Abstract
Background and aim
Resveratrol is a bioactive molecule used in dietary supplements and herbal medicines and consumed worldwide. Prior work showed that resveratrol's anti-atherogenic properties are mediated in part through the adenosine A2A receptor. The present study explores the potential contribution of adenosine A2A receptor activation to neuroprotective action of resveratrol on cognitive deficits in a model of atherosclerosis-prone systemic lupus erythematosus.
Experimental procedure
Using behavioral analysis (open field, static rod, novel object recognition) and QRT-PCR, this study measured working memory, anxiety, motor coordination, and expression of mRNA in the brain.
Results and conclusion
Data indicate that resveratrol increases working memory, on average but not statistically, and shows a trend towards improved motor coordination (p = 0.07) in atherosclerosis-prone lupus mice. Additionally, resveratrol tends to increase mRNA levels of SIRT1, decrease vascular endothelial growth factor and CX3CL1 mRNA in the hippocampus. Istradefylline, an adenosine A2A receptor antagonist, antagonizes the effects of resveratrol on working memory (p = 0.04) and the expression of SIRT1 (p = 0.03), vascular endothelial growth factor (p = 0.04), and CX3CL1 (p = 0.03) in the hippocampus.
This study demonstrates that resveratrol could potentially be a therapeutic candidate in the modulation of cognitive dysfunction in neuropsychiatric lupus, especially motor incoordination. Further human studies, as well as optimization of resveratrol administration, could confirm whether resveratrol may be an additional resource available to reduce the burden of cognitive impairment associated with lupus. Additionally, further studies need to address the role of A2A blockade in cognitive function among the autoimmune population.
Section
3. Dietary therapy/nutrients supplements.
Taxonomy (classification by EVISE)
autoimmunity, inflammation, neurology.
Keywords: Systemic lupus erythematosus, Cognition, Neurolupus, Nutraceutical, Cardiovascular
Graphical abstract
Highlights
-
•
Increased working memory by resveratrol partially mediated by adenosine A2A.
-
•
Motor coordination improvement by resveratrol partially mediated by adenosine A2A.
-
•
A2A blockade accompanied by changes in brain SIRT1, neurotactin, and VEGF mRNA.
List of abbreviations
- ANOVA
analysis of variance
- APOE KO
atherosclerosis model
- CI
confidence interval
- Ct
cycle threshold
- CX3CL1
neurotactin ligand
- CX3CR1
neurotactin receptor
- ERα
estrogen receptor alpha
- F4/80
macrophage/microglia
- FAS KO
lupus model
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IACUC
institutional animal care and use committee
- ICAM-1
intercellular adhesion molecule-1
- IL-1
interleukin-1
- LTP
long term potentiation
- NAD
nicotinamide adenine dinucleotide
- NOR
novel object recognition
- NPSLE
neurolupus
- PBS
phosphate buffered saline
- PGC1-α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- QRT-PCR
quantitative real time polymerase chain reaction
- SIRT
sirtuin
- SLE
lupus
- VEGF
vascular endothelial growth factor
- ZO1
zona occludens-1
1. Introduction
Neuropsychiatric lupus (NPSLE, neurolupus), a complication of systemic lupus erythematosus (SLE, lupus), can present with diverse deficits such as headaches, seizures, cerebrovascular disease, psychosis, movement disorders, mood disorders, confusional state and cognitive dysfunction.1,2 Neurolupus is extremely difficult to treat and its occurrence does not correlate with severity of lupus flare.3, 4, 5 Cognitive dysfunction related to NPSLE can severely impact young people, thus significantly reducing their quality of life.5,6 Further, SLE patients have a 50 times higher risk of developing cardiovascular complications such as atherosclerosis, stroke, and myocardial infarction, than the general population7 and vascular health is extremely important in the maintenance of normal cognitive function.8, 9, 10 Patients presenting with acute neurologic symptoms as a result of NPSLE do not always respond to typical anti-inflammatory medications used in the SLE standard of care such as steroids and hydroxychloroquine.11 This non-responsiveness to anti-inflammatory treatments may result from the idea that cognitive changes in NPSLE are not due to inflammation alone but to the interaction between vascular disease and chronic inflammation. This intersection presents an opportunity to develop a novel model and treatment for neurologic complications of lupus.
Research shows that resveratrol, a polyphenolic compound found in grapes and berries, can protect against vascular disease and prolong the lifespan in animal models.12,13 Resveratrol not only protects against vascular disease, it also decreases immune responsiveness in autoimmune diseases,14 increases cerebral blood flow and reduces subjective ratings of fatigue.15 The protective effects of resveratrol may be due, in part, to its inhibitory effect on monocyte differentiation and pro-inflammatory cytokine production16 via the adenosine receptor.17 Our prior work showed that the adenosine A2A receptor blocks the atheroprotective effect of resveratrol. Further, we recently demonstrated that resveratrol has atheroprotective effects in the lupus setting.18 Because neurolupus may be due to the intersection of pro-inflammatory and atherogenic processes, we are interested in studying the effect of, and mechanism of action for, resveratrol on cognitive changes in atherosclerosis-prone lupus mice.19 Our hypothesis is that resveratrol will improve cognitive function and reduce pro-inflammatory markers in atherosclerosis prone lupus mice through the adenosine A2A receptor.
