Abstract
Background
Among the manifestations of COVID-19 are taste and smell disorders (TSDs).
Aim
To evaluate the sensitivity and specificity of TSDs and other associated symptoms to estimate predictive values for determining SARS-CoV-2 infection.
Design & setting
A retrospective observational study of healthcare professionals in Catalonia, Spain.
Method
A study of the sensitivity and specificity of TSDs has been carried out using the polymerase chain reaction (PCR) test for the diagnosis of SARS-CoV-2 as the gold standard value. Logistic regressions adjusted for age and sex were performed to identify additional symptoms that might be associated with COVID-19.
Results
The results are based on 226 healthcare workers with clinical symptoms suggestive of COVID-19, 116 with positive PCR and 110 with negative PCR. TSDs had an odds ratio (OR) of 12.4 (95% confidence interval [CI] = 6.3 to 26.2), sensitivity 60.3% and specificity 89.1%. In the logistic regression model, the association of TSD, fever or low-grade fever, shivering, dyspnoea, arthralgia, and myalgia obtained an area under the curve (AUC) of 85.7% (95% CI = 80.7 % to 90.7 %), sensitivity 82.8 %, specificity 80.0%, and positive predictive values 81.4% and negative 81.5%.
Conclusion
TSDs are a strong predictor of COVID-19. The association of TSD, fever, low-grade fever or shivering, dyspnoea, arthralgia, and myalgia correctly predicts 85.7% of the results of the COVID-19 test.
How this fits in
Initial Asian studies did not refer TSDs as a common symptom of COVID-19. TSDs seem to be a strong predictor of a positive COVID-19 result in the European population. The association of TSD, shivering, dyspnoea, arthralgia, and myalgia correctly predicted 85.7% of positive results of the COVID-19 test in Spanish patients with mild-to-moderate clinical symptoms. TSDs could be included as part of routine screening for COVID-19.
Introduction
After severe acute respiratory syndrome caused by SARS-CoV coronavirus in 2002 and Middle East acute respiratory syndrome caused by MERS-CoV in 2012, 1 another highly pathogenic coronavirus called SARS-CoV-2 appeared in December 2019 in Wuhan, China, and has spread rapidly around the world. On 11 March 2020, the World Health Organization (WHO) called this new coronavirus outbreak a pandemic. 2 Currently this virus has infected more than 174 million people worldwide. 3 SARS-CoV-2 is a single-stranded RNA (ssRNA), betacoronavirus, and the disease it causes is COVID-19.
According to initial clinical studies from Asia, the most common symptoms of COVID-19 were fever, cough, dyspnoea, myalgia, arthralgia, headache, odynophagia, and less frequently rhinorrhoea and nasal congestion. 4–6 But as the disease has spread around the world, a more varied and complex clinical spectrum has been described. According to European data, there is increasing evidence that patients with COVID-19 may present with a heterogeneous spectrum. Many affected people with mild-to-moderate forms showed TSDs even without fever, cough, or other systemic abnormalities. 7–9 It is common for many viruses, such as rhinoviruses, parainfluenza virus, and some coronaviruses 10,11 to cause olfactory dysfunction through an inflammatory reaction of the nasal mucosa and the development of rhinorrhoea; however, the olfactory dysfunction associated with COVID-19 infection seems peculiar, as it is not associated with rhinorrhoea.
Initial studies identified fever, fatigue, cough, and dyspnoea as predictors of COVID-19. 12 Subsequently, the TSDs were also associated with COVID-19. 7,13–15
On observing clinical differences between populations, the aim of the study was to evaluate the sensitivity and specificity of TSDs and associated symptoms to estimate predictive values for SARS-CoV-2 infection in a sample of Spanish healthcare professionals from the central region of Catalonia, an autonomous community in north-western Spain.
Method
A study of sensitivity and specificity of TSDs was designed using the reverse transcription-polymerase chain reaction (RT-PCR) test as the gold standard value, the diagnostic test for SARS-CoV-2 coronavirus.
A telephone interview was carried out with nurses and doctors in primary care of the Territorial Management of Central Catalonia (in the counties of Bages, Berguedà, Anoia, Solsonès, Moianès, and Osona) with clinical symptoms suggestive of COVID-19. Healthcare professionals were included with at least one positive PCR performed during the period from 10 March–10 April 2020, and who had presented with mild-to-moderate COVID-1 (defined as patients who did not require admission to intensive care units). Interviews were conducted between 4 May23 May 2020. There was a 95% response rate. The duration of the interview was 5–10 minutes. All data were extracted from the telephone interview.
