Abstract
Background
Novel developmental mutations associated with disease are a continuous challenge in medicine. Clinical consequences caused by these mutations include neuron and cognitive alterations that can lead to epilepsy or autism spectrum disorders. Often, it is difficult to identify the physiological defects and the appropriate treatments.
Results
We have isolated and cultured primary cells from the skin of a patient with combined epilepsy and autism syndrome. A mutation in the potassium channel protein Kv10.2 was identified. We have characterised the alteration of the mutant channel and found that it causes loss of function (LOF). Primary cells from the skin displayed a very striking growth defect and increased differentiation. In vitro treatment with various carbonic anhydrase inhibitors with various degrees of specificity for potassium channels, (Brinzolamide, Acetazolamide, Retigabine) restored the activation capacity of the mutated channel. Interestingly, the drugs also recovered in vitro the expansion capacity of the mutated skin cells. Furthermore, treatment with Acetazolamide clearly improved the patient regarding epilepsy and cognitive skills. When the treatment was temporarily halted the syndrome worsened again.
Conclusions
By in vitro studying primary cells from the patient and the activation capacity of the mutated protein, we could first, find a readout for the cellular defects and second, test pharmaceutical treatments that proved to be beneficial. The results show the involvement of a novel LOF mutation of a Potassium channel in autism syndrome with epilepsy and the great potential of in vitro cultures of primary cells in personalised medicine of rare diseases.
Introduction
Autism spectrum disorders (ASD) affect normal brain development. ASD is characterised by deficits in social interaction, delayed communication skills and repetitive patterns of behaviour [1, 2]. Epilepsy encompasses a group of neurological disorders characterised by recurrent epileptic seizures. These seizures cause abnormal neuroelectric flows, resulting in physical injuries and neuronal disabilities [3]. The co-existence of epilepsy and ASD has long been recognised, frequently presented with other developmental disabilities such as developmental delay or intellectual disability [4, 5]. However, the molecular mechanisms involved in the combined development of epilepsy and ASD are unclear. The identification of associated genetic mutations plays a key role in the understanding, diagnosis and treatment of these disorders.
The term channelopathy refers to a spectrum of disorders caused by dysfunction of ion channels [6]. Ion channels are transmembrane proteins that facilitate a specific inflow/outflow of ions according to their electrochemical gradient. Genetic brain channelopathies arise from inherited or de novo mutations of ion channel genes within the central nervous system. These mutations elicit homeostatic imbalances in membrane excitability, commonly related with epilepsy, ASDs, intellectual disability or ataxia. The role of potassium channels has been highlighted within the described channelopathies, because of their function in the recovery of the resting membrane potential [7–9].
The Kv10.2 channel (also known as Eag2, encoded by the KNCH5 gene) belongs to the ether-à-go-go (Eag) potassium channel family, within the superfamily of voltage-gated potassium (Kv) channels. Eag channel family consists of two known members in human, Kv10.1 and Kv10.2 [10, 11]. Kv10.2 is widely expressed in the central nervous system and organs such as skeletal muscle, heart and lung, playing a key role in setting membrane potentials and action potential repolarisation [12]. Kv10.2 has been reported to participate in tumour growth, cell size control and mitotic entry in medulloblastoma and has been proposed as a tumour marker and a therapeutic target [13–15]. However, its physiological function and role in neurological disorders is not fully understood.
A mutation in Kv10.2 has only been reported once before in human, a Kv10.2 gain of function (GOF) mutation was identified in a child with epileptic encephalopathy [16, 17]. Here we report a case of a child that presented at the age of two years old epilepsy and mild developmental delay. Although epilepsy was under apparent good clinical control, the child displayed language and motor regression, ataxia and a progressive autistic behaviour. A genetic study identified a mutation in the KCNH5 gene, resulting in a punctual substitution in the Kv10.2 channel. By using cDNA constructs of the wild type or the mutated protein, we performed electrophysiological analyses in cellula. This demonstrated a loss of function (LOF) of the mutant channel. In addition, we isolated and cultured cells from the patient displaying significant growth and differentiation defects. Proliferation was not seemingly affected therefore we hypothesise that the channel dysfunction might affect the shape and spreading of cells via the cytoskeleton. Interestingly, pharmacological chemicals that promote potassium intake recovered growth expansion proprieties of the skin cells and also the activation capacity of mutant Kv10.2. Following the in vitro observations, the patient was treated with one such drugs, what achieved significant improvement. Therefore, we show that pharmaceutical rescue of a Kv10.2 mutant channel improved the clinical evolution of an infant with an epilepsy-cognitive syndrome. We discuss the potential implications of the cellular defects observed into the molecular biology of rare genetic neurological disorders.
Results
An 11-year-old boy patient displayed moderate to severe developmental delay, autism, epilepsy and minimal physical dysmorphia and hyperlaxity during physical exploration. From 2 years of age onwards, after childhood episodic epilepsy attacks, he presented a severe chronic neuro-developmental complex disorder phenotype with intellectual disability and signs of the autism spectrum. The patient also displayed spinocerebellar ataxia, losses of motor skills and language regression until total loss at the age of 6. No structural alterations were observed during brain MRI (Magnetic Resonance Imaging; not shown). The karyotype test did not show any disturbance and biochemical and metabolic profiles were normal.
The patient received various antiepileptic treatments with variable impact (see Materials and methods). An interesting observation was the differential response to the same compound in different drug vehicles. While valproate (VPA) treatment of the commercial brand Depakine® (Sanofi S.A., France) was effective in controlling epilepsy, a generic VPA preparation increased the frequency of epileptic seizures and deteriorated neurodevelopmental parameters. The different response to a same active principle just depending on the drug vehicle was a determining hint to suspect about a possible channelopathy.
Next-generation sequencing (NGS), in particular Exome sequencing (ES), is a useful tool to identify genetic mutations that may cause neurologic diseases [18–20]. To identify candidate variants with possible functional significance, we performed two different ES analyses, one of them in a trio framework covering the unaffected parents. We found five interesting non-synonymous mutations: two compound heterozygous mutations with one allele inherited from each parent, and one heterozygous mutation inherited from the mother in a gene with a potential role in neurodevelopment disorders (Table 1). The frequency of the Calcium Voltage-Gated Channel Subunit CACNA1I A583T variant is relatively common (gnomAD: 0.002421; TOPMED: 0.003763), close to be considered as a polymorphism (frequency ≥ 0.01). Both variants in Glycogen Synthase Kinase 3 Beta-Interacting NIN gene are already registered in gnomAD and TOPMED database. The only variant not previously reported was the one in KCNH5 gene.
Table 1.
Summary of candidate variants identified and in silico prediction scores in the patient
| Gene | Protein ID | DNA change | AA change | gnomAD | TOPMED | GERP | CADD | REVEL |
|---|---|---|---|---|---|---|---|---|
| CACNA1I | Q9P0X4 | 82C>T | P28S | n.d | 8.00E−06 | 1.27 | 14.69 | 0.350 |
| 1747G>A | A583T | 2.42E−03 | 3.76E−03 | −0.55 | 0.67 | 0.244 | ||
| NIN | Q8N4C6 | 5264C>T | A1755V | 2.10E−05 | 1.10E−05 | 4.50 | 19.36 | 0.078 |
| 6348A>G | I2116M | 2.10E−05 | 1.10E−05 | 1.79 | 13.81 | 0.218 | ||
| KCNH5 | Q8NCM2 | 2566A>C | N856H | n.d | n.d | 5.92 | 21.50 | 0.531 |
gnomAD and TOPMED databases gather previously reported variation frequencies. GERP tool display a conservation score for a nucleotide position, values above 3 are considered as highly conserved. CADD tool scores the predicted deleteriousness of a variant (SNP or indels), values above 20 can be considered as “likely deleterious”. REVEL method combines 13 individual tools to predict the pathogenicity of missense variants, with scores from 0 to 1, it is estimated “likely disease causing” when above 0.5
We performed in silico predictions to evaluate the deleteriousness of the observed mutations (GERP, CADD and REVEL; Table 1). Scores obtained for NIN and CACNA1I mutations did not seem to be deleterious. Furthermore, neither the biological role of NIN nor NIN variant phenotypes reported in ClinVar (219 results) seems to be related with the disease of the patient. With regard to CACNA1I, none of the 47 results gathered in ClinVar were related with epilepsy, just one result was found as ‘Global developmental delay’. Otherwise, all the prediction scores for KCNH5 variant displayed values above the threshold considered as deleterious. Furthermore, 368 of 395 KCNH5 transcript variants found in ClinVar were reported as ‘Early infantile epileptic encephalopathy with suppression bursts’. Therefore, although the healthy mother contained this variant, we considered that KCNH5 mutation holds a role in the disease of the patient, possibly bolstered by another genetic alteration that we did not detect.
