Abstract
Background
In low- and middle-income countries, surveillance of antimicrobial resistance (AMR) is mostly hospital-based and, in view of poor access to clinical microbiology, biased to more resistant pathogens. We aimed to assess AMR among Escherichia coli isolates obtained from urine cultures of pregnant women as an indicator for community AMR and compared the AMR results with those from E. coli isolates obtained from febrile patients in previously published clinical surveillance studies conducted within the same population in Nanoro, rural Burkina Faso. We furthermore explored feasibility of adding urine culture to standard antenatal care in a rural sub-Saharan African setting.
Methods
Between October 2016–September 2018, midstream urine samples collected as part of routine antenatal care in Nanoro district were cultured by a dipslide method and screened for antibiotic residues. Significant growth was defined as a pure culture of Enterobacterales at counts of ≥ 104 colony forming units/ml.
Results
Significant growth was observed in 202/5934 (3.4%) cultures; E. coli represented 155 (76.7%) of isolates. Among E. coli isolates, resistance rates to ampicillin, cotrimoxazole and ciprofloxacin were respectively 65.8%, 64.4% 16.2%, compared to 89.5%, 89.5% and 62.5% among E. coli from clinical isolates (n = 48 of which 45 from blood cultures). Proportions of extended spectrum beta-lactamase producers and multidrug resistance were 3.2% and 5.2% among E. coli isolates from urine in pregnant women versus 35.4%, and 60.4% respectively among clinical isolates.
Conclusions
The E. coli isolates obtained from healthy pregnant women had significantly lower AMR rates compared to clinical E. coli isolates, probably reflecting the lower antibiotic pressure in the pregnant women population. Adding urine culture to the routine urine analysis (dipstick) of antenatal care was feasible. The dipslide culture method was affordable and user-friendly and allowed on-site inoculation and easy transport; challenges were contamination (midstream urine sampling) and the semi-quantitative reading. Provided confirmation of the present findings in other settings, E. coli from urine samples in pregnant women may be a potential indicator for benchmarking, comparing, and monitoring community AMR rates across populations over different countries and regions.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13756-022-01142-7.
Keywords: Antimicrobial resistance, Community, Asymptomatic bacteriuria, ANC, Pregnancy, Escherichia coli, Rural Africa, Burkina Faso
Introduction
Antimicrobial resistance (AMR) rises globally and is a threat to public health, particularly in low- and middle-income countries (LMIC) [1]. Surveillance is one of the five domains of the World Health Organization's Global Action plan against AMR [2]. The Global Antimicrobial Resistance Surveillance System (GLASS) set outs standards for collection, analysis and sharing of AMR data at a worldwide level. Aggregated surveillance data reported to GLASS rely on antibiotic susceptibility testing (AST) results of clinical samples [3].
However, surveillance of clinical samples has shortcomings particularly when applied in LMIC. First, LMIC face problems of access to competent and quality-assured clinical bacteriology [4]. As a result, samples processed in LMIC settings may be biased to more advanced disease stages and collected under coverage of empiric antibiotic treatment. Second, surveillance by clinical samples may be influenced by the type of clinical specimen (e.g. blood vs. respiratory tract secretions), previous antibiotic use as well as indications for sampling, factors that are often not standardized in LMIC [5]. Finally, clinical bacteriology in LMIC is typically implemented at the second level of care, i.e. the district referral hospital [6]. Although samples may be collected at the primary level of care (health post, health center), expertise and skills for sampling as well as reliable transport systems may be lacking [1]. By consequence, the deducted surveillance data may tend towards an overestimation of AMR rates and may not reflect AMR rates at community level.
Using Escherichia coli isolates obtained from pregnant women could be a way to circumvent these shortcomings. First, urine is already routinely collected at antenatal care visits at all healthcare levels throughout LMIC. Second, the collection method and specimen are universally comparable. Third, pregnant women tend to use fewer antibiotics (for concerns of safety for the unborn child), which means that there is a relatively low antibiotic pressure among this population compared to patients. And finally, urine cultures, once inoculated during ANC visit, can be transported to a larger healthcare facility with microbiology lab with minimal effect on the reliability of the culture result.
The primary objective of this study was to assess if E. coli isolates recovered from urine samples of healthy pregnant women can serve as a proxy for AMR surveillance at the community level in rural West-Africa. We focused on E. coli as it is the most frequent isolate in asymptomatic bacteriuria in pregnancy [7]. In addition, E. coli is a key pathogen in current programs that monitor AMR in humans [3] and in One Health populations (AGISAR) [8]. Our secondary objective was to explore whether adding bacteriological culture of urine to the routine antenatal care is feasible in a rural West-African setting.
Methods
Study design
We conducted a cross-sectional study recruiting pregnant women attending routine antenatal care (ANC) in rural Burkina Faso. Urine samples routinely obtained for dipstick analysis (glucose and protein) were semi-quantitatively cultured by dipslide technique to assess for asymptomatic bacteriuria (ASB). For the purpose of this study, isolates growing in counts of ≥ 104 colony forming units (CFU/ml) belonging to the Enterobacterales species and Enterococcus faecalis were considered as "significant growth". Antibiotic susceptibility testing was done for E. coli isolates. AMR profiles were compared to E. coli isolates obtained from blood culture surveillance studies performed in the same district and the same laboratory. Women were asked about recent antibiotic use prior to sampling and urine samples were screened for antibiotic residues.
Study site, period and participants, routine antenatal care
The study was conducted from October 2016 to September 2018 at the Clinical Research Unit of Nanoro (CRUN) in the Center-West Region of Burkina Faso. Samples were obtained in 9 health centers within the Health and Demographic Surveillance System (HDSS) of CRUN, at 11–38 km from CRUN (Fig. 1). The HDSS monitors changes in a total population of 60,000 persons distributed over 24 villages [9]. Routine ANC is provided at the health centers and is organized in morning hours between 7 and 12 a.m. A total of 4 ANC visits are recommended during each pregnancy [10] and comprise collection of demographic data (age, week of pregnancy and Gestation Parity Abortion score [GPA score]) and uranalysis for glucose and protein by dipstick test.
