Abstract
Background
Every year, an estimated one million children and young adolescents become ill with tuberculosis, and around 226,000 of those children die. Xpert MTB/RIF Ultra (Xpert Ultra) is a molecular World Health Organization (WHO)‐recommended rapid diagnostic test that simultaneously detects Mycobacterium tuberculosis complex and rifampicin resistance. We previously published a Cochrane Review 'Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for tuberculosis disease and rifampicin resistance in children'. The current review updates evidence on the diagnostic accuracy of Xpert Ultra in children presumed to have tuberculosis disease. Parts of this review update informed the 2022 WHO updated guidance on management of tuberculosis in children and adolescents.
Objectives
To assess the diagnostic accuracy of Xpert Ultra for detecting: pulmonary tuberculosis, tuberculous meningitis, lymph node tuberculosis, and rifampicin resistance, in children with presumed tuberculosis.
Secondary objectives
To investigate potential sources of heterogeneity in accuracy estimates. For detection of tuberculosis, we considered age, comorbidity (HIV, severe pneumonia, and severe malnutrition), and specimen type as potential sources.
To summarize the frequency of Xpert Ultra trace results.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, MEDLINE, Embase, three other databases, and three trial registers without language restrictions to 9 March 2021.
Selection criteria
Cross‐sectional and cohort studies and randomized trials that evaluated Xpert Ultra in HIV‐positive and HIV‐negative children under 15 years of age. We included ongoing studies that helped us address the review objectives. We included studies evaluating sputum, gastric, stool, or nasopharyngeal specimens (pulmonary tuberculosis), cerebrospinal fluid (tuberculous meningitis), and fine needle aspirate or surgical biopsy tissue (lymph node tuberculosis). For detecting tuberculosis, reference standards were microbiological (culture) or composite reference standard; for stool, we also included Xpert Ultra performed on a routine respiratory specimen. For detecting rifampicin resistance, reference standards were drug susceptibility testing or MTBDRplus.
Data collection and analysis
Two review authors independently extracted data and, using QUADAS‐2, assessed methodological quality judging risk of bias separately for each target condition and reference standard. For each target condition, we used the bivariate model to estimate summary sensitivity and specificity with 95% confidence intervals (CIs). We stratified all analyses by type of reference standard. We summarized the frequency of Xpert Ultra trace results; trace represents detection of a very low quantity of Mycobacterium tuberculosis DNA. We assessed certainty of evidence using GRADE.
Main results
We identified 14 studies (11 new studies since the previous review). For detection of pulmonary tuberculosis, 335 data sets (25,937 participants) were available for analysis. We did not identify any studies that evaluated Xpert Ultra accuracy for tuberculous meningitis or lymph node tuberculosis. Three studies evaluated Xpert Ultra for detection of rifampicin resistance. Ten studies (71%) took place in countries with a high tuberculosis burden based on WHO classification. Overall, risk of bias was low.
Detection of pulmonary tuberculosis
Sputum, 5 studies
Xpert Ultra summary sensitivity verified by culture was 75.3% (95% CI 64.3 to 83.8; 127 participants; high‐certainty evidence), and specificity was 97.1% (95% CI 94.7 to 98.5; 1054 participants; high‐certainty evidence).
Gastric aspirate, 7 studies
Xpert Ultra summary sensitivity verified by culture was 70.4% (95% CI 53.9 to 82.9; 120 participants; moderate‐certainty evidence), and specificity was 94.1% (95% CI 84.8 to 97.8; 870 participants; moderate‐certainty evidence).
Stool, 6 studies
Xpert Ultra summary sensitivity verified by culture was 56.1% (95% CI 39.1 to 71.7; 200 participants; moderate‐certainty evidence), and specificity was 98.0% (95% CI 93.3 to 99.4; 1232 participants; high certainty‐evidence).
Nasopharyngeal aspirate, 4 studies
Xpert Ultra summary sensitivity verified by culture was 43.7% (95% CI 26.7 to 62.2; 46 participants; very low‐certainty evidence), and specificity was 97.5% (95% CI 93.6 to 99.0; 489 participants; high‐certainty evidence).
Xpert Ultra sensitivity was lower against a composite than a culture reference standard for all specimen types other than nasopharyngeal aspirate, while specificity was similar against both reference standards.
Interpretation of results
In theory, for a population of 1000 children:
• where 100 have pulmonary tuberculosis in sputum (by culture):
‐ 101 would be Xpert Ultra‐positive, and of these, 26 (26%) would not have pulmonary tuberculosis (false positive); and ‐ 899 would be Xpert Ultra‐negative, and of these, 25 (3%) would have tuberculosis (false negative).
• where 100 have pulmonary tuberculosis in gastric aspirate (by culture):
‐ 123 would be Xpert Ultra‐positive, and of these, 53 (43%) would not have pulmonary tuberculosis (false positive); and ‐ 877 would be Xpert Ultra‐negative, and of these, 30 (3%) would have tuberculosis (false negative).
• where 100 have pulmonary tuberculosis in stool (by culture):
‐ 74 would be Xpert Ultra‐positive, and of these, 18 (24%) would not have pulmonary tuberculosis (false positive); and ‐ 926 would be Xpert Ultra‐negative, and of these, 44 (5%) would have tuberculosis (false negative).
• where 100 have pulmonary tuberculosis in nasopharyngeal aspirate (by culture):
‐ 66 would be Xpert Ultra‐positive, and of these, 22 (33%) would not have pulmonary tuberculosis (false positive); and ‐ 934 would be Xpert Ultra‐negative, and of these, 56 (6%) would have tuberculosis (false negative).
Detection of rifampicin resistance
Xpert Ultra sensitivity was 100% (3 studies, 3 participants; very low‐certainty evidence), and specificity range was 97% to 100% (3 studies, 128 participants; low‐certainty evidence).
Trace results
Xpert Ultra trace results, regarded as positive in children by WHO standards, were common. Xpert Ultra specificity remained high in children, despite the frequency of trace results.
Authors' conclusions
We found Xpert Ultra sensitivity to vary by specimen type, with sputum having the highest sensitivity, followed by gastric aspirate and stool. Nasopharyngeal aspirate had the lowest sensitivity. Xpert Ultra specificity was high against both microbiological and composite reference standards. However, the evidence base is still limited, and findings may be imprecise and vary by study setting. Although we found Xpert Ultra accurate for detection of rifampicin resistance, results were based on a very small number of studies that included only three children with rifampicin resistance. Therefore, findings should be interpreted with caution. Our findings provide support for the use of Xpert Ultra as an initial rapid molecular diagnostic in children being evaluated for tuberculosis.
Keywords: Adolescent; Child; Humans; Antibiotics, Antitubercular; Antibiotics, Antitubercular/therapeutic use; Cross-Sectional Studies; HIV Infections; HIV Infections/drug therapy; Microbial Sensitivity Tests; Mycobacterium tuberculosis; Mycobacterium tuberculosis/genetics; Rifampin; Rifampin/pharmacology; Sensitivity and Specificity; Sputum; Sputum/microbiology; Tuberculosis, Lymph Node; Tuberculosis, Lymph Node/diagnosis; Tuberculosis, Lymph Node/drug therapy; Tuberculosis, Meningeal; Tuberculosis, Meningeal/cerebrospinal fluid; Tuberculosis, Meningeal/diagnosis; Tuberculosis, Meningeal/drug therapy; Tuberculosis, Pulmonary; Tuberculosis, Pulmonary/diagnosis; Tuberculosis, Pulmonary/drug therapy; Tuberculosis, Pulmonary/microbiology
Plain language summary
Xpert Ultra for diagnosing tuberculosis and rifampicin resistance in children
Why is improving the diagnosis of tuberculosis important?
Every year, an estimated one million children and young adolescents become ill with tuberculosis, and around 226,000 die from the disease. Tuberculosis is caused by the bacterium Mycobacterium tuberculosis and mostly affects the lungs (pulmonary tuberculosis), though it can affect other sites in the body (extrapulmonary tuberculosis). Signs and symptoms of pulmonary tuberculosis include cough, fever, night sweats, and weight loss. Signs and symptoms of extrapulmonary tuberculosis depend on the site of disease. When detected early and treated effectively, tuberculosis is largely curable.
Not recognizing tuberculosis (false negative) early may result in delayed diagnosis and treatment, severe illness, and death. An incorrect tuberculosis diagnosis (false positive) may result in anxiety, unnecessary treatment (which can involve medication side effects), and the possibility of missing alternative diagnoses which warrant treatment.
What was the aim of this review?
To determine the accuracy of Xpert Ultra in children with symptoms of tuberculosis for diagnosing pulmonary tuberculosis, tuberculous meningitis (affecting membranes that surround the brain and spinal cord), lymph node tuberculosis (a painful swelling of one or more lymph nodes, which are bean‐shaped structures that help fight infection), and rifampicin resistance.
What did this review study?
Xpert Ultra, a World Health Organization‐recommended rapid test that simultaneously detects tuberculosis and rifampicin resistance in adults and children with tuberculosis symptoms. Rifampicin is an important medicine used to treat tuberculosis. For tuberculosis diagnosis, we assessed results against two different benchmarks: tuberculosis culture (a method used to grow bacteria on nutrient‐rich media) and a composite definition based on symptoms, chest X‐ray, sputum microscopy (examination under a microscope of mucus and other matter coughed up from the lungs), and culture. For rifampicin resistance detection, we assessed results against drug susceptibility testing or line probe assay (a rapid laboratory‐based test for detecting tuberculosis bacteria).
What were the main results in this review?
We included 14 studies. For pulmonary tuberculosis, we analysed 335 data sets (around 26,000 participants). No studies evaluated Xpert Ultra accuracy for tuberculous meningitis or lymph node tuberculosis. Three studies evaluated Xpert Ultra accuracy for detection of rifampicin resistance.
For a population of 1000 children:
• where 100 have pulmonary tuberculosis in sputum according to culture results:
‐ 101 would be Xpert Ultra‐positive, and of these, 26 (26%) would not have pulmonary tuberculosis (false positive); and ‐ 899 would be Xpert Ultra‐negative, and of these, 25 (3%) would have tuberculosis (false negative).
• where 100 have pulmonary tuberculosis in gastric aspirate (collection of lung and oral secretions from the stomach) according to culture results:
‐ 97 would be Xpert Ultra‐positive, and of these, 27 (28%) would not have pulmonary tuberculosis (false positive); and ‐ 903 would be Xpert Ultra‐negative, and of these, 30 (3%) would have tuberculosis (false negative).
• where 100 have pulmonary tuberculosis in stool according to culture results:
‐ 74 would be Xpert Ultra‐positive and of these, 18 (24%) would not have pulmonary tuberculosis (false positive); and ‐ 926 would be Xpert Ultra‐negative, and of these, 44 (5%) would have tuberculosis (false negative).
• where 100 have pulmonary tuberculosis in nasopharyngeal aspirate (secretions from the uppermost part of the throat, behind the nose) according to culture results:
‐ 66 would be Xpert Ultra‐positive, and of these, 22 (33%) would not have pulmonary tuberculosis (false positive); and ‐ 934 would be Xpert Ultra‐negative, and of these, 56 (6%) would have tuberculosis (false negative).
Xpert Ultra accurately detected rifampicin resistance, but there were few studies and only three children with rifampicin resistance included.
How confident are we in the results of this review?
For pulmonary tuberculosis, we are fairly confident because we included studies from different countries and used two different benchmarks, though neither is perfect. However, the evidence base is still limited and there were few studies with few children for one of the specimen types (nasopharyngeal aspirate).
For rifampicin resistance, we identified few studies with very few children with rifampicin resistance, so we are less confident.
What children do the results of this review apply to?
Children and young adolescents (birth to 14 years) who are HIV‐positive or HIV‐negative, with signs or symptoms of pulmonary tuberculosis. The results also apply to children with severe pneumonia or malnutrition and tuberculosis symptoms. In this review, we did not identify any studies that evaluated Xpert Ultra accuracy for tuberculous meningitis or lymph node tuberculosis.
What are the implications of this review?
The results suggest that Xpert Ultra in sputum, gastric aspirate, stool, and nasopharyngeal aspirate is an accurate method for detecting pulmonary tuberculosis and rifampicin resistance in children.
Using Xpert Ultra in sputum, gastric aspirate, stool, and nasopharyngeal aspirate, the risk of missing a diagnosis of pulmonary tuberculosis (confirmed by culture) is low, suggesting that only a small number of children will not receive treatment. The risk of incorrectly diagnosing a child as having pulmonary tuberculosis is slightly higher. This may result in some children receiving unnecessary treatment.
How up to date is this review?
This review updates our previous review and includes evidence published up to 9 March 2021.
Summary of findings
Summary of findings 1. Xpert Ultra for pulmonary tuberculosis in childrena.
| Review question: what is the diagnostic accuracy of Xpert Ultra for pulmonary tuberculosis in children with signs and symptoms of pulmonary tuberculosis? | |||||||
|
Patients/population: children with presumed pulmonary tuberculosis Index tests: Xpert Ultra Role: an initial test Threshold for index tests: an automated result is provided Reference standard: culture Types of studies: cross‐sectional and cohort studies Setting: primary care facilities and local hospitals | |||||||
| Specimen | Effect (95% Cl) | Number of participants (studies) | Test result | Number of results per 1000 patients tested(95% CI)b | Certainty of the evidence (GRADE) | ||
| Prevalence 1% | Prevalence 10% | Prevalence 20% | |||||
| Sputum | Summary sensitivity 75.3% (64.3 to 83.8) | 127 (5) | True positive | 8 (6 to 8) |
75 (64 to 84) |
151 (129 to 168) |
⊕⊕⊕⊕ High |
| False negative | 2 (2 to 4) |
25 (16 to 36) |
49 (32 to 71) |
||||
| Summary specificity 97.1% (94.7 to 98.5) | 1054 (5) | True negative | 961 (938 to 975) |
874 (852 to 887) |
777 (758 to 788) |
⊕⊕⊕⊕ High |
|
| False positive | 29 (15 to 52) |
26 (13 to 48) |
23 (12 to 42) |
||||
| Gastric aspirate | Summary sensitivity 70.4% (53.9 to 82.9) | 120 (7) | True positive | 7 (5 to 8) |
70 (54 to 83) |
141 (108) |
⊕⊕⊕⊖ Moderatec |
| False negative | 3 (2 to 5) |
30 (17 to 46) |
59 (34 to 92) |
||||
| Summary specificity 94.1% (84.8 to 97.8) | 870 (7) | True negative | 932 (840 to 968) |
847 (763 to 880) |
753 (678 to 782) |
⊕⊕⊕⊖ Moderated |
|
| False positive | 58 (22 to 150) |
53 (20 to 137) |
47 (18 to 122) |
||||
| Stool | Summary sensitivity 56.1% (39.1 to 71.7) | 200 (6) | True positive | 6 (4 to 7) |
56 (39 to 72) |
112 (78 to 143) |
⊕⊕⊕⊖ Moderatec |
| False negative | 4 (3 to 6) |
44 (28 to 61) |
88 (57 to 122) |
||||
| Summary specificity 98.0% (93.3 to 99.4) | 1232 (6) | True negative | 970 (924 to 984) |
882 (840 to 895) |
784 (746 to 795) |
⊕⊕⊕⊕ High |
|
| False positive | 20 (6 to 66) |
18 (5 to 60) |
16 (5 to 54) |
||||
| Nasopharyngeal aspirate | Summary sensitivity 43.7% (26.7 to 62.2) |
46 (4) | True positive | 4 (3 to 6) |
44 (27 to 62) |
87 (53 to 124) |
⊕⊖⊖⊖ Very lowe,f |
| False negative | 6 (4 to 7) |
56 (38 to 73) |
113 (76 to 147) |
||||
| Summary specificity 97.5 (93.6 to 99.0) |
489 (4) | True negative | 965 (927 to 980) |
878 (842 to 891) |
780 (749 to 792) |
⊕⊕⊕⊕ High |
|
| False positive | 25 (10 to 63) |
22 (9 to 58) |
20 (8 to 51) |
||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
CI: confidence interval. aThe results presented in this table should not be interpreted in isolation from the results of individual included studies contributing to each summary test accuracy measure. bPrevalence levels were suggested by the WHO Global Tuberculosis Programme. cDowngraded one level for imprecision due to wide 95% CI. dDowngraded one level for inconsistency as specificity ranged from 78% to 100%, and several 95% CIs did not overlap. eDowngraded one level for indirectness as only two studies (50%) were of low concern regarding applicability (patients enrolled from outpatient or non‐referral settings). fDowngraded two levels for imprecision due to wide 95% CI and because a small number of participants contributed to the analysis for sensitivity.
Summary of findings 2. Xpert Ultra for rifampicin resistancea.
| Review question: what is the diagnostic accuracy of Xpert Ultra for rifampicin resistance in children with signs and symptoms of pulmonary tuberculosis? | |||||||
|
Patients/population: children with presumed pulmonary tuberculosis Index tests: Xpert Ultra Role: an initial test Threshold for index tests: an automated result is provided Reference standard: culture‐based phenotypic drug susceptibility testing and MTBDRplus Types of studies: cross‐sectional and cohort studies Setting: primary care facilities and local hospitals Limitations: the findings are based on 3 studies. Each study included only 1 participant with rifampicin resistance | |||||||
| Specimen | Effect (95% Cl) | Number of participants (studies) | Test result | Number of results per 1000 patients tested(95% CI)b | Certainty of the evidence (GRADE) | ||
| Prevalence 2% | Prevalence 10% | Prevalence 15% | |||||
| All specimens | Sensitivity range 100% to 100% | 3 (3) | True positive | 20 to 20 | 100 to 100 | 150 to 150 | ⊕⊖⊖⊖ Very lowc,d,e |
| False negative | 0 to 0 | 0 to 0 | 0 to 0 | ||||
| Specificity range 97% to 100% | 128 (3) | True negative | 951 to 980 | 873 to 900 | 825 to 850 | ⊕⊕⊖⊖ Lowc,d |
|
| False positive | 0 to 29 | 0 to 27 | 0 to 25 | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
CI: confidence interval. aThe results presented in this table should not be interpreted in isolation from the results of individual included studies contributing to each summary test accuracy measure. bPrevalence levels were suggested by the WHO Global Tuberculosis Programme. cDowngraded one level for risk of bias because in one study the manner of participant selection was unclear, and in another, not all participants were included in the analysis. dDowngraded one level for indirectness because thethree included studies took place in China, Italy, and South Africa, and applicability to other settings is uncertain. eDowngraded two levels for imprecision because only three participants with rifampicin resistance contributed to this analysis for the observed sensitivity.
Background
Tuberculosis is the 13th leading cause of death and the second leading cause of death from a single infectious agent after COVID‐19 (WHO Global Tuberculosis Report 2021). Globally, in 2020, an estimated 10 million people developed tuberculosis disease, including around 1.1 million children younger than 15 years of age, and 226,100 children (205,000 HIV‐negative and 21,100 HIV‐positive children) died from the disease (WHO Global Tuberculosis Report 2021). Globally, in 2020, 53% of HIV‐negative people who died from tuberculosis were men, 32% were women, and 16% were children younger than 15 years of age. The higher proportion of children who die from tuberculosis compared with their estimated share of cases (11%) suggests poorer access to diagnosis and treatment (WHO Global Tuberculosis Report 2021). The toll on younger children is especially tragic. One systematic review that investigated tuberculosis mortality in children found higher case fatality ratios in children from birth to four years of age compared with children aged five to 14 years (Jenkins 2017). Recent epidemiological models that have been accepted and supported by the World Health Organization (WHO) suggest that there is substantial under‐reporting as well as underdiagnosis of tuberculosis in children (Dodd 2017).
Tuberculosis treatment for children follows the same principles as for adults, and the same drugs are used in most cases. The standard treatment for drug‐susceptible tuberculosis – both pulmonary and extrapulmonary forms – is a four‐drug combination regimen of isoniazid, rifampicin, pyrazinamide, and ethambutol given daily for two months, followed by isoniazid and rifampicin given daily for an additional two to four months. Central nervous system and osteoarticular tuberculosis constitute an exception in that treatment with isoniazid and rifampicin is extended for a total of 12 months. The introduction of paediatric fixed‐dose combinations with optimized dosing and taste masking has improved the efficiency of treatment (Wademan 2019). Treatment of drug‐resistant tuberculosis in children generally has better outcomes than in adults (Harausz 2018). Of note, in 2020, the WHO released consolidated guidelines on the treatment of drug‐resistant tuberculosis in children and adults, containing new recommendations for the treatment of child drug‐resistant tuberculosis, including the use of all‐oral regimens (Furin 2019; WHO Consolidated Guidelines (Module 4) 2020).
The diagnosis of child tuberculosis relies on a mix of clinical, epidemiological, radiological, and laboratory information. Child tuberculosis is typically paucibacillary (tuberculosis disease caused by a smaller number of bacteria), and young children cannot voluntarily produce sputum specimens (Marais 2005; Theart 2005). Hence, even under ideal clinical and laboratory conditions, only 30% to 40% of children with tuberculosis have bacteriological confirmation of disease (Dunn 2016). The probability of microbiological confirmation is increased in children with more severe or advanced disease (Marais 2006a; Marais 2006b). However, the diagnostic gap is perpetuated because conventional smear microscopy, which is of limited value in diagnosing child tuberculosis and is no longer recommended by the WHO for diagnosis, remains the most used and most widely available tuberculosis diagnostic method in low‐ and middle‐income countries. Further, the clinical skills and equipment needed for sputum induction and gastric aspiration are often not available in peripheral (subdistrict and community level) health clinics (Reid 2012). Compared with microscopy, tuberculosis culture methods have shown greater, yet highly variable, sensitivity in child tuberculosis (Chiang 2017; Frigati 2015). Unfortunately, tuberculosis culture to support diagnosis is not widely available in high‐burden settings.
The development of Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA), a rapid molecular diagnostic test that simultaneously detects Mycobacterium tuberculosis (M tuberculosis) complex and rifampicin resistance), was a major step towards improving detection of tuberculosis and rifampicin resistance worldwide. However, Xpert MTB/RIF sensitivity is suboptimal in people with smear‐negative tuberculosis, and particularly in children, people living with HIV, and people with extrapulmonary tuberculosis (Horne 2019;Kay 2020; Kohli 2021; Zifodya 2021). To overcome these limitations, Cepheid developed Xpert MTB/RIF Ultra (Xpert Ultra), a re‐engineered assay using a newly developed cartridge that is run on the same device (GeneXpert) after a software upgrade (see Index test(s)).
This Cochrane Review update assessed the accuracy of Xpert Ultra for detecting pulmonary tuberculosis, specific forms of extrapulmonary tuberculosis (i.e. tuberculous meningitis and lymph node tuberculosis), and rifampicin resistance in children presumed to have tuberculosis, using sputum, gastric aspirate, nasopharyngeal aspirate, or stool specimens.
Current WHO recommendations on the use of Xpert Ultra related to this review update are presented in Table 3 and the WHO Consolidated Guidelines (WHO Consolidated Guidelines (Module 3) 2021; WHO Consolidated Guidelines (Module 5) 2022).
1. Current World Health Organization (WHO) diagnostic recommendations in children.
| WHO Consolidated Guidelines (Module 3) 2021a |
| In children with signs and symptoms of pulmonary tuberculosis, Xpert Ultra should be used as the initial diagnostic test for tuberculosis and detection of rifampicin resistance in sputum or nasopharyngeal aspirate, rather than smear microscopy/culture and phenotypic drug susceptibility testing (strong recommendation, low certainty of evidence for test accuracy in sputum; very low certainty of evidence for test accuracy in nasopharyngeal aspirate). |
| In children with signs and symptoms of tuberculous meningitis, Xpert MTB/RIF or Xpert Ultra should be used in cerebrospinal fluid (CSF) as an initial diagnostic test for tuberculous meningitis rather than smear microscopy/culture (strong recommendation, moderate certainty of evidence for test accuracy for Xpert MTB/RIF; low certainty of evidence for test accuracy for Xpert Ultra). |
| In children with signs and symptoms of extrapulmonary tuberculosis, Xpert Ultra may be used in lymph node aspirate and lymph node biopsy as the initial diagnostic test rather than smear microscopy/culture (conditional recommendation, low certainty of evidence). |
| In children with presumed pulmonary tuberculosis and an initial Xpert Ultra‐negative result, in settings with a pretest probability of 5% or more, the WHO recommends a repeat Xpert Ultra test (for a total of two tests). Sputum and nasopharyngeal aspirate specimens may be used (conditional recommendation, very low certainty of evidence for test accuracy). |
| WHO Consolidated Guidelines (Module 5) 2022b |
| In children aged below 10 years with signs and symptoms of pulmonary tuberculosis, Xpert MTB/RIF Ultra should be used in gastric aspirate or stool specimens as the initial diagnostic test for tuberculosis and the detection of rifampicin resistance, rather than smear microscopy/culture and phenotypic drug susceptibility testing. |
aThe findings from Kay 2020 informed development of the guidelines. bThe findings from this review update informed development of the guidelines.
Target condition being diagnosed
There are four target conditions: active pulmonary tuberculosis, tuberculous meningitis, lymph node tuberculosis, and rifampicin resistance.
Tuberculosis
Tuberculosis is an infectious disease caused by bacteria within the M tuberculosis complex, most commonly M tuberculosis. Typically disseminated through the air, M tuberculosis predominantly affects the lungs, causing pulmonary tuberculosis, and less typically can cause disease in other organs of the body in extrapulmonary tuberculosis forms. For this review, we limited evaluation of extrapulmonary tuberculosis to lymph node tuberculosis and tuberculous meningitis. Lymph node tuberculosis is the most common form of extrapulmonary tuberculosis in children (Marais 2006e), and tuberculous meningitis results in the highest morbidity and mortality (Marais S 2010).
The natural history of tuberculosis in children is distinct from that in adults, due to more frequent progression to primary tuberculosis disease (Marais 2004). Children younger than five years of age are at particularly high risk of progression to tuberculous disease following infection, but the risk for older children and adolescents is also higher than in adults. Overall, it is estimated that 90% of tuberculous disease in young children occurs within one year of infection (Marais 2014). In addition to age, factors such as nutritional status, immune‐compromising conditions (e.g. HIV infection), bacillus Calmette‐Guérin (BCG)‐vaccination status, and genetic susceptibility contribute to children's risk of disease progression. Immediately following infection with M tuberculosis in a child, haematogenous spread (by way of the bloodstream) can occur. The period of highest risk for presentation with tuberculous meningitis and miliary tuberculosis is one to three months following primary infection. Children between six months and two years of age are at particularly high risk of these severe forms of tuberculous disease. Approximately 50% of children in this age range progress to tuberculous disease following infection, and 20% to 40% of those children will present with disseminated disease (Marais 2004; Marais 2014). Children younger than five years of age most commonly present with hilar lymph node forms of intrathoracic tuberculous disease. Older children and adolescents more commonly manifest adult‐type disease, including pleural tuberculosis and upper lobe consolidations (Marais 2004).
Laboratory confirmation of tuberculosis in children is challenging for two reasons. First, child tuberculosis most commonly represents as a primary disease process, without the formation of cavities (Marais 2006c). The number of acid‐fast bacilli (the presence of acid‐fast bacilli on a sputum smear or other specimen usually indicates tuberculous disease) present in forms of primary tuberculosis such as hilar lymph node or bronchial tuberculosis is substantially lower than the number present in a pulmonary cavity. Consequently, child tuberculosis is often referred to as 'paucibacillary', and it is more difficult to obtain the organisms needed to confirm disease via conventional smear (no longer recommended) or culture (Dunn 2016). Second, most children younger than six years of age lack the ability to expectorate sputum and are unable to voluntarily produce good‐quality specimens. Therefore, respiratory specimens are often obtained through sputum induction. As children swallow respiratory secretions, early‐morning gastric aspiration is another well‐established (yet still invasive) approach to specimen collection. In one study, the yield of three consecutive morning gastric aspirates was similar to the yield of one induced sputum specimen (Zar 2005). Nasopharyngeal aspiration for respiratory specimens is a less invasive mode of specimen collection (Zar 2012). Stool has also been studied as a child tuberculosis diagnostic specimen; although sensitivity has been lower than with traditional specimens, this specimen has great appeal because collection is non‐invasive and requires no training (Nicol 2014). Because laboratory diagnostics for tuberculosis perform poorly in children, algorithms involving signs, symptoms, tuberculosis exposure, HIV status, laboratory tests, and radiographic findings are commonly used to make a clinical diagnosis of child tuberculosis. However, these algorithms have been shown to perform differently across settings, and their sensitivity and specificity may be site‐specific (David 2017).
Rifampicin resistance
Rifampicin‐resistant tuberculosis is caused by M tuberculosis strains resistant to rifampicin, a critical first‐line tuberculosis drug (see Index test(s)). These strains may be susceptible or resistant to isoniazid (i.e. multidrug‐resistant (MDR) tuberculosis), or resistant to other first‐line or second‐line tuberculosis drugs (WHO Consolidated Guidelines (Module 4) 2020). People with drug‐resistant tuberculosis can transmit the infection to others. The drugs used to treat drug‐resistant tuberculosis are less potent and more toxic than the drugs used to treat drug‐susceptible tuberculosis. The WHO has issued recommendations that all individuals with MDR or rifampicin‐resistant tuberculosis, including those who are also resistant to fluoroquinolones, may benefit from all‐oral treatment regimens (WHO Consolidated Guidelines (Module 4) 2020).
Index test(s)
The index test is Xpert Ultra (Cepheid Inc, Sunnyvale, CA, USA). Xpert Ultra is a nucleic acid amplification test (NAAT) that functions as an automated closed system that performs real‐time polymerase chain reaction (PCR). Specimens are processed using Xpert Sample Reagent and are incubated for 15 minutes, after which the processed samples are pipetted into the cartridge. These tests can be run by operators (such as laboratory technicians and nurses) with minimal technical expertise. Within two hours, the test detects both live and dead M tuberculosis complex DNA and simultaneously recognizes mutations in the M tuberculosis gene encoding the beta subunit of the ribonucleic acid (RNA) polymerase (rpoB) gene, which is the most common site of M tuberculosis mutations leading to rifampicin resistance. Xpert Ultra uses the same platform (GeneXpert) as Xpert MTB/RIF. Xpert Ultra requires an uninterrupted and stable electrical power supply, temperature control, and yearly calibration of the cartridge modules. The WHO has published extensive guidance and practical information on implementing the test (WHO Operational handbook on tuberculosis 2021).
Xpert Ultra was designed to improve the sensitivity to detect M tuberculosis complex and reliability for detection of rifampicin resistance (WHO Operational handbook on tuberculosis 2021). To improve tuberculosis detection, Xpert Ultra incorporates two different multicopy amplification targets (IS6110 and IS1081) and a larger chamber for the PCR reaction. To improve rifampicin resistance detection, Xpert Ultra is based on melting temperature analysis. These revisions have resulted in an approximately 1‐log improvement in the lower limit of detection compared with Xpert MTB/RIF, as well as improved differentiation of certain silent mutations and improved detection of rifampicin resistance in mixed infections (Chakravorty 2017; WHO Operational handbook on tuberculosis 2021). At very low bacterial loads, Xpert Ultra can give a trace result (considered a positive bacteriologic result in children and people living with HIV), though trace does not provide a result for rifampicin susceptibility or resistance. Studies have found that the increase in Xpert Ultra sensitivity for tuberculosis detection has been accompanied by a decrease in specificity, and that Xpert Ultra may be more likely to identify M tuberculosis DNA from prior episodes of tuberculosis, particularly in people with a trace result (Dorman 2018; Mishra 2020). Despite clear guidance in children, Xpert Ultra trace results can complicate decision‐making, and clinical management of trace results is rarely straightforward.
Clinical pathway
Figure 1 presents an example of the clinical pathway and placement of the index test. A careful clinical history of tuberculosis exposure and symptoms is the first step in the diagnostic pathway for child tuberculosis. Children with household or other close and persistent exposure to a person with tuberculosis are at increased risk of tuberculosis infection and resultant progression to tuberculosis disease. All children with recent exposure to tuberculosis must be evaluated for clinical symptoms and for examination findings consistent with tuberculous disease. Additional testing depends on the context but may include chest radiography and a test of tuberculosis infection. Symptoms of tuberculosis disease generally persist for longer than two weeks and are unremitting (Marais 2005). The most common symptoms are cough, fever, decreased appetite, weight loss or failure to thrive, and fatigue or reduced playfulness. Symptoms of extrapulmonary tuberculosis are typically localized, and diagnostic findings are generally obtained from the site of disease (Figure 1). However, no symptom‐based diagnostic algorithms have been validated or shown to be reliable in multiple contexts. Symptom‐based diagnostic algorithms tend to perform poorly in children younger than three years of age and in HIV‐positive children: two populations at high risk for disease progression (Marais 2006d).
1.
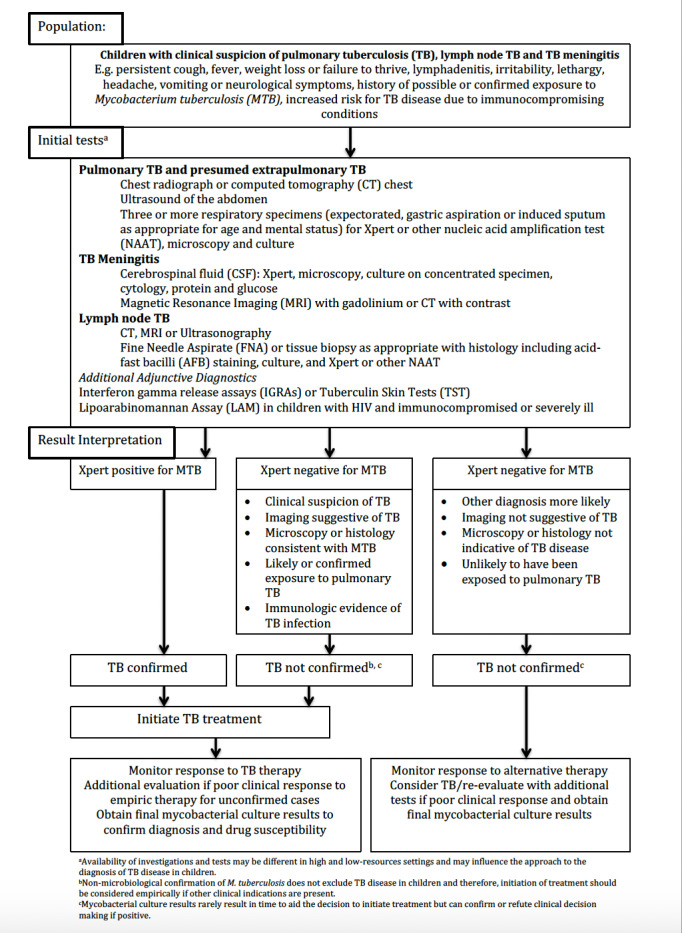
Clinical pathway of Xpert Ultra in children presumed to have tuberculosis
Unfortunately, no clinical examination features are specific to pulmonary tuberculosis in children. However, examination findings in extrapulmonary tuberculosis can be quite specific when identified. Clinicians should consider medical comorbidities that increase the risk for tuberculous disease, and should modify diagnostic algorithms accordingly. HIV infection not only significantly increases risk of tuberculosis in children, it also raises the risk of increased disease severity. HIV‐positive children, especially before effective antiretroviral therapy is established, often present with advanced tuberculosis, such as disseminated disease, and have high levels of immunosuppression, further complicating diagnosis and management.
Additional diagnostic imaging studies can assist in the diagnosis of pulmonary tuberculosis and nearly all forms of extrapulmonary tuberculosis. Tests for tuberculosis infection, such as interferon gamma release assays or tuberculin skin tests, can also aid in establishing the probability of tuberculosis (disease) in a child but are not necessary to make the diagnosis. Diagnostic recommendations strongly suggest collecting appropriate specimens from suspected sites of involvement in both pulmonary and extrapulmonary tuberculosis for microbiological examination. The preferred specimen in pulmonary tuberculosis is sputum; however, in young children who cannot expectorate, the specimen is commonly obtained via a gastric aspirate or induced sputum, and stool is increasingly used. To diagnose extrapulmonary tuberculosis, sample collection targets the affected site of disease.
The purpose of Xpert Ultra testing is diagnosis of pulmonary and extrapulmonary tuberculosis and detection of rifampicin resistance. Results of Xpert Ultra can be used as a decision‐making tool in the following ways.
M tuberculosis detected and rifampicin resistance not detected: child would start treatment for drug‐sensitive tuberculosis.
M tuberculosis detected and rifampicin resistance detected: child would need further testing for drug resistance and would start treatment for drug‐resistant tuberculosis according to country guidelines.
M tuberculosis not detected: a negative Xpert Ultra result does not rule out tuberculosis disease; therefore, clinicians should still consider initiation of tuberculosis treatment in children with history and clinical or radiological features suggestive of tuberculosis disease despite a negative Xpert Ultra result. A negative Xpert Ultra result may also represent a true negative.
Possible consequences of a false‐positive and a false‐negative result may include the following.
False positive: children (and their families) would likely experience anxiety and morbidity caused by additional testing, unnecessary treatment, and possible adverse effects; as well as missed time from school, possible stigma associated with tuberculosis or a diagnosis of drug‐resistant tuberculosis, and the chance that a false‐positive may halt further diagnostic evaluation for other causes of illness. Families also experience unnecessary expense, as well as the risk of missing an important alternative diagnosis.
False negative: would imply increased risk of morbidity and mortality and delayed start of treatment.
Role of index test(s)
For tuberculosis detection, the index test would be used as an initial test, replacing standard practice (i.e. smear microscopy or culture). For detection of rifampicin resistance, the index test would replace culture‐based drug susceptibility testing as the initial test.
Alternative test(s)
Here we summarize selected alternative tests.
Truenat technologies (Molbio Diagnostics, Goa, India) are rapid molecular assays that can detect tuberculosis (Truenat MTB and MTB Plus assays) and rifampicin resistance (Truenat MTB‐RIF Dx assay) from sputum specimens with results reported in less than one hour (WHO Operational handbook on tuberculosis 2021). Truenat MTB and MTB Plus assays use chip‐based PCR for detection of M tuberculosis complex; if a result is positive, a sample of the already extracted DNA may be run on the chip‐based Truenat MTB‐RIF Dx assay to detect mutations associated with rifampicin resistance (WHO Operational handbook on tuberculosis 2021). The assays use portable, battery‐operated devices. The WHO includes Truenat assays in the category 'molecular WHO‐recommended rapid diagnostic tests that can detect tuberculosis (mWRD)' and recommends their use as follows (WHO Consolidated Guidelines (Module 3) 2021).
In adults and children with signs and symptoms of pulmonary tuberculosis, the Truenat MTB or MTB Plus may be used as an initial diagnostic test for tuberculosis rather than smear microscopy/culture (conditional recommendation, moderate certainty of evidence for test accuracy).
In adults and children with signs and symptoms of pulmonary tuberculosis and a Truenat MTB or MTB Plus positive result, Truenat MTB‐RIF Dx may be used as an initial test for rifampicin resistance rather than culture and phenotypic drug susceptibility testing (conditional recommendation, very low certainty of evidence for test accuracy).
Additional alternative approaches for diagnosis of tuberculosis are still used extensively world. Main tests include examination of smear for acid‐fast bacilli (tuberculosis bacteria) under a microscope (light microscopy, using the classical Ziehl‐Neelsen staining technique), fluorescence microscopy, and light‐emitting diode (LED)‐based fluorescence microscopy (no longer recommended by the WHO for diagnosis but used for monitoring in adults). The sensitivity of smear microscopy ranges from 0% to 10% in children (Kunkel 2016). Examination of histology specimens under a microscope following a tissue biopsy targets acid‐fast bacilli and granulomatous inflammation, frequently with caseous necrosis (necrotizing granulomas); however these options are seldom pursued to diagnose child tuberculosis in low‐resource settings due to the invasive nature of the procedures and the technical expertise required.
Lipoarabinomannan (LAM) antigen is a lipopolysaccharide present in the mycobacterial cell wall that can be detected in the urine of people with tuberculous disease (Bjerrum 2019). This urine test offers potential advantages over sputum‐based testing due to ease of sample collection. The accuracy of urinary LAM detection is improved among people living with HIV with advanced immunosuppression (Bjerrum 2019;; Nicol 2014; Shah 2016a). One Cochrane Review found that in inpatient settings, the use of lateral flow (LF)‐LAM as part of a tuberculosis diagnostic testing strategy likely reduces mortality and probably results in a slight increase in tuberculosis treatment initiation in people living with HIV (Nathavitharana 2021). The WHO recommends that LF‐LAM (Alere Determine™ TB LAM Ag, Alere Inc, Waltham, MA, USA), the only product available at the time of this recommendation, should be used to assist in the diagnosis of tuberculosis disease in HIV‐positive adults, adolescents, and children. The full recommendations, which differ for inpatients and outpatients, are described in the WHO Consolidated guidelines for rapid diagnostics for tuberculosis detection (WHO Consolidated Guidelines (Module 3) 2021). However, the evidence for LF‐LAM in children is limited and is primarily extrapolated from adults. A new urinary, point‐of‐care LAM test, Fujifilm SILVAMP TB LAM (FujiLAM, co‐developed by FIND, Geneva, Switzerland, and Fujifilm, Tokyo, Japan), for diagnosis of tuberculosis, is currently under investigation and has the potential to increase sensitivity in children (Broger 2019).
Line probe assays are a category of molecular tests for drug‐resistant tuberculosis that offer speed of diagnosis (one or two days), standardized testing, and potential for high through‐put. Drawbacks are that line probe assays require skills and infrastructure only available in intermediate and central laboratories. Line probe assays for first‐line drugs (which include rifampicin) include GenoType MTBDRplus assay (MTBDRplus, Bruker‐Hain Lifescience, Nehren, Germany), and the Nipro NTM+MDRTB detection kit 2 (Nipro, Tokyo, Japan). These assays detect the presence of mutations associated with drug resistance to isoniazid and rifampicin. MTBDRplus is the most widely studied line probe assay. The WHO recommends that for people with a sputum smear‐positive specimen or a culture isolate of M tuberculosis complex, commercial molecular line probe assays may be used as the initial test instead of phenotypic drug susceptibility testing to detect resistance to rifampicin and isoniazid (conditional recommendation, moderate certainty in the evidence for the test's accuracy; WHO Consolidated Guidelines (Module 3) 2021).
The quest for novel and more efficient technologies for diagnosis of tuberculosis is a cornerstone of current efforts to reduce the burden of disease worldwide. Over the past decade, unprecedented activity has focused on the development of new tools for diagnosis of extrapulmonary tuberculosis, largely supported by the engagement of global agencies. As a result, a strong pipeline of new tools for diagnosis of tuberculosis will complement the use of existing ones and will offer improved options. 'The Tuberculosis Diagnostics Pipeline Report: Advancing the Next Generation of Tools' describes tuberculosis tests in development (Branigan 2021).
Rationale
Timely and reliable diagnosis of tuberculosis in children remains challenging due to both difficulties in collecting sputum samples and the paucibacillary nature of the disease. Under‐diagnosis may lead to increased morbidity, mortality, and disease transmission in this key group.
Our previously published Cochrane Review assessed the accuracy of both Xpert MTB/RIF and Xpert Ultra (Kay 2020). We limited the current review update to the diagnostic accuracy of Xpert Ultra for several reasons. Xpert Ultra has superseded Xpert MTB/RIF, and the manufacturer will be discontinuing Xpert MTB/RIF in most countries in 2023. Given the available evidence about Xpert MTB/RIF from our previous review, we therefore only updated Xpert Ultra as requested by the WHO. The Xpert MTB/RIF text and analyses are available in the last published version of the review (Kay 2020).
Regarding Xpert Ultra, in the original Cochrane Review, we identified few published studies: three studies on Xpert Ultra in sputum (697 participants) and no studies in gastric aspirate and stool specimens. In addition, we had limited data in children younger than 10 years of age, an area of considerable interest for the WHO.
In the current review update, we aimed to determine the diagnostic accuracy of Xpert Ultra for pulmonary tuberculosis, tuberculosis meningitis, lymph node tuberculosis, and rifampicin resistance in children. Parts of the review update, particularly the analyses of gastric aspirate and stool specimens, were used to inform the 2022 WHO updated guidance on the management of tuberculosis in children and adolescents (WHO Consolidated Guidelines (Module 5) 2022; see Table 3).
Objectives
To assess the diagnostic accuracy of Xpert Ultra for detecting: pulmonary tuberculosis, tuberculous meningitis, lymph node tuberculosis, and rifampicin resistance, in children with presumed tuberculosis.
Secondary objectives
To investigate potential sources of heterogeneity in accuracy estimates. For detection of tuberculosis, we considered age, comorbidity (HIV, severe pneumonia, and severe malnutrition), and specimen type as potential sources.
To summarize the frequency of Xpert Ultra trace results.
Methods
Criteria for considering studies for this review
Types of studies
We included cross‐sectional studies, cohort studies, and randomized controlled trials (RCTs) from all settings. We included RCTs that evaluated use of the test for patient health outcomes but also reported sensitivity and specificity. Although we utilized RCTs for the purpose of determining the impact of the test versus a comparator (e.g. usual practice, another test) on health outcomes, the study design was interpreted as a cross‐sectional design for the purpose of determining diagnostic accuracy for the index tests in this review. We included only studies from which we could extract or derive data on the index test giving true positives, false positives, true negatives, or false negatives, as assessed against the reference standards specified below. We included abstracts with sufficient data. In addition, we included ongoing studies that helped us to address the review objectives (see Data collection and analysis). For each of the ongoing studies, we recorded the stage of the study at the time of data extraction for this review (e.g. recruitment completed, recruitment completed and data cleaned, or recruitment ongoing and number (%) of the target sample size recruited) in the Characteristics of included studies table. We excluded case‐control studies and case reports.
Participants
We included studies that evaluated the index tests for pulmonary or extrapulmonary tuberculosis in HIV‐positive and HIV‐negative children and young adolescents aged 0 to 14 years (collectively referred to as children), presumed to have tuberculosis. Studies were eligible for inclusion if they described the use of Xpert Ultra on routine respiratory specimens such as expectorated or induced sputum and gastric and nasopharyngeal specimens. Gastric specimens could be obtained via gastric aspiration, lavage, or washing, as described by study authors. In addition, we included studies evaluating stool specimens, because tuberculosis bacilli are present in swallowed sputum and are recoverable from stool samples using Xpert Ultra. We also included studies that assessed several different specimen types.
Index tests
The index test was Xpert Ultra.
Index test results are automatically generated, and the user is provided with a printable test result as follows.
MTB (M tuberculosis) DETECTED HIGH; RIF (rifampicin) Resistance DETECTED.
MTB DETECTED MEDIUM; RIF Resistance DETECTED.
MTB DETECTED LOW; RIF Resistance DETECTED.
MTB DETECTED VERY LOW; RIF Resistance DETECTED.
MTB DETECTED HIGH; RIF Resistance NOT DETECTED.
MTB DETECTED MEDIUM; RIF Resistance NOT DETECTED.
MTB DETECTED LOW; RIF Resistance NOT DETECTED.
MTB DETECTED VERY LOW; RIF Resistance NOT DETECTED.
MTB DETECTED HIGH; RIF Resistance INDETERMINATE.
MTB DETECTED MEDIUM; RIF Resistance INDETERMINATE.
MTB DETECTED LOW; RIF Resistance INDETERMINATE.
MTB DETECTED VERY LOW; RIF Resistance INDETERMINATE.
MTB Trace DETECTED; RIF Resistance INDETERMINATE.
INVALID (the presence or absence of MTB cannot be determined).
ERROR (the presence or absence of MTB cannot be determined).
NO RESULT (the presence or absence of MTB cannot be determined).
Xpert Ultra incorporates a semi‐quantitative classification for results: trace, very low, low, moderate, and high. Trace corresponds to the lowest bacterial burden for detection of M tuberculosis (Chakravorty 2017). Although no rifampicin resistance results are available for people with trace results, a trace‐positive result is sufficient to initiate tuberculosis therapy in children or people living with HIV, according to the WHO (WHO Consolidated Guidelines (Module 3) 2021). Hence, we considered a trace result to mean M tuberculosis DETECTED.
Target conditions
The target conditions were active pulmonary tuberculosis; two forms of extrapulmonary tuberculosis, tuberculous meningitis and lymph node tuberculosis; and rifampicin resistance.
Reference standards
For detection of pulmonary tuberculosis, tuberculous meningitis, and lymph node tuberculosis, we included two reference standards (see below regarding stool samples).
Culture: tuberculosis was defined as a positive culture on solid or liquid medium from a respiratory sample.
Composite reference standard: tuberculosis was defined as a positive culture or a clinical decision, based on clinical features, to initiate treatment for tuberculosis (i.e. clinically diagnosed tuberculosis). Clinical features might include cough longer than two weeks, fever, or weight loss; pneumonia that did not improve with antibiotics; or a history of close contact with an adult who had tuberculosis.
For the composite reference standard, in the absence of information on tuberculosis treatment, we accepted a study‐specific definition (i.e. a standardized definition of tuberculosis defined by the primary study authors), if available. We also accepted the uniform research definition (Graham 2012; Graham 2015). In these situations, for the older definition (Graham 2012), we defined tuberculosis as 'confirmed, probable, and possible' and not tuberculosis as 'unlikely and not tuberculosis'. For the newer definition (Graham 2015), we defined tuberculosis as 'confirmed and unconfirmed' and not tuberculosis as 'unlikely'.
We included children with unconfirmed tuberculosis in the true‐negative population when evaluating results against a culture reference standard. In contrast, we included children who were not treated for tuberculosis, or who did not meet the study research definition for tuberculosis, in the true‐negative population when evaluating results against a composite reference standard.
Regarding stool specimens (used for the diagnosis of pulmonary tuberculosis), we defined the reference standard similar to MacLean 2019: (1) culture, or (2) Xpert Ultra performed on a routine respiratory specimen, such as sputum or gastric aspirate specimen. We did not include stool Xpert Ultra results in the definition of the reference standard. In addition, none of the included studies used stool culture to verify pulmonary tuberculosis. For these reasons, we thought bias due to incorporation of the index test was unlikely. Hence, tuberculosis was defined as a positive culture or a positive Xpert Ultra on a routine respiratory specimen.
Regarding stool specimens, we also included a composite reference standard as defined above.
Culture is generally considered the best reference standard for tuberculosis diagnosis. However, particularly in children with paucibacillary disease, tuberculosis is verified by culture in only 15% to 50% of cases, depending on disease severity, challenges of obtaining specimens, and resources (Graham 2015). Evaluation of multiple specimens, of the same or different types, may increase the yield of culture for confirming tuberculosis (Cruz 2012;Zar 2012). Therefore, we considered a higher‐quality reference standard to be one in which more than one specimen was used to confirm tuberculosis. We considered a lower‐quality reference standard to be one in which only one specimen was used for tuberculosis diagnosis. We reflected these considerations in the Quality Assessment of Studies of Diagnostic Accuracy – Revised (QUADAS‐2) reference standard domain.
For rifampicin resistance, the reference standards were phenotypic drug susceptibility testing and MTBDRplus. MTBDRplus is a molecular line probe assay designed to detect the presence of multiple mutations causing resistance to isoniazid and rifampicin.
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
We searched the following databases up to 9 March 2021 using the search terms and strategy described in Appendix 1.
Cochrane Infectious Diseases Group Specialized Register.
MEDLINE (Ovid, from 1966).
Embase (Ovid, from 1974).
Cumulative Index to Nursing and Allied Health Literature (CINAHL (EBSCOHost), from 1982).
Science Citation Index – Expanded (from 1900), Conference Proceedings Citation Index – Science (CPCI‐S, from 1990), from the Web of Science (Clarivate Analytics).
Scopus (Elsevier, from 1970).
We also searched ClinicalTrials.gov (clinicaltrials.gov), the WHO International Clinical Trials Registry Platform (ICTRP; who.int/clinical-trials-registry-platform), and the International Standard Randomized Controlled Trials Number (ISRCTN) Registry (www.isrctn.com) for trials in progress, up to 9 March 2021.
Searching other resources
We contacted researchers and experts in the field to identify additional eligible studies. This included sharing the list of included and excluded studies with the WHO Guideline Development Group on the management of tuberculosis in children and adolescents prior to a preparatory webinar for input and feedback. We also checked the references of relevant reviews and studies to identify additional studies.
Data collection and analysis
Selection of studies
We used Covidence to manage the selection of studies (Covidence). Two review authors (AWK and TN, or AWK and LFG) independently screened all titles and abstracts to identify potentially eligible studies. We then obtained the full‐text articles of potentially eligible studies, and two review authors (AWK and TN, or AWK and LFG) independently assessed whether they should be included based on predefined inclusion and exclusion criteria. We resolved disagreements by discussion or by consulting a third review author (AMM or KRS), if necessary. We contacted primary study authors for clarification of methods and other information, as needed. We recorded and summarized reasons for excluding studies in the Characteristics of excluded studies table. We illustrated the study selection process in a PRISMA diagram (Page 2021).
Data extraction and management
We designed a data extraction form and piloted it on two included studies (Appendix 2). We then finalized the form based on the pilot test. Two review authors (AWK and TN or AWK and LFG) independently extracted data using this data extraction form and discussed inconsistencies to achieve consensus. We consulted a third review author (AMM or KRS) to resolve discrepancies, as needed. We entered abstracted data into Google sheets on password‐protected computers. We secured the data set in a cloud storage workspace and we stored extracted data for future review updates. Selected details of data extraction are listed below.
Study details
Number of participants after screening for exclusion and inclusion criteria
Total number of children included in the analysis
Specimen collection methods
Unit of sample collection: one specimen, multiple specimens, unknown, or unclear
Target condition(s)? – pulmonary tuberculosis, tuberculous meningitis, lymph node tuberculosis, rifampicin resistance
For ongoing studies, we recorded the stage of the study at the time of data extraction for this review (e.g. recruitment completed, recruitment completed and data cleaned, or recruitment ongoing and number (%) of the target sample size recruited) in the Characteristics of included studies table.
Patient characteristics and setting
Description of study population
Age: median, mean, range
Sex
HIV status
Percentage and number of HIV‐positive or HIV‐negative participants, if both were included in the study
Type of respiratory specimen included: sputum, gastric aspirate or lavage, stool, nasopharyngeal aspirate
Type of non‐respiratory specimen included: cerebrospinal fluid, fine‐needle aspirate, lymph node biopsy, multiple types, other, unknown
Number of cultures performed per child to exclude tuberculosis
Data on culture performance: number of contaminated cultures with respect to total cultures performed
Clinical setting: outpatient, inpatient, or both
Description of radiographic findings
Information on tuberculosis burden in the country
We classified countries as being high‐burden or not high‐burden for tuberculosis, HIV‐associated tuberculosis, and MDR or rifampicin‐resistant tuberculosis based on the WHO classification for 2021 to 2025 (WHO Global Tuberculosis Report 2021). A country may be classified as high‐burden for one, two, or all three of the high‐burden categories.
We contacted the authors of all included studies for data on specific age ranges and subpopulations and for clarification on study characteristics.
Index test
Pretreatment processing procedure for specimens used for Xpert Ultra
Specimen condition: fresh, frozen, or both
Numbers of true positives, false positives, false negatives, and true negatives by age group (all ages, under one year, one to four years, five to nine years, 10 to 14 years, and birth to 9 years; (see example tables in Appendix 3)
Uninterpretable results for tuberculosis detection (invalid, error, or no result)
Indeterminate results for detection of rifampicin resistance
Xpert Ultra trace results
Reference standards
Details of culture: solid or liquid
Composite reference standard
Rifampicin resistance: phenotypic drug susceptibility testing or MTBDRplus
For each target condition and specimen type, we considered one index test result per child. Hence, the primary unit of analysis was the child. If studies evaluated more than one specimen type, we extracted data for each specimen. Hence, a study may have contributed more than one 2 × 2 table (data set): one for each type of specimen evaluated.
Assessment of methodological quality
We assessed the methodological quality of included studies using the QUADAS‐2 instrument, which we adapted for this review (Whiting 2011). The QUADAS‐2 tool consists of four domains:
patient selection;
index test(s);
reference standard(s); and
flow and timing.
All domains are assessed for risk of bias, and the first three domains are assessed for concerns regarding applicability. We first developed guidance on how to appraise each signalling question within the domains and how to make the overall judgement for each domain. One review author piloted the tool with two of the included studies. We finalized the guidance based on experience gained from the pilot. Appendix 4 presents the QUADAS‐2 tool with signalling questions tailored to this review. Two review authors (AK and LFG or AK and TN) independently completed QUADAS‐2. We resolved disagreements through discussion or by arbitration with a third review author (KRS or AMM), when necessary. We presented results of the quality assessment in the text, in tables, and in graphs.
Statistical analysis and data synthesis
We performed descriptive analyses of the included studies and presented their key characteristics in the Characteristics of included studies table. We stratified all analyses by type of specimen and type of reference standard. We presented individual study estimates of sensitivity and specificity graphically in forest plots and in receiver operating characteristics (ROC) space using Review Manager 5 (Review Manager 2020).
When data were sufficient, we performed meta‐analyses to estimate average sensitivities and specificities using a bivariate model (Chu 2006; Reitsma 2015). We used the bivariate model because the index test, Xpert Ultra, applies a common positivity criterion (Macaskill 2010). When we were unable to fit a bivariate model due to sparse data, few studies, or limited variability in specificity, we simplified the model to a univariate random‐effects or fixed‐effect logistic regression model to pool sensitivity and specificity separately, as appropriate given the observed data (Takwoingi 2015). We performed meta‐analyses using the meqrlogit command for models that included random effects and the blogit command for fixed‐effect meta‐analyses in Stata version 16 (Stata 16). Meta‐analysis using univariate fixed‐effect or random‐effects logistic regression models is not possible when all studies in a meta‐analysis report 100% specificity. For such analyses, we calculated summary specificity by dividing the total number of non‐cases by the total number of true negatives, and we computed the 95% confidence interval (CI) using the Wilson method (Newcombe 1998).
Approach to non‐determinate and trace index test results
Non‐determinate Xpert Ultra test results include 'Error', 'Invalid', and 'No Result', and may be due to an operator error, instrument, or cartridge issue. For each included study that reported the number of non‐determinate results for tuberculosis detection, we estimated the proportion of non‐determinate Xpert Ultra results. As recommended by the WHO, trace results were included in the primary analyses as Xpert Ultra‐positive results. For each included study that provided data on trace results, we calculated the percentage of test positives that were trace results (i.e. number of trace results/number of test positives).
Investigations of heterogeneity
We visually inspected forest and summary ROC (SROC) plots for heterogeneity. When data allowed, we evaluated sources of heterogeneity using subgroup analyses. We were unable to perform meta‐regression because of the number of studies available. For tuberculosis detection, we investigated key subgroups of children: aged under 1 year, aged 1 to 4 years, aged 5 to 9 years, aged 10 to 14 years, HIV‐positive, HIV‐negative, with severe pneumonia, and with severe malnutrition.
Sensitivity analyses
We performed sensitivity analyses excluding data from ongoing studies in the primary analyses.
Assessment of reporting bias
We did not formally assess reporting bias using funnel plots or regression tests because these have not been reported as helpful for diagnostic test accuracy studies (Macaskill 2010).
Assessment of certainty of the evidence
We assessed certainty of the evidence using the GRADE approach for diagnostic studies (Balshem 2011; Schünemann 2008; Schünemann 2016). As recommended, we rated certainty of the evidence as high (not downgraded), moderate (downgraded one level), low (downgraded two levels), or very low (downgraded more than two levels) based on five domains: risk of bias, indirectness, inconsistency, imprecision, and publication bias. For each outcome, certainty of the evidence started as high when high‐quality studies (cross‐sectional or cohort studies) enrolled participants with diagnostic uncertainty. If we found a reason for downgrading, we used our judgement to classify the reason as serious (downgraded one level) or very serious (downgraded two levels).
Three review authors (AWK, TN, and KRS) discussed judgements and applied GRADE (Schünemann 2020a; Schünemann 2020b).
Risk of bias
We used QUADAS‐2 to assess risk of bias.
Indirectness
We assessed indirectness in relation to the population (including disease spectrum), setting, interventions, and outcomes (accuracy measures). We also used prevalence (proportion) of the target condition in the included studies as a guide to whether there was indirectness in the population.
Inconsistency
GRADE recommends downgrading for unexplained inconsistency in sensitivity and specificity estimates. We carried out pre‐specified analyses to investigate potential sources of heterogeneity and downgraded when we could not explain inconsistency in accuracy estimates. We looked at the individual point estimates in the forest plots and judged whether they were more or less the same, as well as the CIs to see if they overlapped.
Imprecision
We considered the width of the 95% CI. In addition, we determined projected ranges for two categories of test results that have the most important consequences for patients – the number of false negatives and the number of false positives – and made judgements on imprecision from these calculations. Imprecision also depends on the number of participants included to determine sensitivity and specificity. We took note of the uncertainty around point estimates along with the number of participants providing those data. We acknowledge the judgement of imprecision is subjective.
Publication bias
We considered the comprehensiveness of the literature search and outreach to researchers in tuberculosis, the presence of only studies that produce precise estimates of high accuracy despite small sample size, and knowledge about studies that were conducted but not published.
The summary of findings tables include the following details:
The review question and its components, population, setting, index test, and reference standards.
Summary estimates of sensitivity and specificity with 95% CIs.
The number of included studies and participants contributing to the estimates of sensitivity and specificity.
Prevalences of the target condition with an explanation of why the prevalences have been chosen.
An assessment of the certainty of the evidence (GRADE).
Explanations for downgrading, as needed.
Results
Results of the search
We identified 2174 records through database searches conducted up to 29 April 2019. An updated search to 9 March 2021 identified 115 records. We found one additional record by contacting researchers at the Foundation for Innovative New Diagnostics (FIND). After removing duplicates, we screened 51 records by title and abstract. We excluded 35 of these, leaving 16 reports, which we retrieved for full text review. We identified 14 unique studies (including one from a source outside of our database searches), integrating 11 new studies since publication of the Cochrane Review (Kay 2020). All studies were written in English. Figure 2 shows the flow of studies in the review. We recorded the excluded studies, including those listed in the previous Cochrane Review (Kay 2020), with reasons for their exclusion in the Characteristics of excluded studies table.
2.

