Abstract
Rationale
Patients with hospital-acquired sepsis (HAS) experience higher mortality and delayed care compared with those with community-acquired sepsis. Capacity strain, the extent to which demand for hospital resources exceeds availability, thus impacting patient care, is a possible mechanism underlying antimicrobial delays for HAS but has not been studied.
Objectives
Assess the association of ward census with the timing of antimicrobial initiation among ward patients with HAS.
Methods
This retrospective cohort study included adult patients hospitalized at five acute care hospitals between July 2017 and December 2019 who developed ward-onset HAS, distinguished from community-acquired sepsis by onset after 48 hours of hospitalization. The primary exposure was ward census, measured as the number of patients present in each ward at each hour, standardized by quarter and year. The primary outcome was time from sepsis onset to antimicrobial initiation. We used quantile regression to assess the association between ward census at sepsis onset and time to antimicrobial initiation among patients with HAS defined by Centers for Disease Control and Prevention Adult Sepsis Event criteria. We adjusted for hospital, year, quarter, age, sex, race, ethnicity, severity of illness, admission diagnosis, and service type.
Results
A total of 1,672 hospitalizations included at least one ward-onset HAS episode. Median time to antimicrobial initiation after HAS onset was 4.1 hours (interquartile range, 0.4–22.3). Marginal adjusted time to antimicrobial initiation ranged from 3.6 hours (95% confidence interval [CI], 2.4–4.8 h) to 6.8 hours (95% CI, 5.3–8.4 h) at census levels 2 standard deviations (SDs) below and above the ward-specific mean, respectively. Each 1-SD increase in ward census at sepsis onset, representing a median of 2.4 patients, was associated with an increase in time to antimicrobial initiation of 0.80 hours (95% CI, 0.32–1.29 h). In sensitivity analyses, results were consistent across severity of illness and electronic health record–based sepsis definitions.
Conclusions
Time to antimicrobial initiation increased with increasing census among ward patients with HAS. These findings suggest that delays in care for HAS may be related to ward capacity strain as measured by census. Additional work is needed to validate these findings and identify potential mechanisms operating through clinician behavior and care delivery processes.
Keywords: time-to-treatment, clinical decision-making, critical care, bed occupancy, risk factors
Sepsis affects more than 350,000 patients annually in the United States, accounting for approximately 6% of hospital admissions and contributing to 35% of deaths among hospitalized patients (1, 2). Although community-acquired sepsis accounts for a majority of cases, patients with hospital-acquired sepsis (HAS) have nearly threefold higher mortality (3–6). These poor outcomes may be in part due to delays in care, as patients with HAS infrequently achieve quality targets for antimicrobial timing despite evidence in community-acquired sepsis consistently demonstrating reduced mortality from early antimicrobial treatment in severe illness (6–16).
Although time to antimicrobial initiation has decreased over the last decade among patients with community-acquired sepsis, significant hospital-level variation suggests that hospital factors may play an important role in antimicrobial timing and may be worthwhile targets to minimize delays (17). Few studies have examined contributors to treatment delays in HAS (18), and, to our knowledge, none have focused on ward patients.
Capacity strain, or the extent to which demand for hospital resources exceeds availability and thus impacts patient care (19), is one possible contributor to care delays for patients with HAS. Previous studies have demonstrated the association of capacity strain with processes of care and patient outcomes in several clinical contexts including community-acquired sepsis, but its effects on timing of antimicrobial initiation for HAS are unknown (20–26). Patients with HAS may be particularly vulnerable to capacity strain–related delays in treatment given diagnostic complexity and uncertainty in the setting of multiple acute and chronic physiologic derangements as well as atypical manifestations of infection seen in patients with HAS (6, 9).
Our aim for this study was to assess the association of ward census, an established marker of degree of capacity strain, with time to antimicrobial initiation among ward patients with HAS. We hypothesized that increasing ward census is associated with increased delays in antimicrobial initiation.
Methods
Study Design, Participants, and Setting
For this retrospective cohort study, we included all patients with an episode of HAS on ward services at five diverse acute care hospitals (a quaternary referral center; a tertiary referral center; an urban, academic-affiliated community hospital; and two rural and suburban community hospitals) in the University of Pennsylvania Health System (UPHS). We included patients admitted after July 1, 2017 and discharged before December 31, 2019.
Data Source
Data for this study were obtained from electronic health records (EHRs) stored in Epic Clarity Database, a UPHS clinical data warehouse.
Sepsis Definitions
To identify episodes of HAS, we implemented the Centers for Disease Control and Prevention (CDC) Adult Sepsis Event (ASE) criteria, operationalized by 1) a blood culture draw; 2) new antimicrobial initiated within 2 days before or after the blood culture draw day (a 5-day suspected or confirmed infection window) and continued for at least 4 days; and 3) new organ dysfunction with onset within the 5-day suspected infection window (as measured by one or more eSOFA points, a simplified adaptation of the sequential organ failure assessment (SOFA) score optimized for EHR data) (see E-Methods and Table E1 in the data supplement) (27). Consistent with prior published approaches, the time at which the first of these three criteria were met was used to define sepsis onset (28–30).
We included only the index (first) HAS episode for each hospitalization to avoid double-counting bias and restricted to episodes with onset after the first 48 hours of hospitalization to distinguish HAS from community-acquired sepsis (27). We also included only sepsis episodes in which sepsis onset occurred on ward units—excluding episodes with onset in intensive care units (ICUs), operating rooms, or the emergency department—to examine comparable care delivery contexts. We excluded patients with sepsis onset on wards with fewer than five HAS cases over the study period (n = 37) to minimize bias related to clinician inexperience with treating sepsis.
Given the use of multiple definitions of sepsis in the literature, we conducted sensitivity analyses using the Third International Consensus Definition for Sepsis (Sepsis-3) to identify patients with HAS, again defining sepsis onset as the time at which the first of the defining criteria occurred (27–30). The E-Methods in the data supplement detail our implementation of sepsis definitions using hour-level EHR data.
