Abstract
Intestinal microbiota have profound effects on viral infections locally and systemically. While they can directly influence enteric virus infections, there is also an increasing appreciation for the role of microbiota-derived metabolites in regulating virus infections. Because metabolites diffuse across the intestinal epithelium and enter circulation, they can influence host response to pathogens at extraintestinal sites. In this review, we summarize the effects of three types of microbiota-derived metabolites on virus infections. While short-chain fatty acids serve to regulate the extent of inflammation associated with viral infections, the flavonoid desaminotyrosine and bile acids generally regulate interferon responses. A common theme that emerges is that microbiota-derived metabolites can have proviral and antiviral effects depending on the virus in question. Understanding the molecular mechanisms by which microbiota-derived metabolites impact viral infections and the highly conditional nature of these responses should pave the way to developing novel rational antivirals.
Introduction
Gut microbiota are a vast population of microorganisms residing in the gastrointestinal tract that play a central role in maintaining metabolic and immune homeostasis. In recent years, it has become widely appreciated that these microbial communities enhance enteric viral infections through a multitude of direct and indirect mechanisms [1]. For example, bacterial surface glycans bound by an enteric virus can promote virion stabilization [2], host cell attachment and receptor binding [2,3], immune skewing in a proviral manner [4,5], and viral recombination [6]. Yet recent work in the murine norovirus (MNV) model system reveals a more nuanced situation where gut microbiota have opposing regional effects on an enteric virus, inhibiting proximal gut infection while simultaneously promoting distal gut infection [7•]. Moreover, gut microbiota also influence viral infections at extraintestinal sites where they generally have inhibitory effects instead of promoting ones [8-11]. Accumulating evidence indicates that inhibition of viral infections at local and distal sites results from microbial metabolite-mediated signaling events in contrast to the stimulatory effects of microbial constituents (e.g. LPS). The focus of this review will be the mechanisms by which microbiota-derived metabolites modulate antiviral immune responses in vivo. Understanding the molecular details of these events could pave the way to developing novel rational antivirals.
Gut microbiota-derived metabolites
It is well-established that gut microbiota shape the development and function of the host immune system. For example, host recognition of bacterial ligands via pattern recognition receptors contributes to immune homeostasis [12]. In addition, microbiota-derived metabolites continuously interact with metabolite-specific receptors and regulate immune responses [13]. Microbial metabolites segregate into three broad categories: (1) metabolites produced from dietary components, (2) metabolites derived from the host and modified by microbes, and (3) metabolites produced de novo by gut bacteria [13]. Metabolites from all three categories are produced in the gut lumen and can diffuse across the intestinal epithelium and enter circulation to access extraintestinal tissues throughout the body. Expression of metabolite-specific receptors is generally broad, enabling these small molecules to shape activities in many cell populations. Herein we will summarize the effects of microbiota-derived metabolites on viral infections as we currently understand them. These interactions are likely complex and nuanced for several reasons. First, their production is influenced by the environment of the gut lumen with a cyclical relationship between metabolite levels and environmental conditions. Second, metabolite-mediated signaling events serve to fine-tune activating and inhibitory immune responses shaped by the local microenvironment. This review is intended to serve as a framework for dissecting these nuanced interactions as they pertain to influencing viral infections as we anticipate many new examples will be uncovered in the future. The conditional nature of these responses could result in seemingly contradictory findings across research labs so it will be important for the field to recognize and report key variables regulating these effects. We will focus on the most well-characterized examples of microbiota-derived metabolites that regulate viral infections, specifically short-chain fatty acids (SCFAs), flavonoids, and bile acids.
Short-chain fatty acids
One category of dietary-derived metabolites with established effects on viral infections are SCFAs like acetate, butyrate, and propionate which are produced during bacterial fermentation of undigested carbohydrates. They are generally considered to be anti-inflammatory due to their ability to inhibit histone deacetylases (HDACs) and their activation of G protein-coupled receptors GPR41/FFAR3 and GPR43/FFAR2 [14,15]. SCFAs can regulate viruses in vitro. For example, the HDAC inhibitory activity of butyrate has been linked to reactivation of latent HIV-1 and Epstein-Barr virus (EBV) in cell culture models [16,17]. Butyrate has also recently been shown to suppress expression of IFN-stimulated genes (ISGs) in vitro, leading to increased susceptibility of cells to infection by a range of IFN-sensitive viruses [18•] whereas SCFAs had no effect on SARS-CoV-2 replication in cultured intestinal biopsies [19].
