Abstract
The three-component naphthalene dioxygenase (NDO) enzyme system carries out the first step in the aerobic degradation of naphthalene by Pseudomonas sp. strain NCIB 9816-4. The three-dimensional structure of NDO revealed that several of the amino acids at the active site of the oxygenase are hydrophobic, which is consistent with the enzyme's preference for aromatic hydrocarbon substrates. Although NDO catalyzes cis-dihydroxylation of a wide range of substrates, it is highly regio- and enantioselective. Site-directed mutagenesis was used to determine the contributions of several active-site residues to these aspects of catalysis. Amino acid substitutions at Asn-201, Phe-202, Val-260, Trp-316, Thr-351, Trp-358, and Met-366 had little or no effect on product formation with naphthalene or biphenyl as substrates and had slight but significant effects on product formation from phenanthrene. Amino acid substitutions at Phe-352 resulted in the formation of cis-naphthalene dihydrodiol with altered stereochemistry [92 to 96% (+)-1R,2S], compared to the enantiomerically pure [>99% (+)-1R,2S] product formed by the wild-type enzyme. Substitutions at position 352 changed the site of oxidation of biphenyl and phenanthrene. Substitution of alanine for Asp-362, a ligand to the active-site iron, resulted in a completely inactive enzyme.
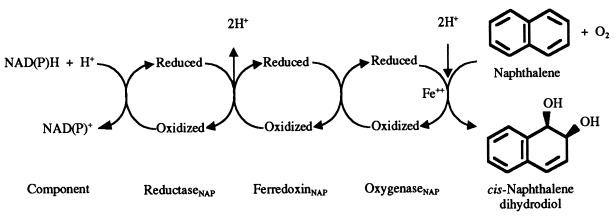
The naphthalene dioxygenase (NDO) enzyme system (EC 1.14.12.12) from Pseudomonas sp. strain NCIB 9816-4 catalyzes the first step in the aerobic degradation of naphthalene. In this reaction (Fig. 1), NDO adds both atoms of oxygen to the aromatic nucleus of naphthalene, forming homochiral (+)-cis-(1R,2S)-dihydroxy-1,2-dihydronaphthalene (cis-naphthalene dihydrodiol) (30, 31). In addition, NDO catalyzes the oxidation of a wide variety of aromatic compounds to enantiomerically pure chiral products (8, 56). The NDO system consists of three components, each of which has been purified and characterized. An iron-sulfur flavoprotein reductase and an iron-sulfur ferredoxin transfer electrons from NAD(P)H to the catalytic oxygenase component (14, 15, 23, 24). The oxygenase is composed of large and small subunits, α and β, respectively, that are in an α3β3 configuration (35). NDO is a member of a large family of oxygenases whose α subunits contain a Rieske [2Fe-2S] center and mononuclear nonheme iron (10). In the NDO system, electrons are transferred from the Rieske center of the ferredoxin to the Rieske center of the oxygenase α subunit. The reduced Rieske center in one α subunit transfers an electron to mononuclear iron at the active site in an adjacent α subunit (35, 50). His-208, His-213, and Asp-362 coordinate the active-site iron, forming a 2-His-1-carboxylate facial triad. This structural motif is found in other mononuclear nonheme iron enzymes, including tyrosine hydroxylase, isopenicillin synthetase, and 2,3-dihydroxybiphenyl 1,2-dioxygenase (26, 40). Asp-205 in the catalytic domain of the NDO α subunit is hydrogen bonded to His-208 and to His-104 in the adjacent α subunit (Fig. 2). His-104 is one of the Rieske center ligands. Asp-205 has been shown to be required for efficient electron transfer from the Rieske center to the active-site iron (50).
FIG. 1.
Reaction catalyzed by the three-component NDO system.
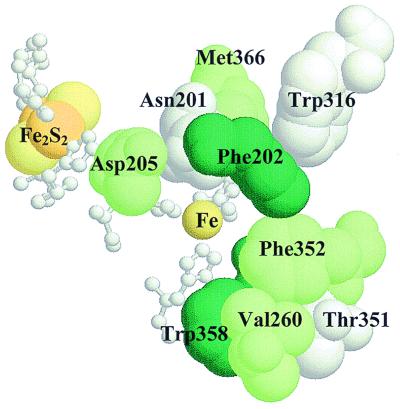
FIG. 2.
Structure of the active site of NDO, showing the Rieske [2Fe-2S] center and mononuclear iron in adjacent α subunits with their coordinating amino acids (shown in white ball-and-stick format). Also shown are Asp-205, an amino acid important for efficient electron transfer between the two redox centers, and the amino acids that were targets for site-directed mutagenesis in this study. RasMol (version 2.6) was used to generate the view of the active site.
Recent studies have shown that the oxygenase α subunits are responsible for determining the substrate specificities of NDO and the related enzymes 2-nitrotoluene dioxygenase (2NTDO) from Pseudomonas sp. strain JS42 and 2,4-dinitrotoluene dioxygenase (DNTDO) from Burkholderia sp. strain DNT (48, 49). The crystal structure of NDO allowed the identification of amino acids near the active-site iron atom in the catalytic domain of the α subunit (35) (Fig. 2). We have used this information to design site-directed mutations in the α subunit of NDO in order to identify amino acids near the active site that control the regioselectivity and enantioselectivity of NDO. Several variants of NDO with amino acid substitutions at the active site were generated and characterized. Those with substitutions at position 352 resulted in the largest changes in NDO specificity.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli strains DH5α and JM109(DE3) were used for subcloning and gene expression experiments, respectively. Competent E. coli strains ES1301 mutS and JM109 were purchased from Promega Corp., Madison, Wis., and used in the site-directed mutagenesis procedure described below.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α | Δ(lacZYA-argF)U169 hsdR17 relA1 supE44 endA1 recA1 thi gyrA96 φ80dlacZΔM15 | Life Technologies, Gaithersburg, Md. |
| JM109 | endA1 recA1 gyrA96 thi hsdR17 relA1 supE44 Δ(lac-proAB) mcrA [F′ traD36 proAB+ lacIqZΔM15] | 65 |
| JM109(DE3) | endA1 recA1 gyrA96 thi hsdR17 relA1 supE44 Δ(lac-proAB) mcrA [F′ traD36 proAB+ lacIqZΔM15] λ(DE3) | Promega Corp. |
| ES1301 mutS | KmrlacZ53 mutS201::Tn5 thyA36 rha-5 metB1 deoC IN(rrnD-rrnE) | Promega Corp. |
| Plasmids | ||
| pDTG141 | Apr; nahAaAbAcAd (encoding the naphthalene dioxygenase components reductaseNAP, ferredoxinNAP, and large and small subunits of the oxygenase, respectively) under the control of the T7 promoter of pT7-5 | 61 |
| pMASTER-1 | Tcr Aps; pALTER-1 carrying the KpnI-XbaI fragment of pDTG141 (nahAc′Ad) | 50 |
Kmr, kanamycin resistance; Apr, ampicillin resistance; Tcr, tetracycline resistance.
Media and growth conditions.
E. coli strains were grown at 37°C in Luria-Bertani medium (12) or Terrific broth medium (42). Antibiotics were added to the following final concentrations as appropriate: ampicillin, 150 μg/ml; tetracycline, 12.5 μg/ml. To produce induced cells for biotransformation studies, JM109(DE3) strains carrying plasmids of interest were grown at 30°C in minimal salts medium (MSB) (60) containing 10 mM glucose, 0.1 mM thiamine, and ampicillin. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 100 μM when the culture turbidity reached 0.6 to 0.8 at 660 nm. After a 2-h induction, biotransformations were initiated as described below. For plates, MSB was solidified with 1.8% Noble agar (Difco Laboratories, Detroit, Mich.) and Luria-Bertani medium was solidified with 1.5% Bacto Agar (Difco Laboratories).
