Abstract
Background
Hyperthyroidism has a significant, well-established impact on the cardiovascular system on both a molecular and circulatory level. The cardiac consequences of thyrotoxicosis are not uncommon, indicated by a 1.2% prevalence of this disorder in the United States. However, our case describes the less widely observed association between thyrotoxicosis and valvulopathy.
Case summary
A 69-year-old Hispanic male presented with a 3-week history of shortness of breath, intermittent chest pain, and lower extremity swelling. Transthoracic echocardiogram revealed a dilated left and right atrium with severe tricuspid regurgitation, moderate mitral regurgitation, malcoaptation of the tricuspid valve leaflets, and a myxomatous mitral valve. In addition, right ventricular systolic function was moderately reduced. A right and left heart catheterization was performed with findings of normal right heart pressures and normal coronary arteries, respectively. To further evaluate the aetiology of the patient’s heart failure, thyroid studies were sent, revealing a thyroid-stimulating hormone value of <0.010 uIU/mL and a free T4 of 1.96 ng/dL. A 4.9 cm lesion was seen on thyroid ultrasound. We concluded that the patient’s heart failure and notable valvular abnormalities were likely as a result of thyrotoxic heart disease. Furosemide and methimazole were initiated while inpatient, and the patient was discharged with close follow-up.
Discussion
We demonstrate a unique case of the possible hemodynamic and cellular effects of thyroid hormone on the development of primary and secondary valve dysfunction. This association is important for clinicians to be aware of, as treatment of its underlying aetiology can lead to improvement in a patient’s cardiac outcomes.
Keywords: Heart failure, Valvular heart disease, Cardiomyopathies, Cardiac imaging, Echocardiography, Case report
Learning points.
Thyrotoxicosis can result in cardiac effects on both a haemodynamic and cellular level that can lead to primary and secondary valvular changes.
Recognize thyrotoxicosis as an aetiology for right-sided heart failure and valvular regurgitation, as its treatment can reverse cardiac findings.
Introduction
A well-established association exists between the thyroid gland and the cardiovascular system. The effect of hyperthyroidism on the heart is demonstrated in prior studies both on a molecular and circulatory level; however, this diagnosis is often neglected.1,2 Thyroid hormone regulates the handling of intracellular calcium and, in excess, results in changes to contractility, cardiac output, and systemic vascular resistance through a tachycardia-mediated mechanism.3,4 Hyperthyroidism carries a 1.2% prevalence in the United States and thus cardiovascular manifestations of thyrotoxicosis, such as high-output heart failure, pulmonary artery hypertension, and atrial fibrillation, are not uncommon.4,5,6,7 However, less widely described is the relationship between hyperthyroidism, right heart failure, and cardiac valvulopathy. We present a case of a patient with right heart failure with severe tricuspid valve regurgitation and mitral valve regurgitation with myxomatous valves. Complete cardiac workup eventually led to the likely diagnosis of right heart failure and valvular disease in association with newly diagnosed thyrotoxicosis.
Timeline
