Abstract
The interrelationship between matrix degradation, oxidative stress, inflammation and trace elements can be speculated in COVID-19. The objective of the study was to evaluate the oxidative stress, inflammation and matrix degradation markers and trace elements in COVID-19 positive patients. A group of confirmed severe COVID-19 positive patients (n = 30) along with COVID-19 negative patients (n = 30) with similar symptoms were included. Both group of patients were assessed for oxidative stress markers, inflammatory cytokines, matrix metalloproteinase (MMP)s and their inhibitors along with trace elements in blood. All the data were subjected to univariate as well as multivariate analysis including PCA, PLS-DA, OPLS-DA. Diagnostic accuracy was tested by ROC curve analysis. Further relationship with Neutrophil/ lymphocyte (N/L) ratio was established if any. Increased oxidative stress, inflammation and matrix degradation is evidenced by significant rise in oxidative markers, inflammatory cytokines and MMP9/TIMP-1 ratio. Decreased Cu/Zn ratio is also observed in COVID-19 positive patients. Multivariate analysis identified SOD, Cu/Zn ratio, IL-6 and TOS, as effective discriminant among the two groups of patients. Further, accuracy was confirmed by ROC curves. Neutrophil/ lymphocyte (N/L) ratio, shows significant negative association with SOD (r= -0.75, p < 0.005) and Cu/Zn ratio (r = -0.88, p < 0.005). These data suggest the attributes of these biomarkers in disease severity. The potential use of these blood-based laboratory markers in disease prognosis seems promising and warrants further attention. Given by the symptoms and severity of the disease, it will be promising to monitor Cu/Zn ratio along with other prognostic indicators.
Keywords: SARS-CoV-2, Matrix Metalloproteinase 9, Interleukin-6, Trace Elements, Oxidative stress
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, previously 2019-nCoV), first time detected in China, is severely contagious. The pathophysiology of COVID-19 is less studied [1, 2]. The virus invades type 2 alveolar epithelial cells, releases several cytokines and inflammatory markers. This ‘cytokine storm’ along with neutrophils, CD4 helper T cells and CD8 cytotoxic T cells cause lung inflammation [3].
Decreased bronchial wall elasticity of the bronchial wall associated with extracellular matrix (ECM) degradation, is reported earlier in COVID-19 [4]. Matrix metalloproteinases (MMP), the major ECM degrading enzymes, help in normal remodeling and repair processes of the lungs. The regulation of MMPs activity is mainly in balance with several tissue inhibitors of matrix metalloproteinases (TIMP). These proteases are over-expressed and cause lung tissue damage in several respiratory diseases. Particularly the role MMP-2, 9 and TIMPs in chronic obstructive lung disease is discussed earlier by our group [5, 6]. Similarly, there is only one study reported so far to cite MMP-9 as an early indicator of respiratory failure in COVID-19 patients [7]. Airway modeling usually involves increased MMPs action induced by pro-inflammatory cytokines. Reactive oxygen species (ROS) is shown to activate MMPs and inactivate TIMPs [5]. Cytokines such as IL-1, IL-6, and TNF-α are shown to upregulate MMPs, which can affect inflammation [8].
Zn cations are shown to inhibit SARS-coronavirus RNA polymerase activity by decreasing its replication. Higher mortality among COVID-19 patients is reported to be associated with low Zn level [9]. Similarly, role of copper (Cu) in COVID-19 is published elsewhere [10]. The balance between Zn and Cu is very vital as Zn neutralizes the inflammatory effect of Cu. Magnesium (Mg), another mineral having anti-inflammation, anti-oxidant property, is reported to have therapeutic beneficial effect in other lung diseases including COVID-19 [11, 12]. Neutrophil lymphocyte (N/L) ratio, a potential prognostic biomarker in COVID-19. might aid early detection of severe COVID-19 cases, thereby reducing the overall mortality [13, 14].
Although the interrelationship between matrix degradation, oxidative stress, inflammation and trace elements can be speculated in COVID-19, not much data is available till date. MMP-9/TIMP-1 ratio, a marker for airway severity is reported earlier to be associated with total oxidative stress in chronic obstructive pulmonary disease (COPD) [5], hence it would be tempting to see its role in COVID-19.
Multivariate statistical method effectively identifies the interactions between the potential biomarkers and ensures diagnostic and prognostic performances in terms of specificity, sensitivity and reliability. Principal Component Analysis (PCA), Partial Least-Squares Discriminant Analysis (PLS-DA) and Variable Importance in Projection (VIP) score is used to discriminate inflammatory biomarkers in several diseases [5, 15].
The present study is aimed to observe the changes in oxidative stress, matrix degradation, inflammation, trace elements in COVID-19 confirmed patients. The study also highlights the most useful potential marker (s) to discriminate the COVID-19 positive patients from COVID-19 negative cases using multivariate analysis followed by receiver operator characteristic (ROC) curve analysis. Further association with N/L ratio is also established, if any.
Materials and methods
Patient selection
The study screened all male patients (Jun 2020 to Sep 2020), reported at cough clinic of a tertiary care institute in India. Out of them 103 patients presented with symptoms of fever, cough, fatigue, headache or suspected to be infected with COVID-19, were randomly selected. The laboratory confirmation was done by real-time reverse transcriptase-polymerase chain reaction based positive SARS-CoV-2 RNA with throat swab samples (Genes2Me Pvt Ltd, India). Apart from patient history with fever or any respiratory illness, CT image finding for viral pneumonia, RT-PCR positivity for SARS-CoV-2 RNA, any one of the conditions such as shortness of breath, RR ≥ 30 times/min, (2) Oxygen saturation (Resting state) ≤ 93%, (3) PaO2/FiO2 ≤ 300 mmHg were the characteristics for severe patients. The details are given in flow-chart (Fig. 1). The institutional ethical permission was taken prior to the study and informed consent was taken from the patients.
