Abstract
Astrocytes have become established as an important regulator of neuronal activity in the brain. Accumulating literature demonstrates that cocaine self-administration in rodent models induces structural changes within astrocytes that may influence their interaction with the surrounding neurons. Here, we provide evidence that cocaine impacts astrocytes at the functional level and alters neuronal sensitivity to astrocyte-derived glutamate. We report that a fourteen-day period of short access to cocaine (2-hour/day) decreases spontaneous astrocytic Ca2+ transients and precipitates changes in astrocyte network activity in the nucleus accumbens shell. This is accompanied by increased prevalence of slow inward currents, a physiological marker of neuronal activation by astrocytic glutamate, in a subset of medium spiny neurons. Within, but not outside, of this subset, we observe an increase in duration and frequency of NMDA receptor-mediated synaptic events. Additionally, we find that the link between synaptic NMDA receptor plasticity and neuron sensitivity to astrocytic glutamate is maintained independent of drug exposure, and is observed in both cocaine and saline control animals. Imaging analyses of neuronal Ca2+ activity show no effect of cocaine self-administration at individual cells or on neuronal network activity in brain slices. Therefore, our data indicate that cocaine self-administration promotes astrocyte-specific functional changes that can be linked to increased glutamate-mediated coupling with principal neurons in the nucleus accumbens. Such coupling may be spatially restricted as it does not result in a broad impact on network structure of local neuronal circuits.
Introduction
Astrocytes play diverse roles in regulation of synaptic transmission and have been proposed to coordinate synaptic plasticity and organization of neuronal microcircuits1,2. Astrocytes may exercise control over neuronal signaling via glutamate recycling2, regulation of neuronal energy demands by lactate glycolysis3,4, buffering of excess K+ released during periods of sustained neuronal activity5,6, and through the release of neuroactive molecules7.
The mechanisms underlying astrocytic release of neuromodulators, gliotransmission, remain subjects of active debate8–14. However, considering the lack of evidence for specialized gliotransmitter release sites and the fact that only the finest of astrocytic processes penetrate into the synaptic cleft, the bulk of gliotransmitter exocytosis is thought to occur outside the synapse15,16. Accordingly, neuronal response to gliotransmitters has been linked to the pool of extrasynaptic receptors. In the case of astrocyte-derived glutamate, extrasynaptic NMDARs are of particular interest because of their sensitivity to low glutamate concentrations and relatively high permeability to Ca2+ 17–19. The electrophysiological hallmark of the NMDAR-mediated response to astrocytic glutamate are slow inward currents (SICs), that are prominently distinguished from synaptic NMDAR-mediated events by their large amplitudes and slow kinetics17,20,21. SICs arise spontaneously in subpopulations of cells, but can also be elicited or inhibited by pharmacological, optical, and mechanical stimulation of astrocytes18,19,22–26. NMDA-mediated SICs may therefore serve as a useful proxy of neuronal sensitivity to glutamate released from the surrounding astrocytes. Indeed, a number of studies have highlighted the possibility that SICs may be involved in synchronization of local neuronal microcircuits in the hippocampus, the somatosensory cortex and the NAc17–19,27–29.
Multiple behavioral outputs have been reported to result from astrocyte-selective manipulations. These include locomotor effects, sleep regulation, affective processing, memory encoding, and food intake30. Mounting evidence indicates that substance use triggers changes within astrocytes that impact drug-evoked behavior. For example, chemogenetic activation of astrocytes has been shown to attenuate motivation for ethanol, an effect that may involve signaling through astrocytic gap-junctions31. Astrocyte-released adenosine has been found to depress excitatory synaptic transmission and regulate effects of amphetamine32 and ethanol (32464636) while reduced association of astrocyte processes with synapses may play a role in heroin seeking33.
Cocaine exposure has been shown to elicit morphological changes in astrocytes34,35 as well as downregulation of both the astrocyte glutamate transporter GLT-136, and the cystine/glutamate exchanger with the latter proposed to underlie reduction in basal glutamate levels reported in cocaine self-administration models37. Mice with astrocytes lacking putative gliotransmitter release machinery displayed attenuated cocaine-induced reinstatement of conditioned place preference and cue-induced reinstatement of cocaine self-administration in a transgenic mouse model38. However, induction of gliotransmitter release in the nucleus accumbens (NAc) by selective elevation of astrocytic Ca2+ also attenuated cue-induced reinstatement of cocaine self-administration in the rat39.
Despite evidence that astrocyte-specific manipulations may influence cocaine-seeking, it is not known how cocaine experience affects baseline levels of astrocyte activity and functional interactions with neuronal targets. The goal of this study was to evaluate whether a history of cocaine self-administration impacts measures of astrocyte signaling that may be linked to synaptic plasticity of medium spiny neurons (MSNs) in the NAc shell. We demonstrate that cocaine-induced changes in astrocytic Ca2+ within single cells and astrocyte networks occur without an effect on astrocyte membrane excitability or potassium current rectification. We further demonstrate that these changes are associated with increased sensitivity of NAc shell MSNs to astrocytic glutamate and with NMDA receptor-mediated synaptic plasticity, but that they do not impact broader measures of neuronal Ca2+ signaling.
Materials and Methods
Animals and housing
Male Sprague-Dawley rats (Rattus norvegicus) weighing 225–250 g were obtained from Taconic Laboratories. Rats were individually housed with food and water available ad libitum in their home cages. A 12/12 hr light/dark cycle was used with the lights on at 7:00 a.m. All experimental procedures were performed during the light cycle. The experimental protocols were consistent with the guidelines issued by the U.S. National Institutes of Health and were approved by the Institutional Animal Care and Use Committee.
Jugular catheterization and stereotaxic surgeries
Under isoflurane anesthesia (1.5–2.5% isoflurane in O2) an indwelling silastic catheter (SAI infusion technologies, Lake Villa, IL, USA) was placed into the right jugular vein and sutured in place. The catheter was routed subcutaneously to a mesh platform placed between the shoulder blades. Catheters were flushed daily with 0.3 ml of the antibiotic Timentin (0.93 mg/ml) dissolved in heparinized saline. The catheters were sealed with plastic obturators when not in use. Following catheter implantation, the rats were placed in a stereotaxic apparatus under isoflurane anesthesia. Genetically encoded calcium indicator, GCaMP6f, targeting either neurons (AAV9.Syn.GCaMP6f.WPRE.SV40, Addgene 100837) or astrocytes (pZac2.1.gfaABC1D.cytoGCamp6f.SV40, Addgene 52925) was injected bilaterally (2 μl/side) into the NAc shell using the following coordinates (relative to bregma, in mm): AP +1, ML ± 1, DV −7) via a Neuros syringe (Hamilton). At 14–20 days after virus microinjections, the rats began cocaine self-administration training.
Cocaine Self-Administration Training
The rats were placed in operant chambers (Med Associates) and allowed to lever-press for intravenous cocaine infusions (0.21 mg cocaine per 50 μl saline over a 2.8 s infusion) during daily 2-hour self-administration sessions as previously described40. Briefly, each cocaine infusion was followed by a 20-s timeout period during which responses had no scheduled consequences. The rats were initially trained using a fixed ratio 1 (FR1) schedule of reinforcement. When stable responding was achieved under the FR1 schedule, they were switched to an FR5 schedule. Rats self-administered cocaine for 14 days and were paired with yoked saline controls. The yoked animals received an infusion of saline every time their pair received cocaine. There were no consequences to lever pressing by the yoked saline animals.
