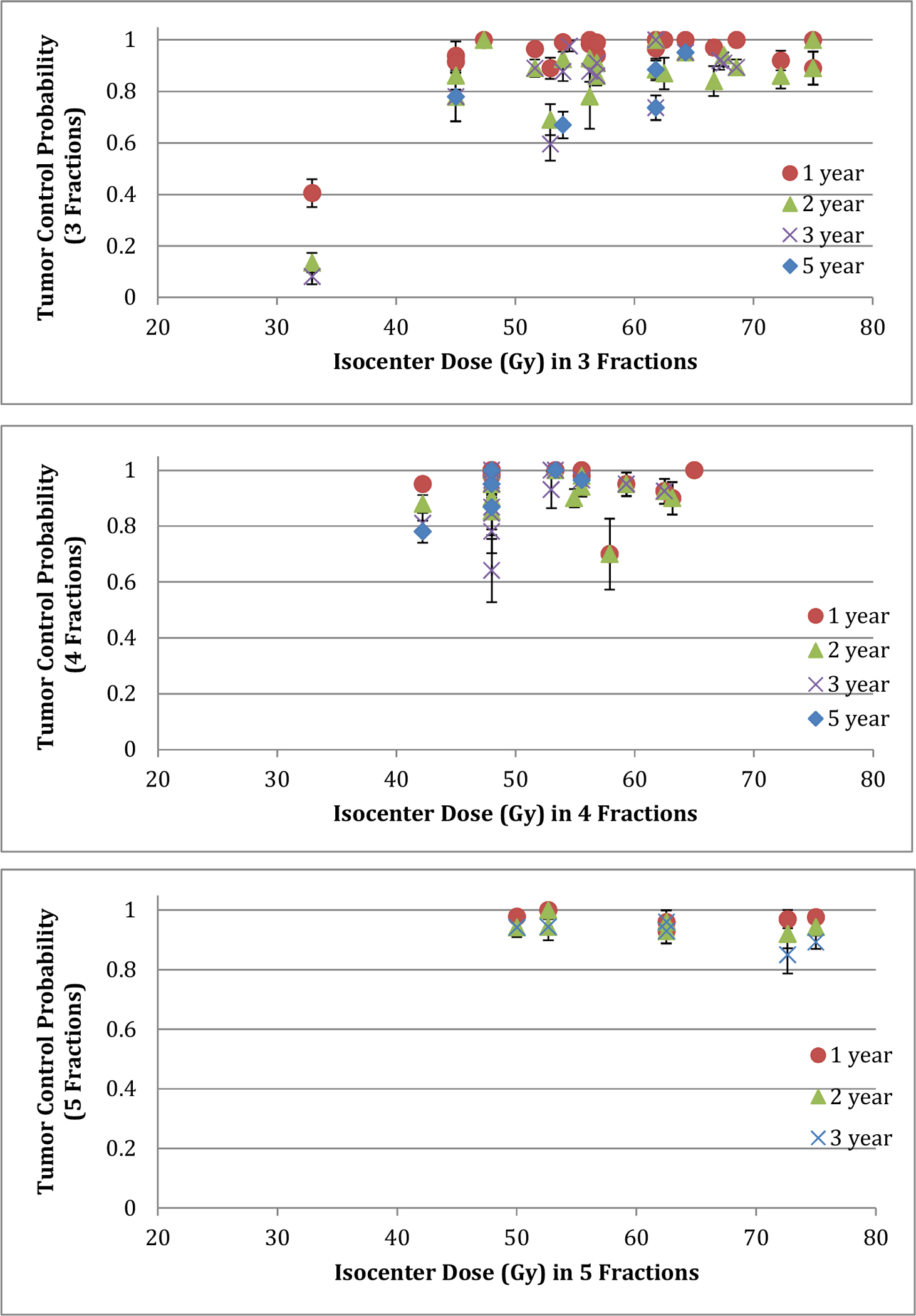
Tumor control probability of 3- (top), 4- (middle) and 5- (bottom) fraction SBRT for T1- and T2-stage NSCLC as a function of physical dose at isocenter. A large error bar for a data point represents a small number of patients associated with that data point.
5. FACTORS AFFECTING OUTCOMES
Using data from the reviewed literature, we studied the dependence of LC on total isocenter dose and number of fractions. We converted dose to biological effective doses using the three models described below. The available large pool of clinical data shows a steep dose-response for local tumor control for SBRT for early stage lung cancer (Figure 1). An additional factor that affects LC is tumor size based on T stage. To achieve a maximum TCP, T2 lesions consistently required a higher physical dose at the isocenter than T1 lesions (approximately 1.3 Gy per fraction higher based on the regrowth model) (Table 2).
6. MATHEMATICAL/BIOLOGICAL MODELS
Three biophysical models were considered and fit to the collected clinical data: the linear-quadratic (LQ) model, the universal survival curve (USC) model[65], and the regrowth model[85]. These were chosen due either to common usage (LQ and USC), or having the best fit to the data (regrowth). For the LQ model, BED is expressed: , where α and β characterize the intrinsic radiosensitivity of cells and D and d are the total and fractional doses, respectively. For the USC model, where −1/D0 and Dq are the slope and x-intercept of the logarithm survival curve, n is the number of fractions, and dT is the transition dose where the LQ model smoothly transitions to the terminal asymptote of the multitarget model. For these two models, TCP can be expressed as: , where K0. is the number of clonogenic cells at the beginning of radiotherapy.