Fig. 1.
Flow chart for subject selection and study design
Sample collection and preparation
Early morning overnight fasting venous EDTA and serum sample was collected. Complete blood cell count blood cell count was performed using an electronic cell counter (XT 2000i; Sysmex, Kobe, Japan). The plasma was used for measuring oxidative markers, cytokines and MMPs. Likewise, the serum was used to measure Cu, Zn and Mg.
Biochemical assay of plasma oxidative stress parameters
Malondialdehyde (MDA) in plasma was measured as per method suggested by Buege et al. (1978) [16]. Total oxidative stress (TOS) levels in plasma were based on the principle of ferrous ion oxidation to ferric ion [17]. Nitrate and nitrite (NOx) in plasma was measured by Griess reaction in which a deep purple azo compound was formed by Griess Reagent converting nitrate to nitrite by nitrate reductase. Similarly, plasma peroxynitrite was measured as described by VanUffelen and colleagues [18]. Uric acid under the enzymatic action of uricase is converted to allantoin and measured at 293 and 700 nm. Total bilirubin in plasma was estimated using Doumas reference method [19] (Siemens Healthcare, India). Glutathione S-transferases (GST) activity in plasma was assayed at 340 nm using substrates such as (1-chloro-2, 4-dinitrobenzene) and reduced glutathione (GSH), as described earlier [20, 21]. The activity of glutathione peroxidase (GSH-Px) was analyzed by Buege et al. (1978) and expressed in µmol/min/ml [16]. Plasma Superoxide dismutase (SOD) was measured by enzyme linked immunosorbent assay (ELISA) (Thermofischer, USA) (Thermofisher Scientific, USA), as per the instructions by manufacturer. The analytical sensitivity of the assay is 0.044 U/ml. The intra- and inter assay sensitivities CV were < 10%.
Assessment of inflammatory cytokines
Quantification of tumour necrosis factor (TNF)-α, IL-6 and IL-10 in plasma samples was done by ELISA (Thermofisher Scientific, USA), as per the instructions by manufacturer. The assay sensitivities were as follows: IL-1 β, 0.09 pg/ml; IL-6, 0.07 pg/ml; TNF-α, <3pg/ml; IL-10, 0.09 pg/ml.
Analysis of the MMP-9, MMP-2, MMP-12, TIMP-1, TIMP-2 expression levels
ELISA commercial kits (Quantikine ELISA Kit; R&D Systems, USA) were used to measure MMP- 2,9,12 and TIMP-1&2 levels in plasma. The assay sensitivities for MMP-9, MMP-2, MMP-12, TIMP-1 and TIMP-2 were 0.15 ng/ml, 0.28 ng/ml, < 10 pg/ ml, < 0.08 ng/ml and 0.10 ng/ml respectively.
Assessment of trace elements (Cu, Zn) and mg
Serum copper and zinc was estimated by kit method (Randox, UK). Zinc forms a chelation with 5-Br-PAPS 2-(5-bromo-2-pyridylazo)-5-(N-propyl-N-sulfopropylamino)-phenol and copper reacts with a specific color reagent, 3.5-Di-Br-PAESA 4-(3,5-Dibromo-2-pyridylazo)-N-Ethyl-N-(3-sulphopropyl) aniline, to form a stable, colored chelate. Mg was estimated by the end point calorimetric method using xylidyl blue.
Statistical analysis
Univariate analysis:
The results were presented as a mean ± standard deviation (SD). To analyze the statistical differences between patients and control, Mann-Whitney U test was carried out using GraphPad Prism (v.6.04 GraphPad software). Spearman’s Rank Correlation was used for correlation analysis. Statistical significance was achieved when the p value was less than 0.05.
Multivariate analysis.
In order to reduce possible concentration differences between samples, normalization (by sum) and scaling by mean-centering was done. All the data sets, were subjected to multivariate statistics such as unsupervised classification modeling by PCA along with PLS-DA and orthogonal projection to latent structure with discriminant analysis (OPLS-DA), using metaboanalyst [22]. Separation of classes, pattern observation and outliers were identified from the PCA score plots. Further class separation was maximized using supervised classification models including PLS-DA and OPLS-DA. The goodness of fit and predictive ability in the PLS-DA and OPLS-DA models is quantitatively measured by the parameter R2, and Q2 respectively. Both the values usually vary within 0 and 1; with a perfect fit (R2) and (Q2) corresponding to 1 indicates 100% goodness of fit and predictive ability. The VIP score of a variable is calculated as a weighted sum of the squared correlations between the PLS-DA components and the original variable. A cut-off of VIP score > 1.5 was considered as the threshold for selection of the most significant variables that best correlated with the discriminative scores of PLS-DA. The cross-validation of PLS-DA model was done by permutation of the best two components and compared with 100 different permutated models. The accuracy of diagnostic model was also used to evaluate ROC curve. The sensitivity and specificity and AUC of the selected parameters were determined using this curve.
Results
The baseline characteristics of the patients and healthy volunteers are summarized in Table 1. No significant change was noticed for age and BMI of the patients. Whereas, absolute neutrophil count (ANC), absolute leukocyte count (ALC), N/L ratio, platelet count, international normalized ratio (INR) was found to be significantly changed (p < 0.005) in COVID-19 patients.
Table 1.
