Abstract
In the modern world, myocardial infarction is one of the most common cardiovascular diseases, which are responsible for around 18 million deaths every year or almost 32% of all deaths. Due to the detrimental effects of COVID-19 on the cardiovascular system, this rate is expected to increase in the coming years. Although there has been some progress in myocardial infarction treatment, translating pre-clinical findings to the clinic remains a major challenge. One reason for this is the lack of reliable and human representative healthy and fibrotic cardiac tissue models that can be used to understand the fundamentals of ischemic/reperfusion injury caused by myocardial infarction and to test new drugs and therapeutic strategies. In this review, we first present an overview of the anatomy of the heart and the pathophysiology of myocardial infarction, and then discuss the recent developments on pre-clinical infarct models, focusing mainly on the engineered three-dimensional cardiac ischemic/reperfusion injury and fibrosis models developed using different engineering methods such as organoids, microfluidic devices, and bioprinted constructs. We also present the benefits and limitations of emerging and promising regenerative therapy treatments for myocardial infarction such as cell therapies, extracellular vesicles, and cardiac patches. This review aims to overview recent advances in three-dimensional engineered infarct models and current regenerative therapeutic options, which can be used as a guide for developing new models and treatment strategies.
I. INTRODUCTION
Cardiovascular diseases (CVD) have been the leading cause of death in the developed world for over 100 years, responsible for more deaths than all forms of cancer combined.1 In the United States alone, CVDs are responsible for over 650 000 deaths yearly.2 In addition to the burden on human health, CVDs cost the United States more than $350 billion every year, causing a massive economic burden.2 Moreover, with the onset of the COVID-19 pandemic, cardiovascular complications arising from COVID-19 have added an extra burden to the healthcare systems.3 Furthermore, with the average age of the population steadily increasing, the risk and costs of CVD related diseases have risen dramatically over the last decades, and will continue to rise in the future.4
Of the deaths caused by CVDs, myocardial infarction (MI) is responsible for over 50%.1,2 The main mechanism of damage during MI is ischemic/reperfusion injury (I/R).5 During MI, ischemia, or the lack of oxygen to the affected myocardium, leads to necrosis and cell death. This damage is further exacerbated following reperfusion of the damaged tissue, as the sudden influx of oxygenated blood leads to the development of reactive oxygen species (ROS) causing oxidative stress and additional cell death.6–8 The limited healing capacity of cardiomyocytes (CMs) and the formation of fibrotic scar tissue produced by the activated cardiac fibroblasts (CFs) to repair damaged tissue leads to the loss of heart function and eventually heart failure. Accordingly, there is a significant amount of ongoing research across various disciplines to prevent and treat MI.9–11 Although many approaches have been used to treat MI, limited success has been achieved due to the limited self-repair ability and regenerative capacity of native heart cells.12
Currently, heart transplantation is the only long-term therapeutic option for patients once end stage heart failure has been reached.1,2,9,10 Unfortunately, the current demand for donor hearts significantly exceeds the amount available, leading to the death of many patients before this therapeutic option becomes available.13,14 Additionally, many potential hearts are deemed unusable for transplant, with the major reasons including age and geographical distance from donor to recipient.15 Therefore, it is not ethical or possible to experiment on a great number of these donor organs as they are vital for transplant patients.
With the inability to use human hearts at a rate required for experimental research, the majority of our current knowledge on how the constituents of the cardiovascular system (CVS) function has been obtained through the use of animal models.5 Similarly, much of our understanding of heart disease comes from carefully manipulated animal models that possess a desired phenotype, which is not always attained in a physiologically realistic manner. Even though these experiments include carefully selected control groups, it is impossible to consider all potential variables, which may lead to contradictory outcomes.16–19 Furthermore, due to the differences in human and animal physiology and pathology, there are often drastic differences between animal studies and human trials leading to poor success in clinical translation.20
Over the past few decades, tissue engineering has proven itself as a useful in vitro option for the study of heart disease under controlled conditions using human cells.21–25 Such systems allow for the study of cellular responses to various stressors without the disadvantages of traditional in vitro, ex vivo, and in vivo models.26 Furthermore, these engineered models facilitate direct testing on human tissue-like structures, which are invaluable for discovering preventive approaches and treatments while avoiding possible discordances seen when using non-human models. Additionally, tissue engineered models allow for the high-throughput investigation of physiological and pathological phenomena on local levels, while systemic influences seen in in vivo models could be antagonistic.17,18,27–30 Additionally, the use of model tissues allows for selective inclusion, exclusion, or direct manipulation of individual cell types comprising the tissue.
Many current in vitro tissue models utilize biological scaffolds, hydrogels, or decellularized matrices to provide a 3D environment for cardiovascular cells that mimic their native environment.17,24,25,27,29–36 The application of these novel engineering methods has led to the creation of heart tissue models that have enabled an understanding of MI that was not previously possible with the traditional methods.17,18 Specifically, many of these models have been utilized to study the diagnosis, pathogenesis, and recovery before, during, and after I/R, as well as potential novel therapeutics for this pathology.17,25,27,28,37
Current therapeutics to treat the MI-induced damaged cardiac tissue include cellular therapies,38–40 biomaterial-based therapies41–43 or cell incorporated biomaterials, and direct reprogramming of fibroblasts into CMs.44 Although cellular therapies have shown some success, recently the focus has been shifted to the paracrine signaling in the infarct tissue and therapies using cell secreted factors such as cytokines, growth factors, and microRNAs (miRNAs) packaged in extracellular vesicles (EVs) and exosomes.45,46 Integration of exosomes with biological scaffolds such as cardiac patches has proven effective in preclinical trials and is under investigation in multiple ongoing clinical trials.47
In this review, we compile studies that engineered cardiac tissue models as platforms to study MI and fibrotic tissue models to investigate the mechanisms of MI and review the available regenerative therapeutic options for treating the infarct tissue. We briefly discuss the current understanding of MI pathogenesis as well as the subsequent endogenous response, both with and without clinical intervention. We then summarize the current limitations of in vivo and ex vivo models to better understand the need for tissue engineered models. Next, we review numerous 3D cardiac tissue models to study MI, as well as the infarct tissue models, based on fabrication method and discuss how they provide new insights into MI compared with current pre-clinical models. Then, we look at the current state of regenerative therapies applied for healing the infarct tissue. Finally, we discuss the current limitations of these tissue engineered models and therapies and how they can be addressed to further advance the field and subsequently patient care.
II. PHYSIOLOGICAL CHANGES IN THE HEART DURING AND AFTER MI
The main role of the heart is to provide blood to the entire body, including itself, via coronary arteries.6 Through both genetic background and lifestyle choices, excess plaque can build up in these arteries leading to atherosclerosis.48 This can in turn lead to complications in blood delivery to the myocardium.6 If an extensive blockage occurs, oxygenated blood can no longer be provided to the tissue, leading to MI and I/R.
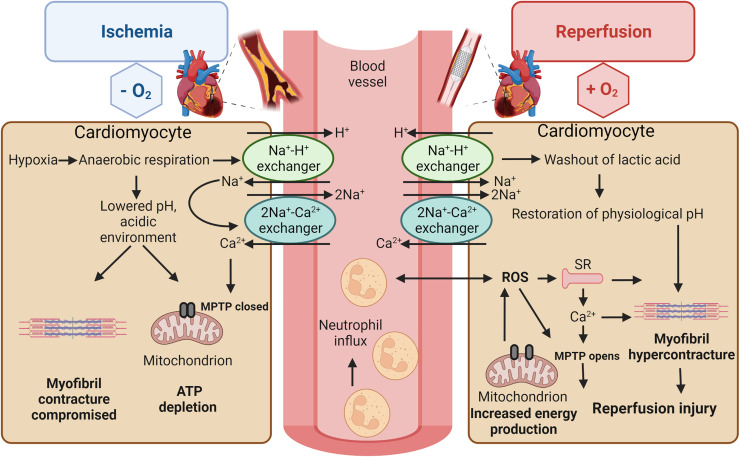
MI is the process of heart tissue necrosis from either a complete or a partial coronary blockage of downstream CMs. Without oxygen, CMs halt oxidative phosphorylation which in turn leads to adenosine triphosphate (ATP) depletion, the breakdown of mitochondrial membrane depolarization, and compromised contraction.5 Cellular metabolism switches to glycolysis, creating an acidic (low pH) environment.6 With the decreased pH, the Na+–H+ ion exchanger is activated causing an increase in intracellular Na+. However, due to a lack of ATP during ischemia, the cell is unable to remove excess sodium through the Na+–K+ pump and is thus forced to run the Na+–Ca2+ ion exchanger in reverse causing a cellular overload of Ca2+.6 If this cascade is allowed to continue unhindered for longer than 20 min, a wave of cell death begins at the infarct site traveling out.5,6
In order to prevent MI complications such as heart failure or patient death, the obstructed coronaries must be opened via either thrombolytic therapy or percutaneous coronary intervention (PCI).49 However, the reperfusion of ischemic myocardium can itself induce CM death, typically increasing the final infarct size by 50%.6–8 In the first few minutes following reperfusion, a large production of ROS occurs from a variety of sources.5–8 This induces opening of the mitochondrial permeability transition pore (MPTP),6,7 which contributes to the Ca2+ overload, damaging the cell membrane via lipid peroxidation.5–8 Finally, the increased ROS levels cause enzyme denaturation and DNA damage6 (Fig. 1).
FIG. 1.
Governing intracellular, extracellular, and molecular mechanisms in myocardial ischemia/reperfusion injury. During ischemia (on the left), lactate production results in reduction in intracellular pH. Na+–H+ and Na+–Ca2+ exchangers are activated causing the overload in intracellular Ca2+, restricting the MPTP opening on the mitochondrial membrane and cardiomyocyte contractility. During reperfusion (on the right), physiological intracellular pH is quickly restored, MPTP reopens, and cardiomyocytes contract abnormally due to increased ROS production and ion influx.
Following the initial onset of MI, a systemic response and remodeling process begins. Immune cells play a significant role in the initial systemic response.50–53 Neutrophils and macrophages infiltrate the wounded area within several hours of the infarct and remain there for several days to digest necrotic tissue. Afterward, macrophage-mediated cytokine release initiates remodeling, apoptosis, necroptosis, and neovascularization.50,51 In addition to the immune response following MI, scar formation plays a significant role in cardiovascular remodeling. As CMs are incapable of proliferating, once the necrotic tissue has been successfully digested, it is replaced by activated CFs, which immediately begin to close the resulting wound.50,51,54 This new fibrotic tissue contains high levels of collagen, which leads to an increase in heart stiffness at the injury site.50,51,54 The increased stiffness, along with the inability of CFs to beat, results in decreased cardiac function and output, significantly increasing the risk of death from subsequent MIs.6 Since MI contributes to heart disease progression and probable patient death, there has been tremendous research across many fields aimed at treating, mitigating, as well as modeling the pathophysiology of MI both in vivo and in vitro.
III. MI MODELS
Due to the limited regenerative capacity of the human heart, few therapeutic options are available that can be used to treat MI. Therefore, preclinical models used to mimic MI conditions are extremely beneficial for studying the biology of MI and testing new drugs and therapeutic options to treat the post-MI damaged tissue. This section is dedicated to reviewing the in vivo, ex vivo, and in vitro models that have been developed to better understand the outcomes of MI and I/R and on some occasions, used as platforms to test new drugs and therapies.
A. In vivo models
In vivo models are one of the most commonly used models to study MI and are greatly valuable for testing drug efficacy and safety, as well as the systemic response of the body under physiological conditions, which is not possible to do in vitro or ex vivo. With the help of in vivo MI models, researchers can create clinically relevant infarct sites, assess infarct size and microvascular damage, and evaluate the neovascularization, scar formation, inflammation or immune response, and changes in physiology and blood biomarker levels in the body in response to MI and treatments.55 Methods to create MI models in animals are primarily surgical ligation (of left coronary artery), occlusion/constriction of arteries, intubation, chemical exposure (isoproterenol), cryoinjury, and genetic modification.55,56
Typically, MI is studied in small animal models like mice56–59 and rats,60–63 but larger animals such as rabbits,64–67 dogs,68–70 sheep,71–73 pigs,74–77 and minipigs78,79 have also been used. Although small animal models are logistically convenient and easier to handle, they are limited by their different anatomy, physiology, and histology compared with humans. On the other hand, although larger animal models better mimic the anatomy and physiology of humans, they are difficult to work with due to their high cost, risk of infection, problems with arrhythmia and inconsistent perfusion, and ethical concerns.55,57 Finally, due to the complexity of the in vivo environment, it is not possible to do mechanistic studies or dissect the effect of a treatment on a specific pathway.80,81
B. Ex vivo models
Ex vivo models involve the use of animal or human hearts outside the body or in culture conditions to evaluate the heart physiology in response to treatment. Several approaches have been used to create ex vivo models. In one approach, the whole heart is harvested and subjected to normal (working heart) or retrograde (Langendorff) perfusion through a pulmonary vein or coronary vasculature, respectively, using a special buffer/medium.82–84 To create MI conditions, the perfusion is blocked for up to 3 h (ischemia) and restored (reperfusion), and the changes in heart physiology are recorded.82–84 In another approach, the living heart tissue is cut into thin slices using a high-precision vibratome and maintained in culture.85–90 The heart slices retain their viability and beat for up to 28 days in culture. Ex vivo models are beneficial because they allow for the accurate measurement of the infarct size, easy and reproducible assessment of left ventricular (LV) function and troponin levels, and high-throughput analysis.55,91,92 However, these models eliminate the effect of systemic components like immune response, circulation, and interaction with other tissues in the body, which can make interpretation of the findings more difficult. Though less complex than in vivo, ex vivo models are still not simple enough for parametric studies; it is impossible to dissect the effect of one individual parameter or know the reasons for the observed outcome. Moreover, the risk of edema, the limited source of energy in culture conditions, and the limited stability of the tissues outside the body prevent these models from being preferable models to study MI.55
C. In vitro models
In vitro cardiac models involve the use of primary cells and/or cell lines, in the presence or absence of cytokines and biomaterials, in a controlled environment. The cells are usually obtained by isolating CMs from animal or human hearts or by differentiating stem cells. Although there is an ongoing discussion on the benefits and limitations of using cells isolated from neonatal or adult donors, primary CMs are the main cells used to study cardiac cell physiology;93,94 however, their usage is restricted due to their limited proliferative capacity.95,96 Moreover, adult donor cells require a technically challenging isolation process, transfection with viral vectors, a short-term culture-period after isolation, and are unable to spontaneously beat in culture.97 Therefore, as an alternative, mesenchymal stem cells (MSCs),98–100 embryonic stem cells (ESCs),101 or induced pluripotent stem cells (iPSCs)24,31,102 can be differentiated into CMs after amplification. These cells are especially valuable when creating MI models for high throughput assays or for engineering 3D models, particularly if they are from human-origin, allowing for patient-specific models.
Two-dimensional (2D) in vitro models are simple models obtained by culturing cells on a flat surface such as the bottom of culture plates or petri dishes. 2D culture is cost-efficient, easy to use and manipulate, and can be high throughput. It allows for better analysis of the cells and dissection of the factors that play roles in a particular mechanism.103 Therefore, many studies choose to evaluate the effect of ischemic and reperfusion mimicking conditions, using 2D cardiac models.104–106 However, 2D models overlook cell–matrix interactions and fail to account for the effects of other tissues or other systemic effects (circulatory, immune, and endocrine systems). Moreover, cell morphology in 2D is different than in vivo, leading to differences in the cell physiology and function compared to native tissue.107 For instance, primary CMs change their phenotype when cultured in 2D, while stem cell (usually iPSC)-derived CMs (iCMs) are not as mature compared with 3D cultured cells.108 Furthermore, 2D models usually include CMs alone; therefore, they lack the interaction between different cell types that are prominent in the heart. Additionally, 2D cultures are more sensitive to changes in oxygen levels compared with 3D models, which is an important parameter to study in MI models. These limitations prevent 2D models from closely recapitulating in vivo conditions, making them inadequate platforms to study I/R and result in poor clinical translation.109
The heart microenvironment is a dynamic 3D network, where the CMs and the stromal, endothelial (EC), and immune cells interact with each other, as well as with the extracellular matrix (ECM). Engineered models aim to recapitulate this 3D microenvironment as closely as possible. Since 3D constructs support the cell–cell and cell–ECM interactions, they yield more physiologically relevant results than 2D models. As the characteristics of each component can be controlled, the effect of each factor in these components on both normal heart physiology and function, and MI progression can easily be studied. Recent advances in tissue engineering, microfluidics, and stem cell technologies have made it possible to obtain human-representative and reliable results from engineered 3D MI models.24,31,94,102 On the other hand, the inability to account for the in vivo systemic changes that occur after MI, as well as the immaturity of iCMs, the main cell type used in engineered cardiac models, compared with native CMs (which will be discussed in detail in Secs. III C 1 and III C 2), are limiting factors for 3D in vitro models. Another challenge in 3D engineered models is the restricted nutrient and gas transfer within these constructs, which limits the size and thickness of the construct that can be achieved. To address this problem, constructs that allow controlled ingrowth of blood vessels within the engineered tissue should be designed.110,111 Hence, scaffolds should be made with large pores and contain growth factors to induce angiogenesis. Blood vessel formation is essential for the success of these constructs as these vessels help maintain cell viability and are critical for integration of the construct with the host tissue, and thus allow for the treatment of larger defects in the heart.
Regardless of these limitations, these models are becoming the platform of choice to study MI. However, it should be noted that 3D in vitro models aim to be complementary to in vivo models, as in vivo models are crucial to evaluate the safety, biodistribution, and efficacy of a drug/treatment, and the systemic response of the body against it.
1. Engineered 3D models to study I/R
To engineer more biomimetic 3D in vitro models that recapitulate cell–cell and cell–ECM interactions, various methods have been utilized including cell sheets, spheroids/organoids, microfluidic devices, and bioprinting (Fig. 2). Section III C 1 brings together recent studies on 3D cardiac tissue models using these techniques, specifically focusing on the effect of I/R using these models.
FIG. 2.
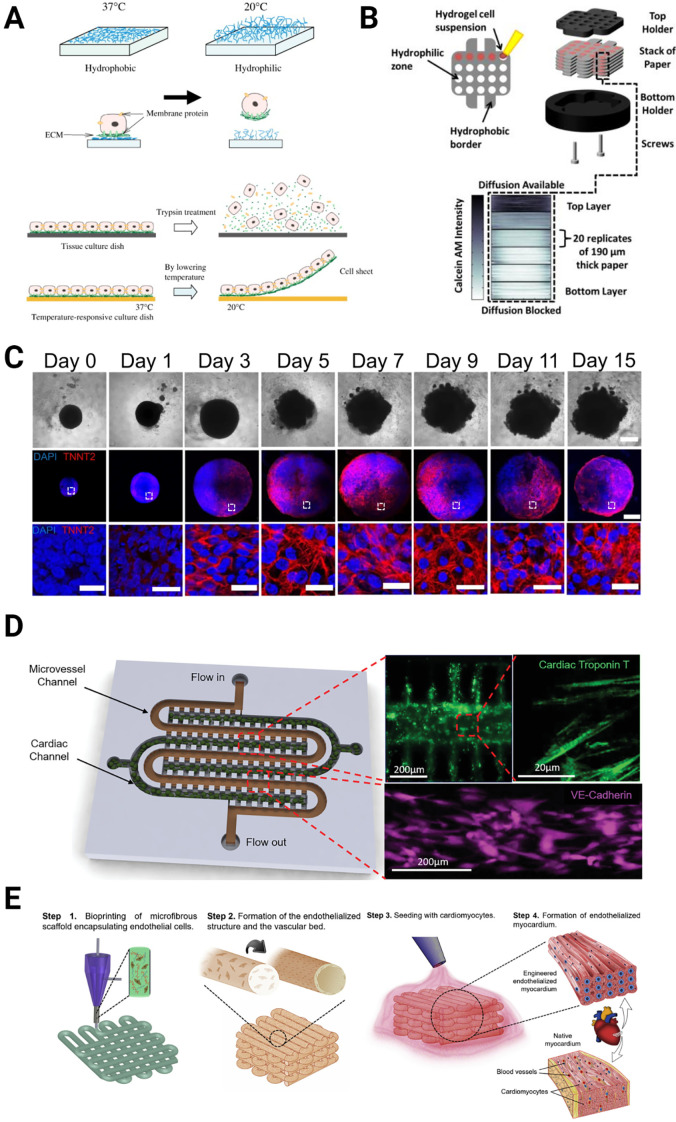
Various methods used to create cardiac tissue models in vitro. (a) Cell sheet [Reproduced with permission from Masuda et al., Adv. Drug Delivery Rev. 60, 277–285 (2008). Copyright 2008 Elsevier], (b) paper-based model of cardiac ischemia [Reproduced with permission from Mosadegh et al., Adv. Healthcare Mater. 3, 1036–1043 (2014). Copyright 2014, Wiley], (c) organoids [Reproduced with permission from Lewis-Israeli et al., Nat. Commun. 12, 5142 (2021). Copyright 2021 Author(s), licensed under a Creative Commons Attribution (CC BY) License], (d) microfluidic devices [Reproduced with permission from Ellis et al., Small 18, 2201330 (2022). Copyright 2022 Wiley], and (e) bioprinting [Reproduced with permission from Zhang et al., Biomaterials 110, 45 (2016). Copyright 2016 Elsevier].
a. Cell sheets, spheroids and organoids
Cell sheets are usually engineered by seeding cells on a temperature-sensitive polymer such as poly (N-isopropylacrylamide) (PIPAAm), which changes its adhesive properties in response to temperature.112–114 At normal culture conditions (37 °C), the surface is slightly hydrophobic and cell-adhesive, and at lower temperatures (<32 °C), the surface is highly hydrophilic and non-adhesive. CMs are seeded on the polymer at normal culture temperature when the substrate is adhesive, allowing for cell attachment. After the cells form a monolayer, the temperature is lowered to make the surface non-adhesive. As a result, the cells detach from the surface as a layer to form the cell sheet [Fig. 2(a)]. Alternatively, a soluble polymer can be used to form the cell sheets before the polymer is dissolved away. Cell sheets are widely used in cardiac tissue engineering applications,115,116 specifically as cardiac patches;112,117,118 however, due to the lack of 3D structure and limited cell types within the sheets, their use for I/R modeling is limited. In one such study, a human iCM-based cell sheet platform was developed to model I/R.104 iCMs were first seeded on gelatin, and following cell-sheet formation, the gelatin was dissolved. Cell sheets were exposed to anoxia or normoxia for up to 24 h, to create I/R conditions. The researchers showed that the beating rate decreased under anoxia and reperfusion, but the cells adapted to the anoxic conditions in time and started to beat at a higher rate after 15 h in anoxia. This indicates that the CM physiology might have changed in CVD patients with coronary artery occlusion. Similarly, Yamasaki et al. engineered human cardiac cell sheets using human iCMs and evaluated contractile force under either constant hypoxia for long-period (8 days), or short-period hypoxia (4 days) followed by normoxia (8 days) to mimic I/R conditions.119 They reported decreased contractile force, contraction velocity, Ca2+ transient kinetics, and ATP levels, yet unchanged sarcomere structure and cell number following hypoxia.119
While cell sheets support cell–cell interactions and improve the beating of the cells, they do not take into account the effect of the microenvironment, which limits their use alone as I/R models. Spheroids are 3D cell aggregates, usually created in a scaffold-free environment, that may consist of single or multiple cell types with strong cell–cell interactions.120 Similarly, organoids are three-dimensional (3D) organ-like multicellular models that are derived from stem cells121,122 [Fig. 2(c)]. The intrinsic ability of stem cells to self-assemble allows researchers to study natural development, structural organization, regeneration, and disease progression in vitro using spheroids and organoids.123
To date, successful organoid models of many organ systems including brain, kidney, liver, and heart have been reported.124–128 However, the use of organoids in cardiac tissue engineering is relatively new,123,129,130 and only a few of them have been dedicated to studying disease models,131 such as congenital heart disease,132 arrythmia,133 short QT syndrome (SQTS),133 familial cardiomyopathy,134 and I/R in MI.135 In the study of interest, Voges et al. combined collagen and iCMs to create human cardiac organoids (hCOs) and studied the regenerative potential of the hCOs following cryoinjury.135 They reported for the first time that hCOs were recapitulating fetal cardiac tissue characteristics, and that increased CM proliferation led to regeneration and functional recovery of MI-mimicking local cryoinjury within 2 weeks. Similarly, in a recent study, Sharma et al. engineered mouse and human cardiac spheroids and exposed them to I/R mimicking conditions in vitro.136 Under these conditions, they observed that a high percentage of the cardiomyocytes died whereas only some portion of the fibroblasts and endothelial cells died. Moreover, they evaluated the cardiac damage-related gene expressions for in vivo, in vitro human cardiac spheroids (hCSs), and in vitro mouse cardiac spheroids I/R models, and reported that the gene expression of hCSs was more similar to in vivo.136
Additionally, hCOs have also been used to model contractile pathophysiology, especially with patient-specific iPSCs that might carry genetic defects such as short QT syndrome (SQTS) and familial cardiomyopathy.133,134 Researchers used CRISPR-Cas9 to correct the disease-related mutations to obtain isogenic control and compared disease vs healthy phenotype in vitro. Such patient-specific organoids hold great potential to provide new mechanistic insights to explain comorbidities in heart-related pathologies.