KCNH channels are included into the cyclic nucleotide-binding domain (CNBD) channels family [21]. However, KCNH channels do not bind cyclin nucleotides, although they have a cyclic nucleotide-binding homology domain (CNBHD) [22]. The C-terminal CNBD/CNBHD domain interacts with pore opening [23]. Also, the distal C-terminal portion of the CNBHD forms a coiled-coil domain which seems to be necessary for channel tetramerisation. In analogy with the structure of Kv10.1 channel, the Kv10.2 N856H mutation, located closely to the intracellular C-terminal domain, might be part of the CNBHD domain or the terminal coiled-coil domain.
With the above premises, we aimed to investigate whether the KCNH5 mutation affected the function of the channel. We performed in vitro electrophysiological experiments to compare the properties of N856H Kv10.2 mutant channel to the wild type (WT) Kv10.2 one. To this end, we cloned the mutated form into a mammalian expressing vector. We then overexpressed this construct, or the wild type gene, in COS7 cells, as described in Material and Methods.
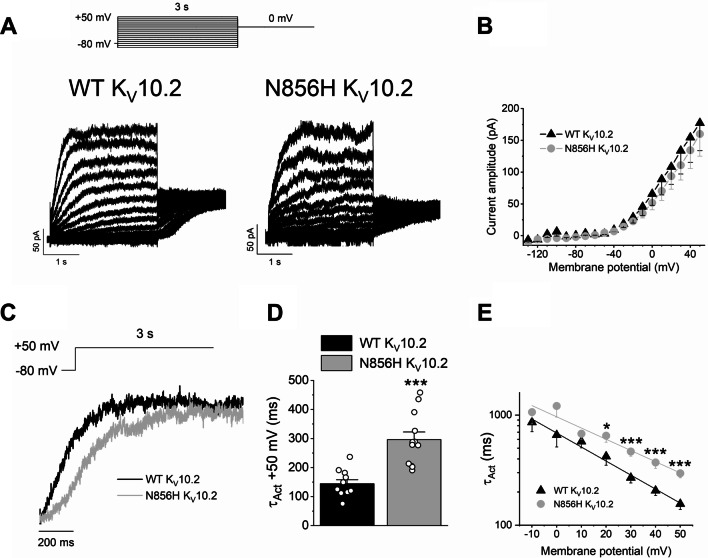
Voltage currents were elicited by 3-s pulses to potentials between −130 and + 50 mV from a holding potential of −80 mV in 10 mV steps, followed by a pulse to 0 mV. After applying this voltage pulse protocol, the slowly activating outward current generated by N856H Kv10.2 mutant channels resulted to be slower than in WT Kv10.2 channels (Fig. 1A). The current–voltage relationship (I-V) was obtained after plotting the amplitude of the current measured at the end of the 3-s pulses versus the membrane potential (Fig. 1B). The magnitude of the current resulted to be similar for both channels. However, the activation kinetics of the current generated by N856H Kv10.2 channels resulted significantly slower than that generated by the activation of WT channels (Fig. 1C, D). This effect was significant at membrane potentials positive to + 20 mV (p < 0.05) (Fig. 1E). These results show that the mutation causes a LOF of the channel.
Fig. 1.
Electrophysiological properties of WT and N856H Kv10.2 channels expressed in COS7 cells. A Current recordings obtained in COS7 cells transfected with WT or N856H Kv10.2, as indicated, after applying the pulse protocol shown on the top. B Current–voltage relationship obtained after plotting the amplitude of the current measured at the end of the 3-s pulses versus membrane potential, as in A. C Normalised current recordings obtained after depolarising cells expressing WT or N856H from −80 mV to + 50 mV. D Time constant of activation of WT or N856H at + 50 mV. E Voltage dependence of the time constant of activation of WT or N856H. n = 12 for WT Kv10.2, n = 11 for N856H Kv10.2. *p < 0.05, **p < 0.01, ***p < 0.001
We questioned whether the mutation of the Kv10.2 channel resulted in cellular defects. To this aim, we isolated cells from the epidermis of the skin of the patient and established primary cultures. We then studied the morphology and behaviour of the mutant keratinocytes. The epidermis is easily accessible and allows isolation and expansion of keratinocytes to establish an in vitro model of the disorder. Primary keratinocytes from the patient (Kmut) were compared with control keratinocytes from the skin of two healthy children with no alterations detected (KCT).
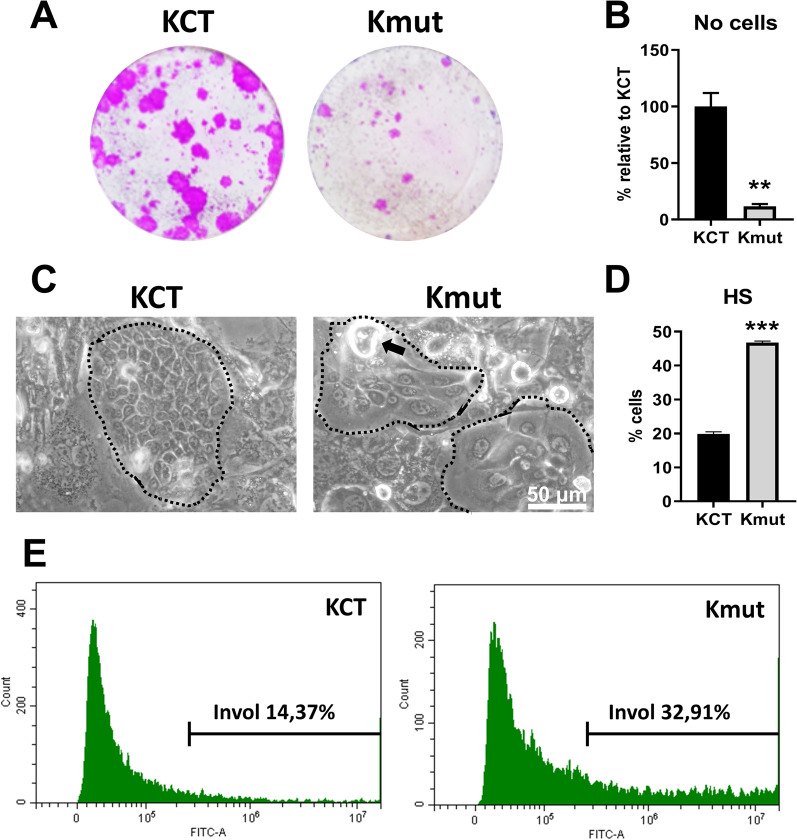
The growth capacity of Kmut was greatly reduced, as monitored by clonogenicity assays (clonal expansion; Fig. 2A) and by the number of growing cells harvested from the plate at the control confluence (Fig. 2B). The in vitro phenotype of primary keratinocytes in these conditions is a useful indicative of the proliferation/differentiation potential of the original tissue. While KCT cells grew tightly packed within large colonies, Kmut cells displayed an altered morphology growing in small colonies. During terminal squamous differentiation, keratinocytes lose their proliferative capacity, increase in size and stratify before turning into corneocytes and shed from the surface [24]. Kmut colonies were composed by large differentiating-like cells less tightly attached to each other (Fig. 2C). Kmut also cells stratified prematurely and more profusely (Fig. 2C; black arrow).
Fig. 2.
N856H Kv10.2 mutant primary cells isolated from the patient display a strong growth defect and enhanced terminal differentiation. Healthy keratinocytes from two healthy infantile donors were used as control (KCT) compared with mut keratinocytes containing the mutation (Kmut). A Representative clonal expansion, as monitored by clonogenicity assays (n = 3 triplicates per essay, 2 essays per healthy strain). B Number of cells harvested in A at confluence of healthy strains, relative to KCT (n = 2, 2 essays per healthy strain). C Representative phase contrast microphotograph of KCT or Kmut cells as indicated. Dotted lines enclose keratinocyte colonies, surrounded by a fibroblastic feeder layer (see “Materials and methods”). Black arrow points to a typical detaching differentiated cell that were very frequent in the mutant cultures. Scale bar, 50 μm. D Bar histogram shows the percentage of cells with high size and complexity, by light scattering parameters (HS), typical of differentiated keratinocytes, as quantified by flow-cytometry (n = 2, as in A). E Expression of the epidermoid differentiation marker involucrin n KCT or Kmut, as indicated, measured by immunodetection and flow cytometry (representative of 3 independent essays). **p < 0.01, ***p < 0.001
To monitor and quantitate the differences in cellular morphology and behaviour, cell size and complexity was analysed by flow cytometry and light scattering parameters. It is well established that keratinocytes become larger and more complex and acquire high light scatter proprieties (HS) as they differentiate [25]. As shown in Fig. 2D, the percentage of Kmut cells with HS values doubled the KCT reference measurements. Involucrin, a precursor protein of the cornified cell envelope, broadly used as a terminal squamous differentiation marker [25, 26], was also increased in Kmut compared to KCT (Fig. 2E). All measured parameters consistently demonstrated a growth defect and an enhanced index of terminal differentiation in the mutant cells.
