Abstract
Several endocrine therapies are currently available for the treatment of estrogen receptor (ER) positive breast cancer, but the clinical benefit of these agents is limited by endocrine therapy drug resistance. A common mechanism of endocrine therapy resistance is ESR1 mutations. The first-generation selective estrogen receptor degrader (SERD) fulvestrant has activity against ESR1 mutant tumors but requires intramuscular injection and has poor bioavailability that precludes optimal drug dosing. This led to the development of second-generation SERDs which are potent and have improved oral bioavailability and pharmacokinetics. Several of these oral SERDs are now in phase III trials in both the early and advanced ER positive breast cancer settings. This review summarizes the background of oral SERD development, the current status and future perspectives.
Keywords: selective estrogen receptor degraders, breast cancer, estrogen receptor
Introduction
Breast cancer is the most common cancer diagnosed globally,1 and more than 70% of breast cancers are estrogen receptor (ER) positive and human epidermal growth factor-2 (HER2) negative.2 Endocrine therapy (ET) forms the backbone of systemic therapy for these cancers. Currently available ET agents include luteinizing hormone-releasing hormone (LHRH) agonists, selective estrogen receptor modulators (SERMs) such as tamoxifen, aromatase inhibitors (AIs) including letrozole, anastrozole, and exemestane, and the first-generation selective estrogen receptor degrader (SERD) fulvestrant. This review summarizes the history and current status of a novel group of new ET agents, the oral, second-generation SERDs, several of which are already in advanced stages of clinical development.
Fulvestrant, the First SERD
In the 1980s, a series of steroidal 7α-alkylamide analogs of 17β-estradiol were developed that had pure antagonist actions at the ER, in contrast to tamoxifen which has mixed ER agonist and antagonist actions.3 The lead compound in this series was ICI 164,384 which achieved 89% ligand-ER binding affinity,4 disrupted ligand-ER dimerization,5 completely blocked trophic action of estradiol in the rat uterus6 and increased ER turnover in the cytoplasm via ER protein degradation and reduced ER half-life.7,8 However, ICI 164,384 had poor in vivo potency and was subsequently modified by fluorinating the terminal alkyl group and substituting the amide moiety in the 7α-side chain with polar groups, to produce ICI 182,780.3
ICI 182,780 competes with estradiol to bind at the ER, and subsequently inhibit ER dimerization, increase ER degradation, and reduce ER translocation to the nucleus and ER target gene transcription.9 The side chain of ICI 182,780 was designed long enough with at least 15 atoms to interact with the coactivator binding groove of ER, interfering with recruitment of coactivators for gene transcription.10,11 Compared with ICI 164,384 and tamoxifen, ICI 182,780 had greater ER binding affinity and inhibited the growth of ER positive breast cancer cells to a greater extent in vitro and in vivo. In animal studies, ICI 182,780 demonstrated poor oral bioavailability, and improved potency was achieved by administering the drug as a subcutaneous arachis oil depot.3
ICI 182,780 was later renamed fulvestrant and formulated with castor oil and alcohols for intramuscular injection. Fulvestrant was approved for clinical use in 2002 as a 4-weekly 250 mg intramuscular injection, after it was shown to be non-inferior to anastrozole in post-menopausal women with advanced breast cancer who had progressed on first-line ET, which in the majority of cases was tamoxifen at that time.12,13 With this dose regimen though, steady-state plasma concentrations were not reached for several months leaving patients vulnerable to early disease progression.14 Preoperative studies also suggested that greater efficacy could be achieved with a higher dose, as fulvestrant reduced ER and progesterone receptor (PR) expression and Ki67 scores in a dose-dependent manner,15,16 and lack of change in these markers were seen with the 250 mg dose.17
