In a large cross-sectional study done in 114 centers in 10 countries, the authors assessed whether there was an association of an elevated SARS-CoV-2 plasma antigen level at presentation with a variety of patient characteristics, clinical outcomes, and viral factors.
Visual Abstract. Baseline Plasma Antigen and Outcomes in Patients Hospitalized With COVID-19.
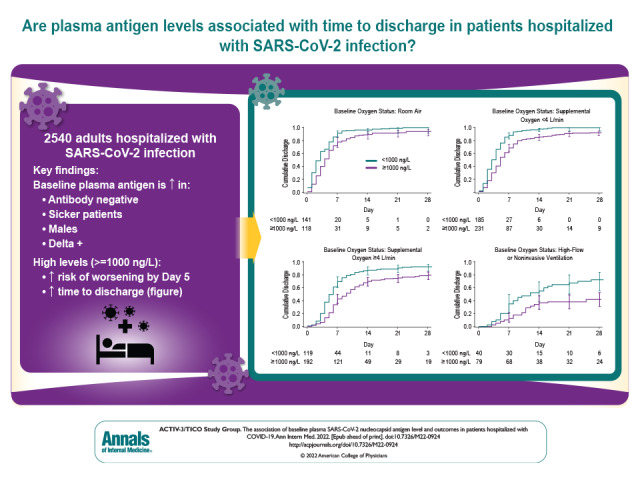
In a large cross-sectional study done in 114 centers in 10 countries, the authors assessed whether there was an association of an elevated SARS-CoV-2 plasma antigen level at presentation with a variety of patient characteristics, clinical outcomes, and viral factors.
Abstract
Background:
Levels of plasma SARS-CoV-2 nucleocapsid (N) antigen may be an important biomarker in patients with COVID-19 and enhance our understanding of the pathogenesis of COVID-19.
Objective:
To evaluate whether levels of plasma antigen can predict short-term clinical outcomes and identify clinical and viral factors associated with plasma antigen levels in hospitalized patients with SARS-CoV-2.
Design:
Cross-sectional study of baseline plasma antigen level from 2540 participants enrolled in the TICO (Therapeutics for Inpatients With COVID-19) platform trial from August 2020 to November 2021, with additional data on day 5 outcome and time to discharge.
Setting:
114 centers in 10 countries.
Participants:
Adults hospitalized for acute SARS-CoV-2 infection with 12 days or less of symptoms.
Measurements:
Baseline plasma viral N antigen level was measured at a central laboratory. Delta variant status was determined from baseline nasal swabs using reverse transcriptase polymerase chain reaction. Associations between baseline patient characteristics and viral factors and baseline plasma antigen levels were assessed using both unadjusted and multivariable modeling. Association between elevated baseline antigen level of 1000 ng/L or greater and outcomes, including worsening of ordinal pulmonary scale at day 5 and time to hospital discharge, were evaluated using logistic regression and Fine–Gray regression models, respectively.
Results:
Plasma antigen was below the level of quantification in 5% of participants at enrollment, and 1000 ng/L or greater in 57%. Baseline pulmonary severity of illness was strongly associated with plasma antigen level, with mean plasma antigen level 3.10-fold higher among those requiring noninvasive ventilation or high-flow nasal cannula compared with room air (95% CI, 2.22 to 4.34). Plasma antigen level was higher in those who lacked antispike antibodies (6.42 fold; CI, 5.37 to 7.66) and in those with the Delta variant (1.73 fold; CI, 1.41 to 2.13). Additional factors associated with higher baseline antigen level included male sex, shorter time since hospital admission, decreased days of remdesivir, and renal impairment. In contrast, race, ethnicity, body mass index, and immunocompromising conditions were not associated with plasma antigen levels. Plasma antigen level of 1000 ng/L or greater was associated with a markedly higher odds of worsened pulmonary status at day 5 (odds ratio, 5.06 [CI, 3.41 to 7.50]) and longer time to hospital discharge (median, 7 vs. 4 days; subhazard ratio, 0.51 [CI, 0.45 to 0.57]), with subhazard ratios similar across all levels of baseline pulmonary severity.
Limitations:
Plasma samples were drawn at enrollment, not hospital presentation. No point-of-care test to measure plasma antigen is currently available.
Conclusion:
Elevated plasma antigen is highly associated with both severity of pulmonary illness and clinically important patient outcomes. Multiple clinical and viral factors are associated with plasma antigen level at presentation. These data support a potential role of ongoing viral replication in the pathogenesis of SARS-CoV-2 in hospitalized patients.
Primary Funding Source:
U.S. government Operation Warp Speed and National Institute of Allergy and Infectious Diseases.
Mortality rates for adults hospitalized with COVID-19 remain high, ranging from approximately 6% to 27% from large published clinical trials (1–3). The extent to which ongoing SARS-CoV-2 viral replication versus the host immune system contributes to the severity of disease, pathogenesis, and mortality of SARS-CoV-2 infection in hospitalized patients is uncertain (4). Although some antivirals have shown promise in hospitalized patients (5, 6), several monoclonal antibodies and other antiviral treatments have failed in this population while improving outcomes in the outpatient setting (2, 7–9); in contrast, immunomodulatory drugs (for example, corticosteroids, Janus kinase inhibitors, and tocilizumab) are only efficacious in more advanced disease (3, 10, 11). However, small studies have shown that higher levels of plasma viral RNA near the time of hospital admission are associated with increased prevalence of nonpulmonary organ failure (12), critical illness and both intensive care unit and hospital mortality (13–15), and lower chance of sustained recovery (16). Although factors such as male sex, older age, and diabetes are established risk factors for poor outcomes from COVID-19 (17), how such characteristics relate to plasma viral burden in recently hospitalized persons has not been described to date.
Immune factors play a role in the control of acute SARS-CoV-2 viral replication (18), as the body's immune system can usually mount a response sufficient for the vast majority of SARS-CoV-2 infections from requiring hospitalization (19). A core aspect of this response includes the production of neutralizing antibodies, most of which are aimed at epitopes in the receptor-binding domain of the viral spike protein (20). Thus, the kinetics and magnitude of the immune response may partially control viral replication in the acutely infected host (21) and have implications for potential benefit of antiviral therapies (1, 16).
