BACKGROUND:
Neural components of the fibrous filum terminale (FT) are well known but are considered as embryonic remnants without functionality.
OBJECTIVE:
To investigate the ultrastructure of human FT specimens for sensory nerve endings and record paraspinal muscle activity on electrostimulation of the FT.
METHODS:
We prospectively investigated a cohort of 53 patients who underwent excision of the FT for the treatment of tethered cord syndrome. Surgical FT specimens were investigated by light and transmission electron microscopy. Intraoperative electrophysiological routine monitoring was extended by recording paraspinal muscles above and below the laminotomy level.
RESULTS:
Light microscopy revealed tiny peripheral nerves piercing the pia mater of the FT and entering its fibrous core. Transmission electron microscopy unveiled within the fibrous core of the FT myelinated nerve structures in 8 of the 53 patients and unmyelinated ones in 10 of the 53 patients. Both nerve endings encapsulated in fibrous tissue or unencapsulated nonmyelinated Schwann cell nerve bundles, that is, Remak cells, were found. Those nerve endings resembled mechanoreceptor and nociceptive receptor structures found in human skin, muscle tendons, and skeletal ligaments. Specifically, we found Ruffini mechanoreceptors and in addition nerve endings which resembled nociceptive glioneural structures of the skin. Bipolar electrostimulation of the FT was associated with paraspinal muscle activity above and below the spinal segment at which the FT was stimulated.
CONCLUSION:
Morphological and electrophysiological results indicate the presence of functional sensory nerve endings in the FT. Like other spine ligaments, the FT may serve as a proprioceptive element but may also contribute to back pain in spine disorders.
KEY WORDS: Filum terminale, Sensory nerve endings, Morphology, Electrophysiology
ABBREVIATIONS:
- CMAP
compound muscle action potential
- EAS
external anal sphincter
- EMG
electromyography
- FT
filum terminale
- H&E
hematoxylin and eosin
- PGP
protein gene product
- TCS
tethered cord syndrome
- TEM
transmission electron microscopy.
The human filum terminale (FT) has been understood as an extension of the spinal pia mater containing fibrovascular tissue, ependyma, glial tissue, myelinated axons, and dorsal root ganglion cells.1 Today, the FT is seen as an elastic fibrous band that anchors the conus medullaris to the coccyx.2-6 Still, the neural elements of the FT are commonly understood as embryonic remnants occurring in the conversion of the caudal segments of the human fetal spinal cord into conus and filum.7
However, from observations of intact nerve cell bodies, axons, dendrites, and synapses within the filum terminale in adult cats and squirrel monkeys, it was supposed that there is an anatomical basis for nerve impulse conduction and transmission.8 But how this might function remains obscure.7 Others observed an abundance of peripheral nerve fibers in surgical specimens of the adult FT but concluded that these fibers were probably not functional because surgical transection of the FT did not cause any symptoms of neurologic deterioration.9
In skin, skeletal tendons, and ligaments, a variety of peripheral nerve ending structures have been recognized serving as mechanoreceptors or nociceptive receptors. Unencapsulated nerve endings enveloped by unmyelinated Schwann cells, that is, Remak cells, were shown to serve as nociceptors causing neuropathic pain.10,11 Encapsulated structures, such as the Golgi tendon organ and Ruffini corpuscles, serve as mechanoreceptors and have been distinguished from unencapsulated nerve endings.11,12 Of note, mechanoreceptors have also been found in interspinous ligaments, in facet joint ligaments, and even in intervertebral disks.13,14 Electrical stimulation of interspinous ligaments and facet joint ligaments activated paraspinal muscles above and below the stimulation site.15-18
We theorized that the neural elements of the FT may include structures which can serve as mechanoreceptors and/or nociceptive receptors. In search for such receptors, we performed light and transmission electron microscopy (TEM) of FT specimens obtained during surgery for tethered cord syndrome. Hypothesizing that such receptors may be functionally active, we stimulated the FT during surgery for tethered cord syndrome and recorded, in addition to routine monitoring of sphincter and leg myotomes, paraspinal muscle activity above and below the laminotomy level of the stimulation site.
