Abstract
Rationale
Vaccination is the most effective protection against influenza. Patients with interstitial lung diseases (ILDs) represent a high-risk group for influenza complications. Thus, yearly influenza vaccination is recommended, but evidence on its effects is sparse.
Objectives
This study aimed to compare all-cause mortality and all-cause and respiratory-related hospitalization between vaccinated and unvaccinated patients with ILD.
Methods
Using data from the largest German statutory health insurance fund (about 27 million insurees in 2020), we analyzed four influenza seasons from 2014–2015 to 2017–2018 and compared vaccinated with unvaccinated patients with ILD. Starting from September 1 of each year, we matched vaccinated and unvaccinated patients in a 1:1 ratio using a rolling cohort design. Mortality and hospitalization were compared with Kaplan-Meier plots, and effects were calculated during the influenza season (in season) with risk ratios.
Results
Both the vaccinated and the unvaccinated cohorts included 7,503 patients in 2014–2015, 10,318 in 2015–2016, 12,723 in 2016–2017, and 13,927 in 2017–2018. Vaccination rates were low at 43.2% in season 2014–2015 and decreased over time to 39.9% in season 2017–2018. The risk ratios for all-cause mortality were 0.79 (95% confidence interval [CI], 0.65–0.97; P = 0.02) in season 2014–2015, 0.66 (95% CI, 0.54–0.80; P < 0.001) in 2015–2016, 0.89 (95% CI, 0.76–1.04; P = 0.15) in 2016–2017, and 0.95 (95% CI, 0.81–1.12; P = 0.57) in 2017–2018. The effects on all-cause hospitalization and respiratory-related hospitalization were similar in all seasons.
Conclusions
Although an unequivocally beneficial impact of influenza vaccination in patients with ILD could not be demonstrated, we observed promising results regarding avoidance of all-cause mortality in half of the seasons observed. Given the low vaccination rates, further efforts are necessary to improve vaccination rates in patients with ILD.
Keywords: administrative data, Germany, statutory health insurance, survival, vaccination rate
Interstitial lung diseases (ILDs) comprise a diverse group of pulmonary diseases summarized on the basis of similar clinical, physiologic, or pathologic characteristics and characterized by high mortality (1, 2). Influenza has a high public health relevance and is associated with an increased risk of hospitalization and death, especially in high-risk groups based on their underlying health conditions (3–5). Information on influenza in patients with ILD is scarce. However, a seasonality in hospitalizations and mortality in ILD was reported, with peaks in the winter, which might be related to the influenza season (6, 7). The most effective protection is vaccination (8). According to the World Health Organization, all individuals with chronic pulmonary diseases should receive an influenza vaccination (8, 9). Therefore, yearly vaccination of patients with ILD is highly recommended. However, vaccination rates appear to be rather low among patients with ILD. For example, Mohr and colleagues reported vaccination rates of less than 30% in a single-center study in southeast Germany for the 2016–2017 to 2019–2020 seasons (10). Because evidence of the impact of influenza vaccination on individuals with ILD is clearly lacking, we aimed to compare all-cause mortality as well as all-cause and respiratory-related hospitalization in influenza-vaccinated versus -unvaccinated patients with ILD in Germany.
Methods
Data Set and Sample Selection
We performed an epidemiological claims data analysis with completely anonymized statutory health insurance (SHI) data. According to national guidelines on secondary data analysis, ethical approval and consent to participate is not required (11). In Germany, health insurance is mandatory, with approximately 87% of the resident population being insured by statutory funds (12). Access to membership in the SHI is available to everyone, regardless of factors such as professional affiliation, income, age, or comorbidities (12). SHI is financed via risk-independent, income-dependent contributions and offers free access to a wide range of services. Particularly for outpatient physician care, no copayments are due, whereas for in-hospital care, a flat rate of €10/d is charged (12). The present analyses refer to data from the largest German SHI fund (Allgemeine Ortskrankenkasse [AOK]), which insures approximately one-third of the German resident population (13), and were provided by the AOK Research Institute (WIdO).
The initial data set contained all insured adults with an International Classification of Diseases, 10th Revision (ICD-10), diagnosis of ILD, including idiopathic interstitial pneumonia (J84.1), other fibrosing ILD (J84.0, J84.8, J84.9, or D48.1), sarcoidosis (D86.0–D86.9), drug-associated ILD (J70.2–J70.4), pneumoconiosis (J62.0–J62.8 or J63.0–J63.8), radiation-associated pneumonitis (J70.1), eosinophilic pneumonia (J82), hypersensitivity pneumonitis (J67.9), and connective tissue–associated ILD (CTD) (J99.1), from January 1, 2013, to December 31, 2018.
To ensure the best possible identification of patients with true ILD and, therefore, to minimize the risk of false-positive classifications, we used a previously applied algorithm (14) (Figure 1). Accordingly, we excluded individuals without a confirmed outpatient diagnosis (outpatient ICD diagnoses in Germany have to be categorized as Z = condition after; A = exclusion diagnosis; V = suspected diagnosis; and G = confirmed diagnosis) by a pulmonologist, an internal medicine specialist, or a rheumatologist (the latter for CTD only) or any inpatient diagnosis. Furthermore, we omitted individuals who did not have at least one relevant diagnostic procedure (bronchoscopy, computed tomography of the lungs, pulmonary function testing, and assessment of antibodies). Individuals with implausible ILD diagnoses were also disregarded. First, this group contained patients who received an exclusion diagnosis of ILD after a confirmed ILD diagnosis. Second, it included patients either with radiation-associated pneumonitis but without a diagnosis of malignancy or with CTD but without a diagnosis of autoimmune disease. Finally, we left out patients assigned to different ILDs simultaneously as well as individuals without continuous enrollment with the AOK.
Figure 1.