Despite the difficulty of controlling organoid size, the lack of support in the form of vasculature, and the difficulty of integrating various cells into the organoids, these studies showed that organoids can be a promising tool for developing preclinical treatment and disease profiling.122,123
b. Scaffold-based 3D models
In order to most closely recapitulate the native cardiac tissue in the 3D in vitro models, researchers took into consideration different fundamental properties of the heart, such as stiffness, age, multiple cell types, and CM alignment. In that regard, the most commonly used biomaterials to construct the microporous and hydrogel-based 3D MI models are collagen,137,138 collagen–fibrinogen,139 Matrigel,140,141 gelatin methacryloyl (GelMA),142,143 poly(ethylene glycol)–(arginine–glycine–aspartic acid) (PEG–RGD),142,144 and chitosan.145
Acun et al. created young and aged tissue models by encapsulating young (day 35–55) and aged (days 100–120) iCMs in GelMA-PEG hydrogels (with three different stiffnesses representing fetal, adult, and aged heart tissue).142 They evaluated the stress response of each model when exposed to MI mimicking conditions.142 They reported a significant decrease in cell viability for aged cardiac tissue constructs compared with young tissue constructs after going through conditions representing ischemia and reperfusion.142 Similarly, Chen and Vunjak-Novakovic also utilized iCMs to engineer 3D tissue models via encapsulation in collagen–fibrinogen hydrogels.139 Similar to the previous study, they showed that the 3D model recapitulated the in vivo conditions; more cell death was observed through apoptosis in the reperfusion phase than in the ischemia phase, and the addition of cardioprotective therapeutics (cyclosporine A and N-acetyl-l-cysteine) or lowering the pH, reduced the detrimental effects of I/R. Interestingly, lowering the pH (pH 6.4) showed the most effective protection against the injury caused by the rapid normalization of intercellular pH that occurs during reperfusion.139 In one of the early studies, Liu et al. engineered a 3D tissue model by embedding adipose derived stem cells (ADSCs) in chitosan hydrogel, and mimicked I/R by inducing ROS production through hydrogen peroxide treatment.145 They showed that the chitosan components, (1→4)-2-acetamido-2-deoxy-β-d-glucan (N-AC-Glu) and (1→4)-2-amino-2-deoxy-b-d-glucan (d-Glu), reduced the effect of I/R and verified this in vivo, where ADSCs engraftment, survival, and endogenous stem cell homing in the ischemic heart were enhanced in the presence of chitosan.
In another study utilizing rhesus monkey-derived iCMs in collagen gels to study I/R, researchers found that the 3D model was more sensitive to ischemic conditions than the 2D model.138 Transcriptomic and pathway analyses revealed that pathways related to cell–cell and cell–ECM interactions, energy metabolism, and paracrine signaling were similar in the engineered 3D model compared to the native rhesus monkey myocardium, showing that these models can recreate the in vivo conditions.
In their study, Katare et al. preferred to use primary CMs, and they engineered a 3D model by seeding neonatal rat CMs onto a ring-shaped collagen scaffold and cultured the construct under hypoxic conditions (1% O2) for 6 h.137 The engineered constructs showed conduction defects, dephosphorylation of connexin-43, and down-regulation of cell survival proteins similar to the infarcted adult heart. When they treated the 3D MI model with cell protective agents, cyclosporine A and acetylcholine, they showed reduction in the ischemia-induced effects. Recently, Funcke et al. engineered 3D MI models by mixing all isolated rat heart cells with fibrinogen and thrombin and placing them in agarose molds and in between two silicone posts and analyzed the effect of two different hypoxic conditions on the contraction force and release of cardiac troponin I.146 Then, they created an engineered human heart model by using iCMs instead of rat CMs and exposed them to the same hypoxic treatment conditions and used them to assess the cardioprotective property of DOR agonist [d-Ala2, d-Leu5]-enkephalin (DADLE); for which they observed a positive effect for engineered rat hearts, and not with engineered human heart models.
Hidalgo et al. engineered tissue models incorporating CM alignment in the model by constructing Matrigel-coated microgroove patterns to align immature and metabolically matured human iCMs, and tested their response to I/R conditions.141 They showed that mature iCMs were more sensitive to I/R than immature cells, with a 5%–30% increase in cell death in response to I/R after the cells were metabolically matured. Apoptosis was reduced under ischemic/reperfusion conditions after the addition of cyclosporine A into the media.
To engineer more physiologically relevant and mature cardiac tissue models, some studies incorporated other relevant cells including CFs,140,147 endothelial cells144 or a combination of these cells148 with CMs.149 Mosadegh et al. engineered multiple layers of paper coated with Matrigel containing rat neonatal CMs, CFs, or 3T3 fibroblasts and stacked these paper layers such that the CMs were in the bottom layer where oxygen diffusion was blocked, mimicking the ischemic conditions that occur as a result of MI [Fig. 2(b)].140 To reduce oxygen diffusion from the top, more paper layers with 3T3 cells were added to the 3D model. They showed that the more hypoxic the CMs became, the more fibroblasts migrated toward the bottom layers containing the CMs, mimicking the native tissue environment.140 Recently, del Campo et al. engineered human myocardium by combining CMs and human foreskin fibroblasts (7:3 ratio) with collagen, and performed cryoinjury on this model to mimic the heart attack.150 After identifying the effect of the cryoinjury on the tissue model, they used it as a platform to analyze the efficacy of an extracellular vesicle (EV)-based treatment.150 In another study, Yue et al. created 3D co-cultured platforms of rat CMs and iECs by encapsulating them in PEG-RGD hydrogels and investigated the effect of CM-EC crosstalk on the viability of CMs when exposed to MI mimicking conditions.144 They observed improved CM viability in the presence of iECs as well as significantly different gene expression under oxidative stress indicating that iECs protected CMs from oxidative stress.144 In a previous study, they also investigated the ischemia induced deterioration of endothelium by encapsulating CRISPR/Cas9 edited iECs in GelMA hydrogels and maintaining the 3D constructs under hypoxic conditions.143
c. Microfluidic devices
A different approach to modeling cardiac systems is using microfluidic systems. Recent advances in microfluidic systems and lab-on-a-chip models have allowed for the development of biomimetic organ-on-a-chip models, which are powerful tools for addressing limitations in typical 2D cell cultures, such as 3D cell–cell interactions, fluid flow, and ECM/environmental interactions.151–153 Organ-on-a-chip models enable high control over cell placement, perfusion parameters, and vascularization to mimic a wide variety of biological systems such as the heart, lungs, and kidneys, for purposes such as drug development, disease modeling, and even general physiology studies.154
For heart models in particular, the degree of control over the 3D environment, tissue perfusion, and cell placement allows for greatly enhanced modeling of cardiac injuries and CVDs21,155 as well as drug testing,156 mechanistic studies of regenerative therapies such as EVs,157 and general cardio physiological studies,158–161 given the complexity and importance of vascularization of cardiac tissue in many healthy and diseased states.17,162
Heart-on-a-chip models have become a preferred tool to model disease conditions, such as I/R during MI. Martewicz et al. engineered a microfluidic system using neonatal rat CMs to observe the effect of hypoxic conditions on the intercellular Ca+2 handling properties.163 Ellis et al. developed and fully characterized a myocardium-on-chip model by encapsulating iCMs in GelMA hydrogel in the middle channel, representing the cardiac muscle, and seeding iECs on the side channels, representing the microvasculature, which can be used as a platform to study I/R in vitro.24 In a recent study, Ellis et al. improved this myocardium-on-chip model and showed that it can mimic physiological miRNA expression during both the ischemic and reperfusion phases of MI in vitro by controlling cell placement, perfusion, and oxygen availability, showing similar results to time matched clinical plasma samples [Fig. 2(d)].164 In a similar manner, Veldhuizen et al. engineered a heart-on-a-chip model using stem cell-derived CMs and CFs encapsulated in collagen hydrogels and used it as a platform to observe the effect of hypoxic culture conditions.165 Recently in a similar study, Liu et al. developed a heart-on-a-chip model integrated with bioelectronic devices to measure the intra- or extracellular electrophysiological readouts under ischemic conditions using immortalized mouse atrial HL-1 cells.166
While microfluidic devices are a powerful tool for modeling tissues, there are also inherent limitations to this methodology that should be considered. First, organ-on-a-chip microfluidic devices still utilize cultured cells and thus inherit many of the limitations of cell culture.155 These include control of cell maturity, stress due to culturing, variances due to cell passaging, and, in the case of stem cell-derived cells, sometimes poorly defined properties and mechanisms of physiological activity. Second, by modeling a single tissue, many microfluidic devices also fail to account for systemic factors in disease and drug response, as well as in normal tissue homeostasis.155,167 This can result in non-physiological responses to certain treatments or disease states. Finally, specifically with regard to printed microfluidic devices, resolution can be a significant limiting factor, especially when attempting to model microvasculature effects.167 While the resolution has drastically improved over the years, the highest resolution obtained under ideal conditions is approximately 20 μm, as opposed to the maximum 10 μm diameter of a typical capillary.168 These limitations currently control the degree to which in vivo systems can be accurately modeled in microfluidic systems.
d. Three-dimensional (3D) bioprinting
Three-dimensional (3D) bioprinting is a layer-by-layer additive manufacturing technology allowing accurate spatial deposition of the biological materials and active cells in a pre-designed pattern and is thus considered a promising technique for fabricating biomimetic cardiac tissue that can be used to study I/R in vitro. In order to have functional bioprinted cardiac tissue constructs, it is crucial to mimic the organized structure of cardiomyocytes in the native heart.169,170 Resolution is very important for the precise control of the location and organization of the printed tissue construct, which is critical for the functionality of the printed cardiac tissue model. Unfortunately, one of the limitations of the extrusion based bioprinting is the relatively low resolution (around couple hundred micrometers171) which is a function of various parameters such as the nozzle diameter, the distance between nozzle and the stage, printing speed, bioprinted material, and its flow rate.172 Although the resolution for the extrusion based printing does not allow for cell-scale fabrication, recent studies have shown that CM alignment can be achieved by utilizing conductive nanoparticles (NPs) with an electric field,173 and modified nozzles.174 Even though there are many studies that focus on engineering cardiac tissue constructs using 3D bioprinting [Fig. 2(e)] (reviewed in detail by Sharma et al.,109 Liu et al.,175 and Wang et al.176) up until today, none of them used the bioprinted constructs to study I/R.109,158,177
Using bioprinting is beneficial for creating complex tissue constructs with different cell types. However, for the long-term viability of the printed constructs, it is necessary to include a functional vasculature network. Although some studies have included the vasculature network,178–180 it is difficult to print the vasculature system with the required dimensions due to the relatively low resolution of 3D bioprinting, especially for extrusion-based 3D printers. Another limitation is introducing flow through the vasculature. It is crucial to design the vasculature network to have one inlet and one outlet in order to easily combine it with a pump to constantly supply the media through the constructs. Moreover, how to combine the tubing to the construct is another point to take into consideration. Another difficulty that comes with a relatively larger scale of 3D printed models is the challenges with imaging. Due to the thickness of the 3D-printed constructs, it can be difficult to use the current imaging tools efficiently while analyzing the condition of the construct. Given these limitations, a 3D printed construct has never been used to study I/R before. Microfluidics devices were preferred over 3D printed constructs due to their benefits mentioned previously, even though they lack the scale of the 3D printed constructs.
2. Engineered 3D post-MI tissue models
Although many animal models have been established to study the changes in post-MI tissue when treated using different therapies, few studies have focused on in vitro models. A few studies included the scar tissue formed after MI while engineering the 3D models and used them either to evaluate the efficacy of therapeutics or observe the effect of the scar (fibrotic) tissue on the beating function of the heart. Numerous methods have been used to create such in vitro models, including organoids, bioprinting, and microfluidics (Fig. 3). This section is dedicated to reviewing the recent developments in materials and methods used for creating 3D infarct tissue models in vitro.
FIG. 3.
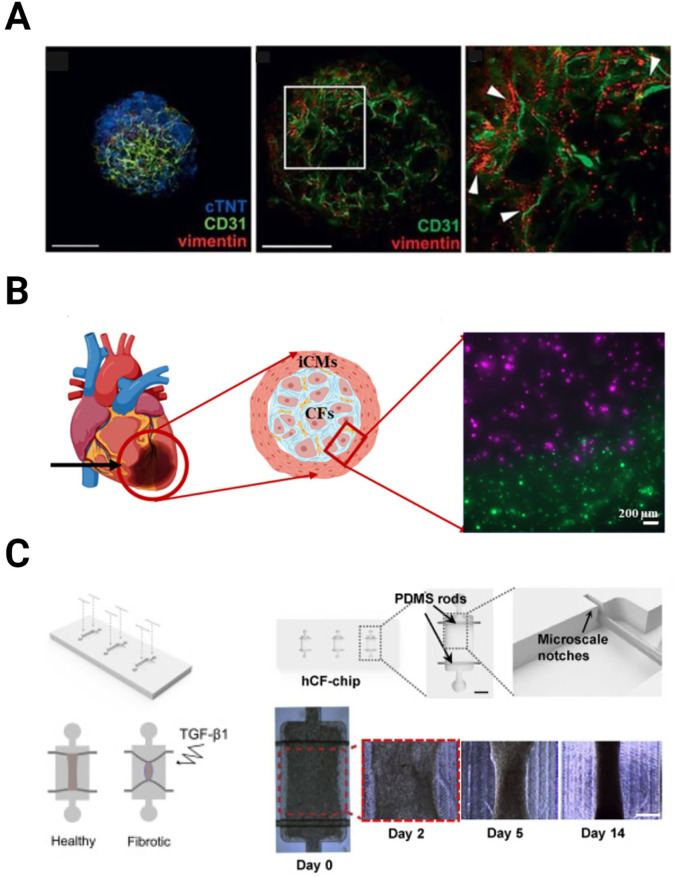
Different methods used to create the fibrosis models. (a) Organoids. Laser scanning confocal microscopy collapsed Z-stack of vascularized cardiac spheroids stained with antibodies against markers of cardiac fibroblasts (CFs), endothelial cells (ECs), and cardiomyocytes (CMs) (vimentin, CD31, and cTNT, respectively) (left). A single laser scanning confocal microscopy image showing vimentin-positive CFs are positioned immediately next to CD31-positive ECs (middle and right). Reproduced with permission from Figtree et al., Cells Tissues Organs 204, 191 (2017). Copyright 2017 Karger Publishers. (b) 3D bioprinting. Schematic showing the infarct region model and corresponding cell types. Inset picture showing the successful infarct region printing using stained iCMs (green) and hCFs (magenta) [Reproduced from Basara et al., Gels 7, 70 (2021). Copyright 2021, MDPI], (c) microfluidic devices. A schematic showing the healthy and TGF-β treated fibrotic tissue-on-chip (left). Display of the hCF-chip containing three microwells (top-middle). Top view image showing a well with two elastomeric rods (arrows) suspended on each end (inset-top-middle). (Scale bar: 1 mm). Schematic of the microscale notches on each end of the well, help hold the elastomeric rod in place (top-right). Representative images for control tissues at days 0, 2, 5, and 14 post-seeding (bottom right). (Scale bar: 500 μm) [Reproduced with permission from Mastikhina et al., Biomaterials 233, 119741 (2020). Copyright 2020 Elsevier].
a. Spheroids and organoids
As mentioned earlier, cardiac spheroids and organoids have been shown to be promising tools to create in vitro models to study the human heart microenvironment and drug cytotoxicity.181 Recently organoids have been employed to investigate fibrosis development and as a platform for the assessment of therapeutic treatments.182,183 Richards et al. developed a 3D in vitro post-MI myocardial tissue model by mixing human iCMs and non-CMs (4:2:1 ratio of cardiac ventricular fibroblasts: umbilical vein endothelial cells (HUVECs): adipose-derived stem cells (hADSC), without the use of any ECM proteins, and placed this cell mixture in agarose molds to form hCOs. By leveraging nutrient transport principles (e.g., oxygen diffusion) through the organoid, they created a gradient throughout the spheroid which included an apoptotic center resembling the infarct region, a boundary region, and a healthy region.182 Hallmarks of MI such as metabolic changes, impaired calcium handling, and increased fibrosis was detected at the transcriptomic, structural, and functional levels. Moreover, they reported an upregulation in the fibrosis-related genes and an increase in elastic modulus similar to the fibrosis development in vivo.182 In another study, Figtree et al. developed cardiac spheroids using hanging drop cultures of freshly isolated rat primary ventricular cardiac cells and used TGFβ1 treatment to create the fibrotic tissue [Fig. 3(a)].183 As expected, TGFβ1 treatment increased cardiac fibrotic markers and ECM deposition in the spheroids, making them adequate post-MI in vitro fibrosis models. Using this model, they evaluated the cytotoxic effect of doxorubicin (DOX) treatment and observed that DOX treatment increased the apoptosis marker expression, as well as the spheroid volume.183
b. Scaffold based 3D models and 3D bioprinting
Direct encapsulation of the cells in hydrogels along with micro molding techniques have been widely used in cardiac tissue engineering applications.184 For post-MI cardiac fibrosis models, however, it is not a preferred method as it is more challenging to spatially pattern the different cell types and/or materials compared to 3D bioprinting or using microfluidic devices. Sadeghi et al. developed a 3D hydrogel-platform consisting of primary rat CMs and CFs and tuned the mechanical properties of the GelMA hydrogel to match the native cardiac tissue.185 To create the fibrotic tissue microenvironment, they stimulated this construct with TGFβ1 to activate the CFs.185
As mentioned in Sec. III C 1 d, 3D bioprinting has become a powerful tool for cardiac tissue engineering applications, because it enables accurate spatial control of different materials and cell types, and thus is a promising method to create a 3D cardiac fibrosis model. Basara et al. and Koti et al. utilized multi-material printing to create the infarct region using CMs and fibroblasts in different printheads [Fig. 3(b)].31,186 Both studies showed that 3D bioprinting can be used to engineer viable infarct region models, by characterizing the mechanical properties and printability of the used bioink, evaluating their cytocompatibility, and printing the scar tissue and healthy tissue together by utilizing two printheads.31,186
c. Microfluidic devices
Microfluidic devices have become a preferred tool to model post-MI infarct region in vitro and the subsequent cardiac fibrosis187–189 because they not only allow for spatially patterning of different cell types in the channels, but also enable observation of changes in biochemical and biomechanical properties over time. Modeling cardiac fibrosis, in particular, has gained increased interest recently, due to the increasing awareness of the role that cardiac fibrosis plays in post-MI remodeling [Fig. 3(c)].188 As such, developing complex biomimetic models has become imperative for both understanding and developing effective treatments for MI.157 The application of modern bioprinting techniques has allowed for the development of some models with sophisticated cell positioning or vasculature that would be difficult to achieve otherwise,190,191 making it possible to engineer heart-on-a-chip models with complex microvasculature.192 While this remains an existing challenge, the use of novel techniques such as multi-nozzle printing makes it possible to create complex ex vivo models of post-MI or fibrotic tissue to study the progression of fibrosis and various intervention strategies.193 These models can be used to identify contributing pathways and biomarkers of a pro-fibrotic state through various techniques, including clinical imaging techniques, effluent biomarker analysis, and effluent EV analysis, as well as investigating the mechanics behind these changes.154,155 Furthermore, bioprinted microfluidic organ-on-a-chip models are gaining recognition as alternative pre-clinical drug testing platforms in terms of predicting drug toxicity.194 Many drugs fail Phase 1 clinical trials due to the inability of 2D cell culture and existing models to accurately recapitulate a drug's full effect on an organ, and bioprinted organ-on-a-chip models provide an attractive and cost-effective alternative to increase the success rate of drug trials. The ability to generate such a wide breadth of data for both preclinical studies and physiological understanding of relatively understudied aspects of MI, such as cardiac fibrosis and microenvironment alterations, demonstrates the versatility and utility of these models.
IV. REGENERATIVE THERAPIES FOR TREATING POST-MI TISSUE
The development of improved pharmaceutical and therapeutic intervention methods for MI-related damage has become a major motivator for the development of many biomimetic cardiac models, in large part due to the prevalence of MI in worldwide deaths and medical costs. With the aforementioned advances in modeling cardiac behavior in both healthy and disease states, recent novel interventions have gained traction after success in these models. These novel therapies have been developed to treat damaged tissue following MI, including stem cell (SC) therapies,195,196 extracellular vesicle (EV) or exosome treatments,45,46 direct reprogramming of fibroblasts into CMs,44 biomaterial-based therapies,197,198 and cardiac patches.118,199 In this section, recent developments for each of these therapeutics used for treating post-MI tissue will be reviewed (Table I).
TABLE I.
Comparison of different techniques used for treating MI considering the selected cell type, biomaterials, methods for evaluation, and limitations.
| Treatment | Cell source | Biomaterials | Assessment Method in vivo | Limitations |
|---|---|---|---|---|
| Cell therapies | MSCs,205,207,210,213,216,217,233 iPSCs,221–224 iCMs205,233–235 | – | LVEF, infarct size, electric activity, angiogenesis | Tumor formation, low retention, low cell survival, low cell maturity |
| Biomaterials based | MSCs,270,294 endothelial progenitor cells (EPCs),293,334 iPS-cardiac progenitor cells (iPS-CPCs),295 iCMs296 | dECM,197,269–271,292 adamantane-modified HA (Ad-HA) and β-cyclodextrin-modified HA (CD-HA),293,334 sodium alginate,294 MeHA,295 gold nanoparticle (AuNP)-hyaluronic acid (HA),296 elastin-like recombinamers (ELRs)297 | Walk distance test (clinical),197 LVEF, fractional shortening, cardiac muscle area angiogenesis, infarct size | Variations in biological response depending on the post-MI delivery time point, immune response |
| EVs | MSCs,294,295 cardiac stromal cells (CSCs),253 iPSCs,250 iCMs,250 EPCs,293,334 epicardial cells150 | MeHA,295 dECM,253 collagen,250 Ad-HA and CD-HA,293,334 sodium alginate294 | Cardiac function assessment, LVEF, fractional shortening, angiogenesis | Low retention, not yet mechanistically well-understood |
| Cardiac patches | Primary rat CMs,298,309,311,312,316,317,324,331–333 iCMs,36,41,60,177,299,300,306,320,321 iECs,300,306,320,321 FBs,41,60,300,306 human coronary artery endothelial cells,305,317,333 H9C2 cardiomyoblasts,307,319 rat bone marrow-derived stem cells (r-BMSCs),310 ESCs,313 CPCs,318 smooth muscle cells,320 CSCs,253,322 human dermal fibroblasts333 | Fibrinogen–thrombin–matrigel,298 decellularized placenta,299 fibrin–matrigel–thrombin,300 collagen,41,304 Ti3C2Tx MXene-PEG,36 decellularized porcine myocardium slide,303 carbon nanotubes (CNTs) incorporated methacrylated collagen (MeCol)–alginate,305 nanocellulose-PGS-PPy,307 PU-PANI-SiO2,308 nCe-PCL-gelatin,309 decellularized bovine pericardium,310 oxidized alginate–gelatin–polyacrylic acid,311 cellulose,312 fibrin,313,332 spider silk reinforced fibrin,314 PCL-both ends capped with nitrates,315 CNT-reinforced non-mulberry silk,316 GelMA-colagen,60 alginate–gelatin,317 gelatin-HA,318 PCL-PGS,319 GelMA,320 decellularized human omental tissue,321 fibrinogen–thrombin–aprotinin,322 PPy-silk fibroin,324 methacrylated elastin-gelatin-CNTs,331 decellularized porcine myocardium253 | Infarct size, cardiac functionality LV wall thickness, LVEF, angiogenesis | Arrhythmias, endurance integration, immune response |
A. Cell therapies
There has been extensive effort to develop cell therapies to treat MI patients. Two decades ago, the first generation of cardiac cell therapies started with adult cell types such as bone marrow-derived mesenchymal stem cells (MSCs), skeletal myoblasts, and cardiac progenitor cells (CPCs).200 With the advancements in iPSCs in the 2000s, the second generation of cardiac cell therapies arose including iPSCs, ESCs, and iPSC-derived CPCs and CMs.201 Most recently, because the cardiac cell makeup consists of more than one cell type and MI is molecularly complex, the third generation of cardiac cell therapies has emerged with combinational cell therapies using more than one cell type with complementary roles.202 Overall encouraging results regarding efficacy, safety, and functional and structural improvements were reported upon cell therapy.200 However, the mechanism of action of these cell therapies and whether the cells or the secreted paracrine factors facilitate the therapeutic effects is still subject to debate.203 Here, we focus on recent advancements in stem cell therapies as they are the most favored post-MI cell therapies.