Carbonic anhydrase inhibitors (CAIs) have been used in the clinic for a wide range of disorders. Among CAIs, sulphonamides are used as diuretics, antiglaucoma or antiepileptic [27]. Acetazolamide (AZA) is a sulphonamide approved as CAI by the Food and Drug Administration (FDA) for the treatment of epilepsy [28, 29]. The inhibition of carbonic anhydrase by AZA might change intracellular pH, with an effect over the transmembrane potential. Among AZA physiological effects, a drop in serum potassium level in the extracellular environment was described, suggesting an increase in the cellular potassium intake [30, 31]. Closely related to AZA, brinzolamide (BZA) is a sulphonamide drug used as CAI with potential use as antiepileptic [32, 33]. In an initial phase to search for a drug efficient in treating the patient for the Kv10.2 channel dysfunction, we went on treating primary skin keratinocytes of the patient with these drugs. In addition, we were interested in testing the retigabine (RTG) compound. This drug has proven to specifically open another closely related family of voltage-gated potassium channels, Kv7.2–5 [34–37], and allows higher dosed treatments. However, RTG is not authorised to treat minors.
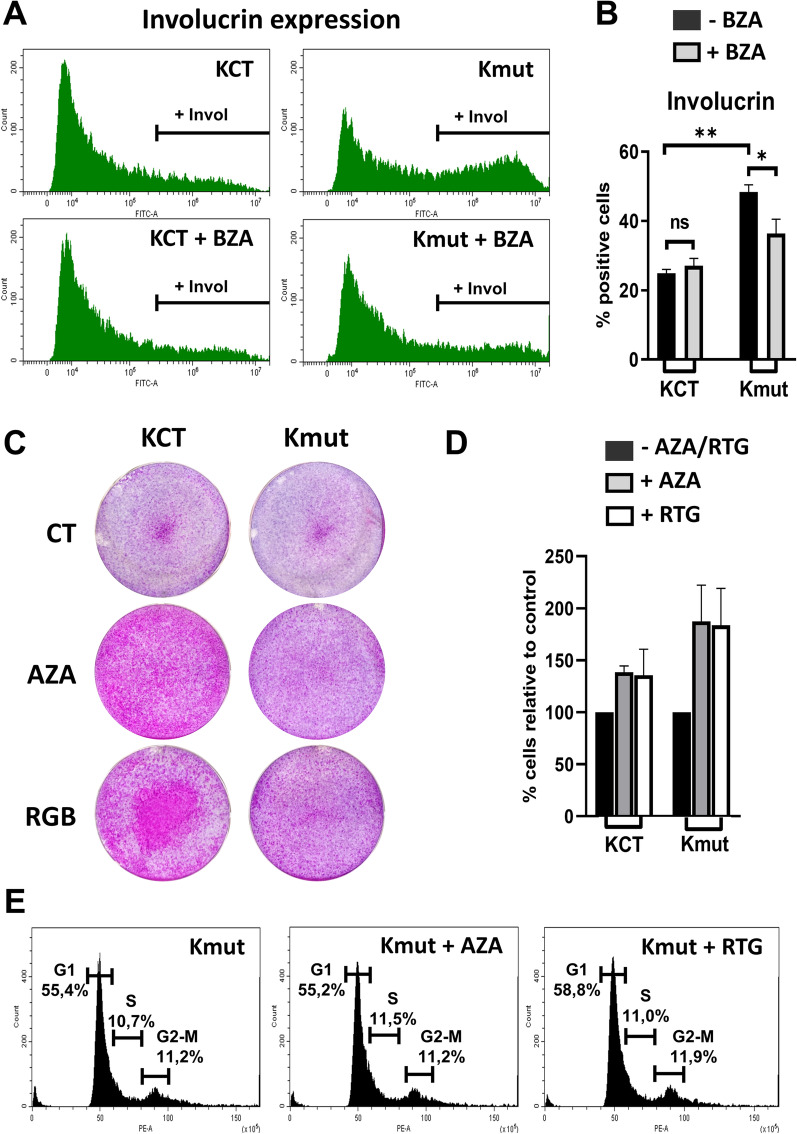
Treatment for 6 days with BZA did not cause significant changes in the growth of control KCT cultures. However, BZA caused a decrease of the involucrin positive Kmut cell population (Fig. 3A, B). AZA and RTG very significantly enhanced clonal expansion (Fig. 3C). However, the difference in the number of cells after treatments was not as striking (Fig. 3D). Furthermore, analyses of DNA content revealed no differences in cell cycle dynamics between KCT and Kmut cells (Fig. 3E). Therefore, AZA and at a higher extent RTG rescued at least in part the growth defect of Kmut cells, although did not influence proliferation rates. Other cellular processes, such as cell shape, adhesion or spreading, might be affected by the Kv10.2 LOF mutation. To note, cell shape and adhesion are critical to keratinocyte growth and differentiation [38].
Fig. 3.
Pharmacological treatments with CAIs recover expansion capacity of the mutant primary cells isolated from the patient without significantly altered the cell cycle. A Representative flow cytometry histograms for the epidermoid differentiation marker involucrin of KCT or mutant Kmut cells as indicated. Cells were treated with 10 μM brinzolamide (BZA) for 6 days as indicated. B Bar histogram shows percentage of KCT or Kmut involucrin positive cells, as quantified by flow cytometry (n = 2). ns: non-significant. C Clonal expansion capacity of KCT upon 10 μM AZA or RGB 7 days treatments. Cells plated at high density, Kmut double number of cells than KCT, colonies in pink (n = 3 in each essay, four essays, with KCT cells from two different individuals). D Number of cells in C, harvested at confluence of KCT. E cell cycle analyses of cells in D, as determined by DNA content stained with Propidium Iodide. *p < 0.05, **p < 0.01
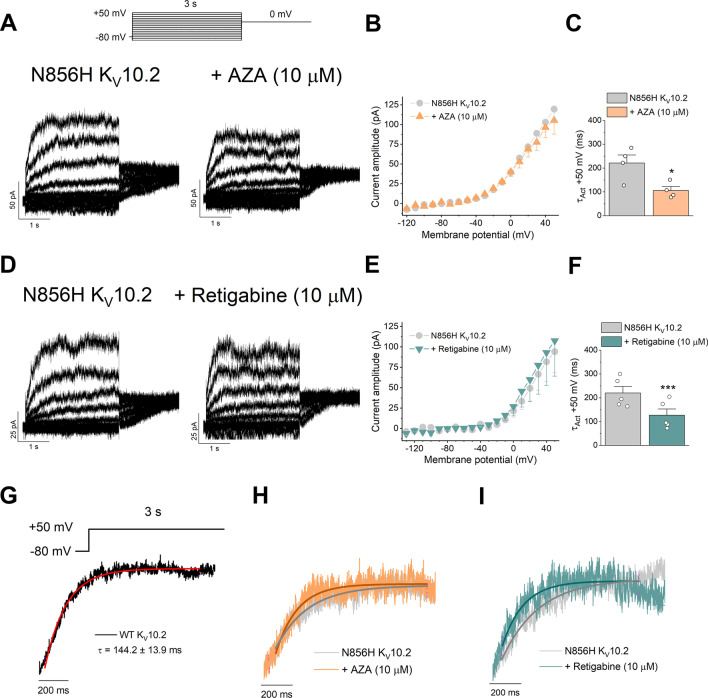
We wanted to determine whether the function of the N856H Kv10.2 was improved by the drugs described above, or the beneficial effect of treatments on Kmut cells was due to the boost of other potassium channels raising the cellular potassium influx. To that aim, we transfected again COS7 cells with the N856H Kv10.2 mutant channel protein and we performed electrophysiological analyses in the absence, or after perfusing transfected cells with AZA or RTG, at 10 μM. Figure 4 shows that N856H Kv10.2 current recordings in the absence and in the presence of AZA (Fig. 4A) or retigabine (Fig. 4D). The magnitude of the current was not modified by either drug (Fig. 4B, E). However, the time constant of activation was significantly shorter in the presence of either AZA or RTG (Fig. 4C, F). Remarkably, either AZA or RTG restored the activation kinetics of N856H Kv10.2, to levels similar of the WT Kv10.2 (Fig. 4G-I).