Study Variables
The primary exposure was ward census, measured as the number of patients present on a given ward at each hour, standardized by subtracting the mean and dividing by standard deviation (SD) for each ward by quarter-year. The primary outcome was time from sepsis onset to antimicrobial initiation measured in hours. Secondary outcomes included antimicrobial initiation within 1 hour of sepsis recognition (a Surviving Sepsis Campaign recommendation) (31), antimicrobial initiation within 3 hours of sepsis onset (a component of the Centers for Medicare and Medicaid Services [CMS] SEP-1 quality measure) (32), antimicrobial initiation at each hour after sepsis onset, and in-hospital mortality (E-Methods and Table E1). Included qualifying antimicrobials were adapted from the CDC Hospital Toolkit for Adult Sepsis Surveillance (27).
All models were adjusted for hospital, year, and quarter fixed effects, as well as potentially confounding patient factors believed to theoretically or demonstratively contribute to degree of capacity strain and/or antimicrobial timing, including age, sex, race, ethnicity, admission diagnosis category (from International Classification of Diseases-10 codes), service type at sepsis onset (i.e., medicine vs. surgery), and severity of illness as measured by SOFA score at sepsis onset (18, 33, 34).
Analyses
We analyzed the association of ward census with each outcome among patients identified by the CDC ASE definition, first controlling for fixed effects and then in addition adjusting for the above patient-level covariates in separate multivariable regression models. We did not adjust for or stratify by sepsis onset subgroup (categorized by first criterion fulfilled to define sepsis onset—antimicrobial initiation, blood culture draw, or organ dysfunction start) in any model, as this variable was considered a collider, and thus conditioning analyses on this variable would introduce selection bias (Figure E1) (35–37). We also did not adjust for antimicrobial initiation in our mortality analysis because we hypothesized that antimicrobial timing is a causal intermediate in the relationship between ward census and mortality.
For the primary analysis, we fit quantile regression models for median time to antimicrobial initiation to assess the association of ward census at the hour of sepsis onset with the number of hours to antimicrobial initiation.
We used quantile regression rather than traditional linear regression, given the right-skewed distribution of time to antimicrobial initiation and the ability to contrast medians of a predicted outcome with this methodology (38).
In secondary analyses, we fit multivariable logistic regression models to assess the association of ward census at the hour of sepsis onset with odds of antimicrobial initiation within 1 or 3 hours of sepsis onset.
To assess the association between time-varying ward census at any hour after sepsis onset and the probability of antimicrobial initiation at each hour, we fit discrete-time time-to-event models using the complementary log-log link (39). Discrete-time time-to-event models have two distinct advantages for our study design. First, given that some EHR data are documented at the patient-hour level rather than continuously, this approach has the advantage of allowing for modeling of interval-censored data. Second, hazard ratio estimates from this model can be interpreted as the hourly probability of antimicrobial initiation, given that the patient meets criteria for sepsis and has not yet received antimicrobials (40).
In addition, we fit a multivariable logistic regression model to assess the association of ward census at sepsis onset with in-hospital mortality. Finally, to determine if severity of illness modifies the relationship between ward census and the primary and secondary outcomes, we repeated the primary and secondary analyses adjusting for the interaction between initial SOFA score and census level at sepsis onset.
For all secondary analyses, we calculated the adjusted probability of antimicrobial initiation or mortality across levels of ward-specific standardized census. To enhance clinical interpretability of the time-to-event model, we also calculated cumulative predicted probabilities of antimicrobial initiation at 3, 6, and 12 hours after sepsis onset for varying levels of ward census from sepsis onset until antimicrobial initiation.
In sensitivity analyses, we repeated all adjusted models among patients with HAS identified using the Sepsis-3 definition. We also performed a post hoc analysis to compare study cohort subgroups categorized by sepsis onset–defining criteria and a post hoc exploratory analysis repeating the primary adjusted model using restricted cubic splines with five knots to account for nonlinearity at the extremes of census.
All analyses were done using Stata 17 (StataCorp LP) and R version 4.1.0 (R Foundation for Statistical Computing). This protocol was deemed exempt by The University of Pennsylvania Institutional Review Board (#832918).
Results
Sepsis Cohorts
Using the CDC ASE definition to examine 222,102 UPHS hospital admissions over the 2.5-year study period, we identified 1,672 (0.75%) visits associated with an index episode of ward-onset HAS. The median age among patients with ward-onset HAS was 63 years (interquartile range [IQR], 54–71 yr) and a majority 1,095 (66%) were white (Table 1). The median time from admission to onset of first HAS episode was 7.3 days (IQR, 3.8–12.7 d), and the median hospital length of stay was 24 days (IQR, 15–36 d). The median initial SOFA score at sepsis onset was 4 (IQR, 2–6). Antimicrobials were initiated at a median of 4.1 hours (IQR, 0.4–23.3 h) after sepsis onset. Less than one-third (479 [29%]) and less than one-half (751 [45%]) of patients with HAS received antimicrobials within 1 or 3 hours of sepsis onset, respectively. In-hospital mortality was 19% (n = 320).
Table 1.
