Abstract
Background:
Retrospective studies suggest that conditioning therapy with busulfan (Bu) plus melphalan (Mel) may result in longer progression-free survival (PFS) compared to Mel alone in patients with multiple myeloma undergoing autologous hematopoietic cell transplantation (auto-HCT). We aimed to test this hypothesis in a randomized trial.
Methods:
The primary objective of the study was to compare PFS with Bu-Mel to Mel alone conditioning in patients with multiple myeloma. Transplant eligible patients with newly diagnosed multiple myeloma, age ≤ 70-years, with at least stable disease, were randomized between October 2011 and April 2017, 1:1 to receive: 1) Bu-Mel, with a test dose of Bu 32 mg/m2followed by pharmacokinetically adjusted doses on days −7, −6, −5, and −4 to achieve target daily area under the curve (AUC) 5000 mmol-minute and Mel 70 mg/m2/day on days −2 and −1 (total Mel dose 140 mg/m2), or 2) Mel 200 mg/m2 on day −2. Randomization of this single-center, open-labeled, phase III trial was performed via a Clinical Trial Conduct Website at the University of Texas MD Anderson Cancer Center. The primary analysis was based on 202 treated patients (n=104; n=98) comparing PFS in the two treatment groups. The accrual is complete and final results are presented here. The study is registered with ClinicalTrials.gov, number NCT01413178.
Findings:
There were no treatment-related mortalities by day 100 in either arm. The incidence of grade II-III mucositis was 74% (77 of 104) with Bu-Mel vs. 14% (14 of 98) with Mel. At day-90 after auto-HCT, 102 (98%) patients given Bu-Mel and 95 (97%) patients given Mel achieved partial response (PR) or better. The median follow up for all patients was 21.9 months (IQR: 12.0-46.9 months). Median PFS was 64.7 months (IQR: 32.9, 64.7 months) with Bu-Mel vs. 43.5 months (IQR: 19.9 months, not estimated) with Mel, with HR=0.53, 95% CI [0.30, 0.91] (p=0.022). In a multivariable regression model, Bu-Mel conditioning was significantly associated with superior PFS.
Interpretation:
These findings, if confirmed in other ongoing studies, suggest that Bu-Mel may replace Mel alone as the conditioning regimen for auto-HCT in newly diagnosed myeloma patients.
Funding:
The National Institutes of Health (NIH) in part funded the study through MD Anderson's Cancer Center Support Grant (CA016672). The NIH employs no author.
INTRODUCTION
Autologous hematopoietic stem cell transplantation (auto-HCT), in combination with immunomodulatory drug- (IMiD) and proteasome inhibitor- (PI) based induction is associated with one of the highest response rates in multiple myeloma and currently is accepted as part of upfront therapy in eligible patients with newly diagnosed multiple myeloma(1). The outcomes have consistently improved over time, owing primarily to the advent of novel agents like IMiDs and PIs, and the use of post-transplant maintenance therapy(2). Considerable efforts have been made to improve the efficacy of pre-transplant conditioning regimens. In this context, several drug combinations have shown promise, but none have convincingly demonstrated superiority over melphalan (Mel) alone, and this remains the conditioning regimen of choice(3). Nevertheless, existing data suggest that intensifying pre-transplant conditioning chemotherapy may decrease the relapse rate and thus prolong progression-free survival (PFS) after auto-HCT(4).
Among various drugs, busulfan (Bu) has demonstrated substantial efficacy either alone(5) or in combination with other drugs as conditioning for auto-HCT(6). Pre-clinical data suggest that the cytotoxicity of the combination of Bu-Mel is synergistic in myeloma cell lines (Supplemental data. Figures S1A & S1B). A Spanish Registry analysis of auto-HCT for multiple myeloma between 1990 and 2000 concluded that the combination of oral Bu 12 mg/kg plus Mel 140 mg/m2 (Bu-Mel) resulted in a superior complete remission (CR) rate and possibly longer PFS compared to other preparative regimens, which also included Mel 200 mg/m2 (MEL200)(7, 8). Encouraged by these results, the Programa Español de Tratamientos en Hematología (PETHEMA)/Grupo Español de Mieloma (GEM) conducted a prospective, non-randomized GEM2000 study of oral Bu-Mel conditioning in patients with newly diagnosed multiple myeloma(9). An interim analysis performed two years after study activation (N=225) demonstrated a high incidence (8%) of veno-occlusive disease (VOD)(10). Therefore, the trial’s conditioning regimen was changed from Bu-Mel to Mel200(10). The final analysis after the enrollment of 542 additional patients showed that Bu-Mel resulted in higher treatment-related mortality (TRM) of 8.4% vs. 3.5% with Mel200 (p=0.002) due to higher a frequency of VOD(9). The median PFS was significantly longer with Bu-Mel (41 vs. 31 months with Mel200; p=0.009), but the overall survival (OS) was similar.
Concerns regarding VOD with oral Bu largely have been ameliorated with the introduction of intravenous (IV) Bu, which results in linear pharmacokinetics (PK) and more reproducible systemic exposure(11). Patient safety can be further enhanced using individualized dose adjustment based on pharmacokinetic studies to target a specific systemic exposure (area under the curve). Recent studies have focused on combining IV Bu with Mel(12, 13) and/or novel agents(6, 14) in myeloma patients. Although these studies underscored the safety and efficacy of the IV Bu-based combinations, a prospective, randomized trial comparing IV Bu-Mel with Mel conditioning has not been conducted to reach a definitive conclusion in auto-HCT.
The primary objective of our study was to compare PFS between the two treatment groups. In order to address this, we conducted an open-labeled, randomized, single-center phase III trial to compare IV Bu plus Mel (Bu-Mel) to Mel alone as conditioning regimens in myeloma patients undergoing auto-HCT. The primary endpoint was PFS time. The secondary endpoints included overall survival (OS) time, response rate, toxicities including VOD, and quality of life (QOL).
METHODS
Study design and participants
This open label, phase 3 randomized trial was conducted at The University of Texas MD Anderson Cancer Center (Houston, TX, USA). All patients were enrolled through the Department of Stem Cell Transplantation. Patients with newly diagnosed symptomatic multiple myeloma, age ≤ 70-years, with at least stable disease (SD) after a minimum of 2 cycles of induction therapy were eligible. Other inclusion criteria were: Karnofsky performance score ≥ 70% and adequate cardiac, pulmonary, hepatic, and renal function. Patients were excluded if they had relapsed/progressive disease, an uncontrolled infection, were positive for the human immunodeficiency virus, or had received a prior allogeneic or auto-HCT. All patients provided written informed consent before enrolling in the study.