1. Mesenchymal stem cells (MSCs)
MSCs are multipotent adult stem cells that can differentiate into multiple lineages, including endothelial and CM-like cells and can be derived from a variety of tissues including bone marrow and adipose tissue, expanded extensively in vitro, and exhibit so low immunogenicity that even allogeneic MSCs would not cause any immune reaction. Most of the MSC studies date back to the early 2000s, yet MSC therapy is still one of the most popular post-MI treatment methods.200,204,205 In preclinical animal studies, MSCs have been shown to integrate into the host tissue, support cardiac repair and function, and reduce infarct size, while also enhancing vascular density.200,206,207 Especially regarding vascular density, the mode of action of MSCs appeared to be primarily paracrine.208–210 MSCs are known to secrete a variety of regulatory and trophic factors such as growth factors (i.e., SDF-1α, HGF-1, ILGF-1, VEGF, FGF, and PGF) and cytokines (i.e., ANG-1, MMPs, IL-1, IL-6, and PLAT), especially when subjected to hypoxic conditions.211–214 Considering that post-MI cell death results from a deficiency of oxygenation, MSCs are valuable cells to promote angiogenesis and vasculogenesis to oxygenate the infarct zone. To show the efficacy of the MSCs for treating post-MI tissue, Guo et al. recently formed a human umbilical cord mesenchymal stem cell (hUCMSC) sheet and applied it to in vivo post-MI mouse model.215 They reported that applying the hUCMSC sheet reduced fibrosis in the border zone and increased the LV wall thickness compared to untreated and cell suspension-treated controls.215
Chen et al. conducted the first clinical trial with 69 post-MI patients, in which intracoronary injection of autologous bone marrow-derived MSCs significantly improved cardiac function and reduced scar size, showing their regenerative and restorative potential.216 Recently, Florea et al. isolated human MSCs (hMSCs) from four male donors (median age: 24-year-old) and delivered either low or high numbers (20 × 106 or 100 × 106) of hMSCs to the border zones of the chronically infarcted myocardial territory of 15 patients (median age: 66-year-old).217 They reported reduced scar size with both doses, while increased ejection fraction (EF) was reported only in the high cell dose group, highlighting the importance of using high cell doses and concentrations in cell therapies.217 Although MSC therapy has been proven to improve cardiac repair, there remains challenges with its use due to low retention rate, slow migration to the infarct zone, and low survival rate.218,219
2. Induced pluripotent stem cells (iPSCs)
iPSCs are produced by reprogramming somatic cells, allowing the use of the patient's own cells and reducing the rejection risk.220 Similar to MSCs, iPSCs can also be expanded extensively for large-scale cardiac repair purposes. Nelson et al. were the first to intramyocardially deliver iPSCs to immunodeficient mice,221 reporting significant improvement in ventricular function and reduced post-MI adverse remodeling, such as fibrosis, and confirmed the cardiac differentiation of the injected iPSCs in vivo. However, the injected iPSCs formed tumors in the immunodeficient mice, ultimately leading to reduced cardiac function due to the stress created by the tumor. Many other studies also verified the tumorigenic potential of the transplanted iPSCs in post-MI murine hearts.222,223 On the other hand, Templin et al. assessed the therapeutic potential of iPSCs in a large animal MI model for the first time and reported vascular differentiation and durability of iPSCs engraftment.224 Even though the tumor formation is a risk with iPSC treatments, there are a few ongoing clinical trials investigating the safety and efficacy of iPSCs.38
3. Stem cell-derived cardiomyocytes (ESC-CMs, iPSC-CMs, or iCMs)
Early studies regarding stem cell-based MI treatment strategies heavily relied on the use of ESC-derived CMs. ESC-CMs significantly improved electromechanical properties with regular calcium transient and regenerated the infarcted hearts in both small and large animal studies including nonhuman primates.225,226 However, ESCs and ESC-derived cells are ethically controversial and have the risk of rejection as well as tumorigenesis or teratoma formation once transplanted.227–230 Therefore, iCMs emerged as an attractive therapeutic option to replace the lost CMs without having the risks of rejection or tumorigenesis and have been the focus of many studies in recent years.39,231,232 Citro et al. were the first to compare the therapeutic efficacy of iCMs to the “gold standard” MSCs in 2014.233 Their results revealed that iCMs were equipotent with MSCs in improving cardiac function, while iCM therapy also attenuated cardiac fibrosis in rat hearts. Similarly, in large animal studies, iCMs significantly improved post-MI contractile function and cardiac bioenergetic efficiency.234 However, few iCMs were reported to survive in the long term (8 weeks post-transplantation).201,234 Recently, Shiba et al. delivered allogeneic iCMs into the infarct and border zones of non-human primates (cynomolgus monkey) and reported improved contractility for at least 12 weeks, but also an increased risk of non-lethal tachycardia.235
Overall, preclinical iCM therapies have proven to be effective in post-MI hearts, but challenges such as low retention rate, cardiac immaturity, population heterogeneity, and possible arrhythmia and tumorigenicity risks remain unsolved.39,220 Several strategies have been offered to mature iCMs, including biochemical manipulations,236–238 mechanical,239–241 and electrical stimuli.242–244 Even though structural maturity is often achieved, the contractile properties of iCMs remain at suboptimal levels, which limits the translation of iCMs to the clinic.231
4. Combinational cell therapies
Novel combinational cell therapies aim to leverage the synergistic effects of more than one cell type in repairing both the myocardium and the vasculature of the post-MI hearts. Park et al. reported that human iCMs combined with human MSC-loaded porcine heart ECM patches amplified cardiac repair in the rat MI model.40 MSC patches not only enhanced the retention and the engraftment of the iCMs but also constituted pleiotropic effects to promote iCM maturity. Moreover, researchers identified the key secreted factor(s) from hMSCs to be pro-angiogenetic (VEGFa, IGF-1, FGF2), anti-inflammatory (TGFβ1, IL-10), and anti-fibrotic (TIMP-2). Similarly, Natsumeda et al. reported that allogeneic MSCs and cardiac stem cells (CSCs) synergistically reduced scar size and improved cardiac function.245 Göttingen swine (minipig) were subjected to MI and administered either MSCs (200m/injection), CSCs (1m/injection), or the combination of these two cell types (200m MSCs:1m CSCs). This allogeneic cell combination therapy (ACCT) was reported to be safe and regenerative with no arrhythmogenicity and immune response up to 3 months post-therapy. In terms of cardiac function improvement, ACCT increased the perfusion in the infarct region, prevented post-MI adverse remodeling and enhanced cardiac contractility compared with either cell type alone. Additionally, the combination of MSCs and their derived exosomes have been reported to improve heart performance.246 In both in vitro and rat models, sequential delivery of exosomes, followed by MSCs (2 × 106 MSCs) three days later, improved the MSC retention and survival at the infarct region, reduced the scar size, promoted neovascularization, and improved cardiac function (i.e., LVEF). Overall, combinational cell therapies hold great potential as they benefit from the pleiotropic effects of the additional cells and/or their secretome.
5. Mechanisms of action
There are two proposed mechanisms of action for the cell therapies: First is that the transplanted cells will proliferate and repopulate the damaged myocardium. This proposed mechanism is, however, seriously challenged by studies, showing that transplanted cell survival and retention is extremely low and the surviving cells cannot be the source of cardiac recovery.247 The second, and currently prevailing, mechanism of action is that secreted biomolecules from transplanted cells promote endogenous repair through stimulating pro-angiogenic and anti-inflammatory pathways and recruiting immune cells to the myocardium.247,248 In a pilot study, immune modulation therapy (i.e., altering immune response) was shown to significantly reduce the risk of death and hospitalization following MI.249 Moreover, a recent study revealed the underlying reason to be the regional accumulation of CCR2+ and CX3CR1+ macrophages.248 This once again highlights the effect of the paracrine factors secreted by the transplanted cells in modulating the post-MI recovery.
B. Extracellular vesicles (EVs)
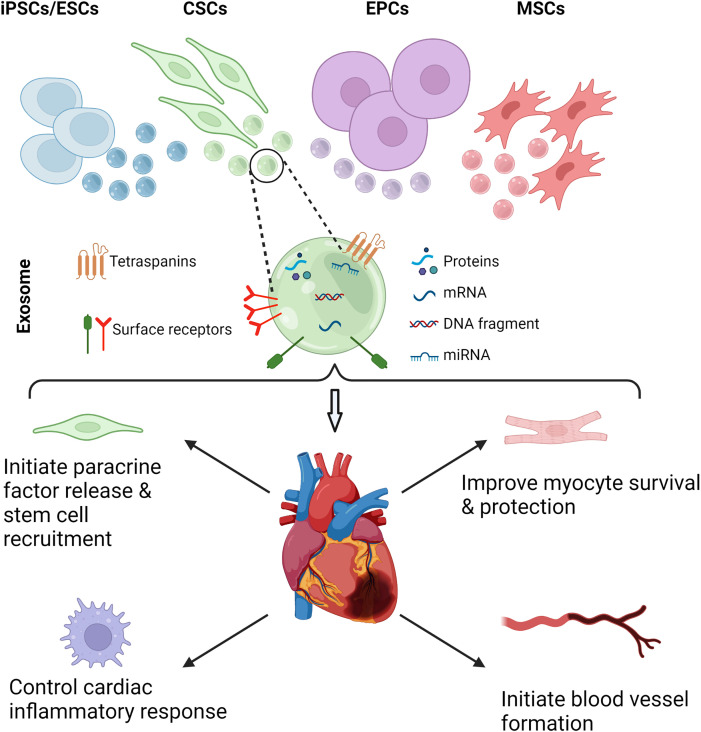
While the exact mechanisms by which stem cells promote cardiac repair and immunomodulation in vivo are not well understood, recent studies suggest that paracrine signaling pathways stimulated by molecules such as cytokines, growth factors, and microRNAs (miRNAs) are major effectors of both pro- and anti-fibrotic signaling pathways post-MI.208–210 Cytokines and miRNAs are attractive targets for intervention because these signaling molecules are often packaged in EVs in a highly controlled way, enabling efficient targeting of these biomolecules to designated cell types. Additionally, EVs can be isolated from blood plasma and other biofluids, from individual patients, or from cell effluent, using stem cells or in vitro models, with relative ease compared to the complex processes required for other methods.250 Exosomes, an EV subgroup with diameters typically between 30 and 200 nm, are commonly released from most cell types and are major vehicles for cytokines, chemokines, miRNA, and other miscellaneous signaling molecules that can be internalized by and affect function in recipient cells (Fig. 4). These contents are known to influence many diverse and pathologically relevant biological processes, including angiogenesis, immune cell phenotype, immunomodulation, endothelial, and epithelial to mesenchymal transition, and cell differentiation, and as such, exosomes are both packaged and released from cells in a highly controlled manner, often as a response to detected stimuli.251
FIG. 4.
Exosomes are excreted from numerous stem cells such as induced pluripotent stem cells (iPSCs), embryonic stem cells (ESCs), cardiac stem cells (CSCs), endothelial progenitor cells (EPCs), and mesenchymal stem cells (MSCs) (top). Exosomes consist of proteins, mRNA, miRNA, and DNA fragments (middle). When applied on the damaged heart tissue, exosomes initiate paracrine factor release leading to stem cell recruitment, modulate immune response, improve myocyte survival and protection, and initiate new blood vessel formation.
The usage of exosomes secreted from stem cells as a cell-free therapy has recently come under investigation, both as an independent treatment and in conjunction with injectable biomaterials. Stem cell exosomes have been shown to improve cardiac recovery during both ischemia and reperfusion phases in MI for CMs, endothelial cells, and CFs in vitro, and reducing infarct size and adverse inflammation and improving functional recovery post-MI in in vivo studies, with more in vivo and human clinical studies under way.252 While this effect appears to be independent of the stem cell type, MSCs, particularly bone-marrow-derived MSCs (BMMSCs) have been most widely investigated.197,252 Integration of BMMSC exosomes with existing cardiac patch strategies has proven effective in a host of preclinical trials and is under investigation in a number of ongoing clinical investigations, as demonstrated by Fields et al.47 Completed preclinical studies by Huang et al. suggest that this integrated, cell-free cardiac patch can provide similar or improved cardioprotective effects compared with existing treatments and that the use of this cell-free type of patch reduces the risk of autoimmune response to the patch, increases viable storage time, and is easily produced, combining the benefits of exosomal treatment with the utility of a cardiac patch.253
C. Direct reprogramming of cardiac fibroblasts into cardiomyocytes
With the discovery of the prospect of direct differentiation of CFs to CMs, a novel method for treating the infarct region has emerged.44 This reprogramming is attainable through introducing three cardiogenic transcription factors, namely, Gata4, Mef2c, and Tbx5 (GMT).254 Over the last decade, studies were dedicated to investigating the effect and role of using different miRNAs,255,256 culture conditions,257,258 epigenetic factors,259,260 transcription factors257,261,262 and mechanobiology in direct reprogramming.263,264
Although direct reprogramming has a great potential for treating the fibrotic tissue formed after MI, there are some limitations toward translation to clinical trials. First, the reprogramming efficiency was reported to be insufficient in humans, compared to small animal in vivo models, and it must be enhanced. Second, the safety and efficacy of this treatment should be verified with large animal in vivo models. Finally, the underlying molecular mechanism of the direct reprogramming has not been fully understood yet, and therefore, more studies should be dedicated to investigating it.44
D. Biomaterials-based therapies
Biomaterials-based tissue engineering approaches combine natural or synthetic polymers, bioactive molecules, and/or cells for post-MI recovery purposes. Previously, natural polymers such as collagen, alginate, hyaluronic acid (HA), and decellularized ECM showed high biocompatibility and improved therapeutic function.197,265,266 Specifically, decellularized ECM and collagen scaffolds have been shown to enhance endogenous cardiac repair while also providing mechanical and electrical support for the damaged cardiac tissue.267–271 Similarly, in their study Yokoyama et al. used fibronectin, which is an ECM protein, to create 3D iCM cardiac tissues through a special layer-by-layer fabrication method and reported improved cell viability under in vitro hypoxic conditions, as well-improved LVEF when implanted in a rat MI model.272 Another protein used to induce CM regeneration is agrin. Agrin was shown to induce CM proliferation in vitro,273 and promote regeneration of infarcted heart in mice273 and pigs.274 It was also shown that periostin-loaded gelatin foams induced cardiac regeneration with improved myocardial function in a rat model by reintroducing mononucleated CMs to the cell cycle without inducing fibrosis.275 Alternatively, synthetic polymers such as poly(lactic-co-glycolic) acid (PLGA) and poly-(L-lactic) acid (PLLA) show controllable and strong mechanical and physical properties.43 To benefit the robust biocompatibility of the natural polymers along with the strength and consistency of the synthetic materials, researchers have been using hybrid biomaterials with or without cells.276,277 On the other hand, the use of non-conductive biomaterials may hinder electrical signal propagation in scar tissue, resulting in non-synchronous contraction of CMs.278 Therefore, employing electroconductive materials that complement the conductive nature of heart tissue have been shown to be promising in improving the electrophysiological properties after MI.278,279 Additionally, composites of non-conductive polymers with conductive nanoparticles such as carbon nanotubes (CNTs),280,281 graphene,282,283 gold nanoparticles,284,285 or MXene286 have been shown to improve the electroconductive behavior of the polymers they were blended in, and enhanced cell–cell communication and functional properties.284
The mode of delivery is usually injection to the infarct zone to attenuate the adverse cardiac remodeling and increase cell retention in the damaged heart.287 Alternatively, engineered 3D constructs (i.e., cardiac patches) can be delivered through a catheter for minimally invasive percutaneous therapies, which is explained in more detail in Secs. IV D 1 and IV E.
1. Injectable hydrogels
Hydrogels are crosslinked 3D polymer networks that can absorb a large amount of water. The polymer can be locally injected and then crosslinked in situ to form a stable hydrogel via physical and chemical stimuli, without the need for any major surgery.268,288
ECM has been an attractive biomaterial for regeneration and functional recovery and is shown to be more efficacious when derived from younger tissues.265,289,290 Preclinical studies showed the feasibility of ECM use to mediate post-MI ventricular remodeling, improve function, and increase the number of endogenous CMs.42,291,292 In 2019, Traverse et al. conducted a first-in-man study and transendocardially injected decellularized porcine cardiac ECM-derived hydrogel (Ventri-Gel) into 15 post-MI patients (57–62 years old). Through LV volumes, EF, and scar size evaluation by cardiac magnetic resonance (CMR) imaging, serum B-type natriuretic peptide (BNP) level, the 6-min walk test distance, and quality of life using New York Heart Association (NYHA) functional classification assessment at 3- and 6-month post-treatment, researchers verified the safety, feasibility, and effectiveness of injecting ECM hydrogels in the clinic.197 A recent approach for post-MI recovery is local and sustained delivery of ECM subsets such as EVs and nucleic acids via injected hydrogels. Methacrylated-HA (MeHA) hydrogel-based miRNA delivery showed improved outcomes in a mouse model, ensuring sustained CM proliferation for two weeks and functional improvements for four weeks with a single injection.293 Similarly, in both small and large animal models, natural hydrogels improved the therapeutic effect of EVs due to precise localization and increased retention.293–295
Additionally, researchers have engineered hydrogels for enhanced mechanical and/or electrical performance. Li et al. combined gold nanoparticles (AuNP) with RGD functionalized MeHA hydrogel (AuNP-HA) and encapsulated iCMs for post-MI cardiac recovery.296 They reported enhanced angiogenic effects exerted by encapsulated iCMs. Additionally, due to the conductive nature of AuNP-HA, it increased gap junction expression and resynchronized electrical conduction in the mouse heart. In a sheep model, Contessotto et al. developed a degradable elastin-like recombinamer or peptide (ELR or ELP) hydrogel with mechanical and bioactive customization. Intramyocardial injection of ELR hydrogels resulted in a less fibrotic and more angiogenic ischemic core, as well as CM functional improvement.297
The efficacy of injectable biomaterials has spurred interest in the clinical applications of individual bio-components while improving the storage capability and production efficiency of these biomaterials. In particular, the combination of stem cells and collagen-based hydrogels has reliably improved cardiac function post-MI in porcine models without any adverse inflammatory effect, showing promising potential for translation to clinical trials.295 Additionally, this methodology is further refined to develop a cell-free patch that recapitulates this success, in order to provide demonstrated clinical benefits while minimizing the risk of an immune response or other negative effects associated with cell-dependent therapies.253
E. Cardiac patches
Cardiac patches are porous matrices that aim to physically support cardiac function, and repair damaged cardiac tissue.298 Although the main purpose of placing a cardiac patch on the scar tissue is to promote functional CM migration toward the damaged tissue and repair it, the patch can also be used as a delivery vehicle to protect the therapeutic cargo molecules and improve their survival and stability, as explained in Secs. IV B and IV D.299 In both regards, therapeutic cardiac patches have shown great potential in improving cardiac function.41,298,300
Cellular cardiac patches have been engineered by combining different cell types such as stem cells299,301 and iCMs41,299 with natural and synthetic biomaterials as well as conductive hydrogels or conductive nanoparticle-hydrogel composites.302 Recently, EVs, growth factors, and miRNAs have become the preferred factors encapsulated in biomaterials, replacing cells.45,46 The common approach to determining the effectiveness of the therapeutic patches is evaluating angiogenesis, infarct region size reduction, LV wall thickness, and cardiac contractility properties in animal models.298,299,303 As mentioned previously, rat models have been the platform of choice for such evaluations, yet there are some studies that are done with other rodents and macaques, while some have recently transitioned to clinical trials.199 Various techniques have been used to fabricate the cardiac patches such as direct encapsulation,41,304 3D bioprinting,305–307 and electrospinning.308,309
Tens of papers have been published on therapeutic cardiac patches in 2021 alone (reviewed in Ref. 43). Here, we will focus on the most recent studies (published in 2021 and 2022) that have developed cardiac patches using various methods and evaluated their potential as a cardiac patch by assessing their mechanical and physical properties, stability, and cytotoxicity using in vitro models and/or their therapeutic potential using in vivo models.
1. Direct seeding or encapsulation of the cells
Numerous studies have developed cardiac patches by directly seeding the cells on biomaterials. Ozturk et al. seeded rat bone marrow-derived stem cells on the decellularized bovine pericardium, evaluated the effect of the electromechanically stimulated patch on the infarct region, and showed the patch was successfully integrated with the infarct region and that cell migration took place.310 Similarly, Jiang et al. seeded iCMs on decellularized rat placenta and showed that compared to an acellular patch and iCMs alone, the cellularized patch was more effective in reducing the infarct region and improving neovascularization in a post-MI rat model.299 Pretorius et al. engineered thick (∼2.12 mm), viable, and functional cardiac patches using a layer-by-layer assembly of the iEC, iCM, and CF-seeded fibrin layers.300 Through the 4 weeks of culture, they observed that the iCMs were synchronized with a beating frequency comparable to the human heart (55 beats per minute) and cardiac-specific marker expression increased. On the other hand, they also reported an increase in apoptosis/necrosis markers throughout the culture period, but the change was not significant.300 Song et al. developed conductive cardiac patches by incorporating polyacrylic acid into alginate/gelatin hydrogel and seeding rat CMs on top.311 They reported improved CM sarcomere alignment, improved cardiac function restoration, and new vessel formation in an in vivo rat MI model.311 Distinctively, He et al. engineered cardiac patches by seeding rat CMs on cellulose obtained from sea squirts, which are responsible for biofouling in marine aquaculture, with the goal of converting “trash” to “treasure.” They showed that the patches obtained were functional in vitro and improved cardiac function of MI rats in vivo.312
Menasche et al. conducted the first in human study using fibrin scaffold-based cardiac patches with embedded hESC-derived cardiac progenitor cells and showed restored heart function after implantation of the patches on to the fibrotic heart tissue.313 Bobylev et al. suggested improving the poor mechanical properties of fibrin by reinforcing it with spider silk and reported that the obtained material is mechanically stable enough to be used as a cardiac patch.314
As briefly discussed in Sec. IV B, acellular cardiac patches have been used as a tool to deliver therapeutic factors to the infarct region. Liu et al. encapsulated an extracellular protein, reelin, in collagen patches and showed that it improves heart function, protects CMs from apoptosis, and reduces the infarct area in adult mice after MI.304 In an interesting study, Zhu et al. designed a nitrate-functionalized patch by covalently binding nitrate groups to the biodegradable poly-(ε-caprolactone) (PCL) polymer so that small molecule drugs could be loaded onto the patch. As it is applied to the myocardium, nitric oxide (NO) is released from the patch, which provided effective cardioprotection, improved heart function, and decreased adverse remodeling.315
Most recently, Tiburcy et al. started clinical trials with 53 patients for the cardiac patch that they have developed over the last 20 years, in which they focused on creating ESC- and iPSC-engineered human myocardium using a repeatable manufacturing protocol.41 They were able to create mature patches by co-culturing ESC-CMs or iCMs and hCFs (70% to 30% ratio), develop a special media to enhance iCM viability, establish a serum-free protocol, and scale up cardiac patch production.
2. 3D bioprinting
3D bioprinting is one of the most preferred tools to create cardiac patches (reviewed in Refs. 301 and 176). Here, we will focus on recent developments in bioprinted cardiac patches, which utilized different biomaterials and methods.
