Abstract
Unruptured intracranial aneurysms (UIA) occur in around 3% of the population. Important management questions concern if and how to perform preventive UIA occlusion; if, how and when to perform follow up imaging and non-interventional means to reduce the risk of rupture. Using the Standard Operational Procedure of ESO we prepared guidelines according to GRADE methodology. Since no completed randomised trials exist, we used interim analyses of trials, and meta-analyses of observational and case-control studies to provide recommendations to guide UIA management. All recommendations were based on very low evidence. We suggest preventive occlusion if the estimated 5-year rupture risk exceeds the risk of preventive treatment. In general, we cannot recommend endovascular over microsurgical treatment, but suggest flow diverting stents as option only when there are no other low-risk options for UIA repair. To detect UIA recurrence we suggest radiological follow up after occlusion. In patients who are initially observed, we suggest radiological monitoring to detect future UIA growth, smoking cessation, treatment of hypertension, but not treatment with statins or acetylsalicylic acid with the indication to reduce the risk of aneurysm rupture. Additionally, we formulated 15 expert-consensus statements. All experts suggest to assess UIA patients within a multidisciplinary setting (neurosurgery, neuroradiology and neurology) at centres consulting >100 UIA patients per year, to use a shared decision-making process based on the team recommendation and patient preferences, and to repair UIA only in centres performing the proposed treatment in >30 patients with (ruptured or unruptured) aneurysms per year per neurosurgeon or neurointerventionalist. These UIA guidelines provide contemporary recommendations and consensus statement on important aspects of UIA management until more robust data come available.
Keywords: Unruptured intracranial aneurysms, risk of rupture, coiling, clipping, endovascular repair, risk factors, medical management, aneurysm growth, grading of recommendations, assessment, management, guidelines
Introduction
Unruptured intracranial aneurysms (UIA) are acquired, pathological dilations at major branching brain vessels and have a prevalence of up to 3% in the adult population. 1 The prevalence of UIA is 1.6-fold higher in women, but is similar across Europe, Northern America Japan. In persons with two or more first-degree relatives with subarachnoid haemorrhage from a ruptured aneurysm, and in patients with polycystic kidney disease the risk for having or developing UIA is considerably higher. 1
UIA are commonly diagnosed incidentally in imaging for non-related symptoms (e.g. headache of dizziness), or in the setting of other entities (e.g. trauma, ischaemic stroke, malignancies or psychiatric diseases) or during screening for aneurysms or for (brain) diseases in general. The radiological detection rate of UIA is increasing due to the higher availability, increased quality more frequent use of cranial imaging. After radiological detection, UIA can remain stable, grow without or with subsequent rupture or rupture without concomitant aneurysm growth. Aneurysm growth during follow-up is associated with an increased risk of rupture, with a 1 year risk of rupture ranging from 2% to 10% according to the size, site and shape of the aneurysm. 2 Rupture of a UIA causes aneurysmal subarachnoid haemorrhage (SAH), which is a rare but serious form of stroke: the case-fatality of SAH remains up to 35% to 45% and about 30% of patients survive with severe neurological and/or neurocognitive deficits. 3 The mean age of SAH patients is 52 years. 4
Several prospective cohort studies or case-control studies within different geographic populations or time periods have studied the risk of aneurysm rupture. A prediction model based on easily retrievable risk factors derived from a systematic review of the literature and meta-analysis of data estimated that the 5-year risk of aneurysm rupture ranges from 0.4% to 17.8%. 5 A comparable prediction model found similar results. 6 However, considerable uncertainty remains regarding the individual risk of rupture in a given patient, because several important risk factors, such as aneurysm morphology, positive family history, smoking at baseline and change of smoking and hypertension over time were not included. Another limitation is that the prediction models are built on cohorts of patients selected for not undergoing preventive aneurysm occlusion. Thus, the data cannot be extrapolated to all patients with an unruptured aneurysm. Moreover, most patients in whom an aneurysm is detected are relatively young and have a life expectancy that is much longer than the horizon of the 5-year risk prediction. Several comprehensive reviews have discussed these issues in detail over the last years.7–9
If an UIA is detected, patients and their treating team are confronted with many questions, such as (1) should preventive occlusion be performed? (2) If so, by microsurgical or endovascular means? (3) if no preventive occlusion is done, should follow-up imaging be done to detect aneurysm growth? (4) can we advise medical management or life style changes to reduce the risk of rupture? and (5) should follow-up imaging be performed after preventive occlusion? Since the last European Stroke Organization Guidelines for the Management of Intracranial Aneurysms and Subarachnoid Haemorrhage published in 2013, 10 many new data, with varying levels of evidence, have been reported. These include the risk of rupture of UIA, functional outcome after preventive UIA repair, especially in the setting of novel endovascular devices, and on decision making. Fewer data exist for UIA patients who are managed other than with preventive repair. The aim of this guideline document is to provide contemporary evidence-based recommendations for the management of patients with UIA. For areas where robust evidence is lacking we provide consensus-based suggestions. In this guideline document we focus on adult patients with asymptomatic UIA.
Methods
Composition and approval of the module working group
These guidelines were initiated by the European Stroke Organisation. Two chairpersons were selected to assemble and coordinate the Guideline Module Working Group (MWG). The final group consisted of two interdisciplinary co-chairs (GR and NE) and seven additional experts in the field (RB, HD, TK, AL, DN, SP and MV) from six different European countries. The MWG initially included five neurosurgeons, of whom two also perform endovascular aneurysm treatment, two interventional radiologists two neurologists. During the process two neurologists (DAS and CT) were added to the MWG. The ESO Guideline Board and Executive Committee reviewed the intellectual and financial disclosures of all MWG members and approved the composition of the group. The full details of all MWG members and their disclosures are included in Supplemental Table 1.
Development and approval of clinical questions
The guidelines were developed using GRADE methodology and the European Stroke Organisation Standard Operating Procedure, as described previously.11,12 In brief, the MWG developed a list of topics, and corresponding questions of greatest clinical interest. Questions were developed in the Population, Intervention, Comparator, Outcome (PICO) format. We formulated five PICO questions, refined following comments from the ESO Guidelines board and the ESO Executive Committee, and the revised versions were approved by the board and committee.
The outcomes were rated by members of the MWG as: critical, important or of limited importance according to GRADE criteria. Final decision on outcomes used a Delphi approach. Results of the outcomes rating for each PICO question are included in the supplement.
We considered separate outcomes for the different PICOs due to the different nature of the underlying research questions. Using the Delphi method, the MWG voted in a closed ballot to identify which outcomes were of highest priority, according to the GRADE methodology using a 9-point scale (7–9: critical; 4–6: important; 1–3: of limited importance). We present the final scores per outcome, based on the mean votes from all participants (Supplemental Table 2). The final recommendations are based on those outcomes that have been rated as critical according to the GRADE approach.
Literature search
For each PICO question, systematic searches of the PubMed, EMBASE and Cochrane databases covering the period from the inception of each database to November 2020 were conducted by one of the ESO Guidelines methodologist (AvL). NE, GR and AvL agreed on the search terms for each PICO question. We also searched one of the authors’ (GR) personal reference libraries, which is prospectively built by a daily PubMed search (search terms are listed in the Supplemental Materials) and included selected studies of interest from this database that were published until October 2021. Search results were loaded into the web-based Covidence platform (Health Innovation, Melbourne, Australia) for assessment by the MWG. Per PICO question two MWG members formed a ‘PICO group’. The PICO groups consisted of: PICO 1 NE, HD; PICO 2 GR, SP; PICO 3 RB, AL; PICO 4 GR, MV and PICO 5 TK, DN. These two MWG members were assigned to independently screen the titles and abstracts of publications registered in Covidence and then assess the full text of studies determined to be potentially relevant. All disagreements were resolved by discussion between the two reviewers or by the MWG chairs. We prioritised randomised controlled trials (RCTs) but where data were limited, we considered systematic reviews or meta-analyses of observational studies, large observational studies and health registry data analyses.
A study was included if it reported the components of predetermined inclusion criteria. The inclusion criteria varied between PICO1 and 2 versus PICO 3, 4 and 5, because of the different nature of the underlying PICOs and/or underlying research questions and clinical implications (e.g. risk of aneurysm rupture as opposed to functional outcome in PICO 1 or functional as opposed to only radiological outcomes in PICO 2). A study was excluded if it had one or more exclusion reasons or did not consider the components of inclusion criteria. A study was considered potentially relevant if few components of the inclusion criteria were reported in the title or abstract or if there was insufficient information to exclude the study. Full texts of all the included or potentially relevant articles were loaded into Covidence software. ‘Second level selection’ or ‘Full text screening’ of these articles was performed by reading the full text of the articles selected at ‘First level selection’ and following the same inclusion and exclusion criteria. Second level selection was performed in duplicate by two assessors independently. Discrepancies or conflicts in selection or rejection of studies were resolved by consensus or by a third reviewer when needed. This was done both at the first and second level selection.
In studies with duplicate data (companion publications), the original study or the study reporting detailed or recent data (with a greater number of subjects) was included. When different outcomes were reported in the studies, all of these were included. Further details on the literature search are provided in the Supplemental Materials.
In the first-round, titles and abstracts of the corresponding references were reviewed for relevance to the predefined PICOs and the inclusion or exclusion criteria listed in Table 1.
Table 1.
Inclusion and exclusion criteria for the PICO questions.
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| PICO 1 | (1) Observational/cohort studies a | (1) Studies with retrospective case finding (2) studies on >10% fusiform or dissecting aneurysms |
| (2) Prospective registry studies | (3) Studies reporting exclusively on aneurysms associated with arteriovenous malformations or in populations with specific diseases (such as collagen disorders, Moyamoya disease or syndrome, dwarfism or autoimmune disorders) | |
| (3) Meta-analyses | (4) Studies in which previously ruptured aneurysms could not be distinguished from additional UIA | |
| (4) Studies with >50 patients | ||
| (5) Studies on saccular unruptured aneurysms | ||
| PICO 2 | (1) Observational a or cohort studies reporting on a functional outcome scale | (1) Retrospective studies |
| (2) Case-control studies (prospective) reporting on a functional outcome scale | (2) Single-centre studies | |
| (3) Randomised studies reporting on a functional outcome scale (4) prospective registry studies (5) meta-analyses reporting on a functional outcome scale | (3) Studies on >10% fusiform or dissecting aneurysms | |
| (4) Studies reporting on aneurysms associated with arteriovenous malformations or in populations with specific diseases (such as collagen disorders, Moyamoya disease or syndrome, dwarfism or autoimmune disorders) | (6) Studies with >50 patients | |
| (7) Studies on saccular unruptured aneurysms | (5) Studies in which previously ruptured aneurysms could not be distinguished from additional UIA | |
| (6) Studies in which treatment outcome was not reported separately for ruptured and unruptured aneurysms | ||
| PICO 3–5 | (1) Observational/cohort studies a (2) prospective case-control studies | (1) Retrospective patient identification |
| (3) Randomised studies | (2) Studies on >10% fusiform or dissecting aneurysms | |
| (4) Prospective registry studies; (5) meta-analyses of studies mentioned under 1–4 | (3) Studies reporting on aneurysms associated with arteriovenous malformations or in populations with specific diseases (such as collagen disorders, Moyamoya disease or syndrome, dwarfism or autoimmune disorders) | |
| (6) Cohort studies including >50 patients (RCTs can have less patients) | ||
| (7) Studies on saccular unruptured aneurysms | (4) Studies in which patients with previously ruptured aneurysms could not be distinguished from those with only unruptured aneurysms | |
| (5) Studies in which outcomes were not reported separately for ruptured and unruptured aneurysms |
If observational studies included a low proportion of patients with aneurysms related to other diseases and/or these were reported separately, such studies were also considered.
The PRISMA flow charts for all PICOs are given in the Supplemental Materials.
Data analysis
AvL, DAS CT performed data extraction and analysis. DAS, CT and the members of the appropriate PICO group evaluated the available evidence. The results were discussed via regular meetings with the two MWG chairs (NE, GR), and discrepancies were resolved in these meetings. Where appropriate, fixed or random-effects meta-analyses were conducted using Review Manager (RevMan) software. 13 Results were presented as estimates of effect with associated 95% confidence interval (95% CIs). Statistical heterogeneity across studies was assessed using the I2 statistic, and classified as moderate (⩾30%), substantial (⩾50%) or considerable (⩾75%). 14
Evaluation of the quality of evidence and formulation of recommendations
The risk of selection, performance, detection, attrition reporting biases in each randomised trial was assessed using the Cochrane Collaboration’s tool. 13
Quality assessment (according to GRADE) was performed by the two ESO methodologists (AvL and SL). The GRADE system method was applied for each PICO question to analyse the body of evidence for each outcome and by assessing all factors that may increase or decrease the level of evidence. For each PICO question and each outcome, the quality of evidence was rated using the (GRADEpro Guideline Development Tool (McMaster University, 2015; developed by Evidence Prime, Inc.)) as high, moderate, low or very low. 11 Factors that decreased level of evidence include study limitations, inconsistency of results, indirectness of evidence, imprecision publication bias. Factors that might increase level of evidence were large magnitude of effect, plausible confounding, which would reduce a demonstrated effect and dose-response gradient. The relevant PICO group was responsible for formulating an evidence-based recommendation according to the GRADE evidence profiles and the ESO standard operating procedure.
The phrasing of the recommendations was extensively discussed between all MWG members until consensus was reached. For each recommendation the quality of evidence and the strength of the recommendation were assessed. Expert Consensus Statements were formulated where the MWG members considered that not enough evidence was available to provide evidence-based recommendations for situations in which practical guidance was needed for everyday clinical practice. For Expert Consensus Statements that formulated specific data, for example, minimum case volumes, the entire MWG first discussed the actual amount in an open discussion and then agreed on the specific data after anonymous voting, Expert Consensus Statements were modified using the Delphi method until consensus was reached regarding the phrasing of the statement. The Expert Consensus Statements were then sent to all MWG members for anonymous voting. In the main document we list only those Expert Consensus Statements where all experts agreed upon. In the Supplemental Material we provide all Expert Opinion statements that were voted upon, plus the votings (Supplemental Table 3). Importantly, these Expert Consensus Statements should not be regarded as evidence-based recommendations, since they only reflect the opinion of the MWG.