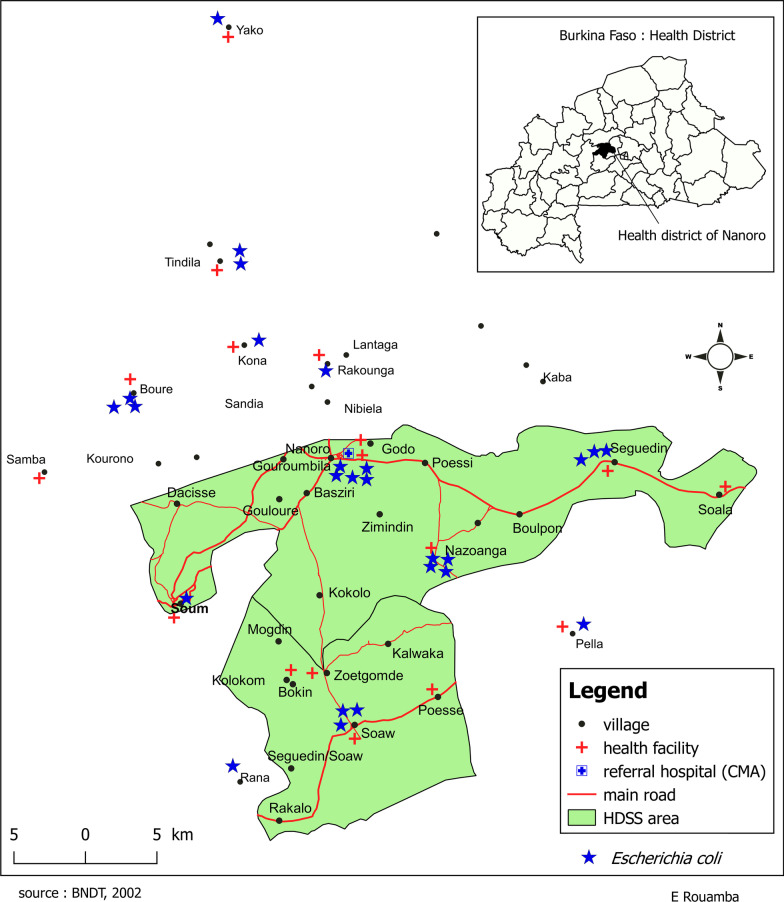
Fig. 1.
Map of the Health and Demographic Surveillance Site of Nanoro. The blue stars indicate residences of women with cultured E. coli. There were no evident clusters. Cases were living within and just outside the health and demographic surveillance site
Study intervention: urine culture and collection of demographic and clinical data
Informed consent was sought from all women attending routine ANC by the ANC nurse. After written consent was obtained, a study nurse provided a sterile cup and instructions on how to obtain a clean midstream urine sample. Apart from routinely collected ANC data, information on antibiotic use in the past 2 weeks, and signs and symptoms of urinary tract infection were collected. For semi-quantitative culture, dipslide devices (Uricult MC/CLED, International Medical Products, Brussels, Belgium and Servocults, Meus S.R.L, Padova, Italy) were used. The dipslide consisted of cysteine-, lactose, and electrolyte-deficient (CLED) agar on one side and MacConkey agar on the other side. Inoculation was done by the study nurse: dipslides were fully submerged in the urine sample, alternatively, a sterile pipette was used to inoculate both agar slides. The dipslide was then placed straight-up on a piece of absorbent paper to allow excess urine to leak off. Subsequently, the urine was tested for presence of glucose and protein using a dipstick analysis (UroColor strips [Standard Diagnostics, Gyeonggi-do, Republic of Korea] or Urine-10 strips [Cypress Diagnostics, Hulshout, Belgium]). Leukocyturia was quantified according to the manufacturers’ instruction as negative (−), + (25–74 cells/µL), ++ (75–499 cells/µL), or +++ (500 or more cells/µL). The urine dipslides and the left-over urine samples were stored in the fridge (2–8 °C). Transport to the laboratory was done in a light protected box by motorcycle, within 24 h after collection, at room temperature.
Semi-quantitative culture, bacterial identification and antibiotic susceptibility testing
Upon reception at the laboratory of CRUN, dipslides were incubated for 16–24 h at 35 °C. Grown cultures were assessed for colony counts by comparing the number of colonies to the figure provided in the product's instructions for use. Bacterial isolates were identified using standardized biochemical techniques and API (bioMérieux, Marcy l’Etoile, France) in case of doubtful test reactions. The isolates were subsequently stored in Tryptic Soy Agar (CM0131, Oxoid Ltd). All bacteria growing in counts of ≥ 104 colony forming units/ml (CFU/ml) except for non-fermentative Gram-negative bacteria and bacteria considered as contaminants [Bacillus spp. and coagulase-negative staphylococci (CNS)] were shipped to the Institute of Tropical Medicine in Antwerp (Belgium) for confirmation of identification by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) technology (Bruker MALDI Biotyper, Bruker, Billerica, MA, US) at the University Hospital of Leuven (Belgium). Isolates identified as E. coli were processed for AST by disk diffusion (Neo-Sensitabs, Rosco Diagnostica A/S, Taastrup, Denmark) according to Clinical and Laboratory Standards Institute (CLSI) guidelines [11]. Combination disk tests (Neo-Sensitabs, Rosco Diagnostica A/S, Taastrup, Denmark) were performed to assess the production of Extended Spectrum ß-lactamases (ESBL) [11]. Neo-Sensitabs disk contents were as follows: nitrofurantoin 300 µg, fosfomycin 200 µg, ampicillin 10 µg, cotrimoxazole (trimethoprim 1.25 µg + sulfamethoxazole 23.75 µg), ciprofloxacin 5 µg, gentamicin 10 µg, ceftriaxone 30 µg, ceftazidime 30 µg, ceftriaxone-clavulanic acid 30 + 10 µg and ceftazidime-clavulanic acid 30 + 10 µg.