In terms of in vivo effects of SCFAs on virus infections, there is evidence that they can be both antiviral and proviral depending on the virus in question. For example, butyrate provides protection against influenza virus disease in mice by reducing the infiltration of neutrophils into the airways where they contribute to tissue damage [20] (Figure 1): Reduced neutrophil recruitment was linked to butyrate-mediated activation of FFAR3 on bone marrow cells, leading to the differentiation of monocytes predisposed to become alternatively activated macrophages (AAMs) in the lung instead of inflammatory macrophages. AAMs produce less neutrophil chemoattractant CXCL1 than inflammatory macrophages. Butyrate treatment also enhanced the CD8 T cell response to influenza virus, specifically by accelerating their metabolism and effector function [20]. These functional changes in CD8 T cells were partially FFAR3-dependent although the underlying mechanism is unclear.
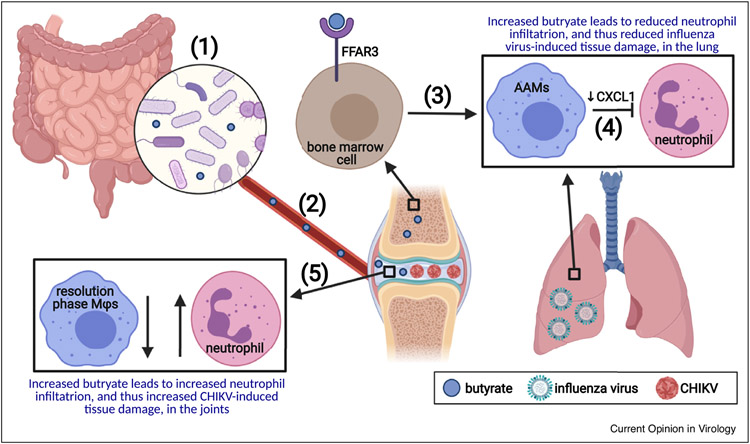
Figure 1.
The SCFA butyrate has opposing effects on inflammatory disease associated with influenza virus infection of the lung and CHIKV infection of joints.
(1) Gut microbes produce SCFAs including butyrate through fermentation of undigested carbohydrates in the intestinal lumen. (2) SCFAs enter circulation, reaching a wide range of peripheral tissues including bone and joints. (3) Butyrate promotes differentiation of bone marrow cells into monocytes predisposed to become tissue repair AAMs in a FFAR3-dependent process. (4) During influenza virus infection of the lung, mice fed a high fiber diet or supplemented with butyrate contain a higher proportion of AAMs in the lung macrophage population, resulting in reduced levels of the neutrophil chemoattractant CXCL1 and hence reduced tissue damage resulting from neutrophilia. (5) Conversely, mice fed a high fiber diet or supplemented with butyrate develop more severe inflammatory arthropathy during CHIKV infection, correlating with a reduced signature of resolution phase macrophages (Mfs) which function to prevent neutrophil infiltration and initiate tissue repair. Created with BioRender.com.
While butyrate and a high fiber diet served to blunt the inflammatory response during influenza virus infection of mice and thereby reduce disease, they exacerbated the inflammatory arthropathy caused by chikungunya virus (CHIKV) infection in a mouse model [21] (Figure 1): Both a high-fiber diet and butyrate supplementation of drinking water resulted in increased foot swelling in CHIKV-infected mice compared to controls in spite of comparable virus titers. This phenotype correlated with an increase in edema and neutrophil inflammation at the peak of arthritis although mechanisms underlying these pathologies were not determined. RNAseq-based analysis of mice fed a high fiber diet uncovered a reduced signature of resolution phase macrophages which function to prevent further neutrophil infiltration and initiate tissue repair, suggesting impaired resolution of inflammation under high-fiber conditions.