Fig. 4.
Electrophysiological measurements of N856H Kv10.2 channel expressed in COS7 cells, in presence or absence of AZA or RTG drugs. A Original current recordings of N856H Kv10.2 channels in the absence or presence of AZA, as indicated, after applying the pulse protocol shown on the top. Current traces are shown in 20-mV increments for clarity. B Current–voltage relationship obtained after plotting the amplitude of the current measured at the end of the 3-s pulses versus membrane potential in the absence and in the presence of AZA. C Time constant of activation of N856H Kv10.2 mutant channels at + 50 mV cells in the absence or in the presence of AZA. D–F As in A–C but with RTG instead of AZA. G For comparison, WT (wild type protein) Kv10.2 currents with the exponential fit of activation. H Current recordings elicited after the activation of N856H Kv10.2 mutant channels in the absence or presence of AZA. I Same as in H but with RTG instead of AZA. Data are expressed as the mean ± SEM of n = 4 (for AZA) and n = 5 (for RTG). *p < 0.05, ***p < 0.001
Finally, encouraged by the beneficial in vitro results with the CAI drugs, given that the effect of BRZ on growth was less significant (not shown) and that RTG is not authorised on minors, we introduced AZA in a combined therapeutic treatment of the patient. The aim was to improve the Kv10.2 mutated channel and to stimulate the potassium intake with AZA (250 mg every 12 h) while maintaining VPA and terbutaline treatment as described in “Materials and methods”.
To note, the clinical response to the AZA treatment by the patient was favourable with epilepsy control and significant improvement in mood (personal-social, adaptative), communication, motor and cognitive skills with no adverse effects. The treatment started at a chronological age of 10 years but a calculated neurodevelopment age of 2 to 3 year. The age-equivalent score was obtained from the development quotient (DQ) within the Battelle Developmental Inventory (BDI-2) [39]. This battery of tests analyses 5 areas of neurodevelopment: personal-social, adaptive, motor, communicative and cognitive. BDI-2 allows evaluating children from birth to a chronological age of 8 years, with some special considerations for children with developmental disorders, as in the present case [40, 41].
In 2–4 weeks of AZA combined treatment, the age equivalent representing the raw score earned by children that age, improved in all areas of neurodevelopment, being more impressive in the personal-social, communicative and cognitive skills (Table 2), as noted both by the clinician and the parents. The social, communicative, adaptive, motor and cognitive competences improved from a global DQ of average 2.3 years old (pointed out objects, interacted with the environment, poor social interaction and poor speech until complete loss) to an age-equivalent score of average 5.5 years (recovery of speech, recognised letters and completed interactive games and puzzles). The speech, which had been lost for more than 3 years, improved to 1–3 words phrases and a broad vocabulary. The social behaviour improved from a frequent apathy to being more affectionate with the family and a slight interaction with other children. As for motor skills, the patient improved from reduced walking ability to stair climbing with railing without external help. These improvements partially diminished after two months possibly due to a tolerance effect. However, 6 months after starting the treatment, in order to reduce medication, AZA intake was suppressed and 2 weeks later, the neurodevelopment significantly worsened again with epileptic seizures frequency increased. Then the treatment was re-introduced up to date.
Table 2.
Development quotient quantification initially and after AZA treatment, according to the Battelle Developmental Inventory (BDI‐2), years of cognitive age
| Gobal domains | Area assessed | Initical score | Score after treatment |
|---|---|---|---|
| Adaptative | Self‐care | 2 | 4 |
| Personal responsibility | 2 | 4 | |
| Adult interaction | 3 | 6 | |
| Personal‐social | Peer interaction | 2 | 4 |
| Self‐concept & social role | 2 | 6 | |
| Receptive | 3 | 6 | |
| Communication | Expressive | 2 | 6 |
| Gross motor | 2 | 6 | |
| Motor | Fine motor | 3 | 5 |
| Perceptual motor | 2 | 6 | |
| Attention & memory | 2 | 6 | |
| Cognitive | Reasoning & academic skills | 2 | 6 |
| Perception & concepts | 3 | 6 | |
| Total average improvement | 2.3 | 5.5 |
Discussion
Here we present functional, cellular and clinical evidence for the involvement of a LOF mutation of the Kv10.2 channel in a complex syndrome including mild to severe development delay, epilepsy, language and motor regression and progressive autistic behaviour. The genetic characterisation revealed a non-previously described mutation in KCNH5 gene. This is, to our knowledge, the first LOF mutation reported in human Kv10.2 with clinical consequences. The involvement of this ion channel in epilepsy is further supported by a previously identified GOF mutation in Kv10.2 [16]. It is not striking that either loss or gain of function in a protein channel leads to a dysfunction of the channel, as the activation dynamics are anyhow affected. Recently, Kv10.2 deletion was shown to increase epilepsy susceptibility in a rat model, the authors proposing a role for Kv10.2 in the development of epileptic disorders [42]. Our results also further support suggestions that the chromosomal region around 14q23.2, where KCNH5 is localised, might be a 'hot spot' for neurological diseases. Various clinical features associated with alterations in this chromosomal region have been described, including craniofacial abnormalities, mental retardation and epilepsy [43–45].
What caught our interest in the patient with the Kv10.2 mutation was the autistic regression with progressive language loss and the differential response to antiepileptic treatments with the same active principle (VPA) but different drug vehicles. This fact made that after discarding structural or metabolic diseases, the patient was subjected to a genetic study. Punctual mutations or hemizygous deletion of one or more critical gene(s) controlling neuronal excitability have been associated with the epilepsy phenotype [46]. Because ion channels are important determinants of seizure susceptibility and the Eag‐related voltage‐gated potassium channel Kv10.2 has been previously linked with epileptic disorders, we hypothesised that mutation of this gene may be responsible for the epilepsy phenotype.
An electrophysiological characterisation of mutated N856H Kv10.2 is the inception to elucidate its role in neurological disorder development. Even though the steady-state current amplitude was not altered in the mutant channels, their slower activation kinetics induced a significantly decreased current at short times, what would translate in a LOF of the channels during the neuron action potentials. This decrease of the current magnitude is more relevant at increased firing rates. Since the mutant channel activates more slowly, there should be less potassium efflux, promoting depolarisation of the neurons and likely, epileptic seizures. Just a few milliseconds delay in a channel opening might be enough to change the duration and rate of action potential firing, consequently disturbing the neural balance state thus leading to neurodevelopmental disorders such as epilepsy. The fact that the activation speed was restored by the AZA and RTG drugs further demonstrates that the p.Asn856His mutation causes LOF of the channel.
Interestingly, the Kv10.2 channel is widely expressed in the brain [47, 48]. Frequently, pathogenic potassium channel alterations give rise to a LOF, with reduced activity resulting in changes in the resting membrane potential and the repolarisation process [49]. As the Kv10.2 channel is expressed in pyramidal and excitatory neurons, we suggest that the mutation now identified can enhance their firing frequency. These findings would explain why restoring the potassium intake was an effective therapeutic approach for the patient.
In vitro primary skin keratinocytes characterisation showed a striking growth defect in the mutant cells freshly isolated from the patient. Keratinocyte post-mitotic differentiation is induced by various intracellular pathways, responding to growth factors, loss of cell adhesion, DNA damage or mitotic defects [50, 51]. We therefore hypothesise that the LOF mutation in Kv10.2 might indirectly disrupt some of these cellular processes. Interestingly, although the growth of mutant cells was significantly improved by the CAIs AZA and RTG, the proliferation rate (cell cycle) was not proportionally increased. Therefore, we are tempted to speculate that the LOF of the Kv10.2 channel might affect cell shape, spreading and maybe adhesion capacity. This would explain why mutant keratinocytes detached and stratified prematurely. Changes in cell shape are critical for keratinocyte homeostasis and for the axons of neurons that make synaptic connexions. Interestingly, a crosstalk between ion channel activity and the cytoskeleton has been reported and a physiological role in mechano-signalling proposed [52, 53]. Therefore, a speculative model can be drawn whereby genetic alterations might cause cognitive and epileptic disorders due to their effect on the cytoskeleton, cell shape and spreading, ultimately affecting neuron communication.
