Abstract
Background and Aim
Trends in steroid use and the effects of the initial dose, duration of use, and tapering schedule on clinical efficacy were assessed in Japanese patients with ulcerative colitis (UC) undergoing steroid treatment.
Methods
We enrolled 191 cases with UC who underwent steroid treatment between 2006 and 2020. We assessed the difference in clinical remission rates in cases with different initial doses of steroid. Clinical factors for clinical remission at week 4 and discontinuation of corticosteroid within 12 weeks were also assessed.
Results
Clinical remission and response at week 4 were obtained in 107 (56.0%) and 58 cases (30.4%), respectively. In hospitalized patients, male sex (odds ratio [OR], 0.373; 95% confidence interval [CI], 0.146–0.956) and younger age (OR, 0.974; 95% CI, 0.951–0.998) were associated with clinical remission at week 4. Partial Mayo score (OR, 0.643; 95% CI, 0.451–0.918) and initial steroid dose of ≥30 mg (OR, 3.278; 95% CI, 1.274–8.435) were associated with clinical remission at week 4 in outpatients. Clinical remission at week 4 (OR, 0.300; (95% CI, 0.126–0.718)) and the steroid dose reduction rate at week 4 (OR, 0.092; 95% CI, 0.036–0.234) were associated with treatment discontinuation within 12 weeks. The proportion of patients in whom corticosteroids were discontinued at week 12 was significantly higher (P = 0.006) in 2016–2020 (28/52; 53.8%) than in 2006–2010 (15/54; 27.8%).
Conclusion
The steroid reduction rate at week 4 may be critical for discontinuation within 12 weeks. Withdrawal of corticosteroids has been becoming more appropriate in the last 5 years than before.
Keywords: corticosteroid, initial dose of corticosteroid, reduction rate of corticosteroid, ulcerative colitis

Initial steroid dose of ≥30 mg was associated with clinical remission at week 4 in outpatients. Clinical remission at week 4 and the steroid dose reduction rate at week 4 were associated with treatment discontinuation within 12 weeks. Withdrawal of corticosteroids has been become more appropriate in the last 5 years.
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disease, which includes ulcerative colitis (UC) and Crohn's disease. Although the etiology of UC is unknown and a fundamental treatment has not been established, the pathophysiology of UC has been extensively studied, and involves host genetic factors, immune system dysregulation, and environmental factors. 1 Patients often experience intermittent flare‐ups of disease, and abdominal symptoms are continuously observed for an extended period in some cases.
Therapeutic treatments for UC include 5‐aminosalicylic acids (5‐ASAs), corticosteroids, thiopurine, calcineurin inhibitors, antitumor necrosis factor (TNF)‐α antibody, Janus kinase inhibitor, anti‐a4b7 integrin antibody, and anti‐IL‐12/23 antibody. Although many medical treatments have been recently developed, 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 the fundamental treatment for active UC includes 5‐ASA and corticosteroids. Steroid treatment is considered for patients with more severe symptoms when 5‐ASAs are not effective in inducing remission, or for hospitalized patients with acute severe UC. 10 , 11 , 12 , 13 , 14 A meta‐analysis indicated that corticosteroids are more effective than placebo in inducing clinical remission. 15 Further, previous studies have indicated that oral corticosteroids are superior to 5‐ASA in active UC with extensive colitis. 16 , 17
Although the effectiveness of corticosteroids is clear, due to potential adverse events, caution should be exercised with steroid use. Physicians must be mindful of the initial dose and the period of administration. It is also critical to reduce the steroid dose after inducing clinical remission. Most guidelines recommend a dose of 40–60 mg/day in hospitalized patients. 12 , 13 However, no differences in clinical efficacy were found in cases with initial doses of ≥60 mg daily, and the risk of colectomy was not correlated with the daily dose. 18 Few studies have examined the therapeutic effects of the initial steroid dose. Moreover, there is no clear evidence to indicate whether the short‐term efficacy of oral corticosteroids and that of intravenous corticosteroids are comparable in hospitalized patients. 14
For outpatients with active UC, budesonide multi‐matrix (MMX) can be used, and previous studies have shown that budesonide MMX (9 mg/day) is more effective than placebo for inducing clinical remission. 19 , 20 , 21 However, oral corticosteroids are also used for outpatients with moderately active UC who do not require hospitalization. Usually, the daily steroid dose is 30–40 mg. However, there are scarce data that can be used to determine the optimum dose of corticosteroids in outpatients, except that 40 mg of steroid daily is more effective than 20 mg of steroid daily. 22 The use of ≥40 mg/day is associated with increased adverse effects 22 ; therefore, it is desirable to use the lowest effective dose possible if the short‐term efficacy is comparable between patients on low doses and those on high doses of steroid. Unfortunately, there are scarce recent studies regarding the clinical efficacy and safety of the initial dose of steroid in outpatients with active UC.
Of note, there is less evidence regarding the steroid administration period and the tapering schedule after inducing remission, than that available on the initial dose of steroid. The American College of Gastroenterology (ACG) guidelines indicate that the duration of treatment with systemic corticosteroids should be as short as possible, and recommend early initiation of corticosteroid sparing therapy. 12 However, there is no clear description of the steroid reduction protocol. The tapering schedule should be guided by clinical symptoms, cumulative steroid exposure, and the onset of action of alternative therapies. British guidelines recommend that the dose of corticosteroid should be tapered over 6–8 weeks. 13 To ensure safety, corticosteroids should be tapered as early as possible. However, few studies have assessed the differences in recurrence rates with different tapering schedules.