Study flow diagram. aKay 2020. bStudies only evaluated Xpert MTB/RIF and other reasons: not a diagnostic study; study did not include children; case‐control study; abstract; index test not studied.
Description of included studies
We describe key characteristics in the Characteristics of included studies table and Table 4. All were cross‐sectional or cohort studies, with the exception of one, which had an unclear study design. The studies were conducted in both inpatient and outpatient settings; seven took place in tuberculosis high‐burden countries.
2. Key characteristics of included studies.
| Study | Reference standard | Study design | HIV status | Clinical setting | High tuberculosis burden | Type of specimens | Xpert Ultra non‐determinatea% (number/total) | Xpert Ultra traceb% (number) |
| Barcellini 2019 | Culture | Cross‐sectional | Negative | Outpatient | No | Sputum | None | None |
| Jaganath 2021 | Culture, composite |
Cohort | Both | Both | No | Sputum, gastric, nasopharyngeal | Not reported | Sputum: 12% (2); gastric: 67% (2); nasopharyngeal: 40% (2) |
| Kabir 2020 | Culturec, composite |
Cross‐sectional | Yes | Inpatient | Yes | Sputum, stool | < 1% (1/446) | Sputum: 39% (11); stool: 80% (48) |
| Liu 2021 | Culturec | Cohort | Negative | Both | Yes | Sputum, gastric, stool, nasopharyngeal | Not reported | Sputum: 0%; gastric: 30% (8); stool: 38.% (16); nasopharyngeal: 0% |
| NCT04121026 | Culture | Cohort | Positive | Both | Yes | Sputum, gastric, stool, nasopharyngeal | 4% (5/114) | Sputum: 0%; gastric: 25% (1); nasopharyngeal: 0%; stool: 0% |
| NCT04203628 | Culture | Cohort | Both | Both | Yes | Stool | 3% (2/76) | Stool: 40% (2) |
| NCT04240990 | Culture | Cohort | Both | Inpatient | Yes | Gastric, stool, nasopharyngeal | 1% (2/237) | Stool: 60% (3) |
| NCT04899076 | Culturec | Cohort | Both | Both | Yes | Stool | 10% (42/434) | Stool: 39% (12) |
| Nicol 2018 | Culture, composite |
Cohort | Both | Inpatient | Yes | Sputum | 11% (50/453) | Sputum: 26% (8) |
| Parigi 2021 | Culture, composite | Unclear | Not reported | Inpatient | No | Gastric | Not reported | NA |
| Sabi 2018 | Culture, composite |
Cohort | Both | Both | Yes | Sputum | 0% (0/215) | Sputum: 19% (3) |
| Ssengooba 2020 | Culture | Cohort | Both | Unclear | No | Sputum, gastric | Not reported | Sputum: 67% (4); gastric: 57% (13) |
| Sun 2020 | Culture, composite |
Cohort | Not reported | Unclear | Yes | Gastric | Not reported | Gastric: 26% (20) |
| Zar 2019 | Culture, composite |
Cohort | Both | Inpatient | Yes | Nasopharyngeal | Not reported | Nasopharyngeal: 45% (9) |
aNon‐determinate results are Error, Invalid, or No Result. bCalculated as percentage of total number of positive tests. cFor stool, Xpert on respiratory specimens was accepted as part of the reference standard.
For pulmonary tuberculosis, 108 data sets (20,407 participants) were available for analysis; for rifampicin resistance, three data sets (131 participants) were available.
We did not identify any studies that evaluated Xpert Ultra accuracy for tuberculous meningitis or lymph node tuberculosis.
Methodological quality of included studies
Pulmonary tuberculosis
Figure 3 and Appendix 5 show risk of bias and applicability concerns for 14 studies that evaluated Xpert Ultra for detection of pulmonary tuberculosis.
3.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
In the patient selection domain, we considered 13 studies (93%) at low risk of bias because they enrolled a consecutive or random sample of eligible participants and avoided inappropriate exclusions. We considered one study (7%) at unclear risk of bias because the manner of patient sampling was unclear (Parigi 2021). With respect to applicability, we considered 10 studies (71%) of low concern because participants in these studies were evaluated in primary care facilities, in local hospitals, or in both settings (Barcellini 2019; Jaganath 2021; Liu 2021; NCT04121026; NCT04203628; NCT04240990; NCT04899076; Nicol 2018; Sabi 2018; Ssengooba 2020). We considered three studies (21%) of high concern because participants were evaluated exclusively as inpatients in tertiary care centres (Kabir 2020; Parigi 2021; Zar 2019). We considered one study of unclear concern because we were unsure about the clinical setting (Sun 2020).
In the index test domain, we considered all studies at low risk of bias. With respect to applicability, we considered eight studies (73%) to of low concern (Barcellini 2019; Jaganath 2021; Nicol 2018; Parigi 2021; Sabi 2018; Ssengooba 2020; Sun 2020; Zar 2019). We considered all studies that evaluated stool specimens of unclear concern because of the absence of an established protocol for stool processing before Xpert Ultra testing.
In the reference standard domain, we considered 13 studies (93%) at low risk of bias and one study at unclear risk of bias because the ability of the reference standard to appropriately classify child tuberculosis was uncertain (Kabir 2020). With respect to applicability, we considered nine (64%) studies of low concern because speciation was performed, confirming M tuberculosis instead of other mycobacterial species (Barcellini 2019; Jaganath 2021; NCT04121026; NCT04203628; NCT04899076; Nicol 2018; Sabi 2018; Ssengooba 2020; Zar 2019, and five studies of unclear concern because we could not tell whether speciation was performed (Kabir 2020; Liu 2021; NCT04240990; Parigi 2021; Sun 2020).
In the flow and timing domain, we considered 13 studies (93%) at low risk of bias because all participants were included in the analysis. We considered one study at high risk of bias (Liu 2021) because most enrolled children were not included in the analysis.
Rifampicin resistance
In the patient selection domain, we judged two studies at low risk of bias (Liu 2021; Parigi 2021), and one study at unclear risk of bias because the manner of patient selection was not reported (Parigi 2021). Regarding applicability, in the patient selection domain we had low concern for one study (Liu 2021), and high concern for two studies because all patients were recruited from an inpatient setting (Parigi 2021; Zar 2019). In the index test and reference standard domains, we judged all studies at low risk of bias and of low concern regarding applicability. In the flow and timing domain, we judged one study at high risk of bias because not all participants were included in the analysis (Liu 2021).
Findings
I. Detection of pulmonary tuberculosis
Due to little observed variability in specificity and in the volume of analyses, we chose to present only forest plots, as such plots were more informative than corresponding SROC plots.
Xpert Ultra for pulmonary tuberculosis
Xpert Ultra in sputum specimens
Culture reference standard
Eight studies (1216 participants) evaluated Xpert Ultra in sputum specimens against culture (Barcellini 2019; Jaganath 2021; Kabir 2020; Liu 2021; NCT04121026; Nicol 2018; Sabi 2018; Ssengooba 2020). Xpert Ultra sensitivity ranged from 62% to 89%, and specificity from 86% to 100% (Figure 4). Three studies did not contribute data to the meta‐analysis because sensitivity was not estimable (Barcellini 2019; Liu 2021; NCT04121026). In the remaining five studies (1181 participants), Xpert Ultra summary sensitivity was 75.3% (95% CI 64.3 to 83.8), and summary specificity was 97.1% (95% CI 94.7 to 98.5) (Table 5).
4.

