Abstract
The CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018 comprehensively describes the current population-based incidence of primary malignant and non-malignant brain and other CNS tumors in children and adolescents ages 0–19 years, collected and reported by central cancer registries covering approximately 100% of the United States population. Overall, brain and other CNS tumors are the most common solid tumor, the most common cancer, and the most common cause of cancer death in children and adolescents ages 0–19 years. This report aims to serve as a useful resource for researchers, clinicians, patients, and families.
Executive Summary
The Central Brain Tumor Registry of the United States (CBTRUS), in collaboration with the Centers for Disease Control and Prevention (CDC) and National Cancer Institute (NCI), is the largest population-based registry focused exclusively on primary brain and other central nervous system (CNS) tumors in the United States and represents the entire United States population. The CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018 contains the most up-to-date population-based data on primary brain and other CNS tumors in children and adolescents available through the surveillance system in the United States and supersedes all previous reports in terms of completeness and accuracy, thereby providing a current comprehensive source for the descriptive epidemiology of these tumors.
Incidence
The annual average age-specific incidence rate of all malignant and non-malignant brain and other CNS tumors in children and adolescents ages 0–19 years was 6.23 per 100,000 population between 2014 and 2018. When stratified by behavior, incidence was 3.55 per 100,000 population for malignant tumors only, and 2.67 for non-malignant tumors only.
This overall rate was higher in females compared to males (6.35 versus 6.11 per 100,000) and non-Hispanics (of any race) compared to Hispanics (6.52 versus 5.33 per 100,000).
An estimated 5,260 new cases of malignant and non-malignant brain and other CNS tumors are expected to be diagnosed in children and adolescents ages 0–19 years in the United States in 2023.
Mortality
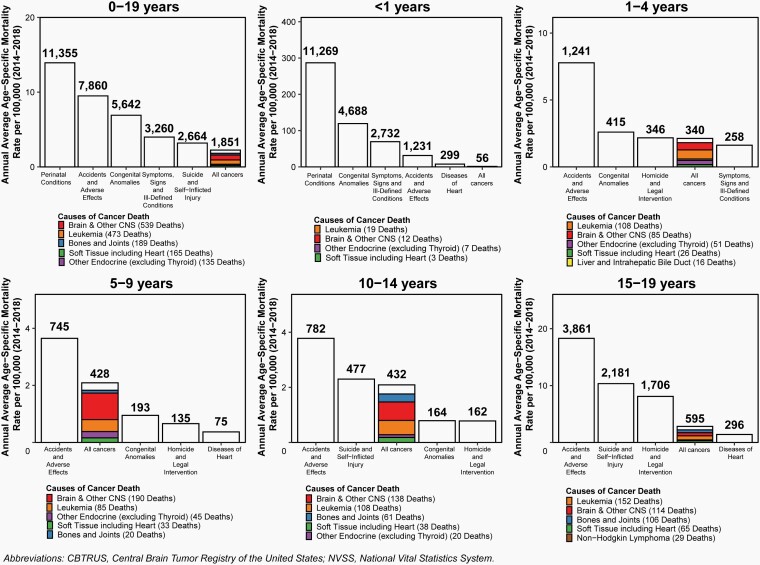
There were 2,693 deaths attributed to malignant brain and other CNS tumors between 2014 and 2018 in children and adolescents ages 0–19 years. This represents an annual average mortality rate of 0.66 per 100,000 population, and an average of 539 deaths per year caused by malignant brain and other CNS tumors.
Survival
The five-year relative survival rate following diagnosis of a malignant or non-malignant brain or other CNS tumor was 83.9%. Survival following diagnosis with a brain and other CNS tumor was highest in adolescents ages 15–19 years (90.5%) and lowest in children less than one year old (71.9%).
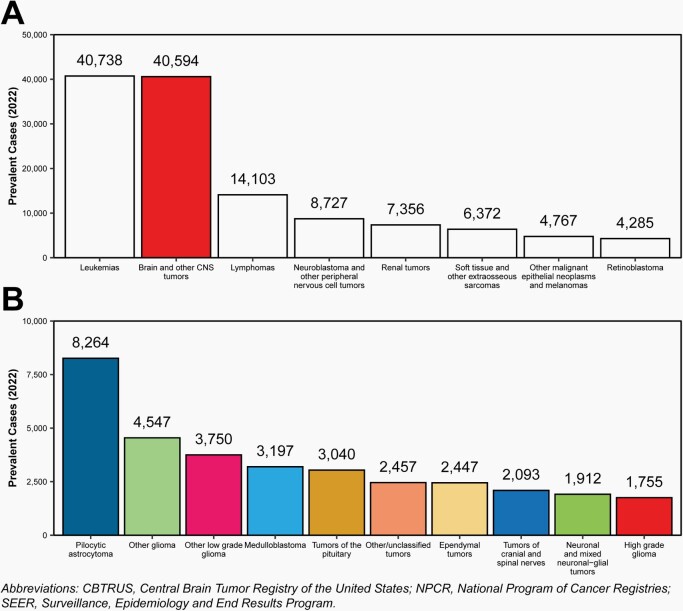
Prevalence
There were an estimated 40,594 children and adolescents ages 0–19 years living with a primary brain and other CNS tumor diagnosis in 2022. This is comparable to leukemia, where there are an estimated 40,738 children and adolescents ages 0–19 years living with a leukemia diagnosis.
The most prevalent histopathologic group was pilocytic astrocytoma (estimated 8,264 cases).
Introduction
Brain tumors are a significant source of cancer-related morbidity and mortality in children and adolescents. This age group is diagnosed with unique groups of cancers and requires separate reporting in order to accurately portray the state of brain tumors in these populations.
The Central Brain Tumor Registry of the United States (CBTRUS) is the largest population-based registry of primary brain and other central nervous system (CNS) tumors in the United States and covers ~100% of the United States (US) population for the period between 2014 and 2018. The objective of the CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018 is to provide a comprehensive summary of the current descriptive epidemiology of primary brain and other CNS tumors of childhood and adolescence (ages 0–19 years) in the US population. CBTRUS obtained all newly diagnosed primary brain and other CNS tumors data submitted to the Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries (NPCR) and the National Cancer Institute’s (NCI) Surveillance, Epidemiology and Results (SEER) Program in November 2020 and covered diagnosis years 2000–2018. Incidence counts and rates of primary brain and other CNS tumors are documented by histopathology, sex, age, race, and Hispanic ethnicity. Mortality and relative survival rates calculated using NPCR data for the period 2001–2017 are also presented.
Background
CBTRUS is currently the only population-based site-specific registry in the United States that works in partnership with a public cancer surveillance organization, the CDC’s NPCR, and from which data are directly received under a special agreement. This agreement permits transfer of data through the National Program of Central Registries Cancer Surveillance System (NPCR-CSS) Submission Specifications mechanism. CBTRUS researchers combine the NPCR data with data from the SEER Program1 of the NCI, which was established for national cancer surveillance in the early 1970s. All data from NPCR and SEER originate from tumor registrars who adhere to the Uniform Data Standards (UDS) for malignant and non-malignant brain and other CNS tumors as directed by the North American Association of Central Cancer Registries (NAACCR) (http://www.naaccr.org). Along with the UDS, there are quality control checks and a system for rating each central cancer registry (CCR) to further ensure that these data are reported as accurately and completely as possible. As a surveillance partner, CBTRUS can therefore report high quality data on brain and CNS tumors with histopathological specificity useful to the communities it serves. Its database represents the largest aggregation of population-based data on the incidence of primary brain and other CNS tumors in the United States.
Technical Notes
Data Collection
CBTRUS contains incidence data from 52 independent CCRs (48 NPCR and 4 SEER registries) representing ~98% of the US population for the time period examined in this report. Please see the 2021 CBTRUS Statistical Report for additional information about the way these data are obtained and processed. These cases are classified using the International Classification of Diseases for Oncology, Third edition (ICD-O-3) for assignment of histopathology, behavior, and site codes. These codes are grouped using a modified version of the CBTRUS Grouping (Supplementary Table 1).2 As there is no standard definition for glioma, CBTRUS defines glioma as ICD-O-3 histopathology codes 9380–9384, and 9391–9460. It is also important to note that the statistics for lymphomas and hematopoietic neoplasms contained in this report refer only to those lymphomas and hematopoietic neoplasms that arise in the brain and other CNS ICD-O-3 topography codes.
Primary brain and other CNS tumors can be broadly classified in non-malignant (ICD-O-3 behavior codes of /0 for benign and /1 for uncertain) and malignant (ICD-O-3 behavior code of /3). Collection of central (state) cancer data was mandated in 1992 by Public Law 102–515 for all primary malignant tumors (ICD-O-3 behavior code of /3) (Supplementary Table 1), the Cancer Registries Amendment Act.3 This mandate was expanded to include non-malignant brain and other CNS tumors (ICD-O-3 behavior code of /0 and /1) with the 2002 passage of Public Law 107–260, starting January 1, 2004.4 See Supplementary Table 3 for a summary of specific glioma histopathologies included in glioma groupings.
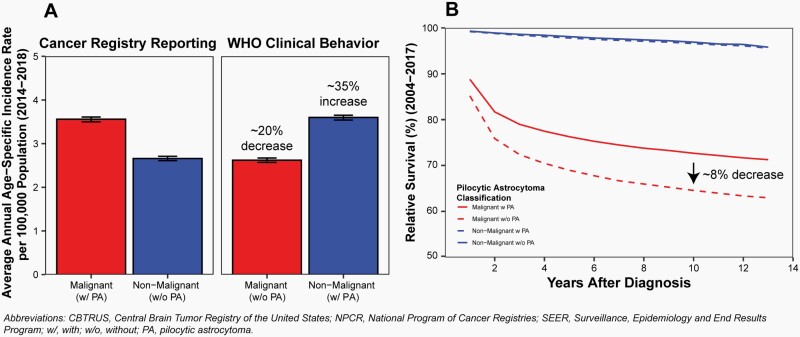
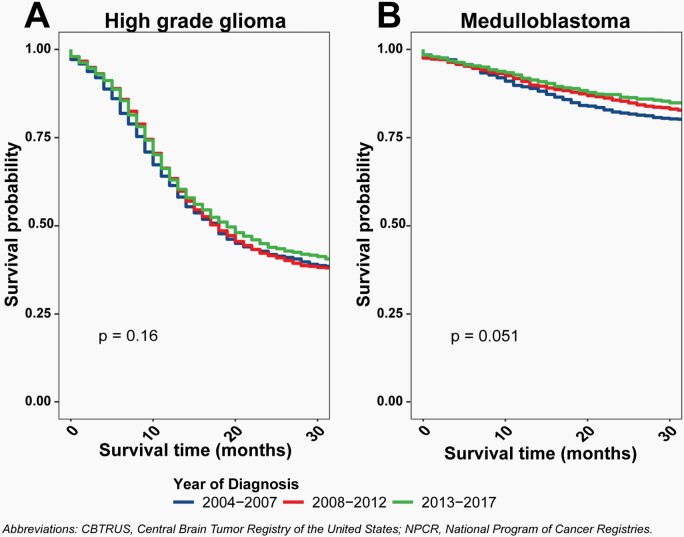
Pilocytic astrocytoma is clinically considered and classified as a Grade I, non-malignant (ICD-O-3 behavior code of /1) tumor by the World Health Organization (WHO) guidelines for brain and other CNS tumors.5 For the purposes of cancer registration, these tumors have historically been reported as malignant (ICD-O-3 behavior code of /3) tumors both in the United States and by the International Agency for Research on Cancer (IARC) and International Association of Cancer Registries.6,7 Classification of these tumors as malignant has been followed by CBTRUS in its reporting unless otherwise stated. Classification of these tumors as malignant has a significant impact on both incidence and survival estimates (Figure 1), including an upward bias for both incidence of malignant tumors and survival estimates for malignant brain tumors. Please see a recent publication for additional discussion of the effect of this classification on cancer incidence and survival reporting.8
Fig. 1.
Effect of Reclassification of Pilocytic Astrocytoma from Malignant to Non-Malignant Behavior for Diagnoses in Children and Adolescents Ages 0–19 Years on A) Average Annual Incidence from 2014–2018 and B) Relative Survival after Diagnosis from 2004–2017, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2014–2018.
Age-specific incidence rates per 100,000 population for the entire United States for selected other cancers were obtained from the United States Cancer Statistics (USCS), produced by CDC and NCI, for the purpose of comparison with brain and other CNS tumor incidence rates.9 This database includes both NPCR and SEER data and represents the entire US population. Comparison cancers are classified using the International Classification of Childhood Cancer (ICCC) grouping system. ICCC categories for this report were generated using the SEER Main and Extended Classification for ICCC Recode ICD-O-3/WHO 200810 based on the ICCC, Third edition11,12 and 2007 WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues13
De-identified survival data for malignant brain and other CNS tumors were obtained from the USCS program for 42 NPCR registries with available survival data for the years 2001 to 2017 and for non-malignant brain and other CNS tumors for the years 2004 to 2017 (data collection for non-malignant tumors began in 2004). This dataset provides population-based information for 82% of the US population for the years 2001 to 2017 and is a subset of the data used for the incidence calculations presented in this report. Survival information is derived from both active and passive follow-up.
Mortality data for the years 2014 to 2018 used in this report are from the National Vital Statistics System (NVSS) and include deaths where primary brain or other CNS tumor was listed as primary cause of death on the death certificate for individuals from all 50 states and the District of Columbia. These NVSS14 (includes death certification data for 100% of the US population) data were obtained from the NCI via SEER*Stat malignant brain and other CNS tumors and comparison (for malignant brain tumors and comparison cancers). NVSS data are not collected through the cancer registration system. These data represent the primary cause of death listed on each individual death certificate, and as a result, deaths in persons with cancer may be recorded as non-cancer deaths.
Methods
Counts, means, medians, rates, ratios, proportions, and other relevant statistics were calculated using R 4.1.3 statistical software15 and/or SEER*Stat 8.4.0.16 Figures and tables were created in R 4.1.3 using the following packages: flextable, officer, orca, plotly, SEER2R, sf, tigris, and tidyverse.17–25 Rates are suppressed when counts are fewer than 16 within a cell but included in totals, except when data are suppressed from only one cell to prevent identification of the number in the suppressed cell. NOTE: reported percentages may not add up to 100% due to rounding.
Incidence and Mortality Rates
.— Age-specific incidence and mortality rates and 95% confidence intervals26 (CI) were estimated for malignant and non-malignant tumors and for selected histopathology groupings by sex, race, Hispanic ethnicity, and age groups. Estimates are presented by age groups < 1, 1–4, 5–9, 10–14, and 15–19 years. Race categories in this report are all races, White, Black, American Indian/Alaskan Native (AIAN), and Asian/Pacific Islander (API). Other race, unspecified, and unknown race are included in statistics that are not race-specific. Hispanic ethnicity was defined using the NAACCR Hispanic Identification Algorithm, version 2, data element, which utilizes a combination of cancer registry data fields (Spanish/Hispanic Origin data element, birthplace, race, and surnames) to directly and indirectly classify cases as Hispanic or non-Hispanic.27 The NAACCR regional scheme (http://faststats.naaccr.org/usregions.php) was used for statistics reported by region of the United States.
Incidence rate ratios (IRR) were generated based on these age-specific incidence rates. These IRR were used to compare groups, using the formulas described by Fay et al. to calculate p-values.28 IRR were considered statistically significantly different when the p-value was less than 0.05.
Estimated Future Cases
.— Estimated numbers of expected malignant and non-malignant brain and other CNS tumors were calculated for 2023–2025. To project estimates of newly diagnosed brain and other CNS tumors, age-specific annual brain tumor incidence rates were generated for 2000–2018 for malignant tumors, and 2006–2018 (years 2004–2005 excluded as these were the first few years of data collection during which incidence increased significantly) for non-malignant tumors. These were generated by state, age, and histopathologic type. Joinpoint 4.9.0.029 was used to fit regression models to these incidence rates,30 which were used to predict numbers of cases in future years using the parameter from the selected models. Modified Bayesian Information Criterion procedures included in Joinpoint were used to select the best fitting model. The overall totals presented are based on total malignant and non-malignant incidence, and the presented stratified rates may not add up to these totals. Estimated numbers of cases are highly dependent on input data. Different patterns of incidence within strata can significantly affect the projected estimates, especially when the number of cases within a stratum is low. Estimates are generated with the assumption of consistent trends in cases and population. Caution should be used when utilizing these estimates.
Estimation of Relative Survival
.— SEER*Stat 8.4.0 statistical software was used to estimate one-, two-, three-, four-, five-, and ten-year relative survival rates for primary malignant and non-malignant brain and other CNS tumor cases diagnosed between 2004–2017 in 42 NPCR CCRs. This software utilizes life-table (actuarial) methods to compute survival estimates and accounts for current follow-up. Second or later primary tumors, cases diagnosed at autopsy, cases in which race or sex is coded as other or unknown, and cases known to be alive but for whom follow-up time could not be calculated, were excluded from survival data analyses prior to release of the survival dataset to CBTRUS by NPCR.
Prevalence Estimation
.— For estimation of brain and other CNS tumor prevalence, new case count data by histopathology and single age at diagnosis for malignant and non-malignant brain tumors (2004–2018 for non-malignant tumors) were extracted from CBTRUS from 2000–2018 and from SEER 9 for 1975–2018. For comparison cancers, new case count data by ICCC-defined histopathology and single age at diagnosis from USCS for 2001–2018 and from SEER 9 for 1975–2018. New case diagnoses and survival were projected from 2019–2022 using prevEst in R 4.0, which were then used to estimate total number of prevalent cases by histopathology and age for the year at prevalence, 2022.
Incidence and Mortality Trends
.— Joinpoint 4.9.0.029 was used to estimate incidence and mortality time trends and generate annual percentage changes (APC) and 95% CI. Rather than calculating a single consistent slope of change over an entire period of time, Joinpoint allows for points where the slope of the trend can change during the time period (joinpoints). This method starts with a model that assumes one consistent trend over time, and tests whether the addition of these “joinpoints” result in a model which has a fit that represents a statistically significant improvement over the model with no joinpoints. These models are tested through use of Monte Carlo permutations, e.g., the program repeats the same analysis multiple times using random samples to identify the “true” proportion of times that a comparison is statistically significant. The models allowed for a maximum of three joinpoints (two for non-malignant tumors), a minimum of three years from a joinpoint to either end of the time-period, and a minimum of three years between joinpoints.31
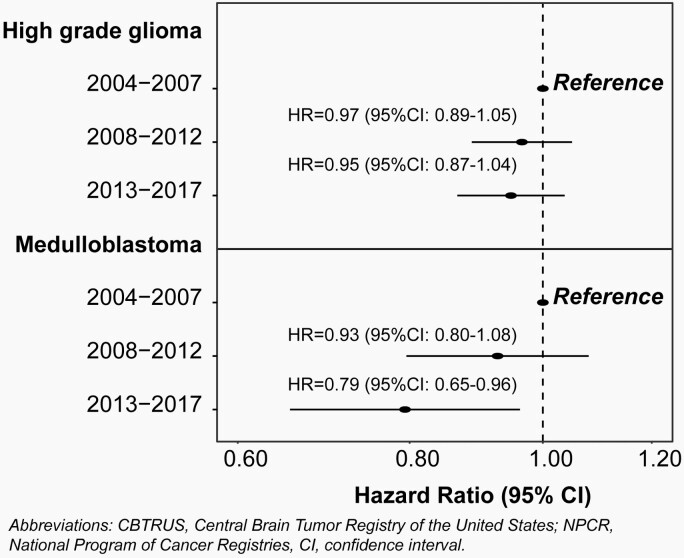
Survival Trends
.— For analysis of survival trends, year of diagnosis was divided into 3, equal time periods: 2004–2007, 2008–2012, and 2013–2017. Univariate Kaplan-Meier analysis was performed to assess differences in overall survival by time-period in individuals 0–19 years of age. Kaplan-Meier survival curves were generated for patients with a high-grade glioma and then specifically for patients with a medulloblastoma. Log rank tests were performed to evaluate differences in survival curves. Age-stratified multivariable Cox proportional hazard models, adjusted for sex, race/ethnicity, and treatment, were performed for each time-period, hazard ratios (HR) and associated 95% CI are reported. Surgery subgroups were defined by SEER site specific surgery codes for primary brain and CNS: no surgery (00), excisional/subtotal resection (20, 21, 40), and gross total resection (30, 55). Treatment was defined on the basis of radiation and surgery received. The Cox proportional hazard assumptions were tested and models were not found to be in violation.
Results
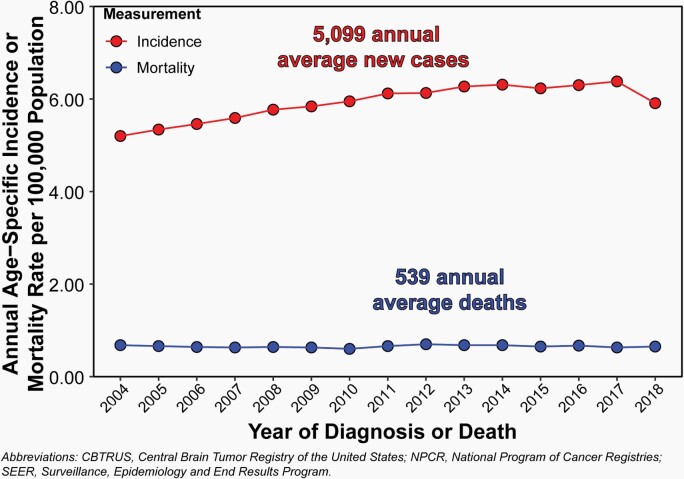
Overall, the annual average age-specific incidence rate of all malignant and non-malignant brain and other CNS tumors in children and adolescents ages 0–19 years was 6.23 per 100,000 population between 2014 and 2018, for an average of 5,099 newly diagnosed cases per year (Figure 2). During the same period, there were 2,693 deaths attributed to malignant brain and other CNS tumors in children and adolescents ages 0–19 years. This represents an annual average mortality rate of 0.66 per 100,000 population, and an average of 539 deaths per year caused by malignant brain and other CNS tumors (Figure 2).
Fig. 2.
Annual Incidence Rates of All Primary Brain and Other Central Nervous System Tumors and Mortality Rates of Malignant Primary Brain and Other Central Nervous System Tumors in Children and Adolescents Ages 0–19 Years, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2004–2018.
Central Cancer (State) Registry-Specific and Regional Brain Tumor Incidence Rates
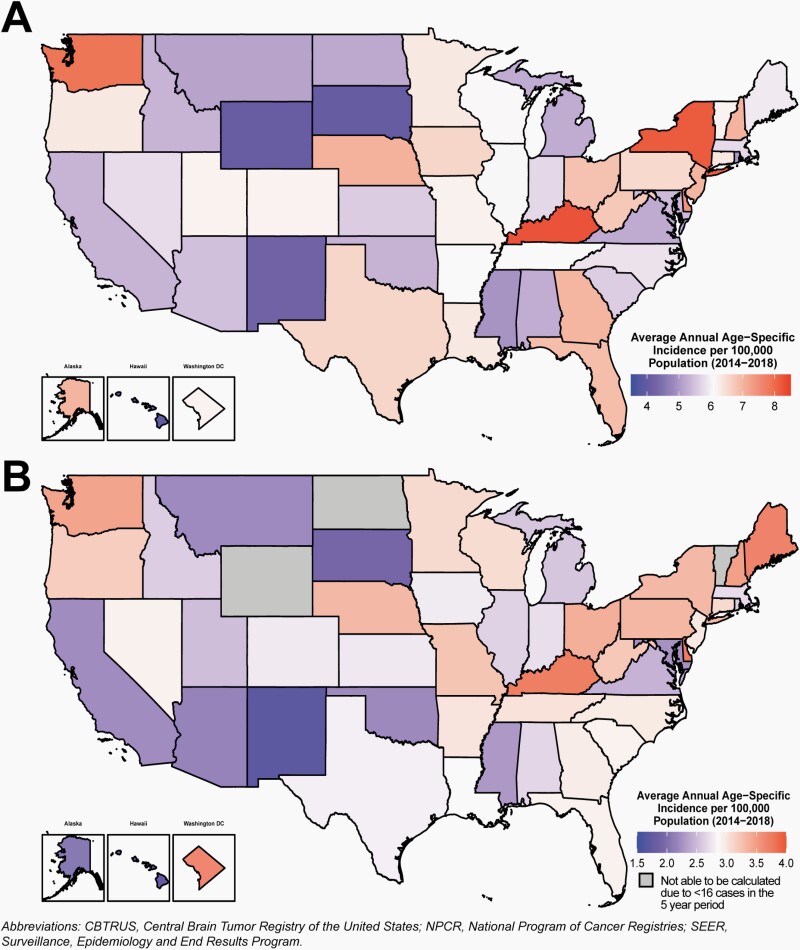
The overall incidence rates for all primary brain and other central nervous system tumors and glioma in children and adolescents by CCR are shown in Figure 3 and Supplementary Table 4.
