Abstract
The csrA gene encodes a small RNA-binding protein, which acts as a global regulator in Escherichia coli and other bacteria (T. Romeo, Mol. Microbiol. 29:1321–1330, 1998). Its key regulatory role in central carbon metabolism, both as an activator of glycolysis and as a potent repressor of glycogen biosynthesis and gluconeogenesis, prompted us to examine the involvement of csrA in acetate metabolism and the tricarboxylic acid (TCA) cycle. We found that growth of csrA rpoS mutant strains was very poor on acetate as a sole carbon source. Surprisingly, growth also was inhibited specifically by the addition of modest amounts of acetate to rich media (e.g., tryptone broth). Cultures grown in the presence of ≥25 mM acetate consisted substantially of glycogen biosynthesis (glg) mutants, which were no longer inhibited by acetate. Several classes of glg mutations were mapped to known and novel loci. Several hypotheses were examined to provide further insight into the effects of acetate on growth and metabolism in these strains. We determined that csrA positively regulates acs (acetyl-coenzyme A synthetase; Acs) expression and isocitrate lyase activity without affecting key TCA cycle enzymes or phosphotransacetylase. TCA cycle intermediates or pyruvate, but not glucose, galactose, or glycerol, restored growth and prevented the glg mutations in the presence of acetate. Furthermore, amino acid uptake was inhibited by acetate specifically in the csrA rpoS strain. We conclude that central carbon flux imbalance, inhibition of amino acid uptake, and a deficiency in acetate metabolism apparently are combined to cause metabolic stress by depleting the TCA cycle.
Acetate metabolism is probably important for the survival of Escherichia coli in the mammalian intestine, since a large amount of acetate (up to 70 mM) is produced through fermentation of carbohydrate by enteric anaerobes (5). In the laboratory, growth in liquid media, such as tryptone broth, leads to the secretion of ∼1 to 2 mM acetate in the late exponential phase. This acetate is subsequently taken up and metabolized (14, 36). No transporter for acetate has been identified (3, 34), although acetate uptake is saturable, suggesting that one may exist (11). Metabolism of acetate requires its activation to acetyl-coenzyme A (CoA). In E. coli, two pathways exist for the metabolic interconversion of acetate and acetyl-CoA. Acetyl-CoA synthetase (EC 6.2.1.1) (Acs pathway) produces acetyl-CoA directly from acetate, while acetate kinase (EC 2.7.2.1) and phosphotransacetylase (EC 2.3.1.8) (AckA-Pta pathway) produce acetyl phosphate as an intermediate. The Acs pathway is a catabolite-repressible, acetate-inducible, and high-affinity system, ideally suited for scavenging extracellular acetate present at physiological concentrations. On the other hand, the reversible AckA-Pta pathway functions primarily in generating acetate (2). The AckA-Pta pathway is considered to be constitutive (2, 14), while acs requires ςS, cyclic AMP receptor protein, and Fnr for full expression (36; unpublished observations).
Growth on acetate also requires the glyoxylate shunt, which bypasses the decarboxylation steps of the tricarboxylic acid (TCA) cycle, allowing net synthesis of biosynthetic precursors from acetate. The two enzymes of the glyoxylate shunt, isocitrate lyase (EC 4.1.3.1) and malate synthase (EC 4.1.3.2), are synthesized when E. coli is grown on acetate. These two proteins are encoded by aceA and aceB, respectively. These genes, together with aceK, which encodes isocitrate dehydrogenase (IDH) kinase/phosphatase, form the aceBAK operon. Expression of aceBAK is affected by several regulatory factors, including IclR, FadR, integration host factor, and ArcAB (reviewed in reference 4). Transcriptional regulation is not the only mechanism by which cells modulate flux through the glyoxylate shunt. Posttranslational modification of IDH (EC 1.1.1.42) by the bifunctional enzyme IDH kinase/phosphatase is also important in allowing the glyoxylate shunt to compete effectively with the TCA cycle (4).
Previously, we elucidated a novel bacterial global regulator, a small RNA-binding protein called CsrA (carbon storage regulator A) (17–19; reviewed in reference 27). In E. coli, CsrA represses a number of stationary-phase functions and activates certain exponential-phase functions. CsrA represses gluconeogenesis, glycogen biosynthesis, and glycogen catabolism; it activates glycolysis (30, 33, 44). Thus, a mutation in csrA exerts a dramatic effect on the flow of carbon into glycogen, causing mutant cells to accumulate ≥20-fold higher levels of glycogen than the wild-type cells. Glycogen can constitute greater than 50% of the dry weight of a csrA mutant harvested in the early stationary phase of growth (44). Glycogen synthesis in E. coli requires three essential enzymes, ADP-glucose pyrophosphorylase (EC 2.7.7.27), glycogen synthase (EC 2.4.1.21), and glycogen branching enzyme (EC 2.4.1.18), encoded by glgC, glgA, and glgB, respectively. These genes are clustered in two tandem operons, glgBX and glgCAP, which also include genes encoding the catabolic enzymes glycogen phosphorylase (EC 2.4.1.1) (glgP) and glycogen debranching enzyme (EC 3.2.1.−) (glgX) (reviewed in references 24 and 44). CsrA negatively regulates these three glg biosynthetic genes, glgP, and the monocistronic gene glgS, which stimulates glycogen synthesis by an undefined mechanism (30, 44). A mutation in csrA results in decreased adenylate energy charge and altered levels of the central carbon metabolites fructose-1,6-bisphosphate and phosphoenolpyruvate (33). Nevertheless, in a variety of media, the growth rate of a csrA mutant is indistinguishable from that of its isogenic parent.
In this study, we examined the regulatory role of the csrA gene in acetate metabolism, including its effects on the acetate activation pathways and the glyoxylate shunt. We demonstrated that the csrA gene positively regulates Acs and the glyoxylate shunt enzyme isocitrate lyase but does not affect Pta or certain TCA cycle enzymes. Interestingly, modest levels of acetate cause a dramatic growth defect in csrA rpoS mutant strains. Suppressor mutations that restored growth in the presence of acetate were observed in abundance and generally were found to decrease glycogen biosynthesis. Insight into the nature of this surprising stress caused by acetate was sought by examining genetic factors and metabolites that either favor or suppress the acetate-induced growth defect and the appearance of glycogen mutations. Our results indicate that the central problem is insufficient TCA cycle flux. This is apparently caused by greatly enhanced carbon flux away from the TCA cycle and towards glycogen biosynthesis in conjunction with decreased uptake of amino acids.
(The experiments described here were conducted in partial fulfillment of requirements for the Ph.D. degree by B. Wei at the University of North Texas Health Science Center at Fort Worth.)
MATERIALS AND METHODS
Chemicals and reagents.