Hybrid biomaterials have been created by mixing various hydrogels and sometimes conductive materials to fabricate patches that are strong, conductive, and printable, to improve cellular response.302 3D bioprinted CNT-reinforced alginate and methacrylated collagen were combined with human coronary artery endothelial cells (HCAEC) to improve electrical conductivity, compressive modulus, and cell proliferation and migration, showing potential for future in vivo studies.305 Similarly, Mehrotra et al. created a hybrid conductive bioink by mixing non-mulberry-silk, GelMA, PEGDA, and CNTs and bioprinted anisotropic vascularized tissue constructs by mixing this bioink with HUVECs prior to printing. They kept this construct in culture in a perfusion-based reactor for 14 days to ensure vasculature formation, and then seeded it with neonatal rat CMs and incorporated the construct with calcium peroxide (CPO) and IL-10-based GelMA microspheres, hypothesizing that the release of oxygen from CPO microspheres would support CM and HUVEC viability after implantation at the MI region.316 Ten days after implantation to a rat MI model, they observed neovascularization near the patch.316 Basara et al. created conductive hybrid cardiac patches by printing Ti3C2Tx MXene on PEG constructs using an aerosol jet printer and seeding iCMs on them, and reported that viable, patternable, functional tissue constructs can be developed using this method with improved contraction kinetics and cardiac related protein and gene expression.36 In a similar manner, hybrid bioinks were created and characterized by different groups using GelMA and collagen with ventricle-type human CMs and CFs,60 alginate and gelatin with endothelial cells,317 HA and gelatin with human cardiac-derived progenitor cells (hCMPCs).318 On the other hand, instead of utilizing hydrogels, Yang et al. created a different type of cardiac scaffold composing of poly-(glycerol sebacate) (PGS) (a thermoset polymer) and PCL (a thermoplastic polymer).319 By utilizing fused deposition modeling 3D printing technology, they printed this construct, and seeded it with H9C2 rat CMs. In their in vivo rat MI experiments, they showed that application of this cardiac patch increased LV wall thickness, reduced infarct region size, improved vascularization, and promoted tissue repair.319
A number of studies have focused on the 3D bioprinting of scaffold-free cardiac patches by printing cardiac spheroids on needle arrays according to the desired 3D pattern.177,306 Both studies reported enhanced vascularization, smaller scar tissue area and improved cardiac function when these patches were tested in rat MI models, indicating the potential of cardiac spheroids formed patches as a therapeutic treatment for MI.177,306
Gao et al. utilized a different method called multiphoton-excited 3D printing (MPE-3DP), to create a micropatterned scaffold with biomimetic cardiac ECM structure using a photoactive gelatin polymer solution and seeded this construct with iCMs, iECs and iPSCs-derived smooth muscle cells. The therapeutic effect of this cardiac patch was then evaluated in an in vivo MI murine model, which revealed elevated cardiac function, increase in wall thickness, better vascularization, and fewer apoptotic cells.320
In their study, Noor et al. created vascularized cardiac patches by mixing iCMs with decellularized omental tissue to create the cardiac tissue construct, and by mixing iECs with sacrificial bioink (gelatin).321 They confirmed the functionality of the printed cardiac patches by evaluating the calcium transient properties and performing immunostaining. One main advantage of this method is the ability to create patient-specific cardiac patches with a thickness of a couple millimeters. Distinctively, Sue et al. utilized hydrodynamic focusing to create the cardiac patches.322 They printed free-standing biomimetic microvessels using hydrodynamic focusing of HUVECs with gelatin, and Tisseel® fibrin gel with human cardiosphere-derived stromal cells (hCSCs) cast on top.322 In vivo experiments with rat and pig MI models revealed that the patch promoted CM proliferation, new blood vessel formation, and cardiac function recovery.322
3. Electrospinning
In addition to 3D bioprinting, electrospinning has been widely utilized to engineer cardiac patches.323 To create conductive cardiac patches, Feng et al. electrospun polyurethane/polyaniline/silicon oxide polymers and showed improved electrical signal transduction.308 They performed an adhesion experiment using the patch and a porcine heart and reported adequate self-adhesion that can endure multiple diastolic and systolic movements of the heart.308 Similarly, Liang et al. blended silk fibroin with conductive polypyrrole prior to electrospinning to create conductive biocompatible cardiac patches and reported improved Ca+2 kinetics, sarcomere structure, and cardiac-specific protein expression of the seeded neonatal rat CMs, showing a potential for future in vivo studies.324 In another study, Jain et al. created a cell-free patch by electrospinning PCL and PCL blended with gelatin (PCLG) and coating it with cerium oxide nanoparticles (nCe). They showed that nCe/PCLG nanofibers reduced ROS production and prevented agonist-induced cardiac hypertrophy in neonatal rat CMs.309
4. Injectable cardiac patches
As discussed in Sec. IV E 3, various techniques have been used to create biomimetic 3D patches with fixed geometry and size.325–327 Although these patches can range from 100 μm to a couple millimeters thickness with aligned and spontaneously beating CMs, traditional cardiac patch placement on the heart usually needs an open-chest surgery and suturing to the epicardial surface of the heart, and hence is traumatizing for the patient.328,329 Recent studies aim for minimally invasive procedures that improve the translational capacity of the cardiac patches. Soft and flexible cardiac patches can be injected rather than implanted. Recently developed cardiac patches have shape memory due to their microfabricated lattice design that allow them to be folded into a needle as small as 1 mm to be delivered by injection. Montgomery et al. reported minimally invasive delivery of 1 cm x 1 cm cell-laden cardiac patch made of ultraviolet (UV)-crosslinkable and elastomeric polymer poly[octamethylene maleate (anhydride) citrate] (POMAC) on the porcine heart without open-chest surgery.330 The effective elasticity of the POMAC patches allowed them to maintain size and shape as well as to integrate into the host heart with high cellular viability of the delivered CMs in the patch. Although patch delivery was not inferior to the surgical placed patches, the small size of the patch would necessitate delivery of multiple patches in the case of a large injury. Wang et al. used methacrylated elastin, gelatin, and carbon nanotubes to develop injectable and conductive cardiac patches.331 In both rat and minipig MI models, both acellular and CM-loaded patches have maintained their shape and led to functional repair for up to 4 weeks. Focusing more on commercialization, Huang et al. developed cell-free, off-the-shelf patches by cryopreservation.253 They combined the benefits of the native ultrastructural properties of the decellularized porcine myocardium and bioactivity of human cardiac stromal cell secretome for post-MI cardiac repair in both rodent and porcine models. As a novel approach, Tang et al. developed a regenerative fibrin patch by injecting in situ polymerizable biomaterials directly on the damaged tissue using a double-lumen syringe with the help of pressurized carbon dioxide, referred to as a spray painting device by the authors.332 The platelet fibrin patch which is created by injecting platelet-rich plasma and media solution with calcium attenuated adverse remodeling, reduced the scar size, and preserved post-MI cardiac function in a mouse MI model via the release of regenerative growth factors.
Cardiac patches are one of the most preferred tools to treat the damaged myocardium after MI, with some studies going through clinical trials.41 Recent developments allow researchers to create complex cardiac patches including cell types that are primary for heart tissue and biomimetic materials, as well as vasculature network and biochemical factors. On the other hand, smooth integration of the cardiac patch with the heart tissue in terms of beating synchronously, withstanding the cyclic motion, and not creating arrhythmias still remains as limitations, and developing cardiac patches that meet these imperative requirements remains a challenge.333
V. CURRENT LIMITATIONS AND PROSPECTS
In this review, we looked at MI from a tissue engineering and regenerative medicine point of view and put together recent developments on different methods to engineer I/R models and post-MI tissue models as well as the regenerative therapies available to treat the long-term outcomes of MI. We highlighted the materials and methods used for each section and talked about their advantages and limitations. This review aims to provide the big picture of what has been studied and discovered regarding MI from modeling to treating, with a goal of guiding the present researchers through the materials and methods that can be used.
Despite the advancements in the tissue-engineering field, to date, some challenges remain to be resolved to create biomimetic in vitro cardiac tissue models. These challenges result from the human heart being a very complex organ.109 In order to properly create the heart tissue in vitro, the model should have the aligned myofibril structure of the heart with longitudinal shape of ventricular myocytes, and should allow for the cells to communicate for authentic contraction.335 Although some studies have been able to develop models addressing these considerations, creating functional models on a larger scale still remains a challenge due to difficulty in incorporating vasculature together with fabricated cardiac tissue.336 Larger constructs engineered using mature cardiac cells and quick fabrication methods integrated with the vasculature and blood mimetic flow would be the next step of in vitro cardiac tissue models to study I/R.
Aside from modeling the complex heart tissue, it is near impossible to completely mimic the cascade of mechanisms that lead to scar tissue formation following MI. On the other hand, engineering fibrotic tissue models in vitro is less challenging than creating animal models, and it is important to create platforms for testing potential therapies in place of or before in vivo studies. Creating the fibrotic tissue models shares similar limitations as mentioned previously for engineering the cardiac tissue models. Additionally, taking into account the effect of the immune system as well as patient-specific variances, such as age, gender, and genetic makeup, remain a challenge.337,338
To date, not many studies have utilized in vitro models as a platform to evaluate the efficacy of the therapeutic options. As researchers develop more biomimetic, reliable, and clinically relevant models, it will become preferable to use these tools to test new therapies. Developing novel therapies for injured heart tissue has been the focus of numerous studies. However, due to the limited regenerative capacity of the human heart, it is challenging to find methods to fully repair and regenerate the infarct region post-MI. Cell therapies alone are limited due to their low retention and very short cell survival period after injection. For the biomaterials-based methods and cardiac patches, their biocompatibility as well as integration with host myocardium must be further improved. Additionally, the implementation of most cardiac patches is invasive and would require open heart surgery, which might pose a risk for patients.43 In terms of therapeutics, future studies will most likely shift their focus from cell therapies to cell-secreted factor therapies (EVs, cytokines), which eliminate the risk of an inflammatory reaction of the host and are less invasive compared to placing cardiac patches. These factors may also be combined with injectable biomaterials to enhance the local retention and the effect of the factors, while keeping the procedure minimally invasive.
VI. CONCLUSIONS
Cardiac tissue engineering has developed profoundly in recent years, and with the currently available tools, it is possible to engineer more representative cardiac models to reliably study I/R as well as to create the fibrotic tissue after MI. However, researchers are still far from fully recapitulating the response of the human body and the human heart microenvironment in vitro. Engineering in vitro models is quite crucial for developing and testing new therapeutic options before animal studies. In that regard, understanding the benefits and drawbacks of the materials and methods used by seeing the broad view is essential to develop better models and therapies. An ideal model should recapitulate the native cardiac tissue as comprehensively as possible both in healthy and diseased states, and it should incorporate all the cell types in the heart at the correct ratios, densities, and organization, as well as the appropriate biomaterials with the necessary biochemical, biophysical, and biomechanical cues, so that functional tissues with functional vasculature are formed. With the developments in the tissue engineering field, researchers are getting closer to creating more biomimetic healthy cardiac tissue and infarct tissue models. From the regenerative therapy point of view, researchers are utilizing different tools to create the best treatment to recover the heart from the effects of MI. For the therapy to be successful, it should initiate regeneration, recover cardiac functionality, and prevent arrhythmias without triggering an immune response. Although some therapies are still due for primary results, there are potential therapies that have been translated into clinical trials reporting hopeful results.
ACKNOWLEDGMENTS
This work was supported by NIH (Award No. R01HL141909), NSF CAREER Award (No. 1651385), and the Naughton Fellowship. All figures are created with BioRender.com.
AUTHOR DECLARATIONS
Conflict of Interest
The authors have no conflicts to disclose.
Author Contributions
Gozde Basara: Conceptualization (lead); Writing – original draft (lead); Writing – review & editing (lead). Gokhan Bahcecioglu: Conceptualization (equal); Supervision (lead); Writing – original draft (equal); Writing – review & editing (equal). S. Gulberk Ozcebe: Writing – original draft (equal); Writing – review & editing (equal). Bradley W Ellis: Writing – original draft (equal); Writing – review & editing (equal). George Ronan: Writing – original draft (supporting); Writing – review & editing (equal). Pinar Zorlutuna: Conceptualization (equal); Funding acquisition (lead); Project administration (lead); Resources (lead); Supervision (lead); Writing – original draft (equal); Writing – review & editing (equal).
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Laflamme M. A. and Murry C. E., “ Regenerating the heart,” Nat. Biotechnol. 23, 845–856 (2005). 10.1038/nbt1117 [DOI] [PubMed] [Google Scholar]
- 2. Virani S. S. et al. , “ Heart disease and stroke statistics—2021 Update,” Circulation 143, e254 (2021). 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 3. Chung M. K., Zidar D. A., Bristow M. R., Cameron S. J., Chan T., Harding C. V., Kwon D. H., Singh T., Tilton J. C., Tsai E. J., Tucker N. R., Barnard J., and Loscalzo J., “ COVID-19 and cardiovascular disease,” Circ. Res. 128, 1214–1236 (2021). 10.1161/CIRCRESAHA.121.317997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodgers J. L., Jones J., Bolleddu S. I., Vanthenapalli S., Rodgers L. E., Shah K., Karia K., and Panguluri S. K., “ Cardiovascular risks associated with gender and aging,” J. Cardiovascu. Dev. Dis. 6, 19 (2019). 10.3390/jcdd6020019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reddy K., Khaliq A., and Henning R. J., “ Recent advances in the diagnosis and treatment of acute myocardial infarction,” World J. Cardiol. 7, 243–276 (2015). 10.4330/wjc.v7.i5.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalogeris T., Baines C. P., Krenz M., and Korthuis R. J., “ Cell biology of ischemia/reperfusion injury,” Int. Rev. Cell Mol. Biol. 298, 229 (2012). 10.1016/B978-0-12-394309-5.00006-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hausenloy D. J., Barrabes J. A., Bøtker H. E., Davidson S. M., Lisa F. D., Downey J., Engstrom T., Ferdinandy P., Carbrera-Fuentes H. A., Heusch G., Ibanez B., Iliodromitis E. K., Inserte J., Jennings R., Kalia N., Kharbanda R., Lecour S., Marber M., Miura T., Ovize M., Perez-Pinzon M. A., Piper H. M., Przyklenk K., Schmidt M. R., Redington A., Ruiz-Meana M., Vilahur G., Vinten-Johansen J., Yellon D. M., and Garcia-Dorado D., “ Ischaemic conditioning and targeting reperfusion injury: A 30 year voyage of discovery,” Basic Res. Cardiol. 111, 70 (2016). 10.1007/s00395-016-0588-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Granger D. N. and Kvietys P. R., “ Reperfusion injury and reactive oxygen species: The evolution of a concept,” Redox Biol. 6, 524–551 (2015). 10.1016/j.redox.2015.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kathiresan S. and Srivastava D., “ Genetics of human cardiovascular disease,” Cell 148, 1242–1257 (2012). 10.1016/j.cell.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu B., Yu H., Zwartbol M., Ruifrok W. P., van Gilst W. H., de Boer R. A., and Silljé H. H. W., “ Identification of hypertrophy- and heart failure-associated genes by combining in vitro and in vivo models,” Physiol. Genomics 44, 443–454 (2012). 10.1152/physiolgenomics.00148.2011 [DOI] [PubMed] [Google Scholar]
- 11. Sarkar K., Cai Z., Gupta R., Parajuli N., Fox-Talbot K., Darshan M. S., Gonzalez F. J., and Semenza G. L., “ Hypoxia-inducible factor 1 transcriptional activity in endothelial cells is required for acute phase cardioprotection induced by ischemic preconditioning,” Proc. Natl. Acad. Sci. U. S. A. 109, 10504–10509 (2012). 10.1073/pnas.1208314109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tenreiro M. F., Louro A. F., Alves P. M., and Serra M., “ Next generation of heart regenerative therapies: Progress and promise of cardiac tissue engineering,” Npj Regener. Med. 6, 30 (2021). 10.1038/s41536-021-00140-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singhal A. K., Abrams J. D., Mohara J., Hasz R. D., Nathan H. M., Fisher C. A., Furukawa S., and Goldman B. I., “ Potential suitability for transplantation of hearts from human non-heart-beating donors: Data review from the gift of life donor program,” J. Heart Lung Transplant 24, 1657–1664 (2005). 10.1016/j.healun.2004.11.043 [DOI] [PubMed] [Google Scholar]
- 14. Taylor D. O., Stehlik J., Edwards L. B., Aurora P., Christie J. D., Dobbels F., Kirk R., Kucheryavaya A. Y., Rahmel A. O., and Hertz M. I., “ Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth official adult heart transplant report—2009,” J. Heart Lung Transplant 28, 1007–1022 (2009). 10.1016/j.healun.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 15.See http://www.organdonor.gov/statistics-stories/statistics.html for “ Organ Donation Statistics—Organ Donor ” (accessed February 20, 2020).
- 16. Leung C. M., de Haan P., and Ronaldson-Bouchard K. et al., “ A guide to the organ-on-a-chip,” Nat. Rev. Methods Primers 2, 33 (2022). 10.1038/s43586-022-00118-6 [DOI] [Google Scholar]
- 17. Zhang Y. S., Aleman J., Arneri A., Bersini S., Piraino F., Shin S. R., Dokmeci M. R., and Khademhosseini A., “ From cardiac tissue engineering to heart-on-a-chip: Beating challenges,” Biomed. Mater. 10, 034006 (2015). 10.1088/1748-6041/10/3/034006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ribas J., Sadeghi H., Manbachi A., Leijten J., Brinegar K., Zhang Y. S., Ferreira L., and Khademhosseini A., “ Cardiovascular organ-on-a-chip platforms for drug discovery and development,” Appl. Vitro Toxicol. 2, 82–96 (2016). 10.1089/aivt.2016.0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hay M., Thomas D. W., Craighead J. L., Economides C., and Rosenthal J., “ Clinical development success rates for investigational drugs,” Nat. Biotechnol. 32, 40 (2014). 10.1038/nbt.2786 [DOI] [PubMed] [Google Scholar]
- 20. Perel P., Roberts I., Sena E., Wheble P., Briscoe C., Sandercock P., Macleod M., Mignini L. E., Jayaram P., and Khan K. S., “ Comparison of treatment effects between animal experiments and clinical trials: Systematic review,” BMJ 334, 197 (2007). 10.1136/bmj.39048.407928.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang G., McCain M. L., Yang L., He A., Pasqualini F. S., Agarwal A., Yuan H., Jiang D., Zhang D., Zangi L., Geva J., Roberts A. E., Ma Q., Ding J., Chen J., Wang D.-Z., Li K., Wang J., Wanders R. J. A., Kulik W., Vaz F. M., Laflamme M. A., Murry C. E., Chien K. R., Kelley R. I., Church G. M., Parker K. K., and Pu W. T., “ Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies,” Nat. Med. 20, 616–623 (2014). 10.1038/nm.3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skolimowski M., Nielsen M. W., Abeille F., Skafte-Pedersen P., Sabourin D., Fercher A., Papkovsky D., Molin S., Taboryski R., Sternberg C., Dufva M., Geschke O., and Emnéus J., “ Modular microfluidic system as a model of cystic fibrosis airways,” Biomicrofluidics 6, 034109 (2012). 10.1063/1.4742911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Israel M. A., Yuan S. H., Bardy C., Reyna S. M., Mu Y., Herrera C., Hefferan M. P., Van Gorp S., Nazor K. L., Boscolo F. S., Carson C. T., Laurent L. C., Marsala M., Gage F. H., Remes A. M., Koo E. H., and Goldstein L. S. B., “ Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells,” Nature 482, 216–220 (2012). 10.1038/nature10821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ellis B. W., Acun A., Can U. I., and Zorlutuna P., “ Human iPSC-derived myocardium-on-chip with capillary-like flow for personalized medicine,” Biomicrofluidics 11, 024105 (2017). 10.1063/1.4978468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Acun A. and Zorlutuna P., “ Engineered myocardium model to study the roles of HIF-1α and HIF1A-AS1 in paracrine-only signaling under pathological level oxidative stress,” Acta Biomater. 58, 323–336 (2017). 10.1016/j.actbio.2017.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhatia S. N. and Ingber D. E., “ Microfluidic organs-on-chips,” Nat. Biotechnol. 32, 760–772 (2014). 10.1038/nbt.2989 [DOI] [PubMed] [Google Scholar]
- 27. Jastrzebska E., Tomecka E., and Jesion I., “ Heart-on-a-chip based on stem cell biology,” Biosens. Bioelectron. 75, 67–81 (2016). 10.1016/j.bios.2015.08.012 [DOI] [PubMed] [Google Scholar]
- 28. Zhang Y. S. and Khademhosseini A., “ Seeking the right context for evaluating nanomedicine: From tissue models in petri dishes to microfluidic organs-on-a-chip,” Nanomedicine 10, 685–688 (2015). 10.2217/nnm.15.18 [DOI] [PubMed] [Google Scholar]
- 29. Simmons C. S., Petzold B. C., and Pruitt B. L., “ Microsystems for biomimetic stimulation of cardiac cells,” Lab Chip 12, 3235–3248 (2012). 10.1039/c2lc40308k [DOI] [PubMed] [Google Scholar]
- 30. Yang H. and Ma Z., “ Microsystem for stem cell-based cardiovascular research,” BioNanoSci. 2, 305–315 (2012). 10.1007/s12668-012-0064-3 [DOI] [Google Scholar]
