Abstract
Introduction and objectives:
Hepatocellular carcinoma (HCC) is the 6th cause of cancer and hepatitis C (HCV) and B (HBV) viruses are the most frequent risk factors for HCC. Patients co-infected with HCV or HBV and human immunodeficiency virus (HIV) present a faster progression to liver fibrosis and higher incidence of HCC. The aim of this study was to evaluate the survival and clinical outcomes of co-infected patients with HCC comparing with non-HIV patients.
Methods:
We conducted a retrospective cohort study including 267 HCC patients with HCV or HBV infection with or without HIV. The primary endpoint was overall survival. A Kaplan-Meier curve was presented to assess survival function. Clinical and radiologic variables according to HIV status were compared by logistic regression.
Results:
Among 267 HCC patients, 25 (9.3%) were HIV positive. In the co-infected group, patients were younger (49.8 vs 61.2 years, p<0.001), cirrhosis was less predominant (88% vs 96.7%, p=0.05), a smaller proportion received HCC treatment (60% vs 86.3%, p=0.001) and the frequency of portal vein tumoral thrombosis was higher (32% vs 11.1%, p=0.003). The overall mortality rate was higher in the HIV positive group (92% vs 74.3%), independently of clinical and tumoral variables.
Conclusion:
Co-infected patients with HCC presented a higher mortality, tumor diagnosis in a younger age, less underlying cirrhosis and a higher frequency of tumoral thrombosis. Further studies are warranted to better understand the role of HIV in hepatocarcinogenesis, in order to improve the management of those patients, particularly regarding screening programs.
Keywords: Hepatocellular carcinoma, Cirrhosis, Hepatitis C Virus, Hepatitis B virus, human immunodeficiency virus, Highly active antiretroviral
INTRODUCTION
Hepatocellular carcinoma (HCC) is the 6th cause of cancer and the 4th leading cause of cancer-related mortality worldwide, with 841,000 new cases in 2018 and 782,000 deaths annually [1]. Cirrhosis is the main risk factor for HCC and its diagnosis is important for prevention, detection and treatment of this tumor [2]. The Hepatitis C (HCV) and B (HBV) viruses are the most frequent causes of cirrhosis, followed by nonalcoholic steatohepatitis (NASH) and alcohol intake [3].
HCV, HBV and human immunodeficiency virus (HIV) share the same transmission routes. Since the 1990s, this co-infection has acquired more relevance since it may lead to a faster progression to fibrosis and cirrhosis [4]. Highly active antiretroviral (HAART) has changed the survival of HIV patients,extending the life expectancy. Therefore, increasing the risk of developing complications due to other associated chronic diseases, such as cirrhosis and HCC [5,6]. HIV has a cocarcinogenic effect in synergy with viral hepatitis, which associated with immunodeficiency, induces a faster progression of liver disease and increases the risk of HCC [5].
Patients with HIV and liver disease have a higher mortality and the most common etiologies are HCV (33%) and HBV (6–10%) [7]. In co-infected patients, liver diseases are responsible for the second cause of death, behind only AIDS (Acquired Immunodeficiency Syndrome) [8].
Despite the relevance of the topic and the increasing number of co-infected patients with HCC, studies in the literature are scarce and most are cross sectional or have a small sample size. In this context, the aim of this study was to evaluate survival and clinical outcomes in co-infected patients with HIV and HCV/HBV comparing with non-HIV patients. We hypothesized that co-infected patients have more aggressive tumor behavior and higher mortality rates than those without HIV.
MATERIAL AND METHODS
Study Design
We conducted a retrospective cohort study on a tertiary care hospital. Among 364 patients diagnosed with HCC between April 2007 and December 2019, 267 patients with HCV or HBV infection with or without HIV were included. Demographic, clinical, laboratory and radiological data were obtained by reviewing medical records.
HCC diagnosis was based on EASL diagnostic criteria [9]. Patients were excluded from the study for the following reasons: incomplete tumor or patient data (n=1) and another liver disease etiology (n=97).
All included patients had HCC and HCV or HBV. The diagnosis of chronic hepatitis B was established by the presence of positive serology for HBV [HBsAg (+), antiHBC (+)] and positive HBV PCR (HBV DNA / Viral Load test) for more than 6 months. Chronic hepatitis C was defined by the presence of serum HCV antibody (anti-HCV) and HCV PCR (HCV RNA) positive for more than 6 months. HIV was diagnosed with HIV positive serology and then the presence of HIV RNA. The patients were divided into two groups HIV (+) and HIV (−).The patient accrual is summarized in the flowchart (Figure 1).
