Abstract
Objective:
The primary purpose of the study was to determine whether Multisystemic Therapy adapted for health care settings (MST-HC) improved asthma management and health outcomes in high-risk African American adolescents with asthma.
Method:
Eligibility included self-reported African American ethnicity, age 12 to 16, moderate to severe asthma, and an inpatient hospitalization or at least two emergency department visits for asthma in the last 12 months. Adolescents and their families (N=170) were randomized to MST-HC or in-home family support (FS). Data were collected at baseline and post-treatment (7 months) including an asthma management interview, medication adherence phone diary, and lung function biomarker (FEV1). Analyses were conducted using linear mixed modeling for continuous outcomes and generalized linear mixed modeling for binary outcomes.
Results:
In intent-to-treat analyses, adolescents randomized to MST-HC were more likely to improve on two the measures of medication adherence and FEV1. Per protocol analysis demonstrated that MST-HC had a medium effect on adherence measures, and had a small to medium effect on lung function and the adolescent’s response to asthma exacerbations.
Conclusion:
There are few interventions that have been shown to successfully improve asthma management in minority youth at highest risk for poor morbidity and mortality. MST, a home-based psychotherapy originally developed to target behavior problems in youth, improved asthma management and lung function compared to a strong comparison condition. Further follow-up is necessary to determine whether MST-HC reduces health care utilization accounting for seasonal variability. A limitation to the study is that there was a greater number of participants in the control group coming from single parent families than in the MST group.
Keywords: Multisystemic Therapy, Asthma, Adolescents, Health Disparities
Asthma is the most common cause of hospitalization in children other than infections. Pediatric asthma accounts for more missed school days than any other chronic condition Centers for Disease Control and Prevention (CDC, 2011, 2013a, 2013b). Although rates of childhood asthma are increasing worldwide (World Health Organization, 2011) there has been a disproportionate and alarming rise in rates of asthma among urban, disadvantaged, minority children (Agency for Healthcare Research and Quality; AHRQ, 2011; CDC, 2011, 2012, 2013b). Inner city children, and adolescents in particular, appear to be most at risk for morbidity and mortality as a result of asthma (CDC, 2010; 2013b). Rates of hospitalizations and emergency department visits for asthma care are also high in this group (AHRQ, 2011; CDC, 2013b).
Poor asthma management is thought to be a major driver of asthma morbidity and mortality (Braman, 2006; Bruzzese et al., 2012; Gustafsson, Watson, Davis, & Rabe, 2006; Rabe et al., 2004). African American children and adolescents appear to be at highest risk for poor adherence to a variety of asthma management tasks (Drotar & Bonner, 2009), including adherence to asthma controller medications (medications prescribed daily to prevent asthma exacerbations) and responding to asthma symptoms (usually with quick relief inhalers) (McDaniel & Waldfogel, 2012; Rohan et al., 2010). Therefore, improving illness management may be one way to improve health outcomes among African American adolescents with asthma and reduce asthma-related health disparities.
Social-ecological theory provides a guiding framework (Bronfenbrenner, 1979) for conceptualizing the multiple factors involved in poor illness management (Brown, 2002; Burgess, Sly, & Devadason, 2011; Naar-King, Podolski, Ellis, & Frey, 2006; Yinusa-Nyahkoon, Cohn, Cortes, & Bokhour, 2010). Social-ecological theory posits that complex problem behaviors, such as poor illness management, are multiply determined and reflect difficulties within many systems in which the child and family are embedded. Extra-familial systems such as school, peers, and community institutions such as the health care system are seen as interconnected with the individual and his or her family and therefore as also affecting illness management.
Because of these multiple factors that affect whether or not the adolescent is able to adequately manage asthma, educational interventions alone are typically insufficient to improve asthma management and health outcomes, (Clark, Griffiths, Keteyian, & Partridge, 2010; Crocker et al., 2011; Kahana, Drotar, & Frazier, 2008). This may be particularly true within urban minority populations of youth with asthma who have high rates of health care utilization such as emergency department (ED) and inpatient visits. Not only are these markers of asthma morbidity, but they are also a major driver of health care costs. More intensive, multi-component interventions may be necessary to improve asthma management and improve outcomes for such high-risk youth with asthma. Despite this, there are limited studies investigating such multi-component interventions, and few have exclusively targeted minority adolescents with asthma (AHRQ, 2007; Rhee, Belyea, Hunt, & Brasch, 2011). Only one study to date has targeted minority youth with moderate to severe asthma (requiring daily controller medications). Bruzzese et al. (2011) found that a multi-session, school-based intervention with a physician education component improved the percentage of urban minority youth who were prescribed asthma controller medications. However rates of adherence to these controller medications were not formally assessed. In addition, this study did not focus upon high-risk minority youth with multiple admissions or ED visits.
The purpose of this study was to test whether Multisystemic Therapy (MST) (Henggeler, Schoenwald, & Borduin, 2009), a home and community-based family therapy grounded in the social ecological model, could improve asthma management (particularly adherence to daily controller medications and responding to asthma exacerbations) and lung functioning in high-risk urban adolescents with moderate to severe persistent asthma. MST was originally developed and empirically validated for the treatment of severe behavior problems in youth and has been successfully adapted as MST-HC (MST Health Care) to target severe problems with illness management in adolescents with chronic medical conditions. In particular, MST-HC has been shown to improve health outcomes in youth with type 1 diabetes (Ellis et al., 2012), with HIV (Ellis, Naar-King, Cunningham, & Secord, 2006; Letourneau et al., 2012), and with obesity (Naar-King, Ellis, Kolmodin, Cunningham, Jen, et al., 2009). We hypothesized that high-risk African American adolescents with poorly controlled asthma would show greater improvements in asthma management and lung functioning when receiving MST-HC compared to in-home family support (FS).
Method
Participants
The study was a randomized trial comparing MST-HC to FS (comparison condition) to improve asthma management in high-risk African American youth. In order to be eligible, adolescents had to be between 12 years, 0 months and 16 years, 11 months old, to be diagnosed with moderate to severe persistent asthma, to self-identify as African American and be residing in a home setting (e.g. not in residential treatment) with a caregiver who was willing to participate in treatment. Having moderate to severe persistent asthma ensured that enrolled participants would be expected to be prescribed a daily asthma controller medication based on national standards of care (National Heart Lung & Blood Institute, 2007). High-risk was defined as having at least one asthma-related hospitalization or at least two asthma-related emergency department (ED) visits in the last 12 months at an urban children’s hospital. Exclusion criteria included thought disorder, suicidality, mental retardation, having another chronic health condition, or unable to complete assessments or interventions in English.