The regrowth model links the population averaged TCP and biologically effective dose (BED) as , where , , and ; α and β are radiobiological parameters[86]; τ is follow-up time starting from the beginning of radiation treatment; T is the elapsed treatment time for the SBRT treatment course; Td is the potential tumor doubling time; δ is a fitting parameter characterizing the speed of tumor cell regrowth after SBRT; D and d are the total and fractional doses, respectively; K0 is the number of clonogenic cells at the beginning of radiation; Kcr is the critical clonogenic cell number that defines control of an individual tumor;σk is the Gaussian width of the distribution of tumor cell numbers. The independent model parameters (α, α/β, Td, Kcr/K0, σk/K0, and δ) were determined from a simultaneous fit to the 1-, 2-, 3-, and 5-year actuarial or Kaplan-Meier TCP data. Clinical data including 1, 2, 3, and 5 year TCP for T1, T2, and combined T1 and T2 stage as a function of the biological effective dose to isocenter were fitted to each of the above models allowing all model parameters to freely float to achieve the best of fit. The studies with fractional doses of greater than 3 Gy were considered [3,4,6,9–14,18,19,21,24,26,27,31–33,38,39,44,47,48,52,55,56,59–61,69–71,73,75,81,84,87,88,90,94,97,98,101,103,106,109]. The TCP data were separated for Stage T1 and T2 tumors if data were available; otherwise analysis was performed for mixed stages. The elapsed treatment time of 7/5 times the number of fractions was used if not reported. The least chi-square (χ2) method was used to fit the data with a single set of parameters for all data and two sets for T1 and T2 separately. The goodness of fit was measured by χ2/ndf, where ndf is the number of degrees of freedom, defined as the total number of data points minus the number of free parameters in the fit. Table 1 presents the goodness fit and the model parameters determined from the data fitting. Notice that the regrowth model leads to a lower χ2/ndf value, compared to the LQ and USC models. The fitting with the regrowth model extracted the model parameters separately for T1 and T2. This is because the regrowth model considers the tumor regrowth after the treatment, allowing fitting of all the TCP data collected at different follow-up times. In contrast, the simpler versions of the LQ and USC models that are more often used clinically do not account for post-treatment regrowth and can only be used to fit the TCP data for a given follow-up time, which could not lead to a convergent fitting for T1 and T2 separately.
Table 1:
The goodness of fit (χ2/ndf) and model parameters determined from simultaneous fits to 1−, 2−, 3−, and 5-year TCP data for stage T1 and T2 lung tumors using the regrowth model and from the fits to the 3-year TCP data for combined stages T1 and T2 tumors using the LQ and USC models. The α/β value in the USC model equals to 34.1, which was calculated by .
| Parameters | χ2/ndf | α (Gy-1) | α/β (Gy) | Various parameters | |
|---|---|---|---|---|---|
|
| |||||
| Regrowth | T1 | 3.8 | 0.129±0.004 | 24.8±1.9 | Td=47.1±16.2 days, |
| δ=0.267±0.041, | |||||
| Kcr/K0=0.010±0.002 | |||||
| σK/K0=0.005±0.001 | |||||
|
| |||||
| T2 | 3.8 | 0.110±0.004 | 19.3±2.3 | Td=95.1±31.0 days | |
| δ=0.278±0.035 | |||||
| Kcr/K0=0.012±0.001 | |||||
| σK/K0=0.007±0.001 | |||||
|
| |||||
| T1+T2 | 3.8 | 0.123±0.007 | 20.7±1.0 | Td=63.8±5.8 days | |
| δ=0.253±0.025 | |||||
| Kcr/K0=0.008±0.003 | |||||
| σK/K0=0.004±0.002 | |||||
|
| |||||
| LQ | T1+T2 | 7.1 | 0.163±0.010 | 32.5±3.5 | K0=(1.07±0.07)×104 |
|
| |||||
| USC | T1+T2 | 7.2 | 0.163±0.005 | D0=1.7±0.1 Gy | |
| Dq=16.1±1.2 Gy | |||||
| K0=(1.06±0.07)×104 | |||||
Figure 2 presents examples of fitting the TCP data with the regrowth, LQ, and USC models. The fittings of combined T1 and T2 data yield large α/β values (> 20 Gy) based on the regrowth and LQ models (Table 1). The results indicate that TCP has a steep dose response, reaching the maximum TCP at BED ≥ 90 and 110 for T1 and T2 tumors, respectively. As indicated by the value of χ2/ndf in Figure 2, the regrowth model yields slightly better fitting than the LQ and USC models (4.9 vs. 7.1 and 7.2) to the TCP data. Details on the methods and results for the TCP modeling have been reported separately[43].
Fig.2.


Fitting tumor control probability data of SBRT for T1- and T2-stage NSCLC with the regrowth, USC, and LQ models: (a) fitting 1-, 2-, 3-, and 5-year TCP data simultaneously using the regrowth model; fitting 3-year TCP data with the (b) regrowth model, (c) - LQ model, and (d) USC model.
7. SPECIAL SITUATIONS
The summarized data and model-based results are derived entirely from patients with T1 and T2 medically inoperable early stage NSCLC treated with 3–5 fractions of SBRT, without prior radiation. The degree to which these data are applicable to operable patients, those with metastatic disease to the lung from other primary sites (e.g. breast, colorectal, sarcoma), those with previous lung radiation therapy, or those treated with hypofractionation schedules less than three or greater than five fractions, is not well known. For example, data from patients with lung metastases from primary colorectal tumors suggests an inferior local control[62]. There may be meaningful clinical endpoints beyond local control that were not addressed (e.g. recurrence rate in the same lobe, hilar and/or mediastinal lymph nodes, distant metastases and overall survival). The heterogeneity of the published data and lack of consistent reporting for these end-points precludes a rigorous assessment of this relationship at present.
