Abstract
CTXφ is a lysogenic filamentous bacteriophage that encodes cholera toxin. Filamentous phages that infect Escherichia coli require both a pilus and the products of tolQRA in order to enter host cells. We have previously shown that toxin-coregulated pilus (TCP), a type IV pilus that is an essential Vibrio cholerae intestinal colonization factor, serves as a receptor for CTXφ. To test whether CTXφ also depends upon tol gene products to infect V. cholerae, we identified and inactivated the V. cholerae tolQRAB orthologues. The predicted amino acid sequences of V. cholerae TolQ, TolR, TolA, and TolB showed significant similarity to the corresponding E. coli sequences. V. cholerae strains with insertion mutations in tolQ, tolR, or tolA were reduced in their efficiency of CTXφ uptake by 4 orders of magnitude, whereas a strain with an insertion mutation in tolB showed no reduction in CTXφ entry. We could detect CTXφ infection of TCP− V. cholerae, albeit at very low frequencies. However, strains with mutations in both tcpA and either tolQ, tolR, or tolA were completely resistant to CTXφ infection. Thus, CTXφ, like the E. coli filamentous phages, uses both a pilus and TolQRA to enter its host. This suggests that the pathway for filamentous phage entry into cells is conserved between host bacterial species.
Vibrio cholerae is a gram-negative bacterium that causes cholera, a severe and sometimes lethal diarrheal disease. Humans become infected with V. cholerae after ingesting food or water that has been contaminated with the pathogen. V. cholerae is capable of colonizing and multiplying within the small intestine. This colonization requires production of a bundle-forming pilus, called toxin-coregulated pilus (TCP) (34). In addition to TCP, other virulence factors are expressed once the pathogen reaches the small intestine. One of these virulence factors is cholera toxin, a potent protein exotoxin that elicits a secretory response from intestinal epithelial cells. This response is the principle basis for the secretory diarrhea that is the hallmark of cholera (30).
Cholera toxin is an A-B-type toxin encoded by the ctxAB operon. This operon is part of the genome of CTXφ, a 7-kb lysogenic filamentous bacteriophage (37). Lysogenic conversion of nontoxigenic strains to toxigenicity by CTXφ infection appears to be a critical step in the evolution of fully pathogenic V. cholerae. The CTXφ genome is subdivided into two regions: a 4.6-kb core region that includes ctxAB and a 2.4-kb region designated RS2 (38). The organization of the core-encoded genes and the deduced amino acid sequences of their products (with the exception of ctxAB) resemble those of filamentous phages derived from a variety of bacterial species. These similarities, along with experimental evidence, suggests that the CTXφ core genes encode proteins required for virion morphogenesis. The CTXφ core gene products include Cep, which is thought to be the virion major coat protein, and Psh, OrfU, and Ace, which are thought to be minor coat proteins. The core-encoded Zot protein is similar to protein pI of coliphage M13 (15) and is required for virion assembly and secretion but is not part of the phage particle. Although lacking similarity to any Escherichia coli filamentous phage DNA sequences, our data indicate that the RS2 region of the CTXφ genome encodes the genes and noncoding sequences required for phage replication, integration, and transcriptional repression (14, 38).
The molecular steps involved in infection of E. coli by F pilus-specific filamentous phages (the Ff phages) such as f1 and M13 have been well characterized. The process begins when a domain of a minor coat protein (pIII) located on one end of the phage particle binds to the tip of the conjugative F pilus of E. coli (13). This interaction between pIII and F is thought to result in pilus retraction, which draws the phage through the bacterium's outer membrane. Subsequent phage translocation through the periplasmic space requires the tolQRA gene products (32, 33). Recent studies indicate that the periplasmic part of TolA binds to pIII and thereby serves as a coreceptor for phage entry into the bacterium (26). TolQ and TolR appear to interact with TolA via their inner membrane domains (8, 19), although their exact function in filamentous phage uptake remains unknown. Ff phage can infect E. coli lacking the F pilus, albeit at much lower frequencies than infection of F+ cells (29). Following translocation of the phage through the periplasm via the TolQRA complex, the phage major capsid protein, pVIII, inserts into the inner membrane (4) and the single-stranded phage genome enters the cytoplasm and begins a new cycle of phage replication and infection.
The physiological role of the tolQRAB gene products remains uncertain. The tolQRA gene products of E. coli are thought to contribute to maintaining the integrity of the outer bacterial membrane. Disruption of these tol genes enhances the sensitivity of the bacteria to certain antibiotics and detergents and leads to leakage of periplasmic proteins into the extracellular surroundings (17, 18, 39). Mutations also prevent transfer of certain colicins into the cell (16). Disruption of a fourth tol gene, tolB, located immediately 3′ of tolQRA, generates cells with comparable membrane deficiencies; however, mutation of this tol gene has no detectable effect upon Ff phage uptake (32).
For filamentous phages that infect hosts other than E. coli, little is known concerning the molecular aspects of phage entry. We previously found that V. cholerae cells harboring deletions or particular amino acid substitutions in tcpA, which encodes the major subunit of TCP, are resistant to CTXφ infection. This finding suggested that this type IV pilus serves as a receptor for CTXφ (37). The CTXφ ligand that binds TCP has been hypothesized to be the core-encoded protein OrfU, based both upon the size and relative position of this gene within the CTXφ genome (37). Although OrfU does not have significant sequence similarity to Ff pIII, Holliger and Riechmann have predicted that the N-terminal portion of OrfU has structural similarity to the domain of pIII that interacts with E. coli TolA (11). In the current study, we investigated whether orthologues of the E. coli tolQRAB genes are encoded in the V. cholerae genome and whether the products of these genes are required for CTXφ infection. We found that the V. cholerae genome contains four contiguous open reading frames (ORFs) predicted to encode proteins similar to E. coli TolQRAB and that disruption of the V. cholerae tolQRA genes severely reduces the efficiency of V. cholerae CTXφ uptake. Further supporting the importance of V. cholerae tolQRA in CTXφ uptake, we found that TCP− strains of V. cholerae can be infected by CTXφ, albeit at greatly reduced frequencies, and that TolQRA are absolutely required for phage entry into TCP− cells.
MATERIALS AND METHODS
Strains, media, and antibiotics.
The bacterial strains used in this study are listed in Table 1. Bacteria were grown in Luria-Bertani (LB) broth (2) at 37°C. To induce TCP expression and the concomitant autoagglutination of classical V. cholerae strain O395, bacteria were cultured on a roller drum shaker at 30°C overnight as previously described (34). Antibiotics were used at the following concentrations: ampicillin, 50 μg/ml (V. cholerae) and 100 μg/ml (E. coli); streptomycin, 200 μg/ml; kanamycin, 50 μg/ml; chloramphenicol, 15 μg/ml (E. coli) and 1 μg/ml (V. cholerae); spectinomycin, 50 μg/ml; and rifampicin, 40 μg/ml. Arabinose (Ara) (0.02%) was added to LB broth to induce expression of genes under the control of the E. coli promoter, pBAD (9).
TABLE 1.