Drafting of the document, revision and approval
Each PICO question was addressed in distinct sections, in line with the updated ESO SOP. First, ‘Analysis of current evidence’ summarised current pathophysiological considerations followed by a summary and discussion of the results of the identified RCTs and other studies. Second, ‘Additional information’ was added when more details on the studies referred to in the first section were needed to provide information on key subgroup analyses of the included studies, on ongoing or future RCTs, and on other studies which can provide important clinical guidance on the topic.
The Guideline document was reviewed several times by all MWG members and modified using the Delphi method until consensus was reached. The final submitted document was peer-reviewed by two external reviewers, two members of the ESO Guideline Board and one member of the Executive Committee.
Results
PICO 1: In adult patients with unruptured intracranial aneurysms does any type of microsurgical or endovascular aneurysm occlusion compared to no aneurysm occlusion improve outcomes?
The aim of preventive aneurysm occlusion in a patient with an unruptured intracranial aneurysm is to prevent poor functional outcome or death from a future SAH. However, there is continued uncertainty on the risk and benefit of preventive aneurysm repair versus observation: Preventive aneurysm repair but also observation is associated with a risk of poor functional outcome or death from complications or due to aneurysm rupture. Thus, it is important to balance the risk of immediate reduced functional outcome from complications and the long-term risk of poor functional outcome from future SAH. Key factors in this risk balance are the risk of rupture of an individual aneurysm and the risk of preventive occlusion of the same aneurysm.
Analysis of current evidence
There are no completed RCTs comparing preventive aneurysm occlusion versus observation in patients with UIA and quality of life or death plus dependency as an outcome measure. One RCT comparing endovascular treatment versus observation was stopped because of poor recruitment when 80 patients of the planned 2000 patients had been enrolled. 15
Most cohort studies on future risk of SAH in patients with UIA did not use quality of life or clinical outcome as an outcome measurement, but the incidence of aneurysm rupture as a surrogate endpoint instead, likely because a cohort study with a clinical outcome measure requires even longer follow-up periods. To provide guidance on management of UIA patients we therefore analyse the current evidence on the risk of aneurysm rupture and the risk of preventive aneurysm repair, irrespective of modality (i.e. microsurgery or endovascular treatment). It should be noted that the risk of rupture data in the prospective cohort studies is inevitably based on only a selection of patients: UIA patients were selected by for example, treatment decisions (those with the highest expected risk of rupture were often offered preventive treatment), or geographic location (whereas the risk of rupture likely differs according to geographic differences). Moreover, most cohort studies had incomplete data on risk factors for aneurysm rupture, such as aneurysm morphology, or blood pressure and smoking status during follow up.
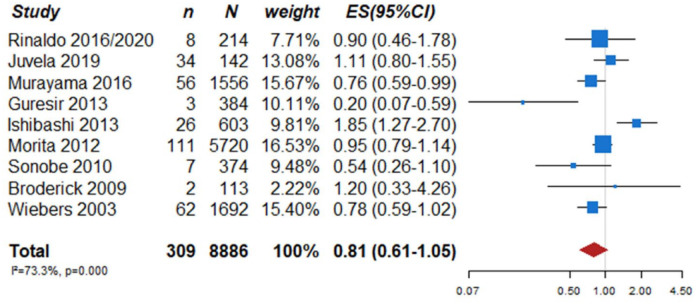
In total, nine prospective cohort studies on UIA patients with aneurysm rupture as the primary endpoint met the inclusion criteria (Table 2).5,16–24 In the pooled analysis of these nine studies including a total of 309 events during a follow-up of 33,923 patient-years, the 1-year risk of rupture was estimated 0.81% (95% CI 0.61–1.05) (Figure 1). Further, a previous meta-analysis of six cohort studies including data from 8382 patients and 10,272 UIA estimated an overall 5-year risk of aneurysm rupture of 3.4% (95% CI 2.9–4.0). 5 Independent risk factors for rupture in that data set were patient age (⩾70 years), hypertension, previous history of SAH, aneurysm size, aneurysm location and geographic region. According to the presence of these risk factors, the absolute 5-year risk of rupture ranged from 0.3% to over 15%. In addition to these six risk factors, irregular aneurysm morphology has been identified in the largest prospective UIA cohort study in Japanese patients. In that study on 5720 patients and 6697 aneurysms, aneurysms with irregular protrusions of the aneurysm wall were more likely to rupture, compared to regularly shaped aneurysms (hazard ratio (HR) 1.63, 95% CI 1.08–2.48). 21
Table 2.
Prospective (observational) cohort studies evaluating rate of rupture of intracranial aneurysms across time.
| Study | Participants (n) | Women (%) | Proportion of patients not treated with aneurysm occlusion (%) | Population | Minimum and/or maximum aneurysm size (mm) | Mean/median aneurysm size (diameter, mm) | Rupture events | Patient-years |
|---|---|---|---|---|---|---|---|---|
| Wiebers et al. 24 a | 1692 | 75 | 42% (1692/4060) | European and North American | ≥2 b | 7.4 ± 6.9 | 51 | 6544 |
| Broderick et al. 23 | 113 | 66 | – | North American, Australian, Austra-Asian | ≥2 | NA c | 2 | 167 |
| Sonobe et al. 22 | 374 | 64 | 84% (374/446) | Japanese | <5 | 3.3 ± 0.9 | 7 | 1306 |
| Morita et al. 21 | 5720 | 68 | 63% (4195/6697) | Japanese | >3 | 5.7 ± 3.6 | 111 | 11,684 |
| Güresir et al. 20 | 263 | 78 | Germans | <7 | NA d | 3 | 1500 | |
| Ishibashi et al. 19 | 603 | 71 | – | Japanese | ≥2 | NA e | 19 | 1406 |
| Rinaldo et al. 16 | 214 | 66 | – | North American | 2–45 | NA f | 8 | 884 |
| Murayama et al. 18 | 1556 | 68 | 87% (1960/2252) | Japanese | >2 | 3.4 ± 2.2 | 56 | 7368 |
| Juvela et al. 17 | 142 | 54 | – | Finnish | 2–26 | 5.1 ± 3.7 | 34 | 3064 |
A small proportion of patients had an associated condition, such as arteriovenous malformation (2.0%) or polycystic kidney disease (1.6%).
85% of patients had aneurysms with size ≤12 mm.
Only five patients had an aneurysm ≥7 mm.
Mean diameter was 3.6 ± 1.7 mm in patients without aneurysm rupture during follow-up and 3 ± 1 mm in ruptured aneurysms.
74% of all aneurysms were small (<5 mm).
13.1% of all aneurysms within the size category of ≥10 mm.
Figure 1.
PICO 1 aneurysm rupture rates at 1 year in single arm studies.
n: number of events; N: number of patients; CI: confidence interval; ES: estimated 1-year rupture rate.
Additional information
Recently detected aneurysm growth, defined as ⩾1 mm growth in any direction, is another important and strong risk factor for aneurysm rupture. A recent study of individual patient data estimated the absolute risk of rupture after observation of growth 2.9% (95% CI 0.9–4.9) at 6 months, 4.3% (95% CI 1.9–6.7) at 1 year and 6.0% (95% CI 2.9–9.1) at 2 years. 2 Other risk factors that were identified in studies with varying levels of evidence include cigarette smoking, 25 and a positive family history of SAH. 26
Regarding the risk of preventive aneurysm repair, there are preliminary data from one ongoing RCT (ClinicalTrials.gov Identifier: NCT01139892) that compares surgical clipping with endovascular coiling for UIA. 27 That trial used the composite endpoint ‘treatment failure’, defined as: (A) the inability to repair the aneurysm with the allocated modality; (B) aneurysm rupture and (C) residual aneurysm at 1-year follow-up. Secondary outcomes included neurological deficits and overall morbidity/mortality according to the modified Rankin Scale (mRS). In a published interim analysis after enrolment of 136 of the planned 260 patients, treatment failure occurred in 10% to 18% of patients, in relation to treatment modality (see PICO 2). The 1-year risk of poor outcome (mRS > 2) after preventive UIA occlusion ranged from 3.6% to 4.2% for both modalities, without a statistically significant difference between the modalities.
Additional data on UIA treatment risk factors per treatment modality (microsurgery or endovascular repair) were identified in a meta-analysis of 114 retrospective and prospective studies including data from 106,433 patients with 108,263 aneurysms. 28 In that study, risk factors for clinical complications defined as a new neurological deficit were comorbid diseases (diabetes, cardiac comorbidity or congestive heart failure and coagulopathies), wide aneurysm neck (>4 mm or dome-to-neck ratio >1.5) posterior circulation aneurysms. Modality-specific risk factors were stent-assisted coiling or flow diverting stents for endovascular UIA occlusion and aneurysm calcification for microsurgical UIA occlusion.
In conclusion, a completed RCT comparing clinical outcomes in UIA patients who undergo preventive aneurysm repair versus observation is lacking. At present, the overall estimated 1-year risk of rupture of aneurysms that undergo follow-up is estimated 0.8%. The available data on the risk of rupture and additional risk factors for rupture enable the estimation of the risk of aneurysm rupture, with considerable variability between patients based on patient- and aneurysm characteristics. The risk of aneurysm rupture is increased after detection of UIA growth on follow up imaging. The overall risk of preventive aneurysm repair (defined as neurological worsening to a mRS score >2) for aneurysms that can be treated by both treatment modalities (microsurgical or endovascular aneurysm repair) currently ranges from 3.6% to 4.2% and varies according to aneurysm complexity (morphology and calcifications) or location, use of specific endovascular devices/or comorbid diseases. 27 The quality of the evidence for treatment of aneurysms is very low (decreased due to serious bias in the studies, inconsistency indirectness) and that for treatment of aneurysms after detection of growth is also very low, due to very serious indirectness, bias in studies.
The number of patients treated per centre has been identified as an important factor for outcome after preventive UIA treatment already more than 25 years ago, 29 and this relation has in recent years been reported again, both in patients with ruptured and unruptured aneurysms.30,31 Experts in the field consider many features important when evaluating UIA, which makes a multidisciplinary approach pivotal. 32

PICO 2: In adult patients with unruptured intracranial aneurysms does any type of microsurgical occlusion compared to any type of endovascular occlusion improve outcome?
For patients in a good clinical condition after rupture of an intracranial aneurysm, there is evidence from RCTs that standard coiling results in better functional outcome than microsurgical treatment for aneurysm where both treatment modalities are considered feasible. The disadvantage of endovascular treatment is that it carries a considerable risk of reopening of the aneurysms, thereby necessitating follow-up imaging in all patients and retreatment in around 10%. 33 Data from patients with ruptured intracranial aneurysms can however not be applied to those with UIA, since patients with SAH from a ruptured intracranial aneurysm carry a higher risk of treatment complications and a higher risk of rupture in the initial weeks after initial treatment. Thus, the risk-benefit ratio differs in these two patient populations.
Analysis of current evidence and evidence-based recommendation
There are no completed RCTs comparing microsurgical versus endovascular occlusion of UIA with quality of life or death plus dependency as relevant outcome measure.
One ongoing RCT (ClinicalTrials.gov Identifier: NCT01139892) that randomised patients with an UIA to microsurgical clipping or endovascular treatment (standard coiling, coiling with modified coils or stent assisted coiling) reported interim data after enrolment of 136 of the 260 planned patients and 1-year results were known for 104 patients. 27 The primary outcome measure in this trial was the composite endpoint ‘treatment failure’ at 1 year, with treatment failure defined as: (A) initial failure of aneurysm treatment using the allocated modality; (B) intracranial haemorrhage during follow up or (C) a residual or recurrent aneurysm found during follow-up. Treatment failure at 1 year occurred in 5 of 48 (10%; 95% CI 3–23) microsurgically and 10 of 56 (18%; 95% CI 9–30) endovascular treated patients (OR 0.54; 95% CI 0.1–1.9). Secondary outcomes included neurological deficits following treatment, which occurred in 16 of 65 (25%; 95% CI 15–37) microsurgically and 7 of 69 (10%; 95% CI 4–20) endovascularly treated patients, and 1-year poor outcome (defined as mRS > 2), which occurred in 2 of 48 (4.2%; 95% CI 1–14) microsurgically and 2 of 56 (3.6%; 95% CI 0–12) endovascularly treated patients.
Another RCT was prematurely stopped because of safety reasons after randomisation of 78 of the planned 450 patients. 34 This trial compared treatment with flow diversion (any type of flow diverter) versus the ‘best standard option’, which could consist of coiling (with or without stenting), parent vessel occlusion (PVO), microsurgical clipping or conservative management. Patients for whom no standard option but only flow diversion was deemed suitable, were included in a registry. The study had a primary safety outcome and a primary efficacy outcome. The primary safety outcome was death or dependency (mRS > 2) at 3 months. The primary efficacy outcome was a composite of complete or near-complete occlusion of the aneurysm (3–12 months) combined with an independent functional outcome (mRS score ⩽2). The trial was halted when 12 (16%) of 75 patients who were allocated to or received flow diversion at any time were dead (n = 8) or dependent (n = 4) at 3 months. At that time 39 patients had been randomised to flow diversion and 39 to best standard option, that consisted of coiling (n = 25), parent vessel occlusion (n = 10) and no intervention (n = 4). Efficacy was below expectations of the trial hypothesis: 16 (42.1%) of 38 patients (95% CI 26.7–59.1) randomly allocated to flow diversion failed to reach the primary outcome, as compared with 14 (35.9%) of 39 patients allocated to standard treatment (95% CI 21.7–52.9).