See Figure 1 for Consort Diagram showing participant flow through the study. Medical record review and direct contact with clinical staff identified 399 patients who were hospitalized or admitted to the ED twice in one year for asthma. Of these, 196 were contacted by research staff to further screen for eligibility. Twelve refused to be screened, and 1 refused to participate in the study after screening (Refusal rate = 7% refusal). One family did not meet eligibility criteria, and 12 families could not be reached between screening and consent. A total of 170 families consented and completed baseline data collection (87% recruitment rate). Eighty-four were randomized to MST-HC and 86 to FS. Two families randomized to FS were removed from the study due to safety concerns that developed during treatment that interfered with the delivery of a home-based intervention; and another family was removed when it was discovered that they did not meet study eligibility criteria. Thus the final analyzed sample was 167 (84 in MST-HC and 83 in FS). In MST, 85% of families received the allocated intervention [at least 3 sessions; (Ellis et al., 2005)]. In FS, 71% received the allocated intervention.
Figure 1.
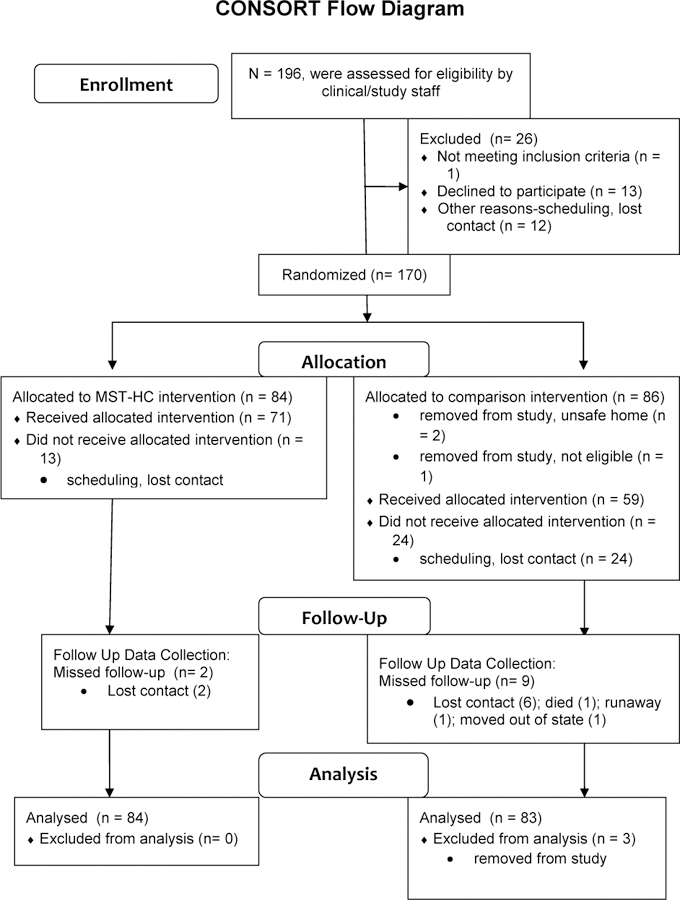
Consort Diagram showing participant flow through the study.
Procedure
The study was approved by the university’s Internal Review Board. Participants were initially approached in person by medical staff at the time of a regularly scheduled visit to a university-affiliated pediatric asthma clinic or during an inpatient hospitalization that described the study or informed of the study by letters sent to their homes. Letters and staff contacts were followed up by phone contacts from study research staff; home-based consent visits were subsequently conducted if families indicated an interest in participating. Baseline data collection, including spirometry, subsequently occurred in the home by trained research assistants. All data collectors were blind to the participant’s study condition. Post-test data collection took place seven months after baseline data collection. Families were provided $50 to compensate them for participating in each data collection session. Randomization was stratified based on 1) severity of asthma complications as indicated by number of recent hospitalizations or ED visits (three or more hospitalizations/ED visits in the previous 12 months versus zero to two hospitalizations/ED visits) and 2) receipt of asthma specialty care (visit to hospital-based multidisciplinary asthma specialty clinic in the last 13 months or not).
Multisystemic Therapy – Health Care (MST-HC).
Adolescents assigned to the intervention condition received MST-HC as adapted for the treatment of poor self-management in youth with asthma (Naar-King, Ellis, Kolmodin, Cunningham, & Secord, 2009). MST includes several key features: (a) A comprehensive set of identified risk factors (e.g., across individual, family, peer, school, and neighborhood domains) associated with the problem behavior is targeted through interventions that are individualized for each adolescent; (b) These interventions integrate empirically-based clinical treatments (e.g., cognitive–behavioral therapy), which historically have been used to focus on a limited aspect of the adolescent’s social ecology (typically only the individual adolescent or at most the adolescent and family), into a broad-based ecological framework that addresses relevant risk factors across family, school, and community contexts; (c) Interventions focus on promoting behavioral changes in the adolescent’s natural ecology by empowering caregivers with skills and resources to address difficulties inherent in raising adolescents, and empowering adolescents to cope with medical, family, school, and neighborhood problems; (d) Services are delivered via a home-based model, which facilitates high engagement and low dropout rate and are delivered in home, school, and/or neighborhood settings at times convenient to the family; and (e), MST includes an intensive quality assurance system that aims to optimize youth outcomes by supporting therapist fidelity to MST treatment principles (Henggeler et al., 2006).
Although it is well-specified and operationalized using MST treatment principles, MST is not a typical, manualized “one size fits all” intervention where the therapist follows a set of pre-arranged tasks in a time-limited sequence. Instead, MST-HC therapists began with an initial multisystemic assessment designed to identify the strengths and weaknesses of the adolescent, the family, and their transactions with extra-familial systems (e.g., peers, school, community, medical treatment team). A functional assessment of non-adherent behavior, using interviews and in-vivo observations) was used to identify setting events, antecedents and consequences of inadequate asthma management across the family, peer, school and community settings. Based upon this assessment, the MST-HC therapist chooses from a menu of evidence-based interventions that best treat the identified problem behaviors (e.g. underuse of preventive medications, poor identification of asthma triggers, not carrying rescue medications at all times) and their particular causes in each family. The MST-HC therapist provided treatment to families and their related contacts (extended family members, school personnel, medical team contacts), with the number of sessions per week dependent upon clinical need. MST sessions could take place several times per week (or day) initially and then only weekly once the adolescent’s asthma management had improved. MST-HC treatment goals identified conjointly by family members and the MST-HC therapist during the assessment phase were explicitly targeted for change during the treatment phase. For the proposed study, treatment goals were typically illness management-related (e.g. “takes 90% of controller medications based on medication counts”, “carries inhaler when out of the home”).
