Abstract
Repetitive transcranial magnetic stimulation (rTMS) is a promising, innovative, and non-invasive therapy used clinically. Efficacy of rTMS has been demonstrated to ameliorate psychiatric disorders and neuropathic pain through neuromodulation of affected neural circuits. However, little is known about the mechanisms and the specific neural circuits via which rTMS facilitates these functional effects. The aim of this study was to begin revealing the mechanisms by which rTMS may tap into existing neural circuits, by using a well characterized spinal motor circuit – the phrenic circuit. Here we hypothesized that rTMS can be used to enhance phrenic motoneuron excitability in anesthetized Sprague Dawley rats. Multiple acute rTMS protocols were used revealing 10 Hz rTMS protocol induced a robust, long-lasting increase in phrenic motoneuron excitability, functionally evaluated by diaphragm motor evoked potentials (59.1 ± 21.1 % of increase compared to baseline 60 min after 10 Hz protocol against 6.0 ± 5.8 % (p = 0.007) for Time Control, −5.8 ± 7.4 % (p < 0.001) for 3 Hz, and 5.2 ± 12.5 % (p = 0.008) for 30 Hz protocols). A deeper analyze allowed to discriminate “responder” and “non-responder” subgroups among 10 Hz rTMS treated animals. Intravenous injections of GABAA and GABAB receptor agonists prior to 10 Hz rTMS treatment, abolished the enhanced phrenic motoneuron excitability, suggesting GABAergic input plays a mechanistic role in rTMS-induced phrenic excitability. These data demonstrate that a single high frequency rTMS protocol at 10 Hz increases phrenic motoneuron excitability, mediated by a local GABAergic “disinhibition”. By understanding how rTMS can be used to affect neural circuits non-invasively we can begin to harness the therapeutic potential of this neuromodulatory strategy to promote recovery after disease or injury to the central nervous system.
Keywords: rTMS, Preclinical model, Motoneuron excitability, GABAergic modulation
1. Introduction
One of the major challenges in the medical community is to develop reliable, non-invasive, and painless therapeutics for treating neurological disorders. Repetitive transcranial magnetic stimulation (rTMS) is a promising therapeutic already implemented in the clinic to treat many disorders including depression (Jassova et al., 2019; Martin et al., 2017; McClintock et al., 2018), stroke (Sebastianelli et al., 2017) or post-traumatic stress disorder (Kozel, 2018; Yan et al., 2017). High frequency rTMS, delivered below the motor threshold has also been shown to induce long lasting neuromodulatory behavioral effects (Ziemann et al., 2008). Apart from using high frequency rTMS as a therapeutic, a single pulse of TMS delivered above the motor threshold on the primary cortical areas has been used to evaluate muscle function by recording specific motor evoked potentials (MEP) in humans (Petrosyan et al., 2017), including diaphragmatic MEP (MEPdia) during stimulation of diaphragm motor cortex (Davey et al., 1996; Khedr and Trakhan, 2001; Lissens, 1994; Maskill et al., 1991; Mehiri et al., 2006; Sharshar et al., 2003). Despite these beneficial effects, little is known about how rTMS or single pulse TMS results in these neurological outcomes.
We have previously used a preclinical model of respiratory neurobiology to explore the effects of TMS on the phrenic motor circuit (Vinit et al., 2016). The phrenic motoneuron pool located within the spinal cord (cervical segments C3-C6) is innervated by supraspinal connections from cortical and brainstem neurons in human (Gandevia and Rothwell, 1987; Maskill et al., 1991), cat (Lipski et al., 1986) and rat (Alexandrov et al., 2007). The spinal phrenic network (moto- and interneurons) receives excitatory and inhibitory input from supraspinal and spinal interneurons, which can directly modulate phrenic motor output and diaphragm activity (Lane et al., 2008; Tian et al., 1998; Zholudeva et al., 2018). The neurorespiratory circuit is well known for being sensitive to neuromodulation, which can be elicited by a variety of factors including injury, hypoxia, hypercapnia or even stress (Mitchell and Johnson, 2003; Richard et al., 2013). Using a human-adapted figure-of-eight coil in naive and cervical spinal cord-injured Sprague Dawley rats, we demonstrated that a single pulse of TMS through the skull results in neuronal depolarization within cortical and subcortical areas (Vinit et al., 2016, 2014). The elicited action potential volleys propagate through corticospinal pathways to several muscles, including the diaphragm.
The neuromodulating effects of rTMS depends on stimulation frequency, number of pulses and intensity. Traditionally, low-frequency rTMS protocols (≤5 Hz) induce a reduction in excitability (long-term depression-like effect) (Trippe et al., 2009) whereas high-frequency protocols (≥10 Hz) lead to an increase in neuronal excitability (long--term potentiation-like effect) (Hoogendam et al., 2010; Tang et al., 2017), the latter being of high interest for enhancing neuroplasticity. However, only a few studies have tried to decipher the molecular effect of high frequency rTMS protocols. In fact, 10 Hz rTMS has proven to attenuate inhibitory neuron activity and decrease inhibitory synaptic strength. This effect was accompanied by a destabilization of gephyrin clusters (scaffolding protein that bind to ionotropic γ-aminobutyric acid (GABA) receptor) in vitro and this observation was validated in vivo in anesthetized mice (Lenz et al., 2016). Taken together, molecular studies on rTMS show that a 10 Hz rTMS modifies the excitation/inhibition balance which leads to a “local disinhibition”, and increased excitability (Lenz and Vlachos, 2016). Interestingly, theta burst stimulation (TBS) protocols also induce a decrease of activity markers in excitatory and inhibitory neurons whereas synaptic activity markers increased following repeated stimulation (Volz et al., 2013), and this magnetic stimulating protocol can modulate the activity of some classes of inhibitory cells in rats (Benali et al., 2011).
The present work tests several rTMS protocols in vivo in naïve Sprague Dawley rats in their potential for increasing phrenic motoneuron excitability and investigates the role of GABAergic networks within this neuromodulatory pathway. Increasing the neurorespiratory excitability with an adequate rTMS protocol could be used as a therapeutic to improve ventilation in breathing disorders such as respiratory deficits caused by cervical spinal cord injury.
2. Material and methods
2.1. Ethics statement
Experiments reported in this manuscript were performed at two institutions, adhering to their respective policies. Adult female, Sprague-Dawley rats (Envigo; n = 6, 250—280 g) were used for neuroanatomical tracing, adhering to policies laid out by the National Research Council Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at Drexel University (USA). Animals were housed in groups of three, with access to food and water ad libitum on a 12 -h light/dark cycle. Adult Sprague–Dawley male rats (Janvier, France; n = 55, 350–450 g) were used for transcranial magnetic stimulation experiments, approved by the Ethics Committee of the University of Versailles Saint-Quentin-en-Yvelines and complied with the French and European laws (EU Directive 2010/63/EU) regarding animal experimentation (Apafis #2017111516297308_v3). The animals were dual housed in individually ventilated cages in a state-of-the-art animal care facility (2CARE animal facility, accreditation A78-322-3, France), with access to food and water ad libitum on a 12 -h light/dark cycle.
2.2. Transcranial magnetic stimulation (TMS)
TMS was performed using the magnetic stimulator MAGPRO R30 (Magventure, Farum, Denmark) connected to a figure-of-eight coil (Cool-B65) delivering a unique biphasic pulse with an intensity of the stimulus expressed as a percentage of maximum output of the stimulator (% MO). The MO was set at 90 %, and the center of the coil was placed at 6 mm caudal to Bregma, above the animal’s skull. This position and the MO have been shown to always elicit a specific diaphragmatic Motor Evoked Potential (MEPdia) from supraspinal stimulation, as we previously published (Vinit et al., 2014). In total, each animal received no more than 100 single pulses of TMS with an interpulse duration above 15 s to avoid low frequency repetitive TMS-like effects. This protocol was used to elicit specific MEPdia in order to evaluate the excitability of the phrenic motor circuit.
2.3. Acute repetitive TMS (rTMS) protocols
rTMS protocols were also applied in anesthetized animals, at −6 mm caudal to Bregma. They consisted of 9 trains of 100 biphasic pulses (900 stimulations per protocol) at 3 different frequencies [low frequency (3 Hz), high frequency (10 Hz) and very high frequency (30 Hz)]. Each train was separated by 30 s intervals delivered at 50 % of MO [below the motor threshold, as we previously published (Vinit et al., 2014)]. Control animals received a sham rTMS protocol (e.g. no stimulation), with total duration the same as rTMS treated animals.