- 31. Basara G., Ozcebe S. G., Ellis B. W., and Zorlutuna P., “ Tunable human myocardium derived decellularized extracellular matrix for 3D bioprinting and cardiac tissue engineering,” Gels 7, 70 (2021). 10.3390/gels7020070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Engelmayr G. C., Cheng M., Bettinger C. J., Borenstein J. T., Langer R., and Freed L. E., “ Accordion-like honeycombs for tissue engineering of cardiac anisotropy,” Nat. Mater. 7, 1003–1010 (2008). 10.1038/nmat2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kai D., Prabhakaran M. P., Jin G., and Ramakrishna S., “ Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering,” J. Biomed. Mater. Res. A 99, 376–385 (2011). 10.1002/jbm.a.33200 [DOI] [PubMed] [Google Scholar]
- 34. Neal R. A., Jean A., Park H., Wu P. B., Hsiao J., Engelmayr G. C., Langer R., and Freed L. E., “ Three-dimensional elastomeric scaffolds designed with cardiac-mimetic structural and mechanical features,” Tissue Eng., Part A 19, 793–807 (2013). 10.1089/ten.tea.2012.0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agarwal A., Farouz Y., Nesmith A. P., Deravi L. F., McCain M. L., and Parker K. K., “ Micropatterning alginate substrates for in vitro cardiovascular muscle on a chip,” Adv. Funct. Mater. 23, 3738–3746 (2013). 10.1002/adfm.201203319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Basara G., Saeidi-Javash M., Ren X., Bahcecioglu G., Wyatt B. C., Anasori B., Zhang Y., and Zorlutuna P., “ Electrically conductive 3D printed Ti3C2Tx MXene-PEG composite constructs for cardiac tissue engineering,” Acta Biomater. 139, 179 (2022). 10.1016/j.actbio.2020.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Björnmalm M., Yan Y., and Caruso F., “ Engineering and evaluating drug delivery particles in microfluidic devices,” J. Controlled Release 190, 139–149 (2014). 10.1016/j.jconrel.2014.04.030 [DOI] [PubMed] [Google Scholar]
- 38. Kim J. Y., Nam Y., Rim Y. A., and Ju J. H., “ Review of the current trends in clinical trials involving induced pluripotent stem cells,” Stem Cell Rev. Rep. 18, 142–154 (2022). 10.1007/s12015-021-10262-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tu C. and Zoldan J., “ Moving iPSC-derived cardiomyocytes forward to treat myocardial infarction,” Cell Stem Cell 23, 322–323 (2018). 10.1016/j.stem.2018.08.011 [DOI] [PubMed] [Google Scholar]
- 40. Park S.-J., Kim R. Y., Park B.-W., Lee S., Choi S. W., Park J.-H., Choi J. J., Kim S.-W., Jang J., Cho D.-W., Chung H.-M., Moon S.-H., Ban K., and Park H.-J., “ Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction,” Nat. Commun. 10, 3123 (2019). 10.1038/s41467-019-11091-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tiburcy M., Hudson J. E., Balfanz P., Schlick S., Meyer T., Liao M.-L. C., Levent E., Raad F., Zeidler S., Wingender E., Riegler J., Wang M., Gold J. D., Kehat I., Wettwer E., Ravens U., Dierickx P., van Laake L. W., Goumans M. J., Khadjeh S., Toischer K., Hasenfuss G., Couture L. A., Unger A., Linke W. A., Araki T., Neel B., Keller G., Gepstein L., Wu J. C., and Zimmermann W.-H., “ Defined engineered human myocardium with advanced maturation for applications in heart failure modelling and repair,” Circulation 135, 1832–1847 (2017). 10.1161/CIRCULATIONAHA.116.024145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spang M. T. and Christman K. L., “ Extracellular matrix hydrogel therapies: In vivo applications and development,” Acta Biomater. 68, 1–14 (2018). 10.1016/j.actbio.2017.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mei X. and Cheng K., “ Recent development in therapeutic cardiac patches,” Front. Cardiovasc. Med. 7, 294 (2020). 10.3389/fcvm.2020.610364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yamakawa H. and Ieda M., “ Cardiac regeneration by direct reprogramming in this decade and beyond,” Inflammation Regener. 41, 20 (2021). 10.1186/s41232-021-00168-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Abreu R. C., Fernandes H., da Costa Martins P. A., Sahoo S., Emanueli C., and Ferreira L., “ Native and engineered extracellular vesicles for cardiovascular therapeutics,” Nat. Rev. Cardiol. 17, 685–697 (2020). 10.1038/s41569-020-0389-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Firoozi S., Pahlavan S., Ghanian M.-H., Rabbani S., Barekat M., Nazari A., Pakzad M., Shekari F., Hassani S.-N., Moslem F., Lahrood F. N., Soleimani M., and Baharvand H., “ Mesenchymal stem cell-derived extracellular vesicles alone or in conjunction with a SDKP-conjugated self-assembling peptide improve a rat model of myocardial infarction,” Biochem. Biophys. Res. Commun. 524, 903–909 (2020). 10.1016/j.bbrc.2020.02.009 [DOI] [PubMed] [Google Scholar]
- 47. Fields L., Ito T., Kobayashi K., Ichihara Y., Podaru M.-N., Hussain M., Yamashita K., Machado V., Lewis-McDougall F., and Suzuki K., “ Epicardial placement of human MSC-loaded fibrin sealant films for heart failure: Preclinical efficacy and mechanistic data,” Mol. Ther. 29, 2554–2570 (2021). 10.1016/j.ymthe.2021.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moghaddam A. S., Afshari J. T., Esmaeili S.-A., Saburi E., Joneidi Z., and Momtazi-Borojeni A. A., “ Cardioprotective microRNAs: Lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease,” Atherosclerosis 285, 1–9 (2019). 10.1016/j.atherosclerosis.2019.03.016 [DOI] [PubMed] [Google Scholar]
- 49. Ozaki Y., Katagiri Y., Onuma Y., Amano T., Muramatsu T., Kozuma K., Otsuji S., Ueno T., Shiode N., Kawai K., Tanaka N., Ueda K., Akasaka T., Hanaoka K. I., Uemura S., Oda H., Katahira Y., Kadota K., Kyo E., Sato K., Sato T., Shite J., Nakao K., Nishino M., Hikichi Y., Honye J., Matsubara T., Mizuno S., Muramatsu T., Inohara T., Kohsaka S., Michishita I., Yokoi H., Serruys P. W., Ikari Y., and Nakamura M., “ CVIT expert consensus document on primary percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI) in 2018,” Cardiovasc. Intervention Ther. 33, 178–203 (2018). 10.1007/s12928-018-0516-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Turillazzi E., Pomara C., Bello S., Neri M., Riezzo I., and Fineschi V., “ The meaning of different forms of structural myocardial injury, immune response and timing of infarct necrosis and cardiac repair,” Curr. Vasc. Pharmacol. 13, 6–19 (2015). 10.2174/15701611113119990008 [DOI] [PubMed] [Google Scholar]
- 51. Epelman S., Liu P. P., and Mann D. L., “ Role of innate and adaptive immune mechanisms in cardiac injury and repair,” Nat. Rev. Immunol. 15(15), 117–129 (2015). 10.1038/nri3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fan Z.-X. and Yang J., “ The role of microRNAs in regulating myocardial ischemia reperfusion injury,” Saudi Med. J. 36, 787–793 (2015). 10.15537/smj.2015.7.11089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boon R. A. and Dimmeler S., “ MicroRNAs in myocardial infarction,” Nat. Rev. Cardiol. 12, 198 (2014). 10.1038/nrcardio.2014.207 [DOI] [PubMed] [Google Scholar]
- 54. Gentek R. and Hoeffel G., “ The innate immune response in myocardial infarction, repair, and regeneration,” Adv. Exp. Med. Biol. 1003, 251–272 (2017). 10.1007/978-3-319-57613-8 [DOI] [PubMed] [Google Scholar]
- 55. Lindsey M. L., Bolli R., Canty J. M., Du X.-J., Frangogiannis N. G., Frantz S., Gourdie R. G., Holmes J. W., Jones S. P., Kloner R. A., Lefer D. J., Liao R., Murphy E., Ping P., Przyklenk K., Recchia F. A., Longacre L. S., Ripplinger C. M., Eyk J. E. V., and Heusch G., “ Guidelines for experimental models of myocardial ischemia and infarction,” Am. J. Physiol.: Heart Circ. Physiol. 314, H812 (2018). 10.1152/ajpheart.00335.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kumar M., Kasala E. R., Bodduluru L. N., Dahiya V., Sharma D., Kumar V., and Lahkar M., “ Animal models of myocardial infarction: Mainstay in clinical translation,” Regul. Toxicol. Pharmacol. 76, 221–230 (2016). 10.1016/j.yrtph.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 57. Shin H. S., Shin H. H., and Shudo Y., “ Current status and limitations of myocardial infarction large animal models in cardiovascular translational research,” Front. Bioeng. Biotechnol. 9, 673683 (2021). 10.3389/fbioe.2021.673683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Villiers C. D. and Riley P. R., “ Mouse models of myocardial infarction: Comparing permanent ligation and ischaemia-reperfusion,” Dis. Model. Mech. 13, dmm046565 (2020). 10.1242/dmm.046565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Golforoush P., Yellon D. M., and Davidson S. M., “ Mouse models of atherosclerosis and their suitability for the study of myocardial infarction,” Basic Res. Cardiol. 115, 73 (2020). 10.1007/s00395-020-00829-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim K. S., Joo H. J., Choi S.-C., Kim J.-H., Park C.-Y., Song M.-H., Noh J.-M., Cha J.-J., Hong S. J., Ahn T. H., Kim M.-N., Na J. E., Rhyu I. J., and Lim D.-S., “ Transplantation of 3D bio-printed cardiac mesh improves cardiac function and vessel formation via ANGPT1/Tie2 pathway in rats with acute myocardial infarction,” Biofabrication 13, 045014 (2021). 10.1088/1758-5090/ac1e78 [DOI] [PubMed] [Google Scholar]
- 61. Srikanth G., Prakash P., Tripathy N., Dikshit M., and Nityanand S., “ Establishment of a rat model of myocardial infarction with a high survival rate: A suitable model for evaluation of efficacy of stem cell therapy,” J. Stem Cells Regener. Med. 5, 30–36 (2009). 10.46582/jsrm.0501006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wu Y., Yin X., Wijaya C., Huang M.-H., and McConnell B. K., “ Acute myocardial infarction in rats,” J. Vis. Exp. 48, 2464 (2011). 10.3791/2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Huang Z., Shen Y., Sun A., Huang G., Zhu H., Huang B., Xu J., Song Y., Pei N., Ma J., Yang X., Zou Y., Qian J., and Ge J., “ Magnetic targeting enhances retrograde cell retention in a rat model of myocardial infarction,” Stem Cell Res. Ther. 4, 149 (2013). 10.1186/scrt360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tan M.-Y., Xia B., Xiao Z., Fan Z.-W., Zhou H., Guo X., and Huang Y.-C., “ Development of a new model for acute myocardial infarction in rabbits,” J. Vet. Med. Sci. 79, 467–473 (2017). 10.1292/jvms.16-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Morrissey P. J., Murphy K. R., Daley J. M., Schofield L., Turan N. N., Arunachalam K., Abbott J. D., and Koren G., “ A novel method of standardized myocardial infarction in aged rabbits,” Am. J. Physiol.: Heart Circ. Physiol. 312, H959–H967 (2017). 10.1152/ajpheart.00582.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fujita M., Morimoto Y., Ishihara M., Shimizu M., Takase B., Maehara T., and Kikuchi M., “ A new rabbit model of myocardial infarction without endotracheal intubation,” J. Surg. Res. 116, 124–128 (2004). 10.1016/S0022-4804(03)00304-4 [DOI] [PubMed] [Google Scholar]
- 67. Wang F., Wen L., Liu J., Peng W., Meng Z., Chen Q., Wang Y., Ke B., Guo Y., and Mi P., “ Albumin nanocomposites with MnO2/Gd2O3 motifs for precise MR imaging of acute myocardial infarction in rabbit models,” Biomaterials 230, 119614 (2020). 10.1016/j.biomaterials.2019.119614 [DOI] [PubMed] [Google Scholar]
- 68. Wu G., Du Z., Hu C., Zheng Z., Zhan C., Ma H., Fang D., Ahmed K. T., Laham R. J., Hui J. C. K., and Lawson W. E., “ Angiogenic effects of long-term enhanced external counterpulsation in a dog model of myocardial infarction,” Am. J. Physiol.: Heart Circ. Physiol. 290, H248–H254 (2006). [DOI] [PubMed] [Google Scholar]
- 69. Zhang H., Cui Y., Tian Y., Yuan W., Yang J., Peng P., Li K., Liu X., Zhang D., Wu A., Zhou Z., and Tang Y., “ A novel model for evaluating thrombolytic therapy in dogs with ST-elevation myocardial infarction,” BMC Cardiovasc. Disord. 16, 21 (2016). 10.1186/s12872-016-0194-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Guerrero-Miranda C. Y. and Hall S. A., “ Dog model holds promise for early mechanical unloading in patients with acute myocardial infarction,” Circ.: Heart Failure 11, e004972 (2018). 10.1161/CIRCHEARTFAILURE.118.004972 [DOI] [PubMed] [Google Scholar]
- 71. Rabbani S., Ahmadi H., Fayazzadeh E., Sahebjam M., Boroumand M. A., Sotudeh M., and Nassiri S. M., “ Development of an ovine model of myocardial infarction,” ANZ J. Surg. 78, 78–81 (2008). 10.1111/j.1445-2197.2007.04359.x [DOI] [PubMed] [Google Scholar]
- 72. Rienzo M., Imbault J., Boustani Y. E., Beurton A., Sampedrano C. C., Pasdois P., Pernot M., Bernus O., Haïssaguerre M., Couffinhal T., and Ouattara A., “ A total closed chest sheep model of cardiogenic shock by percutaneous intracoronary ethanol injection,” Sci. Rep. 10, 12417 (2020). 10.1038/s41598-020-68571-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lock M. C., Darby J. R. T., Soo J. Y., Brooks D. A., Perumal S. R., Selvanayagam J. B., Seed M., Macgowan C. K., Porrello E. R., Tellam R. L., and Morrison J. L., “ Differential response to injury in fetal and adolescent sheep hearts in the immediate post-myocardial infarction period,” Front. Physiol. 10, 208 (2019). 10.3389/fphys.2019.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu S., Li K., Wagner Florencio L., Tang L., Heallen T. R., Leach J. P., Wang Y., Grisanti F., Willerson J. T., Perin E. C., Zhang S., and Martin J. F., “ Gene therapy knockdown of Hippo signaling induces cardiomyocyte renewal in pigs after myocardial infarction,” Sci. Transl. Med. 13, eabd6892 (2021). 10.1126/scitranslmed.abd6892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bikou O., Watanabe S., Hajjar R. J., and Ishikawa K., “ A pig model of myocardial infarction: Catheter-based approaches,” in Experimental Models of Cardiovascular Diseases: Methods and Protocols, edited by Ishikawa K. ( Springer, New York, NY, 2018), pp. 281–294. [DOI] [PubMed] [Google Scholar]
- 76. McCall F. C., Telukuntla K. S., Karantalis V., Suncion V. Y., Heldman A. W., Mushtaq M., Williams A. R., and Hare J. M., “ Myocardial infarction and intramyocardial injection models in swine,” Nat. Protoc. 7, 1479–1496 (2012). 10.1038/nprot.2012.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Teramoto N., Koshino K., Yokoyama I., Miyagawa S., Zeniya T., Hirano Y., Fukuda H., Enmi J., Sawa Y., Knuuti J., and Iida H., “ Experimental pig model of old myocardial infarction with long survival leading to chronic left ventricular dysfunction and remodeling as evaluated by PET,” J. Nucl. Med. 52, 761–768 (2011). 10.2967/jnumed.110.084848 [DOI] [PubMed] [Google Scholar]
- 78. Li Y., Chen X., Jin R., Chen L., Dang M., Cao H., Dong Y., Cai B., Bai G., Gooding J. J., Liu S., Zou D., Zhang Z., and Yang C., “ Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs,” Sci. Adv. 7, eabd6740 (2021). 10.1126/sciadv.abd6740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hirano A., Fujita J., Kanazawa H., Kawaguchi S., Handa N., Yamada Y., Okuda S., Hishikawa S., Teratani T., Kunita S., Tohyama S., Seki T., Tabei R., Nakajima K., Kishino Y., Okada M., Okamoto K., Shimizu H., Kobayashi E., and Fukuda K., “ Cryoinjury-induced acute myocardial infarction model and ameroid constrictor-induced ischemic heart disease model in adult micro-mini pigs for preclinical studies,” Transl. Med. Commun. 2, 1 (2017). 10.1186/s41231-017-0011-y [DOI] [Google Scholar]
- 80. Sayour A. A., Celeng C., Oláh A., Ruppert M., Merkely B., and Radovits T., “ Sodium–glucose cotransporter 2 inhibitors reduce myocardial infarct size in preclinical animal models of myocardial ischaemia–reperfusion injury: A meta-analysis,” Diabetologia 64, 737–748 (2021). 10.1007/s00125-020-05359-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Raposo L., Lourenço A. P., Nascimento D. S., Cerqueira R., Cardim N., and Leite-Moreira A., “ Human umbilical cord tissue-derived mesenchymal stromal cells as adjuvant therapy for myocardial infarction: A review of current evidence focusing on pre-clinical large animal models and early human trials,” Cytotherapy 23, 974–979 (2021). 10.1016/j.jcyt.2021.05.002 [DOI] [PubMed] [Google Scholar]
- 82. Zhang H., Dvornikov A. V., Huttner I. G., Ma X., Santiago C. F., Fatkin D., and Xu X., “ A Langendorff-like system to quantify cardiac pump function in adult zebrafish,” Dis. Model. Mech. 11, dmm034819 (2018). 10.1242/dmm.034819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li H., Liu C., Bao M., Liu W., Nie Y., Lian H., and Hu S., “ Optimized Langendorff perfusion system for cardiomyocyte isolation in adult mouse heart,” J. Cell. Mol. Med. 24, 14619–14625 (2020). 10.1111/jcmm.15773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rossello X., Hall A. R., Bell R. M., and Yellon D. M., “ Characterization of the Langendorff perfused isolated mouse heart model of global ischemia–reperfusion injury: impact of ischemia and reperfusion length on infarct size and LDH release,” J. Cardiovasc. Pharmacol. Ther. 21, 286–295 (2016). 10.1177/1074248415604462 [DOI] [PubMed] [Google Scholar]
- 85. Wang K., Terrar D., Gavaghan D. J., Mu-u-min R., Kohl P., and Bollensdorff C., “ Living cardiac tissue slices: An organotypic pseudo two-dimensional model for cardiac biophysics research,” Prog. Biophys. Mol. Biol. 115, 314–327 (2014). 10.1016/j.pbiomolbio.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 86. Ou Q., Jacobson Z., Abouleisa R. R. E., Tang X.-L., Hindi S. M., Kumar A., Ivey K. N., Giridharan G., El-Baz A., Brittian K., Rood B., Lin Y.-H., Watson S. A., Perbellini F., McKinsey T. A., Hill B. G., Jones S. P., Terracciano C. M., Bolli R., and Mohamed T. M. A., “ Physiological biomimetic culture system for pig and human heart slices,” Circ. Res. 125, 628–642 (2019). 10.1161/CIRCRESAHA.119.314996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pitoulis F. G., Watson S. A., Perbellini F., and Terracciano C. M., “ Myocardial slices come to age: An intermediate complexity in vitro cardiac model for translational research,” Cardiovasc. Res. 116, 1275–1287 (2020). 10.1093/cvr/cvz341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fischer C., Milting H., Fein E., Reiser E., Lu K., Seidel T., Schinner C., Schwarzmayr T., Schramm R., Tomasi R., Husse B., Cao-Ehlker X., Pohl U., and Dendorfer A., “ Long-term functional and structural preservation of precision-cut human myocardium under continuous electromechanical stimulation in vitro,” Nat. Commun. 10, 117 (2019). 10.1038/s41467-018-08003-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kang C., Qiao Y., Li G., Baechle K., Camelliti P., Rentschler S., and Efimov I. R., “ Human organotypic cultured cardiac slices: New platform for high throughput preclinical human trials,” Sci. Rep. 6, 28798 (2016). 10.1038/srep28798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Watson S. A., Scigliano M., Bardi I., Ascione R., Terracciano C. M., and Perbellini F., “ Preparation of viable adult ventricular myocardial slices from large and small mammals,” Nat. Protoc. 12, 2623–2639 (2017). 10.1038/nprot.2017.139 [DOI] [PubMed] [Google Scholar]
- 91. Alqarni F., Alsaadi M., and Karem F., “ MR image analysis of ex vivo mouse model of heart ischemia,” Saudi J. Biol. Sci. 28, 1990–1998 (2021). 10.1016/j.sjbs.2020.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Montero-Bullon J.-F., Aveiro S. S., Melo T., Martins-Marques T., Lopes D., Neves B., Girão H., Rosário M Domingues M., and Domingues P., “ Cardiac phospholipidome is altered during ischemia and reperfusion in an ex vivo rat model,” Biochem. Biophys. Rep. 27, 101037 (2021). 10.1016/j.bbrep.2021.101037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yadav V., Chong N., Ellis B., Ren X., Senapati S., Chang H.-C., and Zorlutuna P., “ Constant-potential environment for activating and synchronizing cardiomyocyte colonies with on-chip ion-depleting perm-selective membranes,” Lab Chip 20, 4273–4284 (2020). 10.1039/D0LC00809E [DOI] [PubMed] [Google Scholar]
- 94. Ellis B. W., Traktuev D. O., Merfeld-Clauss S., Can U. I., Wang M., Bergeron R., Zorlutuna P., and March K. L., “ Adipose stem cell secretome markedly improves rodent heart and hiPSC-derived cardiomyocyte recovery from cardioplegic transport solution exposure,” Stem Cells 39, 170–182 (2021). 10.1002/stem.3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. He L. and Zhou B., “ Cardiomyocyte proliferation: Remove brakes and push accelerators,” Cell Res. 27, 959–960 (2017). 10.1038/cr.2017.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhao M.-T., Ye S., Su J., and Garg V., “ Cardiomyocyte proliferation and maturation: Two sides of the same coin for heart regeneration,” Front. Cell Dev. Biol. 8, 594226 (2020). 10.3389/fcell.2020.594226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Peter A. K., Bjerke M. A., and Leinwand L. A., “ Biology of the cardiac myocyte in heart disease,” Mol. Biol. Cell 27, 2149–2160 (2016). 10.1091/mbc.E16-01-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Watanabe M., Horie H., Kurata Y., Inoue Y., Notsu T., Wakimizu T., Adachi M., Yamamoto K., Morikawa K., Kuwabara M., Sakaguchi T., Morisaki T., Miake J., Nishimura M., Tsuneto M., Shirayoshi Y., Ito S., Kitakaze M., Ninomiya H., Yamamoto K., and Hisatome I., “ Esm1 and Stc1 as angiogenic factors responsible for protective actions of adipose-derived stem cell sheets on chronic heart failure after rat myocardial infarction,” Circ. J. 85, 657–666 (2021). 10.1253/circj.CJ-20-0877 [DOI] [PubMed] [Google Scholar]
- 99. Szaraz P., Gratch Y. S., Iqbal F., and Librach C. L., “ In vitro differentiation of human mesenchymal stem cells into functional cardiomyocyte-like cells,” J. Visualized Exp. 126, e55757 (2017). 10.3791/55757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Guo X., Bai Y., Zhang L., Zhang B., Zagidullin N., Carvalho K., Du Z., and Cai B., “ Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: New regulators and its implications,” Stem Cell Res. Ther. 9, 44 (2018). 10.1186/s13287-018-0773-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Choi S.-C., Seo H.-R., Cui L.-H., Song M.-H., Noh J.-M., Kim K.-S., Choi J.-H., Kim J.-H., Park C.-Y., Joo H. J., Hong S. J., Ko T. H., Choi J.-I., Kim H. J., Kim J.-H., Paek S.-H., Park J.-N., Kim D.-H., Jang Y., Park Y., and Lim D.-S., “ Modeling hypoxic stress in vitro using human embryonic stem cells derived cardiomyocytes matured by FGF4 and ascorbic acid treatment,” Cells 10, 2741 (2021). 10.3390/cells10102741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ren X., Ellis B. W., Ronan G., Blood S. R., DeShetler C., Senapati S., March K. L., Handberg E., Anderson D., Pepine C., Chang H.-C., and Zorlutuna P., “ A multiplexed ion-exchange membrane-based miRNA (MIX·miR) detection platform for rapid diagnosis of myocardial infarction,” Lab Chip 21, 3876–3887 (2021). 10.1039/D1LC00685A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sebastião M. J., Serra M., Pereira R., Palacios I., Gomes-Alves P., and Alves P. M., “ Human cardiac progenitor cell activation and regeneration mechanisms: Exploring a novel myocardial ischemia/reperfusion in vitro model,” Stem Cell Res. Ther. 10, 77 (2019). 10.1186/s13287-019-1174-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Häkli M., Kreutzer J., Mäki A.-J., Välimäki H., Lappi H., Huhtala H., Kallio P., Aalto-Setälä K., and Pekkanen-Mattila M., “ Human induced pluripotent stem cell-based platform for modeling cardiac ischemia,” Sci. Rep. 11, 4153 (2021). 10.1038/s41598-021-83740-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Clerk A., Kemp T. J., Zoumpoulidou G., and Sugden P. H., “ Cardiac myocyte gene expression profiling during H2O2-induced apoptosis,” Physiol. Genomics 29, 118–127 (2007). 10.1152/physiolgenomics.00168.2006 [DOI] [PubMed] [Google Scholar]
- 106. Son E., Lee D., Woo C.-W., and Kim Y.-H., “ The optimal model of reperfusion injury in vitro using H9c2 transformed cardiac myoblasts,” Korean J. Physiol. Pharmacol. 24, 173–183 (2020). 10.4196/kjpp.2020.24.2.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gentile C., “ Filling the gaps between the in vivo and in vitro microenvironment: engineering of spheroids for stem cell technology,” Curr. Stem Cell Res. Ther. 11, 652–665 (2016). 10.2174/1574888X10666151001114848 [DOI] [PubMed] [Google Scholar]