Figure 1:
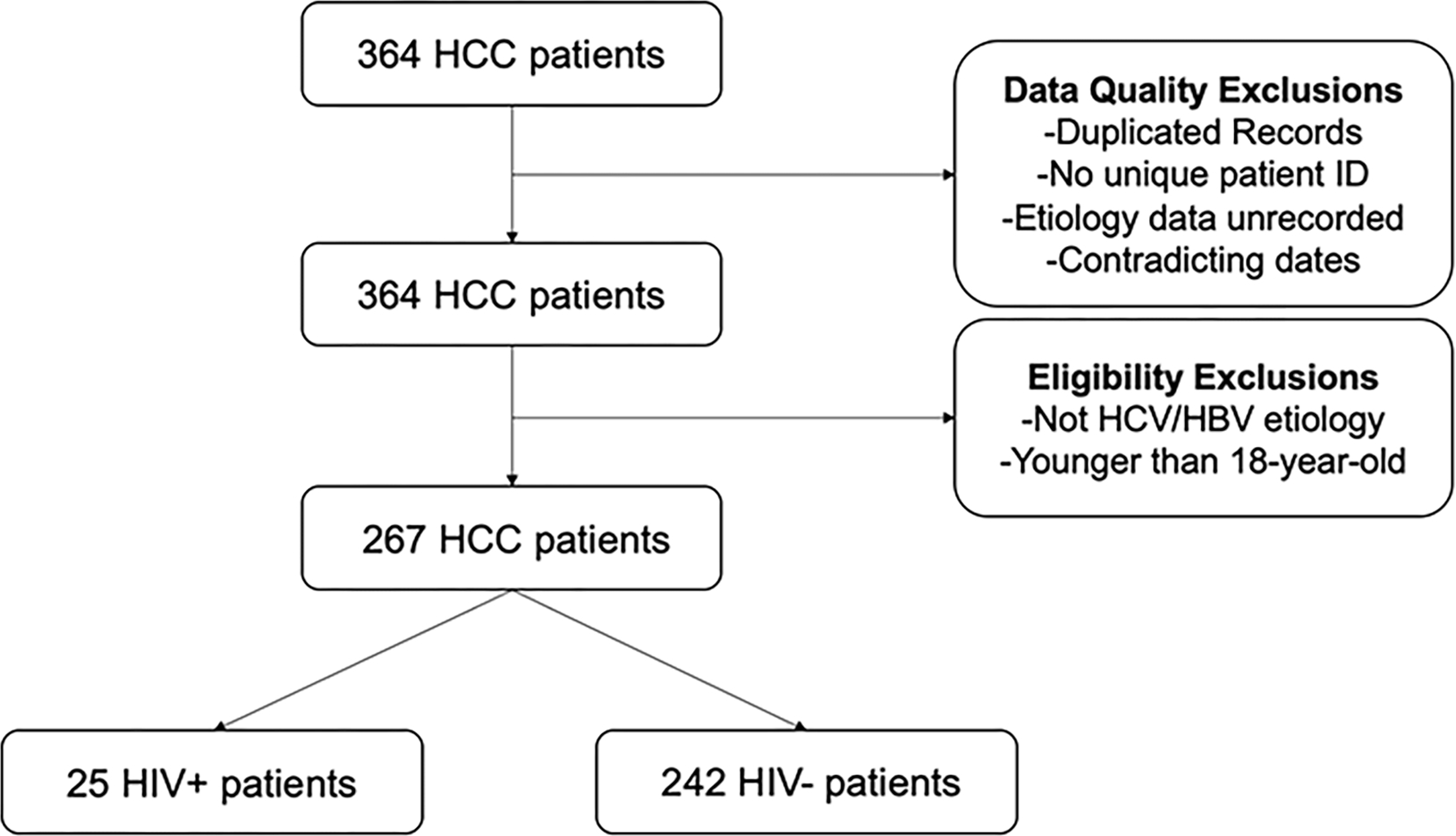
Patients selection flowchart
The study protocol is complying ethical standards from the revised Helsinki Declaration in 2008 and it was approved by our Institutional Review Board, which waived the requirement for informed consent.
Demographic, clinical and laboratorial data
A detailed medical record review of the included patients was made by a hepatologist with 5 years’ experience.
The following variables were evaluated: age at HCC diagnosis, gender, etiology of chronic liver disease, alcohol consumption, the presence of cirrhosis, Child-Pugh score, serum alpha-fetoprotein (AFP) levels and BCLC staging system at HCC diagnosis, HCC treatment, tumoral portal vein thrombosis, date of the last follow-up, date and cause of death.
Radiological data
Radiological reports of contrast-enhanced computed tomography or magnetic resonance imaging were retrospectively reviewed [9]. The radiological reports were done by gastrointestinal radiologists with at least 5 years’ experience.
The following variables were analyzed in imaging studies of HCC diagnosis: number and size of tumor nodules, presence of tumoral portal vein thrombosis and presence of extrahepatic metastases.