2.4. GABA agonist administration
To investigate the role of the GABAergic system in plasticity elicited by rTMS, two GABA receptor agonists were used in this study: 0.05 mg of Clonazepam (Rivotril), a GABAA receptor agonist delivered at 1 mg/ml as a single intravenous injection, and 0.3 mg of Baclofen (BACLOFENE AGUETTANT), a GABAB receptor agonist delivered at 2 mg/ml as a single intravenous injection. Blood samples (1 mL) were drawn 5 min after agonist injection, but prior to rTMS, and at the end of each experiment (60 min after rTMS) to evaluate plasma concentration of agonists throughout the experiment. Blood samples in heparin tubes were centrifuged for 5 min at 0.8 x g (room temperature). The plasma was collected (around 400 μL per sample) and stored at −80 °C for further analysis of drug dose.
Baclofen and Clonazepam were quantified in rat plasma using liquid chromatography coupled to tandem mass spectrometry detection (LC–MS/MS TSQ Endura, ThermoFischer Scientific, Les Ulis, France) derived from a method previously published (Imbert et al., 2015) using baclofen-D4 and alprazolam-D5 as internal standard (IS), respectively. Briefly, 10 μl of IS and 100 μl of a mixture of methanol/H2O (70/30, v/v) was added to 50 μl of plasma. After agitation and centrifugation, supernatant was directly injected in the chromatogram. Data were collected in selected reaction monitoring mode using the transition m/z 214.1 → m/z 151.1 and m/z 218.1 → m/z 154.1 for baclofen and its IS, and m/z 316.1 → m/z 270.1 and m/z 314.5 → m/z 286.2 for clonazepam and its IS. Intra and inter-day precisions of the method were below 12.7 % and accuracy between 95 % and 107 %. Limits of quantification were 20 ng/ml and 5 ng/ml for baclofen and clonazepam, respectively.
2.5. Electrophysiological recordings
55 animals were used for electrophysiological recordings. They were randomly divided into 8 groups: Sham rTMS (n = 6), 3 Hz rTMS (n = 5), 10 Hz rTMS (n = 22), 30 Hz rTMS (n = 6), Sham rTMS + Clonazepam (n = 3), Sham rTMS + Baclofen (n = 3), 10 Hz rTMS + Clonazepam (n = 4) and 10 Hz rTMS + Baclofen (n = 6). Electrophysiological recordings of diaphragm activity were used to functionally evaluate the effects of rTMS on the phrenic motor circuit. Briefly, anesthesia was induced using isoflurane (5% in 21 % O2 balanced) in an anesthesia chamber and maintained through a nose cone (2.5 % in 100 % air balanced). A 25 G catheter was placed into the tail vein and the animals were tracheotomized and pump ventilated (Rodent Ventilator, model 683; Harvard Apparatus, South Natick, MA, USA). The ventilation rate (frequency > 72 breaths per minute, tidal volume: 2.5 ml2.5 mL) was adjusted to reduce the end-tidal CO2 value below the animals’ central apneic threshold throughout the experiment to avoid recording spontaneous diaphragm contractions. During the recordings, animals were placed on a heating pad to maintain a constant body temperature (37.5 ± 0.5 °C) and their rectal temperature was continuously monitored throughout the experiment. Arterial pressure was measured through a catheter inserted into the right femoral artery. Arterial and tracheal pressures were monitored continuously with transducers connected to a bridge amplifier (AD Instruments, Dunedin, New Zealand). Isoflurane anesthesia was then slowly converted to urethane anesthesia (1.8 g/kg, i.v.; Sigma-Aldrich). The depth of anesthesia was confirmed by the absence of response to toe pinch. A laparotomy was performed, and the liver was gently moved caudally to access the diaphragm. Gauze soaked with warm phosphate-buffered saline was placed on the liver to prevent dehydration. The head of the animal was placed on a non-magnetic custom-made stereotaxic apparatus which allowed its positioning from the center of the figure-of-eight coil to −6 mm from Bregma, at an angle of 0°, as previously described (Vinit et al., 2014). Two custom-made hooked bipolar electrodes were placed on each mid-costal part of the diaphragm and left in place for the entire duration of the experiment. MEPdia induced by a single pulse of TMS was recorded (summation of 5–10 TMS pulses between 2 heart beats, max 10 trials at 90 % of MO). Then rTMS (3, 10 or 30 Hz at 50 % MO) protocol or Sham rTMS (i.e. same duration without stimulation) was applied and MEPdia was recorded at 1, 10, 20, 40 and 60 min post-rTMS (with a summation of 10 pulses of TMS at 90 % MO). Recipients of GABA agonists received MEPdia recording before and 5 min after the injection (just prior to rTMS protocol). These electromyographic signals were amplified (gain, 1k; A–M Systems, Everett, WA, USA) and band pass-filtered (100 Hz to 10 kHz). The signals were then digitized with an 8-channel Powerlab data acquisition device (Acquisition rate: 100 k/s; AD Instruments, Dunedin, New Zealand) connected to a computer and analyzed using LabChart 8 Pro software (AD Instruments, Dunedin, New Zealand).
2.6. Neuroanatomical tracing
A retrograde, transynaptic viral tracing technique (pseudorabies virus, PRV) was used to label the phrenic motor circuit in naïve, Sprague Dawley rats as previously described (Lane et al., 2008). Briefly, animals were anesthetized with isoflurane (4% induction, 2% maintenance in 100 % O2), ventral side shaved and sterilized with alternating wipes of ethanol and betadine prior to a laparotomy to expose the diaphragm. PRV was then topically applied to the left hemi-diaphragm (50 μL). Overlying muscle was sutured, skin was closed with wound clips and analgesic (Buprenorphine, 0.025 mg/kg s.q.) and Lactated Ringers (5 mL, s.q.) were given post operatively. Animals were sacrificed 72 h after PRV tracing by an overdose of Beuthanasia-D Solution, and transcardially perfused with ice-cold physiological saline (0.9 % NaCl in water) followed by ice-cold paraformaldehyde (PFA, 4% w/v in 0.1 M PBS, pH 7.4). The brain and spinal cord were dissected and post-fixed in 4% PFA until further processing. The PRV recombinant used in this study (PRV614, 1 × 108 pfu) was provided by Dr. David Bloom (University of Florida). Primary antibodies (polyclonal rabbit) to PRV were generously donated by Dr. Lyn Enquist (Princeton University).
2.7. Immunohistochemistry
Spinal cord tissue spanning caudal brainstem to beginning of thoracic spinal cord (T1) was blocked and cryoprotected (15 % then 30 % sucrose w/v in PBS, overnight), and sectioned (horizontal, on-slide 30 μm). Sections were rehydrated in 0.1 M PBS for 15 min and blocked against endogenous peroxidase activity (30 % methanol, 0.6 % hydrogen peroxide in 0.1 M PBS) for 1 h at room temperature. Sections were then washed for 15 min in 0.1 M PBS and blocked against non-specific protein labeling (10 % normal donkey serum in 0.1 M PBS with 0.02 % Triton-X) for 1 h at room temperature. Primary antibodies (Table 1) were applied to tissue in blocking solution overnight at room temperature. The following day, tissues were washed for 15 min (3 × 5 min of 0.1 M PBS) prior to application of secondary antibodies (Table 1) in blocking solution for 2 h at room temperature. Stained sections were then washed for 15 min (3 × 5 min of 0.1 M PBS), allowed to dry, and cover-slipped with fluorescence mounting medium (Fluoromount-G, SouthernBiotech). Sections were imaged using a Zeiss AxioImager microscope with Apotome 2 and digital camera (Zeiss Axiocam MRm) attached to a Dell PC.
Table 1.
