Abstract
RosR is a determinant of nodulation competitiveness and cell surface characteristics of Rhizobium etli and has sequence similarity to a family of transcriptional repressors. To understand how RosR affects these phenotypes, we mutagenized a rosR mutant derivative of R. etli strain CE3 with a mini-Tn5 that contains a promoterless gusA gene at one end, which acts as a transcriptional reporter. Using a mass-mating technique, we introduced rosR into each mutant in trans and screened for mutants that expressed different levels of β-glucuronidase activity in the presence and absence of rosR. A screen of 18,000 mutants identified 52 insertions in genes negatively regulated by RosR and 1 insertion in a gene positively regulated by RosR. Nucleotide sequence analysis of the regions flanking the insertions suggests that RosR regulates genes of diverse function, including those involved in polysaccharide production and in carbohydrate metabolism and those in a region containing sequence similarity to virC1 and virD3 from Agrobacterium tumefaciens. Two of the mutants produced colonies with altered morphology and were more competitive in nodulation than was CE3ΔrosR, the rosR parent. One mutant that contained an insertion in a gene with similarity to exsH of Sinorhizobium meliloti did not nodulate the plant host Phaseolus vulgaris without rosR. These results indicate that RosR directly or indirectly influences expression of diverse genes in R. etli, some of which affect the cell surface and nodulation competitiveness.
Competition among microorganisms determines the outcome of many biological events in nature, and yet competitiveness is poorly understood. Lack of knowledge of the mechanistic basis for competitiveness is due, in large part, to the difficulty in conducting a genetic analysis of this quantitative trait. Identification of mutants affected in competitiveness is challenging in many microbial systems due to variability and the need for a high degree of replication in mutant screens (26, 32).
The Rhizobium-legume symbiosis provides a good model system with which to study the molecular basis of bacterial competitiveness, because nodulation competitiveness is a readily quantifiable trait. Rhizobial species establish mutualistic relationships with specific leguminous plants by initiating the development of a specialized plant organ, the root nodule, in which the bacteria fix atmospheric nitrogen. The ability of a particular rhizobial strain to establish this in the presence of other strains is known as nodulation competitiveness. Nodulation competitiveness is measured by comparing the proportions of rhizobial strains that are initially applied to the seed with the proportion of the nodules that are later occupied by each strain.
The Rhizobium etli-bean symbiosis is well suited to the study of the genetic basis of nodulation competitiveness. Variability can be minimized by using a genetically homogeneous plant host population, such as common bean (Phaseolus vulgaris), which is highly inbred. Moreover, the nodules usually contain a pure culture of the successful competitor, which distinguishes this microbial competition from many others in which detection of competitive success is difficult.
The rosR gene likely encodes a regulator that plays a critical role in both nodulation competitiveness and determination of cell surface characteristics in R. etli (2, 10). A rosR mutant was originally identified by its distinctive domed colony morphology that results from its hydrophobic cell surface. The rosR mutant nodulates and fixes nitrogen, but when the mutant and the parent are coinoculated in equal amounts, nearly all of the root nodules are occupied by the parent strain. A vast (approximately 17,000-fold) excess of the mutant is required to achieve equal nodule occupancy, indicating that the rosR mutant is drastically reduced in nodulation competitiveness.
RosR is 80% identical to MucR from Sinorhizobium meliloti and Ros from Agrobacterium tumefaciens (16, 24). Ros and MucR act as transcriptional repressors by binding DNA sequences in the promoter regions of regulated genes via putative zinc finger motifs (15, 24). MucR affects the production of an alternative exopolysaccharide (EPS), EPS II (galactoglucan), in place of the normal EPS I (succinoglycan) by repressing transcription of the genes involved in EPS II synthesis (24, 39). Ros represses the virC and virD operons in A. tumefaciens, which are involved in determining virulence, as well as ipt, which is involved in cytokinin production (13, 16). In addition, ros mutants do not produce succinoglycan, and the repressive activity of Ros in Agrobacterium radiobacter is enhanced by Fe3+ and glucose in the culture medium (11, 14).
To elucidate the role of RosR in R. etli, we developed a genetic screen for genes transcriptionally regulated by RosR. RosR-regulated genes were identified by randomly inserting a reporter gene throughout the genome of a rosR mutant of R. etli and then comparing reporter gene expression in the presence and absence of rosR in trans. We identified the subset of those RosR-regulated genes involved in determining cell surface characteristics and nodulation competitiveness. This study represents the first broad-based screen of the entire genome to identify genes regulated by a member of this family of transcriptional regulators.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli strains were grown in Luria-Bertani broth at 37°C, and R. etli strains were grown at 28°C in yeast extract mannitol (YEM) (35), tryptone-yeast extract (TY) (8), or Bergersen's synthetic medium supplemented with 1 mM methionine (BSM) (7). Solid media contained 1.5% agar, and antibiotics were used at the following concentrations: streptomycin, 200 μg/ml; spectinomycin, 100 μg/ml; nalidixic acid, 15 μg/ml; ampicillin, 50 μg/ml; tetracycline, 12 μg/ml; and kanamycin, 50 μg/ml. X-GlcA (5-bromo-4-chloro-3-indolyl glucuronic acid) was used at 50 μg/ml. Plasmid DNA was isolated from E. coli using the Qiaprep Kit (Qiagen Inc.). Restriction and modification enzymes were used according to the directions of the manufacturers (Promega Corp. and New England Biolabs). Plasmids were introduced into R. etli either by triparental mating using the helper plasmid pRK2013 or by biparental mating using E. coli strain S17-1 λpir as the donor strain.
TABLE 1.
Characteristics of bacterial strains and plasmids
| Strain(s) or plasmid | Descriptiona | Reference |
|---|---|---|
| Strains | ||
| R. etli CE3 | Spontaneous Smr mutant of CFN42; Nxr Smr | 29 |
| R. etli CE3013 | Tn5 derivative of CE3 unaffected in nodulation competitiveness; Nxr Smr Kmr | 5 |
| R. etli CE3003 | rosR::Tn5 Nxr Smr Kmr | 2 |
| R. etli CE3ΔrosR | Derivative of CE3 with rosR deleted; Nxr Smr | 10 |
| R. etli MB001 to MB065 | mTn5SSgusA40 derivative of CE3ΔrosR; Nxr Smr Spr | This study |
| E. coli DH5α | φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 deoR | 21 |
| E. coli S17-1 λpir | thi pro hsdR hsdM+ recA RP4 2-Tc::Mu-Km::Tn7Smr λpir | 36 |
| Plasmids | ||
| pLAFR3 | Broad-host-range cosmid cloning vector; Tcr | 33 |
| pH8B1 | 1.2-kb rosR subclone in pLAFR3; Tcr | 10 |
| pRK2013 | Tra+ helper plasmid; Kmr | 18 |
| pCAM140 | mTn5SSgusA40 (promoterless gusA transposon) in pUT; Smr Spr Apr | 36 |
| pBBR1MCS-3 | Broad-host-range cloning vector; Tcr | 25 |
| pMB001 to pMB065 | SacI fragment containing mTn5SSgusA40 insertion from corresponding MB strain cloned into pBBR1MCS-3; Spr Tcr | This study |
Antibiotic resistances are abbreviated as follows: ampicillin, Apr; nalidixic acid, Nxr; gentamicin, Gmr; kanamycin, Kmr; streptomycin, Smr; spectinomycin, Spr; and tetracycline, Tcr.
