Abstract
Gonadotropin-regulated testicular RNA helicase (GRTH)/DDX25 is a DEAD-box RNA helicase essential for the completion of spermatogenesis. Our previous studies indicated that blocking the GRTH phospho-site or perturbing the GRTH/protein kinase A (PKA) interface could provide an avenue for developing a nonhormonal male contraceptive. In this study, cyclic peptides were rationally designed and synthesized as promising therapeutic agents. The peptides showed effective delivery into COS-1 and germ cells and a dose-dependent inhibitory effect on GRTH phosphorylation. The peptides inhibit GRTH phosphorylation in the presence of PKA, and binding to the helicase resulted in thermal stabilization of non-phospho GRTH. Increased efficiency in fluorescence resonance energy transfer (FRET) assay revealed their interaction with GRTH. Cyclic peptide exposure of cultures from mice seminiferous tubules resulted in significant inhibition of phospho GRTH. These peptides did not exhibit toxicity. Effective delivery and targeted decrease of in vitro expression of phospho GRTH by cyclic peptides provide a promising angle to develop effective compounds as a nonhormonal male contraceptive.
Graphical Abstract
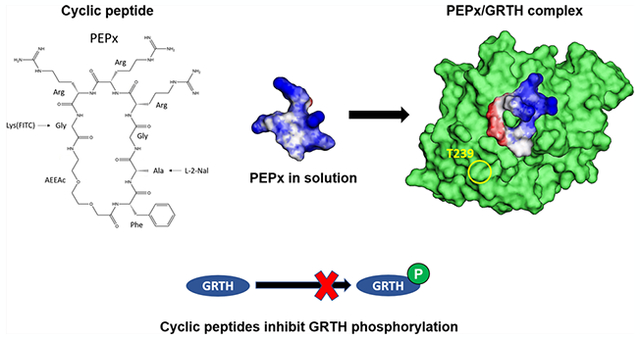
INTRODUCTION
Gonadotropin-regulated testicular RNA helicase (GRTH/DDX25) is a testis-specific member of the DEAD-box family of RNA helicases expressed in Leydig and germ cells of the male gonad and found to have an essential role in spermatid development during spermatogenesis. GRTH is expressed in rat, mouse, and human testis and acts as a post-transcriptional regulator of genes essential for the completion of spermatogenesis.1–4 GRTH knockout mice are sterile and lack spermatozoa due to failure of elongation of round spermatids during spermiogenesis.2 There are two species of GRTH, a 56 kDa non-phosphorylated form, predominantly found in the nucleus where it interacts with chromosomal region maintenance 1 protein (CRM1) and participates in mRNA transport to cytoplasmic sites, and a 61 kDa phosphorylated GRTH (pGRTH) species which is present exclusively in the cytosol and found to reside in chromatoid bodies5,6 and to be associated with polyribosomes where it may be involved in the translation of germ cell-specific genes.3 Transient expression of GRTH cDNA in the COS-1 cells showed a similar pattern of expression as observed in mice.7 Our previous studies found a missense mutation of arginine to histidine at position 242 (R242H) in exon 8 of GRTH in 5.8% of Japanese patients with azoospermia. In vitro experiments using GRTH (R242H) mutant plasmid in COS-1 cells resulted in the loss of pGRTH form with preservation of the non-phospho GRTH species.7 We recently showed that GRTH knock-in (KI) mice model carrying the human GRTH gene mutation R242H are sterile, lack sperm due to arrest at step 8 of round spermatids, and display complete loss of pGRTH, revealing the functional relevance of pGRTH.8
We have recently identified residue T239, structurally adjacent to the patient mutant at R242 as the GRTH phosphorylation site. Molecular modeling and biochemical studies allowed us to determine the role of R242 and other critical solvent-exposed residues in its vicinity (hereafter, referred to as the GRTH/protein kinase A (PKA) interface) that controls the phosphorylation status of the protein.9 These include residues E165, K240, and D237, which are all structurally distant from the conserved motifs involved in the ATPase and helicase activities. Site-directed mutagenesis of these residues resulted in marked reduction or abolition of pGRTH species. Computer simulations showed that critical disruptions of intramolecular H-bonds at the GRTH/PKA interface lead to modest but consequential structural changes that can affect the PKAα catalytic efficiency. The unique distribution of basic and acidic residues at the GRTH/PKA interface9 provides a strong motivation to derive pharmacophores for developing nonhormonal contraceptive compounds. Computational modeling also showed that the surface topography of the GRTH/PKA interface is relatively shallow, lacking the desirable deep crevices typically targeted as potential binding pockets for small druglike molecules. Cyclic peptides are an alternative for such (nondruggable) surfaces, and the GRTH/PKA interface appears to have unique features that could be exploited for selectivity, specificity, and high-affinity binding. A cyclic peptide that binds at or near the phospho-site can potentially perturb PKA activity or block the phosphorylation of GRTH, thereby inhibiting spermatogenesis.
In their simplest form, cyclic peptides consist of amino acids (typically, ≤10 aa) linked together to form a macrocyclic ring structure. Many biologically active cyclic peptides are linked through head-to-tail cyclization, where an amide bond is formed between the amino and carboxy termini of each end. There are several advantages of using cyclic peptides for the development of therapeutics.10 First, cyclic peptides show better biological activity than linear peptides because of their conformational rigidity, and their small ring structure provides resistance toward proteolytic degradation.11,12 Also, cyclic peptides have larger surface areas, leading to higher affinity and selectivity of protein targets.13 Second, the toxicity of cyclic peptides is less compared to the other drugs, as their degradation products are amino acids. However, the major challenge of cyclic peptides as therapeutics is the poor membrane permeability.14 Thus, a great deal of effort has been devoted to designing cell-penetrating cyclic peptides for efficient delivery into cells. Cyclic peptides can be categorized based on their physicochemical properties or conformations. Several cyclic peptides containing L-amino acids were developed and synthesized to investigate their cell-penetrating properties and examine their role as molecular transporters.15 Cyclic peptides rich in arginine residues can be effectively delivered into mammalian cells.16 The addition of l-2-naphthylalanine in the peptides facilitates plasma-membrane binding and internalization. In another study, a cyclic peptide containing arginine and tryptophan was conjugated to a drug, showing its potential use as an intracellular drug delivery platform.17 Arginine-rich cyclic peptides were shown to display higher structural rigidity and enhanced transduction efficiency;18 this study also suggested that cyclic peptides have higher cellular uptake efficiency compared to their linear forms, indicating that the cyclization strategy could be employed for the delivery of various potential drug candidates. Considering these facts and the structural features of the GRTH surrounding the exposed site at T239, we rationally designed cyclic peptides that meet the above requirements, such as effective ingress into cells and binding to the target protein at the expected binding interface. We have evaluated the efficacy of cyclic peptides using an FITC tag to show that they effectively enter COS-1 cells and mice seminiferous tubules. Cellular thermal shift assay (CETSA) and fluorescence resonance energy transfer (FRET) assays were performed to assess the binding ability of the cyclic peptide to GRTH protein in COS-1 cells. Further, the efficiency of the cyclic peptide binding to the PKA-binding site was assessed in the presence of PKA. Treatment of both cyclic peptides in COS-1 stable cells expressing GRTH and seminiferous tubules endogenously expressing GRTH showed a significant reduction in pGRTH species, indicating their effectiveness in preventing phosphorylation of GRTH by PKA.
RESULTS
Computational Design of Cyclic Peptides.
Despite the shallowness of the GRTH surface at the PKA-binding interface, there is a clear electrostatic pattern radiating from the center, with a region of negative potential surrounded by a nonpolar transition halo and regions of positive potential further away (Figure 1A). This pattern is consistent with the hotspots distribution obtained from the single-analogue simulations (Figure 1B; cf. the Experimental Section and the Supporting Information for details): some areas are targeted preferentially by basic groups, others by acidic groups, and others by nonpolar (in this context, hydrophobic) or netneutral polar groups; other areas are avoided altogether by all of the analogues (cf. Figure S4). This pattern is significant and may function as recognition sites for specific, high-affinity binding of cyclic peptides. One possible pharmacophore chosen here as the basis for the design of all of the peptides is shown in Figure 1C. Although some leeway exists in the choice of residues matching the pharmacophore, some requirements are imposed a priori, which restrict the options: (i) the cyclic peptide must contain fewer than 10 residues (or being of similar length if nonpeptidic spacers, such as poly(ethylene glycol) (PEG), are used to separate functional groups), (ii) incorporate local moieties that facilitate penetration into the cell membrane, (iii) accommodate a fluorescence probe for in vitro monitoring of cellular localization without disrupting GRTH binding, and (iv) have enough structural flexibility in solution to adapt to the shallow, flexible surface upon binding. One of the simplest cyclic peptides that accommodates these stringent conditions and the main elements of the pharmacophore is cyclo(1FAGRRRG7-AEEAc), referred to as PEPx (all residues in the L configuration, unless otherwise stated).