List of Primary and Secondary Antibodies.
| Primary Antibodies | ||||
|---|---|---|---|---|
| Antigen | Host | Concentration | Manufacturer | Cat Number |
| GABA A Receptor alpha 1 | Rabbit | 1:750 | Abcam | ab33299 |
| GABA B Receptor 2 | Rabbit | 1:750 | Abcam | ab75838 |
| PRV | Goat | 1:10000 | courtesy of Lynn Enquist, Princeton University | |
| Secondary Antibodies | ||||
| Rabbit - AF488 | Donkey | 1:400 | ThermoFischer Scientific | A-21206 |
| Goat - AF594 | Donkey | 1:400 | Jackson ImmunoResearch | 705-585-003 |
2.8. Data processing and statistical analyses
Diaphragm electromyography traces for each side (at least 5 MEPdia) were averaged and superimposed using LabChart Pro software (AD Instruments) prior to, 1, 10, 20, 40 and 60 min post-rTMS treatment. The baseline-to-peak amplitude of the first wave of each superimposed MEPdia was calculated. The mean arterial pressure (MAP) for each time point was also evaluated. Two-way ANOVA (Fisher LSD Method for multiple comparisons) was used to compare MEPdia throughout the experiment and between different rTMS protocols. To compare GABA agonist recipients, a Student’s paired t-test or a Wilcoxon t-test was used to compare MEPdia amplitude and MAP before and after delivery of agonists to determine statistical differences between animals, over duration of the experiment.
An exponential regression was used on the MEPdia data collected from animals stimulated with 10 Hz rTMS to evaluate the percent of animals that responded to the rTMS treatment compared to Sham rTMS. Regression coefficient R2 was calculated. These groups have been designed by comparing the 10 Hz treated animals’ individual percentage of variation MEPdia values with the Mean percentage of variation MEPdia of the Sham rTMS group ± a given Standard Deviation (SD) at 60 min post-rTMS. Calculation of the given SD that can be considered as the threshold from “non-responder” to “responder” used Chebyshev’s Theorem applied to the Sham rTMS population. According to this theorem, at least 75 % of the Sham rTMS group values are between the mean ± 2SD. In the present study, all the Sham rTMS values lay between Mean ± 2SD, giving the guarantee that 10 Hz group values, which are above this threshold of Sham rTMS Mean ± 2SD, do not overlap with Sham rTMS group values. Thus, 10 Hz treated rats presenting values above this threshold are considered as “responder” to this treatment, whereas others below the threshold are considered as “non-responders”. For evaluating the significant effect of rTMS treatment on MEPdia percentage variation at 60 min compared to baseline, a Kruskal-Wallis One-way ANOVA (Dunn’s Method) was performed.
All the data were presented as mean ± SEM and statistics were considered significant when p < 0.05. SigmaPlot 12.5 software was used for all analyses.
3. Results
3.1. rTMS results in increased excitability of diaphragm muscle
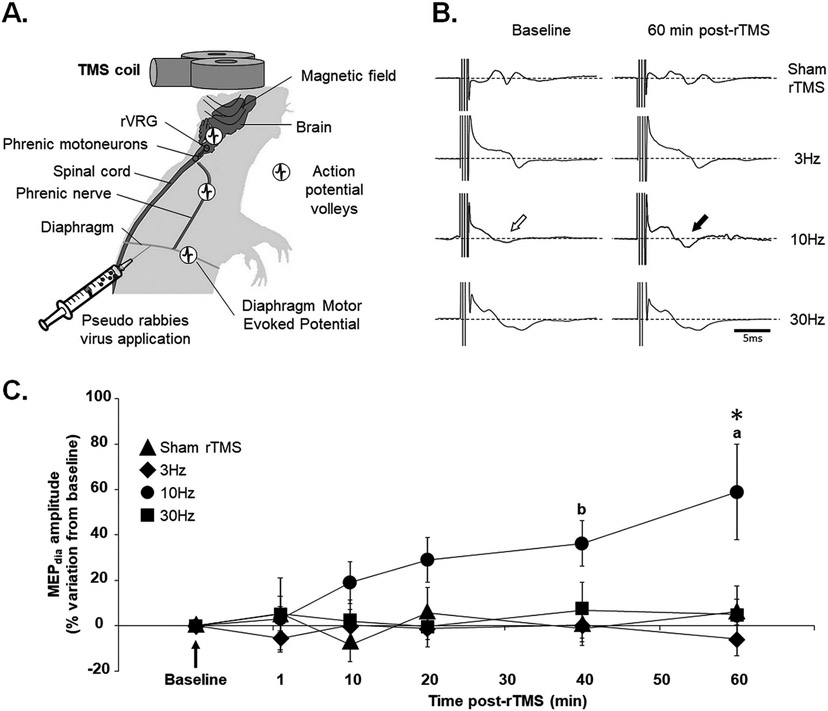
A single pulse of TMS delivered above the motor threshold (90 % of MO) ~6 mm above the Bregma always induces a MEPdia that can be recorded by electrodes implanted into the diaphragm for each animal (Fig. 1A, B). All values taken before rTMS treatment were normalized to the baseline. Then the percentage of variation for each group at each time post-rTMS was evaluated. No significant differences in MEPdia amplitude were detected between Sham rTMS, 3 Hz, 10 Hz and 30 Hz groups at 1 min, 10 min, 20 min and 40 min post-rTMS. However, the 10 Hz treated group presented a significant increase in MEPdia amplitude compared to Sham rTMS, 3 Hz and 30 Hz groups at 60 min post-stimulation (Fig. 1C). There were no differences in MEPdia amplitude during the 60 min post-rTMS in any of the other groups (Sham rTMS, 3 Hz and 30 Hz). Interestingly, the 10 Hz group presented a significant increase in MEPdia amplitude at 40 min compared to 1 min post-rTMS (p = 0.014), and at 60 min compared to 1 (p < 0.001), 10 (p = 0.003) and 20 (p = 0.025) min post-rTMS (Fig. 1C).
Fig. 1. Effect of different rTMS protocols on phrenic motoneuron excitability.
(A) Schematic of magnetic coil used for Transcranial Magnetic Stimulation (TMS) positioned over the rat’s head. The proposed descending pathways that are stimulated are represented with action potential volleys. Pseudorabies virus is applied to the diaphragm for retrograde labeling of phrenic motor network. (B) Representative diaphragmatic motor evoked potentials (MEPdia) recordings before and 60 min after 3 Hz, 10 Hz and 30 Hz rTMS or Sham rTMS. The white arrow shows the MEPdia at baseline, the black arrow indicates the increase in MEPdia response, corresponding to an increase in phrenic motoneuron excitability. (C) Time course showing the evolution of the percentage of MEPdia compared to baseline values following Sham, 3 Hz, 10 Hz or 30 Hz rTMS treatment. * p < 0.01 compared to Sham rTMS, 3 Hz and 30 Hz groups; a p < 0.05 compared to 1, 10 and 20 min post-rTMS; b p < 0.05 compared to 1 min post-rTMS (Two-way ANOVA, Fisher LSD post-hoc test).
3.2. Responders and non-responders to 10 Hz rTMS
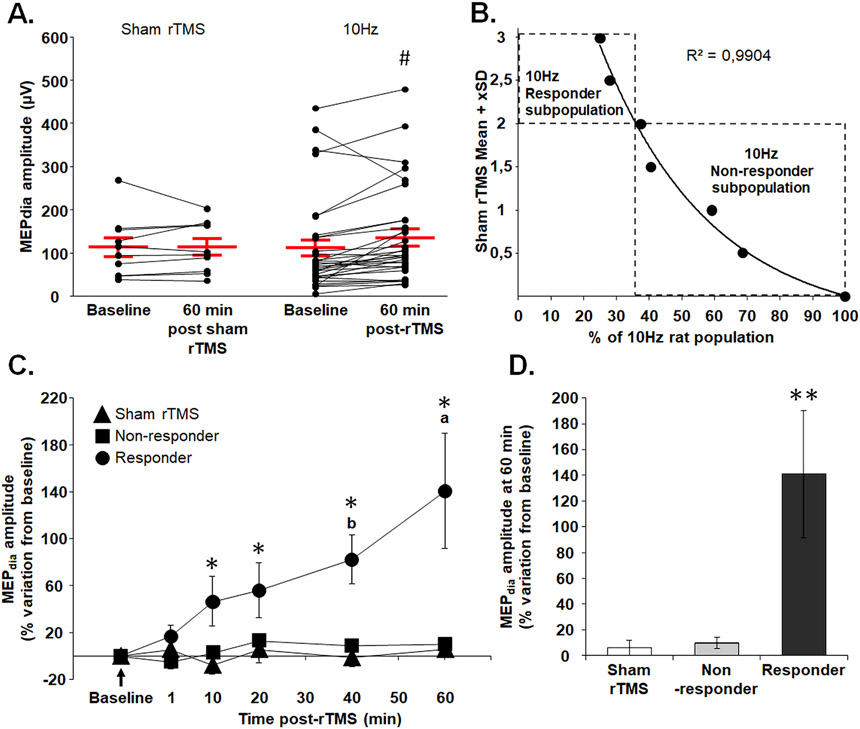
To evaluate the heterogeneity in phrenic excitability elicited by 10 Hz rTMS, raw MEPdia amplitudes 60 min post-sham and 10 Hz rTMS were plotted and compared to baseline (Fig. 2A). These data revealed a significant increase in MEPdia 60 min post-10 Hz rTMS, compared to baseline, but the increase was not observed in all 10 Hz rTMS animals. A regression analysis was then conducted on individual 10 Hz rTMS data points, revealing a statistical separation of animals with rTMS-mediated increase in MEPdia at least by 2 standard deviations (e.g. at least 40.68 % higher than Sham rTMS). This analysis resulted in separation of “Responders,” which comprised 37.5 % of the 10 Hz rTMS group, and “Non-responders,” representing 62.5 % of the group (Fig. 2B).