The present study evaluates trends of steroid use and the effect of the initial dose, duration of steroid use, and tapering schedule on clinical efficacy at a single institution to compare the differences between clinical guidelines and real‐world data.
Methods
Patients
Patients with UC undergoing steroid treatment at Third Department of Internal Medicine of the Kansai Medical University Hospital between 2006 and 2020 were retrieved from the institutional clinical database for UC. Each course of steroid use was analyzed as a case, even in patients who had repeated steroid courses. A total of 107 patients were enrolled, with 191 courses of steroid administered. The use of corticosteroid was defined as the use of intravenous or oral prednisolone. Patients using only topical corticosteroids were excluded. We retrospectively reviewed the medical records. The study design was reviewed and approved by the Ethics Committees at Kansai Medical University Hospital (2020278). An opt‐out approach was used for consent because this was a retrospective observational study and there was no risk to participants. Patients were given the opportunity to refuse participation in this study by posting their preference on the institutional website (http://www.kmu.ac.jp/hirakata/hospital/2671t800000135zj.html.). If the patients were less than 20 years old, their parents or guardians also had the right to refuse participation.
Data collection
We assessed demographic data at entry, including dates of steroid administration, sex, age, body weight at the steroid administration, disease duration, disease type (first attack, relapsing‐remission, chronic continuous), partial Mayo score (pMayo), Mayo endoscopic subscore (MES), clinical severity by modifying the Truelove–Witts classification (moderate or severe), and requirements for hospitalization (inpatient, outpatient at entry). The following data were determined: the absence of a history of steroid use for UC, the number of steroid courses, the steroid route of administration (oral or intravenous), daily dose at entry and at week 4, and week 12 after steroid use, the duration of steroid use, and the total steroid dose during the course (until steroid discontinuation or until the start of alternative/additional treatment due to clinical symptoms for UC).
Because we did not collect adverse effects prospectively, only clear adverse events, such as pneumonia, Herpes zoster infection, Clostridioides difficile infection (CDI) and cytomegalovirus (CMV) infection, and venous thrombosis, were obtained from the medical charts. Herpes zoster infection was defined as clinical symptoms requiring antiviral treatment. Venous thrombosis was defined by radiological examination or ultrasonography with any clinical symptoms.
Assessment of short‐term clinical outcome
We assessed clinical efficacy at week 4, classified as clinical remission, clinical response, and no response. Clinical remission was defined as a pMayo of ≤1, including no rectal bleeding. Clinical response was defined as a pMayo of every factor exhibiting a decrease of ≥1. All other cases were defined as no response, including the need for treatment for UC symptoms.
Assessment of reduction rate of steroid dose
The rates of steroid reduction at weeks 4 and 12 relative to the initial dose were calculated. At week 4, the proportion of cases with a dose reduction rate of <50% (slow reduction) and those with ≥50% (standard reduction) was assessed. The proportion of cases with or without discontinuation of corticosteroids at week 12 was assessed.
Endpoints and statistical analysis
First, the difference in clinical remission rates at week 4 was assessed in cases with different initial doses of steroid. Clinical remission rates were compared among cases with initiation of steroid doses of <40 mg, 40–59 mg, and ≥60 mg in hospitalized patients. Also, clinical remission rate was also compared between cases receiving ≥1.0 mg/kg or <1.0 mg/kg of corticosteroid as the initial daily dose. For outpatients, clinical remission rates were compared for initial steroid doses of ≥30 mg and <30 mg or initial steroid doses ≥0.5 mg/kg <0.5 mg/kg. Second, clinical characteristics at baseline were compared between the clinical remission and the no remission groups at week 4. Third, clinical factors associated with the discontinuation of steroid dose at week 12 were also assessed. Fourth, clinical characteristics, initial steroid dose, duration of steroid use, and discontinuation of steroid at week 12 were compared between cases with or without adverse effects. Finally, changes in the initial steroid dose, steroid dose reduction rates at week 4, median duration of steroid use, and the median total doses of steroid were compared during the following periods of time: 2006–2010, 2011–2015, and 2016–2020.
Categorical variables were compared using the chi‐squared or Fisher's exact test. Paired variables were evaluated using Mann–Whitney U test or Kruskal–Wallis test. Logistic regression was also used to perform multivariate analysis. The factors for clinical remission at week 4, the factors for discontinuation of steroid at week 12, and the factors for occurrence of adverse events were analyzed using univariate and multivariate analyses. All statistical analyses were performed using IBM SPSS Statistics version 26.0 (IBM Corp., Armonk, NY, USA). Statistical significance was set at P < 0.05. All authors had access to the study data and reviewed and approved the final manuscript.
Results
Clinical characteristics
The patients' clinical characteristics are summarized in Table 1. A total of 144 cases (75.4%) previously used corticosteroids, and 38 cases (19.9%) were treated with thiopurine on entry. The number of cases with a first, second, third, and fourth or more course of corticosteroids was 47, 99, 34, and 11, respectively (Figure 1)). There were 31 (16.2%) patients with severe disease activity according to the modified Truelove–Witts classification. The median partial Mayo score was 7. Eighty‐five cases were hospitalized, and 106 cases were outpatients. Most cases had moderate and severe endoscopic activity with an MES of 2 or 3, whereas an MES of 1 was observed in 12 cases (12/148; 8.1%).
Table 1.
