Abstract
Rationale
It remains unclear whether non-cystic fibrosis bronchiectasis increases the risk of lung cancer, because smoking history was not considered in previous studies.
Objectives
To evaluate whether participants with bronchiectasis have a higher risk of incident lung cancer than those without bronchiectasis with information on smoking status.
Methods
This was a population-based cohort study of 3,858,422 individuals who participated in the 2009 National Health Screening Program. We evaluated the incidence of lung cancer in participants with bronchiectasis (n = 65,305) and those without bronchiectasis (n = 3,793,117). We followed the cohort up until the date of lung cancer diagnosis, date of death, or December 2018. Cox proportional hazard regression models were used to evaluate the relative risk of lung cancer between participants with bronchiectasis and those without bronchiectasis.
Results
The incidence of lung cancer in participants with bronchiectasis was significantly higher than in those without bronchiectasis (2.1 vs. 0.7 per 1,000 person-years; P < 0.001), with an adjusted hazard ratio (aHR) of 1.22 (95% confidence interval [CI], 1.14–1.30) in the model adjusting for potential confounders and accounting for the competing risk of mortality. Regardless of smoking status, the risk of lung cancer was significantly higher in participants with bronchiectasis than in those without bronchiectasis (aHR, 1.28 [95% CI, 1.17–1.41] for never-smokers; aHR, 1.26 [95% CI, 1.10–1.44] for ever-smokers). Although bronchiectasis did not increase the risk of lung cancer among participants with chronic obstructive pulmonary disease (COPD), it significantly increased the risk of lung cancer in participants without COPD (aHR, 1.19 [95% CI, 1.09–1.31]).
Conclusions
The presence of bronchiectasis was associated with a higher risk of lung cancer after considering the smoking status.
Keywords: bronchiectasis, lung cancer, smoking, epidemiology, cohort study
Non–cystic fibrosis bronchiectasis (hereafter referred to as bronchiectasis) has been regarded as an orphan disease (1). However, recent studies have revealed that bronchiectasis is not uncommon, is an important cause of chronic suppurative lung disease, and contributes to a considerable healthcare burden (2–5). Indeed, patients with bronchiectasis have a greater mortality rate than people in the general population (6, 7). In particular, lung cancer development is associated with a lower survival rate in patients with bronchiectasis (6), and previous studies suggest that patients with bronchiectasis have a higher risk of lung cancer than those without bronchiectasis (8, 9).
However, these previous studies did not include smoking status when assessing bronchiectasis for risk of lung cancer development (8, 9). Given that smoking is one of the most important confounding factors that increases the occurrence of lung cancer (10), controlling for smoking is essential when evaluating the causal relationship between chronic lung disease and lung cancer (11–13). Another recent study provided indirect evidence that bronchiectasis may increase the risk of lung cancer by showing a higher lung cancer–related mortality in participants with bronchiectasis relative to those without bronchiectasis (6). Although this study considered smoking status, the study was limited in being a single-center study prone to selection bias (6). Other studies have provided conflicting evidence that bronchiectasis may have a protective effect against lung cancer development; however, studies with small sample sizes and a cross-sectional study design have potential bias, limiting the interpretation of these studies (14, 15).
Another important confounding factor in the evaluation of the association between bronchiectasis and lung cancer is chronic obstructive pulmonary disease (COPD). COPD is a common comorbidity of bronchiectasis, and bronchiectasis–COPD overlap has been increasingly recognized (16, 17). In addition, a recent large population-based longitudinal study suggested that COPD can increase the risk of lung cancer regardless of smoking status (11). Thus, there might be a correlation between COPD and bronchiectasis in the development of lung cancer. Although a previous study evaluated the impact of COPD on the association between bronchiectasis and lung cancer (9), information on this issue is still lacking, and more evidence is needed.
Thus, whether participants with bronchiectasis have a higher risk of lung cancer than those without bronchiectasis is unclear. In this study, we aimed to investigate whether participants with bronchiectasis have a higher risk for developing lung cancer than those without bronchiectasis using data from a large nationwide cohort that collected information on smoking status and COPD.
Methods
Study Population and Design
We conducted a population-based cohort study using the Korean National Health Insurance Service (NHIS) database (18). South Korea has a single-payer universal health system; the NHIS maintains insurance claim data regarding all reimbursed inpatient and outpatient visits, procedures, and prescriptions. In addition, the NHIS database includes data from annual or biennial health screening examination programs for all adults provided free of charge by the Ministry of Health and Welfare. The eligibility for the health screening examination program is as follows: 1) Employees, including self-employed individuals paying insurance premiums, are eligible for health examinations biannually regardless of age, and employees engaged in manual labor receive health examinations annually; and 2) for individuals who do not pay insurance premiums, the health examination is provided to those ⩾40 years of age biannually (19).
From 2009, the health screening exam included the following examinations: anthropometric measurement (height, weight, waist circumference, body mass index [BMI], blood pressure, visual acuity, and auditory acuity), questionnaires (past medical history, smoking, alcohol consumption, physical activity, urinary dysfunction [66 years of age], and a history of any previous falls [66 years of age]), blood tests (hemoglobin, fasting blood glucose, blood lipid concentrations [total cholesterol, triglyceride, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol], a liver panel [alanine aminotransferase, aspartate aminotransferase, and γ glutamyl transferase], hepatitis B antigen/antibody tests [66 years of age], creatinine, and estimated glomerular filtration rate), urine dipstick test for urinary protein, a chest X-ray, imaging tests for osteoporosis (dual-energy X-ray absorptiometry bone densitometry, peripheral bone [calcaneus] ultrasound or quantitative computed tomography [CT] [66 years of age]), Korean Dementia Screening Questionnaire-C (66 years of age), physical function tests (66 years of age), and mental health tests (40 and 66 years of age) (19).
The current participation rate of health screening examination is 70–80%, which makes it representative of the population. The Korean government provides anonymized health examination data to researchers for research purposes (19).
