Abstract
Background
Yoga is an ancient spiritual practice that originated in India and is currently accepted in the Western world as a form of relaxation and exercise. It has been of interest for people with schizophrenia to determine its efficacy as an adjunct to standard‐care treatment.
Objectives
To examine the effects of yoga versus standard care for people with schizophrenia.
Search methods
We searched the Cochrane Schizophrenia Group Trials Register (November 2012 and January 29, 2015), which is based on regular searches of MEDLINE, PubMed, EMBASE, CINAHL, BIOSIS, AMED, PsycINFO, and registries of clinical trials. We searched the references of all included studies. There were no language, date, document type, or publication status limitations for inclusion of records in the register.
Selection criteria
All randomised controlled trials (RCTs) including people with schizophrenia comparing yoga to standard‐care control.
Data collection and analysis
The review team independently selected studies, quality rated these, and extracted data. For binary outcomes, we calculated risk ratio (RR) and its 95% confidence interval (CI), on an intention‐to‐treat basis. For continuous data, we estimated the mean difference (MD) between groups and its 95% CI. We employed mixed‐effect and fixed‐effect models for analyses. We examined data for heterogeneity (I2 technique), assessed risk of bias for included studies, and created 'Summary of findings' tables using GRADE (Grading of Recommendations Assessment, Development and Evaluation).
Main results
We included eight studies in the review. All outcomes were short term (less than six months). There were clear differences in a number of outcomes in favour of the yoga group, although these were based on one study each, with the exception of leaving the study early. These included mental state (improvement in Positive and Negative Syndrome Scale, 1 RCT, n = 83, RR 0.70 CI 0.55 to 0.88, medium‐quality evidence), social functioning (improvement in Social Occupational Functioning Scale, 1 RCT, n = 83, RR 0.88 CI 0.77 to 1, medium‐quality evidence), quality of life (average change 36‐Item Short Form Survey (SF‐36) quality‐of‐life subscale, 1 RCT, n = 60, MD 15.50, 95% CI 4.27 to 26.73, low‐quality evidence), and leaving the study early (8 RCTs, n = 457, RR 0.91 CI 0.6 to 1.37, medium‐quality evidence). For the outcome of physical health, there was not a clear difference between groups (average change SF‐36 physical‐health subscale, 1 RCT, n = 60, MD 6.60, 95% CI ‐2.44 to 15.64, low‐quality evidence). Only one study reported adverse effects, finding no incidence of adverse events in either treatment group. This review was subject to a considerable number of missing outcomes, which included global state, change in cognition, costs of care, effect on standard care, service intervention, disability, and activities of daily living.
Authors' conclusions
Even though we found some positive evidence in favour of yoga over standard‐care control, this should be interpreted cautiously in view of outcomes largely based each on one study with limited sample sizes and short‐term follow‐up. Overall, many outcomes were not reported and evidence presented in this review is of low to moderate quality ‐ ‐too weak to indicate that yoga is superior to standard‐care control for the management of schizophrenia.
Plain language summary
Yoga versus standard care for schizophrenia
Review question
Is yoga effective as an add‐on treatment for people with schizophrenia?
Background
Yoga comes from ancient India and involves physical postures and breathing exercises to promote balance between mind and body. Yoga has now been widely adopted as a method of relaxation and exercise, improving strength, flexibility, co‐ordination, endurance, and breathing control and concentration. Yoga has also been shown to reduce stress and promote health and feelings of well‐being. Yoga has been used as a complementary therapy for many health conditions, including improving blood pressure control as well as mental health conditions such as depression and anxiety disorders.
Some research suggests that yoga could also be of benefit as an add‐on treatment to reduce the complex symptoms of schizophrenia (such as hearing voices, seeing things, lack of interest in people and activities, tiredness, loss of emotions and withdrawal) and improve the quality of life of people with schizophrenia. Yoga and its use specifically for people with schizophrenia is under‐researched.
Study characteristics
We included eight short‐term studies (less than six months) that randomised people with schizophrenia to either receive sessions of yoga or standard care in this review. The yoga programmes described varied from 45 minutes to 1 hour in length, and from 8 sessions to a maximum of 36 sessions. We found these studies by electronic searching of the Cochrane Schizophrenia Group's register in January 2015. All studies continued prescribed antipsychotic treatment for the participants.
Key results
Some results suggest that yoga may be beneficial for people with schizophrenia. Yoga may be beneficial to mental state, social functioning and quality of life but the available evidence is weak and needs to be treated with a good degree of caution. No adverse effects were found by the one trial that reported this outcome. Several other important outcomes were not addressed by the studies, including changes in cognition, economic considerations, and daily living activities. There was not enough good‐quality evidence in this review to claim that yoga should be prescribed as an add‐on to standard care for schizophrenia.
Quality of the evidence
Evidence was limited and weak. The number of included studies was small, and only short‐term follow‐up was reported. More, larger, and long‐term trials that focus on important outcomes are therefore necessary.
Ben Gray, Senior Peer Researcher, McPin Foundation.http://mcpin.org/
Summary of findings
Summary of findings for the main comparison. YOGA versus STANDARD CARE for schizophrenia.
| YOGA versus STANDARD CARE for schizophrenia | ||||||
| Patient or population: people with schizophrenia Settings: Intervention: YOGA versus STANDARD‐CARE CONTROL | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | YOGA versus STANDARD‐CARE CONTROL | |||||
| Mental state: Overall ‐ not improved PANSS Follow‐up: 4 months | Low | RR 0.70 (0.55 to 0.88) | 83 (1 study) | ⊕⊕⊕⊝ medium2 | ||
| 800 per 1000 | 560 per 1000 (440 to 704) | |||||
| Moderate | ||||||
| 900 per 1000 | 630 per 1000 (495 to 792) | |||||
| High | ||||||
| 1000 per 1000 | 700 per 1000 (550 to 880) | |||||
| Social functioning: Overall ‐ not improved SOFS Follow‐up: 4 months | Low1 | RR 0.88 (0.77 to 1) | 83 (1 study) | ⊕⊕⊕⊝ medium2 | ||
| 800 per 1000 | 704 per 1000 (616 to 800) | |||||
| Moderate1 | ||||||
| 900 per 1000 | 792 per 1000 (693 to 900) | |||||
| High1 | ||||||
| 1000 per 1000 | 880 per 1000 (770 to 1000) | |||||
| Quality of life: Average change ‐ mental health SF‐36 Follow‐up: 12 weeks | The mean quality of life: average change ‐ mental health in the intervention groups was 15.5 higher (4.27 to 26.73 higher) | 60 (1 study) | ⊕⊕⊝⊝ low3,4 | Protocol pre‐stated "Improvement or deterioration in quality of life" ‐ no trial reported binary data; we chose 1 of 2 QOL measures to report here. | ||
| Physical health: Average change SF‐36 | The mean physical health: average change in the intervention groups was 6.6 higher (2.44 lower to 15.64 higher) | 60 (1 study) | ⊕⊕⊝⊝ low5,6 | Protocol pre‐stated "Improvement or deterioration in physical health" ‐ no trial reported binary data; we chose physical‐health dimension of QOL measure. | ||
| Adverse events: any | See comment | See comment | See comment | 94 (1 study) | ⊕⊕⊕⊝ medium7 | Risks were calculated from pooled risk differences. The study reported no adverse effects. |
| Costs of care | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported this outcome. |
|
Leaving the study early Leaving the study early: participants lost to follow‐up ‐ short term (low = good) |
Low1 | RR 0.91 (0.6 to 1.37) | 457 (8 studies) |
⊕⊕⊕⊝ medium8 | ||
| 800 per 1000 |
728 per 1000 (480 to 1000) |
|||||
| Medium1 | ||||||
| 900 per 1000 | 819 per 1000 (540 to 1000) | |||||
| High1 | ||||||
| 1000 per 1000 | 910 per 1000 (600 to 1000) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Moderate risk ‐ that of control group in trial.
2 Imprecision ‐ rated 'serious' due to small sample size. 3 Indirectness: rated 'serious' ‐ no trial reported binary data, and review authors had to use one of two submeasures. 4 Imprecision: rated 'serious' ‐ unclear of clinical meaning of scores from 4 to 26. 5 Indirectness: rated 'very serious' ‐ no trial reported binary data, and review authors had to use one of two submeasures from quality‐of‐life scale. 6 Imprecision: rated 'serious' ‐ unclear of clinical meaning of scores from 2 to 16.
7 Imprecision: rated 'serious'‐ based on one study with no reported adverse events.
8 Risk of bias: rated 'serious' ‐ a number of participants withdrew from one trial, and it was not clear to which group they were randomised.
Background
Description of the condition
Schizophrenia is a relatively common mental disorder with a lifetime prevalence of 0.3% to 0.6% and an incidence of 10.2 to 22.0 per 100,000 (McGrath 2008). Schizophrenia has many different clinical presentations, with patients suffering both positive and negative symptoms. Positive symptoms reflect an excess or distortion of normal functions such as delusions, hallucinations, and disorganised speech and behaviour. Negative symptoms reflect a reduction or loss of normal functions such as affective flattening, apathy, avolition, and social withdrawal. The pathophysiology of the manifestation of negative symptoms is not yet fully understood, whilst positive symptoms are thought to be due to mesolimbic dopaminergic overactivity (Stahl 2007).
Schizophrenia has been identified as a serious public health concern, ranking 11th in the causes of years lived with disability worldwide (Global Burden of Disease Study 2013 Collaborators). The various symptoms of schizophrenia can have a significant impact on a person's ability to function within society.
The current emphasis of treatment for the management of schizophrenia is antipsychotic medication. Although antipsychotic medication is effective in reducing positive symptoms, usually within the early stages of treatment (Leucht 2013), it is of less benefit for negative symptoms and cognitive deficits (Fusar‐Poli 2014; Nielsen 2015). Unfortunately, it is the negative symptoms that cause the most disability (Vancampfort 2011b; Vancampfort 2012b). Therapies such as Cognitive‐behavioural therapy are often used as adjunct treatments to antipsychotic medicuation but has limited beneficial effects on negative and cognitive symptoms (Jauhar 2014), these interventions also have societal costs (van der Gaag 2011). Other low‐cost treatments that decrease negative symptoms, reduce cognitive deficits, and promote mental and physical quality of life and functional recovery are thus warranted.
Antipsychotic medications are classed into older typicals and more recent atypical drugs. Both classes are not without potential considerable side effects, including extrapyramidal side effects for typicals and weight gain or sedation for atypicals. This side effect profile and patient preference to avoid these wherever possible has resulted in additional non‐pharmacological interventions being utilised as either an adjunct or alternative to medication therapy (Kern 2009).
Description of the intervention
Yoga originates from India as an ancient Hindu practice incorporating physical postures with breathing exercises with the goal of achieving a balance between the mental and physical state (Bussing 2012; Ross 2012; Sherman 2012). The principles behind its practice were first described by Patanjali, and were believed to prepare the mind and the body for spiritual development (Ross 2012). Yoga has been widely adopted in the Western world as both a method of relaxation and exercise. The most widely used yoga practice in the Western world is hatha yoga (Collins 1998). Its use of yoga postures, or asanas, improves strength, flexibility, co‐ordination, and endurance, and its use of breathing exercises, or pranayama, improves respiratory control and concentration. Mantra yoga is another well‐known and widely practiced form of Hindu yoga that focuses on the use of chants to achieve mental and spiritual transformation (Sherman 2012).
As the popularity of yoga has increased, research into its effect on both physical and mental health has identified key benefits of yoga. It has been shown to both reduce stress and improve cognitive function in healthy individuals (Bangalore 2012), and to be useful as a complementary therapy for many health conditions, such as blood pressure control and mental health conditions including depression and anxiety disorders (Bussing 2012).
The benefits of yoga in other mental health conditions led to research into its role as a complementary therapy for the management of schizophrenia. A systematic review of randomised control trials (RCTs) indicated that yoga could be beneficial as an add‐on treatment to reduce both positive and negative symptoms of schizophrenia and improve the health‐related quality of life of people with schizophrenia (Vancampfort 2012a).
How the intervention might work
Research has found that yoga has a role in regulating the autonomic nervous system (Varambally 2012b), decreasing sympathetic tone, creating a reaction the opposite to fight or flight reaction. There is a subsequent effect on the limbic system and hypothalamic pituitary axis resulting in a reduction in blood cortisol levels. This leads to a regulation of heart rate and blood pressure, which has obvious cardiovascular benefits (Damodaran 2002). Yoga also focuses on relaxed breathing, and this internal concentration is thought to reduce stress by minimising mental focus on external stressors or threats (Bangalore 2012). This decrease in cortisol levels is also thought to result in better control of blood glucose, cholesterol, and total lipids. Since antipsychotic medication for the treatment of schizophrenia is associated with dyslipidaemia, diabetes, and obesity (Mitchell 2013), yoga may be a useful adjuvant to therapy to minimise these effects (Bangalore 2012). The improvement in the physical health of people with schizophrenia could have a direct improvement on their mental health. Research has also found that yoga is has a role in improving sleep (Collins 1998). There is also thought to be a role in yoga of oxytocin, a hormone related to improved mood, analogues of which have been suggested as possible treatment of schizophrenia (Bangalore 2012; Feifel 2011). Research has found that plasma levels of oxytocin are higher in people after practice of yoga (Varambally 2012b).
Why it is important to do this review
Yoga and its use for people with schizophrenia is under‐researched when compared with many other physical and mental health conditions. The practice of yoga has shown promising results in other areas for benefiting health, and this report will assess whether or not yoga has a place in the treatment of schizophrenia.
Due to an increasing demand from patients for alternatives or adjunct treatment to their medication and a prevalence of poor antipsychotic compliance (Elkins 2005; Van Os 2009), the utilisation of yoga could go some way to benefit patients and indeed the economic burden of treatment. In a time of increasing patient choice, this review aimed to investigate the potential benefits of yoga and expectantly aid the integration of yoga into clinical practice.
This review has built on systematic reviews of the last few years (Cramer 2013; Vancampfort 2012a; Vancampfort 2012b). The Vancampfort review concluded that there is a place for yoga as an add‐on treatment for schizophrenia, but Cramer concluded that no recommendation could be made regarding yoga as a routine intervention for people with schizophrenia. The somewhat diverging views highlight the need to continue to observe this area for emerging data to help provide clear guidance. Several studies have compared the effects of yoga and other forms of exercise as add‐on therapies for the management of schizophrenia, however in this review we focused only on comparisons of yoga with standard‐care control to gain some insight into the absolute effects of this approach in helping people with schizophrenia.
Objectives
To examine the effects of yoga versus standard‐care control for people with schizophrenia.
Methods
Criteria for considering studies for this review
Types of studies
All relevant RCTs. If a trial was described as 'double blind' but implied randomisation, we would have included such trials in a sensitivity analysis (see Sensitivity analysis). If their inclusion did not result in a substantive difference, they would have remained in the analyses. If their inclusion had resulted in important clinically significant but not necessarily statistically significant differences, we would not have added the data from these lower‐quality studies to the results of the better trials, but presented such data within a subcategory. We excluded quasi‐randomised studies, such as those allocating by alternate days of the week. Where people within the group receiving yoga were given additional treatments, we only included data if the adjunct treatment was evenly distributed between groups and it was only the allocation of yoga that was randomised.
Types of participants
We considered all people with a diagnosis of schizophrenia or related disorders, including schizophreniform disorder, schizoaffective disorder, and delusional disorder, regardless of their gender, age, or severity of illness, whose diagnosis was made by any means. We were interested in ensuring that information was as relevant to the current care of people with schizophrenia as possible, and so proposed, if possible, to clearly highlight the current clinical state (acute, early postacute, partial remission, remission) as well as the stage (prodromal, first episode, early illness, persistent) and as to whether the studies primarily focused on people with particular problems (for example negative symptoms, treatment‐resistant illnesses).
Types of interventions
1. Yoga therapy
Yoga, however defined by the study, incorporating any of the major subtypes such as mantra, laya, hatha, and raja and including breathing exercises and/or meditation and/or body postures.
2. Standard‐care control group
We defined standard care as the care participants would normally receive or had previously received for the management of their schizophrenia, without yoga intervention. This could also include wait‐list control.
Types of outcome measures
If possible, we proposed to divide all outcomes into short term (less than 6 months), medium term (7 to 12 months), and long term (over 1 year).
