Dear Editor,
We read with interest the Article by Höglinger and colleagues reporting the results of phase 2, randomised, placebo-controlled trial of tilavonemab for patients with progressive supranuclear palsy.1 We wish to report the neuropathologic findings of two patients who came to autopsy after treatment with this humanised recombinant IgG4 anti-human tau antibody.2 One was enrolled in the phase 2 trial described by Höglinger and colleagues,1 and the other in a phase 1 trial with the same antibody.3
Patient 1 was a 67-year-old man who had a 5-year history of progressive supranuclear palsy, diagnosed according to the Movement Disorder Society criteria.4 He was enrolled in the phase 2 trial of tilavonemab at age 64 years and received 14 doses, each of 2000 mg, over a 48-week period.1 After completing the trial, he was enrolled in the extension study and continued to receive a 2000 mg dose every 28 days for 13 doses.5 In total, he received 27 doses (54 000 mg). The interval from the last infusion to death was 57 weeks. Patient 2 was an 87-year-old man who had a 5-year history of progressive supranuclear palsy with apraxia. He was enrolled in a phase 1 trial of tilavonemab and received a single dose of tilavonemab (50 mg/kg; 4510 mg dose) at age 86 years. 6 The interval from infusion to death was 50 weeks. Neither adverse events nor obvious clinical alterations were observed in either patient.
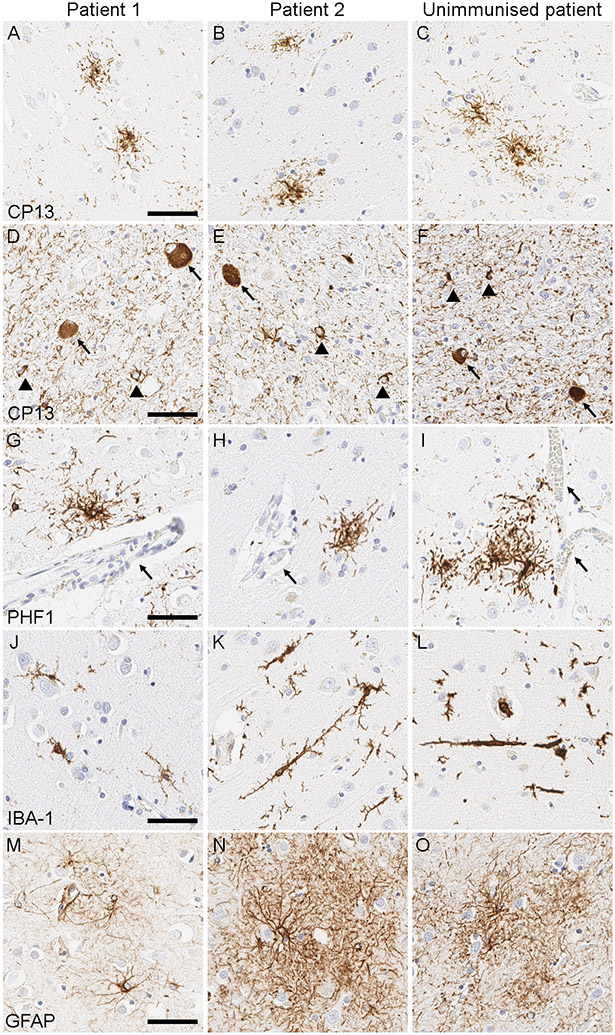
Brain autopsies were performed with the consent of the legal next-of-kin or an individual with power-of-attorney for the patients. Progressive supranuclear palsy pathology was confirmed in both patients by phosphorylated-tau (CP13) immunohistochemistry (Figure 1), with coincidental Lewy body pathology in patient 1. Tau burden in these two patients was compared with that in ten patients with autopsy-confirmed progressive supranuclear palsy (mean age 73·7 years [SD 8·4]) for eight vulnerable brain regions, but we found no difference in density or distribution of tau pathology (Supplementary Table 1). A study of patients with autopsy-confirmed progressive supranuclear palsy and corticobasal degeneration who had received the anti-tau antibody gosuranemab reported that perivascular vesicular astrocytes (PVAs) with tau accumulation within lysosomes might be a glial response associated with antibody treatment.7 However, we did not detect PVAs in the midfrontal gyrus, where PVAs were most frequently found in the literature, by phosphorylated-tau (PHF1) immunohistochemistry used in their study (Figure 1).7 Immunohistochemistry for IBA-1 and GFAP revealed microgliosis and astrogliosis that was mild in patient 1 and moderate in patient 2, although morphology and severity of microgliosis and astrogliosis were highly variable in unimmunised patients (Supplementary Table 1). Bushy astrocytosis and rod-shaped microglia were reported as potential glial responses after tau immunotherapy,7 but we believe that these findings are nonspecific because we also observed them in unimmunised patients (Figure 1).
Figure 1:
Representative images of phosphorylated-tau immunohistochemistry. (A-F) Phosphorylated-tau (CP13) immunohistochemistry shows tufted astrocytes (A-C) in the caudate nucleus, as well as globose tangles (arrows; D-F) and coiled bodies (arrowheads; D-F) in the subthalamic nucleus, indicating the neuropathologic diagnosis of progressive supranuclear palsy. (G-I) Phosphorylated-tau (PHF1) immunohistochemistry shows tufted astrocytes but does not identify perivascular vesicular astrocytes in the midfrontal cortex in either immunised or unimmunised patients (arrows indicate blood vessels). (J-L) IBA-1 immunohistochemistry shows microgliosis in the midfrontal cortex: mild in patient 1 (J), moderate in patient 2 (K), and of variable severity in unimmunised patients (L). Rod-shaped microglia are present in patient 2 (K) and unimmunised patients (L). (M-O) GFAP immunohistochemistry shows astrogliosis in the midfrontal cortex: mild in patient 1 (M) and moderate in patient 2 (N). Unimmunised patients show variable severity of astrogliosis (O). Abbreviations: GFAP, glial fibrillary acidic protein IBA-1, ionized calcium binding adaptor molecule 1. Scale bars = 50 μm.
