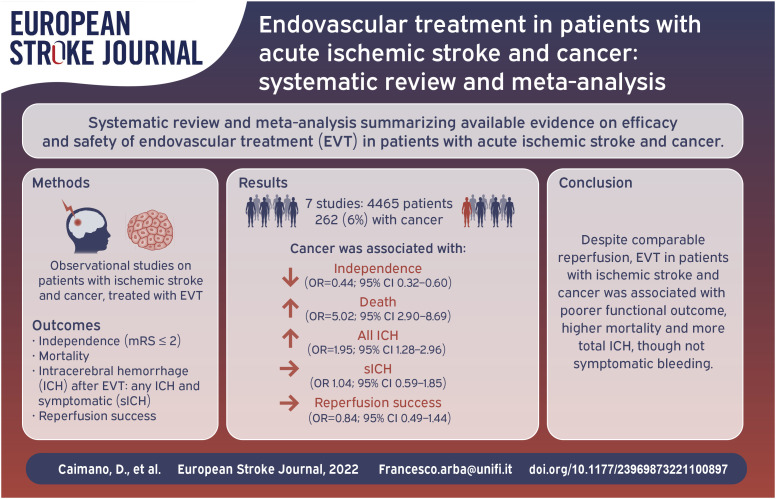
Abstract
Introduction:
Although stroke occurs frequently in patients with cancer, there is scarce evidence regarding the safety and efficacy of endovascular treatment (EVT) in patients with acute ischemic stroke and concurrent cancer. We performed a systematic review and meta-analysis to summarize the existing literature.
Methods:
We searched for English written observational studies reporting data on safety and efficacy of EVT in patients with acute ischemic stroke and concurrent cancer. Outcomes of interest were: functional independence (modified Rankin Scale (mRS) ⩽ 2); mortality at 3 months; rate of successful recanalization (modified Treatment In Cerebral Ischemia (mTICI) 2b or 3); occurrence of any hemorrhagic transformation (both symptomatic and asymptomatic). We pooled data with Maentel-Haenszel model to calculate cumulative odds ratios (ORs).
Results:
We included seven studies with a total of 4465 patients, of whom 262 (6%) with cancer. We observed various definitions of cancer across included studies. Patients with cancer had less likely mRS⩽2 at 3 months (24% vs 42%, OR = 0.44; 95% CI = 0.32–0.60) and increased probability of death (43% vs 19%, OR = 5.02; 95% CI = 2.90–8.69). There was no difference in successful recanalization (70% vs 75%, OR = 0.84; 95% CI = 0.49–1.44); patients with cancer had increased risk of any intracerebral hemorrhage after treatment (49% vs 34%, OR = 1.95; 95% CI = 1.28–2.96), though not for symptomatic ICH (OR 1.04; 95% CI = 0.59–1.85).
Conclusion:
Patients with acute ischemic stroke and cancer have similar EVT recanalization but higher probability of functional dependence, death, and any hemorrhagic transformation, though not necessarily symptomatic, compared with patients without cancer. Our results may help communication with patients and carers.
Keywords: Ischemic stroke, endovascular treatment, active malignancy, cancer, outcome, disability, mortality
Graphical abstract.
Introduction
Stroke is a leading cause of death and disability and represents a relevant health and social issue.1,2 Reperfusion therapy with intravenous thrombolysis and/or endovascular treatment (EVT) is the mainstay of acute stroke treatment. Emerging evidence has shown that approximately one in ten patients diagnosed with ischemic stroke have a comorbid cancer, and this figure will likely increase with advances in cancer treatments and consequent longer life expectancy. 2 It has been postulated that cancer has a similar role to vascular risk factors, exposing patients to increased risk of developing cerebrovascular disease in the lifetime.3,4
The efficacy and safety of reperfusion therapy in patients with acute stroke has been investigated for intravenous thrombolysis. A meta-analysis showed no difference in efficacy and safety of intravenous thrombolysis in patients with acute stroke and cancer compared to those without, 4 supporting the use of thrombolytic treatment in this subgroup of patients. EVT for acute ischemic stroke has extended the efficacy of treatment of patients with large vessel occlusion,5,6 however, in randomized controlled trials there is a lack of data for patients with cancer. 7 As a consequence, efficacy and safety in patients with acute ischemic stroke and cancer treated with EVT are scarce, conflicting and available only from observational studies.
Hence, we conducted a systematic review and meta-analysis to evaluate the efficacy and safety of EVT in patients with ischemic stroke and cancer.
Methods
This review was performed according to PRISMA 8 and MOOSE 9 recommendations and the Cochrane Handbook for Systematic Review of Interventions (https://training.cochrane.org/handbook). Data search, extraction, analysis and interpretation were performed following a pre-specified study protocol developed by the investigators (not registered or published).