Additional information
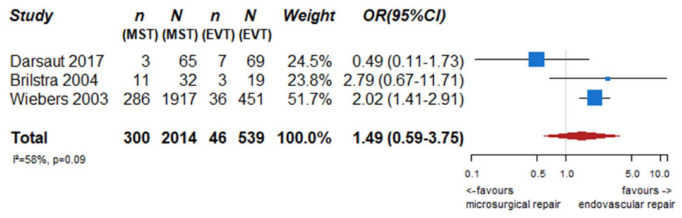
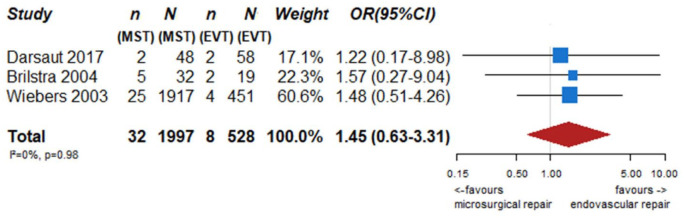
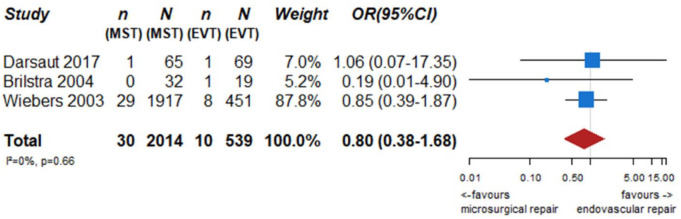
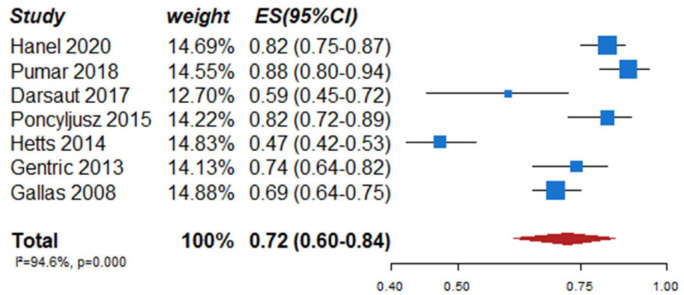
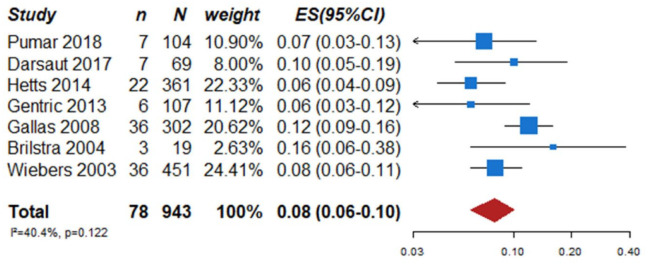
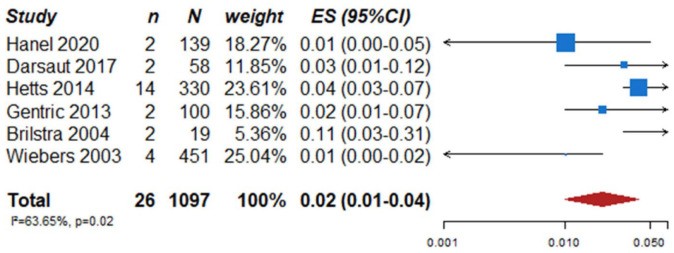
We identified two observational studies that fulfilled the predefined selection criteria describing relevant outcomes for microsurgical and endovascular UIA occlusion but without comparison of the two treatment modalities,24,35 six observational studies reporting on relevant outcomes after endovascular treatment only.36–41 (Table 2) In the three studies with both a microsurgical and endovascular treatment group, the OR of microsurgical versus endovascular treatment were for periprocedural stroke 1.49 (95% CI 0.59–3.75), for poor functional outcome (defined as mRS 3–6) after more than 3 months follow up 1.45 (95% CI 0.63–3.31) and for death within 3 months 0.80 (0.38–1.68) (Figures 2–4; Tables 3 and 4). Complete aneurysm occlusion after surgery was not reported in any of these observational studies. For any endovascular treatment, complete aneurysm occlusion was reported in seven studies with an overall occlusion rate of 72% (95% CI 60–84), periprocedural stroke in seven studies (8%; 95% CI 6–10) poor outcome (defined as mRS 3–6) 3 to 12 months after the procedure in six studies (2.4%; 95% CI 1.6–3.6) (Figures 5–7). There were no good data on chance of aneurysm rupture after microsurgical or endovascular occlusion of the aneurysm.
Figure 2.
PICO 2 – association between microsurgical aneurysm repair, compared to endovascular aneurysm repair and risk of periprocedural stroke.
n: number of events; N: number of patients; MST: microsurgical treatment; EVT: endovascular treatment; CI: confidence interval; OR: odds ratio.
Figure 3.
PICO 2 – association between microsurgical aneurysm repair, compared to endovascular aneurysm repair and poor neurological outcome or death (mRS 3–6) after at least 3 months of follow-up.
n: number of events; N: number of patients; MST: microsurgical treatment; EVT: endovascular treatment; CI: confidence interval; OR: odds ratio.
Figure 4.
PICO 2 – association between microsurgical aneurysm repair, compared to endovascular aneurysm repair, and death within 3 months of follow-up.
n: number of events; N: number of patients; MST: microsurgical treatment; EVT: endovascular treatment; CI: confidence interval; OR: odds ratio.
Table 3.
Baseline characteristics of participants in the studies included in the quantitative analysis performed for PICO 2.
| Study | Study design | Treatment | Participants (n) | Women (%) | Aneurysm size range (mm) | Periprocedural stroke |
|---|---|---|---|---|---|---|
| Wiebers et al. 24 a | Observational | No treatment, EVT, MST | 4060 | 76 | 2–≥25 mm (range) | MST: 286/1917 |
| EVT: 36/451 | ||||||
| Brilstra et al. 35 | Observational | EVT, MST | 51 | 75 | 0–>25 (range) | MST: 11/32 |
| EVT: 3/19 | ||||||
| Gallas et al. 36 | Observational | EVT | 302 | 63 | NA b | EVT: 36/302 |
| Guglielmi detachable coils | ||||||
| Gentric et al. 37 | Observational | EVT | 107 | 69 | NA c | EVT: 6/107 |
| Stent assisted coilling | ||||||
| Hetts et al. 38 | Observational | EVT | 361 | 77 | 4–20 (range) | EVT: 22/361 |
| Stent assisted coil, simple coil | ||||||
| Poncyljusz et al. 39 | Observational | EVT | 78 | 73 | NA | – |
| LVIS and LVIS Jr. stents | ||||||
| Darsaut et al. 27 | RCT | EVT, MST | 136 | 69 | 3–25 (range) | MST: 3/65 |
| EVT: 7/69 | ||||||
| Pumar et al. 40 | Observational | EVT | 104 | 75 | <10 mm | EVT: 7/104 |
| SILK flow diverter | ||||||
| Hanel et al. 41 | Observational | EVT | 141 | 88 | ≤12 mm d | – |
| Pipeline embolisation device for wide necked aneurysms |
EVT: endovascular treatment; MST: microsurgical treatment; NA: not available; RCT: randomised controlled trial.
A small proportion of patients had an associated condition, such as arteriovenous malformation (2.0%) or polycystic kidney disease (1.6%).
Aneurysms <10 mm represented 77% of the participants.
Aneurysms <6 mm represented 52% of the participants.
Aneurysms 7 to 12 mm represented 16% of the participants.
Table 4.
GRADE table PICO 2.
| Question: Microsurgical compared to endovascular intervention and outcome | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Setting: UIA patients | ||||||||||||
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Neurosurgical | Endovascular intervention | Relative (95% CI) | Absolute (95% CI) | ||
| Poor outcome (mRS 3–6) | ||||||||||||
| 3 | Observational studies | Serious a | Not serious | Not serious | Serious c | None | 32/1997 (1.6%) | 8/528 (1.5%) | OR 1.45 (0.63–3.31) | Seven more per 1000 (from 6 fewer to 33 more) | ⨁◯◯◯ Very low | Critical |
| Mortality within 3 months | ||||||||||||
| 3 | Observational studies | Serious a | Not serious | Not serious | Serious c | None | 30/2014 (1.5%) | 10/539 (1.9%) | OR 0.80 (0.38–1.68) | Four fewer per 1000 (from 11 fewer to 12 more) | ⨁◯◯◯ Very low | Critical |
| Periprocedural stroke | ||||||||||||
| 3 | Observational studies | Serious a | Not serious | Serious b | Serious c | None | 300/2014 (14.9%) | 46/539 (8.5%) | OR 1.49 (0.59–3.75) | 37 more per 1000 (from 33 fewer to 174 more) | ⨁◯◯◯ Very low | Critical |
CI: confidence interval; OR: odds ratio.
All studies rated serious with Robins-I tool.
Outcomes had various definitions across studies.
Confidence interval unable to exclude substantial benefit or harm.
Figure 5.
PICO 2 – occlusion rates at 3 months in studies assessing endovascular treatment in patients with unruptured intracranial aneurysm.
n: number of events; N: number of patients; CI: confidence interval; ES: estimated rate of complete aneurysm occlusion.
Figure 6.
Periprocedural stroke events rates in studies assessing endovascular treatment in patients with unruptured intracranial aneurysm.
n: number of events; N: number of patients; CI: confidence interval; ES: estimated rate of periprocedural stroke events.
Figure 7.
PICO 2 – poor outcome events rates in studies assessing endovascular treatment in patients with unruptured intracranial aneurysm.
n: number of events; N: number of patients; CI: confidence interval; ES: estimated rate of poor outcome events.
We retrieved additional data on risk of treatment complications from a recent systematic review and meta-analysis of 114 retrospective and prospective studies including data from 106,433 patients with 108,263 aneurysms. 28 For endovascular treatment (74 studies), the pooled risk for clinical complications was 4.96% (95% CI 4.00–6.12), and that for case fatality 0.30% (95% CI 0.20−0.40). Risk factors for complications included female sex (pooled OR, 1.06 (95% CI 1.01–1.11)), diabetes (OR 1.81 (95% CI 1.05–3.13)), hyperlipidaemia (OR 1.76 (95% CI 1.3–2.37)), cardiac comorbidity (OR 2.27 (95% CI 1.53–3.37)), wide aneurysm neck (>4 mm or dome-to-neck ratio >1.5; OR 1.71 (95% CI 1.38–2.11)), posterior circulation aneurysm (OR 1.42 (95% CI 1.15–1.74)), stent-assisted coiling (OR 1.82 (95% CI 1.16–2.85)) and stenting (OR 3.43 (95% CI 1.45–8.09)).
For microsurgical treatment (54 studies), the pooled risk for clinical complications was 8.34% (95% CI 6.25−11.10) and that for case-fatality 0.10% (95% CI 0.00−0.20). Factors associated with risk of complications for microsurgical therapy were age (OR per year increase, 1.02 (95% CI 1.01–1.02)), female sex (OR 0.43 (95% CI 0.32–0.85)), coagulopathy (OR 2.14 (95% CI 1.13–4.06)), use of anticoagulation (OR 6.36 (95% CI 2.55–15.85)), smoking (OR 1.95 (95% CI 1.36–2.79)), hypertension (OR 1.45 (95% CI 1.03–2.03)), diabetes (OR 2.38 (95% CI 1.54–3.67)), congestive heart failure (OR 2.71 (95% CI 1.57–4.69)), posterior aneurysm location (OR 7.25 (95% CI 3.70–14.20)) and aneurysm calcification (OR 2.89 (95% CI 1.35–6.18)).
With the advent of new endovascular techniques, it is important that the team deciding on which treatment to offer to patients has ample experience in microsurgical and all endovascular treatment options, which makes a multidisciplinary approach pivotal. 32
When a medical decision is preference sensitive, meaning that more than one management strategy is reasonable, shared decision making can offer a solution. Shared decision making is a process whereby physician and patient decide together recognising each other’s expertise. After being properly informed, patients can actively participate in the decision-making process with their healthcare providers. The information given to the patient should: (1) provide information on the condition, options, benefits, harms, scientific uncertainties; (2) clarify values by describing outcomes and/or asking the patient to rate the importance of benefits and harms; (3) make the decision explicit. 42
In conclusion, there are no data from completed RCTs or other comparative studies on clinically relevant outcomes for UIA patients undergoing microsurgical or endovascular repair. The available data from the non-completed RCT do not show a superiority on clinical outcome for either one of the treatment-modalities, but lack power because the published data were interim results. The quality of evidence is therefore very low. The other trial, comparing flow diversion versus any other type of management, including no aneurysm treatment, was halted prematurely because of safety reasons. The results of this trial suggest that treatment with flow diverting stents should only be considered if no other treatment options are available that can occlude the aneurysm with a risk lower than the expected 5-year risk of rupture, but again evidence is very low. We could not include the data from this trial in our meta-analysis because the comparator consisted of entirely different treatment options (coiling with or without stenting, parent vessel occlusion, microsurgical clipping, or conservative management).
All other data come from case series or device specific registries of patients selected for a specific type of treatment or device. Because of this selection bias, the data cannot be used to directly compare the two treatment modalities regarding efficacy or treatment complications. The observational data suggest that patients selected for endovascular therapy have a lower risk of clinical complications than patients selected for microsurgical therapy, and that in patients selected for regular coiling the risk of complications is lower than in patients selected for more advanced endovascular techniques. However, since these were data from observational studies and not RCTs, the treatment risks cannot be directly compared, and quality of evidence if very low because of very serious indirectness and bias in studies. The risk factor profile (including aneurysm characteristics) for treatment complications differs between endovascular and microsurgical treatment, which may guide decisions on treatment modality. Female sex is associated with an increased risk for endovascular therapy and a decreased risk for microsurgical therapy. A wide neck is a risk factor only found for endovascular therapy. Increasing age, coagulopathy and use of anticoagulation, and aneurysm calcification are risk factors only found for microsurgical therapy, and the increased risk for complications in posterior circulation (i.e. vertebral, basilar and posterior cerebral arteries and their branches but excluding the posterior communicating artery) aneurysms is much higher for microsurgical than for endovascular therapy.
PICO 3. In adult patients with unruptured intracranial aneurysms does any type and frequency of follow-up imaging followed by aneurysm occlusion in case of aneurysm growth or other change compared to no follow-up imaging improve outcome?