Identification of RosR-regulated insertions.
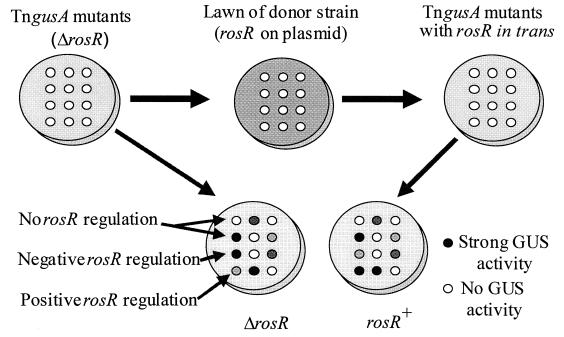
R. etli strain CE3ΔrosR was mutagenized with mTn5SSgusA40 by a previously described method (36), and transposon mutants were selected on BSM with appropriate antibiotics. Individual mutants were patched onto master plates in a grid of 48 mutants per plate (Fig. 1). These mutants were replicated onto TY without antibiotics, to avoid antibiotic carryover to the mating plates, using 48-prong metal replicators. E. coli S17-1 λpir(pH8B1) was grown in Luria-Bertani broth overnight, and cells were pelleted, washed twice with TY to remove antibiotics, and resuspended in the original volume of TY. Two hundred microliters of the resuspended cells was plated onto TY plates to form the lawn of the donor strain. The R. etli mutants were replicated onto the lawn of S17-1 λpir(pH8B1) and grown overnight at 28°C. These mating spots were then replicated onto BSM with appropriate antibiotics to select for the R. etli mutants carrying pH8B1. Both collections of strains (with and without pH8B1) were replicated into 96-well microtiter plates in which each well contained 150 μl of YEM with appropriate antibiotics. The microtiter plates were incubated at 28°C until both sets of plates contained dense cultures. The microtiter plates of the mutants with and without plasmid were replicated onto BSM containing X-GlcA and appropriate antibiotics. These plates were placed at 28°C, and corresponding colonies from each collection of strains were monitored for the appearance of a blue product over several days. Mutants that displayed different levels of accumulation of the blue color with and without pH8B1 were identified, the corresponding patch on the master plate was picked, and a single colony was isolated and retested for RosR regulation in quantitative enzyme assays.
FIG. 1.
Schematic diagram of the genetic screen used to identify RosR-regulated genes.
Quantitative GUS assays.
Strains to be assayed were grown for 3 days in YEM broth. Fifty microliters of the culture was used to inoculate 2 ml of YEM broth and grown overnight. Enzyme assays were carried out on the overnight cultures by using 4-methylumbelliferyl-β-glucuronide as a substrate for the β-glucuronidase (GUS) enzyme (22). Product accumulation was monitored using a TKO-100 fluorometer (Hoefer Scientific Instruments), and the bicinchoninic acid protein assay reagent (Pierce) was used to determine protein concentrations in calculating enzyme activities. Enzyme assays were repeated at least three times using independent cultures. Repression by RosR was calculated by dividing the average GUS activity of the mutant with pLAFR3 by the average GUS activity of the mutant with pH8B1. Activation by RosR was calculated by dividing the average activity of the mutant with pH8B1 by the average activity of the mutant with pLAFR3.
DNA sequencing of regions flanking the transposon insertions.
Genomic DNA from each transposon mutant was cut with SacI and cloned into pBBR1MCS-3, selecting for the spectinomycin resistance gene carried on the transposon. The sequence of the flanking region was obtained by sequencing from the I end of the transposon with the primer 5′GGG AAT TCG GCC TAG GCG G3′ and from the O end (the end with the promoterless gusA gene) with the primer 5′TTT CTA CAG GAC GTA ACA TAA GGG3′. The Big-dye cycle sequencing kit (Applied Biosystems, Inc.) was used, and the resulting reactions were analyzed at the University of Wisconsin—Madison Biotechnology Center. Sequences from both ends of the transposon were trimmed of transposon sequence and fused to obtain a single sequence of the insertion site. To avoid removal of any biologically relevant sequence information, we did not remove the sequences of small duplications at the site of the transposon insertion, which may have occurred as a result of the transposition event. The DNA sequences of the regions identified by more than one insertion were assembled into a single contig, which was used for sequence analysis. Sequence analysis was carried out in July 1999 using the BLASTn and BLASTx algorithms at the National Center for Biotechnology Information via the worldwide web (http://www.ncbi.nlm.nih.gov/) (1).
Screening of mutants for altered competitiveness.
P. vulgaris cultivar Black Turtle seeds (Park Seed Co.) were surface disinfected by treatment with 95% ethanol for 30 s and with 1.6% sodium hypoclorite for 3 min and were planted in a sterilized sand-vermiculite (1.5:1) mixture. Bacterial strains were grown on TY plates with appropriate antibiotics, scraped from the plates, and resuspended in sterile water to an A600 of 0.1 (approximately 108 cells/ml). Either CE3003 or CE3013 was used as a kanamycin-resistant competitor strain. Inoculum mixtures were made by mixing the strains in 1:1 ratios. One milliliter of inoculum was applied to each planted bean seed. Serial dilutions of inocula were plated to determine cell numbers. Beans were placed in a growth chamber and watered with sterile nitrogen-free plant nutrient solution as needed for 21 days (2). Each treatment was applied to six plants, eight nodules were harvested from each plant, and bacterial strains in the nodules were identified by antibiotic resistance (6). Each nodulation competitiveness assay was repeated at least twice.
Nucleotide sequence accession numbers.