Search strategy and selection criteria
Potentially eligible studies were identified using PubMed and EMBASE databases by two independent investigators (CD and LF). Discrepancies were solved by consensus among three authors (CD, LF, and AF). We searched for eligible published studies in English, from January 2015 to January 2022, using the following search strategy: “(‘acute ischemic stroke’/exp OR ‘acute ischemic stroke’ OR ((‘stroke’ OR ‘stroke’/exp OR stroke OR ‘acute cerebrovascular accident’ OR ‘acute cerebrovascular accidents’) AND (ischemic OR ischemic))) AND (endovascular OR ‘thrombectomy’ OR ‘thrombectomy’/exp OR thrombectomy OR (thromb* AND (‘aspiration’ OR ‘aspiration’/exp OR aspiration))) AND (‘cancer’ OR ‘cancer’/exp OR cancer OR ‘tumor’ OR ‘tumor’/exp OR tumor OR ‘carcinoma’ OR ‘carcinoma’/exp OR carcinoma OR ‘malignancy’ OR ‘malignancy’/exp OR malignancy).”
The reference list of eligible studies was screened to identify additional publications suitable for our purposes not included in the original list.
We applied the following inclusion criteria: (1) English written articles; (2) patients with acute ischemic stroke; (3) Observational design of the study; (4) Treatment with mechanical thrombectomy (with or without intravenous thrombolysis); (5) Included patients with cancer, defined as: patients with stroke within 12 months from diagnosis of cancer, OR patients who were administered or refused of any treatment for cancer, OR patients with metastatic cancer; or cancer-related stroke (defined as patients with cryptogenic stroke and concurrent diagnosis of cancer); six-assessment of at least one of the outcomes of interest. We excluded studies that included patients with stroke and history of cancer (i.e. patients who did not have a current cancer at the time of stroke onset). Case reports, conference abstracts, and study protocols were not included. We also excluded experimental or animal studies. Where relevant data were not available from the published papers, we contacted corresponding authors.
Outcomes
As efficacy outcomes we evaluated: (1) functional outcome at 3 months assessed with the Modified Rankin Scale (mRS), and defined as Good Functional Outcome a score of mRS ⩽2; (2) recanalization rate assessed using the modified Treatment In Cerebral Ischemia (mTICI), 10 we defined recanalization of the vessel as mTICI score of 2b or 3. As safety outcomes we evaluated: (1) death occurrence at 3 months; (2) intracerebral hemorrhage (ICH) after treatment, defined either as symptomatic or asymptomatic in index studies. We performed a sensitivity analysis for studies that reported symptomatic ICH (sICH).
Risk of bias assessment
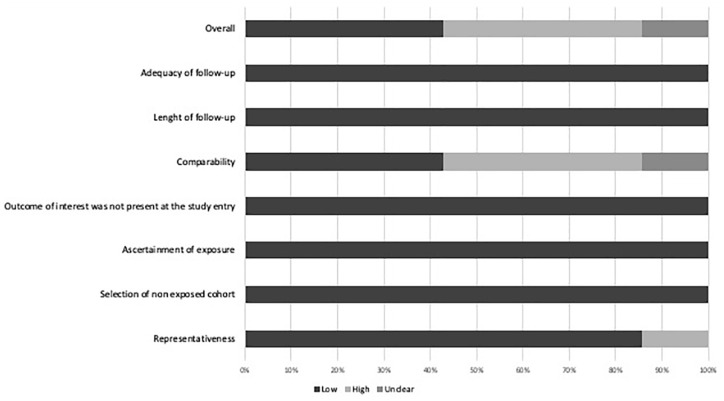
Two investigators (CD, LF) independently extracted data from relevant studies using a predefined form including the following sections: (1) Year of publication and study period; (2) Study design; (3) Inclusion and exclusion criteria; (4) Clinical data; (5) Definition of cancer; 6-Outcome data. The same two investigators assessed independently study quality and risk of bias using the Newcastle-Ottawa Scale for cohort studies. 11 In case of uncertainty, the final decision was made by consensus among three authors (CD, LF, AF).
Statistical analysis
We extracted data from single studies and calculated crude Odds Ratio (OR) between patients with and without cancer. Individual data on patients with and without cancer from each study were used to calculate the combined ORs with 95% CIs by meta-analysis for the outcomes of interest. Pooled effect size was estimated using crude ORs with the Maentel-Haenszel model. We used the DerSimonian-Laird weights (random effects) in all the analysis. Statistical heterogeneity was assessed with I2 statistic and visual inspection of forest plots. Publication bias was assessed by visual inspection of funnel plots. All the analyses were performed in January 2022 using RevMan 5 (https://community.cochrane.org/) and STATA 12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
Results
The initial search retrieved 906 results. After removing 115 duplicates, we screened 791 titles and abstracts and excluded 772 articles and seven congress posters or abstracts. We therefore examined 12 full-text articles and excluded four articles for insufficient data and two articles where outcomes of interest were not reported. We further retrieved one study from snowballing and therefore included seven studies in the final analysis, with a total of 4465 patients, of whom 262 (6%) with cancer. The study selection process is illustrated in Figure 1.