- 108. Chen T. and Vunjak-Novakovic G., “ In vitro models of ischemia-reperfusion injury,” Regener. Eng. Transl. Med. 4, 142–153 (2018). 10.1007/s40883-018-0056-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sharma P., Wang X., Ming C. L. C., Vettori L., Figtree G., Boyle A., and Gentile C., “ Considerations for the bioengineering of advanced cardiac in vitro models of myocardial infarction,” Small 17, 2003765 (2021). 10.1002/smll.202003765 [DOI] [PubMed] [Google Scholar]
- 110. Williams M. A. C., Mair D. B., Lee W., Lee E., and Kim D.-H., “ Engineering three-dimensional vascularized cardiac tissues,” Tissue Eng., Part B 28, 336–350 (2022). 10.1089/ten.teb.2020.0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wanjare M., Kawamura M., Hu C., Alcazar C., Wang H., Woo Y. J., and Huang N. F., “ Vascularization of engineered spatially patterned myocardial tissue derived from human pluripotent stem cells in vivo,” Front. Bioeng. Biotechnol. 7, 208 (2019). 10.3389/fbioe.2019.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Haraguchi Y., Shimizu T., Yamato M., and Okano T., “ Regenerative therapies using cell sheet-based tissue engineering for cardiac disease,” Cardiol. Res. Pract. 2011, 845170. 10.4061/2011/845170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yamato M. and Okano T., “ Cell sheet engineering,” Mater. Today 7, 42–47 (2004). 10.1016/S1369-7021(04)00234-2 [DOI] [Google Scholar]
- 114. Masuda S., Shimizu T., Yamato M., and Okano T., “ Cell sheet engineering for heart tissue repair,” Adv. Drug Delivery Rev. 60, 277–285 (2008). 10.1016/j.addr.2007.08.031 [DOI] [PubMed] [Google Scholar]
- 115. Sasaki D., Matsuura K., Seta H., Haraguchi Y., Okano T., and Shimizu T., “ Contractile force measurement of human induced pluripotent stem cell-derived cardiac cell sheet-tissue,” PLOS ONE 13, e0198026 (2018). 10.1371/journal.pone.0198026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sakaguchi K., Takahashi H., Tobe Y., Sasaki D., Matsuura K., Iwasaki K., Shimizu T., and Umezu M., “ Measuring the contractile force of multilayered human cardiac cell sheets,” Tissue Eng., Part C 26, 485–492 (2020). 10.1089/ten.tec.2020.0164 [DOI] [PubMed] [Google Scholar]
- 117. Haraguchi Y., Shimizu T., Matsuura K., Sekine H., Tanaka N., Tadakuma K., Yamato M., Kaneko M., and Okano T. III, “ Cell sheet technology for cardiac tissue engineering,” in Cardiac Tissue Engineering: Methods and Protocols, edited by Radisic M. and Black L. D. ( Springer, New York, NY, 2014), pp. 139–155. [DOI] [PubMed] [Google Scholar]
- 118. Wang L., Serpooshan V., and Zhang J., “ Engineering human cardiac muscle patch constructs for prevention of post-infarction LV remodeling,” Front. Cardiovasc. Med. 8, 111 (2021). 10.3389/fcvm.2021.621781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yamasaki Y., Matsuura K., Sasaki D., and Shimizu T., “ Assessment of human bioengineered cardiac tissue function in hypoxic and re-oxygenized environments to understand functional recovery in heart failure,” Regener. Ther. 18, 66–75 (2021). 10.1016/j.reth.2021.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zuppinger C., “ 3D cardiac cell culture: A critical review of current technologies and applications,” Front. Cardiovasc. Med. 6, 87 (2019). 10.3389/fcvm.2019.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hofer M. and Lutolf M. P., “ Engineering organoids,” Nat. Rev. Mater. 6, 402–420 (2021). 10.1038/s41578-021-00279-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kim J., Koo B.-K., and Knoblich J. A., “ Human organoids: Model systems for human biology and medicine,” Nat. Rev. Mol. Cell Biol. 21, 571–584 (2020). 10.1038/s41580-020-0259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhao D., Lei W., and Hu S., “ Cardiac organoid—A promising perspective of preclinical model,” Stem Cell Res. Ther. 12, 272 (2021). 10.1186/s13287-021-02340-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fujii M., Matano M., Toshimitsu K., Takano A., Mikami Y., Nishikori S., Sugimoto S., and Sato T., “ Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition,” Cell Stem Cell 23, 787 (2018). 10.1016/j.stem.2018.11.016 [DOI] [PubMed] [Google Scholar]
- 125. Lancaster M. A., Renner M., Martin C.-A., Wenzel D., Bicknell L. S., Hurles M. E., Homfray T., Penninger J. M., Jackson A. P., and Knoblich J. A., “ Cerebral organoids model human brain development and microcephaly,” Nature 501, 373–379 (2013). 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Takasato M., Er P. X., Chiu H. S., Maier B., Baillie G. J., Ferguson C., Parton R. G., Wolvetang E. J., Roost M. S., Chuva de Sousa Lopes S. M., and Little M. H., “ Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis,” Nature 526, 564–568 (2015). 10.1038/nature15695 [DOI] [PubMed] [Google Scholar]
- 127. Hu H., Gehart H., Artegiani B., LÖpez-Iglesias C., Dekkers F., Basak O., van Es J., Chuva de Sousa Lopes S. M., Begthel H., Korving J., van den Born M., Zou C., Quirk C., Chiriboga L., Rice C. M., Ma S., Rios A., Peters P. J., de Jong Y. P., and Clevers H., “ Long-term expansion of functional mouse and human hepatocytes as 3D organoids,” Cell 175, 1591 (2018). 10.1016/j.cell.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 128. Turco M. Y., Gardner L., Hughes J., Cindrova-Davies T., Gomez M. J., Farrell L., Hollinshead M., Marsh S. G. E., Brosens J. J., Critchley H. O., Simons B. D., Hemberger M., Koo B.-K., Moffett A., and Burton G. J., “ Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium,” Nat. Cell Biol. 19, 568–577 (2017). 10.1038/ncb3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Arai K., Murata D., Verissimo A. R., Mukae Y., Itoh M., Nakamura A., Morita S., and Nakayama K., “ Fabrication of scaffold-free tubular cardiac constructs using a Bio-3D printer,” PLOS ONE 13, e0209162 (2018). 10.1371/journal.pone.0209162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Drakhlis L., Biswanath S., Farr C.-M., Lupanow V., Teske J., Ritzenhoff K., Franke A., Manstein F., Bolesani E., Kempf H., Liebscher S., Schenke-Layland K., Hegermann J., Nolte L., Meyer H., de la Roche J., Thiemann S., Wahl-Schott C., Martin U., and Zweigerdt R., “ Human heart-forming organoids recapitulate early heart and foregut development,” Nat. Biotechnol. 39, 737–746 (2021). 10.1038/s41587-021-00815-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Nugraha B., Buono M. F., von Boehmer L., Hoerstrup S. P., and Emmert M. Y., “ Human cardiac organoids for disease modeling,” Clin. Pharmacol. Ther. 105, 79–85 (2019). 10.1002/cpt.1286 [DOI] [PubMed] [Google Scholar]
- 132. Lewis-Israeli Y. R., Wasserman A. H., Gabalski M. A., Volmert B. D., Ming Y., Ball K. A., Yang W., Zou J., Ni G., Pajares N., Chatzistavrou X., Li W., Zhou C., and Aguirre A., “ Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease,” Nat. Commun. 12, 5142 (2021). 10.1038/s41467-021-25329-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hulot J.-S., “ Modeling cardiac arrhythmias with organoids∗,” J. Am. Coll. Cardiol. 73, 2325–2327 (2019). 10.1016/j.jacc.2019.01.076 [DOI] [PubMed] [Google Scholar]
- 134. Stillitano F., Turnbull I. C., Karakikes I., Nonnenmacher M., Backeris P., Hulot J.-S., Kranias E. G., Hajjar R. J., and Costa K. D., “ Genomic correction of familial cardiomyopathy in human engineered cardiac tissues,” Eur. Heart J. 37, 3282–3284 (2016). 10.1093/eurheartj/ehw307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Voges H. K., Mills R. J., Elliott D. A., Parton R. G., Porrello E. R., and Hudson J. E., “ Development of a human cardiac organoid injury model reveals innate regenerative potential,” Development 144, 1118–1127 (2017). 10.1242/dev.143966 [DOI] [PubMed] [Google Scholar]
- 136. Sharma P., Ming C. L. C., Wang X., Bienvenu L. A., Beck D., Figtree G., Boyle A., and Gentile C., “ Biofabrication of advanced in vitro 3D models to study ischaemic and doxorubicin-induced myocardial damage,” Biofabrication 14, 025003 (2022). 10.1088/1758-5090/ac47d8 [DOI] [PubMed] [Google Scholar]
- 137. Katare R. G., Ando M., Kakinuma Y., and Sato T., “ Engineered heart tissue: A novel tool to study the ischemic changes of the heart in vitro,” PLOS ONE 5, e9275 (2010). 10.1371/journal.pone.0009275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yang H., Shao N., Holmström A., Zhao X., Chour T., Chen H., Itzhaki I., Wu H., Ameen M., Cunningham N. J., Tu C., Zhao M.-T., Tarantal A. F., Abilez O. J., and Wu J. C., “ Transcriptome analysis of non human primate-induced pluripotent stem cell-derived cardiomyocytes in 2D monolayer culture vs. 3D engineered heart tissue,” Cardiovasc. Res. 117, 2125–2136 (2020). 10.1093/cvr/cvaa281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Chen T. and Vunjak-Novakovic G., “ Human tissue-engineered model of myocardial ischemia–reperfusion injury,” Tissue Eng., Part A 25, 711–724 (2019). 10.1089/ten.tea.2018.0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Mosadegh B., Dabiri B. E., Lockett M. R., Derda R., Campbell P., Parker K. K., and Whitesides G. M., “ Three-dimensional paper-based model for cardiac ischemia,” Adv. Healthcare Mater. 3, 1036–1043 (2014). 10.1002/adhm.201300575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hidalgo A., Glass N., Ovchinnikov D., Yang S.-K., Zhang X., Mazzone S., Chen C., Wolvetang E., and Cooper-White J., “ Modelling ischemia-reperfusion injury (IRI) in vitro using metabolically matured induced pluripotent stem cell-derived cardiomyocytes,” APL Bioeng. 2, 026102 (2018). 10.1063/1.5000746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Acun A., Nguyen T. D., and Zorlutuna P., “ In vitro aged, hiPSC-origin engineered heart tissue models with age-dependent functional deterioration to study myocardial infarction,” Acta Biomater. 94, 372–391 (2019). 10.1016/j.actbio.2019.05.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Acun A. and Zorlutuna P., “ CRISPR/Cas9 edited induced pluripotent stem cell-based vascular tissues to model aging and disease-dependent impairment,” Tissue Eng., Part A 25, 759–772 (2019). 10.1089/ten.tea.2018.0271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yue X., Acun A., and Zorlutuna P., “ Transcriptome profiling of 3D co-cultured cardiomyocytes and endothelial cells under oxidative stress using a photocrosslinkable hydrogel system,” Acta Biomater. 58, 337–348 (2017). 10.1016/j.actbio.2017.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Liu Z., Wang H., Wang Y., Lin Q., Yao A., Cao F., Li D., Zhou J., Duan C., Du Z., Wang Y., and Wang C., “ The influence of chitosan hydrogel on stem cell engraftment, survival and homing in the ischemic myocardial microenvironment,” Biomaterials 33, 3093–3106 (2012). 10.1016/j.biomaterials.2011.12.044 [DOI] [PubMed] [Google Scholar]
- 146. Funcke S., Werner T. R., Hein M., Ulmer B. M., Hansen A., Eschenhagen T., and Hirt M. N., “ Effects of the delta opioid receptor agonist dadle in a novel hypoxia-reoxygenation model on human and rat-engineered heart tissue: A pilot study,” Biomolecules 10, 1309 (2020). 10.3390/biom10091309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rupert C. E., Kim T. Y., Choi B.-R., and Coulombe K. L. K., “ Human cardiac fibroblast number and activation state modulate electromechanical function of hiPSC-cardiomyocytes in engineered myocardium,” Stem Cells Int. 2020, e9363809. 10.1155/2020/9363809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Giacomelli E., Meraviglia V., Campostrini G., Cochrane A., Cao X., van Helden R. W. J., Krotenberg Garcia A., Mircea M., Kostidis S., Davis R. P., van Meer B. J., Jost C. R., Koster A. J., Mei H., Míguez D. G., Mulder A. A., Ledesma-Terrón M., Pompilio G., Sala L., Salvatori D. C. F., Slieker R. C., Sommariva E., de Vries A. A. F., Giera M., Semrau S., Tertoolen L. G. J., Orlova V. V., Bellin M., and Mummery C. L., “ Human-iPSC-derived cardiac stromal cells enhance maturation in 3d cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease,” Cell Stem Cell 26, 862 (2020). 10.1016/j.stem.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Soon K., Mourad O., and Nunes S. S., “ Engineered human cardiac microtissues: The state-of-the-(He)art,” Stem Cells 39, 1008–1016 (2021). 10.1002/stem.3376 [DOI] [PubMed] [Google Scholar]
- 150. del Campo C. V., Liaw N. Y., Gunadasa-Rohling M., Matthaei M., Braga L., Kennedy T., Salinas G., Voigt N., Giacca M., Zimmermann W.-H., and Riley P. R., “ Regenerative potential of epicardium-derived extracellular vesicles mediated by conserved miRNA transfer,” Cardiovasc. Res. 118, 597 (2021). 10.1093/cvr/cvab054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Pampaloni F., Reynaud E. G., and Stelzer E. H. K., “ The third dimension bridges the gap between cell culture and live tissue,” Nat. Rev. Mol. Cell Biol. 8, 839–845 (2007). 10.1038/nrm2236 [DOI] [PubMed] [Google Scholar]
- 152. Bhagat S. and Singh S., “ Cultivating human tissues and organs over lab-on-a-chip models: Recent progress and applications,” in Progress in Molecular Biology and Translational Science, edited by Pandya A. and Singh V. ( Academic Press, 2022), Chap. 7, pp. 205–240. [DOI] [PubMed] [Google Scholar]
- 153. Uppal G., Bahcecioglu G., Zorlutuna P., and Vural D. C., “ Tissue failure propagation as mediated by circulatory flow,” Biophys. J. 119, 2573–2583 (2020). 10.1016/j.bpj.2020.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Yang Q., Xiao Z., Lv X., Zhang T., and Liu H., “ Fabrication and biomedical applications of heart-on-a-chip,” Int. J. Bioprint. 7, 370 (2021). 10.18063/ijb.v7i3.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Paloschi V., Sabater-Lleal M., Middelkamp H., Vivas A., Johansson S., van der Meer A., Tenje M., and Maegdefessel L., “ Organ-on-a-chip technology: A novel approach to investigate cardiovascular diseases,” Cardiovasc. Res. 117, 2742–2754 (2021). 10.1093/cvr/cvab088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ronaldson-Bouchard K. and Vunjak-Novakovic G., “ Organs-on-a-Chip: A fast track for engineered human tissues in drug development,” Cell Stem Cell 22, 310–324 (2018). 10.1016/j.stem.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Wagner K. T., Nash T. R., Liu B., Vunjak-Novakovic G., and Radisic M., “ Extracellular vesicles in cardiac regeneration: potential applications for tissues-on-a-chip,” Trends Biotechnol. 39, 755–773 (2021). 10.1016/j.tibtech.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhang Y. S., Arneri A., Bersini S., Shin S.-R., Zhu K., Goli-Malekabadi Z., Aleman J., Colosi C., Busignani F., Dell'Erba V., Bishop C., Shupe T., Demarchi D., Moretti M., Rasponi M., Dokmeci M. R., Atala A., and Khademhosseini A., “ Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip,” Biomaterials 110, 45–59 (2016). 10.1016/j.biomaterials.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Beverung S., Wu J., and Steward R., “ Lab-on-a-chip for cardiovascular physiology and pathology,” Micromachines 11, 898 (2020). 10.3390/mi11100898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Cruz-Moreira D., Visone R., Vasques-Nóvoa F., Barros A. S., Leite-Moreira A., Redaelli A., Moretti M., and Rasponi M., “ Assessing the influence of perfusion on cardiac microtissue maturation: A heart-on-chip platform embedding peristaltic pump capabilities,” Biotechnol. Bioeng. 118, 3128–3137 (2021). 10.1002/bit.27836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kolanowski T. J., Busek M., Schubert M., Dmitrieva A., Binnewerg B., Pöche J., Fisher K., Schmieder F., Grünzner S., Hansen S., Richter A., El-Armouche A., Sonntag F., and Guan K., “ Enhanced structural maturation of human induced pluripotent stem cell-derived cardiomyocytes under a controlled microenvironment in a microfluidic system,” Acta Biomater. 102, 273–286 (2020). 10.1016/j.actbio.2019.11.044 [DOI] [PubMed] [Google Scholar]
- 162. Abulaiti M., Yalikun Y., Murata K., Sato A., Sami M. M., Sasaki Y., Fujiwara Y., Minatoya K., Shiba Y., Tanaka Y., and Masumoto H., “ Establishment of a heart-on-a-chip microdevice based on human iPS cells for the evaluation of human heart tissue function,” Sci. Rep. 10, 19201 (2020). 10.1038/s41598-020-76062-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Martewicz S., Michielin F., Serena E., Zambon A., Mongillo M., and Elvassore N., “ Reversible alteration of calcium dynamics in cardiomyocytes during acute hypoxia transient in a microfluidic platform,” Integr. Biol. 4, 153–164 (2012). 10.1039/C1IB00087J [DOI] [PubMed] [Google Scholar]
- 164. Ellis B. W., Ronan G., Ren X., Bahcecioglu G., Senapati S., Anderson D., Handberg E., March K. L., Chang H.-C., and Zorlutuna P., “ Human heart anoxia and reperfusion tissue (HEART) model for the rapid study of exosome bound miRNA expression as biomarkers for myocardial infarction,” Small 18, 2201330 (2022). 10.1002/smll.202201330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Veldhuizen J., Chavan R., Moghadas B., Park J. G., Kodibagkar V. D., Migrino R. Q., and Nikkhah M., “ Cardiac ischemia on-a-chip to investigate cellular and molecular response of myocardial tissue under hypoxia,” Biomaterials 281, 121336 (2022). 10.1016/j.biomaterials.2021.121336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Liu H., Bolonduro O. A., Hu N., Ju J., Rao A. A., Duffy B. M., Huang Z., Black L. D., and Timko B. P., “ Heart-on-a-chip model with integrated extra- and intracellular bioelectronics for monitoring cardiac electrophysiology under acute hypoxia,” Nano Lett. 20, 2585–2593 (2020). 10.1021/acs.nanolett.0c00076 [DOI] [PubMed] [Google Scholar]
- 167. Chen C., Mehl B. T., Munshi A. S., Townsend A. D., Spence D. M., and Martin R. S., “ 3D-printed microfluidic devices: Fabrication, advantages and limitations—A mini review,” Anal. Methods 8, 6005–6012 (2016). 10.1039/C6AY01671E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Xie Z.-T., Kang D.-H., and Matsusaki M., “ Resolution of 3D bioprinting inside bulk gel and granular gel baths,” Soft Matter 17, 8769–8785 (2021). 10.1039/D1SM00926E [DOI] [PubMed] [Google Scholar]
- 169. Sriphutkiat Y., Kasetsirikul S., Ketpun D., and Zhou Y., “ Cell alignment and accumulation using acoustic nozzle for bioprinting,” Sci. Rep. 9, 17774 (2019). 10.1038/s41598-019-54330-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Qasim M., Haq F., Kang M.-H., and Kim J.-H., “ 3D printing approaches for cardiac tissue engineering and role of immune modulation in tissue regeneration,” Int. J. Nanomed. 14, 1311–1333 (2019). 10.2147/IJN.S189587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Malekpour A. and Chen X., “ Printability and cell viability in extrusion-based bioprinting from experimental, computational, and machine learning views,” J. Funct. Biomater. 13, 40 (2022). 10.3390/jfb13020040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Ng W. L., Chua C. K., and Shen Y.-F., “ Print me an organ! Why we are not there yet,” Prog. Polym. Sci. 97, 101145 (2019). 10.1016/j.progpolymsci.2019.101145 [DOI] [Google Scholar]
- 173. Kim W., Jang C. H., and Kim G. H., “ A myoblast-laden collagen bioink with fully aligned au nanowires for muscle-tissue regeneration,” Nano Lett. 19, 8612–8620 (2019). 10.1021/acs.nanolett.9b03182 [DOI] [PubMed] [Google Scholar]
- 174. Samandari M., Alipanah F., Majidzadeh-A K., Alvarez M. M., Trujillo-de Santiago G., and Tamayol A., “ Controlling cellular organization in bioprinting through designed 3D microcompartmentalization,” Appl. Phys. Rev. 8, 021404 (2021). 10.1063/5.0040732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Liu N., Ye X., Yao B., Zhao M., Wu P., Liu G., Zhuang D., Jiang H., Chen X., He Y., Huang S., and Zhu P., “ Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration,” Bioact. Mater. 6, 1388–1401 (2021). 10.1016/j.bioactmat.2020.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Wang Z., Wang L., Li T., Liu S., Guo B., Huang W., and Wu Y., “ 3D bioprinting in cardiac tissue engineering,” Theranostics 11, 7948–7969 (2021). 10.7150/thno.61621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ong C. S., Fukunishi T., Zhang H., Huang C. Y., Nashed A., Blazeski A., DiSilvestre D., Vricella L., Conte J., Tung L., Tomaselli G. F., and Hibino N., “ Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes,” Sci. Rep. 7, 4566 (2017). 10.1038/s41598-017-05018-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Skylar-Scott M. A., Uzel S. G. M., Nam L. L., Ahrens J. H., Truby R. L., Damaraju S., and Lewis J. A., “ Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels,” Sci. Adv. 5, eaaw2459 (2019). 10.1126/sciadv.aaw2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Bova L., Billi F., and Cimetta E., “ Mini-review: Advances in 3D bioprinting of vascularized constructs,” Biol. Direct 15, 22 (2020). 10.1186/s13062-020-00273-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Tsukamoto Y., Akagi T., and Akashi M., “ Vascularized cardiac tissue construction with orientation by layer-by-layer method and 3D printer,” Sci. Rep. 10, 5484 (2020). 10.1038/s41598-020-59371-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Polonchuk L., Chabria M., Badi L., Hoflack J.-C., Figtree G., Davies M. J., and Gentile C., “ Cardiac spheroids as promising in vitro models to study the human heart microenvironment,” Sci. Rep. 7, 7005 (2017). 10.1038/s41598-017-06385-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Richards D. J., Li Y., Kerr C. M., Yao J., Beeson G. C., Coyle R. C., Chen X., Jia J., Damon B., Wilson R., Starr Hazard E., Hardiman G., Menick D. R., Beeson C. C., Yao H., Ye T., and Mei Y., “ Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity,” Nat. Biomed. Eng. 4, 446–462 (2020). 10.1038/s41551-020-0539-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Figtree G. A., Bubb K. J., Tang O., Kizana E., and Gentile C., “ Vascularized cardiac spheroids as novel 3D in vitro models to study cardiac fibrosis,” Cells Tissues Organs 204, 191–198 (2017). 10.1159/000477436 [DOI] [PubMed] [Google Scholar]
- 184. McCain M. L., Agarwal A., Nesmith H. W., Nesmith A. P., and Parker K. K., “ Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues,” Biomaterials 35, 5462–5471 (2014). 10.1016/j.biomaterials.2014.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Sadeghi A. H., Shin S. R., Deddens J. C., Fratta G., Mandla S., Yazdi I. K., Prakash G., Antona S., Demarchi D., Buijsrogge M. P., Sluijter J. P. G., Hjortnaes J., and Khademhosseini A., “ Engineered three-dimensional cardiac fibrotic tissue to study fibrotic remodeling,” Adv. Healthcare Mater. 6, 1601434 (2017). 10.1002/adhm.201601434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Koti P., Muselimyan N., Mirdamadi E., Asfour H., and Sarvazyan N. A., “ Use of GelMA for 3D printing of cardiac myocytes and fibroblasts,” J. 3D Print. Med. 3, 11 (2019). 10.2217/3dp-2018-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Khalil N. N. and McCain M. L., “ Engineering the cellular microenvironment of post-infarct myocardium on a chip,” Front. Cardiovasc. Med. 8, 709871 (2021). 10.3389/fcvm.2021.709871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Mastikhina O., Moon B.-U., Williams K., Hatkar R., Gustafson D., Mourad O., Sun X., Koo M., Lam A. Y. L., Sun Y., Fish J. E., Young E. W. K., and Nunes S. S., “ Human cardiac fibrosis-on-a-chip model recapitulates disease hallmarks and can serve as a platform for drug testing,” Biomaterials 233, 119741 (2020). 10.1016/j.biomaterials.2019.119741 [DOI] [PubMed] [Google Scholar]
- 189. Mainardi A., Carminati F., Ugolini G. S., Occhetta P., Isu G., Diaz D. R., Reid G., Visone R., Rasponi M., and Marsano A., “ A dynamic microscale mid-throughput fibrosis model to investigate the effects of different ratios of cardiomyocytes and fibroblasts,” Lab Chip 21, 4177–4195 (2021). 10.1039/D1LC00092F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Daly A. C., Pitacco P., Nulty J., Cunniffe G. M., and Kelly D. J., “ 3D printed microchannel networks to direct vascularisation during endochondral bone repair,” Biomaterials 162, 34–46 (2018). 10.1016/j.biomaterials.2018.01.057 [DOI] [PubMed] [Google Scholar]