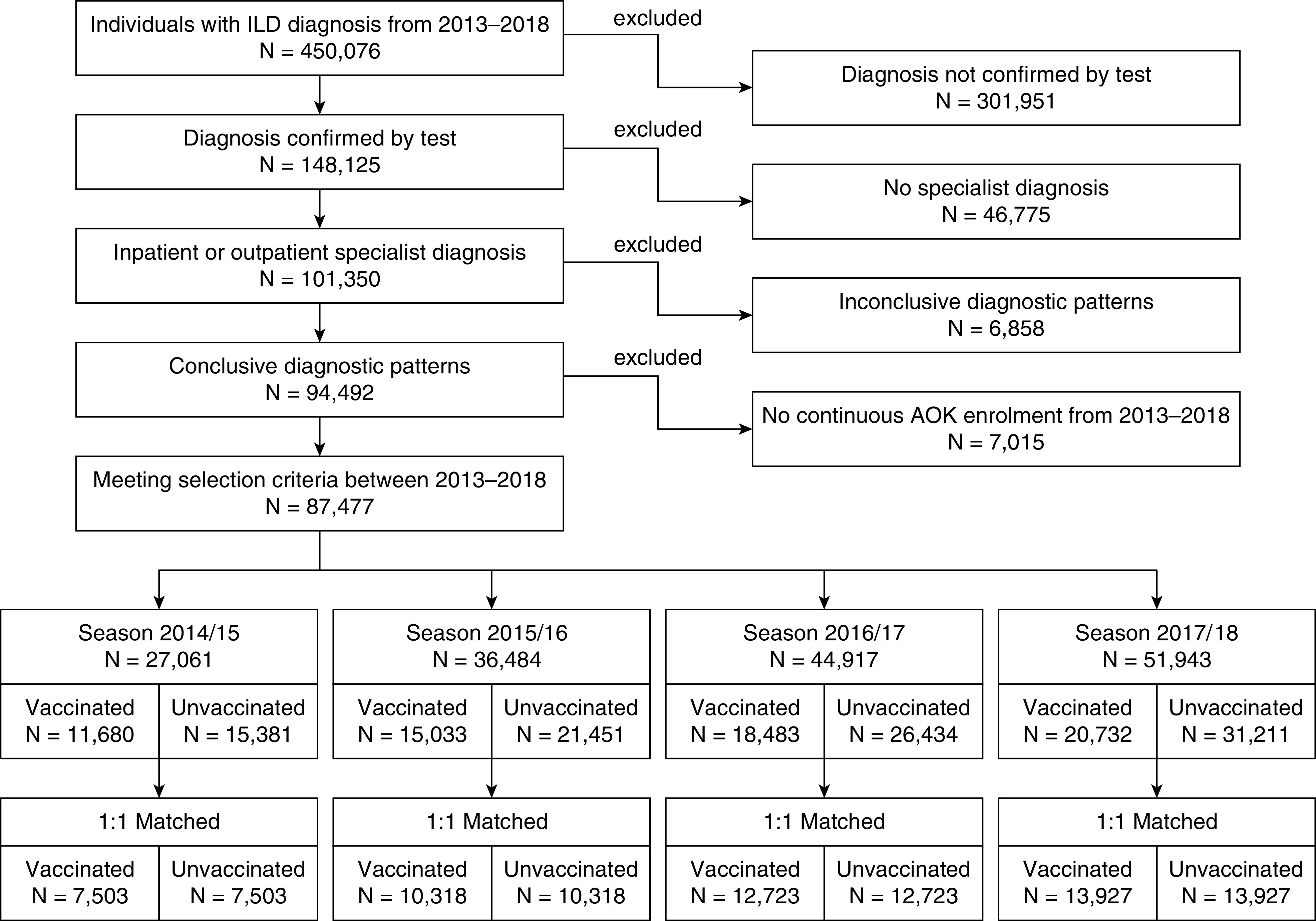
Participant flow of the study population. AOK = Allgemeine Ortskrankenkasse; ILD = interstitial lung disease.
Influenza vaccination was identified via codes from the physician’s fee scale (Einheitlicher Bewertungsmaßstab) in the outpatient setting, because influenza vaccination is only provided in outpatient care and not in hospitals (see Table E1 in the data supplement). The four influenza seasons investigated included 2014–2015, 2015–2016, 2016–2017, and 2017–2018.
From among the identified patients with ILD, we selected patients for each influenza season separately because the seasons were analyzed independently. The German federal government agency for disease control and prevention (Robert Koch Institute [RKI]) suggests yearly vaccination in October or November, but provision is also feasible during an influenza wave (15). Against this background, for each season, we set the period between September 1 and January 31 of the next year as the vaccination season. Patients vaccinated outside this time frame were excluded. To be eligible for selection in a vaccination season, only patients who had received their ILD diagnosis before the beginning of the respective season were considered. In addition, we excluded patients with palliative treatment, which was identified via outpatient and inpatient treatment and diagnosis (Table E1), during the 3 months before the matching date. Such patients are at high risk of experiencing adverse outcomes but receive vaccination only if their state of health allows it. Consequently, they would increase a healthy vaccinee bias (16). Finally, patients with missing data on matching variables were excluded.
Matching Procedure and Outcome Variables
We performed a day-specific exact matching using a rolling cohort design (17). Starting from the beginning of each season on September 1, we matched patients receiving influenza vaccination to patients without vaccination in a 1:1 ratio. For each individual patient, follow-up ended in case of an event (hospitalization or death) or at the end of the influenza season. Furthermore, both patients of a matched pair were censored if the previously unvaccinated control patient received vaccination during the observation period. With vaccination, the previously unvaccinated control patients were included in the treatment group on the day of vaccination and thus eligible for matching. Because influenza vaccination is only provided in the outpatient setting, hospitalized patients were not eligible for matching during the hospital stay. After discharge, they were considered again for matching.
By using a rolling cohort study design, our methodological approach enabled us to partially overcome some weaknesses of previous vaccination studies. More specifically, because of the day-specific exact matching of patients in the treatment and control groups, covariates were exactly balanced, which reduced biases such as confounding by indication and the healthy vaccinee bias (16). Furthermore, we were able to take a prospective view of the data and thus to account for the timing of the vaccination (before or after an outcome) as well as the occurrence of the outcomes (within or outside the influenza season). Finally, our methodological approach enabled us to visualize outcomes by Kaplan-Meier curves.