Antibiotic residue testing
Urine samples were tested for the presence of antibiotic residues as part of the work-up at the laboratory of CRUN. For each urine sample, a Mueller–Hinton plate was inoculated with 0.5 McFarland saline solutions of Bacillus spizizenii (ATCC 6633). An absorbent paper disk was saturated with urine and placed on the Mueller–Hinton agar. Plates were incubated at 35 °C for 18–24 h. The appearance of an inhibition zone around the urine disk was considered positive for the presence of residue antibiotics [12].
Comparison with clinical isolates
For comparison, AST results from clinical isolates of urine and blood cultures collected under different study protocols in the Nanoro district hospital and processed at CRUN during the same period were used. Two studies assessed the proportion and differentiation of malaria and bacteremia in the Nanoro district hospital [13, 14], a third study assessed the incidence and reservoir of non-typhoidal Salmonella bloodstream infection [15, 16] and finally, several isolates were obtained from a blood culture surveillance study for follow-up of antimicrobial resistance (unpublished data).
Definitions
For the purpose for this study, single-organism cultures with counts of ≥ 104 CFU/ml belonging to the Enterobacterales species or Enterococcus faecalis were considered as "significant growth" and the isolates were considered as “pathogens”. Isolates obtained from febrile patients are referred to as “clinical samples”. Asymptomatic bacteriuria was defined as the presence of actively multiplying bacteria in the urinary tract in patients that have no obvious symptoms of urinary tract infection (UTI) [17]. Skin- or environmental bacteria (CNS, Bacillus spp.), non-fermentative Gram-negative bacteria and bacteria growing as mixed flora (≥ 2 different isolates) were considered as contaminants [12]. In case a culture grew with mixed isolates including Enterobacterales, the latter were also subcultured for the purpose of antibiotic susceptibility testing (AST). Staphylococcus aureus isolates were not considered for antibiotic susceptibility testing.
Multi-drug resistance (MDR) for Enterobacterales was defined as resistance to the three principal oral antibiotic categories for urinary tract infection (penicillins, cotrimoxazole and fluoroquinolones). The number of parities of each participant was categorized as in nullipara (never given birth), primipara (given birth once), multipara (≥ 2 births) or grand multipara (≥ 5 births) [18].
Sample size, data registration and statistical analysis
In line with the CLSI M39 [19], a minimum number of 30 E. coli isolates was targeted for separate antibiotic susceptibility reporting. Assuming a prevalence of 5–10% asymptomatic bacteriuria with 10% contamination rate and E. coli being 40% of retrieved isolates, 6000 women were targeted. Data were recorded in a coded database (Microsoft Excel, Redmond, US). Differences in proportions were compared using as appropriate a Mann–Whitney-u test, a Kruskall Wallis test or a Chi-square test. For smaller sample sizes (value in one of the cells ≤ 5), the Fischer exact test was used. A p value of 0.05 was considered as statistically significant. Reporting of the methods and results was done according to the STROBE guidelines for cross-sectional studies [20].
Ethics
The study was approved by the national ethics committee of Burkina Faso (Comité d’Ethique pour la Recherche en Santé (Reference No. 2015-7-96 July 1st, 2015), the institutional review board of ITM, Antwerp (Reference 1008/15 from December 15th, 2015) and the ethics committee of the University Hospital of Antwerp (Reference 15/51/563, January 4th, 2016). Written informed consent was obtained before participation in the study. A screening log with reasons for refusal was completed at each health center included in the study. If ASB was diagnosed, laboratory staff of the study site communicated the recovery of clinically significant isolates and their AST results to the study investigator, who informed the ANC nurse or the clinician responsible of the ANC. Participants were treated according to national treatment guidelines.
Results
Characteristics of study participants
Over a time-period of 2 years (October 2016 to September 2018) a total of 6018 urine samples were collected. A total of 84 (1.4%) samples were excluded from analysis because the participant reported symptoms suggestive of urinary tract infection, leaving 5934 samples representing 5907 unique participants (27 participants were sampled on two separate ANC visits). Secondary analyses concerning parity and dipstick analyses and prior antibiotic use were done on samples for which data were complete (n = 5741 [96.7%]). A cluster of missing data included 36 consecutive dipstick analyses obtained between June 11th and August 8th from Healthcare centre Seguedin.
The age of participants for whom full data were available ranged from 14 to 50 years. Overall, the median (interquartile range [IQR]) age was 25 (20–30) years. In total 23.9% of samples were obtained from nulli- or primipara. All other cases were multi (43.4%) or grand multipara (32.6%) (Additional file 1: Table S1). Most samples were obtained from women in the second (32.9%) or third (63.1%) trimester of pregnancy (Table 1).
Table 1.