Primary outcomes
1. Mental state
1.1 Clinically significant response in mental state (as defined by individual studies) 1.2 Average endpoint score on mental state scales 1.3 Average change scores on mental state scales
2. Global state
2.1 Relapse 2.2 Clinically significant change in global state (as defined by each study) 2.3 Any change in global state 2.4 Average endpoint or change scores from global state scales
3. Social functioning
3.1 Clinically significant response in social functioning (as defined by individual studies) 3.2 Average endpoint score on social functioning scales 3.3 Average change scores on social functioning scales
4. Adverse effects
4.1 Any significant adverse effects of yoga 4.2 Any significant adverse effects of standard care
Secondary outcomes
5. Quality of life
5.1 Clinically significant response in quality of life functioning (as defined by individual studies) 5.2 Average endpoint score on quality‐of‐life scales 5.3 Average change scores on quality‐of‐life scales
6. Cognitive functioning
6.1 Clinically significant response in cognitive functioning (as defined by individual studies) 6.2 Average endpoint score on cognitive‐functioning scales 6.3 Average change scores on cognitive‐functioning scales
7. Leaving the study early
7.1 Any reason 7.2 Due to adverse effects of intervention 7.3 Due to lack of engagement with intervention 7.4 Due to death (suicide, natural causes, other)
8. Costs of care
8.1 Direct costs of care 8.2 Indirect costs of care
9. Effect on standard care
9.1 Reduction in reported adverse effects of standard care 9.2 Change in the level of standard care required to manage condition
10. Physical health
10.1 Clinically significant change in physical health (as defined by individual studies) 10.2 Any change in physical health
11. Service use
11.1 Acute hospital admissions 11.2 Length of stay in hospital
12. Disability
12.1 Significant change in disability (as defined by individual studies)
13. Daily living
13.1 Clinically significant change in daily‐living skills (as defined by individual studies) 13.2 Average endpoint score daily‐living scales 13.3 Average change scores on daily‐living scales
Summary of findings table
We used the GRADE approach to interpret findings, in Schünemann 2008, and GRADE profiler (GRADEPRO) to import data from RevMan 5.1 (Review Manager) to create Table 1. This table provided outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient care and decision making. We aimed* to select the following main outcomes for inclusion in the 'Summary of findings' table:
Mental state: overall improvement (as defined by studies)
Relapse*
Social functioning: overall improvement (as defined by studies)
Quality of life: overall improvement (as defined by studies)
Change in physical health
Adverse effects
Costs of care: direct and indirect
Search methods for identification of studies
Electronic searches
Cochrane Schizophrenia Group Trials Register
The Trials Search Co‐ordinator searched the Cochrane Schizophrenia Group’s Trials Register (November 2012 and January 29, 2015) using the following search strategy:
*Yoga* in Title OR Abstract OR Index Terms of REFERENCE OR in Interventions of STUDY
The Cochrane Schizophrenia Group’s Register of Trials is compiled by systematic searches of major resources (including MEDLINE, PubMed, EMBASE, CINAHL, BIOSIS, AMED, PsycINFO, and registries of clinical trials) and their monthly updates, handsearches, grey literature, and conference proceedings (see Group Module). There were no language, date, document type, or publication status limitations for inclusion of records into the register.
Searching other resources
1. Reference searching
We inspected references of all included studies for further relevant studies.
2. Personal contact
We contacted the first author of each included study for information regarding unpublished trials.
Data collection and analysis
Selection of studies
Review authors AK and JC independently inspected citations from the 2012 search and JB inspected citations from the 2015 search and identified relevant abstracts. We compared findings to ensure reliability. In case of disputes, we would have acquired the full report for more detailed scrutiny.
JB obtained full reports of the abstracts meeting the review criteria, which JB, AK, and JC independently inspected. CEA (see Acknowledgements) re‐inspected all identified studies in order to ensure reliable selection. We did not disagree on selection; in future versions, if it is not possible to resolve disagreements by discussion, we will attempt to contact the study authors for clarification.
Data extraction and management
1. Extraction
Review authors JB, AK, and JC independently extracted data from all included studies and compared results of the data extraction. We discussed any disagreements and documented decisions; if necessary, we contacted authors of studies for clarification. CEA helped clarify issues with any remaining problems, and we documented these final decisions. We extracted data presented only in graphs and figures whenever possible, but included this data in the review only if two review authors independently had the same result. We attempted to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary. If studies were multicentre, we would have extracted data relevant to each component centre separately. Where possible, we reported total end‐scale measures, as opposed to subscale measures. We had two exceptions (see Differences between protocol and review).
2. Management
2.1 Forms
We extracted data onto simple standard forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if: a) the psychometric properties of the measuring instrument were described in a peer‐reviewed journal (Marshall 2000); and b) the measuring instrument had not been written or modified by one of the trialists for that particular trial. Ideally, the measuring instrument should have been either i) a self report or ii) completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly; in Description of studies we noted if this was the case or not.
2.3 Endpoint versus change data
Both endpoint and change data have advantages. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult‐to‐measure conditions such as schizophrenia. We decided to use primarily endpoint data, and only use change data if the former were not available. We combined endpoint and change data in the analysis, as we preferred to use mean differences rather than standardised mean differences throughout (Higgins 2011).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to all data before inclusion.
For change data:
We entered change data, as when continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. We presented and entered change data into statistical analyses.
For endpoint data:
a) When a scale started from the finite number 0, we subtracted the lowest possible value from the mean and divided this by the standard deviation. If this value was lower than 1, it strongly suggested a skew, and we would exclude the study. If this ratio was higher than 1 but below 2, there was suggestion of skew. We would enter the study and test whether its inclusion or exclusion would change the results substantially. Finally, if the ratio was larger than 2, we would include the study, because skew was less likely (Altman 1996; Higgins 2011).
b) If a scale started from a positive value (such as the Positive and Negative Syndrome Scale, which can have values from 30 to 210) (Kay 1986), we would modify the calculation described above to take into account the scale starting point. In such cases skew is present if 2 standard deviations > (S ‐ S min), where S is the mean score and S min is the minimum score.
(Please note, irrespective of the above rules, we would enter endpoint data from studies of at least 200 participants in the analysis because skewed data pose less of a problem in large studies.)
2.5 Common measure
To facilitate comparison between trials, we intended to convert variables that could be reported in different metrics, such as days in hospital (mean days per year, per week, or per month) to a common metric (for example mean days per month).
2.6 Conversion of continuous to binary
Where possible, we made efforts made to convert outcome measures to dichotomous data. We did this by identifying cutoff points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale, in Overall 1962, or the Positive and Negative Syndrome Scale, in Kay 1986, this could be considered to be a clinically significant response (Leucht 2005; Leucht 2005a). If data based on these thresholds were not available, we used the primary cutoff presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for yoga intervention. Where keeping to this made it impossible to avoid outcome titles with clumsy double‐negatives (for example 'Not un‐improved'), we reported data where the left of the line indicated an unfavourable outcome. We noted this in the relevant graphs.
Assessment of risk of bias in included studies
Review authors JB, AK, and JC worked independently to assess risk of bias by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions to assess trial quality (Higgins 2011). This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting.
If the raters had disagreed, we would have made the final rating by consensus with the involvement of another member of the review group. Where a study provided inadequate details of randomisation and other characteristics of the trial, we attempted to contact the study authors in order to obtain further information. We would have reported non‐concurrence in quality assessment, but if disputes had arisen as to which category a trial was to be allocated, again, we would have resolved this by discussion.
We noted the level of risk of bias in both the text of the review and in the 'Risk of bias' table within the Characteristics of included studies and Table 1.
Measures of treatment effect
1. Binary data
For binary outcomes, we calculated a standard estimation of the risk ratio and its 95% confidence interval. It has been shown that risk ratio is more intuitive than odds ratios, and that odds ratios tend to be interpreted as risk ratio by clinicians (Boissel 1999; Deeks 2000). The number needed to treat for an additional beneficial outcome/number needed to treat for an additional harmful outcome statistic with its confidence intervals is intuitively attractive to clinicians but is problematic both in its accurate calculation in meta‐analyses and its interpretation (Hutton 2009). For binary data presented in the Table 1, where possible, we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes, we estimated mean difference between groups. We preferred not to calculate effect size measures (standardised mean difference). However, if scales of very considerable similarity had been used, we presumed there was a small difference in measurement, and we calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data pose problems. Firstly, authors often fail to account for intraclass correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992), whereby P values are spuriously low, confidence intervals unduly narrow, and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
If clustering had not been accounted for in primary studies, we would have presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review, we will seek to contact first authors of studies to obtain intraclass correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). If clustering was incorporated into the analysis of primary studies, we would have presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We sought statistical advice and were advised that the binary data as presented in a report should be divided by a 'design effect'. We calculated this using the mean number of participants per cluster (m) and the ICC (Design effect = 1 + (m ‐ 1)*ICC) (Donner 2002). If the ICC was not reported, we would have assumed it to be 0.1 (Ukoumunne 1999).
If cluster studies had been appropriately analysed taking into account ICCs and relevant data documented in the report, we would have synthesised these with other studies using the generic inverse‐variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (for example pharmacological, physiological, or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason, cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we had planned to use only the data of the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data were binary, we simply added these and combined within the two‐by‐two table. If data were continuous, we combined data following the formula in Section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where the additional treatment arms were not relevant, we did not use these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of the data be unaccounted for, we would not reproduce these data or use them within analyses (except for the outcome 'leaving the study early'). If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we would have marked such data with (*) to indicate that such a result may well be prone to bias.
2. Binary
In the case where attrition for a binary outcome was between 0 and 50% and where these data were not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis). We assumed all those leaving the study early to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes, we used the rate of those who stayed in the study ‐‐ in that particular arm of the trial ‐‐ for those who did not. We undertook a sensitivity analysis to test how prone the primary outcomes were to change when data only from people who completed the study to that point were compared to the intention‐to‐treat analysis using the above assumptions.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome was between 0 and 50%, and data only from people who completed the study to that point were reported, we used these data.
3.2 Standard deviations
If in future updates standard deviations are not reported, we will first try to obtain the missing values from the authors. If these are not available, where measures of variance for continuous data are missing, but an exact standard error and confidence intervals are available for group means, and either P value or t value is available for differences in mean, we can calculate them according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When only the standard error (SE) is reported, standard deviations (SDs) can be calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions present detailed formulae for estimating SDs from P values, t or F values, confidence intervals, ranges, or other statistics (Higgins 2011). If these formulae do not apply, we will calculate the SDs according to a validated imputation method that is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We nevertheless will examine the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Last observation carried forward
We anticipated that in some studies the method of last observation carried forward (LOCF) would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, where LOCF data were used in the trial, if less than 50% of the data were assumed, we presented and used these data and indicated that they were the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, in order to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations that we had not predicted would arise. If such situations or participant groups arose, we would have fully discussed these.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, in order to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods that we had not predicted would arise. If such methodological outliers had been present, we would have fully discussed these.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs in order to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We investigated heterogeneity between studies by considering the I2 method alongside the Chi2 P value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i) magnitude and direction of effects and ii) strength of evidence for heterogeneity (for example P value from Chi2 test, or a confidence interval for I2). We will interpret an I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic as evidence of substantial levels of heterogeneity (Higgins 2011). When we found substantial levels of heterogeneity in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
1. Protocol versus full study
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in Section 10.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We tried to locate protocols of included randomised trials. If the protocol was available, we compared outcomes in the protocol with those in the published report. If the protocol was not available, we compared outcomes listed in the methods section of the trial report with actually reported results.
2. Funnel plot
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are again described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar size. In future updates of this review, if funnel plots are possible, we will seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seemed to be true to us, and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model, in that it puts added weight onto small studies, which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose the fixed‐effect model for all analyses. The reader is, however, able to choose to inspect the data using the random‐effects model.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
1.1 Primary outcomes
We did not anticipate a need for any subgroup analysis.
1.2 Clinical state, stage or problem
We proposed to undertake this review as part of a family of similar reviews that will provide an overview of the effects of yoga for people with schizophrenia in general. In addition, we aimed to report data on subgroups of people in the same clinical state, stage, and with similar problems.
2. Investigation of heterogeneity
If inconsistency was high, we reported this. We first investigated whether data had been entered correctly. Secondly, if data were correct, we visually inspected the graph and successively removed outlying studies to see if homogeneity was restored. For this review, we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we would present the data. If not, we would not pool the data and we would discuss these issues. We know of no supporting research for this 10% cutoff, but we used prediction intervals as an alternative to this unsatisfactory state.
If in future updates of this review unanticipated clinical or methodological heterogeneity is obvious, we will simply state hypotheses regarding these. We do not anticipate undertaking analyses relating to such situations.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in such a way as to imply randomisation. For the primary outcomes we would have included these studies, and if there was no substantive difference when the implied randomised studies were added to those with better description of randomisation, then we would have employed all data from these studies.
2. Assumptions for lost binary data
Where we had to make assumptions regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption/s and when we used data only from people who completed the study to that point. If there was a substantial difference, we would have reported results and discussed them but continued to employ our assumption.
If we needed to make assumptions regarding missing SDs data (see Dealing with missing data), we would have compared the findings of the primary outcomes when we used our assumption/s and when we used data only from people who completed the study to that point. We would have undertaken a sensitivity analysis testing how prone results were to change when completer‐only data only were compared to imputed data using the above assumption. If there was a substantial difference, we would have reported results and discussed them but continued to employ our assumption.
3. Risk of bias
For the primary outcome, we analysed the effects of excluding trials that we judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available) allocation concealment, blinding, and outcome reporting. If the exclusion of trials at high risk of bias had substantially altered the direction of effect or the precision of the effect estimates, then we would not have included data from these trials in the analysis.
4. Imputed values
We had intended to undertake a sensitivity analysis to assess, if necessary, the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster randomised trials.
If we had noted substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we would not pool data from the excluded trials with the other trials contributing to the outcome, but would have presented them separately.
5. Fixed effect and random effects
We synthesised all data using a fixed‐effect model, however we also aimed to synthesise data for the primary outcome using a random‐effects model to evaluate whether this altered the significance of the results. If the significance of results changed we would have noted this in the text.
Results
Description of studies
Please see Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies.
Results of the search
In the search we undertook for this review, we found 1019 papers that were potentially relevant. We identified no duplicates. After removing 727 articles that were clearly irrelevant, we inspected 292 abstracts. From these, we selected 27 reports to further assess for inclusion. We then grouped these into 'studies' where several of the reports referred to the same trial. We had to exclude 11 of these studies. So, the search generated 15 reports of 9 trials, of which 8 trials (14 reports) were included in the meta‐analysis. The PRISMA table shows results of our search (see Figure 1).
1.
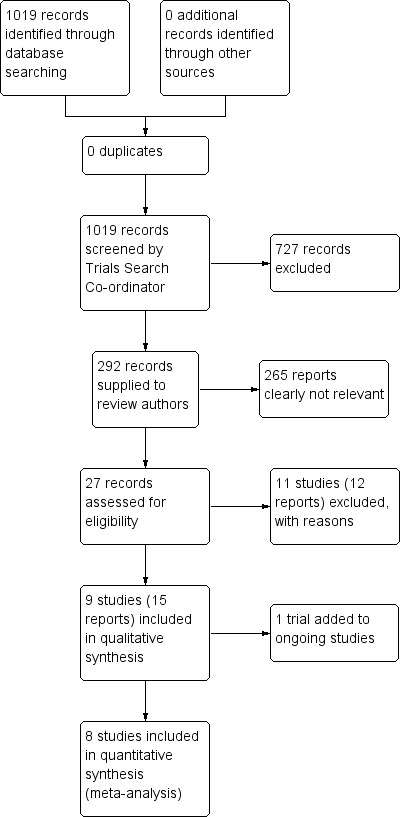
Study flow diagram.
Included studies
1. Methods
No studies were double‐blind due to the nature of the intervention. In an effort to minimise bias, a number of studies (six out of eight) stated that the outcomes assessor was blind to group allocation (Behere 2011; Ikai 2013; Ikai 2014; Lin 2013; Varambally 2012; Visceglia 2011). However, two of the included studies reported no details of blinding (Jayaram 2013; Lin 2006). All studies were parallel studies and were described as randomised. Five studies reported outcomes immediately after intervention, with the exception of two studies that reported outcomes after a follow‐up period of no treatment (Behere 2011; Varambally 2012).
Two studies reported outcomes on completion of the yoga intervention (8 weeks) and a further follow‐up at the 16‐week time point, but in the yoga group only (Ikai 2013; Ikai 2014). We assumed that the standard‐care group results were constant, so we included results from the 8‐week time point in the standard‐care group and 16‐week time point in the yoga group for the Ikai 2013 study. However, it is not known whether differing time points between study arms may have biased results from this study. In the Ikai 2014 study, follow‐up at 16 weeks was reported, but as no data was reported for the standard‐care group and the Functional Assessment for Comprehensive Treatment of Schizophrenia and Positive and Negative Syndrome Scale in the yoga group, we included only the 8‐week follow‐up data.
2. Length of trials
The overall duration of all included trials was short term, varying in length from one to four months. Within this short‐term time‐frame, the duration of studies ranged from 1 month, in Jayaram 2013, to 8 weeks/2 months (Ikai 2014; Lin 2006; Visceglia 2011), 12 weeks (Lin 2013), and 4 months (Behere 2011; Ikai 2013; Varambally 2012). One study, Lin 2013, did provide an 18‐month follow‐up, however it could not be included as a "compensatory" yoga or exercise programme was offered to control participants before the final follow‐up, which systematically negated the control‐group condition. No viable medium‐ or long‐term data were therefore presented by the included trials.
3. Participants
A total of 457 people participated in the 8 studies. The total sample size ranged from 18 to 140. The majority of studies included less than 100 participants (Behere 2011; Ikai 2013; Ikai 2014; Jayaram 2013; Lin 2006; Visceglia 2011), with the exception of two studies (Lin 2013; Varambally 2012), which included 140 and 120 participants respectively, although both of these studies included exercise arms that were not considered as part of this review. Both males and females were included in all studies, with the exception of one study that exclusively included females (Lin 2013).
