Abstract
Axillary lymphadenopathy caused by the high immunogenicity of messenger RNA (mRNA) COVID-19 vaccines presents radiologists with new diagnostic dilemmas in differentiating vaccine-related benign reactive lymphadenopathy from that due to malignant causes. Understanding axillary anatomy and lymphatic drainage is key to radiologic evaluation of the axilla. US plays a critical role in evaluation and classification of axillary lymph nodes on the basis of their cortical and hilar morphology, which allows prediction of metastatic disease. Guidelines for evaluation and management of axillary lymphadenopathy continue to evolve as radiologists gain more experience with axillary lymphadenopathy related to COVID-19 vaccines. General guidelines recommend documenting vaccination dates and laterality and administering all vaccine doses contralateral to the site of primary malignancy whenever applicable. Guidelines also recommend against postponing imaging for urgent clinical indications or for treatment planning in patients with newly diagnosed breast cancer. Although conservative management approaches to axillary lymphadenopathy initially recommended universal short-interval imaging follow-up, updates to those approaches as well as risk-stratified approaches recommend interpreting lymphadenopathy in the context of both vaccination timing and the patient’s overall risk of metastatic disease. Patients with active breast cancer in the pretreatment or peritreatment phase should be evaluated with standard imaging protocols regardless of vaccination status. Tissue sampling and multidisciplinary discussion remain useful in management of complex cases, including increasing lymphadenopathy at follow-up imaging, MRI evaluation of extent of disease, response to neoadjuvant treatment, and potentially confounding cases.
An invited commentary by Weinstein is available online.
©RSNA, 2022
Introduction
In December 2020, approximately 1 year after identification of the first cases of COVID-19, the U.S. Food and Drug Administration (FDA) granted emergency use authorization for the Pfizer-BioNTech and Moderna two-dose messenger RNA (mRNA)–based vaccines, followed by emergency use authorization for Johnson & Johnson’s single-dose viral vector vaccine in February 2021 (1–3). As of February 2022, over 215 million people have been fully vaccinated in the United States, with an additional 93 million having received a booster dose (4).
Axillary symptoms attributed to the mRNA-based vaccines, including axillary swelling, tenderness, and lymphadenopathy, were commonly reported in FDA submission documents. Specifically, axillary swelling or tenderness in the vaccinated arm was the second most frequently reported solicited (specifically queried) local reaction in the Moderna clinical trials, after injection site pain. Up to 16% of Moderna vaccine recipients reported axillary symptoms compared with 4.3% of placebo recipients after the second dose (5). Although an unsolicited (not specifically queried) reaction in the Pfizer-BioNTech clinical trials, axillary symptoms were more common in vaccinated recipients, with 64 vaccine recipients reporting lymphadenopathy after vaccination compared with six placebo recipients (6).
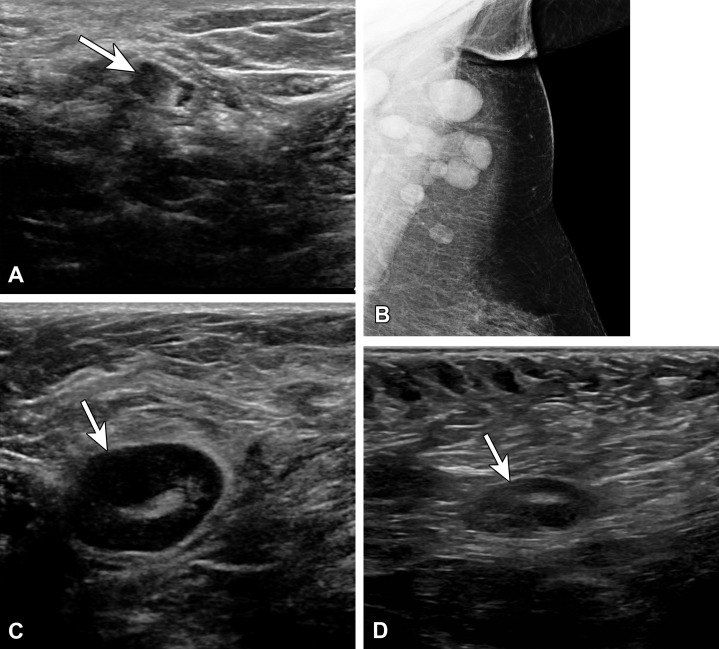
Unilateral axillary lymphadenopathy has multiple causes, but before the mass COVID-19 vaccination effort, vaccine-related axillary lymphadenopathy was a rarely reported finding at breast imaging (7). Vaccine-related lymphadenopathy has been reported for different vaccines, including the bacillus Calmette-Guérin (BCG), human papillomavirus (HPV), and influenza vaccines (Fig 1) (8–10).
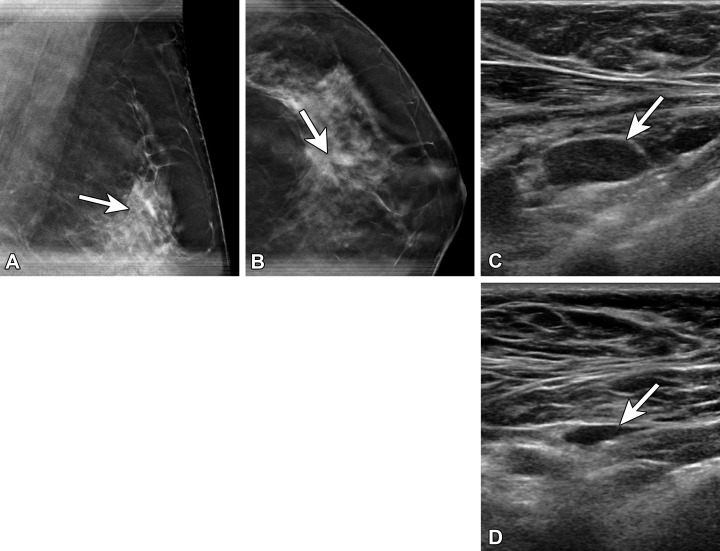
Figure 1.
(A) Vaccine-related lymphadenopathy in a 57-year-old man who presented for evaluation of the left axilla after surgical resection of melanoma along his back and positive axillary sentinel lymph node biopsy (SLNB). US image several weeks after an influenza vaccination shows a type 5 lymph node with asymmetric nodular cortical thickening (arrow). Results of core needle biopsy were benign, with no evidence of melanoma. (B–D) Vaccine-related lymphadenopathy in a 59-year-old woman who presented for evaluation of left axillary swelling after recently receiving a tetanus, diphtheria, and pertussis (Tdap) vaccination. Mammogram (B) shows multiple enlarged lymph nodes superiorly within the axilla. US images (C, D) show a type 5 lymph node with asymmetric nodular cortical thickening (arrow in C), which resolved at 3-month follow-up (arrow in D).
More common causes of unilateral axillary lymphadenopathy include axillary spread of invasive breast cancer and reactive lymphadenopathy. Reactive lymphadenopathy is often seen with infectious conditions (breast or skin abscess) or inflammatory conditions (mastitis and idiopathic granulomatous mastitis). Lymphadenopathy that is considered reactive owing to infectious or inflammatory conditions can be assessed as Breast Imaging Reporting and Data System (BI-RADS) category 2 without additional evaluation.
Unilateral lymphadenopathy without a clear cause can be assessed as BI-RADS 3 with short-interval imaging follow-up or as BI-RADS 4 with tissue sampling recommended, depending on the morphologic appearance of the lymph node and the radiologist’s level of concern after factoring in the clinical history and examination results. Benign silicone axillary lymphadenopathy related to breast augmentation can manifest unilaterally and is associated with characteristic snowstorm shadowing (11).
Causes of bilateral axillary lymphadenopathy include benign and malignant entities. Benign causes include autoimmune diseases (rheumatoid arthritis, systemic lupus erythematosus) (12), granulomatous diseases (tuberculosis, sarcoidosis) (13), and systemic infections (HIV, mononucleosis). Malignant causes include lymphoma, leukemia, and metastatic disease (thyroid, lung, gastrointestinal, pancreas, ovarian) (14). Although this is less common, any of the systemic processes listed can manifest asymmetrically or unilaterally.