On the basis of preexisting literature and clinical expertise, covariates included in the matching process considered categorized age in years (18–29, 30–39, 40–49, 50–59, 60–69, 70–79, 80–89, >90), sex (male, female), German federal state, ILD diagnosis, and Elixhauser comorbidity score (18). The Elixhauser comorbidity index includes a set of 31 comorbidity categories, which were dummy coded. The index was implemented using the ICD-10 coding algorithm of Quan and colleagues (19). To avoid false-positive comorbidity diagnosis, at least two confirmed outpatient diagnoses in two separate quarters or one primary inpatient diagnosis in the year before matching were necessary. To avoid double-counting for the categories of hypertension (complicated and uncomplicated), diabetes (complicated and uncomplicated), and cancer (solid tumor without metastasis and metastasis cancer), only the more severe category was counted. The Elixhauser score per person was built by summing up individual comorbidities. Furthermore, we coded a dummy for nursing home residency and the need for nursing care according to degrees of care, because these patients are considered a highly vulnerable population (20). Before 2017, need for nursing care was operationalized by three and thereafter by five degrees of care. The classification of care is based on the need for assistance with activities of daily living measured in terms of time, with higher degrees indicating a higher need for care (21). In addition, we included information on receipt of in or outpatient radiotherapy or chemotherapy in the 3 months before matching, because these variables indicate severe conditions and therefore are closely linked to short-term mortality. Standardized mean differences were used to assess group balancing, with differences less than 0.1 indicating a good balance (22).
All-cause mortality and all-cause as well as respiratory-related hospitalization were defined as outcomes for each season. Respiratory-related hospitalizations included all hospital stays with a primary or secondary ICD-10 diagnosis of “acute upper respiratory infections” (J00–J06), “influenza and pneumonia” (J09–J18), or “other acute lower respiratory infections” (J20–J22) or a primary ILD-specific diagnosis.
Definition of Influenza Periods
For the analysis, the observation period was divided into time intervals before (preseason), during (in-season), and after (postseason) the influenza season. The periods varied for each season and were defined on the basis of influenza surveillance data from the RKI (23). The preseason started on the day of matching and lasted until the beginning of the influenza season. This beginning was set at the time when influenza viruses were described as being detected continuously. The end of in-season coincided with the beginning of postseason and was set at Calendar Week 20 each year (15). Each postseason lasted until August 31 because September 1 marks the beginning of the vaccination period and, therewith, the start of the next preseason. Exact dates for all seasons are reported in Table E2.
Statistical Analysis
Each influenza season was analyzed separately to capture potential differences in the effects between the different seasons. We visualized the overall observation time for mortality and hospitalization from matching until August 31 of each season with Kaplan-Meier curves. The association between influenza vaccination and mortality as well as hospitalization was estimated during the influenza seasons (in-season period) by risk ratios (RRs) with bootstrapped 95% confidence intervals (95% CIs) with 1,000 replications. Because the effect of the vaccine is only gradually building, the first 14 days after matching were disregarded for the estimation.
Within a first sensitivity analysis, we analyzed effects of vaccination for preseason, in-season, and postseason to check our results for residual confounding (24). Here, vaccination should not have any effect in pre- and postseason. The impact of biases on the estimation of effects of influenza vaccination in observational studies has been described before (16, 25, 26). The most important biases are the healthy vaccinee bias and confounding by indication. It is suspected that a small subset of frail and terminally ill patients induces the healthy vaccinee bias (16), which we tried to control for by excluding palliative patients. Furthermore, we considered important variables with high influence on the short-term mortality, such as chemotherapy/radiotherapy, nursing home residency, and the patients’ degree of care. With sensitivity analysis 1, however, we can check whether residual confounding nevertheless remains.
In a second sensitivity analysis, we redefined the periods and set the beginning of in-season at the time when 10% of the specimens were classified as positive according to the RKI influenza surveillance data. The dates for all seasons are reported in Table E1.
In addition to the main analysis, we performed subgroup analyses for patients with idiopathic interstitial pneumonia and patients aged 60 years and older (data not shown). No noteworthy differences between the groups appeared compared with the main analyses. All analyses were performed at a significance level of 5% using R version 4.0.3 software (R Foundation for Statistical Computing).
Results
Population Characteristics
Of the 450,076 patients in the initial data set, 87,477 individuals met the selection criteria and were identified as patients having an ILD (Figure 1). The vaccination coverage rate was 43.2% in season 2014–2015, 41.2% in 2015–2016, 41.2% in 2016–2017, and 39.9% in 2017–2018. The season-specific participant flow with detailed information on exclusion characteristics, eligibility, and matching is provided in Figures E1–E4. Table 1 displays selected baseline characteristics of the matched population for each season. All other variables are presented in Table E3. Here, we also included all Elixhauser categories in dummy-coded format to check for group differences. Balance, indicated by standardized mean differences less than 0.1, was achieved for all variables in all seasons covered, including the Elixhauser categories that were not included in the matching procedure, suggesting similar comorbidity burden in vaccinated and unvaccinated patients.
Table 1.