Positive cases were considered to be those patients with at least one positive PCR for COVID-19 and negative cases were those patients with all negative PCRs. Exclusion criteria were patients who were asymptomatic but had a PCR because of close contact with a sick person; patients with olfactory or gustatory dysfunction before the epidemic; and patients hospitalised in the intensive care unit at the time of the study.
A total of 226 participants were included, randomly selected from a list of healthcare professionals symptomatic with flu-like symptoms or COVID-19 presumption provided by the technical unit of the Territorial Management of Central Catalonia of the Catalan Institute of Health. Of these, 116 were considered positive cases and 110 were considered negative cases.
The following sociodemographic variables were studied: age; sex; and professional category. The clinical variables were: previous changes in sense of taste and/or smell; smoking (does not smoke, smokes [number of cigarettes]); allergies (only environmental allergies such as to pollen, mites, and animal hair were considered); hospital admission; number of admission days; and laboratory results (PCR). For each clinical symptom it was asked whether it had occurred (yes/no) and for how long. The following were studied: fever (temperature ≥38°C); low-grade fever (temperature 37°C–37.9°C); cough; asthenia; anorexia; diarrhoea; odynophagia; abdominal pain; vomiting and/or nausea; dyspnoea; chest pain; shivering; conjunctival hyperaemia; lacrimation; dry eyes; blurred vision; sneezing; rhinorrhoea; nasal congestion and/or obstruction; epistaxis; tinnitus; hearing loss; sputum production; haemoptysis; tachycardia; headache; dizziness; impaired consciousness; ataxia; acute cerebrovascular disease; convulsions; change in sense of taste; change in sense of smell; neuralgia; myalgia; arthralgia; skin rash; vesicular lesions; maculopapules; itchy skin; and pseudo-chilblains.
Statistical analysis
The sociodemographic variables and clinical characteristics of the sample were described using absolute frequencies and percentages. Mean and standard deviation together with minimum and maximum were used to describe the duration of symptoms. The χ² comparison was used to analyse the relationship between two categorical variables. The OR was estimated with its CI as a measure of association between the main symptoms and the PCR result, with the latter considered as the gold standard.
From the results of the bivariate analyses, logistic regressions were performed using the stepwise forward selection method to determine the best predictor symptoms, in addition to TSD, of a positive PCR result. To analyse the classification capacity of the models, the AUC was used together with the values of sensitivity, specificity, positive predictive value, and negative predictive value. The receiver operating characteristic (ROC) was used to compare and graphically represent the models.
All CIs are 95% CIs and a significance level of 5% was set. R statistical software (version 4.0.3) and SPSS (version 27) were used.
Results
Of the 226 health professionals included, 116 (51.3%) had a positive PCR and were considered positive cases (COVID-19 positive) and 110 were considered negative cases (COVID-19 negative). The cohort was predominantly female and a significantly greater amount of COVID-19 positive participants smoked (P = 0.026) and were admitted more (0.002).
COVID-19 positive patients had more fever, low-grade fever, shivering, asthenia, dyspnoea, change in sense of taste and smell, arthralgia, myalgia, anorexia, nasal obstruction, sneezing, epistaxis, urticarial lesions or itching, , tachycardia, chest pain, diarrhoea, sputum production, and headache (P<0.005) (Table 1).
Table 1. Clinical characteristics of the sample, at any time.
