Abstract
Background and purpose:
With high survival rates for pediatric Hodgkin lymphoma (HL), attention has turned to minimizing treatment-related morbidity and mortality. Chemotherapy and dose of radiation to organs at risk (OARs) contribute to elevated risks of secondary malignancy and cardiopulmonary disease. We sought to characterize the radiation dose to OARs, toxicities, and outcomes for pediatric HL patients treated with proton therapy (PT).
Materials and methods:
Fifty patients aged 11–21 with HL consecutively treated with PT were evaluated 1–2 months following completion of PT and every 6 months thereafter. Acute and late toxicities were captured retrospectively using CTCAE v5. Patterns of relapse were characterized, and survival was assessed using Kaplan-Meier method.
Results:
Most (47, 94%) patients received PT to the mediastinum. Median mean heart dose was 4.3 Gy (RBE) and median bilateral lung V20Gy was 5.8%. Median integral dose was 1.7 Gy. For the 27 female patients, a median mean dose of 0.4 and 0.3 Gy (RBE) was delivered to ipsilateral and contralateral breast tissue, respectively. No on-treatment grade 3–5 toxicities were seen. At a median follow-up of 5.3 years, no PT-related grade 3–5 toxicities or secondary malignancies developed. Five patients relapsed at a median time of 9.2 months after PT (range 2.5–24.9 months; 5-year recurrence free survival 90%). Recurrences were both in- and out-of-field in all 5 cases with no marginal failures. All relapsed patients were successfully salvaged (5-year overall survival 100%).
Conclusion:
For pediatric HL patients, proton treatment resulted in marked dose sparing of OARs with low rates of toxicity, no marginal failures, and excellent 5-year survival.
Keywords: Hodgkin, protons, radiation, toxicity, secondary malignancy
Introduction
Hodgkin lymphoma (HL) is the most common adolescent cancer. Survival rates now exceed 95% with contemporary combined modality treatment across all stages (Smith, Altekruse, Adamson, Reaman, & Seibel, 2014). There are over 35,000 survivors of pediatric and adolescent HL in the United States (Phillips et al., 2015). Attention has turned to minimizing long-term morbidity and mortality associated with chemotherapy and radiation treatment (RT), including cardiovascular, pulmonary, endocrine, and gonadal damage. Higher radiation dose and larger radiation treatment fields have been independently associated with increased risk for overall mortality in HL survivors (Castellino et al., 2011). Radiation dose is also associated with increased risk of secondary malignancy, which ultimately surpasses risk of HL relapse at 20 years and joins cardiovascular disease as the leading causes of death in pediatric HL survivors.
Despite lower prescribed radiation doses in more recent series, the absolute excess risk of breast cancer remains elevated above 50% and is the most common secondary malignancy in female patients (O’Brien, Donaldson, Balise, Whittemore, & Link, 2010). Cardiac toxicity, including coronary artery disease, delayed radiation pericarditis, and fatal myocardial infarction, is increased even within 2 years after mediastinal radiation and persists for at least 20 years in pediatric HL survivors (Hancock, Donaldson, & Hoppe, 1993). Advances in treatment planning design, dose reduction, and smaller treatment volume with transformation from mantle field to involved-node RT have reduced mean heart dose from 27.5 Gy to 7.7 Gy (Maraldo et al., 2012), yet the risk of cardiac mortality remains increased for pediatric patients receiving an average cardiac dose above 5 Gy (Tukenova et al., 2010). By 50 years of age, 45% of survivors experience one grade 3–5 cardiovascular condition (Bhakta et al., 2016). Pulmonary toxicity, including pulmonary fibrosis, interstitial pneumonitis, and restrictive or obstructive lung diseases, has been associated with younger age at radiation, dose >15 Gy, and chemoradiotherapy (Huang et al., 2011). In all, chronic medical conditions are reported by 70% of pediatric HL survivors, most frequently thyroid, cardiac, and pulmonary (Castellino et al., 2011).
The literature demonstrates the importance of dose reduction to OARs as a means of mitigating treatment-related toxicity risk in pediatric patients with HL. Protons, as opposed to photons, have the dosimetric benefit of lacking exit dose, therefore decreasing the volume of OARs exposed to radiation. This significant reduction in dose to vital OARs, such as the heart, breast, and lungs, is expected to decrease the risk of late toxicities. In this series of pediatric HL patients consecutively treated with PT, we sought to characterize the dose distributions to OARs, toxicities, and treatment outcomes.
Methods
Population
Between 2012 and 2018, 50 consecutive patients aged 11–21 with HL were treated with PT at a single center and were enrolled on an institutional review board-approved outcomes monitoring protocol. Patient characteristics, toxicities, patterns of relapse, and survival data were collected.
PT Planning
Patients were simulated with a CT scan along with fusion of diagnostic PET/CT scans. They were treated with involved site radiation therapy (ISRT) per Children’s Oncology Group (COG) and International Lymphoma Radiation Oncology Group (ILROG) guidelines (Hodgson, Dieckmann, Terezakis, Constine, & International Lymphoma Radiation Oncology, 2015). Treatment was delivered with uniform scanning or pencil beam techniques. Pencil beam scanning (PBS) became available in 2016, so patients treated after its implementation typically received PT with PBS depending on machine availability. Daily kV x-ray verification based on bony anatomy was used for treatment setup accuracy. Plans were created using Velocity, and dose is reported in Gy equivalents of relative biological effectiveness (RBE). The doses quoted are weighted by biological response with generic proton RBE of 1.1 and permit direct comparison with photon doses (Paganetti et al., 2019). Select representative comparison plans were generated to visualize dosimetric differences between PT and photon RT. Planned target volume (PTV) was a uniform expansion of 0.5cm. Treatment planning prioritized PTV coverage to receive at least 95% of the prescription dose. Following optimization of target coverage, the organs at risk were prioritized including breast, heart, and lung.
Post-PT Follow-up
Patients were scheduled for follow-up at 1–2 months following completion of radiation treatment and every 6 months thereafter. Toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Acute and subacute toxicities were defined as occurring within or beyond 3 months of radiation treatment, respectively. Late toxicities were defined as occurring 1 year or more after RT. Results from pulmonary function tests (PFTs) and echocardiograms were collected when available.
Statistics
Recurrence free survival (RFS) and overall survival (OS) were calculated using Kaplan-Meier method. Analyses were performed in SPSS with two-sided p-values.