METHODS
Patients
We prospectively investigated a cohort of 53 patients (20 male, 33 female, age range 1-79 years) who underwent excision of the FT for the treatment of tethered cord syndrome. Thirty-two cases had pathological MRI findings, that is, a low-lying conus and/or a fatty filum. Twenty-one cases had no MRI findings but a progressive tethered cord syndrome (TCS) and a connective tissue disorder. All cases were recruited according to a recently reported study protocol, which includes comprehensive neurological, radiological, and urological diagnostics.6 Since preliminary TEM results revealed sensory nerve endings in the FT of the first 16 patients, the Institutional Review Board protocol was amended to include the recording of paraspinal muscles above and below the surgical level electrostimulation of the FT in addition to the routine intraoperative electrophysiological monitoring for TCS surgery. Ethical approval for the study was obtained under IRB protocol 1107867-10, including informed consent from patients and/or their legal guardians.
Surgical Procedure
Surgery was performed through an interspinous approach 1 spine segment below the conus level. Patients were maintained on gas anesthesia throughout surgery. Microsurgical resection of approximately 2.5 cm of FT for tethered cord release was performed.
EMG Recordings
Intraoperative neurophysiological monitoring was conducted using a Cadwell monitoring platform (Cadwell). Triggered electromyography recordings were obtained using a Neurosign V4 100 mm × 1 mm and 100 mm × 1 mm disposable concentric bipolar stimulator probe (Neurosign). Train of four responses were obtained after reversal of the neuromuscular blockade with sugammedex from the abductor hallucis after stimulation of the posterior tibial nerve supramaximally. After FT stimulation, CMAP recordings were percutaneously obtained from paraspinal muscles 1 segment above and below the laminotomy, the anal sphincter, and bilaterally the myotomes of the lower extremity. Stimulation was started at 0.5 mA and incrementally increased up to 15 mA. The receptive stimulus was repeated 3 to 5 times until a reproducible pattern was found. For descriptive statistical analysis, the averages of stimulus intensity, latency, and compound muscle action potential (CMAP) were taken.
Histological and Ultrastructural Studies of the Neural Elements in the FT
Fresh specimens were generously trimmed at the ends to remove parts potentially exposed to heat injury from coagulation. Hematoxylin and eosin (H&E) routine histology of the FT was performed in all cases. In 10 consecutive cases, immunohistochemistry was also performed using neurofilament and protein gene product 9.5 (PGP 9.5), polyclonal rabbit anti-PGP 9.5 (Agilent; Cat. No. Z511601-2) and monoclonal mouse anti-neurofilament (Agilent; Cat. No. GA60761-2).
TEM samples of 1 to 2 mm thickness were cut from the end of the specimen, fixed in glutaraldehyde, and embedded in epon-araldite. Ultrathin sections (80 nm) were cut (Reichert-Jung Ultracut E) and poststained with uranyl acetate and lead citrate.
RESULTS
Histopathology and Immunohistochemistry
H&E staining of FT revealed neuropil in 42 of the 53 specimens and nerve twigs in 48 of the 53 specimens (Figure 1A). Immunohistochemical staining for axons revealed reactivity to PGP9.5 in 9 of the 10 specimens, whereas reactivity to neurofilament was found in 10 of the 10 specimens, thus confirming the presence of axons within the FT (Figure 1B).
FIGURE 1.
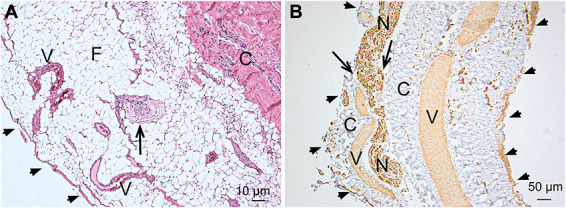
Light microscopic findings. A, H&E staining of a longitudinal section of a filum specimen. A pial cell layer envelopes the FT (arrowheads). Underneath a larger fat pad (F), the collagenous core of the FT can be seen. The fat contains multiple small vessels (V) and, in just 1 specimen, a Pacinian mechanoreceptor (arrow). Pacinian corpuscles are typically found in subcutaneous tissue of the skin and are pressure sensitive.12 B, Immunostaining of a longitudinal section of a filum specimen. The arrowheads mark the outer pial cell layer. A nerve (N) is piercing through the pia into the fibrous core of the FT (arrows). Multiple blood vessels (V) are running in a longitudinal direction within the collagenous tissue C. FT, filum terminale.