Baseline characteristics of the patients
| Parameters | COVID-19 positive patients (n = 30) |
COVID-19 negative patients (n = 30) |
p value |
|---|---|---|---|
| Age (yrs) ± SD | 63 ± 8.3 | 61.3 ± 9.5 | NS |
| Gender | Male | Male | Not applicable |
| Body mass index (BMI) kg/m2 | 21.43 ± 1.62 | 21.35 ± 1.64 | NS |
| Comorbidities |
None − 11 (36%) Hypertension 8 (26%) Diabetes 9 (30%) Osteoarthritis 2 (6%) |
None − 16 (53%) Hypertension 7 (23%) Diabetes 7 (23%) |
- |
| Total leucocyte count (TLC) 1 × 103/µL, median (IQR) | 10,765 (5780) | 14,312 (3700) | p < 0.005 |
| Haemoglobin (Hb) (g/dl), median (IQR) | 12.07 (2.5) | 9.85 (1.12) | p < 0.005 |
| Absolute neutrophil count (ANC), median (IQR) | 7280 (4130) | 4320 (3125) | p < 0.005 |
| Absolute leukocyte count (ALC), median (IQR) | 1070 (630) | 990 (540) | p < 0.005 |
| Neutrophil/lymphocyte (N/L) ratio, median (IQR) | 7.1 (6.2) | 6.8 (4.3) | p < 0.005 |
| Platelet count 1 × 103/µL) median (IQR) | 159.5(128) | 210 (94) | p < 0.005 |
| INR, median (IQR) | 1.11 (0.27) | 0.87 (0.12) | p < 0.005 |
Mann-Whitney U test is used to test the significance; p < 0.05 (significant); NS- not significant
Oxidative stress markers
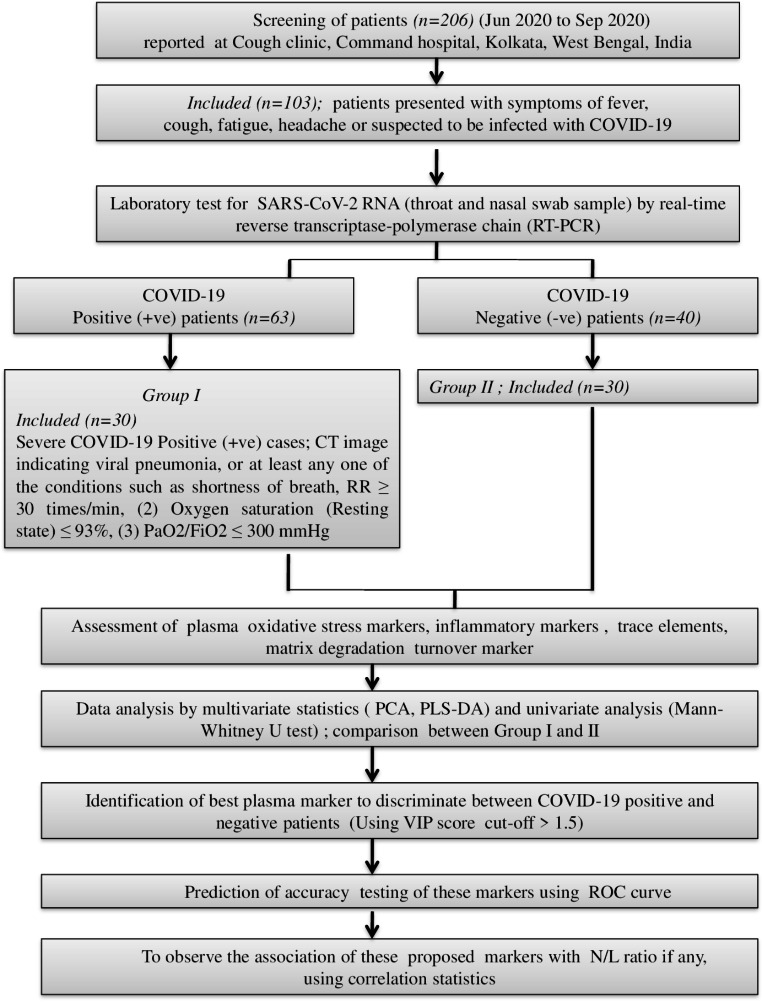
Figure 2 illustrates the plasma oxidative stress parameters for both groups of patients. A significant increase in oxidative parameters such as TOS (p < 0.0001), NOx (p < 0.05), MDA (p < 0.0001) and peroxynitrate (p < 0.005) was seen in COVID-19 positive patients. A significant decrease in antioxidant defence markers including GSH-PX (p < 0.05), GSH (p < 0.05), GST (p < 0.05), is also observed. No significant change for plasma uric acid and bilirubin is observed in these patients.
Fig. 2.
Bar plot comparing oxidative, inflammatory, matrix degradative markers and minerals between COVID-19 positive and negative patients. Mann-Whitney U test is used to test the significance. A p value < 0.05 is considered to be statistically significant
Plasma cytokines
The plasma cytokine levels in both groups of patients are illustrated in Fig. 2. A significant increase in IL-6, TNF-α levels was observed in COVID-19 positive patients (TNF-α; p < 0.05, IL-6; p < 0.0001). No statistical change was observed for plasma IL-10 level.
Plasma MMP-9, 2, 12, TIMP-1 and 2
The levels of MMP-9/TIMP-1, TIMP-2, MMP-2 and MMP-12 in both the groups are shown in Fig. 2. The MMP-9/TIMP-1 ratio, MMP-2 and MMP-12 levels are increased significantly in COVID-19 positive patients as compared to COVID-19 negative patients. No change is observed for TIMP-2 levels.