These two in vivo studies highlight the conditional nature of microbial metabolite-mediated responses: While the SCFA butyrate dampens inflammatory disease during influenza virus infection, it exacerbates immunopathologic levels of inflammation during CHIKV infection. They also underscore the immunoregulatory activity of metabolites that can only be dissected using in vivo model systems.
Flavonoids
Plant-derived polyphenol compounds, or flavonoids, can be processed by commensal bacteria to a variety of bioactive metabolites. One flavonoid degradation product, DAT, has been shown to augment the type I IFN response in the lung and protect mice from influenza virus infection [22]. Extending earlier work demonstrating that gut microbiota provide protection from severe influenza virus infections in mice [9,10], Steed et al. demonstrated that oral DAT supplementation was sufficient to protect antibiotic-treated mice from severe influenza virus disease [22]. This protection was dependent on type I IFN signaling since no protection was observed in DAT-supplemented Ifnar−/− mice. As reported for butyrate-mediated protection of mice from influenza virus disease, DAT treatment reduced tissue damage and apoptosis in the lungs of infected mice although no differences in virus titers were detected. Two lines of evidence suggest that DAT priming of the IFN response before viral infection is important for its protective effect in this model system: First, DAT treatment increased expression of multiple ISGs in the lungs even in the absence of exogenous viral infection. Second, DAT pretreatment for one week before viral infection was sufficient to protect animals while posttreatment for two days after viral infection actually exacerbated viral disease. Clodronate treatment of mice abolished DAT-mediated protection to influenza virus disease, suggesting that DAT primes phagocytes to confer this protection. DAT-mediated enhancement of ISG expression was maintained in Mavs−/− bone marrow-derived macrophages (BMDMs) but was not observed in Stat1−/− BMDMs, demonstrating a role in type I IFN signaling in contrast to induction.
Bile acids
The mammalian host converts cholesterol to the primary bile acids chenodeoxycholic acid (CDCA) and cholic acid (CA) in the liver where they are conjugated with taurine or glycine. Primary conjugated bile acids are stored in the gall bladder until ingestion of a meal, at which time they are released into the gut lumen to aid in digestion. Here they encounter commensal bacteria which deconjugate and dehydroxylate them to the secondary bile acids lithocholic acid (LCA) and deoxycholic acid (DCA) and various other modified forms. Approximately 95% of bile acids are reabsorbed in the distal ileum and transported back to the liver via enterohepatic circulation while only 5% are shed in the feces. Numerous bile acid receptors (BARs) sense bile acid pools and control a tight feedback mechanism to regulate the amounts of various bile acids within the liver and the intestine. These include nuclear receptors like farnesoid X receptor (FXR) and surface-expressed receptors like the G protein-coupled receptor TGR5, each of which interacts differentially with various bile acids [23].
In addition to regulating bile acid metabolism, BAR signaling can have profound effects on immune responses [23,24] including those elicited by viral infections (Figure 2). Bile acids have long been recognized to have proviral activity. For example, it was first reported in 1994 that high serum bile acid concentrations correlated with resistance of hepatitis C virus (HCV) patients to IFN-α treatment [25]. Bile acid treatment of HCV replicon-bearing cells increased viral replication and decreased the antiviral activity of IFN in a FXR-dependent manner, suggesting that bile acid engagement of FXR negatively regulates the IFN signaling pathway [26]. Likewise, bile acids promote replication of porcine enteric calicivirus in vitro, a proviral effect that was associated with reduced STAT-1 phosphorylation [27]. Bile acids also promote replication of human noroviruses and sapoviruses in vitro [28,29]. A recent study proposed a model for bile acid-mediated enhancement of human norovirus infection in vitro in which bile acids increase endosomal/lysosomal acidification, activating the sphingomyelinase ASM which in turn converts sphingomyelin to ceramide in host membranes; ceramide-rich microdomains could result in clustering of a surface protein that serves as the human norovirus receptor [28]. For murine noroviruses, VP1 capsid protein binding to bile salts induces a major conformational change in the virion that both increases receptor binding and infectivity [30,31] as well as blocking antibody recognition [32•]. Overall, bile acids can promote viral infections in a variety of ways ranging from suppressing antiviral immune responses to directly enhancing viral binding to host receptors.