Figure 1.

Computational design of GRTH-binding cyclic peptides: (A) Electrostatic potential on the solvent-accessible surface of GRTH at the PKA-binding interface (blue: positive potential; red: negative; white: neutral); yellow circle indicates location of T239. (B) GRTH surface representation (same orientation as in (A) with the single amino acid analogues hotspots indicated in green (each point corresponds to the location of the Cβ atom in the corresponding residue)); T239 shown in yellow, basic residues in blue (R242 is occluded but indicated by the arrow); acidic residues in red. (C) Pharmacophore derived from the hotspot distribution of functional groups used here (two others can be derived; cf. Supporting Information). F: Phe; Q: Gln; b (basic): Arg+, His+, or Lys+; a (acidic): Glu− or Asp−; hyd (nonpolar/hydrophobic): Ala, Ile, Val, or Leu; pol (net-neutral polar): Gln or Asn. Circles containing more than one symbol means that any of the indicated groups can be used. Solid lines indicate a single peptide bond; dashed lines, one or more (indicated by the adjacent numbers); double arrow indicates allowed permutation. (D) Computational prediction of PEPx conformations in solution (see text and Table 1); each structure is a representative member of a conformational family (cf. Supporting Information for details). Backbone ring in yellow; only nonhydrogen atoms shown; the backbone RMSD (including PEG2) between any two conformers is in the 1.5–3 Å range. (E) Electrostatic potential on the solvent-accessible surface of two representative PEPx conformers shown in (D). (F) Superposition of a selected set of PEPx conformers upon binding to the GRTH/PKA interface (circle indicates the position of T239; GRTH orientation similar to that in (A)); only one GRTH surface shown for clarity. All of the conformers find favorable interactions with the protein, albeit with significant variability in binding modes due to the flexibility of both PEPx and protein surface. (G) Mutual structural adaptation upon binding enables PEPx to find its way into the shallow crevices at the GRTH surface; solvent-accessible surface of PEPx colored green; only three conformers are shown to illustrate. See the Supporting Information for computational details.
To obtain evidence that this molecular scaffold can provide the basis for the design of GRTH-binding cyclic peptides, we followed a computational multistage cyclization procedure and predicted the binding modes at the interface (cf. details in the Supporting Information). The simulations suggest that, in solution, the peptide is quite flexible, with several coexisting conformational families, presumably, in fast interconversion (Figure 1D; coordinates of representative members in the Supporting Information). Certain structural flexibility is desirable to allow local adaptation to the interface and may be controlled by changing the length or composition of the linker (e.g., flexibility may be reduced with Gly2 instead of PEG2; not tested). The molecular surface representation of the conformers in solution (two shown in Figure 1E) reveals a common feature: a pronged cationic structure with basic residues on one end of the ring and a hydrophobic region on the other, giving the peptide the cationic/amphipathic character desirable for membrane translocation and cytosolic localization. Furthermore, analysis of the predicted binding modes (cf. details in the Supporting Information) shows that all of the conformers are attracted to GRTH at or near the PKA-binding interface, although with significant structural variability (Figure 1F) and mutual adaptation upon binding (Figure 1G). Such changes in the local GRTH structure are the desired perturbations hypothesized above as a possible inhibitory mechanism of T239 phosphorylation by PKA, barring a direct competitive binding inhibition of the kinase. Although every motif in the pharmacophore plays a part in binding and stabilization, not all of the groups interact simultaneously with the protein, suggesting potential improvement in affinity and specificity.
This computational analysis suggests that the proposed PEPx can indeed be used as the basic molecular scaffold for the synthesis of a series of cell-penetrating cyclic peptides. We took advantage of the pharmacophore itself to incorporate some features known to facilitate membrane crossing in vitro. Studies have shown that having 3–4 arginine residues and at least one aromatic or hydrophobic group are common characteristics of cell-penetrating peptides, enabling effective permeabilization into cells.16,19 Given the uncertainty in the optimal length of the linkers and the local flexibility they introduce, which must be kept within limits for both enthalpic and entropic reasons, we propose to use either polyglycine or poly(ethylene glycol) of two units in length at most. Based on these considerations, we synthesized the set of cyclic peptides shown in Table 1, which incorporate relatively minor side-chain modifications to PEPx: PEP0 replaces A2 by l-2-naphthylalanine (cf. Scheme 1), whereas PEP1 and PEP2 replace G7 by l- and d-Lys(FITC), respectively (we tested both configurations because of the unknown effects of the conjugated fluorophore size on GRTH binding; smaller alternatives have been proposed).16 As controls, we designed a linear peptide (CP1) with the same amino acid composition as PEP1 to prove the need for cyclization, and two additional control cyclic peptides to study the effects of individual moieties: inversion of charge at the polar end of the ring (CP2) and reduction in the size of the hydrophobic/nonpolar groups at the nonpolar end (CP3).
Table 1.
Peptides Used in the Experiments
| sl. no. | name of the peptide (abbreviation) | sequence | modification |
|---|---|---|---|
| 1 | cyclic peptide without FITC (PEP0) | cyclo(Phe-Nal-Gly-Arg-Arg-Arg-Gly-AEEAc) | head-to-tail cyclization |
| 2 | cyclic peptide 1 (PEP1) | cyclo(-Phe-Nal-Gly-Arg-Arg-Arg-Lys(FITC)-AEEAc) | head-to-tail cyclization |
| 3 | cyclic peptide 2 (PEP2) | cyclo(-Phe-Nal-Gly-Arg-Arg-Arg-{D-Lys(FITC)}-AEEAc) | head-to-tail cyclization |
| 4 | control peptide 1 (CP1) | Phe-Nal-Gly-Arg-Arg-Arg-Lys(FITC)-AEEAc | linear |
| 5 | control peptide 2 (CP2) | Cyclo(-Phe-Nal-Gly-Glu-Glu-Glu-Lys(FITC)-AEEAc) | head-to-tail cyclization |
| 6 | control peptide 3 (CP3) | cyclo(-Gly-Gly-Gly-Arg-Arg-Arg-Lys(FITC)-AEEAc) | head-to-tail cyclization |
Scheme 1.

Structure of PEP0 Showing the Position of FITC in PEP1 and PEP2, Based on PEPx (Figure 7)
Cytotoxicity Analysis of Cyclic Peptides.
MTT cell cytotoxicity assay was performed to determine the IC50 values of cyclic and control peptides used in this study. The cell viability was not significantly altered at higher concentrations of cyclic peptides. The IC50 value for each peptide was calculated from the dose–response plot and data fit with a linear regression (Figure S5). The IC50 values of PEP0, PEP1, PEP2, CP1, CP2, and CP3 were 392, 312.30, 333.06, 314.06, 173.77, and 256.66 μM, respectively. Higher IC50 values indicate less toxicity and hence the maximum concentration of cyclic peptides for the experiments were fixed as 100 μM.
Effective Delivery of Cyclic Peptides into COS-1 Cells.
The PEP1, PEP2, CP1, CP2, and CP3 tagged with FITC were utilized to assess the effective delivery in COS-1 cells. To determine the uptake of the cyclic peptides, stable COS-1 cells expressing GRTH (see the Methods section) were incubated with the respective cyclic peptides for 4 h, and DAPI was used as counterstain. Compared to CP1 (linear peptide), all of the cyclic peptides gained entry into cells and accumulated in both the cytoplasm and nucleus, as shown by intracellular FITC (Figure 2). However, it is interesting to note that not all of the peptides displayed the same uptake efficiency, presumably because of their specific properties, three-dimensional structure, and amino acid compositions. Nevertheless, these initial findings confirmed the efficient delivery of cyclic peptides into mammalian cells, thus deemed suitable for subsequent studies.
Figure 2.