Clinical characteristics of patients treated with corticosteroids
| Clinical characteristics | number |
|---|---|
| Gender (male:female) | 88:103 |
| Age, years; median (IQR) | 40 (25–58) |
| Body weight (kg); median (IQR) | 51.7 (46.7–59.8) |
| Duration of disease, years; median (IQR) | 3.84 (1.06–9.23) |
| Disease Severity* | |
| Moderate | 156 (81.7%) |
| Severe | 31 (16.2%) |
| Previous use of systemic corticosteroids; n (%) | 144 (75.4%) |
| Number of steroid courses | |
| 1 | 47 (24.6%) |
| 2 | 99 (51.8%) |
| ≥3 | 45 (23.6%) |
| In hospital; n (%) | 85 (44.5%) |
| Partial Mayo score; median (IQR) | 6 (5–7) |
| Mayo endoscopic score; median (IQR) | 3 (2–3) |
| 1 | 12 |
| 2 | 52 |
| 3 | 84 |
| Not performed | 43 |
| Mesalamine use (oral and/or rectal) | 119 (62.3%) |
| Thiopurine use: n (%) | 38 (19.9%) |
IQR, interquartile range.
Japanese classification by modified Truelove–Witts classification
Figure 1.
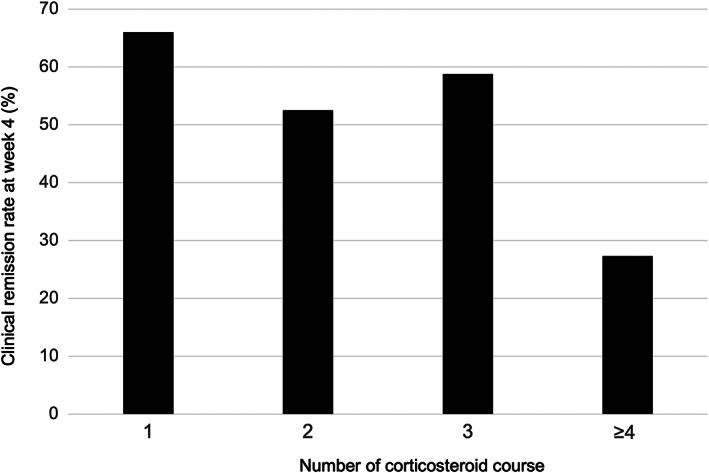
The rate of clinical remission at week 4 by number of steroid courses
Short‐term outcomes in patients undergoing corticosteroid treatment
To induce remission, corticosteroids were administered orally and intravenously in 138 and 53 cases, respectively, and the median initial daily steroid doses were 30 mg (excluding seven patients who were treated with pulse steroid therapy). Clinical remission and response at week 4 were obtained in 107 (56.0%) and 58 cases (30.4%), respectively. The rates of clinical remission in hospitalized cases (55.3% [95% CI, 50.0–60.7%]) were almost equal to those in cases of outpatients (56.6% [95% CI, 51.8–61.4%]) (P = 0.856) (Figure S1, Supporting information). The rate of clinical remission tended to be higher in cases with first use of corticosteroids (steroid naïve cases) (31/47; 66.0%) than in cases with previous use of corticosteroids (75/144; 52.1%) (P = 0.097) (Fig. 1). Furthermore, the rate of clinical remission decreased to 27.3% in cases undergoing a fourth or more course of corticosteroids.
Short‐term outcomes in hospitalized patients undergoing corticosteroid treatment
In hospitalized cases, the median partial Mayo score was 7 (interquartile range [IQR]: 6–8) and the median steroid dose was 45.0 mg (IQR: 30.0–50.0). The rate of clinical remission was comparable between cases undergoing oral corticosteroid (21/35, 60.0%) treatment and intravenous steroid (26/50, 52.0%) treatment (P = 0.727). The rate of clinical remission at week 4 was not significantly different (P = 0.39) in cases treated with an initial steroid dose of ≥60 mg, including those who underwent pulse therapy (12/26, 46.2%), 40–59 mg (24/38, 63.2%), and <40 mg (11/21, 52.3%) (Fig. 2a). In hospitalized cases, the median pMayo tended to be higher in cases with an initial steroid dose of ≥60 mg (pMayo 7 [IQR: 6–8.25]) and 40–59 mg (pMayo 7 [IQR: 5.75–8]) than in cases with an initial steroid dose <40 mg (pMayo 6 [IQR: 5–8]). However, this difference was not significant (P = 0.209). In contrast, the proportion of cases with an MES of 3 in patients treated with initial steroid dose ≥60 mg (70.4% [19/27]) was significantly higher than that in patients treated with an initial steroid dose <40 mg (35.0% [7/20]) (P = 0.016). We also confirmed that the rate of clinical remission at week 4 was not significantly different (P = 0.29) between cases treated with an initial steroid dose of ≥1 mg/kg (18/35; 51.4%) and <1.0 mg/kg (22/37; 59.5%). However, it was only 33% in cases treated with an initial steroid dose of <0.5 mg/kg.
Figure 2.