Although detection of nasal swab nucleocapsid (N) antigen is a reflection of viral replication in the upper airway and is used for the diagnosis of acute SARS-CoV-2 infection, plasma N antigen (plasma antigen) level may be more likely to reflect systemic viral replication. The ACTIV-3 (Accelerating COVID-19 Therapeutic Interventions and Vaccines, Inpatient Monoclonal Antibodies and Other Therapies) platform trial, also called TICO (Therapeutics for Inpatients With COVID-19), assesses the clinical effect of novel anti–SARS-CoV-2 viral agents in hospitalized patients with acute COVID-19 (22). In this trial, plasma antigen level and antispike and anti-N antibody status at enrollment are determined centrally using stored specimens. In addition, polymerase chain reaction (PCR) testing of nasal swab material retrieved at baseline is used to determine the viral variant causing the infection. The study enrolled inpatients from August 2020 through mid-November 2021, spanning the emergence of the Delta variant as the dominant variant before its replacement with Omicron. The primary objective of this report is to describe the clinical characteristics and viral factors, such as variant type, associated with antigen level at enrollment (baseline plasma antigen level) and to assess the association of the antigen level with subsequent short-term prognosis.
Methods
Participants
The study population includes those who were randomly assigned in the TICO platform trial to receive 1 of 5 antiviral products (bamlanivimab [8], sotrovimab [2], amubarvimab–romlusevimab [2], tixagevimab–cilgavimab [23], and Molecular Partners MP0420 [24]) or matched placebo (22) and for whom a baseline assessment of the relevant biomarkers of interest was available.
Biomarkers of Interest
Quantitative plasma SARS-CoV-2 N antigen was measured using a microbead-based immunoassay (Quanterix). The lower limit of quantification of this assay is 3 ng/L; 2.9 ng/L was imputed for samples below this level. A level of 1000 ng/L or greater was a prespecified binary cutoff, reflecting the median plasma level in the TICO trial of bamlanivimab (16). Antispike neutralizing antibody was determined using a surrogate viral neutralization test (GenScript cPass). This assay provides an assessment of the percentage of binding that is inhibited by the participant's antibodies (ranging from 0% to 100%); results of 30% or more were considered positive (25). Anti-N pan-immunoglobulin positivity was determined according to the manufacturer's directions (Bio-Rad Platelia SARS-CoV-2 Total Antibody Test). The SARS-CoV-2 viral RNA was extracted from a mid-turbinate nasal swab collected at the same time as the plasma sample. Beginning in February 2021, the presence of the Delta variant was determined using an reverse transcriptase PCR (RT-PCR) assay specifically designed to the N-terminal domain region of the spike gene. The RT-PCR of N was used as a positive control. If RT-PCR was negative for spike but positive for N, it was considered non-Delta. Among 811 samples that were characterized as Delta via PCR, 810 (99.9%) were confirmed as Delta by either Pangolin or Nextclade sequencing; 3 samples among 364 identified as non-Delta by quantitative PCR were found to be Delta by sequence analysis.
Clinical Data
To be eligible for inclusion in TICO, participants needed to be hospitalized for acute COVID-19 within 12 days of symptom onset. The cohort was characterized for dates of enrollment (August 2020 to 15 November 2021, corresponding to the emergence of the Delta variant but before Omicron), demographic characteristics (age, sex, race, or ethnicity), geographic location of recruitment (United States, Europe, Asia, Africa), coexisting chronic illness (yes or no: hypertension, diabetes, renal impairment, asthma and/or chronic obstructive pulmonary disease, obesity, cardiovascular disease, hepatic impairment, cancer, and disorders associated with immune dysfunction [that is, HIV, other immunosuppressive conditions, and/or medicines for antirejection, immunomodulation, or biologics]), prior SARS-CoV-2 vaccination (fully vaccinated [full course completed—2 doses of messenger RNA vaccine or single dose of Johnson & Johnson vaccine—and symptoms started ≥14 days after last dose], partially vaccinated [symptoms within 14 days of a last dose or only 1 of 2-dose course received], and not vaccinated), number of days since symptom onset, pulmonary disease severity (no supplemental oxygen, low-flow supplementary oxygen <4 L/min, low-flow supplementary oxygen ≥4 L/min, high-flow nasal cannula [HFNC] or noninvasive ventilation [NIV]), and receipt of remdesivir in days before enrollment (0, 1, 2, or more days).
Statistical Analysis
Participant characteristics were described using basic descriptive statistics. Associations between patient characteristics and the plasma antigen level were assessed using linear regression models with log10-transformed antigen level as the dependent variable. Geometric mean titers were calculated from these models by back-transforming least-squares mean results for each dependent variable. Coefficient estimates from these models to the power 10 represent the “fold-difference” antigen level according to the reference level of the variable of interest.
Associations with baseline plasma antigen level geometric mean titers were assessed using both univariable and multivariable models. In the multivariable model, covariates included only premorbid attributes—for example, demographic characteristics, comorbidities, and vaccine status. Similarly, the association of baseline variables with elevated plasma antigen level was assessed using univariable and multivariable logistic regression. Elevated plasma antigen was defined by a dichotomous value of antigen level 1000 ng/L or greater, which was identified as the median value in the bamlanivimab study (16).
The association between antigen level of 1000 ng/L or greater and 2 patient outcomes was also evaluated. Both outcomes were evaluated across subgroups defined by baseline oxygen status and are restricted to participants in the placebo group. One outcome—worsening of pulmonary status at day 5—used a 7-point ordinal scale defined as back to normal activities; mild symptoms; conventional oxygen supplementation less than 4 L/min; conventional oxygen supplementation 4 L/min or more; HFNC or NIV; mechanical ventilation, extracorporeal membrane oxygenation, or new hemodialysis; or death. The participant's status on this scale on day 5 was dichotomized as either worse or same/better than baseline. We used logistic regression with and without adjustment for baseline premorbid attributes and baseline oxygen status and a further analysis in which we additionally adjusted for baseline antibody status.