Isopropyl-β-d-thiogalactopyranoside (IPTG), o-nitrophenol-β-d-galactopyranoside, l-amino acids, CoA, acetyl-CoA, acetyl phosphate, propionic acid, benzoic acid, 2,4-dinitrophenol, malate dehydrogenase (EC 1.1.1.37), β-nicotinamide adenine dinucleotide (β-NAD), β-NAD phosphate, and palmitic acid were purchased from Sigma Chemical Co. (St. Louis, Mo.). The palmitic acid was suspended in 10% Brij 58, saponified with KOH, and filter sterilized before use (38). Citrate synthase (EC 4.1.3.7) was from Boehringer Mannheim Biochemicals (Indianapolis, Ind.). The compound 5-bromo-4-chloro-3-indolyl-d-galactopyranoside (X-Gal) was from U.S. Biochemical Corp. (Cleveland, Ohio). 14C-radiolabeled l(U)-amino acids (54.2 mCi/mmol) were purchased from NEN Life Science Products, Inc. (Boston, Mass.). All other biochemical reagents were purchased from commercial sources and were of the highest quality available.
Bacterial strains and plasmids.
Table 1 lists the strains, plasmids, and phages that were used in this study, their sources, and the relevant genotypes. Strain designations that contain the prefix TR1-5 indicate that the wild-type csrA allele has been replaced by the TR1-5 mutant allele (csrA::kanR) by P1vir transduction.
TABLE 1.
Bacterial strains, plasmids and phages used in this study
| Strain, plasmid, or phage | Description | Source or reference |
|---|---|---|
| E. coli strains | ||
| BW3414 | ΔlacU169 rpoS(Am) | Barry Wanner |
| EG3-153 | BW3414 glg::kanR; a polar insertion in a 3.4-kb EcoRI fragment; ′glgBXC′ | This study |
| MG1655 | Prototrophic | Michael Cashel |
| CAG strains | Strain collection used for transduction mapping | 39 |
| CAG18450 | MG1655 (Tn10 at 76.5 min, near glgCAP) | 39 |
| CAG18500 | MG1655 (Tn10 at 92 min, near aceBAK) | 39 |
| RH106 | rpoS::Tn10 | 9 |
| MG1655rpoS | MG1655 rpoS::Tn10 (from RH106) | This study |
| W3110 | F− prototrophic | Richard Wolf |
| SS364 | W3110 Δ(lacZ)58 | This study |
| SS412 | SS364 pta::lacZ | This study |
| SS413 | SS364 ackA-pta::lacZ | This study |
| SS414 | SS364 acs::lacZ | This study |
| BWacs | BW3414 acs::lacZ | This study |
| CP875 | ΔlacX74 thi-1 thr-1(Am) leuB6 metF159(Am) rpsL136 λlacY | 26 |
| AJW803 | CP875 φ(Δacs::Km-1) | 14 |
| AJW-BW | BW3414 φ(Δacs::Km-1) | This study |
| CP911 | CP875 Δ(ackA pta hisJ hisP dhu) | 14 |
| PLWT | aceB::lacZ (at lac) zah-281::Tn10 | David LaPorte |
| BW3414ace | BW3414 aceB::lacZ zah-281::Tn10 | This study |
| K8-5m | aceA3 iclR13 | 42 |
| TR1-5 | csrA::kanR | 30 |
| Mutants isolated from TR1-5BW or TR1-5BWacs after acetate treatment: | ||
| B1-TR | TR1-5BW3414 glgB | |
| L9-1 | TR1-5BW3414; no glycogen | |
| MD-1 | TR1-5BWacs; medium glycogen | |
| BL-1 | TR1-5BWacs glgB | |
| LT-1 | TR1-5BWacs; no glycogen | |
| Plasmids | ||
| pUC19 | Cloning vector; Ampr | 45 |
| pPOP245 | glgA in pBR322; Tetr | 13 |
| pPRC1 | glgC in pUC19; Ampr | This study |
| pCZ3-3 | φglgC::lacZ in pMLB1034; Ampr | 28 |
| pRS415 | For construction of ′lacZ operon fusions; Ampr | 37 |
| Bacteriophages | ||
| P1vir | Strictly lytic P1; forms clear plaques | Carol Gross |
| λRS45 | For transferring ′lac fusions to single copy | 37 |
| λSS118 | λRS45 carrying pta::lacZ fusion | This study |
| λSS120 | λRS45 carrying ackA::lacZ fusion | This study |
| λSS121 | λRS45 carrying acs::lacZ fusion | This study |
Growth conditions.
Luria-Bertani medium (1% tryptone, 1% NaCl, 0.5% yeast extract, 0.2% glucose [pH 7.4] [22]) was used for routine laboratory cultures. Kornberg medium (1.1% K2HPO4, 0.85% KH2PO4, 0.6% yeast extract [pH 6.8], and 0.5% glucose for liquid or 1% glucose for solid medium) was used for evaluating the capacity of colonies to synthesize glycogen after being stained over iodine vapor (8, 18). Tryptone broth contained 1% tryptone and 0.5% NaCl, pH 7.4. Potassium morpholinopropane sulfate (MOPS) medium (23) supplemented with l-amino acids (2 mg/liter), nitrogenous bases (0.2 mM), and vitamins (0.01 mM) was used in studies of the glyoxylate shunt enzymes and in gene expression experiments. All organic acids were added as sodium salts. Media were supplemented with the following compounds as required: kanamycin, 100 μg/ml; tetracycline, 10 μg/ml; ampicillin, 100 μg/ml; and X-Gal, 40 μg/ml. Sodium acetate was added to the media at a final concentration of 50 mM unless otherwise indicated. Cultures were inoculated with 1 volume of overnight culture per 500 volumes of freshly prepared medium and were grown at 37°C on a gyratory shaker at 250 rpm.
Preparation of cell extracts.
Cell-free extracts for assays of isocitrate lyase, isocitrate dehydrogenase, and citrate synthase were prepared from the mid-exponential-phase cultures according to the method of Maloy et al. (20). Extracts for assays of acetate kinase and phosphotransacetylase were prepared from late-exponential-phase cultures according to the method of Brown et al. (2), except that a French pressure cell was used to disrupt cells instead of sonication.
Enzyme assays.
Acs and Pta were assayed according to the method of Brown et al. (2). The reaction of acetyl-CoA with oxaloacetate to form citrate was coupled to the oxidation of malate, with the concomitant production of NADH, which was monitored spectrophotometrically. Acs activity was determined in an ackA-pta genetic background to avoid interference by AckA and Pta. These reaction mixtures contained 100 mM Tris-HCl at pH 8.0, 0.5 mM MgCl2, 0.5 mM β-NAD, 0.5 mM CoA, 50 mM l-malate, 12.5 μg of crystalline malate dehydrogenase (5,300 U/mg of protein), 25 μg of crystalline citrate synthase (110 U/mg of protein), cell extract, 10 mM acetate, and 10 mM ATP for the Acs assay or 10 mM lithium acetyl phosphate instead of acetate and ATP for the Pta assay.
Isocitrate lyase was assayed at 25°C by the method of Maloy et al. (20). The reaction mixtures contained 100 mM potassium phosphate buffer (pH 7.0), 6 mM MgCl2, 4 mM phenyl hydrazine HCl, 12 mM cysteine HCl, 8 mM trisodium dl-isocitrate, and cell extract. IDH was assayed at 25°C according to the method of LaPorte et al. (15). The reaction mixtures contained 25 mM MOPS at pH 7.5, 250 μM β-NAD phosphate, 500 μM dl-isocitrate, 5 mM MgCl2, and cell extract.