Ultrastructure
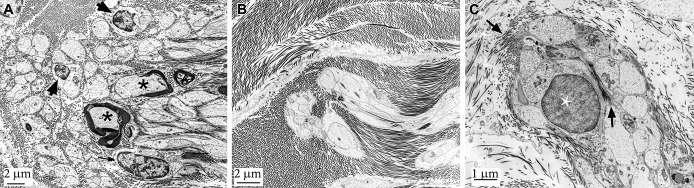
Transmission electron microscopy revealed myelinated structures in 8 of the 53 FT specimens and unmyelinated nerve structures in 10 of the 53 specimens. The unmyelinated structures resembled sensory nerve endings, that is, Remak cell bundles (Figure 2), and encapsulated nerve endings, that is, Ruffini mechanoreceptors (Figure 3).
FIGURE 2.
TEM findings of unencapsulated nerve endings. A, TEM of a FT specimen showing multiple Remak cells within the collagenous core of the FT. Arrows indicate Schwann cell nuclei, asterisks myelinated nerve structures. B, Magnification of 2 Remak cell axon bundles, each accommodating multiple small-caliber axons. Those nerve endings are bulb-shaped and strictly aligned to the course of the surrounding collagenous fibers. C, Larger magnification of a bulbous Remak cell nerve ending. Multiple axons are embedded within the Remak Schwann cell. Note that at the tip of the nerve bulb, collagen fibers are entering the inner core of the nerve ending (arrows). This seems to connect the surrounding collagen tissue to collagen fibrils within the Remak cell axon bundle possibly transmitting stretch forces of the filum to the axons. FT, filum terminale; TEM, transmission electron microscopy.
FIGURE 3.
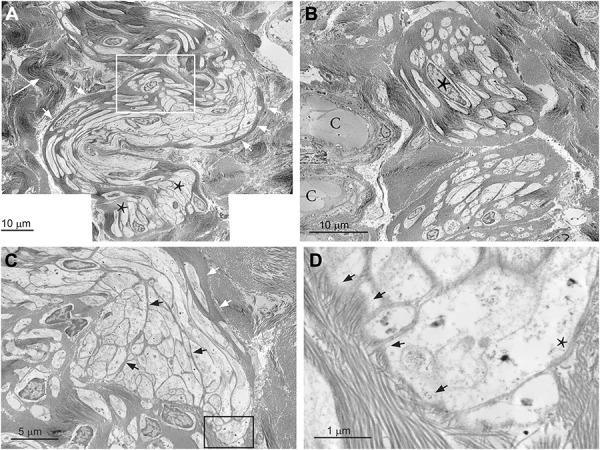
TEM findings of encapsulated nerve endings. A, TEM of FT specimens revealed Ruffini structures showing a cylindrical capsule structure containing multiple Remak cell axon bundles. The capsule (short white arrows) is crimped like an inverse-S similar to the surrounding collagen fiber bundles (large white arrow). The white rectangle marks a corpuscular structure embedded in the fibrous capsule and is shown in more detail in B. At its end, the cylinder is bulged and compartmentalized carrying multiple axon bundles (asterisks), which are shown more in detail in C and D. B, 2 corpuscular structures containing Remak cells embedded within fibrous tissue. It seems that the Remak cells are arranged in a spiral manner around a virtual longitudinal axis. A Remak cell nucleus (black asterisk) can be seen at the bottom of a corpuscle. Two capillaries (C) run near the corpuscles. C, A bulged nerve end structure as marked with an asterisk in A is shown. The nerve ending is compartmentalized by fibrous septa (black arrows). The fibrous capsule is marked with white arrows. The black rectangle marks a clip shown in D. D, Toward its end, the cylinder loses its fibrous capsule and eventually even its basal membrane (asterisks). Collagen fibrils of the surrounding fibrous tissue connect with the septal structures within the bulb (arrows). FT, filum terminale; TEM, transmission electron microscopy.