Outcomes and statistical analysis
Continuous variables were expressed as mean ± standard deviation or range, while qualitative variables were expressed as frequency (percentage). The primary endpoint was overall survival (OS). There were no significant follow-up losses, and patients without ascertained death date were regarded as alive and followed until the last clinical appointment.
Demographic characteristics and HCC related variables were present in correspondent tables. The comparison of these variables according to HIV status was done by logistic regression. For better understanding, the variables were categorized binarily, using clinically relevant cut-off points. Continuous variables were expressed as mean ± standard deviation and range, while qualitative variables were expressed as frequency (percentage).
For the assessment and comparison of mortality rates according to HIV status we assumed a Poisson distribution. A Kaplan-Meier curve was presented to assess survival function. Selection of variables composing the final models was based on predetermined conceptual frameworks well established on literature and on clinical relevance. For all statistical analyses, a p-value < 0.05 was considered statistically significant.
RESULTS
Baseline clinical and demographic characteristics
A total of 267 patients with HCC and HBV or HCV were included in the study, of which 25 (9.4%) were HIV positive. The median age was 60.1 years, predominant male (72%). The clinical and demographic characteristics of the general population in the trial are summarized in Table 1.
Table 1:
Clinical and demographic baseline characteristics of general population
| Variables | N = 267 |
|---|---|
|
| |
| HIV, n (%) | |
| Positive | 25 (9.4) |
| Negative | 242 (90.6) |
|
| |
| Age (years old), n (%) | |
| < 60 | 134 (51.6)) |
| >60 | 133 (49.4) |
|
| |
| Male Gender, n (%) | 194 (72) |
|
| |
| Cirrhosis, n(%) | 256 (96) |
|
| |
| Child-Pugh, n (%) | |
| A | 176 (65.9) |
| B | 81 (30.3) |
| C | 10 (3.7) |
|
| |
| Etiology, n (%) | 218 (81.6) 49 (18.3) |
| HCV | 218 (81.6) |
| HBV | 49 (18.3) |
|
| |
| Alcohol Consumption, n (%) | 92 (34.4) |
|
| |
| Symptoms at Diagnosis, n (%) | 79 (29.5) |
|
| |
| BCLC, n (%) | |
| A–0 | 109 (40.8) |
| B | 56 (20.9) |
| C | 83 (31.1) |
| D | 19 (7.1) |
|
| |
| Number of lesions, n (%) | |
| 1 | 152 (56.9) |
| 2 | 50 (18.7) |
| 3 | 33 (12.3) |
| +4 | 32 (12.1) |
|
| |
| Largest Lesion Size (cm), n (%) | |
| < 3 | 93 (34.8) |
| 3 – 5 | 83 (31.1) |
| >5 | 91 (34.1) |
|
| |
| AFP (ng/mL), n (%) | |
| <10 | 76 (28.4) |
| 10 – 500 | 121 (45.3) |
| >500 | 70 (26.2) |
|
| |
| Extravascular metastasis, n (%) | 21 (7.8) |
|
| |
| Tumoral Portal Vein Thrombosis, n (%) | 35 (13) |
|
| |
| Treatment, n (%) | 224 (83.9) |
Note: HIV: Human immunodeficiency virus; HCV: Hepatitis C virus; HBV: Hepatitis B virus; BCLC: Barcelona Clinic Liver Cancer; AFP: alpha- fetoprotein
Etiology was either HCV (81.6%) or HBV (18.3%), and 96% had cirrhosis, alcohol consumption was present in 33.4% of patients. Regarding tumor characteristics at diagnosis, 56.9% had a single lesion, with a mean size of the largest nodule of 56 mm (9 – 218 mm). 13% of patients presented tumoral portal vein thrombosis and 7.8% extravascular metastasis. In terms of tumor staging, 40.8% presented as early stage (BCLC 0-A), 20.9% intermediate stage (BCLC B) and 38.7% advanced or terminal stage (BCLC C-D).
The mean AFP level at diagnosis was 3,904 ng/mL (1.2 – 60,050 ng/mL) with 26.2% of patients presenting AFP level > 500 ng/ml. HCC treatment was performed in 84% of the patients and the most common treatment was transarterial chemoembolization (TACE).
In HIV patients, 90% were using antiretrovirals drugs, with undetectable viral load (<50 copies/ml) in 88%. The mean CD4+ lymphocyte count was 411 (76/mm3 to 701/mm3) and 68.75% of patients presented CD4 above 250/mm3.