Patient characteristics in the matched population for each season
| Season 2014–2015 |
Season 2015–2016 |
Season 2016–2017 |
Season 2017–2018 |
|||||
|---|---|---|---|---|---|---|---|---|
| Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | |
| No. of patients | 7,503 | 7,503 | 10,318 | 10,318 | 12,723 | 12,723 | 13,927 | 13,927 |
| Age, yr, mean (SD) | 68.9 (11.7) | 69.0 (11.7) | 69.4 (11.6) | 69.5 (11.6) | 69.8 (11.7) | 69.9 (11.6) | 69.8 (11.8) | 70.0 (11.7) |
| Age groups, yr, n (%) | ||||||||
| 20–29 | 23 (0.3) | 23 (0.3) | 13 (0.1) | 13 (0.1) | 22 (0.2) | 22 (0.2) | 20 (0.1) | 20 (0.1) |
| 30–39 | 101 (1.3) | 101 (1.3) | 105 (1.0) | 105 (1.0) | 135 (1.1) | 135 (1.1) | 163 (1.2) | 163 (1.2) |
| 40–49 | 359 (4.8) | 359 (4.8) | 499 (4.8) | 499 (4.8) | 558 (4.4) | 558 (4.4) | 573 (4.1) | 573 (4.1) |
| 50–59 | 1,079 (14.4) | 1,079 (14.4) | 1,470 (14.2) | 1,470 (14.2) | 1,743 (13.7) | 1,743 (13.7) | 1,912 (13.7) | 1,912 (13.7) |
| 60–69 | 1,669 (22.2) | 1,669 (22.2) | 2,445 (23.7) | 2,445 (23.7) | 3,081 (24.2) | 3,081 (24.2) | 3,456 (24.8) | 3,456 (24.8) |
| 70–79 | 2,998 (40.0) | 2,998 (40.0) | 3,770 (36.5) | 3,770 (36.5) | 4,400 (34.6) | 4,400 (34.6) | 4,616 (33.1) | 4,616 (33.1) |
| 80–89 | 1,261 (16.8) | 1,261 (16.8) | 1,970 (19.1) | 1,970 (19.1) | 2,717 (21.4) | 2,717 (21.4) | 3,109 (22.3) | 3,109 (22.3) |
| 90 + | 13 (0.2) | 13 (0.2) | 46 (0.4) | 46 (0.4) | 67 (0.5) | 67 (0.5) | 78 (0.6) | 78 (0.6) |
| Sex, male, n (%) | 3,613 (48.2) | 3,613 (48.2) | 4,909 (47.6) | 4,909 (47.6) | 6,090 (47.9) | 6,090 (47.9) | 6,632 (47.6) | 6,632 (47.6) |
| ILD diagnosis (ICD-10), n (%) | ||||||||
| Idiopathic interstitial pneumonia | 3,306 (44.1) | 3,306 (44.1) | 4,443 (43.1) | 4,443 (43.1) | 5,440 (42.8) | 5,440 (42.8) | 5,767 (41.4) | 5,767 (41.4) |
| Other fibrosing ILDs | 853 (11.4) | 853 (11.4) | 1,428 (13.8) | 1,428 (13.8) | 2,004 (15.8) | 2,004 (15.8) | 2,380 (17.1) | 2,380 (17.1) |
| Sarcoidosis | 2,866 (38.2) | 2,866 (38.2) | 3,678 (35.6) | 3,678 (35.6) | 4,284 (33.7) | 4,284 (33.7) | 4,631 (33.3) | 4,631 (33.3) |
| Drug-associated ILDs | 6 (0.1) | 6 (0.1) | 10 (0.1) | 10 (0.1) | 22 (0.2) | 22 (0.2) | 25 (0.2) | 25 (0.2) |
| Pneumoconiosis | 212 (2.8) | 212 (2.8) | 286 (2.8) | 286 (2.8) | 311 (2.4) | 311 (2.4) | 318 (2.3) | 318 (2.3) |
| Radiation-associated pneumonitis | 6 (0.1) | 6 (0.1) | 18 (0.2) | 18 (0.2) | 19 (0.1) | 19 (0.1) | 20 (0.1) | 20 (0.1) |
| Eosinophilic pneumonia | 24 (0.3) | 24 (0.3) | 71 (0.7) | 71 (0.7) | 115 (0.9) | 115 (0.9) | 155 (1.1) | 155 (1.1) |
| Hypersensitivity pneumonitis | 162 (2.2) | 162 (2.2) | 249 (2.4) | 249 (2.4) | 304 (2.4) | 304 (2.4) | 336 (2.4) | 336 (2.4) |
| Connective tissue–associated ILD | 68 (0.9) | 68 (0.9) | 135 (1.3) | 135 (1.3) | 224 (1.8) | 224 (1.8) | 295 (2.1) | 295 (2.1) |
| Elixhauser comorbidity score, mean (SD) | 3.6 (2.0) | 3.6 (2.0) | 3.8 (2.1) | 3.8 (2.1) | 4.0 (2.1) | 4.0 (2.1) | 4.0 (2.2) | 4.0 (2.2) |
| Care dependency,* n (%) | ||||||||
| No care level | 6,953 (92.7) | 6,953 (92.7) | 9,408 (91.2) | 9,408 (91.2) | 11,342 (89.1) | 11,342 (89.1) | 12,150 (87.2) | 12,150 (87.2) |
| Care level 1 | 463 (6.2) | 463 (6.2) | 736 (7.1) | 736 (7.1) | 1,088 (8.6) | 1,088 (8.6) | 86 (0.6) | 86 (0.6) |
| Care level 2 | 83 (1.1) | 83 (1.1) | 172 (1.7) | 172 (1.7) | 279 (2.2) | 279 (2.2) | 1,205 (8.7) | 1,205 (8.7) |
| Care level 3 | 4 (0.1) | 4 (0.1) | 2 (0.0) | 2 (0.0) | 14 (0.1) | 14 (0.1) | 403 (2.9) | 403 (2.9) |
| Care level 4 | — | — | — | — | 0 (0.0) | 0 (0.0) | 79 (0.6) | 79 (0.6) |
| Care level 5 | — | — | — | — | 0 (0.0) | 0 (0.0) | 4 (0.0) | 4 (0.0) |
| Nursing home residency, n (%) | 15 (0.2) | 15 (0.2) | 47 (0.5) | 47 (0.5) | 77 (0.6) | 77 (0.6) | 80 (0.6) | 80 (0.6) |
| Radiotherapy or chemotherapy in 3 mo before matching, n (%) | 10 (0.1) | 10 (0.1) | 39 (0.4) | 39 (0.4) | 80 (0.6) | 80 (0.6) | 106 (0.8) | 106 (0.8) |
Definition of abbreviations: ICD-10 = International Classification of Diseases, Tenth Revision; ILD = interstitial lung disease; SD = standard deviation.
Care dependency until 2017 in three levels and after 2017 in five levels.
Mortality
Kaplan-Meier curves for mortality from the day of matching until August 31 of the following year (Figure 2) were very similar in the immediate time after the matching and diverged later at different time points per season. The mean follow-up times were 210 days in season 2014–2015, 216 days in 2015–2016, 213 days in 2016–2017, and 218 days in 2017–2018. The in-season RR estimates for the effects of vaccination were 0.79 (95% CI, 0.65–0.97; P = 0.02) in season 2014–2015, 0.66 (95% CI, 0.54–0.80; P < 0.001) in 2015–2016, 0.89 (95% CI, 0.76–1.04; P = 0.15) in 2016–2017, and 0.95 (95% CI, 0.81–1.12; P = 0.57) in 2017–2018. More specifically, in-season mortality rates per 100 person-years were 6.0 (95% CI, 5.1–7.0) for vaccinated patients and 7.5 (95% CI, 6.6–8.7) for unvaccinated patients in season 2014–2015, 4.8 (95% CI, 4.1–5.6) and 7.3 (95% CI, 6.5–8.3) in 2015–2016, 6.4 (95% CI, 5.6–7.1) and 7.2 (95% CI, 6.4–8.0) in 2016–2017, and 5.3 (95% CI, 4.7–6.0) and 5.6 (95% CI, 5.0; 6.3) in 2017–2018.