Total numbers of samples with numbers of significant growth for 5741 healthy pregnant women, matched by parity and trimester of pregnancy
| Patients for whom complete data were available | Total | Trimester 1 | Trimester 2 | Trimester 3 | ||||
|---|---|---|---|---|---|---|---|---|
| n = 5741 | n = 5741 | n = 227 | n = 1889 | n = 3625 | ||||
| nr. Cases | % growth | nr. Cases | % growth | nr. Cases | % growth | nr. Cases | % growth | |
| Gestation | ||||||||
| Nullipara (percentage significant growth) | 7 | 0% | 1 | 0% | 1 | 0% | 5 | 0% |
| Primipara (percentage significant growth) | 1367 | 3.9% | 65 | 4.6% | 538 | 3.9% | 784 | 3.7% |
| Multipara (percentage significant growth) | 2493 | 3.0% | 99 | 4.0% | 832 | 3.5% | 1562 | 2.7% |
| Grand multipara (percentage significant growth) | 1874 | 2.4% | 62 | 0% | 518 | 2.9% | 1294 | 2.3% |
| Age distribution (years [IQR]) | 25 (20–30) | 24 (20–30) | 24 (19–30) | 26 (21–30) | ||||
| Dipstick results | ||||||||
| Nitrite* (n [%]) | 68 (1.2%) | 7 (3.1%) | 29 (1.5%) | 32 (0.9%) | ||||
| Leukocytes* (n [%]) | 1055 (18.4%) | 44 (19.4%) | 318 (16.8%) | 717 (19.1%) | ||||
| Dipslide results** | ||||||||
| < 104 CFU/ml | 2865 (49.9%) | 125 (55.1%) | 905 (47.9%) | 1835 (50.6%) | ||||
| ≥ 104 CFU/ml | 643 (11.2%) | 18 (7.9%) | 184 (9.7%) | 441 (12.1%) | ||||
| Clinically significant growth | 173 (3.4%) | 7 (3.1%) | 65 (3.4%) | 101 (2.8%) | ||||
| Asymptomatic bacteriuria | 97 (1.7%) | 4 (1.8%) | 37 (2.0%) | 56 (1.5%) | ||||
Significant growth is defined as growth of Enterobacterales in counts of ≥ 104 CFU/ml. Parity was defined as follows: nullipara = never give birth, primipara = given birth once, multipara = parity ≥ 2, grand multipara = parity ≥ 5 [18]
Data differ slightly from those in Fig. 2 where data are presented for all samples
Clinically significant growth is defined as growth with an Enterobacterales species in counts of ≥ 104 CFU/ml
Asymptomatic bacteriuria is defined as clinically significant growth with counts of ≥ 105 CFU/ml
Breakdown of samples, proportions of significant growth, species recovered
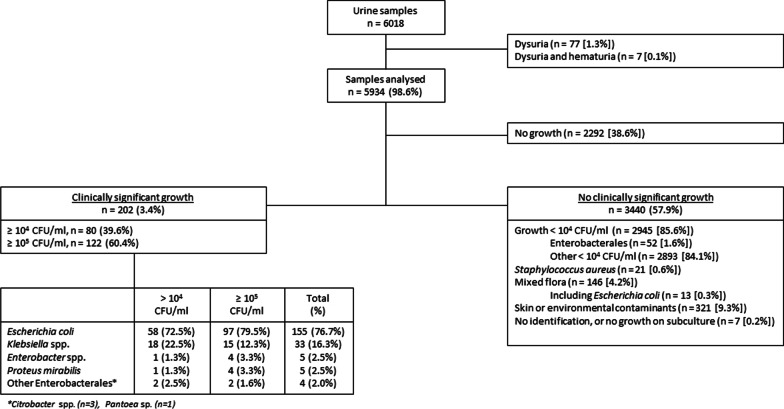
A breakdown of samples and significant growth is presented in Fig. 2. In total 2292 (38.6%) of dipslides did not have any growth, 2945 (49.6%) had growth of < 104 CFU/ml and 697 (11.6%) had growth of ≥ 104 CFU/ml. In total 202 samples (28.9% of grown cultures and 3.4% of all samples) showed significant growth (growth ≥ 104 CFU/ml), of which 122 (2.1%) reached ≥ 105 CFU/ml and therefore qualified as ASB.
Fig. 2.
Clinically significant growth was defined as isolates growing in counts of ≥ 104 CFU/ml belonging to the Enterobacterales species or Enterococcus faecalis. Clinically significant isolates growing in counts of ≥ 105 CFU/ml were defined as ASB
Escherichia coli accounted for 76.7% of pathogens obtained (155/202) from cultures with ≥ 104 CFU/ml. Other species were Klebsiella spp. (n = 32 [15.8%]), Proteus mirabilis (n = 5 [2.5%]), Enterobacter spp. (n = 5 [2.5%]), Citrobacter spp. (n = 3 [1.5%]) and Pantoea (n = 1 [0.5%]). Species distribution between samples with ≥ 104 CFU/ml and ≥ 105 CFU/ml did not differ significantly (Mann–Whitney p < 0.001).
Escherichia coli from clinical samples
A total of 48 E. coli isolates were obtained from clinical samples in the Nanoro district hospital between 2012 and 2019. Forty-five isolates were retrieved from blood cultures and 3 isolates from urine cultures. None of the isolates from clinical samples were obtained from pregnant women.
Antibiotic resistance among E. coli from healthy pregnant women and febrile patients
AMR profiles of both the clinical isolates and isolates from healthy pregnant women are presented in Table 2. Resistance to ampicillin, cotrimoxazole and ciprofloxacin were respectively 65.8%, 64.4% 16.2%. MDR was observed in 5.2% isolates; resistance to gentamicin occurred in 3.9% isolates. There were 5 (3.2%) ESBL producing E. coli isolates; 2 of which were co-resistant to cotrimoxazole and ciprofloxacin, of which one was co-resistant to gentamicin as well. There was no resistance to fosfomycin and only 3.9% resistance to nitrofurantoin among E. coli recovered from urine in healthy pregnant women.
Table 2.