The present study not only shows a novel mutation involved in a human rare disease. It also demonstrates the utility of primary cell cultures in personalised medicine. Primary in vitro cell models can emerge as powerful systems to identify genetic mutations, cellular defects and pharmacological treatments in rare (and other) diseases. Skin or oral cell types can be isolated, expand and frozen at significant rates, without the need of repetitive biopsies [54]. As described in Results, the in vitro study due to the use of the BZA substitute equivalent in this case prompted us to modify the clinical treatment and to design a therapeutic combined strategy. The result was a remarkable improvement of the patient in oral and emotional communication, motor skills and epilepsy control. The fact that stoppage of the AZA treatment led to a behaviour regression, further points out the role of the compound in the disease control. Therefore, a general potassium intake strategy elicited good results. In order to optimise this in a more specific treatment and in view of the in vitro results, we are now considering more specific treatments by drugs such as RTG that specifically open another closely related family of voltage-gated potassium channels, Kv7.2–5 [34–37]. This might attenuate the tolerance effect observed with AZA.
The rescue of orphan drugs is an interesting strategy that may lead to finding new applications for disused compounds. An example of this is the use of CAIs in epilepsy while they were initially designed for glaucoma [28, 29, 32, 33]. However, when as in this case, personalised medicine can be implemented, a customised treatment can be designed and in vitro results can put forward the new use. Nowadays, compounds such as RTG have not been tested for its use in minors. One of the key applications of in vitro personalised studies is to validate or not the efficiency of still poorly characterised pharmaceuticals on the actual cells from the patient. These models should in the future provide significant advance in the diagnosis and treatment of rare diseases.
Conclusions
By in vitro studying primary cells from the mutant Kv10.2 patient, we could find a readout for the cellular defects and second, test a pharmaceutical treatment that proved to be beneficial. The results show the involvement of a novel LOF mutation of a Potassium channel in an epilepsy/autism syndrome and provide potential new understanding into neurological genetic rare diseases. In summary, our study proves the great potential of in vitro cultures of primary cells in personalised medicine of rare (and other) diseases that should be further exploited.
Materials and methods
Clinical treatments
The patient received various antiepileptic treatments with variable impact not only in epilepsy control but also in the neurodevelopment and autistic behaviour. Treatment with valproate (400 mg every 12 h), drug enhancing GABAergic transmission, was associated with good epilepsy control and acquisition of new skills. Treatment with terbutaline (3 mg every 12 h) was applied to improve potassium transport via the sodium–potassium pump β-adrenergic-mediated [55–58]. Otherwise, we tested and suppressed treatment with levetiracetam, antiepileptic that inhibits the synaptic vesicle 2A, because of sudden epileptic seizures followed by periods of stagnation or regression in neurodevelopment.
Exome sequencing, processing and analysis
First, we performed an exome sequencing for > 1,870 genes associated with neuropediatric diseases using a Neuroexome gene panel provided by CGPP-IBMC (Spain), followed by a Trio Exome sequencing, with samples of the patient and both parents. For the Neuroexome panel, the next-generation sequencing (NGS) library was prepared using a customized SureSelect XT kit from Agilent (ref. S3133972, Santa Clara, CA, USA) followed by paired-end sequencing on a NexSeq500 from Illumina (San Diego, CA, USA). For the Trio Exome, the NGS library was prepared using a SureSelect Human All Exon V6 kit from Agilent (ref. S07604514), which capture 60 MB of the human exome target sequence, subsequently sequenced on a Novaseq 6000 from Illumina.
For the Neuroexomegene panel, the average exome sequence coverage per nucleotide was 353X, a 98.36% of the target exome sequences captured a minimum of 20 × reads, and a 99.22% were higher to 10x. For the Trio Exome, the average exome sequence coverage per nucleotide was 292X, a 97.4% of the sequences captured a minimum of 20x, and a 97.9% were higher to 10x. To search possible deletions/insertions a personalised Software was used, PattRec [59].
Allele frequencies of the previously known variants were annotated according to gnomAD and TOPMED databases. We displayed GERP score to measure the conservation of the mutated base [60], and CADD tool to measure the predicted deleteriousness of the nucleotide variants [61]. To predict the pathogenicity of missense variants we used the REVEL method, which integrates scores from 13 different prediction tools [62, 63]. Finally, we used ClinVar database to search relationships between genetic variations reported for a gene and associated patient phenotypes [64].
Plasmids and site-directed mutagenesis
The human Kv10.2 cDNA cloned in pcDNA3.1 vector was kindly provided by Luis A. Pardo (Max Planck Institute for Multidisciplinary Sciences, Göttingen, Germany). Point mutation in wild-type Kv10.2 was introduced by PCR, using Herculase II Fusion DNA polymerase (Agilent, Santa Clara, CA USA). PCR cycle programs were as follows: initial denaturation for 4 min at 96 °C, followed by 30 cycles consisting of: 30 s at 96 °C, 1 min at 55 °C and 8 min at 72 °C. After these cycles, another period of 30 s at 72 °C. After these cycles, another period of 30 s at 72 °C. Reference nucleotide sequence used was NG_034062. The patient exhibited a heterozygous potentially pathogenic missense mutation (c.2566A > C; p.Asn856His) affecting a potassium channel (Kv10.2). To that end, the following primers were used (modified sequence is shown in underlined letters):
Forward: 5′ CCAAACACCCACTAAGAAAAACAG 3′
Reverse: 5′ GTGGGTGTTTGGTCACACTG 3′
The product of the PCR was purified with QIAquick Gel Extraction Kit (Qiagen, Germany) and then digested with DpnI (New England Biolab, Ipswich, MA, USA) for 1 h at 37 °C and 15 min at 70 °C in order to remove the traces of the original vector and obtain only the DNA resultant of the PCR. Wild-type and mutant constructs were verified by DNA sequencing.
Cell culture and transient transfection
Primary keratinocytes were isolated and cultured in the presence of a mouse fibroblast feeder layer (inactivated by mitomycin C), in Rheinwald FAD medium supplemented with 10% (v/v) foetal bovine serum (FBS; Gibco, Paisley, UK), 1.2 mM Ca2+, 5 ng/ml Epidermal growth factor (EGF) and 100 U/ml of penicillin/streptomycin (P/S, Gibco), as described previously [65, 66]. Low passages (2–4) keratinocytes from the patient and two independent healthy individuals were included in the studies. Mouse fibroblast 3T3-J2 cell line used as feeder layer was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco) supplemented with 10% (v/v) of donor calf serum and 100 U/ml of P/S. Brinzolamide solution (AZOPT, Novartis, Switzerland), Acetazolamide (AZA, ref. A6011, Sigma-Aldrich) and retigabine (RTG, ref. 90,221, Supelco) were used in cultures at 10 μM.
For electrophysiology experiments, COS7 cells were cultured in DMEM supplemented with 10% (v/v) of FBS and 100 U/ml of P/S and plated in 35-mm culture dishes. Cells were transiently cotransfected with WT Kv10.2 or N856H Kv10.2 cloned into pCDNA3.1, and EBO-pcDLeu2 as reporter gene, codifying CD8. This was performed by using Fugene-6 (Merck, Germany) at 60% confluence and following manufacturer’s instructions [67, 68]. The amount of cDNA transfected was 4 μg for the channel and 2 μg for EBO and the ratio of DNA:Fugene was 1:3. After 48 h transfection, cells were removed from culture plates using TrypLE™ Express (Gibco), after exposing cells to polystyrene microspheres bound to anti-CD8 (Dynabeads M-450; Thermo Fisher Scientific, Waltham, MA, USA) [67–69].
Electrophysiology
Potassium currents were recorded from COS7 cells using the whole-cell configuration of the patch-clamp technique with an Axopatch 200B amplifier and a Digidata 1322A (Molecular Devices, Silicon Valley, CA, USA) as previously described [70–72]. The intracellular pipette filling solution contained: 80 mM K-aspartate, 50 mM KCl, 3 mM phosphocreatine, 10 mM KH2PO4, 3 mM MgATP, 5 mM HEPES-K, 5 mM EGTA and was adjusted to pH 7.25 with KOH. The bath solution contained: 145 mM NaCl, 4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM HEPES-Na, 10 mM glucose and was adjusted to pH 7.40 by NaOH.