MST-HC interventions targeted asthma management problems within the family system, peer network, and the broader community systems within which the family was embedded. MST-HC therapists drew upon a menu of evidence-based intervention techniques that included cognitive-behavioral therapy, behavior therapy, parent training and behavioral family systems therapy. Individual interventions with adolescents included addressing asthma knowledge deficits or skills deficits such as improper use of inhalers. Family interventions in MST-HC include introducing systematic monitoring, reward, and discipline systems in order to decrease caregiver disengagement from the asthma regimen, developing family organizational routines such as regular controller medication administration times, and helping caregivers to communicate effectively with each other about asthma care and avoidance of triggers. School interventions included helping caregivers improve communication strategies with school personnel such as teachers, counselors and school nurses regarding their child’s asthma care needs, and increasing the accessibility of medications to youth while in school (e.g. ensuring youth could carry their inhaler rather than keeping it in the school office). Interventions within the health care system were also critical and included helping the family resolve barriers to keeping medical appointments and promoting positive family- physician communication and relationships. Families who indicated that they had no regular asthma care provider or were interested in making changes in their asthma care were assisted in accessing asthma care as part of the intervention. Based on our previous MST-HC trials, treatment was planned to last for six months. Mean length of treatment, excluding drop-outs, was 5.14 months (SD=1.25) and mean number of sessions was 27.09 (SD = 12.03; range 4 to 62 sessions). MST-HC was provided by four masters-level therapists with varied backgrounds (one psychologist, three social workers). Three therapists were African-American and one was White.
Family Support (FS).
Families randomized to the comparison condition received weekly, home-based, client-centered, non-directive supportive family counseling. Home-based delivery of services was chosen so as to avoid inequity of treatment dose due to ease of access to services (e.g. home versus office). The comparison condition was intended to control for improvement due to non-MST specific treatment factors such as positive family expectancies due to entering treatment, receiving positive regard and encouragement for completing asthma care from therapists, and providing family members with opportunities to discuss asthma care. Therefore, the weekly visits had three goals: 1) to provide empathic support to the youth and caregivers regarding the adolescent’s asthma and related care needs 2) to provide the family with opportunities to discuss barriers they identified to the completion of asthma care, and 3) to discuss the availability of supports to help the family with asthma management. Non-asthma related problems such as family relationship problems could also be discussed during the visits if requested by the family. Therapists accomplished these goals by providing Rogerian, client-centered, non-directive counseling (Rogers, 1951). This counseling emphasizes empathic and reflective listening in order to facilitate growth that stems from within the individual. In order to provide support in the areas that were most difficult for the family, therapists began each session by asking open-ended questions regarding asthma management during the prior week. Youth completed a checklist of their asthma symptoms (if any) during the prior week to guide the conversation. Family members were then verbally reinforced for what was going well; when barriers to care were raised, the therapist did not address these concerns with skills building or problem solving interventions, but rather supported the family to come up with their own ideas regarding ways to address such challenges.
The FS intervention was six months in length and hence was matched to MST-HC for length of treatment. Since MST session dose is flexible, matching the control condition for dose was not possible. However, an approximate dose of weekly 45-minute session consistent with traditional outpatient therapy approaches (and therefore with what would be provided in a real-world setting) was chosen. Mean length of treatment, excluding drop-outs, was 4.20 months (SD = 1.78) and mean number of sessions was 11.03 (SD = 5.74; range 3 to 24). FS was provided by six masters-level clinicians and one bachelor’s level clinician. Five therapists were African-American and two were White.
Treatment Fidelity.
In order to promote fidelity to the MST-HC model, state-of-the-art quality assurance protocols were used that included an initial five day training, weekly on-site clinical supervision from a Ph.D. level supervisor with an extensive background with MST-HC, weekly phone consultation with an MST expert with experience with the application of MST to chronic health conditions, and quarterly booster trainings. The initial standard MST five-day orientation training was adapted by the research team to include formal asthma education for MST therapists as well as education regarding factors that are predictive of poor treatment adherence and symptom exacerbations among adolescents with asthma. MST therapists were trained to have sufficient knowledge regarding asthma to enable them to conduct asthma adherence interventions with families (e.g. methods of environmental control, differences between use of rescue and controller medications, using asthma action plans for symptom management, etc.). Quality assurance protocols also included use of a manual on use of MST-HC with youth with high-risk asthma developed during a feasibility trial (Naar-King, Ellis, Kolmodin, Cunningham, & Secord, 2009), and feedback on therapist and supervisor fidelity to MST procedures (Henggeler & Schoenwald, 1998; Schoenwald, 1998). All sessions were audio-recorded, and independent coders rated one randomly selected session per month per therapist using the MST code scheme (Huey, 2001) adapted for MST-HC (Ellis, Naar-King, Templin, Frey, & Cunningham, 2007).
For the FS condition, quality assurance protocols included a detailed manual, an initial three-day training, and a minimum of bi-weekly on-site clinical supervision from a Ph.D.-level supervisor with experience with Rogerian psychotherapy. All sessions were audio-recorded, and supervisors reviewed one tape per month. To ensure that elements of MST-HC were not present in the comparison condition, 15 FS tapes (approximately one per quarter during the active intervention phase) were randomly selected and coded by trained MST coders who were blind to treatment condition.
Measures
Asthma Management.
The Family Asthma Management System Scale (FAMSS) (McQuaid, Walders, Kopel, Fritz, & Klinnert, 2005) is a clinical interview completed conjointly with caregivers and teens. Questions are open ended and the interviewer must resolve any discrepancies between the reporters by making standardized judgments regarding degree of asthma management on a 9-point scale (1=poor management, 9=excellent management). The interview takes approximately 45 minutes to complete. The measure has been found to be correlated with objective measures of asthma management such as electronic monitors and accounted for a significant percentage of variance in asthma morbidity in a sample of youth ages 7 to 17 (McQuaid et al., 2005), and has demonstrated validity and sensitivity to intervention effects in low income children (M. Celano, Klinnert, Holsey, & McQuaid, 2011; M. P. Celano, Holsey, & Kobrynski, 2012). Scale developers trained study raters, and inter-rater reliability between raters and scale developers was high (ICC=.933). Three illness management-specific subscales, Medication Adherence, Child Response to Symptoms and Exacerbations, and Family Response to Symptoms and Exacerbations, were used in the present study and have been shown to be related to objective measures of medication adherence and asthma morbidity (McQuaid et al., 2005).