Forest plots of Xpert Ultra sensitivity and specificity in sputum for pulmonary tuberculosis in children by type of specimen and reference standard. The squares represent the sensitivity and specificity of each study, the black lines their confidence intervals (CIs). FN: false negative; FP: false positive; TN: true negative; TP: true positive.
3. Xpert Ultra summary sensitivity and specificity for pulmonary tuberculosis, by type of specimen.
| Analysis group | Reference standard | Studies | Number of children (TB cases) | Summary sensitivity % (95% CI) | Summary specificity % (95% CI) | Positive predictive value % (95% CI)a | Negative predictive value % (95% CI)a |
| Sputum | Culture | 5 | 1181 (127) | 75.3 (64.3 to 83.8) | 97.1 (94.7 to 98.5) | 74.4 (61.9 to 84.0) | 97.3 (95.9 to 98.1) |
| Sputum | Composite | 5 | 1108 (527) | 23.5 (20.1 to 27.3) | 99.8 (98.8 to 100) | 93.8 (68.1 to 99.1) | 92.1 (91.8 to 92.5) |
| Gastric aspirate | Culture | 7 | 990 (120) | 70.4 (53.9 to 82.9) | 94.1 (84.8 to 97.8) | 56.9 (34.9 to 76.6) | 96.7 (94.6 to 97.8) |
| Gastric aspirate | Composite | 4 | 448 (229) | 46.5 (29.7 to 64.1) | 98.4 (91.4 to 99.7) | 76.9 (31.6 to 96.0) | 94.3 (92.1 to 95.9) |
| Stool | Culture | 6 | 1432 (200) | 56.1 (39.1 to 71.7) | 98.0 (93.3 to 99.4) | 75.3 (45.5 to 91.7) | 95.2 (93.2 to 96.8) |
| Stool | Composite | 2 | 572 (199) | 50.3 (43.3 to 57.1) | 99.5 (97.9 to 99.9) | 91.2 (72.2 to 97.7) | 94.7 (93.9 to 95.4) |
| Nasopharyngeal aspirate | Culture | 4 | 535 (46) | 43.7 (26.7 to 62.2) | 97.5 (93.6 to 99.0) | 65.8 (45.3 to 81.7) | 93.9 (91.8 to 95.5) |
| Nasopharyngeal aspirate | Composite | 2 | 222 (24) | 50.0 (31.0 to 69.0) | 98.2 (95.4 to 99.3) | 75.9 (52.4 to 90.0) | 94.6 (92.2 to 96.4) |
CI: confidence interval; TB: tuberculosis. aPredictive values were determined at a pretest probability of 10%.
Composite reference standard
Five studies (1108 participants) evaluated Xpert Ultra in sputum specimens against a composite reference standard (Jaganath 2021; Kabir 2020; Liu 2021; Nicol 2018; Sabi 2018). Xpert Ultra sensitivity ranged from 20% to 50%, and specificity from 98% to 100% (Figure 4). Xpert Ultra summary sensitivity was 23.5% (95% CI 20.1 to 27.3), and summary specificity was 99.8% (95% CI 98.8 to 100.0) (Table 5).
Xpert Ultra in gastric aspirate specimens
Culture reference standard
Seven studies (990 participants) evaluated Xpert Ultra in gastric aspirate specimens against culture (Jaganath 2021; Liu 2021; NCT04121026; NCT04240990; Parigi 2021; Ssengooba 2020; Sun 2020). Xpert Ultra sensitivity ranged from 0% to 100%, and specificity from 78% to 100% (Figure 4). The low sensitivity in Jaganath 2021 could be due to having only one culture positive case in the study. Xpert Ultra summary sensitivity was 70.4% (95% CI 53.9 to 82.9), and summary specificity was 94.1% (95% CI 84.8 to 97.8) (Table 5).
Composite reference standard
Four studies (448 participants) evaluated Xpert Ultra in gastric aspirate specimens against a composite reference standard (Jaganath 2021; Liu 2021; Parigi 2021; Sun 2020). Xpert Ultra sensitivity ranged from 10% to 60%, and specificity from 88% to 100% (Figure 4). Xpert Ultra summary sensitivity was 46.5% (95% CI 29.7 to 64.1), and summary specificity was 98.4% (95% CI 91.4 to 99.7) (Table 5).
Xpert Ultra in stool specimens
Culture reference standard
Six studies (1432 participants) evaluated Xpert Ultra in stool specimens against culture (Kabir 2020; Liu 2021; NCT04121026; NCT04203628; NCT04240990; NCT04899076). Xpert Ultra sensitivity ranged from 39% to 100%, and specificity from 90% to 100% (Figure 4). Xpert Ultra summary sensitivity was 56.1% (95% CI 39.1 to 71.7), and summary specificity was 98.0% (95% CI 93.3 to 99.4) (Table 5).
Composite reference standard
Two studies (572 participants) evaluated Xpert Ultra in stool specimens against a composite reference standard (Kabir 2020; Liu 2021). In Kabir 2020, Xpert Ultra sensitivity was 54% (95% CI 44 to 64), and specificity was 100% (95% CI 99 to 100); while in Liu 2021, Xpert Ultra sensitivity was 45% (95% CI 35 to 56), and specificity was 95% (95% CI 82 to 99) (Figure 4). Xpert Ultra summary sensitivity was 50.3% (95% CI 43.3 to 57.1), and summary specificity was 99.5% (95% CI 97.9 to 99.9) (Table 5).
Xpert Ultra in nasopharyngeal aspirate specimens
Culture reference standard
Five studies (537 participants) evaluated Xpert Ultra in nasopharyngeal aspirate against culture (Jaganath 2021; Liu 2021; NCT04121026; NCT04240990; Zar 2019). Xpert Ultra sensitivity ranged from 20% to 67%, and specificity from 50% to 99% (Figure 4). In Liu 2021, only one participant did not have tuberculosis, and sensitivity was not estimable. In the remaining four studies (535 participants), Xpert Ultra summary sensitivity was 43.7% (95% CI 26.7 to 62.2), and summary specificity was 97.5% (95% CI 93.6 to 99.0) (Table 5).
Composite reference standard
Two studies (222 participants) evaluated Xpert Ultra in nasopharyngeal aspirate against a composite reference standard (Jaganath 2021; Zar 2019). In Jaganath 2021, Xpert Ultra sensitivity was 23% (95% CI 5 to 54), and specificity was 86% (95% CI 57 to 98); while in Zar 2019, Xpert Ultra sensitivity was 14% (95% CI 9 to 21), and specificity was 100% (95% CI 93 to 100) (Figure 4). Xpert Ultra summary sensitivity was 50.0% (95% CI 31.0 to 69.0), and summary specificity was 98.2% (95% CI 95.4 to 99.3) (Table 5).
Investigations of heterogeneity
Xpert Ultra accuracy by age group
The analyses for Xpert Ultra sensitivity and specificity by specimen type and age group are based on a small number of studies. For sensitivity and specificity estimates for individual studies, refer to Figure 5 (sputum and gastric aspirate) and Figure 6 (stool and nasopharyngeal aspirate). For summary sensitivity and specificity estimates by specimen type and age group, see Table 6. We describe several key analyses below.
5.

Forest plots of Xpert Ultra sensitivity and specificity in sputum and gastric aspirate for pulmonary tuberculosis by age group, culture reference standard. The squares represent the sensitivity and specificity of each study, the black lines their confidence intervals (CIs). FN: false negative; FP: false positive; TN: true negative; TP: true positive.
6.

Forest plots of Xpert Ultra sensitivity and specificity in stool and nasopharyngeal specimens for pulmonary tuberculosis by age group, culture reference standard. The squares represent the sensitivity and specificity of each study, the black lines their confidence intervals (CIs). FN: false negative; FP: false positive; TN: true negative; TP: true positive.
4. Xpert Ultra summary sensitivity and specificity for pulmonary tuberculosis, by type of specimen and age group.
| Analysis group | Reference standard | Studies | Number of children (TB cases) | Summary sensitivity % (95% CI) | Summary specificity % (95% CI) | Positive predictive value % (95% CI) a | Negative predictive value % (95% CI) a |
| Sputum specimen | |||||||
| < 1 year | Culture | 4 | 257 (16) | 75.0 (49.2 to 90.3) | 97.9 (95.1 to 99.1) | 80.0 (61.7 to 90.9) | 97.2 (93.8 to 98.8) |
| <1 year | Composite | 5 | 260 (103) | 15.5 (9.74 to 23.9) | 100 (97.6 to 100)b | —c | —c |
| 1–4 years | Culture | 5 | 468 (43) | 69.8 (54.6 to 81.6) | 96.2 (93.9 to 97.7) | 67.3 (55.0 to 77.6) | 96.7 (94.8 to 97.8) |
| 1–4 years | Composite | 4 | 420 (213) | 20.7 (15.7 to 26.6) | 100 (98.2 to 100)b | —c | —c |
| 5–9 years | Culture | 5 | 282 (39) | 66.7 (50.7 to 79.6) | 96.8 (87.6 to 99.2) | 70.0 (35.8 to 90.7) | 96.4 (94.3 to 97.6) |
| 5–9 years | Composite | 4 | 263 (134) | 25.4 (16.6 to 36.8) | 100 (97.1 to 100)b | —c | —c |
| 10–14 years | Culture | 5 | 135 (23) | 91.9 (68.7 to 98.3) | 97.7 (77.2 to 99.8) | 81.7 (26.8 to 98.2) | 99.1 (95.9 to 99.8) |
| 10–14 years | Composite | 4 | 129 (62) | 40.3 (28.9 to 52.9) | 100 (94.6 to 100)b | —c | —c |
| 0–9 years | Culture | 5 | 1012 (98) | 69.7 (58.1 to 79.3) | 97.2 (94.5 to 98.6) | 73.4 (58.7 to 84.2) | 96.7 (95.3 to 97.6) |
| 0–9 years | Composite | 5 | 943 (450) | 21.1 (17.6 to 25.1) | 100 (99.2 to 100)b | —c | —c |
| Gastric aspirate specimen | |||||||
| < 1 year | Culture | 5 | 182 (26) | 67.3 (43.5 to 84.6) | 94.0 (84.7 to 97.8) | 55.4 (31.5 to 77.1) | 96.3 (93.1 to 98.0) |
| 1–4 years | Culture | 4 | 327 (30) | 71.5 (40.0 to 90.4) | 94.0 (73.8 to 98.9) | 57.1 (25.1 to 84.1) | 96.8 (92.5 to 98.6) |
| 1–4 year | Composite | 3 | 72 (52) | 50.0 (36.7 to 63.3) | 100 (83.9 to 100)b | —c | —c |
| 0–9 years | Culture | 6 | 659 (70) | 63.6 (47.7 to 77.0) | 94.9 (83.8 to 98.5) | 57.9 (31.0 to 80.9) | 95.9 (94.1 to 97.2) |
| 0–9 years | Composite | 3 | 142 (101) | 47.5 (38.0 to 57.2) | 100 (91.4 to 100)b | 100 (32.9 to 100) | 94.5 (93.0 to 95.5) |
| Stool specimen | |||||||
| < 1 year | Culture | 4 | 295 (31) | 65.2 (33.7 to 87.3) | 96.2 (88.9 to 98.7) | 65.3 (40.2 to 84.0) | 96.2 (91.5 to 98.3) |
| < 1 year | Composite | 2 | 199 (65) | 50.8 (38.8 to 62.6) | 100 (97.2 to 100)b | —c | —c |
| 1–4 years | Culture | 3 | 331 (30) | 43.3 (27.1 to 61.2) | 97.1 (74.8 to 99.7) | 62.7 (13.2 to 94.9) | 93.9 (91.8 to 95.5) |
| 1–4 years | Composite | 2 | 186 (62) | 42.8 (28.4 to 58.6) | 99.3 (77.2 to 100) | 87.0 (14.1 to 99.6) | 93.9 (92.2 to 95.3) |
| 5–9 years | Culture | 3 | 145 (19) | 57.9 (35.6 to 77.4) | 89.7 (83.0 to 93.9) | 38.4 (24.7 to 54.3) | 95.0 (91.8 to 97.0) |
| 5–9 years | Composite | 2 | 126 (47) | 46.8 (33.2 to 60.9) | 100 (95.4 to 100)b | —c | —c |
| 10–14 years | Composite | 2 | 61 (25) | 72.0 (51.8 to 86.0) | 97.5 (38.9 to 100) | 76.4 (5.50 to 99.5) | 96.9 (94.3 to 98.4) |
| 0–9 years | Culture | 6 | 1279 (154) | 52.8 (35.0 to 69.9) | 98.0 (93.4 to 99.4) | 74.1 (55.2 to 96.6) | 94.9 (92.7 to 96.6) |
| 0–9 years | Composite | 2 | 511 (174) | 47.1 (39.8 to 54.6) | 99.7 (97.9 to 100) | 94.6 (71.2 to 99.2) | 94.4 (93.7 to 95.1) |
| 1–4 years | Culture | 2 | 251 (24) | 50.0 (31.0 to 69.0) | 98.2 (95.4 to 99.3) | 75.9 (52.4 to 90.0) | 94.6 (92.2 to 96.4) |
| Nasopharyngeal aspirate specimen | |||||||
| 0–9 years | Culture | 3 | 478 (42) | 42.6 (26.0 to 61.1) | 98.6 (96.7 to 99.4) | 77.7 (58.5 to 89.5) | 93.9 (91.8 to 95.5) |
| 0–9 years | Composite | 2 | 205 (148) | 14.2 (9.43 to 20.8) | 98.5 (74.3 to 99.9) | 50.5 (04.6 to 95.7) | 91.2 (90.5 to 91.8) |
CI: confidence interval; TB: tuberculosis. aPredictive values were determined at a pre‐test probability of 10%. bMeta‐analysis using univariate fixed‐effect or random‐effects logistic regression models is not possible when all studies in a meta‐analysis report 100% specificity. Therefore, the summary specificity was calculated by dividing the total number of non‐cases by the total number of true negatives. cCould not be determined. It was not possible to compute likelihood ratios post‐estimation because different models were fitted separately for sensitivity and specificity.
Sputum specimens, 10 to 14 years
In children aged 10 to 14 years, seven studies (151 participants) evaluated Xpert Ultra in sputum against culture (Barcellini 2019; Jaganath 2021; Kabir 2020; NCT04121026; Nicol 2018; Sabi 2018; Ssengooba 2020). Two studies did not contribute data to the meta‐analysis because sensitivity was not estimable (Barcellini 2019; NCT04121026). In the remaining five studies (135 participants), Xpert Ultra summary sensitivity was 91.9% (95% CI 68.7 to 98.3), and summary specificity was 97.7% (95% CI 77.2 to 99.8).
Gastric aspirate specimens, under one year
In children under one year of age, six studies (185 participants) evaluated Xpert Ultra on gastric aspirate specimens against culture (Jaganath 2021; Liu 2021; NCT04121026; NCT04240990; Parigi 2021; Ssengooba 2020). One study did not contribute data to the meta‐analysis because sensitivity was not estimable (Jaganath 2021). In the remaining five studies (182 participants), Xpert Ultra summary sensitivity was 67.3% (95% CI 43.5 to 84.6), and summary specificity was 94.0% (95% CI 84.7 to 97.8).
Gastric aspirate specimens, one to four years
In children aged one to four years, six studies (384 participants) evaluated Xpert Ultra on gastric aspirate specimens against culture (Jaganath 2021; Liu 2021; NCT04121026; NCT04240990; Parigi 2021; Ssengooba 2020). Two studies did not contribute data to the meta‐analysis because sensitivity was not estimable (Jaganath 2021; NCT04121026). In the remaining four studies (327 participants), Xpert Ultra summary sensitivity was 71.5% (95% CI 40.0 to 90.4), and summary specificity was 94.0% (95% CI 73.8 to 98.9).
Stool specimens, under one year
In children under one year of age, four studies (295 participants) evaluated Xpert Ultra in stool against culture (Kabir 2020; Liu 2021; NCT04121026; NCT04240990). Xpert Ultra summary sensitivity was 65.2% (95% CI 33.7 to 87.3), and summary specificity was 96.2% (95% CI 88.9 to 98.7).
Stool specimens, one to four years
In children aged one to four years, four studies (372 participants) evaluated Xpert Ultra in stool against culture (Kabir 2020; Liu 2021; NCT04121026; NCT04240990). One study did not contribute data to the meta‐analysis because sensitivity was not estimable (NCT04121026). In the remaining three studies (331 participants), Xpert Ultra summary sensitivity was 43.3% (95% CI 27.1 to 61.2), and summary specificity was 97.1% (95% CI 74.8 to 99.7).
Xpert Ultra accuracy by HIV status
We identified few studies that determined Xpert Ultra accuracy for pulmonary tuberculosis by specimen type in HIV‐positive and HIV‐negative children. For sensitivity and specificity estimates for individual studies, refer to Figure 7 and for summary sensitivity and specificity, to Table 7. We describe two analyses below.
7.