All studies included people with schizophrenia. The diagnostic criteria employed varied between studies. Four studies used the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (Behere 2011; Jayaram 2013; Lin 2013; Varambally 2012), two studies used the 10th revision of the International Statistical Classification of Diseases (Ikai 2013; Ikai 2014), and one study specified Axis I diagnosis of schizophrenia (Visceglia 2011). One study did not specify the diagnostic criteria (Lin 2006). As well as schizophrenia, one study, Lin 2006, included mixed diagnoses (18/60 of sample) including affective psychosis, neurotic disorder, and reactive psychosis. One study, Visceglia 2011, specified that a number of participants had multiple diagnoses (such as mild mental retardation, borderline personality disorder, and antisocial personality disorder); it is unknown whether the extent of multimorbidity in this study influenced the validity of findings.
No report referred to the current clinical state of participants (acute, early postacute, partial remission, remission), and similarly no report focused on people with particular problems, for example negative symptoms or treatment illnesses. A number of studies did not specify length of illness, although two studies included inpatients, one with a duration of illness from 2 months to 20 years (Lin 2006); two studies specified outpatients with a mean duration of illness of approximately 25 years (Ikai 2013; Ikai 2014); and another stated that participants were ''state‐hospitalised'' (Visceglia 2011), presumably reflecting a population with greater disease burden than the outpatient setting.
4. Setting
Two studies took place in an inpatient setting (Lin 2006; Visceglia 2011), five in an outpatient setting (Behere 2011; Ikai 2013; Ikai 2014; Lin 2013; Varambally 2012), and one in both inpatient and outpatient settings (Jayaram 2013). Three studies took place in India within the same institute (National Institute of Mental Health and Neurosciences, Bangalore) (Behere 2011; Jayaram 2013; Varambally 2013), two in China (Lin 2006; Lin 2013), two in Japan (Ikai 2013; Ikai 2014), and one in the United States (Visceglia 2011).
5. Interventions
5.1 Yoga
In all studies the yoga intervention was delivered by trained or certified yoga instructors with the exception of one study (Lin 2006), which did not specify the qualification of instructors. Two studies stated that the instructor held a master's degree in yoga (Ikai 2013; Ikai 2014). All studies employed supervised group yoga sessions with yoga therapist:participant ratios of 1:5 (Visceglia 2011), 1:5‐10 (Lin 2013), or unclear/non‐specified (Behere 2011; Ikai 2013; Ikai 2014; Jayaram 2013; Lin 2006; Varambally 2012).
The yoga programmes described in the included studies were heterogeneous. The frequency of yoga sessions provided varied from 1 per week (over 8 weeks) (Ikai 2013; Ikai 2014), to twice weekly over 8 weeks (Visceglia 2011), to 3 times weekly over 12 weeks (Lin 2013), to 4 times per week over 2 months (Lin 2006), to 25 sessions over 1 month (Varambally 2012), or daily for 1 month (Visceglia 2011). Two studies specified that yoga was delivered over one month, but frequency was not specified (Behere 2011; Jayaram 2013). The number of sessions provided therefore ranged from 8, in Ikai 2013 and Ikai 2014, to 36 sessions, in Lin 2013. All studies assessed follow‐up after completion of the yoga intervention with the exception of one study, which assessed after two months of ''self‐practice'' (Behere 2011), and similarly, two further studies re‐assessed participants after eight weeks (Ikai 2013; Ikai 2014). In one study (Ikai 2014), participants were asked not to perform self practice of yoga at home.
Two studies mentioned the yoga discipline (Ikai 2014; Lin 2013), which referred to hatha yoga. For three studies (Behere 2011; Jayaram 2013; Varambally 2012), the yoga practice was developed by the same school (Swami Vivekananda Yoga Anusandhana Samsthana), and these studies took place in the same centre (National Institute of Mental Health and Neurosciences in Bangalore, India). Two of these studies implemented the same yoga intervention consisting of "loosening exercises" for 10 minutes, yoga postures (asanas) for 20 minutes, breathing exercises for 18 minutes, and relaxation for 3 minutes (Behere 2011; Jayaram 2013). Three further studies described a similar class structure (Lin 2006; Lin 2013; Varambally 2012), with some variability in the relative time spent in individual components of the class; Ikai 2013; Ikai 2014 [7 minutes of warming‐ and loosening‐up exercises (including guided meditation for 3 minutes), 28 minutes of yoga postures (asanas), 7 minutes of relaxation, 8 minutes of breathing exercises], Lin 2006 (10 minutes warm‐up, 40 minutes yoga, 10 minutes relaxation); Lin 2013 (10 minutes breathing control, 10 minutes warm‐up, 30 minutes yoga postures, 10 minutes relaxation); Varambally 2012 (10 minutes ''loosening exercises'', 20 minutes yoga postures, 8 minutes breathing exercises, 3 minutes relaxation). Similarly, Visceglia 2011 describes a comparable class structure, but individual timing of each component was not specified as this was matched to participant‐related factors such as energy level and mood. The time therefore for the yoga intervention in these studies varied between 45 minutes to 1 hour. Meditation was included in three studies (Ikai 2013; Ikai 2014; Lin 2006).
In two studies participants were expected to adhere to 70% to 75% of supervised sessions (Lin 2013; Varambally 2012). Particpants in the Varambally 2012 study were expected to maintain an exercise log of self practice for the two‐month follow‐up home programme, but this was ''poorly followed''. No studies described any feasibility outcomes, with the exception of Lin 2013, which specified adherence to the prescribed supervised classes of 51.1%, and Ikai 2013, which reported that the mean number of sessions attended among randomised participants was 7.8.
5.2 Standard‐care control
Three studies compared yoga intervention against standard care (Jayaram 2013; Lin 2006; Visceglia 2011). Two studies had an additional intervention group, comparing yoga, exercise (type not specified), and standard care (Behere 2011; Varambally 2012), and one study compared yoga to ''aerobic'' exercise and standard care (Lin 2013). Lin 2013 and Varambally 2012 used wait‐lists, with the participants being offered yoga after the study had ended. Behere 2011, Jayaram 2013, and Visceglia 2011 stated comparison groups to be ''waiting list group'' but gave no further details.
In two studies (Ikai 2013; Ikai 2014), standard care consisted of a weekly regular day‐care programme consisting of social‐skills training and psycho‐education. In addition, yoga and standard‐care groups were registered in the regular day‐care programme and could avail of ''ambulatory treatment'', which consisted of non‐structured clinical management such as pharmacotherapy, and very brief psychotherapy by participant's treating psychiatrist (from personal communication with study author 6 August 2015).
6. Outcomes
The following outcomes for which we could obtain useable data are listed below, followed by a summary of data that we could not use in this review as well as missing outcomes.
6.1 Outcome scales
6.1.1 Mental state
i. Positive and Negative Syndrome Scale (PANSS) (Kay 1986)
This 30‐item scale assesses severity of psychotic symptomology in general. It consists of three subscales: positive symptoms, negative symptoms general psychopathology, and a total score. Scoring ranges from 1 to 7, with a low score indicating a lesser severity of symptoms (1 = absent, 2 = minimal, 3 = mild, 4 = moderate, 5 = moderate severe, 6 = severe, 7 = extreme). We included positive symptoms, negative symptoms, and total score in this review. There was no agreement in how these were measured in the three trials that included PANSS; each was measured in a different way: binary, average change score, and average endpoint.
ii. Schedule for Assessment of Negative Symptoms (SANS) (Andreasen 1983)
This 6‐point scale assesses the negative symptoms of schizophrenia, rating alogia, affective blunting, avolition‐apathy, anhedonia‐apathy, anhedonia‐asociliaty, and attention impairment. Higher scores indicate more severe symptoms.
iii. Schedule for Assessment of Positive Symptoms (SAPS) (Andreasen 1984)
This scale selectively assesses the positive symptoms of psychosis; the higher the score, the more severe the symptoms.
iv. Calgary Depression Scale (CDS) (Addington 1994)
This 9‐point scale measures depression in schizophrenia rated from 0‐3 (0 = symptom is absent). The total score includes the following nine items; depression, hopelessness, self depreciation, guilty ideas of reference, pathological guilt, morning depression, waking early, suicide, and observed depression.
v. 25‐Item Resilience Scale (Wagnild 1993)
This scale measures the degree of individual resilience. The scale covers five factors of resilience: purpose, perseverance, self reliance, equanimity, and existential aloneness. Items are scored on a 7‐point scale ranging from 1 = disagree to 7 = agree, with possible scores ranging from 25 to 175; a higher score indicates greater individual resilience.
6.1.2 Social functioning
i. Socio‐Occupational Functioning Scale (SOFS) (Saraswat 2006)
This scale assesses various aspects of social functioning and incorporates 14 domains (bathing and grooming; clothing and dressing; eating, feeding and diet; neatness and maintenance activities; conversational skills; social appropriateness/politeness; social engagement; money management; orientation/mobility; instrumental social skills; recreation/leisure; work; respect for property; independence/responsibility), each being graded on a 5‐point Likert scale (1 = no impairment, 2 = mild impairment, 3 = moderate impairment, 4 = severe impairment, 5 = extreme impairment) with a high score indicating greater severity of social impairment. The sum of individual domains gives an overall score.
ii. TRENDS Accuracy Score (TRACS) (TRENDS: Tool for Recognition of Emotions in Neuropsychiatric Disorders) (Behere 2008)
This scale assesses emotional recognition abilities. It consists of 80 images (52 static (still) and 28 dynamic (video clip) images) of six basic emotions, happy, sad, fear, anger, surprise, disgust, and a neutral expression, emoted by four actors. A higher score indicates a higher number of correctly identified emotions out of a maximum of 80.
iii. Functional Assessment for Comprehensive Treatment of Schizophrenia (FACT‐Sz) (Suzuki 2008)
This scale evaluates psychosocial functioning of patients with a score of 0–100, and is judged entirely on an objective basis. A cutoff score of 60 is intended to indicate somewhat acceptable functioning; scores of 70‐80 would indicate minimal impairments, and greater than 80 indicates recovery. Scores of 40 or below would indicate marked impairment.
6.1.3 Quality of life
i. World Health Organization Quality of Life BREF questionnaire (WHOQOL‐BREF) (Skevington, 2004)
This scale assesses an individual's quality of life and consists of 26 questions based on four domains: physical health, psychological, social relationships, and environment. No total or composite score is generated. Each question is rated from 1 to 5, raw scores are converted to transformed scores. The mean score of items within each domain is used to calculate the domain score, with a maximum possible score of 100, a higher score indicating a higher quality of life.
ii. Short‐Form 36 (SF‐36) (Ware 1993)
This 36‐point questionnaire evaluates quality of life and consists of an eight‐scale profile of scores and a summary of physical and mental measures. The summary scores of physical and mental health are the weighted sums of the eight dimensions of physical health (physical functioning, physical role, pain, and general health) and mental health (energy, social functioning, emotional role, and emotional well‐being). Higher scores indicate better physical or mental health.
iii. EuroQoL 5 Dimensions (EQ‐5D) (Brooks 1996)
This generic, non‐disease‐specific scale evaluates health‐related quality of life and is self completed by the respondent. The EQ‐5D descriptive system evaluates the following five dimensions: mobility, self care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has three levels: no problems, some problems, extreme problems. The digits for dimensions of this scale can be combined in a five‐digit number describing the respondent’s health state and can also be converted into a single index value by applying a formula that essentially attaches values (also called weights) to each of the levels in each dimension.
6.2 Missing outcomes
Overall, this review was subject to a considerable number of missing outcomes. No studies reported data on key outcomes of global state, costs of care, effect on standard care, service use, disability, or activities of daily living. One study presented a number of scales that investigated dimensions of cognition functioning (Lin 2013), but no total end scores were provided and therefore were not included in this review.
A dissertation generated from a final search just prior to completion of this review included 18‐month follow‐up data for the Lin 2013 study. Closer examination unfortunately revealed that at some time point between the 12‐week and 18‐month time points the control group received a "compensated" 12‐week yoga or exercise programme, which systematically negated the control‐group condition. We were therefore unable to include this long‐term follow up in our review.
Excluded studies
We generated over 1,000 potential studies from this search. The Trials Search Co‐ordinator excluded 727 studies, and the review authors excluded a further 265 reports as they did not meet study criteria. We examined a further 27 reports in detail. Eleven studies (12 reports) have now been excluded. One study was excluded as it was not randomised (Bhatia 2012). The majority of studies were excluded because it was not clear they included a yoga versus standard‐care control group comparison (Duraiswamy 2007; JPRN‐UMIN000013746; Mahal 1976; Manjunath 2013; Paikkatt 2012; Ramu 1999; SLCTR‐2013‐008; Vancampfort 2011a; Xie 2006). One study investigated the effects of yoga on caregivers, rather than on people with a diagnosis of schizophrenia themselves (Varambally 2013). We have provided details of excluded studies in the Characteristics of excluded studies table.
Awaiting assessment
No studies are currently awaiting assessment.
Ongoing studies
One study was ongoing and was published in protocol format only (Bhatia 2014). This appears to be a comprehensive study with more than 300 participants randomised to date and outcomes detailed including mental state, cognitive function, and general function. We eagerly await data are for this study.
Risk of bias in included studies
See also 'Risk of bias' tables in Characteristics of included studies and Figure 2 and Figure 3.
2.
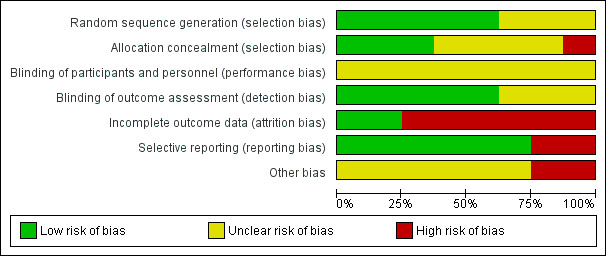
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
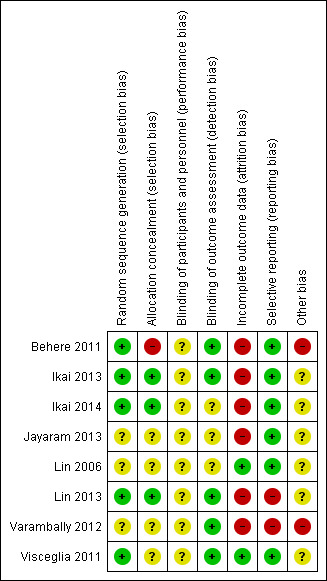
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies were RCTs. A number of studies did not provide detail on how the randomisation method was executed and were therefore deemed as at unclear risk of bias. Five studies had adequately described randomisation methods (Behere 2011; Ikai 2013; Ikai 2014; Lin 2013; Visceglia 2011), utilising computer‐generated random numbers, with one of these, Lin 2013, using block randomisation with a block size of 12; we rated these studies as at low risk of bias.
Concealment bias varied. We rated three studies as low risk for adequate concealment (Ikai 2013; Ikai 2014; Lin 2013), as it was reported that a randomisation list was kept concealed from research staff involved in recruitment, assessment, and the study intervention. Varambally 2012 reported that "subjects" allocation was "kept concealed", although no concealment strategy was described; we therefore rated this study as unclear risk. We rated three studies as unclear risk because they reported no explicit detail on concealment approach (Jayaram 2013; Lin 2006; Visceglia 2011). We rated one study as at high risk of bias due to inadequate allocation concealment, as one of the study authors performed group randomisation (Behere 2011).
Blinding
Due to the nature of the intervention, none of the studies were able to use a double‐blind technique, since it would not be possible for yoga/control participants or practitioners delivering the yoga intervention to be blind to group allocation. The risk of performance bias implications were therefore unclear. A number of studies provided explicit detail on blinding of the outcome assessor; we rated these as at low risk of bias for detection (Behere 2011; Ikai 2013; Ikai 2014; Lin 2013; Varambally 2012; Visceglia 2011). We rated the remaining studies as at unclear risk of bias due to inadequate detail on blinding of outcome assessor.
Incomplete outcome data
We rated only two studies, Lin 2006 and Visceglia 2011, as at low risk bias with regard to attrition bias, as all participants were continued to follow‐up. The remaining studies did not include all randomised participants in the analysis, and we noted that two studies had systematic differences between the yoga and control groups (Jayaram 2013; Varambally 2012), which we therefore rated as at high risk of bias. We also rated Lin 2013 as high risk, as the group allocation of participants who withdrew was unclear. We rated Behere 2011 as high risk due to the relatively high proportion of participants randomised who were not included in the analysis (27.5%). We rated Ikai 2013 and Ikai 2014 as high risk of bias, as 16‐week follow‐up data were not provided for the standard‐care group. Intervention group 16‐week follow up data for the PANSS and FACT‐Sz was also not provided in the Ikai 2014 study'.
Selective reporting
We rated two studies as at high risk of bias with regard to selective reporting, as no data were reported for one or more outcomes listed (Lin 2013; Varambally 2012). All remaining studies reported data for all outcomes listed and were therefore rated as at low risk of bias.
Other potential sources of bias
The majority of studies appeared to have other potential sources of bias. We ranked two studies, Behere 2011 and Varambally 2012, as at high risk of other bias, as one of the authors may have been invested in the yoga intervention, due to their affiliation with Swami Vivekananda Yoga Anusandhana Samsthana. Yoga for these studies was developed from this school. Data extraction from the Lin 2006 study relied on translation from an outside source. Visceglia 2011 reported that a number of participants had multiple diagnoses, which may have influenced outcomes. Adherence of groups to day‐care programme and ''ambulatory treatment'' was not specified by Ikai 2013 or Ikai 2014, which may have been a potential source of bias.