The ACR BI-RADS Atlas (fifth edition) (15) offers general guidance on management of axillary lymphadenopathy, advising that “enlarged axillary lymph nodes may warrant comment, clinical correlation, and additional evaluation, especially if they are new or considerably larger or rounder when compared to previous examination.” The BI-RADS atlas concurrently acknowledges that “a review of the patient’s medical history may elucidate the cause for axillary adenopathy, averting recommendation for additional evaluation” (15).
The high immunogenicity of mRNA COVID-19 vaccines presents radiologists with new diagnostic dilemmas in differentiating benign reactive lymphadenopathy due to vaccination from that potentially due to malignant causes, especially in high-risk oncologic patients. Radiologists should be familiar with axillary anatomy and lymphatic drainage patterns, as well as with the spectrum of benign and pathologic lymph node morphologies. Moreover, careful review of patients’ clinical and vaccination history, as well as ancillary imaging findings, will help inform management decisions of reassurance, short-term surveillance, or further workup including biopsy. Awareness of evolving data and practice guidelines regarding vaccine-induced lymphadenopathy, with special consideration of risk-based management algorithms, can reduce unnecessary biopsies in low-risk patients and avoid potential diagnostic delays in oncologic patients.
The purpose of this article is to review essential aspects of evaluation of axillary lymphadenopathy, illustrate clinical scenarios, and discuss management of axillary lymphadenopathy in the setting of COVID-19 vaccinations and oncologic risk factors.
Axillary Anatomy and Lymphatic Drainage of Breast and Upper Extremity
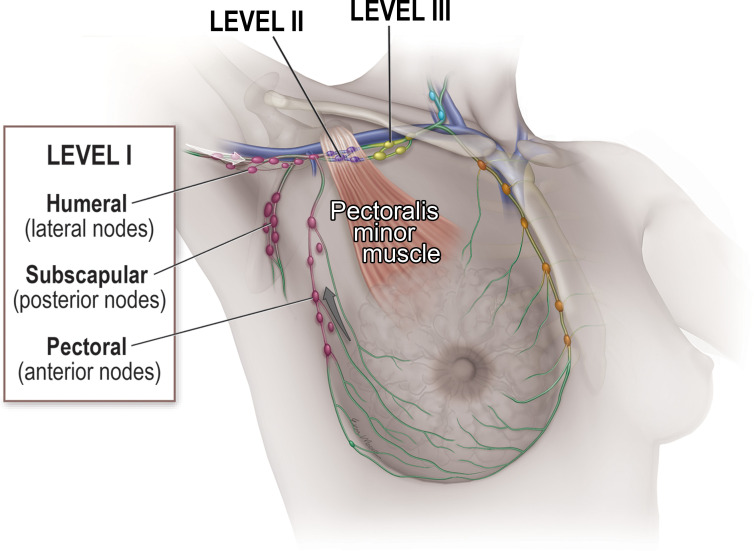
Understanding axillary anatomy and lymphatic drainage is key to radiologic evaluation of the axilla. There are three anatomic levels within the axilla, defined by their relationship to the pectoralis minor muscle. Level I lymph nodes are inferolateral to the pectoralis minor and are divided into three groups: the pectoral (anterior) group (located near the lateral thoracic vessels along the inferior border of the pectoralis minor), the subscapular (posterior) group (located along the inferior border of the subscapularis on the posterior wall of the axilla), and the humeral (lateral) group (located on the lateral wall of the axilla) (16).
Level II lymph nodes are posterior or deep to the pectoralis minor. In addition, interpectoral (Rotter) lymph nodes located between the pectoralis minor and pectoralis major muscles are considered level II nodes. Level III lymph nodes are superomedial to the pectoralis minor (Fig 2).
Figure 2.
Axillary nodal anatomy and lymphatic drainage. The relationship to the pectoralis minor muscle defines the three anatomic levels of lymph nodes within the axilla. Initial lymphatic drainage of the breast (gray arrow) is predominantly to the level I pectoral group, while initial lymphatic drainage of the upper extremity (white arrow) is predominantly to the level I humeral group.
Breast lymphatic drainage is predominantly to the pectoral (anterior) group of level I, while upper extremity lymphatic drainage is predominantly to the humeral (lateral) group of level I. However, primary lymphatic drainage of the breast and of the upper extremity are both typically to the level I axillary lymph nodes before proceeding to the level II nodes, to the level III nodes, and ultimately into the thorax (16).
Cortical Morphologic Features of Axillary Lymph Nodes
Although short-axis measurements are used with other imaging modalities and intrathoracic or intra-abdominal lymph node locations to classify normal and abnormal lymph nodes (17–19), axillary nodal morphology including shape, cortical thickness and uniformity, and presence or absence of a central fatty hilum are considered the most important criteria for distinguishing normal from abnormal axillary lymph nodes (20). Normal and benign nodes appear oval or reniform, with cortical thickness less than or equal to 3 mm and a preserved fatty hilum (20).
US evaluation and classification of axillary lymph nodes based on cortical thickness and appearance of the hilum have been shown to be more accurate predictors of malignancy than the overall size of the lymph node (21–23). Nodal vascularity at color Doppler US also helps distinguish benign from metastatic lymph nodes, with benign nodes demonstrating hilar perfusion and most metastatic nodes demonstrating eccentric or peripheral perfusion (24,25).
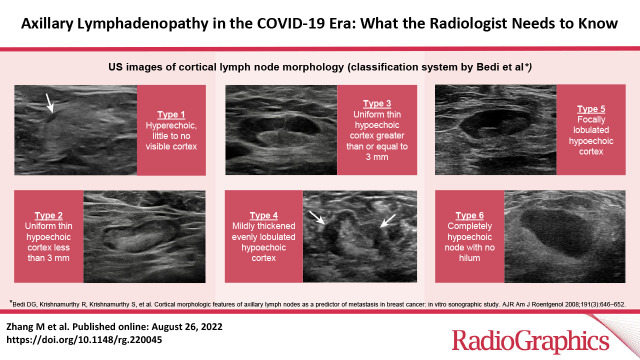
Cortical morphologic features as described by Bedi et al (21) are used in a six-type classification system, as follows: type 1 = hyperechoic, little to no visible cortex; type 2 = uniform thin hypoechoic cortex less than 3 mm; type 3 = uniform hypoechoic cortex greater than or equal to 3 mm; type 4 = mildly thickened evenly lobulated hypoechoic cortex; type 5 = focally lobulated hypoechoic cortex; and type 6 = completely hypoechoic node with no hilum (21,26) (Fig 3). Use of this classification system showed negative predictive values for malignancy for type 1–4 lymph nodes of 89%–100% and positive predictive values for malignancy for type 5 and type 6 lymph nodes of 29% and 58%, respectively. This classification system had 80% overall accuracy (21).
Figure 3.
US images of lymph node morphology classified according to the system of Bedi et al (21). (A) Type 1 has a very thin almost imperceptible cortex (arrow). (B) Type 2 has a cortex of 3 mm or less. (C) Type 3 has a diffusely thickened cortex greater than 3 mm. (D) Type 4 has a lobulated cortex (arrows). (E) Type 5 has eccentric cortical thickening or focal lobulation with displacement of the fatty hilum. (F) Type 6 has a completely effaced fatty hilum.
In a prospective study that compared axillary US evaluations of the ipsilateral vaccinated arm at three distinct time points (before vaccination, the week after the first dose, and the week after the second dose), a statistically significant increase in number of total nodes, maximum diameter, cortical thickness, Bedi classification grade, and Doppler signal was noted in COVID-19 disease-naive patients compared with previously infected patients, indicating a greater lymph node response to the COVID-19 vaccine in patients without previous COVID-19 infection (27). More specifically, this study reported that the most common lymph node morphology in disease-naive patients after vaccination was benign Bedi classification type 3 morphology compared with type 2 morphology in patients with prior COVID-19 infection (27).