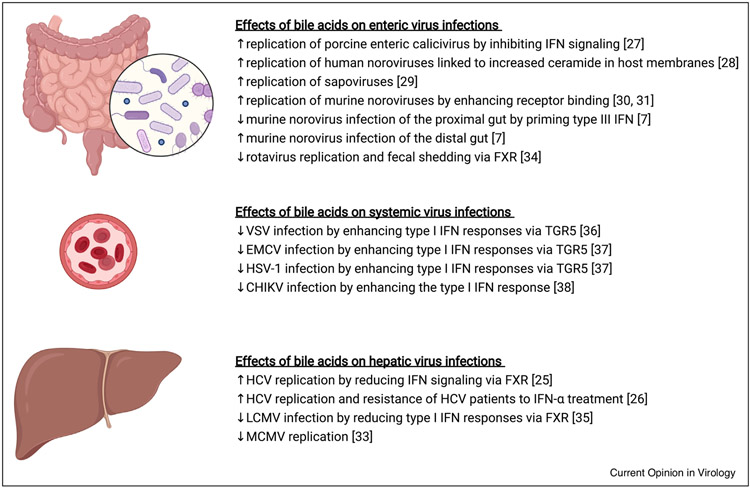
Figure 2.
Bile acids have opposing effects on virus infections in local and systemic sites.
Numerous studies have reported a role for bile acids in promoting and inhibiting viral infections, as summarized here. In general, bile acids regulate viral infections by either promoting or inhibiting IFN responses. Yet additional mechanisms are emerging, including enhancement of virus-receptor binding (e.g. murine norovirus) and increased ceramide-rich microdomains in host membranes which could cause clustering of the viral receptor (e.g. human norovirus). Created with BioRender.com.
In apparent contradiction to the studies described above, bile acids can also trigger antiviral responses. For example, bile acids reduce replication of murine cytomegalovirus (MCMV) in primary hepatocytes [33]. Interestingly, this phenotype displayed cell type specificity since MCMV replication in murine fibroblasts was unaffected by bile acid treatment. Similar to the proviral effects of bile acids, their antiviral activity has been linked to engagement of specific BARs: Bile acids and FXR agonists can reduce rotavirus replication in vitro and virus shedding in vivo [34]. Furthermore, Fxr−/− mice infected with lymphocytic choriomeningitis virus (LCMV) had higher viral loads and reduced type I IFN gene expression in their livers compared to wild-type controls [35]. TGR5 has also been linked to promotion of type I IFN responses. Xiong et al. discovered that TGR5 is an ISG since its expression was enhanced in response to viral infection and exogenous IFN-β treatment in a STAT1-dependent manner [36]. Moreover, TGR5 overexpression in macrophages inhibited replication of a variety of viruses including vesicular stomatitis virus (VSV), New-castle disease virus, and herpes simplex virus 1 (HSV-1) while bile acid or TGR5 agonist treatment potentiated IFN expression and blocked viral replication [36,37]. Mechanistically, two studies have reported that TGR5 promotes IRF3 phosphorylation although Xiong et al. discovered a key role for AKT in TGR5-dependent IRF3 activation [36] while Hu et al. discovered a key role for the nonreceptor tyrosine kinase SRC and downstream phosphorylation of multiple signaling components in the IFN induction pathway, including RIG-1, MAVS, STING, TBK1, and IRF3 [37]. The in vivo relevance of TGR5-mediated IFN enhancement has been demonstrated by reduced serum IFN-β and IFN-α levels, increased viral loads and reduced survival in Tgr5−/− mice infected with EMCV, VSV, or HSV-1 compared to wild-type controls [36,37]. Furthermore, injection of wild-type, but not Tgr5−/−, mice with the bile acid CDCA was sufficient to increase serum IFN-β, reduce viral loads, and increase survival following HSV-1 infection [37]. Collectively, these studies reveal a key role for the BAR TGR5 in potentiating type I IFN responses during viral infections.