Figure 1.
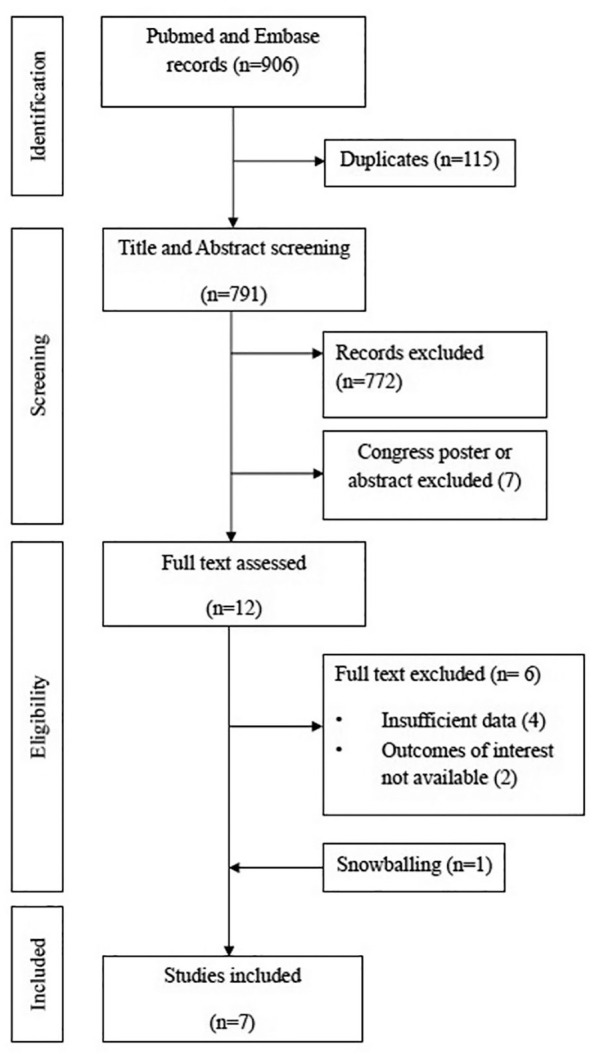
Flowchart of study selection.
The general characteristics of the included studies are summarized in Table 1. All studies had a retrospective design, five from single center clinical cohorts12–16 and two from multicentre clinical cohorts.17,18 Included studies were published from 2018 to 2022 and were from Korea,12,13,15,17 Canada, 14 Netherlands, 18 and Italy. 16 Three studies had a low risk of bias,13,15,18 three a high risk of bias14,16,17 and one an unclear risk 12 (Supplemental Table 1 and Figure 2). A total of 2771 (62%) patients (of whom 111 with cancer) received both intravenous thrombolysis and mechanical thrombectomy, whereas 1694 (38%) patients (of whom 151 with cancer) received direct endovascular treatment. Mean age ranged was similar in patients with and without cancer, median stroke severity measured with the National Institutes of Health Stroke Scale (NIHSS) ranged between 12 and 16 in patients without cancer and between 11and 20 in patients with cancer. Data for onset-to-groin-puncture-time (OTT) were present in four studies13,15,16,18 with similar the median OTT time between the two groups. Definition and localization of cancer across studies are summarized in Table 2 and Table 3, respectively.
Table 1.
Summary of included studies.
| Author | Design | Country and inclusion period | Sample size, N | Patients with cancer, N | Group type | Age, Median (IQR) or Mean (±SD) | NIHSS, Median (IQR) or Mean (±SD) | treated with IVT, N (%) # | Any ICH, N(%) | Anterior circulation stroke, N (%) | Onset to Groin Puncture time, min (IQR) | Successful recanalization, N (%)* | Three months mortality, N (%) | Good functional outcome, N (%)** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jung et al. 12 | Retrospective, SC | Korea, 2011–2015 |
329 | 19 | Cancer | 69 (58–75) | 16 (6–20) | 3 (16) | 4 | 19 (100) | NA | 12 (63) | 12 (63) | 3 (16) |
| Control | NA | NA | 154 (50) | NA | 272 (88) | 261 (84) | 27 (10) | 135 (48) | ||||||
| Cho et al. 17 | Retrospective, MC | Korea, 2011–2016 |
378 | 27 | Cancer | 69 (9.95) | 11 (7–14) | 17 (63) | 12 (44) | All | NA | 23 (85) | 9 (33) | 10 (37) |
| Control | 70.1 (11.46) | 12 (9–15) | 203 (58) | 115 (33) | 290 (83) | 29 (8) | 139 (40) | |||||||
| Lee et al. 2019 13 | Retrospective, SC | Korea, 2012–2017 |
253 | 26 | Cancer | 63.2 (11.6) | 14 (10–18) | 5 (19) | 17 (62) | NA | 232 (154–504) | 23 (88) | 8 (31) | 6 (23) |
| Control | 68.8 (11.3) | 13 (9–17) | 79 (35) | 92 (41) | 293 (208–547) | 205 (91) | 20 (9) | 95 (42) | ||||||
| Ozaki et al. 14 | Retrospective, SC | Canada, 2015–2019 |
300 | 18 | Cancer | 68.5 (26.3) | 16.5 (6.5) | 3 (17) | 4 (22) | 16 (84) | NA | 18 (100) | 3 (17) | 4 (22) |
| Control | 71.4 (15.3) | 15.8 (7.8) | 155 (57) | NA | 255 (90) | 238 (84) | 39 (14) | 114 (40) | ||||||
| Lee et al. 2021 15 | Retrospective, SC | Korea, 2014–2020 |
341 | 34 | Cancer | 64.5 (11.4) | 18 (11–23) | 9 (27) | 14 (41) | 30 (88) | 238 (170–508) | 26 (77) | 9 (27) | 11 (32) |