Although the Bedi classification system is not widely used in clinical practice and is not included in the fifth edition of the BI-RADS atlas, cortical morphology—on which the Bedi classification is based—is an important aspect of assessing lymph nodes. Therefore, critical evaluation of cortical-hilar morphology combined with the anatomic location of lymph node involvement will aid radiologists in deciding appropriate management of axillary lymphadenopathy. Lymph nodes with type 1–4 morphology located in the humeral (lateral) group are most consistent with reactive lymphadenopathy in the setting of ipsilateral COVID-19 vaccination, whereas lymph nodes with type 5 or type 6 morphology located in the pectoral (anterior) group are concerning for malignancy.
General Considerations regarding COVID-19 Vaccination and Imaging Examinations
As COVID-19 vaccinations continue, vaccine-induced axillary lymphadenopathy should be considered a frequent and expected imaging finding. In a published study of 1217 patients who received a COVID-19 vaccine, subsequent breast imaging revealed axillary lymphadenopathy in 44% of patients with at least one modality (28).
In the months after the rollout of the Pfizer-BioNTech and Moderna vaccines, multiple authors, professional societies, and expert panels released general guidelines for the timing of imaging in patients vaccinated for COVID-19. Imaging persistence of axillary lymphadenopathy from COVID-19 vaccination has been reported in the literature, with one retrospective study of 23 women after recent COVID-19 vaccination reporting a median interval between the first vaccine dose and abnormal imaging results of 9.5 days (range, 2–29 days) (29). Wolfson et al (28) reported persistent lymphadenopathy up to 43 weeks after vaccination, although the authors showed that lymphadenopathy was more likely to be seen within 14 days of vaccination and rarely after 50 days following the second vaccine dose (28).
Of patients who underwent follow-up examinations within 12 weeks, 25% were given BI-RADS category 3 recommendations; no patient in this group was diagnosed with a subsequent malignancy (28). A separate retrospective study of patients presenting with axillary lymphadenopathy after the second dose reported benign results for all biopsies recommended within 12 weeks of vaccination (30).
All guidelines recommend that vaccination date and laterality be documented on intake forms or be readily available to radiologists in the electronic medical record (31–34). Guidelines also recommend that screening mammography be performed without regard to vaccination status or timing of vaccinations (33,35). Given the concern for decreased use of breast cancer screening during the pandemic, every effort should be made to minimize or reduce barriers to screening and to have women return to screening without delay.
At our institution, screening mammography is recommended regardless of recent vaccination. In addition, imaging for urgent clinical indications including acute breast or axillary symptoms or urgent treatment planning for patients with newly diagnosed breast cancer should not be postponed owing to vaccination timing (32–34). Finally, when applicable, both vaccine doses should be administered on the side contralateral to the primary malignancy (32–34).
Axillary Lymphadenopathy at Screening and Surveillance Mammography
Management approaches to axillary lymphadenopathy in patients who have recently received a COVID-19 vaccine are found in Tables 1 and 2. Although an expert opinion initially released by the Society of Breast Imaging (SBI) in January 2021 was “by design a conservative one, which stressed an abundance of caution” and recommended assigning a BI-RADS category 0 for all unilateral axillary adenopathy at screening examinations to allow further assessment and documentation of medical and COVID-19 vaccination history (31), these conservative guidelines have since been updated. Updated guidelines issued by the SBI in February 2022 now recommend that radiologists consider giving a BI-RADS 2 to average-risk women presenting with unilateral axillary lymphadenopathy at screening mammography without suspicious findings in the breast after recent COVID-19 vaccination in the ipsilateral arm (35).
Table 1:
Risk-stratified Management Guidelines Issued between February 2021 and March 2021
Table 2:
Risk-stratified Management Guidelines Issued between August 2021 and February 2022
Risk-stratified approaches interpret lymphadenopathy in the context of both vaccination timing and the patient’s overall risk of metastatic disease. For patients with a COVID-19 vaccination within the preceding 6 weeks who present with isolated ipsilateral axillary lymphadenopathy and no other health concerns, Lehman et al (33) recommend clinical follow-up in lieu of additional imaging. Similarly, risk-based recommendations released by the European Society of Breast Imaging advise clinical management and benign classification (BI-RADS 2) for imaging-detected axillary lymphadenopathy ipsilateral to the injection site in asymptomatic patients without a breast cancer history or suspicious breast imaging findings (34) (Fig 4).
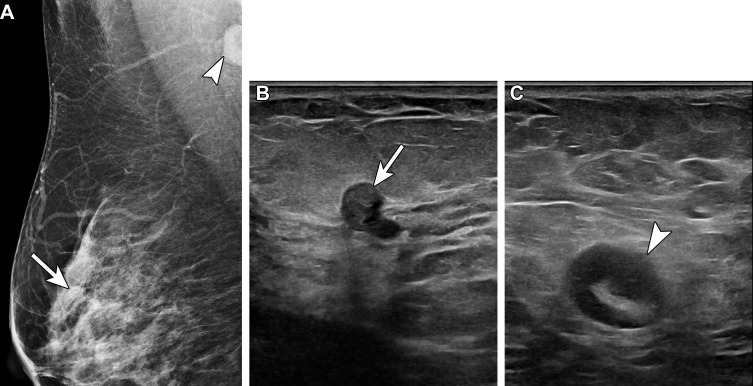
Figure 4.
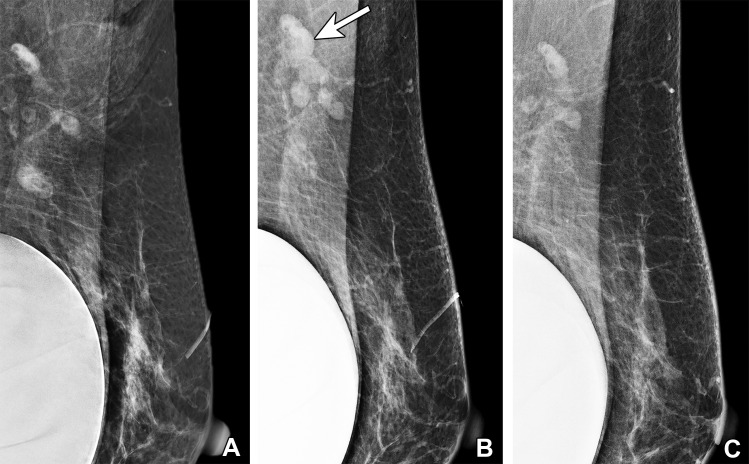
Vaccine-induced axillary lymphadenopathy in a 63-year-old woman with a retropectoral silicone gel implant who underwent screening mammography over 3 different years. (A) Left mediolateral oblique (MLO) mammogram in 2019 shows normal left axillary lymph nodes. (B) Left MLO mammogram in 2021, 2 weeks after she received the first dose of Pfizer-BioNTech SARS-CoV-2 vaccine in the left arm, shows several mildly enlarged superiorly located left axillary lymph nodes (arrow), considered to reflect vaccine-induced lymphadenopathy and assessed as benign (BI-RADS 2). The inferiorly located lymph nodes are unchanged. (C) Left MLO mammogram in 2022 shows normalization of the left axillary lymph nodes.
In our experience, lymphadenopathy superiorly located on the mediolateral oblique (MLO) view, with normal inferiorly located lymph nodes, is often seen with vaccination-induced reactive lymphadenopathy (Fig 4). This risk-based approach allows evaluation of patients at high risk for axillary metastatic disease (eg, breast, head and neck malignancy, upper extremity or trunk melanoma, lymphoma) (32) while decreasing the number of unnecessary imaging examinations and possible biopsies in low-risk patients.
In addition to axillary lymphadenopathy, imaging-detected ipsilateral axillary edema that can also extend into the axillary tail and breast after COVID-19 vaccination has also been reported (36–39). Axillary edema in the setting of recent COVID-19 vaccination without associated lymphadenopathy can be considered benign (BI-RADS 2) (Fig 5), while risk-based stratification should be used for axillary edema with concurrent lymphadenopathy.
Figure 5.