Adherence to daily corticosteroid medication (i.e. controller medication) was also assessed with the Daily Phone Diary (Modi & Quittner, 2006), a cued recall procedure that collects information about participants’ activities, companions, and moods during the previous 24 hours. Adolescents completed two DPD assessments within a two-week period. For all activities lasting five minutes or more, participants reported the type of activity, duration, and who was present. Interviewers assisted participants in reconstructing their day as accurately as possible by providing prompts such as time of day or information about the previous activity (e.g., “After you finished dinner, what did you do next?”). For the current study, information from the two DPD assessments was combined to determine use of controller medication in either of the 24-hour periods (1 = participant took controller medication on at least one of the two days, 0 = participant did not take controller medication on either day).
Lung Functioning.
Pulmonary functioning was assessed using forced expiratory maneuvers obtained using a portable calibrated recording spirometer (KoKo ®) at the time of the research interview. Forced expiratory volume in one second (FEV1) provides reliable and reproducible information about airflow in health and disease and has been found to correlate with clinical outcomes (Knudson, Kaltenborn, Knudson, & Burrows, 1987). While standing, the subject was encouraged to perform between 5 and 8 maneuvers to obtain 3 acceptable tracings. Forced Expiratory Volume in one second (FEV-1) was measured. All research assistants were trained by the KoKo ® spirometry’s training specialist. Then the asthma specialty clinic’s respiratory therapist evaluated all research assistants performing spirometry measurements based on ATS standards.
Statistical Analyses
Using t-tests for continuous variables and chi-square tests for categorical variables, demographic and other baseline variables were compared between the treatment and control groups, and between those who completed the 7-month follow-up assessment and those who did not. The primary outcomes at the follow-up were analyzed for both the intent-to-treat sample (all randomized participants) and per-protocol sample (participants who received a pre-defined minimum dose of treatment) Methodologists have increasingly argued that adopting intent-to-treat approaches as the sole analytical strategy can ignore valuable information available in the other strata of participants (Amico, 2009). Instead, a “profile approach” for outcomes addresses the impact of offering the intervention program within a given existing service (intent-to-treat) as well as the impact of the intervention when received. Thus, intent-to-treat analyses included all participants who were randomized into either the MST-HC group (n=84) or the FS group (n=83) and not removed during the trial, and per-protocol analyses included participants in MST-HC (n=71) and FS (n=59) groups who received the minimum treatment dosage.
Analyses were conducted using linear mixed modeling for continuous outcomes (SAS PROC MIXED) and generalized linear mixed modeling for binary outcomes (i.e. DPD; PROC GLIMMMIX). Each of the mixed models controlled for gender, age at baseline, family income, number of treatment sessions, and single-parent household, and examined the effects of time, treatment group, and the time by treatment group interaction. Models predicting FEV1 also controlled for adolescents’ height. Missing data at follow-up were accounted for in the mixed effects models using maximum likelihood estimation (13.25% in MST, 2.38% in Control).
Results
Study Retention
At follow-up, 92.22% of the sample (n=154) completed assessments. Participants in FS were more likely than those in the MST group to have missed the seven-month follow-up (χ2 = 6.87, p < .01). Participants who completed the follow-up assessment did not differ from non-completers on baseline demographics, asthma management measures, or FEV1 (an indicator of lung functioning) (p > .05).
Baseline Data
Descriptive statistics for the MST-HC and FS groups are presented in Table 1. Adolescents in the FS group were more likely than those in the MST-HC group to live in single-parent households (χ2 = 4.66, p = .03).
Table 1.
Baseline Characteristics of Adolescents Randomized to MST and Control Groups
| Characteristics | Control (n =83) | MST (n =84) | |||
|---|---|---|---|---|---|
|
| |||||
| n | % | n | % | ||
|
|
|
||||
| Gender (% Female) | 32 | 0.38 | 33 | 39.29 | |
| Single-parent household | 54 | 67.96 | 41 | 50.00 | * |
| Completed six-month assessment | 72 | 86.75 | 82 | 97.62 | * |
| DPDa medication adherence (% Yes) | 22 | 30.56 | 15 | 19.74 | * |
|
|
|
||||
| M | SD | M | SD | ||
|
|
|
||||
| Age (years) | 13.64 | 1.41 | 13.32 | 1.28 | |
| Annual family income (1–10; 4=$13K–$15,999) | 4.16 | 2.24 | 4.17 | 2.23 | |
| Sessions attended | 7.99 | 6.84 | 22.94 | 14.76 | * |
| Height (cm) | 161.39 | 11.00 | 160.10 | 8.44 | |
| FEV1 | 2.21 | 0.60 | 2.05 | 0.56 | |
| Family response to symptoms | 3.89 | 1.66 | 3.75 | 1.61 | |
| Child response to symptoms | 3.24 | 1.82 | 2.83 | 1.50 | |
| FAMSSb medication adherence | 4.61 | 2.24 | 4.17 | 2.23 | |
indicates a significant difference between the groups at p <.05.
Daily Phone Diary.
Family Asthma Management System Scale.
Intent-to-Treat Analyses
As shown in Figures 2 – 4, results of intent-to-treat analyses indicated significant time by treatment interactions for FEV1 (b=.02 [95% C.I. .0004,.04], SE=.01, p<.05), FAMSS Medication Adherence (b=.14 [95% C.I. .02,.26], SE=.06, p=.03) and DPD Medication Adherence (b=.18 [95% C.I. .02,.34], SE=.08, p=.03), with the MST-HC group demonstrating greater improvement in lung function and controller medication adherence than the FS group. There were no differences in Child or Family Response to Symptoms. As seen in Table 2, treatment had a medium effect on changes in FAMSS and DPD adherence to medication, and a small effect on changes in FEV1.
Figure 2.