Fig. 2. Meta-analysis of 10 Hz rTMS treated animals resulted in statistical separation of “responder” and “non-responder” subpopulations.
(A) Diaphragm motor evoked potential (MEPdia) amplitude before and 60 min following Sham and 10 Hz rTMS treatment groups. (B) Exponential regression of 10 Hz rTMS treated group statistically separated into 2 subpopulations (“Responder” and “Non-responder”) based on the Mean + Standard Deviation (SD) values of the Sham rTMS group. Each dot represents the percent of animals considered as “Responder” for Mean + given SD. The threshold for discriminating the two populations was set at Sham rTMS Mean + 2SD. (C) Time-course of percent change in MEPdia amplitude following indicated protocols compared to baseline, with 10 Hz rTMS separated into Responder and Non-responder groups. (D) Percent change from baseline in MEPdia amplitude at 60 min post-rTMS for Sham, Non-responder and Responder 10 Hz rTMS groups. # p < 0.001 compared to baseline (Wilcoxon t-test); * p < 0.05 compared to Sham rTMS and Non-responder groups; a p < 0.01 compared to 1, 10, 20 and 40 min post-rTMS; b p < 0.005 compared to 1 min post-rTMS (Two-way ANOVA, Fisher LSD post-hoc test); ** p < 0.05 compared to Sham rTMS and Non-responder groups (Kruskal-Wallis One-way ANOVA (Dunn’s Method)).
The raw MEPdia amplitudes for Sham rTMS, “Responder” and “Non-responder” of 10 Hz rTMS were then plotted with time. There was no significant difference between Sham rTMS, Non-responder and Responder groups at 1 min post-rTMS. Starting at 10 min rTMS, however, MEPdia of the “Responders” group significantly increased and maintained elevated up to 60 min post-rTMS compared to Sham rTMS and “Non-responders” (Fig. 2C, D). No difference was observed between the Sham rTMS group and Non-responder group at all time points.
3.3. GABAergic networks play a role in diaphragmatic excitability induced by rTMS
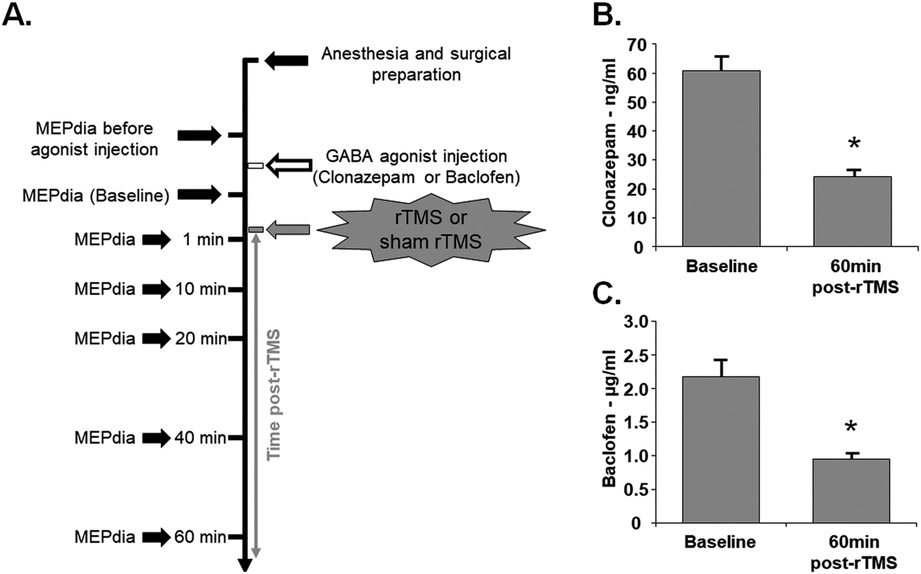
Two GABAergic agonists were used to test the hypothesis that GABAergic networks play a role in enhanced diaphragm excitability induced by 10 Hz rTMS. Clonazepam (GABAA agonist) or Baclofen (GABAB agonist), were intravenously injected prior to sham or 10 Hz rTMS protocol (Fig. 3A). Drug concentration in blood plasma were measured over time to ensure physiological relevance (according to the manufacturer). There was no statistical difference in drug concentration between sham or 10 Hz rTMS groups, so the data were pooled together and compared over time. Concentrations of clonazepam (Fig. 3B) and Baclofen (Fig. 3C) significantly decreased 60 min following rTMS or sham rTMS compared to plasma levels immediately post injection. While we observed an overall decrease with time, these molecules remained in blood plasma at effective concentrations at the end of the experiment.
Fig. 3. Experimental timeline and GABA agonists dosage.
(A) GABAergic agonists were delivered prior to Sham or rTMS treatment. The phrenic motoneuronal excitability was assessed 7 times (MEPdia). (B) Plasma concentration of GABAA agonist (Clonazepam) and (C) GABAB agonist (Baclofen) immediately after drug injection (Baseline) and 60 min post-rTMS. Paired t-test, * p < 0.001 compared to baseline.
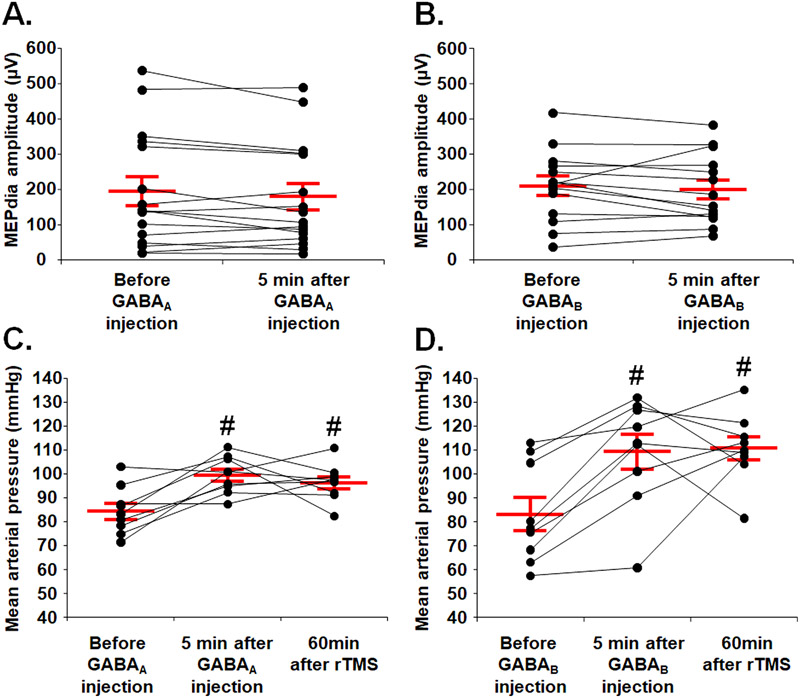
In order to evaluate the neuromodulatory effect of each GABAergic agonist on rTMS treatment and exclude a direct physiological effect on the motoneuron excitability, MEPdia were recorded before and 5 min after i.v. injection. There was no difference in raw MEPdia amplitude within each group before and after GABA agonist injection (Fig. 4A and B). There was, however, a significant increase in mean arterial pressure (MAP) 5 min post-injection in both groups compared to the pre-injection values (Fig. 4C and D). We observed no significant difference in MAP in either group 60 min post-drug injection compared to values measured 5 min post-injection (Fig. 4C and D).