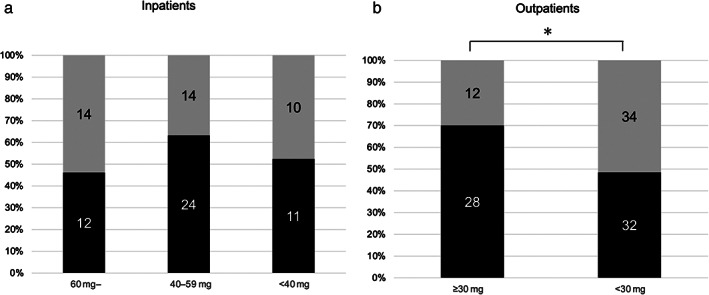
Differences of clinical efficacy at week 4 in hospitalized patients (a), and in outpatients (b) who received various initial dose of corticosteroids. *P value < 0.05. ( ), CR (+); (
), CR (+); ( ), CR (−)
), CR (−)
Clinical characteristics of cases with and without clinical remission at week 4 are shown in Table 2. The median age of cases with clinical remission was significantly lower than that of patients without clinical remission (P = 0.039). The proportion of cases in clinical remission among men tended to be higher than that among women (P = 0.054). Other factors such as disease duration, partial Mayo score, and MES at baseline were comparable between cases with or without clinical remission at week 4. Multiple logistic regression analysis indicated that male sex (odds ratio [OR], 0.373; 95% confidence interval [CI], 0.146–0.956) and younger age (OR, 0.974; 95% CI, 0.951–0.998) were associated with clinical remission at week 4.
Table 2.
Factors associated with clinical remission at week 4 after initiation of steroid in hospitalized patients with ulcerative colitis
| Remission (+) (n = 47) | Remission (−) (n = 38) | Univariate analysis | Multivariate analysis | |
|---|---|---|---|---|
| P value | P value | |||
| OR (95% CI) | ||||
| Gender (male) | 32 (68.1%) | 18 (36.0%) | 0.054 |
P = 0.040 OR, 0.373 (0.146–0.956) |
| Age, years; median (IQR) | 32 (23–48) | 46 (30–65) | 0.039 |
P = 0.034 OR 0.974 (0.951–0.998) |
| Duration of disease, years; median (IQR) | 1.80 (0.33–5.80) | 2.22 (0.62–5.76) | 0.639 | |
| Previous use of systemic corticosteroids | 26 (55.3%) | 28 (73.7%) | 0.080 |
P = 0.090 OR 0.430 (0.162–1.140) |
| Clinical severity (severe) | 13 (27.7%) | 12 (31.6%) | 0.693 | |
| Partial Mayo score at baseline; median (IQR) | 7 (6–8) | 7 (5–8) | 0.953 | |
| Mayo Endoscopic score at baseline; median (IQR) | 3 (2–3) | 3 (2–3) | 0.432 | |
| Initial corticosteroid dose; median (IQR) | 40 (30–50) | 50 (30–60) | 0.590 | |
| <40 mg | 11 (23.4%) | 10 (26.3%) | 0.386 | |
| 40–60 mg | 24 (51.1%) | 14 (36.8%) | ||
| ≧60 mg | 12 (25.5%) | 14 (36.8%) |
CI, confidence interval; IQR, interquartile range; OR, odds ratio.
Short‐term outcomes in outpatients undergoing steroid treatment
In outpatients, the median pMayo and initial steroid dose at week 0 were 6.0 (IQR: 6.0–7.0) and 20.0 (IQR: 20.0–30.0) mg, respectively. Although disease severity was greater in cases treated with an initial steroid dose of ≥30 mg (partial Mayo score 6 [IQR: 5–7]) than in those treated with an initial dose of <30 mg (partial Mayo score 5 [IQR: 4–6]) (P = 0.046), the rate of clinical remission at week 4 was 70.0% (28/40) in patients treated with steroid doses ≥30 mg and 48.5% (32/66) in cases treated with steroid doses <30 mg (P = 0.030) (Fig. 2b). Although body weight was collected only in 20 cases of outpatients, the rate of clinical remission was significantly higher (P = 0.043) in cases treated with an initial steroid dose of ≥0.5 mg/kg (10/10; 100%) than others (6/10; 60.6%) and this result is consistent with those of comparison of daily corticosteroid dose. Table 3 shows the clinical characteristics of cases with or without clinical remission at week 4 in outpatients. Median pMayo was lower in cases with clinical remission than that in cases without clinical remission at week 4 (Table 3). Other factors such as gender, disease duration, and previous use of corticosteroid at baseline were comparable between outpatients with or those without clinical remission at week 4 (Table 3). Multiple logistic regression analysis indicated that the pMayo (OR, 0.643; 95% CI, 0.451–0.918) and initial steroid dose of ≥30 mg (OR, 3.278; 95% CI, 1.274–8.435) were associated with clinical remission at week 4 in outpatients.
Table 3.
Factors for clinical remission at week 4 after initiation of corticosteroid treatment in outpatients with ulcerative colitis
| Remission (+) (n = 60) | Remission (−) (n = 46) | Univariate analysis | Multivariate analysis | |
|---|---|---|---|---|
| P value | P value | |||
| OR (95% CI) | ||||
| Gender (male) | 20 (33.3%) | 18 (39.3%) | 0.537 | |
| Age, years; median (IQR) | 42 (24–56) | 40 (29–59) | 0.630 | |
| Duration of disease, years; median (range) | 6.27 (2.24–20.9) | 5.91 (1.67–9.24) | 0.167 | |
| Previous use of systemic steroid | 49 (81.7%) | 41 (89.7%) | 0.287 | |
| Previous/present use of thiopurine | 12 (20.0%) | 15 (32.6%) | 0.171 | |
| Partial Mayo score at baseline; median (IQR) | 5 (5–6.25) | 6 (5–7) | 0.035 |
P = 0.015 OR 0.643 (0.451–0.918) |
| Mayo Endoscopic score at baseline; median (IQR) | 2 (1.25–3) | 3 (2–3) | 0.144 | |
| Initial corticosteroid dose | 0.03 |
P = 0.014 OR 3.278 (1.274–8.435) |
||
| <30 mg | 32 (53.3%) | 34 (74.0%) | ||
| ≥30 mg | 28 (46.7%) | 12 (26.0%) | ||
CI, confidence interval; IQR, interquartile range; OR, odds ratio.