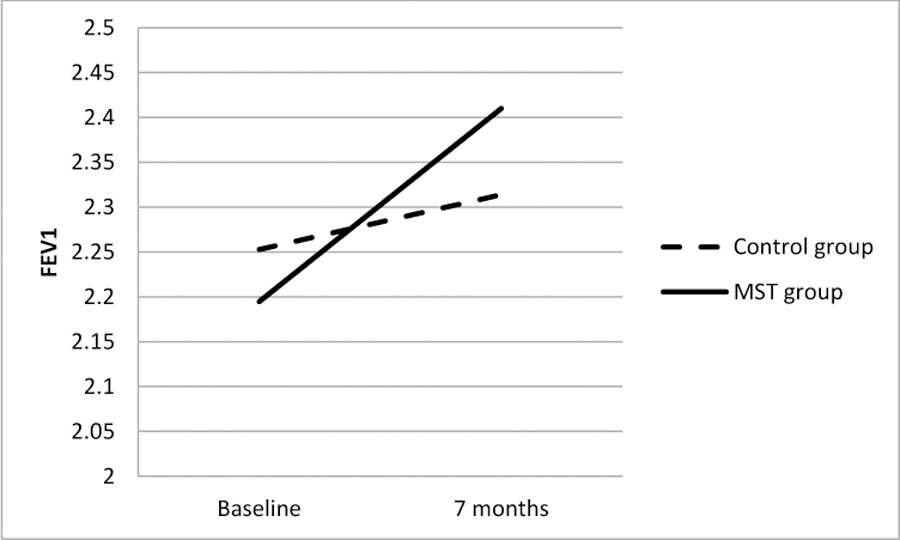
Difference between MST and Control Groups in Change in Lung Function (FEV1) from Baseline to the 7-Month Follow-Up.
Figure 4.
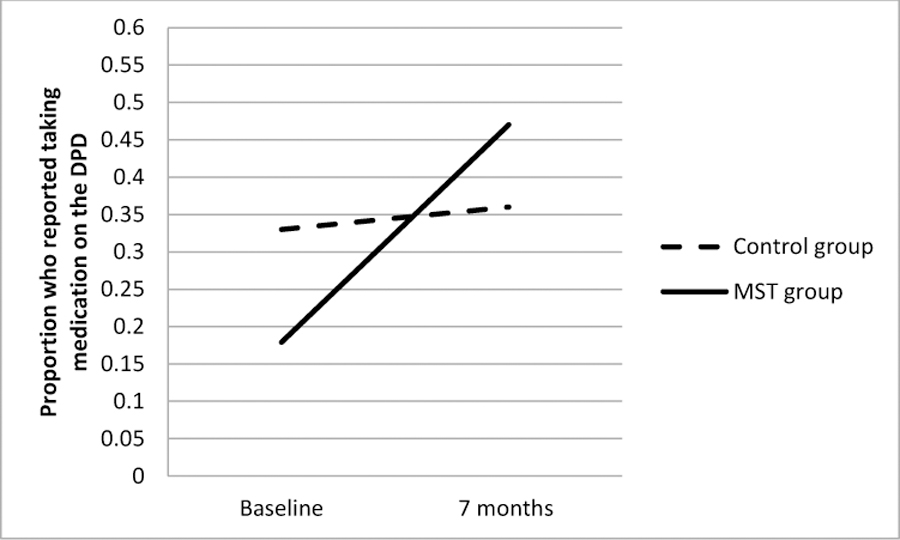
Difference between MST and Control Groups in Change in the Proportion of Participants Reporting Adherence to Controller Medication on the Daily Phone Diary (DPD) from Baseline to the 7-Month Follow-Up.
Table 2.
Mean Change in Continuous Outcome Variables from Baseline to Six Months for MST and Control Groups, and Magnitude (Effect Size) of Differences in Change Scores between Groups
| Average Change Scores | |||||
|---|---|---|---|---|---|
| Control Group |
MST Group |
dGMA-RAWa | |||
| Asthma Outcomes | M | SD | M | SD | or Odds Ratiob |
| INTENT-TO-TREAT | |||||
| FEV1 | 0.07 | 0.40 | 0.20 | 0.43 | 0.24 |
| Caregiver symptom response | 0.56 | 2.10 | 0.83 | 2.15 | 0.17 |
| Child symptom response | 0.21 | 1.87 | 0.74 | 2.15 | 0.34 |
| FAMSS medication adherencec | 0.07 | 2.47 | 1.20 | 2.80 | 0.46 |
| DPD medication adherenced | 0.04 | 0.25 | 8.00 | ||
| PER-PROTOCOL | |||||
| FEV1 | 0.03 | 0.41 | 0.22 | 0.45 | 0.36 |
| Caregiver symptom response | 0.44 | 2.16 | 1.09 | 2.15 | 0.41 |
| Child symptom response | 0.09 | 1.76 | 0.90 | 2.17 | 0.49 |
| FAMSS medication adherencec | 0.39 | 2.23 | 1.66 | 2.36 | 0.50 |
| DPD medication adherenced | 0.04 | 0.29 | 9.80 | ||
Effect size for growth modeling analysis in the same metric as Cohen’s d (Feingold, 2009).
Odds ratio for DPD (change in odds of MST group reporting medication use on DPD compared to change in odds for control group).
Family Asthma Management System Scale
Daily Phone Diary; numbers indicate change in proportion of group reporting medication use on DPD.
Per-Protocol Analyses
The per-protocol analyses indicated that, unlike the intent-to-treat analyses, youth receiving at least a minimum dose of MST-HC had significantly greater improvements in Child Response to Symptoms (b=.11 [95% C.I. .01,.21], SE=.05, p=.04). Similar to the intent-to treat analyses, participants receiving MST-HC also had greater improvement in lung function (FEV1; b=.03 [95% C.I. .01,.05], SE=.01, p=.01) and adherence to controller medication, as measured by the FAMSS (b=.15 [95% C.I. .03,.27], SE=.06, p=.02) and by the DPD (b= .21 [95% C.I. .03,.39], SE=.09, p=.03). As seen in Table 2, the size of the effect of MST-HC was small to medium on changes in lung function and medium on medication adherence when the treatment sample was considered.