Fig. 4. Phrenic motoneuron excitability and mean arterial pressure following delivery of GABA agonist.
(A) Phrenic motoneuron excitability evaluated by MEPdia amplitude before and 5 min following GABAA agonist injection (Clonazepam). (B) Phrenic motoneuron excitability evaluated by MEPdia amplitude before and 5 min following GABAB agonist injection (Baclofen). (C) Mean arterial pressure before, 5 min and 60 min after 10 Hz or sham rTMS protocol with Clonazepam injection. (D) Mean arterial pressure before, 5 min and 60 min after 10 Hz or sham rTMS protocol with Baclofen injection. Paired t-test, # p < 0.01 compared to before GABA agonist injection.
We then tested the hypothesis that GABAergic networks play a role in rTMS-mediated phrenic motoneuron excitability. GABA agonists were administered before the rTMS or sham rTMS protocol (Fig. 3A) and MEPdia was recorded immediately post-drug administration and compared to 1, 10, 20, 40 and 60 min post-sham or 10 Hz rTMS (Fig. 5A). Administration of GABA agonists did not significantly affect MEPdia amplitude in any of the groups up to 20 min post sham or rTMS. At 40 and 60 min post-drug injection, however, MEPdia of all conditions were significantly diminished compared to 10 Hz rTMS (Fig. 5B). This suggests that GABA agonist dampens phrenic motoneuron excitability elicited by 10 Hz rTMS.
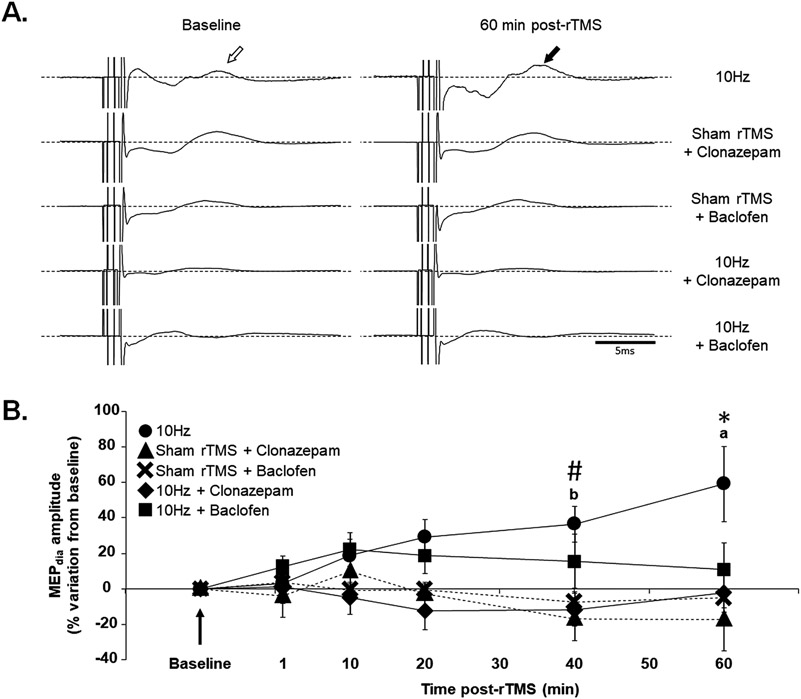
Fig. 5. 10 Hz rTMS protocol and phrenic motoneuronal excitability with GABA agonists.
(A) Representative MEPdia traces prior to and 60 min post-10 Hz rTMS, Sham rTMS + GABAA agonist (Clonazepam), Sham rTMS + GABAB agonist (Baclofen), 10 Hz rTMS + Clonazepam and 10 Hz rTMS + Baclofen protocols. The white arrow shows the MEPdia at baseline, the black arrows show the MEPdia increase following 10 Hz rTMS protocol. (B) Time-course of percent change in MEPdia amplitude following indicated protocols compared to baseline. # p < 0.05 compared to Sham rTMS + GABAA agonist and 10 Hz rTMS + GABAA agonist groups; * p < 0.009 compared to Sham rTMS + GABAA agonist, Sham rTMS + GABAB agonist, 10 Hz rTMS + GABAA agonist and 10 Hz + GABAB agonist; a p < 0.05 compared to 1, 10 and 20 min post-rTMS; b p < 0.05 compared to 1 min post-rTMS (Two-way ANOVA, Fisher LSD post-hoc test).
Moreover, time post-treatment (or sham rTMS) was also evaluated. No difference in MEPdia amplitude was observed over time with treatment (rTMS or sham rTMS) for Sham rTMS + Clonazepam, Sham rTMS + Baclofen, 10 Hz + Clonazepam and 10 Hz + Baclofen groups. However, 10 Hz group showed a significant increase in MEPdia amplitude at 40 min compared to 1 min post-rTMS (p = 0.014) and at 60 min compared to 1 (p < 0.001), 10 (p = 0.003) and 20 (p = 0.027) min post-rTMS (Fig. 5B).
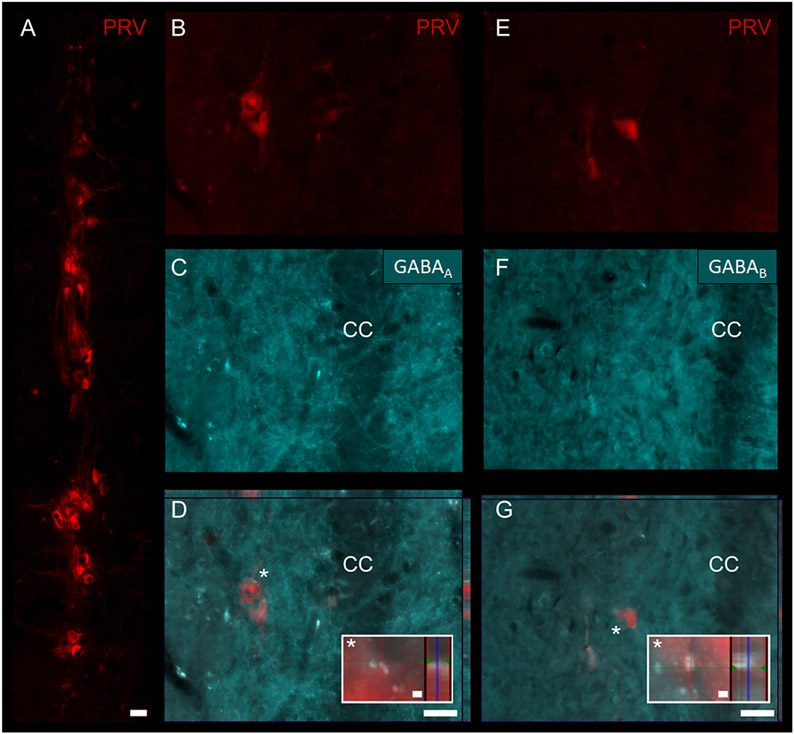
To qualitatively validate GABAergic influence on the phrenic network, we used the neuroanatomical tracer, pseudorabies virus (PRV), to identify phrenic motoneurons (Fig. 6A) and interneurons (Fig. 6B and E) located throughout the cervical spinal cord (C3–6). Immunohistochemistry of GABAA and GABAB receptors (Fig. 6C and F, respectively) on PRV traced spinal sections demonstrated the expression of these receptors on the phrenic motor network (Fig. 6D and G). An example of these receptors closely adjacent to phrenic interneurons is demonstrated in orthogonal projections (insets in Fig. 6D and G).
Fig. 6. Anatomical localization of GABAA and GABAB on phrenic motor network identified using transsynaptic pseudorabies virus (PRV).
When applied onto the diaphragm, PRV infects phrenic motoneurons (A) (located in the cervical spinal cord level 3–5 in the rat). PRV is then retrogradely transported and trans-synaptically labels phrenic interneurons (B, E). Immunohistochemistry for GABAA (C) and GABAB (F) receptors used to demonstrate examples of phrenic GABAergic receptor expression (D, G). Orthogonal projections of overlays are demonstrated in D and G. White asterisk (*) in D and G denote high-magnification (40x) insets. CC: central canal. Scale bars: 50 μm in A, D, G; 2 μm in insets.