The aim of radiological monitoring of an UIA is to identify growth or morphological change of the UIA. Since aneurysms that show growth or morphological change during follow-up are at increased risk of rupture, preventive treatment of these aneurysms should be reconsidered. Although patients often experience the outcome of a follow-up scan reassuring in case no growth or morphological change is detected, patients may experience anxiety in the period around the scan. Thus, follow-up imaging in UIA patients has downsides, such as patient anxiety (before every scan) and health care costs but also the risk of neurological deficits due to treatment complications in case of preventive aneurysm treatment. However, benefits of follow-up imaging could be reassurance for UIA patient (after every negative scan) and prevention of poor outcome from SAH 43 in case of preventive aneurysm treatment following detection of aneurysm growth.

Analysis of current evidence and evidence-based recommendations
There are no completed RCTs or other controlled studies with death or dependency as relevant outcome measure that compare radiological monitoring of patients with an UIA with no radiological monitoring. Therefore, we could only analyse single-arm observational studies that mostly considered growth or aneurysm rupture as outcome measures rather than clinical outcome.
In a pooled analysis of 10 international cohorts of radiologically followed UIA patients, UIA growth was seen in 17% of the followed 1507 patients and in 14% of the 1909 aneurysms during 5782 patient-years of follow-up. 44 Predictors for aneurysm growth were Earlier subarachnoid haemorrhage, Location of the aneurysm, Age >60 years, Population, Size of the aneurysm Shape of the aneurysm(=ELAPSS score). The 3-year growth risk ranged from 5% to 42% and the 5-year growth risk from 9% to 60%, depending on the risk factor status. 43
The absolute risk of rupture of an aneurysm with detected growth has recently been investigated in a multicentre study including individual patient data from a total of 312 patients with 329 growing aneurysms, that did not receive preventive repair despite aneurysm growth. 2 During the 864 aneurysm-years of follow-up, 25 (7.6%) of the aneurysms ruptured in 24 patients. The absolute risk of rupture after growth was 2.9% (95% CI 0.9–4.9) at 6 months, 4.3% (95% CI 1.9–6.7) at 1 yearand 6.0% (95% CI 2.9–9.1) at 2 years. 2 In multivariable analyses, predictors of rupture were aneurysm size (7 mm or larger HR 3.1; 95% CI 1.4–7.2), shape (irregular HR 2.9; 95% CI 1.3–6.5), site (middle cerebral artery HR 3.6; 95% CI 0.8–16.3; anterior cerebral artery, posterior communicating artery, or posterior circulation HR 2.8; 95% CI 0.6–13.0). In the triple-S (size, site, shape) prediction model, the 1-year risk of aneurysm rupture after growth ranged from 2.1% to 10.6%.
In a series of 118 patients with an UIA from an era before preventive UIA repair was performed and who had a mean follow-up of 18.5 years (range 0.8–52.3), 29% of UIA ruptured after a mean follow-up time of 13.6 years (range 1.2–51.0). 25 It is worth noting that the included patients may have had a higher rupture risk, because the risk of rupture is higher in Finnish populations compared to other Caucasian populations, and because a large majority of UIA in that study was detected during DSA in patients with SAH from rupture of another aneurysm, which is another risk factor of rupture.
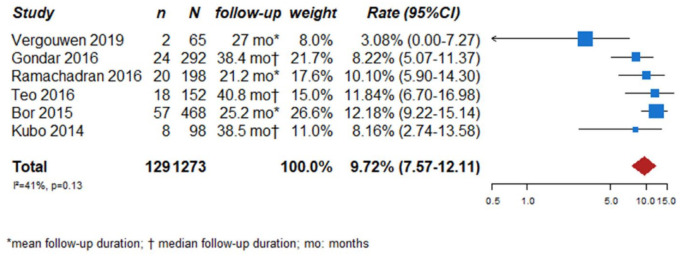
We searched for observational single-arm studies of radiologically followed aneurysms that reported growth as outcome measure. We identified six studies, with a total of 1273 followed UIA.45–50 Follow-up duration ranged from 21.2 to 40.8 months. The total aneurysm growth rate of the included studies was 9.7% (129 of the 1273 UIA, 95% CI 7.6–12.1) (Figure 8).
Figure 8.
PICO 3 – aneurysm growth rates in observational single arm studies.
n: number of events; N: number of patients; CI: confidence interval; rate: estimated rate of aneurysm growth during the follow-up period.
Additional information
The effectiveness of radiological monitoring followed by treatment of aneurysms that show growth has not been studied in cohorts with clinical outcome. The efficacy in terms of preventing rupture of such an approach has been the subject of two recent studies.51,52 Both studies only included patients with small UIA (⩽7 mm in 95%), the oldest included 292 patients with 368 UIA 51 and the more recent 545 patients with 671 aneurysms. 52 The investigators in both studies proceeded with treatment only in case of rupture or growth. This management strategy in this population with small aneurysms led to a small number of ruptured aneurysms. The annual risk of rupture was in the oldest study 0.24% (n = 3, 95% CI 0.17−2.40) 51 and in the more recent 0.1% (n = 3, 95% CI 0−0.24). 52 However, the safety of such a strategy in terms of treatment complications was not studied in these two studies.
CTA and MRA are sensitive measures to detect and follow intracranial aneurysms, and obviate the need for serial DSA in patients undergoing follow-up imaging for UIA.53–55
Several cross-sectional studies and two longitudinal studies showed that aneurysm wall enhancement, using high-resolution vessel wall MR imaging, is more common in unstable (i.e. ruptured, symptomatic, or having a morphologic structure that changes over time) than in stable (i.e. incidental or non-evolving) UIA.49,56
When a medical decision is preference sensitive, meaning that more than one management strategy is reasonable, shared decision making can offer a solution. Shared decision making is a process whereby physician and patient decide together recognising each other’s expertise. After being properly informed, patients can actively participate in the decision-making process with their healthcare providers. The information given to the patient should: (1) provide information on the condition, options, benefits, harms, scientific uncertainties, (2) clarify values by describing outcomes and/or asking the patient to rate the importance of benefits and harms and (3) make the decision explicit. 42
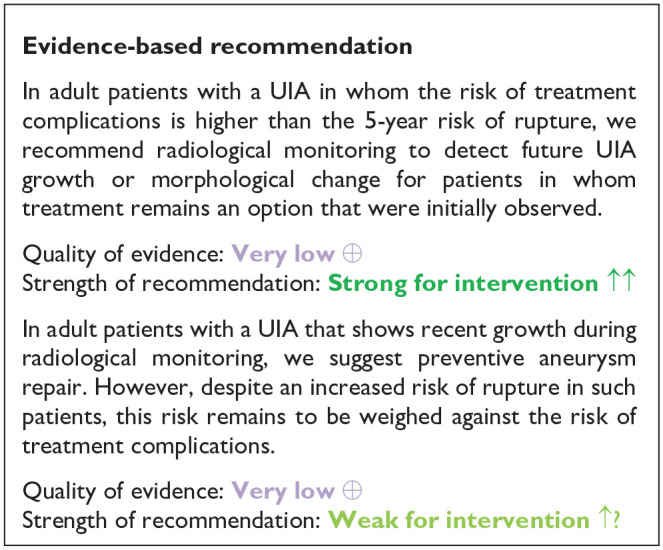
In conclusion, evidence from RCTs comparing (clinical) outcomes in UIA patients who undergo radiological monitoring for a UIA versus no monitoring is lacking, and quality of the evidence provided above is very low (decreased due to serious bias in the studies, and indirectness). Aneurysm growth is a risk factor for aneurysm rupture, but other risk factors, such as aneurysm diameter and location remain equally important. The evidence for treating aneurysms after detection of growth is very low due to serious imprecision, indirectness and bias in the study. The risk of future aneurysm growth can be estimated using the ELAPPS score; this enables clinicians to plan timing of follow-up imaging in an individual UIA patient. 44 Here is insufficient evidence to advise on frequency of follow up imaging for UIA in general. The frequency depends on many patient and aneurysm characteristics and on the results of previous follow up images. In UIA that have remained stable over years the interval may be enlarged, but data supporting enlarging interval in stable aneurysms are lacking. In case of aneurysm growth, the risk of rupture can be estimated using the triple-S prediction score and should be weighed against the risk of treatment complications. 2 Future studies need to determine the role of aneurysm wall enhancement as a biomarker for aneurysm stability.

PICO 4: In adult patients with unruptured intracranial aneurysms does any life-style modification or any medical treatment (e.g. anti-inflammatory drugs, antihypertensive drugs, statins) in comparison to no treatment improve outcome?
Smoking and hypertension are risk factors for aneurysm formation, growth and rupture.5,7,25 Moreover, inflammation has been suggested as a modifiable pathway in UIA development and rupture. 7 Thus, treatment with anti-inflammatory drugs may decrease the risk of rupture of UIA. Two such treatments that are widely available, cheap and have a beneficial risk profile are acetylsalicylic acid and statins. Small controlled series have indeed suggested an anti-inflammatory effect of acetylsalicylic acid treatment in the aneurysmal wall. 57 For statins, several animal studies showed a protective effect of statin treatment in experimental UIA studies. We therefore searched the literature to investigate if treatment of hypertension, smoking cessation or treatment with acetylsalicylic acid or statins decreases the risk of growth or rupture.
Analysis of current evidence
See Table 5 for GRADE table of RCTs included for PICO 4 (quality of evidence for all recommendations in PICO 4 is very low)
Table 5.
GRADE table PICO 4.
| Question: Lifestyle modification or medical treatment compared to no modification or treatment for improve outcome | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Setting: UIA patients | ||||||||||||
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Lifestyle modification or medical treatment | No modification or treatment | Relative (95% CI) | Absolute (95% CI) | ||
| Smoking cessation and aneurysm rupture | ||||||||||||
| 1 | Observational studies | Not serious a | Not serious | Not serious | Very seriousb,c | None | 0/11 (0.0%) | 8/26 (30.8%) | Not estimable | ⨁◯◯◯ Very low | Critical | |
| Smoking cessation and aneurysm growth rupture | ||||||||||||
| 1 | Observational studies | Not serious a | Not serious | Not serious | Very seriousb,c | None | 3/11 (27.3%) | 12/26 (46.2%) | Not estimable | ⨁◯◯◯ Very low | Critical | |
| Aspirin intake and aneurysm rupture | ||||||||||||
| 1 | Observational studies | Serious d | Not serious | Not serious | Not serious | None | 11/1087 (1%) | 1/643 (0.2%) | OR 0.11 (0.01–0.86) | – per 1000 (from – to –) | ⨁◯◯◯ Very low | Critical |
| Aspirin intake and aneurysm growth | ||||||||||||
| 1 | Observational studies | Not serious a | Not serious | Not serious | Serious c | None | –/113 | −/159 | HR 0.29 (0.11–0.77) | – per 1000 (from – to –) | ⨁◯◯◯ Very low | Critical |
| Controlled hypertension and aneurysm rupture | ||||||||||||
| 1 | Observational studies | Not serious a | Not serious | Not serious | Seriousc,e | None | 2/– | HR 16.66 (2.10–132.90) | 17 Fewer per 1000 (from 133 fewer to 2 fewer) | ⨁◯◯◯ Very low | Critical | |
| Controlled hypertension and aneurysm growth | ||||||||||||
| 1 | Observational studies | Not serious a | Not serious | Not serious | Seriousc,e | None | HR 1.55 (0.63–3.80) | Two fewer per 1000 (from 4 fewer to 1 fewer) | ⨁◯◯◯ Very low | Critical | ||
| Statin intake and aneurysm rupture or rupture | ||||||||||||
| 1 | Randomised trials | Serious f | Not serious | Not serious | Very seriousc,g | None | −/93 | −/116 | HR 0.759 (0.415–1.386) | – per 1000 (from – to –) | ⨁◯◯◯ Very low | Critical |
CI: confidence interval; HR: hazard Ratio; OR: odds ratio.
Using Robins-I: overall risk of bias is moderate.
Small sample size and number of cases.
Only one study.
At least one study has an overall serious risk of bias using Robins-I.
Wide confidence interval.
Using ROB-2: randomisation raised some concerns; deviation high concerns; missing outcomes and measuring outcome low concerns; and selection of reported results raised some concerns.
Confidence interval not allowing to exclude substantial benefit or harm.
Smoking
We found no RCTs comparing smoking cessation versus smoking on the risk of quality of life, death or dependence or growth and rupture of an intracranial aneurysm. In the absence of RCTs, we searched for observational studies comparing the risk of growth or rupture between UIA patients who continued smoking and those who quitted smoking during follow-up. We excluded observational studies comparing the risk of growth or rupture between patients who were smoking at baseline and those who were former smokers. We identified two overlapping studies,58,59 and only included the most recent study, because of a longer follow-up duration and more outcomes. 58 This study included 87 patients (with 111 aneurysms) with a median follow-up time between aneurysm measurements of 21.7 years (range 1.2 and 51.0 years). The results showed that 14/34 (41%) patients who continued smoking during follow-up had aneurysm rupture and 5/34 (15%) had aneurysm growth without rupture. Of the 11 patients who stopped smoking during follow-up, none (0%) had aneurysm rupture and 3 (27%) had aneurysm growth without rupture.
Hypertension
There are no RCTs comparing the effect of treatment of hypertension versus no treatment of hypertension on the quality of life, death or dependency or risk of growth and rupture of an intracranial aneurysm as outcome measure. In the absence of RCTs, we searched for observational studies comparing the risk of growth or rupture between UIA patients who had uncontrolled hypertension and those with controlled hypertension during follow-up. We excluded case-control studies (patients with SAH versus patients with an UIA) and observational studies comparing the risk of growth or rupture between patients with and without hypertension at baseline, but without data on blood pressure during follow up. We identified two studies describing patients from the same prospective, multicentre cohort study that consecutively enrolled 1866 eligible patients with ischaemic cerebrovascular disease and an UIA < 7 mm.60,61 Since one study had aneurysm growth as an outcome measure and the other aneurysm rupture, we included both studies.