2. Material and methods
2.1. Mice
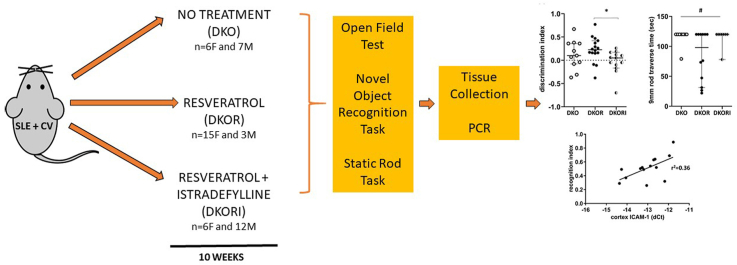
In order to produce atherosclerosis prone lupus mice, B6.129P2-Apoetm1Unc/J (APOE KO; atherosclerosis model) and MRL/MpJ-Faslpr/J (FAS KO; lupus model) mice on the B6 background were purchased from the Jackson Laboratories (Bar Harbor, ME). Single knockout mice were intercrossed to produce three groups of mice with the following genotypes: apoE−/- Fas+/+, apoE−/- Fas+/- and apoE−/- Fas−/-. DNA was extracted from tail using the Qiagen DNeasy Tissue Kit. Genotyping of the wild-type versus the apoE knockout allele20 and the lpr allele21 was performed as described. Beginning at 15 weeks of age, apoE−/- Fas−/- (representing atherosclerosis-prone lupus mice) male and female mice were split into three treatment conditions: the control group (n = 13 [7 M, 6F]) was fed a regular chow diet, the resveratrol group (n = 18 [3 M, 15F]) was fed regular chow and given water containing 0.01% resveratrol dissolved in ethanol in a light-protected water bottle, and the resveratrol + istradefylline group (n = 18 [12 M, 6F]) was fed a diet of regular chow supplemented with 2 mg/kg of the adenosine A2A receptor antagonist istradefylline (Teklad) and given water containing 0.01% resveratrol dissolved in ethanol in a light-protected water bottle. Animals were co-housed in single-sex groups based on birth cohort, up to a maximum of five animals per cage. All animals were maintained on their respective treatment regimens for 10 weeks in a temperature-controlled room with 12 h light/dark cycle according to the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) and a protocol approved by the NYU Winthrop Hospital IACUC.
2.2. Behavioral testing
Before splitting the mice into their groups (at 15 weeks old) and at the end of the above outlined treatments (at 25 weeks old), animals were tested on tasks related to anxiety, memory, and motor coordination.
Open Field Test: Acclimated mice were brought to the test room singly and placed in the center of a plexiglass chamber 45 cm × 45 cm x 25 cm with 15 cm × 15 cm grids. Mice were allowed to freely explore the chamber for the duration of the test session which was 1 trial for 10 min. Upon completion of the test, each mouse was returned to the home cage. In addition to horizontal units of activity, time spent in center, rearing behavior, defecation, and grooming activity was measured. Total locomotor activity was calculated by summing the total number of lines crossed and total number of rears.
Novel Object Recognition (NOR) Task: On the day after open field testing (also used as habituation trial for NOR), each mouse was familiarized to two equal objects placed in opposing corners of the open field arena for 5 min, with the mouse allowed to freely explore during the session (familiarization phase). Then, each mouse was sent back to its home cage for 5 min (for short-term memory assessment) and reintroduced to the arena after one object was exchanged (novel object; test phase). Duration and number of object contacts were measured. To prevent coercion to explore the objects, each mouse was released against the center of the opposite wall with its back to the objects. During both the familiarization and the test phase, objects were located in opposite and symmetrical corners of the arena and location of novel versus familiar object were counterbalanced. The discrimination index was calculated by subtracting the total time spent with the familiar object from the total time spent with the novel object and then dividing the result by the total time spent with both objects.
Static Rod Task: Each mouse, one at a time, was placed at the far end of the widest of three rods (600 mm × 35 mm; suspended 250 mm from the ground) first, facing the end of the rod away from the bench. The time it took for the mouse to orient towards the bench and total transit time to the 100 mm mark were recorded. A maximum time of 120 s on the rod was allowed for each mouse. If the mouse turned upside down on the rod it was arbitrarily assigned a time of 120 s and was not placed on narrower rods. Each mouse was returned to a clean cage and allowed to rest for at least 5 min before being placed on successively narrower rods (22 mm and 9 mm diameters). If the mouse fell within the first 5 s of being placed on a rod it was replaced on the rod without penalty 2 times before it was assigned the maximum value of 120 s.
2.3. Tissue collection
After 10 weeks of exposure to treatment (at 25 week old), mice were euthanized by CO2 inhalation. After euthanasia, each mouse was perfused with sterile PBS and the brain was collected. The entire brain was removed from the skull and hemi-sected. One half of the brain (left side) was flash frozen in 2-methyl butane in a methanol/dry ice slurry. The other half of the brain (right side) was dissected for collection of hippocampus and cortex; each brain region was flash frozen in 2-methyl butane. Hippocampus and cortex were chosen as a reflection of the behavioral testing done on the mice, i.e. testing memory and motor function.
2.4. RNA isolation and gene expression analysis by QRT-PCR
Total RNA was isolated from hippocampus and cortex by homogenizing tissue with Trizol reagent (Life Technologies) and dissolving in nuclease-free water. The quantity of total RNA from each condition was measured by absorption at 260 and 280 nm wavelengths by ultraviolet spectrophotometry (Hitachi U2010 spectrophotometer).
QRT-PCR analysis was performed using the FastStart SYBR Green Reagents Kit according to the manufacturers' instructions on the Roche Light Cycler 480 (Roche Applied Science, Indianapolis, IN). cDNA was copied from 1 μg of total RNA using Murine Leukemia Virus reverse transcriptase primed with oligo dT. Equal amounts of cDNA were taken from each reverse transcription reaction mixture for real-time PCR amplification using gene specific primers for vascular endothelial cell growth factor (VEGF), macrophage/microglia (F4/80), intercellular adhesion molecule-1 (ICAM-1), interleukin-1 (IL-1), neurotactin (CX3CL1), neurotactin receptor (CX3CR1), sirtuin 1 (SIRT1), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), estrogen receptor alpha (ERα), claudin, occludin, and zonula occludens-1 (ZO1).
QRT-PCR was performed using techniques standardized in our laboratory. Each reaction was done in triplicate. To correct for differences in cDNA load among samples, the target PCRs were normalized to a reference PCR involving the endogenous housekeeping gene GAPDH. Non-template controls were included for each primer pair to check for significant levels of any contaminants. A melting-curve analysis was performed to assess the specificity of the amplified PCR products.