Currents were filtered at 1 kHz (4-pole Bessel filter) and sampled at 2 kHz. Micropipettes were pulled from borosilicate glass capillary tubes (GD-1; Narishige, Japan) on a programmable horizontal puller P-87 (Sutter Instruments Co., Novato, CA, USA) and heat-polished with a microforge (Narishige). Micropipette resistance ranged between 2–4 MΩ. pClamp version 10.6 software (Molecular Devices) was used for data acquisition and analysis. Currents were recorded at room temperature (21–23 °C) at a stimulation frequency of 0.03 Hz. OriginPro 2018 (OriginLab Corporation, Northampton, MA, USA.) and Clampfit 10.6 programs were used to perform least-squares fitting and for data presentation. All the compounds used for the external and internal solutions were from Merck.
Clonogenicity assays
For clonogenicity assays, 5,000 keratinocytes per well were plated per triplicate in 6-well plates and grown in high-calcium FAD medium. About 14 days later, the cultures were fixed with 3.7% formaldehyde in PBS for 10 min and stained with rhodanile blue as described previously [25].
Flow cytometry and Antibodies
Keratinocytes were harvested, fixed in 3.7% formaldehyde in PBS and stained for involucrin as previously described [38]. Primary anti-involucrin antibody (SY5, I-9018, Sigma-Aldrich, Inc., St Louis, MO, USA) produced in mouse, then revealed with Alexa Fluor® 488-conjugated goat anti-mouse (115-547-003) from Jackson ImmunoResearch (Philadelphia, PA, USA). Antibody staining was controlled by the use of a similar concentration of isotype negative immunoglobulin IgG from mouse serum (I-5381, Sigma-Aldrich). After staining, cells were firmly resuspended and filtered through a 70 µM mesh to minimize the presence of aggregates and then analysed on a CytoFLEX from Beckman Coulter (Brea, CA, USA). 10,000 events were gated and acquired to analyse.
Statistical analyses
Data are presented as mean ± standard deviation from two or more independent culture dishes conditions (n) and at least two independent experiments (N). Data sets were compared using an unpaired two-tailed Student’s t test when two data sets or one-way ANOVA when more than two data sets were analysed (GraphPad Prism 8). For multiple comparison, Tukey test was used. A p value of < 0.05 was considered statistically significant. In every case sample size was chosen accordingly. Damaged samples were excluded from analyses.
Acknowledgements
We are grateful to the patient’s parents for their consent for the study and the publication. We thank Dr Luis A. Pardo for kindly provide us with the unpublished human Kv10.2 cDNA cloned in pcDNA3.1 vector and Rosa Ayesa for advice, Iris Aja, Ángel Saiz and the Technical Services of the IIB-Alberto Sols (CSIC-UAM, Madrid) for technical assistance. JG was supported by the PI17/01307 grant and currently is recipient of the predoctoral scholarship from AECC PRDCA19003GALA (Spain). LC is recipient of a predoctoral scholarship from the Consejo Nacional de Ciencia y Tecnología (709426; Mexico), PGS is recipient of a predoctoral contract FPU (FPU17/02731; Spain) and MBN is recipient of a predoctoral contract from CSIC (Spain).
Abbreviations
- ASD
Autism spectrum disorder
- AZA
Acetazolamide
- BZA
Brinzolamide
- CAI
Carbonic anhydrase inhibitor
- DMEM
Dulbecco’s Modified Eagle’s Medium
- Eag
Ether-à-go-go
- EGF
Epidermal growth factor
- ES
Exome sequencing
- FBS
Fetal bovine serum
- FDA
Food and Drug Administration
- GOF
Gain of function
- HS
High scatter
- LOF
Loss of function
- MRI
Magnetic Resonance Imaging
- NGS
Next-generation sequencing
- P/S
Penicillin/streptomycin
- RTG
Retigabine
- VPA
Valproate
- WT
Wildtype
Author contributions
JG: Data curation, data analyses, manuscript writing and figure making and review. PGS, MBN and LC: data curation, figure making and review. TG: supervising, funding and review. MSPP: Clinical characterisation and treatment. CV: conceptualization, supervision, experimental design, funding, writing and review. DGL: patient recruitment, clinical characterisation and treatment, study design, writing and review. AG: study and experimental design, manuscript writing and review, funding. All authors read and approved the final manuscript.
Funding
The study was funded by ISCIII-FIS/FEDER grants PI14/00900, PI17/01307 and PI20/00880 (AG), CIBERCV program CB/11/00222, CSIC 2019AEP148 (CV) and MINECO/AEI-FEDER PID2019-104366RB-C21 (CV and TG). The funding bodies did not have a role in the study, collection, analysis, interpretation of data or in writing the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The research obtained approval by the ethical committee for Clinical Research and Studies in Cantabria, Spain (CEIC, Research Institute and Hospital Universitario Marqués de Valdecilla). The study is included in the research network RD16/0022/0010. We obtained informed consent from both parents for the skin biopsy, in vitro cultures and molecular studies (codes HUMV-651681831 and NM_129318.4) that are available in the clinical files of the patient at the Regional Health System.
Consent for publication
Parents of the participant had given specific informed consent both to the study and its publication. Written informed consent for publication of the clinical details was obtained from the parents, and a copy of the consent form is available for review by the Editor of the journal.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Carmen Valenzuela, Email: cvalenzuela@iib.uam.es.
Domingo González-Lamuño, Email: gonzaleld@unican.es.
Alberto Gandarillas, Email: agandarillas@idival.org.
References
- 1.Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, Park BY, Snyder NW, Schendel D, Volk H, Windham GC, Newschaffer C. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health. 2017;38:81–102. doi: 10.1146/annurev-publhealth-031816-044318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma SR, Gonda X, Tarazi FI. Autism spectrum disorder: classification, diagnosis and therapy. Pharmacol Ther. 2018;190:91–104. doi: 10.1016/j.pharmthera.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Chang BS, Lowenstein DH. Mechanisms of disease Epilepsy. N Engl J Med. 2003;13:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- 4.Lee BH, Smith T, Paciorkowski AR. Autism spectrum disorder and epilepsy: disorders with a shared biology. Epilepsy Behav. 2015;47:191–201. doi: 10.1016/j.yebeh.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley AW, Holmes GL. Epilepsy and autism. Cold Spring Harbor Perspect Med. 2016;6(4):a022749. doi: 10.1101/cshperspect.a022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J. Channelopathies. 2014;57(1):1–18. doi: 10.3345/kjp.2014.57.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartolini E, Campostrini R, Kiferle L, Pradella S, Rosati E, Chinthapalli K, Palumbo P. Epilepsy and brain channelopathies from infancy to adulthood. Neurol Sci. 2020;41(4):749–761. doi: 10.1007/s10072-019-04190-x. [DOI] [PubMed] [Google Scholar]
- 8.Schmunk G, Gargus JJ. Channelopathy pathogenesis in autism spectrum disorders. Front Genet. 2013;4:1–20. doi: 10.3389/fgene.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luca G, Ilenio S, Martino C, Luigi C, Fabio F, Maria CD, Mauro P. Update on the implication of potassium channels in autism: K+ channelautism spectrum disorder. Front Cell Neurosci. 2015;9:1–14. doi: 10.3389/fncel.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saganich MJ, Vega-Saenz De Miera E, Nadal MS, Baker H, Coetzee WA, Rudy B. Cloning of components of a novel subthreshold-activating K+ channel with a unique pattern of expression in the cerebral cortex. J Neurosci. 1999;19(24):10789–10802. doi: 10.1523/JNEUROSCI.19-24-10789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.González C, Baez-Nieto D, Valencia I, Oyarzún I, Rojas P, Naranjo D, Latorre R. K+ channels: function-structural overview. Compr Physiol. 2012;2(3):2087–2149. doi: 10.1002/cphy.c110047. [DOI] [PubMed] [Google Scholar]
- 12.Ju M, Wray D. Molecular identification and characterisation of the human eag2 potassium channel. FEBS Lett. 2002;524(1–3):204–210. doi: 10.1016/S0014-5793(02)03055-7. [DOI] [PubMed] [Google Scholar]