Proportions of antibiotic resistance and combined antibiotic resistance for Escherichia coli obtained from urine samples of healthy pregnant women compared to clinical samples from febrile patients
| Resistance per antibiotic | Healthy pregnant women | Febrile patients |
|---|---|---|
| Urine culture (n = 155) | Urine culture (n = 3) blood culture (n = 45) | |
| n (%) | n = 48 (%) | |
| Nitrofurantoin | 6 (3.9%) | Not done |
| Fosfomycin | 0 (0%) | Not done |
| Ampicillin | 102 (65.8%) | 43 (89.6%) |
| Cotrimoxazole | 97 (64.4%) | 43 (89.6%) |
| Ciprofloxacin | 25 (16.2%) | 30 (62.5%) |
| Gentamicin | 6 (3.9%) | 11 (22.9%) |
| Ceftriaxone | 5 (3.2%) | 18 (37.5%) |
| ESBL producers | 5 (3.2%) | 17 (35.4%) |
| Combined resistance | n (%) | n (%) |
|---|---|---|
| Ampicillin + cotrimoxazole | 80 (51.6%) | 41 (85.4%) |
| Ampicillin + cotrimoxazole + ciprofloxacin | 8 (5.2%) | 29 (60.4%) |
| Ampicillin + cotrimoxazole + gentamicin | 3 (1.9%) | 11 (22.9%) |
| Ampicillin + cotrimoxazole + ciprofloxacin + gentamicin | 2 (1.3%) | 11 (22.9%) |
| ESBL + cotrimoxazole | 2 (1.3%) | 17 (35.4%) |
| ESBL + cotrimoxazole + ciprofloxacin | 2 (1.3%) | 16 (33.3%) |
| ESBL + cotrimoxazole + gentamicin | 1 (0.6%) | 7 (14.5%) |
| ESBL + cotrimoxazole + ciprofloxacin + gentamicin | 1 (0.6%) | 7 (14.5%) |
Differences in proportions of clinical and urine isolates were assessed using chi-square test. For smaller sample sizes (value in one of the cells ≤ 5), the Fischer exact test was used. All differences between isolates obtained from healthy pregnant women and febrile patients were statistically significant (p < 0.001)
There was no statistical difference in resistance patterns between isolates growing in counts of 104 CFU/ml and 105 CFU/ml
In contrast, resistance rates among isolates obtained from febrile patients were significantly higher for all individual antibiotics as well as combinations. Out of 48 isolates, 43 (89.5%) were resistant to each ampicillin and cotrimoxazole, and 30 (62.5%) were resistant to ciprofloxacin; MDR was observed in 60.4% of isolates. Resistance to gentamicin was lowest at 22.9%. In total 18 isolates showed resistance to ceftriaxone; all but one (35.4% of total) were confirmed as ESBL producers. All ESBL producing isolates were co-resistant to cotrimoxazole and all but one were co-resistant to ciprofloxacin.
Additional file 1: Table S2 shows an overview of antibiotic susceptibility profiles of other major pathogens obtained from healthy pregnant women; other Enterobacterales growing in ≥ 104 CFU/ml, E. coli obtained from urine samples growing in mixed flora. This overview shows that antibiotic resistance among other potential pathogens (i.e. Klebsiella spp., Enterobacter spp., Proteus spp.) was also relatively low. One Klebsiella pneumoniae and one Enterobacter cloacae were ESBL-producers (2/44, 4.5% of non-E. coli Enterobacterales).
Trimester and parity
Most (120/202 [59.4%]) participants with significant growth were in their third trimester of pregnancy, differences with the first and second trimester were however not significant (Kruskall Wallis, p = 0.6). There was also no relation between proportion of significant growth and parity (Kruskall Wallis, p = 0.07).
Antibiotic use prior to sampling
In total 96 (1.7%) samples screened positive for antibiotic residue. Previous antibiotic use was reported by 29 patients, of whom 2 had antibiotic residues in their urine samples. One participant with significant growth reported antibiotic use prior to sampling and four participants screened positive for antibiotic residues.
Leukocyte esterase and nitrite
Leukocyturia was present in 1084 (18.4%) samples and nitrite in 74 (1.2%) samples. For detection of significant growth, the positive predictive and negative predictive values of leukocyturia were 6.7% and 97.3% respectively; for nitrite they were 37.8% and 97.0% and for leukocyturia and nitrite combined they were 48.7% and 96.9% (Additional file 1: Table S3).
Discussion
Summary of findings
The present study assessed the AMR rates of E. coli present as significant growth (≥ 104 CFU/ml) in the urine of healthy pregnant women in rural Burkina Faso. Among 155 E. coli isolates obtained from 5934 healthy women, AMR rates were significantly lower compared to E. coli isolates obtained from clinical samples (mostly blood cultures) in the same district.
Comparison with other studies
In the present study, ASB was defined as growth of one species of Enterobacterales in counts of 105 CFU/ml or more and was present in 2.1% of women. For antimicrobial susceptibility testing we used the quantitative cut-off of ≥ 104 CFU/ml to define ‘significant growth’, as previously done in an international survey of antimicrobial susceptibility in uncomplicated urinary tract infections [21].
The ASB proportion in the present study was lower compared to some earlier studies from sub-Saharan Africa, citing proportions of 7–40% [17, 22–24], but it was comparable to those reported elsewhere (range 1.9–9.5%) [25, 26]. The presently lower proportions of ASB may be related to the stringent definition, i.e. including only Enterobacterales as significant organisms, whereas in most other studies with higher ASB proportions, Staphylococci represented a substantial number of cases [17, 22–24].
AMR rates among the E. coli isolates obtained from urine of pregnant women were significantly lower compared to AMR rates of clinical isolates. Among the individual antibiotics, this difference was most apparent for ciprofloxacin, i.e. 16.2% for the urine isolates in pregnant women versus over 60% among clinical isolates. Likewise, proportions of ESBL producing and MDR isolates among E. coli from urine in healthy pregnant women were 3.2% and 5.2% versus 35.4%, and 60.4% respectively among clinical isolates. It is tempting to speculate that these differences reflect the use of antibiotics such as ciprofloxacin and third generation cephalosporins in the community setting.