| Symptom | COVID-19 | COVID-19 | P | ||
|---|---|---|---|---|---|
| Positive (n = 116) | Negative (n = 110) | ||||
| n | % | n | % | ||
| Fever (≥38 ºC) | 43 | 37.1 | 12 | 10.9 | 0.000 |
| Febrile (<38 ºC) | 81 | 69.8 | 45 | 40.9 | 0.000 |
| Shivers | 69 | 59.5 | 28 | 25.5 | 0.000 |
| Asthenia | 98 | 84.5 | 70 | 63.6 | 0.000 |
| Dyspnoea | 43 | 37.1 | 18 | 16.4 | 0.000 |
| Alteration of taste | 60 | 51.7 | 11 | 10.0 | 0.000 |
| Alteration of smell | 63 | 54.3 | 9 | 8,2 | 0.000 |
| Arthralgias | 51 | 44.0 | 19 | 17.3 | 0.000 |
| Myalgias | 82 | 70.7 | 10 | 9.1 | 0.000 |
| Anorexia | 44 | 37.9 | 20 | 18.2 | 0.001 |
| Congestion or nasal obstruction | 35 | 30.2 | 13 | 11.8 | 0.001 |
| Sneezing | 22 | 19.0 | 9 | 8.2 | 0.015 |
| Epistaxis | 8 | 6.9 | 1 | 0.9 | 0.021 |
| Hives or itching | 10 | 8.6 | 2 | 1.8 | 0.021 |
| Instability (balance) | 19 | 16.4 | 8 | 7.3 | 0.027 |
| Tachycardia | 16 | 13.8 | 6 | 5.5 | 0.028 |
| Chest pain | 23 | 19.8 | 11 | 10.0 | 0.029 |
| Diarrhoea | 52 | 44.8 | 35 | 31.8 | 0.030 |
| Spit production | 11 | 9.5 | 3 | 2.7 | 0.032 |
| Headache | 80 | 69.0 | 63 | 57.3 | 0.046 |
Only variables with statistically significant differences are shown.
In relation to the symptoms of COVID-19 positive patients, statistically significant differences were observed by sex; women presented more changes in sense of smell and taste, headache, and nasal obstruction.
Statistically significant differences by age group were also observed; those aged >50 years had more fever, cough, and headache, and those aged <40 years had more nasal obstruction and rhinorrhoea.
Figure 1 shows the duration of the most prevalent symptoms. Symptoms with a mean duration of more than 10 days with the mean number of days of duration, standard deviation and range, are presented in a supplementary table.
Figure 1. Duration of the most prevalent symptoms.