Electrophysiology
Twenty-four hours after the last behavioral session, the rats were deeply anesthetized with isoflurane and decapitated. Brains were rapidly removed and coronal slices (300 μm-thick) containing the NAc were cut using a vibratome (VT1200S; Leica Microsystems) in an ice-cold artificial cerebrospinal fluid (aCSF) cutting solution in which NaCl was replaced with an equiosmolar concentration of sucrose. ACSF contained the following (in mM): 130 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, 1 MgCl2, 2 CaCl2, pH 7.2–7.4, when saturated with 95% O2 and 5% CO2; osmolarity was 305–315 mOsm. After cutting, the slices were kept in aCSF heated to 36 °C for 30–40 minutes, following which they were maintained in aCSF at room temperature until transfer to the recording chamber. To facilitate astrocyte visualization, some slices were incubated with sulforhodamine 101 (SR101, 1 μM, Sigma-Aldrich) for 25 min and then transferred to regular aCSF. Composition of aCSF in the recording chamber was altered to enhance detection of SICs. This included lowering Mg2+ concentration to 0.005 mM and supplementing the aCSF with the NMDA receptor co-agonist, d-serine (0.01 mM) and GABAA antagonist, picrotoxin (0.1 mM). Additionally, about ~40% of SIC recordings were done in the presence of the AMPA receptor, antagonist DNQX (10 μM). DNQX did not have a significant effect on any of the measured SIC parameters and these recordings were pooled with DNQX-free recordings for analysis unless otherwise indicated in the text. All recorded SICs were NMDAR-mediated as confirmed by sensitivity to the NMDAR antagonist, DL-AP5. ACSF in the recording chamber was continuously oxygenated, heated to 32±1°C using an automated temperature controller (Warner Instruments) and perfused at a rate of 1–2 ml/min. Brain slices were viewed under an upright microscope (Olympus BX51WI) with infrared differential interference contrast optics and a 40x water-immersion objective. Recording pipettes were pulled from borosilicate glass capillaries (World Precision Instruments) to a resistance of 4–7 MΩ when filled with the intracellular solution. The intracellular solution contained the following (in mM): 145 potassium gluconate, 2 MgCl2, 2.5 KCl, 2.5 NaCl, 0.1 BAPTA, 10 HEPES, 2 Mg-ATP, and 0.5 GTP-Tris, pH 7.2–7.3, with KOH, osmolarity 280–290 mOsm.
Astrocytes were identified as SR101 positive cells under LED illumination captured with the 560/630 nM filter cube. Astrocyte potassium (K+) currents were recorded in voltage-clamp mode (Vholding=−80 mV). Following seal rupture, series resistance was compensated (65–75%). Inward rectifying K+ currents were evoked by a series of 10 mV, 40 ms steps from −180 mV to 0 mV following a 200 ms pre-step to 0 mV as in previous studies41–44. Delayed rectifier K+ currents were measured as outward responses to a series of 10 mV, 500 ms steps from −80 mV to +80 mV, following a 100 ms pre-step to −50 mV. Inward rectifying K+ currents were measured at the end of each 40 ms-long step. Delayed rectifier currents were measured at current peak. NAc shell MSNs were identified by their morphology and presence of spontaneous synaptic activity at a holding voltage of −70 mV.
SICs were selected for analysis based on slow rise kinetics (>10 ms) and large amplitudes (>25 pA). The amplitude of spontaneous excitatory postsynaptic currents (sEPSCs) was computed from an average of 50–100 current traces. For sEPSCs recorded in the absence of DNQX, decay time was expressed as a weighted double exponential fit to the decay phase of an average sEPSC trace as previously described45. For NMDA-mediated sEPSCs recorded in the presence of DNQX, decay was defined as time between 90% and 10% of the maximal amplitude relative to baseline46. Mean sEPSC frequencies were analyzed from 10–20 s long trace segments.
Access resistance (10–30 MΩ) was monitored throughout each recording by injection of 10 mV hyperpolarizing pulses; data were discarded if access resistance changed >25% over the course of data collection. All recordings were low-pass filtered at 2 kHz and digitized at 20 kHz using Digidata 1440A (Molecular Devices) coupled to MultiClamp 700B amplifier (Molecular Devices) and pClamp10 software (Molecular Devices).
Calcium Imaging
Ca2+ imaging videos were undertaken in the same slices, and under the same conditions, as the electrophysiological recordings. Two-minute videos of the NAc shell were acquired with ORCA-Flash 4.0 (V2) digital camera (Hamamatsu) during excitation by an LED light source (X-Cite XLED1, Excelitas Technologies). Videos were binned at 512 × 512 pixels and collected with a 40x objective (0.65 μm/pixel) at 25 frames/second. Videos were analyzed offline with ImageJ and Matlab (Mathworks). Individual cells were manually isolated as regions of interests (ROIs). Relative fluorescence intensity (dF/F0) within each ROI was calculated using Matlab scripts modified from Romano et al.47 as: FROI-αFneuropil/F0, where FROI is the average fluorescence intensity within the ROI at each timepoint, α=0.4, Fneuropil is the average fluorescence intensity of perisomatic neuropil around each ROI at each timepoint, and F0 is fluorescence intensity of slow baseline fluctuations, unrelated to neuronal activity. Amplitude, half-width, and frequency of calcium transients were analyzed with custom-written Matlab scripts. Half-width was defined as event duration at 50% of event amplitude.
Network Analysis
Network properties were analyzed in Matlab using the Brain Connectivity Toolbox48 as follows: Ca2+ transients for each ROI were binarized and transformed binary traces were used to calculate phi coefficients for each ROI pair in each imaged field. The phi coefficient is a measure of correlation between binary series, similar to Pearson’s correlation coefficient. An undirected adjacency matrix was then constructed for each imaged field containing values of phi coefficients between ROI pairs. Adjacency matrices were then thresholded to preserve the largest 25% of phi coefficient values, representing the strongest 25% of connections. Adjacency matrices were used to calculate eight measures of network structure for each imaged field (ROIs are nodes in this analysis). 1. Network averaged (global) clustering coefficient: a single nodes’ clustering coefficient is calculated as the fraction of nodes it is connected to that are also connected to each other and then averaged across all nodes. 2. Communities: the number of non-overlapping groups maximally connected to each other, but minimally connected to other groups. 3. Transitivity: the probability that, if one node is connected to two others, those two nodes are also connected to each other. 4. Assortativity: coefficient that measures the extent to which nodes of similar strength connect to each other. 5. Density: the ratio of existing connections relative to all possible connections in a network; 6. Modularity: the tendency of a network to be segregable into communities with dense intracommunity connections and sparse intercommunity connections; 7. Characteristic path length: the average shortest path between any two nodes in a network. 8. Global efficiency: the inverse of the characteristic path length. More details on calculation, interpretation, and applicability of these and other measures to brain networks can be found in several excellent reviews48,49.
Statistics
All analyses were done in Excel 2016 or GraphPad Prism 8. Categorical data were analyzed using Fisher’s exact test. Comparisons between two groups were carried out with either Student’s t or Mann-Whitney U tests as necessary based on the F-test for homogeneity of variances. For all other comparisons, 2-way ANOVA, followed by Bonferroni post-hocs was used as indicated in the text. All data were reported as mean ± standard error of the mean and statistical significance threshold was set at p<0.05.
Results
Cocaine self-administration alters astrocyte Ca2+ dynamics
Intracellular Ca2+ fluctuations are a prominent marker of astrocyte activity linked to regulation of synaptic transmission and are sensitive to a variety of receptor agonists and antagonists, including dopamine50. However, it is unknown whether astrocytic Ca2+ is influenced by cocaine self-administration. To address this, we measured spontaneous Ca2+ in astrocytic somata and proximal processes in the NAc shell 24 hours or 14 days after the last self-administration session, by monitoring fluorescence of virally expressed Ca2+ reporter, GCamp6f, under control of cytoplasm-targeted GFAP promoter. We found significant decreases in NAc shell Ca2+ event amplitude associated with both 24 hour and 14-day withdrawal timepoints when compared to their respective saline-yoke populations (Fig. 1A,B). There were no significant differences in Ca2+ transient frequency between cocaine and saline populations neither after 24 hours nor after 14 days of withdrawal from self-administration (Fig. 1C). However, Ca2+ transient duration was significantly reduced after cocaine self-administration at both timepoints when compared to the respective saline-yoke populations (Fig 1D). These results indicate that history of cocaine self-administration has a lasting impact on the dynamics of intracellular Ca2+ signaling within individual astrocytes of the NAc shell.
Figure 1. Astrocyte Ca2+ transients after cocaine self-administration.