Molecular techniques.
Plasmid DNA was isolated as described previously (42) or by using a Midi Kit (Qiagen, Inc., Chatsworth, Calif.). For nucleotide sequencing, DNA was further purified using a Centricon 100 filter unit (Amicon, Inc., Beverly, Mass.). Restriction digests were performed as suggested by the enzyme suppliers (New England Biolabs, Inc., Beverly, Mass., and Promega Corp.). DNA fragments were purified from gel slices using the GeneClean Spin Kit according to the instructions of the manufacturer (Bio 101, Vista, Calif.). Ligation reactions, transformation of E. coli strains, and agarose gel electrophoresis were performed by standard procedures (59).
Site-directed mutagenesis.
Mutagenesis of nahAc was carried out with the Altered Sites II in vitro Mutagenesis System according to the instructions of the manufacturer (Promega Corp.). Plasmid pMASTER-1 (50), which contains the 3′ end of the nahAc gene and the complete nahAd gene (which encode the α and β subunits of NDO, respectively), was used as the template for mutagenesis. Each mutagenic oligonucleotide was designed with a silent mutation that altered the restriction pattern of the plasmid (Table 2) to facilitate screening for clones carrying the desired mutation. Phosphorylated oligonucleotides used for mutagenesis were synthesized by Genosys Biotechnologies Inc., Midland, Tex. The nucleotide sequences of both strands of the entire insertion in pMASTER-1 were determined for each mutant. Fluorescent automated DNA sequencing was carried out by the University of Iowa DNA Facility using an Applied Biosystems 373A automated DNA sequencer. After verification of each mutation by restriction digestion and sequence analysis, the 1.5-kb KpnI-XbaI fragments carrying each mutation were individually cloned into KpnI-XbaI-digested pDTG141. After this subcloning step, the presence of each mutation was verified by restriction and sequence analyses. The resulting derivatives of pDTG141 were introduced into JM109(DE3) for expression studies, and in this way each mutant protein was produced from an identical expression system.
TABLE 2.
Amino acid substitutions in the α subunit of NDO generated by site-directed mutagenesis
| Mutation | Mutagenic oligonucleotidea | Restriction site change | Indigo formationb |
|---|---|---|---|
| N201A | 5′-GAGGCACCCGCGGAAGCTTTTGTGGGAGATGCA-3′ | HindIII | + |
| N201Q | 5′-GCACCCGCGGAACAATTTGTGGGAGATGCA-3′ | Tsp509I | + |
| N201S | 5′-CCGCGGAAAGCTTTGTGGGAG-3′ | HindIII | ++ |
| F202L | 5′-CCGCGGAAAAGCTTGTGGGAGATG-3′ | HindIII | − |
| F202V | 5′-CGCGGAAAACGTTGTGGGAGATG-3′ | AclI | ++ |
| V260A | 5′-ATATTCAGGTGCGCATAGCGCAG-3′ | FspI | ++ |
| V260L | 5′-GGACGGATATTCAGGGCTCCATAGCGCAGACTTG-3′ | BanII | ++ |
| V260N | 5′-GACGGATATTCAGGTAACCATAGCGCAGACTTG-3′ | BstEII | ++ |
| W316A | 5′-GGTGTTTTCAAAGTCGCGAACCCGATCGAC-3′ | NruI | +++ |
| T351N | 5′-CTGTTCAGCGAAACTTCGGGCCTGCT-3′ | Remove AclI | ++ |
| T351R | 5′-CTGTTCAGCGAAGGTTCGGGCCTGCT-3′ | Remove AclI | + |
| T351S | 5′-CTGTTCAGCGAAGCTTCGGGCCTGCT-3′ | HindIII | +++ |
| F352L | 5′-TTCAGCGAACGCTCGGGCCTGC-3′ | Remove AclI | ++ |
| F352V | 5′-TTCAGCGAACGGTCGGGCCTGC-3′ | Remove AclI | + |
| W358A | 5′-GGCCTGCTGGCTTCGCGGAAAGCGACGACA-3′ | None | −c |
| D362A | 5′-GAAAGCGACGCCAATGACAAT-3′ | BsaHI | − |
| M366W | 5′-ACGACAATGACAATTGGGAAACAGCTTCGC-3′ | MfeI | ++ |
Underlined bases indicate the position of the introduced or eliminated restriction site. Base changes are in boldface.
Indigo formation was monitored after 8 h as described in Materials and Methods. +++, colonies were dark blue [corresponds to JM109(DE3)(pDTG141), expressing wild-type NDO]; ++, colonies were medium blue; +, colonies were pale blue; −, no blue color [corresponds to negative control, JM109(DE3)(pT7-5)].
Colonies were pale blue after 12 h.
Whole-cell biotransformations.
Induced E. coli cultures (50 ml) were supplemented with 20 mM glucose and 80 mM phosphate buffer (pH 7.2). Solid substrates (naphthalene, biphenyl, or phenanthrene) were added to a final concentration of 0.025% (wt/vol). Cultures were incubated at 30°C with shaking (250 rpm) for 15 to 18 h. To obtain cells for large-scale biotransformations to produce cis-biphenyl 3,4-dihydrodiol, JM109(DE3)(pDTG141-F352V) was grown at 27°C in MSB containing glucose, thiamine, and ampicillin in a 10-liter Biostat B fermentor (B. Braun Biotech International, Melsungen, Germany). Automated addition of NH4OH was used to maintain the pH at 7.3, and a low glucose feed rate was used to maintain the dissolved O2 concentration at approximately 25% saturation. Cultures were induced for 3 h with 150 μM IPTG when the optical density of the culture (660 nm) reached approximately 0.7. Induced cultures (5.5 liters) were incubated at 27°C for 14 to 17 h with 0.025% (wt/vol) substrate (biphenyl or phenanthrene), high agitation (700 rpm), automated pH control (pH 7.3), and a slow glucose feed.
Indigo formation.
JM109(DE3) strains carrying pDTG141 derivatives with the various mutations were grown overnight at 37°C on nitrocellulose filters placed on MSB agar plates containing 10 mM glucose, 0.1 mM thiamine, and 150 μg of ampicillin per ml. Dried Whatman no. 1 filter papers that had been soaked in a 10% solution of indole dissolved in acetone were placed in the petri dish covers after colony formation. Production of indigo from indole vapor by NDO (16) was observed as colonies turned blue. No induction was carried out for these studies.
Separation and identification of products.
Culture supernatants from whole-cell biotransformation experiments were extracted with sodium hydroxide-washed ethyl acetate and analyzed by thin-layer chromatography (TLC) (57). Phenyl boronic acid (PBA) derivatives (27) were prepared as previously described (55). PBA-derivatized extracts were analyzed by gas chromatography-mass spectrometry (GC-MS) as previously described (54). cis-Naphthalene dihydrodiol was purified by preparative-layer chromatography with chloroform-acetone (8:2) (58). Regioisomers of biphenyl dihydrodiol were separated by preparative-layer chromatography (1.0- or 2.0-mm thickness; E. Merck Industries, Inc., Gibbstown, N.J.) using multiple elution (three or four developments) with chloroform-acetone (9:1). cis-Biphenyl 3,4-dihydrodiol was also purified by radial-dispersion chromatography using a Chromatotron (Harrison Research, Palo Alto, Calif.). Extracts in chloroform containing 0.1% triethylamine were applied to 2.0-mm-thick silica plates and eluted at a flow rate of 7 ml/min with a chloroform-acetone step gradient (0 to 15% acetone in 3% steps over 1 h; 0.1% triethylamine was present at each step). Fractions (8 ml) were analyzed by TLC, and those containing cis-biphenyl 3,4-dihydrodiol were combined and concentrated at 35°C under reduced pressure.