Effects of interventions
See: Table 1
1. COMPARISON 1: YOGA versus STANDARD‐CARE CONTROL
We evaluated one single comparison in this review: yoga versus standard‐care control. Eight studies compared yoga to standard‐care control for schizophrenia (Behere 2011; Ikai 2013; Ikai 2014; Jayaram 2013; Lin 2006; Lin 2013; Varambally 2012; Visceglia 2011). A dissertation pertaining to the Lin 2013 study included long‐term follow‐up data. However, prior to the 18‐month time point, the control group was offered a "compensatory" exercise or yoga intervention, which systematically negated the control condition, thereby precluding inclusion of this data. Consequently no useable data was available for medium‐ or long‐term outcomes, so all outcomes listed were short term (less than six months).
1.1 Mental state
Mental state was measured using a number of different outcomes (PANSS, SANS, SAPS, CDS, and Resilience Scale). Even within PANSS, the total score was presented three ways; binary (improved, not improved), average change score, and average endpoint score. There were no subgroups for any of these outcomes.
1.1.1 Mental state: 1. Overall a. Not improved (total PANSS)
For this outcome we found a single study (total n = 83). We found evidence of a clear difference between 'yoga' and 'standard care' (1 RCT, n = 83, risk ratio (RR) 0.70, 95% confidence interval (CI) 0.55 to 0.88; Analysis 1.1).
1.1. Analysis.
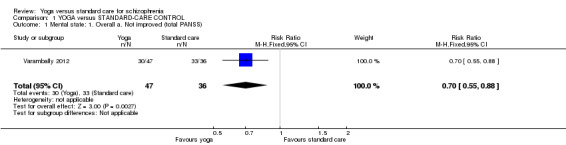
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 1 Mental state: 1. Overall a. Not improved (total PANSS).
1.1.2 Mental state: 1. Overall b. Average change score (PANSS, low = good)
For this outcome we found a single study involving 18 participants. We found a clear difference between 'yoga' and 'standard care' (1 RCT, n = 18, mean difference (MD) ‐26.33, 95% CI ‐37.71 to ‐14.95; Analysis 1.2).
1.2. Analysis.
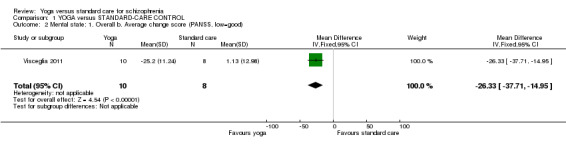
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 2 Mental state: 1. Overall b. Average change score (PANSS, low=good).
1.1.3 Mental state: 1. Overall c. Average endpoint score (PANSS, low = good)
For this outcome we found three studies, with a total of 176 participants. We found evidence of a clear difference between 'yoga' and 'standard care' (3 RCTs, n = 176, MD ‐10.74, 95% CI ‐15.39 to ‐6.09; Analysis 1.3).
1.3. Analysis.
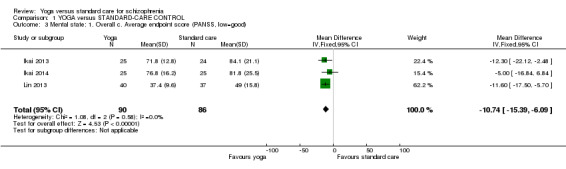
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 3 Mental state: 1. Overall c. Average endpoint score (PANSS, low=good).
1.1.4 Mental state: 2. Negative symptoms a. Not improved (PANSS)
For this outcome we found a single study, with a total of 83 participants. We found evidence of a clear difference between 'yoga' and 'standard care' (1 RCT, n = 83, RR 0.72, 95% CI 0.57 to 0.90; Analysis 1.4).
1.4. Analysis.
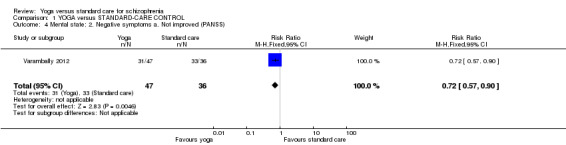
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 4 Mental state: 2. Negative symptoms a. Not improved (PANSS).
1.1.5 Mental state: 2. Negative symptoms b. Average score at endpoint (PANSS, low = good)
For this outcome we found five relevant studies, with a total of 243 participants. We found evidence of a clear difference between 'yoga' and 'standard care' (5 RCTs, n = 243, MD ‐1.92, 95% CI ‐3.06 to ‐0.78). However, this outcome had important levels of heterogeneity (Chi2 = 15.83; df = 4.0; P = 0.003; I2 = 75%; Analysis 1.5).
1.5. Analysis.
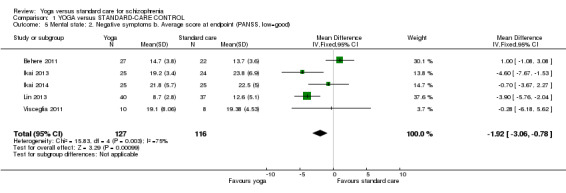
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 5 Mental state: 2. Negative symptoms b. Average score at endpoint (PANSS, low=good).
1.1.6 Mental state: 2. Negative symptoms c. Average score at endpoint (SANS, low = good)
We identified one study relevant to this outcome involving 27 participants. We found no difference between 'yoga' and 'standard care' (1 RCT, n = 27, MD 4.80, 95% CI 0.94 to 8.66; Analysis 1.6).
1.6. Analysis.

Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 6 Mental state: 2. Negative symptoms c. Average score at endpoint (SANS, low=good).
1.1.7 Mental state: 2. Negative symptoms d. Average change score (PANSS, greater decrease = good)
For this outcome we found a single study involving 18 participants. We found evidence of a clear difference between 'yoga' and 'waiting list' (1 RCT, n = 18, MD ‐6.00, 95% CI ‐9.87 to ‐2.13; Analysis 1.7).
1.7. Analysis.
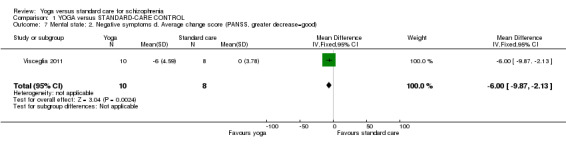
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 7 Mental state: 2. Negative symptoms d. Average change score (PANSS, greater decrease=good).
1.1.8 Mental state: 3. Positive symptoms a. Not improved (PANSS)
For this outcome we found a single study involving 83 participants. We found evidence of a clear difference between 'yoga' and 'waiting list' (1 RCT, n = 83, RR 0.98, 95% CI 0.79 to 1.22; Analysis 1.8).
1.8. Analysis.
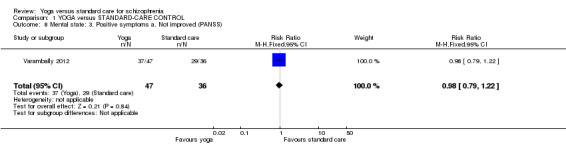
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 8 Mental state: 3. Positive symptoms a. Not improved (PANSS).
1.1.9 Mental state: 3. Positive symptoms b. Average score at endpoint (PANSS, low = good)
For this outcome we found five relevant studies involving 243 participants. We found evidence of a clear difference between 'yoga' and 'standard care' (5 RCTs, n = 243, MD ‐1.46, 95% CI ‐2.50 to ‐0.42; Analysis 1.9).
1.9. Analysis.
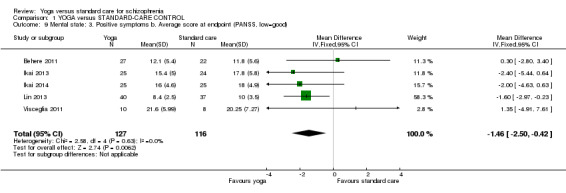
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 9 Mental state: 3. Positive symptoms b. Average score at endpoint (PANSS, low=good).
1.1.10 Mental state: 3. Positive symptoms c. Average score at endpoint (SAPS, low = good)
For this outcome we found a single study involving 27 participants. We did not find evidence of a clear difference between 'yoga' and 'standard care' (1 RCT, n = 27, MD 2.80, 95% CI 0.80 to 4.80; Analysis 1.10).
1.10. Analysis.
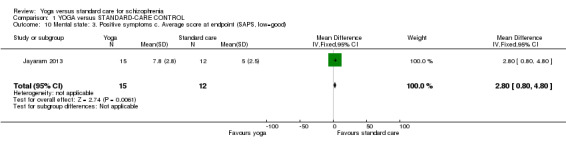
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 10 Mental state: 3. Positive symptoms c. Average score at endpoint (SAPS, low=good).
1.1.11 Mental state: 3. Positive symptoms d. Average change score (PANSS, greater decrease = good)
We identified one study relevant to this outcome involving 18 participants. We found evidence of a clear difference between 'yoga' and 'standard care' (1 RCT, n = 18, MD ‐5.27, 95% CI ‐9.19 to ‐1.35; Analysis 1.11).
1.11. Analysis.
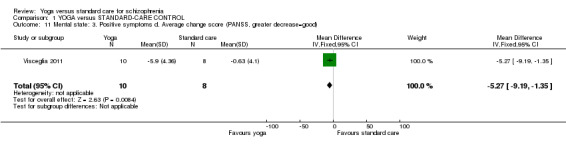
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 11 Mental state: 3. Positive symptoms d. Average change score (PANSS, greater decrease=good).
1.1.12 Mental state: 4. Depresssive symptoms a. Average score (CDS, greater decrease = good)
For this outcome we found a single study involving 54 participants. We found evidence of a clear difference between 'yoga' and 'standard care' (1 RCT, n = 54, MD ‐2.90, 95% CI ‐4.86 to ‐0.94; Analysis 1.12).
1.12. Analysis.
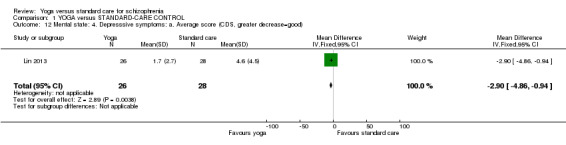
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 12 Mental state: 4. Depresssive symptoms: a. Average score (CDS, greater decrease=good).
1.1.13 Mental state: 5. Resilience a. Average score at endpoint (Resilience Scale, high = good)
One study with 50 participants was relevant to this outcome. We found no difference between 'yoga' and 'standard care' for this outcome (1 RCT, n = 50, MD 3.20, 95% CI ‐11.27 to 17.67; Analysis 1.13).
1.13. Analysis.
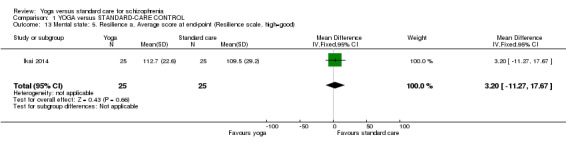
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 13 Mental state: 5. Resilience a. Average score at end‐point (Resilience scale, high=good).
2. Social functioning
This outcome was evaluated using the SOFS, which was presented dichotomously in one study, Varambally 2012, and using average score at endpoint in two further studies (Behere 2011; Jayaram 2013). Social functioning was also evaluated using the FACT‐Sz in two studies (Ikai 2013; Ikai 2014), and emotional recognition was measured using the TRACS (Behere 2011), which we categorised under social functioning.
2.1 Social functioning: 1. Overall a. Not improved
We identified one study relevant to this outcome involving 83 participants. This outcome had no subgroups. For this outcome, we found evidence of a clear difference between 'yoga' and 'standard care' (1 RCT, n = 83, RR 0.88, 95% CI 0.77 to 1.00; Analysis 1.14).
1.14. Analysis.
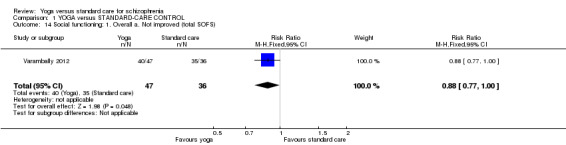
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 14 Social functioning: 1. Overall a. Not improved (total SOFS).
2.2 Social functioning: 1. Overall b. Average score at endpoint (SOFS, high score = good)
For this outcome we found two relevant studies involving 76 participants. This outcome had no subgroups. There was not a clear difference between 'yoga' and 'standard care' (2 RCTs, n = 76, MD 0.64, 95% CI ‐2.12 to 3.39; Analysis 1.15).
1.15. Analysis.
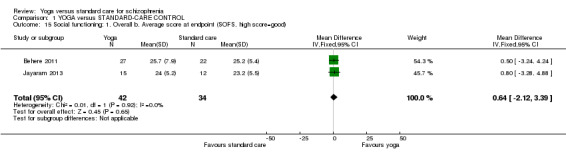
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 15 Social functioning: 1. Overall b. Average score at endpoint (SOFS, high score=good).
2.3 Social functioning 1. Overall c. Average score at endpoint (FACT‐Sz, high score = good)
Two studies were relevant to this outcome involving 99 participants. We did not find evidence of a clear difference between 'yoga' and 'standard care' (2 RCTs, n = 99, MD 4.26, 95% CI 0.81 to 7.71). This outcome had high levels of heterogeneity (Chi2 = 5.89; df = 1; P = 0.02; I2 = 83%; Analysis 1.16).
1.16. Analysis.
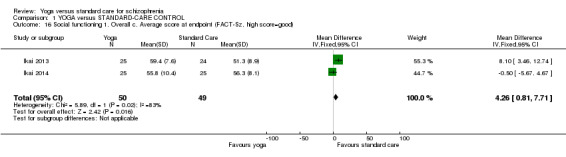
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 16 Social functioning 1. Overall c. Average score at endpoint (FACT‐Sz, high score=good).
2.4 Social functioning: 2. Emotional recognition ‐ average score (TRACS, high score = good)
For this outcome we found a single study involving 49 participants. This outcome had no subgroups. We did not find evidence of a clear difference between 'yoga' and 'standard care' (1 RCT, n = 49, MD ‐4.30, 95% CI ‐10.07 to 1.47; Analysis 1.17).
1.17. Analysis.
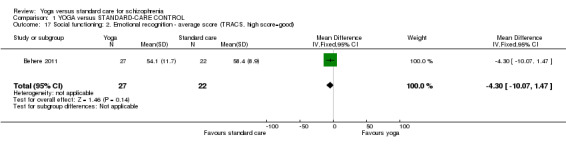
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 17 Social functioning: 2. Emotional recognition ‐ average score (TRACS, high score=good).
3. Adverse events
We identified one study relevant to this outcome (total n = 94, Lin 2013), the data from which we divided into two subgroups.
3.1 Any serious
This result was not estimable, as no serious adverse effects were reported for the yoga or standard‐care group (Analysis 1.18).
1.18. Analysis.
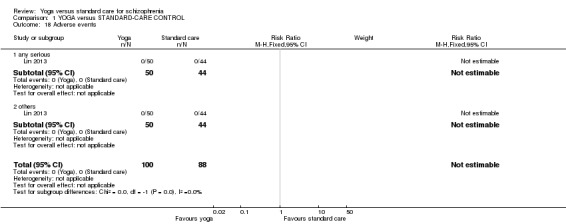
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 18 Adverse events.
3.2 Others
This result was also not estimable, as no other adverse effects were reported for the yoga or standard‐care group (Analysis 1.18).
4. Quality of life
Quality of life was measured using three different outcome measures (WHOQOL‐BREF, SF‐36, and EQ‐5D). As outlined in the Differences between protocol and review section, since total scores were not reported for most of these measures, subscale measures were reported in this review.
4.1 Quality of life: 1. Average change (WHOQOL‐BREF, greater increase = good)
We identified one study relevant to this outcome (Visceglia 2011). This outcome (total n = 18) consisted of the following subgroups: physical health, psychological, social relationships, and environment (Analysis 1.19).
1.19. Analysis.
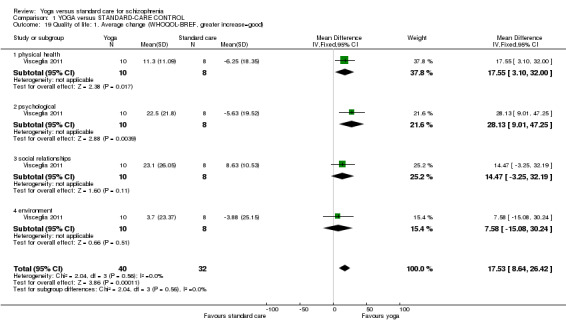
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 19 Quality of life: 1. Average change (WHOQOL‐BREF, greater increase=good).
4.1.1 Physical health
We found evidence of a clear difference between 'yoga' and 'standard care' within this subgroup (1 RCT, n = 18, MD 17.55, 95% CI 3.10 to 32.00).
4.1.2 Psychological
We found evidence of a clear difference between 'yoga' and 'standard care' within this subgroup (1 RCT, n = 18, MD 28.13, 95% CI 9.01 to 47.25).
4.1.3 Social relationships
There was not a clear difference between 'yoga' and 'standard care' within this subgroup (1 RCT, n = 18, MD 14.47, 95% CI ‐3.25 to 32.19).