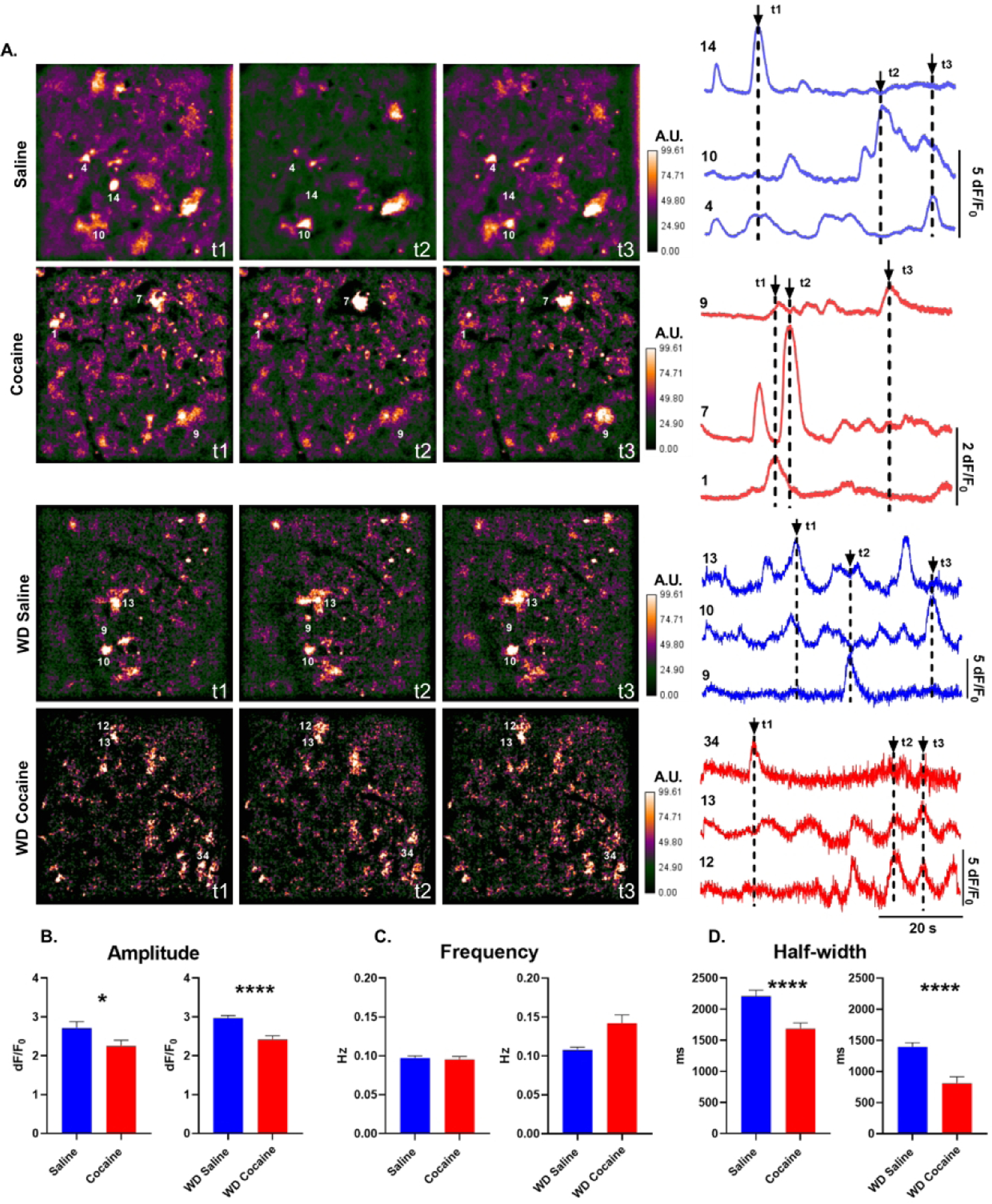
A) Left: False-color GFAP-GCamp6f Ca2+ fluorescence frames from NAc shell astrocyte videos after 24 hours (saline, cocaine) and 14 days (wd saline, wd cocaine) withdrawal from cocaine self-administration. The color scale is in arbitrary units (A.U.) expressed as percent of maximal fluorescence intensity. Right: Representative dF/F0 traces taken from numbered ROIs. Arrows indicate time points (t1–t3) represented by image frames to the left. B-D) Histograms of amplitude, frequency, duration (half-width) of Ca2+ events in NAc shell astrocytes (n=182 saline cells, 145 cocaine cells, 354 wd saline cells, 133 wd cocaine cells *, p<0.05; ****, p<0.0001; Mann-Whitney U tests).
Striatal astrocytes form a syncytium of cells that facilitate propagation of Ca2+ signals across the gap-junction coupled network. We evaluated whether cocaine self-administration had an impact on astrocyte network structure by examining binary series correlations between Ca2+ transients in pairs of imaged cells. We evaluated eight measures of network organization with graph theory tools previously used for analysis of neuronal networks. At 24 hours of withdrawal, cocaine self-administration significantly increased the density of functional NAc shell astrocyte network connections when distributions of density values between saline-yoke and cocaine groups were compared (p = 0.0095, Kolmogorov-Smirnov test) although this failed to reach significance when population means were compared (p = .055, Mann-Whitney U-test). Cocaine self-administration at 24 hours of withdrawal significantly decreased modularity and increased efficiency of astrocyte networks within slices as compared to the saline-yoke population (Fig. 2A,B modularity: p = 0.0444, unpaired T-test with Welch’s correction; efficiency: p = 0.036 Mann-Whitney U-test). None of these significant differences were preserved at 14 days of withdrawal from cocaine self-administration (Fig. 2C). Instead, 14 days of withdrawal was associated with significant decreases in network transitivity and assortativity compared to networks from the respective saline-yoke population, producing disassortative networks (Fig. 2C,D transitivity: p = 0.006; assortativity: p = 0.0302, Mann-Whitney U-test). Overall, these results indicate that duration of withdrawal from cocaine has a disparate impact on astrocytic Ca2+ evaluated at the single cell and astrocyte network level. Further implications of these findings are presented in the Discussion.
Figure 2. Astrocyte network structure after cocaine self-administration.