- 191. Chang C. C., Boland E. D., Williams S. K., and Hoying J. B., “ Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies,” J. Biomed. Mater. Res., Part B 98B, 160–170 (2011). 10.1002/jbm.b.31831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Zhang Y. S., Haghiashtiani G., Hübscher T., Kelly D. J., Lee J. M., Lutolf M., McAlpine M. C., Yeong W. Y., Zenobi-Wong M., and Malda J., “ 3D extrusion bioprinting,” Nat. Rev. Methods Primers 1, 75 (2021). 10.1038/s43586-021-00073-8 [DOI] [Google Scholar]
- 193. Dong G., Hu Y., Huyan Y., Zhang W., Yang C., and Da L., “ 3D-printing of scaffold within bionic vascular network applicable to tissue engineering,” in Proceedings of the Seventh Asia International Symposium on Mechatronics, edited by Duan B., Umeda K., and Hwang W. ( Springer, Singapore, 2020), pp. 917–922. [Google Scholar]
- 194. Nie J., Gao Q., Fu J., and He Y., “ Grafting of 3D bioprinting to in vitro drug screening: A review,” Adv. Healthcare Mater. 9, 1901773 (2020). 10.1002/adhm.201901773 [DOI] [PubMed] [Google Scholar]
- 195. Rojas S. V., Kensah G., Rotaermel A., Baraki H., Kutschka I., Zweigerdt R., Martin U., Haverich A., Gruh I., and Martens A., “ Transplantation of purified iPSC-derived cardiomyocytes in myocardial infarction,” PLoS One 12, e0173222 (2017). 10.1371/journal.pone.0173222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Germena G. and Hinkel R., “ iPSCs and exosomes: Partners in crime fighting cardiovascular diseases,” J. Pers. Med. 11, 529 (2021). 10.3390/jpm11060529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Traverse J. H., Henry T. D., Dib N., Patel A. N., Pepine C., Schaer G. L., DeQuach J. A., Kinsey A. M., Chamberlin P., and Christman K. L., “ First-in-man study of a cardiac extracellular matrix hydrogel in early and late myocardial infarction patients,” JACC Basic Transl. Sci. 4, 659–669 (2019). 10.1016/j.jacbts.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Smagul S., Kim Y., Smagulova A., Raziyeva K., Nurkesh A., and Saparov A., “ Biomaterials loaded with growth factors/cytokines and stem cells for cardiac tissue regeneration,” Int. J. Mol. Sci. 21, 5952 (2020). 10.3390/ijms21175952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Zimmermann W.-H., “ Tissue engineered heart repair from preclinical models to first-in-patient studies,” Curr. Opin. Physiol. 14, 70–77 (2020). 10.1016/j.cophys.2020.02.001 [DOI] [Google Scholar]
- 200. Guo Y., Yu Y., Hu S., Chen Y., and Shen Z., “ The therapeutic potential of mesenchymal stem cells for cardiovascular diseases,” Cell Death Dis. 11, 349 (2020). 10.1038/s41419-020-2542-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Ishida M., Miyagawa S., Saito A., Fukushima S., Harada A., Ito E., Ohashi F., Watabe T., Hatazawa J., Matsuura K., and Sawa Y., “ Transplantation of human-induced pluripotent stem cell-derived cardiomyocytes is superior to somatic stem cell therapy for restoring cardiac function and oxygen consumption in a porcine model of myocardial infarction,” Transplantation 103, 291–298 (2019). 10.1097/TP.0000000000002384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Hatzistergos K. E. and Vedenko A., “ Cardiac cell therapy 3.0,” Circ. Res. 121, 95–97 (2017). 10.1161/CIRCRESAHA.117.311293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Li J., Hu S., Zhu D., Huang K., Mei X., López de Juan Abad B., and Cheng K., “ All roads lead to rome (the heart): Cell retention and outcomes from various delivery routes of cell therapy products to the heart,” J. Am. Heart Assoc. 10, e020402 (2021). 10.1161/JAHA.120.020402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Peng H., Shindo K., Donahue R. R., and Abdel-Latif A., “ Cardiac cell therapy: Insights into the mechanisms of tissue repair,” Int. J. Mol. Sci. 22, 1201 (2021). 10.3390/ijms22031201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Neef K., Drey F., Lepperhof V., Wahlers T., Hescheler J., Choi Y.-H., and Šarić T., “ Co-transplantation of mesenchymal stromal cells and induced pluripotent stem cell-derived cardiomyocytes improves cardiac function after myocardial damage,” Front. Cardiovasc. Med. 8, 794690 (2022). 10.3389/fcvm.2021.794690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Rafii S. and Lyden D., “ Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration,” Nat. Med. 9, 702–712 (2003). 10.1038/nm0603-702 [DOI] [PubMed] [Google Scholar]
- 207. Amado L. C., Saliaris A. P., Schuleri K. H., John M. St., Xie J.-S., Cattaneo S., Durand D. J., Fitton T., Kuang J. Q., Stewart G., Lehrke S., Baumgartner W. W., Martin B. J., Heldman A. W., and Hare J. M., “ Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction,” Proc. Natl. Acad. Sci. U. S. A. 102, 11474–11479 (2005). 10.1073/pnas.0504388102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Zgheib C., Xu J., and Liechty K. W., “ Targeting inflammatory cytokines and extracellular matrix composition to promote wound regeneration,” Adv. Wound Care 3, 344–355 (2014).). 10.1089/wound.2013.0456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Piccinini A. M. and Midwood K. S., “ Illustrating the interplay between the extracellular matrix and microRNAs,” Int. J. Exp. Pathol. 95, 158–180 (2014). 10.1111/iep.12079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Mirotsou M., Zhang Z., Deb A., Zhang L., Gnecchi M., Noiseux N., Mu H., Pachori A., and Dzau V., “ Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair,” Proc. Natl. Acad. Sci. U. S. A. 104, 1643–1648 (2007). 10.1073/pnas.0610024104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Watt S. M., Gullo F., van der Garde M., Markeson D., Camicia R., Khoo C. P., and Zwaginga J. J., “ The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential,” Br. Med. Bull. 108, 25–53 (2013). 10.1093/bmb/ldt031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Hoffmann J., Glassford A. J., Doyle T. C., Robbins R. C., Schrepfer S., and Pelletier M. P., “ Angiogenic effects despite limited cell survival of bone marrow-derived mesenchymal stem cells under ischemia,” Thorac. Cardiovasc. Surg. 58, 136–142 (2010). 10.1055/s-0029-1240758 [DOI] [PubMed] [Google Scholar]
- 213. Premer C., Wanschel A., Porras V., Balkan W., Legendre-Hyldig T., Saltzman R. G., Dong C., Schulman I. H., and Hare J. M., “ Mesenchymal stem cell secretion of SDF-1α modulates endothelial function in dilated cardiomyopathy,” Front. Physiol. 10, 1182 (2019). 10.3389/fphys.2019.01182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Karantalis V. and Hare J. M., “ Use of mesenchymal stem cells for therapy of cardiac disease,” Circu. Res. 116, 1413–1430 (2015). 10.1161/CIRCRESAHA.116.303614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Guo R., Wan F., Morimatsu M., Xu Q., Feng T., Yang H., Gong Y., Ma S., Chang Y., Zhang S., Jiang Y., Wang H., Chang D., Zhang H., Ling Y., and Lan F., “ Cell sheet formation enhances the therapeutic effects of human umbilical cord mesenchymal stem cells on myocardial infarction as a bioactive material,” Bioact. Mater. 6, 2999–3012 (2021). 10.1016/j.bioactmat.2021.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Chen S., Fang W., Ye F., Liu Y.-H., Qian J., Shan S., Zhang J., Chunhua R. Z., Liao L., Lin S., and Sun J., “ Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction,” Am. J. Cardiol. 94, 92–95 (2004). 10.1016/j.amjcard.2004.03.034 [DOI] [PubMed] [Google Scholar]
- 217. Florea V., Rieger A. C., DiFede D. L., El-Khorazaty J., Natsumeda M., Banerjee M. N., Tompkins B. A., Khan A., Schulman I. H., Landin A. M., Mushtaq M., Golpanian S., Lowery M. H., Byrnes J. J., Hendel R. C., Cohen M. G., Valasaki K., Pujol M. V., Ghersin E., Miki R., Delgado C., Abuzeid F., Vidro-Casiano M., Saltzman R. G., DaFonseca D., Caceres L. V., Ramdas K. N., Mendizabal A., Heldman A. W., Mitrani R. D., and Hare J. M., “ Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (the trident study),” Circu. Res. 121, 1279–1290 (2017). 10.1161/CIRCRESAHA.117.311827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Boyle A. J., Schulman S. P., and Hare J. M., “ Stem cell therapy for cardiac repair,” Circulation 114, 339–352 (2006). 10.1161/CIRCULATIONAHA.105.590653 [DOI] [PubMed] [Google Scholar]
- 219. Thakker R. and Yang P., “ Mesenchymal stem cell therapy for cardiac repair,” Curr. Treat. Options Cardiovasc. Med. 16, 323 (2014). 10.1007/s11936-014-0323-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Lalit P. A., Hei D. J., Raval A. N., and Kamp T. J., “ Induced pluripotent stem cells for post–myocardial infarction repair,” Circu. Res. 114, 1328–1345 (2014). 10.1161/CIRCRESAHA.114.300556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Nelson T. J., Martinez-Fernandez A., Yamada S., Perez-Terzic C., Ikeda Y., and Terzic A., “ Repair of acute myocardial infarction with iPS induced by human stemness factors,” Circulation 120, 408–416 (2009). 10.1161/CIRCULATIONAHA.109.865154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Ahmed R. P., Ashraf M., Buccini S., Shujia J., and Haider H. K., “ Cardiac tumorigenic potential of induced pluripotent stem cells in an immunocompetent host with myocardial infarction,” Regener. Med. 6, 171–178 (2011). 10.2217/rme.10.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Zhang Y., Wang D., Chen M., Yang B., Zhang F., and Cao K., “ Intramyocardial transplantation of undifferentiated rat induced pluripotent stem cells causes tumorigenesis in the heart,” PLoS One 6, e19012 (2011). 10.1371/journal.pone.0019012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Templin C., Zweigerdt R., Schwanke K., Olmer R., Ghadri J.-R., Emmert M. Y., Müller E., Küest S. M., Cohrs S., Schibli R., Kronen P., Hilbe M., Reinisch A., Strunk D., Haverich A., Hoerstrup S., Lüscher T. F., Kaufmann P. A., Landmesser U., and Martin U., “ Transplantation and tracking of human-induced pluripotent stem cells in a pig model of myocardial infarction,” Circulation 126, 430–439 (2012). 10.1161/CIRCULATIONAHA.111.087684 [DOI] [PubMed] [Google Scholar]
- 225. Chong J. J. H., Yang X., Don C. W., Minami E., Liu Y.-W., Weyers J. J., Mahoney W. M., Van Biber B., Cook S. M., Palpant N. J., Gantz J. A., Fugate J. A., Muskheli V., Gough G. M., Vogel K. W., Astley C. A., Hotchkiss C. E., Baldessari A., Pabon L., Reinecke H., Gill E. A., Nelson V., Kiem H.-P., Laflamme M. A., and Murry C. E., “ Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts,” Nature 510, 273–277 (2014). 10.1038/nature13233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Shiba Y., Fernandes S., Zhu W.-Z., Filice D., Muskheli V., Kim J., Palpant N. J., Gantz J., Moyes K. W., Reinecke H., Van Biber B., Dardas T., Mignone J. L., Izawa A., Hanna R., Viswanathan M., Gold J. D., Kotlikoff M. I., Sarvazyan N., Kay M. W., Murry C. E., and Laflamme M. A., “ Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts,” Nature 489, 322–325 (2012). 10.1038/nature11317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Taylor C. J., Bolton E. M., and Bradley J. A., “ Immunological considerations for embryonic and induced pluripotent stem cell banking,” Philos. Trans. R. Soc. London B 366, 2312–2322 (2011). 10.1098/rstb.2011.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Volarevic V., Markovic B. S., Gazdic M., Volarevic A., Jovicic N., Arsenijevic N., Armstrong L., Djonov V., Lako M., and Stojkovic M., “ Ethical and safety issues of stem cell-based therapy,” Int. J. Med. Sci. 15, 36–45 (2018). 10.7150/ijms.21666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Hentze H., Soong P. L., Wang S. T., Phillips B. W., Putti T. C., and Dunn N. R., “ Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies,” Stem Cell Res. 2, 198–210 (2009). 10.1016/j.scr.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 230. Behfar A., Perez-Terzic C., Faustino R. S., Arrell D. K., Hodgson D. M., Yamada S., Puceat M., Niederländer N., Alekseev A. E., Zingman L. V., and Terzic A., “ Cardiopoietic programming of embryonic stem cells for tumor-free heart repair,” J. Exp. Med. 204, 405–420 (2007). 10.1084/jem.20061916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Oikonomopoulos A., Kitani T., and Wu J. C., “ pluripotent stem cell-derived cardiomyocytes as a platform for cell therapy applications: Progress and hurdles for clinical translation,” Mol. Ther. 26, 1624–1634 (2018). 10.1016/j.ymthe.2018.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Tohyama S. and Fukuda K., “ Future treatment of heart failure using human iPSC-derived cardiomyocytes,” in Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology, edited by Nakanishi T., Markwald R. R., Baldwin H. S., Keller B. B., Srivastava D., and Yamagishi H. ( Springer, Tokyo, 2016). [PubMed] [Google Scholar]
- 233. Citro L., Naidu S., Hassan F., Kuppusamy M. L., Kuppusamy P., Angelos M. G., and Khan M., “ Comparison of human induced pluripotent stem-cell derived cardiomyocytes with human mesenchymal stem cells following acute myocardial infarction,” PLOS ONE 9, e116281 (2014). 10.1371/journal.pone.0116281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Kawamura M., Miyagawa S., Miki K., Saito A., Fukushima S., Higuchi T., Kawamura T., Kuratani T., Daimon T., Shimizu T., Okano T., and Sawa Y., “ Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model,” Circulation 126, S29–S37 (2012). 10.1161/CIRCULATIONAHA.111.084343 [DOI] [PubMed] [Google Scholar]
- 235. Shiba Y., Gomibuchi T., Seto T., Wada Y., Ichimura H., Tanaka Y., Ogasawara T., Okada K., Shiba N., Sakamoto K., Ido D., Shiina T., Ohkura M., Nakai J., Uno N., Kazuki Y., Oshimura M., Minami I., and Ikeda U., “ Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts,” Nature 538, 388–391 (2016). 10.1038/nature19815 [DOI] [PubMed] [Google Scholar]
- 236. Parikh S. S., Blackwell D. J., Gomez-Hurtado N., Frisk M., Wang L., Kim K., Dahl C. P., Fiane A., Tønnessen T., Kryshtal D. O., Louch W. E., and Knollmann B. C., “ Thyroid and glucocorticoid hormones promote functional T-tubule development in human-induced pluripotent stem cell derived cardiomyocytes,” Circ. Res. 121, 1323–1330 (2017). 10.1161/CIRCRESAHA.117.311920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Lin B., Lin X., Stachel M., Wang E., Luo Y., Lader J., Sun X., Delmar M., and Bu L., “ Culture in glucose-depleted medium supplemented with fatty acid and 3,3′,5-triiodo-l-thyronine facilitates purification and maturation of human pluripotent stem cell-derived cardiomyocytes,” Front. Endocrinol. 8, 253 (2017). 10.3389/fendo.2017.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Yang X., Rodriguez M. L., Leonard A., Sun L., Fischer K. A., Wang Y., Ritterhoff J., Zhao L., Kolwicz S. C., Pabon L., Reinecke H., Sniadecki N. J., Tian R., Ruohola-Baker H., Xu H., and Murry C. E., “ Fatty acids enhance the maturation of cardiomyocytes derived from human pluripotent stem cells,” Stem Cell Rep. 13, 657–668 (2019). 10.1016/j.stemcr.2019.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Boudou T., Legant W. R., Mu A., Borochin M. A., Thavandiran N., Radisic M., Zandstra P. W., Epstein J. A., Margulies K. B., and Chen C. S., “ A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues,” Tissue Eng., Part A 18, 910–919 (2012). 10.1089/ten.tea.2011.0341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Ma R., Liang J., Huang W., Guo L., Cai W., Wang L., Paul C., Yang H.-T., Kim H. W., and Wang Y., “ Electrical stimulation enhances cardiac differentiation of human induced pluripotent stem cells for myocardial infarction therapy,” Antioxid. Redox Signaling 28, 371–384 (2018). 10.1089/ars.2016.6766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Sheehy S. P., Grosberg A., and Parker K. K., “ The contribution of cellular mechanotransduction to cardiomyocyte form and function,” Biomech. Model. Mechanobiol. 11, 1227–1239 (2012). 10.1007/s10237-012-0419-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Nunes S. S., Miklas J. W., Liu J., Aschar-Sobbi R., Xiao Y., Zhang B., Jiang J., Masse S., Gagliardi M., Hsieh A., Thavandiran N., Laflamme M. A., Nanthakumar K., Gross G., Backx P. H., Keller G., and Radisic M., “ Biowire: A new platform for maturation of human pluripotent stem cell derived cardiomyocytes,” Nat. Methods 10, 781–787 (2013). 10.1038/nmeth.2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Ruan J.-L., Tulloch N. L., Razumova M. V., Saiget M., Muskheli V., Pabon L., Reinecke H., Regnier M., and Murry C. E., “ Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue,” Circulation 134, 1557–1567 (2016). 10.1161/CIRCULATIONAHA.114.014998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Au H. T. H., Cui B., Chu Z. E., Veres T., and Radisic M., “ Cell culture chips for simultaneous application of topographical and electrical cues enhance phenotype of cardiomyocytes,” Lab Chip 9, 564–575 (2009). 10.1039/B810034A [DOI] [PubMed] [Google Scholar]
- 245. Natsumeda M., Florea V., Rieger A. C., Tompkins B. A., Banerjee M. N., Golpanian S., Fritsch J., Landin A. M., Kashikar N. D., Karantalis V., Loescher V. Y., Hatzistergos K. E., Bagno L., Sanina C., Mushtaq M., Rodriguez J., Rosado M., Wolf A., Collon K., Vincent L., Kanelidis A. J., Schulman I. H., Mitrani R., Heldman A. W., Balkan W., and Hare J. M., “ A combination of allogeneic stem cells promotes cardiac regeneration,” J. Am. Coll. Cardiol. 70, 2504–2515 (2017). 10.1016/j.jacc.2017.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Huang P., Wang L., Li Q., Xu J., Xu J., Xiong Y., Chen G., Qian H., Jin C., Yu Y., Liu J., Qian L., and Yang Y., “ Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance,” Stem Cell Res. Ther. 10, 300 (2019). 10.1186/s13287-019-1353-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Menasché P., “ Cardiac cell therapy: Current status, challenges and perspectives,” Arch. Cardiovasc. Dis. 113, 285–292 (2020). 10.1016/j.acvd.2020.01.002 [DOI] [PubMed] [Google Scholar]
- 248. Vagnozzi R. J., Maillet M., Sargent M. A., Khalil H., Johansen A. K. Z., Schwanekamp J. A., York A. J., Huang V., Nahrendorf M., Sadayappan S., and Molkentin J. D., “ An acute immune response underlies the benefit of cardiac stem cell therapy,” Nature 577, 405–409 (2020). 10.1038/s41586-019-1802-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Thum T., Bauersachs J., Poole-Wilson P. A., Volk H.-D., and Anker S. D., “ The dying stem cell hypothesis: Immune modulation as a novel mechanism for progenitor cell therapy in cardiac muscle,” J. Am. Coll. Cardiol. 46, 1799–1802 (2005). 10.1016/j.jacc.2005.07.053 [DOI] [PubMed] [Google Scholar]
- 250. Liu B., Lee B. W., Nakanishi K., Villasante A., Williamson R., Metz J., Kim J., Kanai M., Bi L., Brown K., Di Paolo G., Homma S., Sims P. A., Topkara V. K., and Vunjak-Novakovic G., “ Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells,” Nat. Biomed. Eng. 2, 293–303 (2018). 10.1038/s41551-018-0229-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Yáñez-Mó M., Siljander P. R.-M., Andreu Z., Bedina Zavec A., Borràs F. E., Buzas E. I., Buzas K., Casal E., Cappello F., Carvalho J., Colás E., Cordeiro-da Silva A., Fais S., Falcon-Perez J. M., Ghobrial I. M., Giebel B., Gimona M., Graner M., Gursel I., Gursel M., Heegaard N. H. H., Hendrix A., Kierulf P., Kokubun K., Kosanovic M., Kralj-Iglic V., Krämer-Albers E.-M., Laitinen S., Lässer C., Lener T., Ligeti E., Linē A., Lipps G., Llorente A., Lötvall J., Manček-Keber M., Marcilla A., Mittelbrunn M., Nazarenko I., Nolte-'t Hoen E. N. M., Nyman T. A., O'Driscoll L., Olivan M., Oliveira C., Pállinger É., del Portillo H. A., Reventós J., Rigau M., Rohde E., Sammar M., Sánchez-Madrid F., Santarém N., Schallmoser K., Stampe Ostenfeld M., Stoorvogel W., Stukelj R., Van der Grein S. G., Vasconcelos M. H., Wauben M. H. M., and Wever O. D., “ Biological properties of extracellular vesicles and their physiological functions,” J. Extracell. Vesicles 4, 27066 (2015). 10.3402/jev.v4.27066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Zhang D., Shadrin I. Y., Lam J., Xian H.-Q., Snodgrass H. R., and Bursac N., “ Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes,” Biomaterials 34, 5813–5820 (2013). 10.1016/j.biomaterials.2013.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Huang K., Ozpinar E. W., Su T., Tang J., Shen D., Qiao L., Hu S., Li Z., Liang H., Mathews K., Scharf V., Freytes D. O., and Cheng K., “ An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs,” Sci. Transl. Med. 12, eaat9683 (2020). 10.1126/scitranslmed.aat9683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Sadahiro T. and Ieda M., “ Direct cardiac reprogramming for cardiovascular regeneration,” Keio J. Med. 69, 49 (2020). 10.2302/kjm.2019-0008-OA [DOI] [PubMed] [Google Scholar]
- 255. Muraoka N. et al. , “ MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures,” EMBO J. 33, 1565–1581 (2014). 10.15252/embj.201387605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Jayawardena T. M., Finch E. A., Zhang L., Zhang H., Hodgkinson C. P., Pratt R. E., Rosenberg P. B., Mirotsou M., and Dzau V. J., “ MicroRNA induced cardiac reprogramming in vivo,” Circu. Res. 116, 418–424 (2015). 10.1161/CIRCRESAHA.116.304510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Abad M., Hashimoto H., Zhou H., Morales M. G., Chen B., Bassel-Duby R., and Olson E. N., “ Notch inhibition enhances cardiac reprogramming by increasing MEF2C transcriptional activity,” Stem Cell Rep. 8, 548–560 (2017). 10.1016/j.stemcr.2017.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Yamakawa H., Muraoka N., Miyamoto K., Sadahiro T., Isomi M., Haginiwa S., Kojima H., Umei T., Akiyama M., Kuishi Y., Kurokawa J., Furukawa T., Fukuda K., and Ieda M., “ Fibroblast growth factors and vascular endothelial growth factor promote cardiac reprogramming under defined conditions,” Stem Cell Rep. 5, 1128–1142 (2015). 10.1016/j.stemcr.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Zhou Y., Wang L., Vaseghi H. R., Liu Z., Lu R., Alimohamadi S., Yin C., Fu J.-D., Wang G. G., Liu J., and Qian L., “ Bmi1 is a key epigenetic barrier to direct cardiac reprogramming,” Cell Stem Cell 18, 382–395 (2016). 10.1016/j.stem.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Liu Z., Chen O., Zheng M., Wang L., Zhou Y., Yin C., Liu J., and Qian L., “ Re-patterning of H3K27me3, H3K4me3 and DNA methylation during fibroblast conversion into induced cardiomyocytes,” Stem Cell Res. 16, 507–518 (2016). 10.1016/j.scr.2016.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Stone N. R., Gifford C. A., Thomas R., Pratt K. J. B., Samse-Knapp K., Mohamed T. M. A., Radzinsky E. M., Schricker A., Ye L., Yu P., van Bemmel J. G., Ivey K. N., Pollard K. S., and Srivastava D., “ Context-specific transcription factor functions regulate epigenomic and transcriptional dynamics during cardiac reprogramming,” Cell Stem Cell 25, 87 (2019). 10.1016/j.stem.2019.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Hashimoto H., Wang Z., Garry G. A., Malladi V. S., Botten G. A., Ye W., Zhou H., Osterwalder M., Dickel D. E., Visel A., Liu N., Bassel-Duby R., and Olson E. N., “ Cardiac reprogramming factors synergistically activate genome-wide cardiogenic stage-specific enhancers,” Cell Stem Cell 25, 69 (2019). 10.1016/j.stem.2019.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Li Y., Dal-Pra S., Mirotsou M., Jayawardena T. M., Hodgkinson C. P., Bursac N., and Dzau V. J., “ Tissue-engineered three-dimensional (3D) microenvironment enhances the direct reprogramming of fibroblasts into cardiomyocytes by microRNAs,” Sci. Rep. 6, 38815 (2016). 10.1038/srep38815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Kurotsu S., Sadahiro T., Fujita R., Tani H., Yamakawa H., Tamura F., Isomi M., Kojima H., Yamada Y., Abe Y., Murakata Y., Akiyama T., Muraoka N., Harada I., Suzuki T., Fukuda K., and Ieda M., “ Soft matrix promotes cardiac reprogramming via inhibition of YAP/TAZ and suppression of fibroblast signatures,” Stem Cell Rep. 15, 612–628 (2020). 10.1016/j.stemcr.2020.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Badylak S. F., Freytes D. O., and Gilbert T. W., “ Extracellular matrix as a biological scaffold material: Structure and function,” Acta Biomater. 5, 1–13 (2009). 10.1016/j.actbio.2008.09.013 [DOI] [PubMed] [Google Scholar]