Results
Median age at initiation of PT was 17 years (range, 11–21) and 27 patients (54%) were female (Table 1). Most (60%) patients identified as white. Twenty-eight patients (56%) were high risk per COG protocol AHOD1331 defined as stage IIB with bulk, IIIB, IVA, or IVB; 19 patients (38%) were intermediate risk; 3 patients (6%) were favorable risk defined as stage IA/IIA; and 2 patients (4%) were relapsed/refractory. Bulky disease was present in 43 patients (88%), and 26 (52%) had B-symptoms. Classical nodular sclerosing HL was most common (98%) with lymphocyte predominant in 1 (2%) case. Three (6%) patients had a prior history of neoplasm before the HL diagnosis, all of which were in remission at time of treatment: malignant infantile hemangioendothelioma (age 4), pituitary macroadenoma (age 18), and appendiceal carcinoid (age 19). None had received chemotherapy or radiation therapy for these diagnoses.
Table 1:
Patient and treatment characteristics
| n=50 | % | |
|---|---|---|
| Age | ||
| <12 | 3 | 6% |
| 12–18 | 34 | 68% |
| 19–21 | 13 | 26% |
| Sex | ||
| Female | 27 | 54% |
| Male | 23 | 46% |
| Race/Ethnicity | ||
| Asian | 4 | 8% |
| Black | 9 | 18% |
| White | 30 | 60% |
| Hispanic | 3 | 6% |
| Multi-racial/Multi-ethnic | 2 | 4% |
| Other/Unknown | 2 | 4% |
| Stage | ||
| II | 23 | 46% |
| III | 13 | 26% |
| IV | 13 | 26% |
| Relapsed | 1 | 2% |
| Risk | ||
| Low | 3 | 6% |
| Intermediate | 19 | 38% |
| High | 28 | 56% |
| Bulky | ||
| Yes | 44 | 88% |
| No | 6 | 12% |
| B Symptoms | ||
| Yes | 26 | 52% |
| No | 23 | 46% |
| Unknown | 1 | 2% |
| Histologic subtype | ||
| Nodular sclerosing | 49 | 98% |
| Lymphocyte-predominant | 1 | 2% |
| Location | ||
| Mediastinum only | 7 | 14% |
| Non-mediastinum only | 1 | 2% |
| Both | 42 | 84% |
| Proton dose (Gy [RBE]) | ||
| 21–28 | 36 | 72% |
| 29–34 | 9 | 18% |
| 35–36 | 5 | 10% |
| Chemotherapy | ||
| ABVD | 15 | 30% |
| ABVE-PC | 34 | 68% |
| BEACOPP | 9 | 18% |
| Other | 7 | 14% |
The majority of patients were treated according to COG protocols: AHOD0331 (n=20), AHOD0831 (n=3), or were enrolled on AHOD1331 (n=13). The majority of patients received combinations of ABVE-PC (n=34), ABVD (n=15), or BEACOPP (n=9) based regimens. One intermediate risk patient progressed at the end of treatment PET after 4 cycles of ABCE-PC and 2 cycles of DECA, and was treated with brentuximab and autologous stem cell transplant (ASCT) 3 months prior to proton RT. One high risk patient had rapid early response in lymph nodes and neck but slow response in her mediastinum after 5 cycles of ABVE-PC, and was subsequently treated with brentuximab and bendamustine for 4 cycles then bendamustine maintenance prior to consolidative RT. The relapsed/refractory patient was treated with 4 cycles of ABVD, then rituximab with 2 cycles of ifosfamide and vinorelbine.
Most patients (35, 70%) were treated with uniform scanning and the others (15, 30%) were treated with PBS technique. Nearly all patients (47, 94%) were treated to the mediastinum, with many (40, 80%) also treated to an additional site, including neck (n=34), axilla (n=5), spleen (n=4), and abdomen (n=2). Three patients were treated to non-mediastinal sites: one to the left neck for recurrent disease, one to the left anterior ribs, and one to two sites in the thoracic spine (T1-T2, T8-T9). Median prescribed dose was 21.6 Gy (RBE) (range 21–36). Fourteen (28%) patients received a boost. Only one patient had received prior radiation: 8.5 months prior to PT, a male patient presented with a large pericardial effusion causing hemodynamic instability and ICU admission, and emergently received 9 Gy photons delivered in 3 fractions with conventional anterior/posterior radiation at an outside hospital; this photon course was not counted in the dosimetric parameters described below.
Median PTV V95% (volume receiving 95% of the dose) was 96% (range 80–98%). The median mean ipsilateral breast dose was 0.4 Gy (RBE) (range 0–7.0 Gy [RBE]) and contralateral breast dose was 0.3 Gy (RBE) (range 0–2.0 Gy [RBE]). Representative plans for three female patients treated for mediastinal disease along with comparison photon plans are shown in Figure 1 to demonstrate reduction in mean breast dose with PT compared to photons (corresponding detailed dosimetry for these patients are included in the Supplemental table).
Figure 1: Proton therapy dose distributions with comparison photon plans for three female patients treated for Hodgkin lymphoma demonstrating reduced breast dose.