Citrate synthase (EC 4.1.3.7) was assayed by the method of Stitt (41). The reaction mixtures contained 80.6 mM triethanolamine, 3 mM l-malate, 0.22 mM acetylpyridine-adenine dinucleotide, 12.9 kU of malate dehydrogenase/liter, 0.18 mM acetyl-CoA, and cell extract.
Values for enzyme activities were determined within the linear range with respect to the amount of cell extract added, which was experimentally determined for each enzyme. One unit of activity in each case is defined as 1 μmol of product generated per min under the given reaction conditions. Each activity was determined in at least two independent experiments to assure reproducibility.
Uptake of a mixture of amino acids.
Cells were grown in tryptone broth to exponential phase (optical density at 600 nm [OD600], approximately 0.3) and 0.95-ml aliquots were removed and added to sterile tubes containing 0.05 ml of 1 M sodium acetate or water. After a 5-min incubation (37°C; 250 rpm), 5 μCi of the labeled amino acid mixture (54.2 mCi/mmol) was added to each tube. At 0, 1, 2, and 4 min thereafter, 0.2 ml of culture was transferred to centrifuge tubes containing 1 ml of tryptone broth and 200-fold-excess unlabeled amino acids. The cells were immediately washed twice in tryptone broth, and the cell pellet was resuspended in 10 μl of SET buffer (20% sucrose, 50 mM EDTA [pH 8.0], 50 mM Tris-HCl [pH 8.0]) and lysed with 50 μl of lysis solution (0.2 M NaOH, 1% sodium dodecyl sulfate [SDS]). Radioactivity was determined by liquid scintillation counting, and the values were corrected for cell mass at the times of harvest (adjusted to an OD600 of 0.3) and for nonspecific binding (using 0-min time of incubation). Each experiment was conducted at least twice to assure reproducibility.
Protein and β-galactosidase assays.
Total cell protein was measured by the bicinchoninic acid method using bovine serum albumin as the standard (40). β-Galactosidase specific activity was assayed and calculated as described previously (28).
Genetic and molecular biology techniques.
P1vir transduction mapping of glg genotypes and standard molecular biology approaches, such as plasmid isolation and transformation, were conducted as described previously (29, 31).
Construction of gene fusions.
Single-copy chromosomal ′lacZ transcriptional fusions were constructed for ackA pta, pta, and acs in strain W3110 using pRS415 and bacteriophage λRS45 (37). Clone 405 of the Kohara library (12) was the source of a 2,079-bp PvuII-PvuII fragment containing the upstream region of the ackA-pta operon and 84 codons of ackA and of a 1,776-bp ScaI-HpaI fragment containing the putative pta promoter and 171 codons of pta (10). A 1,397-bp Klenow-filled XhoI-ClaI fragment from pSK122, which contained the acs promoter region, was used to construct the acs::lacZ fusion (36). Dideoxy nucleotide sequencing with M13/pUC forward primer, CCCAGTCACGACGTTGTAAAACG, was used to confirm the ′lacZ junctions present in the plasmid clones. The single-copy gene fusions present in the λ lysogens were verified by PCR amplification. The above-mentioned primer was used along with the primer ATCCGGCGATCATCTTCCACC, TATCCAGTTGTTTGAAGGCGCG, or TTTACCAATGGCTTCCATCGCG to amplify the pta, ackA, or acs fusion, respectively.
RESULTS
Growth of csrA rpoS strains is inhibited by acetate.
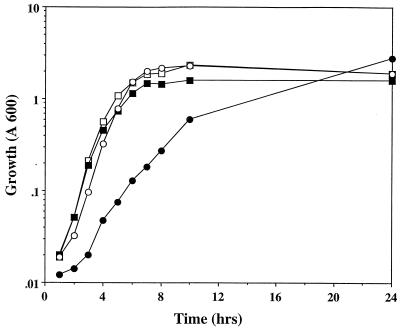
During initial studies to examine the possible regulatory role of csrA in acetate metabolism, we observed that csrA mutants grew poorly in liquid media containing acetate as a sole carbon source and exhibited an extended (up to several hours) and quite variable lag phase (data not shown). Further studies revealed that acetate was not only a poor carbon source for the csrA mutant but also selectively inhibited the growth of csrA mutants when added to rich media (Fig. 1). Whereas the parent strain, BW3414, which we now know carries an rpoS(Am) mutation, and the isogenic csrA::kanR mutant TR1-5BW3414 grew equally well in tryptone broth, 50 mM acetate specifically increased the doubling time of the mutant approximately twofold. In contrast, it had no effect on the growth of the parent. Although other poor sole carbon sources, such as pyruvate and palmitate, also supported slower growth of the csrA mutant strain relative to its parent, they did not inhibit growth on rich media (data not shown). Clearly, the effects of acetate in rich medium could not be explained by a simple inability to metabolize acetate, since sufficient carbon and energy for growth were already available in the rich medium.
FIG. 1.
Growth of csrA+ and csrA::kanR strains in 1% tryptone broth or tryptone broth plus acetate (50 mM). The open circles and squares represent BW3414 (csrA+) grown in tryptone broth with and without acetate, respectively. The solid circles and squares represent TR1-5BW3414 (csrA::kanR) grown in tryptone broth with and without acetate, respectively.
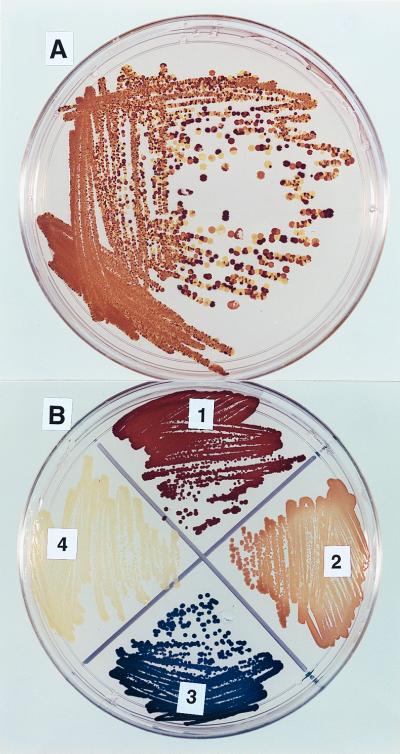
In order to examine the role of csrA in acetate metabolism more fully, we decided to further investigate the stress that is caused by acetate on the csrA mutant. After 24 h in the presence of acetate, each strain was streaked onto Kornberg agar and the resulting colonies were stained with iodine vapor to detect endogenous glycogen. When cultured in tryptone broth, csrA mutants (TR1-5BW3414) yielded colonies that stained a uniform dark brown (17). However, in tryptone plus acetate, they yielded primarily glycogen mutants of a variety of striking phenotypes. These included colonies that stained medium brown, yellow, or blue with iodine vapor (Fig. 2A). These phenotypes indicate moderate synthesis of glycogen, little or no synthesis of glycogen, or synthesis of unbranched glycogen due to the loss of glycogen branching enzyme (6), respectively. The resulting phenotypes were all stable upon repeated subculture (Fig. 2B). In numerous repetitions of this experiment, glycogen mutations always evolved from the csrA mutant grown in the presence of ≥25 mM acetate but never from either the parent strain treated with acetate or the csrA mutant grown in tryptone broth without added acetate. Similar results were observed using medium prepared with 1% Casamino Acids in place of 1% tryptone (data not shown).