The proportion of ESBL producers among the clinical E. coli isolates of the comparator studies (35.4%) was slightly lower compared to the 45% reported for sub-Saharan Africa in recent meta-analyses [27–29]. Carriage rates of ESBL producing E. coli from stool samples ranged from 38% in Chad to 58% in the Central African Republic [30, 31]. For the urine isolates obtained in pregnant women, AMR rates were lower compared to those found in other cross-sectional studies assessing ASB among pregnant women in sub-Saharan Africa. A study from Ghana from 2018 reported high resistance rates among E. coli to nitrofurantoin (35.4%), ciprofloxacin (48.8%), gentamicin (41.5%) and cefuroxime (32.9%) [32]. Two studies from Nigeria (2007 and 2010) reported resistance rates among E. coli of approximately 20% against second generation cephalosporins, 40% against gentamicin and 20–70% against ciprofloxacin [22, 33], which was similar to results from a study performed in Uganda in 2010 [34]. A possible explanation for the observed difference to our results is the fact that we strictly excluded participants with symptoms and signs of urinary tract infection. Likewise, the regional and between-country differences may reflect antibiotic use in hospitals as well as in the community, but also in the sectors of animal health and the environment.
Escherichia coli from urine in healthy pregnant women as an indicator of AMR in the community
In the present cohort of over 6000 pregnant women attending ANC, only 1.5% declared symptoms suggestive of an urinary tract infection and only 1.7% had evidence of antibiotic use as demonstrated by urine analysis. The latter proportion is very low compared to 30–40% antibiotic use (based on parents’ declaration) among children suspected of invasive bacterial infection in three of the comparator studies [13–15]. As such, the presently observed low AMR rates among E. coli isolates from the urine of healthy pregnant women tends to confirm our pre-study assumption, i.e. there may be a risk of overestimation of AMR rates when performing surveillance on selected clinical samples [35]. However, other factors must be taken into account when comparing resistance rates between both groups. First, the species E. coli has distinct pathotypes displaying different degrees in pathogenicity and AMR [36]; further genetic studies are planned to assess the pathotypes of the isolates from pregnant women versus those of the clinical samples. Further, in view of poorly implemented Infection Prevention & Control programmes in healthcare facilities, it is not excluded that part of the clinical isolates were belonging to a particular hospital-associated cluster.
Despite the above considerations, it is tempting to forward E. coli in urine of pregnant women as a potential indicator for benchmarking, comparing and monitoring community AMR rates across communities over different countries and regions. Such community AMR data generate valuable information about the empiric choice of antibiotics in the local context [37, 38] but may also reflect the effect of AMR control measures. As shown at least in this study setting (and to be confirmed in other settings as well), pregnant women have limited illness and antibiotic use and are accessible through ANC clinics. As part of ANC clinics, urine is routinely sampled for dipstick analysis of glucose and protein and WHO recommends midstream urine culture for the diagnosis of ASB [39]. The dipslide devices presently used were affordable (cost approximately 1 €/device) and user-friendly; they have a long shelf-life (6–9 months at room temperature) and allow for reliable inoculation on-site and subsequent transport to the laboratory. At the downside, there are the challenges of midstream-urine sampling (including contamination) and the reading of the colony counts on the dipstick devices as discussed above. Moreover, the proportion of significant growth is low. Leukocyte esterase and nitrite analysis (incorporated in most urine dipsticks) can be used as a screening tool to select samples for culture (40) but in the present study they were not very accurate to predict growth; further research for a reliable biomarker predicting growth is recommended.
Limitations and strengths
As noted above, reading of colony counts on dipslide devices tended to be subject to interpretation and this may have impacted the classification of non-significant growth, significant growth and ASB. However, our results showed that pathogen and AMR profiles were similar between E. coli from the latter two groups. Second, despite well-designed instructions and training, 8.3% of the samples were contaminated, probably related to the less stringent urine sampling in the context of a ANC compared to clinical care. Third, in retrospect, we realized that the GPA system of parity had not been fully understood by all study nurses, leading to possible too low reported numbers of nullipara compared to primipara. Additionally, there were missing data from 36 consecutive participants (0.6% of all included patients) at one of the healthcare centers. Strengths included the systematic methods used to perform this study, with high numbers of participants included and a consistent work-up by a small team of nurses and laboratory staff. The definitions for ASB and contamination were stringent, adding to the robustness of data.
Conclusion
In conclusion, in this cross-sectional study among healthy pregnant women attending ANC in rural Burkina Faso, we retrieved significant growth in 3.4% of urine samples, with E. coli representing over three-quarters of isolates. AMR rates were considerably lower among these urine samples compared to E. coli isolates obtained from clinical isolates in the same study area. Pending further research (geographic generalizability of proportions of growth and predominance of E. coli), E. coli obtained from urine culture during ANC visits has the potential of an indicator organism for benchmarking and monitoring AMR rates across populations worldwide.
Supplementary Information
Additional file 1. Table S1. Overview of demographic data of unique study participants. Table S2. Antibiotic resistance among other significant growth obtained from urine samples of healthy pregnant women. Table S3. Breakdown of leukocyte esterase and nitrite in relation to significant- and not clinically significant growth.
Acknowledgements
The authors are grateful to all participants and ANC nurses for their participation to the study. We would like to thank the laboratory staff of the CRUN unit in Nanoro and to the research nurses of the participating CSPS. Furthermore, a special thanks to Katrien Lagrou from UZ Leuven for assisting in the use of the MALDI-TOF system.
Author contributions
Conceptualization—AP, IG, JJ. Data curation—IG, MP, LP, IK, SY, ZG. Formal analysis—AP, MP, ER, JJ. Methodology—AP, IG, JJ. Supervision—HT, JJ. Validation—MP, JJ. Writing—original draft—AP, MP, SO, JJ. Writing—review and editing—AP, IG, MP, LP, SO, IK, SY, ZG, ER, HT, JJ. All authors read and approved the final manuscript.