Electromyography
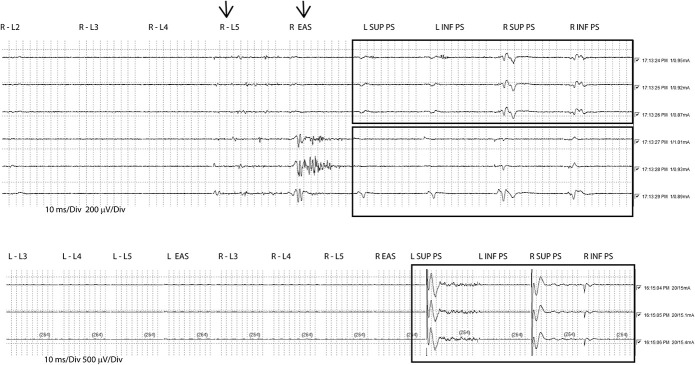
After the stimulation of the FT compound, muscle action potentials were observed intraoperatively in the paraspinal muscles in 22 of the 37 patients. Such response could be elicited only within a small area of the FT, the stimulation electrode had to be moved along the surgically exposed FT to find a sensitive spot. The paraspinal response was stronger in the inferior muscles than in the superior ones. The pattern of response was varying being either bilateral or unilateral. For descriptive statistics of CMAPs and latency, the left and right results were pooled. The lowest stimulus intensity at which CMAPs of the paraspinal muscles were observed was 1.66 ± 1.28 mA (mean ± SD). The latency of inferior paraspinal muscles was 8.9 ± 5.2 ms and the stimulus response 84.6 ± 116.4 mV. The latency of superior muscles was 12.9 ± 5.1 ms and the stimulus response 43.7 ± 57.0 mV. These results are illustrated in Figure 4. Not depending on the stimulus intensity sporadically, the activation of other myotomes of the lower extremities (n = 8) and the sphincter musculature was observed (n = 4). Figure 5 shows typical EMG readings.
FIGURE 4.
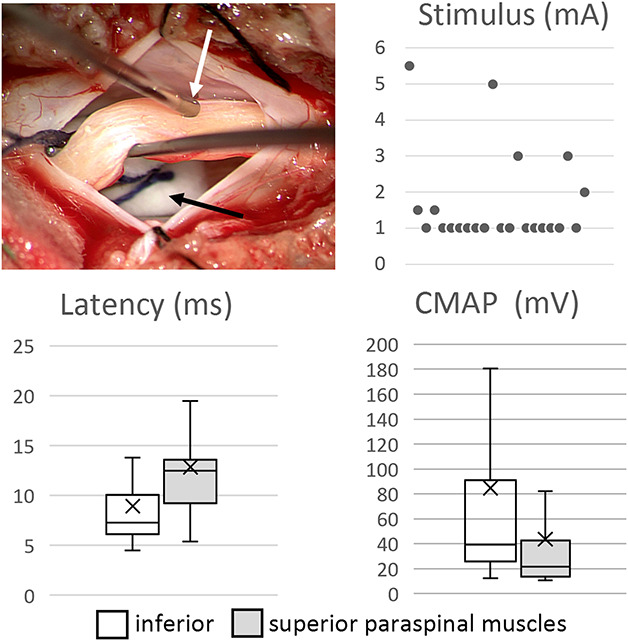
Intraoperative setup showing the positioning of the bipolar electrode (white arrow) with the filum lifted on a hook and the underlying cauda equina insulated with a dry cotton (black arrow). Electrophysiological recordings of paraspinal muscles: lowest stimulus intensity at which the paraspinal muscles were activated (n = 22), latency between stimulus and muscle response, and compound muscle activation potential CMAP of the paraspinal muscles. CMAP, compound muscle action potential.
FIGURE 5.
Typical EMG readings after the electrostimulation of the FT. The upper reading shows that paraspinal musculature is responding already at low stimulus intensity (0.87-1.01 mA). Although some activity was also recorded from the abductor hallucis (arrow). The upper box shows 3 consecutive stimulations with an isolated and reproducible response of paraspinal muscles. The lower box shows the recordings after moving the stimulation electrode. The paraspinal response was weakened, and a reproducible response of the anal sphincter was recorded (arrow). The lower reading shows the reproducible stimulation of the paraspinal musculature without activation of other myotomes even at high stimulus intensity (15-15.4 mA). EAS, external anal sphincter; EMG, electromyography; FT, filum terminale; inf PS, paraspinal muscles inferior to the stimulation level; L, left; L2-L5, leg myotomes; R, right; sup PS, paraspinal muscles superior to the stimulation level.