2.5. Data analysis
Statistical analysis was performed using Graphpad Prism, version 6 (GraphPad Software, San Diego, CA). All normally distributed behavioral data were analyzed by one way ANOVA. Probability values less than 0.05 were regarded as significant. Data are presented as mean ± standard error of the mean unless otherwise specified. QRT-PCR data was analyzed using dCt values (GAPDH Ct – gene of interest Ct) and then the 2ΔΔCt method was used to calculate fold change over control. PCR data are presented as fold change with corresponding 95% confidence intervals. Any non-normal data were analyzed using the appropriate non-parametric tests (e.g. Kruskal-Wallis for equal variances between groups or Welch's ANOVA for unequal variances between groups) and graphed using the median and interquartile range. Pearson correlations were calculated between normally distributed behavioral data and PCR dCt data. All groups contained males and females combined for analysis since stratification by sex was not possible due to low numbers.
Animals were excluded from the open field analysis if they did not explore the chamber at all, i.e. no units of activity. Animals were excluded from the novel-object recognition analysis if they did not explore both objects. Animals were excluded from the static rod task analysis if they did not walk on the rods, i.e. if they remained immobile for the duration of the trial. Finally, animals were excluded from PCR analysis if there was no recoverable RNA.
3. Results
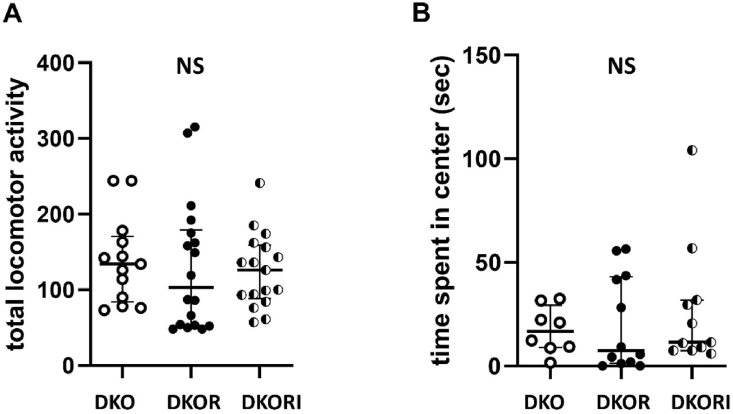
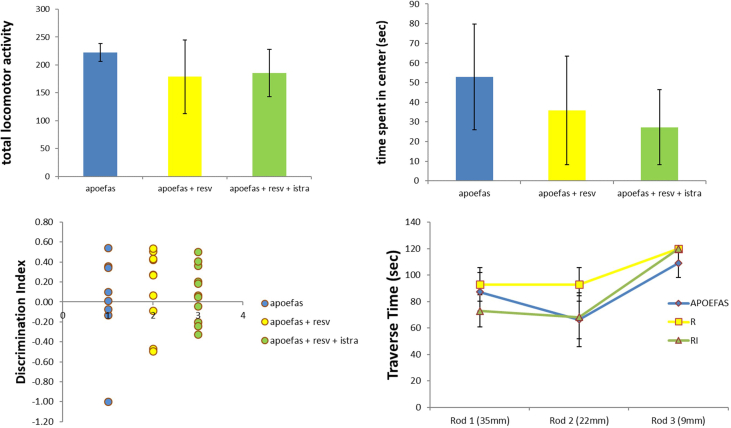
Behavioral Testing: There were no significant differences in behavioral testing between animals before they were divided into their treatment groups (see Supplemental Figure). After 10 weeks of treatment, in the open field test, there were no significant differences in general locomotor activity or time spent in center (H = 0.821, p = 0.66 and F(2,28) = 0.4021, p = 0.67, respectively; Fig. 1A and Fig. 1B).
Fig. 1.
General locomotor activity and anxiety in mice. (A) There was no difference in general locomotor activity in the open field test between groups using a Kruskal-Wallis test. The data represent mean with 95% CI for 13 apoe/fas animals, 18 apoe/fas + resveratrol animals combined and 17 apoe/fas + resveratrol + istradefylline animals. (B) There was no difference in time spent in the center (anxiety) of the open field between groups using a one-way ANOVA. The data represent median with SIQR for 8 apoe/fas animals, 12 apoe/fas + resveratrol animals combined and 11 apoe/fas + resveratrol + istradefylline animals.
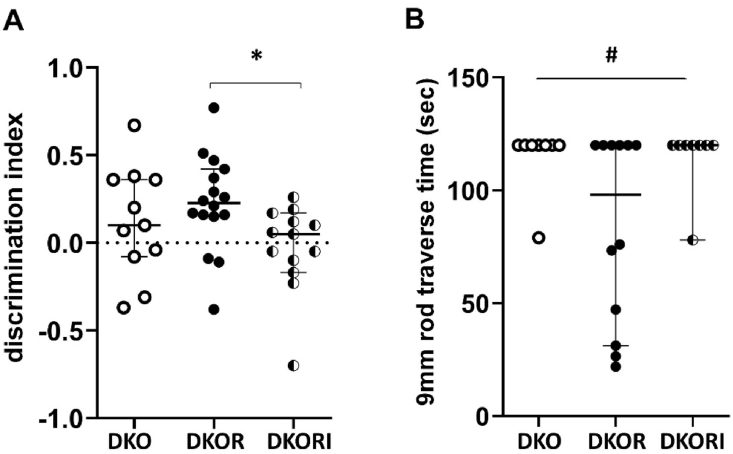
In the novel object recognition task, apoe/fas double knockout mice treated with resveratrol spent more time with a novel object (discrimination index: median = 0.23 ± 0.13) compared to apoe/fas control mice (discrimination index: median = 0.10 ± 0.22), though this increase was not significant, and significantly more time with a novel object compared to apoe/fas mice treated with resveratrol and istradefylline (discrimination index: median = 0.05 ± 0.14; H = 6.32, p = 0.04; Fig. 2A). DKO = double knockout, DKOR = double knockout + resveratrol, DKORI = double knockout + resveratrol + istradefylline.
Fig. 2.
