Extended Data Figure 7. No gross toxicity was observed in mice treated with rAAV9.2xsup-tRNATyr.
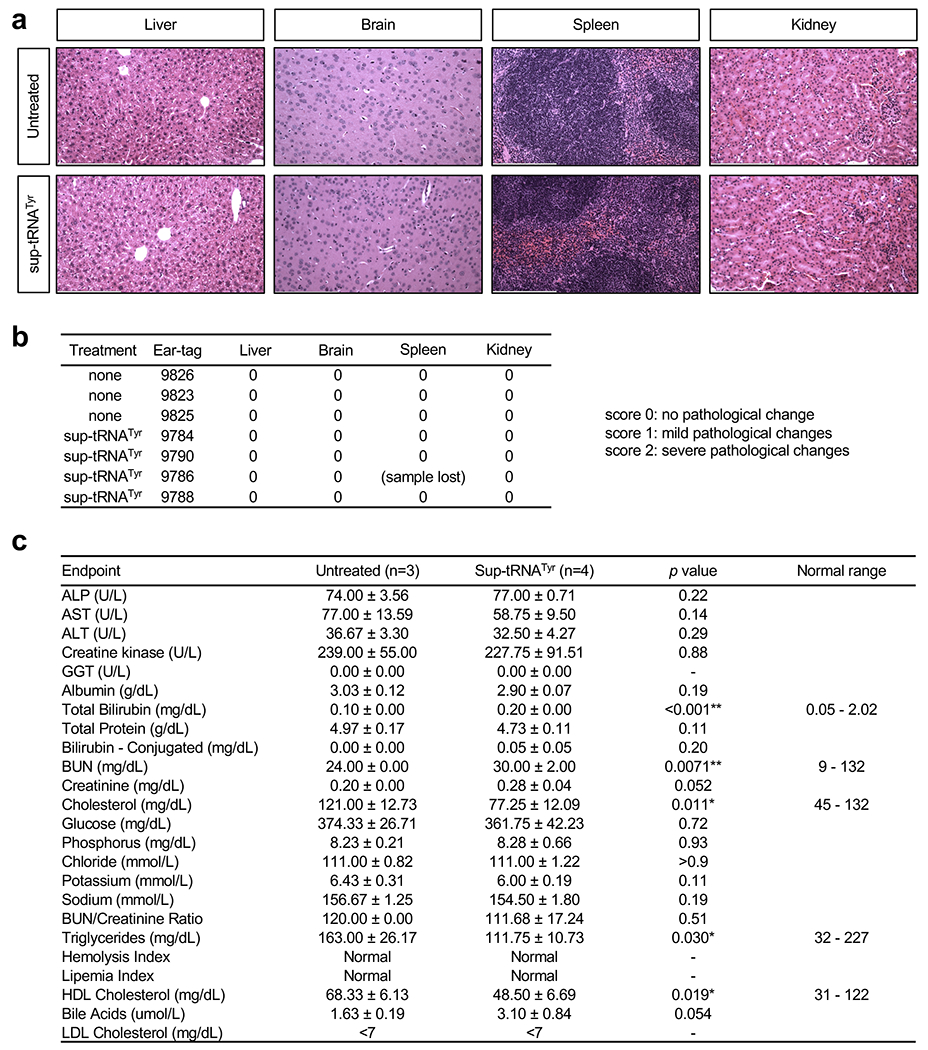
a, Representative H&E staining images of liver, brain, spleen, and kidney sections in untreated (n=3) or sup-tRNATyr-treated (n=4) male KI/+ mice. Mice were treated at 6 weeks old, and euthanized at 16 weeks old. Scale bar=200 μm. b, Blind pathological assessment of the liver, brain, spleen, and kidney H&E slides as described in (a). c, Summary of endpoint serum clinical biochemistry of untreated (n=3) or sup-tRNATyr-treated (n=4) KI/+ mice. Data are mean ± s.d. of biological replicates. Statistical analysis was performed by two-sided Student t-test. *p < 0.05, **p < 0.01. Note that for the endpoints showing statistical significance, values of both groups are within the normal ranges (https://phenome.jax.org/projects/CGDpheno2).