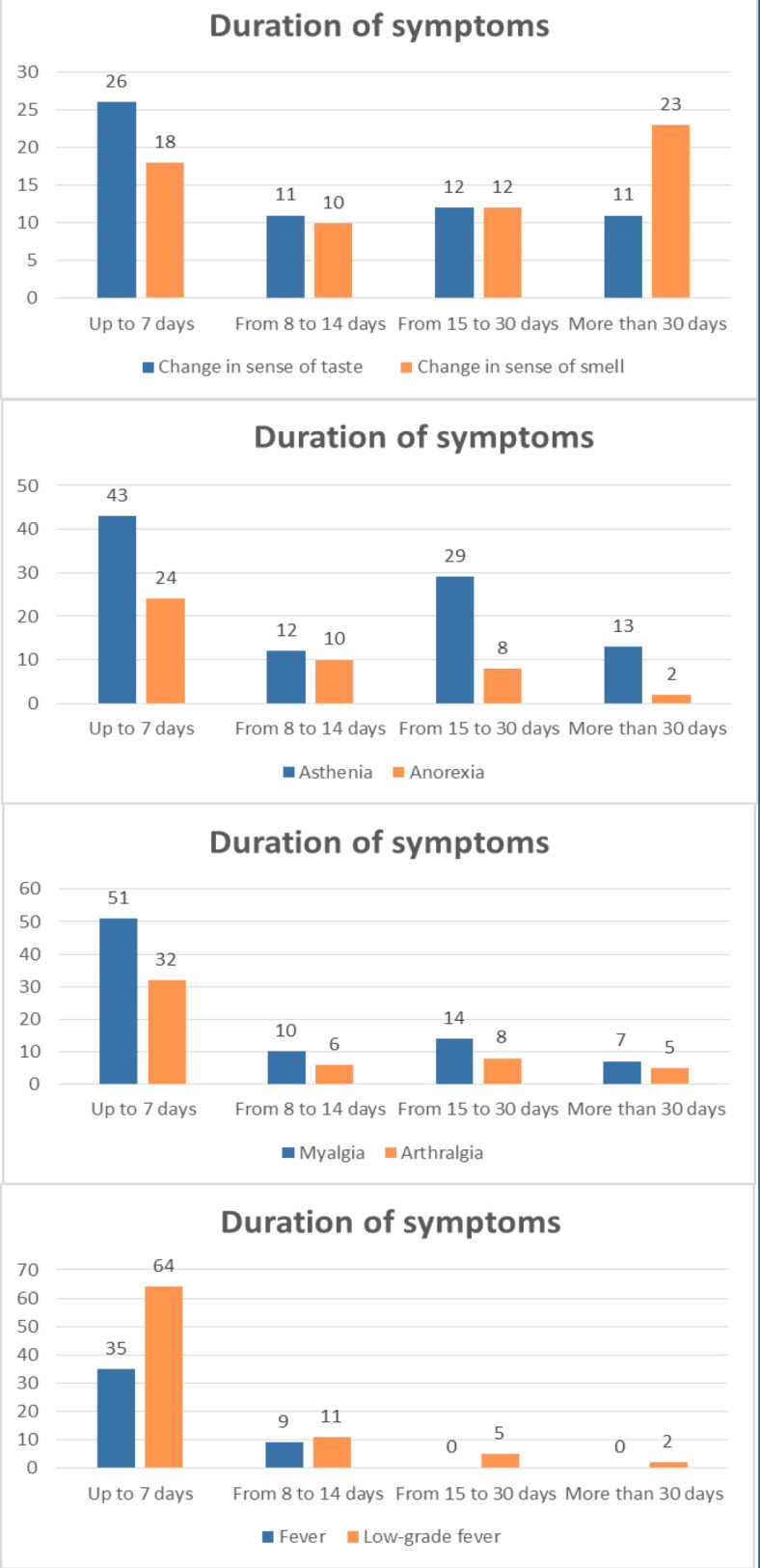
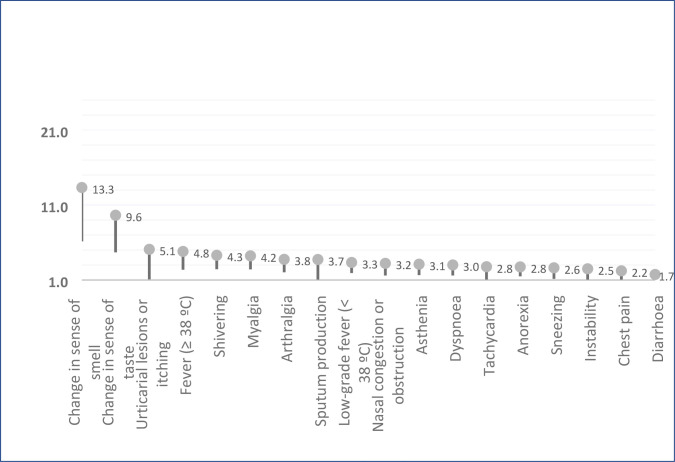
Figure 2 shows the degree of association between symptoms and SARS-CoV-2 infection in the participants studied. Loss of smell stands out with an OR of 13.3 (95% CI = 6.22 to 28.9) as well as change in sense of taste with an OR of 9.6 (95% CI = 4.7 to 19.8).
Figure 2. Association between symptoms and odds ratio of SARS-CoV-2 infection in 226 healthcare workers in central Catalonia who were evaluated by reverse transcription-polymerase chain reaction (RT-PCR).
Finally, with the information from the univariate descriptive and bivariate contrasts, the variables fever, low-grade fever, or shivering and the variables TSDs were grouped for logistic regression; and age, sex, TSDs, urticarial lesions or itching, temperature changes or shivering, myalgia, arthralgia, sputum production, nasal congestion, asthenia, dyspnoea, tachycardia, anorexia, sneezing, chest pain, and diarrhoea were selected as possible predictors. Using the stepwise method, the selection of the best predictors were TSDs, temperature changes or shivering, dyspnoea, myalgia, and arthralgia (Table 2). From the final model (step 5) it was observed that having fever, low-grade fever or shivering corresponded to odds almost three times higher than having a positive CRP compared with those without any temperature change or shivering. Having dyspnoea more than doubles the possibility of presenting a positive test compared with those who do not have dyspnoea; experiencing changes in sense of smell and taste corresponds to an elevenfold increase in the possibility of presenting a positive test compared with not having them; and, on the other hand, having arthralgia or having myalgia does not reach the level of significance in the logistic relationship and therefore, in this model, does not predict the positivity of the test.