Currently, there are few studies reporting local control for large tumors (e.g. > 5 cm). Additional analyses will be necessary to characterize the relationship between local control as a function of tumor size while accounting for the BED.
Data suggest that the therapeutic ratio is likely different for centrally located tumors[12,51] and thus the selection of optimal dose, schedule, technique, and treatment volume might differ in this setting. For example, in a study that included the risks of late toxicity and complications from SBRT in early stage NSCLC by tumor location, there appeared to be an 8-fold increase in grade 3 or higher complications (bacterial pneumonia, radiation pneumonitis, tracheal-bronchial fistula formation, etc.) when the tumor was within or touched a volume within 2 cm of the tracheal-bronchial tree [88] treated in a dose-escalated manner in 3 fractions. This study set the current definition for a centrally located tumor. The finding of unacceptable toxicity for central tumors has been further investigated, including in a cooperative group setting such as RTOG 0813, a phase I/II study designed to determine the maximal tolerated dose and efficacy of SBRT in a dose-escalated 5-fractions regimen from 10 to 12 Gy per fraction[5]. Early reported results showed reasonable overall rates of toxicity even at the highest dose level allowed by the protocol (60 Gy in 5 fractions) which was associated with a 7.2% rate of protocol-specified dose-limiting toxicity; however there were 3 deaths associated with SBRT at the two highest dose-levels. Thus, a patient’s eligibility for SBRT and differences in dose and margin based on whether the tumor is peripherally or centrally located are important and relevant considerations.
8. RECOMMENDED DOSE/VOLUME OBJECTIVES
Based on the data collected and the modeling results generated, Table 2 summarizes the required physical doses at isocenter and at the periphery of the PTV, assuming a prescription isodose line of 80%, to achieve a maximum local control rate with 3, 4 and 5 fraction regimens for T1, T2, and T1+T2 lesions. The derived α/β (Gy) that led to the overall best fit in the entire data set was approximately 21 Gy. Of note, since most of the data analyzed used 3D conformal techniques and not IMRT or VMAT, relationship between the dose distribution within the PTV and the dose at the isocenter or periphery of the PTV may be dependent on the treatment technique utilized and this may influence tumor control probabilities. Another caveat is that because there are fewer data points for isocenter dose < 52 Gy, the recommendation of 42 Gy in 3 fractions should be interpreted with caution and as a minimum dose required. Of note, a recently published guideline from the European Society for Radiotherapy and Oncology Advisory Committee on Radiation Oncology Practice reached a consensus that “risk-adapted” SBRT fractionation was achieved with 3 × 15 Gy for peripherally located lesions. For patients free from severe comorbidities and with favorable long-term OS expectancy, use of the maximum tolerated dose of 3 × 18 Gy should be considered[25].
9. FUTURE STUDIES
Maturation of reported studies with updated outcomes would benefit the robustness of our tumor control models in early stage NSCLC treated with SBRT. Consistent reporting of 5-year outcomes is needed in order to compare to the surgical outcomes, which is often considered the standard of care. For example, the early reports from RTOG 0236 noted 3-year LC rate of 97.6%, and nodal control rate of 87.2%. A subsequent report, with more mature follow-up data noted a 5-year LC rate of 93% and a nodal control rate of 62%[91]. It is reassuring that only 3 additional local recurrences occurred with longer follow-up compared to the 3-year outcomes.
10. REPORTING STANDARDS FOR OUTCOMES
More rigorous and consistent reporting of clinical outcomes together with the doses delivered is needed in order to improve the accuracy of TCP models for early stage NSCLC treated with SBRT (see Table 3 for summary). For example, as mentioned previously, consistent reporting of long-term outcomes of at least 5 years differentiating local control, interlobar control, local regional control (hilar/mediastinal recurrences), as well as incidence of distant metastases and overall survival are needed. In addition, reporting of details regarding the histology of treated tumors, the medical operability status of these patients, and tumor location will help refine TCP models for specific circumstances. Finally, standard dose-reporting metrics are needed. For example, reports should describe how the GTV is defined, how respiratory motion is accounted for at simulation and treatment, and the extent of expansion for the PTV. Doses to the GTV can vary by up to 20–30% depending on whether the dose is prescribed to the isocenter, prescribed to cover a certain percentage of the PTV, or prescribed to a specific isodose line (e.g. 80%). Such dramatic differences in the actual dose delivered may obscure any real dose-response when they are incorrectly represented. With regard to dose calculations, we recommend that modern algorithms be used for future published studies, tissue heterogeneity corrections should be accounted for using modern calculation algorithms.