4. Discussion
Repetitive TMS has been used to treat a wide range of neurological disorders, demonstrating effectiveness in enhancing neural plasticity, but the underlying mechanisms via which rTMS enhances this plasticity are largely unknown. The present study is the first to demonstrate that 1) a robust long-lasting increase in phrenic motoneuron excitability induced by a single acute high frequency treatment of rTMS can be elicited and sustained in a rat model of respiratory neurobiology, and 2) GABAergic neurons within the phrenic network can modulate this therapeutic effect.
Neural stimulation, and especially high frequency electrical stimulation, has been shown to induce long-term potentiation (LTP) in vivo, corresponding to a robust increase in neuronal excitability which can last for hours (Bliss and Gardner-Medwin, 1973; Bliss and Lømo, 1973). Recently, 10 Hz rTMS protocol was investigated in an animal model in vivo and in vitro with the induction of a LTP-like effect in the hippocampus (Lenz et al., 2015; Lenz and Vlachos, 2016; Vlachos et al., 2012). Similarly, in the present study, 10 Hz rTMS treatment induces a robust long-lasting increase in phrenic motoneuron excitability, compared to 3 and 30 Hz protocols which failed to elicit similar effects. Low frequency protocols (<10 Hz) with rTMS are known to reduce cortical excitability and decrease induced MEP (Ziemann et al., 2008). Another explanation could reside in the protocol duration or repetition of this protocol. In fact, the 10 Hz protocol takes longer in time compared to the 30 Hz protocol and shorter than the 3 Hz protocol. The current protocol has been applied once acutely, and more protocol applications (i.e. chronically) at different frequencies could lead to similar effect on the phrenic excitability.
The center of the coil was deliberately positioned at −6 mm from Bregma on the animal’s skull as described previously, since this stimulating position has proven its efficacy to elicit specific diaphragmatic motor evoked potentials in rats (Vinit et al., 2016, 2014). Within this study, rTMS protocols were delivered in the same coil position center at the rat’s Bregma. One improvement in future studies could be a direct application of the rTMS protocol above the phrenic motoneuron pool, i.e. in spinal cord at cervical levels C3-C6 in order to enhance treatment efficacy. The intensity of the protocol (50 % of maximal output of the TMS machine) was deliberately chosen since this intensity does not elicit a diaphragm contraction in our specific experimental design (Vinit et al., 2014). Protocols of subthreshold rTMS are often used in the clinic to neuromodulate cortical structures without inducing action potential volleys, and these have been proven to be effective in humans to improve neurological disorders (Martin et al., 2017). Interestingly, a protocol of repetitive intermittent hypoxia in healthy humans induce a robust increase in corticospinal excitability assessed by MEP in a finger muscle following a single pulse of TMS (Christiansen et al., 2018). In addition, a recent study demonstrated that an acute protocol of high frequency rTMS induce an increase in synaptic plasticity associated proteins (Phosphorylation of S6), known to be involved in neuronal excitability increase, including MAPK/ERK, PI3 kinase and mTOR (Fujiki et al., 2020; Gobert et al., 2008). These subsequent proteins are known to participate in the increase of phrenic motor neuron excitability following intermittent hypoxia (Dougherty et al., 2015; Hoffman et al., 2012).
Another interesting observation was found among the animals treated with 10 Hz rTMS. Individual analyses of the MEPdia changes of the neural excitability bring to light the existence of rats with high response, moderate response or no response to the 10 Hz rTMS treatment. This heterogeneity in terms of efficiency rTMS treatment in the MEP muscular responses in the human population is well documented (Benadhira et al., 2017; Fitzgerald et al., 2016; van de Ruit and Grey, 2019). In human population however, the efficacy of rTMS protocol is generally determined in a qualitative way to determine a patient’s remission (Yip et al., 2017). In the present study, the animals are deeply anesthetized during the procedure and the data obtained are quantitative. The protocol by itself makes qualitative evaluation impossible and separation of the animals by the effectiveness of rTMS treatment on phrenic motoneuronal excitability is impossible. In order to further analyze the efficiency of the 10 Hz rTMS treatment, the Chebyshev’s theorem was applied to our sham rTMS population to categorize the “responder” and “non-responder” subpopulations in our treated animals. Therefore, 37.5 % of 10 Hz treated rats were considered as responders and 62.5 % as non-responders with a high restriction factor. Consequently, the non-responder subgroup presents a similar profile to that of Sham rTMS group, and the responder group shows a robust increase in phrenic motoneuronal excitability with rTMS. This meta-analysis of the data obtained in this study allows us to consider that our 10 Hz rTMS can have a different outcome as a homogenous population. One explanation could be a difference in the biological processes involved in the increase of phrenic motoneuron excitability. High frequency rTMS is also known to induce an increase in brain-derived neurotrophic factor (BDNF) expression in patients who respond to rTMS (Dall’Agnol et al., 2014; Yukimasa et al., 2006) and was also observed in rat central nervous system (Shang et al., 2016). BDNF is known to be involved in respiratory plasticity of phrenic motoneurons, such as the phrenic long-term facilitation following intermittent hypoxia in the rat (Baker-Herman et al., 2004). Conversely, several studies in humans have demonstrated that response to high frequency rTMS depends on a BDNF genotype: patients displaying the Val66Met polymorphism seem to be less sensitive to neuromodulation induced by rTMS (Cheeran et al., 2008). This however cannot be ruled out of our animal population and further studies will be needed to confirm this hypothesis. Other parameters can also be considered in this population heterogeneity. One possibility could be the anesthesia. Under urethane, the animals present different sleep-like brain states similar to rapid eye movement (REM) and non-REM sleep (Zhurakovskaya et al., 2016). Long-term facilitation, which is an increase in phrenic motoneuronal excitability following intermittent hypoxia, is greater during non-REM sleep and abolished during REM sleep (Terada and Mitchell, 2011). The sleep-like state of our animals during the 10 Hz rTMS protocol was not monitored, and even a slight change in sleep-state could affect the subsequent respiratory plasticity.
Another hypothesis supporting the increase in neuronal excitability in the responders group with 10 Hz rTMS treatment is the induction of a “local disinhibition” of the neuronal pathways (Lenz and Vlachos, 2016). One candidate involved in the inhibitory processes is GABA. In this study, GABAA (Clonazepam) and GABAB (Baclofen) agonists were chosen for their clinical relevance. These two compounds are already used in patients to reduce the neuronal excitability in several pathologies such as panic disorders or epilepsy for Clonazepam (Nardi et al., 2013; Pinder et al., 1976), or muscle spasticity for Baclofen (Chang et al., 2013). In addition, a suppression of LTP-like plasticity in motor cortex (paired associative stimulation and Baclofen oral administration) has been observed in clinical study (McDonnell et al., 2007).
The results in this study indicate a significant decrease in GABA agonists concentrations in the plasma samples of our animals at 60 min after sham or 10 Hz rTMS compared to 5 min post-injection (before sham or 10 Hz rTMS). At the dose injected into the animals, no change in phrenic motoneuronal excitability was observed in the min following injection and up to 60 min. The observed increase in MAP right after the injection is a volume compensation of the cardiovascular system. The agonists by themselves at the dose injected has a marginal effect on the cardiovascular system. Interestingly, there is a robust increase in phrenic motoneuronal excitability with the 10 Hz rTMS treatment that is abolished with either the pre-injection of Clonazepam or Baclofen. The neuromodulatory effect of 10 Hz rTMS treatment could play a role in the neuronal control of respiration through GABAergic connections. Indeed, GABAA and GABAB receptors are located on pre-phrenic interneurons. These interneurons are known to be involved in the neural control of phrenic motoneurons (Lane et al., 2008; Zaki Ghali et al., 2019), although we cannot rule out that others spinal pathways could be involved in this excitability (DiMarco and Kowalski, 2013). Nevertheless, the activation of GABAergic receptors with agonists blocks rTMS-mediated phrenic excitability, supporting “local disinhibition” theory induced by high frequency rTMS, as previously described (Benali et al., 2011; Lenz et al., 2016; Lenz and Vlachos, 2016; Levkovitz et al., 1999). In addition, 10 Hz rTMS treatment induced a reduction in the α1 subunit of the GABAA receptors in mouse central nervous system (Zhang et al., 2019), supporting the role of this inhibitory system in neuronal modulation. Conversely, GABAA (Gabazine) and GABAB (Saclofen) antagonists were used in vitro (Kerr et al., 1989; Uchida et al., 1996) and in vivo (Blandizzi et al., 1992; Gleason et al., 2009; Voisin et al., 1996; Wang et al., 2016) to induce a GABAergic disinhibition of the neural system.