- 266. Arnal-Pastor M., Chachques J. C., Pradas M. M., and Vallés-Lluch A., “ Biomaterials for cardiac tissue engineering,” in Regenerative Medicine and Tissue Engineering ( IntechOpen, 2013). [Google Scholar]
- 267. Ye K. Y. and Black L. D., “ Strategies for Tissue engineering cardiac constructs to affect functional repair following myocardial infarction,” J. Cardiovasc. Transl. Res. 4, 575–591 (2011). 10.1007/s12265-011-9303-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Peña B., Jett S., Rowland T. J., Taylor M. R. G., Mestroni L., Laughter M., and Park D., “ Injectable hydrogels for cardiac tissue engineering,” Macromol. Biosci. 18, e1800079 (2018). 10.1002/mabi.201800079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Ungerleider J. L., Johnson T. D., Rao N., and Christman K. L., “ Fabrication and characterization of injectable hydrogels derived from decellularized skeletal and cardiac muscle,” Methods 84, 53–59 (2015). 10.1016/j.ymeth.2015.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Efraim Y., Sarig H., Cohen Anavy N., Sarig U., de Berardinis E., Chaw S.-Y., Krishnamoorthi M., Kalifa J., Bogireddi H., Duc T. V., Kofidis T., Baruch L., Boey F. Y. C., Venkatraman S. S., and Machluf M., “ Biohybrid cardiac ECM-based hydrogels improve long term cardiac function post myocardial infarction,” Acta Biomater. 50, 220–233 (2017). 10.1016/j.actbio.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 271. Seif-Naraghi S. B., Singelyn J. M., Salvatore M. A., Osborn K. G., Wang J. J., Sampat U., Kwan O. L., Strachan G. M., Wong J., Schup-Magoffin P. J., Braden R. L., Bartels K., DeQuach J. A., Preul M., Kinsey A. M., DeMaria A. N., Dib N., and Christman K. L., “ Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction,” Sci. Transl. Med. 5, 173ra25 (2013). 10.1126/scitranslmed.3005503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Yokoyama J., Miyagawa S., Akagi T., Akashi M., and Sawa Y., “ Human induced pluripotent stem cell-derived three-dimensional cardiomyocyte tissues ameliorate the rat ischemic myocardium by remodeling the extracellular matrix and cardiac protein phenotype,” PLOS ONE 16, e0245571 (2021). 10.1371/journal.pone.0245571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Bassat E., Mutlak Y. E., Genzelinakh A., Shadrin I. Y., Baruch Umansky K., Yifa O., Kain D., Rajchman D., Leach J., Riabov Bassat D., Udi Y., Sarig R., Sagi I., Martin J. F., Bursac N., Cohen S., and Tzahor E., “ The extracellular matrix protein agrin promotes heart regeneration in mice,” Nature 547, 179–184 (2017). 10.1038/nature22978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Baehr A., Umansky K. B., Bassat E., Jurisch V., Klett K., Bozoglu T., Hornaschewitz N., Solyanik O., Kain D., Ferraro B., Cohen-Rabi R., Krane M., Cyran C., Soehnlein O., Laugwitz K. L., Hinkel R., Kupatt C., and Tzahor E., “ Agrin promotes coordinated therapeutic processes leading to improved cardiac repair in pigs,” Circulation 142, 868–881 (2020). 10.1161/CIRCULATIONAHA.119.045116 [DOI] [PubMed] [Google Scholar]
- 275. Kühn B., del Monte F., Hajjar R. J., Chang Y.-S., Lebeche D., Arab S., and Keating M. T., “ Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair,” Nat. Med. 13, 962–969 (2007). 10.1038/nm1619 [DOI] [PubMed] [Google Scholar]
- 276. Majid Q. A., Fricker A. T. R., Gregory D. A., Davidenko N., Hernandez Cruz O., Jabbour R. J., Owen T. J., Basnett P., Lukasiewicz B., Stevens M., Best S., Cameron R., Sinha S., Harding S. E., and Roy I., “ Natural biomaterials for cardiac tissue engineering: A highly biocompatible solution,” Front. Cardiovasc. Med. 7, 554597 (2020). 10.3389/fcvm.2020.554597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Trombino S., Curcio F., Cassano R., Curcio M., Cirillo G., and Iemma F., “ Polymeric biomaterials for the treatment of cardiac post-infarction injuries,” Pharmaceutics 13, 1038 (2021). 10.3390/pharmaceutics13071038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Esmaeili H., Patino-Guerrero A., Hasany M., Ansari M. O., Memic A., Dolatshahi-Pirouz A., and Nikkhah M., “ Electroconductive biomaterials for cardiac tissue engineering,” Acta Biomater. 139, 118–140 (2022). 10.1016/j.actbio.2021.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Fu A., Yang Y., Wu J., Li S.-H., Fan Y., Yau T. M., and Li R.-K., “ Bio-conductive polymers for treating myocardial conductive defects: long-term efficacy study,” Adv. Healthcare Mater. 11, 2101838 (2022). 10.1002/adhm.202101838 [DOI] [PubMed] [Google Scholar]
- 280. Shin S. R., Jung S. M., Zalabany M., Kim K., Zorlutuna P., bok Kim S., Nikkhah M., Khabiry M., Azize M., Kong J., Wan K., Palacios T., Dokmeci M. R., Bae H., Tang X., and Khademhosseini A., “ Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators,” ACS Nano 7, 2369–2380 (2013). 10.1021/nn305559j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Scott L., Jurewicz I., Jeevaratnam K., and Lewis R., “ Carbon nanotube-based scaffolds for cardiac tissue engineering—Systematic review and narrative synthesis,” Bioengineering 8, 80 (2021). 10.3390/bioengineering8060080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Hitscherich P., Aphale A., Gordan R., Whitaker R., Singh P., Xie L., Patra P., and Lee E. J., “ Electroactive graphene composite scaffolds for cardiac tissue engineering: Electroactive graphene composite scaffolds for cardiac tissue engineering,” J. Biomed. Mater. Res., Part A 106, 2923–2933 (2018). 10.1002/jbm.a.36481 [DOI] [PubMed] [Google Scholar]
- 283. Zhao L., “ A novel graphene oxide polymer gel platform for cardiac tissue engineering application,” 3 Biotech 9, 401 (2019). 10.1007/s13205-019-1912-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Navaei A., Saini H., Christenson W., Sullivan R. T., Ros R., and Nikkhah M., “ Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs,” Acta Biomater. 41, 133–146 (2016). 10.1016/j.actbio.2016.05.027 [DOI] [PubMed] [Google Scholar]
- 285. Li X.-P., Qu K.-Y., Zhang F., Jiang H.-N., Zhang N., Nihad C., Liu C.-M., Wu K.-H., Wang X.-W., and Huang N.-P., “ High-aspect-ratio water-dispersed gold nanowires incorporated within gelatin methacrylate hydrogels for constructing cardiac tissues in vitro,” J. Mater. Chem. B 8, 7213–7224 (2020). 10.1039/D0TB00768D [DOI] [PubMed] [Google Scholar]
- 286. Ye G., Wen Z., Wen F., Song X., Wang L., Li C., He Y., Prakash S., and Qiu X., “ Mussel-inspired conductive Ti 2 C-cryogel promotes functional maturation of cardiomyocytes and enhances repair of myocardial infarction,” Theranostics 10, 2047–2066 (2020). 10.7150/thno.38876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Ungerleider J. L. and Christman K. L., “ Concise review: Injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease—Translational challenges and progress,” Stem Cells Transl. Med. 3, 1090–1099 (2014). 10.5966/sctm.2014-0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Hasan A., Khattab A., Islam M. A., Hweij K. A., Zeitouny J., Waters R., Sayegh M., Hossain M. M., and Paul A., “ Injectable hydrogels for cardiac tissue repair after myocardial infarction,” Adv. Sci. 2, 1500122 (2015). 10.1002/advs.201500122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Ozcebe S. G., Bahcecioglu G., Yue X. S., and Zorlutuna P., “ Effect of cellular and ECM aging on human iPSC-derived cardiomyocyte performance, maturity and senescence,” Biomaterials 268, 120554 (2021). 10.1016/j.biomaterials.2020.120554 [DOI] [PubMed] [Google Scholar]
- 290. Williams C., Quinn K. P., Georgakoudi I., and Black L. D., “ Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro,” Acta Biomater. 10, 194 (2014). 10.1016/j.actbio.2013.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Pattar S. S., Fatehi Hassanabad A., and Fedak P. W. M., “ Acellular extracellular matrix bioscaffolds for cardiac repair and regeneration,” Front. Cell Dev. Biol. 7, 63 (2019). 10.3389/fcell.2019.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Singelyn J. M., Sundaramurthy P., Johnson T. D., Schup-Magoffin P. J., Hu D. P., Faulk D. M., Wang J., Mayle K. M., Bartels K., Salvatore M., Kinsey A. M., DeMaria A. N., Dib N., and Christman K. L., “ Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction,” J. Am. Coll. Cardiol. 59, 751–763 (2012). 10.1016/j.jacc.2011.10.888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Chen C. W., Wang L. L., Zaman S., Gordon J., Arisi M. F., Venkataraman C. M., Chung J. J., Hung G., Gaffey A. C., Spruce L. A., Fazelinia H., Gorman R. C., Seeholzer S. H., Burdick J. A., and Atluri P., “ Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction,” Cardiovasc. Res. 114, 1029–1040 (2018). 10.1093/cvr/cvy067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Lv K., Li Q., Zhang L., Wang Y., Zhong Z., Zhao J., Lin X., Wang J., Zhu K., Xiao C., Ke C., Zhong S., Wu X., Chen J., Yu H., Zhu W., Li X., Wang B., Tang R., Wang J., Huang J., and Hu X., “ Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction,” Theranostics 9, 7403–7416 (2019). 10.7150/thno.32637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Zhu D., Li Z., Huang K., Caranasos T. G., Rossi J. S., and Cheng K., “ Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair,” Nat. Commun. 12, 1412 (2021). 10.1038/s41467-021-21682-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Li H., Yu B., Yang P., Zhan J., Fan X., Chen P., Liao X., Ou C., Cai Y., and Chen M., “ Injectable AuNP-HA matrix with localized stiffness enhances the formation of gap junction in engrafted human induced pluripotent stem cell-derived cardiomyocytes and promotes cardiac repair,” Biomaterials 279, 121231 (2021). 10.1016/j.biomaterials.2021.121231 [DOI] [PubMed] [Google Scholar]
- 297. Contessotto P., Orbanić D., Da Costa M., Jin C., Owens P., Chantepie S., Chinello C., Newell J., Magni F., Papy-Garcia D., Karlsson N. G., Kilcoyne M., Dockery P., Rodríguez-Cabello J. C., and Pandit A., “ Elastin-like recombinamers-based hydrogel modulates post-ischemic remodeling in a non-transmural myocardial infarction in sheep,” Sci. Transl. Med. 13, eaaz5380 (2021). 10.1126/scitranslmed.aaz5380 [DOI] [PubMed] [Google Scholar]
- 298. Jackman C. P., Ganapathi A. M., Asfour H., Qian Y., Allen B. W., Li Y., and Bursac N., “ Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation,” Biomaterials 159, 48–58 (2018). 10.1016/j.biomaterials.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Jiang Y., Sun S.-J., Zhen Z., Wei R., Zhang N., Liao S.-Y., and Tse H.-F., “ Myocardial repair of bioengineered cardiac patches with decellularized placental scaffold and human-induced pluripotent stem cells in a rat model of myocardial infarction,” Stem Cell Res. Ther. 12, 13 (2021). 10.1186/s13287-020-02066-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Pretorius D., Kahn-Krell A. M., Lou X., Fast V. G., Berry J. L., Kamp T. J., and Zhang J., “ Layer-by-layer fabrication of large and thick human cardiac muscle patch constructs with superior electrophysiological properties,” Front. Cell Dev. Biol. 9, 938 (2021). 10.3389/fcell.2021.670504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Das S., Nam H., and Jang J., “ 3D bioprinting of stem cell-laden cardiac patch: A promising alternative for myocardial repair,” APL Bioeng. 5, 031508 (2021). 10.1063/5.0030353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Guo B. and Ma P. X., “ Conducting polymers for tissue engineering,” Biomacromolecules 19, 1764–1782 (2018). 10.1021/acs.biomac.8b00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Shah M., Kc P., and Zhang G., “ In vivo assessment of decellularized porcine myocardial slice as an acellular cardiac patch,” ACS Appl. Mater. Interfaces 11, 23893–23900 (2019). 10.1021/acsami.9b06453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Liu X., De la Cruz E., Gu X., Balint L., Oxendine-Burns M., Terrones T., Ma W., Kuo H.-H., Lantz C., Bansal T., Thorp E., Burridge P., Jakus Z., Herz J., Cleaver O., Torres M., and Oliver G., “ Lymphoangiocrine signals promote cardiac growth and repair,” Nature 588, 705–711 (2020). 10.1038/s41586-020-2998-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Izadifar M., Chapman D., Babyn P., Chen X., and Kelly M. E., “ UV-assisted 3D bioprinting of nanoreinforced hybrid cardiac patch for myocardial tissue engineering,” Tissue Eng., Part C 24, 74–88 (2018). 10.1089/ten.tec.2017.0346 [DOI] [PubMed] [Google Scholar]
- 306. Yeung E., Fukunishi T., Bai Y., Bedja D., Pitaktong I., Mattson G., Jeyaram A., Lui C., Ong C. S., Inoue T., Matsushita H., Abdollahi S., Jay S. M., and Hibino N., “ Cardiac regeneration using human-induced pluripotent stem cell-derived biomaterial-free 3D-bioprinted cardiac patch in vivo,” J. Tissue Eng. Regener. Med. 13, 2031–2039 (2019). 10.1002/term.2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Ajdary R., Ezazi N. Z., Correia A., Kemell M., Huan S., Ruskoaho H. J., Hirvonen J., Santos H. A., and Rojas O. J., “ Multifunctional 3D-printed patches for long-term drug release therapies after myocardial infarction,” Adv. Funct. Mater. 30, 2003440 (2020). 10.1002/adfm.202003440 [DOI] [Google Scholar]
- 308. Feng J., Shi H., Yang X., and Xiao S., “ Self-adhesion conductive sub-micron fiber cardiac patch from shape memory polymers to promote electrical signal transduction function,” ACS Appl. Mater. Interfaces 13, 19593–19602 (2021). 10.1021/acsami.0c22844 [DOI] [PubMed] [Google Scholar]
- 309. Jain A., Behera M., Mahapatra C., Sundaresan N. R., and Chatterjee K., “ Nanostructured polymer scaffold decorated with cerium oxide nanoparticles toward engineering an antioxidant and anti-hypertrophic cardiac patch,” Mater. Sci. Eng. C 118, 111416 (2021). 10.1016/j.msec.2020.111416 [DOI] [PubMed] [Google Scholar]
- 310. Öztürk Ş., Shahbazi R., Zeybek N. D., Kurum B., Gultekinoglu M., Aksoy E. A., Demircin M., and Ulubayram K., “ Assessment of electromechanically stimulated bone marrow stem cells seeded acellular cardiac patch in a rat myocardial infarct model,” Biomed. Mater. 16, 055012 (2021). 10.1088/1748-605X/ac199a [DOI] [PubMed] [Google Scholar]
- 311. Song X., Wang X., Zhang J., Shen S., Yin W., Ye G., Wang L., Hou H., and Qiu X., “ A tunable self-healing ionic hydrogel with microscopic homogeneous conductivity as a cardiac patch for myocardial infarction repair,” Biomaterials 273, 120811 (2021). 10.1016/j.biomaterials.2021.120811 [DOI] [PubMed] [Google Scholar]
- 312. He Y., Hou H., Wang S., Lin R., Wang L., Yu L., and Qiu X., “ From waste of marine culture to natural patch in cardiac tissue engineering,” Bioact. Mater. 6, 2000–2010 (2021). 10.1016/j.bioactmat.2020.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Menasché P., Vanneaux V., Hagège A., Bel A., Cholley B., Cacciapuoti I., Parouchev A., Benhamouda N., Tachdjian G., Tosca L., Trouvin J.-H., Fabreguettes J.-R., Bellamy V., Guillemain R., Suberbielle Boissel C., Tartour E., Desnos M., and Larghero J., “ Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: First clinical case report,” Eur. Heart J. 36, 2011–2017 (2015). 10.1093/eurheartj/ehv189 [DOI] [PubMed] [Google Scholar]
- 314. Bobylev D., Wilhelmi M., Lau S., Klingenberg M., Mlinaric M., Petená E., Helms F., Hassel T., Haverich A., Horke A., and Böer U., “ Pressure-compacted and spider silk–reinforced fibrin demonstrates sufficient biomechanical stability as cardiac patch in vitro,” J. Biomater. Appl. 36, 1126 (2021). 10.1177/08853282211046800 [DOI] [PubMed] [Google Scholar]
- 315. Zhu D., Hou J., Qian M., Jin D., Hao T., Pan Y., Wang H., Wu S., Liu S., Wang F., Wu L., Zhong Y., Yang Z., Che Y., Shen J., Kong D., Yin M., and Zhao Q., “ Nitrate-functionalized patch confers cardioprotection and improves heart repair after myocardial infarction via local nitric oxide delivery,” Nat. Commun. 12, 4501 (2021). 10.1038/s41467-021-24804-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Mehrotra S., Singh R. D., Bandyopadhyay A., Janani G., Dey S., and Mandal B. B., “ Engineering microsphere-loaded non-mulberry silk-based 3D bioprinted vascularized cardiac patches with oxygen-releasing and immunomodulatory potential,” ACS Appl. Mater. Interfaces 13, 50744–50759 (2021). 10.1021/acsami.1c14118 [DOI] [PubMed] [Google Scholar]
- 317. Roche C. D., Sharma P., Ashton A. W., Jackson C., Xue M., and Gentile C., “ Printability, durability, contractility and vascular network formation in 3d bioprinted cardiac endothelial cells using alginate–gelatin hydrogels,” Front. Bioeng. Biotechnol. 9, 110 (2021). 10.3389/fbioe.2021.636257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Gaetani R., Feyen D. A. M., Verhage V., Slaats R., Messina E., Christman K. L., Giacomello A., Doevendans P., and Sluijter J. P. G., “ Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction,” Biomaterials 61, 339–348 (2015). 10.1016/j.biomaterials.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 319. Yang Y., Lei D., Huang S., Yang Q., Song B., Guo Y., Shen A., Yuan Z., Li S., Qing F.-L., Ye X., You Z., and Zhao Q., “ Elastic 3D-printed hybrid polymeric scaffold improves cardiac remodeling after myocardial infarction,” Adv. Healthcare Mater. 8, 1900065 (2019). 10.1002/adhm.201900065 [DOI] [PubMed] [Google Scholar]
- 320. Gao L., Kupfer M. E., Jung J. P., Yang L., Zhang P., Tran Q., Ajeti V., Freeman B. T., Fast V. G., Campagnola P. J., Ogle B. M., and Zhang J., “ Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, three-dimensionally printed scaffold,” Circ. Res. 120, 1318 (2017). 10.1161/CIRCRESAHA.116.310277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Noor N., Shapira A., Edri R., Gal I., Wertheim L., and Dvir T., “ 3D printing of personalized thick and perfusable cardiac patches and hearts,” Adv. Sci. 6, 1900344 (2019). 10.1002/advs.201900344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Su T., Huang K., Mathews K. G., Scharf V. F., Hu S., Li Z., Frame B. N., Cores J., Dinh P.-U., Daniele M. A., Ligler F. S., and Cheng K., “ Cardiac stromal cell patch integrated with engineered microvessels improves recovery from myocardial infarction in rats and pigs,” ACS Biomater. Sci. Eng. 6, 6309–6320 (2020). 10.1021/acsbiomaterials.0c00942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Beck E. C., Jarrell D. K., Lyons A. C., Vanderslice E. J., VeDepo M. C., and Jacot J. G., “ Assessment of electrospun cardiac patches made with sacrificial particles and polyurethane-polycaprolactone blends,” J. Biomed. Mater. Res., Part A 109, 2154–2163 (2021). 10.1002/jbm.a.37201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Liang Y., Mitriashkin A., Lim T. T., and Goh J. C.-H., “ Conductive polypyrrole-encapsulated silk fibroin fibers for cardiac tissue engineering,” Biomaterials 276, 121008 (2021). 10.1016/j.biomaterials.2021.121008 [DOI] [PubMed] [Google Scholar]
- 325. Bian W., Liau B., Badie N., and Bursac N., “ Mesoscopic hydrogel molding to control the 3D geometry of bioartificial muscle tissues,” Nat. Protoc. 4, 1522–1534 (2009). 10.1038/nprot.2009.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Liau B., Christoforou N., Leong K., and Bursac N., “ Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function,” Biomaterials 32, 9180–9187 (2011). 10.1016/j.biomaterials.2011.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Streeter B. W. and Davis M. E., “ Therapeutic cardiac patches for repairing the myocardium,” in Cell Biology and Translational Medicine, Stem Cells: Translational Science to Therapy Vol. 5, edited by Turksen K. ( Springer International Publishing, Cham, 2019), pp. 1–24. [DOI] [PubMed] [Google Scholar]
- 328. Mancuso A., Barone A., Cristiano M. C., Cianflone E., Fresta M., and Paolino D., “ Cardiac stem cell-loaded delivery systems: A new challenge for myocardial tissue regeneration,” Int. J. Mol. Sci. 21, 7701 (2020). 10.3390/ijms21207701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Riegler J., Gillich A., Shen Q., Gold J. D., and Wu J. C., “ Cardiac tissue slice transplantation as a model to assess tissue-engineered graft thickness, survival, and function,” Circulation 130, S77–S86 (2014). 10.1161/CIRCULATIONAHA.113.007920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Montgomery M., Ahadian S., Davenport Huyer L., Lo Rito M., Civitarese R. A., Vanderlaan R. D., Wu J., Reis L. A., Momen A., Akbari S., Pahnke A., Li R.-K., Caldarone C. A., and Radisic M., “ Flexible shape-memory scaffold for minimally invasive delivery of functional tissues,” Nat. Mater. 16, 1038–1046 (2017). 10.1038/nmat4956 [DOI] [PubMed] [Google Scholar]
- 331. Wang L., Liu Y., Ye G., He Y., Li B., Guan Y., Gong B., Mequanint K., Xing M. M. Q., and Qiu X., “ Injectable and conductive cardiac patches repair infarcted myocardium in rats and minipigs,” Nat. Biomed. Eng. 5, 1157–1173 (2021). 10.1038/s41551-021-00796-9 [DOI] [PubMed] [Google Scholar]
- 332. Tang J., Vandergriff A., Wang Z., Hensley M. T., Cores J., Allen T. A., Dinh P.-U., Zhang J., Caranasos T. G., and Cheng K., “ A regenerative cardiac patch formed by spray painting of biomaterials onto the heart,” Tissue Eng., Part C 23, 146–155 (2017). 10.1089/ten.tec.2016.0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Noguchi R., Nakayama K., Itoh M., Kamohara K., Furukawa K., Oyama J., Node K., and Morita S., “ Development of a three-dimensional pre-vascularized scaffold-free contractile cardiac patch for treating heart disease,” J. Heart Lung Transplant. 35, 137–145 (2016). 10.1016/j.healun.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 334. Chung J. J., Han J., Wang L. L., Arisi M. F., Zaman S., Gordon J., Li E., Kim S. T., Tran Z., Chen C. W., Gaffey A. C., Burdick J. A., and Atluri P., “ Delayed delivery of endothelial progenitor cell-derived extracellular vesicles via shear thinning gel improves postinfarct hemodynamics,” J. Thorac. Cardiovasc. Surg. 159, 1825 (2020). 10.1016/j.jtcvs.2019.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Tsui J. H., Ostrovsky-Snider N. A., Yama D. M. P., Donohue J. D., Choi J. S., Chavanachat R., Larson J. D., Murphy A. R., and Kim D.-H., “ Conductive silk–polypyrrole composite scaffolds with bioinspired nanotopographic cues for cardiac tissue engineering,” J. Mater. Chem. B 6, 7185–7196 (2018). 10.1039/C8TB01116H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Montero P., Flandes-Iparraguirre M., Musquiz S., Pérez Araluce M., Plano D., Sanmartín C., Orive G., Gavira J. J., Prosper F., and Mazo M. M., “ Cells, materials, and fabrication processes for cardiac tissue engineering,” Front. Bioeng. Biotechnol. 8, 955 (2020). 10.3389/fbioe.2020.00955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Huang S. and Frangogiannis N. G., “ Anti-inflammatory therapies in myocardial infarction: Failures, hopes and challenges,” Br. J. Pharmacol. 175, 1377–1400 (2018). 10.1111/bph.14155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Lock R., Al Asafen H., Fleischer S., Tamargo M., Zhao Y., Radisic M., and Vunjak-Novakovic G., “ A framework for developing sex-specific engineered heart models,” Nat. Rev. Mater. 7, 295 (2021). 10.1038/s41578-021-00381-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.