List of strains and plasmids used in this study
| Strain or plasmid | Description or genotype | Source or reference |
|---|---|---|
| Strains | ||
| V. cholerae | ||
| O395 | O1 classical strain; Smr | 23 |
| DH1 | O395 tolQ::pDH235 | This study |
| DH2 | O395 tolR::pDH107 | This study |
| DH3 | O395 tolA::pDH149 | This study |
| DH4 | O395 tolB::pDH270 | This study |
| TCP2 | O395 ΔtcpA | 10 |
| DH5 | TCP2 tolQ::pDH235 | This study |
| DH6 | TCP2 tolR::pDH107 | This study |
| DH7 | TCP2 tolA::pDH149 | This study |
| 468-83 | TCP TLC attRS CTXφ− | 28 |
| RV508 | Rifr Specr; derivative of classical strain 569B | 37 |
| E. coli | ||
| Sm10λpir | thi thr leu tonA lacY supE recA::RP-2Tc::Mu Knr::λpir | 23 |
| TPS66 | Missense mutation in tolQ/F+ | 32 |
| Plasmids | ||
| pGP704 | Suicide vector; oriR6K mobRP4 Apr | 23 |
| pDH235 | Internal fragment of V. cholerae tolQ (bp 72–381) inserted into pGP704 | This study |
| pDH107 | Internal fragment of V. cholerae tolR (bp 22–292) inserted into pGP704 | This study |
| pDH149 | Internal fragment of V. cholerae tolA (bp 79–530) inserted into pGP704 | This study |
| pDH270 | Internal fragment of V. cholerae tolB (bp 36–270) inserted into pGP704 | This study |
| pBAD33 | Ara-inducible promoter vector; Cmr | 9 |
| pDH8 | V. cholerae tolQ cloned into pBAD33 | This study |
| pDH9 | V. cholerae tolQR cloned into pBAD33 | This study |
| pDH10 | V. cholerae tolR cloned into pBAD33 | This study |
| pDH11 | V. cholerae tolA cloned into pBAD33 | This study |
| pCTX-Kn | Replicative form of CTX-Knφ | 37 |
| pMW1 | pCTX-Kn ΔorfU | 37 |
| pMW2 | pCTX-Kn Δzot | 37 |
Construction of O395 tolQRAB mutant strains.
Homologous recombination of suicide vectors containing internal fragments of tolQ, tolR, tolA, and tolB into their respective chromosomal genes was used to inactivate each of these genes in the V. cholerae O395 background. These gene fragments were amplified from O395 genomic DNA by PCR. The sequences of the primers used to amplify these internal tol gene fragments relative to the predicted start codon of each of these genes are shown in Table 2. These PCR products were subsequently cloned into the TA cloning vector pCRII-TOPO vector (Invitrogen, Carlsbad, Calif.) according to the manufacturer's protocol. An EcoRI fragment of each of the resulting plasmids which contained the cloned PCR product was then ligated to EcoRI-digested pGP704, a suicide vector encoding Apr which requires the product of the pir gene for replication (23). The resulting plasmids, pDH235, pDH107, pDH149, and pDH270 (Table 1), were subsequently introduced into E. coli Sm10λpir and then mobilized into V. cholerae O395 and TCP2. Transconjugants (Smr and Apr colonies) were selected, and disruption of each tol gene in all of the resulting strains was confirmed by Southern analyses (data not shown).
TABLE 2.
Sequences of the PCR primers used to generate the internal fragments for insertional mutations of V. cholerae tolQRAB
| Primer name | Sequence | PCR product |
|---|---|---|
| tolQKO-1 | 5′ CCTTTTGGGAATGTCGGTTGC 3′ | Internal fragment of tolQ; bp 72–381 |
| tolQKO-2 | 5′ GCTGGTTTCGAGTGAATCAACTTC 3′ | |
| tolRKO-1 | 5′ AAACGTGAGTGAAAGCAGA 3′ | Internal fragment of tolR; bp 22–292 |
| tolRKO-2 | 5′ GAACAATCACATCTTCGATG 3′ | |
| tolAKO-1 | 5′ GCGATATTGCTCTGGGGAG 3′ | Internal fragment of tolA; bp 79–530 |
| tolAKO-2 | 5′ CGTTGCTGTTCTGCCTTTG 3′ | |
| tolBKO-1 | 5′ TGCGGCATTGGAGCTAGTTATTAC 3′ | Internal fragment of tolB; bp 36–270 |
| tolBKO-2 | 5′ TGAATCGA CCCCCATAGATGTC 3′ |
Construction of tolQRA-complementing plasmids.
The full-length tolQ gene was amplified by PCR with the forward primer tolQ-1 (5′ CCGAGAGCTTTGCCTCAGTTAATC 3′) located 43 bp upstream of the predicted start codon of tolQ and the reverse primer tolQ-2 (5′ TTTGGTTTGATAGCCAGCC 3′) ending 26 bp downstream of the predicted stop codon of tolQ. The PCR product was then cloned into the pCRII-TOPO vector. Following subcloning into pBluescript SK(−) (Stratagene, La Jolla, Calif.), a SacI/KpnI fragment containing the insert was ligated into SacI/KpnI-digested pBAD33 (9), resulting in pDH8.
The tolQR genes were amplified by PCR with the forward primer tolQ-1 Kpn (the tolQ-1 forward primer sequence with a KpnI restriction site at the 5′ end) and the reverse primer tolR-2Xba (5′ TCTAGAATTTAAGGTCCGTGAGTAGCCCTAC 3′) which ends 2 bp downstream from the predicted stop codon of tolR and includes an XbaI site added to the 5′ end. The PCR product was first cloned into the pCRII-TOPO vector, and then a KpnI/XbaI fragment containing the insert was ligated into KpnI/XbaI-digested pBAD33 to yield pDH9.
The tolR gene was amplified by PCR using the forward primer tolR-1 (5′ AGTTTCATACCATTCTCCACCGTC 3′) located 69 bp upstream of the predicted start codon of tolR and the reverse primer tolR-2, which has the same sequence and location as tolR-2Xba but lacks the XbaI site. The PCR product was subsequently cloned into the pCRII-TOPO vector, and then a HindIII/XbaI fragment containing the insert was ligated into HindIII/XbaI-digested pBad33, resulting in pDH10.
The tolA gene was amplified using the forward primer tolA-1 (5′ TCCTAAAGTAGGGCTACTCACGGAC3′) located 51 bp upstream of the predicted start codon of tolA and the reverse primer tolA-2 (5′ ACTAGCTCCAATGCCGCATTC 3′) ending 97 bp downstream of the tolA predicted stop codon. Following cloning of the PCR product into the pCRII-TOPO vector and then subcloning into pBluescript SK(−), a PstI/SalI fragment containing the insert was ligated into PstI/SalI-digested pBAD33, resulting in pDH11.
Assays of efficiency of CTXφ infection.
To compare the efficiency of CTXφ infection of different mutant strains, both a previously described supernatant-based transduction assay (37) and a new coculture transduction assay were used. In the supernatant-based transduction assay, filtered supernatants from a strain harboring the kanamycin-marked CTXφ replicative form, pCTX-Kn, were mixed with different recipients. Seventy-five microliters of recipient cells, which were autoagglutinated after overnight growth at 30°C, was vortexed and mixed with 75 μl of the cell supernatants containing CTX-Knφ particles. The phage and recipient cells were gently mixed for 20 min at room temperature on a shaker. Then, each mixture was plated on LB agar containing streptomycin (for O395) or streptomycin and ampicillin (for the tol mutants) to enumerate the potential recipients and on LB agar containing Kn (for O395) or kanamycin and ampicillin (for the tol mutants) to enumerate the transductants. The frequency of infection was determined by dividing the number of transductants (Knr or Knr Apr CFU) by the number of recipients (Smr or Smr Apr CFU).