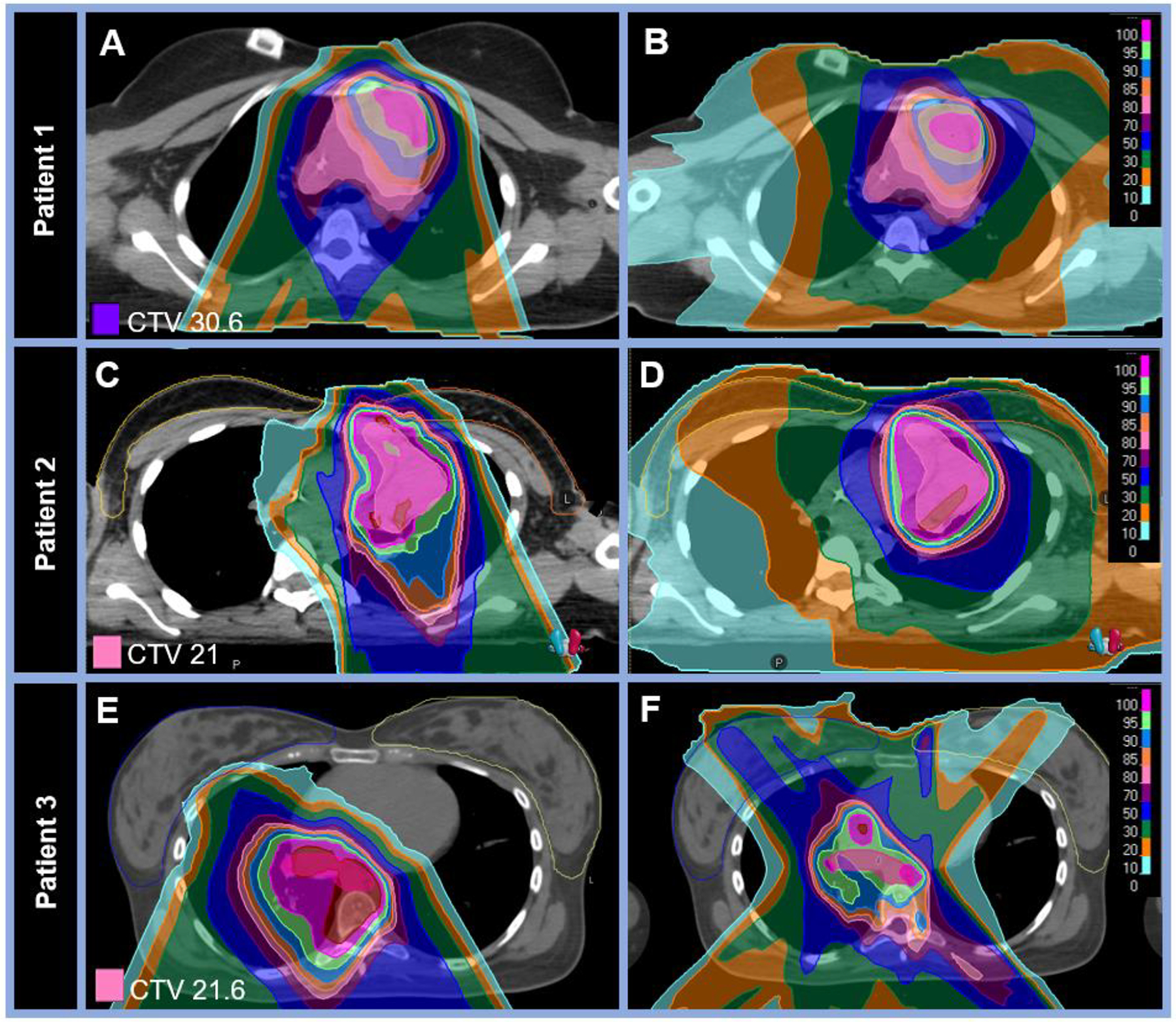
A-B: Comparative dose distributions on two axial planes for a (A) proton and (B) photon plan for an 18-year-old female patient with stage IIA HL of the mediastinum and left supraclavicular nodes treated with pencil-beam scanning protons to 30.6 Gy (RBE) in 17 fractions. Mean ipsilateral breast dose was 0.06 vs 1.53 Gy (RBE) and mean contralateral breast dose was 0.01 vs 0.99 Gy (RBE) for the proton vs photon plans, respectively. C-D: Comparative dose distributions on two axial planes for a (C) proton and (D) photon plan for a 16-year-old female patient with stage IVB HL treated with pencil-bream scanning protons to 21 Gy (RBE) in 14 fractions to the bilateral neck and mediastinum. Mean ipsilateral breast dose was 1.41 vs 2.85 Gy (RBE) and mean contralateral breast dose was 0.26 vs 1.23 Gy (RBE) for the proton vs photon plans, respectively. E-F: Comparative dose distributions on two axial planes for a (E) proton and (F) photon plan for a 21-year-old female patient with stage IVBX HL treated with pencil-bream scanning protons to 21.6 Gy (RBE) in 12 fractions to the mediastinum. Mean ipsilateral breast dose was 0.18 vs 5.62 Gy (RBE) and mean contralateral breast dose was 0.06 vs 3.50 Gy (RBE) for the proton vs photon plans, respectively. Detailed dosimetric data for other organs at risk are outlined in the supplemental Table.
The median mean heart dose was 4.3 Gy (RBE) (range 0–19.1 Gy [RBE]). The dosimetry for the patient with the highest mean heart dose (19.1 Gy [RBE]) is shown in Figure 2 alongside a comparison photon plan. The median heart V5Gy was 23.8% (range 0–91.8%). The median bilateral lung V20Gy (volume receiving 20 Gy) was 5.8% (range 0–17.4%). The median integral mean dose was 1.7 Gy (RBE) (range 0–6.1 Gy [RBE]). Detailed dosimetric parameters are listed in Table 2.
Figure 2: Representative proton plan for the patient with the highest mean heart dose with a comparison photon plan.