Working memory and motor coordination in mice. (A) Apoe/fas animals treated with resveratrol (n = 16) had significantly higher discrimination indices compared to apoe/fas animals treated with resveratrol and istradefylline (n = 13) but not compared to apoe/fas controls (n = 11) using a Kruskal-Wallis test. The data represent median with IQR. (B) Apoe/fas animals treated with resveratrol (n = 12) had a trend towards shorter traverse times on the 9 mm rod compared to apoe/fas controls (n = 8) and apoe/fas animals treated with resveratrol and istradefylline (n = 8) using a Welch's ANOVA test. The data represent median with IQR. ∗ - p < 0.05, # - p < 0.10. DKO = double knockout, DKOR = double knockout + resveratrol, DKORI = double knockout + resveratrol + istradefylline.
In the static rod task, apoe/fas double knockout mice treated with resveratrol showed a trend towards walking across the 9 mm rod faster (traverse time: 83.03 ± 12.07 s) compared to apoe/fas control mice (traverse time: 114.88 ± 5.13 s) and apoe/fas mice treated with resveratrol and istradefylline (traverse time: 114.75 ± 5.25 s; F(2,17) = 3.053, p = 0.07; Fig. 2B).
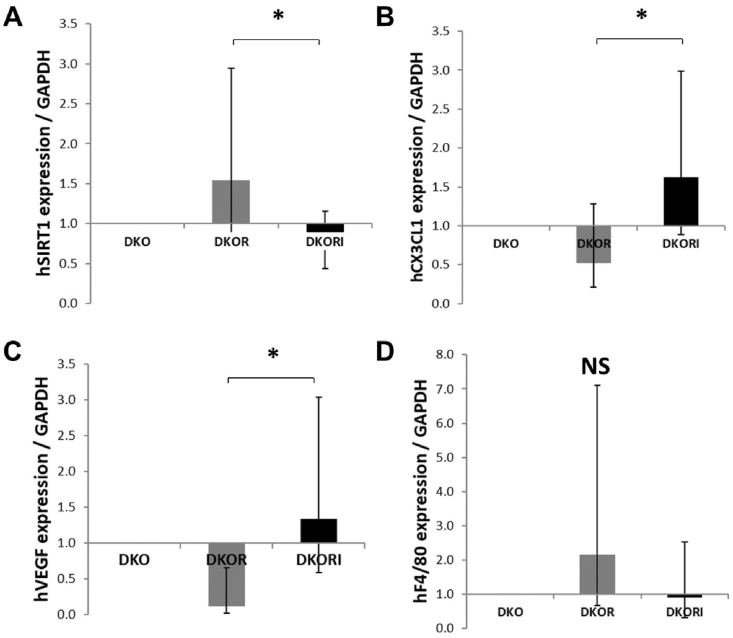
RT-PCR: Apoe/fas double knockout mice treated with resveratrol had increased hippocampal expression of SIRT-1 (1.54, 95%CI [0.84, 2.95]) compared to apoe/fas mice treated with resveratrol and istradefylline (0.71, 95%CI [0.44, 1.15]) but not compared to apoe/fas control mice (1.0, reference group; F(2, 9) = 5.253, p = 0.031; Fig. 3A). Apoe/fas double knockout mice treated with resveratrol had decreased hippocampal expression of CX3CL1 (0.52, 95%CI [0.21, 1.28]) compared to apoe/fas mice treated with resveratrol and istradefylline (1.63, 95%CI [0.89, 3.00]) but not compared to apoe/fas control mice (1.0, reference group; F(2,21) = 3.999, p = 0.03; Fig. 3B). Apoe/fas double knockout mice treated with resveratrol had decreased hippocampal expression of VEGF (0.12, 95%CI [0.02, 0.66]) compared to apoe/fas mice treated with resveratrol and istradefylline (1.34, 95%CI [0.59, 3.04]) but not compared to apoe/fas control mice (1.0, reference group; F(2, 13) = 3.918, p = 0.04; Fig. 3C). There were no significant differences in the hippocampal expression of F4/80 (F(2,14) = 2.814, p = 0.11; Fig. 3D). No differences were seen in PGC-1α, claudin, occludin, ZO1 or CX3CR1.
Fig. 3.
mRNA expression in hippocampus and cortex of mice. (A) Apoe/fas animals treated with resveratrol (n = 10) had significantly higher expression of SIRT1 in the hippocampus compared to apoe/fas animals treated with resveratrol and istradefylline (n = 9) but not compared to apoe/fas controls (n = 6) using a Welch's ANOVA test. (B) Apoe/fas animals treated with resveratrol (n = 10) had significantly lower expression of CX3CL1 in the hippocampus compared to apoe/fas animals treated with resveratrol and istradefylline (n = 9) but not compared to apoe/fas controls (n = 5) using a one-way ANOVA test. (C) Apoe/fas animals treated with resveratrol (n = 14) had significantly lower expression of VEGF in the hippocampus compared to apoe/fas and apoe/fas + resveratrol + istradefylline animals combined (n = 9) using a Welch's ANOVA test. (D) Apoe/fas animals treated with resveratrol (n = 14) showed a trend towards higher expression of F4/80 in the hippocampus compared to apoe/fas and apoe/fas + resveratrol + istradefylline animals combined (n = 10) using a Welch's ANOVA test. The data represent fold change with 95% CI. ∗-p<0.05; NS – not signficant. DKO = double knockout, DKOR = double knockout + resveratrol, DKORI = double knockout + resveratrol + istradefylline.
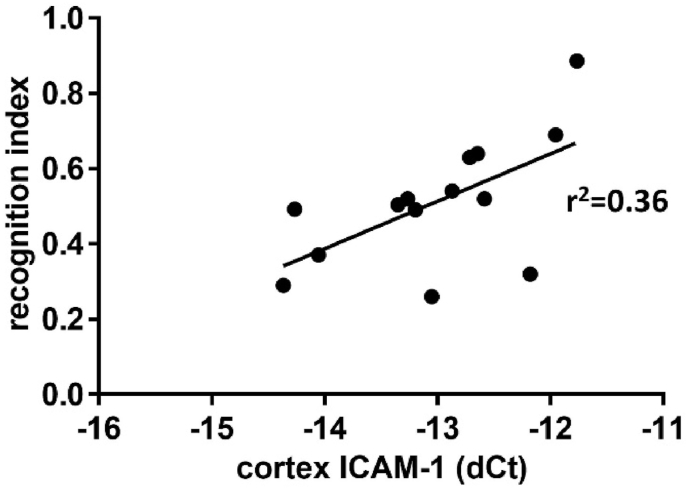
In the cortex, apoe/fas double knockout mice treated with resveratrol had increased cortical expression of ERα (1.25, 95%CI [0.92, 1.70]) compared to apoe/fas mice treated with resveratrol and istradefylline (0.76, 95%CI [0.55, 1.05]) but not compared to apoe/fas control mice (1.0, reference group; F(2, 14) = 4.378, p = 0.034; data not shown). Cortical expression of ICAM-1 was significantly correlated with novel object recognition where higher levels of ICAM-1 expression were associated with better working memory performance (r2 = 0.3619, N = 14, p = 0.0229; Fig. 4).