Fig. 3.
Average Annual Age-Specific Incidence Rates per 100,000 Population of A) All Primary Malignant and Non-Malignant Brain and Other Central Nervous System Tumors, and B) Glioma in Children and Adolescents Ages 0–19 Years by Central Cancer Registry, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2014–2018.
Incidence of brain and other CNS tumors (Figure 3A), and glioma (Figure 3B) varied by CCR. Regional variations between CCR likely reflect differences in reporting and case ascertainment practices, as well as demographic differences in the underlying population that are associated with variation in brain and CNS tumor risk.
There is slight variation by region for all brain and other CNS tumor incidence rates by age group. Please see Supplementary Figure 1 for incidence of children and adolescents ages 0–4, 5–9, 10–14, and 15–19 years.
Internationally, incidence (Supplementary Figure 2A) and mortality (Supplementary Figure 2B) due to primary brain and other CNS tumors in children and adolescents 0–19 years of age varied by country and region.
Higher income countries have higher average annual incidence than their counterparts, with the United States and Canada representing regions with the highest incidence of childhood and adolescent brain and other CNS tumors.
Frequency of Brain and Other CNS Tumor Histopathologies
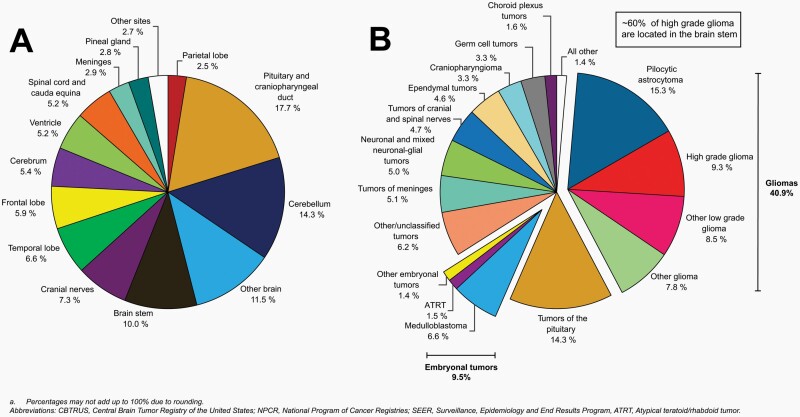
The distribution of brain and other CNS tumors in children and adolescents ages 0–19 years by site is shown in Figure 4A.
Fig. 4.
Distributiona in Children and Adolescents Ages 0–19 Years of All Primary Malignant and Non-Malignant Brain and Other Central Nervous System Tumors (Five-Year Total = 25,497; Annual Average Cases = 5,099) by A) Site and B) Histopathology, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2014–2018.
The most common site was the pituitary and craniopharyngeal duct (17.7%), followed by the cerebellum (14.3%).
While tumors in the brain stem accounted for 10% of all tumors, it is the primary site (~60%) for high grade glioma tumors (Figure 4B).
Cerebrum, ventricle, and brain stem accounted for 5.4%, 5.2%, and 10%.
Other brain is a designation used in cancer registry data when the location of a tumor is not identified in a patient’s record, or when a tumor involves multiple locations in the brain. Please refer to Supplementary Table 1 for more information about the specific sites included in these groups.
The distribution of childhood and adolescent brain and other CNS tumors by histopathology is shown in Figure 4B. Frequencies for each histopathology are presented in Table 2. Frequencies by age groups are presented in Supplementary Figures 3–7.
Table 2.
Annual Average Age-Specific Incidence Ratesa for Brain and Other Central Nervous System Tumors Ages 0–19 Years by Major Histopathology Groupings, Histopathology, and Sex, CBTRUS Childhood and Adolescent Brain Tumor Report: NPCR and SEER, 2014–2018
| Histopathology | Total | Male | Female | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Cases (2014–2018) | Annual Average | Median Age | % All tumors | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | Rate (95% CI) | |
| Gliomasb | 11,474 | 2,295 | 8 | 45.0 | 2.80 (2.75–2.85) | 6,041 | 1,208 | 2.89 (2.82–2.96) | 5,433 | 1,087 | 2.71 (2.64–2.79) |
| Pilocytic astrocytoma | 3,877 | 775 | 7 | 15.2 | 0.95 (0.92–0.98) | 1,983 | 397 | 0.95 (0.91–0.99) | 1,894 | 379 | 0.95 (0.90–0.99) |
| Other Low grade glioma | 2,164 | 433 | 11 | 8.5 | 0.53 (0.51–0.55) | 1,188 | 238 | 0.57 (0.54–0.60) | 976 | 195 | 0.49 (0.46–0.52) |
| High grade glioma | 2,361 | 472 | 9 | 9.3 | 0.58 (0.55–0.60) | 1,214 | 243 | 0.58 (0.55–0.61) | 1,147 | 229 | 0.57 (0.54–0.61) |
| Other glioma | 6,949 | 1,390 | 7 | 27.3 | 1.70 (1.66–1.74) | 3,639 | 728 | 1.74 (1.68–1.80) | 3,310 | 662 | 1.65 (1.60–1.71) |
| Ependymal tumors | 1,176 | 235 | 7 | 4.6 | 0.29 (0.27–0.30) | 664 | 133 | 0.32 (0.29–0.34) | 512 | 102 | 0.26 (0.23–0.28) |
| Choroid plexus tumors | 416 | 83 | 2 | 1.6 | 0.10 (0.09–0.11) | 238 | 48 | 0.11 (0.10–0.13) | 178 | 36 | 0.09 (0.08–0.10) |
| Neuronal and mixed neuronal-glial tumors | 1,282 | 256 | 12 | 5.0 | 0.31 (0.30–0.33) | 697 | 139 | 0.33 (0.31–0.36) | 585 | 117 | 0.29 (0.27–0.32) |
| Tumors of the pineal region | 213 | 43 | 9 | 0.8 | 0.05 (0.05–0.06) | 107 | 21 | 0.05 (0.04–0.06) | 106 | 21 | 0.05 (0.04–0.06) |
| Embryonal tumors | 2,397 | 479 | 5 | 9.4 | 0.59 (0.56–0.61) | 1,431 | 286 | 0.68 (0.65–0.72) | 966 | 193 | 0.48 (0.45–0.51) |
| Medulloblastoma | 1,662 | 332 | 7 | 6.5 | 0.41 (0.39–0.43) | 1,063 | 213 | 0.51 (0.48–0.54) | 599 | 120 | 0.30 (0.28–0.32) |
| ATRT | 383 | 77 | 1 | 1.5 | 0.09 (0.08–0.10) | 191 | 38 | 0.09 (0.08–0.11) | 192 | 38 | 0.10 (0.08–0.11) |
| Other embryonal tumors | 352 | 70 | 3 | 1.4 | 0.09 (0.08–0.10) | 177 | 35 | 0.08 (0.07–0.10) | 175 | 35 | 0.09 (0.07–0.10) |
| Tumors of cranial and spinal nerves | 1,202 | 240 | 12 | 4.7 | 0.29 (0.28–0.31) | 651 | 130 | 0.31 (0.29–0.34) | 551 | 110 | 0.28 (0.25–0.30) |
| Tumors of meninges | 1,291 | 258 | 14 | 5.1 | 0.32 (0.30–0.33) | 622 | 124 | 0.30 (0.27–0.32) | 669 | 134 | 0.33 (0.31–0.36) |
| Lymphomas and hematopoietic neoplasms | 135 | 27 | 11 | 0.5 | 0.03 (0.03–0.04) | 78 | 16 | 0.04 (0.03–0.05) | 57 | 11 | 0.03 (0.02–0.04) |
| Germ cell tumors | 849 | 170 | 12 | 3.3 | 0.21 (0.19–0.22) | 596 | 119 | 0.28 (0.26–0.31) | 253 | 51 | 0.13 (0.11–0.14) |
| Tumors of sellar region | 4,484 | 897 | 16 | 17.6 | 1.10 (1.06–1.13) | 1,420 | 284 | 0.68 (0.64–0.72) | 3,064 | 613 | 1.53 (1.48–1.58) |
| Tumors of the pituitary | 3,639 | 728 | 16 | 14.3 | 0.89 (0.86–0.92) | 946 | 189 | 0.45 (0.42–0.48) | 2,693 | 539 | 1.34 (1.29–1.40) |
| Craniopharyngioma | 845 | 169 | 9 | 3.3 | 0.21 (0.19–0.22) | 474 | 95 | 0.23 (0.21–0.25) | 371 | 74 | 0.19 (0.17–0.21) |
| Unclassified Tumors | 1,570 | 314 | 11 | 6.2 | 0.38 (0.36–0.40) | 808 | 162 | 0.39 (0.36–0.41) | 762 | 152 | 0.38 (0.35–0.41) |
| Totalc | 25,497 | 5,099 | 9 | 100.0 | 6.23 (6.15–6.30) | 12,780 | 2,556 | 6.11 (6.00–6.22) | 12,717 | 2,543 | 6.35 (6.24–6.46) |
aRates are per 100,000.
bCBTRUS defines the broad category of gliomas to include ICD-O-3 histopathology codes 9380–9384, 9391–9460, 9480.
cIncludes histopathologies not listed in this table.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology and End Results Program; CI, confidence interval; NOS, not otherwise specified; ATRT, Atypical teratoid/rhabdoid tumor.
The most frequently reported histopathology in all ages (0–19 years) was pilocytic astrocytoma (15.3%).
Tumors of the pituitary accounted for 14.3% of all childhood and adolescent brain and other CNS tumor histopathologies.
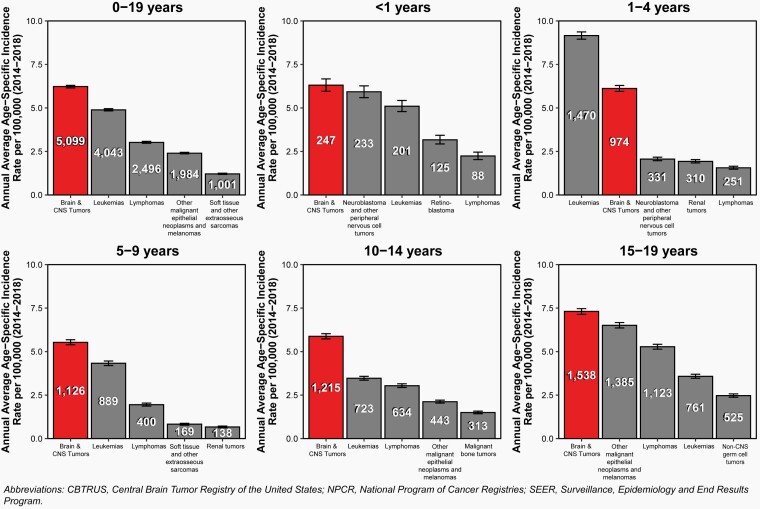
In infants (<1 year of age), gliomas (32.0%) and embryonal tumors (20.8%) were the most commonly occurring tumor type. Of embryonal tumors, 55.6% were atypical teratoid/rhabdoid tumors (ATRT).
In children ages 1–4 years and 5–9 years, gliomas (50.5% and 50.3%, respectively) and embryonal tumors (16.7% and 13.1%, respectively) were the most common tumor types. Among children 1–4 years, 58% of embryonal tumors were medulloblastomas.
In children ages 10–14 years, gliomas (43.0%) and tumors of the pituitary (12.3%) were the most common tumor types.
In adolescents ages 15–19 years, tumors of the pituitary (33.0%) were the most common tumor type, followed by gliomas (27.7%).
Age-Specific Incidence Rates
Incidence Rates by Age at Diagnosis
.— The overall age-specific incidence rate for 2014–2018 for all primary brain and other CNS tumors in children and adolescents (0–19 years of age) was 6.23 per 100,000 population (Table 1). The overall incidence rate was 6.31 per 100,000 population for children < 1 year of age, 6.12 per 100,000 population for children ages 1–4 years, 5.53 per 100,000 population for children ages 5–9 years, 5.88 per 100,000 population for adolescents ages 10–14 years, and 7.31 per 100,000 population for adolescents ages 15–19 years.
Table 1.
Average Casesa, Age-Specific Incidence Ratesb, Average Annual Deaths, Age-Specific Mortality Ratesb, and Five-Year Relative Survival with 95% Confidence Intervals for Brain and Other Central Nervous System Tumors Ages 0–19 Years by Behavior, Sex, Age Groups, Race, and Hispanic Ethnicity, (CBTRUS: Incidence Data provided by CDC’s NPCR and NCI’s SEER Program, 2014–2018; Mortality Data provided by NCHS’s NVSS Program, 2014–2018; Survival Data provided by CDC’s NPCR Program, 2001–2017)
| Group | Incidence Rate (2014–2018) | Mortality Rate (2014–2018) | 5-year Relative Survival (2001–2017) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Malignantc | Non-Malignantd | Malignant Onlyc | Total | Malignantc | Non-Malignantd | |||||
| Annual Average Cases | Rate (95% CI) | Annual Average Cases | Rate (95% CI) | Annual Average Cases | Rate (95% CI) | Annual Average Deaths | Rate (95% CI) | RS (95%CI) | RS (95% CI) | RS (95% CI) | |
| Sex | |||||||||||
| Male | 2,556 | 6.11 (6.00–6.22) | 1,584 | 3.78 (3.70–3.87) | 972 | 2.32 (2.26–2.39) | 297 | 0.71 (0.67–0.75) | 82.8 (82.3–83.2) | 75.7 (75.1–76.3) | 97.7 (97.4–98.1) |
| Female | 2,543 | 6.35 (6.24–6.46) | 1,326 | 3.31 (3.23–3.39) | 1,217 | 3.04 (2.96–3.12) | 242 | 0.60 (0.57–0.64) | 85.1 (84.7–85.5) | 75.6 (74.9–76.2) | 98.4 (98.1–98.6) |
| Age Group | |||||||||||
| <1 year | 247 | 6.31 (5.96–6.67) | 152 | 3.89 (3.62–4.18) | 95 | 2.42 (2.21–2.65) | 12 | 0.31 (0.24–0.40) | 71.9 (70.3–73.4) | 60.3 (58.1–62.3) | 94.1 (92.5–95.4) |
| 1–4 years | 974 | 6.12 (5.95–6.29) | 744 | 4.67 (4.52–4.83) | 230 | 1.44 (1.36–1.53) | 85 | 0.54 (0.49–0.59) | 79.3 (78.5–80.0) | 74.6 (73.7–75.4) | 97.4 (96.7–98.0) |
| 5–9 years | 1,126 | 5.53 (5.39–5.68) | 779 | 3.83 (3.71–3.95) | 347 | 1.71 (1.63–1.79) | 190 | 0.93 (0.87–0.99) | 79.9 (79.2–80.6) | 73.6 (72.7–74.5) | 97.6 (97.0–98.1) |
| 10–14 years | 1,215 | 5.88 (5.73–6.03) | 685 | 3.31 (3.20–3.43) | 530 | 2.56 (2.47–2.66) | 138 | 0.67 (0.62–0.72) | 87.0 (86.4–87.6) | 80.1 (79.3–81.0) | 98.2 (97.8–98.6) |
| 15–19 years | 1,538 | 7.31 (7.14–7.47) | 550 | 2.61 (2.51–2.71) | 988 | 4.69 (4.56–4.83) | 114 | 0.54 (0.50–0.58) | 90.5 (90.0–90.9) | 79.3 (78.3–80.2) | 98.9 (98.6–99.1) |
| Race | |||||||||||
| White | 3,971 | 6.44 (6.35–6.53) | 2,276 | 3.69 (3.62–3.76) | 1,695 | 2.75 (2.69–2.81) | 415 | 0.68 (0.65–0.70) | 84.6 (84.3–84.9) | 76.8 (76.3–77.3) | 98.2 (98.0–98.4) |
| Black | 665 | 4.88 (4.71–5.05) | 373 | 2.73 (2.61–2.86) | 293 | 2.15 (2.04–2.26) | 88 | 0.65 (0.59–0.71) | 79.1 (78.1–80.0) | 68.3 (66.9–69.6) | 96.9 (96.1–97.5) |
| American Indian/Alaska Native | 51 | 3.31 (2.92–3.75) | 27 | 1.79 (1.51–2.12) | 23 | 1.52 (1.26–1.82) | 7 | 0.45 (0.31–0.63) | 83.7 (80.4–86.5) | 71.7 (66.5–76.2) | 99.8 (92.0–100.0) |
| Asian or Pacific Islander | 288 | 5.66 (5.37–5.96) | 166 | 3.26 (3.04–3.49) | 122 | 2.40 (2.21–2.60) | 28 | 0.55 (0.47–0.65) | 80.6 (78.9–82.2) | 71.3 (68.9–73.6) | 98.1 (96.9–98.9) |
| Ethnicity | |||||||||||
| Non-Hispanic | 4,026 | 6.52 (6.43–6.61) | 2,347 | 3.80 (3.73–3.87) | 1,679 | 2.72 (2.66–2.78) | 416 | 0.68 (0.65–0.71) | 84.4 (84.0–84.7) | 76.5 (76.1–77.0) | 98.2 (98.0–98.4) |
| Hispanic | 1,073 | 5.33 (5.19–5.47) | 563 | 2.79 (2.69–2.90) | 510 | 2.53 (2.44–2.63) | 122 | 0.60 (0.55–0.65) | 82.1 (81.4–82.8) | 71.7 (70.6–72.7) | 97.8 (97.3–98.2) |
| Total | 5,099 | 6.23 (6.15–6.30) | 2,910 | 3.55 (3.50–3.61) | 2,190 | 2.67 (2.62–2.72) | 539 | 0.66 (0.63–0.68) | 83.9 (83.6–84.2) | 75.6 (75.2–76.1) | 98.1 (97.9–98.3) |
aAnnual average cases are calculated by dividing the five-year total by five.
bRates are per 100,000.
cAssigned behavior code of/3 by the International Classification for Disease, Oncology 3rd edition (see Supplementary Table 3).
dAssigned behavior code of/0 or/1 by the International Classification for Disease, Oncology 3rd edition (see Supplementary Table 3).
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; CI, confidence interval; NPCR, National Program of Cancer Registries; RS, Relative Survival; SEER, Surveillance, Epidemiology, and End Results Program; NCI, National Cancer Institute; CDC, Centers for Disease Control and Prevention; NCHS, National Center for Health Statistics; NVSS, National Vital Statistics System.
Incidence Rates by Age at Diagnosis and Histopathology
— The age-specific incidence rates by age and histopathology at diagnosis are shown in Table 3.
Table 3.
Annual Average Age-Specific Incidence Ratesa for Childhood Brain and Other Central Nervous System Tumors Ages 0–19 Years by Major Histopathology Groupings, Histopathology, and Age Groups, CBTRUS Childhood and Adolescent Brain Tumor Report: NPCR and SEER, 2014–2018
| Histopathology | <1 year | 1–4 years | 5–9 years | 10–14 years | 15–19 years | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Cases (2014–2018) | Annual Average | % of All Cases in Age Group | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | % of All Cases in Age Group | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | % of All Cases in Age Group | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | % of All Cases in Age Group | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | % of All Cases in Age Group | Rate (95% CI) | |
| Gliomas b | 458 | 92 | 4.0% | 2.34 (2.13–2.57) | 2,824 | 565 | 24.6% | 3.55 (3.42–3.68) | 3,019 | 604 | 26.3% | 2.97 (2.86–3.08) | 2,822 | 564 | 24.6% | 2.73 (2.63–2.83) | 2,351 | 470 | 20.5% | 2.23 (2.14–2.33) |
| Pilocytic astrocytoma | 92 | 18 | 2.4% | 0.47 (0.38–0.58) | 1,135 | 227 | 29.3% | 1.43 (1.34–1.51) | 1,109 | 222 | 28.6% | 1.09 (1.03–1.16) | 914 | 183 | 23.6% | 0.88 (0.83–0.94) | 627 | 125 | 16.2% | 0.60 (0.55–0.64) |
| Other Low grade glioma | 142 | 28 | 6.6% | 0.73 (0.61–0.86) | 347 | 69 | 16.0% | 0.44 (0.39–0.48) | 416 | 83 | 19.2% | 0.41 (0.37–0.45) | 616 | 123 | 28.5% | 0.60 (0.55–0.65) | 643 | 129 | 29.7% | 0.61 (0.56–0.66) |
| High grade glioma | 84 | 17 | 3.6% | 0.43 (0.34–0.53) | 388 | 78 | 16.4% | 0.49 (0.44–0.54) | 763 | 153 | 32.3% | 0.75 (0.70–0.81) | 603 | 121 | 25.5% | 0.58 (0.54–0.63) | 523 | 105 | 22.2% | 0.50 (0.46–0.54) |
| Other glioma | 75 | 15 | 1.1% | 0.38 (0.30–0.48) | 574 | 115 | 8.3% | 0.72 (0.66–0.78) | 534 | 107 | 7.7% | 0.52 (0.48–0.57) | 469 | 94 | 6.7% | 0.45 (0.41–0.50) | 332 | 66 | 4.8% | 0.32 (0.28–0.35) |
| Ependymal tumors | 64 | 13 | 5.4% | 0.33 (0.25–0.42) | 392 | 78 | 33.3% | 0.49 (0.44–0.54) | 242 | 48 | 20.6% | 0.24 (0.21–0.27) | 243 | 49 | 20.7% | 0.24 (0.21–0.27) | 235 | 47 | 20.0% | 0.22 (0.20–0.25) |
| Choroid plexus tumors | 123 | 25 | 29.6% | 0.63 (0.52–0.75) | 138 | 28 | 33.2% | 0.17 (0.15–0.20) | 51 | 10 | 12.3% | 0.05 (0.04–0.07) | 57 | 11 | 13.7% | 0.06 (0.04–0.07) | 47 | 9 | 11.3% | 0.04 (0.03–0.06) |
| Neuronal and mixed neuronal-glial tumors | 31 | 6 | 2.4% | 0.16 (0.11–0.22) | 162 | 32 | 12.6% | 0.20 (0.17–0.24) | 237 | 47 | 18.5% | 0.23 (0.20–0.26) | 404 | 81 | 31.5% | 0.39 (0.35–0.43) | 448 | 90 | 34.9% | 0.43 (0.39–0.47) |
| Tumors of the pineal region | <16 | -- | -- | -- | 49 | 10 | 23.0% | 0.06 (0.05–0.08) | 51 | 10 | 23.9% | 0.05 (0.04–0.07) | -- | -- | -- | -- | 66 | 13 | 31.0% | 0.06 (0.05–0.08) |
| Embryonal tumors | 257 | 51 | 10.7% | 1.31 (1.16–1.48) | 808 | 162 | 33.7% | 1.01 (0.95–1.09) | 731 | 146 | 30.5% | 0.72 (0.67–0.77) | 400 | 80 | 16.7% | 0.39 (0.35–0.43) | 201 | 40 | 8.4% | 0.19 (0.17–0.22) |
| Medulloblastoma | 45 | 9 | 2.7% | 0.23 (0.17–0.31) | 469 | 94 | 28.2% | 0.59 (0.54–0.64) | 637 | 127 | 38.3% | 0.63 (0.58–0.68) | 345 | 69 | 20.8% | 0.33 (0.30–0.37) | 166 | 33 | 10.0% | 0.16 (0.13–0.18) |
| ATRT | 143 | 29 | 37.3% | 0.73 (0.62–0.86) | 190 | 38 | 49.6% | 0.24 (0.21–0.28) | 32 | 6 | 8.4% | 0.03 (0.02–0.04) | <16 | -- | -- | -- | <16 | -- | -- | -- |
| Other embryonal tumors | 69 | 14 | 19.6% | 0.35 (0.27–0.45) | 149 | 30 | 42.3% | 0.19 (0.16–0.22) | 62 | 12 | 17.6% | 0.06 (0.05–0.08) | <16 | -- | -- | -- | <16 | -- | -- | -- |
| Tumors of cranial and spinal nerves | 35 | 7 | 2.9% | 0.18 (0.12–0.25) | 220 | 44 | 18.3% | 0.28 (0.24–0.32) | 206 | 41 | 17.1% | 0.20 (0.18–0.23) | 287 | 57 | 23.9% | 0.28 (0.25–0.31) | 454 | 91 | 37.8% | 0.43 (0.39–0.47) |
| Tumors of meninges | 104 | 21 | 8.1% | 0.53 (0.43–0.64) | 147 | 29 | 11.4% | 0.18 (0.16–0.22) | 156 | 31 | 12.1% | 0.15 (0.13–0.18) | 291 | 58 | 22.5% | 0.28 (0.25–0.32) | 593 | 119 | 45.9% | 0.56 (0.52–0.61) |
| Lymphomas and hematopoietic neoplasms | <16 | -- | -- | -- | -- | -- | -- | -- | 34 | 7 | 25.2% | 0.03 (0.02–0.05) | 35 | 7 | 25.9% | 0.03 (0.02–0.05) | 47 | 9 | 34.8% | 0.04 (0.03–0.06) |
| Germ cell tumors | 62 | 12 | 7.3% | 0.32 (0.24–0.41) | 47 | 9 | 5.5% | 0.06 (0.04–0.08) | 156 | 31 | 18.4% | 0.15 (0.13–0.18) | 318 | 64 | 37.5% | 0.31 (0.27–0.34) | 266 | 53 | 31.3% | 0.25 (0.22–0.29) |
| Tumors of sellar region | 21 | 4 | 0.5% | 0.11 (0.07–0.16) | 173 | 35 | 3.9% | 0.22 (0.19–0.25) | 612 | 122 | 13.6% | 0.60 (0.55–0.65) | 965 | 193 | 21.5% | 0.93 (0.88–0.99) | 2,713 | 543 | 60.5% | 2.58 (2.48–2.68) |
| Tumors of the pituitary | <16 | -- | -- | -- | -- | -- | -- | -- | 308 | 62 | 8.5% | 0.30 (0.27–0.34) | 740 | 148 | 20.3% | 0.72 (0.67–0.77) | 2,543 | 509 | 69.9% | 2.42 (2.32–2.51) |
| Craniopharyngioma | <16 | -- | -- | -- | -- | -- | -- | -- | 304 | 61 | 36.0% | 0.30 (0.27–0.33) | 225 | 45 | 26.6% | 0.22 (0.19–0.25) | 170 | 34 | 20.1% | 0.16 (0.14–0.19) |
| Unclassified Tumors | 131 | 26 | 8.3% | 0.67 (0.56–0.79) | 244 | 49 | 15.5% | 0.31 (0.27–0.35) | 314 | 63 | 20.0% | 0.31 (0.28–0.34) | 409 | 82 | 26.1% | 0.40 (0.36–0.44) | 472 | 94 | 30.1% | 0.45 (0.41–0.49) |
| Totalc | 1,235 | 247 | 4.8% | 6.31 (5.96–6.67) | 4,871 | 974 | 19.1% | 6.12 (5.95–6.29) | 5,628 | 1,126 | 22.1% | 5.53 (5.39–5.68) | 6,074 | 1,215 | 23.8% | 5.88 (5.73–6.03) | 7,689 | 1,538 | 30.2% | 7.31 (7.14–7.47) |
aRates are per 100,000.