Randomization and masking
Patients were randomized 1:1 to the Bu-Mel or Mel alone arms using minimization to balance dynamically on age (≤ 65 versus > 65 years). Randomization was generated using the Clinical Trial Conduct Web Site (https://biostatistics.mdanderson.org/ClinicalTrialConduct/DeskTopDefault.aspx?ReturnUrl=%2fClinicalTrialConduct%2f) in the Department of Biostatistics by research nurses in the Department of Stem Cell Transplantation and Cellular Therapy. The Pocock-Simon algorithm was used for the first 164 patients (Bu-Mel n=100; Mel n=64) with treatment assignment probabilities based on their formula (b), page 107, assigning 1/2 to the constant q, the imbalance of treatment k defined as the unweighted sum on page 106, and using the range as a measure of variability(15). Following the recommendation of The University of Texas MD Anderson Cancer Center’s data safety monitoring board, which monitored the study, the algorithm then was changed to Method 2, where imbalances were based on semi-ranking with probability of 0.99. The research nurse performing the randomization and the patient were masked to treatment assignment before randomization. The study team and the patients were aware of the assigned arm after randomization.
Procedures
Response and progression were defined according to the International Myeloma Working Group (IMWG) criteria(16). The severity of adverse events (AEs) was assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) v3.0 (ctep.cancer.gov). High-risk chromosomal abnormalities were defined per IMWG consensus panel(17). These included detection of t(4; 14), t(14; 16), t(14;20), del(17/17p), or gain(1q) on florescence in situ hybridization (FISH) and/or hypodiploidy or del(13) by conventional karyotyping. The treating physicians completed the evaluations along with the research nurses, who verified and recorded the data for all patients during the inpatient stay and at each clinic study visit for the participating patients.
Patients in the Bu-Mel arm received a test dose of 32 mg/m2 Bu (distributed by Otsuka American Pharmaceuticals, Rockville, MD, USA; manufactured by Patheon Mfg Service LLC, Greenville, NC, USA, or Baxter Oncology, Westfalen, Germany) on day −8 if outpatient, or on day −9 if inpatient, followed by pharmacokinetically (PK) adjusted therapeutic doses of Bu on days −7, −6, −5, and −4, with daily target area under the curve (AUC) 5000 mmol-minute11. Melphalan 70 mg/m2/day (GlaxoSmithKline, Research Triangle Park, North Carolina, USA) was administered on days −2 and −1 (total Mel dose 140 mg/m2). Patients in the Mel arm received Mel 200 mg/m2 on day −2. All patients received supportive care per standard institutional practice. Granulocyte-colony stimulating factor (G-CSF) was administered at a dose of 5 mcg/kg/day subcutaneously beginning on day +5, continuing until evidence of an absolute neutrophil count (ANC) ≥ 0.5 × 109/L.
A subset of 165 patients completed at least one of the 20-symptom severity and six interference items of MD Anderson Symptom Inventory for multiple myeloma (MDASI-MM) before the start of the treatment regimen and weekly for four weeks post auto-HCT(18). The MDASI is a brief, patient-reported outcome measure of the severity of 13 core symptoms and six areas of symptom interference with daily life common to all cancer types(19). Symptom burden, which is the combined impact of disease- and therapy-related symptoms on the ability of persons to function as they did before onset of their disease and/or therapy, is the conceptual framework of the MDASI(20). The MDASI core has been validated for use in multiple cancer diagnoses, is widely used in clinical practice and research, and has specific symptoms added to the core symptoms in some populations to produce disease-and treatment-specific modules, including multiple myeloma(18, 19). The MDASI-MM includes seven symptoms specific to patients with multiple myeloma. Patients rate symptoms and interference on a scale of 0-10 (0 = symptom not present or no interference, 10 = worst imaginable symptom severity or complete interference). Differences between the two arms in individual symptom severity and interference ratings and a composite score of all interference items were assessed at each time point by t-tests, assuming that patient ratings not given at a scheduled time point were missing completely at random.
Multiple myeloma-specific patient assessments were performed at baseline, then every three months during the first year after the transplant. Additional laboratory tests and imaging studies were conducted as clinically necessary. The accrual is complete and final results are presented here.
Outcomes
The primary outcome was PFS rate in the Bu-Mel versus Mel alone conditioning regimen. The secondary objectives were to compare the response rate, incidence of grade 3-4 toxicities, TRM at day-100, and OS between the two treatment arms. The neutrophil engraftment was defined as the first of 3 days when the absolute neutrophil count was ≥ 0.5 × 109/L. The platelet engraftment was defined as platelet count ≥ 20 × 109/L for 7 consecutive days in the absence of platelet transfusion. TRM was defined as death due to any cause in the absence of disease progression. We analyzed patient reported quality of life measures between the two groups using MDASI-MM.
Statistical analysis
The study was approved by the Institutional Review Board and is registered as a randomized trial with clinicaltrials.gov, number NCT01413178. The hypotheses being tested by this randomized group sequential design were H0: λ = 1 vs. H1: λ ≠ 1 based on PFS time, where λ is the hazard ratio of Bu-Mel vs. Mel alone. Assuming a null median PFS time of 20 months with Mel, with two-sided tests having nominal overall type I error 0.029 and power 0.80 to detect a median PFS of 34 months for Bu-Mel, corresponding to hazard ratio 0.59, with up to three tests using O’Brien-Fleming decision boundaries, the anticipated total sample was 205 patients, assuming an accrual rate of 5 patients per month. The tests were conducted when there were totals of 45, 90, and 135 failure events in the two arms, with respective standardized log-rank test Z-score test cutoffs +/− 4.0757, 2.7768, and 2.2077.
Associations between categorical variables and treatment arm stratified by age (≤ 65 versus > 65 years) were assessed using the exact Cochran-Mantel Haenszel test(21), while differences in continuous measures between arms stratified by age were evaluated by the van Elteren test(22). Unadjusted OS and PFS distributions from date of transplant were estimated using the Kaplan-Meier method(23), and the stratified log-rank test(24) was used to test differences between treatment arms. Bayesian piecewise exponential regression(25) was used to assess relationships between PFS and covariates and treatment arm, assuming non-informative N(0,5) prior distributions for the regression coefficients and a gamma (0.001, 0.001) prior for the variance. A chain size of 10,000 was used in the Monte Carlo Markov chains to compute posteriors. Covariates included in the Bayesian regression model were age (> 65 vs. ≤ 65 years), cytogenetic risk (high-risk vs. standard), revised international staging system [(R-ISS) (stage III vs. stage I/II)], disease response to induction therapy [partial response (PR) or better vs. SD or worse], and randomization algorithm (Method 2 vs. Method 1). Longitudinal analysis using mixed models were used to assess covariate effects on repeatedly measured quality of life (QOL) variables. Missing QOL data were assumed to be missing at random in the mixed models. With the exception of QOL, all analyses were produced for per-protocol patients (i.e., those intent-to-treat patients that met all inclusion/exclusion criteria) with non-missing data. For QOL, the subset of 165 patients that completed at least one of the 20-symptom severity and six interference items of MDASI-MM were analyzed. The stratified exact Cochran-Mantel Haenszel test was performed using StatXact 11 while all other statistical analyses were performed using SAS 9.4 for Windows.