Table 2. Classifier performance.
| Predictor(s) | OR | 95% CI | P | AUC | Senstivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|---|
| Step 1 | Changes in sense of smell or taste | 12.4 | (6.3 to 26.2) | <0.001 | 74.7% | 60.3% | 89.1% | 85.4% | 68.1% |
| Step 2 | Changes in sense of smell or taste | 10.6 | (5.3 to 23.1) | <0.001 | 81.3% | 60.3% | 89.1% | 85.4% | 68.1% |
| Fever, low-grade fever or shivering | 4.6 | (2.4 to 10.9) | <0.001 | ||||||
| Step 3 | Changes in sense of smell or taste | 10.2 | (5.0 to 22.6) | <0.001 | 84.0% | 86.2% | 69.1% | 74.6% | 82.6% |
| Fever, low-grade fever or shivering | 3.5 | (1.6 to 8.0) | 0.001 | ||||||
| Myalgia | 2.5 | (1.3 to 4.9) | 0.008 | ||||||
| Step 4 | Changes in sense of smell or taste | 10.4 | (5.0 to 23.0) | <0.001 | 84.5% | 76.7% | 82.7% | 82.4% | 77.1% |
| Fever, low-grade fever or shivering | 3.4 | (1.6 to 7.8) | 0.002 | ||||||
| Myalgia | 1.8 | (0.8 to 3.9) | 0.119 | ||||||
| Arthralgia | 2.1 | (0.9 to 4.7) | 0.082 | ||||||
| Step 5 | Changes in sense of smell or taste | 11.1 | (5.3 to 25.1) | <0.001 | 85.7% | 82.7% | 80.0% | 81.4% | 81.5% |
| Fever, low-grade fever or shivering | 3.0 | (1.4 to 6.8) | 0.007 | ||||||
| Myalgia | 1.6 | (0.7 to 3.5) | 0.234 | ||||||
| Arthralgia | 2.2 | (1.0 to 5.1) | 0.065 | ||||||
| Dyspnoea | 2.4 | (1.1 to 5.3) | 0.030 | ||||||
AUC = area under the curve. NPV = negative predictive value. PPV = positive predictive value.
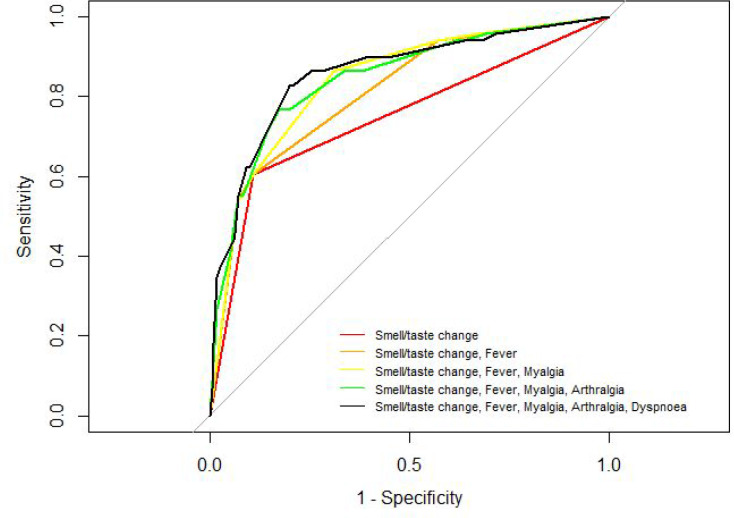
Regarding the discriminative capacity of the models, Figure 3 shows the ROC curves of the variable TSDs and the curves of progressively adding the rest of the selected variables according to the importance in the model and the clinical criterion. The final model has an AUC of 85.7% (95% CI = 80.7% to 90.7%), with a sensitivity of 82.8%, a specificity of 80.0%, a positive predictive value of 81.4%, and a negative predictive value of 81.5%.
Figure 3. Receiver operating characteristic (ROC) plots for symptom classifier models. The dashed diagonal line shows a non-diagnostic result. Area under the curve (AUC) for each symptom classifier group is displayed in Table 2. (A non-colour dependent version of this figure is available in the supplementary materials) .
Discussion
Summary
TSDs are a strong predictor of a positive COVID-19 result. In the study, the association of TSD, fever or low-grade fever, shivering, dyspnoea, arthralgia, and myalgia correctly predicted 85.7% of the results of the COVID-19 test. Further studies are needed to confirm the potential of this association.
Strengths and limitations
The main limitation is the fact that it is a retrospective study, meaning some mild symptoms may have gone unnoticed and participants may have had difficulty in remembering dates.
The authors of this study did not contemplate the use of specific TSD tests for chemosensory assessment, even though such tests would have been ideal. Regarding benefit–risk balance, its use was considered to involve unnecessary additional time and risk of exposure to COVID-19 by clinicians, as well as non-essential discomfort for patients.