In the coculture transduction assay, RV508, a Specr Rifr derivative of 569B (37) harboring pCTX-Kn, was streaked on LB agar plates along with Smr potential recipient strains. After incubating at 30°C for 4.5 h, the cells were recovered from the plates in 3 ml of LB broth. The number of potential recipient cells was determined by counting the number of Smr CFU (the donor strain RV508 is Sms), and the number of transductants was determined by enumerating the Smr Knr CFU (for O395) or Smr Apr Knr CFU (for the tol mutants). Again, the frequency of infection was determined by dividing the number of transductants by the number of potential recipients.
Characterization of other phenotypes of the tolQRAB mutants.
Immunoblot analysis of whole-cell lysates with polyclonal α-TcpA antiserum was carried out as previously described (23). For determination of the growth kinetics of the mutant strains, equivalent dilutions (based on optical density [OD] readings at 600 nm) of overnight LB broth cultures containing the appropriate antibiotics were used as the inocula for cultures. Aliquots were removed from these cultures at 30-min intervals for OD600 determination. At hourly intervals, these aliquots were also plated on LB agar with the appropriate antibiotics to enumerate the number of CFU.
The sensitivity of the tol mutants to deoxycholate (Sigma, St. Louis, Mo.) was assayed by growing the bacteria in LB broth plus the appropriate antibiotics, containing a range of deoxycholate concentrations from 0.025 to 12.4%. After approximately 14-h growth at 37°C, the turbidity of cultures was assayed visually, and cultures without apparent turbidity were scored as sensitive to deoxycholate. The starting inocula for these determinations were mid-log-phase cultures (OD600 of 0.5) of each strain tested.
RNase I leakage from the periplasm was assessed by plating bacteria on LB plates containing 1.0% (wt/vol) type VI RNA from Torula yeast (Sigma) as described by Lazzaroni and Portalier (18). After overnight growth, 0.5 N HCl was added to each plate to precipitate the RNA. Leakage of RNase I was detected by the appearance of a halo surrounding individual colonies after the addition of HCl. Leakage of β-lactamase was determined as follows. Supernatants of overnight cultures were assayed for β-lactamase activity by measuring the color change of nitrocefin (50 μg) (Calbiochem, San Diego, Calif.) per ml, a chromogenic substrate of β-lactamase which turns from yellow (390 nm) to red (486 nm) in the presence of β-lactamase; cleavage of substrate was monitored by a change in absorbance at 486 nm. β-Lactamase activity was measured in both the supernatant and periplasmic extracts of these cells. Periplasmic extracts were prepared by treating cells with NaCl, sucrose, and lysozyme to disrupt the outer membrane (7). The percentage of β-lactamase activity in the supernatant compared to cell associated β-lactamase activity was then calculated.
Molecular biology methods.
Standard molecular biology methods were used in this study (2). Restriction enzymes and ligase were purchased from New England Biolabs (Beverly, Mass.) Southern hybridization was carried out with the ECL direct nucleic acid labelling and detection system (Amersham Pharmacia, Buckinghamshire, England) according to the manufacturer's instructions. The DNA probes for these blots were the internal fragments of the tol genes that were used for targeted disruption of these genes as described above. DNA sequencing was performed by dye terminator cycle sequencing with an Applied Biosystems 373A DNA sequencer at the Tufts Core Facility. The MacVector software package (Oxford Molecular Group) was used to assemble the tolQRAB sequence, and the BLAST programs (1) were used for comparing this sequence to the GenBank database. The hydrophobicity of TolQRAB was calculated with the Kyte-Doolittle algorithm in MacVector. The protein localization program P-sort (25) was used to assess protein localization.
RESULTS
The V. cholerae tolQRAB DNA sequence.
Extrapolating from the model of filamentous phage entry into E. coli, we asked whether the tolQRAB gene cluster could be identified in the V. cholerae genome and if these gene products, TolQRA in particular, were necessary for entry of CTXφ into V. cholerae. To address the first question, the amino acid sequence of each of the E. coli TolQRAB proteins was used to query the partial V. cholerae genome being sequenced by The Institute for Genomic Research (TIGR) for potential V. cholerae tol orthologues. Of the four E. coli sequences, E. coli TolR yielded the most significant similarity (E value, 2e-08) using the BLAST X algorithm (1). No clones overlapped with the relevant contig GVCCN44F at that time; consequently, we used inverse PCR to clone the adjacent sequences from classical V. cholerae strain O395, which we speculated might encode V. cholerae tolQ and tolAB. After several iterations of inverse PCR and DNA sequencing, a sequence spanning 4 kb and containing four ORFs which were similar to E. coli TolQRAB was generated. Our sequence of the O395 tolQRAB cluster has been deposited in GenBank with accession number AF187269. This sequence is virtually identical to the El Tor strain N16961 tolQRAB sequence determined by TIGR (http://www.tigr.org).
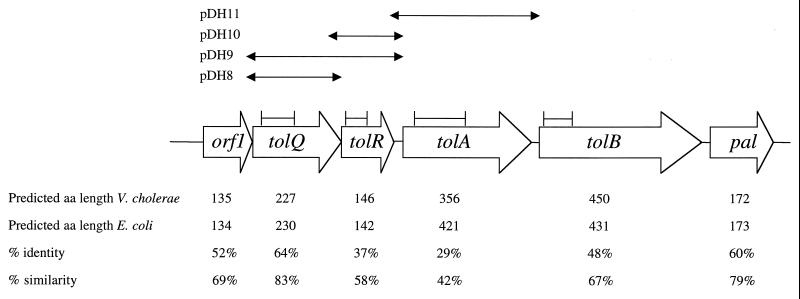
Using the complete V. cholerae genome sequence recently released by TIGR, we found that the genes neighboring the tolQRAB gene cluster in V. cholerae are similar to those in E. coli. In both species, the cluster includes the orf1, tolQ, tolR, tolA, tolB, and pal genes (36), and the predicted V. cholerae TolQRAB sequences bear significant similarity to the E. coli sequences (Fig. 1). The proteins encoded by the V. cholerae tol genes also appear to resemble the E. coli proteins in secondary structure. For example, the Kyte-Doolittle hydrophobicity profiles of V. cholerae and E. coli TolQRAB predict that V. cholerae TolQ, TolR, and TolA, like the E. coli proteins, contain 3, 1, and 1 transmembrane domains, respectively (20, 21, 24, 35). The extended α-helical region with repeats of Lys, Ala, and Glu/Asp found in E. coli TolA (20) also appears to be present in V. cholerae TolA, based on an analysis of secondary structure performed with MacVector. Finally, the protein localization program P-sort (25) predicts that V. cholerae TolB, like E. coli TolB (12), is predominantly periplasmic. These structural similarities suggest that V. cholerae TolQRAB may perform functions similar to those of E. coli TolQRAB.
FIG. 1.
The organization of the tol gene clusters in V. cholerae and E. coli is identical. The predicted lengths of the V. cholerae Tol proteins were derived from an ORF map of the V. cholerae DNA sequence with MacVector. Percent identity and similarity were determined by comparing the predicted amino acid sequences of V. cholerae and E. coli Tol proteins with MacVector. The solid lines flanked by vertical bars represent the positions of the fragments of each tol gene that were used to construct the insertion mutations. The solid lines flanked by arrows represent the sequences cloned into pBAD33 used for the complementation studies.