Fig. 4.
Association between ICAM-1 and working memory. There was a significant positive correlation between expression of ICAM-1 in the cortex and performance in the novel object recognition task independent of treatment group. N pairs = 14; p < 0.05.
4. Discussion
Neuropsychiatric lupus, a complication of the autoimmune disease systemic lupus erythematosus, has few treatment options other than symptomatic treatment, and can severely affect quality of life.22 Often, patients present acutely with cognitive changes that may not respond to anti-inflammatory treatment and can result in lasting cognitive dysfunction.23 Previous work from this laboratory has shown that resveratrol, a nutraceutical found in grapes and berries can ameliorate atherosclerotic-plaque promoting abnormalities in cholesterol handling in a murine model of lupus, in part through the adenosine A2A receptor. This study tested the hypothesis that resveratrol would also improve behavioral deficits in atherosclerosis-prone lupus mice. The results show that atherosclerosis-prone lupus mice treated with resveratrol performed better on a working memory task and showed a trend towards better performance on a motor coordination task, though only the latter showed a trend towards a significant difference. These behavioral improvements were blocked in mice treated with resveratrol and the adenosine A2A receptor blocker, istradefylline, indicating a detrimental effect of istradefylline administration in resveratrol-treated mice. Further, mice treated with resveratrol had, on average, higher expression of SIRT-1 in the hippocampus, which was not observed in mice administered both resveratrol and istradefylline. In addition, resveratrol-treated animals had, on average, lower expression of VEGF and CX3CL1 in the hippocampus, also blocked by istradefylline. In the cortex, there was higher expression of ERα, on average, in resveratrol-treated animals, also affected by the presence of istradefylline. However, in the cortex, increased expression of ICAM-1 was associated with better performance on the novel object recognition task, independent of treatment group.
Neuropsychiatric symptoms, including delirium, memory impairment, and motor incoordination, are found in 25–75% of lupus patients.24,25 NPSLE symptoms are associated with decreased brain white matter integrity and disease severity,26 as well as the presence of neuronal auto-antibodies.27 During acute episodes of neurolupus, there is evidence of increased immunoglobulin in the cerebral spinal fluid and clinical relapses are associated with a breakdown or worsening function of the blood-brain barrier.28,29 Additionally, changes in cerebral vasculature are present in NPSLE including vasculopathy and vasculitis.30 Vascular inflammation is mediated, in part, by vascular endothelial growth factor (VEGF) and recent data showed that a polymorphism in the gene encoding VEGF is associated with increased incidence of neuropsychiatric lupus.31 In some experimental disease models, reduced levels of VEGF in the brain have been associated with reduced inflammation, neuronal survival, and improved memory.32 Interestingly, in humans, although cognitive dysfunction is present in NPSLE, there is limited evidence of correlated anatomical changes.33
This study provides the first evidence that anti-inflammatory properties of resveratrol could play a role in mitigating neurolupus-induced brain dysfunction. Although our findings revealed that resveratrol administration led to a tendency towards increased working memory and a trend towards improved motor coordination in atherosclerosis-prone lupus mice, the biggest impact was seen in the istradefylline group with decreased cognitive function compared to resveratrol-treated mice. These results suggest that any trends seen in resveratrol-treated mice were A2A receptor dependent. Atherosclerosis-prone lupus mice treated with resveratrol have the lowest expression of VEGF and the highest performance on the working memory task, both significantly attenuated by blocking A2A. These findings are consistent with known A2AR engagement in events related to long-term potentiation (LTP) and synaptic plasticity.34,35 Improved coordination related to A2A agonism has also been previously seen in a mouse model of Niemann-Pick C disease, a rare lysosomal storage disorder caused by a missense mutation in the lysosomal cholesterol transport protein NPC1.36 This may be related to A2A effects on lipid handling.37,38
In some studies, adenosine A2A ligation has been found to be detrimental to neuronal health through activation of microglia.39 In fact, istradefylline is used as an adjunct to dopaminergic therapy in humans to treat motor symptoms in Parkinson's disease.40 It is interesting to note that while istradefylline is used to improve cognitive function in Parkinson's, the administration of istradefylline in our model impaired cognitive function in the presence of resveratrol. The opposing effects of A2A antagonism in Parkinson's versus in our atherosclerosis-prone lupus model may reflect the different contribution of A2A receptors in associated cognitive dysfunction across the different disease states.