Role of funding source
The funding source did not play any role in the study design, data collection, analysis, interpretation of the data, or writing of the report. QB, PFT, DRM, PSF, RD, LAW, and MHQ had full access to the raw data. All authors approved the manuscript. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
RESULTS
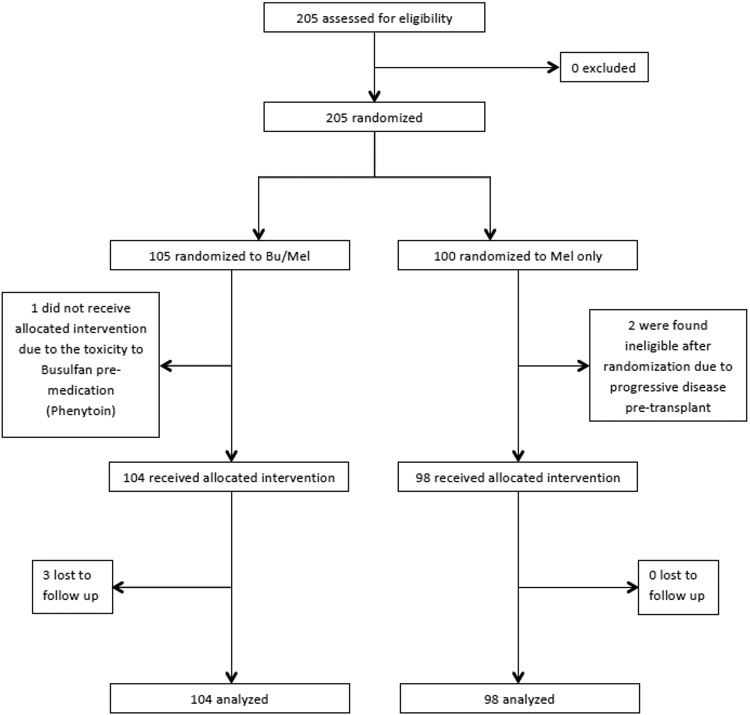
Between October 12, 2011 and March 22, 2017, two hundred and five patients (Bu-Mel, n=105; Mel, n=100) were enrolled (Figure 1). One Bu-Mel patient did not receive the allocated treatment due to the toxicity to pre-medication with phenytoin. Two of the Mel patients were enrolled but did not meet one of the inclusion criterion (i.e., at least SD). These three patients were excluded from all of the analyses. Patient characteristics are summarized in Table 1. The response rate to the induction therapy [≥ PR) was 96% (n=100) in the Bu-Mel arm and 94% (n=92) in the Mel arm. The R-ISS stage, patients with high-risk cytogenetics, type of induction therapy, and type of maintenance therapy after transplant, were comparable between the two arms. Eighty-four percent (n=87) of patients in the Bu-Mel arm and 86% (n=84) of patients in the Mel arm initiated the maintenance therapy. The patients continued maintenance therapy until disease progression or unacceptable toxicity. Eighteen percent (15/84) of patients in the Bu-Mel arm discontinued maintenance therapy due to disease progression (after a median duration of 22.9 months of maintenance) compared to 27% (22/82) in the Mel arm (after a median duration of 17.9 months of maintenance). A higher incidence of earlier disease progression in the Mel arm resulting in early discontinuation of maintenance therapy partly explains the shorter median duration of maintenance in the Mel arm. Furthermore, the median follow up in the Bu-Mel arm was 22.6 months, compared to 20.2 months in the Mel arm, which might also have contributed to a shorter duration of maintenance in the Mel arm.
Figure 1.
Trial profile
Table 1.
Patient Characteristics
| Variable | Bu-Mel (N=104) |
Melphalan (N=98) |
|---|---|---|
| Age at auto-HCT, years (IQR) | 58.9 (53.3-65.0) | 59.5 (51.9-64.2) |
| Median age at auto-HCT, years (range) | 57.9 (31.7-70.9) | 57.6 (34.3-70.6) |
| Gender | ||
| Male | 61 (59%) | 55 (56%) |
| Cytogenetic risk | ||
| High | 32 (31%) | 30 (31%) |
| Standard | 72 (69%) | 68 (69%) |
| Specific abnormalities | ||
| Deletion 17p | 11 (11%) | 8 (8%) |
| Translocation (4; 14) | 6 (6%) | 4 (4%) |
| Amplification 1q | 19 (18%) | 20 (20%) |
| ≥ 3 abnormalities | 3 (3%) | 3 (3%) |
| R-ISS | ||
| I | 33 (39%) | 34 (47%) |
| II | 27 (32%) | 21 (29%) |
| III | 24 (29%) | 18 (25%) |
| Missing | 20 | 25 |
| Serum LDH | ||
| Normal | 63 (89%) | 59 (86%) |
| Abnormal | 8 (11%) | 10 (14%) |
| Missing | 33 | 29 |
| HCT-CI, median (range) | 2 (0-7)* | 1 (0-7) |
| 0 | 35 (35%) | 44 (45%) |
| 1-2 | 23 (23%) | 39 (40%) |
| ≥3 | 43 (43%) | 15 (15%) |
| Response to induction | ||
| sCR + CR | 12 (12%) | 15 (15%) |
| nCR | 10 (10%) | 13 (13%) |
| VGPR | 32 (31%) | 28 (29%) |
| PR | 46 (44%) | 36 (37%) |
| SD | 4 (4%) | 6 (6%) |
| Induction regimen | ||
| VRD | 62 (60%) | 56 (57%) |
| VCD | 18 (17%) | 16 (16%) |
| KRD | 9 (9%) | 15 (15%) |
| VD | 6 (6%) | 7 (7%) |
| CBAD | 3 (3%) | 2 (2%) |
| RD | 5 (5%) | 1 (1%) |
| Other | 1 (1%) | 1 (1%) |
| Maintenance regimen | ||
| Lenalidomide (Len) | 59 (57%) | 57 (58%) |
| Len+Elotuzumab | 12 (12%) | 8 (8%) |
| Len+lxazomib | 6 (6%) | 8 (8%) |
| Len+Dexamethasone | 4 (4%) | 2 (2%) |
| Bortezomib | 6 (6%) | 9 (9%) |
| None | 17 (16%) | 14 (14%) |
| Duration of maintenance therapy (months) | 16.0 (8.5-35.9) | 10.1 (3.7-22.3) |
Data are n (%) or median (IQR) unless otherwise specified.