Role of tolQRAB in CTXφ infection.
To test the role of TolQRAB in CTXφ infection, insertion mutations in each of these genes were generated within the classical biotype V. cholerae strain O395. We found that the strains harboring the tolQ, tolR, and tolB mutations grew heterogeneously on agar plates, forming either large or small colonies. The large colonies reproducibly restreaked only as large colonies, whereas the smaller colonies upon restreaking gave rise to a heterogeneous population of large and small colonies. DH3, which contains the tolA mutation, only grew as large colonies. These large colonies were confirmed by Southern analysis to have the correct integration. We suspect that a spontaneous, secondary mutation that enhances growth has arisen in the large colonies of all four of the strains harboring tol gene mutations. Because of the stability of the large colonies, we chose to use these homogenous populations for the studies of CTXφ infection described below.
The ability of CTXφ to infect each of these four mutant O395 derivatives was tested. Since CTXφ infection of V. cholerae does not result in plaque formation, a transduction assay was initially used. In this assay, cell-free culture supernatants containing a kanamycin-marked CTXφ (CTX-Knφ) were used to transduce recipient strains to kanamycin resistance (Knr). We found that the O395 derivatives containing tolQ, tolR, and tolA mutations were dramatically less susceptible to CTXφ infection than O395 (Table 3). More specifically, the tolA mutant strain could not be infected with this assay, and the tolQ and tolR mutants were approximately 4 orders of magnitude less efficient at CTXφ uptake than wild-type O395. In contrast, the tolB mutation did not confer resistance to CTXφ infection. All four mutant strains showed fewer CFU after overnight culture than the wild-type strain (Table 3, column 3); however, this growth difference is unlikely to account for the resistance of the tolQRA mutant strains to CTXφ infection, since the growth defect of the tolB mutant did not inhibit phage infection. Overall, these results suggest that V. cholerae TolQRA proteins are important for uptake of CTXφ, whereas TolB does not play an essential role in this process.
TABLE 3.
Efficiency of CTXφ infection in V. cholerae tolQRAB mutant strainsa
| Recipientb | Relevant genotype | No. of recipients/ml | No. of transductants/mlc | Frequency of infectiond |
|---|---|---|---|---|
| O395 | Wild type | 3.7 × 109 | 9.0 × 106 | 2.4 × 10−3 |
| DH1 | tolQ | 3.0 × 108 | 1.3 × 102 | 4.3 × 10−7 |
| DH2 | tolR | 8.2 × 108 | 1.1 × 102 | 1.3 × 10−7 |
| DH3 | tolA | 5.9 × 108 | 0 | 0 |
| DH4 | tolB | 1.1 × 108 | 9.1 × 105 | 8.2 × 10−3 |
The transduction assays were conducted at least four times, and the mean number of CFU from these experiments is presented. All standard deviations of these values were less than 10% of the means.
All recipients are derivatives of O395 (see Table 1).
Transductants were counted as the number of Knr CFU per milliliter.
The frequency of infection was calculated by dividing the number of transductants per milliliter by the number of recipients per milliliter.
Complementation studies were performed to verify that the resistance of the tolQ, tolR, and tolA mutant strains to CTXφ infection was due to the disruption of the targeted tol gene and not to polar effects or to spontaneous mutation in another gene. Plasmids containing each of the tol genes under the control of an Ara-inducible promoter, pBAD (9), were introduced into the corresponding mutant strain. Either in the presence or absence of inducer, DH1(pDH8), the tolQ mutant strain harboring the plasmid expressing tolQ, remained resistant to CTXφ infection (Table 4). This suggested the possibility of a secondary mutation, or more likely, that the tolQ mutation in DH1 disrupted the expression of the downstream gene, tolR. To address this latter possibility, a plasmid containing both tolQ and tolR, pDH9, was introduced into the tolQ mutant strain. This plasmid rendered this strain nearly as susceptible to CTXφ infection as the wild-type strain (Table 4). We conclude that the tolQ mutation in DH1 eliminates expression of TolR and that TolR is required for CTXφ infection. To discern whether tolQ is also required in this process, a plasmid encoding a functional TolR, pDH10 (see below), was introduced into the tolQ mutant. Even in the presence of Ara, no complementation was seen (Table 4). Thus, tolQ, like tolR, is necessary for the efficient uptake of CTXφ. Although supplying a functional TolR in trans did not complement the tolQ mutation, it was sufficient to complement the mutation in tolR in DH2 (Table 4). Similarly, expression of TolA rendered the tolA mutant strain, DH3, susceptible to CTXφ (Table 4). The results of these complementation studies confirm that TolQ, TolR, and TolA are required for infection of V. cholerae by CTXφ and that the spontaneous, secondary mutations which allowed these tol mutant strains to grow as large colonies on LB agar plates were not responsible for these strains' resistance to CTXφ infection.
TABLE 4.
tol gene complementation of CTXφ infection defects in V. choleraea
| Recipient strainb | Relevant genotype | Plasmid encoding gene(s) | Arabinosec | No. of recipients/ml | No. of transductants/mld | Frequency of infectione |
|---|---|---|---|---|---|---|
| O395 | Wild type | − | 4.9 × 109 | 5.1 × 106 | 1.0 × 10−3 | |
| DH1(pDH8) | tolQ | tolQ | − | 6.5 × 108 | 1.0 × 102 | 1.5 × 10−7 |
| + | 1.0 × 108 | 1.3 × 102 | 1.3 × 10−6 | |||
| DH1(pDH9) | tolQ | tolQR | − | 3.6 × 108 | 4.4 × 104 | 1.2 × 10−4 |
| + | 4.8 × 109 | 1.6 × 106 | 3.3 × 10−4 | |||
| DH1(pDH10) | tolQ | tolR | − | 5.9 × 108 | 1.4 × 102 | 2.4 × 10−7 |
| + | 6.4 × 108 | 1.1 × 102 | 1.7 × 10−7 | |||
| DH2(pDH10) | tolR | tolR | − | 8.9 × 108 | 1.1 × 103 | 1.2 × 10−6 |
| + | 5.1 × 109 | 7.5 × 106 | 1.5 × 10−3 | |||
| DH3(pDH11) | tolA | tolA | − | 1.7 × 109 | 2.6 × 106 | 1.5 × 10−3 |
| + | 5.2 × 108 | 4.2 × 105 | 8.0 × 10−4 |
The transduction assays were conducted at least three times, and the mean number of CFU from these experiments is presented. All the standard deviations of these values were less than 10% of the means.
All recipients are derivatives of strain O395.
+, addition of 0.02% Ara; −, no Ara.
Transductants were counted as the number of Knr CFU per milliliter.
Frequency of infection is calculated by dividing the number of transductants (Knr recipients per milliliter) by the number of recipients.
Requirement for tolQRA in CTXφ infection of TCP− V. cholerae.