A general theme emerges from this collective body of work that bile acid engagement of BARs can regulate type I IFN responses to either promote or inhibit a range of enteric and hepatic viruses. Several recent studies provide additional context to the relationship between bile acids, IFN, and viral infections. First, Grau et al. discovered that bile acids can promote type III IFN expression and that this response is necessary for the control of murine norovirus infection the proximal small intestine [7•]: Antibiotic treatment (which dramatically reduces levels of unconjugated and secondary bile acids in the intestine due to the depletion of bile acid-biotransforming bacteria) and type III IFN deficiency both resulted in significantly increased viral titers in the proximal small intestine of infected mice. Control of viral infection could be rescued by reconstitution of antibiotic-treated mice with Clostridium scindens, a bacteria with potent bile-acid biotransforming activity, or by oral supplementation with bile acids. Importantly, bile acid supplementation failed to reduce virus titers in Ifnlr1−/− mice, providing a direct link between bile acids and type III IFN (unpublished results). Furthermore, bile acid treatment of cells enhanced type III IFN expression in response to poly(I:C) or murine norovirus infection [7•]. Although the mechanism by which bile acids prime type III IFN remains unresolved, type I and type III induction and signaling pathways are largely overlapping so it would not be surprising if bile acid regulation occurs in a similar manner. Second, Winkler et al. revealed that bile acids can mediate effects on viruses outside of the intestine and liver by demonstrating bile acid-mediated inhibition of circulating Chikungunya virus (CHIKV) [38•]: Similar to findings of Grau et al. for murine norovirus, CHIKV titers were higher in antibiotic-treated mice than in controls and this phenotype could be rescued by colonization with C. scindens or oral bile acid supplementation. Reduced viral titers in the serum of C. scindens-colonized or bile acid-supplemented mice was associated with increased type I IFN levels and was dependent on STAT-1 and MyD88. Third, highlighting the conditional nature of the effect of microbiota-derived metabolites on viral infections, Grau et al. revealed that bile acids play regionally opposing roles during murine norovirus infection of the intestinal tract [7•]: While bile acids prime type III IFN to inhibit infection of the proximal small intestine, they instead enhance murine norovirus infection of distal regions of the gut likely by promoting viral binding to the host receptor CD300lf [30]. It will be fascinating to unravel the regional nature of these interactions.
Conclusions
Over the past decade it has been clearly established that intestinal microbiota have profound effects on viral infections, both locally along the intestinal tract and systemically. While commensal bacteria in the gut lumen can directly influence enteric virus infections in a multitude of ways, there is also an increasing appreciation for the role of microbiota-derived metabolites in regulating virus infections. Because metabolites diffuse across the intestinal epithelium and enter circulation, they can influence host response to pathogens at extraintestinal sites. In this review, we summarize the effects of three types of microbiota-derived metabolites — short-chain fatty acids (SCFAs), flavonoids, and bile acids — on virus infections. While SCFAs serve to regulate the extent of inflammation associated with viral infections, the flavonoid desaminotyrosine and bile acids commonly regulate interferon responses. A common theme that emerges is that individual microbiota-derived metabolites can have proviral and antiviral effects depending on the model system and virus in question. Understanding the molecular mechanisms by which microbiota-derived metabolites impact viral infections and the highly conditional nature of these responses should pave the way to developing novel rational antivirals.
Acknowledgement
This work was funded by N.I.H.1R01AI116892 and 1R01AI141478.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
- 1.Karst SM: The influence of commensal bacteria on infection with enteric viruses. Nat Rev Microbiol 2016, 14:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson CM, Jesudhasan PR, Pfeiffer JK: Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe 2014, 15:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, lovine NM, Wobus CE, Vinjé J et al. : Enteric bacteria promote human and murine norovirus infection of B cells. Science 2014, 346:755–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kane M, Case LK, Kopaskie K, Kozlova A, MacDearmid C, Chervonsky AV, Golovkina TV: Successful transmission of a retrovirus depends on the commensal microbiota. Science 2011, 334:245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilks J, Lien E, Jacobson AN, Fischbach MA, Qureshi N, Chervonsky AV, Golovkina TV: Mammalian lipopolysaccharide receptors incorporated into the retroviral envelope augment virus transmission. Cell Host Microbe 2015, 18:456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson AK, Jesudhasan PR, Mayer MJ, Narbad A, Winter SE, Pfeiffer JK: Bacteria facilitate enteric virus co-infection of mammalian cells and promote genetic recombination. Cell Host Microbe 2018, 23:77–88.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. •. Grau KR, Zhu S, Peterson ST, Helm EW, Philip D, Phillips M, Hernandez A, Turula H, Frasse P, Graziano VR et al. : The intestinal regionalization of acute norovirus infection is regulated by the microbiota via bile acid-mediated priming of type III interferon. Nat Microbiol 2020, 5:84–92 This study reveals that a single class of bacterial metabolites, namely bile acids, have opposing regional effects on an enteric virus.