Finally, the sensitivity and potential false negativity of PCR-based COVID-19 testing must also be considered.
One of the strengths of the study is that it was conducted on health professionals who are knowledgeable regarding the symptoms presented, and through telephone interviews. Other published studies have involved the general population, anonymously and self-reported through smartphone applications. 13–16
Comparison with existing literature
The study aimed to evaluate the symptoms associated with a positive COVID-19 test. TSDs in the COVID-19 positive study sample appeared in similar proportions to 35 other European studies with 8575 patients, with a prevalence of 57.5% of olfactory dysfunction and 53.1% of taste dysfunction. 17
In the sample, patients with severe hyposmia or anosmia usually also complained of loss of taste, as also reported by most articles included in the systematic review and meta-analysis of Ibekwe et al. 18 This is owing to loss in the contribution of smell to their perception of flavour. Paderno et al 19 observed that TSDs were more frequent in participants with mild-to-moderate COVID-19 who did not require admission. The recovery of olfactory and gustatory functions also varies according to studies. In the present study, 61.1% had regained their sense of taste and 44.4% had recovered their sense of smell 14 days after the onset of clinical symptoms. In Barillari et al’s study, 24.3% of patients regained their sense of smell and taste within 9–15 days. 9 And in Lechien et al’s study, 25.5% of patients regained these functions within 2 weeks after resolution of general symptoms. 8
Regarding sex differences, women presented more clinical symptoms of headache, TSD, and nasal obstruction. Other studies agree that the incidence of TSDs is higher in women than in men. 20–22 Women are more likely to develop post-infectious olfactory dysfunction in viral infections related to parainfluenza or Epstein-Barr virus. 10 Looking at age, it is notable that younger patients more often present ear, nose, and throat (ENT) clinical symptoms (nasal obstruction and rhinorrhoea), and these data coincide with the Italian studies of Barillari et al and Lechien et al, 8,9,23 which also found a negative correlation between ENT symptoms and age.
One meta-analysis confirmed that the prevalence rates of olfactory and gustatory dysfunction were different among four geographical regions of the world. The prevalence of olfactory dysfunction in East Asia was significantly lower than that in Europe or the Middle East, and prevalence of gustatory dysfunction in East Asia was significantly lower than that in Europe and North America. 17 A hypothesis proposed by Li et al 24 and Forster et al 25 in relation to the differences between both regions of the world (East Asia, and Europe and North America), on olfactory and taste dysfunctions, indicated that this could be related to differences in the genetic pattern of the virus (potential mutations). Another hypothesis indicated that the variation can be related to angiotensin-converting enzyme 2 (ACE2), a possible host receptor of SARS-CoV-2. 26,27 The presence of a difference in variants of ACE2 according to geographical and ethnic factors has been demonstrated. 28 It is assumed that the difference in variants of ACE2 expressed in olfactory epithelial cells, according to populations from different geographical regions, can influence the prevalence of olfactory and gustatory dysfunction. 17
TSDs were the symptoms with the strongest association for a positive COVID-19 result, with an OR of 13.3 for loss of sense of smell and 9.6 for loss of taste. Overall, TSDs had an OR of 12.4, a sensitivity of 60.3%, and a specificity of 89.1%. These results coincide with Menni’s study, which had a sample of US and UK patients.
The ability of the symptom set to accurately classify subjects as COVID-19 positive was identifed and assessed through logistic regression. Symptoms associated with COVID-19 positivity included TSDs, fever or low-grade fever, shivering, dyspnoea, arthralgia, and myalgia, with an AUC of 85.7% (95% CI = 80.7% to 90.7%). The results are in broad agreement with other studies but with differences: Roland et al 13 also identified odynophagia; Menni et al 15 cough and digestive clinical signs; and the results of Yan et al 14 showed no significance for dyspnoea.
Implications for practice
The findings of this study suggest that TSDs could be included as part of routine screening for COVID-19. These symptoms could be used in mass screening by professionals with limited medical knowledge and using telemedicine. These data could be useful where diagnostic testing for COVID-19 in the general population is difficult and/or in situations with high patient volume and diagnostic testing difficulties. The fact that COVID-19 can be diagnosed without the need for PCR allows early diagnosis and isolation. Future research may be needed in relation to novel or emerging strains.
Funding
This research received no external funding.