| Three weeks before the presentation to the emergency department | Symptoms of shortness of breath, intermittent chest pain, and lower extremity oedema. |
|---|---|
| Day 1 | Physical exam was suggestive of heart failure and BNP was elevated to 881 pg/mL. |
| LVEF was 68%, and TTE showed a dilated left and right atrium with severe TR, moderate MR, malcoaptation of the tricuspid valve leaflets, and a myxomatous mitral valve. | |
| TTE also demonstrated an enlarged right ventricle with moderately reduced systolic function and pulmonary hypertension. | |
| Day 3 | Two days of aggressive diuresis with intravenous furosemide. |
| Right heart catheterization values were elevated, including right atrial pressures of 8 mmHg, right ventricle 31/8 mmHg, pulmonary artery 31/12 mmHg, and pulmonary capillary wedge pressure of 10 mmHg. | |
| Coronary angiogram was normal. | |
| Day 4 | Thyroid-stimulating hormone was significantly low at <0.010 uIU/mL [reference range (RR): 0.45–4.12 uIU/mL], along with an elevated free T4 of 1.96 ng/dL [RR: 0.60–1.12 ng/dL]. |
| Day 5 | Subsequent data revealed slightly elevated thyroid peroxidase antibody of 11 IU/mL [RR: <9 IU/mL], negative thyroid-stimulating immunoglobulin (TSI index <1.0), and negative thyroglobulin antibody (<1 IU/mL). |
| A 4.9 cm lesion in the left thyroid lobe was found on ultrasound. | |
| Day 6 | Methimazole was initiated for the patient’s thyrotoxicosis. |
| Patient was discharged after treatment for heart failure. |
Case presentation
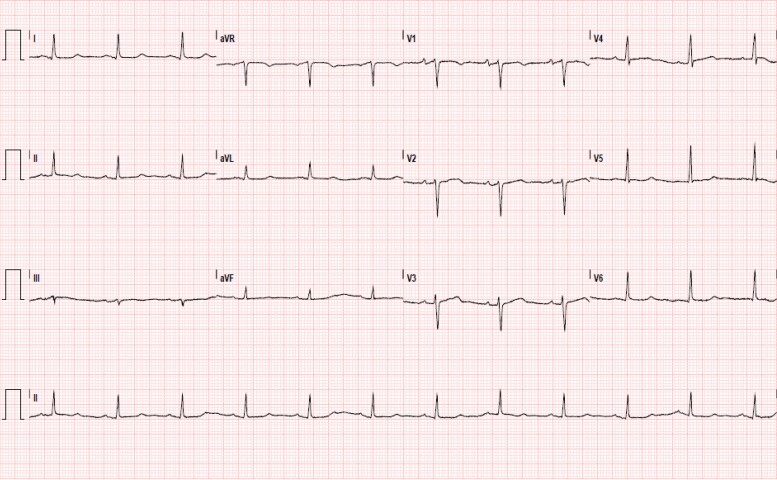
A 69-year-old Hispanic male with no documented past medical history presented to the emergency department with a 3-week history of shortness of breath, intermittent chest pain, and lower extremity edema. Initial vital signs were notable for an elevated blood pressure of 150/86 mmHg and a heart rate of 73 bpm. Jugular venous distention was markedly elevated to 12 cm of water (cm H2O), alongside sparse bibasilar crackles on pulmonary exam and 1 + pitting pedal oedema. Brain natriuretic peptide was as high as 881 pg/mL [reference range (RR): 0–100 pg/mL] with a high sensitivity troponin I of 16 ng/L [RR: 0–20 ng/L], which peaked to 18 ng/L 2 h later. Chest radiograph revealed trace pulmonary vascular congestion and cardiomegaly. Electrocardiogram demonstrated sinus rhythm with non-specific T-wave abnormalities (Figure 1). Transthoracic echocardiogram (TTE) demonstrated a left ventricular systolic ejection fraction (EF) of 68%. Left ventricular diastolic function was found to include an E/A ratio of 2.91, E/e′ ratio of 11.15, and deceleration time of 151 msec. Additional findings included a dilated left and right atrium with severe tricuspid regurgitation (TR), moderate mitral regurgitation (MR), malcoaptation of the tricuspid valve leaflets, and a myxomatous mitral valve (Figures 2–4, Supplementary material online, Videos S1–3). There was a moderately enlarged right ventricle with moderately reduced systolic function and evidence of pulmonary hypertension (see Supplementary material online, Video S4). The patient was subsequently hospitalized for newly diagnosed acute decompensated right heart failure with preserved left ventricular EF.
Figure 1.
Electrocardiogram on patient presentation. Interpretation of sinus rhythm with non-specific T-wave abnormalities.
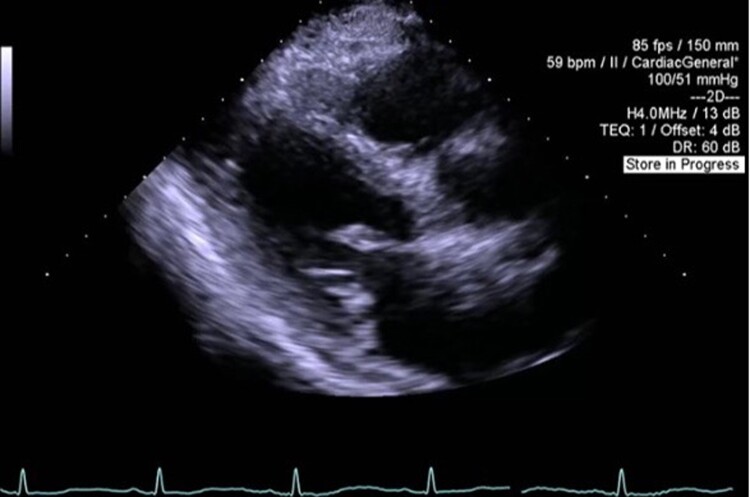
Figure 2.