The sequences from each end of the transposons in the transposon mutants were deposited in the GenBank database, and the accession numbers are indicated in Table 2.
TABLE 2.
Characteristics of mutants with insertions in RosR-regulated loci
| Mutant | rosR genotypeg | GUS activity (nmol of product min−1 mg of protein−1) (mean ± SD) | Flanking DNA sequencesa | Sequence similarity of region flanking transposon insertion [protein, organism (probable function), significance] | Characteristics |
|---|---|---|---|---|---|
| MB001 | rosR mutant | 19,748 ± 690 | AF116349, AF116350 | PssK, R. leguminosarum (polysaccharide polymerization protein)ce E = 3e-29 | 41×h negative regulation |
| rosR+ | 477 ± 81 | ||||
| MB002 | rosR mutant | 4,708 ± 441 | AF116351, AF116352 | Oac, bacteriophage Sf6 (O-antigen acetylase; lipopolysaccharide modification)ce, E = 5e-15; NodX, R. leguminosarum bv. vicea (sugar acetylase)ce, E = 1e-12 | 56× negative regulation |
| rosR+ | 84 ± 6 | ||||
| MB004 | rosR mutant | 3,442 ± 466 | AF116353, AF116354 | AAB63465, Caenorhabditis elegans (O-linked GlcNAc transferase)ce, E = le-08 | 12× negative regulation |
| rosR+ | 290 ± 16 | ||||
| MB005 | rosR mutant | 27 ± 7 | AF116355, AF116356 | No significant similarity | 7× negative regulation |
| rosR+ | 4 ± 3 | ||||
| MB006 | rosR mutant | 70 ± 4 | L13618 | AAB07742; R. etli (putative coproporphyrinogen III oxidase; heme synthesis)ce | 4× positive regulation |
| rosR+ | 247 ± 38 | ||||
| MB009 | rosR mutant | 1,738 ± 64 | AF116357, AF116358 | AAB90655, Archaeoglobus fulgidus (putative dolichol-P-glucose synthase)cf, E = 0.002 | 17× negative regulation |
| rosR+ | 105 ± 5 | ||||
| MB010 | rosR mutant | 1,512 ± 118 | AF116359, AF116360 | CscR, E. coli (repressor of sucrose degradation operon)ce, E = 6e-05 | 8× negative regulation |
| rosR+ | 193 ± 22 | ||||
| MB011 | rosR mutant | 4,336 ± 133 | AF116361, AF116362 | ORF6 in fasciation locus, R. fascians (may be involved in cytokinin synthesis)be, E = 9e-25 | 38× negative regulation |
| rosR+ | 115 ± 9 | ||||
| MB012 | rosR mutant | 1,363 ± 331 | AF116363, AF116364 | AAB63465, C. elegans (O-linked GlcNAc transferase)ce, E = le-08 | 10× negative regulation (217 bp upstream of MB004 insertion) |
| rosR+ | 133 ± 28 | ||||
| MB013 | rosR mutant | 22,936 ± 3,936 | AF116365, AF116366 | No significant similarity | 131× negative regulation, mucoid colony morphology |
| rosR+ | 175 ± 18 | ||||
| MB014 | rosR mutant | 3036 ± 687 | AF116367, AF116368 | LipR, Streptomyces coelicolor (transcriptional activator)be, E = 2e-05 | 13× negative regulation |
| rosR+ | 230 ± 13 | ||||
| MB015 | rosR mutant | 4,746 ± 2,065 | AF116369, AF116370 | ExsH, S. meliloti (endoglycanase; EPS synthesis)ce, E = 5e-19 | 29× negative regulation |
| rosR+ | 162 ± 7 | ||||
| MB016 | rosR mutant | 929 ± 87 | AF116371, AF116372 | AF116463, Streptomyces linocolnesis (hypothetical protein)ce, E = 3e-05 | 16× negative regulation |
| rosR+ | 60 ± 4 | ||||
| MB017 | rosR mutant | 405 ± 73 | AF116373, AF116374 | DegT, Bacillus stearothermophilus (membrane-bound regulatory protein)ce E = 4e-57 | 8× negative regulation |
| rosR+ | 50 ± 14 | ||||
| MB018 | rosR mutant | 467 ± 35 | AF116375, AF116376 | NocM, A. tumefaciens (nopaline permease)de E = 4e-05 | 10× negative regulation |
| rosR+ | 48 ± 20 | ||||
| MB019 | rosR mutant | 13,005 ± 3,157 | AF116377, AF116378 | OtsA, Rhizobium sp. strain NGR234 (trehalose-phosphate synthase)de E, = 5e-06; | 23× negative regulation |
| rosR+ | 569 ± 62 | ||||
| MB020 | rosR mutant | 6,288 ± 171 | AF116379, AF116380 | No significant similarity | 54× negative regulation |
| rosR+ | 116 ± 8 | ||||
| MB021 | rosR mutant | 3,220 ± 502 | AF116381, AF116382 | ExoB, S. meliloti (UDP-glucose epimerase; EPS synthesis)ce, E = 4e-59; AAC07360, Aquifex aeolicus (cellulose synthase)be, E = 2e−08 | 72× negative regulation |
| rosR+ | 45 ± 10 | ||||
| MB022 | rosR mutant | 4,928 ± 376 | AF116383, AF116384 | No significant similarity | 15× negative regulation |
| rosR+ | 338 ± 17 | ||||
| MB023 | rosR mutant | 2,690 ± 710 | AF116385, AF116386 | No significant similarity | 34× negative regulation |
| rosR+ | 80 ± 9 | ||||
| MB024 | rosR mutant | 1,130 ± 275 | AF116387, AF116388 | No significant similarity | 18× negative regulation |
| rosR+ | 62 ± 33 | ||||
| MB025 | rosR mutant | 2,668 ± 247 | AF116389, AF116390 | ExoB; S. meliloti (UDP-glucose epimerase; EPS synthesis)de, E = 4e-59; AAC07360, A. aeolicus (cellulose synthase)ce, E = 2e-08 | 38× negative regulation (602 bp downstream of MB021 insertion) |
| rosR+ | 71 ± 3 | ||||
| MB026 | rosR mutant | 2,112 ± 423 | AF116391, AF116392 | No significant similarity | 28× negative regulation |
| rosR+ | 77 ± 4 | ||||
| MB027 | rosR mutant | 11,674 ± 3,490 | AF116393, AF116394 | ORF2 in picA locus, A. tumefaciens (affects cell surface changes due to plant cell extracts)be, E = 3e-14 | 22× negative regulation |
| rosR+ | 542 ± 75 | ||||
| MB029 | rosR mutant | 3,294 ± 853 | AF116395, AF116396 | No significant similarity | 10× negative regulation |
| rosR+ | 327 ± 41 | ||||
| MB031 | rosR mutant | 2,796 ± 1,127 | AF116397, AF116398 | No significant similarity | 8× negative regulation (49 bp downstream of MB029 insertion) |
| rosR+ | 335 ± 24 | ||||