Bu, busulfan; Mel, melphalan; Auto-HCT, autologous hematopoietic cell transplantation; R-ISS, revised international staging system; LDH, lactate dehydrogenase; HCT-CI, hematopoietic cell transplantation-comorbidity index; CR, complete response; sCR, stringent CR; nCR, near CR; VGPR, very good partial response; PR, partial response; SD, stable disease; PD, progressive disease; VRD, bortezomib-lenalidomide-dexamethasone; VCD, bortezomib-cyclophosphamide-dexamethasone; KRD, carfilzomib-lenalidomide-dexamethasone; VD, bortezomib-dexamethasone; CBAD, cyclophosphamide-bortezomib-adriamycin-dexamethasone; RD, lenalidomide-dexamethasone
n=101
Median time to neutrophil engraftment was 11 days (IQR: 11, 11 days) in the Bu-Mel (n=104) and 11 days (IQR: 11, 12 days) in the Mel arm (n=98). Although median time to neutrophil engraftment was the same in the two arms, the distribution for all patients was significantly lower (i.e., fewer days) for the Bu-Mel arm compared to the Mel arm (p=0.0030). Median times to platelet engraftment were 10 days (IQR: 9, 11 days) and 11 days (IQR: 10, 13 days) in the Bu-Mel (n=104) and the Mel arm (n=98), respectively (p<0.0001). There was no TRM within 100 days in either arm.
Grade II-IV non-hematological toxicity was seen in 103 (99%) of 104 Bu-Mel patients and 82 (84%) of 97 Mel patients (p<0.0001) (Table 2). No patient experienced grade IV mucositis. Only one patient in the entire cohort, who was treated in the Bu-Mel arm, suffered grade IV cardiac toxicity. This patient had an acute myocardial infarction and ventricular fibrillation. However, the patient recovered and received two bare metal stents. At the time of last follow up, this patient was alive and in remission.
Table 2.
Non-hematologic toxicity
| Adverse Event | All Patients (N=202) |
Busulfan+Melphalan (N=104) |
Melphalan (N=98) |
p-value |
|---|---|---|---|---|
| Overall | ||||
| None | 0 | 0 | 0 | <0.0001 |
| Grade 1 | 17 (8%) | 1 (1%) | 16 (16%) | |
| Grade 2 | 66 (33%) | 16 (15%) | 50 (51%) | |
| Grade 3 | 118 (58%) | 86 (83%) | 32 (33%) | |
| Grade 4 | 1 (0.5%) | 1 (1%) | 0 | |
| Diarrhea | ||||
| None | 67 (33%) | 45 (43%) | 22 (22%) | 0.056 |
| Grade 1 | 107 (53%) | 44 (42%) | 63 (64%) | |
| Grade 2 | 21 (10%) | 11 (11%) | 10 (10%) | |
| Grade 3 | 7 (3%) | 4 (4%) | 3 (3%) | |
| Mucositis | ||||
| None | 54 (27%) | 4 (4%) | 50 (51%) | <0.0001 |
| Grade 1 | 57 (28%) | 23 (22%) | 34 (35%) | |
| Grade 2 | 76 (38%) | 62 (60%) | 14 (14%) | |
| Grade 3 | 15 (7%) | 15 (14%) | 0 | |
| Nausea | ||||
| None | 9 (4%) | 7 (7%) | 2 (2%) | 0.91 |
| Grade 1 | 51 (25%) | 22 (21%) | 29 (30%) | |
| Grade 2 | 136 (67%) | 71 (68%) | 65 (66%) | |
| Grade 3 | 6 (3%) | 4 (4%) | 2 (2%) | |
| ALT | ||||
| None | 167 (83%) | 70 (67%) | 97 (99%) | <0.0001 |
| Grade 1 | 25 (12%) | 24 (23%) | 1 (1%) | |
| Grade 2 | 7 (3%) | 7 (7%) | 0 | |
| Grade 3 | 3 (1%) | 3 (3%) | 0 | |
| AST | ||||
| None | 200 (99%) | 103 (99%) | 97 (99%) | 1.00 |
| Grade 1 | 1 (0.5%) | 0 | 1 (1%) | |
| Grade 2 | 1 (0.5%) | 1 (1%) | 0 | |
| Grade 3 | 0 | 0 | 0 | |
| Bilirubin | ||||
| None | 182 (90%) | 94 (90%) | 88 (90%) | 1.00 |
| Grade 1 | 9 (4%) | 5 (5%) | 4 (4%) | |
| Grade 2 | 9 (4%) | 3 (3%) | 6 (6%) | |
| Grade 3 | 2 (1%) | 2 (2%) | 0 | |
| Neutropenic fever | ||||
| None | 99 (49%) | 30 (29%) | 69 (70%) | <0.0001 |
| Grade 1 | 1 (0.5%) | 1 (1%) | 0 | |
| Grade 2 | 0 | 0 | 0 | |
| Grade 3 | 102 (51%) | 73 (70%) | 29 (30%) |
Data are n (%%)
ALT, alanine aminotransferase; AST, aspartate aminotransferase
Overall 5 (2%) of 202 patients, 2 (2%) of 104 in the Bu-Mel arm and 3 (3%) of 98 in the Mel arm, developed second primary malignancies (SPM). In the Bu-Mel arm, 1 patient developed squamous cell skin cancer and rectal adenocarcinoma, and 1 patient developed melanoma and basal cell skin carcinoma. In the Mel arm, 2 developed skin cancers (squamous cell) and one developed myelodysplastic syndrome.
At day-90 after transplant, 102 (98%) of 104 patients in the Bu-Mel arm and 95 (97%) of 98 patients in the Mel arm achieved PR or better. Twenty-eight (27%) of 104 patients in the Bu-Mel arm and 33 (34%) of 98 patients in the Mel arm achieved sCR+CR by day 90. Overall, during the entire study period, 53 (51%) of 104 patients in the Bu-Mel arm and 49 (50%) of 98 patients in the Mel arm achieved sCR+CR.