Image from transthoracic echocardiography. Parasternal long-axis view demonstrating myxomatous changes to the mitral valve.
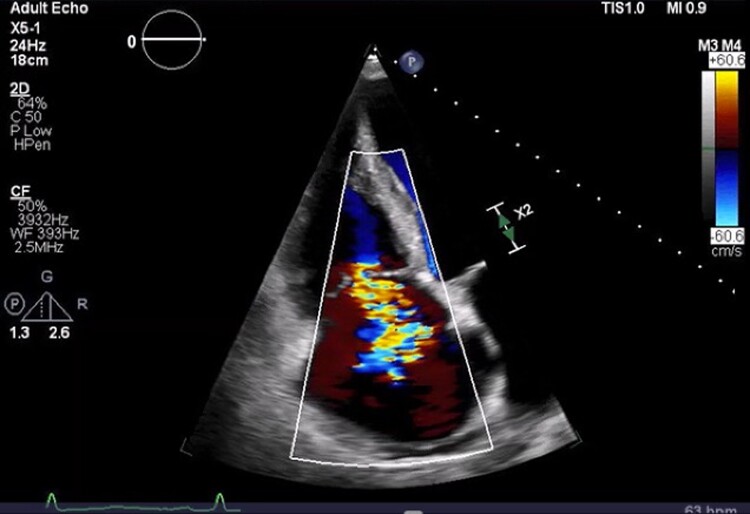
Figure 3.
Image from transthoracic echocardiography. Image of apical four-chamber view with continuous-wave Doppler spectrum depicting severe tricuspid valve insufficiency in the setting of malcoaptation of the tricuspid valve leaflets. The tricuspid regurgitant velocity measured 2.74 m/s.
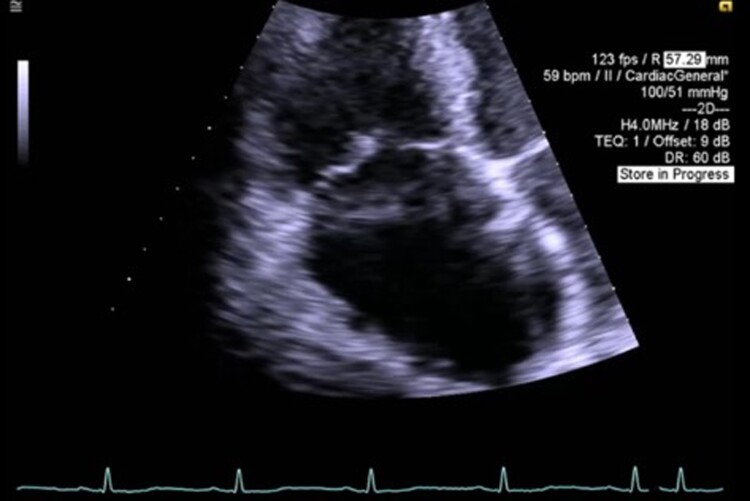
Figure 4.
Image from transthoracic echocardiography. Apical four-chamber view demonstrating malcoaptation of tricuspid valve leaflets.
Two days after the initiation of aggressive diuresis with intravenous furosemide, a right heart catheterization demonstrated mildly elevated right heart pressures (mean right atrial 8 mmHg, right ventricle 31/8 mmHg, pulmonary artery 31/12 mmHg, and pulmonary capillary wedge pressure of 10 mmHg). A coronary angiogram revealed normal coronary arteries. The aetiology of this patient’s heart failure remained unclear. Given the significant valvular abnormalities, thyroid studies were sent for further workup. Thyroid-stimulating hormone (TSH) was significantly low at <0.010 uIU/mL (RR: 0.45–4.12 uIU/mL), along with an elevated free T4 of 1.96 ng/dL (RR: 0.60–1.12 ng/dL).