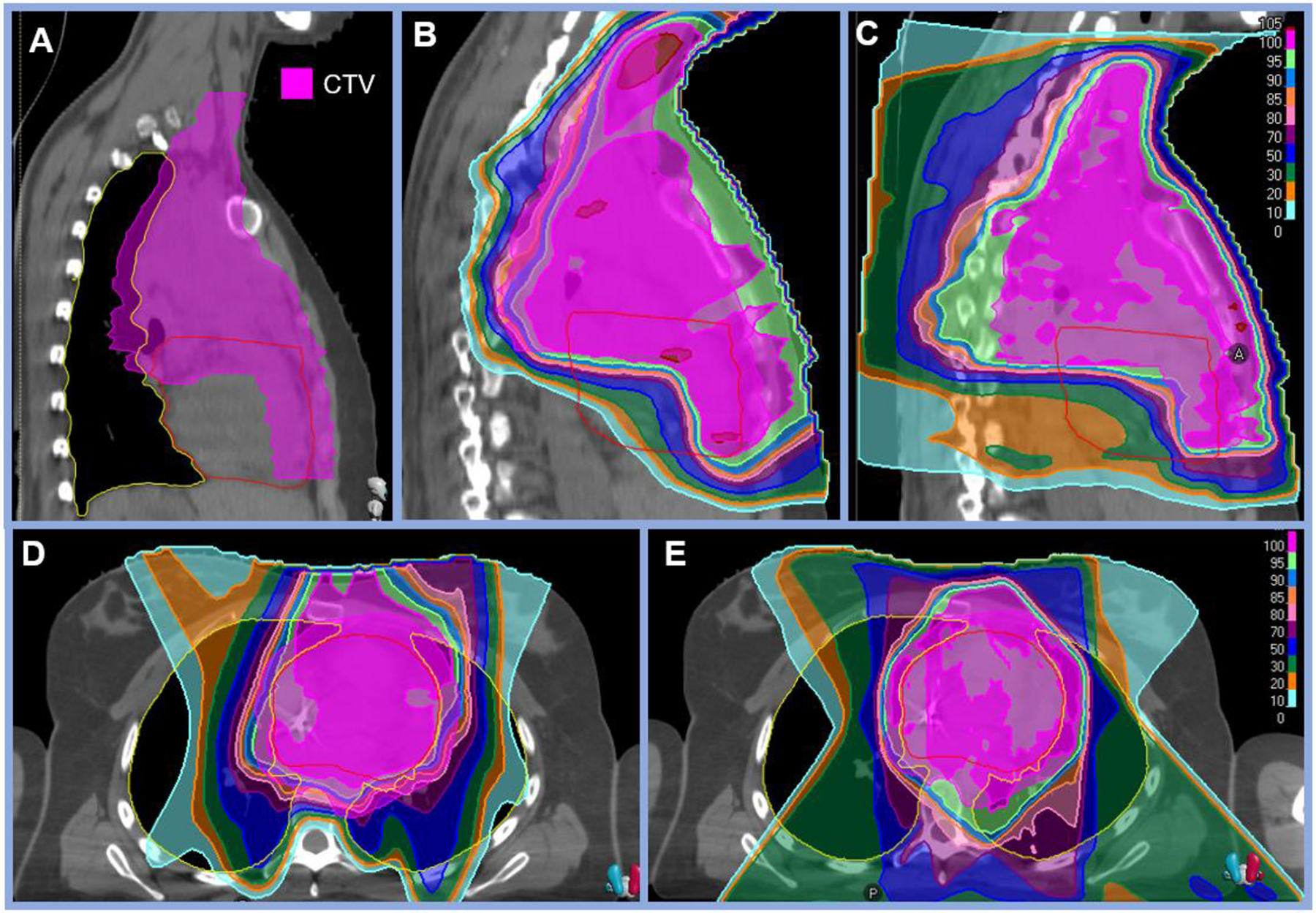
A: Clinical target volume (CTV) shown in magenta demonstrating extensive mediastinal disease in a 19-year-old female with stage IIAX HL treated with uniform scanning protons delivered with three beams (two anterior obliques and one AP) to 21 Gy (RBE). B: Sagittal view of the proton plan delivered with uniform scanning resulting in mean heart dose of 19.1 Gy (RBE), heart V5Gy 91.8%, and bilateral lung V20Gy 10.7%. C: Sagittal view of the comparison photon plan result in mean heart dose of 17.4 Gy, heart V5Gy 94.5%, and bilateral lung V20Gy of 17.3%.
Table 2:
Dosimetric parameters of proton treatment
| Number of Patients (No., %) | Dosimetry | Median | Min | Max | |
|---|---|---|---|---|---|
| PTV | 50 (100) | PTV V95% | 96.2% | 80.0% | 98.3% |
| Heart | 50 (100) | Mean dose (Gy [RBE]) | 4.3 | 0.0 | 19.1 |
| V30Gy | 0.0% | 0.0% | 30.0% | ||
| V15Gy | 13.8% | 0.0% | 81.7% | ||
| V5Gy | 23.8% | 0.0% | 91.8% | ||
| Breast (Ipsilateral) | 27 (54) | Mean dose (Gy [RBE]) | 0.4 | 0.0 | 7.0 |
| Max dose (Gy [RBE]) | 18.4 | 1.8 | 43.0 | ||
| Min dose (Gy [RBE]) | 0 | 0 | 0.01 | ||
| V4Gy | 5.3% | 0.0% | 65.8% | ||
| Breast (Contralateral) | 27 (54) | Mean dose (Gy [RBE]) | 0.3 | 0.0 | 2.0 |
| Max dose (Gy [RBE]) | 29 | 0 | 15.8 | ||
| Min dose (Gy [RBE]) | 0 | 0 | 0 | ||
| V4Gy | 2.0% | 0.0% | 21.1% | ||
| Lung (Bilateral) | 50 (100) | Mean dose (Gy [RBE]) | 4.7 | 0.0 | 22.6 |
| V20Gy | 5.8% | 0.0% | 17.4% | ||
| Esophagus | 50 (100) | Mean dose (Gy [RBE]) | 11.5 | 0.0 | 23.1 |
| Thyroid | 31 (62) | Mean dose (Gy [RBE]) | 15.2 | 0.0 | 25.7 |
| Integral dose | 37 (74) | Mean dose (Gy [RBE]) | 1.5 | 0.0 | 6.1 |
Abbreviations:
PTV: Planned target volume. V95%: Volume receiving 95% of dose.
V30, 20, 15, 5, 4Gy: Volume receiving 30, 20, 15, 5, 4 Gy, respectively.
RBE: Relative biological effectiveness, assumed 1.1 for protons
Eleven (22%) patients had no acute toxicity during treatment; of the 39 patients with toxicity, the highest CTCAE toxicities were grade 1 (n=37) and grade 2 (n=2). No grade 3–5 on-treatment toxicities were seen. Grade 1–2 toxicities were dermatitis (n=24), esophagitis (n=16), fatigue (n=5), dysphagia (n=3), odynophagia (n=2), nausea (n=1), laryngitis (n=1), and dyspnea (n=1). Four patients (8%) had subacute toxicities potentially related to radiation treatment: grade 3 pneumonitis and pleural effusions 7 months post-RT (n=1, resolved), grade 2 pneumonitis 2 months post-PT (n=1, resolved), grade 2 left-ventricular strain (n=1, ongoing), and grade 1 substernal chest pain with panic attacks (n=1, resolved). The patient who developed grade 3 pneumonitis and pleural effusions 1 month after ASCT and 7 months after protons initially presented with grade 2 pleural effusions at diagnosis and was previously treated with 4 cycles of ABVD. At recurrence, he was treated with brentuximab and augmented ICE pre-ASCT. His pneumonitis and effusions resolved after broad-spectrum antibiotics, antifungals, and steroids with return to baseline mild restrictive ventilatory defect. Otherwise, out of the 15 patients with post-RT PFTs available for review, most (n=9) patients had normal PFTs on the most recent available study at a median 2.0 years post-PT (range, 0.1–5.1). Three patients had decreased diffusing capacity: one at 2 months post-PT to the thoracic spine with bilateral lung V20Gy of 2.4% and receipt of 4 cycles of BEACOPP, 1 cycle of ABVD, and 1 of AVD, while the other two had normal PFTs post-PT then developed the abnormality following salvage ASCT. At 5.1 years post-PT, one patient who had received 2 cycles of ABVE-PC and bilateral lung V20Gy of 4.5% had essentially normal PFTs apart from isolated reduction in residual volume.