Patient characteristics and unadjusted outcomes among ward patients with hospital-acquired sepsis using the Centers for Disease Control and Prevention Adult Sepsis Event and Third International Consensus Definition for Sepsis criteria
| CDC ASE | Sepsis-3 | |
|---|---|---|
| No. of subjects | 1,672 | 3,883 |
| Patient characteristics | ||
| Hospital of admission | ||
| 1 | 1,221 (73) | 2,556 (66) |
| 2 | 215 (13) | 562 (14) |
| 3 | 122 (7) | 416 (11) |
| 4 | 69 (4) | 213 (5) |
| 5 | 45 (3) | 136 (4) |
| Admission year | ||
| 2017 | 314 (19) | 760 (20) |
| 2018 | 680 (41) | 1,577 (41) |
| 2019 | 678 (41) | 1,546 (40) |
| Age | 63 (54–71) | 65 (55–73) |
| Male | 910 (54) | 2,109 (54) |
| Race | ||
| White | 1,095 (66) | 2,399 (62) |
| Black | 428 (26) | 1,128 (29) |
| Other* | 149 (9) | 356 (9) |
| Latinx | 45 (3) | 114 (3) |
| Admission diagnosis category | ||
| Symptoms, signs, and abnormal clinical and laboratory findings, NOS | 456 (27) | 1,153 (30) |
| Neoplasms | 291 (17) | 579 (15) |
| Diseases of the circulatory system | 175 (10) | 430 (11) |
| Diseases of the digestive system | 152 (9) | 305 (8) |
| Certain infectious and parasitic diseases | 119 (7) | 281 (7) |
| Injury, poisoning, and certain other consequences of external causes | 97 (6) | 232 (6) |
| Factors influencing health status and contact with health services | 86 (5) | 145 (4) |
| Diseases of the respiratory system | 62 (4) | 137 (4) |
| Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism | 57 (3) | 142 (4) |
| Endocrine, nutritional, and metabolic diseases | 50 (3) | 121 (3) |
| Diseases of the musculoskeletal system and connective tissue | 46 (3) | 135 (3) |
| Diseases of the genitourinary system | 36 (2) | 80 (2) |
| Diseases of the nervous system | 23 (1) | 73 (2) |
| Other† | 22 (1) | 70 (2) |
| Medicine service at time of sepsis onset | 1,372 (82) | 3,208 (83) |
| Days from admission to sepsis onset | 7.3 (3.8–12.7) | 5.8 (3.2–10.5) |
| Sequential Organ Failure Assessment | ||
| At sepsis onset | 4 (2–6) | 4 (2–5) |
| Maximum during sepsis‡ | 8 (5–12) | 6 (4–9) |
| Vasopressors | ||
| At sepsis onset‡ | 29 (2) | 31 (1) |
| Any time during sepsis episode§ | 711 (43) | 979 (25) |
| Mechanical ventilation | ||
| At sepsis onset‡ | 58 (3) | 48 (1) |
| Any time during sepsis episode§ | 612 (37) | 806 (21) |
| Exposure | ||
| No. of ward locations | 43 | 50 |
| SD of standardized ward census,‖ range | 0.85–8.07 | 0.85–8.07 |
| SD of standardized ward census‖ | 2.40 (1.87–3.09) | 2.41 (1.89–3.14) |
| Outcomes | ||
| Time to antimicrobials from sepsis onset, h | 4.1 (0.4–23.3) | 11.3 (2.2–29.3) |
| Received antimicrobials within 1 h of sepsis onset | 479 (29) | 680 (18) |
| Received antimicrobials within 3 h of sepsis onset | 751 (45) | 1,159 (30) |
| In-hospital mortality | 320 (19) | 572 (15) |
Definition of abbreviations: CDC ASE = Centers for Disease Control and Prevention Adult Sepsis Event; IQR = interquartile range; NOS = not otherwise specified; Sepsis-3 = Third International Consensus Definition for Sepsis; SD = standard deviation.
All values presented as n (%) or median (IQR) unless otherwise specified.
Other: Asian, American Indian or Alaskan Native, Pacific Islander, mixed, unknown, other.
Other: mental, behavioral, and neurodevelopmental disorders; diseases of the eye and adnexa; diseases of the ear and mastoid process; pregnancy, childbirth, and the puerperium; diseases of the skin and subcutaneous tissue; congenital malformations, deformations, and chromosomal abnormalities. Each of these diagnoses represented <1% of the overall population and was derived from International Classification of Diseases-Tenth codes.
Includes new start of vasopressors or mechanical ventilation concurrent with sepsis onset.
Sepsis episode: period from sepsis onset to end of antimicrobial therapy.
Standardized for each unit by quarter and year; clinically, SD represents the change in number of patients in a ward.
A larger cohort of patients with ward-onset HAS were identified using Sepsis-3 criteria (n = 3,883), of which 1,330 (34%) overlapped with the CDC ASE cohort. Consistent with prior literature describing CDC ASE criteria as identifying a smaller and sicker population of patients with sepsis (30, 41), patients meeting ASE HAS criteria had higher maximum SOFA scores, more frequently required vasopressors or mechanical ventilation, experienced shorter times to antimicrobial initiation, and experienced higher mortality than those identified using the Sepsis-3 definition (Table 1).
Primary Analysis
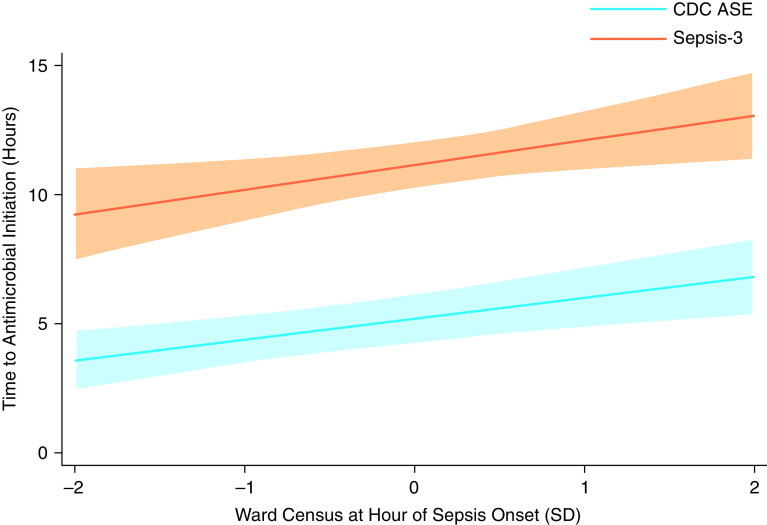
In the primary analysis, every increase in ward census by 1 ward-specific SD at the time of sepsis onset was associated with a 0.80-hour (95% CI, 0.30–1.54 h) increase in median time to antimicrobial initiation. Across quarter-years and all wards included in the study sample (n = 43), median census SD was 2.4 (IQR, 1.9–3.1) patients, ranging from 0.85 for one 12-bed ward to 8.1 in one 39-bed ward (Table E2). Clinically, this translates to a 1-hour increase in median time to antimicrobial initiation associated with every 3-patient increase in ward census. The adjusted predicted median hours from sepsis onset to antimicrobial initiation ranged nearly twofold from 3.6 hours (95% CI, 2.4–4.8 h) to 6.8 hours (95% CI, 5.3–8.4 h) at ward census 2 SDs below and above the ward-specific mean, respectively (Figure 1).