FIG. 2.
Genetic instability of a csrA rpoS strain grown in the presence of acetate. (A) A 24-h culture of TR1-5BWacs was streaked directly from tryptone broth plus 50 mM sodium acetate onto Kornberg agar. The plate was incubated overnight at 37°C, and intracellular glycogen was stained with iodine vapor. (B) Stable glycogen mutants isolated from the plate shown in panel A were streaked onto Kornberg medium and stained with iodine vapor. Strain identities are as follows: 1, TR1-5BWacs; 2, MD-1; 3, BL-1; 4, LT-1.
By plating cells exposed to acetate and harvested at various time points along the growth curve, we observed that glycogen mutants accumulated during the exponential phase of growth (data not shown). Furthermore, the addition of acetate to mid-exponential-phase cultures (OD600 of ∼0.3) caused an immediate decrease in the growth rate (data not shown). These experiments demonstrated that acetate inhibits cell growth and not simply the exit from stationary phase. Incubation of strains for 2 h in the presence of 1 mM acetate, which itself did not result in the appearance of glycogen mutants, did not permit adaptation to 50 mM acetate stress (data not shown).
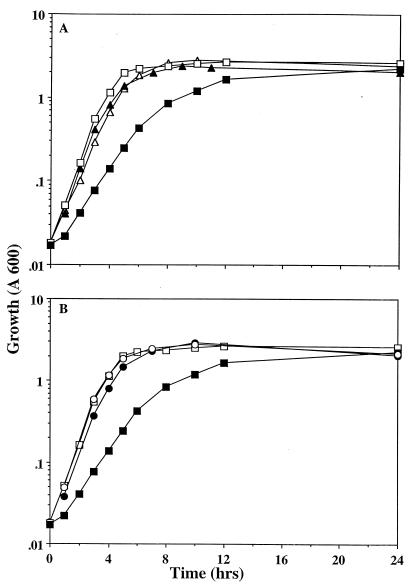
Growth of the mutants that failed to synthesize glycogen or synthesized unbranched glycogen was no longer inhibited by acetate (Fig. 3A), and mutants that accumulated intermediate levels of glycogen exhibited intermediate growth rates (data not shown). P1vir transduction of a transposon mutation (from strain EG3-153) which disrupts glycogen biosynthesis into the csrA mutant also generated a strain that was insensitive to acetate inhibition (Fig. 3B). Strain EG3-153 was isolated as a glycogen-deficient transposon mutant of BW3414 by using previously described methodology (30). The transposition mutation was localized by P1vir transduction and Southern blot analysis to a 3.4-kb EcoRI fragment containing ′glgBXCA′ (see reference 32 for the genomic restriction map). Together, these experiments clearly demonstrated that the growth defect in the presence of acetate occurred, at least in part, because of excessive glycogen synthesis by the csrA mutant.
FIG. 3.
Growth of glycogen-deficient strains in tryptone broth supplemented with acetate (50 mM). (A) Open and solid squares represent BW3414 (csrA+) and TR1-5BW3414 (csrA::kanR), respectively. The open and solid triangles represent two glycogen-deficient mutants, B1-TR and L9-1, respectively, isolated after exposure of TR1-5BW3414 to acetate. (B) Open and solid squares represent BW3414 (csrA+) and TR1-5BW3414 (csrA::kanR), respectively. The open and solid circles represent the glg transposon mutants EG3-153 and TR1-5EG3-153, respectively.
In an attempt to extend these experiments to other E. coli strains, a csrA mutant of the prototrophic strain MG1655 was observed to be genetically stable in the presence of acetate. Because the csrA mutant TR1-5BW3414 is now known to also contain an rpoS(Am) mutation, we constructed single and double csrA::kanR and rpoS::Tn10 derivatives of MG1655. The resultant double mutant grew poorly in the presence of acetate and evolved numerous glycogen mutants. In contrast, the single csrA or single rpoS mutants did not (data not shown). These experiments revealed that sensitivity to acetate requires defects in both csrA and rpoS.
Effects of other compounds.
A variety of other compounds were tested for the ability to inhibit growth of the csrA mutant strain TR1-5BW3414 in tryptone broth and to generate glycogen mutations. Pyruvate, α-ketoglutarate, succinate, fumarate, malate, palmitate, ribose, or glycerol did not yield glycogen mutations at 50 mM concentrations. In contrast, propionate was inhibitory and also caused glycogen mutants to accrue, although not as effectively as acetate. Whereas 100 mM propionate was required to give rise to a significant proportion (more that 70%) of apparent glycogen mutants, 50 mM yielded a significantly smaller proportion of glycogen mutants (≤10%), and 25 mM propionate yielded no mutants (data not shown). Benzoate inhibited the growth of csrA::kanR mutants; at concentrations greater than 25 mM, no growth occurred. However, benzoate failed to yield glycogen mutations at any concentration. Likewise, the uncoupling agent 2,4-dinitrophenol inhibited growth but did not yield glycogen mutants at any concentration tested. These experiments provided evidence that the observed acetate stress does not result from decreased intracellular pH or from depletion of ATP pools.
Mapping of glycogen mutations to three different loci.
P1vir transduction mapping was used to localize several of the glycogen mutations. All 16 independently isolated yellow-staining, glycogen-deficient mutations and two blue-staining, apparent branching enzyme mutations mapped to ∼0.4 min clockwise from the tetR marker of strain CAG18450 (39), which is located at 76.5 min on the most recent E. coli genomic map (32). This result provides evidence that all 18 mutations reside within the glgBX-glgCAP gene cluster. P1 transduction of the 77-min region of the chromosome from CAG18450 restored to the glycogen-deficient mutants both the parental glycogen phenotype and the sensitivity to inhibition by acetate (data not shown).
Since glgC and glgA are both essential for glycogen biosynthesis, mutations completely lacking glycogen could be defective in either glgC, glgA, or both. A complementation experiment was conducted by introducing plasmids carrying wild-type alleles of either glgC or glgA into the mutant strains and testing for restoration of glycogen synthesis. Surprisingly, all 16 of the yellow-staining mutations were complemented by either glgC or glgA. This demonstrated that the underlying mutations did not fully inactivate either of these genes but might have decreased the expression of both genes (e.g., as would be observed for a cis-acting mutation upstream from the glgCAP operon). Furthermore, none of the 16 glycogen-deficient mutations affected the expression of the glgCAP operon in trans (data not shown), as determined by using a glgC::lacZ translational fusion (28).