Subsequent serological data revealed slightly elevated thyroid peroxidase antibody of 11 IU/mL [RR: <9 IU/mL], negative thyroid-stimulating immunoglobulin (TSI index <1.0), and negative thyroglobulin antibody (<1 IU/mL). Thyroid ultrasound showed a 4.9 cm lesion in the left thyroid lobe (Figure 5). The patient’s thyrotoxicosis was believed to likely be attributed to toxic adenoma. It was determined that the patient’s heart failure and valve abnormalities were likely consistent with thyrotoxic heart disease. Methimazole 10 mg once daily was initiated for treatment in addition to furosemide for diuresis and carvedilol and losartan for hypertension. The patient was discharged to a shelter, but unfortunately, he was lost to follow-up and did not obtain a TTE after completion of treatment due to social circumstances.
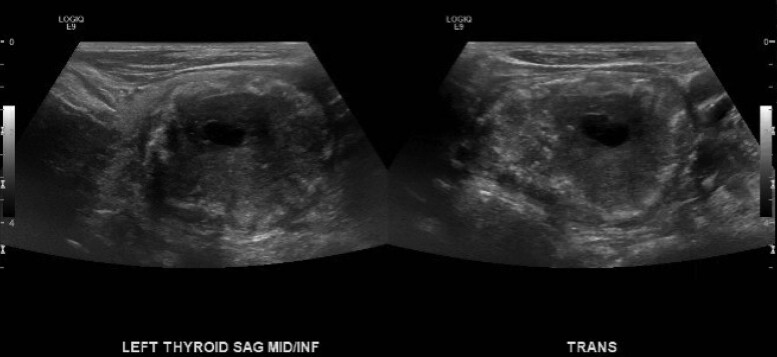
Figure 5.
Image from ultrasound of the left thyroid in sagittal and transverse views. Ultrasound demonstrates a 4.9 cm TI-RADS 5 (mixed cystic and solid, hyperechoic echogenicity, wide shape, ill-defined margin, and punctate echogenic foci) lesion in the left thyroid lobe.
Discussion
The cardiovascular effects of hyperthyroidism have long been recognized and incorporated into clinical practice. One case report identified Grave’s thyrotoxicosis as the cause of their patient’s pulmonary hypertension and significant TR, while another case found severe MR with biventricular failure as a result of toxic Graves’ disease; both cases noted improvement in the patients’ clinical findings after treatment of their hyperthyroid state.8,9 Additional reports demonstrate the reversible nature of heart failure caused by thyrotoxicosis, highlighting the importance of recognizing thyrotoxic cardiomyopathy.10,11 On the other hand, hypothyroidism has also been shown to have a cardiac impact, as demonstrated in characteristic echocardiograph findings, such as larger left atrial diameter in patients with heart valve disease.12 Our patient presented with thyrotoxicosis in the setting of possible toxic adenoma complicated by severe TR due to malcoaptation of the tricuspid valve leaflets and moderate MR due to myxomatous changes of the mitral valve. These findings demonstrate how the effects of hyperthyroidism can impact the cardiovascular system from both a haemodynamic and cellular standpoint.
Valvular regurgitation may be categorized into either primary or secondary etiologies. Primary regurgitation (also known as organic) is caused by congenital or acquired abnormalities of the valve itself. These irregularities lead to dysfunction of the valve and reversal of blood flow. Subsequently, this can cause right heart failure leading to volume overload. In the case of our patient, the echocardiogram revealed myxomatous changes to the mitral valve leading to the development of moderate MR. Ultimately, the myxomatous changes to valve leaflets in conjunction with likely chordae degeneration and intrinsic papillary muscle dysfunction can cause significant valvular dysfunction and regurgitation. Secondary, or functional valvular regurgitation, occurs due to deformation of the valve as a result of elevated intra-cardiac pressure or volume overload.13 The latter likely occurred in our patient with the development of severe TR due to elevated levels of circulating thyroid hormone affecting various hemodynamic components of the cardiovascular system. In a comprehensive review of the literature, Dillmann et al.14 showed that thyroid hormone acts directly on cardiac muscle. This is mediated through the effects of thyroid hormone increasing the cardiac transcription of myosin heavy chain (MHC) α gene, which increases myosin V1, with higher ATPase activity, and decreases myosin V3 isoenzymes through a downregulation in transcription of MHC β gene. The change in ATPase activity leads to an increase in contraction velocity, as a result of faster movement of the globular myosin head along the thin filament. Furthermore, through administration of a beta-adrenergic receptor antagonist in patients with hyperthyroidism, Mintz et al.15 found that hyperthyroidism directly impacts the contractile performance of myocardium. As these changes to haemodynamics occur, there is a direct correlation with the development of secondary TR, as seen in our patient.