Peptide internalization and effect of cyclic and control peptides on GRTH phosphorylation in COS-1 stable cells expressing GRTH: Immunofluorescence images showing the degree of entry of the PEP1, PEP2, CP1, CP2, and CP3 (green) inside the cytoplasm and nucleus of COS-1 cells. DAPI was used as counterstain (blue). Cyclic peptides PEP1, PEP2, and PEP0 (A–C) showed a dose-dependent (5, 20, 60, and 100 μM) inhibitory response, while control peptides CP1, CP2, and CP3 (D–F) showed no effect on GRTH phosphorylation. β-Actin was used as a negative control. The protein band intensities of pGRTH and β-actin from three independent experiments (mean ± SEM) were measured as indicated in the Methods section. Values were normalized by β-actin. *P < 0.05 indicates statistically significant.
Dose-Dependent Effect on GRTH Phosphorylation upon Treatment with Cyclic Peptides.
Here, we show overexpression of pGRTH in COS-1 stable cells using a phospho-specific GRTH antibody (Figure 2). Addition of cyclic peptides PEP0, PEP1, and PEP2 to stable cells expressing pGRTH showed a dose-dependent inhibitory response on phosphorylation of GRTH (Figure 2A–C). A significant dose-dependent decrease on pGRTH protein was observed in the concentration range 20–100 μM (Figure 2A–C). Cyclic peptides showed a maximum inhibition at 100 μM concentration. Effective inhibition of pGRTH upon PEP0 treatment indicated that Lys(FITC) present in the PEP1 did not interfere or participate in the inhibitory effect of cyclic peptide. On the other hand, although effective internalization was achieved with control peptides (CP1, CP2, and CP3), no significant changes were observed in the phosphorylation of GRTH in the COS-1 cells (Figure 2D–F). This demonstrated that cyclic peptides (1 and 2) perturb the GRTH protein at the GRTH/PKA interface and inhibit phosphorylation. The linear peptide, despite its sequence identity to PEP1, was unable to inhibit GRTH phosphorylation. Also, the replacement of Arg with Glu and the introduction of two Gly residues in the other cyclic control peptides (CP2 and CP3) did not influence GRTH phosphorylation. These observations indicate that effective inhibition of GRTH phosphorylation is dependent on the composition of amino acid residues and surface association capability predicted by the pharmacophore and our dynamics simulations.
Time-Dependent Inhibitory Effect of Cyclic Peptides on the pGRTH Expression.
Time-dependent analysis was initiated to determine the effective inhibition of PEP0, PEP1, and PEP2. A significant decrease in pGRTH protein was observed at 8 and 16 h of cyclic peptides PEP1 and PEP2 treatments (Figure 3A,B), whereas PEP0 showed significant decrease of pGRTH at 16 and 24 h time intervals (Figure 3C). PEP0 and PEP1 showed more effective and sustained inhibition of pGRTH compared to PEP2, even after 24 h. These results suggested that PEP0 and PEP1 could act as effective inhibitors of GRTH phosphorylation.
Figure 3.

Time-dependent inhibitory effect of cyclic peptides on pGRTH expression in COS-1 stable cells. Cyclic peptides PEP0, PEP1, and PEP2 (100 μM) showed a time-dependent response on the expression of pGRTH (A–C, respectively). The pGRTH was identified using custom-made GRTH phospho-specific antibody.9 β-Actin was used as a negative control. The protein band intensities of pGRTH and β-actin from three independent experiments (mean ± SEM) are shown. *P < 0.05 indicates statistical different from control.
Inhibitory Effect of Cyclic Peptides on pGRTH in Seminiferous Tubules.
We wanted to study the inhibitory effect of cyclic peptides directly on seminiferous tubules cultures. Immunofluorescence images of seminiferous tubules showed effective uptake of the different all five peptides (PEP1, PEP2, CP1, CP2, CP3) after 16 h of treatment (Figure 4). Exposure of PEP1 and PEP2 (100 μM) to seminiferous tubules showed a significant reduction in pGRTH protein by 60% compared to the untreated sample (Figure 4). While CP1, CP2, and CP3 did not show any effect on pGRTH expression in seminiferous tubules. This study demonstrates that PEP1 and PEP2 inhibit phosphorylation of GRTH, which is endogenously expressed in the seminiferous tubules, while the controls were ineffective.
Figure 4.

Inhibition of pGRTH expression by cyclic peptides in seminiferous tubules. PEP1, PEP2, CP1, CP2, and CP3 showed effective internalization in the seminiferous tubules. Western blot of seminiferous tubules exposed to 100 μM of the respective peptide showed a significant decrease in the PEP1- and PEP2-treated samples compared to those untreated samples or exposed to control peptides. Band intensities were calculated for three independent experiments (n = 3), and the values were plotted in the form of a graph. All data represent mean ± SEM (*P < 0.05).
Assessment of the Interaction of Cyclic Peptides to Non-Phospho GRTH Protein Interface by CETSA.
To assess the binding of cyclic peptides to non-phospho GRTH target protein, PEP1 and PEP2 were added to cell lysates prepared from COS-1 cells expressing GRTH and subjected to heat shock across a range of temperatures, from 40 to 70 °C. CP2 is used as control in this CETSA assay. In the case of CP2, the heat shock effectively denatured all of the proteins, including GRTH, indicating that CP2 did not engage in the thermal stabilization of GRTH (Figure 5). While the addition of PEP1 and PEP2 effectively thermostabilized GRTH significantly in the 50–55 °C temperature range (Figure 5). The interaction of PEP1 and PEP2 at the higher temperature significantly shifted the thermal shift curve between the temperature 45–55 °C compared to the CP2.
Figure 5.

Thermal shift plots (CETSA) showing melting curve profiles of cyclic and control peptides. Western blots showing denaturation pattern of non-pGRTH protein upon treatment with CP2, PEP1, and PEP2. Band intensities were calculated for three independent experiments (n = 3), and the values were plotted in the form of a graph. The sample at a temperature of 40 °C was taken as an initial control for all of the samples. All data represent mean ± SEM.
FRET Analysis Confirms Interactions of Cyclic Peptides with GRTH Protein.
The expression of mCherry-GRTH in COS-1 cells showed a wide distribution throughout the cells. PEP1 and PEP2 showed effective cellular intake compared to CP1, CP2, and CP3 (Figure S6). The acceptor photobleaching method was carried out to observe the intracellular FRET. After photobleaching of the acceptor (mCherry-GRTH), we observed an increase in fluorescence intensity of the donor FITC (PEP1 and PEP2) in the bleached regions, as shown in Figure 6A,B. The enhanced emission of donor FITC indicates that the donor in the excited state transferred energy to the acceptor mCherry in the ground state, hence the observed increase in the donor intensity (Figure S7). This is accomplished only when donor and acceptor are in close proximity, indicating interaction of the cyclic peptide (PEP1 or PEP2) with the GRTH protein. Conversely, the presence of CP2 (donor FITC) in the photobleached region did not show any increase in fluorescence intensity, indicating no interaction between CP2 and GRTH protein (Figure 6A,B). In addition, the increase in the donor FITC fluorescence signal was measured to calculate the FRET efficiency. Selected regions in the bleached sections were taken for the calculation of FRET efficiency (Figure 6C). We observed a significant 4-fold increase in the FRET efficiency for PEP1 and PEP2 compared to the control peptide CP2 (Figure 6D,E).
Figure 6.

Quantitative FRET images on the live COS-1 cells (A) FRET images for acceptor photobleaching in COS-1 cells transiently expressed with mCherry-GRTH and exposed to 15 μM cyclic peptides (PEP1, PEP2, and CP2). Areas were marked and images were acquired before and after the mCherry (acceptor) photobleaching. The cyclic peptides (PEP1 and PEP2) attached with FITC (donor) showed a significant increase in the intensity after the photobleaching, while control peptide (CP2) did not show any increase in the intensity of donor. (B) Intensity of donor and acceptor before and after photobleaching for PEP1, PEP2, and CP2 were represented in the graph. (C) Images of COS-1 cells expressing mCherry-GRTH with PEP1, PEP2, and CP2 and the respective FRET efficiency images. (D) Acceptor photobleaching experiment shown in a plot gave a FRET efficiency of 22% for PEP1, 20% for PEP2, and 5% for CP2; however, the FRET efficiency values change considerably at different locations in the marked regions. (E) Mean values (mean ± SEM) of FRET efficiency % of mCherry-GRTH exposed to 15 μM PEP1, PEP2, and CP2 obtained through the acceptor photobleaching experiment.