4.1.4 Environment
There was not a clear difference between 'yoga' and 'standard care' within this subgroup (1 RCT, n = 18, MD 7.58, 95% CI ‐15.08 to 30.24).
4.2 Quality of life: 2. Average change (SF‐36, greater increase = good)
We found evidence of a clear difference between 'yoga' and 'standard care' (1 RCT, n = 120, MD 10.10, 95% CI 3.06 to 17.15). This outcome had moderate levels of heterogeneity (Chi2 = 1.46; df = 1.0; P = 0.23; I2 = 31%). This outcome consisted of the subgroups physical health and mental health (Analysis 1.20).
1.20. Analysis.
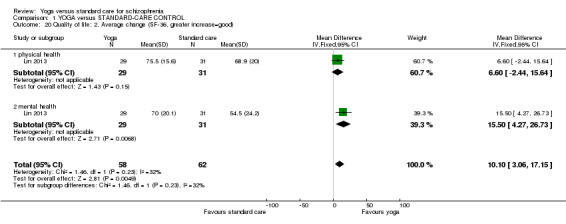
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 20 Quality of life: 2. Average change (SF‐36, greater increase=good).
4.2.1 Physical health
We found one trial relevant to this subgroup, with a total of 60 participants. There was not a clear difference between 'yoga' and 'standard care' within this subgroup (1 RCT, n = 60, MD 6.60, 95% CI ‐2.44 to 15.64).
4.2.2 Mental health
We found one trial relevant to this subgroup, with a total of 60 participants. We found evidence of a clear difference between 'yoga' and 'standard care' within this subgroup (1 RCT, n = 60, MD 15.50, 95% CI 4.27 to 26.73).
4.2.2.1 Quality of life: 3. Average endpoint index scale (EQ‐5D, high score = good)
For this outcome we found two relevant studies (total n = 99). This outcome had no subgroups. There was not a clear difference between 'yoga' and 'standard care' (2 RCTs, n = 99, MD 0.05, 95% CI ‐0.06 to 0.16). Substantial heterogeneity was found for this outcome (Chi2 = 3.15; df = 1; P = 0.08; I2 = 68%; Analysis 1.21).
1.21. Analysis.
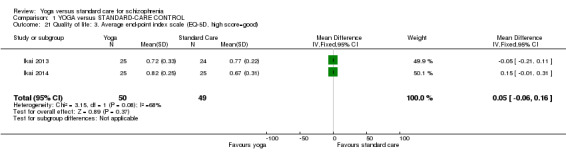
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 21 Quality of life: 3. Average end‐point index scale (EQ‐5D, high score=good).
4.2.2.2 Leaving the study early: Lack of engagement ‐ people lost to follow‐up ‐ short term (low = good)
We included data from all eight studies in this outcome. This outcome had no subgroups. There was a clear difference between 'yoga' and 'standard care' (8 RCTs, n = 457, RR 0.91, 95% CI 0.60 to 1.37). This outcome had reasonably low levels of heterogeneity (Chi2 = 7.88; df = 5.0; P = 0.16; I2 = 37%; Analysis 1.22).
1.22. Analysis.
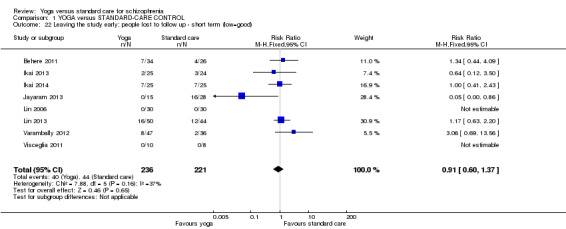
Comparison 1 YOGA versus STANDARD‐CARE CONTROL, Outcome 22 Leaving the study early: people lost to follow up ‐ short term (low=good).
Discussion
Summary of main results
1. YOGA versus STANDARD‐CARE CONTROL for schizophrenia
Overall there was a lack of good‐quality evidence for this comparison with few data available; only eight studies could be included in the review, and the quality of evidence was rated mostly low to moderate. For most outcomes we could not pool data, with many outcomes using data from a single study only. Differences in terms of outcomes used also precluded pooling of data, which significantly weakens the impact of results. For instance, the variable of mental health was measured using four different scales. Even when the same scale was used, for example the PANSS, data were presented in a variety of ways: binary (improved, not improved), average change score, and average endpoint score.
The available data showed a clear difference in favour of the yoga intervention for mental health and social functioning, but this must be interpreted with due caution as outcome results were generated from a single small study (n = 83) that presented data dichotomously, and was ranked high risk for a number of sources of bias including incomplete outcome assessment and selective reporting.
As total scores for the outcome quality of life were lacking, we took the pragmatic approach of including subscore outcomes, which were presented continuously. There was no definitive measure of 'physical health', so we included the physical‐health subscore of a quality‐of‐life measure. Bearing these caveats in mind, some small positive changes were shown for quality of life and physical health, but the clinical implication of these changes is not known. Only one study reported and evaluated adverse effects, finding no events.
Data from eight studies showed a clear difference in favour of yoga for numbers of participants leaving the study early.
Notably, this review was subject to a considerable number of missing outcomes, which included global state, change in cognition, costs of care, effect on standard care, service intervention, effect on disability, and activities of daily living, which weakens the implications of the review.
Overall, differing measures and heterogeneity in data presentation precluded pooling of data for all outcomes included. Despite some small positive changes, data was too sparse to confidently encourage or dissuade the practice of yoga as an adjunct therapy to continuing standard care for people with schizophrenia.
Overall completeness and applicability of evidence
1. Completeness
Evidence was certainly relevant, but overall data were too sparse to extensively address the objectives of this review. The search strategy identified 8 trials involving 459 participants comparing yoga to standard‐care control.
A significant limitation of this review was the absence of medium‐ or long‐term outcomes. It is therefore unknown whether small positive changes seen in some outcomes deteriorated, maintained, or even continued along the same trends, so further studies reporting medium‐ and particularly long‐term follow‐up are necessary. Indeed, the short‐term follow‐up may influence the directness of evidence given the chronic nature of schizophrenia. Even within short‐term outcomes, no data were provided for the outcomes of change in global state, cognition, costs of care, effect on activities of daily living, disability, or service use.
Data extraction for outcomes of interest was also significantly limited by the use of more than one measurement tool for the same outcome and lack of agreement in data presentation, even within the same scale measurement. Other factors were minimal presentation of binary data, significant loss to follow‐up, and lack of intention‐to‐treat analysis. For these reasons, the central question underlying the review, whether yoga confers any advantage over standard care, cannot be confidently answered.
2. Applicability
Entry criteria for studies were mixed, some including psychosis of unknown origin and some a more homogeneous schizophrenia‐only population. Most studies specified diagnostic criteria, but these varied between studies. Settings varied between studies and included a mix of inpatients and outpatients.
One study was conducted in a 'Western' setting (USA), with the rest based in India (three), China (two), and Japan (two). Yoga may be a more accepted mainstream practice in India in particular, where this practice originated, so the wider applicability of these studies is therefore unknown. As resources to obtain yoga training may be more available in some low and middle‐income countries, the implication of these, albeit weak, results may find greater resonance in some non‐'Western' settings.
We excluded any combinations of yoga and other adjunctive practices such as counselling in an attempt to make the 'purest' comparison of yoga versus standard care. By its nature, however, yoga is a heterogeneous practice, intuitively adapted based on factors such as the energy needs of the group as well as training of the yoga instructor. Even though most yoga practice was reasonably consistent between studies and could be usefully grouped into the same core components of 'loosening exercises', yoga postures (asanas), breathing exercises, and relaxation for a duration of 45 minutes to 1 hour in total, exposure varied considerably from 8 sessions to a maximum of 36. This difference in exposure means optimum yoga 'dosage' to effect results is unclear.
All eight studies were relatively small, with 459 participants in total. The small size of these studies significantly weakens the quality of evidence presented, therefore any demonstrated difference between the yoga intervention and control outcomes should be considered in this context. Further studies with a larger sample size should be considered.
Quality of the evidence
See also Risk of bias in included studies and Table 1.
The quality of available data limits our confidence in the small positive changes shown in this review. There was poor consensus between studies on which outcome variables to use, and even when the same outcome measure was used (for example PANSS), this was presented in multiple ways, which precluded pooling of data. Only one study reported the outcome adverse effects, with no reported incidence of this outcome. No other study referred to any adverse effects or harm associated with yoga, but sample sizes were small in general, which may contribute to true harms, or indeed benefits, going undetected.
The quality of the current evidence was low to moderate based on GRADE. One of the fundamental prerequisites of an RCT methodology is random sequence allocation, however this was unclear in three of the eight included studies, raising concern about selection bias. It was accepted due to the nature of the intervention that study participants and yoga practitioners could not be blind to the intervention. In three of the studies performance bias may have been problematic, as blinding of the outcome assessor was unclear. We rated four studies as at high risk of attrition bias due to incomplete outcome data, and in one further study this was unclear. We noted a number of other sources of biases in all studies. Most worryingly, perhaps, is in two of the studies, one of which the main positive findings of improvement in mental state and social functioning were based (Varambally 2012), one of the study authors may have had a potential conflict of interest due to their affiliation with the yoga school involved in the study. These biases should be borne in mind when interpreting the results of this study.
Seven out of eight studies were data extracted from full‐text journal publications. One of these, Lin 2006, relied on translation from an outside source. One further study, Lin 2013, was data extracted from a number of sources pertaining to the same original study, none of which were published as yet in a full‐text journal publication.
Due to the inability to pool data, we generated no I2 for most outcomes. Heterogeneity as judged visually, by examination of the I2 and P value associated with Chi2, was present in one variable only: change in the negative subscale of the PANSS (measured by average score at endpoint). Notably, when we removed the data from Lin 2013 from the pooled analysis, homogeneity was restored, so these values may have been an anomaly or incorrectly reported.
Potential biases in the review process
We aimed the search for studies to be as extensive as possible, and we made every attempt to include global studies and not just those published in the English language. There remains the possibility that there may be other unpublished trials of intervention that the review authors do not currently have access to. This means that the review authors may unwittingly have perpetuated a publication bias.
Agreements and disagreements with other studies or reviews
A previous systematic review, Vancampfort 2012a, covered a similar topic, and the results appear to be broadly similar to the findings of this review. This specifically relates to the reduction of total positive and negative syndrome scale total scores comparing yoga to waiting‐list control. A slightly more recent review, Cramer 2013, echoed findings of this review, namely finding moderate evidence for short‐term effects of yoga on quality of life specifically.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia
A limited number of small studies favoured yoga over standard care, but this was for limited outcomes with short‐term follow‐up. There is currently insufficient evidence to determine whether yoga is beneficial or not for people with schizophrenia. People with schizophrenia may wish to be involved in future trials to help answer this question.
2. For clinicians
There is insufficient evidence in this review to support prescribing yoga for people as an add‐on to standard care for the management of schizophrenia. This uncertainty is not backed up by cost‐benefit analyses, as none of the studies reported financial outcomes. More, larger, and long‐term RCTs reporting these outcomes are therefore necessary.
3. For policymakers
There is insufficient evidence from this review to support a policy change.
Implications for research.
1. General
1.1 Better reporting
These studies did not really follow Guidance of Consolidated Standards of Reporting Trials (CONSORT) statement (Moher 2001). Close adherence to this statement would make future studies more informative for clinicians and people with schizophrenia. Clear description of randomisation, allocation concealment, and blinding would have helped to assure that bias had been minimised.
Aside from two studies with no attrition or withdrawals, two studies performed intention‐to‐treat analysis and used the last‐observation‐carried forward method (Ikai 2013; Ikai 2014), although these studies did not comprehensively report 16‐week follow‐up data, which weakens implications of findings. There should be uniformity in data reporting, for example PANSS and greater use of dichotomous outcomes so that data can be pooled. Unfortunately, due to poor data reporting, we were unable to use most data in the trials.
1.2 Confusion of publication
A requirement to register each trial through a single publicly accessible system would reassure participants that their data would be widely available. Unique study numbers from a single system would prevent the duplication of study reporting and minimise confusion arising from multiple publications referring to the same study.
2. Specific
2.1 Reviews
Many excluded trials could find a place in new or existing systematic reviews. A number of other yoga comparisons need to be made before the completion of a full overview of the effects of yoga for schizophrenia (Table 2).
1. Comparisons relevant to other reviews suggested by excluded and included studies.
| Intervention | Plus | Control | Participants | Reference tag | Proposed relevant Cochrane review |
| Yoga | Nil | Exercise | People with schizophrenia |
Bhatia 2014; Duraiswamy 2007; Lin 2013; Manjunath 2013; Varambally 2012; JPRN‐UMIN000013746 |
Yoga versus non‐standard care for schizophrenia |
| Counselling | Standard care | ||||
| Motivational and feedback session | |||||
| Nil | Caregivers of people with schizophrenia | Varambally 2013 | ‐ | ||
| Yoga | Non‐standard care | People with schizophrenia | SLCTR‐2013‐008*; Paikkatt 2012; Vancampfort 2011a; Xie 2006 | Yoga as part of a package of care versus non‐standard care | |
| Chlorpromazine | Nil | Placebo | Mahal 1976; Ramu 1999 | Chlorpromazine versus placebo for schizophrenia | |
| 'Tagara' (local drug with antipsychotic properties) and 'Brahmyadiyoga' (an herbal compound) | Nil | Chlorpromazine | Chlorpromazine versus herbal compounds for schizophrenia |
* This particular study used yoga combined with relaxation exercises, breathing exercises, body movement exercises, basic acting exercises, Alexander technique, theatre games, exercise ''to build self confidence'', creative work using props, and use of music to enhance creativity and moods.
2.2 Trials
More independent and well‐planned, conducted, and reported RCTs of longer duration are needed to address important, unanswered, and clinically relevant outcomes to be able to extensively answer the question if yoga is a useful add‐on to standard care for people with schizophrenia. Even though we included eight studies in this review, we could present few clinically meaningful results. As a result, we do not really know the medium‐ and long‐term consequences of using this popular treatment and have almost no information that we can confidently trust, on even a few short‐term outcomes.
We are aware that enormous thought and care goes into the design of a trial, and the fact that all trials were from the previous 10 years suggests that this research is in its infancy. Although yoga is an ancient practice, it seemingly has not been subjected to the rigour of scientific scrutiny until relatively recently or extensively explored for use in clinical populations. Indeed the yoga literature seems certainly to lag behind 'exercise' literature. There are many lessons we can therefore learn in terms of important questions from the exercise literature.
Firstly, bearing in mind the chronicity and impact of schizophrenia, the most important outcomes would surely be real‐world, patient‐based meaningful outcomes such 'do you feel better', effect on disability, and activities of daily living, as well as other outcomes outlined in Table 3. These outcomes should be presented dichotomously where possible. Randomisation from a waiting list would seem a sensible study design to provide 'real‐world' evidence.
2. Design of a future study.
| Methods | Allocation: randomised (clearly described). Blinding: single blind (outcomes assessor). Duration: minimum 1 year. Design: parallel. Setting: outpatient and inpatient settings. |
| Participants | Diagnosis: people with a clinical diagnosis of schizophrenia using DSM‐IV criteria. History: patients randomised from waiting list and referred to research staff. N = 300 Age: > 18 years. Sex: males and females. Inclusion criteria: DSM‐IV diagnosis of schizophrenia, age 18 years or older. Exclusion criteria: Presence of physical disability or illness that precludes participation in yoga intervention. |
| Interventions | 1. Yoga: the yoga intervention should be clearly described and consist of the following components: (i) shithileekarana vyayama (loosening exercises) for approximately 10 minutes, (ii) yoga postures (asanas) for approximately 20 minutes, (iii) breathing exercises and relaxation techniques for approximately 20 minutes using a manualised protocol, yoga programme for 12 weeks, 3 times weekly, follow‐up at 6 months and 1 year, yoga delivered by a trained yoga instructor, meditation not included. 2. Standard‐care control. All groups stable pharmacotherapy. |
| Outcomes | Mental state (binary outcomes). Relapses (binary outcomes). Quality of life (binary outcomes). Disability (binary outcomes). Activities of daily living (binary outcomes). Costs: cost of services, cost of care. Adverse events related to yoga (number and type of injuries). Service outcomes: days in hospital, time attending outpatient psychiatric clinic. |
| Notes | Adherence should be logged with participants expected to adhere to 70% to 75% of scheduled sessions. |
DSM‐IV ‐ Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
We also do not know the neurobiological and physiological changes associated with yoga practice. Future trials should examine dose‐response relationships and include process‐evaluation evaluating aspects such as adherence and compliance. The type of yoga should be investigated, as well as the usefulness of adjunctive elements such as meditation. Useful data on effect of service intervention, standard care, and cost‐effectiveness must be a research priority. Studies should be based on sample‐size calculations with blinded assessors. Even though this is not well established in the exercise literature, it would be useful to exclude the Hawthorne effect to design studies so control participants have equitable attention and face‐to‐face feedback in the yoga intervention. Research should examine effectiveness in different stages of the disease, from prodromal, first episode, early illness to persistent illness, and effect on particular problems such as negative symptoms and treatment‐resistance illness.
1. General
Further research should be carried out in order to clarify evidence presented here that shows beneficial effects of yoga in treating schizophrenia. Further trials should include more participants and assess meaningful medium‐ and long‐term outcomes to improve the quality and completeness of data reporting.