P1vir transduction mapping with a collection of Tn10-marked donor strains (39) also determined the genomic locations of 13 independently isolated, medium-brown-staining mutations. Six of these mutations mapped to 77 min (the region of the glg gene cluster), five mutations mapped to ∼42.7 min, and the two remaining mutations mapped to ∼54.0 min. The last two regions of the chromosome do not contain any genes previously known to affect glycogen synthesis.
Effects of csrA on acetate activation enzymes.
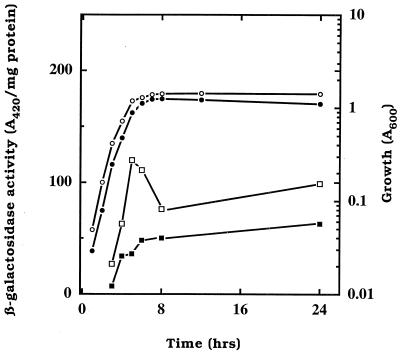
Because strain BW3414 grew well with acetate as the sole carbon source while the csrA mutant did not, it was conceivable that csrA affects either the acetate activation pathway or the glyoxylate shunt. When grown in tryptone broth, wild-type cells (MG1655) exhibited two- to threefold-higher Acs specific activity than did an isogenic csrA mutant (Table 2). This difference was not observed in the BW3414 background, in which Acs was extremely low in both the csrA+ and csrA mutant strains, likely because acs expression also depends upon rpoS (36). In contrast, the csrA mutation exerted little or no effect on the specific activity of Pta. Thus, a csrA mutant was defective in the primary pathway needed for the conversion of acetate to acetyl-CoA. To determine whether this effect occurs at the level of transcription, we measured β-galactosidase activity expressed from the transcriptional fusions acs::lacZ, pta::lacZ, and ackA-pta::lacZ. Induction of acs occurred in the mid-exponential phase and increased to maximal levels during the transition to stationary phase. The expression of the acs::lacZ fusion was higher throughout the growth phase in the csrA+ strain than in the csrA mutant, and the difference during the transition to stationary phase was two- to threefold (Fig. 4). In contrast, csrA did not affect expression of pta::lacZ or ackA::lacZ fusions (data not shown).
TABLE 2.
Specific activities of acetyl-CoA synthetase and phosphotransacetylase from csrA+ and csrA::KanR strainsa
| Strain | Genotype | Sp act (U/mg of protein ± SD; n ≥3)
|
|
|---|---|---|---|
| Acs | Pta | ||
| BW3414 | rpoS csrA+ | 0.008 ± 0.002 | 1.09 ± 0.33 |
| TR1-5BW3414 | rpoS csrA::kanR | 0.005 ± 0.002 | 0.88 ± 0.05 |
| MG1655 | csrA+ | 0.037 ± 0.004 | 0.97 ± 0.25 |
| TR1-5MG1655 | csrA::kanR | 0.011 ± 0.001 | 1.31 ± 0.31 |
Cultures were grown aerobically in 1% tryptone broth to the transition to stationary phase. Enzyme assays were conducted as described in Materials and Methods.
FIG. 4.
Effects of csrA on the expression of an acs::lacZ transcriptional fusion throughout the growth curve. Cultures were grown in 1% tryptone broth. Turbidity readings of cultures of strain SS414 and its isogenic csrA::kanR mutant are indicated by open and solid circles, respectively. β-Galactosidase activities of these two strains are shown as open and solid squares, respectively. Essentially identical results were observed for this experiment using the BW3414 strain background.
Effects of csrA on enzymes of the glyoxylate shunt and Krebs cycle.
Studies to assess the role of csrA in the regulation of the glyoxylate shunt were originally conducted in supplemented MOPS medium containing 50 mM acetate. In this medium, the generation time of the csrA parent strain, BW3414, and its isogenic csrA mutant strain was ∼4 h. Unlike the parent strain, the csrA mutant exhibited an extended lag phase of variable duration, and when stationary-phase cultures were plated onto Kornberg agar, they were found to contain numerous glycogen mutants (data not shown). In mid-exponential phase, isocitrate lyase activities in BW3414 and the isogenic csrA mutant were 0.22 and 0.13 U/mg of protein, respectively. The addition of acetate (25 mM) and succinate (25 mM) to supplemented MOPS medium improved the growth properties of the csrA mutant and prevented the appearance of glycogen mutations. Although the wild-type levels of isocitrate lyase were lower than those in 50 mM acetate medium, the relative levels of this enzyme were still ∼2-fold higher in the parent strain than in the csrA mutant (Table 3). Isocitrate lyase activity was extremely low in media containing glucose (Table 3). The expression of β-galactosidase activities from aceB::lacZ and iclR::lacZ transcriptional fusions in cells growing in MOPS medium supplemented with acetate and succinate (25 mM each) exhibited little or no effects of csrA (data not shown), suggesting that csrA may affect isocitrate lyase activity posttranscriptionally. Finally, the specific activities of two key Krebs cycle enzymes, citrate synthase and IDH, were found to be unaffected by the csrA mutation (Table 3).
TABLE 3.
Specific activities of isocitrate lyase, IDH, and citrate synthase from csrA+ and csrA::kanR strains grown under different conditionsa
| Medium | Sp act (U/mg of protein ± SD; n ≥ 3)
|
|||||
|---|---|---|---|---|---|---|
| Isocitrate lyase
|
IDH
|
Citrate synthase
|
||||
| csrA+ | csrA::kanR | csrA+ | csrA::kanR | csrA+ | csrA::kanR | |
| MOPS | ||||||
| Acetate and succinate | 0.083 ± 0.006 | 0.041 ± 0.006 | 1.23 ± 0.22 | 1.27 ± 0.05 | 0.61 ± 0.05 | 0.51 ± 0.05 |
| Glucose | 0.008 ± 0.000 | 0.010 ± 0.000 | 1.27 ± 0.12 | 1.24 ± 0.08 | 0.20 ± 0.01 | 0.16 ± 0.00 |
| Kornberg + glucose | 0.003 ± 0.000 | 0.003 ± 0.000 | 0.47 ± 0.03 | 0.49 ± 0.04 | 0.03 ± 0.00 | 0.03 ± 0.00 |
Cultures were grown in supplemented MOPS medium containing the indicated carbon sources or in Kornberg medium to mid-exponential phase. Enzyme assays were conducted as described in Materials and Methods.
Disruption of the glyoxylate shunt, Acs pathway, or Pta-Ack pathway in a csrA rpoS strain.