Figure 1.
Adjusted time to antimicrobial initiation after sepsis onset by standardized census among ward patients with hospital-acquired sepsis identified by the Centers for Disease Control and Prevention Adult Sepsis Event (CDC ASE) and Third International Consensus Definition for Sepsis (Sepsis-3) criteria. Shaded areas denote 95% confidence intervals; census standardized for each unit by quarter and year; analyses included hospital, admission-year, and episode-quarter fixed effects and were adjusted for age, sex, race, ethnicity, severity of illness (as measured by Sequential Organ Failure Assessment score at sepsis onset), admission diagnosis category (from International Classification of Diseases-Tenth codes), and service type (i.e., medicine vs. surgery). SD = standard deviation.
Secondary and Sensitivity Analyses
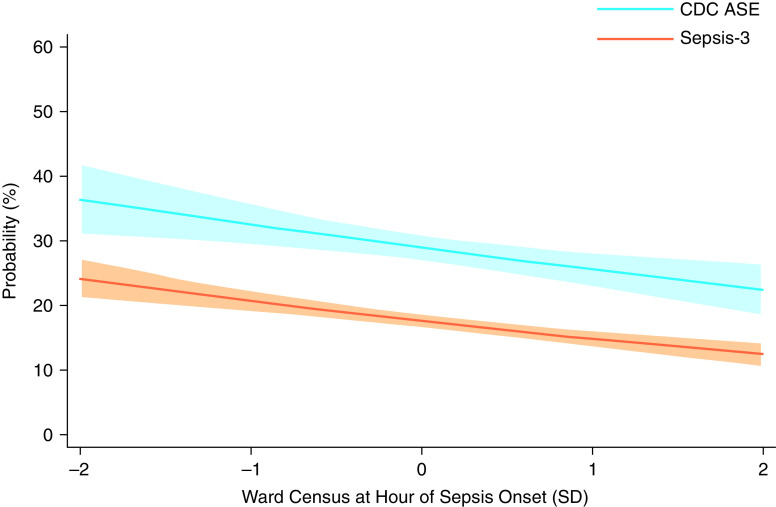
Ward census at the time of sepsis onset was associated with decreased odds of antimicrobial initiation within 1 hour of sepsis onset (adjusted odds ratio [aOR], 0.84; 95% CI, 0.75–0.94 per 1-SD increase in census). The adjusted predicted probability of antimicrobial initiation within 1 hour of sepsis onset ranged from 36.4% (95% CI, 30.8–42.1%) to 22.4% (95% CI, 18.2–26.6%) at ward census 2 SDs below and above the ward-specific mean, respectively (Figure 2).
Figure 2.
Adjusted probability of antimicrobial initiation within 1 hour of sepsis onset by standardized census among ward patients with hospital-acquired sepsis identified by the Centers for Disease Control and Prevention Adult Sepsis Event (CDC ASE) and Third International Consensus Definition for Sepsis (Sepsis-3) criteria. Shaded areas denote 95% confidence intervals; census standardized for each unit by quarter and year; analyses included hospital, admission-year, and episode-quarter fixed effects and were adjusted for age, sex, race, ethnicity, severity of illness (as measured by Sequential Organ Failure Assessment score at sepsis onset), admission diagnosis category (from International Classification of Diseases-Tenth codes), and service type (i.e., medicine vs. surgery). SD = standard deviation.
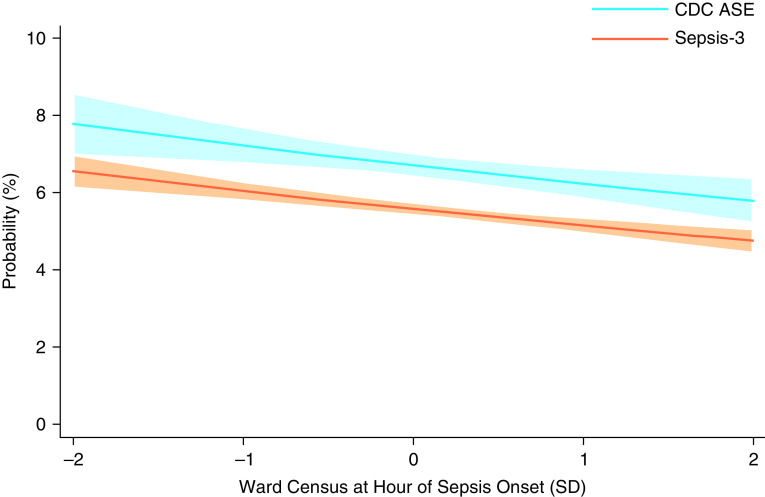
The adjusted predicted probability of antimicrobial initiation within 3 hours of sepsis onset ranged from 49.2% (95% CI, 43.4–55.0%) to 41.2% (95% CI, 36.0–46.4%) at ward census 2, SDs below and above the ward-specific mean, respectively, with aOR of 0.92 (95% CI, 0.83–1.02) (Figure E1).