Comparison of clinical and demographic characteristics according to HIV status
The clinical and demographic characteristics of patients based on HIV status are shown in Table 2. HIV patients were younger at HCC diagnosis compared to monoinfected patients, with a mean age at diagnosis of 49.8 years old (37 – 67.7 yo), versus 61.2 years old (28.6 – 87 yo), respectively (OR = 0.16, CI 0.05 – 0.5; P < 0.001). We also observed a trend towards a higher occurrence of HCC in males in co-infected patients, corresponding to 88% of cases, while in monoinfected males corresponded to 72% of patients. Although, this difference was not statistically significant (OR = 2.98, CI: 0.86 – 10.2; P = 0.08) (Table 3).
Table 2:
Clinical and demographic characteristics according to HIV Status
| Variables | HIV (–) (%) | HIV (+) (%) |
|---|---|---|
|
| ||
| HIV, n (%) | 242 (90.6%) | 25 (9.4%) |
|
| ||
| Age (years old), n (%) | ||
| <60 | 113 (46.7%) | 21 (84%) |
| >60 | 129 (53.3%) | 4 (16%) |
|
| ||
| Male Gender, n(%) | 172 (72%) | 22 (88%) |
|
| ||
| Cirrhosis, n (%) | 234 (96.7%) | 22 (88%) |
|
| ||
| Child Pugh, n(%) | ||
| A | 156 (64.4%) | 20 (80%) |
| B | 76 (31.4%) | 5 (20%) |
| C | 10 (4.1%) | 0 (0%) |
|
| ||
| Etiology, n (%) | ||
| HCV | 199 (82.2%) | 19 (76%) |
| HBV | 43 (17.7%) | 6 (24%) |
|
| ||
| Alcohol consumption, n(%) | 81 (33.4%) | 11 (44%) |
|
| ||
| Symptomatic at diagnosis, n(%) | 169 (69.8%) | 19 (76%) |
|
| ||
| BCLC, n(%) | ||
| A–0 | ||
| B | 52 (21.5%) | 4 (16%) |
| C | 74 (30.5%) | 9 (36%) |
| D | 18 (7.4%) | 1 (4%) |
|
| ||
| Number of lesions, n (%) | ||
| 1 | 135 (55%) | 17 (68%) |
| 2 | 46 (19%) | 4 (16%) |
| 3 | 30 (12%) | 3 (12%) |
| 4+ | 31 (13%) | 11 (4%) |
|
| ||
| Largest lesion size (cm), n(%) | ||
| < 3 | 83 (34%) | 10 (40%) |
| 3 – 5 | 70 (29%) | 7 (28%) |
| > 5 | 89 (36%) | 8 (32%) |
|
| ||
| AFP (ng/mL), n (%) | ||
| <10 | 69 (28%)) | 7 (28%) |
| 10 – 500 | 108 (45%) | 13 (52%) |
| >500 | 63 (26%) | 5 (20%) |
|
| ||
| Tumoral Portal Vein Thrombosis, n (%) | 27 (11%) | 8 (32%) |
|
| ||
| Extravascular metastasis, n (%) | 19 (7.8%) | 2 (8%) |
|
| ||
| Treatment, n (%) | ||
| TACE | 209 (86%) | 15 (60%) |
| PEI/RFA | 41 (17%) | 2 (8%) |
| Ressection/Transplant | 63 (26%) | 4 (16%) |
| Sorafenib | 52 (21%) | 2 (8%) |
Note: HIV: Human immunodeficiency virus; HCV: Hepatitis C virus; HBV: Hepatitis B virus; BCLC: Barcelona Clinic Liver Cancer; AFP: alpha- fetoprotein; TACE: Transarterial chemoembolization; PEI: Percutaneous ethanol injection; RFA: Radiofrequency ablation.
Table 3:
Comparison of baseline characteristics according to HIV status using logistic regression
| Variables | Percentage (%) | Baseline: HIV (−) | P value | |
|---|---|---|---|---|
| HIV (−) | HIV (+) | |||
| Age > 60 yo | 53.3% | 16.0% | OR = 0.16 (0.05 – 0,5) | P < 0.001 |
| Male sex | 72.0% | 88.0% | OR = 2.98 (0.86 – 10.2) | P = 0.08 |
| Cirrhosis | 96.7% | 88.0% | OR = 0.25 (0.06 – 1.0) | P = 0.05 |
| Alcohol consumption | 33.4% | 44.0% | OR = 1.56 (0.67 – 3.59) | P = 0.29 |
| Symptoms at diagnosis | 30.1% | 24.0% | OR = 0.73 (0.28 – 1.9) | P = 0.52 |
| Child B-C | 35.5% | 20.0% | OR = 0.45 (0.16 – 1.25) | P = 0.12 |
| BCLC C-D | 38.0% | 40.0% | OR = 1.08 (0.46 – 2.52) | P = 0.84 |
| ≥ 4 Lesions | 12.8% | 4.0% | OR = 0.28 (0.37 – 2.17) | P = 0.22 |
| Largest lesion ≥ 5 cm | 36.7% | 32.0% | OR = 0.8 (0.33 – 1.95) | P = 0.63 |
| AFP > 500 ng/mL | 26.8% | 20.0% | OR = 0.68 (0.24 – 1.88) | P = 0.46 |
| Extravascular metastasis | 7.8% | 8.0% | OR = 1.02 (0.22 – 4.66) | P = 0.97 |
| Tumoral portal vein thrombosis | 11% | 32.0% | OR = 3.74 (1.47 – 9.50) | P = 0.003 |
| Treatment | 86.3% | 60% | OR = 0.23 (0.09 – 0.57) | P = 0.001 |
Note: HIV: Human immunodeficiency virus; BCLC: Barcelona Clinic Liver Cancer; AFP: alpha- fetoprotein.