No late toxicities were seen after the one-year time point. The patient with ongoing left-ventricular strain has been followed by Cardiology for what has been diagnosed as anthracycline-induced cardiomyopathy and treated with carvedilol and lisinopril. She had received a mean heart dose of 10.2 Gy (RBE) and max heart dose of 23.6 Gy (RBE), in addition to 4 cycles of ABVE-PC and 2 cycles of DECA (cumulatively exceeding 200mg/m2 of doxorubicin). At four years post-PT, one patient had mild global hypokinesis of the left ventricle diagnosed as anthracycline-induced cardiomyopathy (mean heart dose was 0 Gy [RBE]). The remaining patients with post-RT echocardiograms available (n=14) had a normal left ventricular ejection fraction at a median time of 3.3 years from RT completion (range, 0.1–7.1). No secondary malignancies have been reported to date.
At a median follow-up was 5.3 years (range, 2–8.4), all patients were alive. RFS was 90% at 5 years (Figure 3). Five patients had biopsy-proven recurrences, which occurred at a median of 9.2 months after completion of proton treatment (range, 2.5 – 24.9 months) [Table 3]. Recurrences were both in- and out-of-field in all 5 cases; there were no marginal recurrences that could be attributed to proton range uncertainties. Recurrences were treated with induction brentuximab and autologous ASCT in all cases (n=5), and with a combination of augmented ICE (n=1), maintenance brentuximab (n=3), or pembrolizumab (n=1). Following ASCT, consolidation repeat RT was delivered via photons to an out-of-field right lower lobe lung recurrence and an in-field mediastinal recurrence in 1 patient to 30.6 Gy in 17 fractions, completed 8 months after proton RT. All 5 patients are without evidence of disease for a median follow-up of 58.8 months (range 43.4–77.7) since relapse and the patient who initially presented for PT at time of relapse is also without evidence of disease.
Figure 3: Recurrence free survival following proton radiotherapy for pediatric Hodgkin lymphoma.
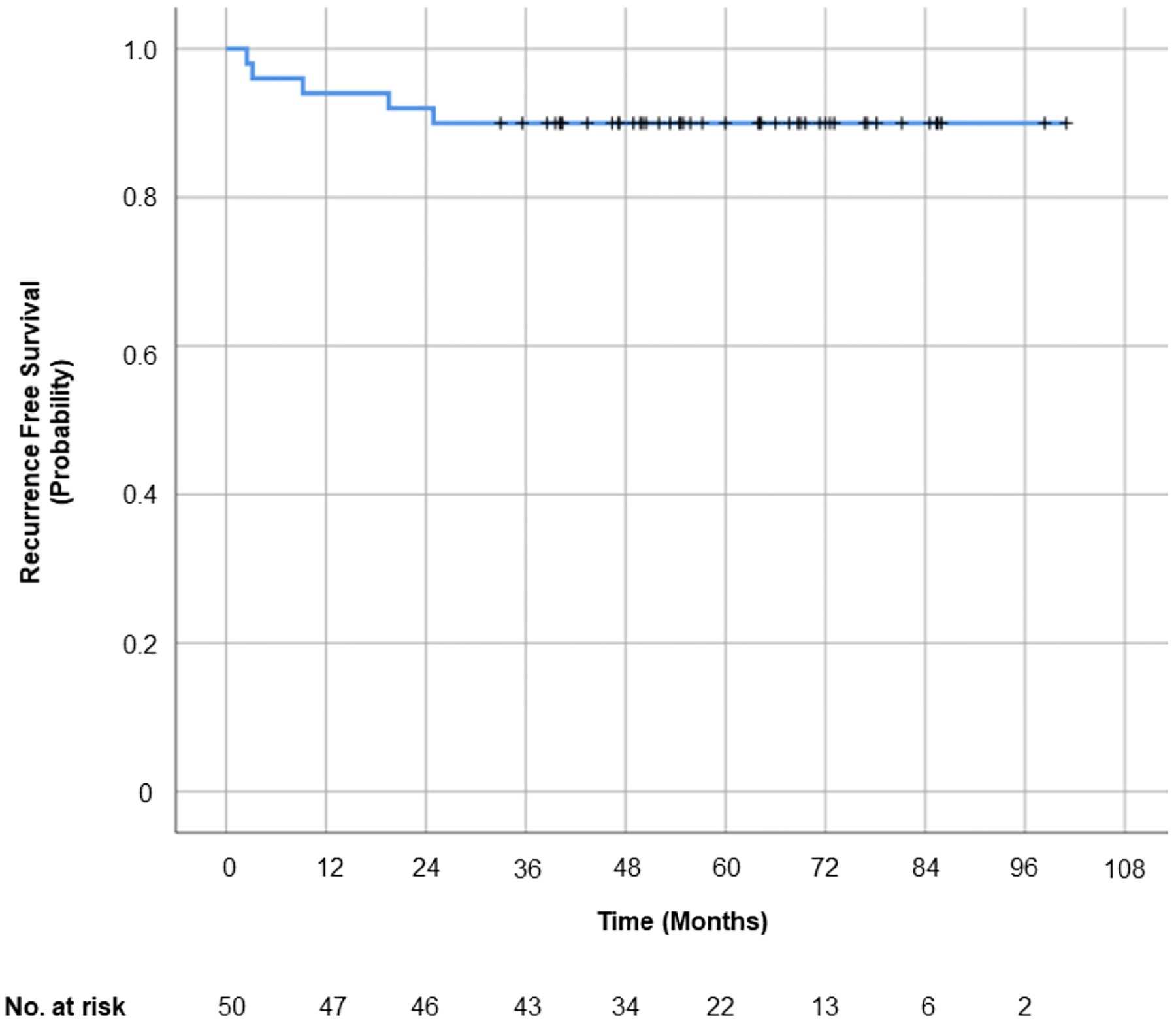
Only 5 patients relapsed over a median follow-up of 5.3 years and all recurrences were both within and outside of the initial treatment field. There were no marginal recurrences. The 5-year recurrence free survival was 90%.