Effect of Cyclic Peptides on pGRTH with or without PKA Induction.
The transfection of PKA in the stable cells increased the phosphorylation of GRTH. However, the increase in phosphorylation of GRTH was significantly reduced/inhibited in the presence of 100 μM concentration of PEP1 and PEP2 with or without the PKA induction. The reduced expression of pGRTH could indicate the competitive binding of cyclic peptides (PEP1 and PEP2) at the PKA site in the presence of PKA. In contrast, the presence of CP2 did not have any effects on the expression of pGRTH with or without PKA induction (Figure 7).
Figure 7.
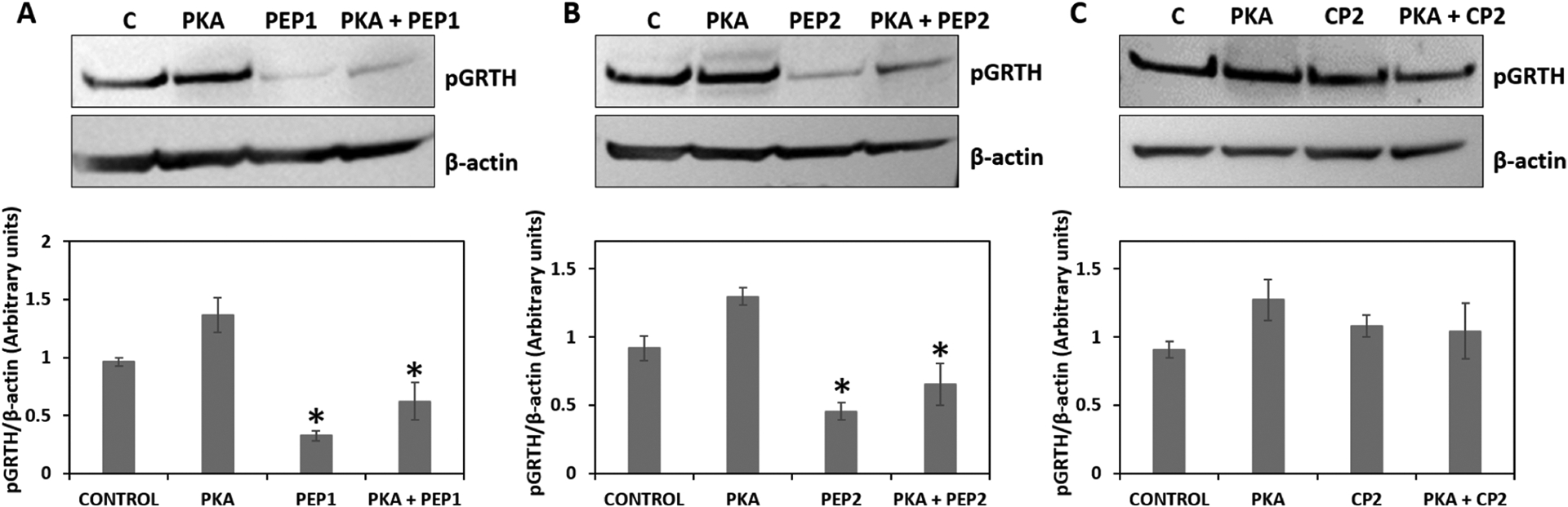
Effect of cyclic peptides on pGRTH with or without PKA induction: Western blot analysis was performed to validate the expression of phospho GRTH in control (C), PKA induction only (PKA), 100 μM cyclic peptide treatment, PEP1 or PEP2, or CP2, and both PKA and cyclic peptide. β-Actin was used as an internal control. The protein band intensities of pGRTH (61 kDa) from three independent experiments (mean ± SEM) were measured using ImageJ software and normalized with the β-actin. All data represent mean ± SEM. *P < 0.05.
DISCUSSION
In this study, we have rationally designed a cyclic peptide that effectively blocks the phosphorylation of GRTH in vitro and has the potential for further developments for its ultimate use as a nonhormonal male contraceptive. Studies from our laboratory highlighted the essential role of GRTH in the completion of spermatogenesis and established the crucial role of the phospho-form of GRTH in spermatid development during spermiogenesis in mice.8 Therefore, designing a small compound capable of inhibiting GRTH phosphorylation by blocking or hampering the PKA-binding recognition site would be one possible strategy toward such a contraceptive agent. However, because the GRTH surface topography at the GRTH/PKA interface appears to be relatively shallow,9 which prevents high-affinity binding of traditional small-molecule drugs, we decided to design a bulkier, flexibly compound that binds to the GRTH interface and acts as a competitor to PKA binding. Our previous study has shown that the GRTH interface has a unique distribution of basic and acidic surface residues surrounding the phosphorylation site (T239) that imparts local structural instability.9 Point mutations of solvent-exposed residues structurally close to T239 partially or completely abolished the expression of phospho GRTH.9 Thus, we hypothesized that the instability could be exploited to design a cyclic peptide that hinders PKA binding or its catalytic efficiency.
We started with the computational derivation of a pharmacophore from which a basic molecular scaffold was proposed. Molecular dynamics simulation of GRTH first provided an ensemble of configurations of the interface. We then used an MC-based computational approach (cf. Supporting Information) to identify the binding regions of amino acid side-chain analogues. The distribution of these motifs led us to propose a general pharmacophore to guide the design of cyclic peptides to target GRTH selectively and the GRTH/PKA interface specifically. These properties are only part of the considerations for developing cyclic peptides for in vivo applications. Effective cell internalization and minimal toxicity are two additional elements we incorporated in the design, which we could accommodate as part of the proposed pharmacophore. The ability of a peptide to cross the cell membrane is a common concern for larger molecules. Although mammalian cell membranes contain many transporters, highly positive charged peptides can lead to endocytic uptake followed by endosomal escape.12 Also, several studies have shown that incorporating aromatic and hydrophobic residues can enhance stability and membrane permeability.16,19 We were able to accommodate these conditions without any significant deviations from the proposed pharmacophore. The basic scaffold we proposed (PEPx) contains only naturally occurring amino acids, is cationic and amphipathic, contains aromatic and hydrophobic residues, and is rich in arginine residues. Computer simulations of PEPx and its minimally modified PEP0 showed that both peptides adopt a family of conformations in solution under physiological conditions, possibly in fast interconversion; all of the conformers display most of the the basic pharmacophoric features. We introduced some structural flexibility through the length and chemical nature of the longer spacer (in our case, G-PEG2), which allows the peptide to adapt to the shallow interface. Computer simulations showed that all of the conformers bind to the expected interface, although with significant structural variations. This binding-mode variability was expected and, within limits, is a desirable feature as it allows the peptide to find the most favorable interactions, albeit at an entropic price.
The results above motivated us to evaluate the effects of the peptides on GRTH phosphorylation in living cells. To monitor its cytosolic localization, we chose G7 as the position for the fluorophore. We chose the Lys(FITC) conjugate in either the l (PEP1) or d (PEP2) configurations, although smaller, more conspicuous probes have been proposed.16 Our study showed that PEP1 and PEP2 contain all of the necessary characteristics that enable effective and easy entry into COS-1 cells and seminiferous tubules. PEP0 is expected to have similar binding features, judging from our computer simulations and its biological inhibitory function on GRTH phosphorylation. In contrast, the linear peptide CP1, which has the same amino acid sequence as PEP1, failed to enter the cells, supporting the notion that cyclization is needed to enhance membrane crossing.
The treatment/exposure of PEP1 and PEP2 to COS-1 cells stably expressing GRTH abolished the expression of pGRTH in vitro significantly, indicating the effective abolition of GRTH phosphorylation by endogenous PKA and authenticating the therapeutic nature of these cyclic peptides; in contrast, the linear control peptide CP1 did not have any effects on the expression of pGRTH in COS-1 cells. Although CP1 may still bind to GRTH at the PKA-binding surface, even when not conforming to the pharmacophore as well as PEP1 does, the observed differences are more likely related to the reduced CP1 entry into the cells, as demonstrated in this study. In contrast, control cyclic peptides CP2, rich in acidic residues Glu, and CP3, which lacks the aromatic side chains of Phe and Nal, did not have any inhibitory effect on the phosphorylation of GRTH despite their surprisingly adequate access to the cells. Both controls lack essential pharmacophore features needed for GRTH binding at the interface, although our fluorescence study shows that both peptides competently reached intracellular sites.