The second outcome evaluated was time to hospital discharge, treating death as a competing risk. To be consistent with other studies (1, 5, 10), the outcome is summarized over 28 days of follow-up and was evaluated within subgroups defined by baseline oxygen status; analyses were restricted to participants in the placebo group. The median days to discharge was calculated on the basis of the cumulative incidence function (26). The association between plasma antigen level and the rate of discharge (subhazard ratio) was estimated using Fine–Gray proportional hazards models (27), with and without adjustment for baseline clinical variables as described earlier. All P values are 2-sided. Analyses were done using SAS, version 9.4 (SAS Institute).
Assessment of the Delta variant was not possible for participants with undetectable nasal swab RNA by RT-PCR. For these participants, as well as those for whom RT-PCR results were unavailable, the variant was imputed as Delta for participants enrolled on or after 20 June 2021 and as non-Delta for those enrolled earlier. This date cutoff corresponds to the date from which Delta became the dominant strain detected in this cohort, an approach used previously (28). A sensitivity analysis removed participants from June and July without imputed RT-PCR results given highest variability in Delta status during that period.
Role of the Funding Source
Investigators from the National Institutes of Health were directly involved in all aspects of this study, including study design, data collection, data analysis, data interpretation, and writing of the report.
Results
Baseline Clinical and Viral Characteristics of Cohort
Between August 2020 and November 2021, 2694 participants were enrolled in 10 countries into 1 of the first 5 interventions in the TICO platform randomized controlled trial. Among these, 2540 participants, from 114 sites, who received the study intervention and had a baseline plasma sample available for antigen measurement are included in this report. As shown in Table 1, 636 participants (25%) were breathing room air at the time of enrollment, 274 (11%) required either HFNC or NIV, and most participants required conventional oxygen supplementation. Most (58%) of the cohort were men, with a median age of 57 years (interquartile range, 46 to 68 years). Eighty-one percent of patients had not received even a single vaccine for SARS-CoV-2. Most participants (78%) were enrolled in centers in the United States.
Table 1.
Baseline Characteristics by Baseline Antigen Level
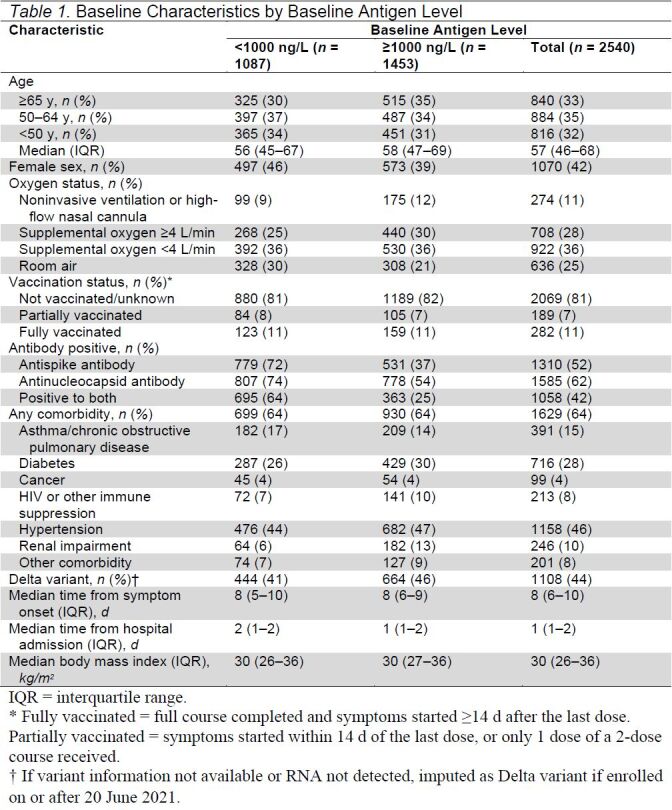
Fifty-two percent of baseline samples were positive for the antispike antibody at enrollment, with slightly more (62%) positive for anti-N antibody; 28% were negative for both antispike and anti-N antibodies. Beginning with participants enrolled in January 2021, nasal swab specimens were tested for the Delta variant; viral RNA was detectable in 88% of the 1675 participants for whom results are available. As shown in Table 1 of Supplement 2, Delta became the dominant strain in circulation in the cohort in late-June 2021.
Patient Clinical Factors Associated With Plasma Antigen Level
Plasma viral antigen level was above the level of quantification (3.0 ng/L) in 95% of baseline samples, with a median of 1442 ng/L (absolute range of 3.0 to 92 404 ng/L). We initially examined the association of 20 baseline clinical and viral factors with plasma antigen level at time of enrollment both without adjustment and with adjustment for all premorbid covariates (Table 2). Plasma antigen level was highly associated with the severity of pulmonary disease, 3.10-fold higher in participants requiring NIV or HFNC (95% CI, 2.22 to 4.34), 2.88-fold higher in those requiring 4 L or more of conventional oxygen supplementation (CI, 2.23 to 3.71), and 1.77-fold higher in those requiring less than 4 L of conventional oxygen supplementation (CI, 1.39 to 2.26) compared with those breathing room air. Plasma antigen level was higher in those with more than 1 week of symptoms but lower with increased days since admission to the hospital and 2 or more days of exposure to the antiviral medication remdesivir. Older age was modestly associated with increased plasma antigen level, particularly in those older than 65 years. Plasma antigen level was also substantially higher in participants with underlying renal impairment (2.63 fold [CI, 1.88 to 3.68]).
Table 2.