Forest plots of Xpert Ultra sensitivity and specificity for pulmonary tuberculosis by specimen type and HIV status, culture reference standard. The squares represent the sensitivity and specificity of each study, the black lines their confidence intervals (CIs). FN: false negative; FP: false positive; TN: true negative; TP: true positive.
5. Xpert Ultra summary sensitivity and specificity by type of specimen and comorbidity.
| Analysis group | Reference standard | Studies | Number of children (TB cases) | Summary sensitivity % (95% CI) | Summary specificity % (95% CI) | Positive predictive value % (95% CI)a | Negative predictive value % (95% CI)a |
| Sputum specimen | |||||||
| HIV‐positive, all ages | Culture | 4 | 181 (29) | 79.5 (59.6 to 91.1) | 98.7 (93.9 to 99.7) | 87.5 (57.7 to 97.3) | 97.7 (95.2 to 98.9) |
| HIV‐positive, all ages | Composite | 3 | 174 (114) | 21.1 (14.5 to 29.5) | 100 (94.0 to 100)b | —c | —c |
| HIV‐positive, 0–9 years | Composite | 2 | 66 (51) | 23.5 (13.9 to 37.0) | 100 (79.6 to 100)b | —c | —c |
| HIV‐negative, all ages | Culture | 4 | 549 (89) | 69.6 (53.3 to 82.1) | 97.3 (94.5 to 98.7) | 74.2 (59.1 to 85.1) | 96.7 (94.7 to 97.9) |
| HIV‐negative, all ages | Composite | 4 | 483 (301) | 24.3 (19.7 to 29.4) | 100 (97.9 to 100)b | —c | —c |
| HIV‐negative, 0–9 years | Culture | 3 | 399 (56) | 73.2 (60.2 to 83.2) | 96.5 (93.9 to 98.0) | 69.9 (56.5 to 80.6) | 97.0 (95.4 to 98.0) |
| HIV‐negative, 0–9 years | Composite | 3 | 337 (232) | 21.1 (16.3 to 26.8) | 100 (96.5 to 100)b | —c | —c |
| Severe malnutrition, all ages | Culture | 5 | 267 (17) | 83.2 (54.2 to 95.5) | 98.5 (62.6 to 100) | 86.1 (14.3 to 99.6) | 98.1 (94.1 to 99.4) |
| Severe malnutrition, all ages | Composite | 4 | 263 (110) | 21.8 (15.1 to 30.5) | 100 (97.6 to 100)b | —c | —c |
| Severe malnutrition, 0–9 years | Culture | 4 | 228 (11) | 81.8 (49.3 to 95.4) | 95.9 (92.2 to 97.8) | 68.6 (52.2 to 81.5) | 97.9 (93.2 to 99.4) |
| Severe malnutrition, 0–9 years | Composite | 3 | 224 (91) | 19.8 (12.8 to 29.2) | 100 (97.2 to 100)b | —c | —c |
| Gastric aspirate specimen | |||||||
| HIV‐negative, all ages | Culture | 3 | 345 (47) | 61.7 (47.2 to 74.4) | 90.8 (82.4 to 95.4) | 42.7 (26.7 to 60.4) | 95.5 (93.7 to 96.9) |
| HIV‐negative, 0–9 years | Culture | 3 | 325 (46) | 63.0 (48.4 to 75.6) | 90.5 (82.5 to 95.1) | 42.5 (27.4 to 59.1) | 95.6 (93.8 to 97.0) |
| Stool specimen | |||||||
| Severe malnutrition, all ages | Culture | 3 | 443 (22) | 68.2 (46.6 to 84.0) | 98.5 (84.2 to 99.9) | 83.5 (29.4 to 98.4) | 96.6 (93.8 to 98.0) |
| Severe malnutrition, 0–9 years | Culture | 3 | 428 (19) | 63.2 (40.3 to 81.3) | 98.5 (84.1 to 99.9) | 82.3 (27.7 to 98.3) | 96.1 (93.1 to 97.7) |
| Nasopharyngeal aspirate specimen | |||||||
| HIV‐negative, all ages | Composite | 2 | 186 (132) | 21.1 (8.5 to 43.5) | 100 (93.4 to 100)b | —c | —c |
| HIV‐negative, 0–9 years | Composite | 2 | 184 (132) | 21.1 (8.5 to 43.5) | 100 (93.1 to 100)b | —c | —c |
CI: confidence interval; TB: tuberculosis. aPredictive values were determined at a pre‐test probability of 10%. bMeta‐analysis using univariate fixed‐effect or random‐effects logistic regression models is not possible when all studies in a meta‐analysis report 100% specificity. Therefore, the summary specificity was calculated by dividing the total number of non‐cases by the total number of true negatives. cCould not be determined. It was not possible to compute likelihood ratios post‐estimation because different models were fitted separately for sensitivity and specificity.
Sputum specimens, HIV‐positive
Five studies (206 participants) evaluated Xpert Ultra in sputum against culture in HIV‐positive children aged birth to 14 years (Jaganath 2021; Nicol 2018; NCT04121026; Sabi 2018; Ssengooba 2020). One study did not contribute data to the meta‐analysis because sensitivity was not estimable (NCT04121026). In the remaining four studies (181 participants), Xpert Ultra summary sensitivity was 79.5% (95% CI 59.6 to 91.1), and summary specificity was 98.7% (95% CI 93.9 to 99.7).
Sputum specimens, HIV‐negative
Six studies (559 participants) evaluated Xpert Ultra in sputum against culture in HIV‐negative children aged birth to 14 years (Barcellini 2019; Jaganath 2021; Liu 2021; Nicol 2018; Sabi 2018; Ssengooba 2020). Two studies did not contribute data to the meta‐analysis because sensitivity was not estimable (Barcellini 2019; Liu 2021). In the remaining four studies (549 participants), Xpert Ultra summary sensitivity was 69.6% (95% CI 53.3 to 82.1), and summary specificity was 97.3% (95% CI 94.5 to 98.7).
Xpert Ultra accuracy in other comorbid conditions
We identified few studies that determined Xpert Ultra accuracy for pulmonary tuberculosis by specimen type in children with severe pneumonia or severe malnutrition. For sensitivity and specificity estimates for individual studies, refer to Figure 8, and for summary sensitivity and specificity, to Table 7. We describe two analyses below.
8.

Forest plots of Xpert Ultra sensitivity and specificity for pulmonary tuberculosis by specimen type and comorbidity, culture reference standard. The squares represent the sensitivity and specificity of each study, the black lines their confidence intervals (CIs). FN: false negative; FP: false positive; TN: true negative; TP: true positive.
Sputum specimens, severe malnutrition
Five studies (267 participants) evaluated Xpert Ultra in sputum against culture in children with severe malnutrition (Jaganath 2021; Kabir 2020; Nicol 2018; Sabi 2018; Ssengooba 2020). Xpert Ultra summary sensitivity was 83.2% (95% CI 54.2 to 95.5), and summary specificity was 98.5% (95% CI 62.6 to 100).
Stool specimens, severe malnutrition
Three studies (443 participants) evaluated Xpert Ultra in stool against culture in children with severe malnutrition (Kabir 2020; NCT04121026; NCT04240990). Xpert Ultra summary sensitivity was 68.2% (46.6 to 84.0) and summary specificity was 98.5% (95% CI 84.2 to 99.9).
Sensitivity analyses
We did not perform a sensitivity analysis for sputum specimens because the only ongoing study (NCT04121026) had no tuberculosis cases and so was not included in the primary analysis. Excluding ongoing studies, meta‐analyses were possible for gastric aspirate specimens (5 studies) and stool specimens (2 studies). As expected, we observed differences owing to the number of studies included in the primary analyses and the sensitivity analyses (Table 8).
6. Sensitivity analyses.
| Analysis group | Reference standard | Studies | Number of children (TB cases) | Summary sensitivity % (95% CI) | Summary specificity % (95% CI) | Positive predictive value % (95% CI)a | Negative predictive value % (95% CI)a |
| Gastric aspirate specimen | Culture | 7 | 990 (120) | 70.4 (53.9 to 82.9) | 94.1 (84.8 to 97.8) | 56.9 (34.9 to 76.6) | 96.7 (94.6 to 97.8) |
| Gastric aspirate specimen | Culture | 5 | 696 (111) | 73.4 (57.3 to 85.5) | 88.1 (81.6 to 92.6) | 40.8 (29.6 to 53.1) | 96.8 (94.5 to 98.1) |
| Stool specimen | Culture | 6 | 1432 (200) | 56.1 (39.1 to 71.7) | 98.0 (93.3 to 99.4) | 75.3 (45.5 to 91.7) | 95.2 (93.2 to 96.8) |
| Stool specimen | Culture | 2 | 572 (89) | 60.7 (50.2 to 70.2) | 90.1 (87.1 to 92.4) | 40.4 (33.1 to 48.2) | 95.3 (94.0 to 96.4) |
| Nasopharyngeal specimen | Culture | 4 | 535 (46) | 43.7 (26.7 to 62.2) | 97.5 (93.6 to 99.0) | 65.8 (45.3 to 81.7) | 93.9 (91.8 to 95.5) |
| Nasopharyngeal specimenb | Culture | 2 | 222 (38) | — | — | — | — |
Sensitivity analyses are indicated in bold. CI: confidence interval; TB: tuberculosis. aPredictive values were determined at a pre‐test probability of 10%. bMeta‐analysis was not performed due to paucity of data and heterogeneity, which precluded the use of univariate fixed‐effect logistic regression as performed for the meta‐analysis of the two studies for stool specimen.
Xpert Ultra trace results
Of the 14 included studies, 13 (93%) reported the number of Xpert Ultra‐positive results that were trace results. In these 13 studies, of the total Xpert Ultra‐positive results, the proportion (expressed as a percentage) of Ultra trace results ranged from 0% to 67% in studies evaluating sputum; 25% to 67% in studies evaluating gastric aspirate; 0% to 45% in studies evaluating nasopharyngeal aspirate; and 0% to 80% in studies evaluating stool (Table 4).
Detection of rifampicin resistance
We identified three studies that evaluated Xpert Ultra for rifampicin resistance (Liu 2021; Parigi 2021; Zar 2019). Each study included only one child with rifampicin resistance (true positive). In Liu 2021, Xpert Ultra sensitivity was 100% (95% CI 3 to 100), and specificity was 97% (95% CI 84 to 100); in Parigi 2021, Xpert Ultra sensitivity was 100% (95% CI 3 to 100), and specificity was 100% (95% CI 95 to 100); and in Zar 2019, Xpert Ultra sensitivity was 100% (95% CI 3 to 100), and sensitivity was 100% (95% CI 84 to 100) (Figure 9).
9.