Funding
This work was funded by the Belgian Directorate of Development Cooperation (DGD) through the Third Framework Agreement between the Belgian DGD and the Institute of Tropical Medicine, Belgium. IG was funded by a scholarship of KU Leuven, Belgium.
Availability of data and materials
All data are available from the researchers upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the national ethics committee of Burkina Faso (Comité d’Ethique pour la Recherche en Santé (Reference No 2015-7-96 July 1st, 2015), the institutional review board of ITM, Antwerp (Reference 1008/15 from December 15th, 2015) and the ethics committee of the University Hospital of Antwerp (Reference 15/51/563, January 4th, 2016). Written informed consent was obtained before participation in the study. A screening log with reasons for refusal was completed at each health center included in the study. If ASB was diagnosed, laboratory staff of the study site communicated the recovery of clinically significant isolates and their AST results to the study investigator, who informed the ANC nurse or the clinician responsible of the ANC. Participants were treated according to national treatment guidelines.
Consent for publication
All authors have read the manuscript and consent to publish.
Competing interests
None of the authors have competing interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Annelies S. Post, Email: annelies.post@gmail.com
Jan Jacobs, Email: jjacobs@itg.be.
References
- 1.Jacobs J, Hardy L, Semret M, Lunguya O, Phe T, Affolabi D, et al. Diagnostic bacteriology in district hospitals in sub-Saharan Africa: at the forefront of the containment of antimicrobial resistance. Front Med. 2019;6:205. doi: 10.3389/fmed.2019.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation. Global action plan on AMR. Geneva: World Health Organization; 2016. Available from: https://www.who.int/publications/i/item/9789241509763.
- 3.World Health Organisation. Global antimicrobial resistance and use surveillance system (GLASS). Geneva: World Health Organisation; 2021. Available from: https://www.who.int/publications/i/item/9789240027336.
- 4.Barbé B, Yansouni CP, Affolabi D, Jacobs J. Implementation of quality management for clinical bacteriology in low-resource settings. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2017;23(7):426–433. doi: 10.1016/j.cmi.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Ombelet S, Ronat J-B, Walsh T, Yansouni CP, Cox J, Vlieghe E, et al. Clinical bacteriology in low-resource settings: today's solutions. Lancet Infect Dis. 2018;18(8):e248–e258. doi: 10.1016/S1473-3099(18)30093-8. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organisation. Second WHO model list of essential in vitro diagnostics. Geneva; 2019. Available from: https://www.who.int/medical_devices/publications/Standalone_document_v8.pdf.
- 7.Nicolle LE. Asymptomatic bacteriuria and bacterial interference. Microbiol Spectr. 2015 doi: 10.1128/microbiolspec.UTI-0001-2012. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organisation. Critically important antimicrobials for human medicine. 2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf.
- 9.Derra K, Rouamba E, Kazienga A, Ouedraogo S, Tahita MC, Sorgho H, et al. Profile: nanoro health and demographic surveillance system. Int J Epidemiol. 2012;41(5):1293–1301. doi: 10.1093/ije/dys159. [DOI] [PubMed] [Google Scholar]
- 10.Rouamba T, Valea I, Bognini JD, Kpoda H, Mens PF, Gomes MF, et al. Safety profile of drug use during pregnancy at peripheral health centres in Burkina Faso: a prospective observational cohort study. Drugs Real World Outcomes. 2018;5(3):193–206. doi: 10.1007/s40801-018-0141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CLSI. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. CLSI supplement M100. 950 West Valley Road, Suite 2500, Wayne, USA: Clinical and Laboratory Standards Institute; 2020. Available from: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf.
- 12.Kouri TT, Gant VA, Fogazzi GB, Hofmann W, Hallander HO, Guder WG. Towards European urinalysis guidelines. Introduction of a project under European Confederation of Laboratory Medicine. Clin Chim Acta Int J Clin Chem. 2000;297(1–2):305–311. doi: 10.1016/S0009-8981(00)00256-4. [DOI] [PubMed] [Google Scholar]
- 13.Kaboré B, Post A, Lompo P, Bognini JD, Diallo S, Kam BTD, et al. Aetiology of acute febrile illness in children in a high malaria transmission area in West Africa. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2021;27(4):590–596. doi: 10.1016/j.cmi.2020.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Maltha J, Guiraud I, Kaboré B, Lompo P, Ley B, Bottieau E, et al. Frequency of severe malaria and invasive bacterial infections among children admitted to a rural hospital in Burkina Faso. PLoS ONE. 2014;9(2):e89103. doi: 10.1371/journal.pone.0089103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guiraud I, Post A, Diallo SN, Lompo P, Maltha J, Thriemer K, et al. Population-based incidence, seasonality and serotype distribution of invasive salmonellosis among children in Nanoro, rural Burkina Faso. PLoS ONE. 2017;12(7):e0178577. doi: 10.1371/journal.pone.0178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Post AS, Diallo SN, Guiraud I, Lompo P, Tahita MC, Maltha J, et al. Supporting evidence for a human reservoir of invasive non-Typhoidal Salmonella from household samples in Burkina Faso. PLoS Negl Trop Dis. 2019;13(10):e0007782. doi: 10.1371/journal.pntd.0007782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ajayi AB, Nwabuisi C, Aboyeji AP, Ajayi NS, Fowotade A, Fakeye OO. Asymptomatic bacteriuria in antenatal patients in Ilorin, Nigeria. Oman Med J. 2012;27(1):31–35. doi: 10.5001/omj.2012.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham FG, Lenveno KJ, Bloom SL, Hauth JC, Gilstrap LC, III, Wenstrom KD. Williams obstetrics. New York: McGraw-Hill Professional; 2005. [Google Scholar]
- 19.CLSI. Analysis and presentation of cumulative antimicrobial susceptibility test data. In: A. J, editor. 4th Edition ed: CLSI; 2014.
- 20.University of Bern. STROBE statement. 2009. Available from: https://www.strobe-statement.org.