A 1-SD increase in time-varying ward census was associated with a 7.4% (95% CI, 2.7–11.8%) decrease in the probability of initiating antimicrobials within any given 1-hour window after sepsis onset (Figure 3). Translating this effect estimate into clinically relevant time frames after sepsis onset, ward census 2 SDs below the mean was associated with cumulative probabilities of antimicrobial initiation of 29.0% (95% CI, 28.7–29.3%) within 3 hours, 47.7% (95% CI, 47.3–48.2%) within 6 hours, and 69.6% (95% CI, 69.1–70.2%) within 12 hours, compared with 22.5% (95% CI, 20.2–24.9%), 38.4% (95% CI, 38.0–38.8%), and 59.0% (95% CI, 58.4–59.5%), respectively, for census levels 2 SDs above the mean (Figure 4).
Figure 3.
Adjusted probability of antimicrobial initiation at any hour after sepsis onset by standardized census among ward patients with hospital-acquired sepsis identified by the Centers for Disease Control and Prevention Adult Sepsis Event (CDC ASE) and Third International Consensus Definition for Sepsis (Sepsis-3) criteria. Shaded areas denote 95% confidence intervals; census standardized for each unit by quarter and year; analyses included hospital, admission-year, and episode-quarter fixed effects and hours from sepsis onset as the time axis and were adjusted for age, sex, race, ethnicity, severity of illness (as measured by Sequential Organ Failure Assessment score at sepsis onset), admission diagnosis category (from International Classification of Diseases-Tenth codes), and service type (i.e., medicine vs. surgery). SD = standard deviation.
Figure 4.
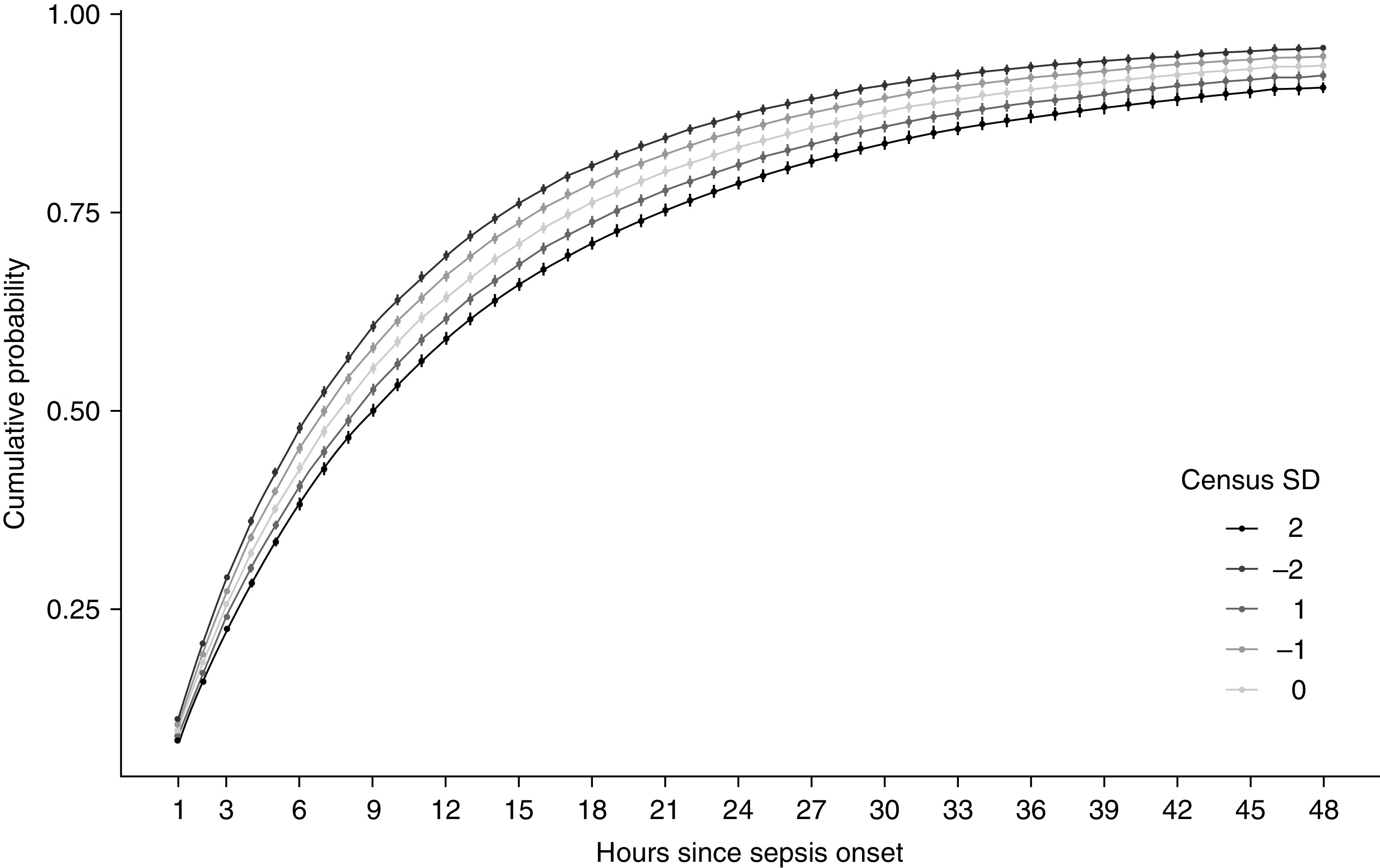
Cumulative probability of antimicrobial initiation by standardized census at sepsis onset among ward patients with hospital-acquired sepsis using Centers for Disease Control and Prevention Adult Sepsis Event criteria. Shaded areas denote 95% confidence intervals; census standardized for each unit by quarter and year; analyses included hospital, admission-year, and episode-quarter fixed effects and were adjusted for age, sex, race, ethnicity, severity of illness (as measured by Sequential Organ Failure Assessment score at sepsis onset), admission diagnosis category (from International Classification of Diseases-Tenth codes), and service type (i.e., medicine vs. surgery). SD = standard deviation.
Ward census at the time of sepsis onset was not associated with in-hospital mortality (aOR, 0.94; 95% CI, 0.83–1.08) (Figure E2).