Discussion
The present study is the largest reported series of pediatric patients with HL consecutively treated with proton therapy at a single institution. With a median follow-up of over 5 years, we investigate the radiation dose to vital organs and clinical outcomes of 50 pediatric patients with HL. Dosimetric analyses of vital OARs demonstrate marked dose sparing of the heart, lungs, and breasts. Overall, treatment was well-tolerated with no grade 3–5 acute toxicities, a low rate of subacute toxicities, and no late toxicities nor development of secondary malignancies. Even with the majority of patients having high risk and/or bulky disease, RFS and OS outcomes were excellent, with only 5 relapses (all both in- and out-of-field) that were all successfully salvaged.
PT enables dose-sparing of the heart, which is particularly relevant given cardiac morbidity is prevalent in over 50% of pediatric HL survivors (Mulrooney et al., 2009). Radiation dose to the heart was minimal in our series, with median mean heart dose of 4.3 Gy (RBE) (range 0–19.1 Gy [RBE]) and median heart V5Gy of 23.8% (range 0–91.8%). Prior studies investigating long-term outcomes of childhood cancer survivors have demonstrated the significance of minimizing heart dose. One large cohort analysis of 14, 358 patients in the Childhood Cancer Survivor Study (CSSS) revealed cardiac radiation exposure >15 Gy increased the relative hazard of congestive heart failure, myocardial infarction, pericardial disease, and valvular abnormalities by two- to sixfold compared to non-irradiated survivors (Mulrooney et al., 2009). A linear dose-risk response for cardiac mortality was shown in a European cohort of 2,870 children irradiated for pediatric cancers before 1986 with a median 26 year follow-up: mean heart dose of 5–14.9 Gy had a standardized mortality ratio (SMR) of 13.7, and dose ≥ 15 Gy had a SMR of 33.3, compared to a SMR of 2.4 for < 5 Gy (Tukenova et al., 2010). The excess relative risk of death due to cardiac disease was 60% at 1 Gy of mean heart dose. It is important to acknowledge the effect of each Gy of radiation dose delivered to the heart, particularly in this population already at risk of cardiomyopathy in the setting of anthracycline use. Our cohort demonstrates that protons can be a tool to further reduce this incremental risk.
While less common than cardiac sequelae, pulmonary morbidity is important to consider when designing radiation fields in pediatric patients. Dose constraints typically include both reduction in mean lung dose as well as reduction in volume of lung receiving low dose (e.g., V20Gy). In our series, the median mean bilateral lung dose was 4.7 Gy [RBE] and bilateral lung V20Gy was 5.8%. Younger age at radiation, chest dose >15 Gy, and chemoradiotherapy have previously been associated with pulmonary fibrosis, interstitial pneumonitis, and restrictive or obstructive lung diseases at 2–20 years of follow-up in pediatric cancer survivors (Huang et al., 2011). One patient in our series developed grade 3 pneumonitis and pleural effusions 7 months following PT, during which he received relatively low lung doses: ipsilateral lung V20Gy of 8.5% and bilateral lung V20Gy of 7.2%. This patient had initiated 6 cycles of brentuximab 5 months before toxicity onset then received 2 cycles of augmented ICE followed by ASCT 1-month preceding toxicity onset. Pulmonary toxicity following ASCT as well as brentuximab is well-documented (Faulk et al., 2018). Fortunately for this patient, the pneumonitis and pleural effusions resolved after broad-spectrum antibiotics, antifungals, and steroids. He has returned to his baseline mild restrictive ventilatory defect present prior to PT and no longer requires medication. It is important in his case, and in the analysis of any potential RT-induced toxicity, to consider the other potential contributing factors, including baseline pulmonary function as well as systemic therapy exposure.
Secondary malignancies, both hematologic and solid tumors, represent potential treatment-related sequelae and are a leading cause of death in pediatric HL survivors (Castellino et al., 2011). Radiation-induced secondary malignancy is typically considered a long-term risk with reported median time to development of 13.6–16.9 years; however, it is possible for secondary solid cancers to develop shortly after treatment with minimum time-to-development previously reported between 1.7–2.8 years (Dorffel, Riepenhausen, Luders, Bramswig, & Schellong, 2015; Wolden, Lamborn, Tate, & Donaldson, 1998). These short-term second cancers may, for instance, be a result of sporadic lesions in patients with tumor predisposition syndromes or hematologic second malignancies after chemotherapy. While it is difficult to clearly ascertain the role of chemotherapy and/or RT in the development of secondary cancers, it is encouraging that there were no secondary malignancies reported at median follow-up of over 5 years in our cohort of patients treated with PT. Although longer follow-up is necessary to adequately assess the risk of second malignancies following PT, a recent comparative modeling study using two prediction models accounting for radiobiological considerations did find that PT for malignant mediastinal lymphoma is expected to reduce the risk for RT-induced second malignancies compared to photon-based IMRT (König et al., 2020).
Breast cancer is the most common secondary cancer in female HL survivors. Despite relatively lower doses of radiation and smaller treatment volumes compared to historical fields, secondary malignancies still developed with photons at a cumulative incidence of 17% at 20 years and 30% at 30 years after low-dose mantle involved field RT to 15–25 Gy treated with MOPP with or without ABVD in a limited-institution prospective cohort of 110 pediatric HL patients (O’Brien et al., 2010). In a cohort of 1230 female childhood cancer survivors from the CCSS who received a median of 14 Gy chest RT (range 2–20 Gy), the standardized incidence ratio (SIR) of breast cancer was 44%, similar to the SIR of 24% for patients who received 40 Gy to the mantle field (Moskowitz et al., 2014). The cumulative incidence of breast cancer by age 50 was 35% among HL survivors and similar to the breast cancer risk among BRCA 1 carriers (31% at age 50). The incidence of breast cancer in young female HL patients increases with absorbed radiation in a dose-dependent manner without a known safe lower limit: even 4 Gy or more is associated with a 3.2-fold increased risk, which increases up to an 8-fold risk with 40 Gy or more (Travis et al., 2003) and these risks persist for more than 25 years after treatment (Schaapveld et al., 2015). Thus, low radiation dose to the breast is of particular importance and these prior studies reveal that even low doses achieved by modern photon treatments delivered as intensity-modulated radiation therapy (IMRT) appear to have equal breast cancer risk. Therefore, the strongest risk-reducing approach is to eliminate dose to most or all breast tissue, which we demonstrate may be achieved with protons. PT is a valuable tool to reduce this risk as shown this report, which describes median ipsilateral and contralateral mean breast doses of 0.4 Gy (RBE) and 0.3 Gy (RBE), respectively. The representative plans shown in Figure 1 and corresponding dosimetry in the Supplement highlight how PT can be exploited to reduce mean breast dose compared to photons in female patients. Although relatively higher ipsilateral mean breast doses may be seen in patients who have more lateralized, anterior mediastinal disease (e.g., Patient 2 in Figure 1 [C-D]), PT still enables lower mean dose compared to photons.