E. coli lacking the F pilus can be infected by Ff phage, although at greatly reduced frequencies than F+ E. coli. F-independent infection of E. coli by Ff phage is dependent on the E. coli tolQRA gene products (29). With our standard liquid suspension CTXφ transduction assay, which relies upon cell-free filtered supernatants containing a marked CTXφ to transduce recipient cells, we have not been able to detect transductants of TCP− V. cholerae recipients (37). However, we found that we could circumvent the requirement for TCP in CTXφ infection if a CTXφ producing donor strain was grown in close proximity to a potential recipient strain. When a strain harboring the CTX-Knφ replicative form was cross-streaked with a TCP− recipient strain, O395 derivative TCP2 (10), we found that CTX-Knφ transfer to the recipient was detectable, albeit approximately 100,000-fold less frequently than CTX-Knφ transfer to O395 in this assay (Table 5). Transfer of the CTXφ genome under these conditions still required formation of functional virions, since mutant forms of CTX-Knφ that are maintained as plasmids but do not give rise to virions (pMW1 and pMW2 [37]) were not transferred. This result indicates that CTXφ transfer was mediated by CTXφ virions rather than by an alternative route, such as conjugation.
TABLE 5.
Influence of tcpA and tol mutations on CTXφ transfer in a coculture transduction assaya
| Recipientb | Mutant gene(s) | No. of recipients/ml | No. of Kanr recipients/ml | Frequency of infectionc |
|---|---|---|---|---|
| O395 | Wild type | 2.2 × 109 | 6.3 × 107 | 2.9 × 10−2 |
| TCP2 | tcpA | 2.1 × 109 | 1.6 × 103 | 7.6 × 10−7 |
| DH1 | tolQ | 1.1 × 109 | 2.9 × 103 | 2.6 × 10−6 |
| DH5 | tcpA and tolQ | 4.9 × 108 | 0 | 0 |
| DH2 | tolR | 7.5 × 108 | 2.1 × 103 | 2.8 × 10−6 |
| DH6 | tcpA and tolR | 1.3 × 108 | 0 | 0 |
| DH3 | tolA | 7.1 × 108 | 4.4 × 102 | 6.2 × 10−7 |
| DH7 | tcpA and tolA | 1.2 × 108 | 0 | 0 |
| DH4 | tolB | 2.2 × 108 | 2.2 × 107 | 1.0 × 10−1 |
Donor strain RV508 (pCTX-Kn) was cross-streaked with the indicated recipient strain on LB agar plates. Transfer of CTX-Knφ to the recipient was then measured after 4.5 h. These assays were conducted at least three times, and the mean number of CFU from these experiments is presented. The standard deviation of these values was less than 10% of the means.
All recipient strains are derivatives of O395.
Frequency of infection was calculated by dividing the number of Knr recipients by the total number of recipients. The ratio of donors (no. of Specr Rifr CFU) to recipients was constant for each assay.
Using this protocol, we found that TCP+ strains containing a mutation in any one of the tolQRA genes could be infected by CTXφ at very low frequencies, similar to those observed for infection of the TCP− strain (Table 5). This suggests that, individually, none of the V. cholerae tolQ, tolR, or tolA gene products is absolutely required for CTXφ infection. However, mutations in any of these three genes in combination with the tcpA mutation rendered the recipient strains completely resistant to CTXφ infection in this assay (Table 5). In contrast, as in the liquid-based transduction assay, a tolB mutation did not significantly affect the ability of the recipient strain to be infected by CTXφ. None of the tol mutations impaired transfer of an RP4-derived conjugal plasmid (5) from E. coli (data not shown). The finding that disruption of the V. cholerae tol genes did not reduce the ability of cells to act as recipients in conjugation is an additional indication that transfer of CTXφ genes under these assay conditions is dependent upon infection of recipients by virions, rather than an alternative mechanism of gene transfer. In conclusion, these experiments demonstrate that, similar to Ff phage infection of E. coli, TCP− cells can be infected by CTXφ and that the tolQRA gene products are absolutely required for infection of TCP− cells. However, unlike E. coli, a single mutation in V. cholerae tolQ, tolR, or tolA does not render these cells completely resistant to CTXφ infection.
Other properties of V. cholerae strains with tolQ, tolR, tolA, and tolB mutations.
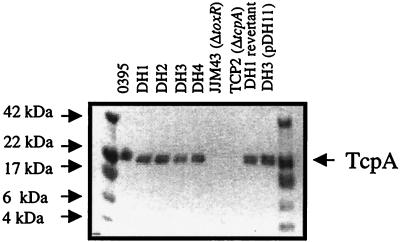
A potential explanation for the inefficiency of CTXφ entry in V. cholerae strains with tolQ, tolR, and tolA mutations is that these strains do not express TCP. This possibility is unlikely because DH1, DH2, and DH3, and DH4 cells autoagglutinated after overnight growth at 30°C, a property dependent on TCP production. To further confirm that these V. cholerae tol mutant strains synthesize TCP, immunoblot assays were carried out. As shown in Fig. 2, all four of the tol mutant strains expressed approximately wild-type amounts of a 20.5-kDa polypeptide that stained with α-TcpA antiserum. This is comparable to E. coli, where tol mutations have been shown not to interfere with production of F pili (32).
FIG. 2.
V. cholerae tolQRAB mutants (DH1, DH2, DH3, and DH4) produce amounts of TcpA similar to those produced by O395. All strains are derivatives of O395. The DH1 revertant was made by growing a small colony of DH1 in LB broth overnight at 37°C in the absence of ampicillin. The excision of pDH235 from tolQ in this revertant strain was confirmed by Southern analysis. All strains were grown in LB broth at 30°C. Whole-cell lysates were prepared in sample buffer as previously described (23) and run on an 4 to 12% Tris-Bis gradient gel (Novex, San Diego, Calif.). The proteins were then transferred to nitrocellulose and probed with anti-TcpA polyclonal antiserum.
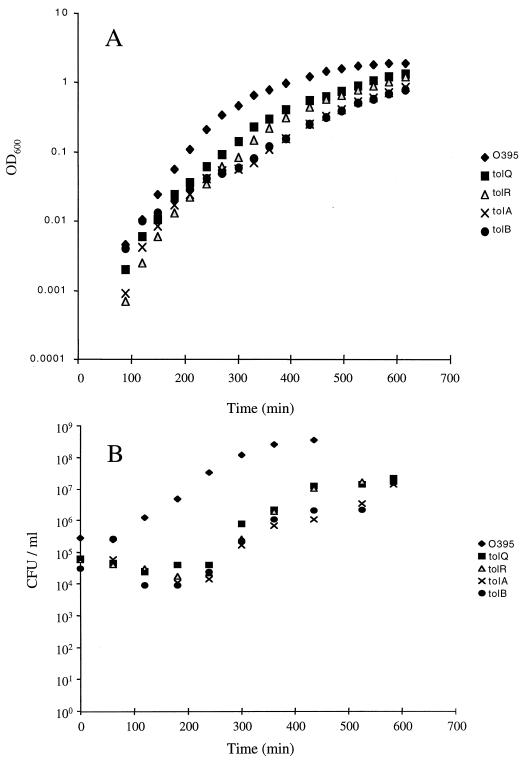
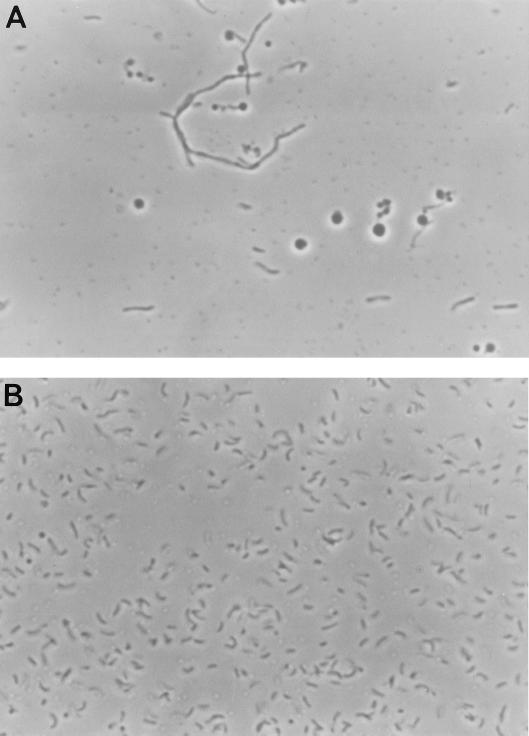
In our studies of the requirement of the tolQRAB gene products in CTXφ infection, we noted that there were reproducibly fewer viable cells in overnight cultures of tol mutant strains than in O395 (Table 3). Further studies were carried out to characterize the growth properties of the V. cholerae tol mutant strains. The change of the OD600 over time of the O395 derivatives with either tolQ, tolR, tolA, or tolB mutations during growth in LB broth did not dramatically differ from O395 (Fig. 3A). However, when these cultures were plated on LB agar to determine the number of CFU, there were far fewer colonies in the tol mutant strains (Fig. 3B). This discrepancy between the OD600 and the recovery of colonies on LB agar plates was most noticeable during the initial lag phase of growth. This reduced plating efficiency may be explained at least in part by the fact that all four V. cholerae tol mutants formed extensive filaments during growth (Fig. 4). This phenotype was most notable during the lag phase of growth. None of the tol-complementing plasmids, with the exception of the tolR-encoding plasmid pDH10, completely eliminated filamentation of the tol mutant strains. Since direct interactions between TolQRA have been demonstrated in E. coli (8, 19), our failure to fully complement the growth defect in the tol mutants may reflect our inability to restore the correct stoichiometry of the TolQRA proteins in these strains.