bCBTRUS defines the broad category of gliomas to include ICD-O-3 histopathology codes 9380–9384, 9391–9460, 9480.
cIncludes histopathologies not listed in this table.
Data are not presented when fewer than 16 cases were reported for the specific category. Average annual counts and associated rates cannot be provided when total cases (2014–2018) are fewer than 16 cases or when a value based on less than 16 cases can be back-calculated using a cell. Suppressed cases are included in the total count.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; CI, confidence interval; CDC, Centers for Disease Control and Prevention; NCI, National Cancer Institute; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology and End Results Program; ATRT, Atypical teratoid/rhabdoid tumor.
Overall incidence rates of all brain and other CNS tumors by histopathology declined with increasing age from < 1 year of age to 10–14 years groups. Adolescents ages 15–19 years had the highest annual average age-specific incidence rate for all primary brain and other CNS tumors (7.31 per 100,000).
The incidence rates of all gliomas were highest in children ages 1–4 years (3.55 per 100,000) and lowest among adolescents ages 15–19 years (2.23 per 100,000).
Incidence rates of choroid plexus tumors, embryonal tumors, choroid plexus tumors decreased with age, respectively.
Incidence rates of neuronal and mixed neuronal-glial tumors, tumors of the cranial and spinal nerves, and tumors of the sellar region increased with age.
Diffuse intrinsic pontine glioma (DIPG) is a particularly devastating type of high-grade glioma that occurs in children. These tumors do not have a distinct ICD-O-3 site code in cancer registry data, but incidence of high-grade glioma of the brain stem is presented in Table 4.
Table 4.
Annual Average Totala, Age-Specific Incidence Ratesb, and One-, Five-, Ten-, and Fifteen-Year Relative Survival with 95% Confidence Intervals for High Grade Glioma in the Brain Stemc Ages 0–19 Years by Sex, Age Groups, Race, and Hispanic Ethnicity, (CBTRUS: Incidence Data provided by CDC’s NPCR and NCI’s SEER Program, 2014–2018; Survival Data provided by CDC’s NPCR Program, 2001–2017)
| Group | Incidence | Relative Survival | ||||
|---|---|---|---|---|---|---|
| Annual Average Cases | Rate (95% CI) | 1-year RS (95% CI) | 5-year RS (95% CI) | 10-year RS (95% CI) | 15-year RS (95% CI) | |
| Sex | ||||||
| Male | 136 | 0.33 (0.30–0.35) | 63.2 (61.0–65.3) | 39.6 (37.3–41.9) | 38.1 (35.7–40.4) | 37.3 (34.9–39.8) |
| Female | 149 | 0.37 (0.34–0.40) | 59.9 (57.7–62.0) | 35.1 (32.9–37.3) | 33.3 (31.1–35.5) | 32.9 (30.6–35.1) |
| Age | ||||||
| <1 year | 6 | 0.15 (0.10–0.22) | 80.5 (69.4–87.9) | 75.8 (64.0–84.2) | 73.6 (61.1–82.6) | 73.6 (61.1–82.6) |
| 1–4 years | 61 | 0.38 (0.34–0.43) | 62.1 (58.9–65.1) | 32.3 (29.2–35.4) | 29.9 (26.8–33.1) | 28.4 (25.2–31.7) |
| 5–9 years | 115 | 0.56 (0.52–0.61) | 49.4 (47.0–51.9) | 25.1 (22.9–27.3) | 24.2 (22.0–26.5) | 24.0 (21.7–26.3) |
| 10–14 years | 67 | 0.33 (0.29–0.36) | 73.4 (70.2–76.3) | 51.7 (48.1–55.2) | 50.4 (46.7–54) | 50.1 (46.3–53.8) |
| 15–19 years | 36 | 0.17 (0.15–0.20) | 79.6 (75.4–83.1) | 60.8 (55.8–65.4) | 56.9 (51.5–61.9) | 56.9 (51.5–61.9) |
| Race | ||||||
| White | 211 | 0.34 (0.32–0.36) | 62.2 (60.4–63.9) | 39.1 (37.3–41.0) | 37.4 (35.5–39.3) | 36.8 (34.9–38.7) |
| Black | 52 | 0.38 (0.34–0.43) | 57.6 (53.7–61.3) | 29.2 (25.6–32.9) | 27.1 (23.5–30.9) | 26.6 (22.8–30.5) |
| American Indian/Alaska Native | <16 total cases from 2014–2018 | <16 total cases from 2014–2018 | < 50 cases | < 50 cases | < 50 cases | < 50 cases |
| Asian or Pacific Islander | 13 | 0.26 (0.20–0.33) | 57.5 (49.5–64.6) | 31.7 (24.5–39.2) | 30.8 (23.6–38.3) | 30.8 (23.6–38.3) |
| Ethnicity | ||||||
| Non-Hispanic | 228 | 0.37 (0.35–0.39) | 63.5 (61.7–65.1) | 39.3 (37.4–41.1) | 37.7 (35.8–39.5) | 37 (35.1–38.9) |
| Hispanic | 56 | 0.28 (0.25–0.31) | 54.2 (50.8–57.6) | 30.1 (26.9–33.4) | 28.0 (24.7–31.4) | 27.7 (24.4–31.1) |
| Total | 285 | 0.35 (0.33–0.37) | 61.5 (59.9–63.0) | 37.3 (35.7–38.9) | 35.6 (34.0–37.3) | 35.1 (33.4–36.7) |
aAnnual average cases are calculated by dividing the five-year total by five.
bRates are per 100,000.
cICD-O-3 site code C71.7 and ICD-O-3 morphology codes 9380, 9381, 9400, 9401, 9440, 9441, 9442/3, 9451, 9460 (See Supplementary Tables 1 and 2 for more information).
Incidence data are not presented when fewer than 16 cases were reported for the specific category and survival data is not presented when fewer than 50 cases were reported for the specific category.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; CI, confidence interval; NOS, not otherwise specified; CDC, Centers for Disease Control and Prevention; NCI, National Cancer Institute; NPCR, National Program of Cancer Registries; RS, Relative Survival; SEER, Surveillance, Epidemiology and End Results Program.
Overall incidence rate of high-grade glioma of the brain stem was 0.35 per 100,000 population.
These tumors occurred more frequently in children ages 5–9 years, where incidence is 0.56 per 100,000 population.
Incidence of these tumors was highest in White (0.34 per 100,000) and Black (0.38 per 100,000) children and adolescents, and was higher in non-Hispanic (0.37 per 100,000) as compared to Hispanic (0.28 per 100,000) children and adolescents.
Age-Specific Incidence Rates by Site and Age Groups
— Incidence rates for each site by age are shown in Table 5.
Table 5.
Annual Average Age-Specific Incidence Ratesa for Childhood Brain and Other Central Nervous System Tumors Ages 0–19 Years by Siteb and Age Groups, CBTRUS Childhood and Adolescent Brain Tumor Report: NPCR and SEER, 2014–2018
| Site | 0–19 years | <1 year | 1–4 years | 5–9 years | 10–14 years | 15–19 years | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Cases (2014–2018) | Annual Average | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | Rate (95% CI) | |
| Frontal, temporal, parietal, and occipital lobes of the brain | 4,128 | 826 | 1.01 (0.98–1.04) | 153 | 31 | 0.78 (0.66–0.92) | 606 | 121 | 0.76 (0.70–0.82) | 712 | 142 | 0.70 (0.65–0.75) | 1,234 | 247 | 1.19 (1.13–1.26) | 1,423 | 285 | 1.35 (1.28–1.42) |
| Frontal lobe | 1,499 | 300 | 0.37 (0.35–0.39) | 49 | 10 | 0.25 (0.19–0.33) | 207 | 41 | 0.26 (0.23–0.30) | 241 | 48 | 0.24 (0.21–0.27) | 450 | 90 | 0.44 (0.40–0.48) | 552 | 110 | 0.52 (0.48–0.57) |
| Temporal lobe | 1,687 | 337 | 0.41 (0.39–0.43) | 59 | 12 | 0.30 (0.23–0.39) | 256 | 51 | 0.32 (0.28–0.36) | 293 | 59 | 0.29 (0.26–0.32) | 507 | 101 | 0.49 (0.45–0.54) | 572 | 114 | 0.54 (0.50–0.59) |
| Parietal lobe | 649 | 130 | 0.16 (0.15–0.17) | 28 | 6 | 0.14 (0.10–0.21) | 115 | 23 | 0.14 (0.12–0.17) | 127 | 25 | 0.12 (0.10–0.15) | 190 | 38 | 0.18 (0.16–0.21) | 189 | 38 | 0.18 (0.15–0.21) |
| Occipital lobe | 293 | 59 | 0.07 (0.06–0.08) | 17 | 3 | 0.09 (0.05–0.14) | 28 | 6 | 0.04 (0.02–0.05) | 51 | 10 | 0.05 (0.04–0.07) | 87 | 17 | 0.08 (0.07–0.10) | 110 | 22 | 0.10 (0.09–0.13) |
| Cerebrum | 1,375 | 275 | 0.34 (0.32–0.35) | 71 | 14 | 0.36 (0.28–0.46) | 260 | 52 | 0.33 (0.29–0.37) | 363 | 73 | 0.36 (0.32–0.40) | 389 | 78 | 0.38 (0.34–0.42) | 292 | 58 | 0.28 (0.25–0.31) |
| Ventricle | 1,333 | 267 | 0.33 (0.31–0.34) | 205 | 41 | 1.05 (0.91–1.20) | 330 | 66 | 0.41 (0.37–0.46) | 241 | 48 | 0.24 (0.21–0.27) | 285 | 57 | 0.28 (0.24–0.31) | 272 | 54 | 0.26 (0.23–0.29) |
| Cerebellum | 3,635 | 727 | 0.89 (0.86–0.92) | 124 | 25 | 0.63 (0.53–0.76) | 980 | 196 | 1.23 (1.16–1.31) | 1,138 | 228 | 1.12 (1.05–1.19) | 803 | 161 | 0.78 (0.72–0.83) | 590 | 118 | 0.56 (0.52–0.61) |
| Brain stem | 2,562 | 512 | 0.63 (0.60–0.65) | 99 | 20 | 0.51 (0.41–0.62) | 642 | 128 | 0.81 (0.75–0.87) | 877 | 175 | 0.86 (0.81–0.92) | 581 | 116 | 0.56 (0.52–0.61) | 363 | 73 | 0.34 (0.31–0.38) |
| Other brain | 2,926 | 585 | 0.71 (0.69–0.74) | 271 | 54 | 1.38 (1.22–1.56) | 663 | 133 | 0.83 (0.77–0.90) | 703 | 141 | 0.69 (0.64–0.74) | 703 | 141 | 0.68 (0.63–0.73) | 586 | 117 | 0.56 (0.51–0.60) |
| Overlapping lesion of brain | 720 | 144 | 0.18 (0.16–0.19) | 62 | 12 | 0.32 (0.24–0.41) | 141 | 28 | 0.18 (0.15–0.21) | 163 | 33 | 0.16 (0.14–0.19) | 190 | 38 | 0.18 (0.16–0.21) | 164 | 33 | 0.16 (0.13–0.18) |
| Brain, NOS | 2,206 | 441 | 0.54 (0.52–0.56) | 209 | 42 | 1.07 (0.93–1.22) | 522 | 104 | 0.66 (0.60–0.71) | 540 | 108 | 0.53 (0.49–0.58) | 513 | 103 | 0.50 (0.45–0.54) | 422 | 84 | 0.40 (0.36–0.44) |
| Spinal cord and cauda equina | 1,330 | 266 | 0.32 (0.31–0.34) | 112 | 22 | 0.57 (0.47–0.69) | 268 | 54 | 0.34 (0.30–0.38) | 222 | 44 | 0.22 (0.19–0.25) | 331 | 66 | 0.32 (0.29–0.36) | 397 | 79 | 0.38 (0.34–0.42) |
| Cranial nerves | 1,859 | 372 | 0.45 (0.43–0.48) | 82 | 16 | 0.42 (0.33–0.52) | 699 | 140 | 0.88 (0.81–0.95) | 448 | 90 | 0.44 (0.40–0.48) | 307 | 61 | 0.30 (0.26–0.33) | 323 | 65 | 0.31 (0.27–0.34) |
| Olfactory nerve | <16 cases | -- | -- | <16 cases | -- | -- | <16 cases | -- | -- | <16 cases | -- | -- | <16 cases | -- | -- | <16 cases | -- | -- |
| Optic nerve | 1,371 | 274 | 0.33 (0.32–0.35) | 73 | 15 | 0.37 (0.29–0.47) | 676 | 135 | 0.85 (0.79–0.92) | 382 | 76 | 0.38 (0.34–0.42) | 157 | 31 | 0.15 (0.13–0.18) | 83 | 17 | 0.08 (0.06–0.10) |
| Acoustic nerve | 288 | 58 | 0.07 (0.06–0.08) | <16 cases | -- | -- | <16 cases | -- | -- | -- | -- | -- | 87 | 17 | 0.08 (0.07–0.10) | 159 | 32 | 0.15 (0.13–0.18) |
| Cranial nerve, NOS | 197 | 39 | 0.05 (0.04–0.06) | <16 cases | -- | -- | 17 | 3 | 0.02 (0.01–0.03) | 33 | 7 | 0.03 (0.02–0.05) | -- | -- | -- | -- | -- | -- |
| Other nervous system | 382 | 76 | 0.09 (0.08–0.10) | 40 | 8 | 0.20 (0.15–0.28) | 135 | 27 | 0.17 (0.14–0.20) | 93 | 19 | 0.09 (0.07–0.11) | 51 | 10 | 0.05 (0.04–0.06) | 63 | 13 | 0.06 (0.05–0.08) |
| Meninges | 738 | 148 | 0.18 (0.17–0.19) | 33 | 7 | 0.17 (0.12–0.24) | 60 | 12 | 0.08 (0.06–0.10) | 94 | 19 | 0.09 (0.07–0.11) | 175 | 35 | 0.17 (0.15–0.20) | 376 | 75 | 0.36 (0.32–0.40) |
| Pituitary and craniopharyngeal duct | 4,508 | 902 | 1.10 (1.07–1.13) | 23 | 5 | 0.12 (0.07–0.18) | 159 | 32 | 0.20 (0.17–0.23) | 603 | 121 | 0.59 (0.55–0.64) | 979 | 196 | 0.95 (0.89–1.01) | 2,744 | 549 | 2.61 (2.51–2.71) |
| Pituitary gland | 3,896 | 779 | 0.95 (0.92–0.98) | -- | -- | -- | -- | -- | -- | 365 | 73 | 0.36 (0.32–0.40) | 821 | 164 | 0.79 (0.74–0.85) | 2,628 | 526 | 2.50 (2.40–2.59) |
| Craniopharyngeal duct | 612 | 122 | 0.15 (0.14–0.16) | <16 cases | -- | -- | -- | -- | -- | 238 | 48 | 0.23 (0.21–0.27) | 158 | 32 | 0.15 (0.13–0.18) | 116 | 23 | 0.11 (0.09–0.13) |
| Pineal gland | 698 | 140 | 0.17 (0.16–0.18) | 22 | 4 | 0.11 (0.07–0.17) | 69 | 14 | 0.09 (0.07–0.11) | 133 | 27 | 0.13 (0.11–0.15) | 229 | 46 | 0.22 (0.19–0.25) | 245 | 49 | 0.23 (0.20–0.26) |
| Olfactory tumors of the nasal cavityc | 23 | 5 | 0.01 (0.00–0.01) | <16 cases | -- | -- | <16 cases | -- | -- | <16 cases | -- | -- | <16 cases | -- | -- | <16 cases | -- | -- |
| Total | 25,497 | 5,099 | 6.23 (6.15–6.30) | 1,235 | 247 | 6.31 (5.96–6.67) | 4,871 | 974 | 6.12 (5.95–6.29) | 5,628 | 1,126 | 5.53 (5.39–5.68) | 6,074 | 1,215 | 5.88 (5.73–6.03) | 7,689 | 1,538 | 7.31 (7.14–7.47) |
aRates are per 100,000.
bThe sites referred to in this table are loosely based on the categories and site codes defined in the SEER site/histology validation list.
cICD-O-3 histology codes 9522–9523 only.
Data are not presented when fewer than 16 cases were reported for the specific category. Average annual counts and associated rates cannot be provided when total cases (2014–2018) are fewer than 16 cases or when a value based on less than 16 cases can be back-calculated using a cell. Suppressed cases are included in the total count.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; CI, confidence interval; CDC, Centers for Disease Control and Prevention; NCI, National Cancer Institute; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology and End Results Program.
The site with the highest incidence of brain and other CNS tumors was the pituitary gland (0.95 per 100,000), followed by cerebellum (0.89 per 100,000).
In infants (< 1 year of age), the site with the highest incidence was brain, NOS (1.07 per 100,000). This may refer to the pons (which does not have a specific location code in the ICD-O-3 system) or to tumors that were otherwise not able to be biopsied. The second most frequently occurring site was the ventricle (1.05 per 100,000).
In children 1–4 years, the most common occurring site was the cerebellum (1.23 per 100,000), followed by the optic nerve (0.85 per 100,000).
In children 5–9 years, the site with highest incidence was cerebellum (1.12 per 100,000), followed by the brain stem (0.86 per 100,000).
In children 10–14 years, the sites with highest incidence were the pituitary gland (0.79 per 100,000) and the cerebellum (0.56 per 100,000).
In adolescents 15–19 years, the sites with highest incidence were the pituitary gland (2.50 per 100,000) and the temporal lobe (0.54 per 100,000).
Median Age at Diagnosis
The median age at diagnosis for all primary brain and other CNS tumors among children and adolescents ages 0–19 years was 9 years old (Table 2).
The histopathology-specific median ages ranged from 1 year for ATRT to 16 years for tumors of the sellar region and subtype tumors of the pituitary.
Choroid plexus tumors, embryonal tumors, ATRT, and other embryonal tumors were histopathologies with median age < 6 years of age at diagnosis.
ATRT and other embryonal tumors were primarily diagnosed in infants and children 0–9 years of age at diagnosis, while tumors of the pineal region, lymphomas and hematopoietic neoplasms, tumors of the pituitary, and craniopharyngioma were rarely diagnosed in infants < 1 year of age.
Sex-, Race-, And Hispanic Ethnicity-Specific Incidence Rates
Distribution and Incidence by Sex, Behavior, and Histopathology
— Overall incidence of primary brain and other CNS tumors by sex and behavior are shown in Table 1. Incidence rates, counts of total cases and annual average cases by sex and histopathology are shown in Table 2 and Figure 5, and are further stratified by age groups in Supplementary Table 4.
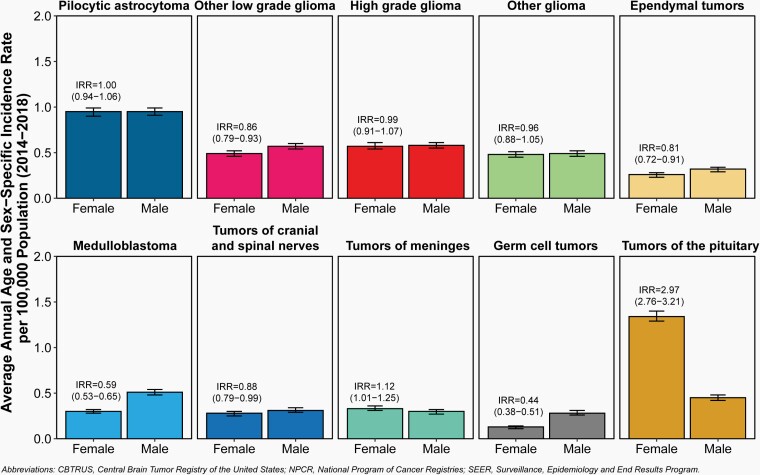
Fig. 5.
Incidence Rates and Incidence Rate Ratios (IRR) with 95% Confidence Intervals by Sex for Selected Primary Brain and Other Central Nervous System Tumor Histopathologies in Children and Adolescents Ages 0–19 Years, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2014–2018.
Overall, 50.1% of all primary brain and other CNS tumors in children and adolescents diagnosed between 2014–2018 occurred in males (12,780 tumors) and 49.9% in females (12,717 tumors) (Table 2).
Approximately 54.4% of the annual average malignant tumor cases occurred in males (1,584 tumors between 2014–2018) and 45.6% in females (1,326 tumors between 2014–2018) (Table 1).
Incidence rates for all primary brain and other CNS tumors combined were higher among females (6.35 per 100,000) than males (6.11 per 100,000).
Incidence was higher in males than females for histopathologies including other low-grade glioma (0.57 vs 0.49 per 100,000), ependymal tumors (0.32 vs 0.26 per 100,000), medulloblastoma (0.51 vs 0.30 per 100,000), and germ cell tumors (0.28 vs 0.13 per 100,000).