Association of Baseline Characteristics With Antigen Level
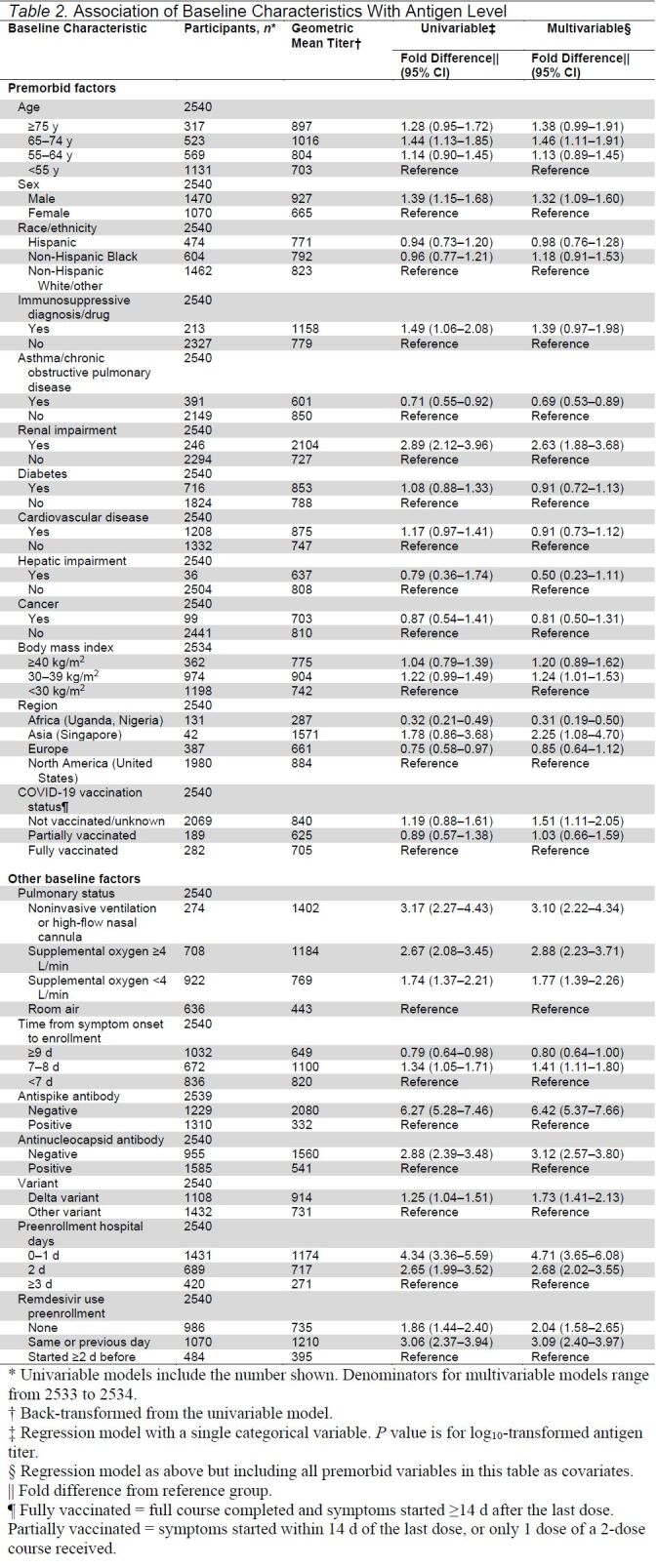
Male sex was associated with a higher level of plasma viral antigen (1.32-fold higher in men than women [CI, 1.09 to 1.60]). As shown in Figure 1 of Supplement 2, these sex differences were seen across all levels of illness. The higher level of viral antigen in men is not explained by differences in vaccination status or differences in length of symptoms before enrollment. Comorbidities that differed by sex included more renal disease and less asthma in men; women more often had obesity, with a body mass index greater than 40 kg/m2 in the cohort. After adjustment for these and other premorbid factors, men were more likely to have antigen levels above this threshold (odds ratio [OR], 1.26 [CI, 1.06 to 1.48]) (Table 2 of Supplement 2).
In contrast, several clinical factors that have been frequently reported in studies to be associated with outcomes in COVID-19, such as race, ethnicity, immunosuppression status, obesity, and presence of other comorbidities were not associated with plasma antigen levels in this analysis.
Vaccination, Antibody, and Viral Variant Characteristics Associated With Plasma Antigen Level
Plasma antigen level was highly associated with antibody status, particularly for antispike receptor-binding domain as measured by surrogate viral neutralization test. Participants with negative antispike antibody status had 6.42-fold higher (CI, 5.37 to 7.66) plasma antigen level than those with positive antibody status; 75% of participants with negative antibody status had plasma antigen level of 1000 ng/L or greater (Table 2 of Supplement 2). Figure 2 of Supplement 2 summarizes the participants in each pulmonary disease severity category in terms of both antibody status and antigen level. The disease categories are heterogeneous with respect to both antibody and antigen status.
The proportion of men and women with positive antispike receptor-binding domain antibody for SARS-CoV-2 was similar (approximately 50%). However, the proportion of men who were antibody negative but had a viral antigen level of 1000 ng/L or greater was higher (OR, 1.41 [CI, 1.07 to 1.85], data not shown). The presence of anti-N antibody was similarly associated with decreased plasma antigen level, although to a lesser extent (3.12 fold [CI, 2.57 to 3.80]). Unvaccinated participants had a higher baseline plasma antigen level than fully vaccinated persons after adjustment for premorbid status (Table 2).
Forty-four percent of participants were infected with the Delta variant, and this group had higher plasma viral antigen levels than those infected with non-Delta variants (1.73 fold [CI, 1.41 to 2.13]). Delta variant status was imputed on the basis of date of enrollment for those patients for whom no nasal swab was available or no viral RNA was detected; excluding these patients did not change these conclusions (data not shown).
Association Between Elevated Plasma Antigen Level and Patient Outcomes
A dichotomous value of antigen level of 1000 ng/L or greater was prespecified on the basis of the median value in the bamlanivimab study (16). As shown in Table 1 as well as Table 2 of Supplement 2, fifty-seven percent of patients (n = 1453) in the cohort had elevated plasma antigen level. Given the strong association between baseline plasma antigen level and pulmonary severity of illness, outcome measures were evaluated separately for the baseline oxygen categories (room air, requiring <4 L/min of conventional oxygen supplementation, requiring ≥4 L/min of conventional oxygen supplementation, and requiring NIV or HFNC).