- 8.Thackray LB, Handley SA, Gorman MJ, Poddar S, Bagadia P, Briseño CG, Theisen DJ, Tan Q, Hykes BL, Lin H et al. : Oral antibiotic treatment of mice exacerbates the disease severity of multiple flavivirus infections. Cell Rep 2018, 22:3440–3453.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J et al. : Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012, 37:158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A: Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A 2011, 108:5354–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S, Jiang Z-Y, Sun Y-F, Yu B, Chen J, Dai C-Q, Wu X-L, Tang X-L, Chen X-Y: Microbiota regulates the TLR7 signaling pathway against respiratory tract influenza A virus infection. Curr Microbiol 2013, 67:414–422. [DOI] [PubMed] [Google Scholar]
- 12.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R: Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118:229–241. [DOI] [PubMed] [Google Scholar]
- 13.Yang W, Cong Y: Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol Immunol 2021, 18:866–877 10.1038/s41423-021-00661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sealy L, Chalkley R: The effect of sodium butyrate on histone modification. Cell 1978, 14:115–121. [DOI] [PubMed] [Google Scholar]
- 15.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D et al. : Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461:1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imai K, Ochiai K, Okamoto T: Reactivation of latent HIV-1 infection by the periodontopathic bacterium Porphyromonas gingivalis involves histone modification. J Immunol Baltim Md 1950 2009, 182:3688–3695. [DOI] [PubMed] [Google Scholar]
- 17.Imai K, Inoue H, Tamura M, Cueno ME, Inoue H, Takeichi O, Kusama K, Saito I, Ochiai K: The periodontal pathogen Porphyromonas gingivalis induces the Epstein-Barr virus lytic switch transactivator ZEBRA by histone modification. Biochimie 2012, 94:839–846. [DOI] [PubMed] [Google Scholar]
- 18. •. Chemudupati M, Kenney AD, Smith AC, Fillinger RJ, Zhang L, Zani A, Liu S-L, Anderson MZ, Sharma A, Yount JS: Butyrate reprograms expression of specific interferon-stimulated genes. J Virol 2020, 94 A bacterial metabolite suppresses the antiviral immune response.
- 19.Pascoal LB, Rodrigues PB, Genaro LM, Dos Santos Pereira Gomes AB, Toledo-Teixeira DA, Parise PL, Bispo-Dos-Santos K, Simeoni CL, Guimarães PV, Buscaratti LI et al. : Microbiota-derived short-chain fatty acids do not interfere with SARS-CoV-2 infection of human colonic samples. Gut Microbes 2021, 13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trompette A, Gollwitzer ES, Pattaroni C, Lopez-Mejia IC, Riva E, Pernot J, Ubags N, Fajas L, Nicod LP, Marsland BJ: Dietary fiber confers protection against flu by shaping Ly6c-patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity 2018, 48:992–1005.e8. [DOI] [PubMed] [Google Scholar]
- 21.Prow NA, Hirata TDC, Tang B, Larcher T, Mukhopadhyay P, Alves TL, Le TT, Gardner J, Poo YS, Nakayama E et al. : Exacerbation of chikungunya virus rheumatic immunopathology by a high fiber diet and butyrate. Front Immunol 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steed AL, Christophi GP, Kaiko GE, Sun L, Goodwin VM, Jain U, Esaulova E, Artyomov MN, Morales DJ, Holtzman MJ et al. : The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017, 357:498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiorucci S, Distrutti E: Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol Med 2015, 21:702–714. [DOI] [PubMed] [Google Scholar]
- 24.Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S: The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol 2009, 183:6251–6261. [DOI] [PubMed] [Google Scholar]
- 25.Serfaty L, Giral P, Loria A, Andréani T, Legendre C, Poupon R: Factors predictive of the response to interferon in patients with chronic hepatitis C. J Hepatol 1994, 21:12–17. [DOI] [PubMed] [Google Scholar]