DISCUSSION
The finding that the intradural FT carries functional sensory nerve endings is novel and sheds a new light on its physiology and pathophysiology. It appears from our morphological and electrophysiological findings that the intradural FT contains encapsulated axon branches, that is, Ruffini mechanoreceptors, which can sense stretch forces and trigger reflexive activation of paraspinal muscles. In addition, we found sensory nerve endings, that is, Remak cell bundles forming glioneural structures, which have been associated with nociceptive functions.
Morphological Findings
Confirming previous observations,4,9,19 our light microscopic studies suggest the natural presence of neural tissue including peripheral nerve components within the intradural FT. In addition, we show that tiny peripheral nerves are not only running near FT20 but can pierce through the pial sheaths into the fibrous tissue of the FT. Here, they form unencapsulated and encapsulated nerve endings, which morphologically classify as “glioneural endorgan”10 and “Ruffini corpuscles.”21
Glioneural End Organ
Unencapsulated nerve endings are composed of axonal structures ensheathed by nonmyelinated Schwann cells better known as “Remak cells.”11 Applying advanced electron microscopy, optogenetic methods, and electrophysiological patch-clamp techniques, it has recently been revealed that networks of Remak cell bundles in the skin of mice provide nociperception. Emphasizing the contribution of both axons and Remak cells, it has been suggested to understand the meshwork of such nerve endings as a nociceptive “glioneural end organ.”10 Accordingly, our morphological findings suggest that the FT is capable of generating pain sensations.
Ruffini Corpuscles
Typical Ruffini corpuscles consist of Remak cell bundles wrapped in a thin fibrous capsule in which the innervating nerve branches extensively.12,22 Collagen fibers associated with the inner core exit the end poles of the Ruffini corpuscle and connect to collagen bundles in the surrounding fibrous tissue allowing for the recording of stretch forces.23 Based on electrophysiological studies, the Ruffini corpuscle has been understood as a fast responding but slowly adapting stretch-sensitive mechanoreceptor. Ruffini corpuscles have been found in skin, joint capsules, and ligaments12,22,24 and in fibrous components of the spine, for example in facet joint ligaments and intervertebral disks.15,16,18,25-28 Our TEM findings revealed that Ruffini receptors are present in the human FT too and may sense stretch forces, for example during spine movements.
Electrophysiological Findings
Intraoperative electrophysiological monitoring during surgery for FT excision in TCS helps to assure that a visually identified FT is not involved in any control of lower extremity muscles, and the anal and urethral sphincters. Typically, the direct bipolar stimulation of cauda equina roots elicits a response of those myotomes already at low stimulus intensities. The stimulation of the FT is expected to cause no response in those muscles even with higher stimulus intensity.9,29 However, others reported an unspecific response of monitored myotomes in 25% of the cases.30 Our study is in accordance with such observations revealing a muscle response of leg myotomes after FT stimulation in 22% of the cases. It is the challenging finding of our study that after FT stimulation, a response of paraspinal muscles was observed in 59% of the cases.
This finding is novel and indicates that the morphologically identified receptor structures of the FT are functional. Interestingly, the activation of paraspinal muscles after FT stimulation is well corresponding to observations after electrostimulation of interspinous ligaments in cats and human spine patients.15,16 In cats, the EMG discharge was strongest in the muscles 1 spine level below of the stimulated ligament, whereas weaker EMG signals were recorded from as far as 2 levels above and below. With a stimulus intensity range of 0 to 12 mA, the mean time delay between the application of the stimulus to the ligament and the resulting EMG ranged from 2.52 to 2.77 ms.16 Our EMG recordings after the electrostimulation of the FT are quite similar, although the muscles responded at lower stimulus intensity and the mean latency was a bit longer. Experimentally, it was shown in cats that the activation of paraspinal muscles can occur through an intersegmental reflex between adjacent lumbar vertebral segments anatomically provided by the intermediate branch plexus of the dorsal rami of the spinal nerves.18,31 The physiological role of such a intersegmental reflex for spinal alignment13 is supported by the observation that knock out mice lacking TrkC neurons connecting between proprioceptive mechanoreceptors and the spinal cord develop scoliosis.27
In cats, it was observed that only narrow active segments of the exposed interspinous ligaments (3-4 mm long and 1 mm wide) were sensitive to the stimulation of paraspinal muscles indicating the inhomogeneous distribution of sensory nerve endings within the ligaments.16 This may explain why we found a paraspinal muscle response after the intraoperative electrostimulation in only 22 of our 37 stimulated patients just because within the surgically exposed FT segment we did not hit such an active zone.