Chiral stationary-phase high-pressure liquid chromatography was used to resolve the enantiomers of cis-naphthalene dihydrodiol. A Chiralcel OJ column (Chiral Technologies, Exton, Pa.) was used as described previously (58). Under these conditions, the (+)-(1R,2S)- and (−)-(1S,2R)-enantiomers of cis-naphthalene dihydrodiol eluted with retention times of 30 and 33 min, respectively. Proton (1H) nuclear magnetic resonance (NMR) spectra were acquired on the Varian UNITY-500 500-MHz spectrometer in the College of Medicine NMR Facility at the University of Iowa. All spectra were obtained using an 8-s relaxation delay, a 5-s acquisition time, a spectral width of 12 ppm, and a 90-degree pulse width of 6.6 μs. Samples were prepared as previously described (58). Optical rotations were determined at 25°C using a Jasco P1020 polarimeter with a 589-nm-wavelength Na lamp. The results are the average of rotations given by three independently purified cis-biphenyl 3,4-dihydrodiol samples. High-resolution mass spectra were recorded (by Lynn Teesch, HR-MS Facility, The University of Iowa) on a VG ZAB-HF mass spectrometer equipped with direct inlet probe. Absorbance spectra (200 to 350 nm) were recorded on a Beckman DU-70 spectrophotometer.
Chemicals.
Naphthalene was obtained from Fisher Scientific Co., Pittsburgh, Pa. Indole, biphenyl, phenanthrene, and 4-hydroxybiphenyl were purchased from Aldrich Chemical Co., Milwaukee, Wis. Synthetic (+/−)-cis-naphthalene dihydrodiol and homochiral (+)-cis-naphthalene dihydrodiol were prepared as previously described (29, 31, 53). Synthetic cis-phenanthrene 9,10-dihydrodiol was provided by Derek Boyd.
Gel electrophoresis and Western blot analyses.
Cell pellets (from 1-ml culture suspensions) were resuspended in 200 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample loading buffer (2) and boiled for 10 min, and samples (15 μl each) were separated by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis (2). Gels were subjected to Western blotting using an antibody specific for the α subunit of NDO (49) as described previously (25, 44). Antigens were visualized using alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (Pierce, Rockford, Ill.).
RESULTS
Construction and preliminary analysis of modified NDO proteins.
Nine positions near the active site in the α subunit of NDO were chosen for site-directed mutagenesis. Based on the crystal structure of NDO (35), Asn-201, Phe-202, Val-260, Trp-316, Phe-352, Trp-358, and Met-366 are located near enough to the mononuclear iron to interact with substrates in the active site (Fig. 2). Asn-201 is positioned too far from the iron atom to be a ligand in the crystallized form of NDO, but was suggested as a possible ligand during some stage of the catalytic cycle. According to the NDO structure, Asp-362 is one of three amino acids that coordinate the iron at the active site (35). Asp-362 was replaced by alanine in order to disrupt iron coordination. Amino acid substitutions were also made at position 351, since the corresponding amino acid has been shown to be critical in determining polychlorinated biphenyl (PCB) congener specificity in biphenyl dioxygenase (36, 45). Site-directed mutations made in the α subunit of NDO are shown in Table 2. In most cases, small hydrophobic amino acids (alanine, valine, and leucine) were substituted for larger hydrophobic amino acids such as phenylalanine and tryptophan in order to change the size and/or shape of the active-site pocket. In some cases, amino acid substitutions were chosen based on alignments of various related dioxygenase sequences (see Discussion).
Indigo formation was used as the first screen for NDO activity. Freshly grown cells of JM109(DE3) carrying modified pDTG141 plasmids were incubated in the presence of indole. Most strains carrying mutant NDO enzymes formed blue colonies in the presence of indole (Table 2). Strains producing F202L and D362A mutant NDO enzymes formed white colonies, suggesting either that these enzymes were inactive or that indole was not a substrate for the modified enzymes. The strain carrying the W358A substitution in NDO formed pale blue colonies upon extended incubation with indole, indicating very weak activity with indole as a substrate.
Production of mutant NDO α subunits.
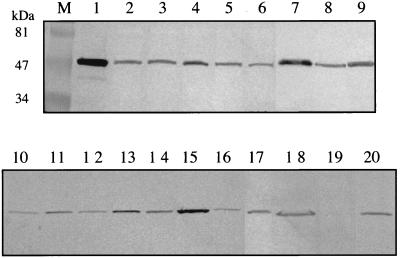
Formation of mutant α subunits was verified in Western blots using whole-cell protein samples from induced JM109(DE3) carrying modified pDTG141 plasmids. A monoclonal antibody specific for the α subunit of NDO was used (49). The results show that all mutant constructs formed full-length α subunits and that there were small variations in the amounts of each mutant protein produced (Fig. 3). More importantly, these results demonstrate that the inability of the D362A and F202L NDO variants to produce products (see below) was not due to the absence of protein. However, we cannot rule out the possibility that the mutations affected the solubility of the proteins or the specificity of the antibody interaction.
FIG. 3.
Western blots showing α subunits formed by JM109(DE3) carrying pDTG141 derivatives with mutations as indicated below. A monoclonal antibody specific for the α subunit of NDO was used as described in Materials and Methods. Lanes: M, prestained molecular mass markers (Bio-Rad Laboratories, Hercules, Calif.); 1, purified wild-type NDO (2 μg); 2, F202L; 3, F202V; 4, V260A; 5, V260L; 6, V260N; 7, N201Q; 8, N201A; 9, N201S; 10, W316A; 11, T351N; 12, T351R; 13, T351S; 14, F352L; 15, F352V; 16, W358A; 17, D362A; 18, M366W; 19, pT7-5 (negative control); 20, wild-type NDO (pDTG141).
Biotransformations with naphthalene as substrate.
Wild-type NDO converts naphthalene to cis-naphthalene 1,2-dihydrodiol (30, 31). Biotransformations with naphthalene resulted in the formation of cis-naphthalene 1,2-dihydrodiol by all NDO variants except those carrying the F202L and D362A substitutions, which formed no product. W358A transformations were very inefficient, with less than 5% of the substrate transformed within 15 h as judged by GC-MS analysis of extracted culture supernatants. In contrast, wild-type NDO transformed >50% of the naphthalene in less than 5 h. The wild-type and all mutant NDO enzymes formed enantiomerically pure (>99%) (+)-(1R,2S)-cis-naphthalene dihydrodiol, except for those with amino acid substitutions at Phe-352. The F352V and F352L variants formed 92 and 96% (+)-(1R,2S)-cis-naphthalene dihydrodiol, respectively. This result provides the first evidence of the importance of a specific amino acid, Phe-352, in determining the enantioselectivity of NDO.
Biotransformations with biphenyl as substrate.