It is of interest that previous studies have shown the effectiveness of Nal and Arg residues of cyclic peptides in conferring inhibitory properties on the phosphorylation of certain proteins.16,19 For example, peptidyl-prolyl isomerase, which specifically binds to phosphorylated Ser/Thr-Pro motifs to regulate post-translational modification, was effectively blocked by cyclic peptides containing Nal and rich in Arg residues.19 Although different considerations have dictated the inclusion of Nal and Arg residues in PEP1 and PEP2, namely, the binding to the GRTH interface and the concomitant structural perturbation affecting PKA efficacy, a different, separate effect may be at play in the blockade of GRTH phosphorylation by PEP0, PEP1, and PEP2. Moreover, PEP0, which is obtained from PEP1 upon removing the Lys(FITC) side chain, also showed similar effects as PEP1, confirming that the presence of the bulky fluorophore did not interfere with binding. A significant reduction of pGRTH was observed at 8 h and 16 h upon treatment with PEP1 and PEP2 of COS-1 cells stably expressing GRTH. Additionally, we observed a reduction in the expression of phospho GRTH upon exposure of PEP1 and PEP2 of COS-1 cells, even when COS1 cells stably expressing GRTH were transiently transfected with PKA catalytic subunit, indicating competitive binding of cyclic peptides at the GRTH/PKA site in the presence of PKA. Having established the inhibitory effect of PEP1 and PEP2 on GRTH phosphorylation, we performed a deeper analysis of the peptide-protein interactions and binding stability. This is exemplified in our CETSA studies, where thermal stabilization of the GRTH protein was effectively retained by PEP1 or PEP2 treatment compared to CP2. The thermal stabilization of proteins by a peptide was successfully demonstrated in recent studies to validate cyclization and binding stability.20,21
In the present study, changes in thermal stability resulted in the presence of soluble protein at a higher temperature. After the heating step, the presence of non-phospho GRTH in solution at 50 and 55 °C upon the exposure of PEP1 and PEP2 indicated that there was protein–ligand interaction, while this was not observed with CP2 due to its inability to bind GRTH. The peptides were also evaluated for their cytotoxic effects. The integrity of the plasma membrane can be disrupted by the entry of molecules, possibly causing cytotoxicity, leading to apoptosis and cell death. This process depends on the specific interactions of the molecule with the membrane and is difficult to predict.22,23 Our studies in vitro showed that exposure to high concentrations of cyclic peptides displayed limited cytotoxicity with high IC50 values. FRET acceptor photobleaching was performed to confirm the interaction of PEP1 and PEP2 with GRTH. An increase in the fluorescence intensity of donor FITC (PEP1 and PEP2) was observed in the photobleached region of acceptor mCherry indicating FRET has occurred due to the proximity of donor FITC and acceptor mCherry. This was further confirmed by the significant increase in the FRET efficiency for PEP1 and PEP2 compared to the linear control peptide (CP1) in COS-1 cells. The interaction of a ligand with a protein by the acceptor photobleaching method was demonstrated in several studies,24,25 showing increases in the donor intensity after photobleaching and FRET efficiency.
This study has demonstrated that specifically tailored cyclic peptides can effectively block phosphorylation of GRTH in vitro, providing the basis for further refinements to enhance its inhibitory efficacy as required for use as a contraceptive in vivo. Our earlier studies revealed the role of phospho GRTH in the spermatogenesis of GRTH-KI generated mice.8 The present study has shown that the proposed synthetic cyclic peptides can enter the germ cells in the seminiferous tubule and decrease the presence of phospho GRTH species. In light of these results, we plan to perform preclinical assays in mice to test the bioavailability and efficacy of PEP1 in target tissue (testis) and its inhibitory effect on GRTH phosphorylation in vivo. We also plan to standardize the dosage and time interval of PEP1 for IP injections in mice and perform testicular bioavailability of PEP1 using confocal imaging and by serum and tissue pharmacokinetic analysis. Assessing the peptide’s effectiveness in blocking GRTH phosphorylation in the testis and the morphological changes induced in the testis, sperm count, and sperm motility will be critical. Likewise, the study of the fertility and mating behavior of male mice during extended periods (4–5 weeks) of treatment (azoospermia or low sperm count will presumably be observed), and the reversibility of such effects will be assessed.
Currently, available male contraceptive pills are hormone-based.26 However, a safe, reversible, effective nonhormonal contraceptive is needed. Future refinements through experimental structure–activity studies combined with computational modeling may help overcome potential limitations in vivo, such as absorption and the ability to cross the blood–testis barrier, paving the way for novel fertility regulators in men.
EXPERIMENTAL SECTION
Molecular Modeling and Simulations.
The computational method for designing cyclic peptides targeting shallow protein surfaces is described in the Supporting Information. We started with the derivation of a pharmacophore and the design of a series of cyclic peptides consistent with both the pharmacophore and a set of chemical features known to facilitate membrane crossing, followed by the prediction of binding modes and the selection of candidates for synthesis and in vitro testing (Figure S1). The structure of GRTH/DDX25 (domain 1) was obtained by homology with DDX19;9 a recent model obtained from a Machine Learning-based approach (AlphaFold2) yielded a similar structure, with only minor conformational differences at the PKA-binding interface, including the side-chain orientations of critical residues (cf. Figure S2B).27 Given the structural uncertainties inherent in this kind of modeling, we performed molecular dynamics (MD) simulations to obtain a family of conformers (substates) of GRTH in an aqueous solution using the TIP3P water model and the all-atom CHARMM forcefield28 (param36) at constant temperature (37 °C) and pressure (1 atm), with protonation states at neutral pH. This procedure avoids potential biases in the derivation of the pharmacophore stemming from the reliance on a single structure. Clustering analysis of GRTH residues at the GRTH/PKA interface yielded 13 distinct substates (Figure S2A), each a potential target of one or more cyclic peptides; conformational selection is thus partially accounted for. Using temperature-annealing Monte Carlo (MC) simulations combined with a residue-titration protocol, we first identified GRTH “hotspots” for high-affinity binding of single amino acid side-chain analogues to the GRTH/PKA interface (Figure S3). Based on the binding patterns (Figure S4), we proposed a general pharmacophore. Once a specific amino acid sequence is selected for a cyclic peptide candidate, a multistage sampling method is used for tail-to-head cyclization (cf. Supporting Information). Structural clustering of the resulting ensemble produced a set of peptide conformers that were subsequently used for binding prediction. The GRTH-cyclic peptide binding modes were obtained by simulated annealing MC followed by structural relaxation through MD simulations (details in the Supporting Information). The method combines MC, MD, and Langevin dynamics (LD) simulations in both implicit29,30 and explicit solvent models, with a technique developed previously to predict strong, weak, and ultraweak protein/peptide associations.31,32
Cell Culture and Plasmid Preparation.
COS-1 cells were obtained from ATCC CRL-1650 and cultured in a T75 flask at 37 °C with 5% CO2, containing Dulbecco modified Eagle medium (DMEM) high glucose, GlutaMax (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Inc., Lawrenceville, GA) and 1× antibiotic–antimycotic (#15240062, Thermo Fisher Scientific). The full-length human GRTH cDNA fragment (GenBank Acc # AF155140) was cloned into pcDNA3.1/V5-His A (Thermo Fisher Scientific, Waltham, MA) at KpnI and XbaI restriction sites, and the sequence was confirmed. This GRTH plasmid construct and COS-1 cells were used to generate a stable cell line expressing human GRTH. The expression of pGRTH in a western blot was assessed using a custom-made affinity-purified phospho-site-specific GRTH polyclonal antibody raised in rabbit to the peptide sequence (CKLIDL[pT239]KIRV) of human GRTH.
Generation of GRTH COS-1 Stable Cell Line.
COS-1 cells were stably transfected with a human GRTH-V5-His vector, essentially as previously described.33 Briefly, following the determination of the optimal concentration of the antibiotic G418 (Geneticin), COS-1 cells were transfected with human GRTH-V5-His construct using Lipofectamine 2000 (Invitrogen) and cultured in DMEM-high glucose media in the presence of 10% FBS. Cells expressing GRTH were selected and cultured for 2 weeks in the presence of Geneticin to obtain stable transfected cells. Single clones were expanded and checked for pGRTH expression. Protein extracts were prepared and checked for pGRTH expression using phospho-specific GRTH antibody.
Treatment of COS-1 Cells with Cyclic Peptides.