Forest plots of Xpert Ultra sensitivity and specificity for rifampicin resistance. The squares represent the sensitivity and specificity of each study, the black lines their confidence intervals (CIs). TP: true positive; FP: false positive; FN: false negative; TN: true negative.
Inconclusive index test results
Non‐determinate results for detection of tuberculosis
The percentage of non‐determinate results ranged from 0% to 11% of Xpert Ultra tests performed in sputum and from 1% to 10% of Xpert Ultra tests performed in stool. Non‐determinate results were not reported or could not be disaggregated from other specimen types for Xpert Ultra tests performed in gastric aspirate and nasopharyngeal aspirate (Table 4).
Indeterminate results for detection of rifampicin resistance
Indeterminate results for detection of rifampicin resistance were common owing to lack of rifampin resistance results with trace results on Xpert Ultra. As a percentage of positive results, trace results represented 0% to 67% for sputum, 26% to 67% for gastric aspirate, 0% to 80% for stool and 0% to 45% for nasopharyngeal aspirate.
Discussion
Summary of main results
This systematic review update summarizes the current literature on the diagnostic accuracy of Xpert Ultra for pulmonary tuberculosis, tuberculous meningitis, lymph node tuberculosis, and rifampicin resistance. Our previously published Cochrane Review assessed the accuracy of both Xpert MTB/RIF and Xpert Ultra (Kay 2020). We limited the current review update to Xpert Ultra for several reasons. Xpert Ultra has superseded Xpert MTB/RIF and the manufacturer will be discontinuing Xpert MTB/RIF in most countries in 2023. Given the available evidence about Xpert MTB/RIF from our previous review, we therefore only updated Xpert Ultra, as requested by the WHO. The Xpert MTB/RIF text and analyses are available in the last published version of the review (Kay 2020).
In this review update, we identified 14 unique studies, integrating 11 new studies since publication of the previous Cochrane Review (Kay 2020). We did not identify any studies that evaluated Xpert Ultra accuracy for tuberculous meningitis or lymph node tuberculosis. The main results from the review are summarized in Table 5, Table 1, and Table 2.
Xpert Ultra accuracy for detection of pulmonary tuberculosis (culture reference standard)
See Table 1
Sputum, 5 studies
Xpert Ultra summary sensitivity verified by culture was 75.3% (95% CI 64.3 to 83.8; 127 participants; high‐certainty evidence), and specificity was 97.1% (95% CI 94.7 to 98.5; 1054 participants; high‐certainty evidence).
Gastric aspirate, 7 studies
Xpert Ultra summary sensitivity verified by culture was 70.4% (95% CI 53.9 to 82.9; 120 participants; moderate‐certainty evidence), and specificity was 94.1% (95% CI 84.8 to 97.8; 870 participants; moderate‐certainty evidence).
Stool, 6 studies
Xpert Ultra summary sensitivity verified by culture was 56.1% (95% CI 39.1 to 71.7; 200 participants; moderate‐certainty evidence), and specificity was 98.0% (95% CI 93.3 to 99.4; 1232 participants; high certainty‐evidence).
Nasopharyngeal aspirate, 4 studies
Xpert Ultra summary sensitivity verified by culture was 43.7% (95% CI 26.7 to 62.2; 46 participants; very low‐certainty evidence), and specificity was 97.5% (95% CI 93.6 to 99.0; 489 participants; high‐certainty evidence).
Interpretation of results
In theory, for a population of 1000 children:
• where 100 have pulmonary tuberculosis in sputum (by culture):
‐ 101 would be Xpert Ultra‐positive, and of these, 26 (26%) would not have pulmonary tuberculosis (false positive); and ‐ 899 would be Xpert Ultra‐negative, and of these, 25 (3%) would have tuberculosis (false negative).
• where 100 have pulmonary tuberculosis in gastric aspirate (by culture):
‐ 123 would be Xpert Ultra‐positive, and of these, 53 (43%) would not have pulmonary tuberculosis (false positive); and ‐ 877 would be Xpert Ultra‐negative, and of these, 30 (3%) would have tuberculosis (false negative).
• where 100 have pulmonary tuberculosis in stool (by culture):
‐ 74 would be Xpert Ultra‐positive, and of these, 18 (24%) would not have pulmonary tuberculosis (false positive); and ‐ 926 would be Xpert Ultra‐negative, and of these, 44 (5%) would have tuberculosis (false negative).
• where 100 have pulmonary tuberculosis in nasopharyngeal aspirate (by culture):
‐ 66 would be Xpert Ultra‐positive, and of these, 22 (33%) would not have pulmonary tuberculosis (false positive); and ‐ 934 would be Xpert Ultra‐negative, and of these, 56 (6%) would have tuberculosis (false negative).
Xpert Ultra accuracy for detection of pulmonary tuberculosis (composite reference standard)
See Table 5.
Sputum, 5 studies
Xpert Ultra summary sensitivity by composite reference standard was 23.5% (95% CI 20.1 to 27.3; 527 participants) and summary specificity was 99.8% (95% CI 98.8 to 100; 581 participants).
Gastric aspirate, 4 studies
Xpert Ultra summary sensitivity by composite reference standard was 46.5% (95% CI 29.7 to 64.1; 229 participants) and summary specificity was 98.4% (95% CI 91.4 to 99.7; 219 participants).
Stool, 2 studies
Xpert Ultra summary sensitivity by composite reference standard was 50.3% (95% CI 43.3 to 57.1; 199 participants) and summary specificity was 99.5% (95% CI 97.9 to 99.9; 373 participants).
Nasopharyngeal aspirate, 2 studies
Xpert Ultra summary sensitivity by composite reference standard was 50.0% (95% CI 31.0 to 69.0; 24 participants) and summary specificity was 98.2% (95% CI 95.4 to 99.3; 198 participants).
Xpert Ultra accuracy for detection of rifampicin resistance
See Table 2.
Xpert Ultra sensitivity was 100% (3 studies, 3 participants; very low‐certainty evidence), and specificity range was 97% to 100% (3 studies, 128 participants; low‐certainty evidence)
Trace results
See Table 4.
Xpert Ultra trace results, regarded as positive in children by WHO standards, were common. Xpert Ultra specificity remained high in children, despite the frequency of trace results.
Xpert Ultra on different types of specimens
Overall, this review adds to the existing body of evidence on the diagnostic accuracy of Xpert Ultra in children. Most notable are the new data on performance of different specimen types that are now being introduced to improve access to diagnostic testing for tuberculosis in children. These findings provide new evidence to shape the development of global practice guidelines for the diagnosis of tuberculosis in children.
Specifically, our review demonstrated differing sensitivities in the different types of specimens. We think that these findings may in part be attributable to differences in the clinical setting and in the quality of the reference standard, mainly the number of cultures used. With respect to the clinical setting, it is more common to collect gastric aspirate specimens in inpatient settings; further, these settings tend to include a higher number of children with advanced disease, which often has a higher microbiological yield (Marais 2006b). Thus, the higher sensitivity against a culture and composite reference standard for gastric aspirate specimens may in part be due to the inpatient setting and higher likelihood of advanced disease. The diagnostic accuracy for Xpert Ultra in sputum and nasopharyngeal aspirate specimens was similar to that presented in the prior review, and the findings would be unlikely to change the current recommendations for these samples (WHO Consolidated Guidelines (Module 3) 2021).
Against a composite reference standard, we found that Xpert Ultra had a sensitivity that ranged from 23.5% to 50.3% and a specificity of greater than 98.2%. These sensitivity estimates were higher than those reported for Xpert MTB/RIF against a composite reference standard in Kay 2020 and may reflect the increased sensitivity of Xpert Ultra compared to Xpert MTB/RIF. In adults, Xpert Ultra trace results may be more likely to reflect false‐positive results, particularly in people with prior tuberculosis (Dorman 2018). Xpert Ultra trace results were common, with the proportion reported in 13 of the 14 studies and recorded in Table 4. Existing guidance in children suggests that trace results should be treated as true‐positive results (WHO Consolidated Guidelines (Module 3) 2021); the test remained highly specific despite the high proportion of trace results.
We found the sensitivity of stool Xpert Ultra to be slightly lower at 56.1% (95% CI 39.1% to 71.7%) than that of sputum Xpert Ultra at 75.3% (95% CI 64.3% to 83.8%) or gastric aspirate Xpert Ultra at 70.4% (95% CI 53.9% to 82.9%). Nonetheless, stool is a promising specimen for diagnosis because, unlike sputum or gastric aspirates, it is non‐invasive. Its greatest benefit may be seen in children younger than five years of age owing to the challenges of collecting specimens through sputum induction and gastric aspiration in this population. The sensitivity of stool was 65.2% (95% CI 33.7% to 87.3%) in children younger than one year of age and 43.3% (95% CI 27.1% to 61.2%) in children aged one to four, suggesting the performance is comparable in younger children. We again noted the lack of standardized procedures for processing stool, with each study using a different approach, and could not evaluate diagnostic accuracy by stool processing procedure.
Subgroup analyses
In subgroup analyses, we were limited by the paucity of data and could not compare diagnostic accuracy in HIV‐positive and HIV‐negative children. Similar to our prior findings with Xpert MTB/RIF (Kay 2020), the limited data did not suggest Xpert Ultra had lower sensitivity in HIV‐positive children. The sensitivity of Xpert Ultra in sputum in HIV‐positive children of all ages was 79.5% (59.6% to 91.1%), compared with 69.6% (53.3% to 82.1%) in HIV‐negative children.
In children with severe malnutrition, the sensitivity of Xpert Ultra was 83.2% (54.2% to 95.5%) in sputum and 68.2% (46.6% to 84.0%) in stool. These estimates were higher than in all children, suggesting that children with severe malnutrition, who, in most settings, are also at high risk for tuberculosis, may represent an ideal population for Xpert Ultra testing. However, these analyses, as well as the analyses in children with severe pneumonia, are based on a small number of studies and participants, so we advise caution in interpretation of the results.
Regarding age group, we found that Xpert Ultra sensitivity in sputum was higher in children aged 10 to 14 years (91.9%, 95% CI 68.7 to 98.3) compared to children of all ages, (75.3%, 95% CI 64.3 to 83.8). We did not find large decreases in the sensitivity of Xpert Ultra in children younger than five years of age in sputum, gastric aspirates or stool compared with all ages. This is an important finding and suggests that Xpert Ultra may perform more comparably in younger children than Xpert MTB/RIF, which has shown decreased sensitivity in younger children (Kay 2020).
Strengths and weaknesses of the review
Completeness of evidence
The data set resulted from comprehensively searching numerous databases, including non‐English studies, handsearching references of included studies, and contacting study authors for additional evidence. We included all identified studies, as well as ongoing studies, from which we could obtain accuracy data. However, we acknowledge that we may have missed some studies despite the comprehensive search and outreach to study authors. We searched for studies for the two most common forms of extrapulmonary tuberculosis (tuberculous meningitis and lymph node tuberculosis) but did not identify studies for these conditions. The review does not include an evaluation of the accuracy of Xpert Ultra in less common forms of child tuberculosis.
Accuracy of the reference standards used
In a systematic review of diagnostic test accuracy studies, the reference standard is the best available test to determine the presence or absence of the target condition. In this review, we included two reference standards: culture and a composite reference standard. Although culture is the best available microbiological reference standard, it is not a perfect reference standard for tuberculosis disease in children owing to the paucibacillary nature of the disease in this population. Some studies performed only one culture and others more than one culture to verify tuberculosis. We considered multiple cultures to be a higher‐quality reference standard. We also evaluated the accuracy of Xpert Ultra against a composite reference standard. The accuracy of composite reference standards is also variable and limited, but may reflect the paucibacillary nature of childhood tuberculosis, which is not taken into account when culture positivity is the reference standard for comparison. For all specimen types, Xpert Ultra sensitivity was lower and specificity similar against a composite reference standard compared with culture. If data on tuberculosis treatment were not provided, we accepted the uniform research definitions or the definition used by the primary study authors (study‐specific definition) for the composite reference standard. Therefore, clinical characteristics and component tests in the composite reference standard differed across studies, and these differences may have contributed to variation in accuracy estimates.
Quality assessment and quality of reporting of the included studies
We considered risk of bias to be low for the patient selection, index test, and flow and timing domains, and low or unclear for the reference standard domain, because some studies collected only a single specimen for culture. In general, studies were fairly well reported. When data were unclear, or when we needed additional information, we corresponded with all primary study authors. Although the quality of the studies was good, for some analyses by age group and comorbidity, the numbers of studies and participants enrolled were small, limiting our ability to draw definitive conclusions in these circumstances.
Comparison with other systematic reviews
We are aware of two previously published systematic reviews that estimated the diagnostic accuracy of Xpert Ultra for pulmonary tuberculosis and rifampicin resistance in children (Signorino 2022; Zhang 2020).
In Signorino 2022, Xpert Ultra accuracy results were: summary sensitivity 74% (95% CI 66 to 81) and specificity 97% (95% CI 95 to 98) in sputum; summary sensitivity 87% (95% CI 76 to 94) and specificity 85% (95% CI 81 to 89) in gastric aspirate; summary sensitivity 73% (95% CI 59 to 85) and specificity 87% (95% CI 84 to 90) in stool; and summary sensitivity 46% (95% CI 29 to 63) and specificity 97% (95% CI 94 to 99) in nasopharyngeal aspirates. These results were similar to ours with the exception of stool and gastric aspirate, which showed higher sensitivity in Signorino 2022 than in our review. For the accuracy estimates in gastric specimens, Signorino 2022 included only two studies (Parigi 2021; Sun 2020), whereas we included these two plus another five studies. The accuracy estimates for Parigi 2021 and Sun 2020 the two included studies were similar in both reviews. For stool specimens, Signorino 2022 again included only two studies (Kabir 2020; Liu 2021), whereas we included these two plus another four studies. The sensitivity estimates for Kabir 2020 and Liu 2021 were higher in Signorino 2022 than in our review; this was likely attributable to the different reference standard for stool. Signorino 2022 used culture on a respiratory specimen as the reference standard for stool, while we used either culture or Xpert Ultra on a respiratory specimen. This difference in reference standard likely also contributed to the lower specificity in Signorino 2022.
Zhang 2020 included only two studies with children, both focused on sputum specimens.
Another review compared the diagnostic accuracy of Xpert Ultra and Xpert MTB/RIF for detection of pulmonary tuberculosis and rifampicin resistance in adults (Zifodya 2021). For detection of rifampicin resistance, Xpert Ultra summary sensitivity was 94.9% (95% credible interval 88.9 to 97.9), and specificity was 99.1% (95% credible interval 97.7 to 99.8) (5 studies, 921 participants in total, 240 with rifampicin resistance).
Applicability of findings to the review question
To assess the applicability of findings to the review question, we considered QUADAS‐2 domains for patient selection, index test, and reference standard. With respect to the patient selection domain, we considered three studies (21%) of high concern because participants were evaluated exclusively as inpatients in tertiary care centres. Studies that take place in referral settings may include patients whose condition is more advanced or more difficult to diagnose than patients seen at lower levels of the health system. With respect to the index test, we considered most studies of low concern regarding applicability. However, we considered applicability of the index test in stool to be unclear, as currently, there is not a standardized protocol for stool testing with Xpert Ultra. With respect to the reference test domain, we considered most studies of low concern regarding applicability.
Authors' conclusions
Implications for practice.
Xpert Ultra sensitivity (defined by culture) for pulmonary tuberculosis was variable across different specimen types, including sputum, gastric aspirate, stool, and nasopharyngeal aspirate. The highest sensitivity was seen with sputum, followed by gastric aspirate, and the lowest in nasopharyngeal aspirate. Xpert Ultra specificity was high in all specimen types. Sensitivity for Xpert Ultra in stool was lower than in sputum or gastric aspirate, but higher than in nasopharyngeal aspirate. However, the evidence base is still limited, and findings may be imprecise and vary by study setting. Additional data are needed on the differential performance of Xpert Ultra by specimen type to guide recommendations for diagnostic algorithms in children. The sensitivity of Xpert Ultra was not dramatically reduced in children aged 0 to 4 years, which differs from our prior findings (Kay 2020), and may represent an advance in the diagnosis of young children with Xpert Ultra compared with Xpert MTB/RIF. Subgroup analyses were limited, but data from sputum and stool specimens in children with severe malnutrition suggest that Xpert Ultra performs well, with a sensitivity that was markedly higher than in all children for both specimens. This high risk group would benefit from access to Xpert Ultra testing to complement treatment for severe malnutrition.
Although we found Xpert Ultra to be accurate for detection of rifampicin resistance, the results were based on a very small number of studies that included only three children with rifampicin resistance. Findings should, therefore, be interpreted with caution.
Evidence in this review is based mainly on culture as the reference standard, and we calculated Xpert Ultra accuracy on the assumption that the reference standard is 100% sensitive and specific. Although culture is acceptable, it is an imperfect reference standard for child tuberculosis. Without a more accurate reference standard, with a limit of detection low enough to detect paucibacillary tuberculosis, the accuracy of novel diagnostic tests for tuberculosis in children will remain difficult to estimate. Despite the presence of a negative Xpert Ultra result, clinicians will still need to consider tuberculosis treatment in children with a high suspicion of tuberculosis or at high risk of a poor outcome. The percentage of non‐determinate results ranged from 0% to 11% in the studies of Xpert Ultra, and tended to be slightly higher in stool specimens. This increased percentage of non‐determinate results should be considered when using stool as a diagnostic specimen.
The evidence from Kay 2020 informed Module 3 of the WHO Consolidated Guidelines on Tuberculosis (WHO Consolidated Guidelines (Module 3) 2021), and the current review update informed module 5 (WHO Consolidated Guidelines (Module 5) 2022). Specific recommendations from those guidelines, with implications for practice, are presented in Table 3.
Implications for research.
There are several areas for which additional research regarding the diagnostic accuracy of molecular tests in children is necessary. There is a need for:
data to evaluate how Xpert Ultra impacts patient‐important outcomes in children and how Xpert Ultra diagnostic accuracy changes when multiple specimen types are evaluated;
studies that evaluate the accuracy of Xpert Ultra for detecting extrapulmonary tuberculosis in children. This is particularly relevant given the encouraging results regarding Xpert Ultra performance in cerebrospinal fluid obtained from adults (Kohli 2021);
more research to identify an improved reference standard that accurately defines tuberculosis in children;
accurate tests performed at the point of care;
additional operational and qualitative research to determine the best approach to less invasive specimen collection;
implementation studies on a method of suction for nasopharyngeal aspiration that is appropriate for low‐skill or low‐resource environments;
additional operational research concerning the use of stool as a diagnostic specimen. These studies should address integration into normal diagnostic clinical pathways, definition of laboratory protocols – including processing methods – that successfully balance ease of implementation and diagnostic performance, and the impact of stool testing on patient‐important outcomes;
qualitative research identifying child and family preferences for and acceptability of comparative diagnostic approaches and specimen collection procedures.
We underscore the continued urgent need to develop new tools that accurately diagnose tuberculosis in children. Ideally, these new tools will be rapid, affordable, feasible, and acceptable to children and their parents.
What's new
| Date | Event | Description |
|---|---|---|
| 31 August 2022 | New search has been performed | The previous published review version assessed the accuracy of both Xpert MTB/RIF and Xpert Ultra. The authors limited this review update to Xpert Ultra, which has superseded Xpert MTB/RIF. The Xpert MTB/RIF text and analyses are available in the previous published review version. |
| 31 August 2022 | New citation required and conclusions have changed | The date of search was updated to 9 March 2021. The authors included 14 unique studies, integrating 11 new studies since the previous published review version. |
History
Protocol first published: Issue 6, 2019
Acknowledgements
Editorial and peer‐reviewer contributions
The following people conducted the editorial process for this review update version.
Contact Editors: Professor Gerry Davies (CIDG); Dr Danielle van der Windt (DTA).
Sign‐off Editor (final editorial decision): Professor Paul Garner (CIDG).
Managing Editor (collated Editors' comments, provided editorial guidance to authors, edited the article): Dr Deirdre Walshe (CIDG).
Copy Editor (copy editing and production): Julia Turner.
The CIDG editorial base is funded by UK aid from the UK Government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed in this review do not necessarily reflect the UK Government's official policies.
We thank Vittoria Lutje (CIDG) for developing the search strategy. We also wish to acknowledge Ryan Vu (Rice University), who contributed to development of the protocol. In addition, we thank Mikashmi Kohli, who provided technical expertise, and Emily MacLean, who provided data on stool specimens; both are at McGill University. We thank Andrew DiNardo (Baylor College of Medicine) for technical assistance. We thank Gemma Villanueva and Hanna Bergman, both with Cochrane Response, who assisted with data entry. We also thank Aakshi Kalra (FIND), who provided data from a large‐scale Xpert MTB/RIF demonstration project conducted in India.
Development of the systematic review was in part made possible with financial support from the US Agency for International Development (USAID) administered by the World Health Organization (WHO) Global Tuberculosis Programme, Switzerland.
We are grateful to Dr Sayera Banu, Programme on Emerging Infections, Infectious Diseases Division, International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), Dhaka, Bangladesh, and her research team for providing additional data on stool specimens for Kabir 2020.
We are appreciative of all study authors, who provided stratified data not available in the published works. Further, we would like to specifically thank the study teams who have shared currently unpublished data for this review: Olivier Marcy and the entire TB‐Speed study team as well as Pamela Nabeta and the entire FIND SPK (Stool Processing Kit) study team.
Appendices
Appendix 1. Detailed search strategies
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions(R) (1946 to present)
1 Mycobacterium tuberculosis/
2 Tuberculosis, Pulmonary/
3 (tuberculosis or TB).tw.
4 ((extrapulmonary or lymph node* or mening* or pulmonary) and TB).ti. or ((extrapulmonary or lymph node* or mening* or pulmonary) and TB).ab.
5 Tuberculosis, Meningeal/
6 Tuberculosis, Lymph Node/
7 1 or 2 or 3 or 4 or 5 or 6
8 Xpert*.ti. or Xpert*.ab.
9 (GeneXpert* or cepheid).ti. or (GeneXpert* or cepheid).ab.
10 Ultra.tw.
11 8 or 9
12 10 and 11
13 7 and 12
14 (child or children* or childhood or infan* or newborn or neonat* or toddler* or adolescen*).mp.
15 exp Child/ or exp Infant/ or Adolescent/ or exp Pediatrics/
16 14 or 15
17 13 and 16
Embase (1947 to present, updated daily)
Search Strategy:
1 Mycobacterium tuberculosis/
2 (tuberculosis or TB).tw.
3 ((extrapulmonary or lymph node* or mening* or pulmonary) and TB).ti. or ((extrapulmonary or lymph node* or mening* or pulmonary) and TB).ab.
4 Xpert*.ti. or Xpert*.ab. (3330)
5 (GeneXpert* or cepheid).ti. or (GeneXpert* or cepheid).ab.
6 Ultra.tw.
7 tuberculous lymphadenitis/ or tuberculous meningitis/
8 lung tuberculosis/
9 1 or 2 or 3 or 7 or 8
10 4 or 5
11 6 and 10
12 9 and 11
13 (child or children* or childhood or infant* or newborn or neonat* or toddler* or adolescen*).mp.
14 exp Child/ or exp Infant/ or Adolescent/ or exp Pediatrics/
15 13 or 14
16 12 and 15
SCI‐EXPANDED, CPCI‐S (Web of Science)
# 7 #6 AND #5
# 6 TOPIC: ((child or children* or childhood or infan* or newborn or neonat* or toddler* or adolescen*) ) OR TOPIC: (pediatric* or paediatric*)
# 5 #4 AND #1
# 4 #3 AND #2
# 3 ALL FIELDS: (Ultra)
# 2 TOPIC: ((Xpert* or Xpert MTB RIF) ) OR TOPIC: (Genexpert* or Cepheid)
# 1 TOPIC: ((Tuberculosis or MDR‐TB or XDR‐TB or tuberculous) ) OR TOPIC: ((mycobacterium tuberculosis) ) OR TOPIC: ((((extrapulmonary or lymph node* or mening* or pulmonary) and TB)))
Scopus
( ( TITLE‐ABS‐KEY ( ( tuberculosis OR mdr‐tb OR xdr‐tb OR tuberculous ) OR ( mycobacterium AND tuberculosis ) OR ( ( ( extrapulmonary OR lymph AND node* OR mening* OR pulmonary ) AND tb ) ) ) ) AND ( TITLE‐ABS‐KEY ( ( xpert* OR xpert AND mtb AND rif ) OR ( xpert* AND ultra OR cepheid OR near* AND patient ) ) ) AND ( TITLE‐ABS‐KEY ( ( child OR children* OR childhood OR infan* OR newborn OR neonat* OR toddler* OR adolescen* ) OR ( pediatric* OR paediatric* ) ) ) ) AND ( ultra ) AND ( LIMIT‐TO ( SUBJAREA , "MEDI" ) OR LIMIT‐TO ( SUBJAREA , "IMMU" ) OR LIMIT‐TO ( SUBJAREA , "BIOC" ) OR LIMIT‐TO ( SUBJAREA, "MULT" ) )
CINAHL, Interface – EBSCOhost
# Query
S7 S5 AND S6
S6 TX child or children* or childhood or infan* or newborn or neonat* or toddler* or adolescen* OR pediatric* or paediatric*
S5 S3 AND S4
S4 TX ( Xpert* or GeneXpert* or cepheid ) AND TX ultra
S3 S1 OR S2
S2 TX "lymph node tuberculosis" OR TX "mening* tuberculosis"
S1 MH mycobacterium tuberculosis OR MH Tuberculosis, Meningeal OR TX "extrapulmonary tuberculosis" OR MH Tuberculosis, Lymph Node
Cochrane Central Register of Controlled Trial (Issue 3 of 12, March 2021)
#1 Mycobacterium tuberculosis
#2 Tuberculosis or tuberculous
#3 MeSH descriptor: [Tuberculosis, Meningeal] explode all trees
#4 MeSH descriptor: [Tuberculosis, Lymph Node] explode all trees=
#5 ((extrapulmonary or lymph node* or mening* or pulmonary) and TB)
#6 #1 or #2 or #3 or #4 or #5
#7 (Xpert* or GeneXpert or cepheid) and Ultra
#8 #6 and #7
#9 child or children* or childhood or infan* or newborn or neonat* or toddler* or adolescen* or pediatric* or paediatric*
#10 #8 and #9
ClinicalTrials.gov, WHO ICTRP, and ISRCTN Registry
tuberculosis and Xpert* and children
Appendix 2. Data extraction form
| Diagnostic accuracy of Xpert in the diagnosis of child tuberculosis: data extraction form | |
| I. ID | |
| Study ID | First Name/Publication Year |
| First author | Name |
| Corresponding author | Name |
| Corresponding author email | |
| Was author contacted? | 1 – Yes 2 – No If yes, dates(s) |
| If yes, author response? | |
| Study data | 1 ‐ Published 2 ‐ In press 3 ‐ Ongoing |
| Title | |
| Year (of publication) | YYYY or 9 – Not reported |
| Year study start date | YYYY or 9 – Not reported |
| Language | 1 – English 2 – Other If other, specify: |
| II. Study details | |
| Country where study was conducted | |
| Country World Bank classification | 1 ‐ Low income
2 ‐ Middle income
3 ‐ High income 4 ‐ Low and high income 5 ‐ Other combination, describe |
| Country tuberculosis burden | 1 ‐ WHO tuberculosis high‐burden 2 ‐ WHO tuberculosis/HIV high‐burden 3 ‐ WHO MDR tuberculosis high‐burden 4 ‐ WHO tuberculosis + MDR tuberculosis high‐burden 5 ‐ WHO tuberculosis + HIV/tuberculosis high‐burden 6 ‐ WHO tuberculosis + HIV/tuberculosis + MDR tuberculosis high‐burden 7 ‐ Not a WHO high‐burden country 8 ‐ Both non‐high‐burden and high‐burden countries included 9 ‐ Other |
| Study design | 1 – Randomized controlled trial 2 – Cross‐sectional 3 – Cohort 4 – Other, specify 9 – Could not tell If other, describe: |
| Participant selection | 1 – Consecutive 2 – Random 3 – Convenience 7 – Other 9 – Unknown/Not reported |
| Direction of study data collection | 1 – Prospective 2 – Retrospective 9 – Unknown/Not reported |
| Inclusion criteria | 1 – Broad 2 – Rigorous 9 – Unknown/Not reported |
| Inclusion criteria for presumptive tuberculosis | 1 – Tuberculosis contact 2 – Cough 3 – Loss of weight 4 – Suggestive chest X‐ray 5 – Immunological evidence of tuberculosis infection (TST/IGRA) 6 ‐ Malnutrition 7 – HIV 8 ‐ Other, describe 9 – Unknown/Not reported |
| Describe inclusion criteria as in study | |
| Number included after recruitment by inclusion and exclusion criteria | Enter number or 9 – Unknown/Not reported |
| Total number of children included in systematic review analysis | Enter number or 9 – Unknown/Not reported |
| Total number of specimens included in analysis with collection method | Enter number or 9 – Unknown/Not reported |
| Unit of analysis (Xpert) | 1 – One specimen per patient 2 – Multiple specimens per patient 3 ‐ Unknown number of specimens per patient 9 – Unknown/Not reported Describe as written in study, if unclear: |
| Did the study include patients with previous tuberculosis history? | 1 – Yes 0 – No 9 – Unknown/Not reported |
| If so, what is the percentage? | Enter % and specify numerator/denominator |
| Target condition? Pulmonary tuberculosis? | 1 ‐ Yes 0 ‐ No |
| Target condition? Rifampicin resistance? | 1 ‐ Yes 0 ‐ No |
| Target condition? Lymph node tuberculosis? | 1 ‐ Yes 0 ‐ No |
| Target condition? Tuberculous meningitis? | 1 ‐ Yes 0 ‐ No |
| Comments about study design | |
| III. Patient characteristics and setting | |
| Description of study population (age, HIV info, etc.) | 1 – All enrolled 2 – All analysed 9 – Unknown/Not reported |
| Age: median, mean, range by months | Enter number or 9 – Unknown/Not reported |
| Gender | ##/total and % female |
| HIV status of participants | 0 – HIV‐ 1 – HIV+ 2 – Both HIV+/‐ 9 – Unknown/Not reported |
| If HIV‐positive participants included, what is the percentage? | % and specify numerator/denominator |
| Type of respiratory specimen included | 1 – All expectorated 2 – All induced 3 – All bronchoalveolar lavage 4 – All gastric lavage 5 – Nasopharyngeal aspirate 6 ‐ Stool 7 – Multiple types 8 – Other 9 – Unknown/Not reported If 7 or 8, describe types and record numbers: |
| Type of non‐respiratory specimen | 1 – Fine needle aspirate 2 – Lymph node biopsy 3 – Cerebrospinal fluid 4 – Multiple types 5 ‐ Other 9 ‐ Unknown/Not reported If 4 or 5, describe types and record numbers: |
| Were Xpert sample and culture obtained from same specimen? | 1 – Yes 0 – No 9 – Unknown/Not reported |
| Number of cultures used to exclude tuberculosis | Describe |
| Information on smear microscopy: was it used? | 1 – Yes 0 – No 9 – Unknown/Not reported |
| Type of microscopy used | 1 – Ziehl‐Neelsen
2 – Fluoresence microscopy
3 ‐ Light emitting diode‐based fluorescence microscopy 4 ‐ Multiple, describe: 9 – Unknown/Not reported |
| Smear type | 1 – Direct 2 – Concentrated (processed) 3 ‐ Both direct and concentrated 9 – Unknown/Not reported |
| Data on culture performance provided? | # of contaminated culture/Total # cultures performed or 9 ‐ Unknown/Not reported |
| Were patient‐important outcomes evaluated? (time to diagnosis, time to treatment, others) | 1 – Yes 2 – No 9 – Unknown/Not reported |
| Time to diagnosis? | Xpert: Culture: 9 – Unknown/Not reported Specify whether time from sample collection to diagnosis in lab or just turnaround time in lab |
| Time to treatment initiation | Xpert: Culture: 9 – Unknown/Not reported |
| Clinical setting, describe as written in the paper | 1 – Outpatient 2 – Inpatient 3 – Both outpatient and inpatient 4 – Other, specify 5 – Laboratory based 9 – Unknown/Not reported Describe as in paper: |
| Laboratory services level | 1 ‐ Central (reference) 2 ‐ Intermediate (regional) 3 ‐ Peripheral (microscopy centre, provincial hospital) 4 – Research laboratory 5 ‐ Other, specify |
| Where were Xpert tests performed? (tests generally available at different laboratory levels, although tests may overlap) Peripheral: acid‐fast bacilli (Ziehl‐Neelsen, Auramine‐rhodamine, Auramine‐O staining) and Xpert MTB/RIF Intermediate: peripheral laboratory tests and culture on solid media and line probe assay (LPA) from smear‐positive sputum Central: intermediate laboratory tests and culture on liquid media and DST (1st‐line and 2nd‐line anti‐tuberculosis drugs) on solid or in liquid media and LPA on positive cultures and rapid speciation tests | 1 ‐ Central (reference) 2 ‐ Intermediate (regional) 3 ‐ Peripheral (microscopy centre, provincial hospital) 4 ‐ Other, specify |
| Was Xpert run outside of a laboratory? | 1 ‐ Yes 0 ‐ No |
| Current treatment: were patients on treatment (defined as tuberculosis drugs for longer than 7 days) for the current tuberculosis episode? (note: may impact culture results) | 1 – Yes 2 – No 9 – Unknown/Not reported |
| If so, what is the percentage? | % Specify numerator/denominator |
| IV. Index test | |
| Xpert cartridge(s) evaluated | 1 ‐ Xpert only 2 ‐ Ultra only 3 ‐ Any combination Xpert and Ultra |
| Xpert platform: was Omni used? Unless Omni was explicitly described, assume standard platform | 1 – Yes, only Omni used for Xpert tests 2 – Yes, both Omni and standard platform used for Xpert tests 3 ‐ No |
| Pretreatment processing procedure for GeneXpert | 1 – None 2 – NALC‐NaOH 3 – NaOH (Petroff) 4 – Other 9 – Unknown/Not reported |
| For Xpert specimen, what was the condition of the specimen when tested? | 1 – Fresh 2 – Frozen 9 – Unknown/Not reported |
| Were uninterpretable (invalid error or no result) results reported for Xpert for tuberculosis detection? | 1 – Yes 9 – Unknown/Not reported If yes, describe numbers: |
| Were indeterminate results reported for Xpert for rifampicin resistance? | 1 – Yes 9 – Unknown/Not reported If yes, describe numbers: |
| V. Reference standard | |
| For tuberculosis detection, what reference standard(s) was used? Respiratory samples? | 1 – Solid culture (specify 1a) 2 – Liquid culture (specify 2a) 3 – Both solid and liquid culture (specify 1a and 2a) 9 – Unknown/Not reported 1a ‐ Solid culture LJ 7H10 7H11 Other 2a – Liquid culture MGIT 960 Other (specify): |
| For tuberculosis detection, what reference standard(s) was used? Lymph node? | 1 – Solid culture (specify 1a) 2 – Liquid culture (specify 2a) 3 – Both solid and liquid culture (specify 1a and 2a) 9 – Unknown/Not reported 1a ‐ Solid culture LJ 7H10 7H11 Other 2a – Liquid culture MGIT 960 Other (specify): |
| For tuberculosis detection, what reference standard(s) was used? Cerebrospinal fluid? | 1 – Solid culture (specify 1a) 2 – Liquid culture (specify 2a) 3 – Both solid and liquid culture (specify 1a and 2a) 9 – Unknown/Not reported 1a ‐ Solid culture LJ 7H10 7H11 Other (specify): 2a – Liquid culture MGIT 960 Other (specify): |
| Reference standard pulmonary tuberculosis: clinical | 1 ‐ Yes 0 – No Multiple answers, list: |
| If clinical, describe as in paper | |
| For rifampicin resistance detection, what reference standard(s) was used? Respiratory samples? |
1 – Solid culture (specify 1a) 2 – Liquid culture (specify 2a) 3 – Both solid and liquid culture (specify 1a and 2a) 4 – M tuberculosis DR plus 9 – Unknown/Not reported 1a ‐ Solid culture LJ 7H10 7H11 Other: Specify method (e.g. proportion): 2a – Liquid culture MGIT 960 Other (specify): |
| For rifampicin resistance detection, what reference standard(s) was used? Lymph node? | 1 – Solid culture (specify 1a) 2 – Liquid culture (specify 2a) 3 – Both solid and liquid culture (specify 1a and 2a) 4 – M tuberculosis DR plus 9 – Unknown/Not reported 1a ‐ Solid culture LJ 7H10 7H11 Other: Specify method (e.g. proportion): 2a – Liquid culture MGIT 960 Other (specify): |
| For rifampicin resistance detection, what reference standard(s) was used? Cerebrospinal fluid? | 1 – Solid culture (specify 1a) 2 – Liquid culture (specify 2a) 3 – Both solid and liquid culture (specify 1a and 2a) 4 – M tuberculosis DR plus 9 – Unknown/Not reported 1a ‐ Solid culture LJ 7H10 7H11 Other: Specify method (e.g. proportion): 2a – Liquid culture MGIT 960 Other (specify): |
| If information is available | |
| Is information on quality assurance of DST available in the study? | 1 – Yes 2 ‐ No 9 – Unknown/Not reported If yes, describe potential sources of bias |
DST: drug susceptibility testing; IGRA: interferon‐gamma release assay; LJ: Löwenstein‐Jensen; LPA: line probe assay; MDR: multidrug‐resistant; MGIT: mycobacterial growth indicator tube; M tuberculosis: Mycobacterium tuberculosis; NALC: N‐acetyl‐L‐cysteine; NaOH: sodium hydroxide; TST: tuberculin skin test; WHO: World Health Organization.
Appendix 3. Example of 2 × 2 result table
| Pulmonary tuberculosis, Xpert Ultra |
Tuberculosis, culture | |||
| Xpert Ultra in sputum | Yes | No | Total | |
| Positive | ||||
| Negative | ||||
| Total | ||||
| Pulmonary tuberculosis, Xpert Ultra | Tuberculosis, CRS | |||
| Xpert Ultra in sputum | Yes | No | Total | |
| Positive | ||||
| Negative | ||||
| Total | ||||
| Pulmonary tuberculosis, Xpert Ultra, < 1 year | Tuberculosis, culture | |||
| Xpert Ultra in sputum | Yes | No | Total | |
| Positive | ||||
| Negative | ||||
| Total | ||||
CRS: composite reference standard.
Appendix 4. QUADAS‐2 review‐specific guidance
Domain 1 – Patient selection
Risk of bias: could the selection of patients have introduced bias?
Signalling question 1: was a consecutive or random sample of patients enrolled?
We answered 'yes' if the study enrolled a consecutive or random sample of eligible patients; 'no' if the study selected patients by convenience; and 'unclear' if the study did not report the manner of patient selection or if we could not tell.
Signalling question 2: did the study avoid inappropriate exclusions?
For pulmonary tuberculosis, we answered 'yes' for all studies because we did not think there were any inappropriate exclusions for children presumed to have pulmonary tuberculosis. For tuberculous meningitis and lymph node tuberculosis, we answered 'no' if the study excluded specimens based on physical appearance (such as purulence) or a biochemical analysis (e.g. adenosine deaminase (ADA) or cell analysis). We answered 'unclear' if we could not tell.
Applicability: are there concerns that the included patients and setting do not match the review question?
We are interested in how Xpert Ultra performs in patients who were evaluated as they would be in routine practice. Paediatric studies conducted in tertiary centres tend to include a larger number of children with advanced disease; therefore, we answered 'low concern' if patients were evaluated in local hospitals or primary care centres; 'high concern' if patients were evaluated exclusively as inpatients in tertiary care centres; and 'unclear concern' if the clinical setting was not reported or if information was insufficient to justify a decision. We also answered 'unclear concern' if Xpert Ultra testing was done at a reference laboratory and the clinical setting was not reported, because it is difficult to tell if a given reference laboratory provides services mainly to very sick patients (inpatients in tertiary care) or to patients with a broad spectrum of disease, including very sick patients and those with less severe disease (primary, secondary, and tertiary care).
Domain 2 – Index test
Risk of bias: could the conduct or interpretation of the index test have introduced bias?
Signalling question 1: were the index test results interpreted without knowledge of results of the reference standard?
We answered 'yes' for all studies because Xpert Ultra test results are automatically generated, and the user is provided with printable test results; thus, there is no room for subjective interpretation of test results.
Signalling question 2: if a threshold was used, was it pre‐specified?
The threshold is pre‐specified in Xpert Ultra. We answered 'yes' for all studies.
Applicability: are there concerns that the index test, its conduct, or its interpretation differs from the review question?
Variations in test technology, execution, or interpretation may affect estimates of the diagnostic accuracy of a test. GeneXpert, the test device platform, simplifies molecular testing by fully integrating and automating the three processes (sample preparation, amplification, and detection) required for real‐time polymerase chain reaction (PCR)‐based molecular testing. All steps in the Xpert Ultra assay are completely automated and self‐contained following sample loading. Minimal training is required for operators such as laboratory technicians and nurses to run the index test.
For pulmonary tuberculosis, we answered 'low concern' if the index test was performed as recommended by the manufacturer. For sputum specimens, we answered 'unclear concern' if the ratio of the Xpert Ultra Sample Reagent to specimen volume was not 2:1 for a raw specimen or 3:1 for a centrifuged sediment, as recommended by the manufacturer, or if we could not tell (Cepheid 2018). Central‐level laboratories use more highly trained staff than peripheral‐ and intermediate‐level laboratories or health facilities. However, we do not consider this to be an applicability concern, as only minimal training is required to run the index tests. We judged unclear concern for all studies that evaluated stool because there is no established technique for stool processing prior to performing Xpert Ultra.
With respect to extrapulmonary specimens, the WHO has provided detailed information about processing steps in the 'Xpert MTB/RIF implementation manual. Technical and operational "how‐to" practical considerations. Annex 2 – Standard operating procedure (SOP) for processing extrapulmonary specimens (CSF, lymph nodes, and other tissues) for "Xpert MTB/RIF assay"' (WHO 2014). We considered these SOPs when addressing concerns about applicability for Xpert Ultra. For extrapulmonary specimens, we answered 'low concern' if the test was performed according to WHO SOPs. We answered 'high concern' if the test was performed in a way that deviated from these recommendations. We answered 'unclear concern' if we could not tell.
Domain 3 – Reference standard
Risk of bias: could the reference standard, its conduct, or its interpretation have introduced bias?
Signalling question 1.a: is a culture reference standard likely to correctly classify the target condition?
For pulmonary tuberculosis, tuberculous meningitis, and lymph node tuberculosis, we anticipated that the vast majority of studies would perform culture. Culture is generally considered the best reference standard for tuberculosis diagnosis. However, particularly in children with paucibacillary disease, tuberculosis is verified by culture in only 15% to 50% of cases, depending on disease severity, challenges of obtaining specimens, and resources (Graham 2015). Evaluation of multiple specimens may increase the yield of culture for confirming tuberculosis (Cruz 2012;Zar 2012). We answered 'yes' for studies using multiple specimens and 'unclear' for studies using only one specimen.
Signalling question 1.b: is the composite reference standard likely to correctly classify the target condition?
A composite reference standard aims to classify children who were not detected by culture. The definition of the composite reference standard is heterogeneous across studies. Irrespective of how tuberculosis was defined in the publications, we classified children as having tuberculosis if they were presumed to have tuberculosis and were started on anti‐tuberculosis treatment. For a composite reference standard, we answered 'unclear" for all studies.
For rifampicin resistance, we answered 'yes' if a study used phenotypic culture‐based drug susceptibility testing or MTBDRplus as the reference standard. As this is an inclusion criterion for the review, we answered 'yes' for all studies.
Signalling question 2: were the reference standard results interpreted without knowledge of results of the index test?
For pulmonary tuberculosis, tuberculous meningitis, and lymph node tuberculosis, we answered 'yes' if the reference test provided an automated result (e.g. MGIT 960); blinding was explicitly stated; or it was clear that the reference standard was performed at a separate laboratory or performed by different people. We answered 'no' if the study stated that the reference standard result was interpreted with knowledge of the Xpert Ultra test result. We answered 'unclear' if we could not tell.
For rifampicin resistance, we answered 'yes' if the reference test provided an automated result (e.g. MGIT 960 SIRE); blinding was explicitly stated; or it was clear that the reference standard was performed at a separate laboratory or performed by different people. We answered 'no' if the study stated that the reference standard result was interpreted with knowledge of the Xpert Ultra test result. We answered 'unclear' if we could not tell.
Applicability: are there concerns that the target condition as defined by the reference standard does not match the question?
For pulmonary tuberculosis, tuberculous meningitis, and lymph node tuberculosis, we answered 'high concern' if the included studies did not differentiate Mycobacterium tuberculosis complex isolated in culture from other mycobacteria using any speciation technique; 'low concern' if speciation was performed using any technique; and 'unclear concern' if we could not tell.
For rifampicin resistance, we considered applicability to be of 'low concern' for all studies because the method used (phenotypic culture‐based drug susceptibility testing or MTBDRplus) is appropriate.
Domain 4 – Flow and timing
Risk of bias: could the patient flow have introduced bias?
Signalling question 1: was there an appropriate interval between the index test and the reference standard?
We expected to find for most included studies that specimens for Xpert and culture were obtained at the same time when patients were evaluated for presumed tuberculosis. Even if there were a delay of several days between index test and reference standards, tuberculosis is a chronic disease, and we consider misclassification of disease status to be unlikely, as long as treatment was not initiated in the interim. We answered 'yes' if the index test and the reference standard were performed at the same time, or if the time interval was less than or equal to seven days; 'no' if the time interval was greater than seven days; and 'unclear' if we could not tell.
Signalling question 2: did all patients receive the same reference standard?
We answered 'yes' if all patients received the same reference standard; 'no' if all patients did not receive the same reference standard; and 'unclear' if we could not tell.
Signalling question 3: were all patients included in the analysis?
We determined the answer to this question by comparing the number of patients enrolled with the number of patients included in the 2 × 2 tables. We answered 'yes' if the numbers matched, and 'no' if there were patients enrolled in the study who were not included in the analysis. We answered 'unclear' if we could not tell.
Judgements for risk of bias assessments for a given domain.
If we answered all signalling questions for a domain 'yes', then judged risk of bias as 'low'.
If we answered all or most signalling questions for a domain 'no', then we judged risk of bias as 'high'.
If we answered only one signalling question for a domain 'no', we discussed further the risk of bias judgement.
If we answered all or most signalling questions for a domain 'unclear', then we judged risk of bias as 'unclear'.
If we answered only one signalling question for a domain 'unclear', we discussed further the risk of bias judgement.
Appendix 5. Risk of bias and applicability concerns graph
10.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.