| MB032 | rosR mutant | 19,776 ± 6,851 | AF116399, AF116400 | PlyA, R. leguminosarum (polysaccharidase)be, E = 3e-11 | 66× negative regulation |
| rosR+ | 301 ± 32 | ||||
| MB033 | rosR mutant | 1,576 ± 320 | AF116401, AF116402 | No significant similarity | 22× negative regulation |
| rosR+ | 71 ± 16 | ||||
| MB034 | rosR mutant | 1,885 ± 590 | AF116403, AF116404 | CbbZ, Rhodobacter sphaeroides (phosphoglycolate phosphatase)be, E = 4e-11 | 11× negative regulation |
| rosR+ | 171 ± 14 | ||||
| MB035 | rosR mutant | 8,728 ± 2,849 | AF116405, AF116406 | Y4JH, Rhizobium sp. strain NGR234 (hypothetical protein on Sym plasmid)ce, E = 5e-44 | 22× negative regulation |
| rosR+ | 398 ± 100 | ||||
| MB036 | rosR mutant | 1,655 ± 397 | AF116407, AF116408 | LipR, Streptomyces coelicolor (transcriptional activator)be, E = 2e-05 | 20× negative regulation (312 bp downstream of MB014 insertion) |
| rosR+ | 84 ± 17 | ||||
| MB037 | rosR mutant | 3,262 ± 938 | AF116409, AF116410 | ExoB, S. meliloti (UDP-glucose epimerase; EPS synthesis)de, E = 4e-59; AAC07360, A. aeolicus (cellulose synthase)ce, E = 2e-08 | 51× negative regulation (1,203 bp downstream of MB021 insertion) |
| rosR+ | 63 ± 30 | ||||
| MB038 | rosR mutant | 6,190 ± 2,527 | AF116411, AF116412 | CelR2, R. leguminosarum bv. trifolii (regulator of genes for cellulose synthesis)ce, E = 2e-22 | 11× negative regulation |
| rosR+ | 561 ± 67 | ||||
| MB039 | rosR mutant | 721 ± 256 | AF116413, AF116414 | PlyA, R. leguminosarum (polysaccharide)be, E = 3e-11 | 105× negative regulation (164 bp upstream of MB032) |
| rosR+ | 7 ± 2 | ||||
| MB040 | rosR mutant | 6,007 ± 3,312 | AF116415, AF116416 | PlyA, R. leguminosarum (polysaccharidase)ce, E = 3e-11 | 198× negative regulation (302 bp downstream of MB032 insertion) |
| rosR+ | 30 ± 6 | ||||
| MB041 | rosR mutant | 2,696 ± 185 | AF116417, AF116418 | PlyA, R. leguminosarum (polysaccharidase)bf, E = 3e-11 | 36× negative regulation (164 bp upstream of MB032 insertion) |
| rosR+ | 75 ± 16 | ||||
| MB042 | rosR mutant | 10,063 ± 1,341 | AF116419, AF116420 | ORF in picA locus, A. tumefaciens (affects cell surface changes due to plant cell extracts)ce, E = 3e-14 | 52× negative regulation (1,012 bp upstream of MB027 insertion) |
| rosR+ | 194 ±54 | ||||
| MB043 | rosR mutant | 4,597 ± 895 | AF116421, AF116422 | VirCl, A. tumefaciens (virulence determinant)bf, E = 8e-27; VirD3, A. tumefaciens (unknown function)be E = 0.36 | 134× negative regulation |
| rosR+ | 34 ± 13 | ||||
| MB045 | rosR mutant | 9,556 ± 1,291 | AF116423, AF116424 | HelE, Dictyostelium discoideum (similar to helicase-like transcription factor)be E = 4e-04; SlpB, Methanosarcina mazei (surface layer protein B)be E = 7e-04; PlyA, R. leguminosarum (polysaccharidase)de E = 0.013 | 86× negative regulation |
| rosR+ | 111 ± 22 | ||||
| MB046 | rosR mutant | 18,870 ± 1,075 | AF116425, AF116426 | Serralysin, Pseudomonas sp. (metalloprotease)ce, E = 1e-07; AlgE1, Azotobacter vinelandii (mannuronan C-5-epimerase)ce, E = 4e-06 | 67× negative regulation |
| rosR+ | 162 ± 26 | ||||
| MB047 | rosR mutant | 3,079 ± 1,165 | AF116427, AF116428 | No significant similarity | 41× negative regulation |
| rosR+ | 75 ± 12 | ||||
| MB048 | rosR mutant | 3,066 ± 286 | AF116429, AF116430 | BAA17459, Synechocystis sp. (hypothetical protein)ce, E = 9e-50 | 12× negative regulation |
| rosR+ | 263 ± 10 | ||||
| MB049 | rosR mutant | 2,456 ± 168 | AF116431, AF116432 | No significant similarity | 9× negative regulation |
| rosR+ | 277 ± 30 | ||||
| MB050 | rosR mutant | 3,514 ± 619 | AF116433, AF116434 | METRS, Methanobacterium thermoautotrophicum (methionyl-tRNA synthetase)cf, E = 4e-29 | 13× negative regulation |
| rosR+ | 265 ± 71 | ||||
| MB051 | rosR mutant | 1,442 ± 186 | AF116435, AF116436 | No significant similarity | 82× negative regulation |
| rosR+ | 18 ± 11 | ||||
| MB052 | rosR mutant | 1,101 ± 218 | AF116437, AF116438 | AF116463, Streptomyces linocolnesis (hypothetical protein)ce, E = 3e-05 | 7× negative regulation (647 bp downstream of MB016 insertion) |
| rosR+ | 155 ± 11 | ||||
| MB054 | rosR mutant | 3,427 ± 904 | AF116439, AF116440 | CAA94113; C. elegans (hypothetical protein)cf, E = 0.015; CAA20616, S. coelicolor (putative lyase) df, E = 0.077 | 7× negative regulation |
| rosR+ | 521 ± 83 | ||||
| MB055 | rosR mutant | 10,025 ± 713 | AF116441AF116442 | PrsD, R. leguminosarum (ATP-binding cassette transporter)ce, E = 3e-86 | 8× negative regulation |
| rosR+ | 1,250 ± 79 | ||||
| MB056 | rosR mutant | 8,995 ± 2,012 | AF116443, AF116444 | No significant similarity | 6× negative regulation |
| rosR+ | 1,493 ± 103 | ||||
| MB057 | rosR mutant | 1,478 ± 289 | AF116445, AF116446 | No significant similarity | 9× negative regulation |
| rosR+ | 159 ± 14 | ||||
| MB059 | rosR mutant | 3,852 ± 1,448 | AF116447, AF116448 | No significant similarity | 6× negative regulation |
| rosR+ | 617 ± 54 | ||||
| MB060 | rosR mutant | 4,365 ± 1,207 | AF116449, AF116450 | Gdh, Pantoea citrea (glucose dehydrogenase)ce, E = 1e-24 | 63× negative regulation |
| rosR+ | 69 ± 11 | ||||
| MB065 | rosR mutant | 19,362 ± 1,718 | AF116451, AF116452 | ExoY, Rhizobium sp. (EPS synthesis)ce, E = 1e-51 | 118× negative regulation, mucoid colony morphology |
| rosR+ | 164 ± 25 |
GenBank accession numbers are indicated, with the first number indicating the sequence from upstream of the gusA gene.