The first study focussed on aneurysm growth and included 272 patients who had radiological monitoring during follow-up (mean follow-up time, 19.6 ± 12.7 months). 61 A total of 141 patients had hypertension of whom 123 used antihypertensive drugs. Multivariate Cox regression analyses were performed to investigate risk factors for aneurysm growth. Using non-hypertensive patients as a reference group, the HRs for risk of growth during follow-up were for uncontrolled hypertension 6.1 (95% CI 2.4–15.4) and for controlled hypertension 1.6 (95% CI 0.6–3.8). No direct comparison was made between patients with uncontrolled and controlled hypertension.
The second study had aneurysm rupture as the primary outcome measure. 60 After a mean follow-up duration of 30.5 ± 12.3 months (range 1.0–45.6 months), 128 (6.9%) patients were lost to follow-up and another eight patients were excluded since they could not give detailed information on acetylsalicylic acid use, leaving 1730 patients for further analysis. Using non-hypertensive patients as a reference group, the HRs for risk of rupture during follow-up were for uncontrolled hypertension 16.7 (95% CI 2.1–132.1) and for controlled hypertension 3.5 (95% CI 0.3–38.5). No direct comparison was made between patients with uncontrolled and controlled hypertension.
Acetylsalicylic acid
There are no completed RCTs comparing treatment with acetylsalicylic acid versus no acetylsalicylic acid on quality of life, death plus dependency or the risk of growth and rupture of an intracranial aneurysm as outcome measure. One ongoing trial (ClinicalTrials.gov Identifier: NCT03063541) investigates whether a treatment strategy of acetylsalicylic acid 100 mg once daily in combination with intensive blood pressure treatment (systolic blood pressure <120 mm Hg) decreases the risk of aneurysm growth or rupture compared to care as usual (no acetylsalicylic acid, systolic blood pressure <140 mm Hg). 62 In the absence of completed RCTs, we searched for observational studies comparing the risk of growth or rupture between UIA patients who used acetylsalicylic acid and those not using acetylsalicylic acid during follow-up. We excluded cross-sectional case-control studies comparing use of acetylsalicylic acid between patients with SAH and those with an UIA. We identified four studies,60,61,63,64 of which we excluded one, 63 since data were derived from the same cohort as from another study, 64 and sex differences on the effect of acetylsalicylic acid on aneurysm rupture was not a predefined analysis.
The first study included patients who were enrolled in the International Study of Unruptured Intracranial Aneurysms. 64 Patients were selected from the prospective untreated cohort (n = 1691) in a nested case-control study. Cases were patients who had a proven aneurysmal subarachnoid haemorrhage during a 5-year follow-up period. Four control subjects were matched to each case by site and size of aneurysm (58 cases, 213 control subjects). Frequency of acetylsalicylic acid use was determined at baseline interview, but not during follow up. In multivariable analyses, patients who used acetylsalicylic acid three times a week to daily had a lower risk of aneurysm rupture (adjusted OR 0.27; 95% CI 0.11–0.67) compared to those who never took acetylsalicylic acid. It may be assumed that patients who were taken acetylsalicylic acid regularly at baseline continue to do so during follow up, but since actual use during follow up was not assessed, we did not include the data of this study in the final analyses
The two other studies described patients from the same prospective, multicentre cohort study that consecutively enrolled 1866 eligible patients with ischaemic cerebrovascular disease and an UIA < 7 mm.60,61 Since one study had aneurysm growth as an outcome measure and the other aneurysm rupture, we included both studies. The first study focussed on aneurysm growth and included 272 patients who had radiological monitoring during follow-up (mean follow-up time 19.6 ± 12.7 months). 61 A total of 113 (42%) patients used acetylsalicylic acid during follow-up. UIA growth occurred in 31 (11%) of the 272 patients. Multivariate Cox regression analyses were performed to investigate risk factors for aneurysm growth. In the multivariate Cox analysis, use of acetylsalicylic acid was associated with a decreased risk of aneurysm growth (HR 0.29 (95% CI 0.11–0.77)). The second study had aneurysm rupture as the primary outcome measure. 60 After a mean follow-up duration of 30.5 ± 12.3 months (range 1.0–45.6 months), 128 (6.9%) patients were lost to follow-up and another eight patients were excluded since they could not give detailed information on acetylsalicylic acid use, leaving 1730 patients for further analysis. Aneurysm rupture during follow-up occurred in 1/643 (0.2%) patients using acetylsalicylic acid and in 11/1087 (1%) patients not using acetylsalicylic acid. In the multivariate Cox analysis, use of acetylsalicylic acid was associated with a decreased risk of aneurysm rupture (HR 0.11 (95% CI 0.01–0.86)).
Statins
There is one completed RCT comparing treatment with a statin versus no statin on the risk of growth and rupture of an intracranial aneurysm, but this trial had no data on death or dependency as outcome measure. 65 In this open-label, multicentre, randomised controlled trial the effect of atorvastatin on the risk of aneurysm growth was studied. The primary endpoint was a composite of aneurysm growth of ⩾0.5 mm, new bleb formation confirmed from magnetic resonance (MR) angiography, and rupture. Enrolment was prematurely terminated due to unexpectedly slow enrolment. Of 231 patients (with 275 intracranial aneurysms), 110 patients (with 128 aneurysms) were randomly assigned to the statin group and 121 patients (with 147 aneurysms) to the control group. Twenty-two dropout patients were excluded. Final analyses were based on 107 aneurysms (in 93 patients) allocated to the statin group and 140 aneurysms (in 116 patients) randomised to the control group. The primary endpoint occurred in 17 aneurysms (16%) in the statin group and in 28 aneurysms (20%) in the control group (log-rank p = 0.359). No aneurysm rupture was observed in both groups.
In addition, we searched for observational studies comparing the risk of growth or rupture between UIA patients who used a statin and those not using a statin during follow-up. We excluded case-control studies comparing use of a statin between patients with SAH and those with an UIA, and observational studies comparing the risk of growth or rupture during follow-up between patients who did and did not use a statin at baseline but without data on the use of statins during follow up. No studies were identified.
Additional information
Apart from exerting a chronic effect, acute hypertension, for example from physical exertion, may also serve as trigger for aneurysmal rupture. We found two studies that assessed trigger factors for aneurysmal rupture.66,67 Both indeed found an increased risk of aneurysm rupture shortly after heavy physical exercise,66,67, one of these studies also found an increased risk shortly after other activities that increase blood pressure such as straining or sexual intercourse, and after cola or coffee consumption. 67 Although the relative risk of aneurysmal rupture is increased shortly after these activities compared to shortly after time periods without these activities, the absolute risk of aneurysmal rupture shortly after these activities is still very low. People at low risk need to avoid 13.4 million cups of coffee or 1.3 million episodes of sexual intercourse to avoid one aneurysmal rupture. 68
The studies on acetylsalicylic acid described above suggest that its use reduces the risk of aneurysm growth or rupture. One might assume that use of antiplatelets agents worsens outcome in patients with aneurysmal rupture. A systematic review and meta-analysis of antiplatelet use in patients with aneurysmal subarachnoid haemorrhage, found a trend towards better outcome in patients treated with antiplatelet agents, possibly due to a reduction in secondary ischaemia. 69 The results were not statistically significant, thus no definite conclusions can be drawn, but the data do not raise a concern on its use in patients with UIA because of a worse outcome after aneurysmal rupture.
In conclusion, there is (very low) evidence that suggests that smoking cessation and blood pressure treatment is beneficial for prevention of aneurysm growth and/or rupture.
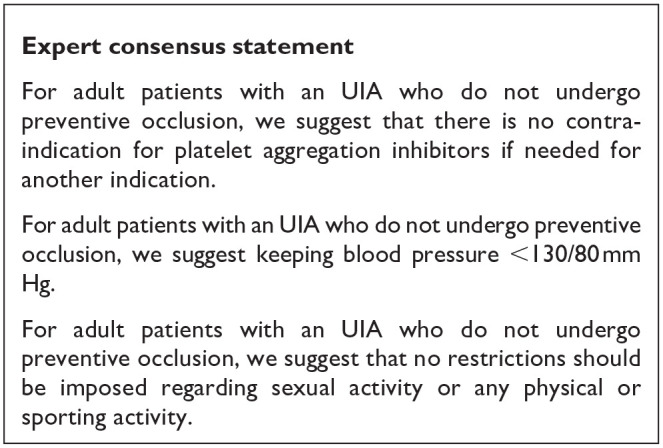
Although there is (very low) evidence that acetylsalicylic acid could be beneficial for prevention of aneurysm growth and/or rupture, we suggest to not start acetylsalicylic acid treatment with the aim to prevent aneurysm growth or rupture since the risk-benefit ratio is uncertain. This may change when data from currently ongoing Phase III RCTs become available. On the other hand, if there is another indication for acetylsalicylic acid treatment (e.g. secondary prevention for coronary heart disease or stroke), we do not suggest cessation of acetylsalicylic acid treatment. As for statins and prevention of aneurysm rupture or growth, the current data do not suggest any beneficial or detrimental effect.

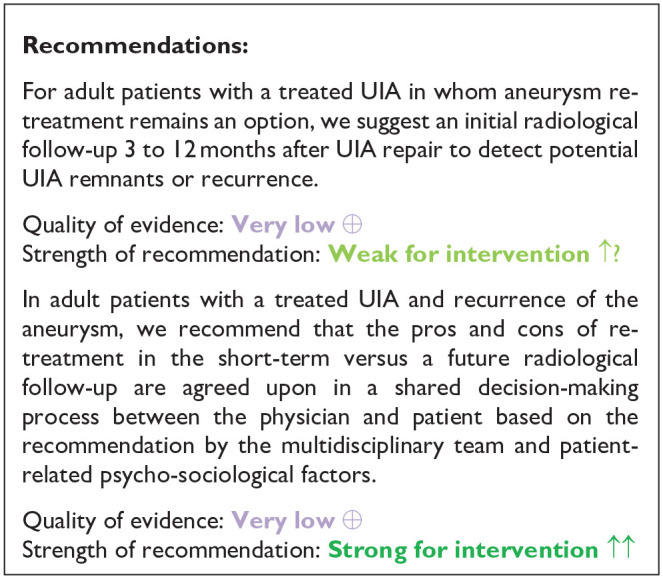
PICO 5: In adult patients with occluded unruptured aneurysms, does any type and frequency of follow-up imaging compared to no follow-up imaging improve outcome?
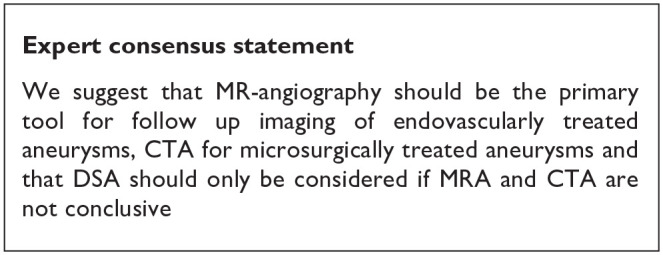
After endovascular or microsurgical treatment of ruptured intracranial aneurysms, aneurysms can reoccur (i.e. re-opening in coiled aneurysms and recurrence in clipped aneurysms) and cause new episodes of subarachnoid haemorrhage.70,71 After preventive occlusion of UIA, aneurysms may therefore also reoccur, with inherent risk of future rupture. The rationale for follow-up imaging after treatment of UIA is to detect aneurysm recurrence, and to treat aneurysms that are no longer occluded to reduce the risk of poor outcome or death as a result of rupture from this aneurysm. Follow-up imaging after aneurysm occlusion may however also induce anxiety and symptoms of depression, thereby reducing quality of life. 72 In general, MR-angio is the preferred imaging modality for UIA patients treated with coiling, 55 whereas CT-angio is preferred for UIA patients having undergone microsurgical clipping. 73 The reasons for these preferences are derived from reduction of coil or clip artefacts by the corresponding modality. Because of its invasiveness, DSA is usually reserved for instances where MR-angio or CT-angio after UIA treatment are not conclusive.
Analysis of current evidence and evidence-based recommendation
We found no RCTs or other controlled studies that compared follow-up imaging with no follow-up imaging, neither in patients with endovascular nor in patients with microsurgical preventive UIA occlusion for any of the relevant outcome measures. We also found no observational studies on clinical outcome or quality of life data of short- and long-term follow-up imaging after endovascular or microsurgical UIA occlusion to detect remnants or recurrence of aneurysms.
We therefore searched for studies (a) on the risk of aneurysmal rupture during follow-up after treatment of UIA and (b) for studies on retreatment after UIA treatment to deduct recommendations for imaging after aneurysm repair from these data.
Additional information
Risk of rupture after preventive UIA occlusion
A systematic review and meta-analysis examining long-term rupture risk over ⩾1-year follow-up duration in patients with UIA who underwent endovascular therapy identified 24 studies describing 4842 patients. During a mean follow-up duration of 3.2 years, 12 patients (0.25%) experienced rupture of a UIA after the initial year of treatment resulting in rupture rate of 0.48 (95% CI 0.45–0.51) per 100 patient-years. 74
Two registry based retrospective studies provide data on risk of aneurysm rerupture or intracranial haemorrhage after microsurgical occlusion or endovascular of UIA. A nationwide retrospective cohort study in South Korea used health insurance data for 11,777 patients undergoing surgical clipping and 14,634 patients undergoing endovascular treatment between 2008 through 2014. 75 The adjusted probabilities of aneurysm rupture at 7 years were 0.7% after surgical clipping and 0.9% after endovascular coiling. The second study was based on the Office of Statewide Health Planning and Development (OSHPD) database in California. 30 In this study, all patients initially treated for a UIA in the period from 1998 to 2005 and with follow-up data through 2009 were analysed. During this period, 1565 patients were treated with surgical clipping and 944 patients with endovascular coiling. The incidence of intracranial haemorrhage during a mean follow-up of 7 years (range 4–12 years) was 5.9% for the clipped patients and 4.8% for the coiled patients.