Incidence was higher in females than males for tumors of the pituitary (1.34 vs 0.45 per 100,000).
Incidence Rates by Race, Behavior, and Histopathology
— Overall incidence of primary brain and other CNS tumors by race and behavior are shown in Table 1. Incidence rates, counts of total cases and annual average cases by race and histopathology are shown in Table 6 and Figure 6, and are further stratified by age groups in Supplementary Table 4.
Table 6.
Annual Average Age-Specific Incidence Ratesa for Brain and Other Central Nervous System Tumors Ages 0–19 Years by Major Histopathology Groupings, Histopathology, Raceb and Hispanic Ethnicity, CBTRUS Childhood and Adolescent Report: NPCR and SEER, 2014–2018
| Histopathology | White | Black | AIAN | API | Hispanic | Non-Hispanic | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Cases (2014–2018) | Annual Average | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | Rate (95% CI) | Total Cases (2014–2018) | Annual Average | Rate (95% CI) | |
| Gliomasc | 9,068 | 1,814 | 2.94 (2.88–3.00) | 1,471 | 294 | 2.16 (2.05–2.27) | 103 | 21 | 1.35 (1.10–1.64) | 576 | 115 | 2.26 (2.08–2.45) | 2,092 | 418 | 2.08 (1.99–2.17) | 9,382 | 1,876 | 3.04 (2.98–3.10) |
| Pilocytic astrocytoma | 3,118 | 624 | 1.01 (0.98–1.05) | 461 | 92 | 0.68 (0.62–0.74) | 36 | 7 | 0.47 (0.33–0.65) | 178 | 36 | 0.70 (0.60–0.81) | 679 | 136 | 0.67 (0.62–0.73) | 3,198 | 640 | 1.04 (1.00–1.07) |
| Other Low grade glioma | 1,699 | 340 | 0.55 (0.53–0.58) | 277 | 55 | 0.41 (0.36–0.46) | 25 | 5 | 0.33 (0.21–0.48) | 118 | 24 | 0.46 (0.38–0.55) | 362 | 72 | 0.36 (0.32–0.40) | 1,802 | 360 | 0.58 (0.56–0.61) |
| High grade glioma | 1,801 | 360 | 0.58 (0.56–0.61) | 379 | 76 | 0.56 (0.50–0.61) | 21 | 4 | 0.27 (0.17–0.42) | 118 | 24 | 0.46 (0.38–0.55) | 475 | 95 | 0.47 (0.43–0.52) | 1,886 | 377 | 0.61 (0.58–0.64) |
| Other glioma | 1,580 | 316 | 0.51 (0.49–0.54) | 215 | 43 | 0.32 (0.27–0.36) | -- | -- | -- | 118 | 24 | 0.46 (0.38–0.55) | 334 | 67 | 0.33 (0.30–0.37) | 1,650 | 330 | 0.53 (0.51–0.56) |
| Ependymal tumors | 932 | 186 | 0.30 (0.28–0.32) | 149 | 30 | 0.22 (0.18–0.26) | <16 | -- | -- | 56 | 11 | 0.22 (0.17–0.29) | 268 | 54 | 0.27 (0.24–0.30) | 908 | 182 | 0.29 (0.28–0.31) |
| Choroid plexus tumors | 325 | 65 | 0.11 (0.09–0.12) | 56 | 11 | 0.08 (0.06–0.11) | <16 | -- | -- | 20 | 4 | 0.08 (0.05–0.12) | 92 | 18 | 0.09 (0.07–0.11) | 324 | 65 | 0.10 (0.09–0.12) |
| Neuronal and mixed neuronal-glial tumors | 1,010 | 202 | 0.33 (0.31–0.35) | 172 | 34 | 0.25 (0.22–0.29) | <16 | -- | -- | 72 | 14 | 0.28 (0.22–0.36) | 232 | 46 | 0.23 (0.20–0.26) | 1,050 | 210 | 0.34 (0.32–0.36) |
| Tumors of the pineal region | 140 | 28 | 0.05 (0.04–0.05) | 53 | 11 | 0.08 (0.06–0.10) | <16 | -- | -- | <16 | -- | -- | 40 | 8 | 0.04 (0.03–0.05) | 173 | 35 | 0.06 (0.05–0.07) |
| Embryonal tumors | 1,856 | 371 | 0.60 (0.58–0.63) | 292 | 58 | 0.43 (0.38–0.48) | 29 | 6 | 0.38 (0.25–0.55) | 159 | 32 | 0.62 (0.53–0.73) | 520 | 104 | 0.52 (0.47–0.56) | 1,877 | 375 | 0.61 (0.58–0.64) |
| Medulloblastoma | 1,306 | 261 | 0.42 (0.40–0.45) | 178 | 36 | 0.26 (0.22–0.30) | 22 | 4 | 0.29 (0.18–0.44) | 115 | 23 | 0.45 (0.37–0.54) | 349 | 70 | 0.35 (0.31–0.38) | 1,313 | 263 | 0.43 (0.40–0.45) |
| ATRT | 281 | 56 | 0.09 (0.08–0.10) | 58 | 12 | 0.09 (0.06–0.11) | <16 | -- | -- | -- | -- | -- | 82 | 16 | 0.08 (0.06–0.10) | 301 | 60 | 0.10 (0.09–0.11) |
| Other embryonal tumors | 269 | 54 | 0.09 (0.08–0.10) | 56 | 11 | 0.08 (0.06–0.11) | <16 | -- | -- | <16 | -- | -- | 89 | 18 | 0.09 (0.07–0.11) | 263 | 53 | 0.09 (0.08–0.10) |
| Tumors of cranial and spinal nerves | 935 | 187 | 0.30 (0.28–0.32) | 141 | 28 | 0.21 (0.17–0.24) | <16 | -- | -- | 79 | 16 | 0.31 (0.25–0.39) | 242 | 48 | 0.24 (0.21–0.27) | 960 | 192 | 0.31 (0.29–0.33) |
| Tumors of meninges | 1,013 | 203 | 0.33 (0.31–0.35) | 169 | 34 | 0.25 (0.21–0.29) | <16 | -- | -- | 69 | 14 | 0.27 (0.21–0.34) | 296 | 59 | 0.29 (0.26–0.33) | 995 | 199 | 0.32 (0.30–0.34) |
| Lymphomas and hematopoietic neoplasms | 97 | 19 | 0.03 (0.03–0.04) | 16 | 3 | 0.02 (0.01–0.04) | <16 | -- | -- | 20 | 4 | 0.08 (0.05–0.12) | 22 | 4 | 0.02 (0.01–0.03) | 113 | 23 | 0.04 (0.03–0.04) |
| Germ cell tumors | 643 | 129 | 0.21 (0.19–0.23) | 90 | 18 | 0.13 (0.11–0.16) | <16 | -- | -- | 86 | 17 | 0.34 (0.27–0.42) | 213 | 43 | 0.21 (0.18–0.24) | 636 | 127 | 0.21 (0.19–0.22) |
| Tumors of sellar region | 3,405 | 681 | 1.10 (1.07–1.14) | 654 | 131 | 0.96 (0.89–1.04) | 52 | 10 | 0.68 (0.51–0.89) | 240 | 48 | 0.94 (0.83–1.07) | 1,228 | 246 | 1.22 (1.15–1.29) | 3,256 | 651 | 1.05 (1.02–1.09) |
| Tumors of the pituitary | 2,783 | 557 | 0.90 (0.87–0.94) | 501 | 100 | 0.73 (0.67–0.80) | -- | -- | -- | 196 | 39 | 0.77 (0.67–0.89) | 1,035 | 207 | 1.03 (0.97–1.09) | 2,604 | 521 | 0.84 (0.81–0.88) |
| Craniopharyngioma | 622 | 124 | 0.20 (0.19–0.22) | 153 | 31 | 0.22 (0.19–0.26) | <16 | -- | -- | 44 | 9 | 0.17 (0.13–0.23) | 193 | 39 | 0.19 (0.17–0.22) | 652 | 130 | 0.21 (0.20–0.23) |
| Unclassified Tumors | 1,228 | 246 | 0.40 (0.38–0.42) | 192 | 38 | 0.28 (0.24–0.32) | 18 | 4 | 0.24 (0.14–0.37) | 90 | 18 | 0.35 (0.28–0.43) | 347 | 69 | 0.34 (0.31–0.38) | 1,223 | 245 | 0.40 (0.37–0.42) |
| Total d | 19,854 | 3,971 | 6.44 (6.35–6.53) | 3,326 | 665 | 4.88 (4.71–5.05) | 253 | 51 | 3.31 (2.92–3.75) | 1,441 | 288 | 5.66 (5.37–5.96) | 5,367 | 1,073 | 5.33 (5.19–5.47) | 20,130 | 4,026 | 6.52 (6.43–6.61) |
aRates are per 100,000.
bIndividuals with unknown race were excluded.
cCBTRUS defines the broad category of gliomas to include ICD-O-3 histopathology codes 9380–9384, 9391–9460, 9480.
dIncludes histopathologies not listed in this table.
Data are not presented when fewer than 16 cases were reported for the specific category. Average annual counts and associated rates cannot be provided when Total Cases (2014–2018) are fewer than 16 cases or when a value based on less than 16 cases can be back-calculated using a cell. Suppressed cases are included in the total count.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology and End Results Program; CI, confidence interval; NOS, not otherwise specified; ATRT, Atypical teratoid/rhabdoid tumor.
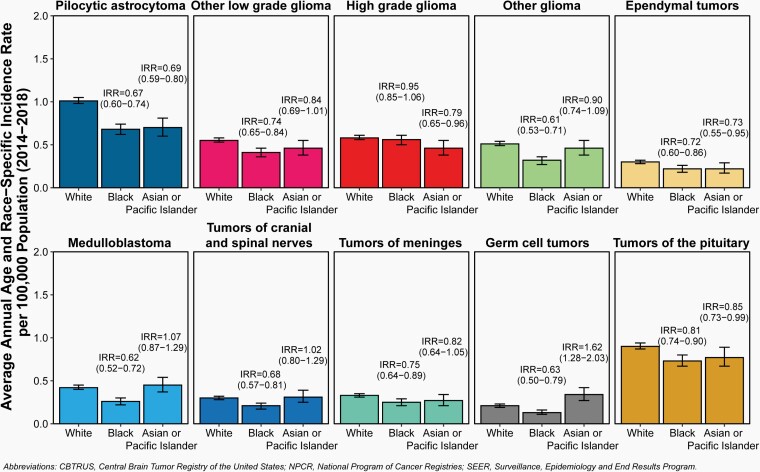
Fig. 6.
Incidence Rate and Incidence Rate Ratios (IRR) with 95% Confidence Intervals by Race for Selected Primary Brain and Other Central Nervous System Tumor Histopathologies in Children and Adolescents Ages 0–19 Years, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2014–2018.
Incidence rates for all primary brain and other CNS tumors combined were lower for AIAN children and adolescents (3.31 per 100,000) compared to White children and adolescents (6.44 per 100,000), Black children and adolescents (4.88 per 100,000), and API (5.66 per 100,000) children and adolescents.
Incidence rates for non-malignant primary brain and other CNS tumors were highest in White children and adolescents (2.75 per 100,000) compared to Black children and adolescents (2.15 per 100,000), AIAN children and adolescents (1.52 per 100,000), and API (2.40 per 100,000) children and adolescents.
Incidence rates for malignant primary brain and other CNS tumors were highest in White children and adolescents (3.69 per 100,000), compared to Black children and adolescents (2.73 per 100,000), AIAN children and adolescents (1.79 per 100,000), and API (3.26 per 100,000) children and adolescents.
AIAN children and adolescents had lowest incidence in all histopathology groups. This may reflect differences in access to diagnostic care.
Incidence of embryonal tumors, medulloblastoma, ATRT, tumors of cranial and spinal nerves, lymphomas and hematopoietic neoplasms, and germ cell tumors observed for API children and adolescents exceeded those observed for White, Black, and AIAN children and adolescents.
While there are histopathologies where significant differences in incidence were observed by race, in most cases the actual difference in incidence races is small and may not be biologically significant.
Incidence Rates by Hispanic Ethnicity, Behavior, and Histopathology
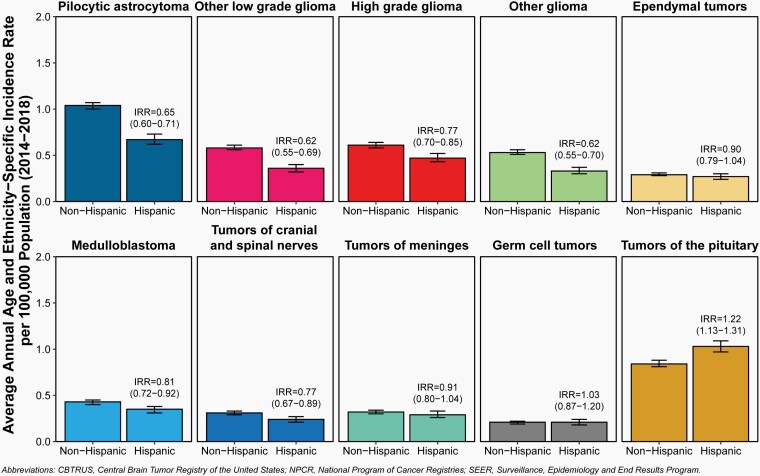
.— Incidence rates by Hispanic ethnicity and histopathology are shown in Table 1. Incidence counts of total cases, average annual age-specific rates, and incidence rate ratios by Hispanic ethnicity and histopathology are shown in Table 6 and Figure 7.
Fig. 7.
Incidence Rates and Incidence Rate Ratios (IRR) with 95% Confidence Intervals by Hispanic Ethnicity for Selected Primary Brain and Other Central Nervous System Tumor Histopathologies in Children and Adolescents Ages 0–19 Years, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2014–2018.
The overall incidence rate for primary brain and other CNS tumors in children and adolescents was 6.52 per 100,000 population among non-Hispanic children and adolescents and 5.33 per 100,000 population among their Hispanic counterparts.
Tumors of the sellar region and tumors of the pituitary were the only histopathologies that were higher in Hispanic than in non-Hispanics children and adolescents.
While there were histopathologies where significant differences in incidence were observed by Hispanic ethnicity, in most cases the actual difference in incidence rates is small and may not be biologically significant.
Frequency of and Incidence of Molecularly-Defined Brain and Other CNS Tumor Histopathologies
Beginning in diagnosis year 2018, US cancer registry systems began collecting data on molecularly defined histopathologies introduced in the 2016 WHO classification of tumours of the CNS, including IDH1/2 mutation and 1p/19q codeletion status for adult-type diffuse glioma, and medulloblastoma subtypes. Total cases of these histopathologies diagnosed in 2018–2019, age-specific incidence rates, median age of diagnosis, and distribution by sex and race/ethnicity are shown in Table 7.
Table 7.
Total Cases, Average Annual Age-Specific Incidencea, Median Age, Sex, and Race/Ethnicity of Molecularly-Defined Brain and Other Central Nervous System Tumors in Children and Adolescents Ages 0–19 Years, CBTRUS Childhood and Adolescent Report: NPCR and SEER, 2018–2019
| Tumor type | ICD-O-3 histopathology codes | Total New Cases (2018–2019) | Rate (95% CI) | Age (median, interquartile range) | Female (%) | Non-Hispanic White (%) | Non-Hispanic Black (%) | Hispanic (%) |
|---|---|---|---|---|---|---|---|---|
| Adult-type diffuse glioma | ||||||||
| IDH1/2 - mutant Astrocytomab | 9400/3, 9401/3, 9445/3 | 142 | 0.09 (0.07–0.10) | 15 (8.75–18) | 45.8 | 70.4 | -- | 16.2 |
| IDH1/2 - wildtype Astrocytoma and Glioblastomac | 9400/3, 9401/3, 9440/3 | 279 | 0.17 (0.15–0.19) | 10 (6–15) | 43.0 | 67.7 | 10.0 | 16.5 |
| IDH1/2 mutant & 1p/19q-codeleted Oligodendrogliomad | 9450/3, 9451/3 | 34 | 0.02 (0.01–0.03) | 15 (9.5–18) | -- | 82.4 | -- | -- |
| Medulloblastoma | ||||||||
| SHH-activated & TP53 wildtypee | 9471/3 | 80 | 0.05 (0.04–0.06) | 4.5 (2–9.25) | 31.3 | 51.2 | -- | 21.2 |
| SHH-activated & TP53 mutant | 9476/3 | < 16 cases | -- | -- | -- | -- | -- | -- |
| WNT-activated | 9475/3 | 25 | 0.02 (0.01–0.02) | 9 (7–11) | -- | 72.0 | -- | -- |
| nonWNT/nonSHH | 9477/3 | 122 | 0.08 (0.06–0.09) | 7 (4–10) | 34.4 | 63.1 | -- | 26.2 |
| Other tumor types | ||||||||
| Embryonal tumor with multilayered rosettes, C19MC-alteredf | 9478/3 | 25 | 0.02 (0.01–0.02) | 2 (1–3) | -- | -- | -- | |
| Ependymoma, RELA fusion | 9396/3 | < 16 cases | -- | -- | -- | -- | -- | |
| Diffuse midline glioma, H3 K27M-mutant | 9385/3 | 197 | 0.12 (0.11–0.14) | 8 (5–12) | 59.9 | 49.2 | 11.7 | 27.9 |
| Pilomyxoid astrocytoma | 9425/3 | 40 | 0.02 (0.02–0.03) | 3 (1–7) | 45.0 | 72.5 | -- | -- |
aRates are per 100,000.
bICD-O-3 histopathology code of 9400/3, 9401/3 and a BMM value of 1 or 3, or ICD-O-3 histopathology code of 9445/3.
cICD-O-3 histopathology code of 9400/3, 9401/3, 9440/3 and a BMM value of 2, 4, or 5.
dICD-O-3 histopathology code of 9450/3, 9451/3 and a BMM of 6 or7.
eICD-O-3 histopathology code of 9471/3 and a BMM value of 8.
fICD-O-3 histopathology code of 9478/3 and a BMM value of 9.
Data are not presented when fewer than 16 cases were reported for the specific category. Counts and associated rates cannot be provided when Total Cases (2018–2019) are fewer than 16 cases or when a value based on less than 16 cases can be back-calculated using a cell. Suppressed cases are included in the total count.
Abbreviations: BMM, brain molecular markers variable; CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology and End Results Program; CI, confidence interval.
While adult-type diffuse glioma is rare in children and adolescents, IDH1/2 - mutant astrocytoma had an incidence rate of 0.09 per 100,000 population, while IDH1/2 - wildtype astrocytoma had an incidence rate of 0.17 per 100,000 population. Median age of diagnosis for these subtypes was 15 and 10 years, respectively.
The most common medulloblastoma subtype is non-WNT/non-SHH, which had an incidence rate of 0.08 per 100,000 population and a median age of diagnosis of 7 years. SHH-activated & TP53 - wildtype medulloblastoma was the second most commonly occurring subtype, with an incidence rate of 0.05 per 100,000 and a median age of diagnosis of 4.5 years. Incidence of WNT-activated medulloblastoma was 0.02 per 100,000 population, with median age of diagnosis of 9 years. SHH-activated and TP53-mutant medulloblastoma was too rare to calculate incidence.
Molecular subtype data was missing for many medulloblastoma cases, but the completeness of these data are expected to increase in future years.
Embryonal tumor with multilayered rosettes, C19MC-altered had an incidence rate of 0.02 per 100,000 population and a median age of diagnosis of 2 years.
Diffuse midline glioma, H3 K27M-mutant had an incidence rate of 0.12 per 100,000 population and a median age of diagnosis of 8 years.
Incidence Time Trends
Time trends in cancer incidence are important measures of the changing burden of cancer in a population over time. Many factors may lead to fluctuations in rates over time, and all of these must be considered when interpreting time trends results. When assessing trends in incidence over time it is critical to use the most recent data available, as delays in reporting may cause small fluctuations in incidence. Time trends analysis methods are used to estimate if the annual percentage change (APC) is significantly different from 0% (meaning no change in incidence from year to year). In addition to assessing statistical significance of changes in incidence over time, the size of this change must also be considered because with datasets as large as CBTRUS very small fluctuations in incidence over time may be statistically significant but not truly represent a large change in proportion of individuals over time.
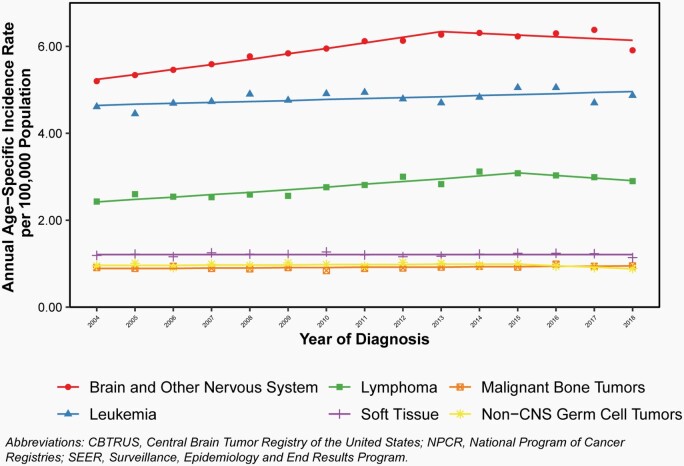
Annual age-specific incidence rates and APC for all primary brain and other CNS tumors in comparison to other common childhood and adolescent cancers are shown in Figure 8.
Fig. 8.
Annual Age-Specific Incidence Rates of All Primary Malignant and Non-Malignant Brain and Other Central Nervous System Tumors in Children and Adolescents Ages 0–19 Years, and Incidence Trends in Comparison to Other Common Childhood and Adolescent Cancers, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2004–2018.
Overall, incidence of all primary malignant and non-malignant brain and other CNS tumors increased significantly from 2004 to 2013 (APC = 2.1%; 95% CI: 1.6% - 2.7%), followed by relatively stable incidence after 2013.
Incidence of leukemia increased slightly over the entire period (APC = 0.5%, 95%CI: 0.1%-0.8%).
Incidence of lymphoma increased from 2004–2015 (APC = 2.2%, 95%CI: 1.6%-2.9%), but was stable thereafter.
Incidence of malignant bone tumors, soft tissue tumors, and non-CNS germ cell tumors remained stable across the entire period.
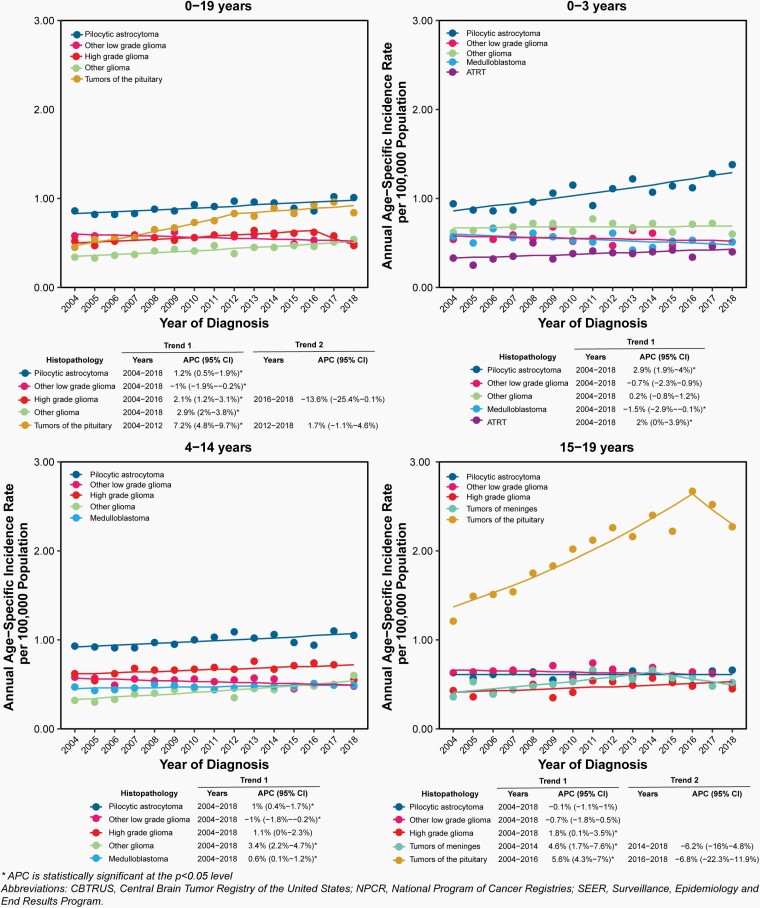
Annual age-specific incidence rates and APC for selected histopathologies overall and by age groups are shown in Figure 9. Complete APC results are available in Supplementary Table 7.