In secondary analyses, the relationship between census and all outcomes did not vary significantly by severity of illness (Table E3). In sensitivity analyses these findings were consistent among the Sepsis-3 cohort of patients with HAS, with similar effect sizes and smaller confidence intervals (Table E4 and Figures 1–3, E2, and E3).
In post hoc comparison of study cohort subgroups categorized by sepsis onset criteria, few patient-level differences were noted, and patients who received antimicrobials first were more likely to have sepsis onset during periods of relatively low ward census, whereas patients with later antimicrobial initiation were more likely to have sepsis onset during periods of relatively high ward census (Table E5). Exploratory sensitivity analysis using restricted cubic splines again demonstrated a consistent association between high census and increased time to antimicrobial initiation, most markedly at census levels higher than the standardized mean, and with a potential threshold effect around 1 SD (Figure E4).
Discussion
In this retrospective cohort study, we found that the time to initiation of antimicrobials lengthened with increasing ward census among inpatients with ward-onset HAS. Specifically, every 3-patient increase in census at the time of sepsis onset corresponded to a 1-hour increase in the median time to antimicrobial initiation. The predicted median time to receipt of a first antimicrobial for patients with HAS onset during times of very high census was nearly two times longer than for patients with HAS onset during times of very low census. To our knowledge, this is the first study to demonstrate that increasing ward census, a measure of capacity strain, is associated with delays in antimicrobial initiation among patients with HAS.
A minority of patients with HAS received antimicrobials within the treatment timing goals recommended by the Surviving Sepsis Campaign (1 h) and CMS SEP-1 quality measures (3 h) (31, 32). Higher census was associated with lower odds of achieving those treatment goals, although with a relatively smaller effect size and wide confidence intervals for antimicrobial initiation within 3 hours of sepsis onset. These findings were consistent in sensitivity analyses using Sepsis-3 criteria. Although this study did not demonstrate an association between ward census and in-hospital mortality, the observed antimicrobial initiation timing was inconsistent with prevailing treatment guidelines, with delays in the range of magnitude associated with increased in-hospital mortality in other clinical settings (11, 12, 14, 15). We suspect there is residual confounding by indication in the relationship between census, timing of antimicrobial initiation, and mortality, which will require more robust severity-of-illness adjustment or causal inference methodology to completely account for. Notably, our secondary analyses suggest that the relationship between census and antimicrobial timing is independent of patients’ severity of illness.
Our findings are consistent with prior studies reporting associations between ward capacity strain and adverse patient outcomes including ICU transfer, cardiac arrest, prolonged length of stay, mortality, and hospital readmission (19, 23–25, 42–44). This study informs our understanding of hospital factors that may be associated with antimicrobial delays for patients with sepsis and particularly poor outcomes for patients with HAS.
Increasing levels of capacity strain related to ward census may contribute to antimicrobial delays through multiple mechanisms, including through diagnostic and/or process delays. As staff resources are strained by high patient census and workload, ordering clinicians’ and nurses’ decision making, diagnostic accuracy, and timeliness of diagnosis and care may be impeded by cognitive strain, decision fatigue, and the need to prioritize among many tasks (22, 23, 45, 46). In addition, high ward census may be associated with a greater demand for hospital-level resources such as phlebotomy, laboratory services, pharmacy approval and dispensing, and nursing medication administration, which may result in related process delays if demand outstrips availability. Additional work is needed to elucidate the specific mechanisms through which capacity strain may contribute to delays in antimicrobial initiation in HAS.
Limitations
This study has important limitations. First, generalizability of this single health system study is limited, although this limitation is mitigated by the diversity of patient demographics and case mixes across our health system, which includes five diverse acute care urban, community, and rural hospitals. Second, as with any observational study, these findings may be biased because of unmeasured or unmeasurable confounders, despite adjustment for patient, service, hospital-level, and time-specific variables as potential confounders. However, we note that our findings are consistent with prior studies linking levels of capacity strain to processes of care and across the most widely used sepsis definitions (22–26). Third, there are multiple potential measures of ward and hospital capacity strain (22), some of which may be particularly relevant to the outcome of antimicrobial initiation (i.e., medication workload) but are not accounted for in this study. Our findings support the need for further studies to examine the role of additional and more nuanced measures of capacity strain in patterns of care and outcomes for patients with HAS.
Last, given that there is no gold standard definition of sepsis onset, and the criteria often used to define sepsis onset for community-acquired cases do not apply for HAS (e.g., time of presentation to the emergency department or time of initial triage), there are limitations to accurate and consistent determination of sepsis onset for this population. Although no consensus approach to identifying sepsis onset for HAS has been established, we have implemented the two most commonly used EHR-based sepsis criteria to identify EHR sepsis onset, which is not to be presumed representative of physiologic sepsis onset. However, our aim was to study a process of care, which in large part depends on clinician recognition of sepsis through data reported in the EHR. Although there are inherent limitations in precision of this approach related to frequency and timing of labs, vital signs, and other documentation, sensitivity analyses demonstrated a consistent pattern of associations in this study across two overlapping but distinct populations of patients with HAS identified by different sepsis definitions. Furthermore, although ASE sepsis onset criteria include antimicrobial initiation, in this cohort few patient-level differences were noted between sepsis onset categories, and relatively low ward census level notably correlated with early antimicrobial initiation.
Future Directions
Future research is needed to identify actionable mechanisms through which care delivery processes are impacted by high ward census. Additional observational studies may fill this gap and inform intervention targets for clinical trials or quality initiatives aimed at mitigating the impact of capacity strain on delays in sepsis care.
Conclusions
Increasing ward census is associated with longer time to antimicrobial initiation among ward patients with HAS. These findings suggest that census may play an important role in the timing of sepsis-related processes of care and that increasing levels of capacity strain may contribute to delays in treatment for ward patients with HAS. Additional research is needed to validate our findings in other health systems and to elucidate underlying operational and/or decision-making mechanisms to guide future interventions to improve delivery of care and outcomes for patients with HAS.