Xpert Ultra, sputum, all ages, culture
2. Test.

Xpert Ultra, sputum, all ages, composite reference standard
3. Test.

Xpert Ultra, sputum, < 1 year, culture
4. Test.

Xpert Ultra, sputum, < 1 year, composite reference standard
5. Test.

Xpert Ultra, sputum, 1–4 years, culture
6. Test.

Xpert Ultra, sputum, 1–4 years, composite reference standard
7. Test.

Xpert Ultra, sputum, 5–9 years, culture
8. Test.

Xpert Ultra, sputum, 5–9 years, composite reference standard
9. Test.

Xpert Ultra, sputum, 10–14 years, culture
10. Test.

Xpert Ultra, sputum, 10–14 years, composite reference standard
11. Test.

Xpert Ultra, sputum, 0–9 years, culture
12. Test.

Xpert Ultra, sputum, 0–9 years, composite reference standard
13. Test.

Xpert Ultra, sputum, HIV‐positive, all ages, culture
14. Test.

Xpert Ultra, sputum, HIV‐positive, all ages, composite reference standard
15. Test.

Xpert Ultra, sputum, HIV‐negative, all ages, culture
16. Test.

Xpert Ultra, sputum, HIV‐negative, all ages, composite reference standard
17. Test.

Xpert Ultra, sputum, HIV‐positive, 0–9 years, culture
18. Test.

Xpert Ultra, sputum, HIV‐positive, 0–9 years, composite reference standard
19. Test.

Xpert Ultra, sputum, HIV‐negative, 0–9 years, culture
20. Test.

Xpert Ultra, sputum, HIV‐negative, 0–9 years, composite reference standard
21. Test.

Xpert Ultra, sputum, severe pneumonia, all ages, culture
22. Test.

Xpert Ultra, sputum, severe pneumonia, all ages, composite reference standard
23. Test.

Xpert Ultra, sputum, severe pneumonia, 0–9 years, culture
24. Test.

Xpert Ultra, sputum, severe pneumonia, 0–9 years, composite reference standard
25. Test.

Xpert Ultra, sputum, severe malnutrition, all ages, culture
26. Test.

Xpert Ultra, sputum, severe malnutrition, all ages, composite reference standard
27. Test.

Xpert Ultra, sputum, severe malnutrition, 0–9 years, culture
28. Test.

Xpert Ultra, sputum, severe malnutrition, 0–9 years, composite reference standard
29. Test.

Xpert Ultra, gastric aspirate, all ages, culture
30. Test.

Xpert Ultra, gastric aspirate, all ages, composite reference standard
31. Test.

Xpert Ultra, gastric aspirate, < 1 year, culture
32. Test.

Xpert Ultra, gastric aspirate, < 1 year, composite reference standard
33. Test.

Xpert Ultra, gastric aspirate, 1–4 years, culture
34. Test.

Xpert Ultra, gastric aspirate, 1–4 years, composite reference standard
35. Test.

Xpert Ultra, gastric aspirate, 5–9 years, culture
36. Test.

Xpert Ultra, gastric aspirate, 5–9 years, composite reference standard
37. Test.

Xpert Ultra, gastric aspirate, 10–14 years, culture
38. Test.

Xpert Ultra, gastric aspirate, 10–14 years, composite reference standard
39. Test.

Xpert Ultra, gastric aspirate, 0–9 years, culture
40. Test.

Xpert Ultra, gastric aspirate, 0–9 years, culture, trace results excluded
41. Test.

Xpert Ultra, gastric aspirate, 0–9 years, composite reference standard
42. Test.

Xpert Ultra, gastric aspirate, HIV‐positive, all ages, culture
43. Test.

Xpert Ultra, gastric aspirate, HIV‐positive, all ages, composite reference standard
44. Test.

Xpert Ultra, gastric aspirate, HIV‐negative, all ages, culture
45. Test.

Xpert Ultra, gastric aspirate, HIV‐negative, all ages, composite reference standard
46. Test.

Xpert Ultra, gastric aspirate, HIV positive, 0–9 years, culture
47. Test.

Xpert Ultra, gastric aspirate, HIV positive, 0–9 years, composite reference standard
48. Test.

Xpert Ultra, gastric aspirate, HIV negative, 0–9 years, culture
49. Test.

Xpert Ultra, gastric aspirate, HIV negative, 0–9 years, composite reference standard
50. Test.

Xpert Ultra, gastric aspirate, severe malnutrition, all ages, culture
51. Test.

Xpert Ultra, gastric aspirate, severe malnutrition, all ages, composite reference standard
52. Test.

Xpert Ultra, gastric aspirate, severe malnutrition, 0–9 years, culture
53. Test.

Xpert Ultra, gastric aspirate, severe malnutrition, 0–9 years, composite reference standard
54. Test.

Xpert Ultra, stool, all ages, culture
55. Test.

Xpert Ultra, stool, all ages, composite reference standard
56. Test.

Xpert Ultra, stool, < 1 year, culture
57. Test.

Xpert Ultra, stool, < 1 year, composite reference standard
58. Test.

Xpert Ultra, stool, 1–4 years, culture
59. Test.

Xpert Ultra, stool, 1–4 years, composite reference standard
60. Test.

Xpert Ultra, stool, 5–9 years, culture
61. Test.

Xpert Ultra, stool, 5–9 years, composite reference standard
62. Test.

Xpert Ultra, stool, 10–14 years, culture
63. Test.

Xpert Ultra, stool, 10–14 years, composite reference standard
64. Test.

Xpert Ultra, stool, 0–9 years, culture
65. Test.

Xpert Ultra, stool, 0–9 years, culture, trace results excluded
66. Test.

Xpert Ultra, stool, 0–9 years, composite reference standard
67. Test.

Xpert Ultra, stool, HIV‐positive, all ages, culture
68. Test.

Xpert Ultra, stool, HIV‐negative, all ages, culture
69. Test.

Xpert Ultra, stool, HIV‐positive, 0–9 years, culture
70. Test.

Xpert Ultra, stool, severe malnutrition, all ages, culture
71. Test.

Xpert Ultra, stool, severe malnutrition, all ages, composite reference standard
72. Test.

Xpert Ultra, stool, severe malnutrition, 0–9 years, culture
73. Test.

Xpert Ultra, stool, severe malnutrition, 0–9 years, composite reference standard
74. Test.

Xpert Ultra, nasopharyngeal aspirate, all ages, culture
75. Test.

Xpert Ultra, nasopharyngeal aspirate, all ages, composite reference standard
76. Test.

Xpert Ultra, nasopharyngeal aspirate, < 1 year, culture
77. Test.

Xpert Ultra, nasopharyngeal aspirate, < 1 year, composite reference standard
78. Test.

Xpert Ultra, nasopharyngeal aspirate, 1–4 years, culture
79. Test.

Xpert Ultra, nasopharyngeal aspirate, 1–4 years, composite reference standard
80. Test.

Xpert Ultra, nasopharyngeal aspirate, 5–9 years, culture
81. Test.

Xpert Ultra, nasopharyngeal aspirate, 5–9 years, composite reference standard
82. Test.

Xpert Ultra, nasopharyngeal aspirate, 10–14 years, culture
83. Test.

Xpert Ultra, nasopharyngeal aspirate, 10–14 years, composite reference standard
84. Test.

Xpert Ultra, nasopharyngeal aspirate, 0–9 years, culture
85. Test.

Xpert Ultra, nasopharyngeal aspirate, 0–9 years, composite reference standard
86. Test.

Xpert Ultra, nasopharyngeal aspirate, HIV‐positive, all ages, culture
87. Test.

Xpert Ultra, nasopharyngeal aspirate, HIV‐positive, all ages composite reference standard
88. Test.

Xpert Ultra, nasopharyngeal aspirate, HIV‐negative, culture
89. Test.

Xpert Ultra, nasopharyngeal aspirate, HIV‐negative, composite reference standard
90. Test.

Xpert Ultra, nasopharyngeal aspirate, HIV‐positive, 0–9 years, culture
91. Test.

Xpert Ultra, nasopharyngeal aspirate, HIV‐positive, 0–9 years, composite reference standard
92. Test.

Xpert Ultra, nasopharyngeal aspirate, HIV‐negative, 0–9 years, culture
93. Test.

Xpert Ultra, nasopharyngeal aspirate, HIV‐negative, 0–9 years, composite reference standard
94. Test.

Xpert Ultra, nasopharyngeal aspirate, severe pneumonia, all ages, culture
95. Test.

Xpert Ultra, nasopharyngeal aspirate, severe pneumonia, all ages, composite reference standard
96. Test.

Xpert Ultra, nasopharyngeal aspirate, severe pneumonia, 0–9 years, culture
97. Test.

Xpert Ultra, nasopharyngeal aspirate, severe pneumonia, 0–9 years, composite reference standard
98. Test.

Xpert Ultra, nasopharyngeal aspirate, severe malnutrition, all ages, culture
99. Test.

Xpert Ultra, nasopharyngeal aspirate, severe malnutrition, all ages, composite reference standard
TST-100. Test.

Xpert Ultra, nasopharyngeal aspirate, severe malnutrition, 0–9 years, culture
TST-101. Test.

Xpert Ultra, nasopharyngeal aspirate, severe malnutrition, 0–9 years, composite reference standard
TST-102. Test.

Xpert Ultra, nasopharyngeal, rifampicin resistance
TST-103. Test.