Fig. 9.
Annual Age-Specific Incidence Rates and Annual Percent Change (APC) with 95% Confidence Intervals (95% CI) of Selected Primary Malignant and Non-Malignant Brain and Other Central Nervous System Tumors and Incidence Trends by the Five Most Common Histopathologies by Age Group in All (Ages 0–19 Years), Infants (0–3 Years), Children (Ages 4–14 Years), and Adolescents (Ages 15–19 Years), CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2004–2018.
Incidence of pilocytic astrocytoma increased significantly from 2004 to 2018 (APC = 1.2%; 95%CI = 0.5% -1.9%). This increase was highest in infants 0–3 years old, where APC was 2.9% (95%CI: 1.9% - 4.0%)
High grade glioma incidence increased slightly, but significantly from 2004 to 2016 (APC = 2.1%; 95%CI: 1.2% - 3.1%).
There was a significant increase in incidence of tumors of the pituitary from 2004 to 2012 (APC = 7.2%; 95%CI: 4.8% - 9.7%) followed by relatively stable incidence after 2012.
The increases in incidence in tumors frequently diagnosed by imaging alone, such as tumors of the pituitary, are partially attributable to improved collection of radiographically diagnosed cases as well as improvement in collection of non-malignant cases in general over time.
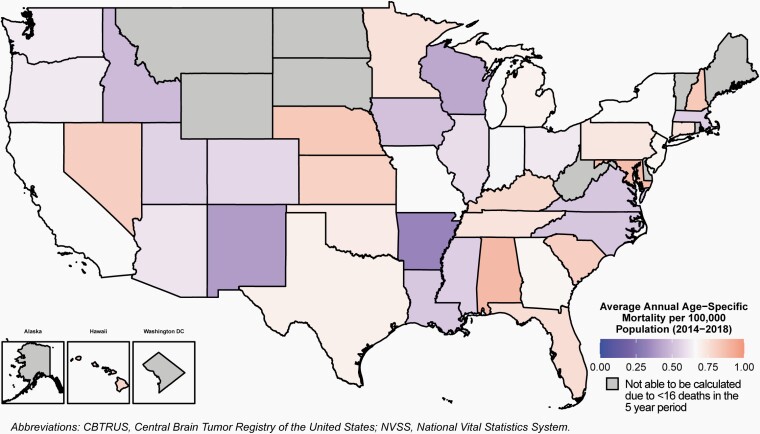
Age-, Sex-, Race-, Ethnicity- and State-Specific Brain Tumor Mortality Rates
Age-specific mortality rates for primary malignant brain and other CNS tumors in the United States during 2014–2018 overall, by sex, race, Hispanic ethnicity, age groups and state are shown in Table 1, Figure 10, and Supplementary Table 4.
Fig. 10.
Average Annual Age-Specific Mortality Rates for Malignant Primary Brain and Other Central Nervous System Tumors in Children and Adolescents Ages 0–19 Years by Central Cancer Registry, CBTRUS Childhood and Adolescent Report: NVSS, 2014–2018.
Mortality due to primary malignant brain tumors was higher in males as compared to females (0.71 per 100,000 as compared to 0.60 per 100,000).
Children ages 5–9 years had the highest mortality of any age group (0.93 per 100,000).
Mortality was highest in White (0.68 per 100,000) and Black (0.65 per 100,000) children and adolescents as compared to other racial groups.
Non-Hispanic children and adolescents had higher mortality as compared to Hispanic children and adolescents (0.68 per 100,000 as compared to 0.60 per 100,000).
Mortality due to primary malignant brain and CNS tumors varied by state, from a minimum of 0.33 per 100,000 to 1.50 per 100,000 population.
Mortality Time Trends
Time trends in cancer mortality are important measures of the changing burden of cancer in a population over time. Many factors may lead to fluctuations in rates over time, and all of these must be considered when interpreting time trends. When assessing trends in mortality over time it is critical to use the most recent data available, as delay in reporting may cause small fluctuations in incidence. Time trends analysis methods are used to estimate if the APC is significantly different from 0% (meaning no change in mortality from year to year).
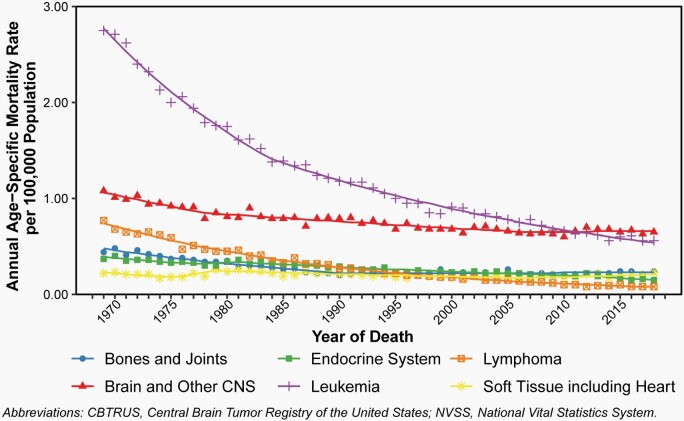
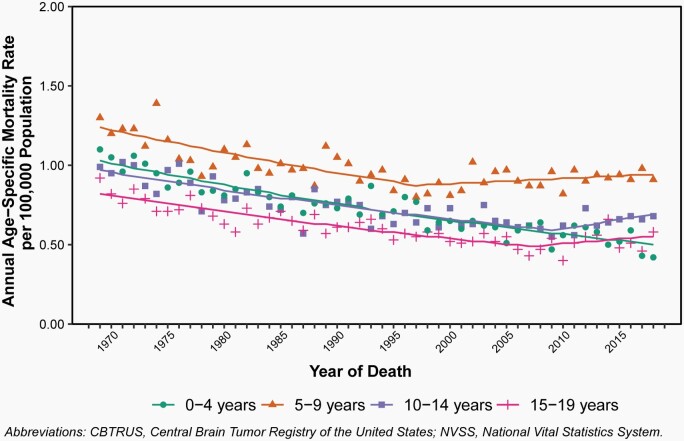
Annual age-specific mortality rates and APC for selected major causes of cancer death as compared to brain and other CNS tumors from 1975 to 2018 are shown in Figure 11 and shown in Figure 12 for annual age-specific mortality rates and APC due to primary malignant brain and other CNS tumors by age groups. Complete APC results are available in Supplementary Table 8.
Fig. 11.
Annual Age-Specific Mortality Rates of Primary Malignant and Non-Malignant Brain and Other Central Nervous System Tumors and Morality Trends in Children and Adolescents Ages 0–19 Years in Comparison to Other Common Childhood and Adolescent Cancers, CBTRUS Childhood and Adolescent Report: NVSS, 1969–2018.
Fig. 12.
Annual Age-Specific Mortality Rates of Primary Malignant and Non-Malignant Brain and Other Central Nervous System Tumors and Mortality Trends in Children and Adolescents Ages 0–19 Years by Age Group, CBTRUS Childhood and Adolescent Report: NVSS, 1969–2018.
Overall, mortality due to cancer has decreased in children and adolescents ages 0–19 years since 1975.
Some of the biggest decreases have come in leukemia, where mortality decreased by 4.4% per year from 1969 to 1984 (95% CI: -4.7% – -4.1%) and by 2.8% per year from 1984 to 2018 (95% CI: -2.9% – -2.7%).
While mortality due to brain and other CNS tumors also decreased by -2.5% from 1969 to 1978 (95% CI: -3.4% – -1.5%) and 0.9% from 1978 to 2007 (95% CI: -1.1% – -0.8%), there has been no significant change in brain and other CNS tumor morality in children and adolescents since 2007.
Although leukemia has historically been the most significant contributor to cancer death in children and adolescents, this trend now means that brain and other CNS tumors are the greatest source of cancer death in this age group.
Only the youngest age group has continued to make improvements in mortality rates due to brain and other CNS tumors. In children 0–4 years old, mortality has decreased 1.4% per year from 1969–2018 (95% CI: -1.6% – -1.3%). Mortality remains stable in all older childhood and adolescent age groups.
Age-, Sex-, Race-, and Ethnicity-Specific Relative Survival
Five-year relative survival estimates for primary malignant and non-malignant brain and other CNS tumors in the United States during 2001–2017 overall, by sex, race, Hispanic ethnicity and age groups are shown in Table 1.
Five-year relative survival was higher in females (85.1%) as compared to males (82.8%). When stratified by behavior, five-year survival estimates did not differ by sex for malignant tumors only.
Adolescents ages 15–19 years had the highest five-year relative survival of any age group (90.5%).
For malignant tumors only, survival was lowest in infants < 1 year old (60.3%), and highest in children and adolescents ages 10–14 (80.1%) and 15–19 years (79.3%).
Five-year relative survival was highest in White children and adolescents (84.6%), and lowest in Black children and adolescents (79.1%). When stratified by behavior, this was also true in malignant tumors only (76.8% as compared to 68.3%).
Non-Hispanic children and adolescents had higher five-year relative survival (84.4%) as compared to Hispanic children and adolescents (82.1%). When stratified by behavior, this was also true in malignant tumors only (76.5% as compared to 71.7%).
Age-, Site-, and Histopathology-Specific Relative Survival
Relative survival estimates for brain and other CNS tumors by histopathology and age at diagnosis are shown in Table 8.
Table 8.
One-, Five-, and Ten-Year Relative Survival Ratesa,b for Selected Malignantc and Non-Malignantd Brain and Other Central Nervous System Tumors Ages 0–19 Years by Age Groups and Histopathology, NPCR, 2001–2017 (varying)
| Histopathology | Age Group | Total Cases (2001–2017) | 1-year RS (95% CI) | 5-year RS (95% CI) | 10-year RS (95% CI) |
|---|---|---|---|---|---|
| Gliomas | 0–19 years | 31,698 | 90.7 (90.4–91.0) | 80.0 (79.5–80.5) | 77.2 (76.6–77.7) |
| <1 year | 1,344 | 88.8 (86.9–90.4) | 78.6 (76.1–80.8) | 73.1 (70.2–75.7) | |
| 1–4 years | 7,919 | 92.1 (91.5–92.7) | 83.1 (82.2–83.9) | 80.2 (79.2–81.2) | |
| 5–9 years | 8,481 | 85.6 (84.8–86.3) | 74.6 (73.7–75.6) | 72.5 (71.5–73.6) | |
| 10–14 years | 7,496 | 92.5 (91.9–93.1) | 82.0 (81.1–82.9) | 79.9 (78.8–80.9) | |
| 15–19 years | 6,458 | 94.1 (93.5–94.6) | 81.2 (80.1–82.2) | 77.2 (76.0–78.4) | |
| Pilocytic astrocytoma | 0–19 years | 10,532 | 98.8 (98.6–99.0) | 96.8 (96.4–97.1) | 95.4 (94.9–95.9) |
| <1 year | 301 | 93.0 (89.2–95.5) | 78.0 (72.4–82.7) | 70.0 (63.3–75.6) | |
| 1–4 years | 2,793 | 99.0 (98.5–99.3) | 97.4 (96.6–97.9) | 95.8 (94.8–96.7) | |
| 5–9 years | 2,971 | 99.0 (98.6–99.3) | 97.5 (96.8–98.1) | 96.4 (95.5–97.1) | |
| 10–14 years | 2,626 | 98.9 (98.4–99.2) | 97.7 (96.9–98.2) | 96.5 (95.4–97.3) | |
| 15–19 years | 1,841 | 99.2 (98.6–99.5) | 96.6 (95.6–97.5) | 95.9 (94.6–96.9) | |
| Other Low grade glioma | 0–19 years | 6,459 | 96.6 (96.2–97.1) | 89.7 (88.9–90.5) | 86.4 (85.4–87.4) |
| <1 year | 367 | 94.7 (91.6–96.7) | 89.2 (85.1–92.2) | 86.8 (82.2–90.3) | |
| 1–4 years | 1,117 | 96.6 (95.4–97.5) | 91.8 (90.0–93.4) | 90.4 (88.3–92.2) | |
| 5–9 years | 1,358 | 95.8 (94.6–96.8) | 89.0 (87.1–90.6) | 86.2 (83.9–88.1) | |
| 10–14 years | 1,706 | 96.6 (95.6–97.4) | 90.1 (88.5–91.5) | 87.9 (86.0–89.6) | |
| 15–19 years | 1,911 | 97.6 (96.8–98.2) | 88.7 (87.0–90.2) | 82.8 (80.6–84.8) | |
| High grade glioma | 0–19 years | 6,551 | 64.7 (63.5–65.9) | 33.6 (32.4–34.8) | 30.5 (29.3–31.8) |
| <1 year | 237 | 71.2 (64.8–76.6) | 64.1 (57.3–70.1) | 61.6 (54.5–68.0) | |
| 1–4 years | 1,245 | 62.6 (59.8–65.2) | 34.0 (31.3–36.8) | 31.1 (28.3–34.0) | |
| 5–9 years | 2,189 | 52.0 (49.9–54.1) | 23.5 (21.6–25.4) | 21.7 (19.8–23.6) | |
| 10–14 years | 1,548 | 71.9 (69.6–74.1) | 38.8 (36.2–41.4) | 36.0 (33.3–38.6) | |
| 15–19 years | 1,332 | 78.2 (75.8–80.3) | 38.3 (35.5–41.2) | 32.4 (29.5–35.4) | |
| Other glioma | 0–19 years | 18,688 | 97.9 (97.7–98.1) | 93.0 (92.5–93.3) | 90.3 (89.8–90.8) |
| <1 year | 740 | 91.5 (89.1–93.4) | 78.0 (74.6–81.1) | 70.1 (66.0–73.9) | |
| 1–4 years | 5,557 | 97.9 (97.5–98.3) | 92.5 (91.7–93.2) | 89.2 (88.2–90.2) | |
| 5–9 years | 4,934 | 97.8 (97.4–98.2) | 93.5 (92.7–94.2) | 91.4 (90.5–92.3) | |
| 10–14 years | 4,242 | 98.4 (98.0–98.8) | 94.5 (93.7–95.2) | 92.6 (91.5–93.5) | |
| 15–19 years | 3,215 | 98.6 (98.1–99.0) | 94.4 (93.4–95.2) | 92.3 (91.0–93.3) | |
| Choroid plexus tumors | 0–19 years | 1,037 | 95.5 (94.0–96.6) | 89.2 (86.9–91.1) | 86.1 (83.4–88.5) |
| <1 year | 321 | 92.8 (89.2–95.3) | 87.1 (82.5–90.6) | 85.0 (79.6–89.0) | |
| 1–4 years | 343 | 94.9 (91.9–96.8) | 85.9 (81.3–89.4) | 83.4 (78.4–87.3) | |
| 5–9 years | 126 | 96.8 (91.6–98.8) | 90.0 (82.5–94.4) | 85.4 (76.0–91.3) | |
| 10–14 years | 128 | 99.2 (94.3–99.9) | 93.7 (87.1–97.0) | 91.5 (81.9–96.1) | |
| 15–19 years | 119 | 99.2 (93.6–99.9) | 98.3 (92.8–99.6) | 91.8 (81.0–96.6) | |
| Neuronal and mixed neuronal-glial tumors | 0–19 years | 2,818 | 98.6 (98.0–99.0) | 95.5 (94.6–96.3) | 94.3 (93.2–95.3) |
| <1 year | 79 | 91.3 (81.9–96.0) | 87.0 (76.6–93.0) | 87.0 (76.6–93.0) | |
| 1–4 years | 367 | 98.1 (95.9–99.1) | 94.4 (91.1–96.6) | 93.4 (89.6–95.8) | |
| 5–9 years | 524 | 98.2 (96.6–99.1) | 93.3 (90.5–95.3) | 92.3 (89.2–94.5) | |
| 10–14 years | 873 | 99.2 (98.3–99.6) | 96.7 (95.0–97.8) | 95.9 (93.9–97.3) | |
| 15–19 years | 975 | 99.0 (98.0–99.5) | 96.8 (95.2–97.9) | 94.9 (92.6–96.5) | |
| Tumors of the pineal region | 0–19 years | 550 | 89.4 (86.5–91.8) | 69.9 (65.5–73.9) | 62.8 (57.8–67.3) |
| <1 year | <50 cases | -- | -- | -- | |
| 1–4 years | 126 | 77.4 (69.0–83.8) | 50.5 (41.0–59.3) | 49.3 (39.7–58.2) | |
| 5–9 years | 131 | 96.8 (91.7–98.8) | 74.2 (64.4–81.6) | 66.4 (55.7–75.0) | |
| 10–14 years | 118 | 98.3 (93.2–99.6) | 83.6 (74.9–89.5) | 69.5 (57.6–78.7) | |
| 15–19 years | 145 | 94.4 (89.0–97.2) | 78.8 (70.2–85.2) | 71.3 (60.9–79.3) | |
| Embryonal tumors | 0–19 years | 7,397 | 82.3 (81.4–83.1) | 63.5 (62.3–64.7) | 58.1 (56.8–59.4) |
| <1 year | 846 | 54.9 (51.4–58.2) | 41.6 (38.2–45.1) | 39.1 (35.6–42.6) | |
| 1–4 years | 2,581 | 74.6 (72.9–76.3) | 55.0 (53.0–57.0) | 51.7 (49.5–53.8) | |
| 5–9 years | 2,124 | 92.7 (91.5–93.7) | 73.5 (71.4–75.5) | 67.7 (65.3–69.9) | |
| 10–14 years | 1,154 | 94.3 (92.8–95.5) | 74.6 (71.8–77.3) | 66.9 (63.5–70.0) | |
| 15–19 years | 692 | 92.6 (90.4–94.4) | 72.9 (69.1–76.3) | 61.7 (57.2–65.8) | |
| Medulloblastoma | 0–19 years | 4,747 | 90.3 (89.4–91.1) | 73.6 (72.2–74.9) | 67.3 (65.7–68.8) |
| <1 year | 160 | 66.9 (58.9–73.7) | 56.6 (48.3–64.1) | 53.3 (44.7–61.2) | |
| 1–4 years | 1,393 | 82.6 (80.5–84.6) | 64.2 (61.5–66.8) | 60.4 (57.5–63.2) | |
| 5–9 years | 1,750 | 94.3 (93.1–95.3) | 76.4 (74.2–78.5) | 70.2 (67.6–72.7) | |
| 10–14 years | 915 | 96.2 (94.7–97.2) | 80.8 (77.8–83.5) | 73.6 (69.9–77.0) | |
| 15–19 years | 529 | 94.4 (92.0–96.1) | 81.4 (77.4–84.7) | 69.4 (64.2–74.0) | |
| ATRT | 0–19 years | 1,000 | 53.2 (50.0–56.3) | 33.3 (30.1–36.4) | 30.9 (27.7–34.1) |
| <1 year | 372 | 39.0 (33.9–44.0) | 19.6 (15.5–24.0) | 16.7 (12.6–21.3) | |
| 1–4 years | 520 | 58.4 (54.0–62.6) | 39.3 (34.7–43.7) | 37.2 (32.6–41.9) | |
| 5–9 years | 67 | 75.2 (62.7–84.0) | 55.4 (41.2–67.6) | 55.4 (41.2–67.6) | |
| 10–14 years | <50 cases | -- | -- | -- | |
| 15–19 years | <50 cases | -- | -- | -- | |
| Other embryonal tumors | 0–19 years | 487 | 80.7 (76.8–84.0) | 67.1 (62.5–71.3) | 64.0 (59.1–68.4) |
| <1 year | 194 | 80.6 (74.1–85.6) | 75.5 (68.5–81.1) | 73.9 (66.6–79.8) | |
| 1–4 years | 195 | 77.5 (70.8–82.8) | 61.5 (53.9–68.3) | 57.7 (49.6–64.9) | |
| 5–9 years | 58 | 85.6 (73.2–92.5) | 57.6 (43.0–69.8) | 55.5 (40.8–67.9) | |
| 10–14 years | <50 cases | -- | -- | -- | |
| 15–19 years | <50 cases | -- | -- | -- | |
| Tumors of cranial and spinal nerves | 0–19 years | 3,120 | 99.7 (99.4–99.9) | 98.6 (98.1–99.0) | 97.4 (96.4–98.0) |
| <1 year | 184 | 100.0 (**-**) | 97.8 (92.6–99.3) | 96.8 (91.0–98.9) | |
| 1–4 years | 651 | 100.0 (**-**) | 99.2 (98.0–99.7) | 98.9 (96.9–99.6) | |
| 5–9 years | 650 | 99.7 (98.7–99.9) | 99.0 (97.7–99.6) | 97.8 (95.8–98.8) | |
| 10–14 years | 659 | 99.4 (98.3–99.8) | 98.0 (96.4–98.9) | 97.3 (95.2–98.5) | |
| 15–19 years | 976 | 99.6 (98.9–99.9) | 98.6 (97.3–99.2) | 96.0 (93.5–97.5) | |
| Tumors of meninges | 0–19 years | 3,078 | 97.2 (96.5–97.7) | 93.7 (92.6–94.5) | 90.6 (89.1–91.9) |
| <1 year | 369 | 95.1 (92.1–97.0) | 92.0 (88.3–94.6) | 91.1 (86.7–94.1) | |
| 1–4 years | 402 | 95.1 (92.4–96.8) | 91.0 (87.4–93.6) | 87.2 (82.3–90.8) | |
| 5–9 years | 377 | 96.7 (94.3–98.1) | 92.4 (88.9–94.8) | 87.8 (82.4–91.6) | |
| 10–14 years | 651 | 97.9 (96.4–98.8) | 94.5 (92.2–96.1) | 91.5 (88.4–93.8) | |
| 15–19 years | 1,279 | 98.2 (97.3–98.8) | 94.9 (93.3–96.1) | 91.9 (89.5–93.7) | |
| Lymphomas and hematopoietic neoplasms | 0–19 years | 288 | 88.6 (84.3–91.8) | 81.6 (76.2–85.8) | 78.3 (72.2–83.2) |
| <1 year | <50 cases | -- | -- | -- | |
| 1–4 years | <50 cases | -- | -- | -- | |
| 5–9 years | 69 | 94.1 (85.0–97.7) | 92.3 (82.4–96.8) | 92.3 (82.4–96.8) | |
| 10–14 years | 70 | 92.6 (83.0–96.8) | 80.0 (67.2–88.3) | 76.0 (60.2–86.2) | |
| 15–19 years | 106 | 83.7 (75.1–89.6) | 74.9 (64.9–82.4) | 73.5 (63.1–81.3) | |
| Germ cell tumors | 0–19 years | 2,274 | 93.7 (92.6–94.7) | 88.6 (87.2–90.0) | 85.7 (83.9–87.3) |
| <1 year | 163 | 65.0 (57.0–72.0) | 61.5 (53.3–68.7) | 61.5 (53.3–68.7) | |
| 1–4 years | 101 | 89.7 (81.7–94.3) | 83.6 (74.1–89.8) | 80.5 (68.8–88.2) | |
| 5–9 years | 379 | 95.9 (93.3–97.5) | 92.3 (88.8–94.7) | 87.2 (82.0–90.9) | |
| 10–14 years | 869 | 97.1 (95.8–98.1) | 91.2 (88.9–93.1) | 87.8 (84.8–90.2) | |
| 15–19 years | 762 | 95.5 (93.7–96.7) | 90.4 (87.9–92.4) | 88.2 (85.3–90.6) | |
| Tumors of the pituitary | 0–19 years | 7,495 | 99.9 (99.8–100.0) | 99.8 (99.5–99.9) | 99.4 (98.9–99.6) |
| <1 year | <50 cases | -- | -- | -- | |
| 1–4 years | 80 | 98.7 (90.9–99.8) | 97.2 (89.1–99.3) | 94.8 (83.7–98.4) | |
| 5–9 years | 552 | 100.0 (**-**) | 100.0 (**-**) | 100.0 (**-**) | |
| 10–14 years | 1,501 | 99.9 (99.3–100.0) | 99.6 (98.9–99.9) | 99.2 (98.1–99.7) | |
| 15–19 years | 5,336 | 99.9 (99.7–100.0) | 99.9 (99.7–100.0) | 99.5 (98.8–99.8) | |
| Craniopharyngioma | 0–19 years | 2,099 | 98.5 (97.9–98.9) | 95.4 (94.3–96.3) | 92.2 (90.6–93.6) |
| <1 year | <50 cases | -- | -- | -- | |
| 1–4 years | 353 | 98.9 (96.9–99.6) | 95.1 (91.8–97.1) | 92.1 (87.5–95.1) | |
| 5–9 years | 743 | 98.5 (97.3–99.2) | 96.0 (94.1–97.3) | 93.1 (90.3–95.1) | |
| 10–14 years | 566 | 99.1 (97.8–99.6) | 96.4 (94.2–97.8) | 93.0 (89.6–95.3) | |
| 15–19 years | 411 | 98.0 (96.0–99.0) | 94.0 (90.8–96.1) | 91.2 (86.7–94.2) | |
| Other/unclassified tumors | 0–19 years | 3,371 | 92.5 (91.6–93.4) | 89.8 (88.7–90.9) | 88.8 (87.5–90.0) |
| <1 year | 257 | 70.1 (63.9–75.4) | 67.8 (61.6–73.3) | 67.8 (61.6–73.3) | |
| 1–4 years | 562 | 88.1 (85.1–90.6) | 83.9 (80.4–86.8) | 83.2 (79.7–86.2) | |
| 5–9 years | 596 | 92.5 (90.0–94.4) | 89.6 (86.7–91.9) | 86.7 (82.9–89.7) | |
| 10–14 years | 858 | 95.3 (93.6–96.5) | 92.3 (90.2–94.0) | 91.8 (89.4–93.6) | |
| 15–19 years | 1,098 | 98.0 (96.9–98.7) | 96.2 (94.7–97.3) | 95.3 (93.3–96.7) | |
| Total e | 0–19 years | 65,402 | 92.5 (92.2–92.7) | 83.9 (83.6–84.2) | 81.1 (80.7–81.4) |
| <1 year | 3,639 | 80.3 (79.0–81.6) | 71.9 (70.3–73.4) | 68.6 (66.9–70.2) | |
| 1–4 years | 13,562 | 89.4 (88.9–89.9) | 79.3 (78.5–80.0) | 76.5 (75.7–77.3) | |
| 5–9 years | 14,774 | 90.0 (89.5–90.5) | 79.9 (79.2–80.6) | 77.1 (76.3–77.9) | |
| 10–14 years | 14,997 | 95.1 (94.7–95.5) | 87.0 (86.4–87.6) | 84.5 (83.8–85.1) | |
| 15–19 years | 18,430 | 96.9 (96.7–97.2) | 90.5 (90.0–90.9) | 87.5 (86.9–88.1) |
aThe cohort analysis of survival rates was utilized for calculating the survival estimates presented in this table. Long-term cohort-based survival estimates reflect the survival experience of individuals diagnosed over the time period, and they may not necessarily reflect the long-term survival outlook of newly diagnosed cases.
bRates are an estimate of the percentage of patients alive at one, two, three, four, five, and ten year, respectively.
cAssigned behavior code of/3 by the International Classification for Disease, Oncology 3rd edition (see Supplementary Table 3).
dAssigned behavior code of/0 or/1 by the International Classification for Disease, Oncology 3rd edition (see Supplementary Table 3).
eIncludes histopathologies not listed in this table.