Rate of dose reduction in patients undergoing corticosteroid treatment
At week 4, clinical remission or response to treatment was observed in 165 cases (86.4%). There was concomitant use of thiopurine in 39 cases (23.6%). The median duration from administration to discontinuation was 100 days, and the recurrence rate until the discontinuation of steroid use was 33.3% (55/165).
In hospitalized patients, the median daily steroid doses at weeks 0, 4, and 12 weeks were 45.0 (IQR: 30.0–50.0) mg, 24.0 (IQR: 15.0–30.0) mg, and 5.0 mg (IQR: 0.0–10.0), respectively. The median duration and total steroid dose administered until discontinuation were 100 (IQR: 62–142) days and 1800 (IQR: 1116–2584) mg, respectively.
In outpatients, the median daily steroid dose at weeks 0, 4 and, 12 were 20.0 (IQR: 20.0–30.0) mg, 15.0 (IQR: 10.0–20.0) mg, and 3.0 (IQR 0–5.0) mg, respectively. The median duration and total amount of steroid until discontinuation were 97 (IQR: 63–174) days and 1053 (IQR: 740–1740) mg, respectively. At week 4, the number of cases with steroid reduction rates of ≥50% was 50 (47.2%). Corticosteroid treatment could not be discontinued in a total of 58 (54.7%) cases within 12 weeks, and 19 cases were still undergoing steroid treatment at week 24.
Clinical factors for discontinuation of corticosteroid within 12 weeks in patients with UC
We evaluated the clinical factors associated with discontinuation within 12 weeks in patients who were clinical responders (Table 4). Univariate analysis indicated that younger patients (P = 0.022), clinical remission (vs clinical response) at week 4 (P < 0.001), and the standard steroid reduction rate at week 4 (P < 0.001) were associated with discontinuation within 12 weeks. Multiple logistic regression analysis indicated that clinical remission at week 4 (OR, 0.300; 95% CI, 0.126–0.718) and corticosteroid reduction rate at week 4 (OR, 0.092; 95% CI, 0.036–0.234) were associated with corticosteroid discontinuation within 12 weeks.
Table 4.
Clinical factors associated with the corticosteroid discontinuation within 12 weeks in patients with ulcerative colitis
| Discontinuation (+) (n = 62) | Discontinuation (−) (n = 103) | Univariate analysis | Multivariate analysis | |
|---|---|---|---|---|
| P value | P value | |||
| OR (95% CI) | ||||
| Inpatients (%) | 26 (41.9%) | 47 (45.6%) | 0.643 | |
| Gender (male) | 24 (38.7%) | 50 (48.5%) | 0.219 | |
| Age, years; median (IQR) | 31 (22–53) | 44 (27–61) | 0.022 |
P = 0.149 OR 0.984 (0.963–1.006) |
| Duration of disease, years; median (IQR) | 4.07 (0.67–9.96) | 3.71 (1.00–9.14) | 0.960 | |
| Previous use of systemic corticosteroids | 42 (67.7%) | 80 (77.7%) | 0.159 | |
| Concomitant use of thiopurine | 12 (19.4%) | 19 (18.4%) | 0.085 |
P = 0.501 OR 0.714 (0.268–1.903) |
| Partial Mayo score; median (IQR) | 6.0 (5.0–6.75) | 6.0 (5.0–7.0) | 0.285 | |
| Mayo Endoscopic score at baseline; median (IQR) | 2 (2–3) | 3 (2–3) | 0.583 | |
| Initial corticosteroid dose; median; median (IQR) | 30 (28.75–40) | 30 (20–50) | 0.411 | |
| Clinical remission at week 4 | 51 (82.3%) | 55 (54.5%) | <0.001 |
P = 0.007 OR 0.300 (0.126–0.718) |
| Corticosteroid dose at week 4; median (IQR) | 10 (10–15) | 20 (15–25) | <0.001 | |
| Corticosteroid dose reduction rate at week 4 | 60.0% (50, 75) | 33.3% (25, 50) | <0.001 |
P < 0.001 OR 0.092 (0.036–0.234) |
CI, confidence interval; IQR, interquartile range, OR, odds ratio.
Adverse events
Adverse events were observed in 31 cases including 2 pneumonia, 5 Herpes zoster infection, 4 venous thrombosis, 5 CDI, and 16 CMV infection cases. Multivariate analysis indicated that age and previous use of systemic corticosteroids were risk factors for adverse effects (Table 5). Fatal adverse effects due to steroid use were not observed in our cohort.
Table 5.
Factors associated with adverse events after initiation of corticosteroid treatment
| Adverse events (+) (n = 31) | Adverse events (−) (n = 160) | Univariate analysis | Multivariate analysis | |
|---|---|---|---|---|
| P value | P value | |||
| OR (95% CI) | ||||
| Gender (male) | 14 (45.2%) | 74 (46.3%) | 0.911 | |
| Age, years; median (IQR) | 62 (34–48) | 37 (24–74) | <0.001 |
P < 0.001 OR 1.042 (1.019–1.065) |
| Duration of disease, years; median (IQR) | 3.57 (0.33–5.80) | 3.90 (1.03–9.07) | 0.826 | |
| Previous use of systemic corticosteroids | 29 (20.1%) | 2 (6.5%) | 0.010 |
P = 0.028 OR 5.38 (1.20–24.39) |
| Clinical severity (severe) | 7 (22.6%) | 24 (15.0%) | 0.497 | |
| Initial corticosteroid dose; median (IQR) | 30 (20–50) | 30 (20–48.75) | 0.804 | |
| Duration of corticosteroid use (day) median (IQR) | 107 (59–163) | 98 (68–168) | 0.286 | |
| Corticosteroid reduction rate at week 4 median (IQR) | 36.7% (25–60) | 50.0% (25–58.75) | 0.714 | |
| Proportion of cases with corticosteroid discontinuation at week 12 | 17/26 (26.9%) | 56/139 (40.3%) | 0.198 |
CI, confidence interval; IQR, interquartile range; OR, odds ratio.