Figure 2.
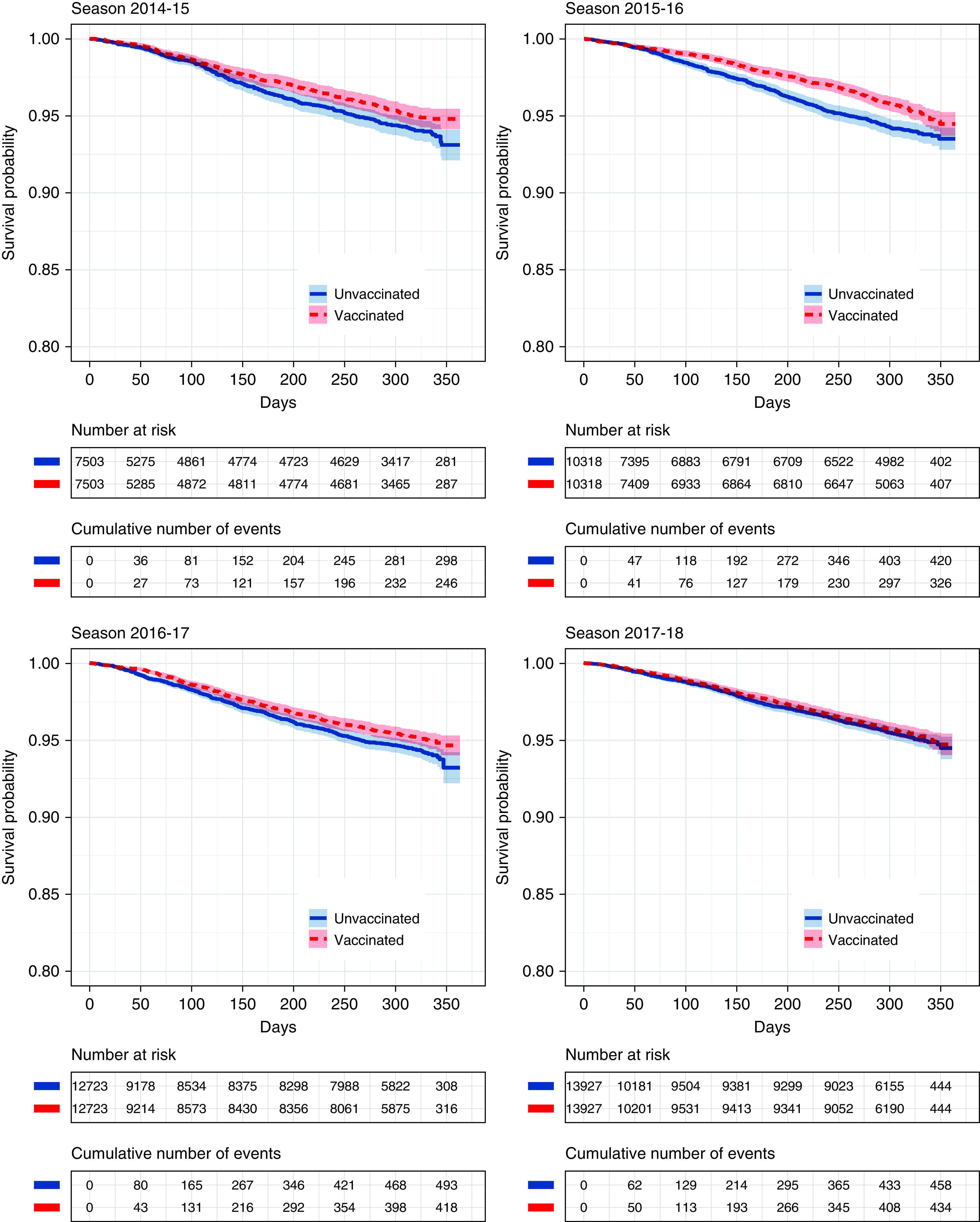
Kaplan-Meier curves for mortality comparison with 95% confidence intervals. Curves depict the whole period from matching until the end of follow-up (maximum August 31). Colored areas represent 95% confidence intervals.
Hospitalization
The course of the Kaplan-Meier curves for all-cause (Figure E5) and respiratory-related (Figure E6) hospitalizations was similar for vaccinated and unvaccinated patients. The corresponding in-season RR estimates for all-cause hospitalization were 0.99 (95% CI, 0.92–1.08; P = 0.46) in season 2014–2015, 1.00 (95% CI, 0.94–1.06; P = 0.99) in 2015–2016, 1.01 (95% CI, 0.96–1.07; P = 0.61) in 2016–2017, and 0.98 (95% CI, 0.93–1.04; P = 0.34) in 2017–2018. The RR estimates for respiratory-related hospitalization were 0.93 (95% CI, 0.82–1.07; P = 0.31) in season 2014–2015, 0.92 (95% CI, 0.82–1.04; P = 0.18) in 2015–2016, 0.99 (95% CI, 0.89–1.11; P = 0.91) in 2016–2017, and 0.99 (95% CI, 0.89–1.09; P = 0.78) in 2017–2018. The in-season hospitalization rates per 100 person-years are included in Table E4.
Sensitivity Analyses
The results of the first sensitivity analysis, in which the influenza seasons were divided into periods to check for residual confounding, are presented in Figure 3 and include the in-season estimates as a comparison. An indication of bias only appeared in the postseason estimate of 2017–2018 for respiratory-related hospitalization. However, especially in mortality analysis, the estimates were less than 1 for three of the four seasons, which might also indicate residual confounding.
Figure 3.