2. Specific
Yoga is well‐established in the Western world as a holistic and spiritual approach to general physical and mental well‐being, however its use and benefits in specific disorders has not yet been fully determined. Since the current available evidence, as highlighted in this review, offers little support for the effectiveness of the practice of yoga in addition to standard‐care treatment of schizophrenia, more well‐designed, conducted, and reported RCTs are needed.
Notes
None.
Acknowledgements
The review authors would like to thank Professor Clive E Adams for the opportunity to perform this study and for his advice and help throughout this review from start to finish, as well as the staff of the Cochrane Schizophrenia Group editorial base, particularly Claire Irving, for their support in the writing of the protocol. The Cochrane Schizophrenia Group Editorial Base in Nottingham, UK, produces and maintains standard text for use in the Methods section of their reviews, and the review authors have used this text as the basis of what appears here, adapting it as required.
The review authors would also like to thank the Trials Search Co‐ordinators of the Cochrane Schizophrenia Group, Samantha Roberts and Farhad Shokraneh, who developed and ran the search. Finally, we thank Jun Xia, Yiu Tsung Ho, and Ting‐ya Yang for their assistance with the translations and Susan Stricklin for peer review.
We generated parts of this review using RevMan HAL v 4.2. You can find more information about RevMan HAL here.
Data and analyses
Comparison 1. YOGA versus STANDARD‐CARE CONTROL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mental state: 1. Overall a. Not improved (total PANSS) | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.55, 0.88] |
| 2 Mental state: 1. Overall b. Average change score (PANSS, low=good) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐26.33 [‐37.71, ‐14.95] |
| 3 Mental state: 1. Overall c. Average endpoint score (PANSS, low=good) | 3 | 176 | Mean Difference (IV, Fixed, 95% CI) | ‐10.74 [‐15.39, ‐6.09] |
| 4 Mental state: 2. Negative symptoms a. Not improved (PANSS) | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.90] |
| 5 Mental state: 2. Negative symptoms b. Average score at endpoint (PANSS, low=good) | 5 | 243 | Mean Difference (IV, Fixed, 95% CI) | ‐1.92 [‐3.06, ‐0.78] |
| 6 Mental state: 2. Negative symptoms c. Average score at endpoint (SANS, low=good) | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 4.80 [0.94, 8.66] |
| 7 Mental state: 2. Negative symptoms d. Average change score (PANSS, greater decrease=good) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐9.87, ‐2.13] |
| 8 Mental state: 3. Positive symptoms a. Not improved (PANSS) | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.79, 1.22] |
| 9 Mental state: 3. Positive symptoms b. Average score at endpoint (PANSS, low=good) | 5 | 243 | Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐2.50, ‐0.42] |
| 10 Mental state: 3. Positive symptoms c. Average score at endpoint (SAPS, low=good) | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [0.80, 4.80] |
| 11 Mental state: 3. Positive symptoms d. Average change score (PANSS, greater decrease=good) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐5.27 [‐9.19, ‐1.35] |
| 12 Mental state: 4. Depresssive symptoms: a. Average score (CDS, greater decrease=good) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐4.86, ‐0.94] |
| 13 Mental state: 5. Resilience a. Average score at end‐point (Resilience scale, high=good) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐11.27, 17.67] |
| 14 Social functioning: 1. Overall a. Not improved (total SOFS) | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 1.00] |
| 15 Social functioning: 1. Overall b. Average score at endpoint (SOFS, high score=good) | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | 0.64 [‐2.12, 3.39] |
| 16 Social functioning 1. Overall c. Average score at endpoint (FACT‐Sz, high score=good) | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | 4.26 [0.81, 7.71] |
| 17 Social functioning: 2. Emotional recognition ‐ average score (TRACS, high score=good) | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐10.07, 1.47] |
| 18 Adverse events | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 any serious | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 others | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Quality of life: 1. Average change (WHOQOL‐BREF, greater increase=good) | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 17.53 [8.64, 26.42] |
| 19.1 physical health | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 17.55 [3.10, 32.00] |
| 19.2 psychological | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 28.13 [9.01, 47.25] |
| 19.3 social relationships | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 14.47 [‐3.25, 32.19] |
| 19.4 environment | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 7.58 [‐15.08, 30.24] |
| 20 Quality of life: 2. Average change (SF‐36, greater increase=good) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 10.10 [3.06, 17.15] |
| 20.1 physical health | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 6.60 [‐2.44, 15.64] |
| 20.2 mental health | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 15.5 [4.27, 26.73] |
| 21 Quality of life: 3. Average end‐point index scale (EQ‐5D, high score=good) | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.06, 0.16] |
| 22 Leaving the study early: people lost to follow up ‐ short term (low=good) | 8 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.60, 1.37] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Behere 2011.
| Methods | Allocation: randomised. Blinding: single blind (raters blind to group status). Duration: 4 months, assessed at baseline, 2 months and 4 months. Design: parallel. Setting: outpatient services of the Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, India. | |
| Participants | Diagnosis: schizophrenia (DSM IV).
History: patients on stabilised antipsychotic medications for 6 weeks or longer before recruitment.
N = 91.
Age: 18 ‐ 60 years.
Sex: 32M,12F
Inclusion criteria: CGI score less than or equal to 3 as assessed by treating psychiatrist. Exclusion criteria: any comorbid psychiatric disorder, medical or neurological illness. |
|
| Interventions | 1.Yoga: 1 month yoga training from a trained yoga instructor developed from a particular school (Swami Vivekananda Yoga Anusandhana Samsthana), followed by 3 months of self practice at home. The techniques consisted of the following components: (i) shithileekarana vyayama (loosening exercises) for 10 minutes, (ii) yoga postures (asanas) for approximately 20 minutes, (iii) breathing exercises for 18 minutes, (iv) quick relaxation techniques for 3 minutes, meditation was not included. (N = 34). 2. Standard‐care control: participants did not receive any add‐on intervention. (N = 26) 3. Exercise: 1 month exercise training from a trained yoga instructor followed by 2 months practice of exercises at home. This consisted of brisk walking, jogging, and exercises in standing and sitting postures and relaxation. No meditation included. Therapist: participant ratio not detailed. (N = 31)* Participants in all the 3 groups continued to receive stable dose of antipsychotic medications until the end of the study. |
|
| Outcomes | Mental state: PANSS. Social functioning: SOFS, emotional recognition (TRACS). Leaving the study early. |
|
| Notes | Same yoga intervention as Jayaram 2013 * Included only data from intervention groups 1 and 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''Using computer‐generated random numbers, 91 patients were allocated to three treatment groups''. Response: Low risk. |
| Allocation concealment (selection bias) | High risk | Quote: "The randomization was performed by one of the authors in the study (Dr JT)." Response: This could potentially be high risk as the order of allocation could be known, which could influence the allocation of participants to either intervention. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel delivering the yoga intervention will be aware they are undertaking or delivering the yoga intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ''The raters were blind to the status, and the raters were not involved in imparting yoga therapy or exercise''. Response: Low risk. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: ''The number of patients who completed the study and included in the final analysis was 27 in the Yoga group, 17 in the Exercise group and 22 in the Waitlist group''. Response: Extent of withdrawal broadly similar between yoga and control group, but rated as high risk as not all participants randomised were included in the final analysis (7 in Yoga group, 14 in Exercise group, and 4 in Waitlist group ‐ 27.5% overall). |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | High risk | Funding: not stated. One of the authors may be invested in intervention due to affiliation with Swami Vivekananda Yoga Anusandhana Samsthana. Yoga for this study was developed from this school. |
Ikai 2013.
| Methods | Allocation: randomised. Blinding: single blind (raters blind to group status). Duration: 8‐week intervention with 8‐week follow‐up. Design: parallel. Setting: Department of Neuropsychiatry, Yamanashi Prefectural Kita Hospital, Yamanashi, Japan. | |
| Participants | Diagnosis: schizophrenia (F20‐F29 according to the International Classification of Diseases, 10th edition).
History: outpatients receiving the same medication for the previous 8 weeks, registered in the day‐care centre.
N = 49.
Age: > 18 years.
Sex: 32M, 18F.
Inclusion criteria: 18 years or older, receiving the same medication for the previous 8 weeks, and registered in the day‐care centre. Exclusion criteria: incapable of providing consent, current substance or alcohol abuse/dependence. |
|
| Interventions | 1. Yoga therapy: 8 weeks training from ''one of the investigators who held a master's degree of yoga''. The techniques consisted of the following components: (i) warming‐up and loosening‐up exercises for 7 minutes (including guided meditation for 3 minutes), (ii) yoga postures (asanas) for 28 minutes, (iii) relaxation for 7 minutes, (iv) breathing exercises for 8 minutes (n = 25). 2. Standard care: a weekly regular day‐care programme consisting of social‐skills training and psycho‐education. Both yoga and standard‐care groups were registered in the regular day‐care programme and could avail ''ambulatory treatment'' that consisted of non‐structured clinical management such as pharmacotherapy, and very brief psychotherapy by participant's treating psychiatrist (from personal communication with study author 06.08.15). Medications were kept constant by participants' treating psychiatrists throughout the study period unless a change was clinically indicated. |
|
| Outcomes | Mental state: PANSS Social functioning: FACT‐Sz Quality of life: EQ‐5D Leaving the study early. Unable to use: Postural sway, flexibility, electrocardiogram, DIEPSS ‐ not listed in protocol |
|
| Notes | Note: same study location and procedure and a number of common authors as Ikai 2014, but the current study took place between June 2012 and October 2012. 16‐week follow‐up data included for yoga group only. 8‐week data included for standard‐care group; assumed 8‐week data for this group was stable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''the randomization list without any stratification or blocks was made with a use of computer program''. Response: Low risk. |
| Allocation concealment (selection bias) | Low risk | Quote: ''opaque envelopes were opened after the baseline assessment'' Response: Likely to be adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel delivering the yoga intervention will be aware they are undertaking or delivering the yoga intervention. Response: Unclear risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ''All assessments were performed by trained psychiatrists who were blind to a subjects' allocation and were not involved in the yoga therapy''. Response: Low risk. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16‐week follow‐up data included for yoga group only. 8‐week data included for standard‐care group. Response: High risk. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed were reported. |
| Other bias | Unclear risk | Adherence of groups to day‐care programme and ''ambulatory treatment'' is not specified. |
Ikai 2014.
| Methods | Allocation: randomised. Blinding: single blind (raters blind to group status). Duration: 8‐week intervention with 8‐week follow‐up. Design: parallel. Setting: Department of Neuropsychiatry, Yamanashi Prefectural Kita Hospital, Yamanashi, Japan. | |
| Participants | Diagnosis: schizophrenia (F20‐F29 according to the International Classification of Diseases, 10th edition).
History: outpatients receiving the same medication for the previous 8 weeks, registered in the day‐care centre.
N = 50.
Age: > 18 years.
Sex: 32M, 17F.
Inclusion criteria: 18 years or older, receiving the same medication for the previous 8 weeks, and registered in the day‐care centre. Exclusion criteria: incapable of providing consent, current substance or alcohol abuse/dependence. |
|
| Interventions | 1. Yoga therapy: 8 weeks hatha yoga in the hospital gymnasium training from ''one of the investigators who held a master's degree of Hatha yoga''. The techniques consisted of the following components: (i) warming‐up and loosening‐up exercises for 7 minutes (including guided meditation for 3 minutes), (ii) yoga postures (asanas) for 28 minutes, (iii) relaxation for 7 minutes, (iv) breathing exercises for 8 minutes; participants were asked to self practice at home (n = 25). 2. Standard care: a weekly regular day‐care programme consisting of social‐skills training and psycho‐education. Both yoga and standard‐care groups were registered in the regular day‐care programme and could avail ''ambulatory treatment'' that consisted of non‐structured clinical management such as pharmacotherapy, and very brief psychotherapy by participant's treating psychiatrist (from personal communication with study author 6 August 2015). Medications were kept constant by participants' treating psychiatrists throughout the study period unless a change was clinically indicated. |
|
| Outcomes | Mental State: 25‐Item Resilience Scale, PANSS Social functioning: FACT‐Sz Quality of life: EQ‐5D Leaving the study early Unable to use: Blood markers, DIEPSS ‐ not specified in protocol. |
|
| Notes | Note: same study location and procedure and a number of common authors as Ikai 2013, but the current study took place between November 2012 and April 2013. Follow‐up was at 16 weeks, but as no data were reported for the standard‐care group and the FACT‐Sz and PANSS in the yoga group, only the 8‐week follow‐up data was included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''A simple randomization list with no special stratification or blocks was made by using a computer program''. Response: Likely to be adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: ''the randomization was performed by using sealed envelopes prepared by physicians at Yamanashi Prefectural Kita Hospital who were not involved in this study'' Response: Likely to be adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel delivering the yoga intervention will be aware they are undertaking or delivering the yoga intervention. Response: Unclear risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: ''All assessments were performed by trained psychiatrists who were blind to the patients' allocations and were not involved in the yoga therapy''. Response: Low risk. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up was at 16 weeks, but no data reported for the standard‐care group and the FACT‐Sz and PANSS in the yoga group. Response: High risk. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed were reported. |
| Other bias | Unclear risk | Adherence of groups to day‐care programme and ''ambulatory treatment'' is not specified. |
Jayaram 2013.
| Methods | Allocation: randomised. Blinding: unclear, no details given. Duration: 1 month, assessed at baseline and at 1 month. Design: parallel. Setting: outpatient and inpatient services of the Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, India. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV), confirmed by 2 independent psychiatrists. History: patients on stabilised antipsychotic medications for 6 weeks or longer before being recruited. N = 43. Age: 18 ‐ 45 years. Sex: 19M, 8F. Inclusion criteria: stable dose antipsychotics for > 6 weeks prior to recruitment, Clinical Global Impression score < 3. Exclusion criteria: psychoactive substance abuse within past 6 months or substance abuse within past month, comorbid neurological or medical disorders. |
|
| Interventions | 1. Yoga: 1 month of specific yoga therapy delivered by a professional yoga therapist. The techniques consisted of the following components: (i) shithileekarana vyayama (loosening exercises) for 10 minutes, (ii) yoga postures (asanas) for approximately 20 minutes, (iii) breathing exercises for 18 minutes, (iv) quick relaxation techniques for 3 minutes, meditation was not included. (N = 15). 2. Standard‐care control: no additional intervention. (N = 28). Participants in both groups continued on unchanged dosage of antipsychotic medication. |
|
| Outcomes | Social functioning: SOFS, TRACS.
Leaving the study early. Unable to use: Physiological measures: plasma oxytocin levels ‐ not specified in protocol. |
|
| Notes | Same yoga intervention as Behere 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ''Subjects were randomized to either yoga group (N = 15) or wait list group (N = 28)" Response: No details given of how randomisation sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants will be aware they are undertaking yoga intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: ''In the yoga group, all 15 patients completed baseline and follow‐up assessments. In the wait‐list group, 12 patients completed both the assessments and were included in the final analysis.'' Response: Systematic differences between groups, as no attrition in yoga group, but 57%* (n = 16) attrition in waiting‐list group that were not included in analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed were reported. |
| Other bias | Unclear risk | *57% data unaccounted for in waiting‐list group, no missing data in yoga group, therefore total loss is less than 50%, but results may be prone to bias. Funding not stated. Trialists not clearly invested in the interventions. |
Lin 2006.
| Methods | Allocation: randomised (no further information given). Blinding: not stated. Duration: 2 months, assessed at baseline and postintervention. Design: parallel. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (n = 42), affective psychosis (n = 12), neurotic disorder (n = 5), reactive psychosis (n = 1) (diagnosed by the Department of Psychiatry in Shantou University).
History: duration of illness of 5.3 ± 4.4 years, range 2 months to 20 years.
N = 60.
Age: 19 ‐ 52 years.
Sex: 24M, 36F.
Inclusion criteria: not stated. Exclusion criteria: complications of the heart, brain and kidney diseases. |
|
| Interventions | 1. Yoga therapy: yoga in addition to occupational and drug therapy, yoga was taught individually to each participant by 2 trainers (one for modelling, one teaching meditation verbally) for 1 hour in total, 4 times a week for 2 months, consisted of a 10‐minute warm‐up, 40‐minute sessions of yoga from ''easy level to hard level'', and 10 minutes of relaxation, included meditation. (N = 30). 2. Standard‐care control: receiving occupational and drug therapy (N = 30). Participants continued on unchanged dosage of antipsychotic medication. |
|
| Outcomes | Leaving the study early. Unable to use: Assessment of adverse events: 10‐item scale ‐ not published in peer‐reviewed journal and also modified by authors. |
|
| Notes | Not specified if yoga trainers were certified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from translation: ''Randomly allocated 60 patients into control group (30) and treatment group (30)''. Response: Participants were randomised into 2 groups, but randomisation method was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants are aware of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals, attrition, or loss to follow‐up mentioned. |
| Selective reporting (reporting bias) | Low risk | All measured outcomes were reported. |
| Other bias | Unclear risk | Funding: not stated, trialists not clearly invested in the interventions. Analysis of this report relied upon translation from an outside source. |
Lin 2013.
| Methods | Allocation: randomised. Blinding: single blind (outcomes assessor). Duration: 12 weeks, assessed at baseline, postintervention at 12 weeks and 18 months. Design: parallel. Setting: recruited from the Early Assessment Service for Young People with Psychosis Program (EASY) in 3 outpatient clinics in Hong Kong. | |
| Participants | Diagnosis: schizophrenia, diagnosis based on DSM‐IV criteria.