Trends in clinical response, initial steroid dose, and rate of steroid dose reduction in patients with UC
Figure 3 indicates the change in short‐term efficacy in patients with UC who underwent corticosteroid treatment every 5 years between 2006 and 2020. The rates of clinical remission and clinical response tended to be higher in 2016–2020 than in other periods (2006–2010, 52.3% [95% CI, 46.5–58.5%]; 2011–2015, 52.4% [95% CI, 46.1–58.7%]; and 2016–2020, 63.4% [95% CI, 57.3–69.5%]) (Fig. 3a). The trend in initial steroid dose changed from the first period (2006–2010) to the third period (2016–2020), as the proportion of cases with a daily dose of steroid ≤20 mg changed from 52.3% (34/65) to 14.3% (9/63), as shown in Figure 3b (P < 0.001). The rate of steroid dose reduction at weeks 4 and 12 was also higher in the more recent periods (Fig. 3c,d). The proportion of patients with a steroid reduction rate of ≥50% at week 4 was significantly higher (P = 0.006) in the third period (46/63, 73.0%) than it was in the first period (32/65, 49.2%) (Fig. 3c). Although the initial dose was higher in the third period than in the first period, the proportion of patients in whom corticosteroids were discontinued at week 12 was significantly higher (P = 0.006) in the third period (28/52; 53.8%) than in the first period (15/54, 27.8%) (Fig. 3d).
Figure 3.
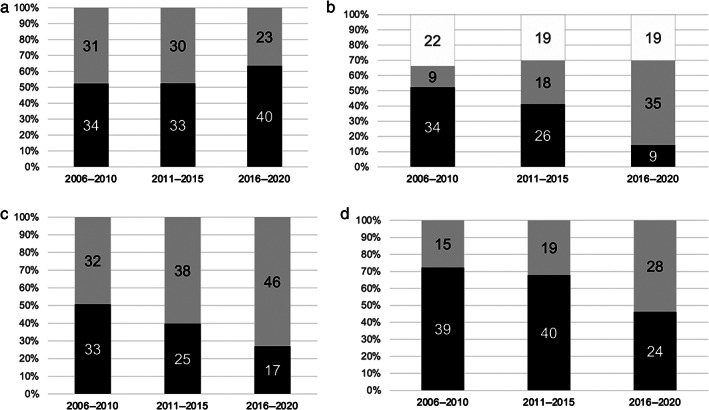
Clinical efficacy of steroid treatment (a), initial dose of steroid (b), rate of decrease in steroid daily dose at week 4 (c) and at week 12 (d) every 5 years between 2006 and 2020. (a): ( ), CR (+); (
), CR (+); ( ), CR (−). (b): (
), CR (−). (b): ( ), ≤20 mg; (
), ≤20 mg; ( ), 21–40 mg; (
), 21–40 mg; ( ), 41 mg. (c): (
), 41 mg. (c): ( ), 0–49%; (
), 0–49%; ( ), 50–100%. (d): (
), 50–100%. (d): ( ), 0–99%; (
), 0–99%; ( ), discontinuation.
), discontinuation.
The median duration of steroid use gradually changed up to the third period (103 [IQR 91–165], 145 [IQR 63–244], and 86 [IQR 61–117] days, P = 0.041). The median total doses of steroid were 1750 (IQR 735–2561) mg, 1447.5 (IQR 945–2514) mg, and 1120 (IQR 878–1902) mg in the first, second, and third periods, respectively (P = 0.217).
Discussion
The short‐ and medium‐term therapeutic effects of the initial corticosteroid dose and the corticosteroid reduction rate in patients with UC were investigated in a single institution. The rate of clinical remission was higher in outpatients undergoing treatment with a corticosteroid dose ≥30 mg. Clinical factors for corticosteroid discontinuation within 12 weeks were associated with clinical remission and a standard corticosteroid dose reduction at week 4. The rate of corticosteroid dose reduction at weeks 4 and 12 were also higher in 2016–2020 than in 2006–2010. Our results also indicated that elder age and previous use of steroid were associated with the occurrence of adverse effects. Finally, the duration of steroid use was also significantly shorter in 2016–2020. This may be due to novel therapeutic developments, such as biologic and small molecule compounds within the last 10 years. As indicated by clinical guidelines, corticosteroid therapy should be discontinued for 3 months, due to adverse events.