There are several considerations with PT planning and important trade-offs need to be considered depending on the priorities of OAR sparing and target coverage. For instance, when sparing ipsilateral mean breast dose, trade-offs may include a higher maximum pixel to the contralateral breast or even slightly higher mean heart dose in the case of posterior beam arrangement, as is commonly implemented in breast-sparing techniques. Elevated mean heart dose may still be seen with PT in cases where patients have extensive, bulky mediastinal disease surrounding the heart, which was seen in the patient with the highest mean heart dose for this cohort (19.1 Gy [RBE]). However, compared to photons as shown in Figure 2, PT in this case did provide the benefit of reduced heart V5Gy (91.8% vs 94.5%) and bilateral lung V20Gy (10.7% vs 17.3%), highlighting these important trade-offs to consider in PT planning. The technique of PT delivery is also an important consideration: uniform scanning was used in this patient since she was treated prior to PBS implementation at our center, but it is likely that PBS could have reduced mean heart dose due to the improved conformality with irregular targets (Thompson et al., 2014).
Only 5 patients relapsed and all were salvaged, with a 5-year recurrence free survival of 90% and an OS of 100% comparing favorably to results for COG studies 0031 and 5942 (Friedman et al., 2014; Wolden et al., 2012), retrospective series (Wray et al., 2016), and a pediatric subgroup of a multi-institutional cohort (Hoppe et al., 2017). All recurrences occurred both within and outside of the initial treatment field with no marginal recurrences that could be attributed to use of protons as opposed to photons. Our outcomes are similar to those of a cohort of 22 pediatric HL patients treated with protons at University of Florida, which demonstrated a 2-year event free survival of 85% and OS 94% (Wray et al., 2016). As in our series, all of their reported 3 recurrences were both in and out of the field.
Our study has several notable strengths: a large cohort of pediatric HL patients consecutively treated with protons at a single institution, detailed radiation dosing parameters, a diverse patient population, and complete follow-up. However, these retrospective data are necessarily limited, for instance in terms of toxicity assessment, and may allow for potential bias and confounding in our report. By including all consecutively treated patients, one male patient who had prior photon RT (9 Gy delivered conventionally in 3 fractions) was also included. It is important to highlight the lack of toxicity he experienced both during and following PT (Grade 0) as well as favorable PT dosimetry in this patient (mean heart dose 0.89 Gy [RBE], bilateral lung V20Gy 6.2%) reflecting the benefit of PT in this setting of prior RT. In addition, our patient cohort is comprised mostly (98%) of nodular sclerosing HL, likely reflecting the indication of RT to bulky disease on COG protocols since these patients often present with bulky mediastinal disease. While this study has the longest single-institution follow-up period of over 5 years, there was a drop in number of patients-at-risk after 5 years, likely due to the fact that many patients were originally from outside our institution and ultimately returned to their home institution for follow-up. While encouraging that there were no second malignancies in the 5-year follow-up period, a longer follow-up will be needed to comprehensively assess long-term rates of secondary malignancy.
Conclusions
Proton treatment resulted in low doses to the heart, lungs, and breasts in a pediatric HL population, with low rates of acute and late toxicity, and favorable RFS and OS. Continued follow-up will be required to detect longer-term secondary malignancies and chronic cardiac and pulmonary morbidity and mortality.
Supplementary Material
Highlights:
Pediatric Hodgkin lymphoma patients are at risk for treatment-related toxicity
Reduced radiation dose to normal tissues can reduce morbidity and mortality
Use of protons (PT) enabled low heart, lung, and breast dose in 50 pediatric patients
No acute and only 1 subacute potentially PT-related grade 3–5 toxicities occurred
Only 5 patients relapsed (all both in- and out-of-field) and all were salvaged
Funding:
NIH/NCATS Grant # TL1-TR-002386 (LAM)
Potential conflicts of interest:
Dr. Modlin received grant funding from the NIH (#TL1-TR-002386). The other authors have no conflicts of interest relevant to this work to disclose.
Abbreviations table:
- ABVD
Doxorubicin hydrochloride (Adriamycin ®), Bleomycin sulfate, Vinblastine sulfate, Dacarbazine
- ABVE-PC
Doxorubicin hydrochloride (Adriamycin ®), Bleomycin sulfate, Vincristine sulfate, Etoposide, Prednisone, Cyclophosphamide
- ASCT
Autologous stem cell transplant
- BEACOPP
Bleomycin sulfate, Etoposide phosphate, Doxorubicin hydrochloride (Adriamycin ®), Cyclophosphamide, Vincristine sulfate (Oncovin ®), Procarbazine hydrochloride, Prednisone
- CCSS
Childhood Cancer Survivor Study
- COG
Children’s Oncology Group
- CTCAE
Common Terminology Criteria for Adverse Events
- Gy
Gray
- HL
Hodgkin lymphoma
- ICE
Ifosfamide, Carboplatin, Etoposide
- ILROG
International Lymphoma Radiation Oncology Group
- IMRT
Intensity-modulated radiation therapy
- ISRT
Involved site radiation therapy
- MOPP
Mechlorethamine hydrochloride, Vincristine sulfate (Oncovin ®), Procarbazine hydrochloride, Prednisone
- OS
Overall survival
- PTV
Planned target volume
- PT
Proton therapy
- RBE
Relative biological effectiveness
- RFS
Recurrence free survival
- RT
Radiation therapy
- SIR
Standardized incidence ratio
- SMR
Standardized mortality ratio
- V4Gy
Volume receiving 4 Gy (RBE)
- V5Gy
Volume receiving 5 Gy (RBE)
- V20Gy
Volume receiving 20 Gy (RBE)
- V95%
Volume receiving 95% of dose
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors do not have conflicts of interest to disclose.