Proportions of retreatment after preventive UIA occlusion
The systematic review on long-term follow-up after endovascular treatment of UIA found a retreatment rate of 4.9% (95% CI 4.3–5.5), but also noted the lack of systematic nature of follow-up and short duration of follow-up (mean 3.2 years) as limitations of the studies included in the review. 74
In a study using administrative data from all non-federal hospitalisations in California (2005–2011) and Florida (2005–2014) that was published after the data search from the above mentioned systematic review, retreatment rates for UIA patients were 4.6% (95% CI 3.9–5.4) after surgical clipping and 10.6% (95% CI 9.8–11.4) after endovascular occlusion. 33 In the retrospective cohort study from South Korea the probabilities of retreatment at 7 years were 3.2% (95% CI 2.9–3.5) in the surgical clipping group and 4.9% (95% CI 4.6–5.3) in the endovascular coiling group. 75 In the OSHPD based study from California, retreatment rates were 8.7% (95% CI 7.4–10.2) after surgical clipping and 20.4% (95% CI 18.0–23.1) after endovascular occlusion of UIA. 30
In summary, there are no studies comparing follow-up imaging with no follow-up imaging in patients treated for UIA. There are also no good data on remnants or recurrence of aneurysms after endovascular or microsurgical UIA treatment. The quality of the evidence underlying the recommendations for this PICO is therefore very due to serious indirectness, serious inconsistency bias in the studies. The risk of aneurysm rupture in the long-term will be smaller than the risk of re-opening, because not all re-opened aneurysms will rupture. The risk of aneurysmal rupture after UIA treatment ranges between 0.1 and 1 per 100 patient-years, without major differences between UIA treated with surgical clipping or endovascular coiling. It should be noted that this risk may be on the one hand an underestimation of the actual risk, because patients underwent surveillance with imaging and retreatment in case of reopening of aneurysms. On the other hand, the risk may be overestimated because from the available data it cannot be distinguished whether the haemorrhage after UIA treatment was caused by rupture of the treated UIA, or by an additional, not treated UIA, or a de novo aneurysm. The proportion of patients undergoing additional treatment varies between 5% and 20% after endovascular UIA occlusion and between 3% and 9% after surgical clipping of UIA. The variation between the studies is probably influenced by different study periods, different durations of follow-up and practice variation.
Discussion
We provided 13 recommendations on UIA management, of which all were derived of very low evidence (Table 6). Additionally, we formulated 15 expert-consensus statements on clinically relevant aspects of UIA to guide clinicians in daily practice until more robust data become available (Table 6).
Table 6.
Synoptic table of all recommendations and expert consensus statements.
| Recommendation | Expert consensus statement |
|---|---|
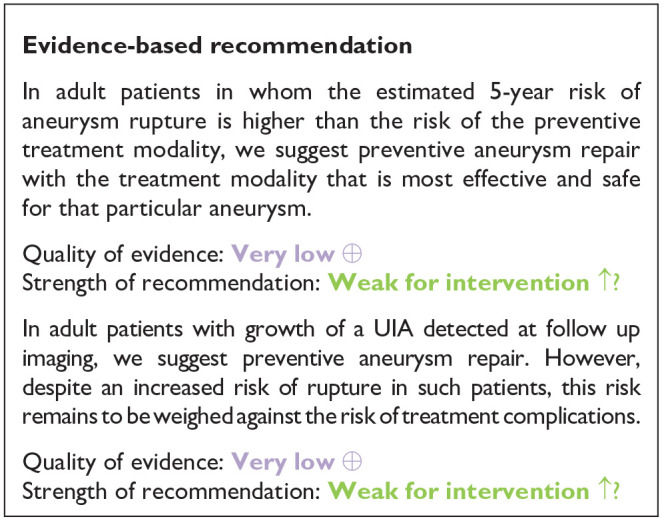
| PICO 1: In adult patients with unruptured intracranial aneurysms does any type of microsurgical or endovascular aneurysm occlusion compared to no aneurysm occlusion improve outcomes? | |
| In adult patients in whom the estimated 5-year risk of aneurysm rupture is higher than the risk of the preventive treatment modality, we suggest preventive aneurysm repair with the treatment modality that is most effective and safe for that particular aneurysm | For adult patients with UIA we suggest assessing such patients within a multidisciplinary setting (i.e. neurosurgery, interventional neuroradiology neurology) at large volume centres (consulting at least 100 UIA patients per year) |
| Quality of evidence: Very low ⊕ | |
| Strength of recommendation: Weak for intervention ↑? | |
| In adult patients with growth of a UIA detected at follow up imaging, we suggest preventive aneurysm repair. However, despite an increased risk of rupture in such patients, this risk remains to be weighed against the risk of treatment complications. | For adult patients with UIA we suggest that the recommendation for versus against preventive aneurysm repair by the multidisciplinary team should be based on: |
| Quality of evidence: Very low ⊕ | – Aneurysm-related risk factors for rupture, that is, UIA size, location and lobulation |
| Strength of recommendation: Weak for intervention ↑? | |
| – Risk factors for rupture, that is, previous SAH from a different aneurysm, family history for UIA or SAH, smoking and hypertension | |
| – UIA growth (1 mm in any diameter) or de novo formation on serial imaging | |
| – Life expectancy | |
| – Risk factors for treatment complications, that is, patient age and comorbid disease, aneurysm morphology and complexity and estimated risk of treatment | |
| In adult patients with UIA who present with clinical symptoms, such as cranial nerve deficits, mass effect and thromboembolic events, we suggest preventive aneurysm repair, taking into account life expectancy and risk of treatment complications | |
| In asymptomatic adult UIA patients with significant comorbid diseases and/or reduced life expectancy (<5 years), we suggest no preventive aneurysm repair | |
| In adult patients with UIA we suggest that the final management decision is made in a shared decision-making process between the physician and the patient, based on the recommendation by the multidisciplinary team and patient-related psycho-sociological factors | |
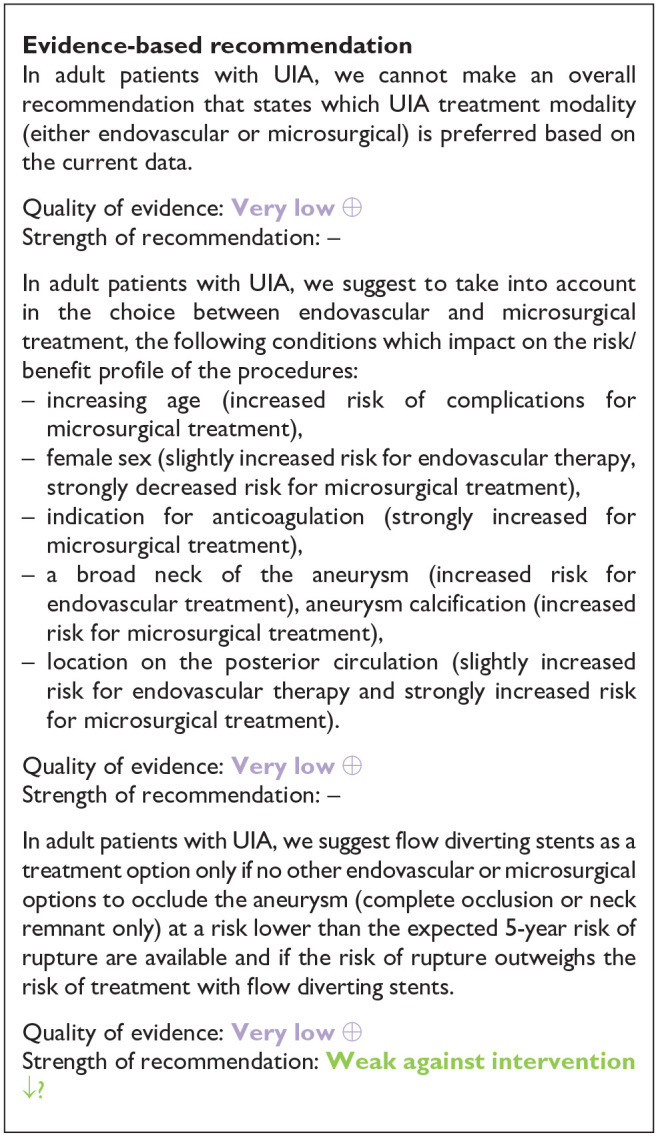
| PICO 2 In adult patients with unruptured intracranial aneurysms does any type of microsurgical occlusion compared to any type of endovascular occlusion improve outcome (decrease proportion of patients remaining dependent on help at time of outcome assessment, decrease case-fatality at time of outcome assessment)? | |
| In adult patients with UIA, we cannot make an overall recommendation that states which UIA treatment modality (either endovascular or microsurgical) is preferred based on the current data. | In adult patients with UIA we suggest that the choice between microsurgical and endovascular treatment should be made in a multidisciplinary setting where the chance of complete aneurysm occlusion and risk of complications of microsurgical and endovascular treatment are openly discussed and compared |
| Quality of evidence: Very low ⊕ | |
| Strength of recommendation: – | |
| In adult patients with UIA, we suggest to take into account, in the choice between endovascular and microsurgical treatment, the following conditions which impact on the risk/benefit profile of the procedures: | In adult patients with UIA we suggest that preventive UIA repair should only be done in centres performing aneurysm treatment in more than 100 patients with ruptured and unruptured aneurysms per year and performing the proposed treatment modality (endovascular or microsurgery) in more than 30 patients with aneurysms (ruptured and unruptured) per year per neurosurgeon or neurointerventionalist |
| – Increasing age (increased risk of complications for microsurgical treatment) | |
| – Female sex (slightly increased risk for endovascular therapy, strongly decreased risk for microsurgical treatment) | |
| – indication for anticoagulation (strongly increased for microsurgical treatment) | |
| – A broad neck of the aneurysm (increased risk for endovascular treatment), aneurysm calcification (increased risk for microsurgical treatment) | |
| – Location on the posterior circulation (slightly increased risk for endovascular therapy and strongly increased risk for microsurgical treatment) | |
| Quality of evidence: Very low ⊕ | |
| Strength of recommendation: – | |
| In adult patients with UIA, we suggest flow diverting stents as a treatment option only if no other endovascular or microsurgical options to occlude the aneurysm (complete occlusion or neck remnant only) at a risk lower than the expected 5-year risk of rupture are available and if the risk of rupture outweighs the risk of treatment with flow diverting stents | In adult patients with UIA in the posterior circulation we suggest endovascular treatment as the first option to consider |
| Quality of evidence: Very low ⊕ | |
| Strength of recommendation: Weak against intervention ↓? | |
| PICO 3 In adult patients with unruptured intracranial aneurysms does any type and frequency of follow-up imaging followed by aneurysm occlusion in case of aneurysm growth or other change compared to no follow-up imaging improve outcome (decrease proportion of patients remaining dependent on help at time of outcome assessment, decrease case-fatality at time of outcome assessment)? | |
| In adult patients with a UIA in whom the risk of treatment complications is higher than the 5-year risk of rupture, we recommend radiological monitoring to detect future UIA growth or morphological change for patients in whom treatment remains an option that were initially observed | In adult patients with UIA undergoing radiological monitoring to detect potential aneurysm growth or morphological change, we suggest that the frequency and duration of follow-up imaging should be based on aneurysm- and patient-related risk factors of growth or rupture and risk of treatment. This should be agreed upon in a shared decision-making process between physician and patient based on the recommendation by the multidisciplinary team and patient-related psycho-sociological factors. |
| Quality of evidence: Very low ⊕ | |
| Strength of recommendation: Strong for intervention ↑↑ | |
| In adult patients with a UIA that shows recent growth during radiological monitoring, we suggest preventive aneurysm repair. However, despite an increased risk of rupture in such patients, this risk remains to be weighed against the risk of treatment complications. | In adult patients with UIA undergoing radiological monitoring to detect potential aneurysm growth or morphological change, we suggest that radiological follow-up should be continued as long as preventive treatment remains an option |
| Quality of evidence: Very low ⊕ | |
| Strength of recommendation: Weak for intervention ↑? | In adult patients with UIA undergoing radiological monitoring to detect potential aneurysm growth or morphological change, we suggest that radiological follow-up should be performed with MRA or CTA |
| PICO 4: In adult patients with unruptured intracranial aneurysms does any life-style modification or any medical treatment (e.g. anti-inflammatory drugs, antihypertensive drugs, statins) in comparison to no treatment improve outcome (increase QALY’s, decrease proportion of patients remaining dependent on help at time of outcome assessment, decrease case-fatality at time of outcome assessment)? | |
| In adult patients with UIA who smoke, we recommend smoking cessation | For adult patients with an UIA who do not undergo preventive occlusion, we suggest that there is no contra-indication for platelet aggregation inhibitors if needed for another indication |
| Quality of evidence: Very low ⊕ | |
| Strength of recommendation: Strong for intervention ↑↑ | |
| In adult patients with UIA and hypertension, we recommend treatment of increased blood pressure | For adult patients with an UIA who do not undergo preventive occlusion, we suggest keeping blood pressure <130/80 mm Hg |
| Quality of evidence: Very low ⊕ | |
| Strength of recommendation: Strong for intervention ↑↑ | |
| In adult patients with UIA, we suggest to not start treatment with acetylsalicylic acid to decrease the risk of aneurysm growth or rupture | For adult patients with an UIA who do not undergo preventive occlusion, we suggest that no restrictions should be imposed regarding sexual activity or any physical or sporting activity |
| Quality of evidence: Very low ⊕ | |
| Strength of recommendation: – | |
| In adult patients with UIA, we suggest to not start treatment with statins to decrease the risk of aneurysm growth or rupture | |
| Quality of evidence: Very low ⊕ | |
| Strength of recommendation: – | |
| PICO 5: In adult patients with occluded unruptured aneurysms, does any type and frequency of follow-up imaging compared to no follow-up imaging improve outcome (increase in QALYs)? | |
| For adult patients with a treated UIA in whom aneurysm re-treatment remains an option, we suggest an initial radiological follow-up 3 to 12 months after UIA repair to detect potential UIA remnants or recurrence | We suggest that MR-angiography should be the primary tool for follow up imaging of endovascularly treated aneurysms, CTA for microsurgically treated aneurysms that DSA should only be considered if MRA and CTA are not conclusive |
| Quality of evidence: Very low ⊕ | |
| Strength of recommendation: Weak for intervention ↑? | |
| In adult patients with a treated UIA and recurrence of the aneurysm, we recommend that the pros and cons of re-treatment in the short-term versus a future radiological follow-up are agreed upon in a shared decision-making process between the physician and patient based on the recommendation by the multidisciplinary team and patient-related psycho-sociological factors | |
| Quality of evidence: Very low ⊕ | |
| Strength of recommendation: Strong for intervention ↑↑ | |
The lack of strong evidence from randomised trials as a fundament for recommendations on preventive UIA treatment, observation, medical management, risk factor control or radiological follow-up predominantly derives from the small risk of rupture for most UIA. The consequences of a UIA rupture are however severe. Despite improvement of outcome over the last decades, still more than half the patients die or remain dependent on help for daily activities after UIA rupture,3,76 and the impact on patients’ quality of life is immense. 77 Size is the most important predictor for future UIA rupture, 5 but most UIA that are identified are small. 1 Moreover, most of the identified UIA are on sites with a relatively low risk of rupture. 1 Hence, for most UIA that are identified the absolute 5-year risk of rupture is not higher than 1% or 2%. 5 Similarly, for most of the UIA, the risk of poor outcome from a treatment complication is around 2% to 4%. These small numbers of ‘events’ make randomised trials using clinical relevant outcomes, such as handicap or quality of life, and comparing preventive occlusion with no preventive occlusion difficult to perform. International, multi-centre trials that include large numbers of patients and have a long-term follow-up are needed, but have been proven difficult to conduct. 15 Even in cohort studies on patients with UIA, clinical relevant outcomes are lacking aneurysmal rupture is used as surrogate outcome. The current evidence comes from indirect comparisons between cohorts of patients with UIA undergoing follow up with UIA rupture as outcome and series of patients undergoing preventive aneurysm occlusion, with treatment complication as outcome. It must be noted that these two endpoints are two different quantities, that is, the former being a risk period and the latter being a one-time risk event. However, decision-making on preventive repair versus observation in daily clinical practice derives from this comparison. There is also a lack of randomised data comparing the risks and benefits of endovascular over microsurgical UIA occlusion. The data on these treatments in patients with ruptured aneurysms cannot be extrapolated to those with UIA, because of the differences in (re)-rupture rates and treatment complications between patients with ruptured and unruptured aneurysms. With the advent of advanced endovascular treatment methods, such as balloon-assisted coiling and flow diverting stents, the lack of sound data has increased even further. The speed of new devices entering this field make it unrealistic that a RCT on UIA patients will ever be completed.