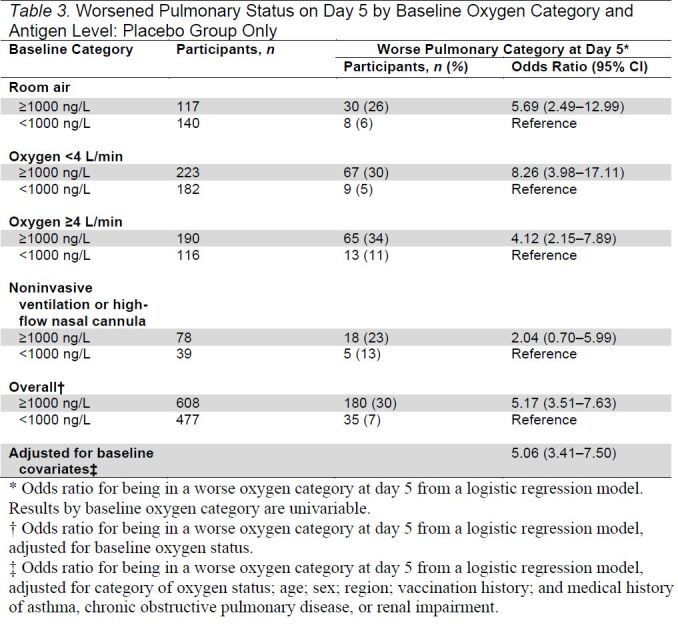
As shown in Table 3, for participants in the placebo group, the odds of pulmonary worsening were substantially higher in every oxygen category for those with a plasma antigen level of 1000 ng/L or greater, with an overall OR, adjusted for baseline oxygen status, of worsening at day 5 of 5.17 (CI, 3.51 to 7.63). This OR estimate was not appreciably changed after additional adjustment for variables reflecting premorbid status (5.06 [CI, 3.41 to 7.50]). Among participants enrolled on room air, 26% of those with plasma levels of 1000 ng/L or greater progressed to requiring oxygen at day 5 versus 6% with antigen level less than 1000 ng/L.
Table 3.
Worsened Pulmonary Status on Day 5 by Baseline Oxygen Category and Antigen Level: Placebo Group Only
Given this marked increased risk for clinical worsening, we next assessed the association between antigen level of 1000 ng/L or greater and time to hospital discharge among participants in the placebo group. As shown in the Figure and Table 3 of Supplement 2, plasma antigen level was associated with duration of hospitalization across all levels of oxygen severity. The median time to discharge was 4 days among participants with antigen level less than 1000 ng/L compared with 7 days among those with antigen level of 1000 ng/L or greater (subhazard ratio, 0.54 [CI, 0.48 to 0.61]). Adjusting for baseline clinical covariates did not change these estimates (subhazard ratio, 0.51 [CI, 0.45 to 0.57]). For participants in the NIV/HFNC group, only 42% of those with antigen level of 1000 ng/L or higher were discharged by day 28 compared with 73% with antigen level less than 1000 ng/L.
Figure. Cumulative incidence of discharge by baseline oxygen status and antigen level: TICO participants assigned to the placebo group.
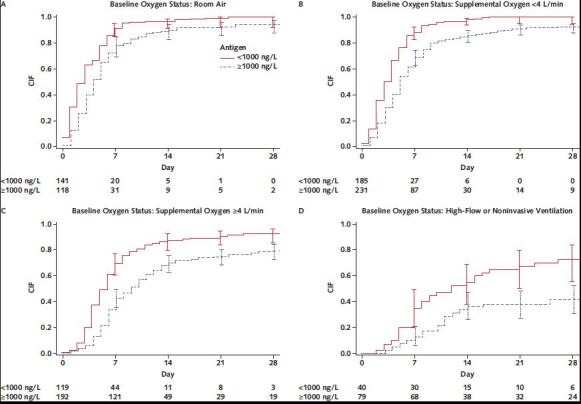
CIF = cumulative incidence function, using Aalen–Johansen estimator of cumulative incidence with 95% CIs.
Table 4, Table 5, and Table 6 of Supplement 2 assess how the association between plasma antigen and outcomes differs with antibody status. The OR of worsening by day 5 was much higher in patients with plasma antigen level of 1000 ng/L or greater in both antibody positive (OR, 3.36 [CI, 1.96 to 5.74]) and antibody negative (OR, 7.92 [CI, 3.67 to 17.07]) (Table 4 of Supplement 2). Similarly, although median days to discharge was shorter for those with positive antibody status versus those with negative antibody status, the associations with elevated antigen levels were similar for these 2 groups; adjusted subhazard ratio for discharge was 0.61 (CI, 0.52 to 0.73) among those with positive antibody status and 0.48 (CI, 0.39 to 0.59) for those with negative antibody status (Table 5 and Table 6 of Supplement 2).
Discussion
In 2540 participants from 114 centers internationally in 10 countries, we found that SARS-CoV-2 N antigen can be detected in plasma in more than 95% of participants at baseline and is highly associated both with the severity of pulmonary illness at presentation and clinically important outcomes. Fifty-seven percent of participants had a baseline plasma antigen level of 1000 ng/L or greater; this group was at markedly increased risk for pulmonary worsening by day 5 and had delayed hospital discharge by day 28, across all levels of severity of illness. Plasma antigen level is 5-fold lower in patients with endogenous neutralizing antibodies, although the prognostic importance of plasma antigen is seen regardless of neutralizing antibody status. Plasma antigen level was higher among men than women across all severities of illness and was lower in patients with more days in hospital and more exposure to remdesivir at enrollment. Taken together, these data support the conclusion that plasma antigen level is not merely a marker of cell injury and ensuing release of antigen but suggests a pathogenic process with ongoing systemic viral replication.