Data are not presented when fewer than 50 cases were reported for the specific category or where less than 16 individuals remain alive at the end of the observation period.
**Not able to be calculated.
Abbreviations: NPCR, National Program of Cancer Registries; RS, Relative Survival; ATRT, Atypical teratoid/rhabdoid tumor.
Overall, one-year survival for all primary brain and other CNS tumors in children and adolescents was 92.5%, this declined to 83.9% for five-year survival, and 81.1% for ten-year survival.
There was large variation in survival estimates depending upon tumor histopathology; five-year survival rates were 96.8% for pilocytic astrocytoma but are 33.3% for ATRT and 33.6% for high grade glioma.
Relative survival in high grade glioma was 64.7% one year after diagnosis, but declined to 33.6% five years after diagnosis, and 30.5% at 10 years after diagnosis.
For embryonal tumors, one-year relative survival was 82.3% but declined to 63.5% five years after diagnosis, and 58.1% at 10 years after diagnosis.
For pilocytic astrocytoma, survival was high across all estimates: one-year relative survival was 98.8%, while five- and ten-year relative survival was 96.8% and 95.4%, respectively.
Within each histopathology, relative survival estimates vary substantially by age group.
Relative survival estimates for brain and other CNS tumors by site and age are shown in Table 9.
Table 9.
One-, Five-, and Ten-Year Relative Survival Ratesa,b for Selected Malignantc and Non-Malignantd Brain and Other Central Nervous System Tumors Sites Ages 0–19 Years by Age Groups and Site, NPCR, 2001–2017 (varying)
| Site | Age Group | Total Cases (2001–2017) | 1-year RS (95% CI) | 5-year RS (95% CI) | 10-year RS (95% CI) |
|---|---|---|---|---|---|
| Frontal lobe | 0–19 years | 3,753 | 93.5 (92.6–94.2) | 81.6 (80.2–82.9) | 77.3 (75.6–78.8) |
| 0 years | 127 | 75.7 (66.9–82.4) | 69.1 (59.7–76.7) | 69.1 (59.7–76.7) | |
| 1–4 years | 584 | 87.1 (84.1–89.6) | 75.8 (71.9–79.2) | 72.3 (68.0–76.2) | |
| 5–9 years | 659 | 94.2 (92.1–95.8) | 83.3 (80.0–86.1) | 79.3 (75.4–82.6) | |
| 10–14 years | 1,002 | 94.9 (93.3–96.2) | 84.9 (82.3–87.2) | 82.4 (79.4–85.0) | |
| 15–19 years | 1,381 | 96.3 (95.1–97.2) | 81.9 (79.5–84.0) | 75.4 (72.4–78.1) | |
| Temporal lobe | 0–19 years | 4,339 | 96.5 (95.9–97.0) | 89.5 (88.5–90.5) | 86.3 (85.0–87.5) |
| 0 years | 159 | 93.5 (88.0–96.5) | 88.5 (81.9–92.8) | 87.6 (80.7–92.1) | |
| 1–4 years | 646 | 94.4 (92.3–96.0) | 90.6 (87.9–92.7) | 87.4 (83.9–90.2) | |
| 5–9 years | 842 | 96.0 (94.4–97.1) | 88.0 (85.4–90.1) | 84.9 (81.8–87.5) | |
| 10–14 years | 1,225 | 96.4 (95.1–97.3) | 90.0 (88.0–91.6) | 87.0 (84.5–89.2) | |
| 15–19 years | 1,467 | 98.1 (97.2–98.7) | 89.8 (87.9–91.4) | 85.8 (83.4–87.8) | |
| Parietal lobe | 0–19 years | 1,847 | 92.8 (91.5–93.9) | 80.6 (78.5–82.4) | 76.6 (74.2–78.7) |
| 0 years | 76 | 77.9 (66.6–85.8) | 70.3 (58.1–79.5) | 67.9 (55.1–77.7) | |
| 1–4 years | 326 | 89.0 (85.0–92.0) | 77.1 (71.9–81.6) | 76.0 (70.6–80.6) | |
| 5–9 years | 346 | 94.0 (90.9–96.1) | 80.9 (75.9–84.9) | 78.5 (73.1–82.9) | |
| 10–14 years | 519 | 94.7 (92.3–96.3) | 84.1 (80.3–87.1) | 80.6 (76.3–84.2) | |
| 15–19 years | 580 | 94.4 (92.2–96.0) | 80.6 (76.9–83.8) | 73.3 (68.5–77.4) | |
| Occipital lobe | 0–19 years | 765 | 96.4 (94.8–97.5) | 88.7 (86.0–90.9) | 85.1 (81.8–87.9) |
| 0 years | <50 cases | -- | -- | -- | |
| 1–4 years | 106 | 92.2 (85.0–96.0) | 82.3 (73.0–88.7) | 77.5 (66.7–85.1) | |
| 5–9 years | 142 | 97.1 (92.5–98.9) | 91.1 (84.4–95.0) | 89.0 (81.5–93.5) | |
| 10–14 years | 228 | 98.6 (95.8–99.6) | 90.0 (84.6–93.5) | 88.4 (82.4–92.4) | |
| 15–19 years | 251 | 96.8 (93.6–98.4) | 89.3 (84.2–92.8) | 83.8 (77.0–88.8) | |
| Cerebrum | 0–19 years | 3,599 | 87.5 (86.4–88.6) | 71.4 (69.8–72.9) | 68.3 (66.6–70.0) |
| 0 years | 216 | 74.9 (68.4–80.2) | 64.9 (57.8–71.1) | 61.7 (54.2–68.3) | |
| 1–4 years | 739 | 89.0 (86.4–91.1) | 78.4 (75.1–81.4) | 74.9 (71.1–78.3) | |
| 5–9 years | 945 | 85.6 (83.2–87.8) | 70.7 (67.5–73.6) | 66.7 (63.2–70.0) | |
| 10–14 years | 980 | 90.2 (88.1–91.9) | 70.9 (67.7–73.8) | 69.0 (65.7–72.1) | |
| 15–19 years | 719 | 88.6 (86.0–90.8) | 67.7 (63.9–71.2) | 64.7 (60.5–68.5) | |
| Ventricle | 0–19 years | 3,446 | 92.9 (91.9–93.7) | 86.1 (84.9–87.3) | 82.5 (81.0–84.0) |
| 0 years | 521 | 86.2 (82.8–89.0) | 80.9 (77.0–84.2) | 79.2 (74.9–82.8) | |
| 1–4 years | 861 | 89.5 (87.2–91.4) | 79.9 (76.9–82.6) | 73.8 (70.2–77.1) | |
| 5–9 years | 639 | 94.5 (92.4–96.1) | 87.3 (84.3–89.8) | 83.3 (79.5–86.4) | |
| 10–14 years | 740 | 96.8 (95.2–97.9) | 91.3 (88.9–93.3) | 89.2 (86.2–91.6) | |
| 15–19 years | 685 | 96.3 (94.5–97.5) | 91.2 (88.6–93.2) | 88.0 (84.6–90.6) | |
| Cerebellum | 0–19 years | 9,201 | 93.5 (93.0–94.0) | 83.6 (82.8–84.4) | 80.3 (79.4–81.2) |
| 0 years | 333 | 62.3 (56.8–67.4) | 47.8 (42.1–53.3) | 45.2 (39.3–50.9) | |
| 1–4 years | 2,532 | 89.7 (88.4–90.8) | 78.6 (76.8–80.2) | 75.7 (73.7–77.5) | |
| 5–9 years | 2,818 | 96.2 (95.4–96.8) | 85.3 (83.8–86.7) | 82.0 (80.2–83.5) | |
| 10–14 years | 1,987 | 97.3 (96.4–97.9) | 89.3 (87.7–90.7) | 86.0 (84.1–87.7) | |
| 15–19 years | 1,531 | 97.2 (96.2–97.9) | 89.4 (87.5–90.9) | 85.3 (82.9–87.3) | |
| Brain stem | 0–19 years | 7,522 | 75.9 (75.0–76.9) | 58.2 (57.0–59.4) | 55.2 (54.0–56.5) |
| 0 years | 263 | 76.2 (70.4–81.0) | 63.4 (56.9–69.3) | 59.0 (52.0–65.4) | |
| 1–4 years | 2,051 | 76.0 (74.1–77.8) | 54.8 (52.5–57.0) | 51.8 (49.4–54.2) | |
| 5–9 years | 2,625 | 66.0 (64.1–67.8) | 46.6 (44.6–48.6) | 44.4 (42.3–46.4) | |
| 10–14 years | 1,581 | 84.1 (82.1–85.8) | 68.9 (66.4–71.3) | 65.6 (62.9–68.2) | |
| 15–19 years | 1,002 | 89.2 (87.1–91.0) | 77.7 (74.8–80.3) | 73.7 (70.4–76.7) | |
| Spinal cord and cauda equina | 0–19 years | 3,326 | 94.6 (93.8–95.4) | 89.1 (87.8–90.2) | 86.4 (84.9–87.7) |
| 0 years | 390 | 93.0 (89.8–95.3) | 90.4 (86.7–93.2) | 90.4 (86.7–93.2) | |
| 1–4 years | 660 | 93.2 (91.0–94.9) | 88.0 (85.1–90.4) | 86.4 (83.0–89.1) | |
| 5–9 years | 546 | 94.7 (92.4–96.3) | 88.4 (85.1–91.0) | 84.6 (80.5–87.9) | |
| 10–14 years | 790 | 94.3 (92.4–95.8) | 87.9 (85.3–90.2) | 84.5 (81.1–87.4) | |
| 15–19 years | 940 | 96.5 (95.1–97.5) | 90.6 (88.3–92.4) | 87.6 (84.6–90.0) | |
| Cranial nerves | 0–19 years | 4,444 | 99.7 (99.5–99.9) | 98.3 (97.8–98.7) | 97.5 (96.8–98.0) |
| 0 years | 252 | 99.4 (94.4–99.9) | 93.2 (88.6–96.0) | 91.3 (86.2–94.6) | |
| 1–4 years | 1,709 | 100.0 (98.8–100.0) | 99.2 (98.4–99.6) | 98.8 (97.9–99.4) | |
| 5–9 years | 1,061 | 99.7 (99.1–99.9) | 98.6 (97.5–99.2) | 97.6 (96.0–98.5) | |
| 10–14 years | 674 | 99.7 (98.8–99.9) | 98.5 (97.0–99.3) | 97.8 (96.0–98.8) | |
| 15–19 years | 748 | 99.4 (98.3–99.7) | 97.4 (95.6–98.4) | 95.8 (93.0–97.5) | |
| Meninges | 0–19 years | 1,557 | 97.3 (96.3–98.0) | 93.9 (92.4–95.1) | 91.0 (88.9–92.6) |
| 0 years | 76 | 85.5 (74.9–91.9) | 82.3 (71.0–89.5) | 79.0 (65.6–87.7) | |
| 1–4 years | 155 | 92.7 (87.2–95.9) | 86.5 (79.6–91.3) | 81.5 (72.9–87.6) | |
| 5–9 years | 189 | 97.8 (94.3–99.2) | 94.0 (89.1–96.8) | 91.6 (84.8–95.4) | |
| 10–14 years | 356 | 98.0 (95.8–99.0) | 95.2 (92.1–97.2) | 91.9 (87.7–94.8) | |
| 15–19 years | 781 | 98.9 (97.8–99.4) | 95.9 (93.9–97.2) | 93.4 (90.3–95.5) | |
| Pituitary and craniopharyngeal duct | 0–19 years | 9,672 | 99.7 (99.5–99.8) | 99.0 (98.8–99.2) | 98.0 (97.5–98.4) |
| 0 years | 50 | 96.4 (83.8–99.2) | 85.5 (69.1–93.6) | 76.9 (57.0–88.5) | |
| 1–4 years | 388 | 98.9 (97.2–99.6) | 96.6 (93.9–98.2) | 93.6 (89.6–96.1) | |
| 5–9 years | 1,256 | 99.3 (98.7–99.7) | 98.3 (97.2–98.9) | 96.6 (94.8–97.8) | |
| 10–14 years | 2,111 | 99.7 (99.3–99.9) | 98.7 (98.0–99.2) | 97.6 (96.4–98.4) | |
| 15–19 years | 5,867 | 99.8 (99.7–99.9) | 99.6 (99.3–99.8) | 98.9 (98.3–99.3) | |
| Pineal gland | 0–19 years | 1,832 | 92.0 (90.7–93.2) | 81.9 (79.9–83.7) | 78.1 (75.8–80.3) |
| 0 years | 65 | 54.7 (41.6–66.0) | 40.1 (27.6–52.3) | 40.1 (27.6–52.3) | |
| 1–4 years | 211 | 79.3 (73.1–84.2) | 59.3 (52.0–65.9) | 56.6 (48.9–63.6) | |
| 5–9 years | 311 | 94.6 (91.4–96.7) | 82.9 (77.6–87.1) | 75.4 (68.6–80.9) | |
| 10–14 years | 600 | 97.2 (95.5–98.3) | 89.0 (85.9–91.5) | 84.9 (80.9–88.1) | |
| 15–19 years | 645 | 94.0 (91.8–95.6) | 86.6 (83.5–89.2) | 84.1 (80.4–87.1) |
aThe cohort analysis of survival rates was utilized for calculating the survival estimates presented in this table. Long-term cohort-based survival estimates reflect the survival experience of individuals diagnosed over the time period, and they may not necessarily reflect the long-term survival outlook of newly diagnosed cases.
bRates are an estimate of the percentage of patients alive at one, two, three, four, five, and ten year, respectively.
cAssigned behavior code of/3 by the International Classification for Disease, Oncology 3rd edition (see Supplementary Table 3).
dAssigned behavior code of/0 or/1 by the International Classification for Disease, Oncology 3rd edition (see Supplementary Table 3).
Abbreviations: NPCR, National Program of Cancer Registries; RS, Relative Survival.
The highest five-year survival was for tumors occurring in the cranial nerves (98.3%).
The lowest five-year survival was for tumors of the brain stem (58.2%).
Within each site, relative survival estimates vary substantially by age group.
Relative survival estimates for high grade glioma of the brain stem are shown in Table 4.
One-year survival after diagnosis with these tumors was 61.5%, while 15-year survival was 35.1%.
Five-year survival is highest in children diagnosed at < 1 year (75.8%) and lowest in children diagnosed at ages 5–9 years (25.1%).
Trends in Survival
Kaplan-Meier survival curves were generated for all high-grade gliomas (Figure 13A) and medulloblastoma (Figure 13B) in individuals 0–19 years of age, using a log rank test to evaluate differences. There were 5,364 high-grade glioma patients and 3,698 medulloblastoma assessed. Although increased survival in high-grade glioma is observed in 2013–2017 compared to 2004–2007, the improvement is not statistically significant (log rank p = 0.16). Similarly, there was a trend towards increased survival for 2013–2017 among medulloblastoma patients, though not statistically significant (log rank p = 0.051).
Fig. 13.
Survival by Year of Diagnosis in Children and Adolescents Ages 0–19 Years for A) High Grade Gliomas and B) Medulloblastoma, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR, 2004–2018.
Age-stratified multivariable Cox proportional hazard models, adjusted for sex, race/ethnicity, and treatment pattern, were generated to assess survival differences by time period of diagnosis. The use of surgery and/or radiation was utilized for the determination of treatment patterns. HR decreased for high grade glioma and medulloblastomas for each time period when compared to 2004–2007 (Figure 14). Significant improvement in survival was observed for patients diagnosed with medulloblastomas in 2013–2017 compared to 2004–2007 (HR = 0.79; 95% CI = 0.65–0.96; p = 0.002).
Fig. 14.
Hazard Ratios and 95% Confidence Intervals by Year of Diagnosis for Death Due to High Grade Glioma and Medulloblastoma in Children and Adolescents Ages 0–19 Years, Adjusted for Sex, Race/Ethnicity, and Treatment, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR, 2004–2017.
Age-specific and Histopathology-Specific Annual Case Projections
The estimated number of cases of all primary brain and other CNS tumors for 2023, 2024, and 2025 are shown by histopathology and age group in Table 10. Estimated numbers of cases are highly dependent on input data. Different patterns of incidence within strata can substantively affect the projected estimates, and strata-specific estimates may not equal the total estimate presented. Caution should be used when utilizing these estimates.
Table 10.
Estimated Number of Newly Diagnosed Casesa,b of Brain and Other Central Nervous System Tumors Ages 0–19 Years Overall, by Age Groups, and by Histopathology Groupings by Year, Diagnosis Years 2023 to 2025, CBTRUS Childhood and Adolescent Report: NPCR and SEER, 2000–2018
| Histopathology | 2023 | 2024 | 2025 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–19 years | 0–4 years | 5–9 years | 10–14 years | 15–19 years | 0–19 years | 0–4 years | 5–9 years | 10–14 years | 15–19 years | 0–19 years | 0–4 years | 5–9 years | 10–14 years | 15–19 years | |
| Gliomas c | 2,160 | 590 | 610 | 560 | 460 | 2,160 | 590 | 620 | 560 | 460 | 2,170 | 600 | 620 | 560 | 470 |
| Pilocytic astrocytoma | 860 | 290 | 250 | 200 | 140 | 870 | 290 | 250 | 200 | 140 | 870 | 290 | 250 | 200 | 140 |
| Other Low grade glioma | 440 | 100 | 90 | 130 | 130 | 440 | 100 | 90 | 130 | 130 | 440 | 100 | 90 | 130 | 130 |
| High grade glioma | 410 | 70 | 160 | 130 | 110 | 410 | 70 | 160 | 130 | 110 | 410 | 70 | 160 | 130 | 110 |
| Other glioma | 440 | 130 | 120 | 100 | 80 | 440 | 130 | 120 | 100 | 80 | 440 | 130 | 120 | 100 | 80 |
| Ependymal tumors | 250 | 100 | 50 | 50 | 50 | 250 | 100 | 50 | 50 | 50 | 250 | 100 | 50 | 50 | 50 |
| Choroid plexus tumors | 80 | 50 | -- | -- | -- | 80 | 50 | -- | -- | -- | 80 | 50 | -- | -- | -- |
| Neuronal and mixed neuronal-glial tumors | 270 | 40 | 50 | 90 | 100 | 270 | 40 | 50 | 90 | 100 | 270 | 40 | 50 | 90 | 100 |
| Tumors of the pineal region | 40 | -- | -- | -- | -- | 40 | -- | -- | -- | -- | 40 | -- | -- | -- | -- |
| Embryonal tumors | 490 | 210 | 140 | 80 | 40 | 490 | 210 | 140 | 80 | 40 | 490 | 210 | 140 | 80 | 40 |
| Medulloblastoma | 340 | 110 | 120 | 70 | 30 | 340 | 110 | 120 | 70 | 30 | 340 | 110 | 120 | 70 | 30 |
| ATRT | 90 | 80 | -- | -- | -- | 90 | 80 | -- | -- | -- | 90 | 80 | -- | -- | -- |
| Other embryonal tumors | 60 | 20 | -- | -- | -- | 60 | 20 | -- | -- | -- | 60 | 20 | -- | -- | -- |
| Tumors of cranial and spinal nerves | 240 | 50 | 40 | 60 | 90 | 240 | 50 | 40 | 60 | 90 | 240 | 50 | 40 | 60 | 90 |
| Tumors of meninges | 270 | 60 | 40 | 60 | 110 | 270 | 60 | 40 | 60 | 110 | 270 | 60 | 40 | 60 | 110 |
| Lymphomas and hematopoietic neoplasms | 30 | -- | -- | -- | -- | 30 | -- | -- | -- | -- | 30 | -- | -- | -- | -- |
| Germ cell tumors | 180 | 20 | 30 | 70 | 60 | 180 | 20 | 30 | 70 | 60 | 180 | 20 | 30 | 70 | 60 |
| Tumors of sellar region | 960 | 40 | 140 | 210 | 590 | 960 | 40 | 140 | 210 | 590 | 960 | 40 | 140 | 210 | 590 |
| Tumors of the pituitary | 800 | -- | -- | 160 | 550 | 800 | -- | -- | 160 | 550 | 800 | -- | -- | 160 | 550 |
| Craniopharyngioma | 170 | -- | -- | 50 | 30 | 170 | -- | -- | 50 | 30 | 170 | -- | -- | 50 | 30 |
| Other/unclassified tumors | 300 | 80 | 70 | 90 | 90 | 300 | 80 | 70 | 90 | 90 | 300 | 80 | 70 | 90 | 90 |
| Total d | 5,260 | 1,220 | 1,180 | 1,290 | 1,660 | 5,260 | 1,220 | 1,180 | 1,290 | 1,660 | 5,260 | 1,220 | 1,180 | 1,290 | 1,660 |
aSource: Estimation based on CBTRUS NPCR and SEER 2000–2018 data for malignant tumors, and NPCR and SEER 2006–2018 data for non-malignant tumors.
bRounded to the nearest 10. Numbers may not add up due to rounding.
cCBTRUS defines the broad category of gliomas to include ICD-O-3 histopathology codes 9380–9384, 9391–9460, 9480.
dIncludes histopathologies not listed in this table.