Despite the lack of data from RCTs, we nevertheless intended to provide clinically relevant recommendations and consensus statements on UIA management, to guide clinicians in daily practice. We derived data from interim analyses of RCTs and meta-analyses of observational and case-control studies. These recommendations or statements pertain to indications and risk factors for preventive UIA repair versus observation (PICO1) or for specific – microsurgical or endovascular – UIA repair modalities (PICO2), for follow-up imaging (PICO 3 and 5) and medical management or risk factor treatment (PICO 4). Additionally, the importance of multidisciplinary, that is, neurosurgery, interventional neuroradiology and neurology, assessment and management of UIA patients at high volume centres (i.e. at least 100 aneurysm patients counselled per year) as well as treatment by experienced neurosurgeons and neurointerventionalists (i.e. at least 30 ruptured or unruptured aneurysm treatments per operator per year) was established, discussed and consented by the MWG.
The present ESO guideline expands upon the previous guidelines on management of UIA patients that were published in 2013 (European Stroke Organisation) and 2015 (American Heart Association/American Stroke Association).10,78 Compared to the 2013 ESO guideline on the management of intracranial aneurysms and subarachnoid haemorrhage, 10 we used the GRADE methodology, restricted the topic to UIA and developed expert consensus statements in areas of distinct uncertainty. Further, our formulated recommendations were mostly derived from recently published data. Compared to the 2015 AHA/ASA guidelines on UIA management, 78 we could include data from large meta-analyses on the risk of and risk factors for aneurysm rupture or for aneurysm repair in relation to UIA repair modality. Because many UIA are nowadays treated with advanced endovascular techniques or devices, such as balloon-assisted coiling, flow-diverting stents or intrasaccular devices, we provided recommendations based on the available literature to guide treatment indications until the currently running randomised trial is eventually completed. 79 An important consideration to keep in mind when assessing the data from the literature on new endovascular devices is the paucity of investigator-initiated data on clinical effectiveness or radiological long-term follow-up of these devices or the lack of controlled case-series. Also, in addition to the previous guidelines, we could for the first time provide recommendations and consensus statements on medical management, such as blood pressure reduction, use of acetylsalicylic acid or statins and life-style changes.
Apart from the lack of data from randomised trials on whether or not to preventively occlude UIA or how to occlude these, and lack of clinically relevant outcome data for certain PICOs, we acknowledge several other limitations. Our search- and inclusion/exclusion criteria prohibited the inclusion of a large body of industry-sponsored or single centre or single arm registry studies on novel endovascular devices, such as intrasaccular flow-disruptors or novel flow-diverting stents. Further, the heterogeneity of previous cohort studies in our analysis on the 1-year risk of aneurysm rupture was 73.3%; this underscores the different nature of the underlying studies, for example, in terms of sample size, study population, follow-up duration. Additionally, the proposed minimum numbers for centres (n = 100 UIA patients per year) and for operators (n = 30 procedures per operator per year) may seem high for individual centres or operators. However, in line with the increasing body of data on improved outcomes in relation to aneurysm treatment numbers (irrespective of treatment modality),31,80 the entire MWG agreed that the introduction of quality measures for the care of UIA patients are required, because this will result in more specialised and centralised care of UIA patients. Since the underlying studies for PICOs were predominantly single-arm studies and lacked a comparison, we were not able to provide risk of bias and/or GRADE tables for all PICOs. Unfortunately, we could not provide an evidence-based recommendation nor consensus statement about the frequency of the follow up imaging of primarily observed UIA. This frequency depends on many aneurysm and patient specific factors and on results of previous follow up imaging, that is, whether or not the UIA is stable over time. As to PICOs 3 to 5, our recommendations were derived from predominantly low evidence, but it is apparent that most of the relevant topics, for example, follow-up imaging versus no follow-up imaging in untreated UIA or the beneficial effect of smoking cessation or blood pressure reduction on the risk of rupture cannot and will not be studied in RCTs.
In conclusion, this guideline provides contemporary recommendations and consensus statements on the management of UIA with respect to treatment indications and choice of treatment modality, risk factors per treatment modality, centre and operator case volume, risk factor management radiological follow-up in patients with an untreated or previously repaired UIA. There is an urgent need for randomised trials or sound comparative studies with relevant clinical outcomes on preventive versus no preventive UIA occlusion, investigator driven studies on new endovascular devices to treat UIA for trials on medical management of UIA.
Plain language summary
Intracranial aneurysms are outpouchings of intracranial arteries. These can be found in around 3% of the adult persons and occur more often in female than in male persons. Aneurysms can tear (rupture), which leads to an arterial bleed in the space between the cerebral membrane and the brain itself, a so-called subarachnoid haemorrhage. This type of stroke occurs at relatively young age, with half the patients being younger than 50 years of age, and two of every three patients are female. The outcome after a subarachnoid haemorrhage is often poor. More than half of the patients die within the first days to weeks after aneurysmal rupture or remain dependent on help for daily life activities. Almost all patients who survive the initial weeks have cognitive complaints, such as poor memory, lack of concentration and reduced energy and most do not return to the activities they did before the haemorrhage.
With the greater availability of brain scanning, the number of persons in whom an unruptured intracranial aneurysm (UIA) is detected has increased and is still increasing. Once an UIA is detected, the question arises how to prevent rupture from it. Currently, the only effective methods are clamping of the neck of the aneurysm (‘clipping’) via a microsurgical operation or occlusion via a catheter inserted in the artery in the groin and navigated into the intracranial arteries (endovascular). Both procedures have a risk of complications, and these risks have to be weighed against the risk of rupture of the UIA. These guidelines provide recommendations and opinions from experts on factors that are relevant for the decision whether or not to advise preventive occlusion, and for the decision on which type of treatment (microsurgical or endovascular) should be advised. Further, it provides guidance on medical management and lifestyle changes to reduce the risk of rupture of an UIA and on follow-up imaging in patients with an UIA.
The guidelines suggest advising preventive aneurysm occlusion in patients in whom the estimated risk of rupture in the next 5 years is higher than the expected risk of complications of preventive UIA occlusion. In general, there is no preference for microsurgical or endovascular treatment, but several factors can guide in the decision, such as age and sex of the patient, the use of anticoagulant medication the site and shape of the aneurysm. Insertion of a stent is a newly developed endovascular method to treat aneurysms, which are associated with a higher risk of complications than regular endovascular treatment. The guidelines advise to use stents only if there are no other treatment options and the risk of rupture of the UIA in the next 5 years is higher than the expected risks of complications from the stent. If no preventive treatment is performed, the guidelines suggest follow up imaging in those patients who are fit enough to undergo a microsurgical or endovascular treatment. If at follow-up imaging the UIA is larger than in the previous imaging, the risk of rupture of that UIA is increased and the decision whether or not to treat the aneurysm should be reconsidered. The guidelines suggest treating hypertension and quit smoking in patient with an UIA, but found no evidence to treat such patients with acetylsalicylic acid or statins (medication that reduces cholesterol levels).
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873221099736 for European Stroke Organisation (ESO) guidelines on management of unruptured intracranial aneurysms by Nima Etminan, Diana Aguiar de Sousa, Cindy Tiseo, Romain Bourcier, Hubert Desal, Anttii Lindgren, Timo Koivisto, David Netuka, Simone Peschillo, Sabrina Lémeret, Avtar Lal, Mervyn DI Vergouwen and Gabriel JE Rinkel in European Stroke Journal
Acknowledgments
We thank ESO staff Luzia Balmer, Yvonne Brüchert and Sabrina Mutter for excellent administrative support as well as the Chair and Co-chair of the Guidelines Board, Guillaume Turc and Simona Sacco, for their support during the conduct of the guideline project. We thank Paut Greebe for textual comments on a pre-final version of the manuscript.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for the development of these guidelines was provided by the European Stroke Organisation, Basel, Switzerland. The authors did not receive financial support for the development, writing or publication of this guideline.
Ethical approval: Ethical approval was not necessary for the work described in this paper.
Informed consent: Not applicable.
Guarantor: A specific guarantor does not exist. The working group has jointly developed the manuscript.
Contributorship: All listed authors have contributed to the preparation and writing of the manuscript.
ORCID iDs: Nima Etminan  https://orcid.org/0000-0003-2832-4018
https://orcid.org/0000-0003-2832-4018
Diana Aguiar de Sousa  https://orcid.org/0000-0002-6702-7924
https://orcid.org/0000-0002-6702-7924
Anttii Lindgren  https://orcid.org/0000-0002-1264-7667
https://orcid.org/0000-0002-1264-7667
Sabrina Lémeret  https://orcid.org/0000-0001-8611-1630
https://orcid.org/0000-0001-8611-1630
Supplemental material: Supplemental material for this article is available online.