Multivariate statistical analysis
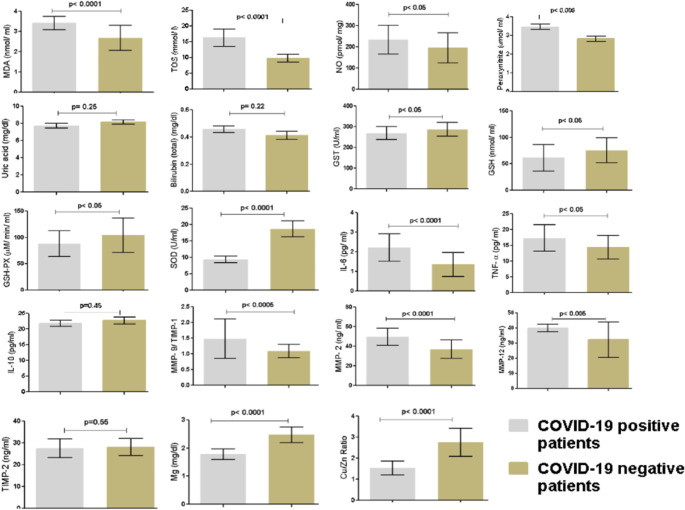
PCA obtained from both group of patients indicate unbiased clustering (Fig. 3a). A clear separation is seen between the two groups of patients by PCA scores scatter plot. Even the PLS-DA (Fig. 3b) signify enhanced class separation.
Fig. 3.
Multivariate analysis; Score scatter plot (a) principal component analysis (PCA) shows discrimination among (a) COVID-19 positive and negative patients (b) Partial least square (PLS) - discriminant analysis (DA) model shows improved discrimination among both group of patients with R2 = 0.91 and Q2 = 0.89 (c) Permutation test statistics for the PLS-DA models indicate that the true model has much higher R2 and Q2 values, and thus is far better than 100 permutated models
The validation of predictive capability of PLS-DA was achieved using permutation tests (Fig. 3c). The permutation tests indicate higher R2 and Q2 values of true model hence far better than permutated models. R2 (goodness of fit) and Q2 (predictive ability) were used to assess the robustness of the PLSDA model. The values of R2 (0.91) and Q2 (0.89) for PLS-DA and OPLS-DA (R2 = 0.89; Q2 = O.87) indicate that the model has good fit and can satisfactorily differentiate the COVID-19 positive from the negative patients. The effective key parameters found to discriminate the two groups of patients, were identified using their VIP values. Variables with VIP scores > 1.5 were considered to be significant for class separation (Fig. 4). With a VIP score cut-off value of 1.5, a total of four parameters such SOD, Cu/Zn ratio, IL-6 and TOS were found to be dysregulated out of which SOD, Cu/Zn ratio were significantly down-regulated along with upregulated IL-6 and TOS levels in COVID-19 patients (Fig. 4).
Fig. 4.
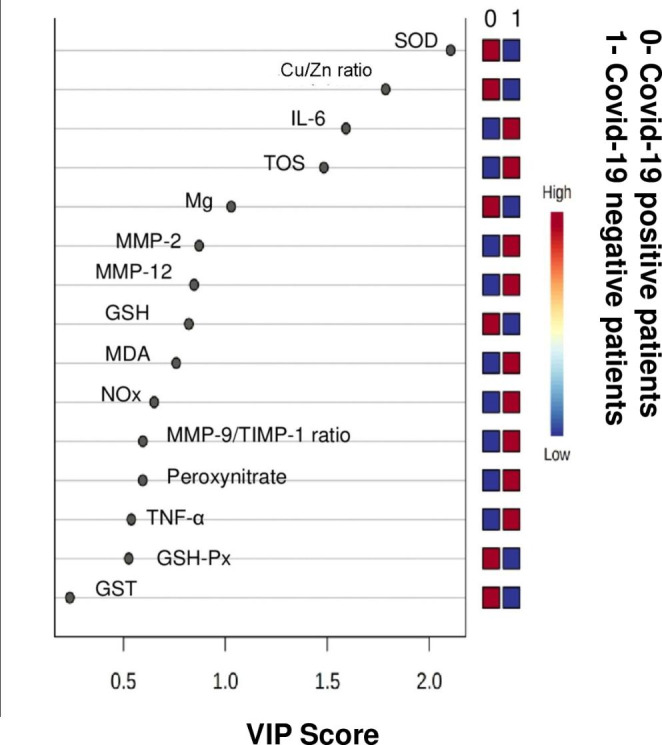
Significantly dysregulated metabolites with variance importance in projection (VIP) scores > 1.5
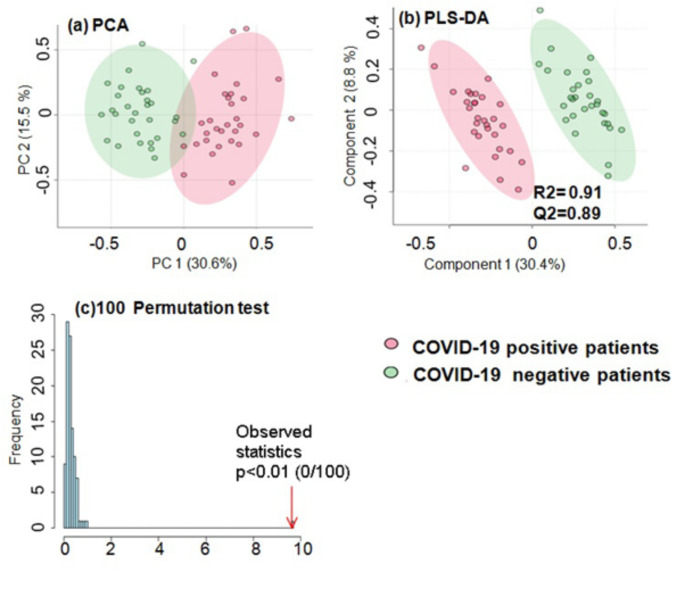
Further, the generated ROC curves for these altered markers indicate these parameters have the highest accuracy in predicting COVID-19 affected patients (Fig. 5). The diagnostic potential of SOD was found to have 90% sensitivity (true positive) and 86.7% specificity (true negative) with criterion < 10.9. Cu/Zn ratio and TOS were having 93.3% and 96.7% of sensitivity with criterion < 1.96 and > 11.12 respectively along with 86.7% specificity each. Similarly, IL-6 was having 83.3% specificity and 76.7% sensitivity with criterion value of > 1.87 (Fig. 5).