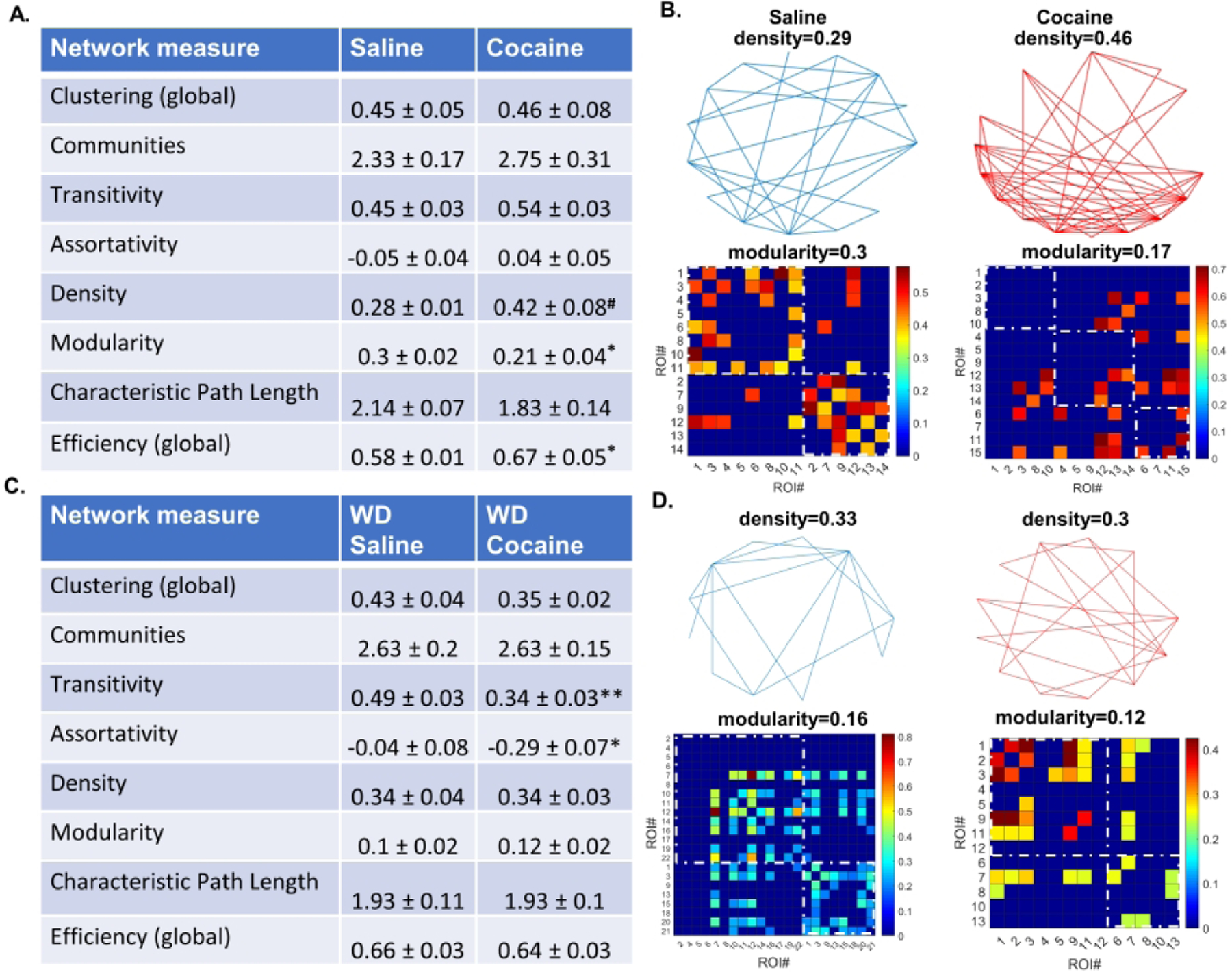
A) A table of network measures for astrocytes in the NAc shell of cocaine and yoked saline rats 24 hours after the last operant session. Cocaine self-administration is associated with increased connection density, decreased modularity, and increased network efficiency (# K-S test, p<0.01, Mann-Whitney U, p=0.055. * Unpaired t-test or Mann-Whitney U, as appropriate, p<0.05). B) Graphical representations of astrocytic networks in a single slice from a yoked saline or a cocaine-experienced rat at 24 hours of withdrawal. Top: Vertices represent individual ROIs, lines represent connections between ROIs. Density is the ratio of existing connections to all possible connections. Bottom: ROI × ROI grids representing connections sorted into communities. Color represents connection strength (phi-coefficient). Two communities are shown in the saline condition and three communities in the cocaine condition (dashed squares). Intra-community connections are inside the dashed squares and all colored slots outside of dashed squares indicate inter-community connections. C) A table of network measures for astrocyte in the NAc shell of cocaine and yoked saline rats 14 days after the last operant session. Cocaine self-administration and withdrawal is associated with decreased transitivity and disassortativity (* p < 0.05, ** p < 0.01 Mann-Whitney U-test). D) Graphical representations of astrocytic networks in a single slice from a yoked saline or a cocaine experienced rat at 14 days of withdrawal.
Cocaine self-administration does not affect potassium current rectification
Buffering of extracellular potassium is one of the main functions attributed to astrocytes throughout the brain. Astrocytic potassium channels are known to be involved in regulation of astrocytic calcium signaling51, astrocytic resting membrane potential52, and astrocytic glutamate release53. To evaluate potential differences in potassium signaling, we used voltage-clamp protocols to examine rectification attributed to inwardly rectifying (Kir) channels that underlie astrocyte regulation of K+ released during periods of neuronal activity54. In whole-cell patch-clamp recordings, we observed weak rectification of Kir amplitude in both saline and cocaine conditions at 24 hours of withdrawal from cocaine. The rectification index, calculated as a ratio of current amplitudes evoked at −10 mV and −150 mV, was 0.9±0.01 for the saline condition and 0.9±0.01 for the cocaine condition. There was no main effect of cocaine exposure across the entire range of evoked Kir current amplitudes (Fig. 3A–B). Similarly, we observed no main effect of cocaine exposure on the amplitude of outward delayed rectifier (Kdr) currents (Fig. 3C–D). Furthermore, no significant differences between saline and cocaine groups were observed in resting membrane potential (saline: −76±1.18 mV; cocaine: −76.2±0.9 mV, p=0.89, unpaired t-test) or input resistance (saline: 18.7±1.6 MΩ; cocaine: 19.6±2.1 MΩ, p=0.71, unpaired t-test). Together, these results show that neither the current rectification attributed to the astrocytic voltage-gated Kir and Kdr currents nor the passive indicators of astrocyte membrane excitability are affected by cocaine self-administration.
Figure 3. Cocaine self-administration does not alter rectification of astroglial potassium currents.

A) Representative traces and B) current-voltage relationship of currents attributed to Kir channels in NAc shell astrocytes from saline control and cocaine-experienced rats. Raw data are presented on the left (main effect of cocaine: p=0.69, two-way RM ANOVA; n=31 saline cells from 7 animals; n=29 cocaine cells from 6 animals). Normalized currents are presented on the right (scale bars are 4 nA and 10 ms; main effect of cocaine: p=0.96, two-way RM ANOVA. C) Representative traces and D) current-voltage relationship of currents attributed to Kdr channels in NAc shell astrocytes from saline control and cocaine-experienced rats. Raw data are presented on the left (scale bars are 4 nA and 200 ms; main effect of cocaine: p=0.21, two-way RM ANOVA). Currents normalized to maximal Kdr are presented on the right (main effect of cocaine: p=0.71, two-way RM ANOVA).
Cocaine exposure increases neuroglial coupling
We next examined whether a combination of changes in some functional signatures of astrocyte activity (i.e. Ca2+ signaling), but not others (i.e. Kir/dr currents, passive membrane properties) at 24 hours of withdrawal influenced neuronal ability to detect astrocyte-derived glutamate. To do so, we monitored NMDA receptor-mediated SICs in whole-cell recordings from NAc shell MSNs. We found that cocaine experience significantly increased SIC prevalence: saline controls displayed SICs in 37% of MSNs (11 out of 30 cells, N=10), while cocaine-experienced animals displayed SICs in 67% of MSNs (26 out of 39 cells, N=10) (Fig. 4A–B; Fisher’s p=0.02, odds ratio (OR)=3.5). Increased prevalence of SICs could be driven by increased number of astrocytic glutamate release events which may be reflected as an increase in SIC frequency. However, the mean frequency of SICs was not affected by cocaine self-administration (saline: 0.48±0.1 events/min, n=11 cells from 10 animals; cocaine: 0.65±0.2 events/min, n=26 cells from 10 animals; U=138, p=0.88; Mann-Whitney U, Fig. 4C). Increased concentration of glutamate per release event may also increase probability of detecting SICs especially in the case of small-amplitude events that are not likely to saturate the pool of available NMDA receptors. If this were the case, an increase in SIC amplitude should also be expected. However, the mean amplitude of SICs was not significantly altered by cocaine experience (saline: 101.3±39.3 pA; cocaine: 109.6±27.5 pA; t35=0.17, p=0.87, unpaired Student’s t; Fig. 4D). These results indicate that short-access cocaine self-administration increases functional coupling between neurons and astrocytes in the NAc shell such that greater number of neurons within the local circuit are responsive to astrocyte-released glutamate. Greater astrocyte-neuron functional coupling is not due to cocaine-induced increase in amount of astrocyte-released glutamate or increase in frequency of astrocytic glutamate release events.
Figure 4. Cocaine self-administration increases prevalence of SICs in NAc shell MSNs.

A) Top, Examples of SICs (arrows) in an MSN from a cocaine-experienced animal. SICs, but not AMPA receptor-mediated synaptic currents, are blocked by an NMDA receptor antagonist, DL-AP5 (50 mM). Bottom, Representative SICs from a cocaine-naïve (saline) and a cocaine-experienced animal. B) Cocaine experience increases the fraction of cells with SICs (* p<0.05, Fisher’s). C-D) SIC frequency and amplitude are not affected by cocaine self-administration. Circles indicate individual MSNs. Black horizontal bars indicate distribution means and standard errors, respectively.