Cirrhosis was present in 88% of HIV positive and 96.7% of HIV negative patients (OR = 0.25, CI: 0.06 – 1.0; P = 0.05), demonstrating a tendency towards a higher occurrence of HCC in co-infected patients at earlier stages of liver disease. Similar to monoinfected patients, most HIV positive patients had preserved liver function, with CHILD A in 80% of cases. Alcohol use was present in 33,4% of those HIV negative and 44% of HIV positive patients [OR = 1.56, CI 0.67 – 3.59; P = 0.29)], with no statistical difference between both group.
Regarding tumor staging, there was also no difference related to HIV status, with the majority of patients being diagnosed at early stage. In HIV positive patients, 44% were BCLC 0-A, 16% BCLC B, 40% BCLC C/D. On the other hand, HIV patients had a higher frequency of tumoral portal vein thrombosis when compared to HIV negative patients [32% vs 11% (OR =3.74, CI 1.47 – 9.5; P = 0.003)] and were less likely to undergo treatment [86% vs 60% (OR = 0.23, CI 0.09 – 0.57; P = 0.001)]. The most common treatment in both groups was TACE, which accounted for 46% of monoinfected and 24% of co-infected patients (Tables 2 and 3).
Survival analysis and prognostic factors related to survival
The average mortality risk was 76% over the whole period. To avoid potential confounding factors, the sample was analyzed by to two conceptual models adjusted for several variables. After accounting for the potentially confound variables, the mortality risk was higher on HIV positive patients on both conceptual models assessed. In model 1, the adjusted variables were age, sex, BCLC, symptoms, Child-Pugh, treatment and AFP (RR = 1.95, CI 1.11 – 3.41; P = 0.01). In model 2 the variables were age, sex, BCLC, symptoms, Child-Pugh, AFP, number of lesions, intravascular metastasis, extravascular metastasis, size of the bigger lesion and treatment (RR = 1.96, CI 1.12 – 3.44; P = 0.01) (Table 4 and Figure 2).
Table 4:
Mortality rate comparison according to HIV status
| HIV status╲Mortality | Frequency (%) | Crude Rate Ratio (95% CI) | Minimally adjusted* Rate Ratio (95% CI) | Model 1** | Model 2*** |
|---|---|---|---|---|---|
| HIV (–) (Baseline) | 180 (74.3%) | RR = 1.36 (0.81 – 2.27) P = 0.23 | RR = 1.61 (0.93 – 2.77) P = 0.08 | RR = 1.95 (1.11 – 3.41) P = 0.01 | RR = 1.96 (1.12 – 3.44) P = 0.01 |
| HIV (+) | 23 (92.0%) |
Note:
Minimally adjusted: Age and sex
Model 1: Adjusted for age, sex, BCLC, symptoms, Child-Pugh, treatment and AFP
Model 2: Adjusted for age, sex, BCLC, symptoms, Child-Pugh, AFP, number of lesions, intravascular metastasis, extravascular metastasis, size of the bigger lesion and treatment.