Fig. 5.
ROC analysis shows area under the curve for SOD, Cu/Zn ratio, TOS, IL-6 to be 0.873, 0.954, 0.974 and 0.838 respectively
Further correlation with N/L ratio, indicates significant association with SOD (r= -0.75, p < 0.005) and Cu/Zn (r = -0.88, p < 0.005) (Fig. 6) unlike TOS and IL-6.
Fig. 6.
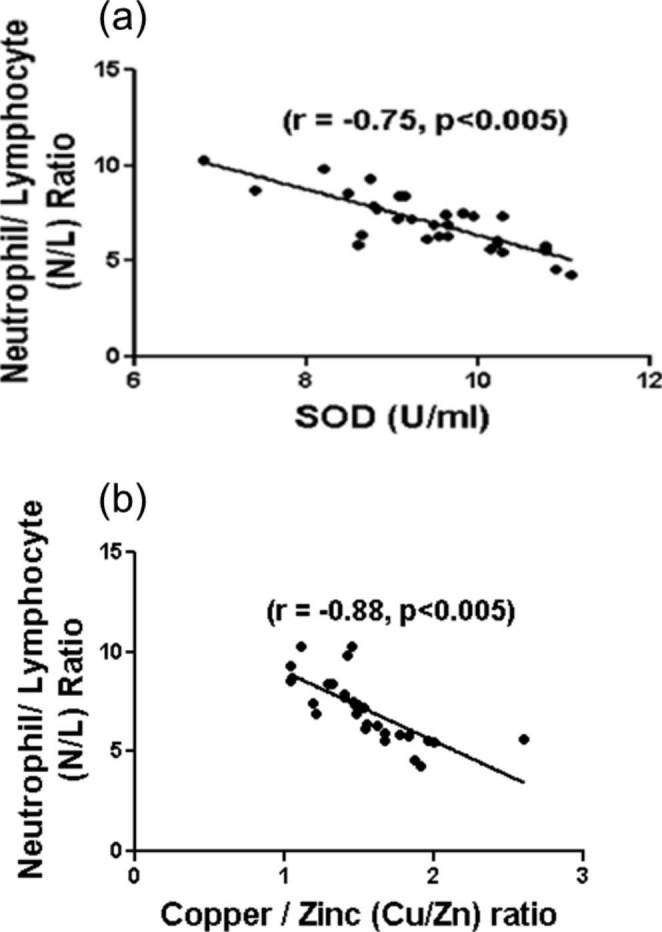
Relationship between (a) plasma superoxide dismutase (SOD) and neutrophil/ lymphocyte (N/L) ratio (b) plasma Cu/Zn ratio and neutrophil/ lymphocyte (N/L) ratio. Spearman’s rank correlation coefficient is denoted by r. A significant negative correlation by Spearman’s rank correlation analysis was observed before and after therapy (p < 0.005)
Discussion
The inter-association between oxidative stress and viral infections associated with respiratory diseases is reported earlier. SARS-CoV2 like other RNA viruses seems to activate oxidative stress [23]. Several inflammatory cells such as monocytes and macrophages release a set of pro-inflammatory cytokines such as IL-1β, IL-6, TNF, IL-8, thereby, play a vital role in COVID-19 infection [24, 25]. These inflammatory cytokines might play an important role in airway remodeling by inducing the MMPs action and causing MMP over-expression and activity [5, 26]. Zinc, an important trace element, regulates proliferation and function of inflammatory cells such as macrophages, neutrophils, T and B cells along with cytokine action [27]. Similarly, Cu, another essential trace element is reported to protect DNA from oxidative stress [28].
ROS, H2O2 are known to produce inflammatory cytokines by triggering NF-κB pathway. This might cause cytokine storm thereby deteriorating prognosis of COVID-19 infection [29]. Our study finds significant increase in total oxidative stress and decrease in antioxidant enzymes These findings are in accordance with the work of Muhammad et al. (2021) [30]. Decreased activities of SOD and GPx are reported in other viral infection [31, 32]. Earlier studies show the role of circulating NOx levels, in lung diseases such as COPD [5] and pulmonary artery hypertension [33]. Our study reports increased NOx and peroxynitrate in COVID-19 patients. This is supported by a prospective observation study conducted by Lorente et al. (2020), which indicates the potential association between high serum nitrate levels and mortality in COVID-19 patients [34]. Similarly, an inverse association between SOD enzyme and N/L ratio (Fig. 6), interprets the relationship between disease severity and antioxidant imbalance.
Increased IL-6, TNF- α in COVID-19 patients (Fig. 2) is also in good agreement with Del Valle et al. (2020) who report them as strong predictors of disease severity and survival [35]. A systematic review intensely describes the importance of IL-6 and other inflammatory cytokines in COVID-19 [36].