SICs are associated with NMDA receptor-mediated synaptic plasticity
Increased prevalence of NMDA receptor-mediated SICs following cocaine exposure suggests an increase in membrane permeability to Ca2+. Given that a large variety of cellular phenomena rely on intracellular Ca2+, we speculated that cells displaying SICs (SIC-positive cells) may exhibit synaptic properties distinct from cells lacking SICs (SIC-negative cells). To examine this, we monitored sEPSCs in the absence and in the presence of the AMPA receptor antagonist, DNQX. In the absence of DNQX, when sEPSCs were mediated by both AMPA and NMDA receptors, we observed no significant differences in amplitude, frequency, or duration of synaptic currents between SIC-positive and SIC-negative cells in either the saline or the cocaine groups (Fig. 5A–D). In the presence of DNQX, the amplitude of NMDAR-mediated sEPSCs also did not differ between SIC-positive and SIC-negative cells and was not affected by cocaine treatment (Fig. 6A–B, p=0.16, 2-way ANOVA). However, frequency of sEPSCs was significantly reduced in SIC-positive cells (Fig. 6C, F1,24=7.99, p<0.01, 2-way ANOVA), but was not affected by cocaine treatment (F1,24=1.26, p=0.28, 2-way ANOVA). Pairwise comparisons revealed no significant differences between any of the groups and there was no interaction between cocaine treatment and presence of SICs (F1,24=0.13, p=0.72, 2-way ANOVA). The decay time of NMDAR-mediated sEPSCs was significantly longer in SIC-positive neurons than in SIC-negative cells (Fig. 6D; F1,24=42.03, p<0.001, 2-way ANOVA). The decay time was not affected by cocaine treatment (F1,24=1.3, p=0.25, 2-way ANOVA), indicating that neuroglial coupling rather than cocaine experience dictated increased sEPSC duration. Pairwise comparisons revealed significant differences between: saline SIC+ vs. saline SIC– (p<0.01), saline SIC+ vs. cocaine SIC– (p<0.05), saline SIC– vs. cocaine SIC+ (p<0.01), and cocaine SIC+ vs cocaine SIC– (p<0.01) groups. There was no significant interaction between the factors of cocaine treatment and SIC presence (F1,24=1.29, p=0.27). Overall, the pattern of changes at NMDA receptor-mediated sEPSCs from SIC-positive and SIC-negative cells was similar between saline and cocaine animals.
Figure 5: Neuroglial coupling does not affect synaptic plasticity of mixed AMPA/NMDA receptor currents.

A) Representative traces of sEPSC averages from SIC-positive and SIC-negative MSNs in saline control and cocaine-experienced animals. B-D) Summary histograms of sEPSC amplitude, sEPSC duration, and sEPSC frequency in SIC-positive and SIC-negative cells (n=6–12 cells; main effect of SICs: amplitude, p=0.89; frequency, p=0.27; decay, p=0.37).
Figure 6: Neuroglial coupling reports plasticity of NMDA receptor-mediated sEPSCs independent of cocaine experience.

A) Representative traces of pharmacologically isolated NMDA receptor-mediated sEPSC averages from SIC-positive and SIC-negative MSN in saline control and cocaine-experienced animals. B-D) Summary histograms of sEPSC amplitude, sEPSC duration, and sEPSC frequency in SIC-positive and SIC-negative cells. (**, p<0.01, 2-way ANOVAs).
Cocaine self-administration does not alter Ca2+ dynamics of NAc shell MSNs
To examine whether cocaine-induced changes in neuronal SICs and NMDA receptor signaling at 24 hours of withdrawal impact neuronal Ca2+ events, we performed Ca2+ imaging of GCamp6f under control of hSyn promoter. We found no significant differences in the amplitude (t269=0.21, p=0.83, unpaired t-test), frequency (t269=1.8, p=0.07, unpaired t-test), or half-width (U=7975, p=0.09, Mann-Whitney U) of neuronal Ca2+ transients between saline- and cocaine-exposed animals (Fig 7A–D). Therefore, increased SIC prevalence and the associated NMDAR-mediated Ca2+ entry does not enhance spontaneous Ca2+ signals in MSNs of the NAc shell. This is, perhaps, not surprising given the low frequency of SICs in slices of both cocaine-naïve and cocaine-experienced animals. Since astrocytic glutamate release underlying the SICs has been proposed to co-ordinate neuronal microcircuit organization in a number of brain regions17–19,27, we followed up with exploration of network structure using graph theory methods as we had done for astrocytic networks. Despite the intriguing relationships that we report for astrocytic networks, none of the eight measures of neuronal network interactions that we examined showed significant differences between saline and cocaine conditions (Table 1). We conclude that cocaine-induced increase of neuroglial coupling is spatially limited and does not influence network structure as reported by Ca2+ imaging in the NAc shell slices.
Figure 7. Spontaneous Ca2+ transients in neurons after cocaine self-administration.

A) Left: False color hSyn-GCamp6f Ca2+ fluorescence images taken from NAc shell neurons of one rat trained to self-administer cocaine and one saline-yoke control. The color scale is in arbitrary units (A.U.) expressed as percent of maximal fluorescence intensity. Right: Representative dF/F0 traces taken from numbered ROIs. Arrows indicate time points (t1–t3) represented by image frames to the left. B-D) Histograms of amplitude, frequency, and half-width of GCamp6f-reported Ca2+ events in NAC shell MSNs.
Table 1. Cocaine self-administration does not impact neuronal network structure.
Measures of network structure calculated for MSNs in the NAc shell of rats trained to self-administer cocaine and their saline-yoke counterparts.
| Network measure | Saline | Cocaine |
|---|---|---|
| Clustering (global) | 0.65 ± 0.04 | 0.67 ± 0.04 |
| Communities | 2.43 ± 0.2 | 2.2 ± 0.2 |
| Transitivity | 0.63 ± 0.03 | 0.66 ± .04 |
| Assortativity | −0.25 ± 0.05 | −0.24 ± 0.06 |
| Density | 0.43 ± 0.02 | 0.48 ± 0.06 |
| Modularity | 0.17 ± 0.02 | 0.16 ± 0.03 |
| Characteristic Path Length | 1.71 ± .05 | 1.66 ± 0.09 |
| Efficiency (global) | 0.69 ± 0.01 | 0.72 ± 0.03 |
Discussion
Our data indicate that cocaine self-administration is linked to a number of novel functional adaptations at NAc shell astrocytes. First, we demonstrate lasting plasticity of Ca2+ transients within individual astrocytes. Second, we identify cocaine-induced plasticity of astrocytic networks supporting the hypothesis of structured communication between astrocytes. Third, we find evidence that sensitivity to astrocyte-derived glutamate isolates a sub-population of NAc shell MSNs that display plasticity of synaptic NMDA receptors. Finally, we report that increased glutamate-mediated neuroglial coupling at 24 hours of withdrawal from cocaine is not associated with altered structure of local MSN circuits in the NAc shell.
Ca2+ signaling in single astrocytes
Astrocyte Ca2+ fluctuations have been linked to gliotransmitter release10,19,22,39 and it is tempting to draw parallels between changes observed in our Ca2+ imaging experiments and incidence of neuronal SICs. Our electrophysiological recordings revealed increased numbers of SIC-positive neurons after cocaine self-administration training while Ca2+ imaging identified shorter and smaller astrocyte Ca2+ transients. This dissociation could arise if Ca2+-independent mechanisms were involved in SIC generation following cocaine experience. Recent work has specifically examined the relationship between astrocytic Ca2+ and SICs in CA1 pyramidal neurons and found that spontaneous SICs are preserved in IP3 knock-out mice as well as in the complete absence of extracellular and intracellular Ca2+, but are eliminated followed blockade of volume-sensitive anion channels14. Indeed, a large body of work has argued both for and against Ca2+-dependence of gliotransmission53,55–58. It is also possible that SICs in our data were triggered by isolated Ca2+ transients intermingled with events that were not related to SIC generation, so that the average values of Ca2+ event amplitude and duration pooled across all events were not affected. In support of this, NAc shell SICs occurred at a very low frequency – ~0.5–2 SICs/minute – while Ca2+ transients in astrocytes were more prevalent with a frequency of ~6 events/minute. Underrepresentation of SIC-linked Ca2+ transients in the averaged data could be further amplified by SIC presence in only a sub-population of all recorded cells.