Our findings revealed no differences in tau burden between immunised and unimmunised patients, which is consistent with the negative results of the clinical trial.1 Since the mouse version of tilavonemab showed a reduction of tau seeding activity in P301S transgenic mice,8 the hypothesis of this clinical trial was that tilavonemab would slow the progression of tau pathology by blocking extracellular spread. Therefore, the lack of reduction of pre-existing intracellular tau pathology is not unexpected, as in the other recent neuropathological study of patients after anti-tau treatment.7
Importantly, progressive supranuclear palsy diagnosis was confirmed at autopsy in both participants. This is consistent with high clinical diagnostic accuracy of progressive supranuclear palsy, which is 81% (1290/1585) in our brain bank as of July 2021 (unpublished data). More importantly, we observed no obvious adverse neuropathologic changes that could be attributed to immunotherapy with tilavonemab.
There are some limitations to our study. First, our pathologic analysis was based on phosphorylated-tau (CP13 and PHF1) immunohistochemistry, which mainly recognize intracellular tau aggregates; therefore, alterations of N-terminal tau species—a target of tilavonemab—in the extracellular space remains unclear. Second, one patient was treated with only one dose, which may not be sufficient to induce morphological alteration of astrocytic tau pathology. Third, the interval from the last infusion to death was about one year in both patients. Given that the half-life of tilavonemab is 27 to 37 days, our negative findings might be explained by this long interval. Finally, we did not do statistical analyses, owing to the small number of immunised patients. Anti-tau antibodies have been used in clinical trials not only for progressive supranuclear palsy, but also Alzheimer’s disease, and as more patients come to autopsy, additional pathologic assessments of study participants will be needed to confirm our findings.
Supplementary Material
Financial Disclosures
Dr. Koga receives research support by a Jaye F. and Betty F. Dyer Foundation Fellowship in progressive supranuclear palsy research and CurePSP.
Dr. Dickson receives support from the NIH (U54 NS100693, UG3-NS104095, R01 AG062348), and Tau Consortium.
Dr. Wszolek is partially supported by the NIH/NIA and NIH/NINDS (1U19AG063911, FAIN: U19AG063911), Mayo Clinic Center for Regenerative Medicine, Mayo Clinic in Florida Focused Research Team Program, the gifts from The Sol Goldman Charitable Trust, and the Donald G. and Jodi P. Heeringa Family, the Haworth Family Professorship in Neurodegenerative Diseases fund, and The Albertson Parkinson's Research Foundation. He serves as PI or Co-PI on Biogen, Inc. (228PD201), Biohaven Pharmaceuticals, Inc. (BHV4157-206 and BHV3241-301), Neuraly, Inc. (NLY01-PD-1), and Vigil Neuroscience, Inc. (VGL101-01.001) grants. He serves as Co-PI of the Mayo Clinic APDA Center for Advanced Research and as an external advisory board member for the Vigil Neuroscience, Inc. He served as Mayo Clinic Florida site P.I. for the protocols: AbbVie, Inc. M15-562 (“A Study to Assess Efficacy, Safety, Tolerability, and Pharmacokinetics of ABBV-8E12 in Subjects With Progressive Supranuclear Palsy (PSP)”), AbbVie, Inc. M15-563 (“An Extension Study of ABBV-8E12 in Progressive Supranuclear Palsy (PSP)”), and C2N Diagnostics C2N-8E12-WW-104 (“Safety, Tolerability, and Pharmacokinetics of C2N-8E12 in Subjects With Progressive Supranuclear Palsy”).
References
- 1.Höglinger GU, Litvan I, Mendonca N, et al. Safety and efficacy of tilavonemab in progressive supranuclear palsy: a phase 2, randomised, placebo-controlled trial. Lancet Neurol 2021; 20(3): 182–92. [DOI] [PubMed] [Google Scholar]
- 2.Plotkin SS, Cashman NR. Passive immunotherapies targeting Abeta and tau in Alzheimer's disease. Neurobiol Dis 2020; 144: 105010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.West T, Hu Y, Verghese PB, et al. Preclinical and Clinical Development of ABBV-8E12, a Humanized Anti-Tau Antibody, for Treatment of Alzheimer's Disease and Other Tauopathies. J Prev Alzheimers Dis 2017; 4(4): 236–41. [DOI] [PubMed] [Google Scholar]
- 4.Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord 2017; 32(6): 853–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An Extension Study of ABBV-8E12 in Progressive Supranuclear Palsy (PSP). https://clinicaltrials.gov/ct2/show/NCT03391765 Date: February 3, 2021, Date accessed: July 13, 2021
- 6.Safety, Tolerability, and Pharmacokinetics of C2N-8E12 in Subjects With Progressive Supranuclear Palsy. https://clinicaltrials.gov/ct2/show/NCT02494024 Date: July 26, 2017, Date accessed: July 13, 2021
- 7.Kim B, Mikytuck B, Suh E, et al. Tau immunotherapy is associated with glial responses in FTLD-tau. Acta Neuropathol 2021; 142(2): 243–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanamandra K, Kfoury N, Jiang H, et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron 2013; 80(2): 402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.