5. Conclusion
In conclusion, this 10 Hz rTMS protocol has proven its efficiency to induce a significant increase in phrenic motoneuronal excitability. These results could lead to a potential application in order to try to enhance respiratory function in a preclinical rat model impacting the respiratory function, such as after a partial cervical injury or cervical contusion.
Acknowledgements
This work was supported by funding from the Chancellerie des Universités de Paris (Legs Poix) (SV, MB), the Fondation de France (SV), the Fondation Médisite (SV), INSERM (MB, SV, AM), Université de Versailles Saint-Quentin-en-Yvelines (SV, AM), the National Institutes of Health, NINDS, R01 NS104291 (MAL) and Ministry of Science and Technology109-2636-B-110-001 (KZL). The supporters had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Declaration of Competing Interest
The authors report no declarations of interest.
References
- Alexandrov VG, Ivanova TG, Alexandrova NP, 2007. Prefrontal control of respiration. J. Physiol. Pharmacol 58 (Suppl 5), 17–23. [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS, 2004. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat. Neurosci 7, 48–55. [DOI] [PubMed] [Google Scholar]
- Benadhira R, Thomas F, Bouaziz N, Braha S, Andrianisaina PS, Isaac C, Moulier V, Januel D, 2017. A randomized, sham-controlled study of maintenance rTMS for treatment-resistant depression (TRD). Psychiatry Res. 258, 226–233. [DOI] [PubMed] [Google Scholar]
- Benali A, Trippe J, Weiler E, Mix A, Petrasch-Parwez E, Girzalsky W, Eysel UT, Erdmann R, Funke K, 2011. Theta-burst transcranial magnetic stimulation alters cortical inhibition. J. Neurosci 31, 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandizzi C, Bernardini MC, Natale G, Martinotti E, Del Tacca M, 1992. Peripheral 2-hydroxy-saclofen-sensitive GABA-B receptors mediate both vagal-dependent and vagal-independent acid secretory responses in rats. J. Auton. Pharmacol 12, 149–156. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Gardner-Medwin AR, 1973. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J. Physiol 232, 357–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Lømo T, 1973. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol 232, 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E, Ghosh N, Yanni D, Lee S, Alexandru D, Mozaffar T, 2013. A review of spasticity treatments: pharmacological and interventional approaches. Crit. Rev. Phys. Rehabil. Med 25, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Houlden H, Bhatia K, Greenwood R, Rothwell JC, 2008. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J. Physiol 586, 5717–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen L, Urbin MA, Mitchell GS, Perez MA, 2018. Acute intermittent hypoxia enhances corticospinal synaptic plasticity in humans. eLife 7, e34304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Agnol L, Medeiros LF, Torres ILS, Deitos A, Brietzke A, Laste G, de Souza A, Vieira JL, Fregni F, Caumo W, 2014. Repetitive transcranial magnetic stimulation increases the corticospinal inhibition and the brain-derived neurotrophic factor in chronic myofascial pain syndrome: an explanatory double-blinded, randomized, sham-controlled trial. J. Pain 15, 845–855. [DOI] [PubMed] [Google Scholar]
- Davey NJ, Murphy K, Maskill DW, Guz A, Ellaway PH, 1996. Site of facilitation of diaphragm EMG to corticospinal stimulation during inspiration. Respir. Physiol 106, 127–135. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, 2013. Activation of inspiratory muscles via spinal cord stimulation. Respir. Physiol. Neurobiol 189, 438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Fields DP, Mitchell GS, 2015. Mammalian target of rapamycin is required for phrenic long-term facilitation following severe but not moderate acute intermittent hypoxia. J. Neurophysiol 114, 1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Hoy KE, Anderson RJ, Daskalakis ZJ, 2016. A study of the pattern of response to rTMS treatment in depression. Depress. Anxiety 33, 746–753. [DOI] [PubMed] [Google Scholar]
- Fujiki M, Yee KM, Steward O, 2020. Non-invasive high frequency repetitive Transcranial Magnetic Stimulation (hfrTMS) robustly activates molecular pathways implicated in neuronal growth and synaptic plasticity in select populations of neurons. Front. Neurosci 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Rothwell JC, 1987. Activation of the human diaphragm from the motor cortex. J. Physiol 384, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason NR, Gallos G, Zhang Y, Emala CW, 2009. The GABAA agonist muscimol attenuates induced airway constriction in guinea pigs in vivo. J. Appl. Physiol 106, 1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert D, Topolnik L, Azzi M, Huang L, Badeaux F, Desgroseillers L, Sossin WS, Lacaille JC, 2008. Forskolin induction of late-LTP and up-regulation of 5’ TOP mRNAs translation via mTOR, ERK, and PI3K in hippocampal pyramidal cells. J. Neurochem 106, 1160–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS, 2012. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J. Appl. Physiol 113, 1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendam JM, Ramakers GM, Di Lazzaro V, 2010. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 3, 95–118. [DOI] [PubMed] [Google Scholar]
- Imbert B, Alvarez J-C, Simon N, 2015. Anticraving effect of baclofen in alcohol-dependent patients. Alcohol. Clin. Exp. Res 39, 1602–1608. [DOI] [PubMed] [Google Scholar]
- Jassova K, Albrecht J, Ceresnakova S, Papezova H, Anders M, 2019. Repetitive transcranial magnetic stimulation significantly influences the eating behavior in depressive patients. Neuropsychiatr. Dis. Treat 15, 2579–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DI, Ong J, Johnston GA, Abbenante J, Prager RH, 1989. Antagonism at GABAB receptors by saclofen and related sulphonic analogues of baclofen and GABA. Neurosci. Lett 107, 239–244. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Trakhan MN, 2001. Localization of diaphragm motor cortical representation and determination of corticodiaphragmatic latencies by using magnetic stimulation in normal adult human subjects. Eur. J. Appl. Physiol 85, 560–566. [DOI] [PubMed] [Google Scholar]
- Kozel FA, 2018. Clinical repetitive transcranial magnetic stimulation for posttraumatic stress disorder, generalized anxiety disorder, and bipolar disorder. Psychiatr. Clin. North Am 41, 433–446. [DOI] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ, 2008. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J. Comp. Neurol 511, 692–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz M, Vlachos A, 2016. Releasing the cortical brake by non-invasive electromagnetic stimulation? rTMS induces LTD of GABAergic neurotransmission. Front. Neural Circuits 10, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz M, Platschek S, Priesemann V, Becker D, Willems LM, Ziemann U, Deller T, Muller-Dahlhaus F, Jedlicka P, Vlachos A, 2015. Repetitive magnetic stimulation induces plasticity of excitatory postsynapses on proximal dendrites of cultured mouse CA1 pyramidal neurons. Brain Struct. Funct 220, 3323–3337. [DOI] [PubMed] [Google Scholar]
- Lenz M, Galanis C, Muller-Dahlhaus F, Opitz A, Wierenga CJ, Szabo G, Ziemann U, Deller T, Funke K, Vlachos A, 2016. Repetitive magnetic stimulation induces plasticity of inhibitory synapses. Nat. Commun 7, 10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkovitz Y, Marx J, Grisaru N, Segal M, 1999. Long-term effects of transcranial magnetic stimulation on hippocampal reactivity to afferent stimulation. J. Neurosci 19, 3198–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Bektas A, Porter R, 1986. Short latency inputs to phrenic motoneurones from the sensorimotor cortex in the cat. Exp. Brain Res 61, 280–290. [DOI] [PubMed] [Google Scholar]
- Lissens MA, 1994. Motor evoked potentials of the human diaphragm elicited through magnetic transcranial brain stimulation. J. Neurol. Sci 124, 204–207. [DOI] [PubMed] [Google Scholar]