- 21.Schito GC, Naber KG, Botto H, Palou J, Mazzei T, Gualco L, et al. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents. 2009;34(5):407–413. doi: 10.1016/j.ijantimicag.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Oli AN, Okafor CI, Ibezim EC, Akujiobi CN, Onwunzo MC. The prevalence and bacteriology of asymptomatic bacteriuria among antenatal patients in Nnamdi Azikiwe University Teaching Hospital Nnewi; South Eastern Nigeria. Niger J Clin Pract. 2010;13(4):409–412. [PubMed] [Google Scholar]
- 23.Turpin C, Minkah B, Danso K, Frimpong E. Asymptomatic bacteriuria in pregnant women attending antenatal clinic at komfo anokye teaching hospital, Kumasi, Ghana. Ghana Med J. 2007;41(1):26. [PMC free article] [PubMed] [Google Scholar]
- 24.Karikari AB, Saba CKS, Yamik DY. Assessment of asymptomatic bacteriuria and sterile pyuria among antenatal attendants in hospitals in northern Ghana. BMC Pregnancy Childbirth. 2020;20(1):239. doi: 10.1186/s12884-020-02936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson TF, Andriole VT. Detection, significance, and therapy of bacteriuria in pregnancy. Update in the managed health care era. Infect Dis Clin N Am. 1997;11(3):593–608. doi: 10.1016/S0891-5520(05)70375-5. [DOI] [PubMed] [Google Scholar]
- 26.Nicolle LE, Gupta K, Bradley SF, Colgan R, DeMuri GP, Drekonja D, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis Off Publ Infect Dis Soc Am. 2019;68(10):e83–e110. doi: 10.1093/cid/ciz021. [DOI] [PubMed] [Google Scholar]
- 27.Moirongo RM, Lorenz E, Ntinginya NE, Dekker D, Fernandes J, Held J, et al. Regional variation of extended-spectrum beta-lactamase (ESBL)-producing enterobacterales, fluoroquinolone-resistant Salmonella enterica and methicillin-resistant Staphylococcus aureus among febrile patients in sub-Saharan Africa. Front Microbiol. 2020;11(2408):567635. doi: 10.3389/fmicb.2020.567235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonda T, Kumburu H, van Zwetselaar M, Alifrangis M, Lund O, Kibiki G, et al. Meta-analysis of proportion estimates of extended-spectrum-beta-lactamase-producing Enterobacteriaceae in east Africa hospitals. Antimicrob Resist Infect Control. 2016;5(1):18. doi: 10.1186/s13756-016-0117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansouri F, Sheibani H, Javedani Masroor M, Afsharian M. Extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae and urinary tract infections in pregnant/postpartum women: a systematic review and meta-analysis. Int J Clin Pract. 2019;22:e13422. doi: 10.1111/ijcp.13422. [DOI] [PubMed] [Google Scholar]
- 30.Farra A, Frank T, Tondeur L, Bata P, Gody JC, Onambele M, et al. High rate of faecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae in healthy children in Bangui, Central African Republic. Clin Microbiol Infect. 2016;22(10):891. doi: 10.1016/j.cmi.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Ouchar Mahamat O, Tidjani A, Lounnas M, Hide M, Benavides J, Somasse C, et al. Fecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae in hospital and community settings in Chad. Antimicrob Resist Infect Control. 2019;8(1):169. doi: 10.1186/s13756-019-0626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forson AO, Tsidi WB, Nana-Adjei D, Quarchie MN, Obeng-Nkrumah N. Escherichia coli bacteriuria in pregnant women in Ghana: antibiotic resistance patterns and virulence factors. BMC Res Notes. 2018;11(1):901. doi: 10.1186/s13104-018-3989-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imade PE, Izekor PE, Eghafona NO, Enabulele OI, Ophori E. Asymptomatic bacteriuria among pregnant women. N Am J Med Sci. 2010;2(6):263–266. doi: 10.4297/najms.2010.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andabati G, Byamugisha J. Microbial aetiology and sensitivity of asymptomatic bacteriuria among ante-natal mothers in Mulago Hospital, Uganda. Afr Health Sci. 2010;10(4):349–352. [PMC free article] [PubMed] [Google Scholar]
- 35.Alós JI, Serrano MG, Gómez-Garcés JL, Perianes J. Antibiotic resistance of Escherichia coli from community-acquired urinary tract infections in relation to demographic and clinical data. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2005;11(3):199–203. doi: 10.1111/j.1469-0691.2004.01057.x. [DOI] [PubMed] [Google Scholar]
- 36.Allocati N, Masulli M, Alexeyev MF, Di Ilio C. Escherichia coli in Europe: an overview. Int J Environ Res Public Health. 2013;10(12):6235–6254. doi: 10.3390/ijerph10126235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schechner V, Temkin E, Harbarth S, Carmeli Y, Schwaber MJ. Epidemiological interpretation of studies examining the effect of antibiotic usage on resistance. Clin Microbiol Rev. 2013;26(2):289–307. doi: 10.1128/CMR.00001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organisation. Surveillance standards for antimicrobial resistance. Geneva: World Health Organisation; 2001. Available from https://www.who.int/csr/resources/publications/drugresist/en/whocdscsrdrs20015.pdf?ua=1.
- 39.World Health Organisation. WHO recommendation on the method for diagnosing anaemia in pregnancy. 2016, p. 1–5. Available from: https://apps.who.int/iris/bitstream/handle/10665/250800/WHO-RHR-16.12-eng.pdf?sequence=1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Overview of demographic data of unique study participants. Table S2. Antibiotic resistance among other significant growth obtained from urine samples of healthy pregnant women. Table S3. Breakdown of leukocyte esterase and nitrite in relation to significant- and not clinically significant growth.
Data Availability Statement
All data are available from the researchers upon reasonable request.