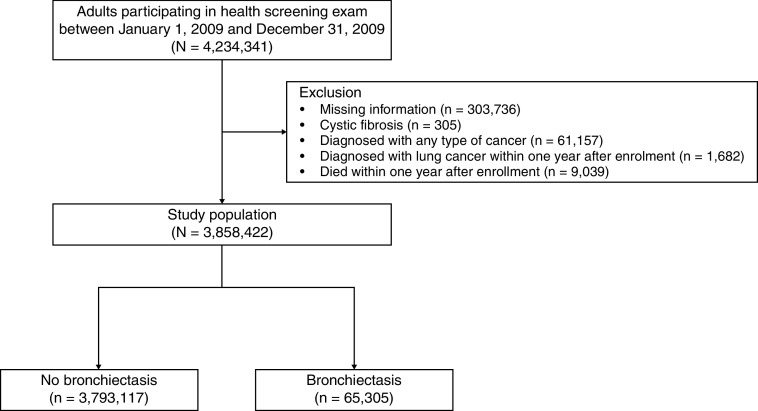
This study initially included 4,234,341 participants aged ⩾20 years who participated in the health screening exam between January 1, 2009, and December 31, 2009. We excluded participants who had missing information on at least one variable among variables analyzed in this study (n = 303,736), those diagnosed with cystic fibrosis (the International Statistical Classification of Diseases and Related Health Problems, 10th revision [ICD-10] code E84) (n = 305), those diagnosed with any type of cancer (ICD-10 codes C00–C97) (n = 61,157), those who were diagnosed with lung cancer within 1 year after enrollment (n = 1,682), and those who died within 1 year after enrollment (n = 9,039). Data from the 3,858,422 remaining participants, composed of those with bronchiectasis (n = 65,305) and those without bronchiectasis (n= 3,793,117), were included in the analysis (Figure 1). The cohort was followed from baseline to the date of diagnosis of lung cancer or death, or until the end of the study period (December 31, 2018), whichever came first.
Figure 1.
Flow chart of the study population.
The institutional review board of Hanyang University College of Medicine approved the study and waived the requirement for informed consent because the NHIS database was constructed after data anonymization (application no. HYUH 2020–11–008).
Definitions of Variables
Adult bronchiectasis was defined by the following criteria: 1) age ⩾ 20 years; 2) at least one claim under ICD-10 code J47 before the date of the health screening exam; and 3) exclusion of those with cystic fibrosis (ICD-10 diagnosis code E84) (2). In addition, we performed further analyses by using an incident bronchiectasis cohort that enrolled participants diagnosed with bronchiectasis within 1 year before the date of the health screening exam. To match the time frame of bronchiectasis and COPD, COPD was defined as at least one claim under ICD codes J42–J44 except for J43.0 (unilateral emphysema) before the date of the health screening exam when analyzing prevalent bronchiectasis and 1 year before the date of the health screening exam when analyzing incident bronchiectasis. Self-administered questionnaires determined smoking status during the health screening exams. Participants were categorized as never-smokers, ever-smokers with <10 pack-years of smoking, ever-smokers with 10–19 pack-years of smoking, and ever-smokers with ⩾20 pack-years of smoking. BMI was calculated as weight in kilograms divided by height in meters squared. Participants were categorized according to Asian-specific criteria as follows: underweight (<18.5 kg/m2), normal weight (18.5–22.9 kg/m2), overweight (23–24.9 kg/m2), or obese (⩾25.0 kg/m2) (20). Income level was dichotomized by percentage with the lowest income bracket at 20%; the low-income category also included Medicaid beneficiaries. The data on alcohol consumption and regular physical activity were also determined using self-administered questionnaires. Categories for alcohol consumption were as follows: none (0 g/d), mild (<30 g/d), and heavy (⩾30 g/d) (21). Categories for exercise were as follows: regular (>30 minutes of moderate physical activity at least five times per week or >20 minutes of strenuous physical activity at least three times per week) and nonregular (22). The number of chest CT scans performed was assessed during 1 year before the time of health screening. Other major comorbidities were also defined using ICD-10 codes during 1 year before the date of health screening exam and summarized using the Charlson Comorbidity Index (CCI) (23, 24).
Study Outcome
The study compared of the risk of lung cancer in participants with bronchiectasis to those without bronchiectasis adjusting for smoking history; the analyses were also stratified by smoking history and COPD. We also evaluated the impact of smoking history or COPD status on the association between bronchiectasis and the development of lung cancer. Lung cancer was defined by ICD-10 diagnosis codes C33–C34 (11) plus the specific insurance code for lung cancer (V193) during the follow-up period. In South Korea, once patients receive the V193 code, they are registered in the National Cancer Registry and receive special insurance benefits. Thus, the validity of lung cancer diagnosis was strictly reviewed by the Korean Health Insurance Review and Assessment Service (25).
Statistical Analyses
Baseline characteristics of participants were compared between those with and without bronchiectasis using a two-tailed Student’s t test for continuous variables and a χ2 test for categorical variables. The incidence rate of lung cancer was calculated by dividing the number of incident cases by the total follow-up duration (1,000 person-years). Cox proportional hazards models were used to evaluate the risk of lung cancer development in participants with bronchiectasis compared with those without bronchiectasis. In multivariable analyses, demographic factors (age, sex, and BMI), lifestyle factors potentially affecting cancer development (smoking amount [never, ever-smokers with <10 pack-years of smoking, ever-smokers with 10–19 pack-years of smoking, and ever-smokers with ⩾20 pack-years of smoking], alcohol consumption history [none, mild, or heavy], income level [low or high], physical activity [regular or nonregular]), and comorbidities (CCI [0, 1, or ⩾2]) were adjusted for in model 1; the number of chest CT scans was further adjusted for in model 2; and a competing risk regression model accounting for competing risk caused by mortality was also used in model 3. The analysis results were further stratified by smoking history and comorbid COPD. A cumulative incidence plot was used to illustrate the incidence of lung cancer according to the presence of bronchiectasis, and a log-rank test was used to evaluate significant differences between the two groups. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc.). All tests were two-sided, and P values of less than 0.05 were considered statistically significant.
Results
Participants
The percentage of participants with bronchiectasis was 1.7% (n = 65,305). Participants with bronchiectasis were more likely to be female, older, and have lower BMIs than those without bronchiectasis. Participants with bronchiectasis were more likely to be never-smokers than those without bronchiectasis (67.4% vs. 60.1%); however, participants with bronchiectasis were more likely to have COPD than those without bronchiectasis (32.9% vs. 4.5%). The number of chest CT scans performed was higher in participants with bronchiectasis than in those without bronchiectasis during 1 year before the time of the health screening (0.33 ± 0.72 vs. 0.03 ± 0.20). Participants with bronchiectasis had a higher CCI than those without bronchiectasis (⩾2 in 33.6% and 1 in 29.8% vs. ⩾2 in 14.7% and 1 in 20.1%) (Table 1). The baseline characteristics of the study population in the incident bronchiectasis cohort are summarized in Table E1 in the data supplement.