- Martin DM, McClintock SM, Forster JJ, Lo TY, Loo CK, 2017. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: a systematic review and meta-analysis of individual task effects. Depress. Anxiety 34, 1029–1039. [DOI] [PubMed] [Google Scholar]
- Maskill D, Murphy K, Mier A, Owen M, Guz A, 1991. Motor cortical representation of the diaphragm in man. J. Physiol 443, 105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF, Cook IA, O’Reardon J, Husain MM, Wall C, Krystal AD, Sampson SM, Morales O, Nelson BG, Latoussakis V, George MS, Lisanby SH, 2018. Consensus recommendations for the clinical application of repetitive Transcranial Magnetic Stimulation (rTMS) in the treatment of depression. J. Clin. Psychiatry 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U, 2007. Suppression of LTP-like plasticity in human motor cortex by the GABAB receptor agonist baclofen. Exp. Brain Res 180, 181–186. [DOI] [PubMed] [Google Scholar]
- Mehiri S, Straus C, Arnulf I, Attali V, Zelter M, Derenne JP, Similowski T, 2006. Responses of the diaphragm to transcranial magnetic stimulation during wake and sleep in humans. Respir. Physiol. Neurobiol 154, 406–418. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM, 2003. Invited Review: neuroplasticity in respiratory motor control. J. Appl. Physiol 94, 358–374. [DOI] [PubMed] [Google Scholar]
- Nardi AE, Machado S, Almada LF, Paes F, Silva AC, Marques RJ, Amrein R, Freire RC, Martin-Santos R, Cosci F, Hallak JE, Crippa JA, Arias-Carrion O, 2013. Clonazepam for the treatment of panic disorder. Curr. Drug Targets 14, 353–364. [DOI] [PubMed] [Google Scholar]
- Petrosyan HA, Alessi V, Sisto SA, Kaufman M, Arvanian VL, 2017. Transcranial magnetic stimulation (TMS) responses elicited in hindlimb muscles as an assessment of synaptic plasticity in spino-muscular circuitry after chronic spinal cord injury. Neurosci. Lett 642, 37–42. [DOI] [PubMed] [Google Scholar]
- Pinder RM, Brogden RN, Speight TM, Avery GS, 1976. Clonazepam: a review of its pharmacological properties and therapeutic efficacy in epilepsy. Drugs 12, 321–361. [DOI] [PubMed] [Google Scholar]
- Richard K, Pierre AG, Roumiana G, 2013. Sex, stress and their influence on respiratory regulation. Curr. Pharm. Des 19, 4471–4484. [DOI] [PubMed] [Google Scholar]
- Sebastianelli L, Versace V, Martignago S, Brigo F, Trinka E, Saltuari L, Nardone R, 2017. Low-frequency rTMS of the unaffected hemisphere in stroke patients: a systematic review. Acta Neurol. Scand 136, 585–605. [DOI] [PubMed] [Google Scholar]
- Shang Y, Wang X, Shang X, Zhang H, Liu Z, Yin T, Zhang T, 2016. Repetitive transcranial magnetic stimulation effectively facilitates spatial cognition and synaptic plasticity associated with increasing the levels of BDNF and synaptic proteins in Wistar rats. Neurobiol. Learn. Mem 134 (Pt B), 369–378. [DOI] [PubMed] [Google Scholar]
- Sharshar T, Ross E, Hopkinson NS, Dayer M, Nickol A, Lofaso F, Moxham J, Similowski T, Polkey MI, 2003. Effect of voluntary facilitation on the diaphragmatic response to transcranial magnetic stimulation. J. Appl. Physiol 95, 26–34. [DOI] [PubMed] [Google Scholar]
- Tang A, Thickbroom G, Rodger J, 2017. Repetitive transcranial magnetic stimulation of the brain: mechanisms from animal and experimental models. Neuroscientist 23, 82–94. [DOI] [PubMed] [Google Scholar]
- Terada J, Mitchell GS, 2011. Diaphragm long-term facilitation following acute intermittent hypoxia during wakefulness and sleep. J. Appl. Physiol 110, 1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF, Peever JH, Duffin J, 1998. Bötzinger-complex expiratory neurons monosynaptically inhibit phrenic motoneurons in the decerebrate rat. Exp. Brain Res 122, 149–156. [DOI] [PubMed] [Google Scholar]
- Trippe J, Mix A, Aydin-Abidin S, Funke K, Benali A, 2009. Theta burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp. Brain Res 199, 411–421. [DOI] [PubMed] [Google Scholar]
- Uchida I, Cestari IN, Yang J, 1996. The differential antagonism by bicuculline and SR95531 of pentobarbitone-induced currents in cultured hippocampal neurons. Eur. J. Pharmacol 307, 89–96. [DOI] [PubMed] [Google Scholar]
- van de Ruit M, Grey MJ, 2019. False positives associated with responder/non-responder analyses based on motor evoked potentials. Brain Stimul. 12, 314–318. [DOI] [PubMed] [Google Scholar]
- Vinit S, Keomani E, Deramaudt TB, Spruance VM, Bezdudnaya T, Lane MA, Bonay M, Petitjean M, 2014. Interdisciplinary approaches of transcranial magnetic stimulation applied to a respiratory neuronal circuitry model. PLoS One 9, e113251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Keomani E, Deramaudt TB, Bonay M, Petitjean M, 2016. Reorganization of respiratory descending pathways following cervical spinal partial section investigated by transcranial magnetic stimulation in the rat. PLoS One 11, e0148180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachos A, Muller-Dahlhaus F, Rosskopp J, Lenz M, Ziemann U, Deller T, 2012. Repetitive magnetic stimulation induces functional and structural plasticity of excitatory postsynapses in mouse organotypic hippocampal slice cultures.J. Neurosci 32, 17514–17523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin DL, Herbison AE, Chapman C, Poulain DA, 1996. Effects of central GABAB receptor modulation upon the milk ejection reflex in the rat. Neuroendocrinology 63, 368–376. [DOI] [PubMed] [Google Scholar]
- Volz LJ, Benali A, Mix A, Neubacher U, Funke K, 2013. Dose-dependence of changes in cortical protein expression induced with repeated transcranial magnetic theta-burst stimulation in the rat. Brain Stimul. 6, 598–606. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yu T, Yuan C, Yuan J, Luo Z, Pan Y, Zhang Y, Zhang Y, Yu B, 2016. Effects of propofol on the dopamine, metabolites and GABAA receptors in media prefrontal cortex in freely moving rats. Am. J. Transl. Res 8, 2301–2308. [PMC free article] [PubMed] [Google Scholar]
- Yan T, Xie Q, Zheng Z, Zou K, Wang L, 2017. Different frequency repetitive transcranial magnetic stimulation (rTMS) for posttraumatic stress disorder (PTSD): a systematic review and meta-analysis. J. Psychiatr. Res 89, 125–135. [DOI] [PubMed] [Google Scholar]
- Yip AG, George MS, Tendler A, Roth Y, Zangen A, Carpenter LL, 2017. 61% of unmedicated treatment resistant depression patients who did not respond to acute TMS treatment responded after four weeks of twice weekly deep TMS in the Brainsway pivotal trial. Brain Stimul. 10, 847–849. [DOI] [PubMed] [Google Scholar]
- Yukimasa T, Yoshimura R, Tamagawa A, Uozumi T, Shinkai K, Ueda N, Tsuji S, Nakamura J, 2006. High-frequency repetitive transcranial magnetic stimulation improves refractory depression by influencing catecholamine and brain-derived neurotrophic factors. Pharmacopsychiatry 39, 52–59. [DOI] [PubMed] [Google Scholar]
- Zaki Ghali MG, Britz G, Lee K-Z, 2019. Pre-phrenic interneurons: characterization and role in phrenic pattern formation and respiratory recovery following spinal cord injury. Respir. Physiol. Neurobiol 265, 24–31. [DOI] [PubMed] [Google Scholar]
- Zhang C, Lu R, Wang L, Yun W, Zhou X, 2019. Restraint devices for repetitive transcranial magnetic stimulation in mice and rats. Brain Behav. 9, e01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholudeva LV, Qiang L, Marchenko V, Dougherty KJ, Sakiyama-Elbert SE, Lane MA, 2018. The neuroplastic and therapeutic potential of spinal interneurons in the injured spinal cord. Trends Neurosci. 41, 625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhurakovskaya E, Paasonen J, Shatillo A, Lipponen A, Salo R, Aliev R, Tanila H, Grohn O, 2016. Global functional connectivity differences between sleep-like states in urethane anesthetized rats measured by fMRI. PLoS One 11, e0155343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, Siebner HR, Classen J, Cohen LG, Rothwell JC, 2008. Consensus: motor cortex plasticity protocols. Brain Stimul. 1, 164–182. [DOI] [PubMed] [Google Scholar]