Wild-type NDO oxidized biphenyl to two metabolites which were detected by TLC. The major metabolite (Rf, 0.2) and the minor metabolite (Rf, 0.18) dehydrated to phenolic products (M+, 170) when analyzed by GC-MS. These results suggested that both metabolites were dihydrodiol isomers, and this was confirmed by GC-MS of their stable respective phenyl boronic acid derivatives, which gave molecular ions at m/z 274. The major metabolite (87% relative yield) had a retention time of 13.8 min and was identical to cis-2,3-dihydroxy-1-phenylcyclohexa-4,6-diene (cis-biphenyl 2,3-dihydrodiol) produced from biphenyl by Sphingomonas yanoikuyae B8/36 (formerly Beijerinckia sp. strain B8/36) (20). The minor PBA-derivatized product (13% relative yield) had a retention time of 14.2 min and was identified as cis-3,4-dihydroxy-1-phenylcyclohexa-1,5-diene (cis-biphenyl 3,4-dihydrodiol; see below).
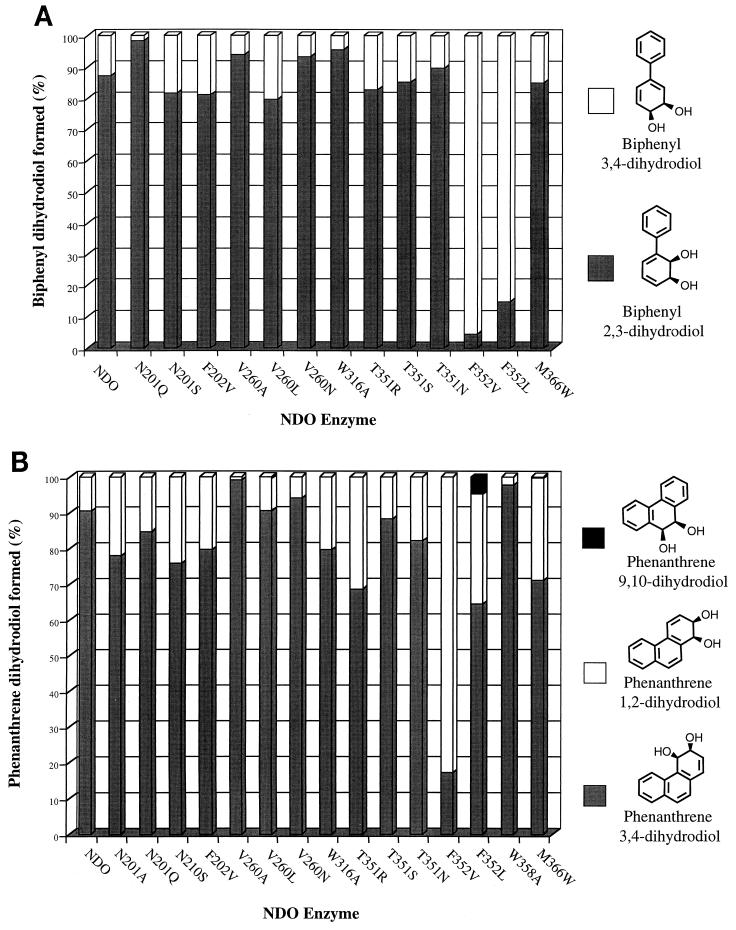
The F202L and D362A mutants formed no products from biphenyl. Mutants N201A and W358A formed only trace amounts of cis-biphenyl 2,3-dihydrodiol. Amino acid substitutions at N201, F202, V260, W316, and T351 had slight effects on the regiospecificity of NDO, as seen by the product distributions shown in Fig. 4A. However, both NDO mutants with changes at position 352 formed cis-biphenyl 3,4-dihydrodiol as the major product (Fig. 4A). The enzyme with the largest specificity change, F352V, formed 96% cis-biphenyl 3,4-dihydrodiol.
FIG. 4.
Product distributions with biphenyl (A) and phenanthrene (B) as substrates. Extracts of culture supernatants from E. coli strains producing wild-type and mutant NDO enzymes were derivatized with PBA and subjected to GC-MS analysis as described in Materials and Methods. Product distributions were determined from the GC-MS peak area integrations of total ion current chromatograms. The data shown are averages from at least three experiments, and standard deviations were 5% or less. Absolute stereochemistry is not intended.
Identification and characterization of cis-biphenyl 3,4-dihydrodiol.
The second biphenyl transformation product ran slightly slower than cis-biphenyl 2,3-dihydrodiol on TLC plates and, when analyzed by GC-MS as its PBA derivative, had a retention time of 14.2 min, compared to 13.8 min for the PBA derivative of cis-biphenyl 2,3-dihydrodiol. The products formed from biphenyl by the F352V mutant were isolated by radial-dispersion chromatography. Approximately 140 mg of crude extract was applied to a 2.0-mm-thick silica Chromatotron plate and eluted as described in Materials and Methods to allow isolation of 40 to 60 mg of pure cis-biphenyl 3,4-dihydrodiol and 1 to 2 mg of cis-biphenyl 2,3-dihydrodiol (fractions eluting before the 3,4-diol). The 3,4-regiochemistry of the diol and 1H NMR shift assignments were established by chemical shift multiplicities and independent H-H decoupling experiments. The 3,4-regiochemistry of the dihydrodiol was apparent by decoupling at H-3 (4.31 ppm), which reduced the multiplicity of the H-2 signal (ddd, 6.16) to a singlet with fine splitting. Acid dehydration resulted in the formation of a product that coeluted with authentic 4-hydroxybiphenyl in TLC and GC-MS analyses.
Physical characteristics of the cis-biphenyl 3,4-dihydrodiol were as follows: λmax (methanol), 204, 228, and 276 nm (ɛ204 = 11,870, ɛ228 = 18,580, and ɛ276 = 4,340 M−1cm−1); calculated mass for the phenylboronate derivative 12C181H1516O211B, 274.1160; found mass, 274.1165; mass spectrum of phenyl boronate derivative m/z (relative intensity), 174 (M+, 100), 170 (55), 152 (11), 142 (84), 115 (22), 77 (6); [α]D −37.5 ± 4.4, n = 3 (c 0.5, methanol); 1H NMR (chloroform), δ 4.21 (ddd, J = 6.4, 4.0, 1.5 Hz, H-4), 4.31 (dd, J = 6.4, 4.2 Hz, H-3), 6.09 (ddd, J = 9.8, 4.0, 0.8 Hz, H-5), 6.16 (ddd, J = 4.2, 1.7, 0.7 Hz, H-2), 6.37 (dt, J = 9.9, 1.6 Hz, H-6), 7.30 (tt, 1H aromatic-p), 7.37 (m, 2H, aromatic-m), 7.46 (m, 2H, aromatic-o).
Biotransformations with phenanthrene as substrate.
Since many of the amino acid substitutions made in this study would be predicted to increase the size of the putative NDO substrate binding site, a larger substrate, phenanthrene, was tested. Identification of the three regioisomers of cis-phenanthrene dihydrodiol was carried out by comparing GC-MS data with cis-3,4-dihydroxy-3,4-dihydrophenanthrene (cis-phenanthrene 3,4-dihydrodiol) and cis-1,2-dihydroxy-1,2-dihydrophenanthrene (cis-phenanthrene 1,2-dihydrodiol) produced by S. yanoikuyae B8/36 (32) and with synthetic cis-9,10-dihydroxy-9,10-dihydrophenanthrene (cis-phenanthrene 9,10-dihydrodiol). The PBA derivatives of cis-phenanthrene 9,10-dihydrodiol, cis-phenanthrene 3,4-dihydrodiol, and cis-phenanthrene 1,2-dihydrodiol had GC retention times of 18.0, 19.1, and 20.2 min, respectively. Wild-type NDO from Pseudomonas sp. strain NCIB 9816-4 formed a 9:1 mixture of cis-phenanthrene 3,4-dihydrodiol and cis-phenanthrene 1,2-dihydrodiol (Fig. 4B). These results are similar to those obtained with NDO from Pseudomonas sp. strain 119 and biphenyl dioxygenase from S. yanoikuyae B8/36 (32).