Early pandemic data from studies using samples from the upper respiratory tract suggested that viral loads peak close to the time of symptom onset and then fall steadily over time, with poor correlation between upper respiratory tract viral load and severity of disease (29, 30). It is important to note that this does not exclude viral replication elsewhere in the body. This report shows that among patients hospitalized with acute COVID-19, with generally more than 7 days of illness duration, plasma provides a readily available, highly relevant specimen to assess for viral activity as it relates to clinically relevant outcomes. The fact that plasma antigen level has striking prognostic value even in patients breathing room air makes it less likely that this simply reflects cellular injury and severity of illness but instead that viral replication is likely ongoing in later stages of disease. The seemingly detrimental host immune responses among patients who progress to require oxygen and become critically ill may be driven in part by ongoing viral replication (31) (whether from viral factors or a dysregulated immune response), and it is plausible that these patients may benefit from both antiviral and immunosuppressive therapy. As shown in Figure 2 of Supplement 2, a substantial portion of critically ill patients requiring HFNC or NIV had elevated plasma antigen level despite having positive antibody status.
Early in the pandemic, positive clinical trials in COVID-19 seemed to mirror this “nasal viral load” timeline, with many successes in antiviral therapies applied early, in the outpatient setting (both monoclonal antibodies and also antiviral drugs) (7, 32); in contrast, many monoclonal antibodies failed to improve outcomes in hospitalized patients when added to remdesivir therapy (2, 8), whereas the successful trials in hospitalized patients requiring oxygen therapy have involved the use of immunosuppressive therapy (3, 10). This has led some to question whether there is an ongoing role for the use of antiviral therapies in hospitalized patients or whether this strategy should be abandoned (4). This report suggests that a precision medicine approach, when feasible, be put to the test in future clinical trials of antivirals in hospitalized patients with COVID-19. The heterogeneity of patients with respect to both antibody status and antigen level supports this, as compared to the current standard of choosing treatments based only on pulmonary disease severity.
The association of male sex with a higher risk for COVID-mortality is widely recognized (33, 34). Although this has been associated with clinical characteristics, including higher prevalence of risk factors such as heart disease, hypertension, and the presence of anti-interferon antibodies (35), we believe this is the first report of an association between male sex and higher viral antigen level. This is not merely a surrogate for severity of illness in this cohort, as these differences occurred even in patients requiring less than 4 L of oxygen. It does not seem to relate to lower vaccination rates or presenting later in the course of illness, as these characteristics did not differ between men and women enrolled in the trial (although >80% of all enrolled patients were unvaccinated so the statistical power to assess for this is limited).
Over the course of the 16-month enrollment into TICO, there was a steady increase in baseline plasma antigen level, most notably as Delta became the dominant variant beginning in June of 2021. This higher level of antigen was seen even with adjustment for antibody status and the emergence of increasing proportions of enrollees who had been fully (2-series) vaccinated during that period. We imputed Delta variant status for the 10% of patients without measurable virus in nasal samples based on timing of Delta variant emergence; excluding these patients did not change these conclusions. It is possible that these higher viral antigen levels may help explain the increased severity of illness seen in Delta infection (36).
Renal insufficiency is a known risk factor for increased mortality in COVID-19 (17, 37). As shown here, those with renal impairment had substantially higher levels of plasma antigen than those without. Whether this reflects increased viral replication or a failure of normal antigen clearance because of renal dysfunction is unknown. The marked regional differences are also striking, with plasma antigen levels in participants from Uganda nearly 3-fold lower and from Singapore nearly twice as high as those recruited from the United States and Europe. Regardless of whether this helps explain why COVID-19 illness is milder in sub-Saharan African populations is unknown. We urge caution in interpreting these findings in Africa and Asia given that both are from single centers and represent less than 10% of the population recruited. Of note, there were no differences in plasma antigen level by self-identified race or ethnicity.
We evaluated the prognostic ability of the plasma antigen level in terms of a short-term clinical outcome and hospital discharge to confirm the clinical relevance of the baseline plasma antigen level and motivate the need to understand clinical correlates at baseline of the plasma antigen level. The ability of the plasma antigen level and other baseline factors to predict longer term outcomes, such as clinical recovery (the primary outcome of the TICO trials) or a differential response to antiviral therapy, will be assessed in future work.
This report has several strengths and limitations. The international, multicenter recruitment of participants coupled with central laboratory measurements of both antigen and antibody status led to reliable estimates of antigen and neutralizing antibody levels in a broadly defined population of persons hospitalized with COVID-19. The plasma antigen assay used here allowed a sensitive assessment of viral level, with detectable antigen in more than 95% of patients, in comparison to previous inpatient cohorts that focused on plasma viral RNA and reported positive values in 20% to 40% of inpatients (13–15, 38). We confirm that plasma antigen level is markedly higher in patients with pulmonary organ dysfunction and also show that high levels of plasma antigen at enrollment are associated with clinically important end points, such as pulmonary deterioration at day 5 and increased time to discharge. Among the shortcomings of the study are that antigen level was measured at the time of study entry as opposed to the time of hospital admission and the fact that some persons had received treatment with remdesivir before study entry, although the median time from hospitalization to enrollment across studies was only 1 day. We have attempted to adjust for these types of potential confounders, although as with any observational comparisons, unmeasured confounders may remain. Although we identified a higher plasma antigen level during the Delta period than in previous variants, this study closed enrollment before the Omicron wave and thus we do not have data on the most current variant. Finally, although we have identified several clinical characteristics associated with baseline antigen level, to design precision trials in COVID-19 will require development and validation of a point-of-care test for measuring plasma antigen.
In conclusion, this large, multicenter cohort confirms that plasma viral antigen can be quantified in early samples in hospitalized patients and is highly associated with both baseline severity of illness and clinically important patient outcomes. Because plasma antigen level is associated with antibody status, time in hospital, and exposure to antiviral therapy, it is likely that it reflects true viral replication. These results suggest that a precision medicine approach to inpatient COVID-19 clinical trials is needed, with a substantial portion of patients hospitalized with acute SARS-CoV-2 infection potentially more likely to benefit from antiviral therapy. Plasma antigen is a practical and clinically meaningful biomarker for hospitalized patients with COVID-19.
Supplementary Material
Footnotes
This article was published at Annals.org on 30 August 2022.
* For the writing group members, see end of text. For a list of all members of the ACTIV-3/TICO Study Group, see Supplement 1.