Table 3:
Recommendations for reporting standards.
| Reporting recommendations | |
|---|---|
| Clinical | Up to 5 years of outcome, including local control, interlobar control, local regional control (hilar/mediastinal recurrences), rates of distant metastases, overall survival, histology, tumor molecular makeup, medical operability status, tumor location, tumor size, and treatment related toxicities |
| Treatment simulation | Type of images, treatment immobilization, motion management strategies (breath-hold, gating, tracking, ITV, etc.) |
| Target volume definitions | Definition of GTV, ITV, CTV (if added), PTV margins |
| Dose calculation | Dose calculation algorithm used, heterogeneity correction, prescription parameters (isocenter, % of PTV, or specific isodose line), minimal GTV, ITV, or PTV coverage, mean doses, maximum doses, and equivalent uniform dose. % of GTV receiving > 110% of prescribed dose, the GTV D95%, Total dose, dose per fraction, total number of fractions, number of elapsed days during treatment |
| Dose delivery | Dose delivery machine, type of image guidance (4D, 3D, 2D, gated), frequency of image guidance, motion management strategies (breath-hold, gating, tracking,compression), motion monitoring |
Perhaps new dose metrics are needed, as current prescription methods were designed for conventionally fractionated 3D conformal radiation therapy designed to deliver more homogenous dose distributions within the PTV. Due to increase heterogeneity of dose delivered with IMRT, the observed dose response (Figure 1) may be related to the GTV minimal dose, mean dose, maximal dose, or possibly to an equivalent uniform dose (with the model parameter ‘a’ to be determined). Other important metrics to report may include the percent of GTV receiving > 110% of the prescribed dose, the GTV D95%, or the percentage of microscopic disease coverage achieved by a particular treatment technique and dose fractionation schedule. In fact, treatment technique and dose fractionation schedule cannot be decoupled from each other but must be considered in combination. For instance, in a planning and modeling study, Arvidson et al., found that dose fractionation schedules and treatment techniques that varied by up to 174 Gy in LQ model 2 Gy fraction equivalents at the edge of the PTV yielding a similar predicted LC did cover more than 80% of possible microscopic disease extensions to a dose of 55 Gy or higher in 2Gy fraction equivalents[2]. They concluded that the high dose per fraction is necessary for some SBRT treatment regimens to obtain adequate microscopic extension coverage, while in other regimens yielding similar local control rates, lower prescribed doses per fraction can be employed since adequate microscopic extension coverage is obtained through added treatment margins. Therefore, a detailed description of target volume definition, treatment margins, and treatment technique is necessary in addition to dose parameters. Further support to reporting the percentage of microscopic disease coverage as a metric for a given dose fractionation schedule and treatment technique is provided by the systematic review of van Baardwijk et al., who found no significant relationship between the dose at the edge of the PTV and freedom of local progression[96]. More recently, Shaverdian et al. reported a clinical series of 120 consecutive stage I NSCLC patients treated with a physical dose regimen of 54 Gy in 3 fractions prescribed to either the PTV (ITV + 3–6 mm) or to the ITV alone[74]. The local control was 100% at 3 years in both clinical scenarios, confirming the hypothesis that doses to adjacent non-target tissue are likely sufficient to sterilize the microscopic disease near the GTV.
These metrics should be consistently reported in all future publications. Moreover, it is essential when different dose schemes are used in a single publication that a breakdown of the patients, tumor characteristics and LC outcomes be reported for each prescription scheme used. Ideally, a prospective web-based dosimetric and outcomes registry that is updated and curated in real-time for the entire country would facilitate future endeavors in analyzing radiation outcomes as a function of treatment parameters.
Supplementary Material
Footnotes
CONFLICTS OF INTEREST:
PL: Viewray Inc.: Research grant, Consultant, Speaking honorarium; Varian Inc.: Consultant, Speaking honorarium; AstraZeneca Inc.: Research grant, Consultant, Advisory board member, Speaking honorarium
BWL: Varian Inc.: Research grant; TibaRay: Board member
TB: None
GXD: None
IME: Endectra LLC: Advisory board; Resero AI LLC: Advisory board; NIH: Research grant
AJ: NIH/NCI grant: P30 CA008748
F-MK: Varian Inc.: Research grant, NIH/NCI grant: R01CA124840
TL: None
MM: None
TS: None
WAT: Varian Inc.: Research grant; WI Alumni Research Foundation: Payment for manuscript preparation, Patents; Viewray Inc.: Scientific advisory board
AT: None
EY: NIH/NCI grant: P30 CA008748
XAL: None
Declaration: None
FINANCIAL DISCLOSURE: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Al-Shahrabani F, et al. Surgical strategies in the therapy of non-small cell lung cancer. World journal of clinical oncology 2014;5:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arvidson NB, Mehta MP Tome WA. Dose coverage beyond the gross tumor volume for various stereotactic body radiotherapy planning techniques reporting similar control rates for stage i non-small-cell lung cancer. International journal of radiation oncology, biology, physics 2008;72:1597–1603. [DOI] [PubMed] [Google Scholar]
- [3].Baumann P, et al. Outcome in a prospective phase ii trial of medically inoperable stage i non-small-cell lung cancer patients treated with stereotactic body radiotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009;27:3290–3296. [DOI] [PubMed] [Google Scholar]
- [4].Baumann P, et al. Factors important for efficacy of stereotactic body radiotherapy of medically inoperable stage i lung cancer. A retrospective analysis of patients treated in the nordic countries. Acta oncologica 2006;45:787–795. [DOI] [PubMed] [Google Scholar]