Table 1.
Characteristics of the study population at the beginning of follow-up
| Total (N = 3,858,422) | Bronchiectasis |
||
|---|---|---|---|
| No (n = 3,793,117) | Yes (n = 65,305) | ||
| Age, years | |||
| <60 | 3,053,882 (79.2) | 3,019,622 (79.6) | 34,260 (52.5) |
| ⩾60 | 804,540 (20.8) | 773,495 (20.4) | 31,045 (47.5) |
| Female | 1,762,757 (45.7) | 1,729,292 (45.6) | 33,465 (51.2) |
| BMI, kg/m2 | 23.7 ± 3.2 | 23.7 ± 3.2 | 23.6 ± 3.2 |
| BMI groups | |||
| <18.5 | 143,365 (3.7) | 140,377 (3.7) | 2,988 (4.6) |
| 18.5–22.9 | 1,507,926 (39.1) | 1,482,814 (39.1) | 25,112 (38.5) |
| 23.0–24.9 | 948,851 (24.6) | 932,559 (24.6) | 16,292 (24.9) |
| ⩾25.0 | 1,258,280 (32.6) | 1,237,367 (32.6) | 20,913 (32.0) |
| Smoking | |||
| Never smoker | 2,323,605 (60.2) | 2,279,598 (60.1) | 44,007 (67.4) |
| Ever smoker | |||
| <10 pack-years | 587,911 (15.2) | 582,471 (15.4) | 5,440 (8.3) |
| 10–19 pack-years | 445,733 (11.6) | 440,473 (11.6) | 5,260 (8.1) |
| ⩾20 pack-years | 501,173 (13.0) | 490,575 (12.9) | 10,598 (16.2) |
| Alcohol consumption | |||
| None | 1,988,595 (51.5) | 1,945,963 (51.3) | 42,632 (65.3) |
| Mild | 1,561,788 (40.5) | 1,542,994 (40.7) | 18,794 (28.8) |
| Heavy | 308,039 (8.0) | 304,160 (8.0) | 3,879 (5.9) |
| Income | |||
| Low | 597,754 (15.5) | 587,702 (15.5) | 10,052 (15.4) |
| High | 3,260,668 (84.5) | 3,205,415 (84.5) | 55,253 (84.6) |
| Physical activity | |||
| Regular | 687,583 (17.8) | 675,259 (17.8) | 12,324 (18.9) |
| Non-regular | 3170,839 (82.2) | 3,117,858 (82.2) | 52,981 (81.1) |
| Number of Chest CT scans | 0.03 ± 0.22 | 0.03 ± 0.20 | 0.33 ± 0.72 |
| Comorbidities | |||
| COPD | 191,662 (5.0) | 170,179 (4.5) | 21,483 (32.9) |
| CCI | |||
| 0 | 2,498,686 (64.8) | 2, 474,818 (65.2) | 23,868 (36.6) |
| 1 | 779,991 (20.2) | 760,512 (20.1) | 19,479 (29.8) |
| ⩾2 | 579,745 (15.0) | 557,787 (14.7) | 21,958 (33.6) |
Definition of abbreviations: BMI = body mass index; CCI = Charlson Comorbidity Index; COPD = chronic obstructive pulmonary disease; CT = computed tomography.
Data are represented as a number (percentage) or mean ± standard deviation.
Association between Bronchiectasis and Lung Cancer Development
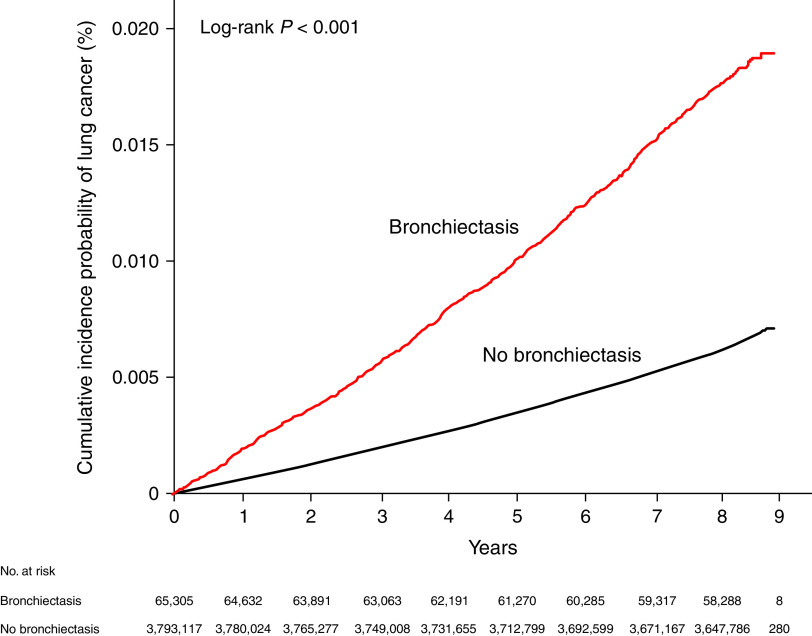
The incidence of lung cancer in participants with bronchiectasis was significantly higher than that in those without bronchiectasis (2.1/1,000 person-years vs. 0.7/1,000 person-years; P < 0.001) (Table 2), which is in line with the cumulative incidence plot (P < 0.001, log-rank test) (Figure 2). Regardless of adjustment for covariables, participants with bronchiectasis had a significantly increased risk of developing lung cancer compared with those without bronchiectasis (unadjusted hazard ratio [HR], 2.83 [95% confidence interval (CI), 2.66–3.01]; adjusted HR in the fully adjusted model 3, 1.22 [95% CI, 1.14–1.30]). Regardless of age and sex groups, participants with bronchiectasis had a significantly higher incidence of lung cancer than those without bronchiectasis (P < 0.001 in the log-rank test for both age and sex groups) (Figure E1). Stratified analyses by subgroups showed that the associations between bronchiectasis and increased risk of lung cancer were significant in participants aged ⩾60 years (adjusted HR in model 3, 1.30 [95% CI, 1.22–1.39]), males (adjusted HR in model 3, 1.20 [95% CI, 1.11–1.29]), and females (adjusted HR in model 3 , 1.25 [95% CI, 1.11–1.41]) but not in participants aged <60 years (Table 2). The risk of lung cancer in participants with bronchiectasis using an incident bronchiectasis cohort relative to those without bronchiectasis and stratified analyses by age group and sex are summarized in Table E2.