Similarly, a recent study showed that resveratrol has anti-inflammatory properties in astroglia, which are blocked by adenosine receptor antagonists.41 In the present study, SIRT1, an NAD + -dependent histone deacetylase, was increased in mice treated with resveratrol and SIRT1 may counter negative effects of A2A receptors, particularly in microglia where it suppresses inflammatory responses,42 allowing beneficial adenosine A2A effects to predominate. Caffeine, a less-specific A2A receptor antagonist than istradefylline, could be used as an exposure in epidemiologic models to determine if caffeine consumption in lupus patients alters neurolupus risk, potentially highlighting the role of A2A modulation as a viable treatment option in targeting cognitive dysfunction in autoimmunity. Alternatively, selective A2A agonists (e.g. CGS) could be studied in mouse models of lupus to determine if stimulation of this receptor is associated with cognitive dysfunction. Although inflammation is typically associated with impaired cognition, this relationship is not always straightforward. For example, obesity, a pro-inflammatory state, is associated with impaired cognition, but not when the neurotactin receptor (CX3CR1) is reduced.43 In atherosclerosis-prone lupus mice, we show a decrease in neurotactin (CX3CL1), possibly indicating that resveratrol is modulating the activation state of microglia, the brain's resident immune cells, through neurotactin. Several studies using mouse models have shown that alternatively activated microglia are associated with improved neuronal or cognitive function.44, 45, 46 In addition, and independent of resveratrol treatment status, higher levels of the pro-inflammatory cell-surface marker ICAM-1 in the brain were associated with better working memory performance. Elevated ICAM-1 in the brain has been shown in other murine neurolupus models.53 Previous work has shown that high levels of ICAM-1 are detrimental to cognition47, 48, 49 but again, this relationship is not clear-cut and neurolupus mice given anti-ICAM antibodies did not show attenuation of inflammation in the choroid plexus.50
There are a few limitations in the current study. First, the administration of resveratrol in vivo presents certain difficulties, as the compound is chemically unstable, highly photosensitive and poorly soluble in water. We have addressed these issues to the extent possible in our facilities, as discussed in the Methods section. Moreover, resveratrol has a short biological half-life, due to fast metabolism in the liver and rapid clearance,51 although despite these limitations, resveratrol has been shown to cross the blood brain barrier.52
Nanotechnology has gained a great deal of attention in recent treatments of neurological disorders and is suggested to be a valuable approach to increase bioavailability of resveratrol, improving its solubility in water and reducing its degradation.53,54 However, we had no resources and access to the nanoencapsulation of resveratrol. Second, this study was a sub-study of an atherosclerosis experiment, so we were not able to include single knock out controls and istradefylline-only controls. Third, the impact of resveratrol may be specific to the highly inflamed and lipid-rich environment resulting when lupus and atherosclerosis co-exist, thus not translating to a single disease state of lupus alone. However, since atherosclerosis is seen commonly in persons with lupus, the overlap of NPSLE with atherosclerosis is substantial.7 Fourth, we only collected hemi-sections of hippocampus and cortex thus we cannot determine any sidedness effects or effects of resveratrol on other brain structures related to memory and coordination such as cerebellum. Fourth, we were not able to stratify analyses by animal sex since we did not have enough power to do so, thus these data do not delineate any specific impact of sex on behavioral or brain gene expression changes resulting from resveratrol treatment. Additionally, neither lipid profiles nor inflammatory markers in the blood or cerebrospinal fluid were quantified, so systemic effects of resveratrol were not determined as they were not the focus of this study. Finally, protein levels in corresponding brain regions were not measured due to limited tissue availability. Future behavioral studies will specifically target the measurement of protein levels.
Further studies are indicated with testing on more cognitive domains along with neuroanatomical changes in resveratrol-treated atherosclerosis-prone lupus mice in order to follow the complex pathways of interaction among atherosclerosis, lupus, and cognition. In summary, the work presented here indicates a potential role for resveratrol as a modulator of cognitive impairment associated with lupus.
Statement of author contributions
All authors participated in the design/interpretation of the study and analysis of the data. IV, IT, SEC, JDL, IHP, AP and SEC made substantial contributions to acquisition and/or interpretation of data. LJK, HAR, and ABR conducted the experiments. LJK and ABR wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by R21 AT007032-01A1 from The National Center for Complementary and Alternative Medicine and by the Elizabeth Daniell Research Fund.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2022.01.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
fig.
s1
References
- 1.Muscal E., Brey R.L. Neurologic manifestations of systemic lupus erythematosus in children and adults. Neurol Clin. 2010 Feb;28(1):61–73. doi: 10.1016/j.ncl.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appenzeller S., Costallat L.T., Cendes F. Neurolupus Arch Neurol. 2006;63(3):458–460. doi: 10.1001/archneur.63.3.458. [DOI] [PubMed] [Google Scholar]
- 3.McGlasson S., Wiseman S., Wardlaw J., Dhaun N., Hunt D.P.J. Neurological disease in lupus: toward a personalized medicine approach. Front Immunol. 2018 Jun 6;9:1146. doi: 10.3389/fimmu.2018.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeltsch-David H., Muller S. Neuropsychiatric systemic lupus erythematosus: pathogenesis and biomarkers. Nat Rev Neurol. 2014 Oct;10(10):579–596. doi: 10.1038/nrneurol.2014.148. [DOI] [PubMed] [Google Scholar]
- 5.Joseph F.G., Scolding N.J. Neurolupus Pract Neurol. 2010 Feb;10(1):4–15. doi: 10.1136/jnnp.2009.200071. [DOI] [PubMed] [Google Scholar]