Cyclic peptides (PEP1 and PEP2) with FITC tag and control peptides (CP1, CP2, and CP3) with FITC tag, and a cyclic peptide without FITC (PEP0) were designed and synthesized commercially (Life-Tein). The purity of all of the peptides used in this study is >95%, as determined by HPLC and mass spectrometry (Figure S8). The obtained lyophilized peptides were dissolved in molecular biology grade water and used for the experiments. All of the peptides were added to the stable cell cultures expressing GRTH (see Table 1). Different concentrations (5, 20, 60, and 100 μM) of peptides (both the cyclic peptides and control) were added into each well of a full-grown (~90% confluency) six-well culture plate seeded at a density of 0.3 × 106 cells/well. The cell culture media containing the respective peptide was kept for up to 16 h. Cells exposed to the peptides were washed with 1× PBS and harvested using trypsin-EDTA. Protein extracts were prepared in the presence of 1× protease phosphatase inhibitor. Similarly, the expression of pGRTH was also analyzed during different times of exposure of cells to the individual peptides to determine the effectiveness of the peptides (time and concentration) in blocking GRTH phosphorylation of cells stably expressing GRTH. Since in initial studies cyclic peptides 1 and 2 at a concentration of 100 μM showed effective decrease in pGRTH in cells exposed for 16 h, protein extracts were prepared at different times of exposure to the peptides (4, 8, 16, and 24 h). Western blots were performed using 30 μg of protein from each sample and the expression of pGRTH was revealed using phospho-specific GRTH antibody. Antibodies used in this study are a custom-made affinity-purified phospho-site-specific GRTH polyclonal antibody raised in rabbit to the peptide sequence (CKLIDL[pT239]KIRV) (1:2000)9 and a GRTH rabbit polyclonal antibody,34 which recognizes the non-phospho of GRTH (non-phospho, 56 kDa).
Immunofluorescence Microscopy to Monitor Effective Delivery of Cyclic Peptides.
COS-1 stable cells expressing GRTH were seeded on sterile coverslips kept inside a six-well cell culture plate at a concentration of 0.3 × 106/well. The plate was kept overnight at 37 °C at 5% CO2 to allow the cells to adhere to the coverslip. Peptides PEP1, PEP2, CP1, CP2, and CP3 at a concentration of 20 μM were added to each well. After 4 h of incubation with respective peptides, coverslips were carefully taken out from the cell culture plate and washed gently with 1× PBS. The cells were further fixed with 50 and 100% methanol for 1 min each and mounted inversely on a microscopic slide using ProLong Diamond Antifade Mountant with DAPI (Thermo Fisher Scientific). The slides were observed under the microscope and cells were microphotographed using Zeiss Axio Observer Z1 Inverted Phase Contrast Fluorescence Microscope and Zen pro software.
Cellular Thermal Shift Assay (CETSA).
CETSA assays were performed as previously described20 with few modifications. For the cell lysate CETSA assay, cell lysates were prepared from the stable cells expressing GRTH in the presence of 1× protease phosphatase inhibitor. Cell debris was removed from the mixture by centrifugation at 10 000 rpm for 5 min at 4 °C and the supernatant was taken for the experiment. The cell lysates were diluted in lysis buffer and 40 μM concentration of either PEP1, PEP2, or CP2 were added to each tube and kept under rotation for 16 h at 4 °C. Then, the tubes were centrifuged at 10 000 rpm for 3 min. Each cell lysates treated with different peptides were divided into 100 μL aliquots and heated individually at different temperatures (40–70 °C) for 3 min in a Veriti thermal cycler (Applied Biosystems) followed by cooling for 3 min at room temperature. The heated lysate samples were centrifuged at 10 000 rpm for 10 min at 4 °C to sediment the precipitates from the soluble fractions. The supernatant containing the remaining soluble proteins was run in a NuPAGE 4–12%, Bis–Tris protein gels and analyzed for the expression of non-phospho GRTH using GRTH rabbit polyclonal antibody1 by western blot.
Analysis of Cyclic Peptides on the Expression of GRTH with or without PKA Transfection.
Stable cells expressing GRTH were cultured in a six-well plate at a seeding density of 0.3 × 106/well. Plasmid expressing PKA α catalytic subunit (PKAα) was used for the experiment. The wells were categorized into four groups: control, only PKAα, only cyclic peptides, and both PKAα + cyclic peptides. PKAα (3 μg/well) were transfected into the respective groups, while for equalization, empty plasmids were transfected into the other groups and cultured further for 24 h. After 24 h transfection, cyclic peptides PEP1, PEP2, and CP2, at a concentration of 100 μM each, were added into the respective wells and kept at 37 °C, 5% CO2 overnight. Protein samples were prepared and run in a PAGE gel for western blotting and developed the blot for phospho GRTH using phospho-specific GRTH antibody (1:1000 dilution) as mentioned earlier.
Western Blots.
All of the protein extracts were resolved in NuPage Novex Bis–Tris 4–12% polyacrylamide gels with NuPAGE MOPS SDS running buffer (Life Technologies). After the gels were run, proteins were transferred to nitrocellulose membranes using the iBlot2 blotting system (Life Technologies). For detection of pGRTH, the custom-made phospho-specific GRTH antibody (1:2000) described above was used and the secondary antibody (1:5000) used is a bovine anti-rabbit HRP-IgG (sc-2374) (Thermo Fisher Scientific). β-Actin Antibody (1:2000; sc-69879) and secondary goat anti-mouse HRP-IgG (1:5000; sc-2055) were purchased from Santa Cruz Biotechnology and used for immunoblotting. All of the membranes were blocked with 5% milk protein dissolved in 1× PBS in 0.1% Tween 20 (PBST) following standard blot transfer and western blot protocols as recommended by the manufacturers. All of the antibodies were diluted in 5% milk protein in PBST. The membranes were developed using Tanon High-sig ECL Western Blotting Substrate solution according to the manufacturer’s recommendations. Band intensities were detected using an iBright chemiluminescence imaging system (Thermo Fisher Scientific) and quantified with ImageJ Software.
Cell Cytotoxicity Assay.
Cell cytotoxicity assay was performed to determine cytotoxic levels (IC50) of the peptides using in vitro CellTiter 96 Non-Radioactive Cell Proliferation Assay (Promega). COS-1 stable cells expressing GRTH were seeded in a 96-well cell culture plate at a seeding density of 0.01 × 106 cells/well and kept for 16 h. PEP0, PEP1, PEP2, CP1, CP2, and CP3 at different concentrations (10, 50, 100, 250, and 500 μM) were added into the cell culture media and incubated for 24 h at 37 °C supplemented by 5% CO2. After incubation, the plates were removed from the wells and 15 μL of dye solution was added to each well and further incubated for 4 h in the humidified chamber at 37 °C, 5% CO2 atmosphere. The reaction was stopped by adding the stop solution supplied with the kit and the color development was recorded using TriStar2 LB 942 Multimode Microplate Reader at 570 nm. Cell viability percentage was calculated by taking the OD value of treatment divided by control and multiplied by 100. The IC50 value for each peptide was calculated from the dose–response plot (concentration of peptide on the x-axis and % cell availability on the y-axis) and data fit with linear regression.
Expression of mCherry-GRTH Plasmid in COS-1 Cells.
Open reading frame (ORF) primers with restriction sites ApaI and AgeI (Fw: CGAGGGCCCTTCATGGTGAGCAAGGGCGAGGAG and Rv: CGAACCGGTTGCTTACTTGTACAGCTCGTCCATGCC) were designed to amplify the mCherry fragment from the commercially ordered plasmid p27-IRES-mCherry (Addgene, MA). The mCherry fragment containing the restriction sites and the human GRTH plasmid (generated previously) was digested using the same restriction enzymes, ApaI and AgeI. After gel run, the digested mCherry fragment and human GRTH plasmid were purified and kept for ligation at 4 °C for 16 h. The ligation mixture was transformed into DH5α competent cells, and positive cells containing plasmid with human GRTH and mCherry were selected and purified. The expression of mCherry was checked by transfecting the plasmid expressing mCherry-GRTH into COS-1 cells. After 24 h transfection, 20 μM PEP1, PEP2, CP1, CP2, and CP3 were added into the COS-1 cells for 4 h, and the images were photographed using EVOS M5000 Imaging System (Thermo Fisher Scientific).
FRET Microscopy and Acceptor Photobleaching.