- 13.Huang X, Dubuc AM, Hashizume R, Berg J, He Y, Wang J, Chiang C, Cooper MK, Northcott PA, Taylor MD, Barnes MJ, Tihan T, Chen J, Hackett CS, Weiss WA, David James C, Rowitch DH, Shuman MA, Jan YN, Jan LY. Voltage-gated potassium channel EAG2 controls mitotic entry and tumor growth in medulloblastoma via regulating cell volume dynamics. Genes Dev. 2012;26(16):1780–1796. doi: 10.1101/gad.193789.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camacho J. Ether à go-go potassium channels and cancer. Cancer Lett. 2006;233(1):1–9. doi: 10.1016/j.canlet.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Stühmer W, Alves F, Hartung F, Zientkowska M, Pardo LA. Potassium channels as tumour markers. FEBS Lett. 2006;580(12):2850–2852. doi: 10.1016/j.febslet.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 16.Veeramah KR, Johnstone L, Karafet TM, Wolf D, Sprissler R, Salogiannis J, Barth-Maron A, Greenberg ME, Stuhlmann T, Weinert S, Jentsch TJ, Pazzi M, Restifo LL, Talwar D, Erickson RP, Hammer MF. Exome sequencing reveals new causal mutations in children with epileptic encephalopathies. Epilepsia. 2013;54(7):1270–1281. doi: 10.1111/epi.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Vasylyev D, Dib-Hajj F, Veeramah KR, Hammer MF, Dib-Hajj SD, Waxman SG. Multistate structural modeling and voltage-clamp analysis of epilepsy/autism mutation Kv10.2-R327H demonstrate the role of this residue in stabilizing the channel closed state. J Neurosci. 2013;33(42):16586–16593. doi: 10.1523/JNEUROSCI.2307-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, Shendure J. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12(11):745–755. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]
- 19.O’Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, Karakoc E, MacKenzie AP, Ng SB, Baker C, Rieder MJ, Nickerson DA, Bernier R, Fisher SE, Shendure J, Eichler EE. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43(6):585–589. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, Stessman HA, Witherspoon KT, Vives L, Patterson KE, Smith JD, Paeper B, Nickerson DA, Dea J, Dong S, Gonzalez LE, Mandell JD, Mane SM, Murtha MT, Sullivan CA, Walker MF, Waqar Z, Wei L, Willsey AJ, Yamrom B, Lee YH, Grabowska E, Dalkic E, Wang Z, Marks S, Andrews P, Leotta A, Kendall J, Hakker I, Rosenbaum J, Ma B, Rodgers L, Troge J, Narzisi G, Yoon S, Schatz MC, Ye K, McCombie WR, Shendure J, Eichler EE, State MW, Wigler M. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515(7526):216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James ZM, Zagotta WN. Structural insights into the mechanisms of CNBD channel function. J Gen Physiol. 2018;150(2):225–244. doi: 10.1085/jgp.201711898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marques-Carvalho MJ, Sahoo N, Muskett FW, Vieira-Pires RS, Gabant G, Cadene M, Schönherr R, Morais-Cabral JH. Structural, biochemical, and functional characterization of the cyclic nucleotide binding homology domain from the mouse EAG1 potassium channel. J Mol Biol. 2012;423(1):34–46. doi: 10.1016/j.jmb.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Dai G, Zagotta WN. Molecular mechanism of voltage-dependent potentiation of KCNH potassium channels. Elife. 2017;6:26355. doi: 10.7554/eLife.26355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rheinwald JG, Green H. Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell. 1975;6(3):317–330. doi: 10.1016/0092-8674(75)90183-X. [DOI] [PubMed] [Google Scholar]
- 25.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73(4):713–724. doi: 10.1016/0092-8674(93)90251-K. [DOI] [PubMed] [Google Scholar]
- 26.Banks-Schlegel S, Green H. Involucrin synthesis and tissue assembly by keratinocytes in natural and cultured human epithelia. J Cell Biol. 1981;90(3):732–737. doi: 10.1083/jcb.90.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar S, Rulhania S, Jaswal S, Monga V. Recent advances in the medicinal chemistry of carbonic anhydrase inhibitors. Eur J Med Chem. 2021;209:112923. doi: 10.1016/j.ejmech.2020.112923. [DOI] [PubMed] [Google Scholar]
- 28.van Berkel MA, Elefritz JL. Evaluating off-label uses of acetazolamide. Am J Health Syst Pharm. 2018;75(8):524–531. doi: 10.2146/ajhp170279. [DOI] [PubMed] [Google Scholar]
- 29.Reiss WG, Oles KS. Acetazolamide in the treatment of seizures. Ann Pharmacother. 1996;30(5):514–518. doi: 10.1177/106002809603000515. [DOI] [PubMed] [Google Scholar]
- 30.Martínez-Monseny AF, Bolasell M, Callejón-Póo L, Cuadras D, Freniche V, Itzep DC, Gassiot S, Arango P, Casas-Alba D, de la Morena E, Corral J, Montero R, Pérez-Cerdá C, Pérez B, Artuch R, Jaeken J, Serrano M, Velázquez-Fragua R, García O, Gutierrez-Solana LG, Macaya A, Pérez-Dueñas B, Aguilera-Albesa S, López L, Miranda MC, Carratala F, Yoldi ME, López-Laso E, Sierra-Córcoles MC, Sebastián-García I, Aísa E, Cancho-Candela R, Carrasco-Marina ML, Couce ML, Roldán S, Muchart J, Morales M, Conde-Lorenzo N. AZATAX: Acetazolamide safety and efficacy in cerebellar syndrome in PMM2 congenital disorder of glycosylation (PMM2-CDG) Ann Neurol. 2019;85(5):740–751. doi: 10.1002/ana.25457. [DOI] [PubMed] [Google Scholar]
- 31.Verbrugge FH, Martens P, Ameloot K, Haemels V, Penders J, Dupont M, Tang WHW, Droogné W, Mullens W. Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. Eur J Heart Fail. 2019;21(11):1415–1422. doi: 10.1002/ejhf.1478. [DOI] [PubMed] [Google Scholar]
- 32.Supuran CT. An update on drug interaction considerations in the therapeutic use of carbonic anhydrase inhibitors. Expert Opin Drug Metab Toxicol. 2020;16(4):297–307. doi: 10.1080/17425255.2020.1743679. [DOI] [PubMed] [Google Scholar]
- 33.Pinard MA, Boone CD, Rife BD, Supuran CT, McKenna R. Structural study of interaction between brinzolamide and dorzolamide inhibition of human carbonic anhydrases. Bioorg Med Chem. 2013;21(22):7210–7215. doi: 10.1016/j.bmc.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 34.Gunthorpe MJ, Large CH, Sankar R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia. 2012;53(3):412–424. doi: 10.1111/j.1528-1167.2011.03365.x. [DOI] [PubMed] [Google Scholar]
- 35.Main MJ, Cryan JE, Dupere JRB, Cox B, Clare JJ, Burbidge SA. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol. 2000;58(2):253–262. doi: 10.1124/mol.58.2.253. [DOI] [PubMed] [Google Scholar]
- 36.Wickenden AD, Yu W, Zou A, Jegla T, Wagoner PK. Retigabine, a novel anti-convulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol Pharmacol. 2000;58(3):591–600. doi: 10.1124/mol.58.3.591. [DOI] [PubMed] [Google Scholar]
- 37.Otto JF, Kimball MM, Wilcox KS. Effects of the anticonvulsant retigabine on cultured cortical neurons: Changes in electroresponsive properties and synaptic transmission. Mol Pharmacol. 2002;61(4):921–927. doi: 10.1124/mol.61.4.921. [DOI] [PubMed] [Google Scholar]
- 38.de Pedro I, Galán-Vidal J, Freije A, de Diego E, Gandarillas A. p21CIP1 controls the squamous differentiation response to replication stress. Oncogene. 2021;40(1):152–162. doi: 10.1038/s41388-020-01520-8. [DOI] [PubMed] [Google Scholar]
- 39.Bliss SL. Test Reviews: Newborg, J. (2005). Battelle Developmental Inventory−Second Edition. Itasca, IL: Riverside. Journal of Psychoeducational Assessment. 2007. p. 409–15.
- 40.Matson JL, Hess JA, Sipes M, Horovitz M. Developmental profiles from the Battelle developmental inventory: a comparison of toddlers diagnosed with Down Syndrome, global developmental delay and premature birth. Dev Neurorehabil. 2010;13(4):234–238. doi: 10.3109/17518421003736032. [DOI] [PubMed] [Google Scholar]
- 41.Goldin RL, Matson JL, Beighley JS, Jang J. Autism spectrum disorder severity as a predictor of Battelle Developmental Inventory-Second Edition (BDI-2) scores in toddlers. Dev Neurorehabil. 2014;17(1):39–43. doi: 10.3109/17518423.2013.839585. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Tang Y, Yan J, Du D, Yang Y, Chen F. Deletion of Kv10.2 causes abnormal dendritic arborization and epilepsy susceptibility. Neurochem Res. 2020;45(12):2949–2958. doi: 10.1007/s11064-020-03143-7. [DOI] [PubMed] [Google Scholar]
- 43.Griswold AJ, Ma D, Sacharow SJ, Robinson JL, Jaworski JM, Wright HH, Abramson RK, Lybæk H, Øyen N, Cuccaro ML, Gilbert JR, Pericak-Vance MA. A de novo 1.5Mb microdeletion on chromosome 14q23.2–23.3 in a patient with autism and spherocytosis. Autism Res. 2011;4(3):221–227. doi: 10.1002/aur.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lybæk H, Øyen N, Fauske L, Houge G. A 2.1 Mb deletion adjacent but distal to a 14q21q23 paracentric inversion in a family with spherocytosis and severe learning difficulties. Clin Genet. 2008;74(6):553–559. doi: 10.1111/j.1399-0004.2008.01072.x. [DOI] [PubMed] [Google Scholar]
- 45.Imbrigiotta N, Lenzo P, Bonsignore M. A Case of deletion of chromosome 14q23.3.