| Control | 68.9 (14) | 15 (8–19) | 80 (26) | 73 (24) | 253 (82) | 330 (185–603) | 269 (88) | 21 (7) | NA | |||||
| Ciolli et al. 16 | Retrospective, SC | Italy, 2008–2016 |
281 | 14 | Cancer | 73 (61–68) | 20 (10–23) | 5 (36) | 6 (43) | NA | 205 (168–368) | 10 (71) | 9 (64) | 3 (21) |
| Control | 72 (60–79) | 16 (10–21) | 127 (48) | 108 (40) | 268 (195–494) | 208 (78) | 38 (14) | 117 (44) | ||||||
| Verschoof et al. 18 | Retrospective, MC | Netherlands, 2014–2017 |
2583 | 124 | CancerControl | 69 (11)70 (14) | 16 (12–19)16 (11–19) | 69 (57)1862 (76) | NA | All | 203 (155–258)200 (153–260) | 82 (68)1451 (61) | 60 (52)614 (27) | 26 (23)976 (42) |
SC = Single Center; MC = Multi Center; IVT = Intravenous Thrombolysis; IQR = Interquartile Range; SD = Standard Deviation; NIHSS = National Institute of Health Stroke Scale; ICH = Intracerebral Hemorrhage.
# = NB. All patients are treated with mechanical thrombectomy.
= Successful recanalization is defined as modified Treatment In Cerebral Ischemia score of 2b or 3.
= Good Functional Outcome is defined as modified Rankin Scale at 3 months ⩽2.
Figure 2.
Graphical representation of Newcastle Ottawa Scale assessment for cohort studies with low, high or unclear risk of bias.
Table 2.
Summary of definition of patients with cancer in included studies.
| Author | Definition of patients with cancer |
|---|---|
| Jung et al. 12 | Patients who had (a) cryptogenic stroke with advanced or metastatic cancer at the time of stroke onset; and (b) elevated D-dimer levels (>1.11 mg/mL) and/or diffusion-restricted lesions in multiple vascular territories. |
| Cho et al. 17 | Patients (1) with any diagnosis of current or previous metastatic disease, (2) undergoing current treatment for malignancy, (3) who refused treatment for current cancer, (4) who received first diagnosis of cancer during hospitalization after the onset of stroke. |
| Lee et al. 2019 13 | Patients (1) with any metastatic disease, (2) undergoing current treatment for a malignancy, (3) offered treatment for a malignancy, but declined. |
| Ozaki et al. 14 | Patients (1) actively receiving treatment (such as radiation, therapy or chemotherapy) for malignancy or (2) being treated conservatively for current active malignancy. |
| Lee et al. 2021 15 | Patients (1) currently undergoing or refusing treatment for malignancy, (2) with cancer diagnosed during hospitalization for stroke. |
| Ciolli et al. 16 | Patients (1) with cancer diagnosis occurred within six months before stroke, (2) treated for cancer within the previous six months, or 3- with recurrent or metastatic cancer. |
| Verschoof et al. 18 | Patients with (1) cancer diagnosis within 12 months prior to stroke, (2) metastatic disease, or (3) cancer treatment in the last 30 days, (4) who declined cancer treatment. |
Table 3.
Frequencies of cancer type.
| Cancer type | Jung et al.
12
N = 19 ǂ |
Lee et al. 2019
13
N = 26 |
Ozaki et al.
14
N = 18 |
Lee et al. 2021
15
N = 34 |
Ciolli et al.
16
N = 14 |
Cho et al.
17
N = 27 |
Verschoof et al.