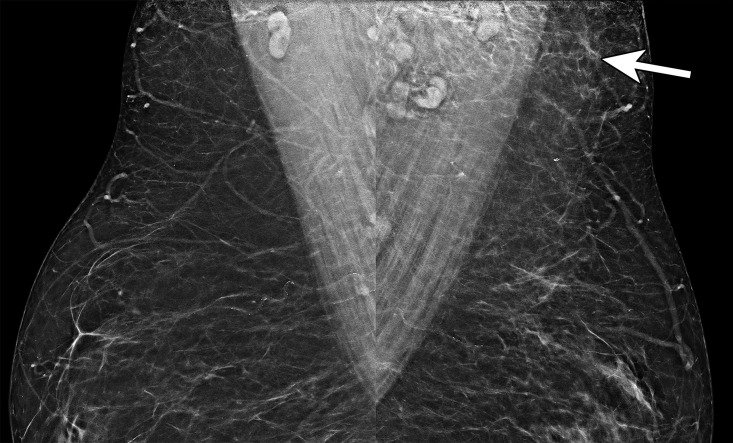
Axillary edema in a 51-year-old woman who underwent screening mammography 3 days after receiving the first dose of the Pfizer-BioNTech SARS-CoV-2 vaccine in the left arm. Bilateral MLO mammograms show asymmetric soft-tissue stranding (arrow) in the left axilla without an associated breast abnormality. This finding is most consistent with axillary edema in the setting of recent vaccination and is benign (BI-RADS 2). Note that the edema is centered around superiorly located lymph nodes, not the inferiorly located lymph nodes.
Patients with suspicious imaging findings in the breast and axillary lymphadenopathy ipsilateral to the vaccination arm should be evaluated with additional diagnostic imaging, including US of the axilla. In the study by Wolfson et al (28), four patients who demonstrated axillary lymphadenopathy and were subsequently diagnosed with metastatic breast cancer all had suspicious concurrent mammographic findings in the ipsilateral breast. For a patient without a history of breast cancer who has axillary lymphadenopathy and an ipsilateral suspicious breast finding, the axilla should be managed on the basis of the level of suspicion for the breast finding (33,34) (Fig 6).
Figure 6.
Atypical lobular hyperplasia and radial scar in a 57-year-old woman recalled from screening for an abnormality in the left breast 6 days after receiving the second dose of the Pfizer-BioNTech SARS-CoV-2 vaccine in the left arm. (A, B) MLO (A) and craniocaudal (B) spot-compression tomosynthesis images show architectural distortion (arrow) at the 1-o’clock position. There was no US correlate for the architectural distortion. (C, D) US images of the axilla show a type 6 lymph node (arrow in C), which decreased in size at 8-week follow-up (arrow in D). Stereotactic biopsy of the distortion yielded atypical ductal hyperplasia, while excision yielded atypical lobular hyperplasia and radial scar.
However, for patients with a history of breast cancer and new axillary lymphadenopathy (ipsilateral or contralateral to the prior breast cancer) who do not have suspicious breast findings, overall nodal metastatic risk based on cancer type, stage, and location as well as the timing of the vaccination must be considered. Patients at low risk for axillary nodal metastases with lymphadenopathy including type 3 and 4 lymph nodes, which can be overwhelmingly attributed to vaccination, should be managed on a case-by-case basis with a cautious strategy (34). For patients at higher risk, short-interval follow-up with axillary US at 12 weeks or lymph node biopsy should be considered (34). High-risk patients, including those with a history of breast cancer, should also be evaluated with diagnostic imaging, including US evaluation of the axilla, with management based on the final BI-RADS assessment category (32–34) (Fig 7).
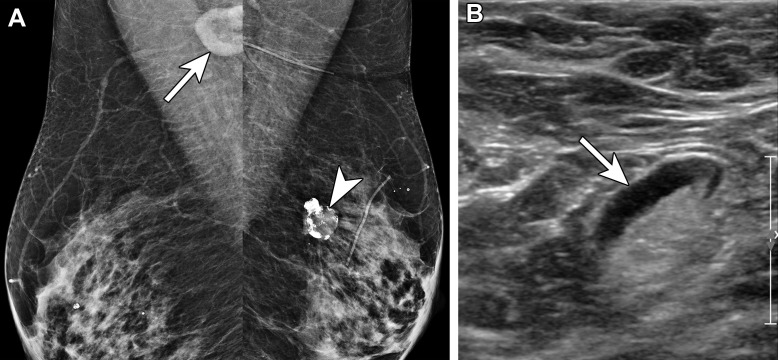
Figure 7.
Sclerosing intraductal papilloma and high-grade metastatic carcinoma in a 48-year-old woman who was recalled from routine screening of the right breast for an asymmetry. She received the second dose of the Pfizer-BioNTech SARS-CoV-2 vaccine in the right arm 1 day earlier. In 2020, she underwent left mastectomy for grade 3 invasive ductal carcinoma. (A) Right MLO mammogram shows an asymmetry (arrow) in the superior right breast, 3 cm from the nipple, and an enlarged right axillary lymph node containing several amorphous calcifications (arrowhead). (B) US image of the right breast shows a complex cystic and solid mass (arrow). Biopsy yielded a sclerosing intraductal papilloma. (C) US image of the right axilla shows a suspicious type 5 lymph node with asymmetric cortical thickening of up to 0.6 cm (arrowhead). Biopsy of the lymph node yielded high-grade metastatic carcinoma, consistent with a breast primary.
Axillary Lymphadenopathy during Diagnostic Evaluation
Patients with active breast cancer in the pretreatment or peritreatment phase should be evaluated with standard imaging protocols regardless of vaccination status. Timely biopsy should be performed when histologic analysis is required for patient management, especially in the setting of type 5 or 6 lymph nodes. However, a lower threshold for timely biopsy of typically less-concerning type 3 or 4 lymph nodes may be required in this population.
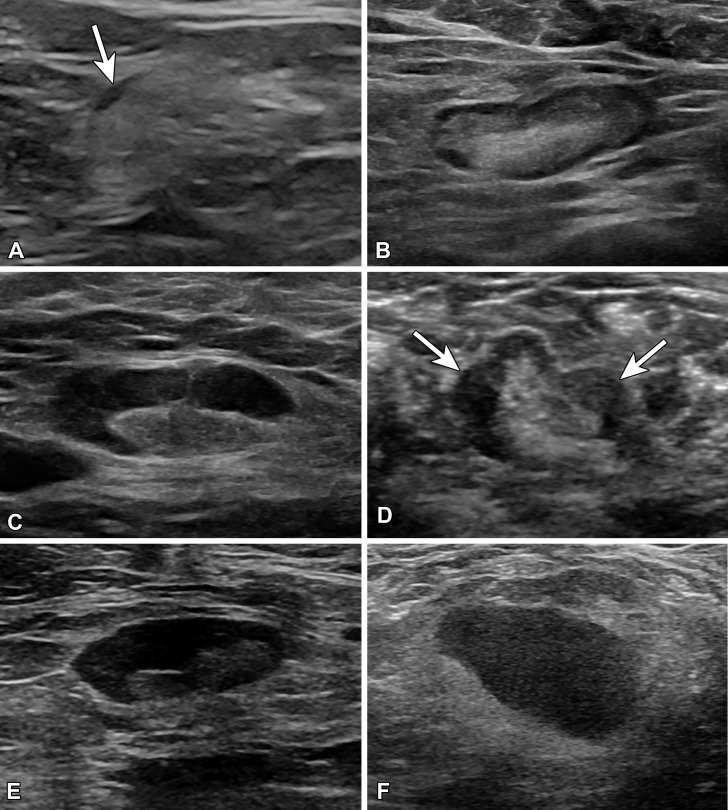
Guidelines for evaluation of painful or palpable lymphadenopathy after recent COVID-19 vaccination are less well-defined. Clinical follow-up alone may be sufficient for certain cases including type 2–4 lymph nodes (Fig 8), while US follow-up may be reserved for persistent clinical or imaging concerns (type 3–5 lymph nodes) (33,40) (Fig 9). In these patients with persistent concerns, axillary US is performed and a final BI-RADS assessment is rendered on the basis of careful consideration of the imaging findings, including cortical-hilar lymph node morphology, clinical presentation, site and timing of vaccinations, and personal risk factors for nodal metastasis (34) (Fig 10).