Conclusions
The association between hyperthyroidism and systemic cardiac effects is well-established. Our patient demonstrates two distinct pathologies that likely resulted in right-sided heart failure, including haemodynamic effects, which can lead to the development of secondary valve dysfunction, and cellular effects of thyroid hormone that can precipitate myxomatous valve changes. It is important for clinicians to understand this established causality between a hyperthyroid state and the development of cardiac pathology, as the treatment of thyrotoxicosis can result in a reversal in cardiac findings.
Supplementary Material
Contributor Information
Sarah Harirforoosh, Department of Medicine, University of California, Irvine, 101 The City Dr S, City Tower Ste 400, Orange, CA 92868-3201, USA.
Garrett Cohen, Department of Medicine, University of California, Irvine, 101 The City Dr S, City Tower Ste 400, Orange, CA 92868-3201, USA.
Diana Glovaci, Department of Cardiology, University of California, Irvine, 101 the City Dr S, Orange, CA 92868, USA.
Pranav M Patel, Department of Cardiology, University of California, Irvine, 101 the City Dr S, Orange, CA 92868, USA.
Lead author biography
 Sarah Harirforoosh graduated from University of Tennessee Health Science Center College of Medicine in 2020. She is currently completing residency in internal medicine at University of California, Irvine and is planning to pursue cardiovascular fellowship.
Sarah Harirforoosh graduated from University of Tennessee Health Science Center College of Medicine in 2020. She is currently completing residency in internal medicine at University of California, Irvine and is planning to pursue cardiovascular fellowship.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementarydata.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared
References
- 1. Klein I, Danzi S. Thyroid disease and the heart. Circulation 2007;116:1725–1735. [DOI] [PubMed] [Google Scholar]
- 2. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001;344:501–509. [DOI] [PubMed] [Google Scholar]
- 3. Moolman JA. Thyroid hormone and the heart. Cardiovasc J S Afr 2002;13:159–163. [PubMed] [Google Scholar]
- 4. Dahl P, Danzi S, Klein I. Thyrotoxic cardiac disease. Curr Heart Fail Rep 2008;5:170–176. [DOI] [PubMed] [Google Scholar]
- 5. Ross D, Burch H, Cooper D, Greenlee MC, Laurbeg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, Walter MA. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016;26:1343–1421. [DOI] [PubMed] [Google Scholar]
- 6. Bonou M, Lampropoulos K, Andriopoulou M, Kotsas D, Lakoumentas J, Barbetseas J. Severe tricuspid regurgitation and isolated right heart failure due to thyrotoxicosis. Indian Heart J 2012;64:600–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faccia M, Porfidia A, Montalto M. Acute right ventricular heart failure: an uncommon case of thyrotoxicosis. Am J Med Sci 2018;356:309–312. [DOI] [PubMed] [Google Scholar]
- 8. Pierre K, Gadde S, Omar B, Awan G, Malozzi C. Thyrotoxic valvulopathy: case report and review of the literature. Cardiol Res 2017;8:134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fekri K, Michel CM, Tamilia M. Reversible, severe mitral regurgitation in thyrotoxic graves’ disease. BMJ Case Rep 2021;14:e239626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alam ST, Zaman J. Case study of thyrotoxic cardiomyopathy. BMJ Case Rep 2019;12:e228896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waqar A, Ansar A, Gudipati S, Liebo M. When it rains it pours: a case of thyroid storm presenting as thyrotoxic cardiomyopathy. J Am Coll Cardiol 2020;75::3277. [Google Scholar]
- 12. Zheng J Z, Ling Y. Impact of hypothyroidism on echocardiographic characteristics of patients with heart valve disease: A single-center propensity score-based study. Front Endocrinol (Lausanne) 2020;11:554762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prihadi EA. Tricuspid valve regurgitation: no longer the “forgotten valve.” Eur Soc Cardiol 2018;16:21–30. [Google Scholar]
- 14. Dillmann W. Biochemical basis of thyroid hormone action in the heart. Am J Med 1990;88:626–630. [DOI] [PubMed] [Google Scholar]
- 15. Mintz G, Pizzarello R, Klein I. Enhanced left ventricular diastolic function in hyperthyroidism: noninvasive assessment and response to treatment. J Clin Endocrinol Metab 1991;73:146–150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.