18
N = 124 |
Total N = 262 |
|---|---|---|---|---|---|---|---|---|
| Hepatobiliary | 5 (26) | 6 (26) | – | 8 (24) | 1 (7) | 7 (26) | – | 27 (10) |
| Lung | 7 (37) | 3 (12) | 2 (10) | 7 (21) | 2 (14) | 6 (22) | 31 (25) | 58 (22) |
| GI | – | 9 (35) | 1 (6) | 5 (15) | 2 (14) | 7 (16) | 41 (33) | 65 (25) |
| GU | – | 3 (12) | 9 (50) | 3 (9) | 5 (36) | 3 (11) | 26 (21) | 49 (19) |
| Breast | – | – | 4 (22) | 2 (6) | 1 (7) | 1 (4) | 16 (13) | 25 (10) |
| Pancreatic | – | 2 (8) | 1 (6) | 9 (26) | 2 (14) | – | – | 14 (5) |
| Hematologic | – | 2 (8) | 1 (6) | – | 1 (7) | 3 (11) | 3 (2) | 10 (4) |
| Thyroid | – | 1 (4) | – | – | – | – | – | 1 (0.4) |
| Other | – | – | – | – | – | – | 7 (6)* | 4 (2) |
Values are Number (%); GI: Gastrointestinal (salivary gland, gastric, colorectal, esophageal); GU: Genitourinary (ovarian, renal, prostate, bladder); Hepatobiliary: gall bladder, bile duct, hepatic.
Metastases from unknown primary tumor (2), malignant tumor lower leg (1, histopathological findings not reported), melanoma (3), sarcoma central pulmonary artery (1).
Seven missings.
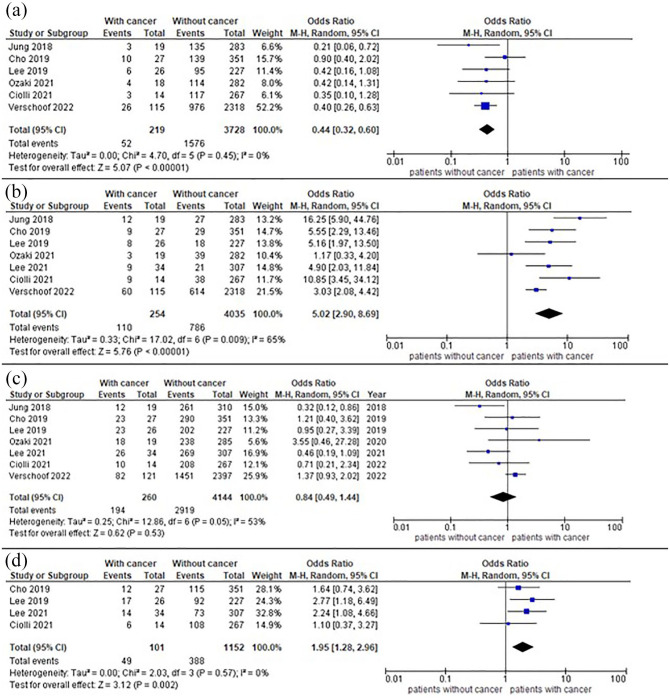
All studies provided mortality and recanalization data, whereas data on functional outcome and intracerebral hemorrhage were present in six13–15,17,18 and four13,15–17 studies, respectively. Outcomes are summarized in Figure 3. Achievement of good functional outcome at 3 months ranged from 16% to 40% in patients with cancer and from 40% to 48% in patients without, and meta-analysis showed a reduction of chances to have good functional outcome in patients with cancer, with 1576/3728 (42%) patients without cancer having mRS⩽2 at 3 months compared with 52/219 (24%) patients with cancer, OR = 0.44 (95%CI = 0.32–0.60). Further results are shown in Supplemental Table 2. Recanalization of the occluded vessel ranged from 63% to 100% in patients with cancer and from 61% to 91% in patients without, 2919/4144 (84%) patients without cancer had a mTICI2b/3 compared with 194/260 (75%) patients with cancer, with pooled OR = 0.84 (95% CI = 0.49-1.44). Mortality rate at 3 months ranged from 17% to 64% in patients with cancer and from 7% to 27% in patients without, pooled data showed that 786/4035 (19%) patients without cancer died within 3 months compared with 110/254 (43%) patients with cancer, OR = 5.02 (95% CI = 2.90–8.69). Where available, we summarized neurological and non-neurological causes of death in Supplemental Table 3. Regarding ICH, three studies13,16,17 adopted the European Co-operative Acute Stroke Study II classification 19 and two studies15,18 used the Heidelberg bleeding classification, 20 for two studies12,14 ICH classification was not available. Occurrence of any ICH ranged from 21% to 62% in patients with cancer and from 24% to 41% in patients without, pooled data showed intracerebral hemorrhage in 388/1152 (34%) patients without cancer and 49/101 (49%) patients with cancer, OR = 1.95 (95% CI = 1.28-2.96). Sensitivity analysis on sICH (data available in four studies14,16–18) revealed that occurrence of sICH ranged from 0% to 14% in patients with cancer and from 6% to 16% in patients without, in total 13/184 (7%) patients with cancer had sICH compared with 238/3362 (7%) patients without, with pooled OR = 1.04 (95% CI = 0.59–1.85) (Supplemental Figure 1). We found moderate statistical heterogeneity across studies mortality and recanalization (I2 = 56% and I2 = 53%, respectively), and none or mild statistical heterogeneity for ICH and functional outcome (I2 = 0% and 30%, respectively). Visual inspection of funnel plots showed slight asymmetry for mortality, recanalization rate and incidence of intracerebral hemorrhage, suggesting presence of publication bias (Supplemental Figure 2–3). We did not investigate publication bias with formal statistical tests due to the small number of studies included.