FIG. 3.
Kinetics of growth of O395 and O395-derived tol mutant strains. All strains were grown in LB broth with the appropriate antibiotics at 37°C and their OD600 (A) and CFU (B) were determined over time.
FIG. 4.
Filamentous morphology of V. cholerae tol mutant strain DH4 (A) compared with O395 (B). O395 derivatives with insertions in tolQ, tolR, and tolA also exhibited filamentation.
In addition to their inability to facilitate entry of filamentous phages, E. coli tol mutants exhibit reduced stability of their outer membranes, resulting in leakage of periplasmic proteins into the extracellular environment (39). In E. coli, this leakiness has been assessed by assays for the extracellular presence of periplasmic proteins, such as β-lactamase and RNase I, and by increased sensitivity to detergents like deoxycholate. The parental strain O395 and its tolQRAB derivates (DH1, DH2, DH3, and DH4) were tested for similar phenotypes. Two E. coli control strains, MG1655 (wild-type E. coli K-12) and TPS66 (32) (an E. coli strain harboring a missense mutation in tolQ), were also analyzed. As reported for E. coli, we saw significant leakage of β-lactamase (about 30% of the total) into the culture supernatants from V. cholerae tol mutants. However, unlike wild-type strains of E. coli containing pBR322 (a plasmid encoding the β-lactamase gene), which have very low levels of β-lactamase (about 1.5%) in supernatants (40), V. cholerae O395, harboring the bla-containing plasmid pCRII, had significant amounts of β-lactamase (about 15 to 20% of the total) present in culture supernatants. Thus, the effect of the tol mutations in leakage of β-lactamase in V. cholerae was difficult to ascertain. To ensure that the β-lactamase detected in the supernatants of O395 and DH1-4 was not due to cell lysis, we also assayed the supernatants for the presence of the cytoplasmic enzyme, β-galactosidase (β-Gal). We found that β-Gal activity in the supernatants of these strains was less than 5% of total β-Gal activity. A similar low percentage of β-Gal activity in supernatants of MG1655 and TPS66 was also measured.
A similar difficulty was encountered when the leakage of RNase I was assayed. Both TPS66 and the four V. cholerae tol mutants (DH1 to DH4) exhibited a halo around colonies indicative of leakage of RNase I. While the wild-type E. coli strain showed no such halo, O395 did, although the size of its halo was slightly smaller than those around the colonies of DH1 to DH4. Thus, the O395 derivatives with tol mutations leaked slightly more RNase I than O395; however, due to the significant basal level of leakiness found in O395, this difference was not as dramatic as that seen between TPS66 and MG1655.
The sensitivity of DH1, DH2, DH3, DH4, and O395 to deoxycholate was compared. The V. cholerae tol mutants showed an increased sensitivity to deoxycholate as these strains had no growth at concentrations of deoxycholate eightfold less than the inhibitory concentration for O395. Thus, as observed in E. coli (39), mutations in the V. cholerae tolQRAB genes increase the sensitivity of these strains to deoxycholate.
DISCUSSION
Our work indicates that there are significant parallels between the pathways used by CTXφ for entry into V. cholerae and by Ff phage for entry into E. coli. Like Ff phage entry into F+ E. coli, we found that efficient entry of CTXφ into V. cholerae requires both a pilus, TCP, and the products of the tolQRA genes but not the product of tolB. Most likely, in both instances, binding of the phages to their respective pilus receptors constitutes the initial event in infection. We found that CTXφ, like the coliphages, does not absolutely require its pilus receptor for uptake into V. cholerae. Therefore, it is reasonable to suggest (as Reichmann and Holliger proposed for the coliphages [26]) that the initial binding of CTXφ to TCP enables the phage to bind to V. cholerae at a significant distance from the cell surface. Binding to TCP somehow then directs CTXφ to the V. cholerae outer membrane where it can interact with the TolQRA complex, which facilitates its traversal of the periplasmic space. In the absence of TCP, however, occasionally CTXφ particles can contact the outer membrane and interact with the TolQRA complex. This model is supported by our data that mutations in both tcpA and one of the tolQRA genes render the target cell resistant to CTXφ infection.
The similarity of V. cholerae TolQRA to E. coli TolQRA, together with the requirement for V. cholerae TolQRA in CTXφ entry into V. cholerae, suggests that elements of the recently proposed model of Ff phage infection of E. coli apply to CTXφ infection of V. cholerae. Based on structural and biochemical data, Riechmann and Holliger have proposed that the requirement for both the F pilus and TolQRA in the entry of Ff phages into E. coli is based on the dual-binding specificities of pIII, a minor coat protein located on one end of Ff phages (26). Domain 2 of pIII (g3p-D2) binds to the F pilus, and domain 1 (g3p-D1) binds to the carboxyl terminus of TolA (TolA-III). Binding of g3p-D2 to F is thought to lead to pilus retraction and to exposure of g3p-D1 for binding to TolA-III. After g3p-D1 binds to TolA-III, the subsequent steps leading to internalization of the Ff phage are not known, though g3p may, along with TolQRA, form a channel in the inner membrane (26).