COS-1 cells expressing mCherry-GRTH cultured in a 35-mm glass-bottom dish were exposed to 15 μM PEP1, PEP2, and CP2 for 16 h and subjected to live-cell imaging. mCherry was excited at 561 nm, and the emission was detected at 570 and 620 nm, whereas FITC was excited at 488 nm, and detection was from 500 to 550 nm. FRET channel was acquired with an excitation at 488 nm and a 570 and 620 nm emission filter. For acceptor photobleaching, steady-state fluorescent images were acquired with an Inverted microscope Zeiss Axio Observer.Z1 with laser scanning unit LSM 780 (Carl Zeiss, Germany) using a 63× oil immersion objective and a 25 mW Argon Diode 405–30. Regions of interest (ROIs) were marked in the cells and bleached in the acceptor channel (mCherry) using 30 cycles time series with 10 bleaching iterations with 100% laser power at 514 nm. Pre- and postbleach images were obtained in the mCherry, FITC, and FRET channels. ROI was marked in the photobleached region intensity of donor and acceptor, and FRET efficiency percentage was calculated using the Zen 2.1 SP3 software. FRET efficiency percentage was calculated from E (%) = 100 (IFITC postbleach − IFITC prebleach)/(IFITC postbleach), where IFITC is the fluorescence intensity of the FITC.
Preparation of Seminiferous Tubule Culture from Adult Mice.
Seminiferous tubules from the testes of adult mice (90 days old) were used for the in vitro culture experiments. Testes were decapsulated and dispersed with sterile forceps in a Petri dish containing ice-cold medium 199 (Cat. No. 11150059; Thermo Fisher Scientific) with 0.1% BSA for 10 min. The dispersed seminiferous tubules were segmented and placed into the 24-well cell culture plate containing medium 199 and incubated in a CO2 incubator. After 4 h, 100 μM cyclic peptides were added and incubated for 16 h. Later the media was removed and tubules were washed with 1× PBS. The tubules were kept on a microscopic slide and mounted with SlowFade Diamond Antifade Mountant (Thermo Fisher Scientific) and imaged using EVOS M5000 fluorescent microscope (Thermo Fisher Scientific). Protein samples prepared from treated and untreated seminiferous tubules were run on a PAGE gel and the expression of pGRTH was detected using the phospho-specific GRTH antibody mentioned above. From at least three independent experiments performed, a graph was plotted by calculating the intensity of pGRTH protein band and normalized using β-actin band.
Analysis of Cyclic Peptides on the Expression of GRTH with or without PKA Transfection.
Stable cells expressing GRTH were cultured in a six-well plate at a seeding density of 0.3 × 106/well. Plasmid expressing PKA α catalytic subunit (PKAα) was used for the experiment.9 The wells were categorized into four groups: control, only PKAα, only cyclic peptides, and both PKAα + cyclic peptides. PKAα (3 μg/well) were transfected into the respective groups, while for equalization, empty plasmids were transfected into the other groups and cultured further for 24 h. After 24 h transfection, cyclic peptides PEP1, PEP2, and CP2 at a concentration of 100 μM each were added into the respective wells and kept at 37 °C, 5% CO2 overnight. Protein samples were prepared and run in a PAGE gel for western blotting and developed the blot for phospho GRTH using a phospho-specific antibody (1:1000 dilution) as mentioned earlier.
Statistical Analysis.
Data are presented as the mean ± SEM of three independent experiments. Mean values of the data were compared and analyzed with one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test using Prism software (GraphPad Prism 7.02 Software, San Diego, CA). A probability of P < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Vincent Schram from Microscopy and Imaging Core, NICHD, for his advice and instruction on FRET technique. This study utilized the high-performance computational capabilities of the Biowulf HPC cluster at the NIH. This work was supported by the NIH Intramural Research Program through the Eunice Kennedy Shriver National Institute of Child Health and Human Development and Center for Information Technology (Program No. 1ZIAHD000150-42).
ABBREVIATIONS
- CETSA
cellular thermal shift assay
- COS 1
CV-1 (Simian) in origin, and carrying the SV40 genetic material (fibroblast-like cell lines derived from monkey kidney tissue)
- CP1
control peptide 1 (linear)
- CP2
control peptide 2 (cyclic)
- CP3
control peptide 3 (cyclic)
- FITC
fluorescein isothiocyanate
- FRET
fluorescence resonance energy transfer
- GRTH
gonadotropin-regulated testicular RNA helicase
- IC50
half-maximal inhibitory concentration
- PEP1
cyclic peptide 1
- PEP2
cyclic peptide 2
- DDX19
DEAD-box helicase 19B
- pGRTH
phospho gonadotropin-regulated testicular RNA helicase
- PKA
protein kinase A
- ROI
region of interest
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.1c01201.
Supporting figures, additional experimental details, and HPLC and mass spectrometry data for all peptides used in this study (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.jmedchem.1c01201
The authors declare no competing financial interest.
Contributor Information
Murugananthkumar Raju, Section on Molecular Endocrinology, Division of Developmental Biology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland 20892, United States.
Raghuveer Kavarthapu, Section on Molecular Endocrinology, Division of Developmental Biology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland 20892, United States.
Rajakumar Anbazhagan, Section on Molecular Endocrinology, Division of Developmental Biology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland 20892, United States.
Sergio A. Hassan, Bioinformatics and Computational Biosciences Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland 20892, United States.
Maria L. Dufau, Section on Molecular Endocrinology, Division of Developmental Biology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland 20892, United States.
REFERENCES
- (1).Tang PZ; Tsai-Morris CH; Dufau ML A novel gonadotropin-regulated testicular RNA helicase. A new member of the dead-box family. J. Biol. Chem 1999, 274, 37932–37940. [DOI] [PubMed] [Google Scholar]
- (2).Tsai-Morris CH; Sheng Y; Lee E; Lei KJ; Dufau ML Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is essential for spermatid development and completion of spermatogenesis. Proc. Natl. Acad. Sci. U.S.A 2004, 101, 6373–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Dufau ML; Tsai-Morris CH Gonadotropin-regulated testicular helicase (GRTH/DDX25): an essential regulator of spermatogenesis. Trends Endocrinol. Metab 2007, 18, 314–320. [DOI] [PubMed] [Google Scholar]
- (4).Dufau ML; Kavarthapu R Gonadotropin regulated testicular RNA helicase, two decades of studies on its structure function and regulation from its discovery opens a window for development of a non-hormonal oral male contraceptive. Front. Endocrinol 2019, 10, No. 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Sato H; Tsai-Morris CH; Dufau ML Relevance of gonadotropin-regulated testicular RNA helicase (GRTH/DDX25) in the structural integrity of the chromatoid body during spermatogenesis. Biochim. Biophys. Acta 2010, 1803, 534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Anbazhagan R; Kavarthapu R; Coon SL; Dufau ML Role of phosphorylated gonadotropin-regulated testicular rna helicase (GRTH/DDX25) in the regulation of germ cell specific mrnas in chromatoid bodies during spermatogenesis. Front. Cell Dev. Biol 2020, 8, No. 580019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Tsai-Morris CH; Koh E; Sheng Y; Maeda Y; Gutti R; Namiki M; Dufau ML Polymorphism of the GRTH/DDX25 gene in normal and infertile Japanese men: a missense mutation associated with loss of GRTH phosphorylation. Mol. Hum. Reprod 2007, 13, 887–892. [DOI] [PubMed] [Google Scholar]