- [5].Bezjak A, et al. Efficacy and toxicity analysis of nrg oncology/rtog 0813 trial of stereotactic body radiation therapy (sbrt) for centrally located non-small cell lung cancer (nsclc). International journal of radiation oncology, biology, physics 2016;96:S8. [Google Scholar]
- [6].Bibault JE, et al. Image-guided robotic stereotactic radiation therapy with fiducial-free tumor tracking for lung cancer. Radiation oncology 2012;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bogart JA. Fractionated radiotherapy for high-risk patients with early-stage non-small cell lung cancer. Seminars in thoracic and cardiovascular surgery 2010;22:44–52. [DOI] [PubMed] [Google Scholar]
- [8].Bouilhol G, et al. Is abdominal compression useful in lung stereotactic body radiation therapy? A 4dct and dosimetric lobe-dependent study. Physica medica : PM : an international journal devoted to the applications of physics to medicine and biology : official journal of the Italian Association of Biomedical Physics 2013;29:333–340. [DOI] [PubMed] [Google Scholar]
- [9].Bradley JD, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung cancer: The pattern of failure is distant. International journal of radiation oncology, biology, physics 2010;77:1146–1150. [DOI] [PubMed] [Google Scholar]
- [10].Bral S, et al. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: Results of a phase ii trial. International journal of radiation oncology, biology, physics 2011;80:1343–1349. [DOI] [PubMed] [Google Scholar]
- [11].Bush DA, et al. High-dose hypofractionated proton beam radiation therapy is safe and effective for central and peripheral early-stage non-small cell lung cancer: Results of a 12-year experience at loma linda university medical center. International journal of radiation oncology, biology, physics 2013;86:964–968. [DOI] [PubMed] [Google Scholar]
- [12].Chang JY, et al. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: How to fly in a “no fly zone”. International journal of radiation oncology, biology, physics 2014;88:1120–1128. [DOI] [PubMed] [Google Scholar]
- [13].Cheung PC, et al. Accelerated hypofractionation for early-stage non-small-cell lung cancer. International journal of radiation oncology, biology, physics 2002;54:1014–1023 [DOI] [PubMed] [Google Scholar]
- [14].Clarke K, et al. Stereotactic body radiotherapy (sbrt) for non-small cell lung cancer (nsclc): Is fdg-pet a predictor of outcome? Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2012;104:62–66. [DOI] [PubMed] [Google Scholar]
- [15].Cykert S, et al. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. Jama 2010;303:2368–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dominioni L, et al. Stage i nonsmall cell lung carcinoma: Analysis of survival and implications for screening. Cancer 2000;89:2334–2344. [DOI] [PubMed] [Google Scholar]
- [17].Donington J, et al. American college of chest physicians and society of thoracic surgeons consensus statement for evaluation and management for high-risk patients with stage i non-small cell lung cancer. Chest 2012;142:1620–1635. [DOI] [PubMed] [Google Scholar]
- [18].Duncker-Rohr V, et al. Stereotactic ablative radiotherapy for small lung tumors with a moderate dose. Favorable results and low toxicity. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft … [et al] 2013;189:33–40. [DOI] [PubMed] [Google Scholar]
- [19].Dunlap NE, et al. Size matters: A comparison of t1 and t2 peripheral non-small-cell lung cancers treated with stereotactic body radiation therapy (sbrt). The Journal of thoracic and cardiovascular surgery 2010;140:583–589. [DOI] [PubMed] [Google Scholar]
- [20].Ezhil M, et al. Determination of patient-specific internal gross tumor volumes for lung cancer using four-dimensional computed tomography. Radiation oncology 2009;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fritz P, et al. Stereotactic, high single-dose irradiation of stage i non-small cell lung cancer (nsclc) using four-dimensional ct scans for treatment planning. Lung cancer 2008;60:193–199. [DOI] [PubMed] [Google Scholar]
- [22].Ginsberg RJ Rubinstein LV. Randomized trial of lobectomy versus limited resection for t1 n0 non-small cell lung cancer. Lung cancer study group. The Annals of thoracic surgery 1995;60:615–622; discussion 622–613. [DOI] [PubMed] [Google Scholar]
- [23].Glide-Hurst CK Chetty IJ. Improving radiotherapy planning, delivery accuracy, and normal tissue sparing using cutting edge technologies. Journal of thoracic disease 2014;6:303–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Grills IS, et al. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone-beam computed tomography image-guided radiotherapy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2012;7:1382–1393. [DOI] [PubMed] [Google Scholar]
- [25].Guckenberger M, et al. Estro acrop consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2017;124:11–17. [DOI] [PubMed] [Google Scholar]
- [26].Haasbeek CJ, et al. Stage i nonsmall cell lung cancer in patients aged > or =75 years: Outcomes after stereotactic radiotherapy. Cancer 2010;116:406–414. [DOI] [PubMed] [Google Scholar]
- [27].Hamamoto Y, et al. Local control of metastatic lung tumors treated with sbrt of 48 gy in four fractions: In comparison with primary lung cancer. Japanese journal of clinical oncology 2010;40:125–129. [DOI] [PubMed] [Google Scholar]
- [28].Hayman JA, et al. Dose escalation in non-small-cell lung cancer using three-dimensional conformal radiation therapy: Update of a phase i trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2001;19:127–136. [DOI] [PubMed] [Google Scholar]
- [29].Heinzerling JH, et al. Four-dimensional computed tomography scan analysis of tumor and organ motion at varying levels of abdominal compression during stereotactic treatment of lung and liver. International journal of radiation oncology, biology, physics 2008;70:1571–1578. [DOI] [PubMed] [Google Scholar]
- [30].Henderson M, et al. Baseline pulmonary function as a predictor for survival and decline in pulmonary function over time in patients undergoing stereotactic body radiotherapy for the treatment of stage i non-small-cell lung cancer. International journal of radiation oncology, biology, physics 2008;72:404–409. [DOI] [PubMed] [Google Scholar]