Significantly fewer ATPs are synthesized when carbon is metabolized through the glyoxylate shunt instead of the Krebs cycle (one acetyl-CoA molecule yields 4 and 12 ATPs, respectively). If this lower capacity for ATP synthesis were involved in acetate stress in csrA rpoS strains, then disruption of the glyoxylate shunt should prevent the appearance of glycogen mutations. However, a csrA rpoS mutant also defective in the glyoxylate shunt remained sensitive to acetate-dependent inhibition of growth and still gave rise to glycogen mutants when grown in the presence of acetate (data not shown). Clearly, diversion of carbon through the glyoxylate shunt was not responsible for acetate stress. Similarly, knocking out the Acs or Pta-AckA pathways in a csrA rpoS mutant did not prevent the appearance of glycogen mutants (data not shown). These experiments, and the observation that palmitic acid, which is metabolized to acetyl-CoA, does not mimic acetate stress, indicated that metabolism of acetate to acetyl-CoA is not required for it to cause metabolic stress. These studies also showed that the effects of acetate are not mediated through its conversion to the intracellular signal molecule acetyl-phosphate, which cannot be synthesized by strains deficient in the Ack-Pta pathway.
Metabolic suppression of acetate-derived glycogen mutations.
We hypothesized that increased gluconeogenesis and glycogen synthesis and decreased glycolytic flux in the csrA mutant may predispose the strain to depletion of the TCA cycle in the presence of acetate. This was further suggested by the finding that succinate plus acetate no longer gave rise to glycogen mutants in MOPS medium. To test this hypothesis more directly, the csrA mutant was cultured in the presence of 50 mM acetate plus 50 mM Krebs cycle intermediates or pyruvate. Each of the compounds α-ketoglutarate, succinate, fumarate, malate, and pyruvate suppressed the appearance of glycogen mutants, while glucose, galactose, or glycerol failed to do so. This provided strong evidence that acetate was depleting the TCA cycle in the csrA rpoS strains.
Uptake of amino acids from the growth medium.
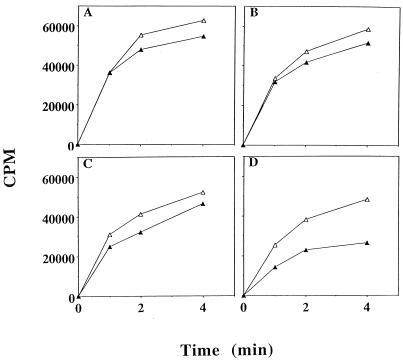
The major carbon and energy sources of cells growing on tryptone broth are amino acids (25), which are metabolized via the TCA cycle (21). Thus, we hypothesized that acetate may affect amino acid uptake in the csrA rpoS strain. Figure 5 shows that the parent strain, MG1655, and csrA and rpoS single mutants exhibited slight inhibition of amino acid uptake, ranging from no inhibition up to ∼20%. However, in four separate experiments, we consistently observed that uptake by the csrA rpoS strain was more sensitive to acetate, exhibiting 35 to 45% inhibition when preincubated for 5 min with 50 mM sodium acetate. Essentially the same results were obtained when pyruvate was added to these strains rather than acetate (data not shown).
FIG. 5.
Uptake of amino acids by mid-exponential-phase cultures in the presence or absence of acetate. 14C-amino acid uptake experiments using strains MG1655 (A), TR1-5MG1655 (csrA::kanR) (B), RHMG1655 (rpoS::Tn10) (C), and RHTR1-5MG1655 (csrA::kanR rpoS::Tn10) (D) were performed as described in Materials and Methods. The open and solid triangles represent control and acetate treatments, respectively.
DISCUSSION
The genetic adaptation of bacteria to their external environment can manifest in striking ways and provide new insights into the physiological complexity of these organisms. The results of the present study show that endogenous stress, resulting from defective regulation of central carbon metabolism, can provide very strong selective pressure for adaptation.
E. coli K-12 strains defective in the csrA and rpoS genes were inhibited by the addition of acetate to the growth medium, which resulted in the rapid appearance of suppressor mutations that disrupt glycogen biosynthesis. Several of the mutations isolated in this study identify two novel genes affecting glycogen synthesis, which we are currently characterizing.
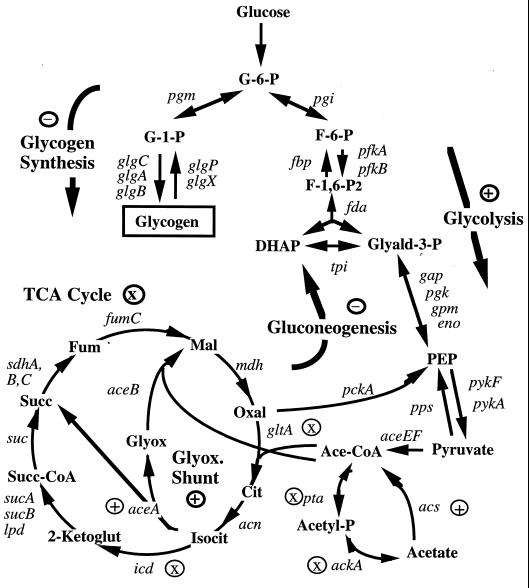
Because central carbon pathways are interconnected, excesses or deficiencies in one pathway should impact upon others (Fig. 6). Considerable evidence indicates that the major metabolic problem caused by adding acetate to csrA rpoS strains is the depletion of the TCA cycle. TCA cycle intermediates or pyruvate, which is a direct precursor of the TCA cycle, restored the growth rate and prevented the appearance of glycogen mutants in the presence of acetate. Furthermore, the contribution of csrA to the underlying stress appears to be readily explained. The csrA gene encodes an RNA-binding protein that is a potent repressor of glycogen synthesis and gluconeogenesis and is an activator of glycolysis (reviewed in reference 27). In a csrA mutant, central carbon metabolism is shifted to favor carbon flow away from the TCA cycle and toward the synthesis of glycogen, which acts as a metabolic sink for carbon and energy (30, 44). The effect of csrA on glycogen synthesis was shown to be a necessary component of acetate-induced stress. Normal growth in the presence of acetate was restored by mutations that decrease glycogen synthesis, and restoration of glycogen synthesis made the latter strains again sensitive to acetate inhibition. Importantly, the csrA mutation did not alter levels of key TCA cycle enzymes.
FIG. 6.
Effects of csrA on intermediary carbon metabolism in E. coli. A summary of previous studies of glycogen metabolism, glycolysis, and gluconeogenesis and results of current studies of the reactions of acetate metabolism and the TCA cycle are shown. Pathways or reactions that are subject to positive regulation, negative regulation, or little or no control are indicated by an encircled +, −, or ×, respectively.
Unlike TCA cycle intermediates, compounds that enter the glycolytic pathway as glucose-6-phosphate (glucose or galactose) or as dihydroxyacetone phosphate (glycerol) (16) could not suppress the effects of acetate. These observations are also consistent with the regulatory effects of a csrA mutation on carbon flux. A csrA mutant is deficient in triose phosphate isomerase, pyruvate kinase F, and other glycolytic enzymes, while it greatly overproduces phosphoglucomutase, glycogen biosynthetic enzymes, and gluconeogenic enzymes (33). Thus, metabolism of glucose-6-phosphate or dihydroxyacetone phosphate is diverted away from the TCA cycle and towards glycogen biosynthesis in a csrA mutant.