Conflicts of Interest: None.
References
- Bhakta N, Liu Q, Yeo F, Baassiri M, Ehrhardt MJ, Srivastava DK, … Robison LL (2016). Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin’s lymphoma: an analysis from the St Jude Lifetime Cohort Study. The Lancet Oncology, 17(9), 1325–1334. doi: 10.1016/s1470-2045(16)30215-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino SM, Geiger AM, Mertens AC, Leisenring WM, Tooze JA, Goodman P, … Hudson MM (2011). Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood, 117(6), 11. doi: 10.1182/blood-2010- [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorffel W, Riepenhausen M, Luders H, Bramswig J, & Schellong G (2015). Secondary Malignancies Following Treatment for Hodgkin’s Lymphoma in Childhood and Adolescence: A Cohort Study With More Than 30 Years’ Follow-up. Dtsh Arztebl Int, 112(18), 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk K, Sopfe J, Campbell K, Liptzin D, Liu A, Franklin A, & Cost C (2018). Pulmonary toxicity in paediatric patients with relapsed or refractory Hodgkin lymphoma receiving brentuximab vedotin. British Journal of Haematology, 183(2), 251–256. [DOI] [PubMed] [Google Scholar]
- Friedman DL, Chen L, Wolden S, Buxton A, McCarten K, FitzGerald TJ, … Schwartz CL (2014). Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children’s Oncology Group Study AHOD0031. J Clin Oncol, 32(32), 3651–3658. doi: 10.1200/JCO.2013.52.5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock SL, Donaldson SS, & Hoppe RT (1993). Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol, 11(7), 8. [DOI] [PubMed] [Google Scholar]
- Hodgson DC, Dieckmann K, Terezakis S, Constine L, & International Lymphoma Radiation Oncology, G. (2015). Implementation of contemporary radiation therapy planning concepts for pediatric Hodgkin lymphoma: Guidelines from the International Lymphoma Radiation Oncology Group. Pract Radiat Oncol, 5(2), 85–92. doi: 10.1016/j.prro.2014.05.003 [DOI] [PubMed] [Google Scholar]
- Hoppe BS, Hill-Kayser C, Tseng Y, Flampouri S, Elmongy h., Cahlon O, … Plastaras J (2017). Consolidative proton therapy after chemotherapy for patients with Hodgkin lymphoma. Annals of Oncology, 28(9), 2179–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TT, Hudson MM, Stokes DC, Krasin MJ, Spunt SL, & Ness KK (2011). Pulmonary outcomes in survivors of childhood cancer: a systematic review. Chest, 140(4), 881–901. doi: 10.1378/chest.10-2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König L, Haering P, Lang C, Splinter M, von Nettelbladt BV, Weykamp F, Hoegen P, Lischalk JW, Herfarth K, Debus J, Hörner-Rieber J (2020). Frontiers in Oncology, 10(989). doi: 10.3389/fonc.2020.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz CS, Chou JF, Wolden SL, Bernstein JL, Malhotra J, Novetsky Friedman D, … Oeffinger KC (2014). Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol, 32(21), 2217–2223. doi: 10.1200/JCO.2013.54.4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, … Leisenring WM (2009). Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ, 339, b4606. doi: 10.1136/bmj.b4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien MM, Donaldson SS, Balise RR, Whittemore AS, & Link MP (2010). Second malignant neoplasms in survivors of pediatric Hodgkin’s lymphoma treated with low-dose radiation and chemotherapy. J Clin Oncol, 28(7), 1232–1239. doi: 10.1200/JCO.2009.24.8062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganetti H, Blakely E, Carabe-Fernandez A, Carlson D, Das I, Dong L, … Willers H (2019). Report of the AAPM TG-256 on the relative biological effectiveness of proton beams in radiation therapy. Medical Physics, 46(3), e53–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Padgett LS, Leisenring WM, Stratton KK, Bishop K, Krull KR, … Mariotto AB (2015). Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev, 24(4), 653–663. doi: 10.1158/1055-9965.EPI-14-1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaapveld M, Aleman BM, van Eggermond AM, Janus CP, Krol AD, van der Maazen RW, … van Leeuwen FE (2015). Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. N Engl J Med, 373(26), 2499–2511. doi: 10.1056/NEJMoa1505949 [DOI] [PubMed] [Google Scholar]
- Smith MA, Altekruse SF, Adamson PC, Reaman GH, & Seibel NL (2014). Declining childhood and adolescent cancer mortality. Cancer, 120(16), 2497–2506. doi: 10.1002/cncr.28748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Mayekar SU, Zhai HF, et al. A dosimetric comparison of proton and photon therapy in unresectable cancers of the head of pancreas (2014). Medical Physics, 41, 081711. doi: 10.1118/1.4887797. [DOI] [PubMed] [Google Scholar]
- Travis LB, Hill DA, Dores GM, Gospodarowicz M, van Leeuwen FE, Holowaty E, … Gilbert E (2003). Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin Disease. JAMA, 290(4), 11. [DOI] [PubMed] [Google Scholar]
- Tukenova M, Guibout C, Oberlin O, Doyon F, Mousannif A, Haddy N, … de Vathaire F (2010). Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol, 28(8), 1308–1315. doi: 10.1200/JCO.2008.20.2267 [DOI] [PubMed] [Google Scholar]
- Wolden S, Lamborn KR, SF C, Tate D, & Donaldson S (1998). Second Cancers Following Pediatric Hodgkin’s Disease. Journal of Clinical Oncology, 16(2), 536–544. [DOI] [PubMed] [Google Scholar]
- Wolden SL, Chen L, Kelly KM, Herzog P, Gilchrist GS, Thomson J, … Nachman J (2012). Long-term results of CCG 5942: a randomized comparison of chemotherapy with and without radiotherapy for children with Hodgkin’s lymphoma--a report from the Children’s Oncology Group. J Clin Oncol, 30(26), 3174–3180. doi: 10.1200/JCO.2011.41.1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray J, Flampouri S, Slayton W, Joyce M, Sandler E, Morris CG, … Hoppe BS (2016). Proton Therapy for Pediatric Hodgkin Lymphoma. Pediatr Blood Cancer, 63(9), 1522–1526. doi: 10.1002/pbc.26044 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