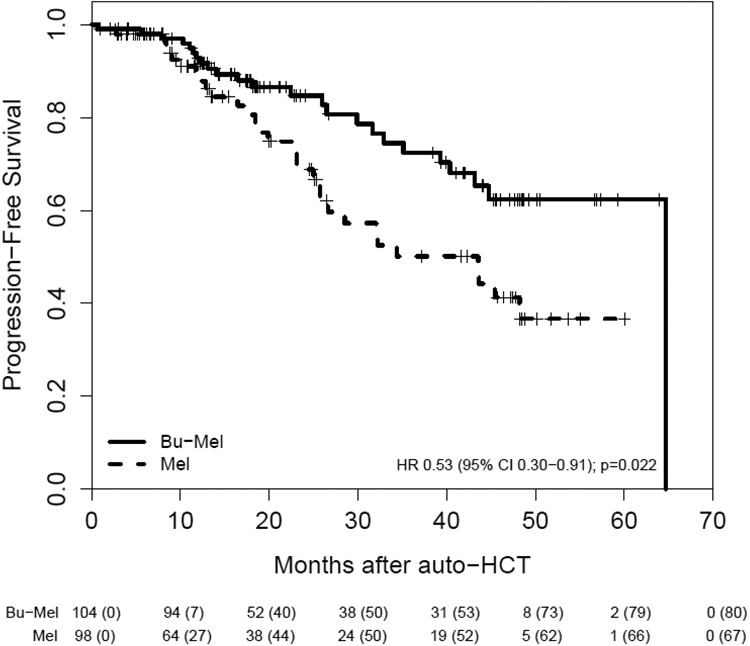
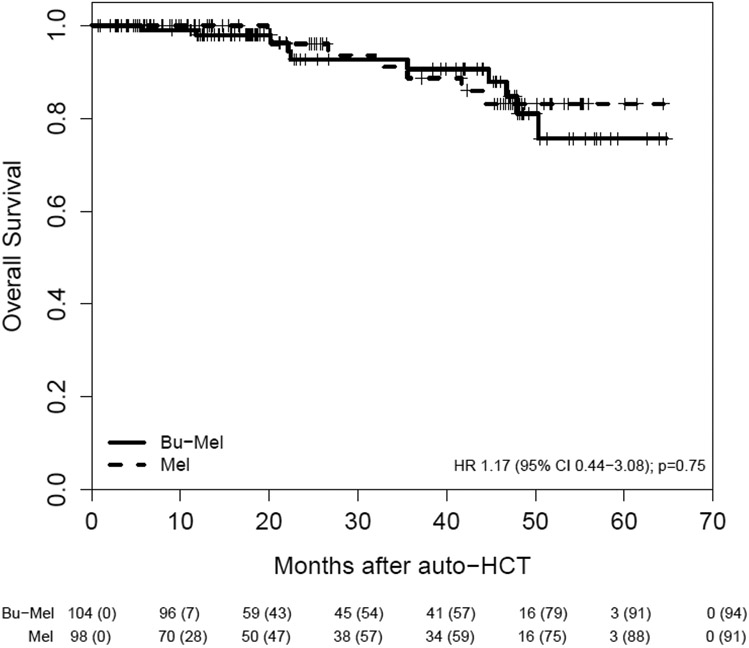
Of the 202 patients, 55 had a PFS event: 24 (23%) of 104 patients in the Bu-Mel arm and 31 (32%) of 98 patients in the Mel arm. The median PFS from auto-HCT in the entire study sample was 64.7 months (IQR: 25.7, 64.7 months). The median PFS was 64.7 months (IQR: 32.9, 64.7 months) in the Bu-Mel vs. 43.5 months (IQR: 19.9 months, not estimated) in the Mel arm, HR = 0.53, 95% CI [0.30, 0.91], (p=0.022). The estimated probability that PFS was at least three years was 0.72 (95% CI: 0.59, 0.82) in the Bu-Mel arm vs. 0.50 (95% CI: 0.35, 0.63) in the Mel arm. Median OS was not reached in either arm. The estimated probability that OS was at least 3 years was 0.91 (95% CI: 0.80, 0.96) with Bu-Mel vs. 0.89 (95% CI: 0.75, 0.95) with Mel. The sample proportions of patients alive at the end of the study follow up were 0.79 (95% CI: 0.67, 0.87) in the entire sample with corresponding proportions 0.76 (95% CI: 0.56, 0.87) in the Bu-Mel arm and 0.83 (95% CI: 0.68, 0.92) in the Mel arm. (Figures 2A & 2B). A total of 17 (8%) patients died during the trial. Ten patients (10%) died in the Bu-Mel arm — seven primarily due to progressive disease and three due to infection at 5.7 months, 1-year, and 22-months after auto-HCT. Seven patients (7%) died in the Mel arm, all due to progressive disease.
Figure 2A.
Progression-Free Survival
Figure 2B.
Overall Survival
When the subset of 62 patients with high-risk cytogenetics was analyzed separately, a significantly longer PFS was observed in the Bu-Mel arm (median not reached vs. median = 25.0 months [IQR: 16.5, 26.6 months] in the Mel arm (HR = 0.23, 95% CI: [0.08, 0.69], p=0.0087). Median OS was not reached in either treatment arm. The estimated probabilities that PFS was at least three years in high-risk patients were 0.93 (95% CI: 0.61, 0.99) and 0.89 (95% CI: 0.43, 0.98) in the Bu-Mel and Mel arms, respectively.
A fitted Bayesian piecewise exponential regression model showed significantly superior PFS time from auto-HCT in the Bu-Mel arm (Table 3). For the Bu-Mel versus Mel effect (n=157), the posterior probability of a beneficial effect (PBE) was 0.956, HR = 0.60 with 95% posterior credible interval 0.25-1.01. Disease response to the induction therapy of PR or better, R-ISS stages I and II, age > 65, presence of high-risk cytogenetic abnormalities and randomization algorithm Method 2 did not have any significant effects on PFS (Table 3).
Table 3.
Fitted Bayesian piecewise exponential survival time regression model for progression-free survival time (N=157, number of events=39)
| Posterior Quantities | |||||
|---|---|---|---|---|---|
| Variable | Mean of β | SD of β | Posterior 95% Credible Interval |
Pr(β > 0 | Data) | |
| Bu-Mel | −0.572 | 0.342 | −1.258 | 0.077 | 0.045 |
| > 65 years of age | −0.007 | 0.418 | −0.826 | 0.795 | 0.513 |
| High-Cytogenetic Risk |
0.200 | 0.395 | −0.555 | 0.983 | 0.697 |
| R-ISS Stage III | 0.235 | 0.389 | −0.563 | 0.957 | 0.730 |
| PR or better to induction therapy | −0.375 | 0.639 | −1.552 | 0.893 | 0.256 |
| 2nd Randomization Algorithm | −0.296 | 0.862 | −2.055 | 1.289 | 0.397 |
R-ISS, revised international staging system; PR, partial response
One hundred and sixty-five patients (Bu-Mel, n=81; Mel, n=84) completed at least one MDASI-MM assessment from before the start of the treatment regimen through the four weeks following the transplant. At baseline, t-tests showed significantly higher mean severity of constipation (1.80, standard deviation [SD] = 2.87 vs. 0.98, SD = 1.94; p=0.036), muscle weakness (2.38, SD=2.49 vs. 1.23, SD=1.86; p=0.0013), diarrhea (1.45, SD=2.43 vs. 0.60, SD=1.10; p=0.0052), and global symptom interference (2.96, SD=2.81 vs. 1.77, SD=2.00; p=0.0030) in the Bu-Mel arm versus the Mel arm. The Bu-Mel patients had a significantly higher mean severity of pain (5.67, SD=2.65 vs. 3.17, SD=3.07; p=0.0043) and mouth sores (7.35, SD=2.41 vs. 1.25, SD=2.22; p<0.0001) than the Mel patients at 7 days post-auto-HCT. Longitudinal analyses of repeatedly measured QOL variables using mixed models showed that the Bu-Mel arm had a significantly higher mean severity of pain (estimated difference [ED]=1.102, p=0.0029), drowsiness (ED=0.674, p=0.039), dry mouth (ED=0.904, p=0.0086), constipation (ED=0.695, p=0.0060), muscle weakness (ED=0.815, p=0.0059), mouth sores (ED=1.683, p<0.0001), rash (ED=0.362, p=0.018), impairment of general activity (ED=1.015, p=0.0095), working (ED=1.229, p=0.0058), and walking, (ED=0.920, p=0.0092) than the Mel arm during the 4 weeks following auto-HCT.