Acetate contributes to depletion of the TCA cycle specifically in the csrA rpoS strain by decreasing the rate of uptake of amino acids, which are metabolized primarily via the TCA cycle (21). Paradoxically, pyruvate also inhibited amino acid uptake in this strain, and both pyruvate and acetate enter the TCA cycle by direct conversion to acetyl-CoA. However, pyruvate also served as a sole carbon source in the csrA rpoS strain without causing the appearance of glycogen mutants and therefore must be able to replenish the TCA cycle. Acetate itself does not serve as a sole carbon source unless suppressor mutations occur, perhaps because the csrA rpoS double mutant is extremely deficient in acetyl-CoA synthetase activity. In addition, the csrA mutant is deficient in isocitrate lyase of the glyoxylate shunt, which is needed for growth on acetate but not pyruvate. However, it is not clear that the latter more modest effect of csrA is enough to inhibit growth.
We have also provided evidence to exclude several potential explanations for the observed acetate stress. It is not caused by bypassing energy-generating steps of the TCA cycle via induction of the glyoxylate shunt, since glyoxylate shunt mutants are still sensitive to acetate stress and generate glycogen mutants. It does not involve conversion of acetate to the intracellular signaling molecule acetyl-phosphate (43), since ack-pta mutants still generated glycogen mutants. Since neither benzoate nor 2,4-dinitrophenol treatment resulted in the appearance of glycogen mutants, acidification of the cytoplasm (as discussed in reference 35) or decreasing the cellular capacity for ATP synthesis does not explain the effects of acetate. Conversion of acetate to acetyl-CoA is apparently not required, because acs and ack-pta mutants still gave rise to glycogen mutations, and palmitic acid and pyruvate, which are also metabolized to acetyl-CoA, neither inhibited growth nor yielded glycogen mutants.
The specific requirement for the rpoS defect in the observed acetate stress is unclear but in part involves sensitization of amino acid uptake to acetate inhibition in the csrA mutant. Interestingly, a variety of connections between rpoS and acetate metabolism have previously been established. Accumulation of acetate in the growth medium has been reported as a signal for increasing rpoS transcription (35). In contrast, acetyl-phosphate appears to be a signal for proteolysis of RpoS, via the direct covalent modification of the protease RssB (1). Therefore, ςS levels appear to respond to acetate metabolism in a complex and dynamic fashion. In addition, rpoS directly or indirectly induces acs expression and therefore promotes acetate metabolism (36).
Glycogen biosynthesis is also stimulated by rpoS via effects on the transcription of glgS, while the glgCAP operon is not regulated via rpoS (9, 24). In this respect, rpoS promotes glycogen synthesis and acts opposite to csrA. Thus, it cannot be argued that the rpoS mutation is required to cause acetate stress because it enhances glycogen biosynthesis. Furthermore, the effects of rpoS on glycogen synthesis are modest in a csrA mutant, and the csrA rpoS double mutant TR1-5BW3414 accumulates very high levels of glycogen (44). It is intriguing to consider that rpoS may have effects on central carbon metabolism that contribute to its involvement in acetate stress, but such a role for rpoS has not been examined.
Finally, it seems unlikely that rpoS specifically affects the mutagenic process, as opposed to the selective process, although this has not been tested. In fact, rpoS has been implicated in the formation of certain types of adaptive mutations that occur in stationary phase (7). However, the acetate-induced mutations in the present study occurred during exponential growth, and of course, a functional rpoS gene actually prevented the appearance of these mutants.
ACKNOWLEDGMENTS
We thank Ely Gordon for the isolation of EG3-153, Woo-Jin Chang for characterization of EG3-153 and for constructing pPRC1, and Harlan Jones for assistance with P1 mapping. We also thank Barry Wanner for providing advice concerning the genotype of BW3414.
Financial support for this research was provided by a National Science Foundation grant to T.R. (MCB9726197).
REFERENCES
- 1.Bouche S, Klauck E, Fischer D, Lucassen M, Jung K, Hengge-Aronis R. Regulation of RssB-dependent proteolysis in Escherichia coli: a role for acetyl phosphate in a response regulator-controlled process. Mol Microbiol. 1998;27:787–795. doi: 10.1046/j.1365-2958.1998.00725.x. [DOI] [PubMed] [Google Scholar]
- 2.Brown T D K, Jones-Mortimer M C, Kornberg H L. The enzymic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J Gen Microbiol. 1977;102:327–336. doi: 10.1099/00221287-102-2-327. [DOI] [PubMed] [Google Scholar]
- 3.Clark D P, Cronan J E., Jr . Two-carbon compounds and fatty acids as carbon sources. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 343–357. [Google Scholar]
- 4.Cronan J E, Jr, LaPorte D. Tricarboxylic acid cycle and glyoxylate bypass. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 206–216. [Google Scholar]
- 5.Cummings J H, Pomare E W, Branch W J, Nalor C P E, MacFarlane G T. Short chain fatty acids in human large intestine, portal, hepatic, and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damotte M, Cattano J, Sigal N, Puig J. Mutants of Escherichia coli K-12 altered in their ability to store glycogen. Biochem Biophys Res Commun. 1968;32:916–920. doi: 10.1016/0006-291x(68)90114-9. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Gomez J M, Blazquez J, Baquero F, Martinez J L. H-NS and RpoS regulate emergence of Lac Ara+ mutants of Escherichia coli MCS2. J Bacteriol. 1997;179:4620–4622. doi: 10.1128/jb.179.14.4620-4622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Govons S, Vinopal R, Ingraham J, Preiss J. Isolation of mutants of Escherichia coli B altered in their ability to synthesize glycogen. J Bacteriol. 1969;97:970–972. doi: 10.1128/jb.97.2.970-972.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hengge-Aronis R, Fisher D. Identification and molecular analysis of glgS, a novel growth-phase-regulated and rpoS-dependent gene involved in glycogen synthesis in E. coli. Mol Microbiol. 1992;6:1877–1886. doi: 10.1111/j.1365-2958.1992.tb01360.x. [DOI] [PubMed] [Google Scholar]
- 10.Kakuda H, Hosono K, Shiroichi K, Ichihara S. Identification and characterization of the ackA (acetate kinase A)-pta (phosphotransacetylase) operon and complementation analysis of acetate utilization by an ackA-pta deletion mutant of Escherichia coli. J Biochem (Tokyo) 1994;116:916–922. doi: 10.1093/oxfordjournals.jbchem.a124616. [DOI] [PubMed] [Google Scholar]
- 11.Kay W W. Genetic control of the metabolism of propionate by Escherichia coli K12. Biochim Biophys Acta. 1972;264:508–521. doi: 10.1016/0304-4165(72)90014-1. [DOI] [PubMed] [Google Scholar]
- 12.Kohara Y, Akiyama K, Isono K. The physical map of the whole Escherichia coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987;50:495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Larsen C E, Preiss J. Biosynthesis of bacterial glycogen. Primary structure of Escherichia coli ADP glucose: α-1,4-glucan, 4-glucosyl transferase as deduced from the nucleotide sequence of the glgA gene. J Biol Chem. 1986;261:16256–16259. [PubMed] [Google Scholar]