Contributor Information
Angela J. Rogers, Division of Pulmonary, Allergy, and Critical Care Medicine, Stanford University, Stanford, California.
Deborah Wentworth, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota.
Andrew Phillips, Institute for Global Health, University College London, London, United Kingdom.
Katy Shaw-Saliba, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland.
Robin L. Dewar, Frederick National Laboratory for Cancer Research, Frederick, Maryland.
Neil R. Aggarwal, Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado, Aurora, Colorado.
Abdel G. Babiker, The Medical Research Council Clinical Trials Unit at UCL, University College London, London, United Kingdom.
Weizhong Chang, Laboratory of Human Retrovirology and Immunoinformatics, Frederick National Laboratory for Cancer Research, Frederick, Maryland.
Nila J. Dharan, Kirby Institute, University of New South Wales Sydney, Sydney, New South Wales, Australia.
Victoria J. Davey, United States Department of Veterans Affairs, Washington, DC.
Elizabeth S. Higgs, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland.
Norman Gerry, Advanced Biomedical Laboratories, Cinnaminson, New Jersey.
Adit A. Ginde, Department of Emergency Medicine, University of Colorado School of Medicine, Aurora, Colorado.
J.W. Awori Hayanga, Department of Cardiovascular Thoracic Surgery, West Virginia University, Morgantown, West Virginia.
Helene Highbarger, Leidos Biomedical Research and AIDS Monitoring Laboratory, Frederick National Laboratory for Cancer Research, Frederick, Maryland.
Jeroen L. Highbarger, Virus Isolation and Serology Laboratory, Frederick National Laboratory, Frederick, Maryland.
Mamta K. Jain, Division of Infectious Diseases, Department of Internal Medicine, UT Southwestern Medical Center, Dallas, Texas.
Virginia Kan, Infectious Diseases Section, VA Medical Center, Washington, DC.
Kami Kim, Division of Infectious Disease and International Medicine, University of South Florida and Global Emerging Diseases Institute, Tampa General Hospital, Tampa, Florida.
Perrine Lallemand, Virus Isolation and Serology Laboratory, Frederick National Laboratory, Frederick, Maryland.
Bradley G. Leshnower, Division of Cardiothoracic Surgery, Department of Surgery, Emory University, Atlanta, Georgia.
Joseph K. Lutaakome, Medical Research Council/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine Uganda Research Unit, Entebbe, Uganda.
Gail Matthews, The Kirby Institute, University of New South Wales, Sydney, Australia.
Ahmad Mourad, Department of Medicine, Division of Infectious Diseases, Duke University Medical Center, Durham, North Carolina.
Eleftherios Mylonakis, Division of Infectious Diseases, Brown University, Providence, Rhode Island.
Ven Natarajan, Frederick National Laboratory for Cancer Research, Frederick, Maryland.
Maria L. Padilla, Mount Sinai School of Medicine, New York, New York.
Lavannya M. Pandit, Michael E. DeBakey Veterans Affairs Medical Center, Baylor College of Medicine, Houston, Texas.
Roger Paredes, Infectious Diseases Department and IrsiCaixa AIDS Research Institute, Hospital Universitari Germans Trias i Pujol, Catalonia, Spain.
Sarah Pett, The Medical Research Council Clinical Trials Unit at UCL, University College London, London, United Kingdom.
Srikanth Ramachandruni, Christus Spohn Health System, Corpus Christi, Texas.
M. Tauseef Rehman, Virus Isolation and Serology Laboratory, Frederick National Laboratory, Frederick, Maryland.
Brad T. Sherman, Laboratory of Human Retrovirology and Immunoinformatics, Frederick National Laboratory for Cancer Research, Frederick, Maryland.
D. Clark Files, Section on Pulmonary, Critical Care, Allergy and Immunologic Disease, Wake Forest Baptist Health, Winston-Salem, North Carolina.
Samuel M. Brown, Division of Pulmonary and Critical Care Medicine, Intermountain Medical Center, and Department of Internal Medicine, University of Utah, Murray, Utah.
Michael A. Matthay, Cardiovascular Research Institute, Departments of Medicine and Anesthesia, University of California, San Francisco, California.
B. Taylor Thompson, Division of Pulmonary and Critical Care, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts.
James D. Neaton, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota.
H. Clifford Lane, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland.
Jens D. Lundgren, CHIP Center of Excellence for Health, Immunity, and Infections and Department of Infectious Diseases, Rigshospitalet, Copenhagen, Denmark.