Given the requirement for V. cholerae TolQRA in CTXφ infection and the predicted structural similarity of domain 1 of the CTXφ g3p orthologue, OrfU, with domain 1 of g3p (11), it is reasonable to suggest that this domain of OrfU binds to V. cholerae TolA after OrfU binds to TCP. If the E. coli model applies, the initial binding of CTXφ to TCP (presumably via OrfU domain 2) results in retraction of TCP and exposes OrfU domain 1 for binding to the C terminus of TolA. TCP retraction has not been demonstrated experimentally in V. cholerae but is suggested by our data. A structural prediction of this model is that after CTXφ particles bind TCP, OrfU is in sufficient proximity to TolA to enable these proteins to interact. If this is the case, then two distinct types of pili, F and TCP, are similarly distributed relative to the TolQ, TolR, and TolA proteins in E. coli and V. cholerae. It remains to be shown whether there is a direct physical interaction between TolQRA and F in E. coli and TolQRA and TCP in V. cholerae.
Our demonstration of CTXφ infection of TCP− V. cholerae cells may provide an explanation for the origin of CTXφ+ TCP− V. cholerae isolates that have been reported. In the laboratory, we were able to infect TCP− CTXφ-V. cholerae O1 isolate 468-83 (28) with CTX-Knφ by coculturing it with a CTXφ donor strain. As with TCP2, a laboratory-derived TCP− strain, the frequency of infection of 468-83 by CTX-Knφ was very low (approximately 2.6 × 10−9) in this assay. Thus, the experimental evidence indicates that infection of TCP− strains by CTXφ is possible, but it is likely to be a rare event. As would be expected in light of these results, relatively few CTXφ+ TCP− strains have been found among environmental isolates.
Although the host molecules required for CTXφ infection of V. cholerae are similar to molecules required for Ff infection of E. coli, our data suggest that there are differences in the importance of TolQRA in CTXφ infection. Unlike E. coli, a mutation in any one of the V. cholerae tolQRA genes, including tolA, did not render the target cell completely resistant to CTXφ. There are a number of potential explanations for this difference. First, there could be an alternate route for uptake of CTXφ, mediated by different proteins. Since cells with mutations in both tcpA and one of the tolQ, tolR, or tolA genes could not be infected with CTXφ, this alternative pathway must be dependent (at least indirectly) on the expression of TCP. Ongoing studies are aimed at determining if V. cholerae carries other genes that might play a role in uptake of CTXφ. Second, because our mutations in tolQ, tolR, and tolA were plasmid integrations into the middle of these genes, it is possible that truncated TolQRA proteins were sufficient to permit the low levels of infection we observed with these mutations. Finally, it may be that our alternative assay for transduction, which relied upon coculture of donor and recipients on a semisolid surface, is more sensitive than the liquid transduction assays used for E. coli and Ff phages. If so, this could account for the residual CTXφ infection of the V. cholerae tol mutant strains.
The similarities between the tol gene products of several gram-negative species, including E. coli (32, 33), Haemophilus influenzae (31), Pseudomonas putida (27), and Pseudomonas aeruginosa (6), suggest that the structure and function of the tol gene products, although as yet not completely defined, is conserved between species. In spite of this similarity, however, differences exist in the phenotypes exhibited by E. coli, P. aeruginosa, and V. cholerae strains containing mutations in their tolQRAB genes. In P. aeruginosa, viable strains containing mutations in the tolQRAB genes could not be obtained, leading to the suggestion that TolQRAB proteins are essential in this bacteria (6). Although the tol genes were not essential in V. cholerae, most mutants grew slowly, giving rise to small colonies in the absence of presumed secondary mutations. In addition, we found that our small-colony V. cholerae strains with mutations in tolQ, tolR, and tolB were not viable at 42°C. In E. coli, TolQRAB proteins have not been reported to be essential for growth at any temperature. Also, the defect in efficiency of plating exhibited by V. cholerae strains with tolQRAB mutations has not been reported in E. coli, although it has recently been reported that a mutation in tolA in E. coli impairs septation and cell division (22). The molecular bases for these differences in phenotypes are not known.
In E. coli, the transcriptional organization of the tolQRAB gene complex consists of two operons: orf1 tolQRA, which has a promoter upstream of orf1, and tolB pal, which has a promoter upstream of tolB (37). Similar to E. coli, the data from our complementation studies suggest that V. cholerae tolQ and tolR are transcriptionally linked, as the insertion mutation in tolQ was polar on tolR and could only be complemented with the addition of both tolQ and tolR. Another possibility, demonstrated for E. coli (36), is that translational control of tolR expression by TolQ exists in V. cholerae. Since the tolR mutation in DH2 was not polar on tolA, these results suggest that in V. cholerae there is no linkage of tolQR transcription to tolA transcription but rather that tolA may have its own promoter. Another explanation is that the mutation introduced in tolR by pDH107 integration is only partially polar on tolA and that the resultant reduced levels of tolA message are sufficient to enable CTXφ infection. Further studies are required to determine the exact transcriptional organization of V. cholerae tolQRAB.
Our finding that the V. cholerae tolQRA products are required for CTXφ uptake suggests a way to improve the biosafety of live-attenuated (CTXφ−) V. cholerae vaccine strains. These strains can be reinfected by CTXφ and thereby revert back to toxigenicity. However, a mutation in either tolQ, tolR, or tolA in a vaccine strain should render it relatively resistant to CTXφ infection. A potential difficulty with this approach is that the pleiotropic effects of these mutations may diminish the strains' capacity to colonize the intestine, a prerequisite for antigenicity (10). Colonization defects have been reported in a Salmonella enterica serovar Typhimurium strain harboring a mutation in tolB (3). Our preliminary results suggest that the tolQRA mutations in DH1, DH2, and DH3 significantly attenuate colonization; however, these results are difficult to interpret given the plating inefficiency of these strains. Even if the V. cholerae tol gene products are required for colonization, it may be possible to isolate specific tolQ, tolR, or tolA mutations which render the resulting strains resistant to CTXφ infection but which do not significantly attenuate intestinal colonization. For this approach to work, the essential functions of TolQRA must be structurally separable from the activity of these proteins as phage coreceptors.
ACKNOWLEDGMENTS
We thank N. Golden for construction of the tolR mutation and A. Kane, B. Davis, B. Hochhut, H. Kimsey, A. Camilli, and C. Moyer for critical reading of the manuscript. We are grateful to Robert Webster for sending strain TPS66. We thank A. Kane and the GRASP Center Intestinal Microbiology Core for preparation of media and M. Berne of the Tufts Core Facility for DNA sequencing.