History: female outpatients with non‐affective functional psychosis within the first 5 years of their illness.
N = 140.*
Age: 18 ‐ 55 years.
Sex: 0M, 140F.
Inclusion criteria: schizophrenia based on DSM‐IV criteria, schizoaffective disorder, schizophreniform psychosis, brief psychotic disorders, psychosis not otherwise specified, and delusional disorder, duration of illness less than 5 years (including 5 years). Exclusion criteria: severe physical illness (myocardial infarction, hypertension, fracture, spinal problem), seizure disorders, intellectual disability or comorbid substance dependence, unstable psychotic symptoms, known pregnancy or other contraindication to MRI, a history of brain trauma or organic brain disease, known history of intellectual disability or special‐school attendance. |
|
| Interventions | 1. Yoga:12 weeks of hatha yoga therapy delivered by certified yoga instructor (3 sessions per week, each 40/50 minutes per session, which included (i) breathing control (10 minutes), (ii) warming up (10 minutes), (iii) yoga postures (asanas) for 30 minutes, (iv) relaxation for 10 minutes, 5 to 10 participants per class, no meditation included, expected adherence to the yoga intervention was > 70%, average yoga class attendance was 51.1%. (N = 50).* 2. Standard‐care control: treatment as usual. (N = 44).* Participants in both groups continued on an unchanged dosage of medication as much as possible, more than a 25% change in dosage in the first 6 weeks after commencement of the intervention was not permitted. 3. Aerobic: 12 weeks (3 sessions per week, each 1 hour) of treadmill walking for 15‐20 minutes and stationary cycling for 25‐30 minutes. (N = 46).** |
|
| Outcomes | Mental state: PANSS.
Quality of life: SF‐36. Adverse effects: routine reporting of physical adverse events. Leaving the study early. Unable to use: Cognitive functioning: (verbal learning, assessed by Hong Kong List Learning test; working memory, assessed by the digit span test; attention and concentration, assessed by the letter cancellation test Q score; cognitive flexibility, assessed by the Stroop Color and Word Test), as no reported total end scale measures. MRI: not listed as an outcome. Physical fitness: (VO2max test), as < 50% data reported (33/94 = 35%). Balance: (SEBT), as < 50% data reported (33/94 = 35%). Flexibility: (Sit‐and‐Reach Test), as < 50% data reported (33/94 = 35%). Standing balance test: no data reported. Body perception and drug adherence measure: (Figure Rating Scale, cognitive attitude towards body size, compliance rating scale, drug attitude inventory), as no data reported. DXA: no data reported. UKU rating scale: no reported total end scale measure. 18‐month follow‐up data, as ''subjects from the wait‐list control group had received a compensated 12‐week yoga or exercise class at some point between T2 and T3'', which was systematically different from yoga group. |
|
| Notes | "Psychosis not otherwise specified" in 13 participants (32.5%) of the yoga group and 15 participants (39.5%) of the waiting‐list control group. *Of 140 randomised participants, 16 withdrew before starting intervention, and it was not clear to which group they were randomised. Of the 16 participants who withdrew, assumed 5 participants each from yoga and control groups and 6 from aerobic exercise group. Data extracted from 4 sources; 2 abstracts, 1 dissertation, and 1 ClinicalTrials.gov protocol. One further dissertation was sourced that contained long‐term follow‐up data, but this revealed that a "compensatory" yoga or exercise programme was offered to the control group at some point between the 12‐week and 18‐month time points. As this systematically negated the control‐group condition, we did not include this long‐term follow‐up data in our review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''A randomization list was created using a random number generator. The random list had a block size of 12 (i.e. for every 12 subjects, 4 would be assigned to the yoga group, 4 to the aerobic group and 4 to the control group''. Response: Low risk. |
| Allocation concealment (selection bias) | Low risk | Quote: ''The randomization list was concealed from research staff involved in recruitment, assessment and intervention.'' Response: Low risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | ''Two investigators will do the yoga training and aerobic exercise without knowing the assessment results.'' Participants aware of group assignment. Response: Unclear risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ''Two research assistants will be well‐trained and recruited to do the assessment, and remains blind to the treatment allocation.'' Response: Low risk. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ''A total of 140 patients were recruited and randomized, 16 of them withdrew before starting intervention. Amongst 124 participants, 9 were excluded from final analysis because of changed diagnosis during study period. 95 of 115 participants completed 12‐week study''. Response: High risk, as not all participants randomised were included in the analysis. Unclear if withdrawals were the same in each study arm as no details of group assignment given of the 16 participants who withdrew. |
| Selective reporting (reporting bias) | High risk | Body perception and drug adherence listed as outcomes in protocol but no results supplied. |
| Other bias | Unclear risk | Funding not stated, but trialists not clearly invested in the interventions. |
Varambally 2012.
| Methods | Allocation: randomised. Blinding: single blind (outcomes assessor). Duration: 4 months, assessed at baseline, 1 month and 4 months. Design: parallel. Setting: National Institute of Mental Health and Neurosciences, India. | |
| Participants | Diagnosis: schizophrenia confirmed by a psychiatrist according to DSM‐IV criteria. History: outpatients on follow‐up. N = 120. Age: of those who completed trial: yoga group: 32.8 (+/‐10.0), waiting list: 33.6 (+/‐ 9.5) years. Sex: 56M, 64F. Inclusion criteria: receiving antipsychotic medication without change in dosages in the last 3 months, rated as moderately symptomatic with a score of 3 or more on Clinical Global Impression. Exclusion criteria: ECT in the past 3 months. | |
| Interventions | 1. Yoga: yoga delivered by a certified yoga trainer from a particular school (Swami Vivekananda Yoga Anusandhana Samsthana). Consisted of shithileekarana vyayama (loosening exercises) for 10 minutes, yoga postures (asanas) for approximately 20 minutes, breathing exercises for 8 minutes, and a quick relaxation technique for 3 minutes, in total 45‐minute session daily for 1 month, no meditation included, expected adherence to the yoga intervention was > 75%. (N = 47).
2. Standard‐care control: receiving no yoga intervention. (N = 36). 3. Exercise: brisk walking, jogging, and exercise in standing. (N = 37). No changes were made to particpant medication status unless absolutely needed. |
|
| Outcomes | Mental state: PANSS. Social functioning: SOFS. Unable to use: Adverse events: extrapyramidal symptoms rating scale (no data reported). |
|
| Notes | Yoga developed from same school as Behere 2011 and Jayaram 2013. Included only data from intervention groups 1 and 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "one investigator (JT) uninvolved in the treatments or assessment generated random numbers". Response: Lacking detail if sequence‐generation strategy was adequate. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "subject's allocation to one of these groups was kept concealed and only ascertained after consent and before he/she was to be randomized." Response: Unclear risk, as concealment strategy was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: ''only the social worker and the yoga therapist were informed to start the corresponding intervention.'' Response: Unclear risk, participants as well as social worker and yoga therapist were aware of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ''the rater was unaware of group allocation’’. Response: Low risk. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: ''some did not turn up at the 4th month follow‐up and therefore final sample was smaller; 39, 22 and 34 in yogasana, exercise and waiting list groups''. Response: High risk, as more participants withdrew from yoga group (17%) than control group (5%), and 21% overall not followed up and not included in analysis. |
| Selective reporting (reporting bias) | High risk | Extrapyramidal symptoms scale (adverse events) listed as an outcome but no data reported. |
| Other bias | High risk | Funding not stated. One of the authors may be invested in intervention due to affiliation with Swami Vivekananda Yoga Anusandhana Samsthana. Yoga for this study was developed from this school. |
Visceglia 2011.
| Methods | Allocation: randomised. Blinding: single blind (outcomes assessor). Duration: 8 weeks. Design: parallel, assessed at baseline and at 8 weeks. Setting: state‐hospitalised psychiatric inpatients at the Bronx Psychiatric Center New York, United States. | |
| Participants | Diagnosis: schizophrenia (Axis 1 diagnosis).
History: inpatients for at least 6 months.
N = 18.
Age: 20 ‐ 60 years.
Sex: 12M, 6F.
Inclusion criteria: patients had to be cleared by the medical director of the hospital. Exclusion criteria: medical director excluded 1 participant with a history of falls. |
|
| Interventions | 1. Yoga therapy: including varying amounts of pranayama (breathing exercises), warm‐ups, yoga postures (asanas), and yoga nidra (deep relaxation) that matched the energy level, attentional ability, and mood state of the group for 45 minutes, twice weekly for 8 weeks, 5 participants per class, no meditation was included. (N = 10). 2. Standard‐care control: receiving no yoga intervention. (N = 8). Any medication changes were noted. |
|
| Outcomes | Mental state: PANSS. Quality of life: WHOQOL‐BREF. Leaving the study early. |
|
| Notes | Pilot study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''subjects were randomly assigned, by computer‐generated random number table" to intervention groups to either YT or WL. Response: Low risk. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants will be aware if undertaking any intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ''psychopathology was assessed using PANSS, administered by a mental health counselor trainee (SL) who had clinical experience in the psychiatric population who was blind to group status ... the same rater assisted participants' completion of the WHOQOL‐BREF''. Response: Low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Unclear risk | Quote: ''the fact that several participants had multiple diagnoses (such as mild mental retardation, borderline personality disorder, and antisocial personality disorder) may have complicated the interpretation of research outcomes''. Response: Unclear risk ‐‐ multiple diagnoses of participants may have compromised the validity of the findings. Funding not stated but trialists not clearly invested in the interventions. |
BDNF ‐ brain‐derived neurotrophic factor CDS ‐ Calgary Depression Scale CG ‐ control group CGI ‐ Clinical Global Impression CSP ‐ clinical stabilometric platform CVRR ‐ coefficient of variation R‐R interval DIEPSS ‐ Drug‐Induced Extrapyramidal Symptoms Scale DSM‐IV ‐ Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition DXA ‐ dual‐energy X‐ray absorptiometry EASY ‐ Early Assessment Service for Young People with Psychosis Program ECT ‐ electro‐convulsive therapy EPS ‐ extrapyramidal symptoms EQ‐5D ‐ EuroQoL 5 dimensions classification system FACT‐Sz ‐ Functional Assessment for Comprehensive Treatment of Schizophrenia GQOLI‐74 ‐ generic quality of life inventory 74 HDL ‐ high‐density lipoprotein IDEAS ‐ Indian Disability Evaluation and Assessment Scale LDL ‐ low‐density lipoprotein MRI ‐ magnetic resonance imaging PANSS ‐ Positive and Negative Syndrome Scale SAA ‐ salivary alpha amylase SEBT‐ Star Excursion Balance Test SOFS ‐ Socio‐Occupational Functioning Scale TRACS ‐ TRENDS Accuracy Score TRENDS ‐ Tool for Recognition of Emotions in Neuropsychiatric Disorders UKU ‐ Udvalg for Kliniske Undersøgelser VAS ‐ visual analogue scale WHOQOL‐BREF ‐ World Health Organization Quality of Life BREF questionnaire WT ‐ wait‐list YT ‐ yoga therapy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bhatia 2012 | Allocation: not randomised. |
| Duraiswamy 2007 | Allocation: randomised. Participants: moderately ill people with schizophrenia attending outpatient and inpatient services. Interventions: yoga versus exercise. Control group was not participants receiving standard care. |
| JPRN‐UMIN000013746 | Allocation: randomised. Participants: people with schizophrenia. Interventions: yoga therapy versus ''A simple exercise'', not standard‐care control. |
| Mahal 1976 | Allocation: ''double blind''. Participants: people with schizophrenia. Interventions: "Tagara" (local drug with antipsychotic properties) and "Brahmyadiyoga" (an herbal compound) versus chlorpromazine versus placebo, not yoga. |
| Manjunath 2013 | Allocation: randomised. Participants: people with schizophrenia or related disorders. Interventions: yoga versus exercise therapy, not standard‐care control. |
| Paikkatt 2012 | Allocation: randomised. Participants: people with schizophrenia. Intervention: yoga plus motivational and feedback session versus waiting list, not yoga alone. |
| Ramu 1999 | Allocation: ''double blind''. Participants: people with schizophrenia. Interventions: "Tagara" (local drug with antipsychotic properties) and "Brahmyadiyoga" (an herbal compound) versus chlorpromazine versus placebo, not yoga. |
| SLCTR‐2013‐008 | Allocation: randomised. Participants: people with schizophrenia. Interventions: yoga combined with relaxation exercises, breathing exercises, body movement exercises, basic acting exercises, Alexander technique, theatre games, exercise ''to build self confidence'', creative work using props, use of music to enhance creativity and moods versus comparison group receiving standard care, which does not include any of the above, not yoga as a stand‐alone intervention versus standard‐care control. |
| Vancampfort 2011a | Allocation: randomised. Participants: people with schizophrenia. Interventions: yoga and aerobic exercise versus control, not yoga alone. |
| Varambally 2013 | Allocation: randomised. Participants: caregivers of people with schizophrenia, not sufferers of schizophrenia. |
| Xie 2006 | Allocation: randomised. Participants: people with schizophrenia. Intervention: yoga plus counselling versus standard care, not yoga alone. |
Characteristics of ongoing studies [ordered by study ID]
Bhatia 2014.
| Trial name or title | Protocol to evaluate the impact of yoga supplementation on cognitive function in schizophrenia: a randomised controlled trial |
| Methods | Allocation: randomised. Blinding: single blind (outcomes assessor). Duration: yoga training daily for 1 hour for 21 days. Design: parallel. Setting: Department of Psychiatry, Post‐Graduate Institute of Medical Education and Research, Dr. Ram Manohar Lohia Hospital, New Delhi, India. |
| Participants | Diagnosis: people with a clinical diagnosis of schizophrenia using DSM‐IV criteria. History: Patients with a clinical diagnosis of schizophrenia were referred to research staff and screened by research personnel. They were then given information on study goals and procedures. N = 234. Age: > 18 years. Sex: males and females. Inclusion criteria: DSM‐IV diagnosis of schizophrenia, age 18 years or older, resident of Delhi. Exclusion criteria: Prior participation in yoga study in research centre, mental ''retardation'' sufficient to impact trial understanding, presence of comorbid conditions that could worsen with exercise, neurological illness that may cause cognitive impairment independent of schizophrenia, presence of physical disability or illness for which yoga or physical exercise are contraindicted. |
| Interventions | 1. Yoga: includes postures or yoga postures (asanas) and pranayama (breathing protocols) using a manualised protocol. 2. Exercise: ''simple'' physical exercise for 1 hour daily, 15 minutes of brisk walking followed by light exercise. 3. Standard care: treatment as usual. All groups stable pharmacotherapy where possible. |
| Outcomes | Cognitive function (Trail Making Test and University of Pennsylvania Computerized Neurocognitive Battery). Clinical severity and daily functioning (Independent Living Skills Survey). Mental state (Schedule for the Assessment of Positive Symptoms and Schedule for the Assessment of Negative Symptoms). General function (Global Assessment of Function). |
| Starting date | August 2010. |
| Contact information | Triptish Bhatia, GRIP‐NIH project, Room #30, Department of Psychiatry, Park Street, Post‐graduate Institute of Medical Education and Research, Dr. Ram Lohia Hospital, New Delhi‐110001, India, Tel +91 11 23404363 bhatiatriptish@yahoo.co.in |
| Notes | Contacted for study data 10 March 2015, no reply. |
Differences between protocol and review
We have changed the title to reflect the interventions presented in the protocol. The protocol stated the control intervention was standard care only, so we have reflected this in the title.
We modified the objective between the protocol and review. The protocol originally stated the objective was to identify if yoga could be used as an effective adjuvant to standard care for the management of schizophrenia. We amended the objective to be more specific to the comparison between yoga and standard care.
We expanded our definition of standard care to acknowledge that trials often use 'wait‐list' as part of their standard care.
We amended some details in the background information slightly to reflect more recent literature.
We amended some of the outcomes between the protocol and review to reflect Cochrane Schizophrenia Group presentation and wording of outcomes, and added two further secondary objectives: disability and activities of daily living. We felt in retrospect that these outcomes were important given the persistent and all‐encompassing nature of schizophrenia. As no relapse data were available, we did not present 'relapse' data in the 'Summary of findings' table, presenting 'leaving the study early' data instead.
The protocol stated that total scores only would be included with the exception of the PANSS. In the review we also included domain scores of the WHOQOL‐BREF (physical health, psychological, social relationships, and environment) and of the SF‐36 (physical health, mental health), as the majority of quality‐of‐life scores in this review did not include a total score. Given the importance of quality of life as a measure in schizophrenia, we felt domain scores were also relevant to include.
Contributions of authors
Julie Broderick: modifying the protocol, extracting data, undertaking the syntheses, and writing the review. Abigail Knowles and Jonathan Chadwick: developing the protocol and assisting in writing the review. Davy Vancampfort: assisting in writing and discussions on the review.
Sources of support
Internal sources
The University of Nottingham, UK.
Nottinghamshire Healthcare NHS Trust, UK.
External sources
-
Health Research Board (HRB), Ireland.
This review was conducted as part of a series of reviews which were funded by a Cochrane Fellowship Grant (CFT‐2014‐880) from the Health Research Board, Ireland (HRB).