Although the role of proteases such as MMP-2, 9, 12 and TIMP-1 are evidenced in several lung diseases [5, 37, 38], very few data are on COVID patients. Our study shows increase in MMP-9/TIMP-1 ratio, MMP-2 and 12 (Fig. 2). Recently, MMP-9 is reported as an early indicator of respiratory failure in COVID-19 patients [8]. MMP-19 is also hypothesized as possible therapeutic target of chloroquine and melatonin in COVID-19 [39]. The suitability of exploring MMPs as therapeutic targets in COVID-19 is reported recently [40]. The positive association between the MMP activity and oxidative stress in COPD is documented by our group recently [5].
Our study finds decreased Cu/Zn ratio in COVID-19 patients (Fig. 2) which is in accordance with the report published by Muhammad et al. (2021) [30]. As these elements are known to be cofactors of SOD, an antioxidant enzyme, hence decreased ratio triggers oxidative stress [41]. Several other studies also recently show decrease in Zn levels in COVID-19 patients [34, 44]. Although mechanism is not clear, surge in inflammatory cytokines seem to cause Cu deficiency [41]. The negative association between Cu/Zn ratio and N/L ratio (Fig. 6.b) narrates the role of trace elements in severity of the disease.
Using univariate analysis, significant role of several indicators related to oxidative stress, inflammation, matrix degradation process, trace elements is observed among COVID-19 patients. Similarly, multivariate approach including VIP score, is able to identify best cut-off dysregulated parameters such as SOD, Cu/Zn ratio, IL-6 and TOS. ROC curves interpret the significant accuracy of the model in terms of sensitivity, specificity. The 90%, 93%, 96.7% sensitivity and 86.7% specificity in respect of parameters such as SOD, Cu/Zn ratio and TOS might enable them to be used as additional laboratory markers for COVID-19. A panel of biomarkers is reported to be preferred over single marker as far as diagnostic utility is concerned [42], which holds good in our finding. Further, significant negative association of SOD and Cu/Zn ratio, with N/L ratio, establishes their role in severity of disease.
Conclusions
Although, there is no in-depth explanation to identify the exact mechanism or pathway involving all these parameters simultaneously during the course of disease, but our finding clearly observes the association among oxidative stress, inflammation, matrix degradation and trace element changes in COVID-19 affected patients. Hence it will be significant to include such parameters in routine lab investigation. Recent data reports partial improvements in clinical parameters and attenuation of cytokine storm among COVID-19 patients supplemented antioxidants orally. It can be speculated that our finding which includes oxidative and inflammatory markers can be helpful for clinicians during follow up of the COVID-19 patients. Given by the symptoms and severity of the disease, it will be promising to monitor blood Cu/Zn ratio along with other prognostic indicators. Four identified parameters such as SOD, Cu/Zn ratio, IL-6 and TOS were found to discriminate the COVID-19 positive patients from the negative ones. Although these were also suitable for diagnostic accuracy, a larger group of patient’s data can validate the finding.
The study is not without limitations. First, the study includes only severe COVID-19 patients. It would be better to investigate in mild cases and healthy controls too. Second, to understand the role of biomarkers we need to have a follow-up post treatment group, which was not possible in the study. Third, N/L ratio, a proposed ideal prognostic marker as per literature survey, was selected to compare with others. Any other relevant clinical parameter assessing the disease progression would be better indicated to assess the interrelationship. Fourth, although the results appeared to be quite encouraging, the sample size was too less (n = 30) to draw any conclusion regarding the mechanism of the disease. It would be worthwhile to study in a larger group of patients involving a multicentric approach. Similarly, it will be ideal to replicate our findings in an independent dataset. Last, it was not possible to rule out any outliers due to the existing co- morbidities such as hypertension, diabetes mellitus and osteoarthritis among these patients.
Acknowledgements
The authors thank the patients who participated in the study.
Abbreviations
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2.
- ECM
extracellular matrix, MMP:Matrix metalloproteinases.
- TIMP
tissue inhibitors of matrix metalloproteinases.
- ROS
Reactive oxygen species.
- COPD
chronic obstructive pulmonary disease.
- PCA
Principal Component Analysis.
- PLS-DA
Partial Least-Squares Discriminant Analysis.
- VIP
Variable Importance in Projection.
- MDA
malondialdehyde.
- TOS
total oxidative stress.
- GST
Glutathione S-transferases.
- GSH-Px
Glutathione peroxidase.
- SOD
Superoxide dismutase.
- TNF
tumour necrosis factor.
- OPLS-DA
orthogonal projection to latent structure with discriminant analysis.
- ANC
absolute neutrophil count.
- ALC
absolute leukocyte count.
- INR
international normalized ratio.
- ROC
Receiver Operator Characteristic.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of Interest
The authors declare that they have no competing interests.