Figure 3.
Forest plot of outcomes of interest: (a) Functional independence (mRS ⩽ 2); (b) Mortality; (c) Successful recanalization, and (d) Any hemorrhagic transformation.
Discussion
We systematically reviewed studies about safety and efficacy of endovascular treatment in patients with acute ischemic stroke and cancer. Our meta-analysis showed that despite similar recanalization rates (i.e. technically successful endovascular treatment), patients with cancer had higher mortality, rate of any intracerebral hemorrhage and reduced functional independence 3 months after the index stroke. Although we found an overall moderate-high risk of bias due to methodological issues across included studies, our results are biologically plausible.
EVT clinical randomized trials did not report data about patients with cancer, and few observational studies focused on this association. Up to 10% of stroke patients have a history of cancer, 3 and those presenting within the therapeutic window are expected to receive reperfusion therapy. Several factors are responsible for the increased incidence of stroke in patients with cancer, ranging from shared risk factors 21 to complications of cancer treatment (e.g. radiation therapy),22,23 blood vessel invasion or compression by the tumor itself, hypercoagulability state due to pro-thrombotic factors.24,25 As a consequence, more precise prognostic information is relevant for clinicians involved in stroke treatment and patients. A small case-control study with around a half of included patients treated with EVT suggested that reperfusion therapy was safe and effective in patients with active malignancy, 26 similar to a further study in patients with active nonmetastatic cancer and remote cancer. 27 However, such studies included also patients treated only with intravenous thrombolysis, whereas our analysis was tailored on EVT with or without intravenous thrombolysis. We showed that patients with cancer had a reduced probability to have functional independence of around 50% and increased risk of death at 3 months after stroke compared to cancer-free patients. Patients with cancer have an expected poor prognosis after stroke: a study reported a median survival of cancer patients after stroke of 4.5 months, with around a fourth of patients died within 30 days. 28 Long-term outcome can be even worse, with more than 90% of death of stroke patients with active malignancy treated with mechanical thrombectomy within 1 year. 29 Recanalization of the occluded vessel is one of the most powerful predictors of prognosis in patients with ischemic stroke treated with reperfusion therapy, 30 and we showed similar recanalization rates between patients with and without cancer. However, we observed a worse prognosis in patients with cancer, suggesting that despite a good technical result of EVT, the poor clinical outcome in patients with cancer is not entirely attributable to the index stroke. In keeping with this assumption, a study reported that more than a half of deaths in patient with cancer were for non-vascular reasons. 12 Although there are factors that could modulate prognosis, such as stage of cancer, treatment of malignancy and baseline functional status, our results confirm that prognosis in stroke patients with cancer is generally poor.
Similarly, the risk of intracerebral hemorrhage in patients with cancer treated with reperfusion therapy has been scarcely investigated. Results from studies about intravenous thrombolysis in patients with cancer are controversial,29,31,32,33 however, the latest American Heart Association guidelines support the use of intravenous thrombolysis in patients with malignancies without other contraindications. 33 Nonetheless, the risk of intracerebral hemorrhage of endovascular treatment in patients with cancer has been scarcely investigated. We showed a nearly two-fold higher risk of any ICH in the cancer group, however, our main outcome included any symptomatic and asymptomatic ICH. In a comprehensive meta-analysis of patients enrolled in clinical trials, 5 the rate of symptomatic ICH from clinical trials on EVT was around 4%, whereas we found approximately twice the amount of symptomatic ICH in cancer patients. 5 Our sensitivity analysis on symptomatic ICH did not confirm the relatively higher risk of hemorrhage in patients with cancer compared to cancer-free patients. Although no definitive conclusions on the risk of symptomatic ICH can be drawn given the small number of cases included in this analysis, the finding has more clinical relevance than the result with any ICH and is reassuring for clinicians. Taken together, such results suggest that the increase in mortality in stroke patients with cancer is not attributable to the increase of ICH risk.