- Data are not presented when fewer than 16 cases were estimated for the specific category. These cases are included in overall rates.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; ATRT, Atypical teratoid/rhabdoid tumor.
The total number of new cases of primary brain and other CNS tumors projected to occur in children and adolescents ages 0–19 years in 2023 was 5,260.
The greatest number of new cases was estimated to be in adolescents ages 15–19 years, in which 1,660 newly diagnosed cases are expected in 2023.
Pilocytic astrocytoma had the highest number of estimated new cases, with 860 new cases expected to be diagnosed in 2023.
The histopathology with the second largest number of estimated new cases was tumors of the pituitary, with 800 cases estimated to be diagnosed in 2023, the majority of which will be in adolescents ages 15–19 years.
Comparing Brain and Other CNS Tumors to Other Common Child and Adolescent Cancers
Average annual age-specific incidence rates for the top five most common cancers in children and adolescents overall and by age groups are shown in Figure 15.
Fig. 15.
Average Annual Age-Specific Incidence Rates with 95% Confidence Intervals and Average Annual Cases for All Primary Malignant and Non-Malignant Brain and Other Central Nervous System Tumors Ages 0–19 Years in Comparison to Top Five Highest Incidence Cancers for Children and Adolescents Overall and by Age Groups, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2014–2018.
The most common cancer overall in children ages 0–19 years was primary brain and other CNS tumors. Brain and other CNS tumors were the most common cause of cancer in all age groups other than ages 1–4 years, where leukemia was the most common cause of cancer.
Average annual age-specific mortality rates for the top five most common causes of death and top five most common causes of cancer death in children and adolescents overall and by age groups are shown in Figure 16.
Fig. 16.
Annual Average Age-Specific Mortality Rates with 95% Confidence Intervals and Average Annual Deaths for Primary Malignant Brain and Other CNS Tumors in Ages 0–19 years as Compared to the Top Five Causes of Death and Top Five Cancer Causes of Death for Children and Adolescents, Overall and by Age Groups, CBTRUS Childhood and Adolescent Report: NVSS, 2014–2018.
The most common cancer death in children ages 0–19 years was primary brain and other CNS tumors. Brain and other CNS tumors were the most common cause of cancer death in children ages 5–14 years, while among children ages 0–4 and 15–19 years, leukemia was the most common cause of cancer death.
Prevalence of Brain and CNS tumors and Other Common Child and Adolescent Cancers
Estimates of the number of prevalent cases of cancer among children ages 0–19 years in the United States in 2022 are shown in Figure 17 and Table 11.
Fig. 17.
Estimated Prevalent Cases in the United States in 2022 in Children and Adolescents Ages 0–19 Years for A) the Eight Most Prevalent Cancers in Children and Adolescents, B) by the Ten Most Prevalent Brain and Other Central Nervous System Histopathologies CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 1975–2018 (varying).
Table 11.
Estimated Prevalent Case Countsa and Crude Prevalence Rate per 100,000 for Brain and Other Central Nervous System Tumors Ages 0–19 Years Overall, by Histopathology Groupings and Age Groups, 2022, CBTRUS Childhood and Adolescent Report: NPCR and SEER, 1975–2018
| Histopathology | 0–19 Years | 0–14 Years | 15–19 Years | |||
|---|---|---|---|---|---|---|
| Prevalent cases | Crude Prevalence Rate per 100,000 | Prevalent cases | Crude Prevalence Rate per 100,000 | Prevalent cases | Crude Prevalence Rate per 100,000 | |
| Gliomasb | 18,334 | 23.31 | 10,644 | 18.53 | 7,690 | 36.28 |
| Pilocytic astrocytoma | 8,264 | 10.51 | 4,907 | 8.54 | 3,357 | 15.84 |
| Other Low grade glioma | 3,750 | 4.77 | 1,991 | 3.47 | 1,759 | 8.30 |
| High grade glioma | 1,755 | 2.23 | 1,011 | 1.76 | 743 | 3.51 |
| Other glioma | 4,547 | 5.78 | 2,735 | 4.76 | 1,812 | 8.55 |
| Ependymal tumors | 2,436 | 3.10 | 1,524 | 2.65 | 912 | 4.30 |
| Choroid plexus tumors | 195 | 0.25 | 112 | 0.19 | 83 | 0.39 |
| Neuronal and mixed neuronal-glial tumors | 1,259 | 1.60 | 608 | 1.06 | 651 | 3.07 |
| Tumors of the pineal region | 195 | 0.25 | 112 | 0.19 | 83 | 0.39 |
| Embryonal tumors | 4,230 | 5.38 | 2,701 | 4.70 | 1,529 | 7.21 |
| Medulloblastoma | 3,197 | 4.07 | 1,936 | 3.37 | 1,260 | 5.95 |
| ATRT | 516 | 0.66 | 423 | 0.74 | 93 | 0.44 |
| Other embryonal tumors | 274 | 0.35 | 200 | 0.35 | 74 | 0.35 |
| Tumors of cranial and spinal nerves | 2,171 | 2.76 | 1,059 | 1.84 | 1,112 | 5.25 |
| Tumors of meninges | 1,516 | 1.93 | 823 | 1.43 | 693 | 3.27 |
| Lymphomas and hematopoietic neoplasms | 195 | 0.25 | 112 | 0.19 | 83 | 0.39 |
| Germ cell tumors | 1,259 | 1.60 | 608 | 1.06 | 651 | 3.07 |
| Tumors of sellar region | 4,589 | 5.84 | 1,659 | 2.89 | 2,929 | 13.82 |
| Tumors of the pituitary | 3,031 | 3.85 | 820 | 1.43 | 2,211 | 10.43 |
| Craniopharyngioma | 1,558 | 1.98 | 839 | 1.46 | 719 | 3.39 |
| Unclassified Tumors | 2,431 | 3.09 | 1,414 | 2.46 | 1,018 | 4.80 |
| Total c | 40,594 | 51.58 | 22,527 | 39.22 | 18,067 | 85.23 |
aSource: Estimation based on CBTRUS NPCR and SEER 2000–2018 data for malignant tumors, and NPCR and SEER 2004–2018 data for non-malignant tumors.
bCBTRUS defines the broad category of gliomas to include ICD-O-3 Histopathology codes 9380–9384, 9391–9460, 9480.
cIncludes histopathologies not listed in this table.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; ATRT, Atypical teratoid/rhabdoid tumor.
There were 40,594 prevalent brain tumor cases in children and adolescents ages 0–19 years in 2022. The most prevalent type of cancer overall was leukemia, which was estimated to have 40,738 prevalent cases.
22,527 of these brain tumor cases are estimated to be in children 0–14 years old, while there were estimated to be 18,067 prevalent cases in adolescents 15–19 years old.
The histopathologic group with the highest prevalence was gliomas, with 18,334 cases.
The next most prevalent histopathologic groups were embryonal tumors (4,230 cases) and tumors of the sellar region (4,589 cases).
As these estimates include only children and adolescents 0–19 years of age at time of prevalence estimate, the total population of childhood and adolescent brain tumor survivors including those that are now in adulthood is significantly larger.
Risk Factors, Long Term, and Late Effects for Childhood and Adolescent Brain and CNS Tumors
Despite being the greatest contributor to cancer mortality in children and adolescents ages 0–19 years, little is known about the etiology of childhood brain and other CNS tumors. There are a few established risk factors for brain and other CNS tumors in children and adolescents, including single gene inherited disorders, genetic syndromes, and ionizing radiation.32,33
Genetic risk factors for child and adolescent brain and CNS tumors
— As compared to adults, there have been minimal germline genetic studies conducted in children and adolescents with brain and other CNS tumors. Approximately 4% of gliomas diagnosed in children and adolescents are attributable to single-gene Mendelian disorders or inherited genetic cancer syndromes, and ~5–10% of children and adolescents diagnosed with brain and other CNS tumors have a family history.32,33 The majority of these inherited syndromes are characterized by loss-of-function mutations in tumor suppressor genes.32,33 A summary of the most commonly occurring syndromes is shown in Supplementary Table 5.
As compared to those in adult brain and other CNS tumors, relatively few candidate gene and genome-wide association studies (GWAS) have been conducted to identify more common (occurring in > 1% of the population) genetic variations that can affect risk of childhood and adolescent brain tumors. A summary of identified single nucleotide polymorphism (SNP) associations is shown in Supplementary Table 6. Similar to prior findings in adult glioma, some implicated genes are involved in processes regulating telomere length (such as regulator of telomere elongation helicase 1 [RTEL1] and telomerase reverse transcriptase [TERT]).34 A genetic predisposition to longer telomere length or more efficient maintenance is a suspected risk factor contributing to incidence of childhood neuroblastoma and ependymoma (statistical significance found only for those ≥ 12 years at age of diagnosis).34,35 GWAS continue to implicate novel genes such as protein-coding gene leucine rich repeat containing 4C (LRRC4C, located at 11q12) and metalloproteinase pappalysin 2 (PAPPA2, located at 1q25.2) which Foss-Skiftesvik et al. observed as associated with increased risk of childhood CNS tumors.36 Further large-scale genetic studies utilizing SNP-array and next-generation sequencing data are needed to identify and validate common and rare germline variants associated with increased risk of primary brain and other CNS tumors in children and adolescents.
Literature regarding the inheritance of primary brain and other CNS tumors remains inconsistent and limited due to the rarity of cases. Recently, a study evaluated incidence rates and family risk of tumors of the CNS and leukemia among individuals from Norway and individuals with Scandinavian ancestry in a single US state, two settings with high quality multigenerational genealogical and cancer registry records.37 Kollerud et al. observed a 3-fold increased risk of developing CNS tumors in Utah and Norway among children with a first-degree relative diagnosed with CNS tumors.37 Another study from France found an increased risk of childhood and adolescent brain tumors with a family history of brain tumor in first- and second-degree relatives.38 While these findings are consistent with some prior studies, the sample may be influenced by the high representation rates of European genetic-ancestry which have been associated with a higher risk of childhood and adolescent brain and other CNS tumors.39
Environmental and Individual Risk Factors for Childhood and Adolescent Brain and Other CNS Tumors
— Numerous potential risk factors for brain and other CNS tumors in children have been examined, but the only consistently validated factor known to increase risk of these tumors is exposure to ionizing radiation. Ionizing radiation has carcinogenic effects which are shown to be stronger in children due to their increased radiosensitivity and longer life expectancy.40 In the United States, the most common man-made radiation exposure to children is from Computed tomography (CT) scan use.41 Associations between CT scans and increased risk of childhood and adolescent brain and other CNS tumors has been reported by research groups in numerous countries, including the United Kingdom (UK), Australia, Taiwan, Germany, the Netherlands, and France.42–48
While it is established that moderate to high doses of ionizing radiation are associated with increased risk of childhood cancers, a recent meta-analysis on low dose exposures of ionizing radiation (such as that from X-ray) suggests that lower levels of ionizing radiation may also cause excess risk for certain types of childhood cancers including brain and other CNS tumors.41 Additionally, this meta-analysis indicated (although not statistically significant) higher risks of brain and other CNS tumors among those exposed in utero via maternal diagnostic imagery than for postnatal exposure.41 A 2021 systematic review of risk of childhood cancers associated with cumulative radiation doses from radiological imaging emphasizes the selective use of CT imaging and the continued need for dose-risk monitoring.49
Non-ionizing radiation, such as that emitted by cellular phones or extremely low-frequency (ELF) magnetic fields, has been repeatedly evaluated as a risk factor for brain and other CNS tumors in children and adolescents. Particular attention has been paid to radiofrequency (RF) radiation which is emitted from technology such as mobile phones and wireless networks, use of which has become much more common among children and young adults in the last several decades. As of 2011, the IARC has classified RF as a possible carcinogen.50,51 A review of risk factors by Vienne-Jumeau et al. found evidence supporting an association between RF exposure and acoustic neuroma in long-term users (adults), but report a lack of significant and consistent evidence for a validated associated between RF exposure and childhood brain and other CNS tumors.50 To date, there is no conclusive evidence that RF or ELF magnetic fields, at the levels emitted by mobile phones may increase the risk brain and other CNS tumors, as reported by Castaño-Vinyals et al. in a large case control study focused on childhood exposures.52
Many factors related to birth, such as gestational age, birth weight, and birth defects have been evaluated for potential associations with risk of childhood and adolescent brain tumors.53 Birth circumstances suspected to be associated with higher risk of developing childhood brain and other CNS tumors include being male, high birth weight (>=4000 g), being born larger for gestational age, and being pre-term (<37 weeks).32 Various studies and meta-analysis support that high birth weight is associated with an increased risk of childhood brain and other CNS tumors overall, particularly astrocytoma and embryonal tumors. A potential association between instrument-assisted delivery and increased risk of childhood brain and other CNS tumors was recently assessed in multiple large population studies, with inconsistent findings. Recent studies done in the United States, Greece, and Denmark found an increased risk of CNS tumors with instrument-assisted delivery.54,55 The United States and Greece studies found effect estimates for instrument-assisted delivery were stronger for astrocytomas.54,55 Approximately 7% of childhood brain and other CNS tumors are thought to be attributable to non-chromosomal structural birth defects (not including known single gene syndromes or chromosomal birth defects such as Down’s syndrome).56 The findings of a recent study using 10 million live birth records showed increased odds of CNS tumors given a non-chromosomal birth defect, but also observed a positive risk association between the total number of major non-chromosomal birth defects per child and the risk of cancer (including CNS tumors) with greater risks among children with two or more major birth defects.56
Many other environmental and parental risk factors have been investigated as contributors to childhood brain and other CNS tumor risk, which remain unvalidated or with inconsistent findings. Some of these evaluated factors include advanced parental age, maternal dietary N-nitroso compounds (NOCs) consumption, and pre-and post-natal exposure to pesticides.32 Currently, studies consistently suggest that children of parents with indicators of lower socioeconomic status (SES) (including lower educational attainment, lower income, and/or participation in public insurance programs) have reduced risk of childhood brain and other CNS tumors.57 Offspring of parents with higher SES appear to be at increased risk of developing childhood and adolescent brain and other CNS tumors. This finding may be due to a yet unknown environmental exposure associated with higher SES but may also be indicative of broader social concerns such as disproportionate access to quality care, which may create differential rates of diagnosis. A meta-analysis of international trends in incidence of brain tumors in children and young adults observed a significant association between childhood brain tumor incidence and gross domestic product per capita, suggesting a positive relationship between incidence and national economic wealth, where high income countries had higher rates compared to low-middle income countries.58 The study posits many reasons for these findings including high income countries having larger populations of European ancestry (a factor independently associated with risk of childhood and adolescent brain and other CNS tumors) and higher rates of CT imaging, while low-middle income countries may be underrepresented due to minimal access to diagnostic healthcare services.58 However, further investigations to identify specific risk factors for each histopathological subtype are required due to the potentially diverse etiologies of these heterogenous malignancies.
Long-Term and Late Effects of Child and Adolescent Brain and CNS Tumors
— Over the last decade, survival rates have slowly improved for children and adolescents diagnosed with brain tumors.59 Although there is variation by tumor type, today, over 80% of children diagnosed with a CNS tumor of any kind will become a long-term survivor (See Table 8). Brain and CNS tumors in children are generally treated with a multimodal approach including surgery, radiation, and chemotherapy.60 Unfortunately, many of these survivors will struggle with long-term and late effects affecting multiple organ systems, that are generally attributable to their curative treatment history.
Long-term effects of brain and other CNS tumor treatment can vary by tumor type, location in the brain, and/or extent of neurosurgical intervention. Long-term effects of a CNS tumor diagnosis can include seizures, neuromuscular deficits, visual impairment, hearing loss, difficult behaviors, and neurocognitive disorders.61
Late effects of brain or other CNS tumor treatment can occur many months or years after a child or adolescent finishes cancer treatment and are often related to radiation or chemotherapy toxicities. Survivors may experience academic or psychosocial difficulties, as well as systemic disorders such as vasculopathies or cerebrovascular accidents, endocrinopathies, infertility, nephropathy, and increased risk of secondary malignancies later in life.62 Children diagnosed with a brain or other CNS tumor at a younger age, treated with cranial radiation therapy, and those with tumors involving eloquent regions of the central nervous system have the highest risk of late effects.63
Recognition and awareness of the long-term and late effects experienced by childhood and adolescent brain tumor survivors are critical to improving their care and quality of life. Through a better understanding of treatment related morbidity, scientists and clinicians can improve future therapeutic interventions for childhood and adolescent brain and other CNS tumor patients with long-term survival in mind. Pediatric oncologists and dedicated research groups, including the North American based Children’s Oncology Group (COG) and the Childhood Cancer Survivor Study (CCSS) are working to improve our understanding of the survivorship experience. More insight can be gained through clinical trial participation and through funding of investigator-initiated research specific to childhood cancer survivors.64
Strengths and Limitations Of Cancer Registry Data
CBTRUS, in collaboration with the CDC and NCI, is the largest population-based registry focused exclusively on primary brain and other CNS tumors in the United States and represents cases collected from the entire US population. The CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018 contains population-based data on all primary brain tumor and other CNS tumors in children and adolescents ages 0–19 years available through the cancer surveillance system in the United States.
Registration of individual cases is conducted by cancer registrars at the institution where diagnosis or treatment occurs and is then transmitted to the CCR, which further transmits this information to NPCR and/or SEER. CCRs, those contributing data to NPCR and to SEER, only report cases to the CDC and NCI for persons who are residents of that particular state, so duplicate records should not occur for persons who may have traveled across state lines for treatment. As a result, the CBTRUS dataset is a complete recording of all cases submitted to CCR for the time period examined, 2014–2018, with minimal duplicates.
Currently, there is no publicly available data source for the collection of survival and outcomes data from all geographic regions in the United States via the cancer registry system. Survival data used for this report are collected by NPCR for 42 of the 51 CCR in the United States primarily through linkage with death certificate and other administrative records. The remaining CCR are collected by SEER through both active and passive methods. The feasibility of these data for use in survival studies has been evaluated65,66 and shown to produce reliable and robust estimates of cancer survival. Use of passive follow-up with record linkage may result in overestimation of survival in some populations, such as those that are more likely to leave the state or country.
No mechanism currently exists for central pathology review of cases within the US cancer registry system, and histopathology code assignment at case registration is based on information contained in the patient’s medical record. The WHO Classification of Tumours of the Central Nervous System was revised in 1993,67 2000,68 2007,69 2016,5 and 2021.70 As of 2018, the US cancer registry system uses the 2016 classification for data abstraction, but tumors included in this report may have been diagnosed using any of the available classifications prior to 2014 due to the variation in adoption of new standards by individual physicians and medical practices. As a result, histopathologies are reflective of the prevailing criteria for the histopathology at the time of case registration. This means that despite changes to the histopathology schema that may occur over time, it is not possible, without additional variables, to go back and reclassify tumors based on the new criteria. In addition to changes in histopathologic criteria over time, there is significant inter-rater variability in histopathological diagnosis of glioma.71,72 This also means that incomplete, incorrect, or alternatively stated diagnoses included in a pathology report or other medical record may result in an incorrect reporting of the details of an individual case.
United States cancer registration requires the reporting of cases that are confirmed by different types of diagnostic procedures, including both microscopic confirmation (where surgery was performed and the diagnosis confirmed by a pathologist) and radiographic confirmation (where diagnosis was made based solely on imaging criteria, such as an magnetic resonance imaging (MRI), CT scan, or X-ray). Only microscopic confirmation allows certainty on the assignment of a specific histopathology as well as for an assignment of a WHO grade. Many tumors have unique characteristics that make them identifiable on imaging, and thereby qualify as a valid type of diagnostic procedure, but it is important to consider the decreased level of certainty of specifying the correct histopathology in these tumors.
Concluding Comment
The CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018 comprehensively describes the population-based incidence, mortality, prevalence, and relative survival of primary brain and other CNS tumors in children and adolescents ages 0–19 years collected and reported by CCRs covering the entire US population. This report aims to serve as a useful resource for researchers, clinicians, patients, and families. CBTRUS continually revises its reports to reflect the current collection and reporting practices of the broader surveillance community in which it works, while integrating the input it receives from the clinical and research communities, especially from neuropathologists, when possible.
CBTRUS Mission
CBTRUS is a not-for-profit corporation committed to providing a resource for gathering and disseminating current epidemiologic data on all primary brain and other central nervous system tumors, benign and malignant, for the purposes of accurately describing their incidence and survival patterns, evaluating diagnosis and treatment, facilitating etiologic studies, establishing awareness of the disease, and ultimately, for the prevention of all brain tumors.
Supplementary Material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Supplementary Table 1. Central Brain Tumor Registry of the United States (CBTRUS), Brain and Other Central Nervous System Tumor Site Groupings
Supplementary Table 2. Childhood and Adolescent Brain and Other Central Nervous System Tumor Histopathology Groupings (Based on 2016 WHO classification)
Supplementary Table 3. ICD-O-3 Morphology Codes for All Histopathologies Included in Glioma and Embryonal Tumor Childhood and Adolescent Report Major Histopathology Groupings (Based on 2016 WHO Classification)
Supplementary Table 4. Total Cases, Annual Average Cases, Age-Specific Incidence Rates, Total Deaths, Annual Average Deaths, and Age-Specific Mortality Rates, for Brain and Other Central Nervous System Tumors Ages 0–19 Years by Central Cancer Registry, (CBTRUS: Incidence Data provided by CDC’s NPCR and NCI’s SEER Program, 2014–2018; Mortality Data provided by NCHS’s NVSS Program, 2014–2018)
Supplementary Table 5. Average Annual Age-Specific Incidence Rates for Brain and Other Central Nervous System Tumors Ages 0–19 Years by Major Histopathology Groupings, Histopathology, Sex, and Age Groups, CBTRUS Childhood and Adolescent Brain Tumor Report: NPCR and SEER, 2014–2018
Supplementary Table 6. Average Annual Age-Specific Incidence Rates for Brain and Other Central Nervous System Tumors Ages 0–19 Years by Major Histopathology Groupings, Histopathology, Race, Hispanic Ethnicity, and Age Group, CBTRUS Childhood and Adolescent Report: NPCR and SEER, 2014–2018
Supplementary Table 7. Annual Percent Change (APC) and 95% Confidence Intervals for Incidence Time Trends in Brain and Other Central Nervous System Tumors by Histopathology Groupings and Age Group, CBTRUS Childhood and Adolescent Report: NPCR and SEER, 2004–2018
Supplementary Table 8. Annual Percent Change (APC) and 95% Confidence Intervals for Cancer Mortality Rates by Cause of Death and Age Group, CBTRUS Childhood and Adolescent Report: NVSS, 1969–2018
Supplementary Table 9. Estimated Prevalent Case Counts and Crude Prevalence Rate per 100,000 for ICCC Defined Cancers Occurring in Locations Other than the Brain or Central Nervous System in Children and Adolescents Ages 0–19 Years at Time of Prevalence in the United States, 2022
Supplementary Table 10. Inherited Syndromes Associated with Childhood and Adolescent Brain and Other Central Nervous System Tumors, Ages 0–19 Years
Supplementary Table 11. Previously Reported Childhood and Adolescent Brain and Other Central Nervous System Tumors Ages 0–19 Years Risk Loci Identified by Genome-wide Association Studies, by Histopathology Including Allele Frequencies, p-values, Odds Ratios (OR) and 95% Confidence Intervals (95% CI)
Supplementary Table 12. A Summary of Recent (2012–2022) Case-control and Cohort Studies Exploring Environmental Exposures and Risk of Childhood and Adolescent Brain and Other Central Nervous System Tumors Ages 0–19 Years
Supplementary Figure 1. Average Annual Age-Specific Incidence Rates per 100,000 Population of Primary Brain and Other Central Nervous System Tumors by Central Cancer Registry and Age Group, in Children and Adolescents Ages A) 0–4 Years, B) 5–9 Years, C) 10–14 Years, and D) 15–19 Years, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2014–2018
Supplementary Figure 2. Country- and Age-Specific A) Incidence and B) Mortality Rates per 100,000 Population due to Primary Malignant Brain and Other Central Nervous System Tumors in Children and Adolescents Ages 0–19 Years (From International Agency for Research on Cancer’s GLOBOCAN 2020)
Supplementary Figure 3. Distribution in Infants Age < 1 Year of All Primary Brain and Other Central Nervous System Tumors (Five-Year Total = 1,235; Annual Average Cases = 247) by A) Site and B) Histopathology, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2014–2018
Supplementary Figure 4. Distribution in Children Ages 1–4 Years of All Primary Brain and Other Central Nervous System Tumors (Five-Year Total = 4,871; Annual Average Cases = 974) by A) Site and B) Histopathology, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2014–2018
Supplementary Figure 5. Distribution in Children Ages 5–9 Years of All Primary Brain and Other Central Nervous System Tumors (Five-Year Total = 5,628; Annual Average Cases = 1,126) by A) Site and B) Histopathology, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2014–2018
Supplementary Figure 6. Distribution in Children Ages 10–14 Years of All Primary Brain and Other Central Nervous System Tumors (Five-Year Total = 6,074; Annual Average Cases = 1,215) by A) Site and B) Histopathology, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2014–2018
Supplementary Figure 7. Distribution in Adolescents Ages 15–19 Years of All Primary Brain and Other Central Nervous System Tumors (Five-Year Total = 7,689; Annual Average Cases = 1,538) by A) Site and B) Histopathology, CBTRUS Childhood and Adolescent Report: US Cancer Statistics—NPCR and SEER, 2014–2018
Acknowledgments
This report was prepared for the Pediatric Brain Tumor Foundation by CBTRUS Co-Scientific Principal Investigator, Quinn T. Ostrom, Ph.D., M.P.H. and her research team from Duke University School of Medicine in collaboration with Co-Scientific Principal Investigator Jill S. Barnholtz-Sloan, Ph.D., the research staff affiliated with the National Cancer Institute (NCI) Division of Cancer Epidemiology and Genetics (DCEG) whose services were provided by the DCEG, and CBTRUS President and Chief Mission Officer Carol Kruchko, collectively known as the CBTRUS Team. The CBTRUS data presented in this report were provided through an agreement with the CDC, NPCR. In addition, CBTRUS used data from the research data files of the NCI, SEER Program. CBTRUS acknowledges and appreciates these contributions to this report and to cancer surveillance in general.