A large number of mechanisms from surface receptors to ion channels to buffering mechanisms have been linked to regulation of astrocyte Ca2+ 59,60,14. Furthermore, cocaine has been shown to drive structural changes of NAc astrocytes as a function of withdrawal time-course which can also be expected to have an impact on Ca2+ signaling34,35,61. Our experiments did not attempt to isolate the source(s) of the observed decreases in amplitude and duration of astrocytic Ca2+. The final determination of whether cocaine-induced increase in SIC-positive MSNs is related to changes in dynamics of astrocyte Ca2+ is thus left to future experiments that combine simultaneous high-resolution imaging with electrophysiological recordings, and pharmacological or genetic manipulations to target mechanisms responsible for generation and propagation of astrocyte Ca2+ transients in the NAc shell.
Extensive literature supports the argument that history of drug exposure is a critical determinant of the molecular and behavioral adaptations to cocaine62–69. In our experiments, we controlled for the environmental stimuli (lights, syringe pump sounds, etc.) and number as well as timing of injections, by using the yoked saline design. This design did not allow for isolation of operant training effects since the saline animals were not given the opportunity to control action-outcome contingencies. Highlighting the importance of behavioral experience with cocaine, however, a recent study found that acute cocaine application increased astrocytic Ca2+ signaling in the NAc shell70, whereas we observed decreases in amplitude and duration of Ca2+ transients following cocaine self-administration. Furthermore, while a decrease in Ca2+ event duration was maintained in the cocaine, relative to saline, group at both 24 hours and 14 days of withdrawal, duration of Ca2+ transients in the saline group was markedly reduced at 14 days of withdrawal, relative to the 24 hour time point (cf. Fig.1 D). This suggests that cessation of daily operant chamber experience may change astrocyte Ca2+ signals independent of cocaine exposure. As the NAc shell is part of a network that processes not only reward signals, but salient environmental cues, environmental changes irrespective of cocaine experience could conceivably impact cellular activity.
Ca2+ signaling in astrocyte networks
Here we examined functional networks, that is, networks whose connections are determined on the basis of correlated activity of their constituent cells. Reduced amplitude of astrocyte Ca2+ transients after cocaine self-administration could impact correlated activity, and therefore network measures, if it reduced the number of identified events. However, we found no significant differences in astrocyte Ca2+ transient frequency between saline-yoke and cocaine self-administering populations. Shorter Ca2+ events could also decrease the likelihood or strength of correlations between cells with similar firing frequencies by decreasing the probability that the events overlap in time. This would be predicted to decrease the fraction of cells with correlated activity, leading to reduced network density. However, we found that network density is increased after cocaine at 24 hours of withdrawal, but is similar between cocaine and saline groups at 14 days of withdrawal. These observations suggest that functional network alterations induced by cocaine may not be solely attributable to changes in activity of single cells.
We propose that cocaine-induced increase in connection density may explain the finding of reduced network modularity at 24 hours of withdrawal. Modularity measures the tendency of a network to be segregable into communities of highly connected nodes that have minimal inter-community connections. Greater connection density can contribute to the breakdown of modularity if new connections are between communities or randomly distributed between and within communities (cf. Fig. 2B). This finding indicates that cocaine self-administration generates less structured (more random) astrocyte functional networks. Increased network density may also contribute to decreased path length, the number of connections that lie between any two cells in a network which, in turn, would increase network efficiency calculated as the inverse of the characteristic path length.
The increases in astrocyte functional network density and efficiency and increase in neuronal SICs after 24 hours of withdrawal from cocaine self-administration suggest that neuronal SICs may be driven by enhanced coordination of activity across the astrocytic network rather than Ca2+ changes within individual astrocytes. Such coordinated activity would be expected to increase connection density and decrease network modularity as observed in our analyses, since higher correlations among all cells in the network would increase the number of functional connections and make segregation of the network into discrete modules less likely. We found no significant differences in network density, modularity, and efficiency between saline and cocaine groups after 14 days of withdrawal. However, within the saline group, network modularity was reduced at 14 days versus 24 hours of withdrawal, similarly to Ca2+ event duration results. This finding, again, suggests that cessation of daily exposure to the operant chamber and non-contingent saline infusions may alter astrocyte responses independent of cocaine.
Significant differences in NAc astrocyte network assortativity and transitivity between the saline and cocaine groups were observed at 14 days of withdrawal. Networks comprised of nodes that are preferentially connected to other nodes with similar numbers of connections are referred to as assortative and have a positive assortativity value. Conversely, networks comprised of nodes that are preferentially connected to nodes with different numbers of connections (either less or more) are described as disassortative. Cocaine self-administration followed by withdrawal, thus, produced strongly disassortative astrocyte networks. Lower transitivity values can be related to this, as disassortative networks are likely to have reduced number of three-node interconnected loops, the basis of transitivity measurements. In neuronal networks, disassortativity has been proposed to improve network stability71 which may indicate reduced sensitivity to weak stimuli72. Interestingly, disassortative neural networks have also been linked to increased synchronization of activity73. Network level relationships have not been previously explored among astrocytes in situ and it is too early to say whether changes that we observed here warrant similar conclusions with regard to stimulus sensitivity, synchronizability, etc. It is nevertheless notable that the absence of changes in network density at 14 days of withdrawal suggests that astrocyte network plasticity after cocaine may not be explainable solely by introduction or deletion of random connections. Network changes do not require structural remodeling72, however, evidence in support of structural plasticity for both neurons and astrocytes after cocaine exposure is extensive and the combined impact of structural and functional connectivity changes on astrocyte network activity is of great interest.
Neuroglial coupling
We find that the population of SIC-positive NAc shell MSNs is significantly larger following cocaine exposure. Increased SIC prevalence could be attributed to increased probability of gliotransmitter release. However, available evidence indicates that extrasynaptic glutamate is decreased in the NAc following chronic cocaine37,74. While glutamate release events associated with SICs may not substantially contribute to ambient extracellular glutamate levels, our finding that the frequency of SICs was not altered by cocaine-experience suggests that the probability of astrocytic glutamate release also remained unchanged. SICs are mediated by NMDA receptors and those receptors located at extrasynaptic sites are ideally situated to detect glia-released glutamate20,21. We and others have previously reported that cocaine self-administration increased contribution of extrasynaptic NMDA receptors in the NAc shell46,75 which is supported by cocaine-induced upregulation of non-canonical NMDA receptors often found at extrasynaptic sites76–78.
We found that the SIC-positive cells displayed longer decay kinetics of NMDA receptor-mediated sEPSCs. This could be attributed to increased expression of ‘slower’ GluN2B-containing NMDA receptors on the cell membrane. Upregulation of GluN2B protein following cocaine self-administration has been previously reported although sensitivity of NMDAR-mediated currents to GluN2B antagonist, Ro 25–6981, remained equivalent between control and cocaine-experienced animals at short withdrawal time points46,69,78, indicating that increased GluN2B expression may not result in functional changes at the synapse. Irrespective of subunit composition, the presence of NMDA receptors at peri- and extra-synaptic sites may be sufficient to prolong decay time of synaptic currents as a result of synaptic glutamate spillover79. Altogether, our data indicate that cocaine-induced redistribution of synaptic NMDARs to extrasynaptic sites facilitates both detection of SICs and slower decay kinetics of synaptic currents in SIC-positive cells.
A range of factors including cocaine dose, total cocaine intake, duration of withdrawal from cocaine and other behavioral parameters could impact neuroglial coupling. We have not evaluated synaptic NMDA receptors signaling or SICs at long withdrawal intervals, but a number of recent studies indicate that cocaine-induced increase in extrasynaptic NMDA signaling may be a transient phenomenon. For example, initial up-regulation of extrasynaptic NMDA receptors was found to dissipate by one month of withdrawal from cocaine75 and 14 days of extinction training was not associated with differences in SIC frequency in the NAc80. Relative abundance of extrasynaptic NMDA receptors may also be synergistic with the effects of dendritic sprouting reported after cocaine since dendritic coverage has been shown to influence duration of synaptic transients81,82. Finally, the association between cocaine self-administration training and SIC prevalence may be amplified by changes in glutamate homeostasis. Both the cocaine-induced decrease in glial glutamate extrusion by system -xc exchangers83 and the increase in glutamate transporter-mediated reuptake46 may be expected to accumulate glutamate inside astrocytes and promote neuronal SICs with possible behavioral effects64,83.