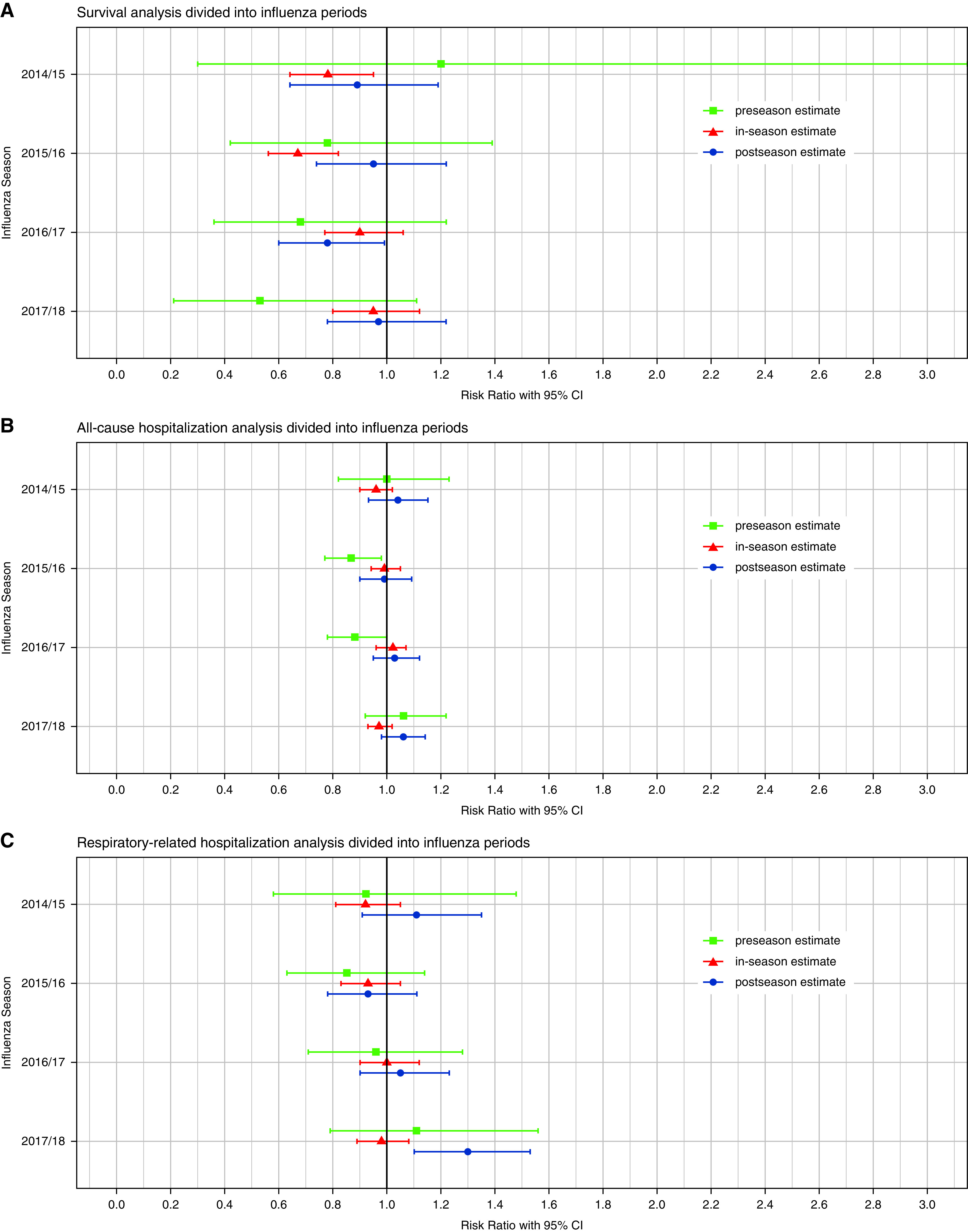
Sensitivity analyses for residual confounding by dividing the influenza season into periods of preseason, in-season, and postseason. Horizontal bars represent 95% confidence intervals (CIs). Preseason extends from September 1 until continuous detection of influenza viruses; in-season extends from continuous detection of influenza viruses until calendar week 20 of the following year; and postseason extends from Calendar Week 20 until August 31.
The second sensitivity analysis, in which we redefined the periods, yielded results similar to the main analysis (see Table E6 and Figure E7). An indication for residual confounding appeared in the preseason estimate for 2016–2017 in the mortality analysis, for 2015–2016 in the all-cause hospitalization analysis, and in the post-season estimate for respiratory-related hospitalization in 2017–2018.
Discussion
In our study, we describe for the first time a comparison of patients with ILD receiving influenza vaccination versus unvaccinated patients for four different influenza seasons. We found a protective effect of influenza vaccination regarding mortality in seasons 2014–2015 and 2015–2016, which, however, was not significant within the other two seasons. In all seasons, all-cause and respiratory-related hospitalizations did not significantly differ between the vaccinated and unvaccinated groups.
The vaccination coverage rate for included patients with ILD was similar to claims data–based estimates of vaccination rates for patients with other severe chronic diseases, such as chronic obstructive pulmonary disease (COPD), in Germany approximately 40% (27). Also, the decline of the vaccination rate observed over time resembles the trends reported for other chronic diseases (27). However, considering the recommendation that all patients with ILD should be vaccinated, the vaccination rates are very low.
Analyses investigating effects of influenza vaccination in patients with ILD have not been published to date. Therefore, we compare our results with studies evaluating the effectiveness of influenza vaccination in patients with COPD. According to a Cochrane review published in 2018 and including six randomized controlled trials (28), vaccinated patients with COPD experience fewer exacerbations than unvaccinated patients with COPD. However, no effect on mortality rates or hospitalizations was reported. Notably, the most recent study included was published in 2004. A recent systematic review and meta-analysis evaluating the effectiveness of influenza vaccination in patients with COPD with severe airflow limitation demonstrated a significant reduction in exacerbations and a tendency toward fewer hospitalizations, but it also found no differences regarding mortality (29). However, the included studies were evaluated to be of low or very low overall quality of evidence for the mortality analysis. Certainly, a direct comparison of our results with these studies is difficult because clinical characteristics differ between patients with ILD and patients with COPD. Nevertheless, viral infections might be associated with acute exacerbations in patients with ILD, especially in patients with idiopathic pulmonary fibrosis (30), and thus influenza should be prevented. An increased risk of death for patients with ILD was also recently reported for coronavirus disease (COVID-19) (31). In addition, patients with ILD in our sample had, on average, four Elixhauser comorbidities, including particularly a high prevalence of cardiovascular diseases and diabetes, which themselves represent risk factors for hospitalization and death after influenza infection.