Xpert Ultra, all specimens, rifampicin resistance
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barcellini 2019.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive enrolment | ||
| Patient characteristics and setting | Presenting signs and symptoms: screening cohort but 891 participants had at least one positive TB symptom, only 3 of whom were children Age: median 10 years Sex, female: 33% HIV infection: 0% Sample size included for analysis: 3 Clinical setting: outpatient Laboratory level where index test was performed: central reference laboratory Country: Italy World Bank income classification: high income TB high‐burden country: no TB/HIV high‐burden country: no MDR‐TB high‐burden country: no Prevalence of TB cases in the study: 0/3 (0%) | ||
| Index tests | Xpert Ultra in sputum | ||
| Target condition and reference standard(s) | Pulmonary TB; microbiological reference standard (liquid and solid culture) | ||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | This was a nested diagnostic evaluation within a screening study. Only 3 children screened positive, and of those, all were evaluated. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Jaganath 2021.
| Study characteristics | |||
| Patient Sampling | Prospective cohort, consecutive enrolment | ||
| Patient characteristics and setting | Presenting signs and symptoms: cough for ≥ 1 week and ≥ 2 of the following: unexplained weight loss or failure to thrive; unexplained fever for ≥ 1 week; unexplained lethargy or reduced playfulness for ≥ 1 week; an abnormal CXR; or contact with an individual with pulmonary TB Age: median 3.9 (IQR 1.5–7) years Sex, female: 47.9% HIV infection: 13% Sample size included for analysis: 213 Clinical setting: outpatient and inpatient Laboratory level where index test was performed: academic hospital Country: Uganda World Bank income classification: low income TB high‐burden country: no TB/HIV high‐burden country: yes MDR‐TB high‐burden country: no Prevalence of TB cases in the study: 23 (10.8%) confirmed TB, 88 (41.3%) unconfirmed TB | ||
| Index tests | Xpert Ultra in sputum, gastric, and nasopharyngeal specimens | ||
| Target condition and reference standard(s) | Pulmonary TB; microbiological reference standard (liquid and solid culture) and composite reference standard | ||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | The exclusions accounted for a low percentage of the total study population | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
Kabir 2020.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive enrolment | ||
| Patient characteristics and setting | Presenting signs and symptoms: persistent, nonremitting cough for > 2 weeks not responding to conventional antibiotics; persistent documented fever (> 38°C/100.4°F) for > 2 weeks; documented weight loss or not gaining weight adequately during the past 3 months; and fatigue, reduced playfulness, and decreased activity Age: 0–4 years: 296 (66.2%) children, 5–9 years: 105 (23.5%) children, 10–14 years: 46 (10.3%) children Sex, female: 193 (42%) HIV infection: not reported Sample size included for analysis: 447 Clinical setting: exclusively inpatient tertiary/specialized hospital Laboratory level where index test was performed: academic hospital Country: Bangladesh World Bank income classification: lower middle income TB high‐burden country: yes TB/HIV high‐burden country: no MDR‐TB high‐burden country: yes Prevalence of TB cases in the study: 29 (6.5%) confirmed by reference standard, 72 (16.1%) confirmed by reference standard or index test, 39 (8.9%) diagnosed by composite reference standard, 111 (24.8%) total cases by microbiologic or composite reference standard | ||
| Index tests | Xpert Ultra in sputum and stool | ||
| Target condition and reference standard(s) | Pulmonary TB; microbiological reference standard (solid culture) and composite reference standard | ||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | Only 7 participants were excluded from the main analysis because they could not provide stool. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
Liu 2021.
| Study characteristics | |||
| Patient Sampling | Prospective cohort, consecutive enrolment | ||
| Patient characteristics and setting | Presenting signs and symptoms: pulmonary lesions in chest imaging (X‐ray or CT scan) including inflammatory infiltration, nodules, cavities, and mediastinal lymphadenopathy, irrespective of close TB exposure history or immunologic evidence (tuberculin skin test or interferon gamma release assay) Age: mean 4.15 (SD 4.16) years Sex, female: 55/126 (44%) HIV infection: 0/126 (0%) Sample size included for analysis: 311 Clinical setting: both inpatient and outpatient Laboratory level where index test was performed: intermediate laboratory Country: China World Bank income classification: upper middle income TB high‐burden country: yes TB/HIV high‐burden country: yes MDR‐TB high‐burden country: yes Prevalence of TB cases in the study: 53.2% confirmed TB, 16.7% unconfirmed | ||
| Index tests | Xpert Ultra in sputum, gastric, stool, and nasopharyngeal specimens | ||
| Target condition and reference standard(s) | Pulmonary TB; microbiological reference standard (liquid culture performed on a sputum or gastric specimen) Rifampicin resistance; MGIT drug susceptibility testing |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | Most enrolled children were not included in the analysis. Participants needed to be willing to provide a stool sample for the purposes of the study and have routine testing respiratory samples, including Xpert, culture, or smear for acid‐fast bacilli. Participants with a definite diagnosis of TB were excluded. Other exclusion criteria included inadequate or invalid sample for the parallel assays, incomplete clinical data or indeterminate clinical diagnosis, and history of anti‐TB therapy | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
NCT04121026.
| Study characteristics | |||
| Patient Sampling | Prospective cohort, consecutive enrolment | ||
| Patient characteristics and setting | Presenting signs and symptoms: ≥ 1 criteria among the following: persistent cough > 2 weeks; persistent fever > 2 weeks; recent failure to thrive (documented clear deviation from a previous growth trajectory in the last 3 months or Z score weight/age < 2); failure of broad spectrum antibiotics for treatment of pneumonia; suggestive CXR features or history of contact with a TB case and any of several symptoms listed above with a shorter duration (< 2 weeks)
Age: median 5.46 (IQR 2.02–9.86) years
Sex, female: 47.60%
HIV infection: 100% (all participants HIV positive)
Sample size included for analysis: 124
Clinical setting: both inpatient and outpatient
Laboratory level where index test was performed: central reference laboratory
Countries: Côte d'Ivoire, Uganda, Mozambique, and Zambia
World Bank income classification: Côte d'Ivoire, Zambia: lower middle income; Mozambique, Uganda: low income
TB high‐burden country: only Mozambique and Zambia
TB/HIV high‐burden country: only Mozambique, Uganda, and Zambia
MDR‐TB high‐burden country: only Mozambique
Prevalence of TB cases in the study: 5% Stage of study when the data were provided: recruitment ongoing |
||
| Index tests | Xpert Ultra in sputum, gastric aspirate, stool, and nasopharyngeal aspirate | ||
| Target condition and reference standard(s) | Pulmonary TB; microbiological reference standard (multiple liquid cultures) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
NCT04203628.
| Study characteristics | |||
| Patient Sampling | Prospective cohort, consecutive enrolment | ||
| Patient characteristics and setting | Presenting signs and symptoms: clinical suspicion of active pulmonary TB, irrespective of extrapulmonary disease (CXR suggestive of TB; or weight loss or failure to thrive within 3 months not solely due to inadequate feeding, or another non‐TB cause; or any cough with loss of weight; or cough alone ≥ 14 days or persistent (> 1 week) and unexplained fever) or microbiological confirmation of active TB disease referred from non‐study health facilities
Age: median 1.5 (IQR 0.9–3.8) years
Sex, female: 47%
HIV infection: 4/111 (3.6%)
Sample size included for analysis: 111
Clinical setting: outpatient and inpatient
Laboratory level where index test was performed: academic hospital
Countries: Uganda and Zambia
World Bank income classification: low income
TB high‐burden country: yes
TB/HIV high‐burden country: yes
MDR‐TB high‐burden country: no
Prevalence of TB cases in the study: 3 (5%) confirmed TB Stage of study when the data were provided: recruitment ongoing |
||
| Index tests | Xpert Ultra in stool | ||
| Target condition and reference standard(s) | Pulmonary TB; microbiological reference standard (multiple liquid cultures) | ||
| Flow and timing | Index and reference tests were performed within the pre‐specified time period | ||
| Comparative | |||
| Notes | The exclusions accounted for a low percentage of the total study population | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
NCT04240990.
| Study characteristics | |||
| Patient Sampling | Prospective cohort, consecutive enrolment | ||
| Patient characteristics and setting | Presenting signs and symptoms: severe acute malnutrition defined as weight‐for‐height Z score (WHZ) < ‐3 SD or mid‐upper arm circumference < 115 mm (in children > 6 months) or clinical signs of bilateral pitting oedema. Usual criteria for hospitalization of children with severe acute malnutrition recommended by the WHO include: medical complications including sepsis and dehydration, severe oedema, poor appetite, and presentation of ≥ 1 'Integrated Management of Childhood Illness' danger signs (unable to drink or breastfeed; vomiting everything; > 1 or prolonged convulsions lasting > 15 min; lethargic or unconscious; convulsing now)
Age: median 1.21 (IQR 0.83–1.60) years
Sex, female: 40.50%
HIV infection: 14.80%
Sample size included for analysis: 257
Clinical setting: exclusively inpatient tertiary/specialized hospital
Laboratory level where index test was performed: central reference laboratory
Countries: Uganda, Zambia
World Bank income classification: Zambia: lower middle income; Uganda: low income
TB high‐burden country: only Zambia
TB/HIV high‐burden country: yes
MDR‐TB high‐burden country: no
Prevalence of TB cases in the study: 3% Stage of study when the data were provided: recruitment ongoing |
||
| Index tests | Xpert Ultra in gastric aspirate, stool, and nasopharyngeal aspirate | ||
| Target condition and reference standard(s) | Pulmonary TB; microbiological reference standard (multiple liquid cultures) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
NCT04899076.
| Study characteristics | |||
| Patient Sampling | Prospective cohort, consecutive enrolment and referral | ||
| Patient characteristics and setting | Presenting signs and symptoms: clinical suspicion of active pulmonary TB, irrespective of extrapulmonary disease (CXR suggestive of TB; or weight loss or failure to thrive within 3 months not solely due to inadequate feeding, or another non‐TB cause; or any cough with loss of weight; or cough alone ≥ 14 days or persistent (> 1 week) and unexplained fever) or microbiological confirmation of active TB disease referred from non‐study health facilities
Age: mean 4.62 years
Sex, female: 48.5%
HIV infection: 19.7%
Sample size included for analysis: 486
Clinical setting: outpatient and inpatient
Laboratory level where index test was performed: academic hospital
Countries: India, Uganda, South Africa
World Bank income classification: India: lower middle income; Uganda: low income; South Africa: upper middle income
TB high‐burden country: only India and South Africa
TB/HIV high‐burden country: yes
MDR‐TB high‐burden country: only India and South Africa
Prevalence of TB cases in the study: 70 (17.8%) confirmed TB Stage of study when the data were provided: recruitment completed |
||
| Index tests | Xpert Ultra in stool | ||
| Target condition and reference standard(s) | Pulmonary TB; microbiological reference standard (liquid culture) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Children had cultures and Xpert Ultra performed in gastric aspirate and sputum specimens as a reference standard | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Nicol 2018.
| Study characteristics | |||
| Patient Sampling | Prospective cohort, manner of selection not reported, with analysis of frozen specimens | ||
| Patient characteristics and setting | Presenting signs and symptoms: cough lasting > 2 weeks and ≥ 1 of the following: household TB contact in previous 3 months; weight loss or failure to gain weight in previous 3 months; positive tuberculin skin test; or chest radiograph suggestive of pulmonary TB Age: median 33 (IQR 15–74) months Sex, female: 49% HIV infection: 19% Sample size included for analysis: 367 Clinical setting: both inpatient and outpatient Laboratory level where index test was performed: research laboratory Country: South Africa World Bank income classification: middle income TB high‐burden country: yes TB/HIV high‐burden country: yes MDR‐TB high‐burden country: yes Prevalence of TB cases in the study: 20% | ||
| Index tests | Xpert Ultra in sputum | ||
| Target condition and reference standard(s) | Pulmonary TB; microbiological reference standard (MGIT) and composite reference standard | ||
| Flow and timing | Index and reference tests were performed within pre‐specified time period | ||
| Comparative | |||
| Notes | The index test was performed on frozen specimens from a previously enrolled cohort | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Parigi 2021.
| Study characteristics | |||
| Patient Sampling | Unclear | ||
| Patient characteristics and setting | Presenting signs and symptoms: clinical suspicion of pulmonary TB Age: median 81.8 months Sex, female: not available HIV infection: not available Sample size included for analysis: 67 Clinical setting: exclusively inpatient tertiary/specialized hospital Laboratory level where index test was performed: academic hospital Country: Italy World Bank income classification: high income TB high‐burden country: no TB/HIV high‐burden country: no MDR‐TB high‐burden country: no Prevalence of TB cases in the study: 82.6% | ||
| Index tests | Xpert Ultra in gastric specimen | ||
| Target condition and reference standard(s) | Pulmonary TB; culture type not specified and composite reference standard Rifampicin resistance; drug susceptibility testing |
||
| Flow and timing | Index and reference tests were performed within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Sabi 2018.
| Study characteristics | |||
| Patient Sampling | Prospective cohort, consecutive enrolment, with analysis of frozen specimens | ||
| Patient characteristics and setting | Presenting signs and symptoms: 1 of the following symptoms: persistent non‐remitting cough > 14 days not responding to antibiotics; repeated episodes of fever within the last 14 days not responding to antibiotics, after malaria has been excluded; weight loss or failure to thrive during previous 3 months; signs and symptoms suggestive of extrapulmonary TB Age: median 65 (IQR 18–120) months Sex, female: 43% HIV infection: 52% Sample size included for analysis: 215 Clinical setting: both inpatient and outpatient Laboratory level where index test was performed: academic hospital Country: Tanzania World Bank income classification: lower middle income TB high‐burden country: no TB/HIV high‐burden country: yes MDR‐TB high‐burden country: no Prevalence of TB cases in the study: 13% | ||
| Index tests | Xpert Ultra in sputum | ||
| Target condition and reference standard(s) | Pulmonary TB; microbiological reference standard (solid (LJ) and liquid (MGIT) culture) and composite reference standard | ||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | Xpert Ultra was performed on frozen specimens | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Ssengooba 2020.
| Study characteristics | |||
| Patient Sampling | Prospective cohort, consecutive enrolment, with analysis of frozen specimens | ||
| Patient characteristics and setting | Presenting signs and symptoms: symptomatic but non‐severe TB including extrathoracic lymph node TB; intrathoracic uncomplicated (hilar) lymph node TB; minimal or no parenchymal abnormality on CXR; smear‐negative on gastric aspirate/other respiratory sample Age: median 2.8 (IQR 1.2–5.3) years Sex, female: 52% HIV infection: 8.5% Sample size included for analysis: 398 Clinical setting: unclear Laboratory level where index test was performed: academic hospital Country: Uganda World Bank income classification: low income TB high‐burden country: no TB/HIV high‐burden country: yes MDR‐TB high‐burden country: no Prevalence of TB cases in the study: 18.7% with confirmed pulmonary TB by the reference tests | ||
| Index tests | Xpert Ultra in sputum and gastric aspirate | ||
| Target condition and reference standard(s) | Pulmonary TB; microbiological reference standard (solid (LJ) and liquid (MGIT) culture) | ||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | Symptomatic children who provided samples in the trial screening process but were not subsequently enrolled in the clinical trial were also eligible for inclusion in this substudy. A low percentage (~10%) of enrolled participants were not included in the analysis | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
Sun 2020.
| Study characteristics | |||
| Patient Sampling | Prospective cohort, consecutive enrolment, with analysis of frozen specimens | ||
| Patient characteristics and setting | Presenting signs and symptoms: thought to have TB based on cough lasting > 2 weeks, weight loss, malnutrition, HIV, TB contact, or positive chest radiograph in accordance with the China and WHO guidelines Age: median 6.4 (IQR 2.1–10.7) years Sex, female: 44% HIV infection: not available Sample size included for analysis: 302 Clinical setting: unclear Laboratory level where index test was performed: unclear Country: China World Bank income classification: upper middle income TB high‐burden country: yes TB/HIV high‐burden country: yes MDR‐TB high‐burden country: yes Prevalence of TB cases in the study: 16% with confirmed pulmonary TB by the reference tests, 43% meeting a composite definition for TB |
||
| Index tests | Xpert Ultra in gastric specimens | ||
| Target condition and reference standard(s) | Pulmonary TB; microbiological reference standard (solid (LJ) and liquid (MGIT) culture) and composite reference standard | ||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
Zar 2019.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective with analysis of frozen specimens | ||
| Patient characteristics and setting | Presenting signs and symptoms: cough lasting > 2 weeks and at least 1 of the following: household TB contact in previous 3 months, weight loss or failure to gain weight in previous 3 months, positive tuberculin skin test, or chest radiograph suggestive of pulmonary TB Age: median 23.3 (IQR 13.5–47.3) months Sex, female: not reported HIV infection: 16% Sample size included for analysis: 195 Clinical setting: inpatient Laboratory level where index test was performed: intermediate Country: South Africa World Bank income classification: upper middle income TB high‐burden country: yes TB/HIV high‐burden country: yes MDR‐TB high‐burden country: yes Prevalence of TB cases in the study: 21% | ||
| Index tests | Xpert Ultra in nasopharyngeal aspirate | ||
| Target condition and reference standard(s) | Pulmonary TB; microbiological reference standard (MGIT) and composite reference standard Rifampicin resistance; LPA (MTBDRplus) |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | Xpert Ultra test was performed on frozen specimens | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
CXR: chest X‐ray; IQR: interquartile range; LJ: Löwenstein‐Jensen; LPA: line probe assay; MDR‐TB: multidrug‐resistant tuberculosis; MGIT: mycobacteria growth indicator tube; SD: standard deviation; TB: tuberculosis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ali 2017 | Unable to separate paediatric data from adult data |
| Atashi 2017 | Adult population |
| Atehortúa Muñoz 2017 | Not a diagnostic accuracy study |
| Azevedo 2018 | Adult population |
| Ballif 2015 | Not a diagnostic accuracy study |
| Banada 2016 | Case‐control study |
| Biadglegne 2014 | Unable to separate paediatric data from adult data |
| Bojang 2016 | Unable to separate paediatric data from adult data |
| Che 2017 | Adult population |
| Cox 2014 | Adult population |
| Cross 2014 | Unable to separate paediatric data from adult data |
| Diallo 2016 | Unable to separate paediatric data from adult data |
| DiNardo 2016 | Not a diagnostic accuracy study |
| DiNardo 2018 | Index test not studied |
| Ejeh 2018 | Unable to separate paediatric data from adult data |
| Gautam 2018 | Unable to separate paediatric data from adult data |
| Gelalcha 2017 | Unable to separate paediatric data from adult data |
| Geleta 2015 | Adult population |
| Ghariani 2015 | Unable to separate paediatric data from adult data |
| Giang 2015 | Unable to extract data by sample type |
| Guajardo‐Lara 2018 | Insufficient data |
| Gulla 2019 | Not a diagnostic accuracy study |
| Hakim 2017 | Not a diagnostic accuracy study |
| Helb 2010 | Adult population |
| Horo 2017 | Unable to separate paediatric data from adult data |
| Huh 2014 | Adult population |
| Kuyinu 2018 | Inappropriate reference standard |
| Lopez 2019 | Index text not studied |
| Lu J 2017 | Screening for clinical tuberculosis before enrolment |
| Lu Y 2018 | Adult population |
| Malik 2018 | Not a diagnostic accuracy study |
| Marcy 2018 | Duplicate data for Marcy 2016 |
| Masenga 2017 | Unable to separate paediatric data from adult data |
| Mekonnen 2015 | Adult population |
| Memon 2018 | Clinical diagnosis of tuberculosis established at enrolment |
| Metaferia 2018 | Inappropriate reference standard |
| Mijovic 2018 | Not a diagnostic accuracy study |
| Modi 2016 | Index test not studied |
| Mulenga 2015 | Index test not studied |
| Naidoo 2016 | Not a diagnostic accuracy study |
| Nair 2016 | Not a diagnostic accuracy study |
| Nansumba 2016 | Index test not studied |
| Nataprawira 2016 | Not a diagnostic accuracy study |
| NCT03831906 | Data is unpublished and incomplete |
| NCT04038632 | Data is unpublished and incomplete |
| Ncube 2017 | Not a diagnostic accuracy study |
| Nduba 2015 | Index text not studied |
| Ngabonziza 2016 | Adult population |
| Nicol 2020 | Index text not studied |
| Ntinginya 2012 | Not a diagnostic accuracy study |
| Opota 2019 | Adult population |
| Pandey 2017 | Unable to separate paediatric data from adult data |
| Pink 2016 | Unable to separate paediatric data from adult data |
| Planting 2014 | Not a diagnostic accuracy study |
| Raizada 2014 | Inappropriate reference standard |
| Raizada 2015a | Inappropriate reference standard |
| Raizada 2015b | Inappropriate reference standard |
| Raizada 2018a | Inappropriate reference standard |
| Raizada 2018b | Inappropriate reference standard |
| Rathour 2019 | Screening for clinical tuberculosis before enrolment |
| Rebecca 2018 | Case‐control study |
| Rivera 2017 | Not a diagnostic accuracy study |
| Sabi 2016 | Not a diagnotic accuracy study |
| Sachdeva 2015 | Not a diagnostic accuracy study |
| Sanchini 2014 | Not a diagnostic accuracy study |
| Sander 2019 | Adult population |
| Sanjuan‐Jimenez 2015 | Adult population |
| Schumacher 2016 | Not a diagnostic accuracy study |
| Scott 2014 | Unable to separate paediatric data from adult data |
| Shah 2016b | Insufficient data |
| Shah 2018 | Not a diagnostic accuracy study |
| Shah 2019 | Case‐control study |
| Sharma 2015 | Unable to separate paediatric data from adult data |
| Sieiro 2018 | Unable to separate paediatric data from adult data |
| Singh 2015 | Clinical diagnosis of tuberculosis established at enrolment |
| Singh 2016 | Adult population |
| Solomons 2016 | Not a diagnostic accuracy study |
| Sun 2019 | Index test not studied on specimen type included in review |
| Sureshbabu 2016 | Unable to separate paediatric data from adult data |
| Tadesse 2015 | Unable to separate paediatric data from adult data |
| Tafur 2018 | Not a diagnostic accuracy study |
| Tang 2017 | Adult population |
| Theron 2011 | Adult population |
| Triasih 2015 | Not a diagnostic accuracy study |
| Ullah 2017 | Unable to separate paediatric data from adult data |
| Walters 2012 | Insufficient data |
| Walters 2017 | Index text not studied |
| Walters 2018 | Not a diagnostic accuracy study |
| Wang 2020 | Adult population |
| Yadav 2020 | Insufficient data |
| Zhang 2016 | Insufficient data |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800015075.
| Study name | Diagnostic accuracy of Xpert MTB/RIF Ultra assay on diagnosing paediatric pulmonary tuberculosis |
| Target condition and reference standard(s) | Pulmonary tuberculosis |
| Index and comparator tests | Xpert Ultra |
| Starting date | 1 January 2018 |
| Contact information | Xuhui Liu; liuxuhui@shaphc.org |
| Notes |
Differences between protocol and review
Differences between review and review update
Title
The title has been updated to reflect the fact that tuberculosis clinicians and researchers are shifting away from latent and active as descriptors of tuberculosis; instead, we have used tuberculosis disease.
Scope of the review
Our previously published Cochrane Review assessed the accuracy of both Xpert MTB/RIF and Xpert Ultra (Kay 2020). We limited the current review update to the diagnostic accuracy of Xpert Ultra for several reasons. Xpert Ultra has superseded Xpert MTB/RIF and the manufacturer will be discontinuing Xpert MTB/RIF in most countries in 2023. Additionally, the WHO specifically requested an updated review on Xpert Ultra to inform gaps in data in children and adolescents. Further, we considered guidance on when to update systematic reviews. An update is suggested if the question is topical for decision‐making for practice, policy, or research priorities, or if the new data will change the findings or credibility of the original review (Garner 2016). We did not believe that an update on Xpert MTB/RIF met these criteria. The Xpert MTB/RIF text and analyses are available in the last published version of the review (Kay 2020).
Objectives
We added a secondary objective: to summarize the frequency of Xpert Ultra trace results. In investigations of heterogeneity, we focused on the age, HIV status, and other comorbid conditions of participants. The prior review evaluated other possible sources of heterogeneity, such as smear and tuberculosis burden, with more included studies on Xpert MTB/RIF (Kay 2020).
Types of studies
We included abstracts with sufficient data. We included ongoing studies that helped us to address the review objectives and recorded the stage of the study at the time data were extracted.
Reference standard
We defined the microbiological reference standard as culture only and did not include smear microscopy, which is less accurate. In addition, we clarified the reference standards as follows. For stool, we accepted as a reference standard a positive result by Xpert Ultra in a sputum specimen. For the composite reference standard, when information about tuberculosis treatment was not available, we accepted the uniform research definition (Graham 2012; Graham 2015). In these situations, using the older definition (Graham 2012), we defined tuberculosis as (1) confirmed, probable, and possible cases; and (2) non‐tuberculosis. For the newer definition (Graham 2015), we used the categories tuberculosis confirmed and not confirmed. In cases where a study‐specific definition for the composite reference standard was applied, this was accepted as well. We added MTBDRplus, a WHO‐recommended test, as a reference standard for rifampicin resistance.
Assessment of methodological quality
Using QUADAS‐2, we judged all studies that evaluated stool as being of unclear concern, because there is no established technique for stool processing prior to performing Xpert Ultra.
Inconclusive results
We had planned to estimate the summary proportion of non‐determinate Xpert Ultra results; however there were few non‐determinate results reported. We have summarized these results in Table 4.
Sensitivity analyses
We had planned to explore the effects of risk of bias items and study characteristics on summary estimates of Xpert Ultra accuracy by excluding the following studies:
studies that used consecutive or random selection of participants;
studies in which the reference standard results were interpreted without knowledge of the index test results; and
studies that included only untreated participants.
We did not perform these sensitivity analyses because all studies satisfied criteria for analyses 1 and 2, and data were insufficient for analysis 3.
Contributions of authors
AWK, TN, and LFG assessed articles for inclusion and extracted data. AMM and KRS resolved disagreements.
AWK, TN, and KRS entered data in Review Manager 2020.
AWK, AMM, KRS, and YT analysed the data and interpreted the analyses. In particular, YT performed statistical analyses.
SEV, KV, AB, and TM reviewed the protocol and co‐ordinated the presentation of the findings to a WHO Guideline Development Group.
AWK, LFG, AMM, KRS, and YT drafted the manuscript.
TN, SEV, KV, AB, TM, ME, and AKD provided critical comments on the manuscript.
All review authors read and approved the final manuscript draft.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK
External sources
-
Foreign, Commonwealth, and Development Office (FCDO), UK
Project number 300342‐104
-
National Institutes of Health (AK), USA
Fogarty International Center (1K01TW0114820)
-
United States Agency for International Development (USAID), USA
Development of the systematic review was in part made possible with financial support from the USAID administered by the World Health Organization (WHO) Global TB Programme, Switzerland. AK, LGF, YT, and AMM received funding from USAID to carry out the review.
-
World Health Organization, Switzerland
WHO Registration 2021/1090755‐0 Purchase Order 202638664
Declarations of interest
AK has conducted prior primary research on tuberculosis diagnostics. The Baylor College of Medicine Children's Foundation‐Swaziland, where Dr Kay is based, received a discount from Cepheid on Xpert MTB/RIF Ultra cartridges for a tuberculosis case finding programme. The Baylor College of Medicine Children's Foundation‐Eswatini is separate from Baylor College of Medicine (AK's employer).
TN has no known conflicts of interest.
SEV is a Medical Officer at the World Health Organization Global Tuberculosis Programme, which commissioned this review update for the 2022 WHO consolidated guidelines on the management of tuberculosis in children and adolescents.
KV is a WHO staff member.
AB works as a technical officer at the WHO Global Tuberculoiss Programme, which commissioned this review update for the 2022 WHO consolidated guidelines on the management of tuberculosis in children and adolescents.
TM is a consultant to the WHO.
LGF has no known conflicts of interest.
ME is a CIDG Editor, and has no known conflicts of interest.
AKD has conducted prior primary research on tuberculosis diagnostics and has no known conflicts of interest. She works for UNICEF, and in her role as child health specialist is sometimes involved in developing recommendations for diagnostic approaches to detect childhood illnesses.
AMM has conducted prior primary research on tuberculosis diagnostics and has no known conflicts of interest. She has undertaken work as an independent contractor for Janssen Global Services, Medscape Independent Contractor, and Oxford Immunotec Inc.
KRS has received financial support for the preparation of systematic reviews and educational materials, consultancy fees from the Foundation for Innovative New Diagnostics (FIND) (for the preparation of systematic reviews), honoraria, and travel support to attend WHO guidelines meetings. KRS is a CIDG and DTA Editor.
YT is a Cochrane Editorial Board Member; a CIDG and DTA Editor; and a Statistical Editor for the Cochrane Bone Joint and Muscle Trauma Group.
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
These authors contributed equally to this work
New
References
References to studies included in this review
Barcellini 2019 {published data only}
- Barcellini L, Borroni E, Cimaglia C, Girardi E, Matteelli A, Marchese V, et al. App-based symptoms screening with Xpert MTB/RIF Ultra assay used for active tuberculosis detection in migrants at point of arrivals in Italy: the E-DETECT TB intervention analysis. PLOS One 2019;14(7):e0218039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaganath 2021 {published data only}
- Jaganath D, Wambi P, Reza TF, Nakafeero J, Aben EO, Kiconco E, et al. A prospective evaluation of Xpert MTB/RIF Ultra for childhood pulmonary tuberculosis in Uganda. Journal of the Pediatric Infectious Diseases Society 2021;10(5):586-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kabir 2020 {published data only}
- Kabir S, Rahman SM, Ahmed S, Islam MS, Banu RS, Shewade HD, et al. Xpert Ultra assay on stool to diagnose pulmonary tuberculosis in children. Clinical Infectious Diseases 2020;73(2):226-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2021 {published data only}
- Liu XH, Xia L, Song B, Wang H, Qian XQ, Wei JH, et al. Stool-based Xpert MTB/RIF Ultra assay as a tool for detecting pulmonary tuberculosis in children with abnormal chest imaging: a prospective cohort study. Journal of Infection 2021;82(1):84-9. [DOI] [PubMed] [Google Scholar]
NCT04121026 {unpublished data only}
- NCT04121026. Validation of a tuberculosis treatment decision algorithm in HIV-infected children (TB-Speed HIV). clinicaltrials.gov/show/NCT04121026 (first received 9 October 2019).
NCT04203628 {unpublished data only}
- NCT04203628. Evaluation of four stool processing methods combined with Xpert MTB/RIF Ultra for diagnosis of intrathoracic paediatric tuberculosis. clinicaltrials.gov/ct2/show/NCT04203628 (first received 18 December 2019).
NCT04240990 {unpublished data only}
- NCT04240990. Development of a diagnostic prediction score for tuberculosis in hospitalized children with severe acute malnutrition (TB-Speed SAM). clinicaltrials.gov/show/NCT04240990 (first received 27 January 2020).
NCT04899076 {published data only}
- NCT04899076. Stool Processing Kit (SPK) evaluation for paediatric TB. clinicaltrials.gov/ct2/show/NCT04899076 (first received 24 May 2021).
Nicol 2018 {published data only}
- Nicol MP, Workman L, Prins M, Bateman L, Ghebrekristos Y, Mbhele S, et al. Accuracy of Xpert MTB/RIF Ultra for the diagnosis of pulmonary tuberculosis in children. Paediatric Infectious Disease Journal 2018;37(10):e261-3. [DOI] [PubMed] [Google Scholar]
Parigi 2021 {published data only}
- Parigi S, Venturini E, Galli L, Chiappini E. Xpert MTB/RIF Ultra performance in diagnosing paediatric pulmonary TB in gastric aspirates. International Journal of Tuberculosis and Lung Disease 2021;25(1):75-7. [DOI] [PubMed] [Google Scholar]
Sabi 2018 {published data only}
- Sabi I, Rachow A, Mapamba D, Clowes P, Ntinginya NE, Sasamalo M, et al. Xpert MTB/RIF Ultra assay for the diagnosis of pulmonary tuberculosis in children: a multicentre comparative accuracy study. Journal of Infection 2018;77(4):321-7. [DOI] [PubMed] [Google Scholar]
Ssengooba 2020 {published data only}
- Ssengooba W, Dieu Iragena J, Nakiyingi L, Mujumbi S, Wobudeya E, Mboizi R, et al. Accuracy of Xpert Ultra in diagnosis of pulmonary tuberculosis among children in Uganda: a substudy from the SHINE trial. Journal of Clinical Microbiology 2020;58(9):e00410-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2020 {published data only}
- Sun L, Zhu Y, Fang M, Shi Y, Peng X, Liao Q, et al. Evaluation of Xpert MTB/RIF Ultra assay for diagnosis of childhood tuberculosis: a multicenter accuracy study. Journal of Clinical Microbiology 2020;58(9):e00702-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zar 2019 {published data only}
- Zar HJ, Workman LJ, Prins M, Bateman LJ, Mbhele SP, Whitman CB, et al. Tuberculosis diagnosis in children using Xpert Ultra on different respiratory specimens. American Journal of Respiratory and Critical Care Medicine 2019;200(12):1531-8. [DOI: 10.1164/rccm.201904-0772OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ali 2017 {published data only}
- Ali RH, Ibrahim NY, Elegail AM, Eltohami NA, Ebraheem RS, Ahmed SF, et al. Evaluation of GeneXpert MTB/RIF and line probe assay for rapid diagnosis of Mycobacterium tuberculosis in Sudanese pulmonary TB patients. Asian Pacific Journal of Tropical Disease 2017;7(7):426-9. [Google Scholar]
Atashi 2017 {published data only}
- Atashi S, Izadi B, Jalilian S, Madani SH, Farahani A, Mohajeri P. Evaluation of GeneXpert MTB/RIF for determination of rifampicin resistance among new tuberculosis cases in west and northwest Iran. New Microbes and New Infections 2017;19:117-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atehortúa Muñoz 2017 {published data only}
- Atehortúa Muñoz SL, Muñoz JR, Cárdenas Moreno SV, Ferreira CA, Cornejo Ochoa JW. Xpert MTB/RIF as a diagnostic tool in a cohort of children under 15 years of age with clinical suspicion of pulmonary tuberculosis in a hospital of high complexity in Medellin [Xpert MTB/RIF® como herramienta diagnóstica en una cohorte de niños menores de 15 años con sospecha clínica de tuberculosis pulmonar en un hospital de alta complejidad de Medellín]. Infectio 2017;21(1):25-31. [Google Scholar]
Azevedo 2018 {published data only}
- Azevedo RG, Dinallo FS, De Laurentis LS, Boulware DR, Vidal JE. Xpert MTB/RIF® assay for the diagnosis of HIV-related tuberculous meningitis in Sao Paulo, Brazil. International Journal of Tuberculosis and Lung Disease 2018;22(6):706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballif 2015 {published data only}
- Ballif M, Renner L, Claude Dusingize J, Leroy V, Ayaya S, Wools-Kaloustian K, et al. Tuberculosis in pediatric antiretroviral therapy programs in low- and middle-income countries: diagnosis and screening practices. Journal of the Pediatric Infectious Diseases Society 2015;4(1):30-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banada 2016 {published data only}
- Banada PP, Naidoo U, Deshpande S, Karim F, Flynn JL, O'Malley M, et al. A novel sample processing method for rapid detection of tuberculosis in the stool of pediatric patients using the Xpert MTB/RIF assay. PLOS One 2016;11(3):e0151980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Biadglegne 2014 {published data only}
- Biadglegne F, Mulu A, Rodloff AC, Sack U. Diagnostic performance of the Xpert MTB/RIF assay for tuberculous lymphadenitis on fine needle aspirates from Ethiopia. Tuberculosis (Edinburgh, Scotland) 2014;94(5):502-5. [DOI] [PubMed] [Google Scholar]
Bojang 2016 {published data only}
- Bojang AL, Mendy FS, Tientcheu LD, Otu J, Antonio M, Kampmann B, et al. Comparison of TB-LAMP, GeneXpert MTB/RIF and culture for diagnosis of pulmonary tuberculosis in The Gambia. Journal of Infection 2016;72(3):332-7. [DOI] [PubMed] [Google Scholar]
Che 2017 {published data only}
- Che NY, Huang SJ, Ma Y, Han Y, Liu ZC, Zhang C, et al. Comparison of histological, microbiological, and molecular methods in diagnosis of patients with TBLN having different anti-TB treatment background. Biomedical and Environmental Sciences 2017;30(6):418-25. [DOI] [PubMed] [Google Scholar]
Cox 2014 {published data only}