This work was supported by NIH grant AI 42347. M.K.W. is a Pew Scholar in the Biomedical Sciences.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidmann J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing and Wiley-Interscience; 1990. [Google Scholar]
- 3.Bowe F, Lipps C J, Tsolis R M, Groisman E, Heffron F, Kusters J G. At least four percent of the Salmonella typhimurium genome is required for fatal infection of mice. Infect Immun. 1998;66:3372–3377. doi: 10.1128/iai.66.7.3372-3377.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Click E M, Webster R E. The TolQRA proteins are required for membrane insertion of the major capsid protein of the filamentous phage f1 during infection. J Bacteriol. 1998;180:1723–1728. doi: 10.1128/jb.180.7.1723-1728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datta N, Hedges R W. Host ranges of R factors. J Gen Microbiol. 1972;70:453–460. doi: 10.1099/00221287-70-3-453. [DOI] [PubMed] [Google Scholar]
- 6.Dennis J J, Lafontaine E R, Sokol P A. Identification and characterization of the tolQRA genes of Pseudomonas aeruginosa. J Bacteriol. 1996;178:7059–7068. doi: 10.1128/jb.178.24.7059-7068.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology; 1994. [Google Scholar]
- 8.Germon P, Clavel T, Vianney A, Portalier R, Lazzaroni J C. Mutational analysis of the Escherichia coli K-12 TolA N-terminal region and characterization of its TolQ-interacting domain by genetic suppression. J Bacteriol. 1998;180:6433–6439. doi: 10.1128/jb.180.24.6433-6439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzman L M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrington D A, Hall R H, Losonsky G, Mekalanos J J, Taylor R K, Levine M M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holliger P, Riechmann L. A conserved infection pathway for filamentous bacteriophages is suggested by the structure of the membrane penetration domain of the minor coat protein g3p from phage fd. Structure. 1997;5:265–275. doi: 10.1016/s0969-2126(97)00184-6. [DOI] [PubMed] [Google Scholar]
- 12.Isnard M, Rigal A, Lazzaroni J C, Lazdunski C, Lloubes R. Maturation and localization of the TolB protein required for colicin import. J Bacteriol. 1994;176:6392–6396. doi: 10.1128/jb.176.20.6392-6396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson A. Role of F pili in the penetration of bacteriophage f1. J Virol. 1972;10:835–843. doi: 10.1128/jvi.10.4.835-843.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimsey H H, Waldor M K. CTXφ immunity: application in the development of cholera vaccines. Proc Natl Acad Sci USA. 1998;95:7035–7039. doi: 10.1073/pnas.95.12.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koonin E V. The second cholera toxin, Zot, and its plasmid-encoded and phage-encoded homologues constitute a group of putative ATPases with an altered purine NTP-binding motif. FEBS Lett. 1992;312:3–6. doi: 10.1016/0014-5793(92)81398-6. [DOI] [PubMed] [Google Scholar]
- 16.Lazdunski C J. Colicin import and pore formation: a system for studying protein transport across membranes? Mol Microbiol. 1995;16:1059–1066. doi: 10.1111/j.1365-2958.1995.tb02331.x. [DOI] [PubMed] [Google Scholar]
- 17.Lazzaroni J C, Fognini-Lefebvre N, Portalier R. Cloning of the excC and excD genes involved in the release of periplasmic proteins by Escherichia coli K12. Mol Gen Genet. 1989;218:460–464. doi: 10.1007/BF00332410. [DOI] [PubMed] [Google Scholar]
- 18.Lazzaroni J C, Portalier R C. Genetic and biochemical characterization of periplasmic-leaky mutants of Escherichia coli K-12. J Bacteriol. 1981;145:1351–1358. doi: 10.1128/jb.145.3.1351-1358.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazzaroni J C, Vianney A, Popot J L, Benedetti H, Samatey F, Lazdunski C, Portalier R, Geli V. Transmembrane alpha-helix interactions are required for the functional assembly of the Escherichia coli Tol complex. J Mol Biol. 1995;246:1–7. doi: 10.1006/jmbi.1994.0058. [DOI] [PubMed] [Google Scholar]
- 20.Levengood S K, Beyer W F, Jr, Webster R E. TolA: a membrane protein involved in colicin uptake contains an extended helical region. Proc Natl Acad Sci USA. 1991;88:5939–5943. doi: 10.1073/pnas.88.14.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewin T M, Webster R E. Membrane insertion characteristics of the various transmembrane domains of the Escherichia coli TolQ protein. J Biol Chem. 1996;271:14143–14149. doi: 10.1074/jbc.271.24.14143. [DOI] [PubMed] [Google Scholar]
- 22.Meury J, Devilliers G. Impairment of cell division in tolA mutants of Escherichia coli at low and high medium osmolarities. Biol Cell. 1999;91:67–75. [PubMed] [Google Scholar]
- 23.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller M M, Vianney A, Lazzaroni J C, Webster R E, Portalier R. Membrane topology of the Escherichia coli TolR protein required for cell envelope integrity. J Bacteriol. 1993;175:6059–6061. doi: 10.1128/jb.175.18.6059-6061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakai K, Kanehisa M. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 26.Riechmann L, Holliger P. The C-terminal domain of TolA is the coreceptor for filamentous phage infection of E. coli. Cell. 1997;90:351–360. doi: 10.1016/s0092-8674(00)80342-6. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Herva J J, Ramos-González M-I, Ramos J L. The Pseudomonas putida peptidoglycan-associated outer membrane lipoprotein is involved in maintenance of the integrity of the cell envelope. J Bacteriol. 1996;178:1699–1706. doi: 10.1128/jb.178.6.1699-1706.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin E J, Lin W, Mekalanos J J, Waldor M K. Replication and integration of a Vibrio cholerae cryptic plasmid linked to the CTX prophage. Mol Microbiol. 1998;28:1247–1254. doi: 10.1046/j.1365-2958.1998.00889.x. [DOI] [PubMed] [Google Scholar]
- 29.Russel M, Whirlow H, Sun T-P, Webster R E. Low-frequency infection of F− bacteria by transducing particles of filamentous bacteriophages. J Bacteriol. 1988;170:5312–5316. doi: 10.1128/jb.170.11.5312-5316.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sears C L, Kaper J B. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sen K, Sikkema D J, Murphy T F. Isolation and characterization of the Haemophilus influenzae tolQ, tolR, tolA, and tolB genes. Gene. 1996;178:75–81. doi: 10.1016/0378-1119(96)00338-1. [DOI] [PubMed] [Google Scholar]
- 32.Sun T P, Webster R E. fii, a bacterial locus required for filamentous phage infection and its relation to colicin-tolerant tolA and tolB. J Bacteriol. 1986;165:107–115. doi: 10.1128/jb.165.1.107-115.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun T P, Webster R E. Nucleotide sequence of a gene cluster involved in entry of E colicins and single-stranded DNA of infecting filamentous bacteriophages into Escherichia coli. J Bacteriol. 1987;169:2667–2674. doi: 10.1128/jb.169.6.2667-2674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vianney A, Lewin T M, Beyer W F, Jr, Lazzaroni J C, Portalier R, Webster R E. Membrane topology and mutational analysis of the TolQ protein of Escherichia coli required for the uptake of macromolecules and cell envelope integrity. J Bacteriol. 1994;176:822–829. doi: 10.1128/jb.176.3.822-829.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vianney A, Muller M M, Clavel T, Lazzaroni J C, Portalier R, Webster R E. Characterization of the tol-pal region of Escherichia coli K-12: translational control of tolR expression by TolQ and identification of a new open reading frame downstream of pal encoding a periplasmic protein. J Bacteriol. 1996;178:4031–4038. doi: 10.1128/jb.178.14.4031-4038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;27:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 38.Waldor M K, Rubin E J, Pearson G D, Kimsey H, Mekalanos J J. Regulation, replication, and integration functions of the Vibrio cholerae CTXφ are encoded by region RS2. Mol Microbiol. 1997;24:917–926. doi: 10.1046/j.1365-2958.1997.3911758.x. [DOI] [PubMed] [Google Scholar]
- 39.Webster R E. The tol gene products and the import of macromolecules into Escherichia coli. Mol Microbiol. 1991;5:1005–1011. doi: 10.1111/j.1365-2958.1991.tb01873.x. [DOI] [PubMed] [Google Scholar]
- 40.Young K, Silver L L. Leakage of periplasmic enzymes from envA1 strains of Escherichia coli. J Bacteriol. 1991;173:3609–3614. doi: 10.1128/jb.173.12.3609-3614.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]