Acute severe hospitalized patients are at a high risk of short‐term colectomy. 18 , 23 Acute severe UC (ASUC) is defined in hospitalized patients using the Truelove–Witts criteria as follows: > 6 bloody stools per day and at least one marker of systemic toxicity including a pulse rate of >90 beats per min, temperature >37.8°C, hemoglobin <10.5 g/dL, and/or an erythrocyte sedimentation rate of >30 mm/h. 23 Although several biologics have been developed, corticosteroids remain the first‐line therapy for hospitalized patients with ASUC. Nevertheless, there is little evidence regarding the appropriate corticosteroid dose in terms of efficacy and safety. A previous systematic review of 32 clinical trials found that colectomy rates in patients treated with daily corticosteroid doses of >60 mg were equivalent to those in patients undergoing treatment with lower doses of corticosteroids. 18 Similar to the findings of a previous study, 18 our study indicated that the short‐term clinical efficacy was comparable regardless of corticosteroid dosage. The rate of clinical remission tended to be lower in patients treated with a higher initial corticosteroid dose (>60 mg daily); however, this difference was not statistically significant. The reason why there were no significant differences in the remission rate by initial corticosteroid dose may be that the dose was adjusted according to disease severity, particularly the endoscopic index of severity in hospitalized cases. Approximately half of the patients in our cohort responded to corticosteroid doses <40 mg/day, which may be explained by the higher proportion of hospitalized patients with moderate severity in our cohort. In Japan, hospitalization is permitted in patients without ASUC if the patient has several episodes of diarrhea with mild to moderate abdominal pain in addition to an elevated C‐reactive protein (CRP). For these inpatients, corticosteroids may be administered at a relatively lower dose.
In patients with active UC, the rate of clinical remission at week 4 was higher among outpatients treated with ≥30 mg of corticosteroids than in those treated with lower doses. This result indicates that an appropriate corticosteroid dose should be used for outpatients according to clinical severity. In contrast, the clinical remission rate was also relatively higher even in patients who were initially treated with a daily corticosteroid dose of 20 mg. Although recent Japanese guidelines recommend that daily corticosteroid doses of 30–40 mg should be used in patients with moderately active disease, more than half of the patients in 2006–2010 were treated with a lower steroid dose (≤20 mg/day). Our results are consistent with those of a recent large Japanese study using claims data, 24 indicating that physicians tended to use a relatively low corticosteroid dose before 2010, and that the proportion of patients with an initial dose of ≥30 mg/day increased between 2011 and 2016. We would like to emphasize that the initial dose of corticosteroids should be appropriately decided according to the clinical guideline because it is not possible to determine whether the steroid is ineffective or the dose is insufficient when lower dose of corticosteroids is given. Furthermore, a low initial dose was most strongly associated with long‐term steroid use. 24 Prolonged steroid use induces critical adverse effects. Because several effective treatments for inducing and maintaining remission are available at present, the long‐term use of lower corticosteroid doses should be avoided.
The tapering schedule for corticosteroid doses should be approached with caution because adverse effects are frequently induced with long‐term use. The steroid dose should be reduced to below the equivalent of 10 mg/day within 3 months of initiation of steroid therapy. 25 Recent European Crohn's and Colitis Organization guidelines have recommended that an appropriate regimen for moderately active disease is prednisolone 40 mg/day for 1 week, and the daily dose should be lowered by 5 mg each week. 9 However, a recent study indicated that corticosteroid was continued for more than 180 days in approximately one‐third of patients receiving corticosteroid. 24 This may be related to the presence of certain number of physicians who still believe that continuation of low dose of steroid (slow reduction of corticosteroid dose) can prevent clinical relapse even after maintaining clinical remission. We would like to emphasize that slow reduction of corticosteroid dose has no benefit.
Our study also indicated that the proportion of patients with a steroid reduction rate of more than half at week 4 was higher in the last 5 years compared with those in other periods. Furthermore, the proportion of patients who discontinued steroid within 12 weeks was also higher in 2016–2020 than in other periods. Several effective treatments are available, such as anti‐TNFα antibody, anti‐α4β7 antibody, Janus kinase inhibitor, and anti‐interleukin 12/23 antibody for patients with steroid dependency, so physicians are more likely to reduce the dose of steroid rapidly. Interestingly, standard reduction of corticosteroid dose at week 4 (≥50%) was associated with discontinuation of corticosteroid therapy within 12 weeks in our study, whereas the dose of corticosteroids, clinical and endoscopic severity at baseline was not associated with corticosteroid discontinuation within 12 weeks. We confirmed that clinical recurrence before corticosteroid discontinuation was not higher in patients with standard corticosteroid reduction at week 4 compared with that observed in other patients (data not shown). Our results suggest that it is important to reduce the corticosteroid dose to less than half of the initial dose at 4 weeks. Patients should not be maintained on the same corticosteroid dose for a prolonged time.
Our study indicated that the median age of patients who discontinued steroid therapy at week 12 was significantly lower than that in patients who did not discontinue treatment. Younger patients may be likely to undergo rapid reduction in steroid doses for many reasons, whereas steroid reduction may be slower in older patients. In terms of adverse effects, corticosteroids should be discontinued as early as possible, particularly in elderly patients.
Our study has several limitations. First, this was a single‐center, retrospective study. Second, endoscopy was not performed on all patients before commencement of corticosteroid therapy. Third, we did not collect data on extraintestinal complications and smoking habits in this study.
Clinical guidelines for the management of UC suggest that an appropriate corticosteroid dose should be used, and the dose should be reduced as early as possible. For mild to moderate recurrence, there are several options that do not involve the use of low doses of systemic corticosteroids, such as increased dose of 5‐ASA, alteration of different 5‐ASA formulations, MMX budesonide, and concomitant use of topical therapy. Regarding the corticosteroid tapering schedule, it may be better to reduce the corticosteroid dose by more than half of the initial dose at week 4, and to discontinue steroid therapy by week 12.
Supporting information
Figure S1. Clinical efficacy of corticosteroids at week 4 in hospitalized patients and outpatients.
Acknowledgment
The authors would like to thank Editage (www.editage.jp) for English language editing.