Discussion
There are few evidence-based interventions to improve asthma management in minority adolescents who are at high risk for morbidity and mortality. Results demonstrate that MST-HC, already shown to be effective in improving medication adherence in youth with diabetes and HIV, successfully improved asthma medication adherence and lung function in this population in an intent-to-treat analysis with a strong comparison condition, home-based supportive family counseling. Furthermore MST-HC improved the adolescent’s response to asthma symptoms and exacerbations and had a small to medium effect on lung function (using an objective health outcome) among youth who received the allocated treatment. Given that FEV1 may take some time to improve following increasing use of daily controller medications (Strunk, 2007), this finding immediately post-treatment is promising, and suggests the possibility of further improvement with longer follow-up. With each 10 percent increase in FEV1, there is a progressive decrease in asthma attacks (Fuhlbrigge et al., 2001) thus decreasing health care utilization. We intend to assess the impact of MST-HC on health care utilization over 12 months of follow-up as it is widely recognized that there are seasonal variations in asthma exacerbations and therefore on rates of hospital or ED visits (National Heart Lung & Blood Institute, 2007).
This study documented the efficacy of MST-HC for youth with asthma compared to a rigorous attention control matched for multiple non-specific treatment effects (e.g., family interactions, home-based, monitoring of symptoms). To date, other MST-HC interventions have utilized standard care controls (Ellis et al., 2008) or comparison treatments provided only to the adolescent (Ellis et al., 2012; Letourneau et al., 2012). The present data suggest that there are crucial aspects of MST-HC that are responsible for outcomes including the provision of evidence-based family interventions and that simple attention, warmth and support to the caregiver of the youth does not account for the findings. Furthermore, MST, which is present focused and action oriented, may be more feasible and acceptable to minority families than simply creating opportunities for families to interact around asthma, as evidenced by increased treatment retention and dose received in the MST-HC group compared to FS.
The dose of MST-HC in the present study was lower than in our prior studies with youth with poorly controlled diabetes (Ellis et al., 2005; Ellis et al., 2012). It is possible that families did not perceive asthma, a common childhood illness, to be a severe as diabetes, and therefore did not participate in as many sessions. Both the MST and FS groups received about half the expected dose of treatment. It is possible that to meet the needs of high-risk families, multi-stressed families, twice the number of expected sessions must be offered to account for frequent cancellations and rescheduling. Alternatively, a lower dose of treatment may have been sufficient to improve adherence to asthma medication, relative to our work with other populations such as diabetes where multiple illness management behaviors (insulin adherence, blood glucose testing, dietary management) were targeted. Future research should test which high-risk families may benefit from a lower dose of treatment and which families require more intensive services. While differential attrition may be a limitation of the study, both study arms achieved high levels of study retention that exceeded published guidelines for acceptability (CDC, 2007; Lyles et al., 2007; Valentine & Cooper, 2008). Another limitation to the study is that there was a greater number of participants in the control group coming from single parent families than in the MST group.
Additional research is also necessary to support the transportability of MST-HC to community settings. Economic analysis is necessary to determine cost-offsets in terms of reduced health care utilization both during the trial and in the future. Future studies can test whether utilizing paraprofessional staff can maintain efficacy and reduce costs. In one version of MST-HC for youth with HIV in rural settings, paraprofessionals were utilized to augment services provided by master’s level clinicians (Letourneau et al., 2012). The Centers for Disease Control has called for expanded use of community health workers in services for chronic disease (Nichols, Ussery-Hall, Griffin-Blake, & Easton, 2012) with careful attention to implementation and training (Lewin et al., 2005). Implementation science is the scientific study of methods to promote the uptake of research findings and evidence-based practice to improve the quality and effectiveness of health care (Eccles et al., 2009). Implementation science studies of MST in mental health are underway (e.g., Glisson et al., 2010; Ogden et al., 2012), and this information can guide similar work in health care, particularly for high-risk minority youth. This work will not only address the science-practice gap but also has the potential to reduce health disparities in chronic illness outcomes.
In summary, there are few interventions that been shown to successfully improve asthma management in minority youth at highest risk for poor morbidity and mortality. MST, a home-based psychotherapy originally developed to target behavior problems in youth, improved adherence to daily controller medications and lung function immediately post-treatment. Further follow-up is necessary to determine whether MST-HC reduces health care utilization accounting for seasonal variability.
Figure 3.
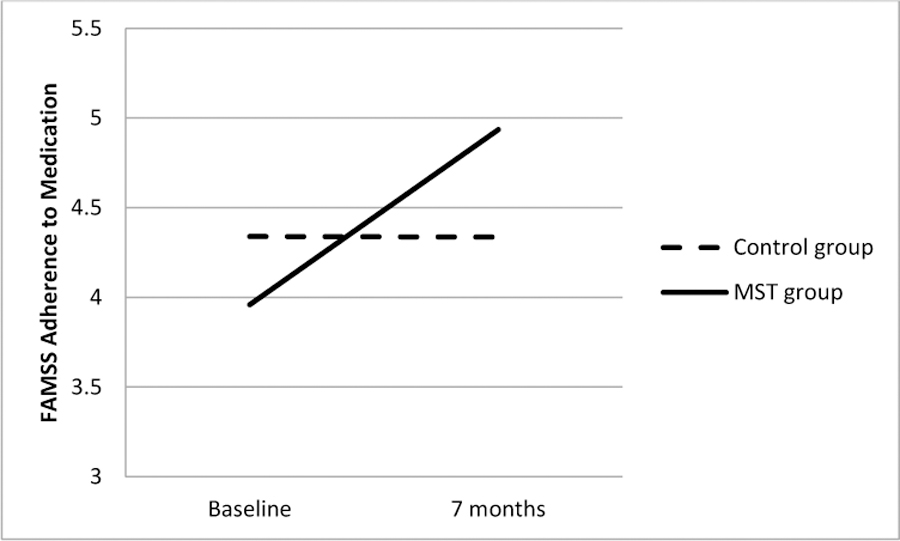
Difference between MST and Control Groups in Change in Adherence to Controller Medication from Baseline to the 7-Month Follow-Up, as reported on the Family Asthma Management System Scale (FAMSS).
Acknowledgments
This research was supported by a grant from the National Institute of Health (1R01AA022891-01).
Contributor Information
Sylvie Naar-King, Wayne State University.
Deborah Ellis, Wayne State University.
Pamela S. King, Wayne State University
Phebe Lam, Wayne State University.
Phillippe Cunningham, Medical University of South Carolina.
Elizabeth Secord, Wayne State University.
Jean-Marie Bruzzese, New York University.
Thomas Templin, Wayne State University.