Compliance with ethical standards
The study protocol was approved by the Institutional Ethics Committee. Informed consent was obtained from all subjects prior to enrolment.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yüce M, Filiztekin E, Özkaya KG. COVID-19 diagnosis —A review of current methods. Biosens Bioelectron. 2021;172:112752. doi: 10.1016/j.bios.2020.112752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parasher A. COVID-19: Current understanding of its pathophysiology, clinical presentation and treatment. Postgrad Med J. 2020;97:postgradmedj–2020. doi: 10.1136/postgradmedj-2020-138577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman S, Montero MTV, Rowe K, Kirton R, Kunik F. Epidemiology, pathogenesis, clinical presentations, diagnosis and treatment of COVID-19: A review of current evidence. Expert Rev Clin Pharmacol. 2021;1:21. doi: 10.1080/17512433.2021.1902303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leng L, Cao R, Ma J, Mou D, Zhu Y, Li W, et al. Pathological features of COVID-19-associated lung injury: a preliminary proteomics report based on clinical samples. Signal Transduction and Targeted Therapy [Internet]. 2020 [cited 2021 Jan 14];5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7557250/. [DOI] [PMC free article] [PubMed]
- 5.Singh B, Ghosh N, Saha D, Sarkar S, Bhattacharyya P, Chaudhury K. Effect of doxycyline in chronic obstructive pulmonary disease - An exploratory study. Pulm Pharmacol Ther. 2019;58:101831. doi: 10.1016/j.pupt.2019.101831. [DOI] [PubMed] [Google Scholar]
- 6.Singh B, Jana SK, Ghosh N, Das SK, Joshi M, Bhattacharyya P, et al. Metabolomic profiling of doxycycline treatment in chronic obstructive pulmonary disease. J Pharm Biomed Anal. 2017;132:103–8. doi: 10.1016/j.jpba.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 7.Ueland T, Holter J, Holten A, Müller K, Lind A, Bekken G, et al. Distinct and early increase in circulating MMP-9 in COVID-19 patients with respiratory failure. The Journal of Infection [Internet]. 2020 [cited 2021 Jan 14];81:e41–3. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7320854/. [DOI] [PMC free article] [PubMed]
- 8.Siwik DA, Colucci WS. Regulation of Matrix Metalloproteinases by Cytokines and Reactive Oxygen/Nitrogen Species in the Myocardium. Heart Failure Reviews [Internet]. 2004 [cited 2019 Jun 12];9:43–51. Available from: https://link.springer.com/article/10.1023/B%3AHREV.0000011393.40674.13. [DOI] [PubMed]
- 9.Anuk AT, Polat N, Akdas S, Erol SA, Tanacan A, Biriken D, et al. The Relation Between Trace Element Status (Zinc, Copper, Magnesium) and Clinical Outcomes in COVID-19 Infection During Pregnancy. Biological Trace Element Research [Internet]. 2020 [cited 2021 Jan 14];1–10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7685187/. [DOI] [PMC free article] [PubMed]
- 10.Hackler J, Heller RA, Sun Q, Schwarzer M, Diegmann J, Bachmann M, et al. Relation of Serum Copper Status to Survival in COVID-19. Nutrients [Internet]. 2021 [cited 2022 Jan 5];13:1898. Available from: https://pubmed.ncbi.nlm.nih.gov/34072977/. [DOI] [PMC free article] [PubMed]
- 11.Tang C-F, Ding H, Jiao R-Q, Wu X-X, Kong L-D. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. European Journal of Pharmacology [Internet]. 2020 [cited 2021 Jan 15];886:173546. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7486870. [DOI] [PMC free article] [PubMed]
- 12.Powell C, Dwan K, Milan SJ, Beasley R, Hughes R, Knopp-Sihota JA, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database of Systematic Reviews. 2012;12:CD003898. doi: 10.1002/14651858.CD003898.pub5. [DOI] [PubMed] [Google Scholar]
- 13.Yang A-P, Liu J, Tao W, Li H. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Liu C, Mao Z, Xiao M, Wang L, Qi S, et al. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Critical Care. 2020;24. [DOI] [PMC free article] [PubMed]
- 15.Patro ARK, Mohanty S, Prusty BK, Singh DK, Gaikwad S, Saswat T, et al Cytokine Signature Associated with Disease Severity in Dengue. Viruses. 2019;11:34. [16] JA, Buege SD, Aust. [30] Microsomal lipid peroxidation, Methods in Enzymology. (1978) 302–310. 10.1016/s0076-6879(78)52032-6.
- 16.Buege JA, Aust SD. [30] Microsomal lipid peroxidation. Methods in Enzymology [Internet]. 1978;302–10. [DOI] [PubMed]
- 17.Jansen E, Ruskovska T, et al. Comparative Analysis of Serum (Anti)oxidative Status Parаmeters in Healthy Persons. International Journal of Molecular Sciences. 2013;14:6106–6115. doi: 10.3390/ijms14036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VanUFFELEN EB, Van der ZEE J, de KOSTER MB, VanSTEVENINCK J, ELFERINK GRJ. Intracellular but not extracellular conversion of nitroxyl anion into nitric oxide leads to stimulation of human neutrophil migration. Biochemical Journal. 1998;330:719–22. doi: 10.1042/bj3300719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doumas BT, Perry BW, Sasse EA, Straumfjord JV. Standardization in Bilirubin Assays: Evaluation of Selected Methods and Stability of Bilirubin Solutions. Clinical Chemistry. 1973;19:984–93. [PubMed]