With the exception of the F202L and D362A mutants, all mutant NDO enzymes formed products with phenanthrene as substrate. Amino acid substitutions at all positions changed product ratios to some extent. V260A and W358A mutants preferentially oxidized phenanthrene at the C-3 and C-4 positions, forming almost no cis-phenanthrene 1,2-dihydrodiol (Fig. 4B). Several variants, including the N201A, N201S, F202V, W316A, T351R, F352V, F352L, and M366W mutants, produced a significantly greater proportion of cis-phenanthrene 1,2-dihydrodiol than did wild-type NDO (Fig. 4B). Of particular interest is the result with the F352V mutant. This enzyme had the opposite regioselectivity to wild-type NDO, forming 83% cis-phenanthrene 1,2-dihydrodiol, in contrast to wild type, which formed 90% cis-phenanthrene 3,4-dihydrodiol. The F352L mutant oxidized phenanthrene to a small amount (5% of the total product) of cis-phenanthrene 9,10-dihydrodiol (Fig. 4B).
DISCUSSION
Biphenyl dioxygenases (BPDOs) from Burkholderia sp. strain LB400 (21), S. yanoikuyae B8/36 (20), and Comamonas testosteroni B-356 (28) form only biphenyl 2,3-dihydrodiol from biphenyl, although various chlorinated biphenyls have been shown to be oxidized at the C-3 and C-4 positions by BPDOLB400 (22). The results presented here provide a detailed characterization of the minor product (13%) formed by NDO from Pseudomonas sp. strain NCIB 9816-4 from biphenyl, cis-biphenyl 3,4-dihydrodiol. This NDO enzyme system is apparently not unique in catalyzing the formation of cis-biphenyl 3,4-dihydrodiol. Recently, NDO from Pseudomonas putida G7 was shown to produce a minor product (3%) from biphenyl that was identified by GC-MS and indirect methods as biphenyl 3,4-dihydrodiol (3). NDO from Pseudomonas fluorescens N3 was also reported to form a small amount (15%) of cis-biphenyl 3,4-dihydrodiol (13). However, the low yield of product and reported instability of the compound precluded extensive characterization. The construction of the F352V NDO variant, which produced 96% cis-biphenyl 3,4-dihydrodiol from biphenyl, allowed the purification of enough product to complete the characterization described in Results. Stability problems were not encountered as long as a pH of >7.0 was maintained during all steps in the biotransformation, extraction, and purification.
The biological formation of cis-3,4-dihydrodiols is rarely observed (8), and synthetic chemists have devised chemoenzymatic methods to obtain this type of compound (7). The enzymes that carry out the next two steps in the naphthalene degradation pathway, the cis-dihydrodiol dehydrogenase and the meta ring cleavage dioxygenase, have been shown to degrade biphenyl and PCB metabolites with substitutions at the 3,4 position (3, 5, 38). Thus, it appears that naphthalene degradation pathway enzymes can carry out the first three steps in biphenyl degradation using an alternative set of intermediates. This observation may have application in the construction of new degradative pathways for biphenyl and PCBs (4).
Single or multiple amino acid substitutions that result in major changes in substrate specificity have been identified in other oxygenase enzyme systems. Single amino acid changes in oxygenases such as toluene 4-monooxygenase and cytochrome P450cam have been reported (1, 34, 43, 46, 51). In the case of toluene dioxygenase, the substitution of a single amino acid in the α subunit (Met-220 changed to Ala) resulted in an enzyme capable of dehalogenating 1,2,4,5-tetrachlorobenzene, a reaction not carried out by wild-type toluene dioxygenase (6). Site-directed and random mutagenesis studies with biphenyl dioxygenases from Burkholderia sp. strain LB400 and Pseudomonas pseudoalcaligenes KF707 have identified amino acids in the oxygenase α subunits that are important in determining PCB congener specificity (9, 17, 36, 39, 45). In particular, replacement of Asn-377 by Thr in BPDOLB400 significantly extended the range of PCBs oxidized (45).
Table 3 shows the amino acids in related dioxygenases that are located at positions corresponding to those mutated in NDO. Some amino acids listed in Table 3 (Phe-202 and Asp-362) are conserved in all of the enzymes shown. In other cases, amino acids are not conserved and an amino acid in NDO was changed to one present in one of the other enzymes. Some of the NDO mutations were chosen based on the identification of amino acids critical for determining substrate specificity in other dioxygenases. In NDO, Thr-351, when changed to Asn, had a minor effect on product formation from phenanthrene. Replacement of this amino acid with Arg in NDO had a slight effect on product formation from biphenyl and a larger effect when phenanthrene was provided as the substrate (Fig. 4). This position corresponds to the critical amino acid in BPDOLB400, Asn-377, that was mentioned above.
TABLE 3.
Comparison of amino acids at the active sites of selected dioxygenase α subunits
| Positionb | Amino acid in the following enzymea:
|
NDO mutation(s) | |||||
|---|---|---|---|---|---|---|---|
| NDO9816-4 | 2NTDOJS42 | DNTDODNT | TDOF1 | BPDOLB400 | BPDOKF707 | ||
| 201 | Asn | Asn | Asn | Gln | Gln | Gln | Ala, Gln, Ser |
| 202 | Phe | Phe | Phe | Phe | Phe | Phe | Leu, Val |
| 260 | Val | Asn | Val | Leu | Ser | Met | Ala, Leu, Asn |
| 316 | Trp | Trp | Phe | Trp | Trp | Trp | Ala |
| 351 | Thr | Ser | Ser | Thr | Asn | Thr | Asn, Arg, Ser |
| 352 | Phe | Ile | Thr | Phe | Phe | Phe | Leu, Val |
| 358 | Trp | Trp | Trp | Phe | Phe | Phe | Ala |
| 362 | Asp | Asp | Asp | Asp | Asp | Asp | Ala |
| 366 | Met | Met | Met | Trp | Trp | Trp | Trp |
2NTDOJS42, 2NTDO from Pseudomonas sp. strain JS42 (47); DNTDODNT, DNTDO from Burkholderia sp. strain DNT (62); TDOF1, toluene dioxygenase from P. putida F1 (66); BPDOLB400, BPDO from Burkholderia sp. strain LB400 (18); BPDOKF707, BPDO from P. pseudoalcaligenes KF707 (63).
Position numbers refer to NDO. Alignments were carried out with the Pileup program (Wisconsin Sequence Analysis Package; Genetics Computer Group, Madison, Wis.) using a gap weight of 3.5 and a gap length of 0.1.
Changes at Val-260 in NDO resulted in minor changes in product formation with biphenyl and phenanthrene. The corresponding amino acid in BPDOKF707, Met-283, when changed to Ser, resulted in an enzyme with no activity (36). The opposite mutation in BPDOLB400, Ser-283 to Met, in the context of two other mutations did not change the substrate specificity (45). However, amino acid changes at this position were shown to affect the regioselectivity and enantioselectivity of 2NTDO (J. V. Parales and D. T. Gibson, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. Q-249, p. 579, 1999). Substitution of Val for Asn-260 in 2NTDO resulted in an enzyme that no longer oxidized the aromatic ring of 2-nitrotoluene, forming only the monooxygenation product 2-nitrobenzyl alcohol (Parales and Gibson, Abstr. 99th Gen. Meet. Am. Soc. Microbiol.). The opposite change in specificity did not occur with the V260N NDO mutant. Like wild-type NDO, the V260N mutant did not oxidize the aromatic ring of 2-nitrotoluene but formed only 2-nitrobenzyl alcohol (data not shown).