- 14.Kumari S, Tishel R, Eisenbach M, Wolfe A J. Cloning, characterization, and functional expression of acs, the gene which encodes acetyl coenzyme A synthetase in Escherichia coli. J Bacteriol. 1995;177:2878–2886. doi: 10.1128/jb.177.10.2878-2886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaPorte D C, Thorsness P E, Koshland D E., Jr Compensatory phosphorylation of isocitrate dehydrogenase. J Biol Chem. 1985;260:10563–10568. [PubMed] [Google Scholar]
- 16.Lin E C C. Dissimilatory pathways for sugars, polyols, and carboxylates. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 307–342. [Google Scholar]
- 17.Liu M Y, Gui G, Wei B, Preston III J F, Oakford L, Yuksel U, Geidroc D P, Romeo T. The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J Biol Chem. 1997;272:17502–17510. doi: 10.1074/jbc.272.28.17502. [DOI] [PubMed] [Google Scholar]
- 18.Liu M Y, Romeo T. The global regulator CsrA of Escherichia coli is a specific mRNA binding protein. J Bacteriol. 1997;179:4639–4642. doi: 10.1128/jb.179.14.4639-4642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M Y, Yang H, Romeo T. The product of the pleiotropic Escherichia coli gene csrA modulates glycogen biosynthesis via effects on mRNA stability. J Bacteriol. 1995;177:2663–2672. doi: 10.1128/jb.177.10.2663-2672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maloy R M, Bohlander M, Nunn W D. Elevated levels of glyoxylate shunt enzymes in Escherichia coli strains constitutive for fatty acid degradation. J Bacteriol. 1980;143:720–725. doi: 10.1128/jb.143.2.720-725.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McFall E, Neuman E B. Amino acids as carbon sources. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 358–379. [Google Scholar]
- 22.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 23.Neidhardt F C, Bloch P L, Smith D F. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preiss J, Romeo T. Molecular biology and regulatory aspects of glycogen biosynthesis in bacteria. Prog Nucleic Acid Res Mol Biol. 1994;47:299–329. doi: 10.1016/s0079-6603(08)60255-x. [DOI] [PubMed] [Google Scholar]
- 25.Pruß B M, Nelms J M, Park C, Wolfe A J. Mutations in NADH:ubiquinone oxidoreductase of Escherichia coli affect growth on mixed amino acids. J Bacteriol. 1994;176:2143–2150. doi: 10.1128/jb.176.8.2143-2150.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pruß B M, Wolfe A J. Regulation of acetyl phosphate synthesis and degradation, and the control of flagellar expression in Escherichia coli. Mol Microbiol. 1994;12:973–984. doi: 10.1111/j.1365-2958.1994.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 27.Romeo T. Global regulation by the small RNA-binding protein CsrA and the non-coding RNA molecule CsrB. Mol Microbiol. 1998;29:1321–1330. doi: 10.1046/j.1365-2958.1998.01021.x. [DOI] [PubMed] [Google Scholar]
- 28.Romeo T, Black J, Preiss J. Genetic regulation of glycogen biosynthesis in Escherichia coli: in vivo effects of the catabolite repression and stringent response systems in glg gene expression. Curr Microbiol. 1990;21:131–137. [Google Scholar]
- 29.Romeo T, Gong M. Genetic and physical mapping of the regulatory gene csrA on the Escherichia coli K-12 chromosome. J Bacteriol. 1993;175:5740–5741. doi: 10.1128/jb.175.17.5740-5741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romeo T, Gong M, Liu M Y, Brun-Zinkernagel A-M. Identification and molecular characterization of csrA, a pleiotropic gene from Escherichia coli that affects glycogen biosynthesis, gluconeogenesis, cell size, and surface properties. J Bacteriol. 1993;175:4744–4755. doi: 10.1128/jb.175.15.4744-4755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romeo T, Preiss J. Genetic regulation of glycogen biosynthesis in Escherichia coli: in vivo effects of cyclic AMP and guanosine 5′-diphosphate 3′-diphosphate and analysis of in vivo transcripts. J Bacteriol. 1989;171:2773–2782. doi: 10.1128/jb.171.5.2773-2782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudd K E. Linkage map of Escherichia coli K-12, edition 10: the physical map. Microbiol Mol Biol Rev. 1998;62:985–1019. doi: 10.1128/mmbr.62.3.985-1019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabnis N A, Yang H, Romeo T. Pleiotropic regulation of central carbohydrate metabolism in Escherichia coli via the gene csrA. J Biol Chem. 1995;270:29096–29104. doi: 10.1074/jbc.270.49.29096. [DOI] [PubMed] [Google Scholar]
- 34.Salmond C V, Kroll R G, Broth I R. The effect of food preservatives on pH homeostasis in Escherichia coli. J Gen Microbiol. 1984;130:2845–2850. doi: 10.1099/00221287-130-11-2845. [DOI] [PubMed] [Google Scholar]
- 35.Schellhorn H E, Stones V L. Regulation of katF and katE in Escherichia coli K-12 by weak acids. J Bacteriol. 1992;174:4769–4776. doi: 10.1128/jb.174.14.4769-4776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin S, Song S G, Lee D S, Pan J G, Park C. Involvement of iclR and rpoS in the induction of acs, the gene for acetyl coenzyme A synthetase of Escherichia coli K-12. FEMS Microbiol Lett. 1997;146:103–108. doi: 10.1111/j.1574-6968.1997.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 37.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 38.Simons R W, Patricia A E, Hilary T C, Nunn W D. Regulation of fatty acid degradation in Escherichia coli: isolation and characterization of strains bearing insertion and temperature-sensitive mutations in the gene fadR. J Bacteriol. 1980;142:621–632. doi: 10.1128/jb.142.2.621-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singer M, Baker T A, Schnitzler G, Deischel S M, Goel M, Dove W, Jaacks K J, Grossman A D, Erickson J W, Gross C A. A collection of genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989;53:1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith P K, Krohn R H, Hermanson G T, Mallie A K, Gartner F H, Provensano J D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 41.Stitt M. Citrate synthase (condensing enzyme) In: Bergmeyer H U, Bergmeyer J, Graßl M, editors. Methods of enzymatic analysis. 3rd ed. IV. Deerfield Beach, Fla: Verlag Chemie; 1984. pp. 353–358. [Google Scholar]
- 42.Vinopal R T, Fraenkel D G. Phenotypic suppression of phosphofructokinase mutations in Escherichia coli by constitutive expression of the glyoxylate shunt. J Bacteriol. 1974;118:1090–1100. doi: 10.1128/jb.118.3.1090-1100.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wanner B L. Signal transduction and cross regulation in the Escherichia coli phosphate regulon by PhoR, CreC, and acetylphosphate. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. pp. 203–221. [Google Scholar]
- 44.Yang H, Liu M Y, Romeo T. Coordinate genetic regulation of glycogen catabolism and biosynthesis in Escherichia coli via the CsrA gene product. J Bacteriol. 1996;178:1012–1017. doi: 10.1128/jb.178.4.1012-1017.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–109. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]