- 20.Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-Induced Liver Necrosis. Protective Role of Glutathione and Evidence for 3,4-Bromobenzene Oxide as the Hepatotoxic Metabolite. Pharmacology. 1974;11:151–69. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 21.MOATAMEDI POUR L, FARAHNAK A, MOLAEI RAD M, GOLMOHAMADI T, ESHRAGHIAN M, Bourque G, et al. <div class=“NodiCopyInline”>Activity Assay of Glutathione S-Transferase (GSTs) Enzyme as a Diagnostic Biomarker for Liver Hydatid Cyst in Vitro. Iranian Journal of Public Health</div> <div class=“NodiCopyInline”>Iranian Journal of Public Health</div> 2014;43:994–999. [PMC free article] [PubMed] [Google Scholar]
- 22.Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Research. 2018;46:W486–94. [DOI] [PMC free article] [PubMed]
- 23.Chernyak BV, Popova EN, Prikhodko AS, Grebenchikov OA, Zinovkina LA, Zinovkin RA. COVID-19 and Oxidative Stress. Biochemistry Biokhimiia [Internet]. 2020;85:1543–53. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7768996. [DOI] [PMC free article] [PubMed]
- 24.Meidaninikjeh S, Sabouni N, Marzouni HZ, Bengar S, Khalili A, Jafari R. Monocytes and macrophages in COVID-19: Friends and foes. Life Sciences. 2021;269:119010. [DOI] [PMC free article] [PubMed]
- 25.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nature Reviews Immunology [Internet]. 2020;20:1–8. Available from: https://www.nature.com/articles/s41577-020-0331-4 [DOI] [PMC free article] [PubMed]
- 26.Solun B, Shoenfeld Y. Inhibition of metalloproteinases in therapy for severe lung injury due to COVID-19. Medicine in Drug Discovery [Internet]. 2020 [cited 2021 Apr 18];7:100052. Available from: https://www.sciencedirect.com/science/article/pii/S2590098620300397 [DOI] [PMC free article] [PubMed]
- 27.Taheri M, Bahrami A, Habibi P, Nouri F. A Review on the Serum Electrolytes and Trace Elements Role in the Pathophysiology of COVID-19. Biological Trace Element Research [Internet]. 2020;1–7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7478435. [DOI] [PMC free article] [PubMed]
- 28.Uriu-Adams JY, Keen CL. Copper, oxidative stress, and human health. Molecular Aspects of Medicine. 2005;26:268–98. [DOI] [PubMed]
- 29.Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Medical Hypotheses. 2020;143:110102. [DOI] [PMC free article] [PubMed]
- 30.Muhammad Y, Kani YA, Iliya S, Muhammad JB, Binji A, El-Fulaty Ahmad A, et al. Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: A cross-sectional comparative study in Jigawa, Northwestern Nigeria. SAGE Open Medicine. 2021;9:205031212199124. [DOI] [PMC free article] [PubMed]
- 31.Dworzański J, Strycharz-Dudziak M, Kliszczewska E, Kiełczykowska M, Dworzańska A, Drop B, et al. Glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity in patients with diabetes mellitus type 2 infected with Epstein-Barr virus. Pagano JS, editor. PLOS ONE. 2020;15:e0230374. [DOI] [PMC free article] [PubMed]
- 32.Strycharz-Dudziak M, Kiełczykowska M, Drop B, Świątek Ł, Kliszczewska E, Musik I, et al. Total Antioxidant Status (TAS), Superoxide Dismutase (SOD), and Glutathione Peroxidase (GPx) in Oropharyngeal Cancer Associated with EBV Infection. Oxidative Medicine and Cellular Longevity. 2019;2019:1–15. [DOI] [PMC free article] [PubMed]
- 33.Klinger JR. Group III Pulmonary Hypertension. Cardiology Clinics. 2016;34:413–33. doi: 10.1016/j.ccl.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Lorente L, Gómez-Bernal F, Martín MM, Navarro-Gonzálvez JA, Argueso M, Perez A, et al. High serum nitrates levels in non-survivor COVID-19 patients. Medicina Intensiva. 2022;43:132-39. [DOI] [PMC free article] [PubMed]
- 35.Del Valle DM, Kim-Schulze S, Huang H-H, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nature Medicine [Internet]. 2020;26:1636–43. Available from: https://www.nature.com/articles/s41591-020-1051-9 [DOI] [PMC free article] [PubMed]
- 36. Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. The Lancet Respiratory Medicine [Internet]. 2020;0. Available from: https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30404-5/fulltext [DOI] [PMC free article] [PubMed]
- 37.NAVRATILOVA Z, ZATLOUKAL J, KRIEGOVA E, KOLEK V, PETREK M. Simultaneous upregulation of matrix metalloproteinases 1, 2, 3, 7, 8, 9 and tissue inhibitors of metalloproteinases 1, 4 in serum of patients with chronic obstructive pulmonary disease. Respirology. 2012;17:1006–12. [DOI] [PubMed]
- 38.Atkinson JJ, Senior RM. Matrix Metalloproteinase-9 in Lung Remodeling. American Journal of Respiratory Cell and Molecular Biology. 2003;28:12–24. [DOI] [PubMed]
- 39.Hazra S, Chaudhuri AG, Tiwary BK, Chakrabarti N. Matrix metallopeptidase 9 as a host protein target ofchloroquine and melatonin for immunoregulation in COVID-19: A network-based meta-analysis. Life Sciences. 2020;257:118096. doi: 10.1016/j.lfs.2020.118096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardy E, Fernandez-Patron C. Targeting MMP-Regulation of Inflammation to Increase Metabolic Tolerance to COVID-19 Pathologies: A Hypothesis. Biomolecules. 2021;11:390. [DOI] [PMC free article] [PubMed]
- 41.Ebrahimi M, Norouzi P, Aazami H, Moosavi-Movahedi AA. Review on oxidative stress relation on COVID-19: Biomolecular and bioanalytical approach. International Journal of Biological Macromolecules [Internet]. 2021 [cited 2021 Oct 28];189:802–18. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8372478. [DOI] [PMC free article] [PubMed]
- 42.Dutta M, Singh B, Joshi M, Das D, Subramani E, Maan M, et al. Metabolomics reveals perturbations in endometrium and serum of minimal and mild endometriosis. Scientific Reports. 2018;8. [DOI] [PMC free article] [PubMed]