References
- Agency for Healthcare Research and Quality (AHRQ). (2007). Closing the quality gap: A critical analysis of quality improvement strategies. 04(07)-0051–5. [Google Scholar]
- Agency for Healthcare Research and Quality (AHRQ). (2011). AHRQ news and numbers. Black children more likely to be hospitalized for severe asthma attacks Retrieved May 2013, from http://www.ahrq.gov/news/newsroom/news-and-numbers/062911.html
- Amico KR (2009). Percent total attrition: A poor metric for study rigor in hosted intervention designs. American Journal of Public Health, 99(9), 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braman SS (2006). The global burden of asthma. CHEST Journal, 130(1_suppl), 4S–12S. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U (1979). The ecology of human development: Experiments by design and nature. Cambridge, MA: Harvard University Press. [Google Scholar]
- Brown RT (2002). Society of pediatric psychology presidential address: Toward a social ecology of pediatric psychology. Journal of Pediatric Psychology, 27(2), 191–201. [DOI] [PubMed] [Google Scholar]
- Bruzzese J-M, Sheares BJ, Vincent EJ, Du Y, Sadeghi H, Levison MJ, … Evans D. (2011). Effects of a school-based intervention for urban adolescents with asthma. A Controlled Trial. American journal of respiratory and critical care medicine, 183(8), 998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzese J-M, Stepney C, Fiorino EK, Bornstein L, Wang J, Petkova E, & Evans D (2012). Asthma self-management is sub-optimal in urban Hispanic and African American/Black early adolescents with uncontrolled persistent asthma. Journal of Asthma, 49(1), 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Sly P, & Devadason S (2011). Adherence with preventive medication in childhood asthma. Pulmonary Medicine, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2010). Youth Risk Behavior Surveillance - United States, 2009. MMWR June 4, 2010, 59(No.SS-5), 1–142. [PubMed] [Google Scholar]
- Celano M, Klinnert MD, Holsey CN, & McQuaid EL (2011). Validity of the Family Asthma Management System Scale with an urban African-American sample. Journal of Pediatric Psychology, 36(5), 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celano MP, Holsey CN, & Kobrynski LJ (2012). Home-based family intervention for low-income children with asthma: a randomized controlled pilot study. Journal of family psychology: JFP: journal of the Division of Family Psychology of the American Psychological Association, 26(2), 171–178. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2007). HIV Prevention Strategic Plan: Extended Through 2010. Retrieved from http://www.cdc.gov/hiv/resources/reports/psp/index.htm.
- Centers for Disease Control and Prevention (CDC). (2011). CDC Vital and Health Statistics. Asthma in the US: Growing Every Year Retrieved May 2013, from http://www.cdc.gov/VitalSigns/Asthma/
- Centers for Disease Control and Prevention (CDC). (2012). CDC Vital and Health Statistics. Summary Health Statistics for U.S. Children: National Health Interview Survey, 2011 Series 10, Number 254. Retrieved May 2013, from http://www.cdc.gov/nchs/data/series/sr_10/sr10_254.pdf
- Centers for Disease Control and Prevention (CDC). (2013a). 24/7: Saving Lives. Protecting People. Asthma and Schools Retrieved May 2013, from http://www.cdc.gov/HealthyYouth/asthma/
- Centers for Disease Control and Prevention (CDC). (2013b). CDC 24/7: Saving Lives. Protecting People. Asthma: Data and Surveillance Retrieved May 2013, from http://www.cdc.gov/asthma/asthmadata.htm
- Clark NM, Griffiths C, Keteyian SR, & Partridge MR (2010). Educational and behavioral interventions for asthma: who achieves which outcomes? A systematic review. Journal of asthma and allergy, 3, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker DD, Kinyota S, Dumitru GG, Ligon CB, Herman EJ, Ferdinands JM, … Sipe TA (2011). Effectiveness of home-based, multi-trigger, multicomponent interventions with an environmental focus for reducing asthma morbidity: a community guide systematic review. American Journal of Preventive Medicine, 41(2), S5–S32. [DOI] [PubMed] [Google Scholar]
- Drotar D, & Bonner MS (2009). Influences on adherence to pediatric asthma treatment: a review of correlates and predictors. Journal of Developmental & Behavioral Pediatrics, 30(6), 574–582. [DOI] [PubMed] [Google Scholar]
- Eccles MP, Armstrong D, Baker R, Cleary K, Davies H, Davies S, … Leng G (2009). An implementation research agenda. Implementation Science, 4(1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D, Naar-King S, Templin T, Frey M, Cunningham P, Sheidow A, … Idalski A (2008). Multisystemic therapy for adolescents with poorly controlled type 1 diabetes. Diabetes Care, 31(9), 1746–1747. doi: 10.2337/dc07-2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis DA, Frey MA, Naar-King S, Templin T, Cunningham PB, & Cakan N (2005). Use of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in chronic poor metabolic control: A randomized controlled trial. [Proceedings of the 65th Annual Scientific Session of the American Diabetes Association]. Diabetes Care, 28(7), 1604–1610. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Naar-King S, Cunningham PB, & Secord E (2006). Use of multisystemic therapy to improve antiretroviral adherence and health outcomes in HIV-infected pediatric patients: evaluation of a pilot program. AIDS Patient Care & STDs, 20(2), 112–121. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Naar-King S, Templin T, Frey MA, & Cunningham PB (2007). Improving health outcomes among youth with poorly controlled type 1 diabetes: The role of treatment fidelity in a randomized clinical trial of multisystemic therapy. Journal of Family Psychology, 21(3), 363–371. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Naar-King S, Xinguang Chen M, Moltz K, Cunningham PB, & Idalski-Carcone A (2012). Multisystemic therapy compared to telephone support for youth with poorly controlled diabetes: Findings from a randomized controlled trial. Annals of Behavioral Medicine, 44(2), 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold A (2009). Effect sizes for growth modeling analysis for controlled trials in the same metric as for classical analysis. Psychological Methods, 14 (1), 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhlbrigge AL, Kitch BT, Paltiel AD, Kuntz KM, Neumann PJ, Dockery DW, & Weiss ST (2001). FEV(1) is associated with risk of asthma attacks in a pediatric population. Journal of Allergy and Clinical Immunology, 107(1), 61–67. [DOI] [PubMed] [Google Scholar]