Discussion
To the best of our knowledge, the current study is the first prospective randomized clinical trial reporting the comparison of Bu-Mel to Mel as conditioning regimens before auto-HCT for multiple myeloma. The result demonstrates that Bu-Mel conditioning is likely to provide significantly superior PFS compared to Mel alone. However, this appears to be a trade-off for significantly higher observed adverse event rates with Bu-Mel.
The linear pharmacokinetics of Mel has allowed dose escalation that has been explored in earlier studies(26). Doses up to 300 mg/m2 have been studied. These studies showed that despite an increase in response rate, the use of higher doses of Mel was associated with increased toxicity, primarily cardiac and gastrointestinal, and lack of a clear survival advantage over Mel 200(3). Our data, in line with the previous report from the Spanish group, indicate that the combination of the two alkylating agents, PK-guided IV-Bu and Mel, provides a useful platform to administer more aggressive conditioning chemotherapy that is associated with superior PFS. The precise mechanism of synergism between Bu and Mel is not clear. The difference in chemical structures of Bu and Mel suggests inherent differences in the type of DNA damage induced by these alkylators. Their synergistic cytotoxicity may be attributed to the formation of complex genomic lesions that are more difficult to repair compared with the type of DNA adducts elicited by each drug alone.
The overall incidence of grade II-IV non-hematological toxicity was higher in the Bu-Mel arm. However, no patient experienced grade IV mucositis and only one patient suffered a grade IV adverse event. That patient recovered and remains in remission. A higher incidence of mucositis with Bu-Mel was not completely unexpected, as this has been demonstrated in previous comparisons of Bu-Mel versus Mel(9, 12). Nevertheless, it was encouraging to notice that the incidence of grade III mucositis was low (14%). Going forward, we are considering the addition of the chemoprotective agent, palifermin, to further reduce the incidence and severity of mucositis in this setting(27).
On symptom analysis, unexpectedly, patients in the Bu-Mel arm reported significantly higher severity of some symptoms and greater overall symptom interference than patients on the Mel arm before the start of treatment. It is unknown if this difference in symptom burden before the start of treatment affected the results of the study. Following auto-HCT, patients on the Bu-Mel arm reported more severe mouth soreness, pain, and interference with daily functioning, mirroring the toxicity rating of more severe mucositis in the Bu-Mel arm. The higher intensity of the double-alkylating agent conditioning regimen of Bu-Mel likely led to these differences. The increased severity of drowsiness, dry mouth, constipation, and muscle weakness experienced by the Bu-Mel arm patients likely was due to an increased need for opioids to control severe pain and mouth sores. More constipation in the Bu-Mel arm may explain the toxicity finding of significantly less diarrhea in the Bu-Mel arm, although patient symptom ratings showed no significant difference in diarrhea between the two arms (ED=0.449, p=0.12). The longer PFS with the Bu-Mel regimen may offset the greater symptom burden early post-auto-HCT. However, the experience of these symptoms is important to patients and should be monitored, managed, and included in descriptions of what to expect during provider-patient treatment decision-making.
There was no TRM at day +100 in the Bu-Mel arm, in contrast with the Spanish study(10). This was primarily due to the absence of VOD, which we attribute to the use of IV Bu with pharmacokinetic dose adjustment, resulting in more predictable pharmacokinetics and a lower intra- and inter-patient variability in exposure(11).
The improvement in PFS with Bu-Mel was observed in the absence of a higher CR rate. This observation is consistent with that of the Spanish group where the PFS benefit with Bu-Mel was observed without any significant improvement in CR rate over Mel alone(9, 12). While a higher depth of response after auto-HCT is associated with longer PFS(28), the durability of response may be equally important. A sustained CR or a lesser response that is continued for a longer duration is associated with improved survival compared to an early loss of CR after transplant(29, 30). Furthermore, among the CR patients, those with undetectable MRD at day 100 had a more durable CR. It, therefore, is possible that synergistic cytotoxicity with Bu-Mel conditioning may yield a deeper and more sustained response, which translates into longer PFS. Since our study was initiated before widespread application of sensitive flow cytometry and next generation sequencing-based assays for MRD detection, we were not able to evaluate MRD status according to current IMWG guidelines. The prolongation of PFS without a discernable difference in initial response rates suggests that the Bu-Mel combination may affect myeloma stem-like cells that contribute to late relapse.
When we separately analyzed the subgroup of cytogenetically defined high-risk myeloma patients, the PFS benefit with the Bu-Mel arm was still significant (not reached vs. 25 months with Mel). The PFS, however, was comparable in both arms in patients with R-ISS stage III disease. The low number of patients with R-ISS stage III disease (24 in Bu-Mel and 18 in Mel) precluded a more robust analysis. Despite an apparent PFS benefit in the cytogenetically characterized high-risk patients, OS was similar in both treatment groups. A longer follow up is needed to appreciate any OS advantage with the Bu-Mel arm.
Improving the efficacy of pre-transplant conditioning regimens remains an essential goal in improving the overall outcomes of myeloma patients. Several trials are ongoing in this context. A randomized trial is comparing Bendamustine plus Mel 200 to Mel 200 in newly diagnosed and relapsed/refractory myeloma (NCT03187223). A phase II trial is comparing Mel 200 with carmustine, etoposide, cytarabine, and melphalan (BEAM) as a preparative regimen for patients with newly diagnosed myeloma (NCT03570983). The Spanish Myeloma Group has completed accrual on a phase III randomized study of induction therapy with bortezomib (velcade), lenalidomide (revlimid), and dexamethasone (VRD) followed by auto-HCT with MEL or Bu-Mel conditioning in newly diagnosed myeloma patients (NCT01916252). The primary outcome measure is PFS assessment between the two arms with MRD immunofixation negative CR assessment as the secondary outcome measure. The results of this trial will help validate the results of our study. Other avenues, which will possibly improve the results of upfront auto-HCT involve induction therapy combining novel agents with monoclonal antibodies. It is conceivable that with newer therapies in the pre-transplant and post-transplant setting the MRD negative remission rate is likely to increase, which is essential for obtaining long-term remission and improving survival after transplant(31, 32).
We acknowledge the several limitations in our study. First, we did not have sufficient data to evaluate the MRD status and its impact on survival. Second, the induction and post-transplant maintenance therapies, although similar in both arms, were not prespecified. Third, this is a study from a single institution with a large volume of myeloma patients. These results should be verified in a cooperative group or a multicenter randomized study to assess the generalizability.
Despite the existing limitations, we have reported the results of a randomized phase III trial, which identifies Bu-Mel as a superior conditioning regimen regarding PFS compared to Mel alone, although with a higher adverse event burden. An OS advantage was not observed. These data suggest that Bu-Mel conditioning can serve as a useful platform for further improvement of transplant outcomes in myeloma patients.