Figure 2:
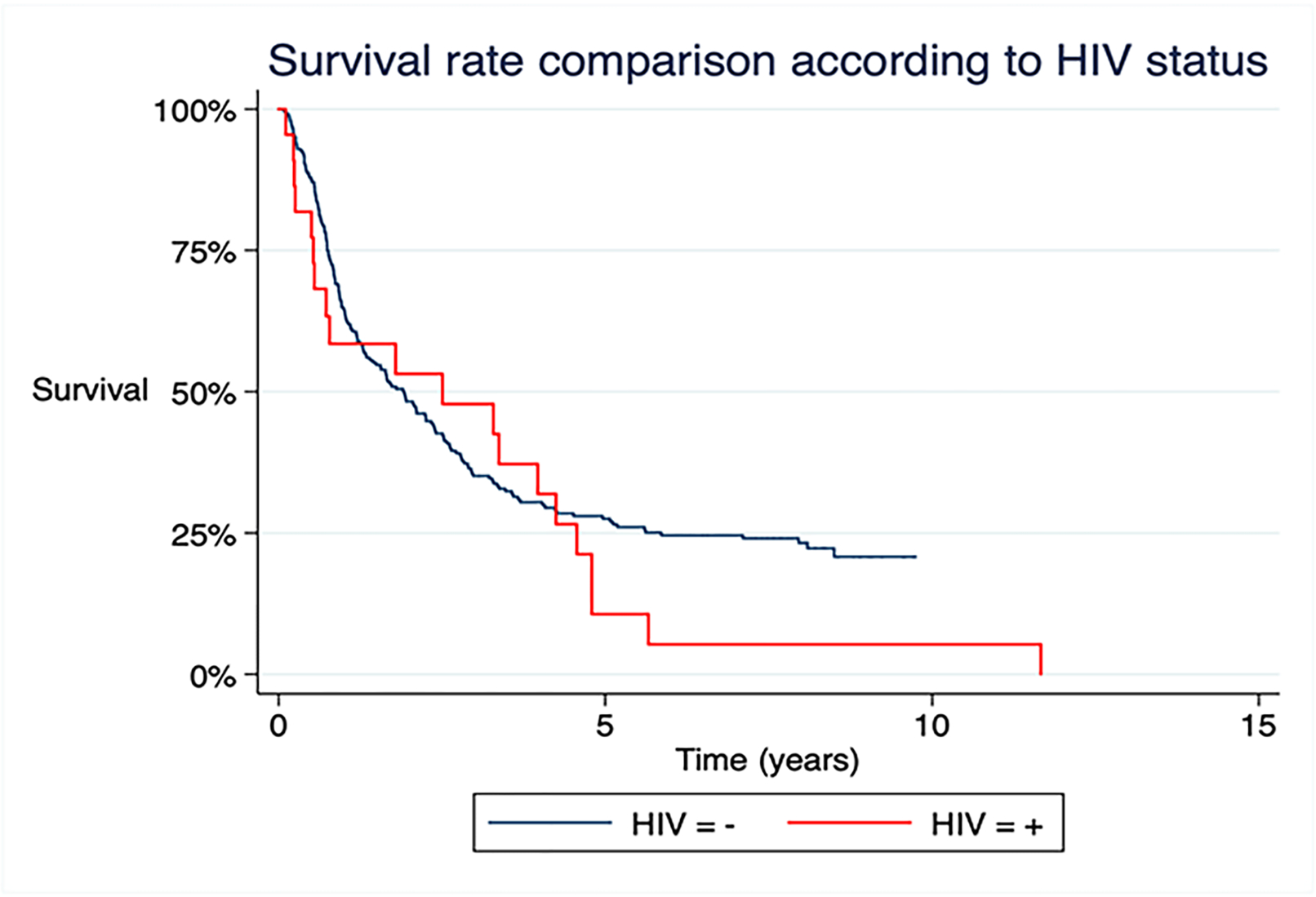
Survival curve in patients according to HIV status
DISCUSSION
HIV is an important public health issue that until the end of 2018 counted with 37.9 million people living with the disease in the world [10]. The introduction of HAART in the 1990s changed the natural history of HIV infection, therefore increasing the number of non-HIV-related deaths [11]. In a multicenter observational study that included prospectively 23,441 HIV positive patients, 50% of deaths were not related to HIV [8]. In co-infected patients, liver disease is the second cause of death, with 66% attributed to HCV, 17% to HBV and 3% directly related to antiviral therapy [8,12,13].
HIV/HCV co-infection is associated with a lower sustained virologic response, with faster progression to fibrosis and cirrhosis, and higher probability of HCC. This outcome is due to multifactorial events, among them a weakened adaptive immune response to HCV infection, activation of hepatic stellate cells by liver damage and production of type I collagen, promoting more pro-inflammatory and profibrogenic cytokines, Also, the reduced ratio of CD4+ to CD8+ cells associated with HIV infection are more fibrogenic [14,15,16].
The interaction between HIV and HBV reduces the ability to eliminate HBV infection after exposure, resulting in increased HBV DNA concentrations, which leads to faster fibrosis and HCC development. A low CD4 count is also associated with a faster progression of fibrosis. Likewise, occult HBV infection, increased ALT and development of AIDS may occur with low CD4 count [17,18]. In our analysis, 90% of co-infected patients were using HAART with adequate viral control in the majority of patients (88% presenting undetectable viral load and 68.75% CD4 above 250/mm3). These results lead to the hypothesis that other mechanisms besides immunodeficiency may also be involved in the higher risk of HCC associated with HIV infection.