The transposon insertion is upstream of the region with similarity to the database member.
The transposon insertion is within the region with similarity to the database member.
The transposon insertion is downstream of the region with similarity to the database member.
gusA is in the same orientation as the region with similarity to the database member.
gusA is in the opposite orientation as the region with similarity to the database member.
rosR mutant, pLAFR3; rosR+, pH8B1.
41×, 41-fold.
RESULTS
Identification of RosR-regulated genes.
We developed a genetic screen to identify RosR-regulated genes (Fig. 1). A derivative of R. etli strain CE3 with the rosR gene deleted, CE3ΔrosR, was mutagenized with a mini-Tn5 containing a promoterless gusA gene, which encodes GUS, at one end acting as a transcriptional reporter. rosR was introduced in trans into each of the mutants by a mass-mating technique. Individual transposon mutants were screened on indicator medium for GUS expression for differences in reporter gene expression with and without rosR in trans. Quantitative GUS enzyme assays confirmed the initial mutant phenotypes (Table 2).
Among 18,000 mutants screened, 53 mutants contained insertions in RosR-regulated genes (Table 2). Fifty-two mutants carried insertions in genes negatively regulated by RosR, and one mutant had an insertion in a gene positively regulated by RosR. Of the insertions in negatively regulated genes, GUS activity ranged from 6-fold repressed in MB059 to 198-fold repressed in MB040. The GUS activity of mutant MB006 was fourfold increased in the presence of RosR.
Sequences of regions flanking transposon insertions.
Analysis of the nucleotide sequences flanking the transposon insertions revealed that each mutant was the result of a unique insertion event. The insertions were in 43 different loci, and 7 loci were identified more than once. Most of the transposon insertions are oriented such that the gusA reporter gene is oriented in the same direction as the portion of the open reading frame (ORF) identified by sequence similarity. The exceptions are mutants MB009, MB041, MB050, and MB054. The RosR-dependent regulation in these mutants may be due to transcription from a RosR-regulated promoter downstream from and oriented convergently to the gusA gene. The transposon in MB039 is inserted in the opposite orientation but in the same site as that of MB041.
Identification of RosR-regulated genes that affect the cell surface.
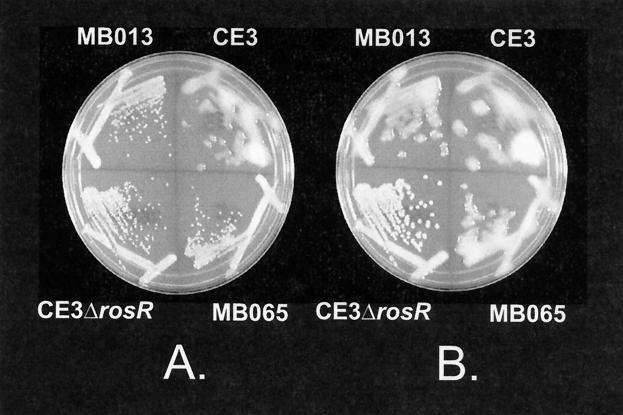
Mutants MB013 and MB065 produced colonies with altered morphology. Both mutants (without rosR in trans) produce colonies on YEM agar that initially appear to be similar to those of the rosR mutant, CE3ΔrosR, but after 4 days of growth the colonies appear to be similar to those of the wild-type strain, CE3 (Fig. 2). When rosR is present in trans in these mutants, they produce colonies that are indistinguishable from the wild type at all times during growth (data not shown). The region flanking the insertion in MB013 has no similarity to any known genes in the database, and the region flanking the insertion in MB065 is similar to the exoY gene from Rhizobium sp. (Table 2).
FIG. 2.
Colony morphologies of mutants MB013 and MB065 in comparison to CE3 and CE3ΔrosR. The same YEM plate is shown at 3 days (A) and 4 days (B) after inoculation.
Identification of RosR-regulated genes involved in nodulation or nodulation competitiveness.
Each mutant strain with either pLAFR3 or pH8B1 in trans was singly inoculated onto beans to determine if each strain could nodulate beans. All mutant strains except MB015 nodulated beans with or without rosR supplied in trans. Mutant MB015 did not nodulate beans unless rosR was provided in trans. When MB015(pLAFR3) (a rosR mutant) was singly inoculated onto beans, either no nodules developed or only a few small white nodules developed on the bean roots. When MB015(pH8B1) (rosR+) was singly inoculated onto beans, normal-appearing nodules developed (data not shown).
We tested the nodulation competitiveness of the mutants that have insertions in RosR-regulated genes to identify the subset of RosR-regulated genes involved in competitiveness. Each of the 52 mutant strains with pLAFR3 was coinoculated with either CE3003 (a rosR mutant competitor strain) or CE3013 (a marked rosR+ competitor with wild-type nodulation competitiveness) in a 1:1 ratio on beans to determine whether the insertion altered the nodulation competitiveness of the mutant. In addition, each mutant strain containing pH8B1 was coinoculated with either CE3003 or CE3013 in a 1:1 ratio to determine whether rosR in trans affected nodulation competitiveness in the mutant strains.