- 6.Mackay M. Lupus brain fog: a biologic perspective on cognitive impairment, depression, and fatigue in systemic lupus erythematosus. Immunol Res. 2015 Dec;63(1-3):26–37. doi: 10.1007/s12026-015-8716-3. [DOI] [PubMed] [Google Scholar]
- 7.Manzi S., Meilahn E.N., Rairie J.E., et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 8.Du S.Q., Wang X.R., Xiao L.Y., et al. Molecular mechanisms of vascular dementia: what can Be learned from animal models of chronic cerebral hypoperfusion? Mol Neurobiol. 2017 Jul;54(5):3670–3682. doi: 10.1007/s12035-016-9915-1. [DOI] [PubMed] [Google Scholar]
- 9.Gorelick P.B., Scuteri A., Black S.E., et al. American heart association stroke council, council on epidemiology and prevention, council on cardiovascular nursing, council on cardiovascular radiology and intervention, and council on cardiovascular surgery and anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011 Sep;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Østergaard L., Engedal T.S., Moreton F., et al. Cerebral small vessel disease: capillary pathways to stroke and cognitive decline. J Cerebr Blood Flow Metabol. 2016 Feb;36(2):302–325. doi: 10.1177/0271678X15606723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pamfil C., Fanouriakis A., Damian L., et al. EULAR recommendations for neuropsychiatric systemic lupus erythematosus vs usual care: results from two European centres. Rheumatology. 2015;54(7):1270–1278. doi: 10.1093/rheumatology/keu482. [DOI] [PubMed] [Google Scholar]
- 12.Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006 Jun;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 13.Hung L.M., Chen J.K., Huang S.S., Lee R.S., Su M.J. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res. 2000 Aug 18;47(3):549–555. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- 14.Singh U.P., Singh N.P., Singh B., et al. Resveratrol (trans-3,5,4'-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Therapeut. 2010 Mar;332(3):829–839. doi: 10.1124/jpet.109.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wightman E.L., Haskell-Ramsay C.F., Reay J.L., et al. The effects of chronic transresveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br J Nutr. 2015 Nov 14;114(9):1427–1437. doi: 10.1017/S0007114515003037. [DOI] [PubMed] [Google Scholar]
- 16.Vasamsetti S.B., Karnewar S., Gopoju R., et al. Resveratrol attenuates monocyte-tomacrophage differentiation and associated inflammation via modulation of intracellular GSH homeostasis: relevance in atherosclerosis. Free Radic Biol Med. 2016 May 5;96:392–405. doi: 10.1016/j.freeradbiomed.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Voloshyna I., Hai O., Littlefield M.J., Carsons S.E., Reiss A.B. Resveratrol mediates anti-atherogenic effects on cholesterol flux in human macrophages and endothelium via PPAR-γ and adenosine. Eur J Pharmacol. 2013;698(1-3):299–309. doi: 10.1016/j.ejphar.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Voloshyna I., Teboul I., Littlefield M.J., et al. Resveratrol counters systemic lupus erythematosus-associated atherogenicity by normalizing cholesterol efflux. Exp Biol Med. 2016 Aug;241(14):1611–1619. doi: 10.1177/1535370216647181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng X., Li H., Rumbin A.A., et al. ApoE−/−Fas−/− C57BL/6 mice: a novel murine model simultaneously exhibits lupus nephritis, atherosclerosis, and osteopenia. J Lipid Res. 2007;48:794–805. doi: 10.1194/jlr.M600512-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Piedrahita J.A., Zhang S.H., Hagaman J.R., Oliver P.M., Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croker B.P., Gilkeson G., Morel L. Genetic interactions between susceptibility loci reveal epistatic pathogenic networks in murine lupus. Gene Immun. 2003;4:575–585. doi: 10.1038/sj.gene.6364028. [DOI] [PubMed] [Google Scholar]
- 22.Bendorius M., Po C., Muller S., Jeltsch-David H. From systemic inflammation to neuroinflammation: the case of neurolupus. Int J Mol Sci. 2018 Nov 13;19(11) doi: 10.3390/ijms19113588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duarte-García A., Romero-Díaz J., Juárez S., et al. Disease activity, autoantibodies, and inflammatory molecules in serum and cerebrospinal fluid of patients with Systemic Lupus Erythematosus and Cognitive Dysfunction. PLoS One. 2018 May 3;13(5) doi: 10.1371/journal.pone.0196487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shastri R., Shah G., Wang P., et al. MR diffusion tractography to identify and characterize microstructural white matter tract changes in systemic lupus erythematosus patients. Acad Radiol. 2016 Nov;23(11):1431–1440. doi: 10.1016/j.acra.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Afeltra A., Garzia P., Mitterhofer A.P., et al. Neuropsychiatric lupus syndromes: relationship with antiphospholipid antibodies. Neurology. 2003;61(1):108–110. doi: 10.1212/01.wnl.0000058904.94330.a7. [DOI] [PubMed] [Google Scholar]; a Brey R.L., Holliday S.L., Saklad A.R., Navarrete M.G., Hermosillo-Romo D., et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology. 2002;58 doi: 10.1212/wnl.58.8.1214. 1214–110. [DOI] [PubMed] [Google Scholar]
- 26.Karaaslan Z., Ekizoğlu E., Tektürk P., et al. Investigation of neuronal auto-antibodies in systemic lupus erythematosus patients with epilepsy. Epilepsy Res. 2017 Jan;129:132–137. doi: 10.1016/j.eplepsyres.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 27.McLean B.N., Miller D., Thompson E.J. Oligoclonal banding of IgG in CSF, blood-brain barrier function, and MRI findings in patients with sarcoidosis, systemic lupus erythematosus, and Behçet's disease involving the nervous system. J Neurol Neurosurg Psychiatry. 1995 May;58(5):548–554. doi: 10.1136/jnnp.58.5.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan Z., Zhou Y., Li X., et al. Brain magnetic resonance imaging, cerebrospinal fluid, and autoantibody profile in 118 patients with neuropsychiatric lupus. Clin Rheumatol. 2018 Jan;37(1):227–233. doi: 10.1007/s10067-017-3891-3. [DOI] [PubMed] [Google Scholar]
- 29.Böckle B.C., Jara D., Aichhorn K., et al. Cerebral large vessel vasculitis in systemic lupus erythematosus. Lupus. 2014 Nov;23(13):1417–1421. doi: 10.1177/0961203314541689. [DOI] [PubMed] [Google Scholar]