- (8).Kavarthapu R; Anbazhagan R; Raju M; Morris CT; Pickel J; Dufau ML Targeted knock-in mice with a human mutation in GRTH/DDX25 reveals the essential role of phosphorylated GRTH in spermatid development during spermatogenesis. Hum. Mol. Genet 2019, 28, 2561–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Raju M; Hassan SA; Kavarthapu R; Anbazhagan R; Dufau ML Characterization of the phosphorylation site of GRTH/DDX25 and protein kinase a binding interface provides structural basis for the design of a non-hormonal male contraceptive. Sci. Rep 2019, 9, No. 6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Zorzi A; Deyle K; Heinis C Cyclic peptide therapeutics: past, present and future. Curr. Opin. Chem. Biol 2017, 38, 24–29. [DOI] [PubMed] [Google Scholar]
- (11).Joo SH Cyclic peptides as therapeutic agents and biochemical tools. Biomol. Ther 2012, 20, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Dougherty PG; Sahni A; Pei D Understanding cell penetration of cyclic peptides. Chem. Rev 2019, 119, 10241–10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Choi JS; Joo SH recent trends in cyclic peptides as therapeutic agents and biochemical tools. Biomol. Ther 2020, 28, 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Tarek M; Maigret B; Chipot C Molecular dynamics investigation of an oriented cyclic peptide nanotube in DMPC bilayers. Biophys. J 2003, 85, 2287–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Park SE; Sajid MI; Parang K; Tiwari RK Cyclic cell-penetrating peptides as efficient intracellular drug delivery tools. Mol. Pharm 2019, 16, 3727–3743. [DOI] [PubMed] [Google Scholar]
- (16).Qian Z; Liu T; Liu YY; Briesewitz R; Barrios AM; Jhiang SM; Pei D Efficient delivery of cyclic peptides into mammalian cells with short sequence motifs. ACS Chem. Biol 2013, 8, 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Shirazi AN; Tiwari R; Chhikara BS; Mandal D; Parang K Design and biological evaluation of cell-penetrating peptide-doxorubicin conjugates as prodrugs. Mol. Pharm 2013, 10, 488–499. [DOI] [PubMed] [Google Scholar]
- (18).Lättig-Tünnemann G; Prinz M; Hoffmann D; Behlke J; Palm-Apergi C; Morano I; Herce HD; Cardoso MC Backbone rigidity and static presentation of guanidinium groups increases cellular uptake of arginine-rich cell-penetrating peptides. Nat. Commun 2011, 2, No. 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Liu T; Liu Y; Kao HY; Pei D Membrane permeable cyclic peptidyl inhibitors against human Peptidylprolyl Isomerase Pin1. J. Med. Chem 2010, 53, 2494–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Almqvist H; Axelsson H; Jafari R; Dan C; Mateus A; Haraldsson M; Larsson A; Martinez Molina D; Artursson P; Lundbäck T; Nordlund P CETSA screening identifies known and novel thymidylate synthase inhibitors and slow intracellular activation of 5-fluorouracil. Nat. Commun 2016, 7, No. 11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Tetley GJN; Murphy NP; Bonetto S; Ivanova-Berndt G; Revell J; Mott HR; Cooley RN; Owen D The discovery and maturation of peptide biologics targeting the small G-protein Cdc42: A bioblockade for ras-driven signaling. J. Biol. Chem 2020, 295, 2866–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Yang NJ; Hinner MJ Getting across the cell membrane: an overview for small molecules, peptides, and proteins. Methods Mol. Biol 2015, 1266, 29–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Galluzzi L; Vitale I; Aaronson SA; Abrams JM; Adam D; Agostinis P; Alnemri ES; Altucci L; Amelio I; Andrews DW; Annicchiarico-Petruzzelli M; Antonov AV; Arama E; Baehrecke EH; Barlev NA; Bazan NG; Bernassola F; Bertrand MJM; Bianchi K; Blagosklonny MV; Blomgren K; Borner C; Boya P; Brenner C; Campanella M; Candi E; Carmona-Gutierrez D; Cecconi F; Chan FK; Chandel NS; Cheng EH; Chipuk JE; Cidlowski JA; Ciechanover A; Cohen GM; Conrad M; Cubillos-Ruiz JR; Czabotar PE; D’Angiolella V; Dawson TM; Dawson VL; De Laurenzi V; De Maria R; Debatin KM; DeBerardinis RJ; Deshmukh M; Di Daniele N; Di Virgilio F; Dixit VM; Dixon SJ; Duckett CS; Dynlacht BD; El-Deiry WS; Elrod JW; Fimia GM; Fulda S; García-Sáez AJ; Garg AD; Garrido C; Gavathiotis E; Golstein P; Gottlieb E; Green DR; Greene LA; Gronemeyer H; Gross A; Hajnoczky G; Hardwick JM; Harris IS; Hengartner MO; Hetz C; Ichijo H; Jäättelä M; Joseph B; Jost PJ; Juin PP; Kaiser WJ; Karin M; Kaufmann T; Kepp O; Kimchi A; Kitsis RN; Klionsky DJ; Knight RA; Kumar S; Lee SW; Lemasters JJ; Levine B; Linkermann A; Lipton SA; Lockshin RA; López-Otín C; Lowe SW; Luedde T; Lugli E; MacFarlane M; Madeo F; Malewicz M; Malorni W; Manic G; Marine JC; Martin SJ; Martinou JC; Medema JP; Mehlen P; Meier P; Melino S; Miao EA; Molkentin JD; Moll UM; Muñoz-Pinedo C; Nagata S; Nuñez G; Oberst A; Oren M; Overholtzer M; Pagano M; Panaretakis T; Pasparakis M; Penninger JM; Pereira DM; Pervaiz S; Peter ME; Piacentini M; Pinton P; Prehn JHM; Puthalakath H; Rabinovich GA; Rehm M; Rizzuto R; Rodrigues CMP; Rubinsztein DC; Rudel T; Ryan KM; Sayan E; Scorrano L; Shao F; Shi Y; Silke J; Simon HU; Sistigu A; Stockwell BR; Strasser A; Szabadkai G; Tait SWG; Tang D; Tavernarakis N; Thorburn A; Tsujimoto Y; Turk B; Vanden Berghe T; Vandenabeele P; Vander Heiden MG; Villunger A; Virgin HW; Vousden KH; Vucic D; Wagner EF; Walczak H; Wallach D; Wang Y; Wells JA; Wood W; Yuan J; Zakeri Z; Zhivotovsky B; Zitvogel L; Melino G; Kroemer G Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death. Cell Death Differ. 2018, 25, 486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Tavares E; Macedo JA; Paulo PM; Tavares C; Lopes C; Melo EP Live-cell FRET imaging reveals clustering of the prion protein at the cell surface induced by infectious prions. Biochim. Biophys. Acta 2014, 1842, 981–991. [DOI] [PubMed] [Google Scholar]
- (25).Bao L; Ding L; Yang M; Ju H Noninvasive imaging of sialyltransferase activity in living cells by chemoselective recognition. Sci. Rep 2015, 5, No. 10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Yuen F; Nguyen BT; Swerdloff RS; Wang C Continuing the search for a hormonal male contraceptive. Best Pract. Res. Clin. Obstet. Gynaecol 2020, 66, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Jumper J; Evans R; Pritzel A; Green T; Figurnov M; Ronneberger O; Tunyasuvunakool K; Bates R; Zidek A; Potapenko A; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Brooks BR; Brooks CL 3rd; Mackerell AD Jr.; Nilsson L; Petrella RJ; Roux B; Won Y; Archontis G; Bartels C; Boresch S; Caflisch A; Caves L; Cui Q; Dinner AR; Feig M; Fischer S; Gao J; Hodoscek M; Im W; Kuczera K; Lazaridis T; Ma J; Ovchinnikov V; Paci E; Pastor RW; Post CB; Pu JZ; Schaefer M; Tidor B; Venable RM; Woodcock HL; Wu X; Yang W; York DM; Karplus M CHARMM: the biomolecular simulation program. J. Comput. Chem 2009, 30, 1545–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Hassan SA; Steinbach PJ Water-exclusion and liquid-structure forces in implicit solvation. J. Phys. Chem. B 2011, 115, 14668–14682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Hassan SA; Mehler EL; Zhang D; Weinstein H Molecular dynamics simulations of peptides and proteins with a continuum electrostatic model based on screened Coulomb potentials. Proteins 2003, 51, 109–125. [DOI] [PubMed] [Google Scholar]
- (31).Cardone A; Bornstein A; Pant HC; Brady M; Sriram R; Hassan SA Detection and characterization of nonspecific, sparsely populated binding modes in the early stages of complexation. J. Comput. Chem 2015, 36, 983–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Cardone A; Pant H; Hassan SA Specific and non-specific protein association in solution: computation of solvent effects and prediction of first-encounter modes for efficient configurational bias Monte Carlo simulations. J. Phys. Chem. B 2013, 117, 12360–12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Villar J; Tsai-Morris CH; Dai L; Dufau ML Androgen-induced activation of gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) transcription: essential role of a nonclassical androgen response element half-site. Mol. Cell. Biol 2012, 32, 1566–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Sheng Y; Tsai-Morris CH; Gutti R; Maeda Y; Dufau ML Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is a transport protein involved in gene-specific mRNA export and protein translation during spermatogenesis. J. Biol. Chem 2006, 281, 35048–35056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