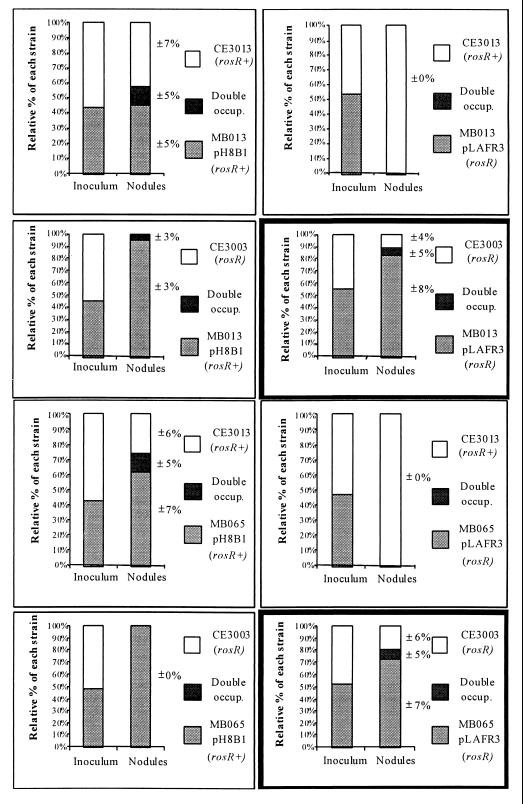
Mutants MB013 and MB065 (without rosR in trans) were more competitive than CE3003 (a rosR mutant competitor) (Fig. 3). For example, when MB013 (a rosR mutant) and CE3003 (a rosR mutant competitor) were coinoculated at approximately a 1:1 ratio, 83% of the nodules were occupied solely by MB013 (Fig. 3). Both MB013 and MB065 were unaffected in nodulation competitiveness when rosR was present in trans (Fig. 3). Although MB015(pLAFR3) (a rosR mutant) displayed a nodulation defect, MB015(pH8B1) (rosR+) was not affected in nodulation or nodulation competitiveness. When MB015(pH8B1) was coinoculated with CE3003 at approximately a 1:1 ratio, all of the nodules were occupied by MB015(pH8B1), and when MB015(pH8B1) was coinoculated with CE3013 at approximately a 1:1 ratio, 48% ± 8% of the nodules were occupied by MB015(pH8B1), 40% ± 6% of the nodules were occupied by CE3013, and 12% ± 5% of the nodules were occupied by both strains. All of the other mutant strains were unaffected in nodulation competitiveness (data not shown).
FIG. 3.
Nodulation competitiveness of mutants MB013 and MB065. Each graph represents a separate treatment. The relative percentage of each strain in the inoculum is indicated by the bar on the left, and the relative percentage of nodules later occupied by each strain (or of nodules occupied by both strains [double occup.]) is indicated by the bar on the right. The treatments that demonstrate the increase in competitiveness of MB013 and MB065 are highlighted by heavy outlining. Standard errors are indicated adjacent to each column division.
DISCUSSION
To understand the role of RosR in R. etli, we developed a genome-wide genetic screen to identify members of the RosR regulon. This screen was designed to identify genes that are negatively or positively regulated by RosR as well as genes directly or indirectly regulated by RosR. Based on the phenotypes of the rosR mutant, we expected to identify three classes of mutants: (i) mutants with an altered cell surface, (ii) mutants with altered nodulation competitiveness, and (iii) mutants with insertions in other RosR-regulated genes. All three classes were identified in the screen.
We identified 43 different RosR-regulated loci, and 7 of the loci were identified by more than one insertion. Two of the RosR-regulated genes affect both the cell surface and nodulation competitiveness of R. etli. Sixteen of the insertions are in regions with no significant sequence similarity to proteins in the sequence databases. Only one gene was positively regulated by RosR.
In the absence of rosR in trans, mutants MB013 and MB065 both produce colonies indistinguishable from the wild type after extended growth on solid media. The region flanking the insertion in MB013 shows no similarity to known genes, while the insertion in MB065 is in a region with similarity to exoY. ExoY is an essential part of the succinoglycan biosynthesis pathway, likely acting as a sugar transferase, and the RosR homolog, MucR, binds upstream of exoY in S. meliloti (9, 30). Assuming that the hydrophobic surface of the rosR mutant is due to derepression of one or more RosR-regulated genes, it is consistent that insertions in some RosR-regulated genes lead to a reversion to hydrophilic cell surfaces.
The two mutants with altered colony morphology were more competitive than the rosR mutant strain. Derepression of many RosR-regulated genes may lead to the great decrease in competitiveness observed in the rosR mutant; therefore, an insertion in any one of those genes would increase the competitiveness of a rosR mutant. The correlation between the subset of RosR-regulated genes that affect the cell surface properties and the subset that affect nodulation competitiveness suggests that the altered competitiveness of the rosR mutant is due to the dramatic changes in cell surface characteristics.
Mutant MB015 without rosR in trans nodulated poorly, yet when rosR was present in trans, the mutant nodulated normally and was unaffected in nodulation competitiveness. The insertion in MB015 is in a region with similarity to exsH from S. meliloti. ExsH is an endoglycanase that cleaves high-molecular-weight EPS into lower-molecular-weight forms (37). In S. meliloti, low-molecular-weight forms of EPS (either EPS I or EPS II) are important for establishing a successful symbiosis, probably acting as a signal molecule to the plant host (4, 20). Interestingly, S. meliloti mucR mutants do not produce low-molecular-weight EPS II, and when mucR mutants are blocked in EPS I production, they do not nodulate the plant host (20). If a low-molecular-weight polysaccharide signal is a common theme in all rhizobial interactions, it is possible that this gene is needed in R. etli to produce such a signal molecule in the rosR mutant background. Further studies need to confirm that it is indeed the gene with similarity to exsH that is responsible for this phenotype and that it is not due to polar effects from the insertion on other downstream genes.
We identified many other RosR-regulated genes that did not affect colony morphology or nodulation competitiveness, and sequence analysis of the regions flanking the insertions suggests hypotheses about the functions of some of these RosR-regulated genes. We identified genes that may be involved in polysaccharide and carbohydrate metabolism, genes that may be involved in survival in the rhizosphere, and genes similar to those that are regulated by Ros in A. tumefaciens (Table 2).