Cocaine exposure has been linked to activation of neuronal subpopulations in the NAc, identified via various characteristics84,85. Here, we present a novel observation that plasticity of synaptic NMDA receptor currents is associated with the presence of SICs, independent of cocaine experience. Adding to prior evidence that extra/peri-synaptic NMDA signaling is increased after cocaine, we hypothesize that such signaling may be a general feature of NAc shell neurons that reflects their sensitivity to astrocytic glutamate. Neuroglial coupling may thus serve as a marker of synapses that display NMDA receptor plasticity. It remains unknown, however, whether changes in SIC-mediated neuroglial coupling are a precursor to or a consequence of NMDA receptor synaptic plasticity.
Surprisingly, we find that synaptic NMDA receptor plasticity does not reflect broader changes in Ca2+ signaling within individual neurons or within neuronal networks of the NAc shell at 24 hours of withdrawal from cocaine. Therefore, increased MSN sensitivity to astrocytic glutamate in the NAc may not directly contribute to Ca2+ mediated organization of cellular and network activity, but rather indicate highly local astrocyte-neuron interactions. Since our data do not allow for identification of SIC-positive cells within the Ca2+ imaging data, it remains to be seen whether MSNs sensitive to astrocyte-derived glutamate contribute to coordinated activity of discrete neuronal populations in the NAc. This may have relevance to cocaine-linked behaviors, as astrocytic control over the relevant neurons may be implemented via precisely targeted, rather than non-discriminate global, interactions.
Funding and Disclosures
The study was funded by DA041513 (PIO). The authors have no competing financial interests to disclose in relation to this work.
Footnotes
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Parpura V, Heneka MT, Montana V, et al. Glial cells in (patho)physiology. Journal of Neurochemistry. 2012;121(1):4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortinski PI, Dong J, Mungenast A, et al. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13(5):584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaczor P, Rakus D, Mozrzymas JW. Neuron-astrocyte interaction enhance GABAergic synaptic transmission in a manner dependent on key metabolic enzymes. Front Cell Neurosci. 2015;9:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–738. [DOI] [PubMed] [Google Scholar]
- 5.Amzica F, Massimini M, Manfridi A. Spatial buffering during slow and paroxysmal sleep oscillations in cortical networks of glial cells in vivo. J Neurosci. 2002;22(3):1042–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellot-Saez A, Kekesi O, Morley JW, Buskila Y. Astrocytic modulation of neuronal excitability through K(+) spatial buffering. Neurosci Biobehav Rev. 2017;77:87–97. [DOI] [PubMed] [Google Scholar]
- 7.Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81(4):728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fellin T, Pozzan T, Carmignoto G. Purinergic Receptors Mediate Two Distinct Glutamate Release Pathways in Hippocampal Astrocytes. J Biol Chem. 2006;281(7):4274–4284. [DOI] [PubMed] [Google Scholar]
- 9.Fiacco TA, Agulhon C, Taves SR, et al. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron. 2007;54(4):611–626. [DOI] [PubMed] [Google Scholar]
- 10.Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28(26):6659–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita T, Chen MJ, Li B, et al. Neuronal Transgene Expression in Dominant-Negative SNARE Mice. J Neurosci. 2014;34(50):16594–16604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sloan SA, Barres BA. Looks Can Be Deceiving: Reconsidering the Evidence for Gliotransmission. Neuron. 2014;84(6):1112–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toth AB, Hori K, Novakovic MM, Bernstein NG, Lambot L, Prakriya M. CRAC channels regulate astrocyte Ca(2+) signaling and gliotransmitter release to modulate hippocampal GABAergic transmission. Sci Signal. 2019;12(582). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez-Gonzalo M, Zehnder T, Requie LM, Bezzi P, Carmignoto G. Insights into the release mechanism of astrocytic glutamate evoking in neurons NMDA receptor-mediated slow depolarizing inward currents. Glia. 2018;66(10):2188–2199. [DOI] [PubMed] [Google Scholar]
- 15.Reichenbach A, Derouiche A, Kirchhoff F. Morphology and dynamics of perisynaptic glia. Brain Res Rev. 2010;63(1–2):11–25. [DOI] [PubMed] [Google Scholar]
- 16.Patrushev I, Gavrilov N, Turlapov V, Semyanov A. Subcellular location of astrocytic calcium stores favors extrasynaptic neuron-astrocyte communication. Cell Calcium. 2013;54(5):343–349. [DOI] [PubMed] [Google Scholar]
- 17.Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal Synchrony Mediated by Astrocytic Glutamate through Activation of Extrasynaptic NMDA Receptors. Neuron. 2004;43(5):729–743. [DOI] [PubMed] [Google Scholar]
- 18.D’Ascenzo M, Fellin T, Terunuma M, et al. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci USA. 2007;104(6):1995–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirttimaki TM, Hall SD, Parri HR. Sustained neuronal activity generated by glial plasticity. J Neurosci. 2011;31(21):7637–7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10(6):2129–2142. [DOI] [PubMed] [Google Scholar]
- 21.Tian G-F, Azmi H, Takano T, et al. An astrocytic basis of epilepsy. Nat Med. 2005;11(9):973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci. 2005;25(9):2192–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bardoni R, Ghirri A, Zonta M, et al. Glutamate-mediated astrocyte-to-neuron signalling in the rat dorsal horn. J Physiol (Lond). 2010;588(Pt 5):831–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen N, Sugihara H, Sharma J, et al. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc Natl Acad Sci USA. 2012;109(41):E2832–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perea G, Yang A, Boyden ES, Sur M. Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nature Communications. 2014;5(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pál B Astrocytic Actions on Extrasynaptic Neuronal Currents. Front Cell Neurosci. 2015;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate Released from Glial Cells Synchronizes Neuronal Activity in the Hippocampus. J Neurosci. 2004;24(31):6920–6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fellin T, D’Ascenzo M, Haydon PG. Astrocytes control neuronal excitability in the nucleus accumbens. ScientificWorldJournal. 2007;7:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pirttimaki TM, Sims RE, Saunders G, Antonio SA, Codadu NK, Parri HR. Astrocyte-Mediated Neuronal Synchronization Properties Revealed by False Gliotransmitter Release. J Neurosci. 2017;37(41):9859–9870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Augusto-Oliveira M, Arrifano GP, Takeda PY, et al. Astroglia-specific contributions to the regulation of synapses, cognition and behaviour. Neurosci Biobehav Rev. 2020;118:331–357. [DOI] [PubMed] [Google Scholar]
- 31.Bull C, Freitas KC, Zou S, et al. Rat nucleus accumbens core astrocytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology. 2014;39(12):2835–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corkrum M, Covelo A, Lines J, et al. Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity. Neuron. 2020;105(6):1036–1047 e1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruyer A, Scofield MD, Wood D, Reissner KJ, Kalivas PW. Heroin Cue-Evoked Astrocytic Structural Plasticity at Nucleus Accumbens Synapses Inhibits Heroin Seeking. Biol Psychiatry. 2019;86(11):811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scofield MD, Li H, Siemsen BM, et al. Cocaine Self-Administration and Extinction Leads to Reduced Glial Fibrillary Acidic Protein Expression and Morphometric Features of Astrocytes in the Nucleus Accumbens Core. Biol Psychiatry. 2016;80(3):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Testen A, Sepulveda-Orengo MT, Gaines CH, Reissner KJ. Region-Specific Reductions in Morphometric Properties and Synaptic Colocalization of Astrocytes Following Cocaine Self-Administration and Extinction. Front Cell Neurosci. 2018;12:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scofield MD, Kalivas PW. Astrocytic Dysfunction and Addiction: Consequences of Impaired Glutamate Homeostasis. Neuroscientist. 2014;20(6):610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker DA, McFarland K, Lake RW, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6(7):743–749. [DOI] [PubMed] [Google Scholar]