Supplementary Material
Research in context.
Evidence before this study
High-dose chemotherapy using melphalan 200 mg/m2 (Mel 200) is considered the standard conditioning regimen for autologous hematopoietic cell transplantation (auto-HCT) in patients with multiple myeloma. This recommendation is based on a large randomized clinical trial that showed the superiority of Mel 200 over a combination of Mel 140 plus total body irradiation (Moreau et al. Blood 2002; 99:731-735). We searched PubMed for randomized clinical trials of conditioning regimens in patients with multiple myeloma using the terms, “conditioning regimen,” “multiple myeloma,” and “randomized” (date restriction: January 1, 1995 -- January 31, 2018) without any language restrictions. We found there is a paucity of data, and only a few randomized clinical trials compared various drug combinations with Mel 200 for pre-transplant conditioning in newly diagnosed myeloma. Notably, none of these combinations demonstrated a survival advantage over Mel 200. Based on the existing evidence, the American Society for Blood and Marrow Transplantation recommends Mel 200 as standard regimen for MM conditioning, outside of a clinical trial (Shah et al. Biol Blood Marrow Transplant 2015; 21:1155-1166). Nonetheless, a retrospective analysis of the Spanish Registry showed that the combination of busulfan plus melphalan (Bu-Mel) resulted in longer event-free survival in myeloma patients undergoing auto-HCT. Based on these results, the Spanish group initiated a prospective study of Bu-Mel conditioning in myeloma patients. However, it could not be completed due to excessive toxicity in the form of veno-occlusive disease (VOD), which was attributed to oral Bu in the Bu-Mel arm (Lahuerta et al. Haematologica 2010; 95: 1913-20). The conditioning regimen was thus changed to Mel alone. The final analysis of this study showed that the combination of Bu-Mel was associated with significantly longer progression-free survival compared to Mel alone. Subsequently, with the development of intravenous busulfan, the incidence of VOD has significantly decreased. Prior to our study, no randomized trial has compared Mel 200 with IV Bu-Mel.
Added value of this study
To our knowledge, this is the first randomized phase 3 trial, which shows a statistically significant progression-free survival benefit of Bu-Mel compared to the widely accepted standard of care Mel 200 pre-transplant conditioning in myeloma patients. These data are a major advance in the field of auto-HCT for multiple myeloma.
Implications of all available evidence
These results highlight the efficacy of Bu-Mel conditioning prior to auto-HCT for multiple myeloma and perhaps establish this combination as a new standard for clinical use and future trials development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in the abstract form at the American Society of Clinical Oncology annual meeting in 2014 (JCO, 2014 ASCO Annual Meeting Abstracts. Vol 32, No 15_suppl), the American Society for Blood and Marrow Transplantation and Center for International Blood & Marrow Transplant Research annual meeting in 2015 (BBMT, 2015, Vol 21, Supplement, Pages S87-S88), the American Society of Hematology annual meeting in 2017 (Blood 2017, 130:399), and the European Hematology Association annual meeting in 2018.
Declaration of interests
We declare no competing interests.
Contributor Information
Qaiser Bashir, Department of Stem Cell Transplantation & Cellular Therapy, The University of Texas MD Anderson Cancer Center.
Prof Peter F. Thall, Department of Biostatistics, The University of Texas MD Anderson Cancer Center
Denái R. Milton, Department of Biostatistics, The University of Texas MD Anderson Cancer Center.
Patricia S. Fox, Department of Biostatistics, The University of Texas MD Anderson Cancer Center.
Jitesh D Kawedia, Department of Stem Cell Transplantation & Cellular Therapy, The University of Texas MD Anderson Cancer Center
Prof Partow Kebriaei, Department of Stem Cell Transplantation & Cellular Therapy, The University of Texas MD Anderson Cancer Center.
Nina Shah, Department of Hematology and Blood and Marrow Transplant, University of California San Francisco.
Krina Patel, Department of Lymphoma/Myeloma, The University of Texas MD Anderson Cancer Center.
Prof Borje S. Andersson, Department of Stem Cell Transplantation & Cellular Therapy, The University of Texas MD Anderson Cancer Center.
Prof Yago L. Nieto, Department of Stem Cell Transplantation & Cellular Therapy, The University of Texas MD Anderson Cancer Center.
Ben C. Valdez, Department of Stem Cell Transplantation & Cellular Therapy, The University of Texas MD Anderson Cancer Center
Simrit Parmar, Department of Lymphoma/Myeloma, The University of Texas MD Anderson Cancer Center.
Gabriela Rondon, Department of Stem Cell Transplantation & Cellular Therapy, The University of Texas MD Anderson Cancer Center.
Ruby Delgado, Department of Stem Cell Transplantation & Cellular Therapy, The University of Texas MD Anderson Cancer Center.
Prof Chitra Hosing, Department of Stem Cell Transplantation & Cellular Therapy, The University of Texas MD Anderson Cancer Center.
Prof Uday R. Popat, Department of Stem Cell Transplantation & Cellular Therapy, The University of Texas MD Anderson Cancer Center.
Betul Oran, Department of Stem Cell Transplantation & Cellular Therapy, The University of Texas MD Anderson Cancer Center.
Stefan O. Ciurea, Department of Stem Cell Transplantation & Cellular Therapy, The University of Texas MD Anderson Cancer Center.
Prof Pei Lin, Department of Hematopathology, The University of Texas MD Anderson Cancer Center.
Prof Donna M. Weber, Department of Lymphoma/Myeloma, The University of Texas MD Anderson Cancer Center.
Sheeba K. Thomas, Department of Lymphoma/Myeloma, The University of Texas MD Anderson Cancer Center.
Hans C. Lee, Department of Lymphoma/Myeloma, The University of Texas MD Anderson Cancer Center.
Elisabet E. Manasanch, Department of Lymphoma/Myeloma, The University of Texas MD Anderson Cancer Center.
Prof Robert Z. Orlowski, Department of Lymphoma/Myeloma, The University of Texas MD Anderson Cancer Center.
Loretta A. Williams, Department of Symptom Research, The University of Texas MD Anderson Cancer Center
Prof Richard E. Champlin, Department of Stem Cell Transplantation & Cellular Therapy, The University of Texas MD Anderson Cancer Center.
Prof Muzaffar H. Qazilbash, Department of Stem Cell Transplantation & Cellular Therapy, The University of Texas MD Anderson Cancer Center.
Data sharing
Additional data are available for this article; details can be found on page # 4 of the appendix.