Author contributions
Conceptualization, A.R.C. and J.M.P.; methodology, A.R.C., J.M.P. and A.R.M.; data collection, A.R.C., A.R.M.; G.S.V. and V.G.F.; validation and formal analysis, P.R.P. and Q.M.C.; investigation, A.R.C. and J.M.P.; data curation, A.R.M., P.R.P. and Q.M.C.; writing—original draft preparation, A.R.C.; writing—review and editing, A.R.C., A.R.M.; G.S.V.; V.G.F. and J.V.A.; supervision, all authors; project administration, A.R.C. All authors have read and agreed to the published version of the manuscript.
Ethical approval
All the data in this study have been treated in a strictly confidential manner following the ethical principles of the Declaration of Helsinki. The regulations established by Organic Law 3/2018, of 5 December, on the Protection of Personal Data and guarantee of digital rights; as well as the European Data Protection Regulation 2016/679, of 27 April 2016, have been followed. The study was approved by the local Ethics Committee Jordi Gol i Gurina Foundation (protocol code 20/094-PCV and date of approval 28/05/2020).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement:
The data presented in this study are available on request from the corresponding author.
Provenance
Freely submitted; externally peer reviewed.
Acknowledgements
The authors would like to thank all the health professionals who participated in the study.
Competing interests
The authors declare that no competing interests exist.
Disclosure
The authors declare no conflict of interest.
References
- 1.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382 (18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Director-General’s opening remarks at the media briefing on COVID-19, 11 March 2020. 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. [10 May 2022]. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 accessed.
- 3.World Health Organization WHO coronavirus (COVID-19) dashboard. 2021. https://covid19.who.int. [20 Jan 2022]. https://covid19.who.int
- 4.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020; 323 (15):1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020; 92 (7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020; 80 (4):388.:S0163-4453(20)30099-2. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passali GC, Bentivoglio AR. Comment to the article "Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study". Eur Arch Otorhinolaryngol. 2020; 277 (8):2391–2392. doi: 10.1007/s00405-020-06024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020; 288 (3):335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barillari MR, Bastiani L, Lechien JR, et al. A structural equation model to examine the clinical features of mild-to-moderate COVID-19: a multicenter Italian study. J Med Virol. 2021; 93 (2):983–994. doi: 10.1002/jmv.26354. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki M, Saito K, Min W-P, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007; 117 (2):272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015; 235 (2):277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- 12.Feng C, Wang L, Chen X, et al. A novel artificial intelligence-assisted triage tool to aid in the diagnosis of suspected COVID-19 pneumonia cases in fever clinics. Ann Transl Med. 2021; 9 (3):201. doi: 10.21037/atm-20-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roland LT, Gurrola JG, Loftus PA, et al. Smell and taste symptom-based predictive model for COVID-19 diagnosis. Int Forum Allergy Rhinol. 2020; 10 (7):832–838. doi: 10.1002/alr.22602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan CH, Faraji F, Prajapati DP, et al. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020; 10 (7):806–813. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020; 26 (7):1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menni C, Valdés AM, Freidin M, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26(7):1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JW, Han SC, Jo HD, et al. Regional and chronological variation of chemosensory dysfunction in COVID-19: a meta-analysis. J Korean Med Sci. 2021; 36 (4):e40. doi: 10.3346/jkms.2021.36.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibekwe TS, Fasunla AJ, Orimadegun AE. Systematic review and meta-analysis of smell and taste disorders in COVID-19. OTO Open. 2020; 4 (3):2473974X2095797. doi: 10.1177/2473974X20957975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paderno A, Schreiber A, Grammatica A, et al. Smell and taste alterations in COVID-19: a cross-sectional analysis of different cohorts. Int Forum Allergy Rhinol. 2020; 10 (8):955–962. doi: 10.1002/alr.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagheri SH, Asghari A, Farhadi M, et al. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak in Iran. Med J Islam Repub Iran. 2020;34:62. doi: 10.34171/mjiri.34.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinato G, Fabbris C, Polesel J, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323(20):2089–2090. doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lechien JR, Chiesa-Estomba CM, Hans S, et al. Loss of smell and taste in 2013 European patients with mild to moderate COVID-19. Ann Intern Med. 2020; 173 (8):672–675. doi: 10.7326/M20-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y-C, Bai W-Z, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020; 92 (6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forster P, Forster L, Renfrew C, Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A. 2020; 117 (17):9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020; 395 (10224):565.:S0140-6736(20)30251-8. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579 (7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Y, Li L, Feng Z, et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020; 6 :11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.