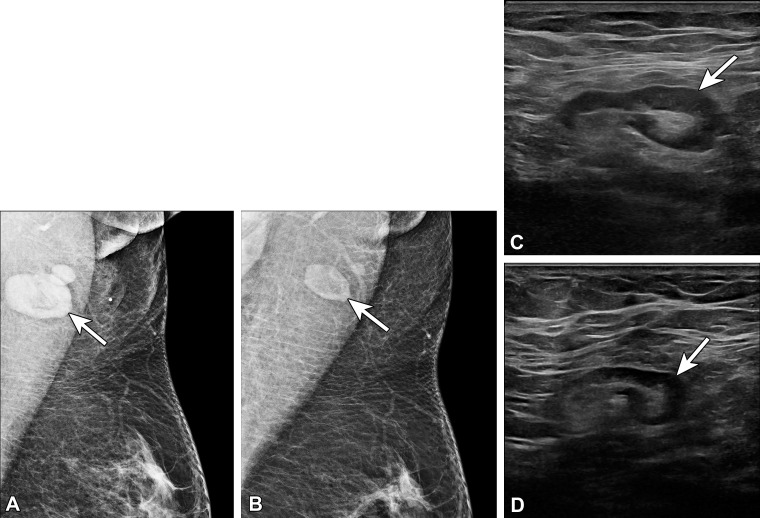
Figure 8.
Type 3 lymph node in a 70-year-old woman with right axillary swelling and pain 1 month after receiving the second dose of the Moderna SARS-CoV-2 vaccine in the right arm. In 2005, she underwent left partial mastectomy and chemoradiation therapy for invasive lobular carcinoma. (A) Bilateral MLO mammograms show lymphadenopathy in the right axilla (arrow) and post–partial mastectomy changes in the superior left breast (arrowhead). (B) US image of the right axilla shows a type 3 lymph node (arrow), with cortical thickness of 3–4 mm. The node was considered reactive in the setting of recent COVID-19 vaccination (BI-RADS 2).
Figure 9.
Type 4 lymph node in a 41-year-old woman with no pertinent medical history who presented with palpable concerns in the left axilla 6 days after receiving the second dose of the Pfizer-BioNTech SARS-CoV-2 vaccine in the left arm. (A, B) MLO mammograms of the left breast show axillary lymphadenopathy (arrow in A), which is new from 1 year earlier (arrow in B). (C, D) US images show a type 4 lymph node (arrow in C), which normalized to a type 2 morphology at 6-week follow-up (arrow in D).
Figure 10.
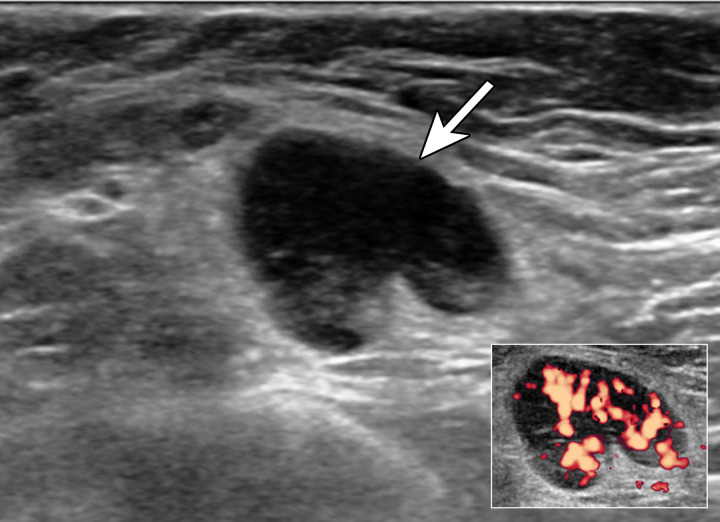
Type 5 lymph node in a 63-year-old BRCA2 mutation carrier with a palpable lump in the left axilla 9 days after receiving the first dose of the Moderna SARS-CoV-2 vaccine in the left arm. In 2006, she underwent left mastectomy for invasive ductal carcinoma. US image shows a type 5 lymph node (arrow), which has associated hyperemia on a power Doppler image (inset) (BI-RADS 4). US-guided core biopsy yielded reactive follicular hyperplasia.
With data showing persistence of axillary lymphadenopathy for up to 43 weeks, current guidelines now support a longer initial follow-up interval of 12 or more weeks for patients assigned a BI-RADS 3 for presumed COVID-19 vaccine–induced lymphadenopathy (28,35). In addition, previous guidelines for biopsy of any persistent unilateral axillary lymphadenopathy seen at short-interval follow-up examinations (31) have now been revised to consider an additional 6-month follow-up, with BI-RADS 3 assessment of unchanged axillary lymphadenopathy after initial presentation and BI-RADS 2 assessment for improving axillary lymphadenopathy (35). Finally, for increasing or enlarging axillary lymphadenopathy, biopsy should be considered (35).
Axillary lymphadenopathy that cannot be attributed to vaccination (eg, bilateral or contralateral) should be managed according to standard diagnostic protocols, which may include short-interval imaging follow-up and, when appropriate, tissue sampling (34,35). Additional cross-sectional chest or body imaging or PET/CT may also be of benefit when there are clinical concerns for systemic processes or malignancy (33).
Lymphadenopathy Identified at Breast MRI
Breast MRI is performed for screening of patients at high lifetime risk of breast cancer, as well as for diagnostic indications such as extent of disease and pathologic nipple discharge. Although there are limited data on the time course of MRI visibility of vaccine-related axillary lymphadenopathy, Wolfson et al (28) showed that lymphadenopathy was rare after 50 days following the second dose but also demonstrated persistent lymphadenopathy for up to 43 weeks. In addition, extrapolation from fluorodeoxyglucose (FDG) PET/CT may offer insight into this clinical question. One retrospective study found persisting axillary nodal FDG uptake 7–10 weeks after the second vaccine dose in 29% of patients (41). In patients undergoing high-risk screening MRI, axillary lymphadenopathy ipsilateral to the site of vaccination without suspicious breast findings may be considered benign (BI-RADS 2) (33).
Unilateral axillary lymphadenopathy at breast MRI may present additional diagnostic challenges, especially in patients with a known cancer. For complex cases such as determining extent of disease (Fig 11) or response to neoadjuvant treatment or for potentially confounding cases, such as axillary lymphadenopathy ipsilateral to the cancer and side of recent vaccination at any imaging modality (Figs 12, 13), radiologists should (a) exercise caution in image interpretation, (b) consider timely tissue sampling (34), and (c) consider multidisciplinary discussion (32) as clinically appropriate. Specifically for these patients, tissue sampling may be required for typically less-concerning type 3–4 axillary lymph nodes out of an abundance of caution.
Figure 11.
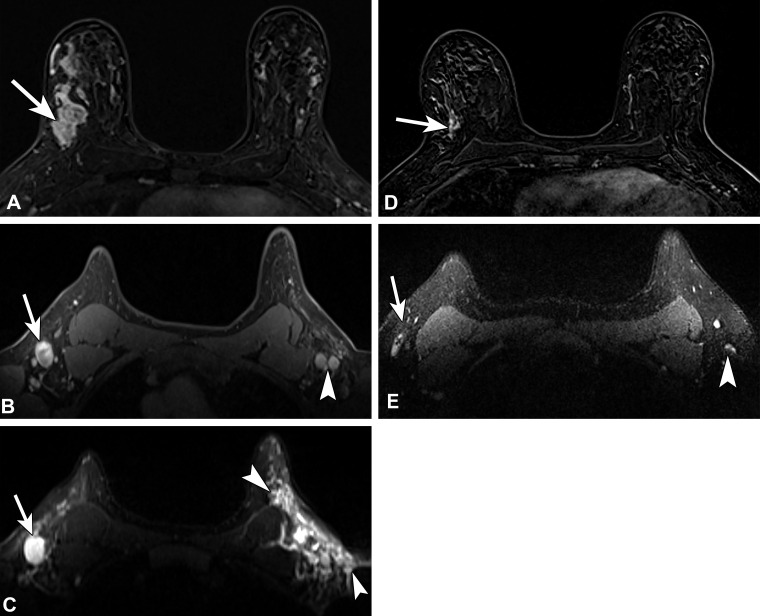
Recently diagnosed invasive ductal carcinoma of the right breast with right axillary nodal metastasis in a 48-year-old woman who presented for MRI for extent of disease evaluation. Three days earlier, she received the first dose of the Pfizer-BioNTech SARS-CoV-2 vaccine in the left arm. (A) Axial contrast-enhanced subtraction MR image shows the known malignancy (arrow) in the right breast. (B, C) Pretreatment axial MR images through the axilla show the known right axillary nodal metastasis (arrow) and left axillary lymphadenopathy (arrowhead in B). On a T2-weighted image (C), there is substantial edema (arrowheads) throughout the left axilla and axillary tail region. US-guided biopsy of an enlarged left axillary lymph node yielded reactive changes. (D) Three-month follow-up axial MR image after neoadjuvant chemotherapy shows decreased extent of disease (arrow). (E) Posttreatment axial MR image through the axilla shows decreased right axillary lymphadenopathy (arrow) and normalized left axillary lymph nodes (arrowhead), as well as resolution of the axillary edema.