- 38.Turner JR, Ecke LE, Briand LA, Haydon PG, Blendy JA. Cocaine-related behaviors in mice with deficient gliotransmission. Psychopharmacology (Berl). 2013;226(1):167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, Kalivas PW. Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biol Psychiatry. 2015;78(7):441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ortinski PI, Briand LA, Pierce RC, Schmidt HD. Cocaine-seeking is associated with PKC-dependent reduction of excitatory signaling in accumbens shell D2 dopamine receptor-expressing neurons. Neuropharmacology. 2015;92:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bordey A, Sontheimer H. Postnatal development of ionic currents in rat hippocampal astrocytes in situ. J Neurophysiol. 1997;78(1):461–477. [DOI] [PubMed] [Google Scholar]
- 42.Standen NB, Stanfield PR. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978;280:169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sontheimer H, Waxman SG. Expression of voltage-activated ion channels by astrocytes and oligodendrocytes in the hippocampal slice. J Neurophysiol. 1993;70(5):1863–1873. [DOI] [PubMed] [Google Scholar]
- 44.D’Ambrosio R, Wenzel J, Schwartzkroin PA, McKhann GM 2nd, Janigro D. Functional specialization and topographic segregation of hippocampal astrocytes. J Neurosci. 1998;18(12):4425–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ortinski PI, Lu C, Takagaki K, Fu Z, Vicini S. Expression of distinct alpha subunits of GABAA receptor regulates inhibitory synaptic strength. J Neurophysiol. 2004;92(3):1718–1727. [DOI] [PubMed] [Google Scholar]
- 46.Ortinski PI, Turner JR, Pierce RC. Extrasynaptic targeting of NMDA receptors following D1 dopamine receptor activation and cocaine self-administration. J Neurosci. 2013;33(22):9451–9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romano SA, Perez-Schuster V, Jouary A, et al. An integrated calcium imaging processing toolbox for the analysis of neuronal population dynamics. PLoS Comput Biol. 2017;13(6):e1005526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. [DOI] [PubMed] [Google Scholar]
- 49.Bassett DS, Sporns O. Network neuroscience. Nat Neurosci. 2017;20(3):353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galloway A, Adeluyi A, O’Donovan B, et al. Dopamine Triggers CTCF-Dependent Morphological and Genomic Remodeling of Astrocytes. J Neurosci. 2018;38(21):4846–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu K-C, Kuo C-S, Chao C-C, et al. Role of voltage-gated K(+) channels in regulating Ca(2+) entry in rat cortical astrocytes. J Physiol Sci. 2015;65(2):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butt AM, Kalsi A. Inwardly rectifying potassium channels (Kir) in central nervous system glia: a special role for Kir4.1 in glial functions. J Cell Mol Med. 2006;10(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woo DH, Han K-S, Shim JW, et al. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell. 2012;151(1):25–40. [DOI] [PubMed] [Google Scholar]
- 54.Sibille J, Dao Duc K, Holcman D, Rouach N. The neuroglial potassium cycle during neurotransmission: role of Kir4.1 channels. PLoS Comput Biol. 2015;11(3):e1004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petravicz J, Boyt KM, McCarthy KD. Astrocyte IP3R2-dependent Ca(2+) signaling is not a major modulator of neuronal pathways governing behavior. Front Behav Neurosci. 2014;8:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci. 2008;28(19):4967–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fiacco TA, McCarthy KD. Multiple Lines of Evidence Indicate That Gliotransmission Does Not Occur under Physiological Conditions. J Neurosci. 2018;38(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Savtchouk I, Volterra A. Gliotransmission: Beyond Black-and-White. J Neurosci. 2018;38(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54(7):716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semyanov A, Henneberger C, Agarwal A. Making sense of astrocytic calcium signals - from acquisition to interpretation. Nat Rev Neurosci. 2020;21(10):551–564. [DOI] [PubMed] [Google Scholar]
- 61.Scofield MD. Exploring the Role of Astroglial Glutamate Release and Association With Synapses in Neuronal Function and Behavior. Biol Psychiatry. 2018;84(11):778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Markou A, Arroyo M, Everitt BJ. Effects of contingent and non-contingent cocaine on drug-seeking behavior measured using a second-order schedule of cocaine reinforcement in rats. Neuropsychopharmacology. 1999;20(6):542–555. [DOI] [PubMed] [Google Scholar]
- 63.Ploense KL, Vieira P, Bubalo L, et al. Contributions of prolonged contingent and non-contingent cocaine exposure to escalation of cocaine intake and glutamatergic gene expression. Psychopharmacology (Berl). 2018;235(5):1347–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou L, Andersen H, Arreola AC, Turner JR, Ortinski PI. Behavioral History of Withdrawal Influences Regulation of Cocaine Seeking by Glutamate Re-Uptake. PLoS One. 2016;11(9):e0163784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Conrad KL, Tseng KY, Uejima JL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31(15):5737–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen BT, Bowers MS, Martin M, et al. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59(2):288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ortinski PI, Vassoler FM, Carlson GC, Pierce RC. Temporally dependent changes in cocaine-induced synaptic plasticity in the nucleus accumbens shell are reversed by D1-like dopamine receptor stimulation. Neuropsychopharmacology. 2012;37(7):1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ortinski PI. Cocaine-induced changes in NMDA receptor signaling. Mol Neurobiol. 2014;50(2):494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Li KL, Shukla A, et al. Cocaine Triggers Astrocyte-Mediated Synaptogenesis. Biol Psychiatry. 2021;89(4):386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vasquez JC, Houweling AR, Tiesinga P. Simultaneous stability and sensitivity in model cortical networks is achieved through anti-correlations between the in- and out-degree of connectivity. Front Comput Neurosci. 2013;7:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmeltzer C, Kihara AH, Sokolov IM, Rudiger S. Degree Correlations Optimize Neuronal Network Sensitivity to Sub-Threshold Stimuli. PLoS One. 2015;10(6):e0121794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gu C, Gu X, Wang P, et al. Disassortative Network Structure Improves the Synchronization between Neurons in the Suprachiasmatic Nucleus. J Biol Rhythms. 2019;34(5):515–524. [DOI] [PubMed] [Google Scholar]
- 74.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–572. [DOI] [PubMed] [Google Scholar]
- 75.Delint-Ramirez I, Segev A, Pavuluri A, Self DW, Kourrich S. Cocaine-Induced Synaptic Redistribution of NMDARs in Striatal Neurons Alters NMDAR-Dependent Signal Transduction. Front Neurosci. 2020;14:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan T, Mameli M, O’Connor EC, et al. Expression of cocaine-evoked synaptic plasticity by GluN3A-containing NMDA receptors. Neuron. 2013;80(4):1025–1038. [DOI] [PubMed] [Google Scholar]
- 77.Creed M, Kaufling J, Fois GR, et al. Cocaine Exposure Enhances the Activity of Ventral Tegmental Area Dopamine Neurons via Calcium-Impermeable NMDARs. J Neurosci. 2016;36(42):10759–10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown TE, Lee BR, Mu P, et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011;31(22):8163–8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina. J Neurosci. 2002;22(6):2165–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang LY, Zhou YQ, Yu ZP, Zhang XQ, Shi J, Shen HW. Restoring glutamate homeostasis in the nucleus accumbens via endocannabinoid-mimetic drug prevents relapse to cocaine seeking behavior in rats. Neuropsychopharmacology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Golden SA, Russo SJ. Mechanisms of psychostimulant-induced structural plasticity. Cold Spring Harb Perspect Med. 2012;2(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arnth-Jensen N, Jabaudon D, Scanziani M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake. Nat Neurosci. 2002;5(4):325–331. [DOI] [PubMed] [Google Scholar]
- 83.Baker DA, Shen H, Kalivas PW. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids. 2002;23(1–3):161–162. [DOI] [PubMed] [Google Scholar]
- 84.Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76(4):790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cruz FC, Babin KR, Leao RM, et al. Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J Neurosci. 2014;34(22):7437–7446. [DOI] [PMC free article] [PubMed] [Google Scholar]