Our study has limitations. Definitions of cancer sensibly differed across studies according to treatment type, presence of metastatic disease, time of the cancer diagnosis and etiology of stroke, possibly introducing a bias in our pooled results. Furthermore, we excluded from our analysis studies with patients with history of cancer, and our results should be interpreted in this regard. All the studies included in the analysis were observational and non-randomized, and we underscore that our results did not provide information about the choice to treat or not patients with cancer and stroke. Furthermore, all the studies were retrospective with a relatively small sample size, and four out of seven studies included patients from Asian ethnicity, suggesting a possible population bias thus limiting the generalizability of the results. Prospective and multicentre studies from different countries may likely provide more precise and less biased data. Finally, most of the included studies have an unclear or high risk of bias, mainly to the lack of adjusted analysis, but this had unlikely affected our results since we used unadjusted data from each study for the pooled results. The main strength of our analysis is that we provided a more precise estimate of the risks and benefits of EVT in patients with acute stroke and cancer. Although our results showed worse outcomes in this subgroup of patients, we emphasize that cancer is not an absolute contraindication to endovascular therapy, and benefit is still relevant, with up to one in four patients achieving good functional outcome at 3 months after stroke. At the same time, clinicians should be aware that despite the technical success of the endovascular therapy (i.e. recanalization of the occluded vessel), patients with cancer have an increased risk of any type of intracerebral hemorrhage, disability, and death compared to patients without cancer. The dramatic improvement in disability and mortality that EVT demonstrated in clinical trials and clinical practice is therefore remarkably reduced, and indications to the treatment should be balanced with life expectation of the patient in each case.
Conclusions
In conclusion, patients with ischemic stroke and cancer treated with EVT have similar recanalization but higher probability of functional dependence, death and any hemorrhagic transformation, though not necessarily symptomatic intracerebral hemorrhage, compared with patients without cancer. Our results may inform clinicians about prognosis of EVT in this subgroup of patients and may help communication with relatives and carers.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873221100897 for Endovascular treatment in patients with acute ischemic stroke and cancer: Systematic review and meta-analysis by Danilo Caimano, Federica Letteri, Francesco Capasso, Nicola Limbucci, Patrizia Nencini, Cristina Sarti, Fana Alemseged, Guido Bigliardi, Andrea Morotti, Danilo Toni, Andrea Zini and Francesco Arba in European Stroke Journal
Acknowledgments
Authors thank Dr. Tessa Piazzini for the help in research strategy.
Footnotes
Contributorship: CD, LF and AF conceived the study, researched the literature, selected the studies and extracted the data. CD and AF performed the statistical analysis. CD, LF and AF drafted the first draft of the manuscript. All authors reviewed and edited the manuscript and provided conceptual insights and approved the final version of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Not applicable.
Ethical approval: Not applicable.
Guarantor: AF
ORCID iDs: Danilo Caimano  https://orcid.org/0000-0003-3906-7686
https://orcid.org/0000-0003-3906-7686
Francesco Arba  https://orcid.org/0000-0003-3941-7383
https://orcid.org/0000-0003-3941-7383
Supplemental material: Supplemental material for this article is available online.
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics’ 2017 update: a report from the American heart association. Circulation 2017; 135: e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schlegel D, Kolb SJ, Luciano JM, et al. Utility of the NIH stroke scale as a predictor of hospital disposition. Stroke 2003; 34: 134–137. [DOI] [PubMed] [Google Scholar]
- 3. Sanossian N, Djabiras C, Mack WJ, et al. Trends in cancer diagnoses among inpatients hospitalized with stroke. J Stroke Cerebrovascular Dis 2013; 22: 1146–1150. [DOI] [PubMed] [Google Scholar]
- 4. Huang S, Lu X, Tang LV, et al. Efficacy and safety of intravenous thrombolysis for acute ischemic stroke in cancer patients: a systemic review and meta-analysis. Am J Transl Res 2020; 12: 4795–4806. [PMC free article] [PubMed] [Google Scholar]
- 5. Goyal M, Menon BK, Van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 6. Badhiwala JH, Nassiri F, Alhazzani W, et al. Endovascular thrombectomy for acute ischemic stroke ameta-analysis. JAMA 2015; 314: 1832–1843. [DOI] [PubMed] [Google Scholar]
- 7. Rothwell PM. External validity of randomised controlled trials: “To whom do the results of this trial apply?” Lancet 2005; 365: 82–93. [DOI] [PubMed] [Google Scholar]
- 8. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. Epub ahead of print 2021. DOI: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting – meta-analysis of observational studies in epidemiology (moose) group B. JAMA Neurol 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 10. Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013; 44: 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wells G, Shea B, O’Connell D, et al. The newcastle-ottawa scale (nos) for assessing the quality of nonrandomised studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2013, accessed 10 January 2022).