Figure 12.
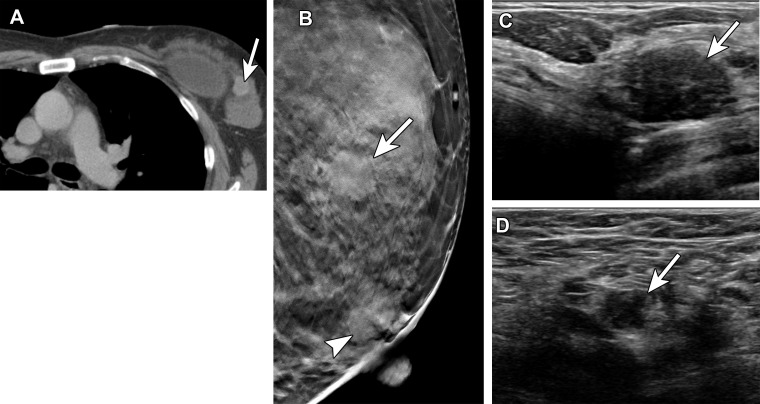
Metastatic carcinoma in a 53-year-old woman with a suspicious mass in the left breast at staging CT, performed after spinal biopsy yielded pathologic findings suggestive of a breast primary. She received the first dose of the Pfizer-BioNTech SARS-CoV-2 vaccine in the left arm 2 days before diagnostic breast imaging. (A) Axial image from staging CT shows the suspicious mass (arrow) in the upper outer left breast. (B) MLO tomosynthesis image shows the corresponding irregular mass (arrow) in the superior left breast. An oval mass (arrowhead) in the subareolar left breast was stable when compared with prior imaging (not shown). (C) US image shows a 1.7-cm mass with irregular margins (arrow) in the left breast at the 2-o’clock position. US-guided core needle biopsy yielded invasive ductal carcinoma. (D) US image of the left axilla shows a corresponding type 6 lymph node (arrow) (BI-RADS 5). Fine-needle aspiration yielded single and clusters of atypical epithelial cells, indicative of metastatic carcinoma.
Figure 13.
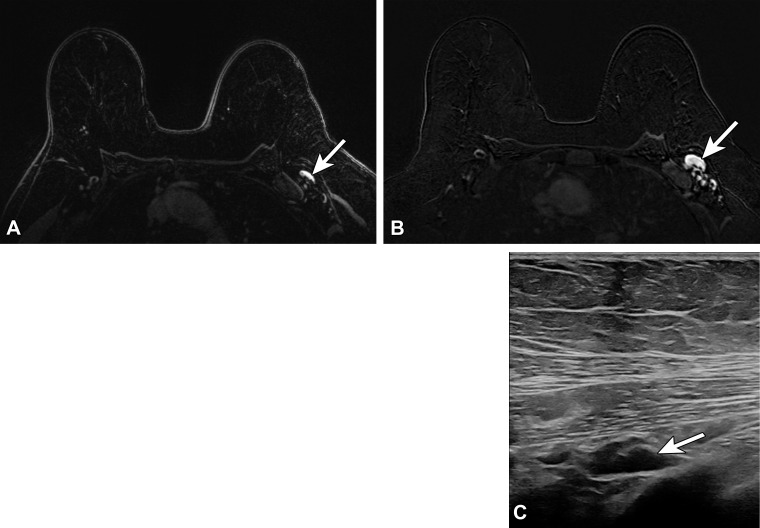
Type 5 lymph node in a 62-year-old woman with human epidermal growth factor receptor 2 (HER2)–positive invasive ductal carcinoma of the left breast, who presented for MRI evaluation of response to neoadjuvant chemotherapy. Three weeks earlier, she received the third (booster) dose of the Moderna SARS-CoV-2 vaccine in the left arm. (A) Pretreatment axial MR image shows a normal-appearing level 1 lymph node (arrow). (B) Posttreatment axial MR image shows enlargement of the lymph node (arrow). (C) Same-day US image of the left axilla shows a type 5 lymph node (arrow) (BI-RADS 4). US-guided core biopsy yielded lymphoid tissue with lymphocytes and histiocytes, without evidence of metastatic carcinoma.
Lymphadenopathy Identified with Other Imaging Modalities
As COVID-19 mRNA vaccination continues, radiologists are increasingly encountering normal or enlarged FDG-avid axillary, supraclavicular, and cervical lymph nodes, potentially confounding interpretation of staging PET/CT studies in oncologic patients. One retrospective cohort study of 650 patients reported the presence of hypermetabolic axillary lymph nodes in 14.5% of recently vaccinated patients after dose 1 and 43.3% of recently vaccinated patients after dose 2 (42). A separate study of 951 patients found that while most cases of hypermetabolic axillary lymphadenopathy at PET/CT could be categorized as malignant or vaccine associated, 14.8% were deemed equivocal, even after consideration of the clinical context, detailed oncologic history, and presence or absence of other abnormal imaging findings (43).
Despite these challenges, general guidelines recommend against unduly delaying COVID-19 vaccination, suggesting postponing PET/CT examinations for nonurgent indications only, such as routine surveillance for low-risk malignancies. In these instances, imaging is ideally performed at least 4–6 weeks after vaccination to decrease confounding findings (44).
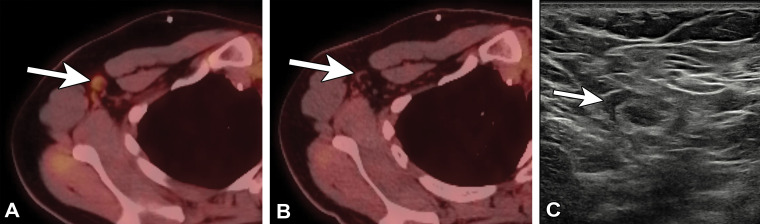
One risk-based institutional management approach recommends no further imaging follow-up for FDG-avid nodes that are unlikely to represent disease or are clinically irrelevant. For clinically relevant morphologically normal FDG-avid nodes, repeat PET/CT in 2–6 weeks or US-guided lymph node sampling is advised. For clinically relevant morphologically abnormal FDG-avid nodes, US or CT follow-up in 2–6 weeks is recommended, followed by biopsy for persistent lymphadenopathy (44) (Fig 14). While no specific guidelines are available regarding other functional imaging modalities, these general management strategies may be reasonably applied to other imaging studies performed to detect metabolic abnormalities, such as SPECT/CT (Fig 15).
Figure 14.
Type 2 axillary lymph node in a 32-year-old woman who underwent neoadjuvant chemotherapy and left mastectomy for invasive ductal carcinoma in 2019 and who presented for restaging with PET/CT. Seven days earlier, she received the first dose of the Pfizer BioNTech SARS-CoV-2 vaccine in the right arm. (A, B) Axial PET/CT images show mild to moderate FDG activity associated with right axillary nodes (arrow in A), new from 4 months earlier (arrow in B). (C) US image of the right axilla 2 months later shows a type 2 axillary lymph node (arrow) (BI-RADS 2).