REFERENCES
- 1.van Rhee F, Giralt S, Barlogie B. The future of autologous stem cell transplantation in myeloma. Blood. 2014. Jul 17;124(3):328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Usmani SZ, Crowley J, Hoering A, Mitchell A, Waheed S, Nair B, et al. Improvement in long-term outcomes with successive Total Therapy trials for multiple myeloma: are patients now being cured? Leukemia. 2013. Jan;27(1):226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayraktar UD, Bashir Q, Qazilbash M, Champlin RE, Ciurea SO. Fifty years of melphalan use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013. Mar;19(3):344–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palumbo A, Bringhen S, Bruno B, Falcone AP, Liberati AM, Grasso M, et al. Melphalan 200 mg/m(2) versus melphalan 100 mg/m(2) in newly diagnosed myeloma patients: a prospective, multicenter phase 3 study. Blood. 2010. Mar 11;115(10):1873–9. [DOI] [PubMed] [Google Scholar]
- 5.Mansi J, da Costa F, Viner C, Judson I, Gore M, Cunningham D. High-dose busulfan in patients with myeloma. J Clin Oncol. 1992. Oct;10(10):1569–73. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez TE, Hari P, Stiff PJ, Smith SE, Sterrenberg D, Vesole DH. Busulfan, Melphalan, and Bortezomib versus High-Dose Melphalan as a Conditioning Regimen for Autologous Hematopoietic Stem Cell Transplantation in Multiple Myeloma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2016. Aug;22(8):1391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahuerta JJ, Martinez-Lopez J, Grande C, Blade J, de la Serna J, Alegre A, et al. Conditioning regimens in autologous stem cell transplantation for multiple myeloma: a comparative study of efficacy and toxicity from the Spanish Registry for Transplantation in Multiple Myeloma. Br J Haematol. 2000. Apr;109(1):138–47. [DOI] [PubMed] [Google Scholar]
- 8.Lahuerta JJ, Grande C, Blade J, Martinez-Lopez J, de la Serna J, Alegre A, et al. Myeloablative treatments for multiple myeloma: update of a comparative study of different regimens used in patients from the Spanish registry for transplantation in multiple myeloma. Leuk Lymphoma. 2002. Jan;43(1):67–74. [DOI] [PubMed] [Google Scholar]
- 9.Lahuerta JJ, Mateos MV, Martinez-Lopez J, Grande C, de la Rubia J, Rosinol L, et al. Busulfan 12 mg/kg plus melphalan 140 mg/m2 versus melphalan 200 mg/m2 as conditioning regimens for autologous transplantation in newly diagnosed multiple myeloma patients included in the PETHEMA/GEM2000 study. Haematologica. 2010. Nov;95(11):1913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carreras E, Rosinol L, Terol MJ, Alegre A, de Arriba F, Garcia-Larana J, et al. Veno-occlusive disease of the liver after high-dose cytoreductive therapy with busulfan and melphalan for autologous blood stem cell transplantation in multiple myeloma patients. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007. Dec;13(12):1448–54. [DOI] [PubMed] [Google Scholar]
- 11.Madden T, de Lima M, Thapar N, Nguyen J, Roberson S, Couriel D, et al. Pharmacokinetics of once-daily IV busulfan as part of pretransplantation preparative regimens: a comparison with an every 6-hour dosing schedule. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007. Jan;13(1):56–64. [DOI] [PubMed] [Google Scholar]
- 12.Blanes M, Lahuerta JJ, Gonzalez JD, Ribas P, Solano C, Alegre A, et al. Intravenous busulfan and melphalan as a conditioning regimen for autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: a matched comparison to a melphalan-only approach. Biol Blood Marrow Transplant. 2013. Jan;19(1):69–74. [DOI] [PubMed] [Google Scholar]
- 13.Blanes M, Gonzalez JD, Lahuerta JJ, Ribas P, Lorenzo I, Boluda B, et al. Bortezomib-based induction therapy followed by intravenous busulfan-melphalan as conditioning regimen for patients with newly diagnosed multiple myeloma. Leuk Lymphoma. 2014. Jul 17:1–5. [DOI] [PubMed] [Google Scholar]
- 14.Freytes CO, Toro JJ, Yeh RF, Stadtmauer EA, Ratanatharathorn V, Akpek G, et al. Safety and Efficacy of Targeted-Dose Busulfan and Bortezomib as a Conditioning Regimen for Patients with Relapsed Multiple Myeloma Undergoing a Second Autologous Blood Progenitor Cell Transplantation. Biol Blood Marrow Transplant. 2014. Aug 16. [DOI] [PubMed] [Google Scholar]
- 15.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975. Mar;31(1):103–15. [PubMed] [Google Scholar]
- 16.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006. Sep;20(9):1467–73. [DOI] [PubMed] [Google Scholar]
- 17.Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016. Jun 16;127(24):2955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones D, Vichaya EG, Wang XS, Williams LA, Shah ND, Thomas SK, et al. Validation of the M. D. Anderson Symptom Inventory multiple myeloma module. Journal of hematology & oncology. 2013. Feb 5;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000. Oct 1;89(7):1634–46. [DOI] [PubMed] [Google Scholar]
- 20.Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. Journal of the National Cancer Institute Monographs. 2007. (37):16–21. [DOI] [PubMed] [Google Scholar]
- 21.Mehta CR PN, Gray R. On computing an exact confidence interval for the common odds ratio in several 2 × 2 contingency tables. Journal of American Statistical Association 1985;80:969–73. [Google Scholar]
- 22.van Elteren PH. On the Combination of Independent Two Sample Tests of wilcoxon. Bulletin of the Institute of International Statistics 1960;37:351–61. [Google Scholar]
- 23.Kaplan EMP. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–81. [Google Scholar]
- 24.Mantel N Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer chemotherapy reports. 1966. Mar;50(3):163–70. [PubMed] [Google Scholar]
- 25.Ibrahim JGCM-H, Sinha D. Bayesian Survival Analysis. Springer, New York. 2004. [Google Scholar]
- 26.Nath CE, Shaw PJ, Trotman J, Zeng L, Duffull SB, Hegarty G, et al. Population pharmacokinetics of melphalan in patients with multiple myeloma undergoing high dose therapy. British journal of clinical pharmacology. 2010. May;69(5):484–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014. May 15;120(10):1453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lonial S, Anderson KC. Association of response endpoints with survival outcomes in multiple myeloma. Leukemia. 2014. Feb;28(2):258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barlogie B, Anaissie E, Haessler J, van Rhee F, Pineda-Roman M, Hollmig K, et al. Complete remission sustained 3 years from treatment initiation is a powerful surrogate for extended survival in multiple myeloma. Cancer. 2008. Jul 15;113(2):355–9. [DOI] [PubMed] [Google Scholar]
- 30.Paiva B, Gutierrez NC, Rosinol L, Vidriales MB, Montalban MA, Martinez-Lopez J, et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012. Jan 19;119(3):687–91. [DOI] [PubMed] [Google Scholar]
- 31.Perrot A, Lauwers-Cances V, Corre J, Robillard N, Hulin C, Chretien ML, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018. Dec 6;132(23):2456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, et al. Association of Minimal Residual Disease With Superior Survival Outcomes in Patients With Multiple Myeloma: A Meta-analysis. JAMA oncology. 2017. Jan 1;3(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data are available for this article; details can be found on page # 4 of the appendix.