- Glisson C, Schoenwald SK, Hemmelgarn A, Green P, Dukes D, Armstrong KS, & Chapman JE (2010). Randomized trial of MST and ARC in a two-level evidence-based treatment implementation strategy. Journal of Consulting and Clinical Psychology, 78(4), 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson PM, Watson L, Davis K, & Rabe K (2006). Poor asthma control in children: evidence from epidemiological surveys and implications for clinical practice. International journal of clinical practice, 60(3), 321–334. [DOI] [PubMed] [Google Scholar]
- Henggeler SW, Halliday-Boykins CA, Cunningham PB, Randall J, Shapiro SB, & Chapman JE (2006). Juvenile drug court: Enhancing outcomes by integrating evidence-based treatments. Journal of Consulting and Clinical Psychology, 74(1), 42. [DOI] [PubMed] [Google Scholar]
- Henggeler SW, & Schoenwald SK (1998). The MST supervisory manual: Promoting quality assurance at the clinical level. Charleston, SC: The MST Institute. [Google Scholar]
- Henggeler SW, Schoenwald SK, & Borduin CM (2009). Multisystemic therapy for antisocial behavior in children and adolescents (second edition). New York: The Guilford Press. [Google Scholar]
- Huey SJ (2001). Adherence training manual for multisystemic therapy (MST): Anchors and guidelines for coding audio taped sessions. Los Angeles: University of Southern California. [Google Scholar]
- Kahana S, Drotar D, & Frazier T (2008). Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. Journal of Pediatric Psychology, 33(6), 590–611. [DOI] [PubMed] [Google Scholar]
- Knudson RJ, Kaltenborn WT, Knudson DE, & Burrows B (1987). The single-breath carbon monoxide diffusing capacity. Reference equations derived from a healthy nonsmoking population and effects of hematocrit. The American Review of Respiratory Disease, 135(4), 805–811. [DOI] [PubMed] [Google Scholar]
- Letourneau EJ, Ellis DA, Naar-King S, Chapman JE, Cunningham PB, & Fowler S (2012). Multisystemic therapy for poorly adherent youth with HIV: Results from a pilot randomized controlled trial. AIDS care, 1–8. doi: 10.1080/09540121.2012.715134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin SA, Dick J, Pound P, Zwarenstein M, Aja G, van Wyk B, & al., e. (2005). Lay health workers in primary and community health care Cochrane Database Systematic Rev.: The Cochrane Library Database; [DOI] [PubMed] [Google Scholar]
- Lyles CM, Kay LS, Crepaz N, Herbst JH, Passin WF, Kim AS, … Mullins MM (2007). Best-evidence interventions: findings from a systematic review of HIV behavioral interventions for US populations at high risk, 2000–2004. Journal Information, 97(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MK, & Waldfogel J (2012). Racial and ethnic differences in the management of childhood asthma in the United States. Journal of Asthma, 49(8), 785–791. [DOI] [PubMed] [Google Scholar]
- McQuaid EL, Walders N, Kopel SJ, Fritz GK, & Klinnert MD (2005). Pediatric asthma management in the family context: The family asthma management system scale. Journal of Pediatric Psychology, 30(6), 492–502. [DOI] [PubMed] [Google Scholar]
- Modi AC, & Quittner AL (2006). Utilizing computerized phone diary procedures to assess health behaviors in family and social contexts. Children’s Health Care, 35, 29–45. [Google Scholar]
- Naar-King S, Ellis D, Kolmodin K, Cunningham P, Jen KLC, Saelens B, & Brogan K (2009). A randomized pilot study of multisystemic therapy targeting obesity in African-American adolescents. Journal of Adolescent Health, 45(4), 417–419. [DOI] [PubMed] [Google Scholar]
- Naar-King S, Ellis D, Kolmodin K, Cunningham P, & Secord E (2009). Feasibility of adapting multisystemic therapy to improve illness management behaviors and reduce asthma morbidity in high risk African American youth: A case series. Journal of Child and Family Studies, 18(5), 564–573. doi: 10.1007/s10826-009-9259-9 [DOI] [Google Scholar]
- Naar-King S, Podolski CL, Ellis DA, & Frey MA (2006). Social ecological model of illness management in high risk youth with type 1 diabetes. Journal of Consulting and Clinical Psychology, 74, 785–789. [DOI] [PubMed] [Google Scholar]
- National Heart Lung & Blood Institute. (2007). National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma, 2007. Available at http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf.
- Nichols P, Ussery-Hall A, Griffin-Blake S, & Easton A (2012). The Evolution of the Steps Program, 2003–2010: Transforming the Federal Public Health Practice of Chronic Disease Prevention. Preventing Chronic Disease, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden T, Bjørnebekk G, Kjøbli J, Patras J, Christiansen T, Taraldsen K, & Tollefsen N (2012). Measurement of implementation components ten years after a nationwide introduction of empirically supported programs–a pilot study. Implementation Science, 7(1), 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe KF, Adachi M, Lai C, Soriano JB, Vermeire PA, Weiss KB, & Weiss ST (2004). Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol, 114(1), 40–47. [DOI] [PubMed] [Google Scholar]
- Rhee H, Belyea MJ, Hunt JF, & Brasch J (2011). Effects of a peer-led asthma self-management program for adolescents. Archives of pediatrics & adolescent medicine, 165(6), 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C (1951). Client-centered therapy: Its current practice, implications and theory. London, UK: Constable. [Google Scholar]
- Rohan J, Drotar D, McNally K, Schluchter M, Riekert K, Vavrek P, … Kercsmar C (2010). Adherence to pediatric asthma treatment in economically disadvantaged African-American children and adolescents: an application of growth curve analysis. Journal of Pediatric Psychology, 35(4), 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwald SK (1998). Multisystemic therapy consultation guidelines. Charleston, SC: MST Institute. [Google Scholar]
- Strunk RC (2007). Childhood Asthma Management Program: lessons learned. The Journal of allergy and clinical immunology, 119(1), 36. [DOI] [PubMed] [Google Scholar]
- Valentine JC, & Cooper H (2008). A systematic and transparent approach for assessing the methodological quality of intervention effectiveness research: The Study Design and Implementation Assessment Device (Study DIAD). Psychological methods, 13(2), 130–149. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2011). Asthma Fact Sheet, number 307 Retrieved May 2013, from http://www.who.int/mediacentre/factsheets/fs307/en/index.html
- Yinusa-Nyahkoon LS, Cohn ES, Cortes DE, & Bokhour BG (2010). Ecological barriers and social forces in childhood asthma management: examining routines of African American families living in the inner city. Journal of Asthma, 47(7), 701–710. [DOI] [PubMed] [Google Scholar]