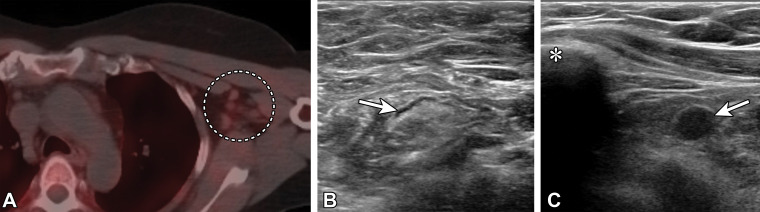
Figure 15.
Type 1 axillary lymph node and type 6 infraclavicular lymph node in a 46-year-old woman who underwent bilateral mastectomy in 2014 for invasive ductal carcinoma in the left breast and who presented for SPECT/CT evaluation of a suspected right parathyroid adenoma. Fourteen days earlier, she received the second dose of the Moderna SARS-CoV-2 vaccine in the left arm. (A) Axial image from technetium 99m–sestamibi SPECT/CT shows mild left axillary lymphadenopathy with mild sestamibi uptake (dashed circle). (B, C) US images from short-term follow-up 5 weeks later show a normalized type 1 axillary lymph node (arrow in B) (BI-RADS 2) and a type 6 left infraclavicular lymph node (arrow in C) (BI-RADS 4A). * in C = clavicle. Fine-needle aspiration of the infraclavicular lymph node yielded benign lymphocytes and macrophages.
Occasionally, axillary lymphadenopathy may be incidentally detected at other CT examinations performed for nonstaging indications, such as in the setting of trauma. For these clinical scenarios, radiologists should apply clinical judgment in deciding to recommend further imaging evaluation or follow-up and—when applicable—biopsy for abnormal lymph nodes or increasing axillary lymphadenopathy seen at short-interval follow-up (Fig 16).
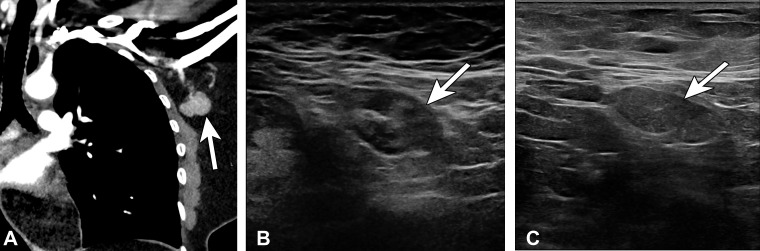
Figure 16.
Type 3 lymph node in a 45-year-old woman with no pertinent medical history in whom left axillary lymphadenopathy was incidentally noted at chest CT. One day earlier, she received the second dose of the Pfizer-BioNTech SARS-CoV-2 vaccine in the left arm. (A) Coronal image from chest CT shows an enlarged abnormal-appearing lymph node (arrow). (B) US image of the axilla 2 days later shows a type 3 lymph node (arrow) (BI-RADS 3). Similar morphology was observed at 6-week follow-up (not shown). (C) US image from 12-week follow-up shows a persistent type 3 lymph node (arrow), which was biopsied at the patient’s request and yielded benign lymphoid tissue.
Conclusion
Management of axillary lymphadenopathy in the COVID-19 era is complex. We discuss a risk-stratified approach that encourages COVID-19 vaccination without any delay in the timing of breast cancer screening with mammography or breast MRI. US evaluation of lymph node morphology and location in combination with the clinical presentation, presence or absence of concomitant breast abnormalities, and overall likelihood of metastatic axillary disease allows prompt biopsy in patients at high risk for metastatic disease, while also reducing the number of unnecessary biopsies of transient benign lymphadenopathy from COVID-19 vaccination. With ongoing implementation of booster vaccines through the course of the evolving COVID-19 pandemic, radiologists will continue to play a pivotal role in implementing and updating guidelines for management of axillary lymphadenopathy.
Acknowledgments
Acknowledgment
We thank Erin E. Moore, MA, for creating Figure 2.
Recipient of a Cum Laude award for an education exhibit at the 2021 RSNA Annual Meeting.
R.W.A. has provided disclosures (see end of article); all other authors have disclosed no relevant relationships.
Disclosures of conflicts of interest.—: R.W.A. Owns common stock in Pfizer.
Abbreviations:
- BI-RADS
- Breast Imaging Reporting and Data System
- FDG
- fluorodeoxyglucose
- MLO
- mediolateral oblique
- mRNA
- messenger RNA
References
- 1. U.S. Food and Drug Administration . Coronavirus (COVID-19) update: December 14, 2020 . https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-december-14-2020. Published December 14, 2020 Accessed March 5, 2022 .
- 2. U.S. Food and Drug Administration . FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine . https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid. Published December 18, 2020. Accessed March 5, 2022 .
- 3. U.S. Food and Drug Administration . FDA issues emergency use authorization for third COVID-19 vaccine . https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine. Published February 27, 2021. Accessed March 5, 2022 .
- 4. U.S. Centers for Disease Control and Prevention . COVID-19 vaccinations in the United States . https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total. Published February 28, 2022. Accessed February 28, 2022 .
- 5. U.S. Centers for Disease Control and Prevention . The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events . https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html. Published August 9, 2021. Accessed January 3, 2022 .
- 6. Centers for Disease Control and Prevention . Pfizer-BioNTech COVID-19 vaccine reactions & adverse events . https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html. Published November 5, 2021. Accessed January 3, 2022 .
- 7. Lee CH , Giurescu ME , Philpotts LE , Horvath LJ , Tocino I . Clinical importance of unilaterally enlarging lymph nodes on otherwise normal mammograms . Radiology 1997. ; 203 ( 2 ): 329 – 334 . [DOI] [PubMed] [Google Scholar]
- 8. Newfield L , Naschitz JE , Yeshurun D . BCG-induced axillary lymphadenitis in the adult [in Hebrew] . Harefuah 1990. ; 119 ( 7-8 ): 199 – 200 . [PubMed] [Google Scholar]
- 9. Shirone N , Shinkai T , Yamane T , et al . Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination . Ann Nucl Med 2012. ; 26 ( 3 ): 248 – 252 . [DOI] [PubMed] [Google Scholar]
- 10. Studdiford J , Lamb K , Horvath K , Altshuler M , Stonehouse A . Development of unilateral cervical and supraclavicular lymphadenopathy after human papilloma virus vaccination . Pharmacotherapy 2008. ; 28 ( 9 ): 1194 – 1197 . [DOI] [PubMed] [Google Scholar]
- 11. Bauer P , Krajicek B , Daniels C , Shah S , Ryu J . Silicone breast implant-induced lymphadenopathy: 18 cases . Respir Med CME 2011. ; 4 ( 3 ): 126 – 130 . [Google Scholar]
- 12. Calgüneri M , Oztürk MA , Ozbalkan Z , et al . Frequency of lymphadenopathy in rheumatoid arthritis and systemic lupus erythematosus . J Int Med Res 2003. ; 31 ( 4 ): 345 – 349 . [DOI] [PubMed] [Google Scholar]
- 13. Petousi N , Mathew J , Thomas EC . A patient presenting with generalised lymphadenopathy: sarcoidosis, lymphoma or tuberculosis? BMJ Case Rep 2012. ; 2012 : bcr1120115150 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeLair DF , Corben AD , Catalano JP , Vallejo CE , Brogi E , Tan LK . Non-mammary metastases to the breast and axilla: a study of 85 cases . Mod Pathol 2013. ; 26 ( 3 ): 343 – 349 . [DOI] [PubMed] [Google Scholar]
- 15. D’Orsi C , Sickles E , Mendelson E , et al . ACR BI-RADS Atlas Breast Imaging Reporting and Data System . 5th ed . Reston, Va: : American College of Radiology; , 2013. . [Google Scholar]
- 16. Ecanow JS , Abe H , Newstead GM , Ecanow DB , Jeske JM . Axillary staging of breast cancer: what the radiologist should know . RadioGraphics 2013. ; 33 ( 6 ): 1589 – 1612 . [DOI] [PubMed] [Google Scholar]