We identified genes that encode proteins with similarity to ExoB, ExoY, ExsH, PrsD, PssK, and PlyA, which are involved in EPS synthesis in other rhizobial species (12, 19, 30, 37). PrsD is a component of a secretion system involved in the export of the ExsH and PlyA proteins in Rhizobium meliloti and Rhizobium leguminosarum, respectively (19, 38). Mutant MB002 has an insertion in a region that encodes a protein with similarity to a sugar acetylase. Other RosR-regulated genes encoding proteins with similarity to glucose dehydrogenase, trehalose-phosphate synthase, and a transcriptional repressor of the sucrose degradation operon may also be involved in carbohydrate metabolism. Altered expression of these genes in rosR mutants is consistent with the activities of Ros in A. tumefaciens and MucR in R. meliloti, both of which affect EPS production.
Other mutants have insertions in genes that may affect bacterial fitness in the rhizosphere. Cellulose synthesis is important for A. tumefaciens attachment to its plant host (27), and mutants MB025 and MB037 contain insertions in a region with similarity to cellulose synthase genes, while the insertion in MB038 is in a region with similarity to celR2, a positive regulator of the genes involved in cellulose synthesis in R. leguminosarum bv. trifolii (3). MB042 has an insertion just upstream of a region with similarity to an ORF in the picA locus of A. tumefaciens, which is involved in altering the cell surface characteristics of the bacterium in response to compounds in plant cell extracts (31). Mutants MB018 and MB019 have insertions adjacent to regions with similarity to genes involved in the synthesis and transport of opines, carbon and nitrogen sources whose production is symbiotically regulated in S. meliloti (28).
Our identification of RosR-regulated genes suggests that the role of RosR in R. etli is similar to that of Ros in A. tumefaciens. Like Ros, RosR is involved in regulation of a region with similarity to vir genes and may affect cytokinin and heme synthesis (11, 13, 16). The predicted product of the ORF upstream from and oriented divergently to the gusA reporter gene in MB043 has a high level of conservation with the VirC1 sequence, containing 87% amino acid identity over 62 amino acids, while the ORF downstream from and in the same orientation as the gusA gene encodes a predicted protein that is 44% identical over 47 amino acids to VirD3. The insertion in MB006 is within an ORF likely to encode a coproporphyrinogen III oxidase, which is involved in the formation of heme (23). MB011 has the transposon insertion in a region with similarity to a gene that may be involved in cytokinin production and virulence in the plant pathogen Rhodococcus fascians, and rhizobial production of cytokinin is implicated in Rhizobium-induced leaf curl syndrome of pigeon pea (17, 34).
Our dissection of the RosR regulon has revealed that RosR affects expression of many functionally diverse genes in R. etli, and it seems likely that the dominant role of RosR is to influence gene expression by transcriptional repression. Surprisingly, the role of RosR shares features of that of Ros in A. tumefaciens, regulating homologs of vir genes as well as affecting polysaccharide production. The presence of vir homologs in a rhizobial species has not been previously reported, and preliminary sequence obtained from pMB043 indicates that homologs of other vir genes are also present on pMB043 (M. A. Bittinger, unpublished data).
As more bacterial genomes are sequenced, there is an increasing need to link an understanding of gene expression with sequence information. DNA sequence alone can be used to predict gene function, but delineation of pathways of coordinate regulation requires functional genetic approaches. Genetic approaches such as we employed in this study allow us to begin to dissect complex regulatory pathways that will complement the anticipated full genome sequence of a rhizobial species.
ACKNOWLEDGMENTS
We thank Patrick Masson, Michelle Rondon, and Susan West for critically reviewing the manuscript.
This work was funded by U.S. Department of Energy grant DE-FG02-96ER20248 and by the College of Agricultural and Life Sciences at the University of Wisconsin—Madison.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Araujo R S, Robleto E A, Handelsman J. A hydrophobic mutant of Rhizobium etli altered in nodulation competitiveness and growth in the rhizosphere. Appl Environ Microbiol. 1994;60:1430–1436. doi: 10.1128/aem.60.5.1430-1436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausmees N, Jonsson H, Hoglund S, Ljunggren H, Lindberg M. Structural and putative regulatory genes involved in cellulose synthesis in Rhizobium leguminosarum bv. trifolii. Microbiology. 1999;145:1253–1262. doi: 10.1099/13500872-145-5-1253. [DOI] [PubMed] [Google Scholar]
- 4.Battisti L, Lara J C, Leigh J A. Specific oligosaccharide form of the Rhizobium meliloti exopolysaccharide promotes nodule invasion in alfalfa. Proc Natl Acad Sci USA. 1992;89:5625–5629. doi: 10.1073/pnas.89.12.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beattie G A, Handelsman J. Evaluation of a strategy for identifying nodulation competitiveness genes in Rhizobium leguminosarum biovar phaseoli. J Gen Microbiol. 1993;139:529–538. doi: 10.1099/00221287-139-3-529. [DOI] [PubMed] [Google Scholar]
- 6.Beattie G A, Handelsman J. A rapid method for the isolation and identification of Rhizobium from root nodules. Microbiol Methods. 1989;9:29–34. [Google Scholar]
- 7.Bergersen F J. Growth of Rhizobium on synthetic medium. Aust J Biol Sci. 1961;14:349–360. [Google Scholar]
- 8.Beringer J E. R-factor transfer in Rhizobium leguminosarum biovar phaseoli. J Gen Microbiol. 1974;84:188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- 9.Bertram-Drogatz P A, Ruberg S, Becker A, Pühler A. The regulatory protein MucR binds to a short DNA region located upstream of the mucR coding region in Rhizobium meliloti. Mol Gen Genet. 1997;254:529–538. doi: 10.1007/s004380050448. [DOI] [PubMed] [Google Scholar]
- 10.Bittinger M A, Milner J L, Saville B J, Handelsman J. RosR, a determinant of nodulation competitiveness in Rhizobium etli. Mol Plant-Microbe Interact. 1997;10:180–186. doi: 10.1094/MPMI.1997.10.2.180. [DOI] [PubMed] [Google Scholar]
- 11.Brightwell G, Hussain H, Tiburtius A, Yeoman K H, Johnston A W B. Pleiotropic effects of regulatory ros mutants of Agrobacterium radiobacter and their interaction with Fe and glucose. Mol Plant-Microbe Interact. 1995;8:747–754. doi: 10.1094/mpmi-8-0747. [DOI] [PubMed] [Google Scholar]