Footnotes
Supported by National Institutes of Health (NIH) grant T32HL098054-11 (J.C.G.); NIH/National Heart, Lung, and Blood Institute (NHLBI) grants F32HL160037-01 (J.C.G.), K23 HL146894 (R.K.), K24 HL143289 (S.D.H.), and K23 HL141639 (G.E.W.); and an American Thoracic Society ATS Foundation Unrestricted Grant (G.E.W.). The views expressed in this article do not communicate an official position of Penn Medicine, the University of Pennsylvania, the NIH, or NHLBI.
Author Contributions: Study concept and design: J.C.G., R.K., A.C.-D., S.D.H., M.P.K., and G.E.W. Data analysis and interpretation: J.C.G., R.K., R.A.H., and G.E.W. Critical review of data, manuscript drafts, and final approvals on submitted manuscript: J.C.G., R.K., R.A.H., A.C.-D., S.D.H., M.P.K., and G.E.W.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E. Epidemiology and costs of sepsis in the United States: an analysis based on timing of diagnosis and severity level. Crit Care Med . 2018;46:1889–1897. doi: 10.1097/CCM.0000000000003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. CDC Prevention Epicenter Program Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA . 2017;318:1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rhee C, Wang R, Zhang Z, Fram D, Kadri SS, Klompas M, CDC Prevention Epicenters Program Epidemiology of hospital-onset versus community-onset sepsis in U.S. hospitals and association with mortality: a retrospective analysis using electronic clinical data. Crit Care Med . 2019;47:1169–1176. doi: 10.1097/CCM.0000000000003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vattanavanit V, Buppodom T, Khwannimit B. Timing of antibiotic administration and lactate measurement in septic shock patients: a comparison between hospital wards and the emergency department. Infect Drug Resist . 2018;11:125–132. doi: 10.2147/IDR.S155099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun A, Netzer G, Small DS, Hanish A, Fuchs BD, Gaieski DF, et al. Association between index hospitalization and hospital readmission in sepsis survivors. Crit Care Med . 2016;44:478–487. doi: 10.1097/CCM.0000000000001464. [DOI] [PubMed] [Google Scholar]
- 6. Leisman DE, Angel C, Schneider SM, D’Amore JA, D’Angelo JK, Doerfler ME. Sepsis presenting in hospitals versus emergency departments: demographic, resuscitation, and outcome patterns in a multicenter retrospective cohort. J Hosp Med . 2019;14:340–348. doi: 10.12788/jhm.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baghdadi JD, Wong MD, Uslan DZ, Bell D, Cunningham WE, Needleman J, et al. Adherence to the SEP-1 sepsis bundle in hospital-onset v. community-onset sepsis: a multicenter retrospective cohort study. J Gen Intern Med . 2020;35:1153–1160. doi: 10.1007/s11606-020-05653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moehring RW, Sloane R, Chen LF, Smathers EC, Schmader KE, Fowler VG, Jr, et al. Delays in appropriate antibiotic therapy for gram-negative bloodstream infections: a multicenter, community hospital study. PLoS One . 2013;8:e76225. doi: 10.1371/journal.pone.0076225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rhee C, Filbin MR, Massaro AF, Bulger AL, McEachern D, Tobin KA, et al. Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program Compliance with the national SEP-1 quality measure and association with sepsis outcomes: a multicenter retrospective cohort study. Crit Care Med . 2018;46:1585–1591. doi: 10.1097/CCM.0000000000003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baghdadi JD, Brook RH, Uslan DZ, Needleman J, Bell DS, Cunningham WE, et al. Association of a care bundle for early sepsis management with mortality among patients with hospital-onset or community-onset sepsis. JAMA Intern Med . 2020;180:707–716. doi: 10.1001/jamainternmed.2020.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med . 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 12. Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med . 2017;376:2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu VX, Fielding-Singh V, Greene JD, Baker JM, Iwashyna TJ, Bhattacharya J, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med . 2017;196:856–863. doi: 10.1164/rccm.201609-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peltan ID, Bledsoe JR, Oniki TA, Sorensen J, Jephson AR, Allen TL, et al. Emergency department crowding is associated with delayed antibiotics for sepsis. Ann Emerg Med . 2019;73:345–355. doi: 10.1016/j.annemergmed.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 15. Seymour CW, Kahn JM, Martin-Gill C, Callaway CW, Yealy DM, Scales D, et al. Delays from first medical contact to antibiotic administration for sepsis. Crit Care Med . 2017;45:759–765. doi: 10.1097/CCM.0000000000002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han X, Spicer A, Carey KA, Gilbert ER, Laiteerapong N, Shah NS, et al. Identifying high-risk subphenotypes and associated harms from delayed antibiotic orders and delivery. Crit Care Med . 2021;49:1694–1705. doi: 10.1097/CCM.0000000000005054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wayne MT, Seelye S, Molling D, Wang XQ, Donnelly JP, Hogan CK, et al. Temporal trends and hospital variation in time-to-antibiotics among veterans hospitalized with sepsis. JAMA Netw Open . 2021;4:e2123950. doi: 10.1001/jamanetworkopen.2021.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amaral AC, Fowler RA, Pinto R, Rubenfeld GD, Ellis P, Bookatz B, et al. Cooperative Antimicrobial Therapy of Septic Shock Database Research Group Patient and organizational factors associated with delays in antimicrobial therapy for septic shock. Crit Care Med . 2016;44:2145–2153. doi: 10.1097/CCM.0000000000001868. [DOI] [PubMed] [Google Scholar]
- 19. Halpern SD. ICU capacity strain and the quality and allocation of critical care. Curr Opin Crit Care . 2011;17:648–657. doi: 10.1097/MCC.0b013e32834c7a53. [DOI] [PubMed] [Google Scholar]