- [31].Hof H, et al. Stereotactic single-dose radiotherapy (radiosurgery) of early stage nonsmall-cell lung cancer (nsclc). Cancer 2007;110:148–155. [DOI] [PubMed] [Google Scholar]
- [32].Hoyer M. RH, Hansen AT, Ohlhuis L, Petersen J. Prospective study of stereotactic radiotherapy of limited-stage non-small-cell lung cancer. International journal of radiation oncology, biology, physics 2006;66:S128–S135 [Google Scholar]
- [33].Inoue T, et al. Stereotactic body radiotherapy using gated radiotherapy with real-time tumor-tracking for stage i non-small cell lung cancer. Radiation oncology 2013;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jang SS, et al. Reconstitution of internal target volumes by combining four-dimensional computed tomography and a modified slow computed tomography scan in stereotactic body radiotherapy planning for lung cancer. Radiation oncology 2014;9:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kavanagh BD, et al. The dosimetric effect of inhomogeneity correction in dynamic conformal arc stereotactic body radiation therapy for lung tumors. Journal of applied clinical medical physics 2006;7:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kimura T, et al. Ct appearance of radiation injury of the lung and clinical symptoms after stereotactic body radiation therapy (sbrt) for lung cancers: Are patients with pulmonary emphysema also candidates for sbrt for lung cancers? International journal of radiation oncology, biology, physics 2006;66:483–491. [DOI] [PubMed] [Google Scholar]
- [37].Kishan AU, et al. Correlation of clinical and dosimetric parameters with radiographic lung injury following stereotactic body radiotherapy. Technology in cancer research & treatment 2014. [DOI] [PubMed] [Google Scholar]
- [38].Kopek N, et al. Co-morbidity index predicts for mortality after stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2009;93:402–407. [DOI] [PubMed] [Google Scholar]
- [39].Koto M, et al. A phase ii study on stereotactic body radiotherapy for stage i non-small cell lung cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2007;85:429–434. [DOI] [PubMed] [Google Scholar]
- [40].Lee P, et al. Current concepts in f18 fdg pet/ct-based radiation therapy planning for lung cancer. Frontiers in oncology 2012;2:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li FX, et al. Comparison of the planning target volume based on three-dimensional ct and four-dimensional ct images of non-small-cell lung cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2011;99:176–180. [DOI] [PubMed] [Google Scholar]
- [42].Linda A, Trovo M Bradley JD. Radiation injury of the lung after stereotactic body radiation therapy (sbrt) for lung cancer: A timeline and pattern of ct changes. European journal of radiology 2011;79:147–154. [DOI] [PubMed] [Google Scholar]
- [43].Liu F, et al. Tumor control probability modeling for stereotactic body radiation therapy of early-stage lung cancer using multiple bio-physical models. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2017;122:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lucas JT Jr., et al. Comparison of accelerated hypofractionation and stereotactic body radiotherapy for stage 1 and node negative stage 2 non-small cell lung cancer (nsclc). Lung cancer 2014;85:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Macpherson RE, et al. Non-small-cell lung cancer dimensions: Ct-pathological correlation and interobserver variation. The British journal of radiology 2009;82:421–425. [DOI] [PubMed] [Google Scholar]
- [46].Matsuo Y, et al. Comparison of long-term survival outcomes between stereotactic body radiotherapy and sublobar resection for stage i non-small-cell lung cancer in patients at high risk for lobectomy: A propensity score matching analysis. European journal of cancer 2014;50:2932–2938. [DOI] [PubMed] [Google Scholar]
- [47].Matsuo Y, et al. Prognostic factors in stereotactic body radiotherapy for non-small-cell lung cancer. International journal of radiation oncology, biology, physics 2011;79:1104–1111. [DOI] [PubMed] [Google Scholar]
- [48].McCammon R, et al. Observation of a dose-control relationship for lung and liver tumors after stereotactic body radiation therapy. International journal of radiation oncology, biology, physics 2009;73:112–118. [DOI] [PubMed] [Google Scholar]
- [49].Mehta HJ, et al. Evaluation and treatment of high-risk patients with early-stage lung cancer. Clinics in chest medicine 2011;32:783–797. [DOI] [PubMed] [Google Scholar]
- [50].Mehta N, et al. Stereotactic body radiation therapy and 3-dimensional conformal radiotherapy for stage i non-small cell lung cancer: A pooled analysis of biological equivalent dose and local control. Practical radiation oncology 2012;2:288–295. [DOI] [PubMed] [Google Scholar]
- [51].Modh A, et al. Local control and toxicity in a large cohort of central lung tumors treated with stereotactic body radiation therapy. International journal of radiation oncology, biology, physics 2014;90:1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mohammed N, et al. Radiographic and metabolic response rates following image-guided stereotactic radiotherapy for lung tumors. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2011;99:18–22. [DOI] [PubMed] [Google Scholar]
- [53].Muirhead R, et al. Use of maximum intensity projections (mips) for target outlining in 4dct radiotherapy planning. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2008;3:1433–1438. [DOI] [PubMed] [Google Scholar]
- [54].Murray B, Forster K Timmerman R. Frame-based immobilization and targeting for stereotactic body radiation therapy. Medical dosimetry : official journal of the American Association of Medical Dosimetrists 2007;32:86–91. [DOI] [PubMed] [Google Scholar]
- [55].Nagata Y, et al. Clinical outcomes of a phase i/ii study of 48 gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. International journal of radiation oncology, biology, physics 2005;63:1427–1431. [DOI] [PubMed] [Google Scholar]