Table 2.
Incidence and hazard ratio of incident lung cancer in participants with bronchiectasis versus those without bronchiectasis stratified by age group and sex
| Number of Participants | Number of Lung Cancer | IR (/1,000 PY) | IRR* (95% Confidence Interval) | Hazard Ratio (95% Confidence Interval) |
||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||
| Overall | ||||||||
| No bronchiectasis | 3,793,117 | 23,115 | 0.742 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Bronchiectasis | 65,305 | 1,092 | 2.099 | 1.24 (1.16–1.32) | 2.83 (2.66–3.01) | 1.37 (1.28–1.45) | 1.24 (1.17–1.32) | 1.22 (1.14–1.30) |
| Age < 60 yr | ||||||||
| No bronchiectasis | 3,019,622 | 7,825 | 0.313 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Bronchiectasis | 34,260 | 168 | 0.593 | 1.04 (0.89–1.21) | 1.90 (1.63–2.21) | 1.16 (0.99–1.36) | 1.04 (0.89–1.21) | 1.04 (0.89–1.21) |
| Age ⩾ 60 yr | ||||||||
| No bronchiectasis | 773,495 | 15,290 | 2.492 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Bronchiectasis | 31,045 | 924 | 3.894 | 1.30 (1.23–1.39) | 1.57 (1.47–1.67) | 1.42 (1.33–1.52) | 1.30 (1.22–1.40) | 1.30 (1.22–1.39) |
| P for interaction | 0.90 | 0.03 | 0.82 | 0.85 | 0.59 | |||
| Male | ||||||||
| No bronchiectasis | 2,063,825 | 16,603 | 0.984 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Bronchiectasis | 31,840 | 802 | 3.230 | 1.23 (1.14–1.32) | 3.29 (3.06–3.53) | 1.34 (1.25–1.44) | 1.23 (1.14–1.32) | 1.20 (1.11–1.29) |
| Female | ||||||||
| No bronchiectasis | 1,729,292 | 6,512 | 0.456 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Bronchiectasis | 33,465 | 290 | 1.066 | 1.25 (1.12–1.41) | 2.34 (2.08–2.63) | 1.39 (1.23–1.56) | 1.25 (1.11–1.41) | 1.25 (1.11–1.41) |
| P for interaction | 0.020 | <0.001 | 0.01 | 0.02 | 0.06 | |||
Definition of abbreviations: IR = incidence rate; IRR = incidence rate ratio; PY = person-years.
Model 1 was adjusted for age, sex, body mass index, smoking history (never-smoker, ever-smoker with <10 pack-years, ever-smoker with 10–19 pack-years, and ever-smoker with ⩾20 pack-years), alcohol consumption (none, mild, or heavy), income level (low or high), physical activity (regular or nonregular), and Charlson Comorbidity Index (0, 1, or ⩾2); model 2 was further adjusted for the number of chest computed tomography scans performed; and model 3 additionally considered mortality as a competing risk.
Variables in model 3 were adjusted.
Figure 2.
Cumulative incidence probability of lung cancer according to bronchiectasis.
Impact of Smoking Status on the Association between Bronchiectasis and Lung Cancer Development
Regardless of smoking status, the incidence of lung cancer in participants with bronchiectasis was significantly higher than in those without bronchiectasis (1.4 vs. 0.5 per 1,000 person-years in never-smokers and 3.2 vs. 0.9 per 1,000 person-years in ever-smokers [1.4 vs. 0.3 per 1,000 person-years in ever-smokers with <10 pack-years; 2.1 vs. 0.6 per 1,000 person-years in ever-smokers with 10–19 pack-years; and 5.5 vs. 2.4 per 1,000 person-years in ever-smokers with ⩾20 pack-years]; P < 0.001 for all) (Table 3). The cumulative incidence plot showed similar results (Figure 3).
Table 3.
Incidence and hazard ratio of incident lung cancer in participants with bronchiectasis versus those without bronchiectasis with stratified analysis by smoking status and chronic obstructive pulmonary disease
| Number of Participants | Number of Lung Cancer | IR (/1,000 PY) | IRR* (95% Confidence Interval) | Hazard Ratio (95% Confidence Interval) |
||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||
| Never-smoker† | ||||||||
| No bronchiectasis | 2,279,598 | 9,907 | 0.527 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Bronchiectasis | 44,007 | 493 | 1.395 | 1.30 (1.18–1.43) | 2.65 (2.42–2.90) | 1.44 (1.31–1.57) | 1.30 (1.18–1.43) | 1.28 (1.17–1.41) |
| Ever-smoker† | ||||||||
| No bronchiectasis | 523,695 | 4,172 | 0.973 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Bronchiectasis | 10,291 | 259 | 3.217 | 1.29 (1.13–1.47) | 3.31(2.92–3.75) | 1.46 (1.28–1.66) | 1.29 (1.13–1.47) | 1.26 (1.10–1.44) |
| Ever smokers†, pack-years | ||||||||
| <10 pack-years | ||||||||
| No bronchiectasis | 582,471 | 1,445 | 0.300 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Bronchiectasis | 5,440 | 62 | 1.412 | 1.25 (0.96–1.63) | 4.70 (3.64–6.06) | 1.45 (1.12–1.88) | 1.25 (0.96–1.64) | 1.23 (0.94–1.61) |
| 10–19 pack-years | ||||||||
| No bronchiectasis | 440,473 | 2,226 | 0.616 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Bronchiectasis | 5,260 | 89 | 2.122 | 1.21 (0.97–1.50) | 3.45 (2.79–4.26) | 1.33 (1.07–1.64) | 1.21 (0.97–1.50) | 1.21 (0.97–1.51) |
| ⩾20 pack-years | ||||||||
| No bronchiectasis | 490,575 | 9,537 | 2.416 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Bronchiectasis | 10,598 | 448 | 5.532 | 1.17 (1.06–1.29) | 2.30 (2.09–2.53) | 1.28 (1.16–1.40) | 1.18 (1.07–1.30) | 1.14 (1.03–1.26) |
| P for interaction | 0.76 | <0.001 | 0.25 | 0.25 | 0.92 | |||
| Without COPD | ||||||||
| No bronchiectasis | 3,622,938 | 19,290 | 0.647 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Bronchiectasis | 43,822 | 489 | 1.372 | 1.19 (1.09–1.30) | 2.12 (1.94–2.32) | 1.25 (1.14–1.37) | 1.19 (1.09–1.30) | 1.19 (1.09–1.31) |
| With COPD | ||||||||
| No bronchiectasis | 170,179 | 3,825 | 2.865 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Bronchiectasis | 21,483 | 603 | 3.680 | 1.08 (0.99–1.18) | 1.29 (1.18–1.40) | 1.17 (1.07–1.27) | 1.08 (0.99–1.18) | 1.06 (0.97–1.16) |
| P for interaction | 0.20 | <0.001 | 0.30 | 0.15 | 0.07 | |||
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; IR = incidence rate; IRR = incidence rate ratio; PY = person-years.