- 26.Chang K-O, George DW: Bile acids promote the expression of hepatitis C virus in replicon-harboring cells. J Virol 2007, 81:9633–9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang K-O, Sosnovtsev SV, Belliot G, Kim Y, Saif LJ, Green KY: Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc Natl Acad Sci U S A 2004, 101:8733–8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murakami K, Tenge VR, Karandikar UC, Lin S-C, Ramani S, Ettayebi K, Crawford SE, Zeng X-L, Neill FH, Ayyar BV et al. : Bile acids and ceramide overcome the entry restriction for GII.3 human norovirus replication in human intestinal enteroids. Proc Natl Acad Sci USA 2020, 117:1700–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takagi H, Oka T, Shimoike T, Saito H, Kobayashi T, Takahashi T, Tatsumi C, Kataoka M, Wang Q, Saif LJ et al. : Human sapovirus propagation in human cell lines supplemented with bile acids. Proc Natl Acad Sci U S A 2020, 117:32078–32085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson CA, Wilen CB, Dai Y-N, Orchard RC, Kim AS, Stegeman RA, Hsieh LL, Smith TJ, Virgin HW, Fremont DH: Structural basis for murine norovirus engagement of bile acids and the CD300lf receptor. Proc Natl Acad Sci U S A 2018, 115:E9201–E9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherman MB, Williams AN, Smith HQ, Nelson C, Wilen CB, Fremont DH, Virgin HW, Smith TJ: Bile salts alter the mouse norovirus capsid conformation: possible implications for cell attachment and immune evasion. J Virol 2019, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. •. Williams AN, Sherman MB, Smith HQ, Taube S, Pettitt BM, Wobus CE, Smith TJ: A norovirus uses bile salts to escape antibody recognition while enhancing receptor binding. J Virol 2021. 10.1128/JVI.00176-21. Online ahead of print Virus binding to bacterial metabolites results in a conformational change in the virion that facilitates receptor binding while simultaneously preventing antibody neutralization.
- 33.Schupp A-K, Trilling M, Rattay S, Le-Trilling VTK, Haselow K, Stindt J, Zimmermann A, Häussinger D, Hengel H, Graf D: Bile acids act as soluble host restriction factors limiting cytomegalovirus replication in hepatocytes. J Virol 2016, 90:6686–6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y, Chang K-O: Inhibitory effects of bile acids and synthetic farnesoid X receptor agonists on rotavirus replication. J Virol 2011, 85:12570–12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honke N, Shaabani N, Hardt C, Krings C, Häussinger D, Lang PA, Keitel V, Lang KS: Farnesoid X receptor in mice prevents severe liver immunopathology during lymphocytic choriomeningitis virus infection. Cell Physiol Biochem 2017, 41:323–338. [DOI] [PubMed] [Google Scholar]
- 36.Xiong Q, Huang H, Wang N, Chen R, Chen N, Han H, Wang Q, Siwko S, Liu M, Qian M et al. : Metabolite-sensing G protein coupled receptor TGR5 protects host from viral infection through amplifying type I interferon responses. Front Immunol 2018, 9:2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu M-M, He W-R, Gao P, Yang Q, He K, Cao L-B, Li S, Feng Y-Q, Shu H-B: Virus-induced accumulation of intracellular bile acids activates the TGR5-β-arrestin-SRC axis to enable innate antiviral immunity. Cell Res 2019, 29:193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. •. Winkler ES, Shrihari S, Hykes BL, Handley SA, Andhey PS, Huang Y-JS, Swain A, Droit L, Chebrolu KK, Mack M et al. : The intestinal microbiome restricts alphavirus infection and dissemination through a bile acid-type I IFN signaling axis. Cell 2020, 182:901–918.e18 Microbiota-derived metabolites, namely bile acids, restrict alphavirus infection by promoting the type I IFN response in an extraintestinal manner.