Limitations
Our studies found sensory nerve endings only in a fraction of the patients possibly because of TEM per case just one 1 to 2 mm thick FT specimen was studied, while the total intradural length of the FT reaches 15 cm.4 However, it has been noted by others that the density of Ruffini corpuscles within fibrous tissue is low and that electrophysiological studies indicate a greater abundancy of such receptors than morphologically confirmed. In addition, the inhomogeneous distribution of Ruffini receptors within ligaments has been noted.12
Previous experimental studies investigating the electrical stimulation of facet joint ligaments could not exclude that the paraspinal muscles were directly activated through the spread of stimulating currents because of the proximity of paraspinal muscle to the tissues being electrically stimulated.18 Such an artefact, that is, EMG activity through volume conduction, is unlikely in our study because the FT was carefully separated from the surrounding tissue, the cauda was insulated with a dry cotton, and a bipolar electrode was used (Figure 4).
That our stimulation reached out to the small nerve rootlets accompanying the FT20 cannot be excluded, although it was avoided to stimulate the FT at sites where such rootlets were observed. Since our study showed that such small nerves pierce into the FT, it is likely that such nerve twigs eventually provide the observed mechanoreceptors and nociceptive receptors. We suggest that those nerves are afferent in nature and connected to the intermediate branch plexus of the dorsal rami of the spinal nerves. Therefore, we believe that the activation of paraspinal muscles after FT stimulation was transmitted through an intersegmental reflex as it has been described in cats.18
The FT is affected in TCS by acquired pathologies, that is, mechanical overload-associated collagen fibril damage, inflammation, and fat infiltration. The comorbidity of connective tissue disorder adds congenital collagen fibril damage.6 Therefore, this study relies on the investigation of a heavily diseased FT.
Whether this might affect the density of sensory nerve endings and receptor structures is not known. However, an experimental study in rabbits has reported, after anterior lumbar spinal fusion, the decrease of sensory nerve endings and receptors in the immobilized rabbit facet joint. In the adjacent not immobilized upper segments, an increase of sensory nerve endings was observed.28 Since our prior work has demonstrated that the FT in TCS is subjected to increased mechanical forces,6 we cannot exclude the possibility that TCS influences the receptor density within the FT. Future anatomical, experimental, and clinical work is needed to investigate this issue.
CONCLUSION
Research over the past 5 decades has revealed that skeletal joint mechanoreceptors provide kinesthetic and proprioceptive data and directly interact with the musculature through reflex arcs to participate in maintaining joint stability.14,32 More recently, it has been recognized that spinal ligament receptors are in a similar fashion involved for the muscular stabilization of the spine.13,15-18
Our findings provide evidence that the FT could be part of a complex sensorimotor system which has been suggested to mastermind the spinal alignment.13 Since the FT is the only axial ligamentous structure directly connecting the spinal cord with the skeleton, that is, the coccyx, it could play a unique physiological role in sensing unphysiological stretch forces from excessive spine movements. Nociceptive sensory structures within the FT may contribute to lower back pain in a variety of spine diseases. This notion is supported by the observation that lower back pain is successfully treated by transection of the FT in tethered cord syndrome, a pathology which is caused by a diseased FT exposing the spinal cord to unphysiological stretch forces.6,33
Acknowledgments
We acknowledge the contribution of Jonathan Mooney for supervision of the intraoperative electrophysiological protocol and Wael Asaad MD, PhD, for his advice for the analysis of the electrophysiological readings. We also acknowledge the work of Grant Jolly for conducting the electron microscopy.