Toluene dioxygenase, which has a Trp residue at the position corresponding to 366 in NDO, dihydroxylates the aromatic ring of toluene to form cis-toluene dihydrodiol (19, 37). However, the M366W variant of NDO oxidized toluene to benzyl alcohol (data not shown), the same product formed by the wild-type enzyme (41). Changing Trp-316 to Ala resulted in a minor change in regioselectivity with phenanthrene. Changing this conserved amino acid to Phe in 2NTDO had a slight effect on the stereochemistry of cis-naphthalene dihydrodiol formed from naphthalene (Parales and Gibson, Abstr. 99th Gen. Meet. Am. Soc. Microbiol.).
The most interesting mutations identified in this study are at position 352. This amino acid appears to play a major role in controlling both the stereochemistry of cis-naphthalene dihydrodiol formed from naphthalene and the regioselectivity with substrates such as biphenyl and phenanthrene (Fig. 4). In addition, a product that is not made by wild-type NDO, cis-phenanthrene 9,10-dihydrodiol, was formed from phenanthrene by the F352L mutant. To compare the substrate specificities of NDO and the new NDO variants with those of the closely related enzymes 2NTDO and DNTDO, we carried out biotransformations with biphenyl and phenanthrene and found that both were very poor substrates for 2NTDO and DNTDO. Both enzymes made a trace amount of cis-biphenyl 2,3-dihydrodiol from biphenyl, and DNTDO made a trace amount of phenanthrene 3,4-dihydrodiol from phenanthrene (data not shown). It is not clear at this time why biphenyl and phenanthrene are such poor substrates for 2NTDO and DNTDO. It is interesting, however, that the only mutation in NDO that affected the stereochemistry of cis-naphthalene dihydrodiol was at position 352. In contrast to NDO and toluene dioxygenase, which make homochiral (+)-cis-naphthalene dihydrodiol (64), DNTDO and 2NTDO form 96 and 70% (+)-cis-naphthalene dihydrodiol, respectively (48, 62). These enzymes do not have Phe at the position corresponding to 352 in NDO (Table 3).
In NDO, Asp-205 is located between the two redox centers at the junction of two adjacent α subunits (35). Replacement of Asp-205 by glutamine resulted in a variant of NDO with no activity (50). In the glutamine-containing enzyme, electron transfer between the Rieske center and the mononuclear iron was shown to be blocked, indicating that Asp-205 is essential for this electron transfer step to occur (50). Iron at the active site of NDO is coordinated by His-208, His-213, and Asp-362 (35). All three of these residues are conserved in the ring-hydroxylating dioxygenases whose sequences have been determined to date. The corresponding histidine residues in toluene dioxygenase from P. putida F1 (His-222 and His-228) were replaced with alanine residues, and these substitutions resulted in completely inactive enzymes (33). The inability to detect products from four different substrates in this study indicates that substitution of Ala at position 362 results in an inactive form of NDO. No activity was detected in crude cell extracts of the D362A mutant with either oxygen uptake assays or product formation assays with [14C]naphthalene (data not shown). These results are consistent with the identification of Asp-362 as a ligand to the mononuclear iron at the active site.
Asn-201, a possible fourth iron-coordinating amino acid, was observed in the crystal structure of NDO. This residue was too far from the iron atom to serve as a ligand in the crystallized form of the enzyme but was suggested as a possible ligand during a step in the catalytic cycle (35). Amino acid substitutions at Asn-201 resulted in enzymes with reduced but significant activity, indicating that this residue does not participate in the coordination of iron at the active site. Crude cell extracts of the N201A and N201Q variants had 5 to 10% of the activity of wild-type NDO (data not shown). The results presented in Fig. 4 suggest that Asn-201 may play a minor role in determining regioselectivity with biphenyl and phenanthrene as substrates. However, Asn-201 may be more important for maintaining appropriate interactions between α subunits through its hydrogen bond with Tyr-103 near the Rieske center in an adjacent α subunit (35). Substitution of an alanine at Asn-201 would disrupt this hydrogen bond and could affect the flow of electrons from the Rieske center to the mononuclear iron, thus reducing enzyme activity. The incorporation of the larger Gln residue at this position may prevent the normal interaction of α subunits even though Gln would be capable of forming a hydrogen bond with Tyr-103. The N201S mutant showed 35 to 40% of the wild-type NDO activity (data not shown), indicating that serine is a reasonably good substitute for Asn at this position, as is commonly the case (52).
Of the three substrates tested, the most significant effects of mutations at the active site were observed with the largest substrate, phenanthrene. This is not an unexpected result, since the substrate pocket is of limited size and larger substrates are likely to come in contact with more amino acids in the active site. Many of the mutations involved the substitution of a small hydrophobic amino acid for a larger one, and in most cases this type of substitution did not severely reduce the activity of the enzyme, as can sometimes occur (11). However, one exception was the replacement of Trp-358 by Ala, which resulted in an enzyme with poor activity with naphthalene, biphenyl, and phenanthrene. In future studies it will be interesting to compare the activities of this and other mutant enzymes with larger polycyclic aromatic compounds. Another exception was the F202L mutant, which failed to form products with all substrates tested. The reason that this substitution resulted in an inactive enzyme while the F202V mutant had good activity is not understood. Somewhat surprisingly, mutations that introduced changes in polarity or charge (V260N and T351R) resulted in enzymes with good activity toward hydrophobic substrates. In general, most changes at the active site, with the exception of those that affect iron binding (Asp-362) and electron transfer (Asp-205 [50]), were tolerated well, suggesting that there is significant flexibility in the range of amino acids that can be introduced at the active site. This suggests that oxygenases with novel catalytic capabilities can be generated by introducing single or multiple mutations near the active site.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant GM29909 from the National Institute of General Medical Sciences.
We thank Derek Boyd, The Queen's University of Belfast, Belfast, United Kingdom, for providing cis-phenanthrene 9,10-dihydrodiol; William Kearney at the University of Iowa College of Medicine NMR Facility for assistance in interpreting NMR data; Juan Parales for assisting with large-scale biotransformations; and Maja Ivkovic-Jensen and Juan Parales for helpful discussions.