- 17. Genereux GP , Howie JL . Normal mediastinal lymph node size and number: CT and anatomic study . AJR Am J Roentgenol 1984. ; 142 ( 6 ): 1095 – 1100 . [DOI] [PubMed] [Google Scholar]
- 18. Glazer GM , Gross BH , Quint LE , Francis IR , Bookstein FL , Orringer MB . Normal mediastinal lymph nodes: number and size according to American Thoracic Society mapping . AJR Am J Roentgenol 1985. ; 144 ( 2 ): 261 – 265 . [DOI] [PubMed] [Google Scholar]
- 19. Dorfman RE , Alpern MB , Gross BH , Sandler MA . Upper abdominal lymph nodes: criteria for normal size determined with CT . Radiology 1991. ; 180 ( 2 ): 319 – 322 . [DOI] [PubMed] [Google Scholar]
- 20. Net JM , Mirpuri TM , Plaza MJ , et al . Resident and fellow education feature: US evaluation of axillary lymph nodes . RadioGraphics 2014. ; 34 ( 7 ): 1817 – 1818 . [DOI] [PubMed] [Google Scholar]
- 21. Bedi DG , Krishnamurthy R , Krishnamurthy S , et al . Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: in vitro sonographic study . AJR Am J Roentgenol 2008. ; 191 ( 3 ): 646 – 652 . [DOI] [PubMed] [Google Scholar]
- 22. Cho N , Moon WK , Han W , Park IA , Cho J , Noh DY . Preoperative sonographic classification of axillary lymph nodes in patients with breast cancer: node-to-node correlation with surgical histology and sentinel node biopsy results . AJR Am J Roentgenol 2009. ; 193 ( 6 ): 1731 – 1737 . [DOI] [PubMed] [Google Scholar]
- 23. Mainiero MB , Cinelli CM , Koelliker SL , Graves TA , Chung MA . Axillary ultrasound and fine-needle aspiration in the preoperative evaluation of the breast cancer patient: an algorithm based on tumor size and lymph node appearance . AJR Am J Roentgenol 2010. ; 195 ( 5 ): 1261 – 1267 . [DOI] [PubMed] [Google Scholar]
- 24. Steinkamp HJ , Mueffelmann M , Böck JC , Thiel T , Kenzel P , Felix R . Differential diagnosis of lymph node lesions: a semiquantitative approach with colour Doppler ultrasound . Br J Radiol 1998. ; 71 ( 848 ): 828 – 833 . [DOI] [PubMed] [Google Scholar]
- 25. Na DG , Lim HK , Byun HS , Kim HD , Ko YH , Baek JH . Differential diagnosis of cervical lymphadenopathy: usefulness of color Doppler sonography . AJR Am J Roentgenol 1997. ; 168 ( 5 ): 1311 – 1316 . [DOI] [PubMed] [Google Scholar]
- 26. Chung HL , Le-Petross HT , Leung JWT . Imaging Updates to Breast Cancer Lymph Node Management . RadioGraphics 2021. ; 41 ( 5 ): 1283 – 1299 . [DOI] [PubMed] [Google Scholar]
- 27. Igual-Rouilleault AC , Soriano I , Quan PL , Fernández-Montero A , Elizalde A , Pina L . Unilateral axillary adenopathy induced by COVID-19 vaccine: US follow-up evaluation . Eur Radiol 2022. ; 32 ( 5 ): 3199 – 3206 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wolfson S , Kim E , Plaunova A , et al . Axillary Adenopathy after COVID-19 Vaccine: No Reason to Delay Screening Mammogram . Radiology 2022. ; 303 ( 2 ): 297 – 299 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mortazavi S . COVID-19 Vaccination-Associated Axillary Adenopathy: Imaging Findings and Follow-Up Recommendations in 23 Women . AJR Am J Roentgenol 2021. ; 217 ( 4 ): 857 – 858 . [DOI] [PubMed] [Google Scholar]
- 30. Nguyen DL , Ambinder EB , Myers KS , Mullen LA , Panigrahi B , Oluyemi E . COVID-19 Vaccine-Related Axillary Adenopathy on Breast Imaging: Follow-Up Recommendations and Histopathologic Findings . AJR Am J Roentgenol 2022. ; 218 ( 6 ): 997 – 998 . [DOI] [PubMed] [Google Scholar]
- 31. Grimm L , Destounis S , Dogan B , et al . SBI recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination . https://www.sbi-online.org/Portals/0/Position%20Statements/2021/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination.pdf. Published January 22, 2021. Accessed January 4, 2022 . [Google Scholar]
- 32. Becker AS , Perez-Johnston R , Chikarmane SA , et al . Multidisciplinary Recommendations Regarding Post-Vaccine Adenopathy and Radiologic Imaging: Radiology Scientific Expert Panel . Radiology 2021. ; 300 ( 2 ): E323 – E327 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lehman CD , D’Alessandro HA , Mendoza DP , Succi MD , Kambadakone A , Lamb LR . Unilateral Lymphadenopathy After COVID-19 Vaccination: A Practical Management Plan for Radiologists Across Specialties . J Am Coll Radiol 2021. ; 18 ( 6 ): 843 – 852 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schiaffino S , Pinker K , Magni V , et al . Axillary lymphadenopathy at the time of COVID-19 vaccination: ten recommendations from the European Society of Breast Imaging (EUSOBI) . Insights Imaging 2021. ; 12 ( 1 ): 119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grimm L , Srini A , Dontchos B , et al . Revised SBI recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination . https://www.sbi-online.org/Portals/0/Position%20Statements/2022/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination_updatedFeb2022.pdf. Published February 28, 2022. Accessed March 10, 2022 . [Google Scholar]
- 36. Woodard S , Zamora K . Axillary edema one day after COVID-19 vaccination . J Breast Imaging 2021. ; 3 ( 4 ): 522 – 523 . [Google Scholar]
- 37. Freitas V , Ghai S , Au F , Kulkarni S , Ruff HM , Bukhanov K . COVID-19 vaccine-related axillary edema in breast imaging setting . Radiol Case Rep 2022. ; 17 ( 3 ): 775 – 778 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Locklin JN , Woodard GA . Mammographic and sonographic findings in the breast and axillary tail following a COVID-19 vaccine . Clin Imaging 2021. ; 80 : 202 – 204 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim E , Reig B . Breast Inflammatory Change Is Transient Following COVID-19 Vaccination . Radiology 2022. . 10.1148/radiol.220321. Published online March 15, 2022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tu W , Gierada DS , Joe BN . COVID-19 Vaccination-Related Lymphadenopathy: What To Be Aware Of . Radiol Imaging Cancer 2021. ; 3 ( 3 ): e210038 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eshet Y , Tau N , Alhoubani Y , Kanana N , Domachevsky L , Eifer M . Prevalence of Increased FDG PET/CT Axillary Lymph Node Uptake Beyond 6 Weeks after mRNA COVID-19 Vaccination . Radiology 2021. ; 300 ( 3 ): E345 – E347 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bernstine H , Priss M , Anati T , et al . Axillary Lymph Nodes Hypermetabolism After BNT162b2 mRNA COVID-19 Vaccination in Cancer Patients Undergoing 18F-FDG PET/CT: A Cohort Study . Clin Nucl Med 2021. ; 46 ( 5 ): 396 – 401 . [DOI] [PubMed] [Google Scholar]
- 43. Cohen D , Krauthammer SH , Wolf I , Even-Sapir E . Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by [18F]FDG PET-CT and relevance to study interpretation . Eur J Nucl Med Mol Imaging 2021. ; 48 ( 6 ): 1854 – 1863 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McIntosh LJ , Bankier AA , Vijayaraghavan GR , Licho R , Rosen MP . COVID-19 Vaccination-Related Uptake on FDG PET/CT: An Emerging Dilemma and Suggestions for Management . AJR Am J Roentgenol 2021. ; 217 ( 4 ): 975 – 983 . [DOI] [PubMed] [Google Scholar]