Mortality analysis unveiled that group differences do not appear immediately after vaccination. This is plausible because immunity is gradually building within the first 14 days after vaccination. To check our results for residual confounding in a sensitivity analysis, we evaluated the effects of vaccination outside the influenza season, when no effect should be present (26). Although there were almost no significant effects in the pre- and postseasons, the point estimates were less than 1 for almost all seasons. Therefore, residual confounding might have remained. However, residual confounding appeared to be higher in those seasons in which no significant effect of the vaccination could be detected. Even though some confounding might remain, we are convinced that the study design is well suited for the evaluation of the vaccination effectiveness, because it is possible to take a prospective view of the data, adjust appropriately for possible confounders, and check the results for residual confounding.
Furthermore, we revealed differences between vaccination seasons, with significant in-season estimates for mortality in 2014–2015 and 2015–2016. Although not significant, the point estimates for respiratory-related hospitalizations were also lower in 2014–2015 and 2015–2016 than in the other seasons, which also indicates differences for these seasons. The population investigated varied slightly between the seasons, because the selection criteria led to an increasing number of patients over time.
Considering excess mortality, the season 2017–2018 was reported to be the worst influenza season since the start of RKI surveillance in 2001. In 2017–2018, the trivalent influenza vaccine did not contain the influenza B/Yamagata lineage, which was the most prevalent in this season (32). The quadrivalent vaccine contained the Yamagata lineage. However, we are not able to identify in our data whether the patient received a trivalent or quadrivalent vaccine. The quadrivalent vaccine has been recommended by the German Standing Committee on Vaccination since the 2018–2019 season (33). Therefore, this might be a possible explanation for the missing effects of the vaccine in the season 2017–2018. However, it does not explain the missing effects in the season 2016–2017. Here the most prevalent type was influenza A (H3N2), which is included in the trivalent vaccine (32). The seasons 2014–2015 and 2016–2017 also had a relatively high excess hospitalization and mortality, whereas the season 2015–2016 was mild in comparison. The season 2015–2016 had the highest vaccination effects in our study. Nevertheless, with our study, we are not able to conclude that this season was mild because of the high impact of the vaccine, because our analysis focuses only on patients with ILD. In addition, influenza vaccination is usually seen as a public health intervention with beneficial effects at the population level rather than at the individual level. Especially, low community prevalence of influenza and a high vaccination rate in the immediate vicinity of the included patients might result in a protective effect also for those without vaccination. This might lead to an underestimation of the positive external effects of influenza vaccination.
Limitations
Our study has several limitations. First, although we used strict selection criteria for the inclusion of patients with ILD, some misidentification cannot be ruled out. However, this uncertainty is present in both groups. Second, we used 1:1 matching in a rolling cohort design to adjust for confounding. Nonetheless, residual confounding might remain, especially because of the healthy vaccinee bias as discussed above. In this context, clinical information that could be used to further minimize the bias is missing in claims databases. Particularly, the forced vital capacity or the diffusing capacity of the lung for carbon monoxide, which are associated with mortality, would have been important parameters to assess disease severity. Third, the definition of respiratory-related hospitalizations might overestimate the occurrence of influenza-caused infections. Infection-related hospitalization in patients with ILD might often be coded as ILD specific, not as influenza specific. Fourth, in some German regions, there are selective contracts that result in different coding of influenza vaccination in the physician’s fee scale (Einheitlicher Bewertungsmaßstab). These specific codes could not be accounted for. However, considering the regional restriction and the vaccination coverage rate, this impact should be very small. Fifth, because of the matching approach, approximately 25% of the vaccinated patients could not be matched despite their eligibility, which reduces to some degree the transferability of our findings to populations with different characteristics.
Our study also has various strengths. It is the first study investigating the effects of influenza vaccination in patients with ILD, which represent an extremely vulnerable group for which respiratory infections should be avoided. Because of the rarity of ILDs, our large data set covering a considerable part of the German population serves as an asset. With our study design, we tried to alleviate important biases that appear in vaccination studies, and our approach might be promising for further studies.
Conclusions
The impact of influenza vaccination has varied across the four seasons investigated, with some years showing very promising results for all-cause mortality. Although not significant, the risk of hospitalization was also lower in vaccinated patients for those seasons, which underlines the clinical relevance of vaccination. Although group differences appeared only after some time rather than immediately after the vaccination and the sensitivity analysis showed no significant off-season effects for most outcomes, our results should be interpreted with caution because residual confounding may remain.
Footnotes
Supported by Stiftung Oskar-Helene-Heim.
Author Contributions: All authors were involved in the conception of the study. M.K. and L. Schwarzkopf initiated the project. P.M. conducted the data preparation and analysis in close coordination with M.K., L. Schwarzkopf, L. Schwettmann, W.M., and F.T. P.M. wrote the first draft of the manuscript, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability statement: The authors confirm that the data used in this study cannot be made available in the manuscript, the supplemental files, or in a public repository owing to German data protection laws (Bundesdatenschutzgesetz). Therefore, they are stored on a secure drive in the Allgemeine Ortskrankenkasse Research Institute to facilitate replication of the results. Generally, access to data of statutory health insurance funds for research purposes is possible only under the conditions defined in German Social Law (SGB V § 287). Requests for data access can be sent as a formal proposal specifying the recipient and purpose of the data transfer to the appropriate data protection agency. Access to the data used in this study can only be provided to external parties under the conditions of the cooperation contract of this research project and after written approval by the health insurance. For assistance in obtaining access to the data, please contact wido@wido.bv.aok.de.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. King TE., Jr Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med . 2005;172:268–279. doi: 10.1164/rccm.200503-483OE. [DOI] [PubMed] [Google Scholar]
- 2. Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet . 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paules C, Subbarao K. Influenza. Lancet . 2017;390:697–708. doi: 10.1016/S0140-6736(17)30129-0. [DOI] [PubMed] [Google Scholar]
- 4. Mauskopf J, Klesse M, Lee S, Herrera-Taracena G. The burden of influenza complications in different high-risk groups: a targeted literature review. J Med Econ . 2013;16:264–277. doi: 10.3111/13696998.2012.752376. [DOI] [PubMed] [Google Scholar]
- 5. Mertz D, Kim TH, Johnstone J, Lam PP, Science M, Kuster SP, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ . 2013;347:f5061. doi: 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olson AL, Swigris JJ, Raghu G, Brown KK. Seasonal variation: mortality from pulmonary fibrosis is greatest in the winter. Chest . 2009;136:16–22. doi: 10.1378/chest.08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. von der Beck D, Grimminger F, Seeger W, Günther A, Löh B. Interstitial lung disease: seasonality of hospitalizations and in-hospital mortality 2005–2015. Respiration . 2022;101:253–261. doi: 10.1159/000519214. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO) 2018. https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
- 9.Robert Koch-Institut. Recommendations of the Standing Committee on Vaccination (STIKO) at the Robert Koch-Institute – 2017/2018. 2017.