REFERENCES
- 1.Atkins W M, Sligar S G. The roles of active site hydrogen bonding in cytochrome P-450cam as revealed by site-directed mutagenesis. J Biol Chem. 1988;263:18842–18849. [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1993. [Google Scholar]
- 3.Barriault D, Durand J, Maaroufi H, Eltis L D, Sylvestre M. Degradation of polychlorinated biphenyl metabolites by naphthalene-catabolizing enzymes. Appl Environ Microbiol. 1998;64:4637–4642. doi: 10.1128/aem.64.12.4637-4642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barriault D, Sylvestre M. Functionality of biphenyl 2,3-dioxygenase components in naphthalene 1,2-dioxygenase. Appl Microbiol Biotechnol. 1999;51:592–597. doi: 10.1007/s002530051437. [DOI] [PubMed] [Google Scholar]
- 5.Barriault D, Vedadi M, Powlowski J, Sylvestre M. cis-2,3-Dihydro-2,3-dihydroxybiphenyl dehydrogenase and cis-1,2-dihydro-1,2-dihydroxynaphthalene dehydrogenase catalyze dehydrogenation of the same range of substrates. Biochem Biophys Res Commun. 1999;260:181–187. doi: 10.1006/bbrc.1999.0706. [DOI] [PubMed] [Google Scholar]
- 6.Beil S, Mason J R, Timmis K N, Pieper D H. Identification of chlorobenzene dioxygenase sequence elements involved in dechlorination of 1,2,4,5-tetrachlorobenzene. J Bacteriol. 1998;180:5520–5528. doi: 10.1128/jb.180.21.5520-5528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyd D R, Sharma N D, Barr S A, Dalton H, Chima J, Whited G, Seemayer R. Chemoenzymatic synthesis of the 2,3- and 3,4-cis-dihydrodiol enantiomers of monosubstituted benzenes. J Am Chem Soc. 1994;116:1147–1148. [Google Scholar]
- 8.Boyd D R, Sheldrake G N. The dioxygenase-catalysed formation of vicinal cis-diols. Nat Prod Rep. 1998;15:309–324. [Google Scholar]
- 9.Brühlmann F, Chen W. Tuning biphenyl dioxygenase for extended specificity. Biotechnol Bioeng. 1999;63:544–551. doi: 10.1002/(sici)1097-0290(19990605)63:5<544::aid-bit4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Butler C S, Mason J R. Structure-function analysis of the bacterial aromatic ring-hydroxylating dioxygenases. Adv Microb Physiol. 1997;38:47–84. doi: 10.1016/s0065-2911(08)60155-1. [DOI] [PubMed] [Google Scholar]
- 11.Caffrey M S. Strategies for the study of cytochrome c structure and function by site-directed mutagenesis. Biochimie. 1994;76:622–630. doi: 10.1016/0300-9084(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 12.Davis R W, Botstein D, Roth J R. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 13.Di Gennaro P, Sello G, Bianchi D, D'Amico P. Specificity of substrate recognition by Pseudomonas fluorescens N3 dioxygenase. J Biol Chem. 1997;272:30254–30260. doi: 10.1074/jbc.272.48.30254. [DOI] [PubMed] [Google Scholar]
- 14.Ensley B D, Gibson D T. Naphthalene dioxygenase: purification and properties of a terminal oxygenase component. J Bacteriol. 1983;155:505–511. doi: 10.1128/jb.155.2.505-511.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ensley B D, Gibson D T, Laborde A L. Oxidation of naphthalene by a multicomponent enzyme system from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1982;149:948–954. doi: 10.1128/jb.149.3.948-954.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ensley B D, Ratzkin B J, Osslund T D, Simon M J, Wackett L P, Gibson D T. Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science. 1983;222:167–169. doi: 10.1126/science.6353574. [DOI] [PubMed] [Google Scholar]
- 17.Erickson B D, Mondello F J. Enhanced biodegradation of polychlorinated biphenyls after site-directed mutagenesis of a biphenyl dioxygenase gene. Appl Environ Microbiol. 1993;59:3858–3862. doi: 10.1128/aem.59.11.3858-3862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erickson B D, Mondello F J. Nucleotide sequencing and transcriptional mapping of the genes encoding biphenyl dioxygenase, a multicomponent polychlorinated biphenyl-degrading enzyme in Pseudomonas strain LB400. J Bacteriol. 1992;174:2903–2912. doi: 10.1128/jb.174.9.2903-2912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson D T, Hensley M, Yoshioka H, Mabry T J. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970;9:1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- 20.Gibson D T, Roberts R L, Wells M C, Kobal V M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973;50:211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- 21.Haddock J D, Gibson D T. Purification and characterization of the oxygenase component of biphenyl 2,3-dioxygenase from Pseudomonas sp. strain LB400. J Bacteriol. 1995;177:5834–5839. doi: 10.1128/jb.177.20.5834-5839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haddock J D, Horton J R, Gibson D T. Dihydroxylation and dechlorination of chlorinated biphenyls by purified biphenyl 2,3-dioxygenase from Pseudomonas sp. strain LB400. J Bacteriol. 1995;177:20–26. doi: 10.1128/jb.177.1.20-26.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haigler B E, Gibson D T. Purification and properties of ferredoxinNAP, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990;172:465–468. doi: 10.1128/jb.172.1.465-468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haigler B E, Gibson D T. Purification and properties of NADH-ferredoxinNAP reductase, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990;172:457–464. doi: 10.1128/jb.172.1.457-464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harlow E, Lane D. Antibodies, a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 26.Hegg E L, Que L J. The 2-His-1-carboxylate facial triad: an emerging structural motif in mononuclear non-heme iron(II) enzymes. Eur J Biochem. 1997;250:625–629. doi: 10.1111/j.1432-1033.1997.t01-1-00625.x. [DOI] [PubMed] [Google Scholar]
- 27.Herbert, A. B., G. N. Sheldrake, P. J. Somers, and J. A. Meredith. January 1990. Separation of 1,2-dihydroxycyclohexa-3,5-diene compounds. European patent EP 0379300A2.
- 28.Hurtubise Y, Barriault D, Sylvestre M. Involvement of the terminal oxygenase β subunit in the biphenyl dioxygenase reactivity pattern toward chlorobiphenyls. J Bacteriol. 1998;180:5828–5835. doi: 10.1128/jb.180.22.5828-5835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeffrey A M, Yeh H J C, Jerina D M. Synthesis of cis-1,2-dihydroxy-1,2-dihydronaphthalene and cis-1,4-dihydroxy-1,4-dihydronaphthalene. J Org Chem. 1974;39:1405–1407. [Google Scholar]
- 30.Jeffrey A M, Yeh H J C, Jerina D M, Patel T R, Davey J F, Gibson D T. Initial reactions in the oxidation of naphthalene by Pseudomonas putida. Biochemistry. 1975;14:575–583. doi: 10.1021/bi00674a018. [DOI] [PubMed] [Google Scholar]
- 31.Jerina D M, Daly J W, Jeffrey A M, Gibson D T. cis-1,2-Dihydroxy-1,2-dihydronaphthalene: a bacterial metabolite from naphthalene. Arch Biochem Biophys. 1971;142:394–396. doi: 10.1016/0003-9861(71)90298-0. [DOI] [PubMed] [Google Scholar]
- 32.Jerina D M, Selander H, Yagi H, Wells M C, Davey J F, Mahadevan V, Gibson D T. Dihydrodiols from anthracene and phenanthrene. J Am Chem Soc. 1976;98:5988–5996. doi: 10.1021/ja00435a035. [DOI] [PubMed] [Google Scholar]
- 33.Jiang H, Parales R E, Lynch N A, Gibson D T. Site-directed mutagenesis of conserved amino acids in the alpha subunit of toluene dioxygenase: potential mononuclear non-heme iron coordination sites. J Bacteriol. 1996;178:3133–3139. doi: 10.1128/jb.178.11.3133-3139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones N E, England P A, Rouch D A, Wong L-L. Engineering the selectivity of aliphatic C-H bond oxidation catalysed by cytochrome P450cam. J. Chem. Soc. Chem. Commun. 1996. pp. 2413–2414. [Google Scholar]