- [56].Nath SK, et al. Locoregional and distant failure following image-guided stereotactic body radiation for early-stage primary lung cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2011;99:12–17. [DOI] [PubMed] [Google Scholar]
- [57].National Lung Screening Trial Research T, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England journal of medicine 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nuyttens JJ van de Pol M. The cyberknife radiosurgery system for lung cancer. Expert review of medical devices 2012;9:465–475. [DOI] [PubMed] [Google Scholar]
- [59].Olsen JR, et al. Dose-response for stereotactic body radiotherapy in early-stage non-small-cell lung cancer. International journal of radiation oncology, biology, physics 2011;81:e299–303. [DOI] [PubMed] [Google Scholar]
- [60].Onimaru R, et al. Steep dose-response relationship for stage i non-small-cell lung cancer using hypofractionated high-dose irradiation by real-time tumor-tracking radiotherapy. International journal of radiation oncology, biology, physics 2008;70:374–381. [DOI] [PubMed] [Google Scholar]
- [61].Onimaru R, et al. Tolerance of organs at risk in small-volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. International journal of radiation oncology, biology, physics 2003;56:126–135. [DOI] [PubMed] [Google Scholar]
- [62].Onishi H MY, Miyakawa A, Yamashita H, Nomiya T, Niibe Y, Nakata K, Kuriyama K, Komiyama T, Marino K, Aoki S, Maehata Y, Araya M, Saito R, Tomoinaga L, Oguri M, Watanabe I, Nonaka H, Sano N. Japanese multi-institutional study of stereotactic body radiation therapy for 380 patients with lung metastases. International journal of radiation oncology, biology, physics 2014;90:S28. [Google Scholar]
- [63].Padda SK, et al. Early-stage non-small cell lung cancer: Surgery, stereotactic radiosurgery, and individualized adjuvant therapy. Seminars in oncology 2014;41:40–56. [DOI] [PubMed] [Google Scholar]
- [64].Papiez L, et al. Extracranial stereotactic radioablation: Physical principles. Acta oncologica 2003;42:882–894. [DOI] [PubMed] [Google Scholar]
- [65].Park C, et al. Universal survival curve and single fraction equivalent dose: Useful tools in understanding potency of ablative radiotherapy. International journal of radiation oncology, biology, physics 2008;70:847–852. [DOI] [PubMed] [Google Scholar]
- [66].Puri V, et al. Treatment outcomes in stage i lung cancer: A comparison of surgery and stereotactic body radiation therapy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2015;10:1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Qiao X, et al. The role of radiotherapy in treatment of stage i non-small cell lung cancer. Lung cancer 2003;41:1–11. [DOI] [PubMed] [Google Scholar]
- [68].Rajagopalan MS Heron DE. Role of pet/ct imaging in stereotactic body radiotherapy. Future oncology 2010;6:305–317. [DOI] [PubMed] [Google Scholar]
- [69].Ricardi U, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: Results of a prospective trial. Lung cancer 2010;68:72–77. [DOI] [PubMed] [Google Scholar]
- [70].Robinson CG, et al. Patterns of failure after stereotactic body radiation therapy or lobar resection for clinical stage i non-small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2013;8:192–201. [DOI] [PubMed] [Google Scholar]
- [71].Rowe BP, et al. Stereotactic body radiotherapy for central lung tumors. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2012;7:1394–1399. [DOI] [PubMed] [Google Scholar]
- [72].Rwigema JC, et al. Incidental mediastinal dose does not explain low mediastinal node recurrence rates in patients with early-stage nsclc treated with stereotactic body radiotherapy. Clinical lung cancer 2014;15:287–293. [DOI] [PubMed] [Google Scholar]
- [73].Salazar OM, et al. Once-weekly, high-dose stereotactic body radiotherapy for lung cancer: 6-year analysis of 60 early-stage, 42 locally advanced, and 7 metastatic lung cancers. International journal of radiation oncology, biology, physics 2008;72:707–715. [DOI] [PubMed] [Google Scholar]
- [74].Shaverdian N, et al. The significance of ptv dose coverage on cancer control outcomes in early stage non-small cell lung cancer patients treated with highly ablative stereotactic body radiation therapy. The British journal of radiology 2016;89:20150963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Shirata Y, et al. Prognostic factors for local control of stage i non-small cell lung cancer in stereotactic radiotherapy: A retrospective analysis. Radiation oncology 2012;7:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Shirvani SM, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. International journal of radiation oncology, biology, physics 2012;84:1060–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Siegel R, Naishadham D Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- [78].Silvestri GA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S–250S. [DOI] [PubMed] [Google Scholar]
- [79].Singhvi M Lee P. Illustrative cases of false positive biopsies after stereotactic body radiation therapy for lung cancer based on abnormal fdg-pet-ct imaging. BMJ case reports 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Snider JW, et al. Cyberknife with tumor tracking: An effective treatment for high-risk surgical patients with single peripheral lung metastases. Frontiers in oncology 2012;2:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Soliman H, et al. Accelerated hypofractionated radiotherapy for early-stage non-small-cell lung cancer: Long-term results. International journal of radiation oncology, biology, physics 2011;79:459–465. [DOI] [PubMed] [Google Scholar]
- [82].Stanzani F, et al. Morbidity, mortality, and categorization of the risk of perioperative complications in lung cancer patients. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia 2014;40:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Steenbakkers RJ, et al. Observer variation in target volume delineation of lung cancer related to radiation oncologist-computer interaction: A ‘big brother’ evaluation. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2005;77:182–190. [DOI] [PubMed] [Google Scholar]