Model 1 was adjusted for age, sex, body mass index, smoking history (never-smoker, ever-smoker with <10 pack-years, ever-smoker with 10–19 pack-years, and ever-smoker with ⩾20 pack-years), alcohol consumption (none, mild, or heavy), income level (low or high), physical activity (regular or nonregular), and Charlson Comorbidity Index (0, 1, or ⩾2); model 2 was further adjusted for the number of chest computed tomography scans performed; and model 3 additionally considered mortality as a competing risk.
Variables in Model 3 were adjusted.
The abovementioned variables, except smoking status, were used in each model.
Figure 3.
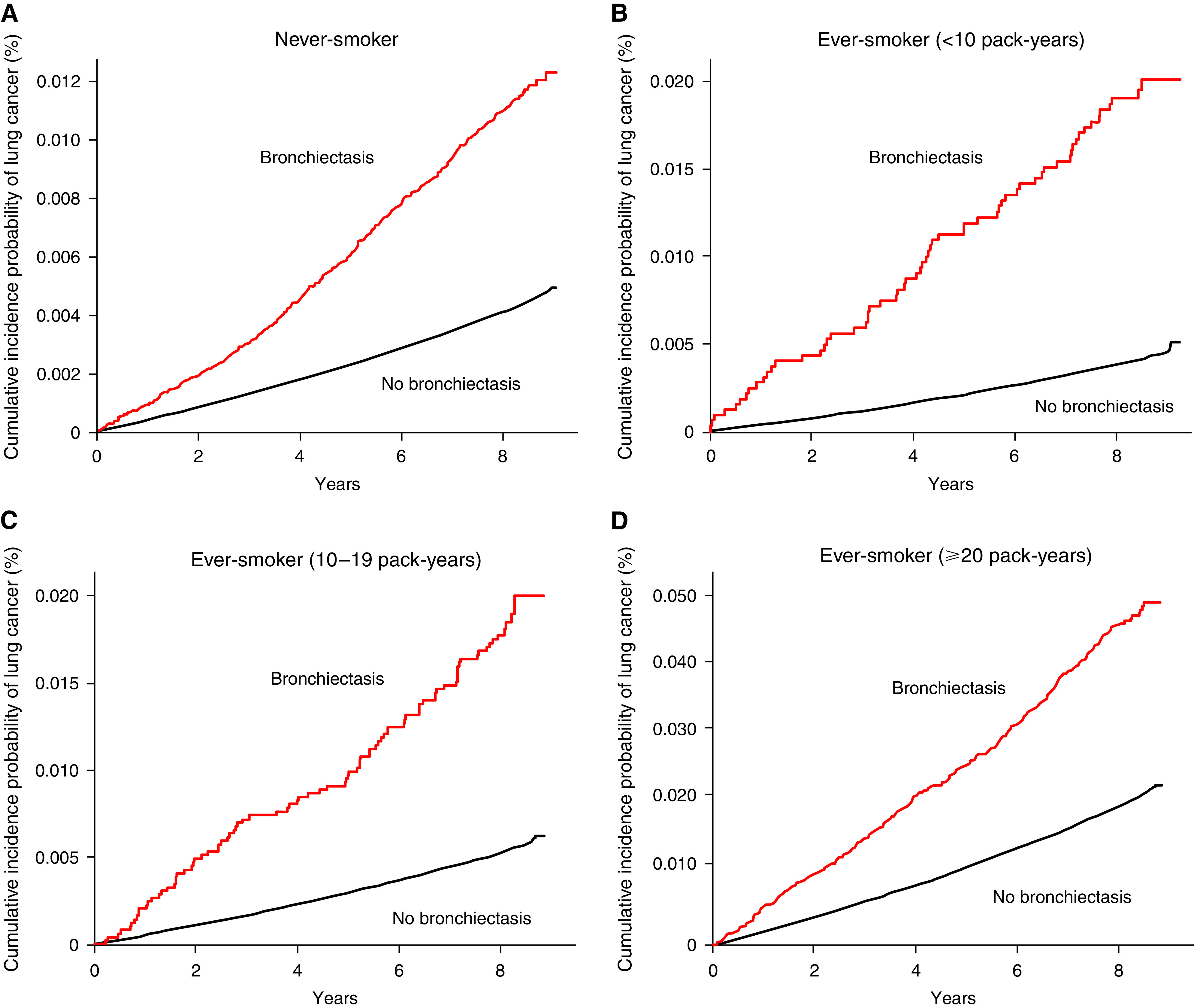
Cumulative incidence probability of lung cancer according to bronchiectasis and smoking status. (A) Never-smoker, (B) ever-smoker with <10 pack-years, (C) ever-smoker with 10–19 pack-years, and (D) ever-smoker with ⩾20 pack-years.
Bronchiectasis significantly increased the risk of lung cancer in participants regardless of smoking history (adjusted HR for never smokers in model 3, 1.28 [95% CI, 1.17–1.41], and adjusted HR for ever-smokers in model 3, 1.26 [95% CI, 1.10–1.44]). Stratified analyses based on smoking amount showed that the risk of lung cancer was increased in ever-smokers with ⩾20 pack-years (adjusted HR in model 3, 1.14 [95% CI, 1.03–1.26]), whereas bronchiectasis did not significantly increase the risk of lung cancer in participants who were ever-smokers with <10 pack-years (adjusted HR in model 3, 1.23 [95% CI, 0.94–1.61]) and ever-smokers with 10–19 pack-years (adjusted HR in model 3, 1.21 [95% CI, 0.97–1.51). There was no significant interaction between smoking status and bronchiectasis in the risk of lung cancer development (P = 0.92 for interaction in model 3). The results of the same analyses performed using an incident bronchiectasis cohort are provided in Table E3.