Footnotes
Petra M. Klinge and Abigail McElroy contributed equally to this work.
Contributor Information
John E. Donahue, Email: John_Donahue@brown.edu.
Andrew Mumford, Email: andrewmumford1993@gmail.com.
Thomas Brinker, Email: thbrinker@gmail.com.
Ziya L. Gokaslan, Email: Ziya_gokaslan@brown.edu.
Funding
This study did not receive any funding or financial support.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1.Harmeier JW. The normal histology of the intradural filum terminale. Arch Neurol Psychiatry. 1933;29(2):308-316. [Google Scholar]
- 2.McElroy A, Klinge PM, Sledge D, Donahue JE, Glabman RA, Rashmir A. Evaluation of the Filum terminale in hereditary equine regional dermal asthenia. Vet Pathol. 2021;58(6):1100-1106. [DOI] [PubMed] [Google Scholar]
- 3.Fontes RB, Saad F, Soares MS, de Oliveira F, Pinto FC, Liberti EA. Ultrastructural study of the filum terminale and its elastic fibers. Neurosurgery. 2006;58(5):978-984; discussion 978-984. [DOI] [PubMed] [Google Scholar]
- 4.Saker E, Henry BM, Tomaszewski KA, et al. The filum terminale internum and externum: a comprehensive review. J Clin Neurosci. 2017;40:6-13. [DOI] [PubMed] [Google Scholar]
- 5.De Vloo P, Monea AG, Sciot R, van Loon J, Van Calenbergh F. The filum terminale: a cadaver study of anatomy, histology, and elastic properties. World Neurosurg. 2016;90:565-573.e1. [DOI] [PubMed] [Google Scholar]
- 6.Klinge PM, Srivastava V, McElroy A, et al. Diseased filum terminale as a cause of tethered cord syndrome in Ehlers–Danlos syndrome: histopathology, biomechanics, clinical presentation, and outcome of filum excision. World Neurosurg. 2022;162:e492-e502. [DOI] [PubMed] [Google Scholar]
- 7.Gamble HJ. Electron microscope observations upon the conus medullaris and filum terminale of human fetuses. J Anat. 1971;110(pt 2):173-179. [PMC free article] [PubMed] [Google Scholar]
- 8.Miller C. The ultrastructure of the conus medullaris and filum terminale. J Comp Neurol. 1968;132(4):547-566. [DOI] [PubMed] [Google Scholar]
- 9.Durdag E, Borcek PB, Ocal O, Borcek AO, Emmez H, Baykaner MK. Pathological evaluation of the filum terminale tissue after surgical excision. Childs Nerv Syst. 2015;31(5):759-763. [DOI] [PubMed] [Google Scholar]
- 10.Abdo H, Calvo-Enrique L, Lopez JM, et al. Specialized cutaneous Schwann cells initiate pain sensation. Science. 2019;365(6454):695-699. [DOI] [PubMed] [Google Scholar]
- 11.Harty BL, Monk KR. Unwrapping the unappreciated: recent progress in Remak Schwann cell biology. Curr Opin Neurobiol. 2017;47:131-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming MS, Luo W. The anatomy, function, and development of mammalian Abeta low-threshold mechanoreceptors. Front Biol (Beijing). 2013;8(4):408-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holm S, Indahl A, Solomonow M. Sensorimotor control of the spine. J Electromyogr Kinesiol. 2002;12(3):219-234. [DOI] [PubMed] [Google Scholar]
- 14.Solomonow M. Sensory-motor control of ligaments and associated neuromuscular disorders. J Electromyogr Kinesiol. 2006;16(6):549-567. [DOI] [PubMed] [Google Scholar]
- 15.Solomonow M, Zhou BH, Harris M, Lu Y, Baratta RV. The ligamento-muscular stabilizing system of the spine. Spine (Phila Pa 1976). 1998;23(23):2552-2562. [DOI] [PubMed] [Google Scholar]
- 16.Stubbs M, Harris M, Solomonow M, Zhou B, Lu Y, Baratta RV. Ligamento-muscular protective reflex in the lumbar spine of the feline. J Electromyogr Kinesiol. 1998;8(4):197-204. [DOI] [PubMed] [Google Scholar]