Declaration of conflict of interest: Makoto Naganuma received the following funding from a commercial source: EA Pharma Co., Ltd., Mochida Pharmaceutical Co., Ltd. for research grants; Takeda Pharmaceutical Co., Ltd., Mitsubishi‐Tanabe Pharmaceutical Co., Ltd., and Pfizer Co., Ltd. for lecture fees. Makoto Naganuma declares that he has no role within the aforementioned companies with regards to employment, consultancy work, patents, products under development, or commercial products.
Financial support: This study was partly funded by a grant from the Japan Agency for Medical Research and Development (20314259).
References
- 1. Naganuma M, Mizuno S, Nanki K, Sugimoto S, Kanai T. Recent trends and future directions for the medical treatment of ulcerative colitis. Clin. J. Gastroenterol. 2016; 9: 329–36. [DOI] [PubMed] [Google Scholar]
- 2. Rutgeerts P, Sandborn WJ, Feagan BG et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005; 353: 2462–76. [DOI] [PubMed] [Google Scholar]
- 3. Sandborn WJ, van Assche G, Reinisch W et al. Adalimumab induces and maintains clinical remission in patients with moderate‐to‐severe ulcerative colitis. Gastroenterology. 2012; 142: 257–65. [DOI] [PubMed] [Google Scholar]
- 4. Sandborn WJ, Feagan BG, Marano C et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate‐to‐severe ulcerative colitis. Gastroenterology. 2014; 146: 85–95. [DOI] [PubMed] [Google Scholar]
- 5. Sandborn WJ, Feagan BG, Marano C et al. Subcutaneous golimumab maintains clinical response in patients with moderate‐to‐severe ulcerative colitis. Gastroenterology. 2014; 146: 96–109.e1. [DOI] [PubMed] [Google Scholar]
- 6. Ogata H, Matsui T, Nakamura M et al. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut. 2006; 55: 1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feagan BG, Rutgeerts P, Sands BE et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- 8. Sandborn WJ, Su C, Sands BE et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2017; 376: 1723–36. [DOI] [PubMed] [Google Scholar]
- 9. Sands BE, Sandborn WJ, Panaccione R et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2019; 381: 1201–14. [DOI] [PubMed] [Google Scholar]
- 10. Harbord M, Eliakim R, Bettenworth D et al. Third European evidence‐based consensus on diagnosis and management of ulcerative colitis. Part 2: Current Management. J. Crohns Colitis. 2017; 11: 769–84. [DOI] [PubMed] [Google Scholar]
- 11. Singh S, Feuerstein JD, Binion DG, Binion DG, Tremaine WJ. A technical review on the management of mild‐to‐moderate ulcerative colitis. Gastroenterology. 2019; 156: 769–808.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am. J. Gastroenterol. 2019; 114: 384–413. [DOI] [PubMed] [Google Scholar]
- 13. Lamb CA, Kennedy NA, Raine T et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019; 68: s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsuoka K, Kobayashi T, Ueno F et al. Evidence‐based clinical practice guidelines for inflammatory bowel disease. J. Gastroenterol. 2018; 53: 305–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ford AC, Bernstein CN, Khan KJ et al. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta‐analysis. Am. J. Gastroenterol. 2011; 106: 590–9. [DOI] [PubMed] [Google Scholar]
- 16. Lennard‐Jones JE, Longmore AJ, Newell AC, Newell AC, Wilson CW, Jones FA. An assessment of prednisone, Salazopyrin, and topical hydrocortisone hemisuccinate used as out‐patient treatment for ulcerative colitis. Gut. 1960; 1: 217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Truelove SC, Watkinson G, Draper G. Comparison of corticosteroid and sulphasalazine therapy in ulcerative colitis. Br. Med. J. 1962; 2: 1708–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticocorticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta‐regression. Clin. Gastroenterol. Hepatol. 2007; 5: 103–10. [DOI] [PubMed] [Google Scholar]
- 19. Sandborn WJ, Travis S, Moro L et al. Once‐daily budesonide MMX® extended‐release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology. 2012; 143: 1218–26. [DOI] [PubMed] [Google Scholar]
- 20. Travis SP, Danese S, Kupcinskas L et al. Once‐daily budesonide MMX in active, mild‐to‐moderate ulcerative colitis: results from the randomised CORE II study. Gut. 2014; 63: 433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sandborn WJ, Danese S, D'Haens G et al. Induction of clinical and colonoscopic remission of mild‐to‐moderate ulcerative colitis with budesonide MMX 9 mg: pooled analysis of two phase 3 studies. Aliment. Pharmacol. Ther. 2015; 41: 409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baron JH, Connell AM, Kanaghinis TG, Lennard‐Jones JE, Jones AF. Out‐patient treatment of ulcerative colitis. Comparison between three doses of oral prednisone. Br. Med. J. 1962; 2: 441–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dinesen LC, Walsh AJ, Protic MN et al. The pattern and outcome of acute severe colitis. J. Crohns Colitis. 2010; 4: 431–7. [DOI] [PubMed] [Google Scholar]
- 24. Matsuoka K, Igarashi A, Sato N et al. Trends in corticosteroid prescriptions for ulcerative colitis and factors associated with long‐term corticosteroid use: analysis using Japanese claims data from 2006 to 2016. J. Crohns Colitis. 2021; 15: 358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dignass A, Eliakim R, Magro F et al. Second European evidence‐based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J. Crohns Colitis. 2012; 6: 965–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Clinical efficacy of corticosteroids at week 4 in hospitalized patients and outpatients.