Impact of COPD on the Association between Bronchiectasis and Lung Cancer Development: Stratified Analyses
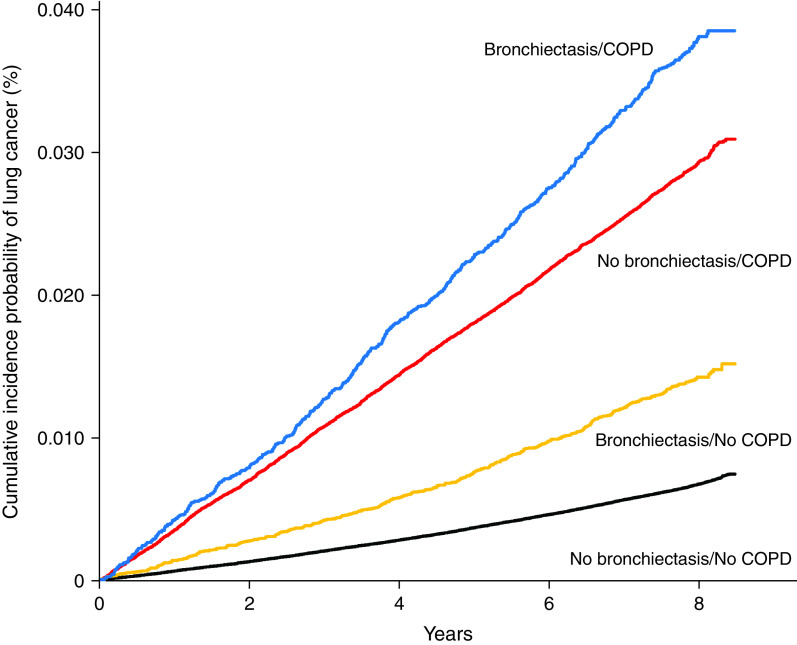
Among participants without COPD, the cumulative incidence of lung cancer was significantly higher in participants with bronchiectasis than in those without bronchiectasis (P < 0.001 in the log-rank test). In contrast, there was no significant difference in the cumulative incidence of lung cancer according to the presence or absence of bronchiectasis among participants with COPD (P = 0.95) (Figure 4). There was a significant interaction between COPD and bronchiectasis in the risk of lung cancer development (P = 0.002 for interaction in model 3), whereas bronchiectasis significantly increased the risk of lung cancer in participants without COPD (adjusted HR in model 3, 1.19 [95% CI, 1.09–1.31]); it did not increase the risk of lung cancer in those with COPD (adjusted HR in model 3, 1.06 [95% CI, 0.97–1.16]) (Table 3).
Figure 4.
Cumulative incidence probability of lung cancer according to bronchiectasis and chronic obstructive pulmonary disease. COPD = chronic obstructive pulmonary disease.
The incidence rates of lung cancer in participants with COPD only, those with bronchiectasis only, those with both bronchiectasis and COPD, and those with neither of the two diseases were 2.9, 1.4, 3.7, and 0.6 per 1,000 person-years, respectively. Compared with participants with neither of two diseases, the risk of lung cancer was significantly increased in participants with bronchiectasis only (adjusted HR in model 3, 1.21 [95% CI, 1.10–1.32]), those with COPD only (adjusted HR, 1.35 [95% CI, 1.30–1.41]), and those with both bronchiectasis and COPD (adjusted HR, 1.43 [95% CI, 1.31–1.56]) (see Table E4).
Discussion
To the best of our knowledge, this is the first and largest population-based cohort study to evaluate the association between bronchiectasis and lung cancer development while adjusting for smoking history. Our study results showed that participants with bronchiectasis had a 1.2-fold increased risk of having lung cancer compared with those without bronchiectasis, even after considering smoking status and mortality as a competing risk. Although bronchiectasis was not associated with an increased risk of lung cancer among participants with COPD, it significantly increased the risk of lung cancer among participants without COPD.
The major strength of this study was the assessment of the risk of lung cancer in participants with incident bronchiectasis while controlling for smoking history, which is the most important risk factor in lung cancer development (26). Previous studies evaluating the relationship between bronchiectasis and the incidence of lung cancer were limited by the absence of information regarding smoking history (8, 9). Thus, whether the impact of bronchiectasis on lung cancer development is independent of smoking history was unclear. This study overcame this limitation by using a national health screening database containing data on smoking history and clearly showed that bronchiectasis, independent of smoking history, is associated with an increased risk of lung cancer.
A possible explanation of the underlying mechanism for the higher risk of lung cancer in participants with bronchiectasis might be a chronic inflammatory process. This is based on evidence for chronic systemic and local inflammation as a mechanism linking COPD and lung cancer development (27). As shown in COPD, participants with bronchiectasis exhibited increased systemic inflammation (28, 29). Furthermore, pulmonary scarring, which results from various infections and injuries and is commonly seen in bronchiectasis (30), has been correlated with the development of carcinomas (31). Hence, chronic inflammation alone or pulmonary scarring caused by recurrent infection may potentially lead to lung cancer development in participants with bronchiectasis. Future research is needed to elucidate the biological link between bronchiectasis and lung cancer development.
The other notable finding of this study was to evaluate the association between bronchiectasis and lung cancer development in the context of COPD. COPD is a common bronchiectasis-related comorbidity (2, 16, 32). It is also known to be one of the most important risk factors for the development of lung cancer (11, 33–35). This phenomenon could be explained as follows: Although COPD and bronchiectasis may share a similar mechanism, chronic inflammation, in the development of lung cancer, the impact of bronchiectasis on lung cancer development may be attenuated by the greater impact of COPD on lung cancer development. However, clinically, it should be interpreted with caution, because COPD had a much more profound effect on the risk of lung cancer than bronchiectasis. In the analyses stratified according to bronchiectasis and COPD, although the risk of lung cancer in participants with both bronchiectasis and COPD (approximately 43% increase compared with those without both diseases) was slightly higher than that in those with COPD only (approximately 35% increase compared with those without both diseases), the risk was much attenuated in participants with bronchiectasis only (approximately 21% increase compared with those without both diseases) (Table E4). These results suggest that the risk of bronchiectasis on lung cancer development appears to be limited to participants without a coexisting diagnosis of COPD, while emphasizing COPD as a potential risk factor for lung cancer in bronchiectasis.