Declarations of interest
Julie Broderick: No conflicts of interest are known at the time of writing the review. Abigail Knowles: No conflicts of interest are known at the time of writing the review. Jonathan Chadwick: No conflicts of interest are known at the time of writing the review. Davy Vancampfort: No conflicts of interest are known at the time of writing the review.
New
References
References to studies included in this review
Behere 2011 {published data only}
- Behere RV, Arasappa R, Jagannathan A, Varambally S, Venkatasubramanian G, Thirthalli J, et al. Effect of yoga therapy on facial emotion recognition deficits, symptoms and functioning in patients with schizophrenia. Acta Psychiatrica Scandinavica 2011;123(2):147‐53. [DOI] [PubMed] [Google Scholar]
Ikai 2013 {published data only}
- Ikai S. Postural sway changes after yoga in schizophrenia. http://bit.ly/1NQhTMU [Date Accessed: January 20, 2013].
- Ikai S, Uchida H, Suzuki T, Tsunoda K, Mimura M, Fujii Y. Effects of yoga therapy on postural stability in patients with schizophrenia‐spectrum disorders: a single‐blind randomized controlled trial. Journal of Psychiatric Research 2013; Vol. 47, issue 11:1744‐50. [DOI] [PubMed]
- Ikai S, Uchida H, Suzuki T, Tsunoda K, Mimura M, Fujii Y. Effects of yoga therapy on postural stability in schizophrenia: A single‐blind randomized controlled trial. Schizophrenia Bulletin 2013; Vol. 39:S351. [DOI] [PubMed]
Ikai 2014 {published data only}
- Ikai S, Suzuki T, Uchida H, Saruta J, Tsukinoki K, Fujii Y, et al. Effects of weekly one‐hour hatha yoga therapy on resilience and stress levels in patients with schizophrenia‐spectrum disorders: an eight‐week randomized controlled trial. Journal of Alternative and Complementary Medicine 2014; Vol. 20, issue 11:823‐30. [DOI] [PubMed]
Jayaram 2013 {published data only}
- Jayaram N, Varambally S, Behere RV, Venkatasubramanian G, Arasappa R, Christopher R, et al. Effect of yoga therapy on plasma oxytocin and facial emotion recognition deficits in patients of schizophrenia. Indian Journal of Psychiatry 2013; Vol. 55, issue 7 Suppl:S409‐14. [DOI] [PMC free article] [PubMed]
Lin 2006 {published data only}
- Lin Y, Wang J, Xie J. Effectiveness of yoga alleviating side effects caused by antipsychotic medications [瑜珈健身法减轻抗精神病药物不良反应的效果观察]. Journal of Nursing Science [护理学杂志] 2006;21(3):56‐7. [Google Scholar]
Lin 2013 {published data only}
- Lin J. The Impacts of Aerobic Exercise and Mind‐Body Exercise (Yoga) on Neuro‐cognition and Clinical Symptoms in Early Psychosis: A Single‐Blind Randomized Controlled Clinical Trial (Dissertation). The University of Hong Kong, 2013. [Google Scholar]
- Lin J. Yoga and exercise in psychosis (YEP). http://ClinicalTrials.gov/show/NCT01207219 [Date Accessed: October 20, 2012].
- Lin J, Lam M, Chiu C, Tse M, Khong PL, Chan C, et al. The impacts of yoga and exercise on neuro‐cognitive function and symptoms in early psychosis. Schizophrenia Bulletin 2011;37:171. [Google Scholar]
- Lin JJ, Lee HM, Chan KW, Chang WC, Su W, Honer WG, et al. The impacts of aerobic exercise and mind‐body exercise (yoga) on neuro‐cognition and clinical symptoms in early psychosis a single‐blind randomized controlled clinical trial. Schizophrenia Research 2014; Vol. 153, issue Suppl 1:S260.
- Pansy CCL. The Long‐Term Effects of Yoga and Aerobic Exercise on Cognitive Function and Clinical Symptoms in Early Psychosis: A Follow‐up Randomized Control Trial (Dissertation). The University of Hong Kong, 2014. [Google Scholar]
Varambally 2012 {published data only}
- Varambally S, Gangadhar BN, Thirthalli J, Jagannathan A, Kumar S, Venkatasubramanian G, et al. Therapeutic efficacy of add‐on yogasana intervention in stabilized outpatient schizophrenia: Randomized controlled comparison with exercise and waiting list. Indian Journal of Psychiatry 2012; Vol. 54, issue 3:227‐32. [DOI] [PMC free article] [PubMed]
Visceglia 2011 {published data only}
- Visceglia E, Lewis S. Yoga therapy as an adjunctive treatment for schizophrenia: A randomized, controlled pilot study. Journal of Alternative and Complementary Medicine 2011;17(7):601‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bhatia 2012 {published data only}
- Bhatia T, Agarwal A, Shah G, Wood J, Richard J, Gur RE, et al. Adjunctive cognitive remediation for schizophrenia using yoga: an open, non‐randomized trial. Acta Neuropsychiatrica 2012;24(2):91‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duraiswamy 2007 {published data only}
- Duraiswamy G, Thirthalli J, Nagendra HR, Gangadhar BN. Yoga therapy as an add‐on treatment in the management of patients with schizophrenia ‐ a randomized controlled trial. Acta Psychiatrica Scandinavica 2007;116(3):226‐32. [DOI] [PubMed] [Google Scholar]
JPRN‐UMIN000013746 {published data only}
- JPRN‐UMIN000013746. Effects study of yoga therapy on the association of mental illness with metabolic disorders, including the carbonyl stress. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000013746 (accessed March 2015).
Mahal 1976 {published data only}
- Mahal AS, Ramu NG, Chaturvedi DD. Double blind controlled study of brahmyadiyoga and tagara in the management of various types of unmada (schizophrenia). Indian Journal of Psychiatry 1976;18(4):283‐92. [Google Scholar]
Manjunath 2013 {published data only}
- Manjunath RB, Varambally S, Thirthalli J, Basavaraddi IV, Gangadhar BN. Efficacy of yoga as an add‐on treatment for in‐patients with functional psychotic disorder. Indian Journal of Psychiatry 2013; Vol. 55, issue 7 Suppl:S374‐8. [DOI] [PMC free article] [PubMed]
Paikkatt 2012 {published data only}
- Paikkatt B, Singh AR, Singh PK, Jahan M. Efficacy of yoga therapy on subjective well‐being and basic living skills of patients having chronic schizophrenia. Industrial Psychiatry Journal 2012; Vol. 21, issue 2:109‐14. [DOI] [PMC free article] [PubMed]
Ramu 1999 {published data only}
- Ramu MG, Chaturvedi DD, Venkataram BS, Shankara MR, Leelavathy S, Janakiramiah N, et al. A DOUBLE BLIND controlled study on the role of brahmyadiyoga and tagara in jirnomada (chronic schizophrenia). Ayurvedic Management of Unmada (Schizophrenia). Central Council for Research in Ayurveda and Siddha, 1999:77‐88. [Google Scholar]
SLCTR‐2013‐008 {published data only}
- SLCTR‐2013‐008. Developing social skills, communication skills and self confidence through dance, drama and yoga in patients with long term mental illness at NIMH, Angoda. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=SLCTR/2013/008 (accessed March 2015).
Vancampfort 2011a {published data only}
- Vancampfort D, Hert M, Knapen J, Wampers M, Demunter H, Deckx S, et al. State anxiety, psychological stress and positive well‐being responses to yoga and aerobic exercise in people with schizophrenia: A pilot study. Disability and Rehabilitation 2011;33(8):684‐9. [DOI] [PubMed] [Google Scholar]
Varambally 2013 {published data only}
- CTRI‐2011‐06‐001792. Efficacy of brief yoga programme for caregivers of out‐patients with schizophrenia. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=2838 [Date Accessed: May 20, 2011].
- Varambally S, Vidyendaran S, Sajjanar M, Thirthalli J, Hamza A, Nagendra HR, et al. Yoga‐based intervention for caregivers of outpatients with psychosis: a randomized controlled pilot study. Asian Journal of Psychiatry 2013;6(2):141‐5. [DOI] [PubMed] [Google Scholar]
Xie 2006 {published data only}
- Xie J, Lin YH, Guo CR, Chen F. Study on influences of yoga on quality of life of schizophrenic inpatients [瑜伽练习对精神分裂症住院患者生活质量的影响]. Nanfang Journal of Nursing [南方护理学报] 2006;13:9‐10. [Google Scholar]
References to ongoing studies
Bhatia 2014 {published data only}
- Bhatia T, Mazumdar S, Mishra NN, Gur RE, Gur RC, Nimgaonkar VL, et al. Protocol to evaluate the impact of yoga supplementation on cognitive function in schizophrenia: a randomised controlled trial. Acta Neuropsychiatrica 2014; Vol. 26, issue 5:280‐90. [DOI] [PMC free article] [PubMed]
Additional references
Addington 1994
- Addington D, Addington D, Schissel B. A depression rating scale for schizophrenics. Schizophrenia Research 1990;3(4):247‐51. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreasen 1983
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS). Iowa: University of Iowa, 1983. [Google Scholar]
Andreasen 1984
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS). Iowa: University of Iowa, 1984. [Google Scholar]
Bangalore 2012
- Bangalore NG, Varambally S. Yoga therapy for schizophrenia. International Journal of Yoga 2012;5(2):85‐91. [PUBMED: 22869990] [DOI] [PMC free article] [PubMed] [Google Scholar]
Behere 2008
- Behere RV, Raghunandan VN, Venkatasubramanian G, Subbakrishna DK, Jayakumar PN, Gangadhar BN. TRENDS: a Tool for Recognition of Emotions in Neuropsychiatric Disorders. Indian Journal of Psychological Medicine 2008;30:32‐8. [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite]. Therapie 1999;54(4):405‐11. [PUBMED: 10667106] [PubMed] [Google Scholar]
Brooks 1996
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37(1):53‐72. [DOI] [PubMed] [Google Scholar]
Bussing 2012
- Bussing A, Michalsen A, Khalsa SBS, Telles S, Sherman KJ. Effects of yoga on mental and physical health: a short summary of reviews. Evidence‐Based Complementary and Alternative Medicine 2012;2012:165410. [PUBMED: 23008738] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 1998
- Collins C. Yoga: intuition, preventive medicine, and treatment. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1998;27(5):563‐8. [PUBMED: 9773368] [DOI] [PubMed] [Google Scholar]
Cramer 2013
- Cramer H, Lauche R, Klose P, Langhorst J, Dobos G. Yoga for schizophrenia: a systematic review and meta‐analysis. BMC Psychiatry 2013;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Damodaran 2002
- Damodaran A, Malathi A, Patil N, Shah N, Suryavansihi, Marathe S. Therapeutic potential of yoga practices in modifying cardiovascular risk profile in middle aged men and women. Journal of the Association of Physicians of India 2002;50(5):633‐40. [PUBMED: 12186115] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town. Cape Town: The Cochrane Collaboration, 2000.
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Elkins 2005
- Elkins G, Rajab MH, Marcus J. Complementary and alternative medicine use by psychiatric inpatients. Psychological Reports 2005;96:163‐6. [DOI] [PubMed] [Google Scholar]
Feifel 2011
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. [DOI] [PubMed] [Google Scholar]
Fusar‐Poli 2014
- Fusar‐Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, et al. Treatments of negative symptoms in schizophrenia: meta‐analysis of 168 randomized placebo‐controlled trials. Schizophrenia Bulletin 2015;41(4):892‐9. [DOI: 10.1093/schbul/sbu170] [DOI] [PMC free article] [PubMed] [Google Scholar]
Global Burden of Disease Study 2013 Collaborators
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet 2015;386(9995):743‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hutton 2009
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. [DOI] [PubMed] [Google Scholar]
Jauhar 2014
- Jauhar S, McKenna PJ, Radua J, Fung E, Salvador R, Laws KR. Cognitive‐behavioural therapy for the symptoms of schizophrenia: systematic review and meta‐analysis with examination of potential bias. British Journal of Psychiatry 2014;204(1):20‐9. [DOI] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986. [Google Scholar]
Kern 2009
- Kern RS, Glynn SM, Horan WP, Marder SR. Psychosocial treatments to promote functional recovery in schizophrenia. Schizophrenia Bulletin 2009;35(2):347‐61. [PUBMED: 19176470] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leucht 2005
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] [DOI] [PubMed] [Google Scholar]
Leucht 2007
- Leucht S, Engel RR, Bauml J, Davis JM. Is the superior efficacy of new generation antipsychotics an artifact of LOCF?. Schizophrenia Bulletin 2007;33(1):183‐91. [PUBMED: 16905632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leucht 2013
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple‐treatments meta‐analysis. The Lancet 2013;382(9896):951‐62. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
McGrath 2008
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiologic Reviews 2008;30:67‐76. [PUBMED: 18480098] [DOI] [PubMed] [Google Scholar]
Mitchell 2013
- Mitchell AJ, Vancampfort D, Sweers K, Winkel R, Yu W, Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders. A systematic review and meta‐analysis. Schizophrenia Bulletin 2013;39(2):306‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285(15):1987‐91. [DOI] [PubMed] [Google Scholar]
Nielsen 2015
- Nielsen R, Levander S, Kjaersdam Telléus G, Jensen S, Østergaard Christensen T, Leucht S. Second‐generation antipsychotic effect on cognition in patients with schizophrenia—a meta‐analysis of randomized clinical trials. Acta Psychiatrica Scandinavica 2015;131(3):185‐96. [DOI] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Ross 2012
- Ross A, Friedmann E, Bevans M, Thomas S. Frequency of yoga practice predicts health: results of a national survey of yoga practitioners. Evidence‐Based Complementary and Alternative Medicine 2012;2012:983258. [PUBMED: 22927885] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saraswat 2006
- Saraswat N, Rao K, Subbakrishna DK, Gangadhar BN. The Social Occupational Functioning scale (SOFS): A brief measure of functional status in persons with schizophrenia. Schizophrenia Research 2006;81:301‐9. [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:359‐83. [Google Scholar]
Sherman 2012
- Sherman KJ. Guidelines for developing yoga interventions for randomized trials. Evidence‐Based Complementary and Alternative Medicine 2012:143271. [PUBMED: 23082079] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skevington, 2004
- Skevington SM, Lofty M, O'Connell KA. The World Health Organization's WHOQOL‐BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Quality of Life Research 2004;13(2):299‐310. [DOI] [PubMed] [Google Scholar]
Stahl 2007
- Stahl SM, Buckley PF. Negative symptoms of schizophrenia: a problem that will not go away. Acta Psychiatrica Scandinavica 2007;115:4‐11. [DOI] [PubMed] [Google Scholar]
Suzuki 2008
- Suzuki T, Uchida H, Nomura K, Takeuchi H, Nakajima S, Tanabe A. Novel rating scales for schizophrenia — Targeted Inventory on Problems in Schizophrenia (TIP‐Sz) and Functional Assessment for Comprehensive Treatment of Schizophrenia (FACT‐Sz). Schizophrenia Research 2008;106:328‐36. [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organistation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. [PubMed] [Google Scholar]
van der Gaag 2011
- Gaag M, Stant AD, Wolters KJK, Buskens E, Wiersma D. Cognitive–behavioural therapy for persistent and recurrent psychosis in people with schizophrenia‐spectrum disorder: cost‐effectiveness analysis. British Journal of Psychiatry 2011;198(1):59‐65. [DOI] [PubMed] [Google Scholar]
Van Os 2009
- Os J, Kapur S. Schizophrenia. The Lancet 2009;374(9690):635‐45. [PUBMED: 19700006] [DOI] [PubMed] [Google Scholar]
Vancampfort 2011b
- Vancampfort D, Probst M, Sweers K, Maurissen K, Knapen J, Hert M. Relationships between obesity, functional exercise capacity, physical activity participation and physical self‐perception in people with schizophrenia. Acta Psychiatrica Scandinavica 2011;123(6):423‐30. [DOI] [PubMed] [Google Scholar]
Vancampfort 2012a
- Vancampfort D, Vansteelandt K, Scheewe T, Probst M, Knapen J, Herdt A, et al. Yoga in schizophrenia: a systematic review of randomised controlled trials. Acta Psychiatrica Scandinavica 2012;126(1):12‐20. [PUBMED: 22486714] [DOI] [PubMed] [Google Scholar]
Vancampfort 2012b
- Vancampfort D, Probst M, Scheewe T, Knapen J, Herdt A, Hert M. The functional exercise capacity is correlated with global functioning in patients with schizophrenia. Acta Psychiatrica Scandinavica 2012;125(5):382‐7. [DOI] [PubMed] [Google Scholar]
Varambally 2012b
- Varambally S, Gangadhar BN. Yoga: a spiritual practice with therapeutic value in psychiatry. Asian Journal of Psychiatry 2012;5(2):186‐9. [PUBMED: 22813667] [DOI] [PubMed] [Google Scholar]
Wagnild 1993
- Wagnild GM, Young HM. Development and psychometric evaluation of the Resilience Scale. Journal of Nursing Measurement 1993;1(2):165‐78. [PubMed] [Google Scholar]
Ware 1993
- Ware JE, Snow KK, Kosinski M, Gandek B. SF‐36 Health Survey. Manual and Interpretation Guide. Boston: The Health Institute, New England Medical Center, 1993. [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]