We acknowledge the efforts of the tumor registrars at hospitals and treatment centers, the CCRs, and the staff from the NPCR and SEER programs, whose efforts to collect accurate and complete data have made this report possible. We are also grateful to our Consulting Neuropathologist, Dr. Janet Bruner, who answered our questions and provided feedback throughout the year, to our Board of Directors and Advisors, and especially Drs. Roberta McKean-Cowdin, Ph.D., Jennifer Cullen, Ph.D., M.P.H., Michael Scheurer, Ph.D., and Diana R. Withrow, Ph.D., to Claudia Smits, L.L.M., Reda J, Wilson, M.P.H., C.T.R., and Mary Elizabeth O’Neil, M.P.H.
Contributor Information
Quinn T Ostrom, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA; The Preston Robert Tisch Brain Tumor Center, Duke University School of Medicine, Durham, North Carolina, USA; Duke Cancer Institute, Duke University School of Medicine, Durham, North Carolina, USA.
Mackenzie Price, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA.
Katherine Ryan, Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA; The Preston Robert Tisch Brain Tumor Center, Duke University School of Medicine, Durham, North Carolina, USA.
Jacob Edelson, Trans Divisional Research Program (TDRP), Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute, Bethesda, Maryland, USA.
Corey Neff, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA.
Gino Cioffi, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Trans Divisional Research Program (TDRP), Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute, Bethesda, Maryland, USA.
Kristin A Waite, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Trans Divisional Research Program (TDRP), Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute, Bethesda, Maryland, USA.
Carol Kruchko, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA.
Jill S Barnholtz-Sloan, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Trans Divisional Research Program (TDRP), Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute, Bethesda, Maryland, USA; Center for Biomedical Informatics & Information Technology (CBIIT), National Cancer Institute, Bethesda, USA.
Disclaimer
CBTRUS is a not-for-profit corporation which gathers and disseminates epidemiologic data on primary brain and other CNS tumors to facilitate research and establish awareness of the disease. CBTRUS makes no representations or warranties, and gives no other assurances or guarantees, express or implied, with respect to the accuracy or completeness of the data presented. The information provided in this report is not intended to assist in the evaluation, diagnosis, or treatment of individual diseases. Persons with questions regarding individual diseases should contact their own physician to obtain medical assistance. The contents in this report are solely the responsibility of the authors and do not necessarily represent the official views of CDC or of NCI.
Funding
Funding for the Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors was provided through a contract with the Pediatric Brain Tumor Foundation. Funding for the CBTRUS analytic files which comprise the CBTRUS database were supported by the following organizations: the Centers for Disease Control and Prevention (CDC) under Contract No.75D30119C06056 Amendment/Modification No:00002, the American Brain Tumor Association, Novocure, the Musella Foundation, The Sontag Foundation, National Brain Tumor Society, the Uncle Kory Foundation, the Pediatric Brain Tumor Foundation, and the Zelda Dorin Tetenbaum Memorial Fund as well as private and in kind donations. CBTRUS is honored to be included among their research awardees. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or of the NCI. The research services of JSB-S, KAW, and GC were provided by the Division of Cancer Epidemiology and Genetics of the NCI.
Conflict of Interest: JSB-S is a full-time paid employee of the NIH/NCI. GC and KAW are full-time contractors of the NIH/NCI. JE is a full-time fellow of the NIH/NCI.
The CBTRUS Scientific Team
Jill S. Barnholtz-Sloan, Ph.D., CBTRUS Co-Scientific Principal Investigator, Associate Director and Senior Investigator, Center for Biomedical Informatics & Information Technology (CBIIT) and Trans Divisional Research Program (TDRP), Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute, Bethesda, MD
Quinn T. Ostrom, Ph.D., M.P.H., CBTRUS Co-Scientific Principal Investigator, Assistant Professor, The Preston Robert Tisch Brain Tumor Center at Duke University Medical Center and Department of Neurosurgery, Duke University School of Medicine, Durham, NC
Gino Cioffi, M.P.H., Trans Divisional Research Program (TDRP), Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute, Bethesda, MD
Kristin A. Waite, Ph.D., Trans Divisional Research Program (TDRP), Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute, Bethesda, MD
Corey Neff, M.P.H., Department of Neurosurgery, Duke University School of Medicine, Durham, NC
Mackenzie Price, M.P.H., Department of Neurosurgery, Duke University School of Medicine, Durham, NC
The CBTRUS Consulting Neuropathologists
Daniel J. Brat, M.D., Ph.D., Professor and Chair, Department of Pathology, Northwestern University Feinberg School of Medicine, Chicago, IL
Janet M. Bruner, M.D., MD Anderson Cancer Center, Houston, TX (Retired as of 2020)
Roger E. McLendon, M.D., Professor, The Preston Robert Tisch Brain Tumor Center at Duke University Medical Center and Department of Pathology, Duke University Medical Center, Durham, NC
Tarik Tihan, M.D., Ph.D., Professor, Neuropathology Division, Department of Pathology, School of Medicine, University of California San Francisco (UCSF), San Francisco, CA
The CBTRUS Board of Directors
Carol Kruchko, President & Chief Mission Officer, Central Brain Tumor Registry of the United States, Hinsdale, IL
Steven Brem, M.D., Vice President, Professor, Penn Neuroscience Center-Neurosurgery, Perelman Center for Advanced Medicine, Philadelphia, PA
Donald Segal, J.D., Treasurer, Segal McCambridge Singer & Mahoney, Ltd., Chicago, IL
Elizabeth B. Claus, M.D., Ph.D., Professor, Departments of Biostatistics and Neurosurgery, Yale University, New Haven, CT, Attending Neurosurgeon, Brigham and Women’s Hospital, Boston, MA
Fred H. Hochberg, M.D., Visiting Scientist, Department of Neurosurgery, University of California at San Diego, San Diego, CA
Margaret Wrensch, Ph.D., Professor, Neuroepidemiology Division, Department of Neurological Surgery, School of Medicine, University of California-San Francisco, San Francisco, CA
L. Lloyd Morgan, Emeritus Member, Patient Advocate, Berkeley, CA.
Darell D. Bigner, M.D., Ph.D. Emeritus Member, Edwin L. Jones, Jr. and Lucille Finch Jones Cancer Research Professor, Department of Pathology, Emeritus Director, The Preston Robert Tisch Brain Tumor Center, Chief, Preuss Laboratory for Brain Tumor Research, Duke, University Medical Center, Durham, NC
The CBTRUS Board of Advisors
Hoda Anton-Culver, Ph.D., Distinguished Professor, Department of Medicine; Principal Investigator, All of Us Precision Medicine Research Program; Director, Genetic Epidemiology Research Institute, University of California-Irvine, Irvine, CA
Melissa Bondy, Ph.D., Professor and Chair, Department of Epidemiology and Population Health, Stanford University School of Medicine, Stanford, CA
Jennifer Cullen, Ph.D., M.P.H., Professor, School of Medicine, Case Western Reserve University, Cleveland, OH
Faith Davis, Ph.D., Professor Emeritus, School of Public Health, University of Alberta, Edmonton, Canada
Roberta McKean-Cowdin, Ph.D., Associate Professor, Department of Epidemiology, University of Southern California, Los Angeles, CA
Nancy Stroup, Ph.D., Epidemiologist, retired.
John Villano, M.D., Ph.D., Professor, Division of Medical Oncology, University of Kentucky Markey Cancer Center, Lexington, KY
Selected CBTRUS Scientific Publications focused on Children and Adolescents
Gittleman H, et al. Descriptive epidemiology of germ cell tumors of the central nervous system diagnosed in the United States from 2006 to 2015. J Neurooncol. 2019 Jun;143(2):251–260. doi: 10.1007/s11060–019–03173–4. Epub 2019 Apr 25. PMID: 31025275.
This manuscript details the descriptive epidemiology, including incidence and survival, for germ cell tumors
Greppin K, et al. Epidemiology of pineoblastoma in the United States, 2000–2017. Neurooncol Pract. 2022 Jan 27;9(2):149–157. doi: 10.1093/nop/npac009. PMID: 35371520; PMCID: PMC8965073.
This manuscript details the descriptive epidemiology, including incidence and survival, for pineoblastoma.
Khanna V, et al. Incidence and survival trends for medulloblastomas in the United States from 2001 to 2013. J Neurooncol. 2017 Dec;135(3):433–441. doi: 10.1007/s11060–017–2594–6. Epub 2017 Aug 21. PMID: 28828582.
This manuscript details the descriptive epidemiology, including incidence, survival, and time trends, for medulloblastoma in children and adolescents.
Ostrom QT, et al. Pilocytic astrocytomas: where do they belong in cancer reporting? Neuro Oncol. 2020 Feb 20;22(2):298–300. doi: 10.1093/neuonc/noz202. PMID: 31637436; PMCID: PMC7442407.
This letter describes the history of inclusion of pilocytic astrocytoma in cancer registry reporting, and the effect of varying behavior classification for these tumors on incidence and survival patterns.
Patil N, et al. Epidemiology of Brainstem High-Grade Gliomas in Children and Adolescents in the United States, 2000–2017. Neuro Oncol. 2020 Dec 21:noaa295. doi: 10.1093/neuonc/noaa295. PMID: 33346835.
This manuscript details the descriptive epidemiology, including incidence, survival, and prevalence, for gliomas of the brain stem in children and adolescents.
References
- 1. National Cancer Institute. Overview of the SEER Program. http://seer.cancer.gov/about/overview.html.
- 2. Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Perkin DM, Whelan S, eds. International Classification of Diseases for Oncology. 3rd ed. World Health Organization; 2000. [Google Scholar]
- 3. Cancer Registries Amendment Act, 102nd Cong. § 515 (1992). https://www.govinfo.gov/content/pkg/STATUTE-106/pdf/STATUTE-106-Pg3372.pdf. Accessed July 21, 2020.
- 4. Benign Brain Tumor Cancer Registries Amendment Act, 107th Cong. § 260 (2002). http://www.gpo.gov/fdsys/pkg/PLAW-107publ260/pdf/PLAW-107publ260.pdf. Accessed July 21, 2020.
- 5. Louis DN, Ohgaki H, Wiestler OD, Cavanee WK, eds. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2016. [Google Scholar]
- 6. Ferlay J, Rous B. Histological groups. In: Bray F, Colombet M, Ferlay J, et al. , eds. Cancer Incidence in Five Continents. Vol. XI. 2019. [Google Scholar]
- 7. Surveillance Epidemiology and End Results (SEER) Program. Solid Tumor Rules: Non-Malignant CNS 2019. https://seer.cancer.gov/tools/solidtumor/Non_Malignant_CNS_STM.pdf. Accessed July 21, 2020.
- 8. Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Pilocytic astrocytomas: where do they belong in cancer reporting? Neuro Oncol. 2020;22(2):298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention National Center for Health Statistics. National Program of Cancer Registries and Surveillance, Epidemiology, and End Results SEER*Stat Database: U.S. Cancer Statistics Incidence Analytic Database – 2001–2018. United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2021. Based on the November 2020 submission. [Google Scholar]
- 10. Surveillance Epidemiology aERSP. ICCC Recode Third Edition ICD-O-3/IARC 2017. 2017. https://seer.cancer.gov/iccc/iccc-iarc-2017.html. Accessed July 1, 2021.
- 11. International Incidence of Childhood Cancer. Vol. III. Lyon, France: International Agency for Research on Cancer; 2017. [Google Scholar]
- 12. Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. 2005;103(7):1457–1467. [DOI] [PubMed] [Google Scholar]
- 13. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. World Health Organization; 2007. [Google Scholar]
- 14. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969–2018) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, released December 2020 Underlying mortality data provided by NCHS. 2020. www.cdc.gov/nchs.
- 15. R Core Team. R: a language and environment for statistical computing. 2021. http://www.R-project.org/. Accessed April 20, 2021.
- 16. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat software version 8.4.0. 2022. www.seer.cancer.gov/seerstat. Accessed May 27, 2022.
- 17. Luo J. SEER2R: reading and writing SEER*STAT data files. R package version 1.0. 2012. http://CRAN.R-project.org/package=SEER2R. Accessed July 21, 2020.
- 18. Gohel D, Hangler F, Sander L, et al. officer: Manipulation of Microsoft Word and PowerPoint Documents. 2020. https://cloud.r-project.org/web/packages/officer/index.html. Accessed July 21, 2020.
- 19. Gohel D, Fazilleau Q, Nazarov M, Robert T, Barrowman M, Yasumoto A. flextable: Functions for Tabular Reporting. 2020. https://davidgohel.github.io/flextable/. Accessed July 21, 2020.
- 20. Hočevar T, Demšar J. Computation of graphlet orbits for nodes and edges in sparse graphs. J Stat Softw. 2016;71(10):24. [Google Scholar]
- 21. Wickham H, Averick M, Bryan J, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4(43):1686. [Google Scholar]
- 22. Sievert C. Interactive Web-Based Data Visualization with R, plotly, and shiny. 2020. https://plotly-r.com. Accessed July 21, 2020.
- 23. Walker K, Rudis B. tigris: Load Census TIGER/Line Shapefiles. 2020. https://github.com/walkerke/tigris. Accessed May 27, 2022.
- 24. Kassambara A, Kosinski M, Biecek P, Fabian S. survminer: Drawing Survival Curves using “ggplot2”. 2020. https://rpkgs.datanovia.com/survminer/index.html. Accessed May 27, 2022.
- 25. Pebesma E. Simple features for R: standardized support for spatial vector data. R J. 2018;10(1):439–446. [Google Scholar]
- 26. Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547–569. [DOI] [PubMed] [Google Scholar]
- 27. NAACCR Race and Ethnicity Work Group. NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1]. 2012.
- 28. Fay MP, Tiwari RC, Feuer EJ, Zou Z. Estimating average annual percent change for disease rates without assuming constant change. Biometrics. 2006;62(3):847–854. [DOI] [PubMed] [Google Scholar]
- 29. Joinpoint Regression Program, Version 4.10.0.0. Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute; 2022; https://surveillance.cancer.gov/joinpoint/. Accessed June 1, 2022. [Google Scholar]
- 30. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 31. Zhu L, Pickle LW, Ghosh K, et al. Predicting US- and state-level cancer counts for the current calendar year: Part II: evaluation of spatiotemporal projection methods for incidence. Cancer. 2012;118(4):1100–1109. [DOI] [PubMed] [Google Scholar]
- 32. Adel Fahmideh M, Scheurer ME. Pediatric brain tumors: descriptive epidemiology, risk factors, and future directions. Cancer Epidemiol Biomarkers Prev. 2021;30(5):813–821. [DOI] [PubMed] [Google Scholar]
- 33. Ostrom QT, Adel Fahmideh M, Cote DJ, et al. Risk factors for childhood and adult primary brain tumors. Neuro Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang C, Ostrom QT, Semmes EC, et al. Genetic predisposition to longer telomere length and risk of childhood, adolescent and adult-onset ependymoma. Acta Neuropathol Commun. 2020;8(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walsh KM, Whitehead TP, de Smith AJ, et al. Common genetic variants associated with telomere length confer risk for neuroblastoma and other childhood cancers. Carcinogenesis. 2016;37(6):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Foss-Skiftesvik J, Hagen CM, Mathiasen R, et al. Genome-wide association study across pediatric central nervous system tumors implicates shared predisposition and points to 1q25.2 (PAPPA2) and 11p12 (LRRC4C) as novel candidate susceptibility loci. Childs Nerv Syst. 2021;37(3):819–830. [DOI] [PubMed] [Google Scholar]
- 37. Del Risco Kollerud R, Cannon-Albright LA, Haugnes HS, et al. Childhood central nervous system tumors and leukemia: Incidence and familial risk. A comparative population-based study in Utah and Norway. Pediatr Blood Cancer. 2020;67(8):e28408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vidart d’Egurbide Bagazgoitia N, Bailey HD, Orsi L, et al. Family history of cancer and the risk of childhood brain tumors: a pooled analysis of the ESCALE and ESTELLE studies (SFCE). Cancer Causes Control. 2019;30(10):1075–1085. [DOI] [PubMed] [Google Scholar]
- 39. Zhang C, Ostrom QT, Hansen HM, et al. European genetic ancestry associated with risk of childhood ependymoma. Neuro Oncol. 2020;22(11):1637–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). UNSCEAR 2013 Report to the General Assembly, Volume 2, Annex B: Effects of Radiation Exposure of Children. New York City, New York, USA: United Nations; 2013. [Google Scholar]
- 41. Little MP, Wakeford R, Bouffler SD, et al. Review of the risk of cancer following low and moderate doses of sparsely ionising radiation received in early life in groups with individually estimated doses. Environ Int. 2022;159:106983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Foucault A, Ancelet S, Dreuil S, et al. Childhood cancer risks estimates following CT scans: an update of the French CT cohort study. Eur Radiol. 2022. [DOI] [PubMed] [Google Scholar]
- 43. Meulepas JM, Ronckers CM, Smets A, et al. Radiation exposure from pediatric CT scans and subsequent cancer risk in the Netherlands. J Natl Cancer Inst. 2019;111(3):256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krille L, Dreger S, Schindel R, et al. Risk of cancer incidence before the age of 15 years after exposure to ionising radiation from computed tomography: results from a German cohort study. Radiat Environ Biophys. 2015;54(1):1–12. [DOI] [PubMed] [Google Scholar]
- 45. Li IG, Yang YH, Li YT, Tsai YH. Paediatric computed tomography and subsequent risk of leukaemia, intracranial malignancy and lymphoma: a nationwide population-based cohort study. Sci Rep. 2020;10(1):7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang WY, Muo CH, Lin CY, et al. Paediatric head CT scan and subsequent risk of malignancy and benign brain tumour: a nation-wide population-based cohort study. Br J Cancer. 2014;110(9):2354–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marcu LG, Chau M, Bezak E. How much is too much? Systematic review of cumulative doses from radiological imaging and the risk of cancer in children and young adults. Crit Rev Oncol Hematol. 2021;160:103292. [DOI] [PubMed] [Google Scholar]
- 50. Vienne-Jumeau A, Tafani C, Ricard D. Environmental risk factors of primary brain tumors: a review. Rev Neurol. 2019;175(10):664–678. [DOI] [PubMed] [Google Scholar]
- 51. Baan R, Grosse Y, Lauby-Secretan B, et al. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 2011;12(7):624–626. [DOI] [PubMed] [Google Scholar]
- 52. Castaño-Vinyals G, Sadetzki S, Vermeulen R, et al. Wireless phone use in childhood and adolescence and neuroepithelial brain tumours: results from the international MOBI-Kids study. Environ Int. 2022;160:107069. [DOI] [PubMed] [Google Scholar]
- 53. Johnson KJ, Cullen J, Barnholtz-Sloan JS, et al. Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2716–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Georgakis MK, Dessypris N, Papadakis V, et al. Perinatal and early life risk factors for childhood brain tumors: Is instrument-assisted delivery associated with higher risk? Cancer Epidemiol. 2019;59:178–184. [DOI] [PubMed] [Google Scholar]
- 55. Yeh KW, He D, Hansen J, et al. The risk of childhood brain tumors associated with delivery interventions: A Danish matched case-control study. Cancer Epidemiol. 2022;76:102077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lupo PJ, Schraw JM, Desrosiers TA, et al. Association between birth defects and cancer risk among children and adolescents in a population-based assessment of 10 million live births. JAMA Oncol. 2019;5(8):1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Francis SS, Wang R, Enders C, et al. Socioeconomic status and childhood central nervous system tumors in California. Cancer Causes Control. 2021;32(1):27–39. [DOI] [PubMed] [Google Scholar]
- 58. Raja N, Hayes L, Basta N, McNally RJQ. International trends in the incidence of brain tumours in children and young-adults and their association with indicators of economic development. Cancer Epidemiol. 2021;74:102006. [DOI] [PubMed] [Google Scholar]
- 59. Pollack IF, Agnihotri S, Broniscer A. Childhood brain tumors: current management, biological insights, and future directions. J. Neurosurg. Pediatr. 2019;23(3):261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Erdmann F, Frederiksen LE, Bonaventure A, et al. Childhood cancer: survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol. 2021;71(Pt B):101733. [DOI] [PubMed] [Google Scholar]
- 61. Fischer C, Petriccione M, Donzelli M, Pottenger E. Improving care in pediatric neuro-oncology patients: an overview of the unique needs of children with brain tumors. J Child Neurol. 2016;31(4):488–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Roddy E, Mueller S. Late effects of treatment of pediatric central nervous system tumors. J Child Neurol. 2016;31(2):237–254. [DOI] [PubMed] [Google Scholar]
- 63. Hobbie WL, Ogle S, Reilly M, et al. Adolescent and young adult survivors of childhood brain tumors: life after treatment in their own words. Cancer Nurs. 2016;39(2):134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Armstrong GT. Long-term survivors of childhood central nervous system malignancies: the experience of the Childhood Cancer Survivor Study. Eur J Paediatr Neurol. 2010;14(4):298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Weir HK, Johnson CJ, Mariotto AB, et al. Evaluation of North American Association of Central Cancer Registries’ (NAACCR) data for use in population-based cancer survival studies. J Natl Cancer Inst Monogr. 2014;2014(49):198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wilson RJ, O’Neil ME, Ntekop E, Zhang K, Ren Y. Coding completeness and quality of relative survival-related variables in the National Program of Cancer Registries Cancer Surveillance System, 1995–2008. J Registry Manag. 2014; 41(2):65–71; quiz 96–67. [PMC free article] [PubMed] [Google Scholar]
- 67. Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993;3(3):255–268. [DOI] [PubMed] [Google Scholar]
- 68. Kleihues P, Cavenee W, eds. Tumours of the Nervous System: World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2000. [Google Scholar]
- 69. Louis D, Wiestler O, Cavanee W, eds. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2007. [Google Scholar]
- 70. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician’s perspective. Acta Neuropathol. 2010;120(3):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Aldape K, Simmons ML, Davis RL, et al. Discrepancies in diagnoses of neuroepithelial neoplasms: the San Francisco Bay Area Adult Glioma Study. Cancer. 2000;88(10):2342–2349. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.