- 10. Mohr A, Plentz A, Sieroslawski A, Pezenburg F, Pfeifer M, Salzberger B, et al. Use of pneumococcal and influenza vaccine in patients with COPD, asthma bronchiale and interstitial lung diseases in south east Germany. Respir Med . 2020;174:106207. doi: 10.1016/j.rmed.2020.106207. [DOI] [PubMed] [Google Scholar]
- 11. Swart E, Gothe H, Geyer S, Jaunzeme J, Maier B, Grobe TG, et al. German Society for Social Medicine and Prevention German Society for Epidemiology. Good Practice of Secondary Data Analysis (GPS): guidelines and recommendations [in German] Gesundheitswesen . 2015;77:120–126. doi: 10.1055/s-0034-1396815. [DOI] [PubMed] [Google Scholar]
- 12. Busse R, Blümel M, Knieps F, Bärnighausen T. Statutory health insurance in Germany: a health system shaped by 135 years of solidarity, self-governance, and competition. Lancet . 2017;390:882–897. doi: 10.1016/S0140-6736(17)31280-1. [DOI] [PubMed] [Google Scholar]
- 13.Bundesministerium für Gesundheit (BMG) 2019. https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/Statistiken/GKV/Mitglieder_Versicherte/KM1_JD_2018.pdf
- 14. Schwarzkopf L, Witt S, Waelscher J, Polke M, Kreuter M. Associations between comorbidities, their treatment and survival in patients with interstitial lung diseases – a claims data analysis. Respir Res . 2018;19:73. doi: 10.1186/s12931-018-0769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert Koch-Institut. 2021. https://www.rki.de/SharedDocs/FAQ/Impfen/Influenza/faq_ges.html
- 16. Remschmidt C, Wichmann O, Harder T. Frequency and impact of confounding by indication and healthy vaccinee bias in observational studies assessing influenza vaccine effectiveness: a systematic review. BMC Infect Dis . 2015;15:429. doi: 10.1186/s12879-015-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med . 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care . 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 19. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care . 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 20. Spreckelsen O, Luque Ramos A, Freitag M, Hoffmann F. Influenza vaccination rates before and after admission to nursing homes in Germany. Aging Clin Exp Res . 2018;30:609–616. doi: 10.1007/s40520-017-0825-5. [DOI] [PubMed] [Google Scholar]
- 21. Nadash P, Doty P, von Schwanenflügel M. The German Long-term care insurance program: evolution and recent developments. Gerontologist . 2018;58:588–597. doi: 10.1093/geront/gnx018. [DOI] [PubMed] [Google Scholar]
- 22. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res . 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robert Koch-Institut. 2021. https://influenza.rki.de/Saisonbericht.aspx
- 24. Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet . 2008;372:398–405. doi: 10.1016/S0140-6736(08)61160-5. [DOI] [PubMed] [Google Scholar]
- 25. Jackson ML, Yu O, Nelson JC, Naleway A, Belongia EA, Baxter R, et al. Further evidence for bias in observational studies of influenza vaccine effectiveness: the 2009 influenza A(H1N1) pandemic. Am J Epidemiol . 2013;178:1327–1336. doi: 10.1093/aje/kwt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol . 2006;35:337–344. doi: 10.1093/ije/dyi274. [DOI] [PubMed] [Google Scholar]
- 27. Akmatov MK, Holstiege J, Steffen A, Bätzing J. Utilization of influenza vaccination among chronically ill individuals in Germany: a nationwide claims-based analysis. Vaccine . 2021;39:952–960. doi: 10.1016/j.vaccine.2020.12.081. [DOI] [PubMed] [Google Scholar]
- 28. Kopsaftis Z, Wood-Baker R, Poole P. Influenza vaccine for chronic obstructive pulmonary disease (COPD) Cochrane Database Syst Rev . 2018;6:CD002733. doi: 10.1002/14651858.CD002733.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bao W, Li Y, Wang T, Li X, He J, Wang Y, et al. Effects of influenza vaccination on clinical outcomes of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ageing Res Rev . 2021;68:101337. doi: 10.1016/j.arr.2021.101337. [DOI] [PubMed] [Google Scholar]
- 30. Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group report. Am J Respir Crit Care Med . 2016;194:265–275. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 31. Drake TM, Docherty AB, Harrison EM, Quint JK, Adamali H, Agnew S, et al. ISARIC4C Investigators Outcome of hospitalization for COVID-19 in patients with interstitial lung disease. An international multicenter study. Am J Respir Crit Care Med . 2020;202:1656–1665. doi: 10.1164/rccm.202007-2794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robert Koch-Institut. Bericht zur Epidemiologie der Influenza in. Berlin, Germany: Robert Koch-Institut; 2018. [Google Scholar]
- 33.Falkenhorst G, Remschmidt C, Weidemann F, Wichmann O, Garbe E, Ledig T, et al. Wissenschaftliche Begründung für die Empfehlung des quadrivalenten saisonalen Influenzaimpfstoffs. Berlin, Germany: Robert Koch-Institut, Epidemiologie und Gesundheitsberichterstattung; 2018. [Google Scholar]