- 12.Buendia A M, Enekel B, Koplin R, Niehaus K, Arnold W, Pühler A. The Rhizobium meliloti exoZ/exoB fragment of megaplasmid 2: ExoB functions as a UDP-glucose 4-epimerase and ExoZ shows homology to NodX of Rhizobium leguminosarum biovar viciae strain TOM. Mol Microbiol. 1991;5:1519–1530. doi: 10.1111/j.1365-2958.1991.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 13.Chou A Y, Archdeacon J, Kado C I. Agrobacterium transcriptional regulator Ros is a prokaryotic zinc finger protein that regulates the plant oncogene ipt. Proc Natl Acad Sci USA. 1998;95:5293–5298. doi: 10.1073/pnas.95.9.5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Close T J, Rogowsky P M, Kado C I, Winans S C, Yanofsky M F, Nester E W. Dual control of Agrobacterium tumefaciens Ti plasmid virulence genes. J Bacteriol. 1987;169:5113–5118. doi: 10.1128/jb.169.11.5113-5118.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Close T J, Tait R C, Kado C I. Regulation of Ti plasmid virulence genes by a chromosomal locus of Agrobacterium tumefaciens. J Bacteriol. 1985;164:774–781. doi: 10.1128/jb.164.2.774-781.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooley M B, D'Souza M R, Kado C I. The virC and virD operons of the Agrobacterium Ti plasmid are regulated by the ros chromosomal gene: analysis of the cloned ros gene. J Bacteriol. 1991;173:2608–2616. doi: 10.1128/jb.173.8.2608-2616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crespi M, Vereecke D, Temmerman W, Van Montagu M, Desomer J. The fas operon of Rhodococcus fascians encodes new genes required for efficient fasciation of host plants. J Bacteriol. 1994;176:2492–2501. doi: 10.1128/jb.176.9.2492-2501.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finnie C, Zorreguieta A, Hartley N M, Downie J A. Characterization of Rhizobium leguminosarum exopolysaccharide glycanases that are secreted via a type I exporter and have a novel heptapeptide repeat motif. J Bacteriol. 1998;180:1691–1699. doi: 10.1128/jb.180.7.1691-1699.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez J E, Reuhs B L, Walker G C. Low molecular weight EPS II of Rhizobium meliloti allows nodule invasion in Medicago sativa. Proc Natl Acad Sci USA. 1996;93:8636–8641. doi: 10.1073/pnas.93.16.8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanahan D. Transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 22.Jefferson R A, Wilson K J. The GUS gene fusion system. In: Gelvin S, Schilperoort R, Verma D P, editors. Plant molecular biology manual. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. pp. B14/1–B14/33. [Google Scholar]
- 23.Keithly J H, Nadler K D. Protoporphyrin formation in Rhizobium japonicum. J Bacteriol. 1983;154:838–845. doi: 10.1128/jb.154.2.838-845.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller M, Roxlau A, Weng W M, Schmidt M, Quandt J, Niehaus K, Jording D, Arnold W, Pühler A. Molecular analysis of the Rhizobium meliloti mucR gene regulating the biosynthesis of the exopolysaccharides succinoglycan and galactoglucan. Mol Plant-Microbe Interact. 1995;8:267–277. doi: 10.1094/mpmi-8-0267. [DOI] [PubMed] [Google Scholar]
- 25.Kovach M E, Elzer P H, Hill D S, Robertson G T, Farris M A, Roop R M I, Peterson K M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 26.Lee K H, Ruby E G. Competition between Vibrio fischeri strains during initiation and maintenance of a light organ symbiosis. J Bacteriol. 1994;176:1985–1991. doi: 10.1128/jb.176.7.1985-1991.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthysse A G. Role of bacterial cellulose fibrils in Agrobacterium tumefaciens infection. J Bacteriol. 1983;154:906–915. doi: 10.1128/jb.154.2.906-915.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy P J, Heycke N, Trenz S P, Ratet P, De Bruijn F J, Schell J. Synthesis of an opine-like compound, a rhizopine, in alfalfa nodules is symbiotically regulated. Proc Natl Acad Sci USA. 1988;85:9133–9137. doi: 10.1073/pnas.85.23.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noel K D, Sanchez A, Fernandez L, Leemans J, Cevallos M A. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J Bacteriol. 1984;158:148–155. doi: 10.1128/jb.158.1.148-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reuber T L, Walker G C. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell. 1993;74:269–280. doi: 10.1016/0092-8674(93)90418-p. [DOI] [PubMed] [Google Scholar]
- 31.Rong L J, Karcher S J, Gelvin S B. Genetic and molecular analyses of picA, a plant-inducible locus on the Agrobacterium tumefaciens chromosome. J Bacteriol. 1991;173:5110–5120. doi: 10.1128/jb.173.16.5110-5120.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simons M, van der Bij A J, Brand I, de Weger L A, Wijffelman C A, Lugtenberg B J J. Gnotobiotic system for studying rhizosphere colonization by plant growth-promoting Pseudomonas bacteria. Mol Plant-Microbe Interact. 1996;9:600–607. doi: 10.1094/mpmi-9-0600. [DOI] [PubMed] [Google Scholar]
- 33.Staskawicz B, Dahlbeck D, Keen N, Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987;169:5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Upadhyaya N M, Parker C W, Letham D S, Scott K F, Dart P J. Evidence for cytokinin involvement in Rhizobium (IC3342)-induced leaf curl syndrome of pigeon-pea (Cajanus cajan Millsp.) Plant Physiol. 1991;95:1019–1025. doi: 10.1104/pp.95.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wacek T J, Brill W J. Simple, rapid assay for screening nitrogen-fixing ability in soybean. Crop Sci. 1976;16:518–523. [Google Scholar]
- 36.Wilson K J, Sessitch A, Corbo J C, Giller K E, Akkermans A D L, Jefferson R A. β-Glucuronidase (GUS) transposons for ecological and genetic studies of rhizobia and other Gram-negative bacteria. Microbiology. 1995;141:1691–1705. doi: 10.1099/13500872-141-7-1691. [DOI] [PubMed] [Google Scholar]
- 37.York G M, Walker G C. The Rhizobium meliloti ExoK and ExsH glycanases specifically depolymerize nascent succinoglycan chains. Proc Natl Acad Sci USA. 1998;95:4912–4917. doi: 10.1073/pnas.95.9.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.York G M, Walker G C. The Rhizobium meliloti exoK gene and prsD/prsE/exsH genes are components of independent degradative pathways which contribute to production of low-molecular-weight succinoglycan. Mol Microbiol. 1997;25:117–134. doi: 10.1046/j.1365-2958.1997.4481804.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhan H, Levery S B, Lee C C, Leigh J A. A second exopolysaccharide of Rhizobium meliloti strain SU47 that can function in root nodule invasion. Proc Natl Acad Sci USA. 1989;86:3055–3059. doi: 10.1073/pnas.86.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]