In the present study, we demonstrated that co-infected patients had a different epidemiological profile than monoinfected, with HCC at younger ages, suggesting a faster progression of liver disease and HCC emergence in this population. We also observed a trend towards increased frequency in male gender when compared to monoinfected patients. These results are in agreement with other studies. In the study performed by Berretta et al. HIV positive patients were 20 years younger than HIV negative patients [19] and Marcon et al. demonstrated that co-infected patients were 10 years younger than monoinfected [20]. Both studies presented a prevalence of male gender [19, 20].
In our experience, HCV was the most frequent etiology of liver disease in HIV patients and the majority presented compensated cirrhosis with Child Pugh A. In a study published in 2013 that sought to determine risk factors for cirrhosis and HCC among HIV patients from the Veterans Affairs Health Care System, the increase in HCC prevalence, from 0.07% to 1.62%, in fifteen years, was the most relevant finding, being higher in patients infected by HCV and with compensated cirrhosis [21]. An Italian and Spanish analysis made by Puoti et al. with 41 HIV positive patients versus 385 HIV negative patients, showed that the patients who developed HCC had underlying chronic hepatitis C more frequently [22].
Most of the HIV co-infected patients in the current study presented an early or intermediate HCC staging at diagnosis, which was not different from the monoinfected patients. These data are similar to those observed by Berretta et al. who reported that 69% of patients were BCLC A or B [19]. In contrast Brau et al. found that in their HIV co-infected population tumors were in a more advanced stage, with 50% classified as BCLC C or D [23].
Despite the earlier stages of both cirrhosis and HCC, co-infected patients had a higher mortality rate (92% vs 74.3%), independently of clinical and tumoral variables. The presence of HIV was an independent risk factor for a worse prognosis, here tumoral portal vein thrombosis was more frequent among HIV-infected patients with HCC and this population was less submitted to treatment. The probable reason is a more aggressive tumor biology in the presence of HIV. This finding is corroborated by other studies that also showed poorer survival in this group of patients. In a single center, Gramenzi et al. reported that in HCC patients, HIV co-infection doubled the risk of death compared to the non-HIV population [24]. A Spanish study published in 2012, which analyzed HCC co-infected patients, a higher mortality rate was observed in the co-infected group, with an average survival of 3 months and a more advanced BCLC stage at diagnosis [25].
The more aggressive nature of HCC in HIV-positive patients seems to be related to HIV features, through the presence of growth signals enhancing HCC cell proliferation and/or a weaker immune response [22]. In other cohort study which purpose was to measure determinants of HCC in HIV, Torgersen et al. showed that a higher viral load and longer duration of HIV viremia had contributed to HCC development. This research suggests that the possible mechanisms associated with this greater viral severity and HCC burden are the progression of liver fibrosis to cirrhosis, hepatocarcinogenesis via immune dysregulation, oxidative stress, hepatocyte apoptosis and CD4+ cell depletion in the gastrointestinal tract with consequent microbial translocation [14,26].
This study has some limitations such as the retrospective model and small population sample. On the other hand, the number of studies in the environment are scare and it is important to demonstrate that co-infected patients presents worse prognosis, which probably is associated with a more aggressive tumoral behavior.
In conclusion, the present study demonstrated that HCC patients co-infected with HBV/HCV and HIV presented younger age, higher mortality, less underlying cirrhosis and higher frequency of tumoral thrombosis. Further studies are warranted for better understanding HIV role in hepatocarcinogenesis, in order to improve the clinical management of these patients, particularly regarding screening program.
Acknowledgments
FINANCIAL SUPPORT: The authors have no support or funding to report
ABBREVIATIONS
- HCC
Hepatocellular carcinoma
- HCV
Viral hepatitis C
- HBV
Viral hepatitis B
- NASH
Nonalcoholic steatohepatitis
- HIV
Human immunodeficiency vírus
- HAART
Highly active antirretroviral
- EASL
European Association for the Study of the Liver
- IRB
Institutional Review Board
- PCR
Polymerase chain reaction
- HBV DNA
Viral Load test
- HIV RNA
Viral Load test
- AFP
Alpha-fetoprotein
- BCLC
Barcelona Clinic Liver Cancer
- OS
Overall survival
- TACE
Transarterial chemoembolization
- OR
Odds Ratio
- AIDS
Acquired Immunodeficiency Syndrome
- PEI