References
- 1. Vlak MH, Algra A, Brandenburg R, et al. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 2011; 10: 626–636. [DOI] [PubMed] [Google Scholar]
- 2. van der Kamp LT, Rinkel GJE, Verbaan D, et al. Risk of rupture after intracranial aneurysm growth. JAMA Neurol 2021; 78: 1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nieuwkamp DJ, Setz LE, Algra A, et al. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 2009; 8: 635–642. [DOI] [PubMed] [Google Scholar]
- 4. Etminan N, Chang HS, Hackenberg K, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol 2019; 76: 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greving JP, Wermer MJ, Brown RD, Jr, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 2014; 13: 59–66. [DOI] [PubMed] [Google Scholar]
- 6. Tominari S, Morita A, Ishibashi T, et al. Prediction model for 3-year rupture risk of unruptured cerebral aneurysms in Japanese patients. Ann Neurol 2015; 77: 1050–1059. [DOI] [PubMed] [Google Scholar]
- 7. Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol 2016; 12: 699–713. [DOI] [PubMed] [Google Scholar]
- 8. Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet 2017; 389: 655–666. [DOI] [PubMed] [Google Scholar]
- 9. Brown RD, Jr, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol 2014; 13: 393–404. [DOI] [PubMed] [Google Scholar]
- 10. Steiner T, Juvela S, Unterberg A, et al. European stroke organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis 2013; 35: 93–112. [DOI] [PubMed] [Google Scholar]
- 11. Guyatt GH, Oxman AD, Schünemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011; 64: 380–382. [DOI] [PubMed] [Google Scholar]
- 12. Steiner T, Dichgans M, Norrving B, et al. European Stroke Organisation (ESO) standard operating procedure for the preparation and publishing of guidelines. Eur Stroke J 2021; 6: cxxii–cxxxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 14. The Cochrane Collaboration, EDS. Cochrane handbook for systematic reviews of interventions. 2nd Ed. Chichester: John Wiley & Sons, The Cochrane Collaboration, 2019. [Google Scholar]
- 15. Raymond J, Darsaut TE, Molyneux AJ. A trial on unruptured intracranial aneurysms (the TEAM trial): results, lessons from a failure and the necessity for clinical care trials. Trials 2011; 12: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rinaldo L, Shepherd DL, Murphy ME, et al. Natural history of untreated unruptured intracranial aneurysms in the elderly. J Neurosurg Sci 2020; 64: 141–146. [DOI] [PubMed] [Google Scholar]
- 17. Juvela S. Treatment scoring of unruptured intracranial aneurysms. Stroke 2019; 50: 2344–2350. [DOI] [PubMed] [Google Scholar]
- 18. Murayama Y, Takao H, Ishibashi T, et al. Risk analysis of unruptured intracranial aneurysms: prospective 10-year cohort study. Stroke 2016; 47: 365–371. [DOI] [PubMed] [Google Scholar]
- 19. Ishibashi T, Murayama Y, Saguchi T, et al. Justification of unruptured intracranial aneurysm repair: a single-center experience. AJNR Am J Neuroradiol 2013; 34: 1600–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Güresir E, Vatter H, Schuss P, et al. Natural History of small unruptured anterior circulation aneurysms: a prospective cohort study. Stroke 2013; 44: 3027–3031. [DOI] [PubMed] [Google Scholar]
- 21. Morita A, Kirino T, Hashi K, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 2012; 366: 2474–2482. [DOI] [PubMed] [Google Scholar]
- 22. Sonobe M, Yamazaki T, Yonekura M, et al. Small unruptured intracranial aneurysm verification study. SUAVe study, Japan. Stroke 2010; 41: 1969–1977. [DOI] [PubMed] [Google Scholar]
- 23. Broderick JP, Brown RD, Jr, Sauerbeck L, et al. Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke 2009; 40: 1952–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wiebers DO, Whisnant JP, Huston J, 3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003; 362: 103–110. [DOI] [PubMed] [Google Scholar]
- 25. Korja M, Lehto H, Juvela S. Lifelong rupture risk of intracranial aneurysms depends on risk factors: a prospective Finnish cohort study. Stroke 2014; 45: 1958–1963. [DOI] [PubMed] [Google Scholar]
- 26. Zuurbier CCM, Mensing LA, Wermer MJH, et al. Difference in rupture risk between familial and sporadic intracranial aneurysms: an individual patient data meta-analysis. Neurology 2021; 97: e2195–e2203. [DOI] [PubMed] [Google Scholar]
- 27. Darsaut TE, Findlay JM, Magro E, et al. Surgical clipping or endovascular coiling for unruptured intracranial aneurysms: a pragmatic randomised trial. J Neurol Neurosurg Psychiatry 2017; 88: 663–668. [DOI] [PubMed] [Google Scholar]
- 28. Algra AM, Lindgren A, Vergouwen MDI, et al. Procedural clinical complications, case-fatality risks, and risk factors in endovascular and neurosurgical treatment of unruptured intracranial aneurysms: a systematic review and meta-analysis. JAMA Neurol 2019; 76: 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Solomon RA, Mayer SA, Tarmey JJ. Relationship between the volume of craniotomies for cerebral aneurysm performed at New York state hospitals and in-hospital mortality. Stroke 1996; 27: 13–17. [DOI] [PubMed] [Google Scholar]
- 30. Gonda DD, Khalessi AA, McCutcheon BA, et al. Long-term follow-up of unruptured intracranial aneurysms repaired in California. J Neurosurg 2014; 120: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 31. Lindgren A, Burt S, Bragan Turner E, et al. Hospital case-volume is associated with case-fatality after aneurysmal subarachnoid hemorrhage. Int J Stroke 2019; 14: 282–289. [DOI] [PubMed] [Google Scholar]
- 32. Etminan N, Beseoglu K, Barrow DL, et al. Multidisciplinary consensus on assessment of unruptured intracranial aneurysms: proposal of an international research group. Stroke 2014; 45: 1523–1530. [DOI] [PubMed] [Google Scholar]
- 33. Daileda T, Vahidy FS, Chen PR, et al. Long-term retreatment rates of cerebral aneurysms in a population-level cohort. J Neurointerv Surg 2019; 11: 367–372. [DOI] [PubMed] [Google Scholar]
- 34. Raymond J, Gentric JC, Darsaut TE, et al. Flow diversion in the treatment of aneurysms: a randomized care trial and registry. J Neurosurg 2017; 127: 454–462. [DOI] [PubMed] [Google Scholar]
- 35. Brilstra EH, Rinkel GJ, van der Graaf Y, et al. Quality of life after treatment of unruptured intracranial aneurysms by neurosurgical clipping or by embolisation with coils. A prospective, observational study. Cerebrovasc Dis 2004; 17: 44–52. [DOI] [PubMed] [Google Scholar]
- 36. Gallas S, Drouineau J, Gabrillargues J, et al. Feasibility, procedural morbidity and mortality, and long-term follow-up of endovascular treatment of 321 unruptured aneurysms. AJNR Am J Neuroradiol 2008; 29: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gentric JC, Biondi A, Piotin M, et al. Safety and efficacy of neuroform for treatment of intracranial aneurysms: a prospective, consecutive, French multicentric study. AJNR Am J Neuroradiol 2013; 34: 1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hetts SW, Turk A, English JD, et al. Stent-assisted coiling versus coiling alone in unruptured intracranial aneurysms in the matrix and platinum science trial: safety, efficacy, and mid-term outcomes. AJNR Am J Neuroradiol 2014; 35: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poncyljusz W, Zarzycki A, Zwarzany Ł, et al. Bare platinum coils vs. hydrocoil in the treatment of unruptured intracranial aneurysms: a single center randomized controlled study. Eur J Radiol 2015; 84: 261–265. [DOI] [PubMed] [Google Scholar]
- 40. Pumar JM, Mosqueira A, Cuellar H, et al. Expanding the use of flow diverters beyond their initial indication: treatment of small unruptured aneurysms. J Neurointerv Surg 2018; 10: 245–248. [DOI] [PubMed] [Google Scholar]
- 41. Hanel RA, Kallmes DF, Lopes DK, et al. Prospective study on embolization of intracranial aneurysms with the pipeline device: the PREMIER study 1 year results. J Neurointerv Surg 2020; 12: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Joseph-Williams N, Newcombe R, Politi M, et al. Toward minimum standards for certifying patient decision aids: a modified Delphi consensus process. Med Decis Making 2014; 34: 699–710. [DOI] [PubMed] [Google Scholar]
- 43. Sánchez van Kammen M, Greving JP, Kuroda S, et al. External validation of the ELAPSS score for prediction of unruptured intracranial aneurysm growth risk. J Stroke 2019; 21: 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Backes D, Rinkel GJE, Greving JP, et al. ELAPSS score for prediction of risk of growth of unruptured intracranial aneurysms. Neurology 2017; 88: 1600–1606. [DOI] [PubMed] [Google Scholar]
- 45. Korja M, Kivisaari R, Rezai Jahromi B, et al. Size of ruptured intracranial aneurysms is decreasing: twenty-year long consecutive series of hospitalized patients. Stroke 2018; 49: 746–749. [DOI] [PubMed] [Google Scholar]
- 46. Ramachandran M, Retarekar R, Raghavan ML, et al. Assessment of image-derived risk factors for natural course of unruptured cerebral aneurysms. J Neurosurg 2016; 124: 288–295. [DOI] [PubMed] [Google Scholar]
- 47. Bor AS, Tiel Groenestege AT, terBrugge KG, et al. Clinical, radiological, and flow-related risk factors for growth of untreated, unruptured intracranial aneurysms. Stroke 2015; 46: 42–48. [DOI] [PubMed] [Google Scholar]
- 48. Kubo Y, Koji T, Kashimura H, et al. Female sex as a risk factor for the growth of asymptomatic unruptured cerebral saccular aneurysms in elderly patients. J Neurosurg 2014; 121: 599–604. [DOI] [PubMed] [Google Scholar]
- 49. Vergouwen MDI, Backes D, van der Schaaf IC, et al. Gadolinium enhancement of the aneurysm wall in unruptured intracranial aneurysms is associated with an increased risk of aneurysm instability: a follow-up study. AJNR Am J Neuroradiol 2019; 40: 1112–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Teo M, St George EJ. Radiologic surveillance of untreated unruptured intracranial aneurysms: a single surgeon’s experience. World Neurosurg 2016; 90: 20–28. [DOI] [PubMed] [Google Scholar]
- 51. Gondar R, Gautschi OP, Cuony J, et al. Unruptured intracranial aneurysm follow-up and treatment after morphological change is safe: observational study and systematic review. J Neurol Neurosurg Psychiatry 2016; 87: 1277–1282. [DOI] [PubMed] [Google Scholar]
- 52. Aubertin M, Jourdaine C, Thépenier C, et al. Results of watchful waiting of unruptured intracranial aneurysms in a Western patient population: a single-center cohort. J Neurointerv Surg 2021: neurintsurg-2021-018151. [DOI] [PubMed] [Google Scholar]
- 53. Menke J, Larsen J, Kallenberg K. Diagnosing cerebral aneurysms by computed tomographic angiography: meta-analysis. Ann Neurol 2011; 69: 646–654. [DOI] [PubMed] [Google Scholar]
- 54. Sailer AM, Wagemans BA, Nelemans PJ, et al. Diagnosing intracranial aneurysms with MR angiography: systematic review and meta-analysis. Stroke 2014; 45: 119–126. [DOI] [PubMed] [Google Scholar]
- 55. Schaafsma JD, Velthuis BK, van den Berg R, et al. Coil-treated aneurysms: decision making regarding additional treatment based on findings of MR angiography and intraarterial DSA. Radiology 2012; 265: 858–863. [DOI] [PubMed] [Google Scholar]
- 56. Omodaka S, Endo H, Niizuma K, et al. Circumferential wall enhancement on magnetic resonance imaging is useful to identify rupture site in patients with multiple cerebral aneurysms. Neurosurg 2018; 82: 638–644. [DOI] [PubMed] [Google Scholar]
- 57. Hasan DM, Chalouhi N, Jabbour P, et al. Evidence that acetylsalicylic acid attenuates inflammation in the walls of human cerebral aneurysms: preliminary results. J Am Heart Assoc 2013; 2: e000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Juvela S. Growth and rupture of unruptured intracranial aneurysms. J Neurosurg 2019; 131: 843–851. [DOI] [PubMed] [Google Scholar]
- 59. Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg 2000; 93: 379–387. [DOI] [PubMed] [Google Scholar]
- 60. Weng JC, Wang J, Du X, et al. Safety of aspirin use in patients with stroke and small unruptured aneurysms. Neurology 2021; 96: e19–e29. [DOI] [PubMed] [Google Scholar]
- 61. Weng JC, Wang J, Li H, et al. Aspirin and growth of small unruptured intracranial aneurysm: results of a prospective cohort study. Stroke 2020; 51: 3045–3054. [DOI] [PubMed] [Google Scholar]
- 62. Vergouwen MD, Rinkel GJ, Algra A, et al. Prospective randomized open-label trial to evaluate risk factor management in patients with unruptured intracranial aneurysms: study protocol. Int J Stroke 2018; 13: 992–998. [DOI] [PubMed] [Google Scholar]
- 63. Chalouhi N, Starke RM, Correa T, et al. Differential sex response to aspirin in decreasing aneurysm rupture in humans and mice. Hypertension 2016; 68: 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hasan DM, Mahaney KB, Brown RD, Jr, et al. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke 2011; 42: 3156–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yoshida K, Uwano I, Sasaki M, et al. Small unruptured aneurysm verification-prevention effect against growth of cerebral aneurysm study using statin. Neurol Med Chir 2021; 61: 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Anderson C, Ni Mhurchu C, Scott D, et al. Triggers of subarachnoid hemorrhage. Role of physical exertion, smoking, and alcohol in the Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS). Stroke 2003; 34: 1771–1776. [DOI] [PubMed] [Google Scholar]
- 67. Vlak MH, Rinkel GJ, Greebe P, et al. Trigger factors and their attributable risk for rupture of intracranial aneurysms: a case-crossover study. Stroke 2011; 42: 1878–1882. [DOI] [PubMed] [Google Scholar]
- 68. Macleod MR, White PM. Not tonight, darling, I might get a headache. Stroke 2011; 42: 1807–1808. [DOI] [PubMed] [Google Scholar]
- 69. Dorhout Mees SM, van den Bergh WM, Algra A, et al. Antiplatelet therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2007; 4: CD006184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rinkel GJ, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol 2011; 10: 349–356. [DOI] [PubMed] [Google Scholar]
- 71. Molyneux AJ, Birks J, Clarke A, et al. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 2015; 385: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ferns SP, Nieuwkerk PT, van Rooij WJ, et al. Long-term MRA follow-up after coiling of intracranial aneurysms: impact on mood and anxiety. Neuroradiol 2011; 53: 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gölitz P, Struffert T, Ganslandt O, et al. Contrast-enhanced angiographic computed tomography for detection of aneurysm remnants after clipping: a comparison with digital subtraction angiography in 112 clipped aneurysms. Neurosurg 2014; 74: 606. [DOI] [PubMed] [Google Scholar]
- 74. Rizvi A, Seyedsaadat SM, Alzuabi M, et al. Long-term rupture risk in patients with unruptured intracranial aneurysms treated with endovascular therapy: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2020; 41: 1043–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kim YD, Bang JS, Lee SU, et al. Long-term outcomes of treatment for unruptured intracranial aneurysms in South Korea: clipping versus coiling. J Neurointerv Surg 2018; 10: 1218–1222. [DOI] [PubMed] [Google Scholar]
- 76. Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: population-based study and systematic review. Neurology 2010; 74: 1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hua X, Gray A, Wolstenholme J, et al. Survival, dependency, and health-related quality of life in patients with ruptured intracranial aneurysm: 10-year follow-up of the United Kingdom cohort of the International Subarachnoid Aneurysm Trial. Neurosurg 2021; 88: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Thompson BG, Brown RD, Jr, Amin-Hanjani S, et al. Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46: 2368–2400. [DOI] [PubMed] [Google Scholar]
- 79. Darsaut TE, Findlay JM, Raymond J. The design of the canadian unruptured endovascular versus surgery (CURES) trial. J Neurol Sci 2011; 38: 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Leifer D, Fonarow GC, Hellkamp A, et al. Association between hospital volumes and clinical outcomes for patients with nontraumatic subarachnoid hemorrhage. J Am Heart Assoc 2021; 10: e018373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873221099736 for European Stroke Organisation (ESO) guidelines on management of unruptured intracranial aneurysms by Nima Etminan, Diana Aguiar de Sousa, Cindy Tiseo, Romain Bourcier, Hubert Desal, Anttii Lindgren, Timo Koivisto, David Netuka, Simone Peschillo, Sabrina Lémeret, Avtar Lal, Mervyn DI Vergouwen and Gabriel JE Rinkel in European Stroke Journal