- 20. Gaieski DF, Agarwal AK, Mikkelsen ME, Drumheller B, Cham Sante S, Shofer FS, et al. The impact of ED crowding on early interventions and mortality in patients with severe sepsis. Am J Emerg Med . 2017;35:953–960. doi: 10.1016/j.ajem.2017.01.061. [DOI] [PubMed] [Google Scholar]
- 21. Pines JM, Localio AR, Hollander JE, Baxt WG, Lee H, Phillips C, et al. The impact of emergency department crowding measures on time to antibiotics for patients with community-acquired pneumonia. Ann Emerg Med . 2007;50:510–516. doi: 10.1016/j.annemergmed.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 22. Kohn R, Harhay MO, Bayes B, Mikkelsen ME, Ratcliffe SJ, Halpern SD, et al. Ward capacity strain: a novel predictor of 30-day hospital readmissions. J Gen Intern Med . 2018;33:1851–1853. doi: 10.1007/s11606-018-4564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kohn R, Harhay MO, Weissman GE, Anesi GL, Bayes B, Greysen SR, et al. Ward capacity strain: a novel predictor of delays in intensive care unit survivor throughput. Ann Am Thorac Soc . 2019;16:387–390. doi: 10.1513/AnnalsATS.201809-621RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anesi GL, Chowdhury M, Small DS, Delgado MK, Kohn R, Bayes B, et al. Association of a novel index of hospital capacity strain with admission to intensive care units. Ann Am Thorac Soc . 2020;17:1440–1447. doi: 10.1513/AnnalsATS.202003-228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anesi GL, Liu VX, Gabler NB, Delgado MK, Kohn R, Weissman GE, et al. Associations of intensive care unit capacity strain with disposition and outcomes of patients with sepsis presenting to the emergency department. Ann Am Thorac Soc . 2018;15:1328–1335. doi: 10.1513/AnnalsATS.201804-241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Terwiesch C, Diwas KC, Kahn JM. Working with capacity limitations: operations management in critical care. Crit Care . 2011;15:308. doi: 10.1186/cc10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention 2018. https://www.cdc.gov/sepsis/pdfs/Sepsis-Surveillance-Toolkit-Mar-2018_508.pdf.
- 28. Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA . 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA . 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rhee C, Zhang Z, Kadri SS, Murphy DJ, Martin GS, Overton E, et al. CDC Prevention Epicenters Program Sepsis surveillance using adult sepsis events simplified eSOFA criteria versus Sepsis-3 Sequential Organ Failure Assessment criteria. Crit Care Med . 2019;47:307–314. doi: 10.1097/CCM.0000000000003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med . 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Specifications manual for national hospital inpatient quality measures https://www.jointcommission.org/-/media/tjc/documents/measurement/specification-manuals/hiqr_specsman_july2019_v5_6.pdf.
- 33. Madsen TE, Napoli AM. The DISPARITY-II study: delays to antibiotic administration in women with severe sepsis or septic shock. Acdad Emerg Med . 2014;21:1499–1502. doi: 10.1111/acem.12546. [DOI] [PubMed] [Google Scholar]
- 34. Engoren M, Arslanian-Engoren C. Race and sex based disparities in sepsis. Heart Lung . 2021;52:37–41. doi: 10.1016/j.hrtlng.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 35. Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, et al. Control of confounding and reporting of results in causal inference studies: guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc . 2019;16:22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 36. Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology . 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 37. Admon AJ, Bohnert ASB, Cooke CR, Taylor SP. Beyond confounding: identifying selection bias in observational pulmonary and critical care research. Ann Am Thorac Soc . 2022 doi: 10.1513/AnnalsATS.202110-1188PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Staffa SJ, Kohane DS, Zurakowski D. Quantile regression and its applications: a primer for anesthesiologists. Anesth Analg . 2019;128:820–830. doi: 10.1213/ANE.0000000000004017. [DOI] [PubMed] [Google Scholar]
- 39. Prentice RL, Gloeckler LA. Regression analysis of grouped survival data with application to breast cancer data. Biometrics . 1978;34:57–67. [PubMed] [Google Scholar]
- 40. Scheike TH, Jensen TK. A discrete survival model with random effects: an application to time to pregnancy. Biometrics . 1997;53:318–329. [PubMed] [Google Scholar]
- 41. Dong R, Tian H, Zhou J, Weng L, Hu X, Peng J, et al. China Critical Care Clinical Trials Group (CCCCTG) External validity of Adult Sepsis Event’s simplified eSOFA criteria: a retrospective analysis of patients with confirmed infection in China. Ann Intensive Care . 2020;10:14. doi: 10.1186/s13613-020-0629-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Volchenboum SL, Mayampurath A, Göksu-Gürsoy G, Edelson DP, Howell MD, Churpek MM. Association between in-hospital critical illness events and outcomes in patients on the same ward. JAMA . 2016;316:2674–2675. doi: 10.1001/jama.2016.15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Needleman J, Buerhaus P, Pankratz VS, Leibson CL, Stevens SR, Harris M. Nurse staffing and inpatient hospital mortality. N Engl J Med . 2011;364:1037–1045. doi: 10.1056/NEJMsa1001025. [DOI] [PubMed] [Google Scholar]
- 44. Elliott DJ, Young RS, Brice J, Aguiar R, Kolm P. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med . 2014;174:786–793. doi: 10.1001/jamainternmed.2014.300. [DOI] [PubMed] [Google Scholar]
- 45. Vohs KD, Baumeister RF, Schmeichel BJ, Twenge JM, Nelson NM, Tice DM. Making choices impairs subsequent self-control: a limited-resource account of decision making, self-regulation, and active initiative. J Pers Soc Psychol . 2008;94:883–898. doi: 10.1037/0022-3514.94.5.883. [DOI] [PubMed] [Google Scholar]
- 46. Mullette-Gillman OA, Leong RL, Kurnianingsih YA. Cognitive fatigue destabilizes economic decision making preferences and strategies. PLoS One . 2015;10:e0132022. doi: 10.1371/journal.pone.0132022. [DOI] [PMC free article] [PubMed] [Google Scholar]