Furthermore, considering the well-established association between COPD and lung cancer (11, 33–35), the independent risk of COPD (e.g., smoking) in the development of lung cancer should be considered when interpreting these study results. The overlap between heavy smoking and COPD is common, and heavy smoking is associated with a substantially increased risk of lung cancer in participants with COPD. Thus, a considerable proportion of the increased risk of lung cancer in bronchiectasis patients with COPD is thought to be attributable to smoking. Identifying these risks conjointly may be important when considering tools to approximate the risk of lung cancer in populations.
There are some potential limitations to this study. First, this study could not provide detailed clinical information; etiologies of bronchiectasis (i.e., etiology such as tuberculosis might have a direct effect on lung cancer development), spirometry results, and histological types of lung cancer were not available. Second, although we used a large nationwide database, the number of participants with bronchiectasis who developed lung cancer was relatively small. This may have resulted in statistical insignificance in some subgroup analyses. For example, in the younger population (age < 60 yr), participants with bronchiectasis had a higher risk of lung cancer than those without bronchiectasis in univariable analysis. However, the significance was not persistent after adjusting for covariates. Given that the number of lung cancer cases was relatively small in the younger population, a future study with a larger population is warranted to evaluate the association between bronchiectasis and lung cancer in this subgroup. Third, this study used Korean national data. Considering that the demographics and clinical characteristics of participants with bronchiectasis are affected by numerous ecological factors, such as ethnicity, the economic status of a country, and exposure to air pollution (36–38), the study results might not be generalizable to other ethnic groups in different countries. Owing to the relatively small number of lung cancer cases and generalizability issues, caution should be exercised when interpreting our study results. Fourth, the diagnoses of bronchiectasis, COPD, and comorbidities were based on ICD-10 codes. Thus, there might be potential misclassification errors in diagnosis. However, given the low prevalence of bronchiectasis, we would like to suggest that our study hypothesis could not be evaluated by using other datasets (e.g., a bronchiectasis cohort other than a large-scale population-based claim database). Fifth, we could not assess the dose–response effect of smoking on the development of lung cancer because smoking was classified into broad groups of pack-years and was not used as a continuous variable. Sixth, our database lacked data on the radiological extent and severity of bronchiectasis, which may be associated with the risk of lung cancer development in participants with bronchiectasis. Further studies are required to understand the role of bronchiectasis in lung cancer development. Nonetheless, our large-scale nationwide dataset may overcome these limitations to a great extent and provide valuable information to clinicians.
In conclusion, this large nationwide study showed that bronchiectasis is associated with a higher risk of lung cancer development after consideration of smoking history.
Footnotes
Supported by the National Research Foundation of Korea and funded by the Ministry of Science, Information and Communications Technologies (grant 2021M3E5D1A01015176 to H.L. and project number 1711138447, KMDF_PR_20200901_0214, the Korea Medical Device Development Fund grant funded by the Ministry of Science, Information and Communications Technologies, the Ministry of Trade, Industry and Energy, the Ministry of Health and Welfare, and the Ministry of Food and Drug Safety) and the Korean Ministry of Education (grant 2021R1I1A3052416 to H.C.). The funders had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Author Contributions: H.L. is the guarantor of this study. All authors listed have provided substantial contributions to the conception, design, data acquisition, analysis, or interpretation of this work. H.C., H.Y.P., K.H., J.W.S., and H.L. drafted the manuscript. All authors participated in revising the manuscript after considerable review.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Keistinen T, Säynäjäkangas O, Tuuponen T, Kivelä SL. Bronchiectasis: an orphan disease with a poorly-understood prognosis. Eur Respir J . 1997;10:2784–2787. doi: 10.1183/09031936.97.10122784. [DOI] [PubMed] [Google Scholar]
- 2. Choi H, Yang B, Nam H, Kyoung DS, Sim YS, Park HY, et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J . 2019;54:1900194. doi: 10.1183/13993003.00194-2019. [DOI] [PubMed] [Google Scholar]
- 3. Quint JK, Millett ER, Joshi M, Navaratnam V, Thomas SL, Hurst JR, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J . 2016;47:186–193. doi: 10.1183/13993003.01033-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joish VN, Spilsbury-Cantalupo M, Operschall E, Luong B, Boklage S. Economic burden of non-cystic fibrosis bronchiectasis in the first year after diagnosis from a US health plan perspective. Appl Health Econ Health Policy . 2013;11:299–304. doi: 10.1007/s40258-013-0027-z. [DOI] [PubMed] [Google Scholar]
- 5. Goeminne PC, Hernandez F, Diel R, Filonenko A, Hughes R, Juelich F, et al. The economic burden of bronchiectasis - known and unknown: a systematic review. BMC Pulm Med . 2019;19:54. doi: 10.1186/s12890-019-0818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sin S, Yun SY, Kim JM, Park CM, Cho J, Choi SM, et al. Mortality risk and causes of death in patients with non-cystic fibrosis bronchiectasis. Respir Res . 2019;20:271. doi: 10.1186/s12931-019-1243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi H, Yang B, Kim YJ, Sin S, Jo YS, Kim Y, et al. Increased mortality in patients with non cystic fibrosis bronchiectasis with respiratory comorbidities. Sci Rep . 2021;11:7126. doi: 10.1038/s41598-021-86407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung WS, Lin CL, Lin CL, Kao CH. Bronchiectasis and the risk of cancer: a nationwide retrospective cohort study. Int J Clin Pract . 2015;69:682–688. doi: 10.1111/ijcp.12599. [DOI] [PubMed] [Google Scholar]
- 9. Chung WS, Lin CL, Hsu WH, Kao CH. Increased risk of lung cancer among patients with bronchiectasis: a nationwide cohort study. QJM . 2016;109:17–25. doi: 10.1093/qjmed/hcu237. [DOI] [PubMed] [Google Scholar]