Percutaneous ethanol injection
- RFA
Radiofrequency ablation
Footnotes
CONFLICT OF INTEREST: Nothing to disclose
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–14. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–36. [DOI] [PubMed] [Google Scholar]
- 4.Joshi D, O’Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet [Internet]. 2011;377(9772):1198–209. Available from: 10.1016/S0140-6736(10)62001-6 [DOI] [PubMed] [Google Scholar]
- 5.Pinato DJ, Pria AD, Sharma R, Bower M. Hepatocellular carcinoma: An evolving challenge in viral hepatitis and HIV coinfection. Aids. 2017;31(5):603–11. [DOI] [PubMed] [Google Scholar]
- 6.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. Hepatology. 1999;30(4):1054–8. [DOI] [PubMed] [Google Scholar]
- 7.Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol. 2008;48(2):353–67. [DOI] [PubMed] [Google Scholar]
- 8.D:A:D Study Group. Liver-Related Deaths in Persons Infected With the Human Immunodeficiency Virus. 2013;166:1632–41. [DOI] [PubMed] [Google Scholar]
- 9.Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol [Internet]. 2018;69(1):182–236. Available from: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 10.UNAIDS. Global HIV and AIDS statistics 2019 Fact sheet. Glob HIV AIDs ststistics, World AIDS day 2019 Fact Sheet. 2019;1(June):1–6. [Google Scholar]
- 11.Article M Causes of Death in HIV-1–Infected Patients Treated with Antiretroviral Therapy, 1996–2006: Collaborative Analysis of 13 HIV Cohort Studies. Clin Infect Dis. 2010;50(10):1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massard J, Ratziu V, Thabut D, Moussalli J, Lebray P, Benhamou Y, et al. Natural history and predictors of disease severity in chronic hepatitis C. J Hepatol. 2006;44(SUPPL. 1):42–7. [DOI] [PubMed] [Google Scholar]
- 13.Puoti M, Torti C, Bruno R, Filice G, Carosi G. Natural history of chronic hepatitis B in co-infected patients. J Hepatol. 2006;44(SUPPL. 1). [DOI] [PubMed] [Google Scholar]
- 14.Torgersen Jessie, Kallan Michael J., Carbonari Dena M., Lesley S Park RLM. HIV RNA, CD4+ Percentage, and Risk of Hepatocellular Carcinoma by Cirrhosis Status. J Chem Inf Model. 2019;53(9):1689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonnard P, Lescure FX, Amiel C, Guiard-Schmid JB, Callard P, Gharakhanian S, et al. Documented rapid course of hepatic fibrosis between two biopsies in patients coinfected by HIV and HCV despite high CD4 cell count. J Viral Hepat. 2007;14(11):806–11. [DOI] [PubMed] [Google Scholar]
- 16.Deng LP, Gui XE, Zhang YX, Gao SC, Yang RR. Impact of human immunodeficiency virus infection on the course of hepatitis C virus infection: A meta-analysis. World J Gastroenterol. 2009;15(8):996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konopnicki D, Mocroft A, De Wit S, Antunes F, Ledergerber B, Katlama C, et al. Hepatitis B and HIV: Prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. Aids. 2005;19(6):593–601. [DOI] [PubMed] [Google Scholar]
- 18.Sarmati L, Malagnino V. HBV infection in HIV-driven immune suppression. Viruses. 2019;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berretta M, Garlassi E, Cacopardo B, Cappellani A, Guaraldi G, Cocchi S, et al. Hepatocellular Carcinoma in HIV-Infected Patients: Check Early, Treat Hard. Oncologist. 2011;16(9):1258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dos Santos Marcon P, Tovo CV, Kliemann DA, Fisch P, Alves De Mattos A. Incidence of hepatocellular carcinoma in patients with chronic liver disease due to hepatitis B or C and coinfected with the human immunodeficiency virus: A retrospective cohort study. World J Gastroenterol. 2018;24(5):613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology. 2013;57(1):249–57. [DOI] [PubMed] [Google Scholar]
- 22.Puoti M, Bruno R, Soriano V, Donato F, Gaeta GB, Quinzan GP, et al. Hepatocellular carcinoma in HIV-infected patients: Epidemiological features, clinical presentation and outcome. Aids. 2004;18(17):2285–93. [DOI] [PubMed] [Google Scholar]
- 23.Bräu N, Fox RK, Xiao P, Marks K, Naqvi Z, Taylor LE, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: A U.S.-Canadian multicenter study. J Hepatol. 2007;47(4):527–37. [DOI] [PubMed] [Google Scholar]
- 24.Merchante N, Merino E, López-Aldeguer J, Jover F, Delgado-Fernández M, Galindo MJ, et al. Increasing incidence of hepatocellular carcinoma in HIV-infected patients in Spain. Clin Infect Dis. 2013;56(1):143–50. [DOI] [PubMed] [Google Scholar]
- 25.Gramenzi A, Tedeschi S, Cantarini MC, Erroi V, Tumietto F, Luciano Attard, et al. Outcome of hepatocellular carcinoma in human immunodeficiency virus-infected patients. Digestive and Liver disease. 2013; 45(6): 516–522. [DOI] [PubMed] [Google Scholar]
- 26.Sherman KE, Peters MG, Thomas D. Human immunodeficiency virus and liver disease: A comprehensive update. Hepatol Commun. 2017;1(10):987–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]