- 35.Kauppi B, Lee K, Carredano E, Parales R E, Gibson D T, Eklund H, Ramaswamy S. Structure of an aromatic ring-hydroxylating dioxygenase-naphthalene 1,2-dioxygenase. Structure. 1998;6:571–586. doi: 10.1016/s0969-2126(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 36.Kimura N, Nishi A, Goto M, Furukawa K. Functional analyses of a variety of chimeric dioxygenases constructed from two biphenyl dioxygenases that are similar structurally but different functionally. J Bacteriol. 1997;179:3936–3943. doi: 10.1128/jb.179.12.3936-3943.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobal V M, Gibson D T, Davis R E, Garza A. X-ray determination of the absolute stereochemistry of the initial oxidation product formed from toluene by Pseudomonas putida 39/D. J Am Chem Soc. 1973;95:4420–4421. doi: 10.1021/ja00794a048. [DOI] [PubMed] [Google Scholar]
- 38.Kuhm A E, Stolz A, Ngai K-L, Knackmuss H-J. Purification and characterization of a 1,2-dihydroxynaphthalene dioxygenase from a bacterium that degrades naphthalenesulfonic acid. J Bacteriol. 1991;173:3795–3802. doi: 10.1128/jb.173.12.3795-3802.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumamaru T, Suenaga H, Mitsuoka M, Watanabe T, Furukawa K. Enhanced degradation of polychlorinated biphenyls by directed evolution of biphenyl dioxygenase. Nat Biotechnol. 1998;16:663–666. doi: 10.1038/nbt0798-663. [DOI] [PubMed] [Google Scholar]
- 40.Lange S J, Que L J. Oxygen activating nonheme iron enzymes. Curr Opin Chem Biol. 1998;2:159–172. doi: 10.1016/s1367-5931(98)80057-4. [DOI] [PubMed] [Google Scholar]
- 41.Lee K, Gibson D T. Toluene and ethylbenzene oxidation by purified naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816-4. Appl Environ Microbiol. 1996;62:3101–3106. doi: 10.1128/aem.62.9.3101-3106.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S-Y, Rasheed S. A simple procedure for maximum yield of high-quality plasmid DNA. BioTechniques. 1990;9:676–679. [PubMed] [Google Scholar]
- 43.Loida P J, Sligar S G. Engineering cytochrome P-450cam to increase the stereospecificity and coupling of aliphatic hydroxylation. Protein Eng. 1993;6:207–212. doi: 10.1093/protein/6.2.207. [DOI] [PubMed] [Google Scholar]
- 44.Lynch N A, Jiang H, Gibson D T. Rapid purification of the oxygenase component of toluene dioxygenase from a polyol-responsive monoclonal antibody. Appl Environ Microbiol. 1996;62:2133–2137. doi: 10.1128/aem.62.6.2133-2137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mondello F J, Turcich M P, Lobos J H, Erickson B D. Identification and modification of biphenyl dioxygenase sequences that determine the specificity of polychlorinated biphenyl degradation. Appl Environ Microbiol. 1997;63:3096–3103. doi: 10.1128/aem.63.8.3096-3103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nickerson D P, Harford-Cross C F, Fulcher S R, Wong L-L. The catalytic activity of cytochrome P450cam towards styrene oxidation is increased by site-directed mutagenesis. FEBS Lett. 1997;405:153–156. doi: 10.1016/s0014-5793(97)00174-9. [DOI] [PubMed] [Google Scholar]
- 47.Parales J V, Kumar A, Parales R E, Gibson D T. Cloning and sequencing of the genes encoding 2-nitrotoluene dioxygenase from Pseudomonas sp. JS42. Gene. 1996;181:57–61. doi: 10.1016/s0378-1119(96)00462-3. [DOI] [PubMed] [Google Scholar]
- 48.Parales J V, Parales R E, Resnick S M, Gibson D T. Enzyme specificity of 2-nitrotoluene 2,3-dioxygenase from Pseudomonas sp. strain JS42 is determined by the C-terminal region of the α subunit of the oxygenase component. J Bacteriol. 1998;180:1194–1199. doi: 10.1128/jb.180.5.1194-1199.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parales R E, Emig M D, Lynch N A, Gibson D T. Substrate specificities of hybrid naphthalene and 2,4-dinitrotoluene dioxygenase enzyme systems. J Bacteriol. 1998;180:2337–2344. doi: 10.1128/jb.180.9.2337-2344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parales R E, Parales J V, Gibson D T. Aspartate 205 in the catalytic domain of naphthalene dioxygenase is essential for activity. J Bacteriol. 1999;181:1831–1837. doi: 10.1128/jb.181.6.1831-1837.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pikus J D, Studts J M, McClay K, Steffan R J, Fox B G. Changes in the regiospecificity of aromatic hydroxylation produced by active site engineering in the diiron enzyme toluene-4-monooxygenase. Biochemistry. 1997;36:9283–9289. doi: 10.1021/bi971049t. [DOI] [PubMed] [Google Scholar]
- 52.Plapp B V. Site-directed mutagenesis: a tool for studying enzyme catalysis. Methods Enzymol. 1995;249:91–119. doi: 10.1016/0076-6879(95)49032-9. [DOI] [PubMed] [Google Scholar]
- 53.Resnick S M, Gibson D T. Biotransformation of anisole and phenetole by aerobic hydrocarbon-oxidizing bacteria. Biodegradation. 1993;4:195–203. [Google Scholar]
- 54.Resnick S M, Gibson D T. Regio- and stereospecific oxidation of 9,10-dihydroanthracene and 9,10-dihydrophenanthrene by naphthalene dioxygenase: structure and absolute stereochemistry of metabolites. Appl Environ Microbiol. 1996;62:3355–3359. doi: 10.1128/aem.62.9.3355-3359.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Resnick S M, Gibson D T. Regio- and stereospecific oxidation of fluorene, dibenzofuran, and dibenzothiophene by naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816-4. Appl Environ Microbiol. 1996;62:4073–4080. doi: 10.1128/aem.62.11.4073-4080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Resnick S M, Lee K, Gibson D T. Diverse reactions catalyzed by naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Ind Microbiol. 1996;17:438–457. [Google Scholar]
- 57.Resnick S M, Torok D S, Gibson D T. Oxidation of carbazole to 3-hydroxycarbazole by naphthalene 1,2-dioxygenase and biphenyl 2,3-dioxygenase. FEMS Microbiol Lett. 1993;113:297–302. doi: 10.1111/j.1574-6968.1993.tb06530.x. [DOI] [PubMed] [Google Scholar]
- 58.Resnick S M, Torok D S, Lee K, Brand J M, Gibson D T. Regiospecific and stereoselective hydroxylation of 1-indanone and 2-indanone by naphthalene dioxygenase and toluene dioxygenase. Appl Environ Microbiol. 1994;60:3323–3328. doi: 10.1128/aem.60.9.3323-3328.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 60.Stanier R Y, Palleroni N J, Doudoroff M. The aerobic pseudomonads; a taxonomic study. J Gen Microbiol. 1966;43:159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- 61.Suen W-C. Ph.D. thesis. Iowa City: The University of Iowa; 1991. [Google Scholar]
- 62.Suen W-C, Haigler B E, Spain J C. 2,4-Dinitrotoluene dioxygenase from Burkholderia sp. strain DNT: similarity to naphthalene dioxygenase. J Bacteriol. 1996;178:4926–4934. doi: 10.1128/jb.178.16.4926-4934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taira K, Hirose J, Hayashida S, Furukawa K. Analysis of bph operon from the polychlorinated biphenyl-degrading strain of Pseudomonas pseudoalcaligenes KF707. J Biol Chem. 1992;267:4844–4853. [PubMed] [Google Scholar]
- 64.Torok D S, Resnick S M, Brand J M, Cruden D L, Gibson D T. Desaturation and oxygenation of 1,2-dihydronaphthalene by toluene and naphthalene dioxygenase. J Bacteriol. 1995;177:5799–5805. doi: 10.1128/jb.177.20.5799-5805.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 66.Zylstra G J, Gibson D T. Toluene degradation by Pseudomonas putida F1: nucleotide sequence of the todC1C2BADE genes and their expression in E. coli. J Biol Chem. 1989;264:14940–14946. [PubMed] [Google Scholar]