- 10. Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE, Lung Health Study Research Group The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med . 2005;142:233–239. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 11. Park HY, Kang D, Shin SH, Yoo KH, Rhee CK, Suh GY, et al. Chronic obstructive pulmonary disease and lung cancer incidence in never smokers: a cohort study. Thorax . 2020;75:506–509. doi: 10.1136/thoraxjnl-2019-213732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woo A, Lee SW, Koh HY, Kim MA, Han MY, Yon DK. Incidence of cancer after asthma development: 2 independent population-based cohort studies. J Allergy Clin Immunol . 2021;147:135–143. doi: 10.1016/j.jaci.2020.04.041. [DOI] [PubMed] [Google Scholar]
- 13. Lee HY, Lee J, Lee C-H, Han K, Choi SM. Risk of cancer incidence in patients with idiopathic pulmonary fibrosis: A nationwide cohort study. Respirology . 2021;26:180–187. doi: 10.1111/resp.13911. [DOI] [PubMed] [Google Scholar]
- 14. Kim YW, Jin KN, Heo EY, Park SS, Chung HS, Kim DK. The association between combined non-cystic fibrosis bronchiectasis and lung cancer in patients with chronic obstructive lung disease. Int J Chron Obstruct Pulmon Dis . 2015;10:873–879. doi: 10.2147/COPD.S80439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim YW, Lee CH, Jin KN, Lee JK, Heo EY, Park SS, et al. The regional association between bronchiectasis and lung cancer in chest CT. BMC Pulm Med . 2016;16:151. doi: 10.1186/s12890-016-0311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee H, Choi H, Chalmers JD, Dhar R, Nguyen TQ, Visser SK, et al. Characteristics of bronchiectasis in Korea: first data from the Korean Multicentre Bronchiectasis Audit and Research Collaboration registry and comparison with other international registries. Respirology . 2021;26:619–621. doi: 10.1111/resp.14059. [DOI] [PubMed] [Google Scholar]
- 17. Hurst JR, Elborn JS, De Soyza A, BRONCH-UK Consortium COPD-bronchiectasis overlap syndrome. Eur Respir J . 2015;45:310–313. doi: 10.1183/09031936.00170014. [DOI] [PubMed] [Google Scholar]
- 18. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J . 2014;38:395–403. doi: 10.4093/dmj.2014.38.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shin DW, Cho J, Park JH, Cho B. National General Health Screening Program in Korea: history, current status, and future direction: a scoping review. Precis Future Med . 2022;6:9–31. [Google Scholar]
- 20. Kim MK, Lee WY, Kang JH, Kang JH, Kim BT, Kim SM, et al. Committee of Clinical Practice Guidelines; Korean Society for the Study of Obesity 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab (Seoul) . 2014;29:405–409. doi: 10.3803/EnM.2014.29.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agarwal DP. Cardioprotective effects of light-moderate consumption of alcohol: a review of putative mechanisms. Alcohol Alcohol . 2002;37:409–415. doi: 10.1093/alcalc/37.5.409. [DOI] [PubMed] [Google Scholar]
- 22. Nam GE, Kim SM, Han K, Kim NH, Chung HS, Kim JW, et al. Metabolic syndrome and risk of Parkinson disease: A nationwide cohort study. PLoS Med . 2018;15:e1002640. doi: 10.1371/journal.pmed.1002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis . 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24. Kim KH. Comparative study on three algorithms of the ICD-10 Charlson comorbidity index with myocardial infarction patients [in Ko] J Prev Med Public Health . 2010;43:42–49. doi: 10.3961/jpmph.2010.43.1.42. [DOI] [PubMed] [Google Scholar]
- 25. Park HY, Kang D, Shin SH, Choi H, Jang SH, Lee CH, et al. Pulmonary tuberculosis and the incidence of lung cancer among patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc . 2022;19:640–648. doi: 10.1513/AnnalsATS.202010-1240OC. [DOI] [PubMed] [Google Scholar]
- 26. Loeb LA, Ernster VL, Warner KE, Abbotts J, Laszlo J. Smoking and lung cancer: an overview. Cancer Res . 1984;44:5940–5958. [PubMed] [Google Scholar]
- 27. Moghaddam SJ, Li H, Cho SN, Dishop MK, Wistuba II, Ji L, et al. Promotion of lung carcinogenesis by chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model. Am J Respir Cell Mol Biol . 2009;40:443–453. doi: 10.1165/rcmb.2008-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martínez-García MA, Perpiñá-Tordera M, Román-Sánchez P, Soler-Cataluña JJ, Carratalá A, Yago M, et al. The association between bronchiectasis, systemic inflammation, and tumor necrosis factor alpha [in Spanish] Arch Bronconeumol . 2008;44:8–14. doi: 10.1016/s1579-2129(08)60003-8. [DOI] [PubMed] [Google Scholar]
- 29. Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer . 2013;13:233–245. doi: 10.1038/nrc3477. [DOI] [PubMed] [Google Scholar]
- 30. Yang B, Ryu J, Kim T, Jo YS, Kim Y, Park HY, et al. Impact of bronchiectasis on incident nontuberculous mycobacterial pulmonary disease: a 10-year national cohort study. Chest . 2021;159:1807–1811. doi: 10.1016/j.chest.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 31. Bobba RK, Holly JS, Loy T, Perry MC. Scar carcinoma of the lung: a historical perspective. Clin Lung Cancer . 2011;12:148–154. doi: 10.1016/j.cllc.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 32. McDonnell MJ, Aliberti S, Goeminne PC, Restrepo MI, Finch S, Pesci A, et al. Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir Med . 2016;4:969–979. doi: 10.1016/S2213-2600(16)30320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Papi A, Casoni G, Caramori G, Guzzinati I, Boschetto P, Ravenna F, et al. COPD increases the risk of squamous histological subtype in smokers who develop non-small cell lung carcinoma. Thorax . 2004;59:679–681. doi: 10.1136/thx.2003.018291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J . 2009;34:380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 35. Young RP, Duan F, Chiles C, Hopkins RJ, Gamble GD, Greco EM, et al. The NLST-ACRIN Cohort Substudy Airflow limitation and histology shift in the national lung screening trial. Am J Respir Crit Care Med . 2015;192:1060–1067. doi: 10.1164/rccm.201505-0894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choi H, Ryu J, Kim Y, Yang B, Hwangbo B, Kong S-Y, et al. Incidence of bronchiectasis concerning tuberculosis epidemiology and other ecological factors: a Korean national cohort study. ERJ Open Res . 2020;6:00097-02020. doi: 10.1183/23120541.00097-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee H, Shin SH, Gu S, Zhao D, Kang D, Joi YR, et al. Racial differences in comorbidity profile among patients with chronic obstructive pulmonary disease. BMC Med . 2018;16:178. doi: 10.1186/s12916-018-1159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dhar R, Singh S, Talwar D, Mohan M, Tripathi SK, Swarnakar R, et al. Bronchiectasis in India: results from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India Registry. Lancet Glob Health . 2019;7:e1269–e1279. doi: 10.1016/S2214-109X(19)30327-4. [DOI] [PubMed] [Google Scholar]