- Cox HS, Mbhele S, Mohess N, Whitelaw A, Muller O, Zemanay W, et al. Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: a pragmatic randomised trial. PLOS Medicine 2014;11(11):e1001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cross 2014 {published data only}
- Cross GB, Coles K, Nikpour M, Moore OA, Denholm J, McBryde ES, et al. TB incidence and characteristics in the remote gulf province of Papua New Guinea: a prospective study. BMC Infectious Diseases 2014;14:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diallo 2016 {published data only}
- Diallo AB, Kollo AI, Camara M, Lo S, Ossoga GW, Mbow M, et al. Performance of GeneXpert MTB/RIF in the diagnosis of extrapulmonary tuberculosis in Dakar: 2010–2015 [Performance du GeneXpert MTB/RIF ® dans le diagnostic de la tuberculose extra-pulmonaire à Dakar: 2010–2015]. Pan African Medical Journal 2016;25:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
DiNardo 2016 {published data only}
- DiNardo AR, Detjen A, Ustero P, Ngo K, Bacha J, Mandalakas AM. Culture is an imperfect and heterogeneous reference standard in pediatric tuberculosis. Tuberculosis (Edinburgh, Scotland) 2016;101S:S105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
DiNardo 2018 {published data only}
- DiNardo AR, Kay AW, Maphalala G, Harris NM, Fung C, Mtetwa G, et al. Diagnostic and treatment monitoring potential of a stool-based quantitative polymerase chain reaction assay for pulmonary tuberculosis. American Journal of Tropical Medicine and Hygiene 2018;99(2):310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ejeh 2018 {published data only}
- Ejeh EF, Undiandeye A, Akinseye VO, Okon KO, Kazeem HM, Kudi CA, et al. Diagnostic performance of GeneXpert and Ziehl-Neelson microscopy in the detection of tuberculosis in Benue State, Nigeria. Alexandria Journal of Medicine 2018;54(4):529-33. [Google Scholar]
Gautam 2018 {published data only}
- Gautam H, Agrawal SK, Verma SK, Singh UB. Cervical tuberculous lymphadenitis: clinical profile and diagnostic modalities. International Journal of Mycobacteriology 2018;7(3):212-6. [DOI] [PubMed] [Google Scholar]
Gelalcha 2017 {published data only}
- Gelalcha AG, Kebede A, Mamo H. Light-emitting diode fluorescent microscopy and Xpert MTB/RIF® assay for diagnosis of pulmonary tuberculosis among patients attending Ambo hospital, west-central Ethiopia. BMC Infectious Diseases 2017;17(1):613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geleta 2015 {published data only}
- Geleta DA, Megerssa YC, Gudeta AN, Akalu GT, Debele MT, Tulu KD. Xpert MTB/RIF assay for diagnosis of pulmonary tuberculosis in sputum specimens in remote health care facility. BMC Microbiology 2015;15:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghariani 2015 {published data only}
- Ghariani A, Jaouadi T, Smaoui S, Mehiri E, Marouane C, Kammoun S, et al. Diagnosis of lymph node tuberculosis using the GeneXpert MTB/RIF in Tunisia. International Journal of Mycobacteriology 2015;4(4):270-5. [DOI] [PubMed] [Google Scholar]
Giang 2015 {published data only}
- Giang DC, Duong TN, Ha DTM, Nhan HT, Wolbers M, Nhu NTQ, et al. Prospective evaluation of GeneXpert for the diagnosis of HIV-negative pediatric TB cases. BMC Infectious Diseases 2015;15:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guajardo‐Lara 2018 {published data only}
- Guajardo-Lara CE, Saldaña-Ramírez MI, Hernández-Galván NN, Dimas-Adame MA, Ayala-Gaytán JJ, Valdovinos-Chávez SB. MGIT and other methods for diagnosing tuberculosis in a private hospital system with low incidence [MGIT y otros métodos para diagnosticar tuberculosis en un sistema hospitalario privado con baja incidencia]. Revista Médica del Instituto Mexicano del Seguro Social 2018;56(2):158-62. [PubMed] [Google Scholar]
Gulla 2019 {published data only}
- Gulla KM, Gunathilaka G, Jat KR, Sankar J, Karan M, Lodha R, et al. Utility and safety of endobronchial ultrasound-guided transbronchial needle aspiration and endoscopic ultrasound with an echobronchoscope-guided fine needle aspiration in children with mediastinal pathology. Pediatric Pulmonology 2019;54(6):881-5. [DOI] [PubMed] [Google Scholar]
Hakim 2017 {published data only}
- Hakim J, Musiime V, Szubert AJ, Mallewa J, Siika A, Agutu C, et al. Enhanced prophylaxis plus antiretroviral therapy for advanced HIV infection in Africa. New England Journal of Medicine 2017;377(3):233-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Helb 2010 {published data only}
- Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. Journal of Clinical Microbiology 2010;48(1):229-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horo 2017 {published data only}
- Horo K, N'Guessan R, Koffi MO, Kouamé-N'Takpé N, Koné A, Samaké K, et al. Use of the Xpert® MTB/RIF test in routine screening of new cases of pulmonary tuberculosis in an endemic area. Revue des Maladies Respiratoires 2017;4(7):749-57. [DOI] [PubMed] [Google Scholar]
Huh 2014 {published data only}
- Huh HJ, Jeong B-H, Jeon K, Koh W-J, Ki C-S, Lee NY. Performance evaluation of the Xpert MTB/RIF assay according to its clinical application. BMC Infectious Diseases 2014;14:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuyinu 2018 {published data only}
- Kuyinu YA, Odugbemi BA, Salisu-Olatunji SO, Adepoju FO, Odusanya OO. Characteristics of Mycobacterium tuberculosis positive patients screened for drug-resistant tuberculosis at a tertiary health facility in Lagos, Nigeria. Journal of the National Medical Association 2018;110(1):88-91. [DOI] [PubMed] [Google Scholar]
Lopez 2019 {published data only}
- Lopez AL, Aldaba JG, Morales-Dizon M, Sarol JN, Daag JV, Ama MC, et al. Urine Xpert MTB/RIF for the diagnosis of childhood tuberculosis. International Journal of Infectious Diseases 2019;79:44-6. [DOI] [PubMed] [Google Scholar]
Lu J 2017 {published data only}
- Lu J, Li H, Dong F, Shi J, Yang H, Han S, et al. The feasibility of Xpert MTB/RIF testing to detect rifampicin resistance among childhood tuberculosis for prevalence surveys in Northern China. BioMed Research International 2017;2017:5857369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu Y 2018 {published data only}
- Lu Y, Zhu Y, Shen N, Tian L, Sun Z. Evaluating the diagnostic accuracy of the Xpert MTB/RIF assay on bronchoalveolar lavage fluid: a retrospective study. International Journal of Infectious Diseases 2018;71:14-9. [DOI] [PubMed] [Google Scholar]
Malik 2018 {published data only}
- Malik AA, Amanullah F, Codlin AJ, Siddiqui S, Jaswal M, Ahmed JF, et al. Improving childhood tuberculosis detection and treatment through facility-based screening in rural Pakistan. International Journal of Tuberculosis and Lung Disease 2018;22(8):851-7. [DOI] [PubMed] [Google Scholar]
Marcy 2018 {published data only}
- Marcy O, Tejiokem M, Msellati P, Truong Huu K, Do Chau V, Tran Ngoc D, et al. Mortality and its determinants in antiretroviral treatment-naive HIV-infected children with suspected tuberculosis: an observational cohort study. Lancet HIV 2018;5(2):e87-95. [DOI] [PubMed] [Google Scholar]
Masenga 2017 {published data only}
- Masenga SK, Mubila H, Hamooya BM. Rifampicin resistance in mycobacterium tuberculosis patients using GeneXpert at Livingstone Central Hospital for the year 2015: a cross sectional explorative study. BMC Infectious Diseases 2017;17(1):640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mekonnen 2015 {published data only}
- Mekonnen F, Tessema B, Moges F, Gelaw A, Eshetie S, Kumera G. Multidrug resistant tuberculosis: prevalence and risk factors in districts of Metema and West Armachiho, Northwest Ethiopia. BMC Infectious Diseases 2015;15:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Memon 2018 {published data only}
- Memon SS, Sinha S, Sharma SK, Kabra SK, Lodha R, Soneja M. Diagnostic accuracy of Xpert Mtb/Rif assay in stool samples in intrathoracic childhood tuberculosis. Journal Tuberculosis and Therapeutics 2018;3(2):115. [Google Scholar]
Metaferia 2018 {published data only}
- Metaferia Y, Seid A, Fenta GM, Gebretsadik D. Assessment of extrapulmonary tuberculosis using Gene Xpert MTB/RIF assay and fluorescent microscopy and its risk factors at Dessie referral hospital, Northeast Ethiopia. BioMed Research International 2018;2018:8207098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mijovic 2018 {published data only}
- Mijovic H, Al-Nasser Y, Al-Rawahi G, Roberts A. Experience with using rapid molecular testing in diagnosing pulmonary and extra-pulmonary pediatric tuberculosis in a non-endemic setting – a retrospective case series. Paediatrics and Child Health (Canada) 2018;23(Suppl 1):e44-5. [Google Scholar]
Modi 2016 {published data only}
- Modi S, Cavanaugh JS, Shiraishi RW, Alexander HL, McCarthy KD, Burmen B, et al. Performance of clinical screening algorithms for tuberculosis intensified case finding among people living with HIV in Western Kenya. PLOS One 2016;11(12):e0167685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulenga 2015 {published data only}
- Mulenga H, Tameris MD, Luabeya KK, Geldenhuys H, Scriba TJ, Hussey GD, et al. The role of clinical symptoms in the diagnosis of intrathoracic tuberculosis in young children. Pediatric Infectious Disease Journal 2015;34(11):1157-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naidoo 2016 {published data only}
- Naidoo P, Dunbar R, Lombard C, du Toit E, Caldwell J, Detjen A, et al. Comparing tuberculosis diagnostic yield in smear/culture and Xpert MTB/RIF-based algorithms using a non-randomised stepped-wedge design. PLOS One 2016;11(3):e0150487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nair 2016 {published data only}
- Nair SA, Raizada N, Sachdeva KS, Denkinger C, Schumacher S, Dewan P, et al. Factors associated with tuberculosis and rifampicin-resistant tuberculosis amongst symptomatic patients in India: a retrospective analysis. PLOS One 2016;11(2):e0150054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nansumba 2016 {published data only}
- Nansumba M, Kumbakumba E, Orikiriza P, Muller Y, Nackers F, Debeaudrap P, et al. Detection yield and tolerability of string test for diagnosis of childhood intrathoracic tuberculosis. Paediatric Infectious Disease Journal 2016;35(2):146-51. [DOI] [PubMed] [Google Scholar]
Nataprawira 2016 {published data only}
- Nataprawira HM, Ruslianti V, Solek R, Hawani D, Mianti M, Anggraeni R, et al. Outcome of tuberculous meningitis in children: the first comprehensive retrospective cohort study in Indonesia. International Journal of Tuberculosis and Lung Disease 2016;20(7):909-14. [DOI] [PubMed] [Google Scholar]
NCT03831906 {unpublished data only}
- NCT03831906. TB-Speed Pneumonia. clinicaltrials.gov/show/NCT03831906 (first received 6 February 2019).
NCT04038632 {unpublished data only}
- NCT04038632. TB-Speed Decentralisation Study. clinicaltrials.gov/show/NCT04038632 (first received 2 August 2019).
Ncube 2017 {published data only}
- Ncube RT, Takarinda KC, Zishiri C, den Boogaard W, Mlilo N, Chiteve C, et al. Age-stratified tuberculosis treatment outcomes in Zimbabwe: are we paying attention to the most vulnerable? Public Health Action 2017;7(3):212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nduba 2015 {published data only}
- Nduba V, Hoog AH, Mitchell E, Onyango P, Laserson K, Borgdorff M. Prevalence of tuberculosis in adolescents, western Kenya: implications for control programs. International Journal of Infectious Diseases 2015;35:11-7. [DOI] [PubMed] [Google Scholar]
Ngabonziza 2016 {published data only}
- Ngabonziza JC, Ssengooba W, Mutua F, Torrea G, Dushime A, Gasana M, et al. Diagnostic performance of smear microscopy and incremental yield of Xpert in detection of pulmonary tuberculosis in Rwanda. BMC Infectious Diseases 2016;16(1):660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicol 2020 {published data only}
- Nicol M, Schumacher S, Workman L, Broger T, Baard C, Prins M, et al. Accuracy of a novel urine test, Fujifilm SILVAMP TB LAM, for the diagnosis of pulmonary tuberculosis in children. Clinical Infectious Diseases 2020;72(9):e280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ntinginya 2012 {published data only}
- Ntinginya EN, Squire SB, Millington KA, Mtafya B, Saathoff E, Heinrich N, et al. Performance of the Xpert® MTB/RIF assay in an active case-finding strategy: a pilot study from Tanzania. International Journal of Tuberculosis and Lung Disease 2012;16(11):1468-70. [DOI] [PubMed] [Google Scholar]
Opota 2019 {published data only}
- Opota O, Zakham F, Mazza-Stalder J, Nicod L, Greub G, Jaton K. Added value of Xpert MTB/RIF Ultra for diagnosis of pulmonary tuberculosis in a low-prevalence setting. Journal of Clinical Microbiology 2019;57(2):pii: e01717-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandey 2017 {published data only}
- Pandey P, Pant ND, Rijal KR, Shrestha B, Kattel S, Banjara MR, et al. Diagnostic accuracy of GeneXpert MTB/RIF assay in comparison to conventional drug susceptibility testing method for the diagnosis of multidrug-resistant tuberculosis. PLOS One 2017;12(1):e0169798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pink 2016 {published data only}
- Pink F, Brown TJ, Kranzer K, Drobniewski F. Evaluation of Xpert MTB/RIF for detection of Mycobacterium tuberculosis in cerebrospinal fluid. Journal of Clinical Microbiology 2016;54(3):809-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Planting 2014 {published data only}
- Planting NS, Visser GL, Nicol MP, Workman L, Isaacs W, Zar HJ. Safety and efficacy of induced sputum in young children hospitalised with suspected pulmonary tuberculosis. International Journal of Tuberculosis and Lung Disease 2014;18(1):8-12. [DOI] [PubMed] [Google Scholar]
Raizada 2014 {published data only}
- Raizada N, Sachdeva KS, Nair SA, Kulsange S, Gupta RS, Thakur R, et al. Enhancing TB case detection: experience in offering upfront Xpert MTB/RIF testing to pediatric presumptive TB and DR TB cases for early rapid diagnosis of drug sensitive and drug resistant TB. PLOS One 2014;9(8):e105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raizada 2015a {published data only}
- Raizada N, Sachdeva KS, Swaminathan S, Kulsange S, Khaparde SD, Nair SA, et al. Piloting upfront Xpert MTB/RIF testing on various specimens under programmatic conditions for diagnosis of TB & DR-TB in paediatric population. PLOS One 2015;10(10):e0140375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raizada 2015b {published data only}
- Raizada N, Sachdeva KS, Sreenivas A, Kulsange S, Gupta RS, Thakur R, et al. Catching the missing million: experiences in enhancing TB & DR-TB detection by providing upfront Xpert MTB/RIF testing for people living with HIV in India. PLOS One 2015;10(2):e0116721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raizada 2018a {published data only}
- Raizada N, Khaparde SD, Salhotra VS, Rao R, Kalra A, Swaminathan S, et al. Accelerating access to quality TB care for pediatric TB cases through better diagnostic strategy in four major cities of India. PLOS One 2018;13(2):e0193194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raizada 2018b {published data only}
- Raizada N, Khaparde SD, Rao R, Kalra A, Sarin S, Salhotra VS, et al. Upfront Xpert MTB/RIF testing on various specimen types for presumptive infant TB cases for early and appropriate treatment initiation. PLOS One 2018;13(8):e0202085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rathour 2019 {published data only}
- Rathour JS, Mantan M, Khanna A, et al. Evaluation of GENE XPERT assay in extrapulmonary tuberculosis in children. Journal of Evolution of Medical and Dental Sciences 2019;8(1):76-80. [Google Scholar]
Rebecca 2018 {published data only}
- Rebecca B, Chacko A, Verghese V, Rose W. Spectrum of pediatric tuberculosis in a tertiary care setting in South India. Journal of Tropical Pediatrics 2018;64(6):544-7. [DOI] [PubMed] [Google Scholar]
Rivera 2017 {published data only}
- Rivera VR, Jean-Juste MA, Gluck SC, Reeder HT, Sainristil J, Julma P, et al. Diagnostic yield of active case finding for tuberculosis and HIV at the household level in slums in Haiti. International Journal of Tuberculosis and Lung Disease 2017;21(11):1140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sabi 2016 {published data only}
- Sabi I, Kabyemera R, Mshana SE, Kidenya BR, Kasanga G, Gerwing-Adima LE, et al. Pulmonary TB bacteriologically confirmed by induced sputum among children at Bugando Medical Centre, Tanzania. International Journal of Tuberculosis and Lung Disease 2016;20(2):228-34. [DOI] [PubMed] [Google Scholar]
Sachdeva 2015 {published data only}
- Sachdeva KS, Raizada N, Sreenivas A, Van't Hoog AH, den Hof S, Dewan PK, et al. Use of Xpert MTB/RIF in decentralized public health settings and its effect on pulmonary TB and DR-TB case finding in India. PLOS One 2015;10(5):e0126065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanchini 2014 {published data only}
- Sanchini A, Fiebig L, Drobniewski F, Haas W, Richter E, Katalinic-Jankovic V, et al. Laboratory diagnosis of paediatric tuberculosis in the European Union/European Economic Area: analysis of routine laboratory data, 2007 to 2011. Eurosurveillance 2014;19(11):pii: 20744. [DOI] [PubMed] [Google Scholar]
Sander 2019 {published data only}
- Sander MS, Laah SN, Titahong CN, Lele C, Kinge T, Jong BC, et al. Systematic screening for tuberculosis among hospital outpatients in Cameroon: the role of screening and testing algorithms to improve case detection. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases 2019;15:100095. [DOI: 10.1016/j.jctube.2019.100095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanjuan‐Jimenez 2015 {published data only}
- Sanjuan-Jimenez R, Toro-Peinado I, Bermudez P, Colmenero JD, Morata P. Comparative study of a real-time PCR assay targeting senX3-regX3 versus other molecular strategies commonly used in the diagnosis of tuberculosis. PLOS One 2015;10(11):e0143025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schumacher 2016 {published data only}
- Schumacher SG, Smeden M, Dendukuri N, Joseph L, Nicol MP, Pai M, et al. Diagnostic test accuracy in childhood pulmonary tuberculosis: a Bayesian latent class analysis. American Journal of Epidemiology 2016;184(9):690-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 2014 {published data only}
- Scott LE, Beylis N, Nicol M, Nkuna G, Molapo S, Berrie L, et al. Diagnostic accuracy of Xpert MTB/RIF for extrapulmonary tuberculosis specimens: establishing a laboratory testing algorithm for South Africa. Journal of Clinical Microbiology 2014;52(6):1818-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2016b {published data only}
- Shah I, Gupta Y. Xpert MTB/RIF for diagnosis of tuberculosis and drug resistance in Indian children. Indian Pediatrics 2016;53(9):837-8. [DOI] [PubMed] [Google Scholar]
Shah 2018 {published data only}
- Shah MA, Shah I. Increasing prevalence of pediatric drug-resistant tuberculosis in Mumbai, India, and its outcome. Pediatric Infectious Disease Journal 2018;37(12):1261-3. [DOI] [PubMed] [Google Scholar]
Shah 2019 {published data only}
- Shah I, Bhamre R, Shetty NS. Accuracy of Xpert® Mycobacterium tuberculosis/rifampicin assay in diagnosis of pulmonary tuberculosis. Infectious Diseases 2019;51(7):550-3. [DOI] [PubMed] [Google Scholar]
Sharma 2015 {published data only}
- Sharma SK, Kohli M, Yadav RN, Chaubey J, Bhasin D, Sreenivas V, et al. Evaluating the diagnostic accuracy of Xpert MTB/RIF assay in pulmonary tuberculosis. PLOS One 2015;10(10):e0141011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sieiro 2018 {published data only}
- Sieiro TL, Aurílio RB, Soares EC, Chiang SS, Sant Anna CC. The role of the Xpert MTB/RIF assay among adolescents suspected of pulmonary tuberculosis in Rio de Janeiro, Brazil. Revista da Sociedade Brasileira de Medicina Tropical 2018;51(2):234-6. [DOI] [PubMed] [Google Scholar]
Singh 2015 {published data only}
- Singh S, Singh A, Prajapati S, Kabra SK, Lodha R, Mukherjee A, et al. Xpert MTB/RIF assay can be used on archived gastric aspirate and induced sputum samples for sensitive diagnosis of paediatric tuberculosis. BMC Microbiology 2015;15:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2016 {published data only}
- Singh UB, Pandey P, Mehta G, Bhatnagar AK, Mohan A, Goyal V, et al. Genotypic, phenotypic and clinical validation of GeneXpert in extra-pulmonary and pulmonary tuberculosis in India. PLOS One 2016;11(2):e0149258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomons 2016 {published data only}
- Solomons RS, Visser DH, Marais BJ, Schoeman JF, Furth AM. Diagnostic accuracy of a uniform research case definition for TBM in children: a prospective study. International Journal of Tuberculosis and Lung Disease 2016;20(7):903-8. [DOI] [PubMed] [Google Scholar]
Sun 2019 {published data only}
- Sun L, Qi X, Liu F, Wu X, Yin Q, Guo Y, et al. A test for more accurate diagnosis of pulmonary tuberculosis. Pediatrics November 2019;144(5):e20190262. [DOI] [PubMed] [Google Scholar]
Sureshbabu 2016 {published data only}
- Sureshbabu R, Lakshmi Murali A, Palaniswamy M. Molecular diagnosis of drug resistance tuberculosis in the Districts Of Tamilnadu. International Journal of Pharma and Bio Sciences 2016;7(4):B42-B6. [Google Scholar]
Tadesse 2015 {published data only}
- Tadesse M, Abebe G, Abdissa K, Aragaw D, Abdella K, Bekele A, et al. GeneXpert MTB/RIF Assay for the diagnosis of tuberculous lymphadenitis on concentrated fine needle aspirates in high tuberculosis burden settings. PLOS One 2015;10(9):e0137471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tafur 2018 {published data only}
- Tafur KT, Coit J, Leon SR, Pinedo C, Chiang SS, Contreras C, et al. Feasibility of the string test for tuberculosis diagnosis in children between 4 and 14 years old. BMC Infectious Diseases 2018;18:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2017 {published data only}
- Tang T, Liu F, Lu X, Huang Q. Evaluation of GeneXpert MTB/RIF for detecting Mycobacterium tuberculosis in a hospital in China. Journal of International Medical Research 2017;45(2):816-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Theron 2011 {published data only}
- Theron G, Peter J, Zyl-Smit R, Mishra H, Streicher E, Murray S, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. American Journal of Respiratory and Critical Care Medicine 2011;184(1):132-40. [DOI] [PubMed] [Google Scholar]
Triasih 2015 {published data only}
- Triasih R, Robertson C, Duke T, Graham SM. Risk of infection and disease with Mycobacterium tuberculosis among children identified through prospective community-based contact screening in Indonesia. Tropical Medicine & International Health 2015;20(6):737-43. [DOI] [PubMed] [Google Scholar]
Ullah 2017 {published data only}
- Ullah I, Javaid A, Masud H, Ali M, Basit A, Ahmad W, et al. Rapid detection of Mycobacterium tuberculosis and rifampicin resistance in extrapulmonary tuberculosis and sputum smear negative pulmonary suspects using Xpert MTB/RIF. Journal of Medical Microbiology 2017;66(4):412-8. [DOI] [PubMed] [Google Scholar]
Walters 2012 {published data only}
- Walters E, Gie RP, Hesseling AC, Friedrich SO, Diacon AH, Gie RP. Rapid diagnosis of pediatric intrathoracic tuberculosis from stool samples using the Xpert MTB/RIF assay: a pilot study. Pediatric Infectious Disease Journal 2012;31(12):1316. [DOI] [PubMed] [Google Scholar]
Walters 2017 {published data only}
- Walters E, Demers AM, Zalm MM, Whitelaw A, Palmer M, Bosch C, et al. Stool culture for diagnosis of pulmonary tuberculosis in children. Journal of Clinical Microbiology 2017;55(12):3355-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2018 {published data only}
- Walters E, Zalm MM, Demers AM, Whitelaw A, Palmer M, Bosch C, et al. Specimen pooling as a diagnostic strategy for microbiologic confirmation in children with intrathoracic tuberculosis. Paediatric Infectious Disease Journal 2018;38(6):e128-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2020 {published data only}
- Wang G, Wang S, Yang X, Sun Q, Jiang G, Huang M, et al. Accuracy of Xpert MTB/RIF Ultra for the diagnosis of pleural TB in a multicenter cohort study. Chest 2020;157(2):268-275. [DOI] [PubMed] [Google Scholar]
Yadav 2020 {published data only}
- Yadav R, Vaidya P, Mathew JL, Singh S, Khaneja R, Agarwal P, et al. Diagnostic accuracy of Xpert MTB/RIF ultra for detection of Mycobacterium tuberculosis in children: a prospective cohort study. Letters in Applied Microbiology 2021;72(3):225-30. [DOI] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang AM, Li F, Liu XH, Xia L, Lu SH. Application of Gene Xpert Mycobacterium tuberculosis DNA and resistance to rifampicin assay in the rapid detection of tuberculosis in children. Zhonghua er ke za zhi [Chinese Journal of Pediatrics] 2016;54(5):370-4. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR1800015075 {unpublished data only}
- ChiCTR1800015075. Diagnostic accuracy of Xpert MTB/RIF Ultra assay on diagnosing pediatric pulmonary tuberculosis. www.chictr.org.cn/showprojen.aspx?proj=25233 (first received 6 March 2018). [ChiCTR1800015075]
Additional references
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [DOI] [PubMed] [Google Scholar]
Bjerrum 2019
- Bjerrum S, Schiller I, Dendukuri N, Kohli M, Nathavitharana RR, Zwerling AA, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database of Systematic Reviews 2019;10(10):CD011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Branigan 2021
- Branigan, D. Tuberculosis Diagnostics Pipeline report 2021. www.treatmentactiongroup.org/wp-content/uploads/2021/11/pipeline_TB_diagnostics_2021_final.pdf (accessed 17 December 2021).
Broger 2019
- Broger T, Sosse B, Du Toit E, Kerkhoff AD, Schutz C, Reipold EI, et al. Novel lipoarabinomannan point-of-care tuberculosis test for people with HIV: a diagnostic accuracy study. Lancet Infectious Diseases 2019;19(8):852-61. [DOI] [PMC free article] [PubMed]
Cepheid 2018
- Cepheid. Brochure: Xpert® MTB/RIF Ultra. www.cepheid.com/en/tests/Critical-Infectious-Diseases/Xpert-MTB-RIF-Ultra (accessed 3 November 2021).
Chakravorty 2017
- Chakravorty S, Simmons AM, Rowneki M, Parmar H, Cao Y, Ryan J, et al. The new Xpert MTB/RIF Ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio 2017;8(4):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiang 2017
- Chiang SS, Swanson DS, Starke JR. New diagnostics for childhood tuberculosis. Infectious Disease Clinics of North America 2015;29(3):477-502. [DOI] [PubMed] [Google Scholar]
Chu 2006
- Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331-2. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, (accessed 1 October 2021). Available at covidence.org.
Cruz 2012
- Cruz AT, Revell PA, Starke JR. Gastric aspirate yield for children with suspected pulmonary tuberculosis. Journal of the Pediatric Infectious Diseases Society 2012;2(2):171-4. [DOI] [PubMed] [Google Scholar]
David 2017
- David SG, Lovero KL, Pombo-March MF, Abreu TG, Ruffino-Netto A, Kritski AL, et al. A comparison of tuberculosis diagnostic systems in a retrospective cohort of HIV-infected children in Rio de Janeiro, Brazil. International Journal of Infectious Diseases 2017;59:150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dodd 2017
- Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Global Health 2017;5(9):e898-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorman 2018
- Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infectious Diseases 2018;18(1):76-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunn 2016
- Dunn JJ, Starke JR, Revell PA. Laboratory diagnosis of Mycobacterium tuberculosis infection and disease in children. Journal of Clinical Microbiology 2016;54(6):1434-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frigati 2015
- Frigati L, Maskew M, Workman L, Munro J, Andronikou S, Nicol MP, et al. Predictors of culture-confirmed pulmonary tuberculosis in children in a high tuberculosis and HIV prevalence area. Pediatric Infectious Disease Journal 2015;34(9):e206-10. [DOI] [PubMed] [Google Scholar]
Furin 2019
- Furin J. Advances in the diagnosis, treatment, and prevention of tuberculosis in children. Expert Review of Respiratory Medicine 2019;13(3):301-11. [DOI] [PubMed] [Google Scholar]
Garner 2016
- Garner P, Hopewell S, Chandler J, MacLehose H, Schünemann HJ, Akl EA, et al. When and how to update systematic reviews: consensus and checklist. BMJ 2016;354:i3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graham 2012
- Graham SM, Ahmed T, Amanullah F, Browning R, Cardenas V, Casenghi M, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. Journal of Infectious Diseases 2012;205(Suppl 2):S199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graham 2015
- Graham SM, Cuevas LE, Jean-Philippe P, Browning R, Casenghi M, Detjen AK. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clinical Infectious Diseases 2015;61(Suppl 3):S179-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harausz 2018
- Harausz EP, Garcia-Prats AJ, Law S, Schaaf HS, Kredo T, Seddon JA, et al. Treatment and outcomes in children with multidrug-resistant tuberculosis: a systematic review and individual patient data meta-analysis. PLOS Medicine 2018;15(7):e1002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horne 2019
- Horne DJ, Kohli M, Zifodya JS, Schiller I, Dendukuri N, Tollefson D, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database of Systematic Reviews 2019;7(6):CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jenkins 2017
- Jenkins HE, Yuen CM, Rodriguez CA, Nathavitharana RR, McLaughlin MM, Donald P, et al. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infectious Diseases 2017;17(3):285-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohli 2021
- Kohli M, Schiller I, Dendukuri N, Yao M, Dheda K, Denkinger CM, et al. Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD012768. [DOI: 10.1002/14651858.CD012768.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunkel 2016
- Kunkel A, Abel Zur Wiesch P, Nathavitharana RR, Marx FM, Jenkins HE, Cohen T. Smear positivity in paediatric and adult tuberculosis: systematic review and meta-analysis. BMC Infectious Diseases 2016;16(282):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10. Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. Cochrane, 2013. Available from srdta.cochrane.org.
MacLean 2019
- MacLean E, Sulis G, Denkinger CM, Johnston JC, Pai M, Khana FA. Diagnostic accuracy of stool Xpert MTB/RIF for detection of pulmonary tuberculosis in children: a systematic review and meta-analysis. Journal of Clinical Microbiology 2019;57(6):e02057-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marais 2004
- Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. International Journal of Tuberculosis and Lung Disease 2004;8(4):392-402. [PubMed] [Google Scholar]
Marais 2005
- Marais BJ, Gie RP, Obihara CC, Hesseling AC, Schaaf HS, Beyers N. Well defined symptoms are of value in the diagnosis of childhood pulmonary tuberculosis. Archives of Disease in Children 2005;90(11):1162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marais 2006a
- Marais BJ, Gie RP, Schaaf HS, Hesseling AS, Enarson DA, Beyers N. The spectrum of disease in children treated for tuberculosis in a highly endemic area. International Journal of Tuberculosis and Lung Disease 2006;10:732–8. [PubMed] [Google Scholar]
Marais 2006b
- Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Enarson DA, Beyers N. The bacteriologic yield in children with intrathoracic tuberculosis. Clinical Infectious Diseases 2006;42:69-71. [DOI] [PubMed] [Google Scholar]
Marais 2006c
- Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Enarson DA, Beyers N. Radiographic signs and symptoms in children treated for tuberculosis: possible implications for symptom-based screening in resource-limited settings. Pediatric Infectious Disease Journal 2006;25(3):237-40. [DOI] [PubMed] [Google Scholar]
Marais 2006d
- Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Lombard C, Enarson DA, et al. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics 2006;118(5):e1350-9. [DOI] [PubMed] [Google Scholar]
Marais 2006e
- Marais BJ, Wright CA, Schaaf HS, Gie RP, Hesseling AC, Enarson DA, et al. Tuberculous lymphadenitis as a cause of persistent cervical lymphadenopathy in children from a tuberculosis-endemic area. Pediatric Infectious Disease Journal 2006;25(2):142-6. [DOI] [PubMed] [Google Scholar]
Marais 2014
- Marais BJ, Schaaf HS. Tuberculosis in children. Cold Spring Harbor Perspectives in Medicine 2014;4:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marais S 2010
- Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infectious Diseases 2010;10(11):803-12. [DOI] [PubMed] [Google Scholar]
Mishra 2020
- Mishra H, Reeve BW, Palmer Z, Caldwell J, Dolby T, Naidoo CC, et al. Xpert MTB/RIF Ultra and Xpert MTB/RIF for diagnosis of tuberculosis in an HIV-endemic setting with a high burden of previous tuberculosis: a two-cohort diagnostic accuracy study. Lancet Respiratory Medicine 2020;8(4):368-82. [DOI] [PubMed] [Google Scholar]
Nathavitharana 2021
- Nathavitharana RR, Lederer P, Chaplin M, Bjerrum S, Steingart KR, Shah M. Impact of diagnostic strategies for tuberculosis using lateral flow urine lipoarabinomannan assay in people living with HIV. Cochrane Database of Systematic Reviews 2021, Issue 8. Art. No: CD014641. [DOI: 10.1002/14651858.CD014641] [DOI] [PMC free article] [PubMed] [Google Scholar]
Newcombe 1998
- Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Statistics in Medicine 1998;17(8):873-90. [DOI] [PubMed] [Google Scholar]
Nicol 2014
- Nicol MP, Allen V, Workman L, Isaacs W, Munro J, Pienaar S, et al. Urine lipoarabinomannan testing for diagnosis of pulmonary tuberculosis in children: a prospective study. Lancet Global Health 2014;2(5):e278-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2021
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reid 2012
- Reid MJ, Saito S, Fayorsey R, Carter RJ, Abrams EJ. Assessing capacity for diagnosing tuberculosis in children in sub-Saharan African HIV care settings. International Journal of Tuberculosis and Lung Disease 2012;16(7):924-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitsma 2015
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58:982-90. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Schünemann 2008
- Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008;336(7653):1106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2016
- Schünemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, et al. GRADE Working Group. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. Journal of Clinical Epidemiology 2016;76:89-98. [DOI: 10.1016/j.jclinepi.2016.01.032] [DOI] [PubMed] [Google Scholar]
Schünemann 2020a
- Schünemann HJ, Mustafa R, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 1. Study design, risk of bias and indirectness in rating the certainty across a body of evidence for test accuracy. Journal of Clinical Epidemiology 2020;122:129-41. [DOI] [PubMed] [Google Scholar]
Schünemann 2020b
- Schünemann HJ, Mustafa R, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 2. Inconsistency, Imprecision, publication bias and other domains for rating the certainty of evidence for test accuracy and presenting it in evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2020;122:142-52. [DOI] [PubMed] [Google Scholar]
Shah 2016a
- Shah M, Hanrahan C, Wang ZY, Dendukuri N, Lawn SD, Denkinger CM, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD011420. [DOI: 10.1002/14651858.CD011420.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Signorino 2022
- Signorino C, Votto M, De Filippo M, Marseglia GL, Galli L, Chiappini E. Diagnostic accuracy of Xpert ultra for childhood tuberculosis: a preliminary systematic review and meta-analysis. Pediatric Allergy and Immunology 2022;33 Suppl 27:80-82:80-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stata 16 [Computer program]
- Stata Statistical Software. StataCorp, Version 16. College Station: StataCorp LP, 2019.
Takwoingi 2015
- Takwoingi Y, Guo B, Riley RD, Deeks JJ. Performance of methods for meta-analysis of diagnostic test accuracy with few studies or sparse data. Statistical Methods in Medical Research 2015;0:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Theart 2005
- Theart AC, Marais BJ, Gie RP, Hesseling AC, Beyers N. Criteria used for the diagnosis of childhood tuberculosis at primary health care level in a high-burden, urban setting. International Journal of Tuberculosis and Lung Disease 2005;9(11):1210-4. [PubMed] [Google Scholar]
Wademan 2019
- Wademan DT, Busakwe L, Nicholson TJ, Zalm M, Palmer M, Workman J, et al. Acceptability of a first-line anti-tuberculosis formulation for children: qualitative data from the SHINE trial. International Journal of Tuberculosis and Lung Disease 2019;12:1263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. [DOI] [PubMed] [Google Scholar]
WHO 2014
- World Health Organization. Xpert MTB/RIF implementation manual. Technical and operational 'how-to' practical considerations. apps.who.int/iris/bitstream/10665/112469/1/9789241506700_eng.pdf 2014 (accessed prior to 29 April 2019).
WHO Consolidated Guidelines (Module 3) 2021
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 3: diagnosis – rapid diagnostics for tuberculosis detection, 2021 update. Licence: CC BY-NC-SA 3.0 IGO. who.int/publications/i/item/who-consolidated-guidelines-on-tuberculosis-module-3-diagnosis---rapid-diagnostics-for-tuberculosis-detection (accessed 12 October 2021).
WHO Consolidated Guidelines (Module 4) 2020
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: treatment – drug-resistant tuberculosis treatment, June 2020. who.int/publications/i/item/9789240007048 (accessed 3 November 2021).
WHO Consolidated Guidelines (Module 5) 2022
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 5: management of tuberculosis in children and adolescents. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. www.who.int/publications/i/item/9789240046764 (accessed 29 April 2022).
WHO Global Tuberculosis Report 2021
- World Health Organization. Global tuberculosis report 2021. www.who.int/publications/digital/global-tuberculosis-report-2021 (accessed 18 October 2021).
WHO Operational handbook on tuberculosis 2021
- World Health Organization. WHO operational handbook on tuberculosis. Module 3: diagnosis - rapid diagnostics for tuberculosis detection, 2021 update. Licence: CC BY-NC-SA 3.0 IGO. www.who.int/publications/i/item/9789240030589 (accessed 2 November 2021).
Zar 2005
- Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet 2005;365(9454):130-4. [DOI] [PubMed] [Google Scholar]
Zar 2012
- Zar HJ, Workman L, Isaacs W, Munro J, Black F, Eley B, et al. Rapid molecular diagnosis of pulmonary tuberculosis in children using nasopharyngeal specimens. Clinical Infectious Diseases 2012;55(8):1088-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2020
- Zhang M, Xue M, He JQ. Diagnostic accuracy of the new Xpert MTB/RIF Ultra for tuberculosis disease: a preliminary systematic review and meta-analysis. International Journal of Infectious Diseases 2020;90:35-45. [DOI] [PubMed] [Google Scholar]
Zifodya 2021
- Zifodya JS, Kreniske JS, Schiller I, Kohli M, Dendukuri N, Schumacher SG, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD009593. [DOI: 10.1002/14651858.CD009593.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kay 2020
- Kay AW, González Fernández L, Takwoingi Y, Eisenhut M, Vu RD, Steingart KR, Detjen AK, Mandalakas AM. Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD013359. [DOI: 10.1002/14651858.CD013359] [DOI] [PMC free article] [PubMed] [Google Scholar]