- 17.Indahl A, Kaigle AM, Reikeräs O, Holm SH. Interaction between the porcine lumbar intervertebral disc, zygapophysial joints, and paraspinal muscles. Spine (Phila Pa 1976). 1997;22(24):2834-2840. [DOI] [PubMed] [Google Scholar]
- 18.Kang YM, Choi WS, Pickar JG. Electrophysiologic evidence for an intersegmental reflex pathway between lumbar paraspinal tissues. Spine (Phila Pa 1976). 2002;27(3):E56-E63. [DOI] [PubMed] [Google Scholar]
- 19.Choi BH, Kim RC, Suzuki M, Choe W. The ventriculus terminalis and filum terminale of the human spinal cord. Hum Pathol. 1992;23(8):916-920. [DOI] [PubMed] [Google Scholar]
- 20.Gaddam SS, Santhi V, Babu S, Chacko G, Baddukonda RA, Rajshekhar V. Gross and microscopic study of the filum terminale: does the filum contain functional neural elements? J Neurosurg Pediatr. 2012;9(1):86-92. [DOI] [PubMed] [Google Scholar]
- 21.Schenk I, Spaethe A, Halata Z. The structure of sensory nerve endings in the knee joint capsule of the dog. Ann Anat. 1996;178(6):515-521. [DOI] [PubMed] [Google Scholar]
- 22.Maeda T, Ochi K, Nakakura-Ohshima K, Youn SH, Wakisaka S. The Ruffini ending as the primary mechanoreceptor in the periodontal ligament: its morphology, cytochemical features, regeneration, and development. Crit Rev Oral Biol Med. 1999;10(3):307-327. [DOI] [PubMed] [Google Scholar]
- 23.Halata Z. The ultrastructure of the sensory nerve endings in the articular capsule of the knee joint of the domestic cat (Ruffini corpuscles and Pacinian corpuscles). J Anat. 1977;124(pt 3):717-729. [PMC free article] [PubMed] [Google Scholar]
- 24.Halata Z, Rettig T, Schulze W. The ultrastructure of sensory nerve endings in the human knee joint capsule. Anat Embryol (Berl). 1985;172(3):265-275. [DOI] [PubMed] [Google Scholar]
- 25.Vandenabeele F, Creemers J, Lambrichts I, Lippens P, Jans M. Encapsulated Ruffini-like endings in human lumbar facet joints. J Anat. 1997;191(pt 4):571-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blecher R, Heinemann-Yerushalmi L, Assaraf E, et al. New functions for the proprioceptive system in skeletal biology. Philos Trans R Soc Lond B Biol Sci. 2018;373(1759):37320170327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blecher R, Krief S, Galili T, et al. The proprioceptive system masterminds spinal alignment: insight into the mechanism of scoliosis. Dev Cell. 2017;42(4):388-399.e3. [DOI] [PubMed] [Google Scholar]
- 28.Onodera T, Shirai Y, Miyamoto M, Genbun Y. Effects of anterior lumbar spinal fusion on the distribution of nerve endings and mechanoreceptors in the rabbit facet joint: quantitative histological analysis. J Orthop Sci. 2003;8(4):567-576. [DOI] [PubMed] [Google Scholar]
- 29.Khealani B, Husain AM. Neurophysiologic intraoperative monitoring during surgery for tethered cord syndrome. J Clin Neurophysiol. 2009;26(2):76-81. [DOI] [PubMed] [Google Scholar]
- 30.Sala F, Tramontano V, Squintani G, et al. Neurophysiology of complex spinal cord untethering. J Clin Neurophysiol. 2014;31(4):326-336. [DOI] [PubMed] [Google Scholar]
- 31.Bogduk N, Wilson AS, Tynan W. The human lumbar dorsal rami. J Anat. 1982;134(pt 2):383-397. [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson H. Role of knee ligaments in proprioception and regulation of muscle stiffness. J Electromyogr Kinesiol. 1991;1(3):158-179. [DOI] [PubMed] [Google Scholar]
- 33.O'Connor KP, Smitherman AD, Milton CK, et al. Surgical treatment of tethered cord syndrome in adults: a systematic review and meta-analysis. World Neurosurg. 2020;137:e221-e241. [DOI] [PubMed] [Google Scholar]