- 12. Jung S, Jung C, Hyoung Kim J, et al. Procedural and clinical outcomes of endovascular recanalization therapy in patients with cancer-related stroke. Intervent Neuroradiol 2018; 24: 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee D, Lee DH, Suh DC, et al. Intra-arterial thrombectomy for acute ischaemic stroke patients with active cancer. J Neurol 2019; 266: 2286–2293. [DOI] [PubMed] [Google Scholar]
- 14. Ozaki T, Nicholson P, Schaafsma JD, et al. Endovascular therapy of acute ischemic stroke in patients with large-vessel occlusion associated with active malignancy. J Stroke Cerebrovascular Dis 2021; 30: 105455. [DOI] [PubMed] [Google Scholar]
- 15. Lee EJ, Bae J, Jeong HB, et al. Effectiveness of mechanical thrombectomy in cancer-related stroke and associated factors with unfavorable outcome. BMC Neurol 2021; 21: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ciolli L, Bigliardi G, Ferraro D, et al. Efficacy of mechanical thrombectomy in patients with ischemic stroke and cancer. J Clin Neurosci 2021; 91: 20–22. [DOI] [PubMed] [Google Scholar]
- 17. Cho BH, Yoon W, Kim JT, et al. Outcomes of endovascular treatment in acute ischemic stroke patients with current malignancy. Neurol Sci 2020; 41: 379–385. [DOI] [PubMed] [Google Scholar]
- 18. Verschoof MA, Groot AE, De Bruijn SFTM, et al. Clinical outcome after endovascular treatment in patients with active cancer and ischemic stroke: a MR CLEAN registry substudy. Neurol 2022; 98(10): e993–e1001. [DOI] [PubMed] [Google Scholar]
- 19. Larrue V, Von Kummer RR, Müller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian acute stroke study (ECASS II). Stroke. 2001; 32: 438–41. [DOI] [PubMed] [Google Scholar]
- 20. Von Kummer R, Broderick JP, Campbell BCV, et al. The heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke 2015; 46: 2981–2986. [DOI] [PubMed] [Google Scholar]
- 21. Bang OY, Chung JW, Lee MJ, et al. Cancer-related stroke: an emerging subtype of ischemic stroke with unique pathomechanisms. J Stroke 2020; 22: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Plummer C, Henderson RD, O’Sullivan JD, et al. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke 2011; 42: 2410–2418. [DOI] [PubMed] [Google Scholar]
- 23. Li SH, Chen WH, Tang Y, et al. Incidence of ischemic stroke post-chemotherapy: a retrospective review of 10,963 patients. Clin Neurol Neurosurg 2006; 108: 150–156. [DOI] [PubMed] [Google Scholar]
- 24. Bang OY, Chung JW, Lee MJ, et al. Cancer cell-derived extracellular vesicles are associated with coagulopathy causing ischemic stroke via tissue factor-independent way: the OASIS-CANCER study. PLoS One 2016; 11: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeh ETH, Chang HM. Cancer and clot: between a rock and a hard place. J Am Coll Cardiol 2017; 70: 939–941. [DOI] [PubMed] [Google Scholar]
- 26. Sallustio F, Mascolo AP, Marrama F, et al. Safety and efficacy of reperfusion therapies for acute ischemic stroke patients with active malignancy. J Stroke Cerebrovascular Dis 2019; 28: 2287–2291. [DOI] [PubMed] [Google Scholar]
- 27. Merlino G, Smeralda C, Gigli GL, et al. Recanalisation theraphy for acute ischemic stroke in cancer patients. Sci Rep 2021; 11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cestari DM, Weine DM, Panageas KS, et al. Stroke in patients with cancer. Neurol 2004; 62: 2025–2030. [DOI] [PubMed] [Google Scholar]
- 29. Oki S, Kawabori M, Echizenya S, et al. Long-term clinical outcome and prognosis after thrombectomy in patients with concomitant malignancy. Front Neurol. Epub ahead of print 2020. DOI: 10.3389/fneur.2020.572589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carvalho A, Santos T, Cunha A, et al. Need for refining successful revascularization in endovascular treatment of acute ischemic stroke: data from real-world. J Neurol Sci 2018; 384: 129–132. [DOI] [PubMed] [Google Scholar]
- 31. Weeda ER, Bohm N. Association between comorbid cancer and outcomes among admissions for acute ischemic stroke receiving systemic thrombolysis. Int J Stroke 2019; 14: 48–52. [DOI] [PubMed] [Google Scholar]
- 32. Masrur S, Abdullah AR, Smith EE, et al. Risk of thrombolytic therapy for acute ischemic stroke in patients with current malignancy. J Stroke Cerebrovascular Dis 2011; 20: 124–130. [DOI] [PubMed] [Google Scholar]
- 33. Seo KD, Suh SH, Heo JH, et al. Increased risk of intracranial hemorrhage and mortality following thrombolysis in patients with stroke and active cancer. J Neurosonol Neuroimaging 2018; 10: 122–132. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873221100897 for Endovascular treatment in patients with acute ischemic stroke and cancer: Systematic review and meta-analysis by Danilo Caimano, Federica Letteri, Francesco Capasso, Nicola Limbucci, Patrizia Nencini, Cristina Sarti, Fana Alemseged, Guido Bigliardi, Andrea Morotti, Danilo Toni, Andrea Zini and Francesco Arba in European Stroke Journal