- 46.de Ligt J, Willemsen MH, van Bon BWM, Kleefstra T, Yntema HG, Kroes T, Vulto-van Silfhout AT, Koolen DA, de Vries P, Gilissen C, del Rosario M, Hoischen A, Scheffer H, de Vries BBA, Brunner HG, Veltman JA, Vissers LELM. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med. 2012;367(20):1921–1929. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- 47.Li M, Wang J, Liu Y, Wu J, Li F, Feng S, Du D, Chen F. Central changes in the Kv10.2 potassium channel in stress-induced hypertension rats. NeuroReport. 2019;30(9):637–644. doi: 10.1097/WNR.0000000000001244. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Feng S, Li M, Liu Y, Yan J, Tang Y, Du D, Chen F. Increased expression of Kv10.2 in the hippocampus attenuates valproic acid-induced autism-like behaviors in rats. Neurochem Res. 2019;44(12):2796–2808. doi: 10.1007/s11064-019-02903-4. [DOI] [PubMed] [Google Scholar]
- 49.Niday Z, Tzingounis A. Potassium channel gain of function in epilepsy: an unresolved paradox. Neuroscientist. 2018;24(4):368–380. doi: 10.1177/1073858418763752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuchs E. Scratching the surface of skin development. Nature. 2007;445(7130):834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gandarillas A. The mysterious human epidermal cell cycle, or an oncogene-induced differentiation checkpoint. Cell Cycle. 2012;11(24):4507–4516. doi: 10.4161/cc.22529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lauritzen I, Chemin J, Honoré E, Jodar M, Guy N, Lazdunski M, et al. Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 2005;6(7):642–648. doi: 10.1038/sj.embor.7400449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinac B. The ion channels to cytoskeleton connection as potential mechanism of mechanosensitivity. Biochim Biophys Acta Biomembranes. 2014;1838:682–691. doi: 10.1016/j.bbamem.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Mollinedo P, Kapitansky O, Gonzalez-Lamuno D, Zaslavsky A, Real P, Gozes I, Gandarillas A, Fernandez-Luna JL. Cellular and animal models of skin alterations in the autism-related ADNP syndrome. Sci Rep. 2019;9(1):736. doi: 10.1038/s41598-018-36859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allon M, Am J. Hyperkalemia in End-Stage and Management1’2. J Am Soc Nephrol. 1995;6(4):1134–1142. doi: 10.1681/ASN.V641134. [DOI] [PubMed] [Google Scholar]
- 56.Sowinski KM, Cronin D, Mueller BA, Kraus MA. Subcutaneous terbutaline use in CKD to reduce potassium concentrations. Am J Kidney Dis. 2005;45(6):1040–1045. doi: 10.1053/j.ajkd.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 57.Clausen T, Everts ME. Regulation of the Na, K-pump in skeletal muscle. Kidney Int. 1989;35(1):1–13. doi: 10.1038/ki.1989.1. [DOI] [PubMed] [Google Scholar]
- 58.Gosmanov AR, Wong JA, Thomason DB. Duality of G protein-coupled mechanisms for β-adrenergic activation of NKCC activity in skeletal muscle. Am J Physiol Cell Physiol. 2002;283(4):1025–1032. doi: 10.1152/ajpcell.00096.2002. [DOI] [PubMed] [Google Scholar]
- 59.Roca I, González-Castro L, Maynou J, Palacios L, Fernández H, Couce ML, Fernández-Marmiesse A. PattRec: an easy-to-use CNV detection tool optimized for targeted NGS assays with diagnostic purposes. Genomics. 2020;112(2):1245–1256. doi: 10.1016/j.ygeno.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 60.Huber CD, Kim Id BY, Lohmueller Id KE. Population genetic models of GERP scores suggest pervasive turnover of constrained sites across mammalian evolution. 2020. [DOI] [PMC free article] [PubMed]
- 61.Kircher M, Witten DM, Jain P, O’roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46(3):310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ioannidis NM, Rothstein JH, Pejaver V, Middha S, McDonnell SK, Baheti S, Musolf A, Li Q, Holzinger E, Karyadi D, Cannon-Albright LA, Teerlink CC, Stanford JL, Isaacs WB, Xu J, Cooney KA, Lange EM, Schleutker J, Carpten JD, Powell IJ, Cussenot O, Cancel-Tassin G, Giles GG, MacInnis RJ, Maier C, Hsieh CL, Wiklund F, Catalona WJ, Foulkes WD, Mandal D, Eeles RA, Kote-Jarai Z, Bustamante CD, Schaid DJ, Hastie T, Ostrander EA, Bailey-Wilson JE, Radivojac P, Thibodeau SN, Whittemore AS, Sieh W. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am J Hum Genet. 2016;99(4):877–885. doi: 10.1016/j.ajhg.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, Maglott DR. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014;42(Database issue). [DOI] [PMC free article] [PubMed]
- 65.Rheinwald JG. Methods for clonal growth and serial cultivation of normal human epidermal keratinocytes and mesothelial cells. In: Baserga R, editor. Cell growth and division. Oxford: IRL Press; 1989. pp. 81–94. [Google Scholar]
- 66.Gandarillas A, Watt FM. c-Myc promotes differentiation of human epidermal stem cells. Genes Dev. 1997;11(21):2869–2882. doi: 10.1101/gad.11.21.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moreno C, Oliveras A, de La Cruz A, Bartolucci C, Muñoz C, Salar E, Gimeno JR, Severi S, Comes N, Felipe A, González T, Lambiase P, Valenzuela C. A new KCNQ1 mutation at the S5 segment that impairs its association with KCNE1 is responsible for short QT syndrome. Cardiovasc Res. 2015;107(4):613–623. doi: 10.1093/cvr/cvv196. [DOI] [PubMed] [Google Scholar]
- 68.Moreno C, Oliveras A, Bartolucci C, Muñoz C, de la Cruz A, Peraza DA, Gimeno JR, Martín-Martínez M, Severi S, Felipe A, Lambiase PD, Gonzalez T, Valenzuela C. D242N, a KV7.1 LQTS mutation uncovers a key residue for Iks voltaje-dependence. J Mol Cell Cardiol. 2017;2017(110):61–69. doi: 10.1016/j.yjmcc.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 69.Macias A, de la Cruz A, Peraza DA, de Benito-Bueno A, Gonzalez T, Valenzuela C. K V 1.5-K V β1.3 Recycling Is PKC-Dependent. Int J Mol Sci. 2021;22(3):1–12. doi: 10.3390/ijms22031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.González T, Arias C, Caballero R, Moreno I, Delpón E, Tamargo J, Valenzuela C. Effects of levobupivacaine, ropivacaine and bupivacaine on HERG channels: Stereoselective bupivacaine block. Br J Pharmacol. 2002;137(8):1269–1279. doi: 10.1038/sj.bjp.0704978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guizy M, David M, Arias C, Zhang L, Cofán M, Ruiz-Gutiérrez V, Ros E, Lillo MP, Martens JR, Valenzuela C. Modulation of the atrial specific Kv1.5 channel by the n-3 polyunsaturated fatty acid, α-linolenic acid. J Mol Cell Cardiol. 2008;44(2):323–335. doi: 10.1016/j.yjmcc.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 72.Lopez-Hurtado A, Peraza DA, Cercos P, Lagartera L, Gonzalez P, Dopazo XM, Herranz R, Gonzalez T, Martin-Martinez M, Mellström B, Naranjo JR, Valenzuela C, Gutierrez-Rodriguez M. Targeting the neuronal calcium sensor DREAM with small-molecules for Huntington’s disease treatment. Sci Rep. 2019;9(1):1–16. doi: 10.1038/s41598-019-43677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.