References
- 1. RECOVERY Collaborative Group.. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:665-76. [PMID: ] doi: 10.1016/S0140-6736(22)00163-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group.. Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2022;22:622-35. [PMID: ] doi: 10.1016/S1473-3099(21)00751-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalil AC , Patterson TF , Mehta AK , et al; ACTT-2 Study Group Members. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795-807. [PMID: ] doi: 10.1056/NEJMoa2031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shapiro AE , Bender Ignacio RA . Time to knock monoclonal antibodies off the platform for patients hospitalised with COVID-19. Lancet Infect Dis. 2022;22:567-9. [PMID: ] doi: 10.1016/S1473-3099(21)00762-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beigel JH , Tomashek KM , Dodd LE . Remdesivir for the treatment of Covid-19 - preliminary report. Reply [Letter]. N Engl J Med. 2020;383:994. [PMID: ] doi: 10.1056/NEJMc2022236 [DOI] [PubMed] [Google Scholar]
- 6. Casirivimab and imdevimab for COVID-19. Aust Prescr. 2022;45:58-9. [PMID: ] doi: 10.18773/austprescr.2022.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen P , Nirula A , Heller B , et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384:229-37. [PMID: ] doi: 10.1056/NEJMoa2029849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lundgren JD , Grund B , Barkauskas CE , et al; ACTIV-3/TICO LY-CoV555 Study Group. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N Engl J Med. 2021;384:905-14. [PMID: ] doi: 10.1056/NEJMoa2033130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta A , Gonzalez-Rojas Y , Juarez E , et al; COMET-ICE Investigators. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941-50. [PMID: ] doi: 10.1056/NEJMoa2107934 [DOI] [PubMed] [Google Scholar]
- 10. Horby P , Lim WS , Emberson JR , et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. [PMID: ] doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salama C , Han J , Yau L , et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384:20-30. [PMID: ] doi: 10.1056/NEJMoa2030340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ram-Mohan N , Kim D , Zudock EJ , et al. SARS-CoV-2 RNAemia predicts clinical deterioration and extrapulmonary complications from COVID-19. Clin Infect Dis. 2022;74:218-26. [PMID: ] doi: 10.1093/cid/ciab394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gutmann C , Takov K , Burnap SA , et al. SARS-CoV-2 RNAemia and proteomic trajectories inform prognostication in COVID-19 patients admitted to intensive care. Nat Commun. 2021;12:3406. [PMID: ] doi: 10.1038/s41467-021-23494-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fajnzylber J , Regan J , Coxen K , et al; Massachusetts Consortium for Pathogen Readiness. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11:5493. [PMID: ] doi: 10.1038/s41467-020-19057-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bermejo-Martin JF , González-Rivera M , Almansa R , et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit Care. 2020;24:691. [PMID: ] doi: 10.1186/s13054-020-03398-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lundgren JD , Grund B , Barkauskas CE , et al; ACTIV-3/TICO Bamlanivimab Study Group. Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels. A randomized controlled trial. Ann Intern Med. 2022; 175:234-43. [PMID: ] doi: 10.7326/M21-3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Williamson EJ , Walker AJ , Bhaskaran K , et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-6. [PMID: ] doi: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diamond MS , Kanneganti TD . Innate immunity: the first line of defense against SARS-CoV-2. Nat Immunol. 2022;23:165-76. [PMID: ] doi: 10.1038/s41590-021-01091-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schultze JL , Aschenbrenner AC . COVID-19 and the human innate immune system. Cell. 2021;184:1671-92. [PMID: ] doi: 10.1016/j.cell.2021.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harvey WT , Carabelli AM , Jackson B , et al; COVID-19 Genomics UK (COG-UK) Consortium. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409-24. [PMID: ] doi: 10.1038/s41579-021-00573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheemarla NR , Watkins TA , Mihaylova VT , et al. Dynamic innate immune response determines susceptibility to SARS-CoV-2 infection and early replication kinetics. J Exp Med. 2021;218. [PMID: ] doi: 10.1084/jem.20210583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murray DD , Babiker AG , Baker JV , et al. Design and implementation of an international, multi-arm, multi-stage platform master protocol for trials of novel SARS-CoV-2 antiviral agents: Therapeutics for Inpatients with COVID-19 (TICO/ACTIV-3). Clin Trials. 2022;19:52-61. [PMID: ] doi: 10.1177/17407745211049829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ACTIV-3—Therapeutics for Inpatients with COVID-19 (TICO) Study Group.. Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial. Lancet Respir Med. 2022. [PMID: ] doi: 10.1016/S2213-2600(22)00215-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. ACTIV-3/TICO Study Group*.. Efficacy and safety of ensovibep for adults hospitalized with COVID-19. A randomized controlled trial. Ann Intern Med. 2022. [PMID: ] doi: 10.7326/M22-1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan CW , Chia WN , Qin X , et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38:1073-8. [PMID: ] doi: 10.1038/s41587-020-0631-z [DOI] [PubMed] [Google Scholar]
- 26. Aalen OO, Johansen S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scandinavian Journal of Statistics. 1978;5:141-50.
- 27. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496-509. doi: 10.1080/01621459.1999.10474144 [DOI]
- 28. Tenforde MW , Self WH , Adams K , et al; Influenza and Other Viruses in the Acutely Ill (IVY) Network. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326:2043-54. [PMID: ] doi: 10.1001/jama.2021.19499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dadras O , Afsahi AM , Pashaei Z , et al. The relationship between COVID-19 viral load and disease severity: a systematic review. Immun Inflamm Dis. 2022;10:e580. [PMID: ] doi: 10.1002/iid3.580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang H , Hogan CA , Verghese M , et al. SARS-CoV-2 nucleocapsid plasma antigen for diagnosis and monitoring of COVID-19. Clin Chem. 2021;68:204-13. [PMID: ] doi: 10.1093/clinchem/hvab216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cevik M , Kuppalli K , Kindrachuk J , et al. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020;371:m3862. [PMID: ] doi: 10.1136/bmj.m3862 [DOI] [PubMed] [Google Scholar]
- 32. Weinreich DM , Sivapalasingam S , Norton T , et al; Trial Investigators. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021;385:e81. [PMID: ] doi: 10.1056/NEJMoa2108163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takahashi T , Ellingson MK , Wong P , et al; Yale IMPACT Research Team. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315-20. [PMID: ] doi: 10.1038/s41586-020-2700-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bechmann N , Barthel A , Schedl A , et al. Sexual dimorphism in COVID-19: potential clinical and public health implications. Lancet Diabetes Endocrinol. 2022;10:221-30. [PMID: ] doi: 10.1016/S2213-8587(21)00346-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bastard P , Rosen LB , Zhang Q , et al; HGID Lab. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370. [PMID: ] doi: 10.1126/science.abd4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Twohig KA , Nyberg T , Zaidi A , et al; COVID-19 Genomics UK (COG-UK) consortium. Hospital admission and emergency care attendance risk for SARS-CoV-2 Delta (B.1.617.2) compared with Alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2022;22:35-42. [PMID: ] doi: 10.1016/S1473-3099(21)00475-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Flythe JE , Assimon MM , Tugman MJ , et al; STOP-COVID Investigators. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis. 2021;77:190-203.e1. [PMID: ] doi: 10.1053/j.ajkd.2020.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ram-Mohan N , Kim D , Zudock EJ , et al; Stanford COVID-19 Biobank Study Group. SARS-CoV-2 RNAemia predicts clinical deterioration and extrapulmonary complications from COVID-19. Clin Infect Dis. 2022;74:218-26. [PMID: ] doi: 10.1093/cid/ciab394 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