- 30.Taha S., Gamal S.M., Nabil M., et al. Vascular endothelial growth factor G1612A (rs10434) gene polymorphism and neuropsychiatric manifestations in systemic lupus erythematosus patients. Rev Bras Reumatol Engl Ed. 2017 Mar - Apr;57(2):149–153. doi: 10.1016/j.rbre.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira W.H., Nunes A.K., França M.E., et al. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res. 2016 Aug 1;1644:149–160. doi: 10.1016/j.brainres.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Kalinowska-Łyszczarz A., Pawlak M.A., Pietrzak A., et al. Subcortical gray matter atrophy is associated with cognitive deficit in multiple sclerosis but not in systemic lupus erythematosus patients. Lupus. 2018 Apr;27(4):610–620. doi: 10.1177/0961203317735186. [DOI] [PubMed] [Google Scholar]
- 33.Li Z., Chen X., Wang T., et al. The corticostriatal adenosine A2A receptor controls maintenance and retrieval of spatial working memory. Biol Psychiatr. 2018;83(6):530–541. doi: 10.1016/j.biopsych.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Cunha R.A. Different cellular sources and different roles of adenosine: a1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem Int. 2008;52:65–72. doi: 10.1016/j.neuint.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Ferrante A., Pezzola A., Matteucci A., et al. The adenosine A2A receptor agonist T1-11 ameliorates neurovisceral symptoms and extends the lifespan of a mouse model of Niemann-Pick type C disease. Neurobiol Dis. 2018 Feb;110:1–11. doi: 10.1016/j.nbd.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Reiss A.B., Cronstein B.N. Regulation of foam cells by adenosine. Arterioscler Thromb Vasc Biol. 2012;32(4):879–886. doi: 10.1161/ATVBAHA.111.226878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiss A.B., Rahman M.M., Chan E.S.L., Montesinos M.C., Awadallah N.W., Cronstein B.N. Adenosine A2A receptor occupancy stimulates expression of proteins involved in reverse cholesterol transport and inhibits foam cell formation in macrophages. J Leukoc Biol. 2004;76(3):727–734. doi: 10.1189/jlb.0204107. [DOI] [PubMed] [Google Scholar]
- 38.Chen P.Z., He W.J., Zhu Z.R., et al. Adenosine A2A receptor involves in neuroinflammation-mediated cognitive decline through activating microglia under acute hypobaric hypoxia. Behav Brain Res. 2018 Mar 6;347:99–107. doi: 10.1016/j.bbr.2018.02.038. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki K., Miyamoto M., Miyamoto T., et al. Istradefylline improves daytime sleepiness in patients with Parkinson's disease: an open-label, 3-month study. J Neurol Sci. 2017 Sep 15;380:230–233. doi: 10.1016/j.jns.2017.07.045. [DOI] [PubMed] [Google Scholar]
- 40.Bobermin L.D., Roppa R.H.A., Quincozes-Santos A. Adenosine receptors as a new target for resveratrol-mediated glioprotection. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2019 Mar 1;1865(3):634–647. doi: 10.1016/j.bbadis.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Zhang S., Gao L., Liu X., Lu T., Xie C., Jia J. Resveratrol attenuates microglial activation via SIRT1-SOCS1 pathway. Evid Based Compl Alternat Med. 2017;2017:8791832. doi: 10.1155/2017/8791832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cope E.C., LaMarca E.A., Monari P.K., et al. Microglia play an active role in obesity-associated cognitive decline. J Neurosci. 2018 Oct 10;38(41):8889–8904. doi: 10.1523/JNEUROSCI.0789-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao Q., Yu W., Tian Q., et al. Chitinase1 contributed to a potential protection via microglia polarization and Aβ oligomer reduction in D-galactose and aluminum-induced rat model with cognitive impairments. Neuroscience. 2017 Jul 4;355:61–70. doi: 10.1016/j.neuroscience.2017.04.050. [DOI] [PubMed] [Google Scholar]
- 44.Hopperton K.E., Trépanier M.O., Giuliano V., Bazinet R.P. Brain omega-3 polyunsaturated fatty acids modulate microglia cell number and morphology in response to intracerebroventricular amyloid-β 1-40 in mice. J Neuroinflammation. 2016 Sep 29;13(1):257. doi: 10.1186/s12974-016-0721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porrini V., Lanzillotta A., Branca C., et al. CHF5074 (CSP-1103) induces microglia alternative activation in plaque-free Tg2576 mice and primary glial cultures exposed to beta-amyloid. Neuroscience. 2015 Aug 27;302:112–120. doi: 10.1016/j.neuroscience.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 46.Zameer A., Hoffman S.A. Increased ICAM-1 and VCAM-1 expression in the brains of autoimmune mice. J Neuroimmunol. 2003 Sep;142(1-2):67–74. doi: 10.1016/s0165-5728(03)00262-5. [DOI] [PubMed] [Google Scholar]
- 47.Pittet F., Van Caenegem N., Hicks-Nelson A.R., et al. Maternal social environment affects offspring cognition through behavioural and immune pathways in rats. J Neuroendocrinol. 2019 Mar 19 doi: 10.1111/jne.12711. [DOI] [PubMed] [Google Scholar]
- 48.Kuban K.C., Joseph R.M., O'Shea T.M., et al. Extremely low gestational age newborn (ELGAN) study investigators. Circulating inflammatory-associated proteins in the first month of life and cognitive impairment at age 10 Years in children born extremely preterm. J Pediatr. 2017 Jan;180 doi: 10.1016/j.jpeds.2016.09.054. 116-123.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Todd M.A. Inflammation and cognition in older adults: evidence from taiwan. Biodemogr Soc Biol. 2017;63(4):309–323. doi: 10.1080/19485565.2017.1403305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brey R.L., Amato A.A., Kagan-Hallet K., Rhine C.B., Stallworth C.L. Anti-intercellular adhesion molecule-1 (ICAM-1) antibody treatment prevents central and peripheral nervous system disease in autoimmune-prone mice. Lupus. 1997;6(8):645–651. doi: 10.1177/096120339700600805. [DOI] [PubMed] [Google Scholar]
- 51.Francioso A., Mastromarino P., Masci A., Erme M., Mosca L. Chemistry, stability and bioavailability of resveratrol. Med Chem. 2014;10:237–245. doi: 10.2174/15734064113096660053. [DOI] [PubMed] [Google Scholar]
- 52.Bastianetto S., Ménard C., Quirion R. Neuroprotective action of resveratrol. Biochim Biophys Acta. 2015;1852(6):1195–1201. doi: 10.1016/j.bbadis.2014.09.011. 2015 Jun. [DOI] [PubMed] [Google Scholar]
- 53.Summerlin N., Soo E., Thakur S., Qu Z., Jambhrunkar S., Popat A. Resveratrol nanoformulations: challenges and opportunities. Int J Pharm. 2015;479:282–290. doi: 10.1016/j.ijpharm.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Andrade S., Ramalho M.J., Pereira M.C., Loureiro J.A. Resveratrol brain delivery for neurological disorders prevention and treatment. Front Pharmacol. 2018;9:1261. doi: 10.3389/fphar.2018.01261. [DOI] [PMC free article] [PubMed] [Google Scholar]