- [84].Stephans KL, et al. A comparison of two stereotactic body radiation fractionation schedules for medically inoperable stage i non-small cell lung cancer: The cleveland clinic experience. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2009;4:976–982. [DOI] [PubMed] [Google Scholar]
- [85].Tai A, et al. Estimate of radiobiologic parameters from clinical data for biologically based treatment planning for liver irradiation. International journal of radiation oncology, biology, physics 2008;70:900–907. [DOI] [PubMed] [Google Scholar]
- [86].Tai A, et al. An analysis of tumor control probability of stereotactic body radiation therapy for lung cancer with a regrowth model. Physics in medicine and biology 2016;61:3903–3913. [DOI] [PubMed] [Google Scholar]
- [87].Takeda A, et al. Stereotactic body radiotherapy for primary lung cancer at a dose of 50 gy total in five fractions to the periphery of the planning target volume calculated using a superposition algorithm. International journal of radiation oncology, biology, physics 2009;73:442–448. [DOI] [PubMed] [Google Scholar]
- [88].Timmerman R, et al. Excessive toxicity when treating central tumors in a phase ii study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2006;24:4833–4839. [DOI] [PubMed] [Google Scholar]
- [89].Timmerman R, et al. Extracranial stereotactic radioablation: Results of a phase i study in medically inoperable stage i non-small cell lung cancer. Chest 2003;124:1946–1955. [DOI] [PubMed] [Google Scholar]
- [90].Timmerman R, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. Jama 2010;303:1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Timmerman RD HC, Michalski J, Straube W, Galvin J, Johnstone D, Bradley J, Barriger R, Bezjak A, Videtic GM, Nedzi L, Werner-Wasik M, Chen Y, Komaki RU, Choy H. Long-term results of rtog 0236: A phase ii trial of stereotactic body radiation therapy (sbrt) in the treatment of patients with medically inoperable stage i non-small cell lung cancer. International journal of radiation oncology, biology, physics 2014;90:S30. [Google Scholar]
- [92].Timmerman RD, Herman J Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014;32:2847–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Timmerman RD, Park C Kavanagh BD. The north american experience with stereotactic body radiation therapy in non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2007;2:S101–112. [DOI] [PubMed] [Google Scholar]
- [94].Trakul N, et al. Tumor volume-adapted dosing in stereotactic ablative radiotherapy of lung tumors. International journal of radiation oncology, biology, physics 2012;84:231–237. [DOI] [PubMed] [Google Scholar]
- [95].Underberg RW, et al. Benefit of respiration-gated stereotactic radiotherapy for stage i lung cancer: An analysis of 4dct datasets. International journal of radiation oncology, biology, physics 2005;62:554–560. [DOI] [PubMed] [Google Scholar]
- [96].van Baardwijk A, et al. Is high-dose stereotactic body radiotherapy (sbrt) for stage i non-small cell lung cancer (nsclc) overkill? A systematic review. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2012;105:145–149. [DOI] [PubMed] [Google Scholar]
- [97].van der Voort van Zyp NC, et al. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: Clinical outcome. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2009;91:296–300. [DOI] [PubMed] [Google Scholar]
- [98].Videtic GM, et al. Intensity-modulated radiotherapy-based stereotactic body radiotherapy for medically inoperable early-stage lung cancer: Excellent local control. International journal of radiation oncology, biology, physics 2010;77:344–349. [DOI] [PubMed] [Google Scholar]
- [99].Videtic GM, et al. 30 gy or 34 gy? Comparing 2 single-fraction sbrt dose schedules for stage i medically inoperable non-small cell lung cancer. International journal of radiation oncology, biology, physics 2014;90:203–208. [DOI] [PubMed] [Google Scholar]
- [100].Videtic GMM, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive summary of an astro evidence-based guideline. Practical radiation oncology 2017;7:295–301. [DOI] [PubMed] [Google Scholar]
- [101].Westover KD, et al. Proton sbrt for medically inoperable stage i nsclc. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2012;7:1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wilcox EE Daskalov GM. Accuracy of dose measurements and calculations within and beyond heterogeneous tissues for 6 mv photon fields smaller than 4 cm produced by cyberknife. Medical physics 2008;35:2259–2266. [DOI] [PubMed] [Google Scholar]
- [103].Xia T, et al. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable stage i/ii non-small-cell lung cancer. International journal of radiation oncology, biology, physics 2006;66:117–125. [DOI] [PubMed] [Google Scholar]
- [104].Xue J, et al. Small field dose measurements using plastic scintillation detector in heterogeneous media. Medical physics 2017;44:3815–3820. [DOI] [PubMed] [Google Scholar]
- [105].Yeo SG Kim ES. Efficient approach for determining four-dimensional computed tomography-based internal target volume in stereotactic radiotherapy of lung cancer. Radiation oncology journal 2013;31:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Yung T, et al. Outcomes of accelerated hypofractionated radiotherapy in stage i non-small-cell lung cancer. Current oncology 2012;19:e264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zhang B, et al. Matched-pair comparisons of stereotactic body radiotherapy (sbrt) versus surgery for the treatment of early stage non-small cell lung cancer: A systematic review and meta-analysis. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2014;112:250–255. [DOI] [PubMed] [Google Scholar]
- [108].Zhang X, et al. Positron emission tomography for assessing local failure after stereotactic body radiotherapy for non-small-cell lung cancer. International journal of radiation oncology, biology, physics 2012;83:1558–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zimmermann FB, et al. Stereotactic hypofractionated radiation therapy for stage i non-small cell lung cancer. Lung cancer 2005;48:107–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