A high-dose regimen that incorporated a loading dose (500 mg on days 0, 14, 28 then every 28 days) was later established as the recommended fulvestrant dosing schedule. The loading dose enabled steady-state plasma levels of fulvestrant to be achieved within the first month of treatment.14 The high-dose regimen was well tolerated and demonstrated superior progression-free survival (PFS) and overall survival (OS) compared with the original dosing in the phase III double-blind placebo-controlled CONFIRM trial which enrolled postmenopausal women with metastatic ER positive breast cancer who had relapse on adjuvant ET or progression after first-line ET for advanced disease.18,19 This new fulvestrant dose showed superior efficacy to AIs in ER positive advanced breast cancer in subsequent randomized controlled trials.20,21
ETs have dramatically improved outcomes for patients with early and advanced ER positive breast cancer, but resistance to ET remains a clinical challenge. An important, and one of the most studied mechanisms of acquired ET resistance is mutations in the ligand-binding domain of the ERα gene (ESR1) which permit estrogen-independent ER activation and signaling.22,23 Structural studies show that these mutations stabilize helix 12 of the ligand-binding domain in an active conformation and computer modeling and in vitro studies suggest that these mutations increase co-activator binding.23–26 The commonest ESR1 mutations have been at the amino acid positions 534–538 of helix 12, as well as at E380 and S463, and may be monoclonal or polyclonal.22,27 Mutations may be detected from tumor tissue or circulating tumor DNA (ctDNA) with good concordance between the two specimen types.28 ESR1 mutations are rare de novo but may be selected for by AI therapy with prevalence of up to 40% in advanced breast cancer patients who have progressed on this treatment.22,27
Fulvestrant has activity in vitro against breast cancer cells with mutant ERα, though the extent of inhibition of mutant ERα is less than the inhibition achieved against wild-type ERα unless much higher drug doses are used.23 The 500 mg fulvestrant dose does not result in maximal ER downregulation in vivo,16,29 nor necessarily provides robust PFS benefit for patients with ESR1 mutant breast cancer.30 Increasing the fulvestrant dose further in an effort to increase efficacy and overcome the relative resistance of ESR1 mutant tumors to treatment has not been practical. Already for the 500 mg dose, patients require two 250 mg/5 mL injections each time, and a higher dose would require multiple intramuscular injections each time and be less tolerable. This gave impetus to research efforts into a new generation of SERDs that could achieve greater potency with the convenience of oral administration.
Second-Generation Oral SERDs
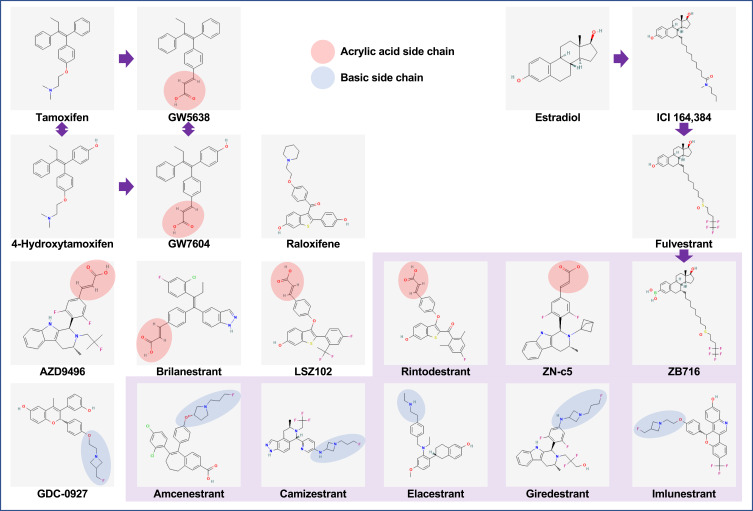
The prodrug GW5638, and its metabolite GW7604, are tamoxifen analogs and were discovered to have SERD-like properties.31 GW5638 binds to ER and sits in the ligand binding pocket with a similar orientation to tamoxifen, but has unique interactions that elicit an increase in surface hydrophobicity of helix 12 which decreases ERα stability.32 Like fulvestrant, GW5638 increased ER turnover and lacked cross-resistance to tamoxifen.33 The non-steroidal chemical structures of GW5638 and raloxifene were used as a template for developing most oral SERDs.34 Fulvestrant was the template for the only steroidal oral SERD candidate ZB716 (fulvestrant-3-boronic acid).35 The chemical structures of these agents, and the novel oral SERD candidates are shown in Figure 1.
Figure 1.
Chemical structures of SERDs. GW5638 and its metabolite GW7604 are tamoxifen analogs which, with raloxifene, became templates for the next generation of oral SERDs. ICI 164,384 and fulvestrant are estradiol analogs and first-generation SERDs, and were templates for oral SERD ZB716. Oral SERDs highlighted in purple are in active clinical development. Chemical structures are from PubChem https://pubchem.ncbi.nlm.nih.gov (Ref. Kim S, Chen J, Cheng T, et al. PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res. 2021;49(D1):1388–1395).
GDC-0810 (brilanestrant),36 AZD9496,37 and LSZ10238 were among the first oral SERD candidates and all had acrylic acid side chains similar to GW5638. In contrast, a few early oral SERD candidates, including GDC-0927 and RAD1901 (elacestrant) had basic side chains. The activity of fulvestrant and some of these candidate oral SERDs were assessed in vitro and in vivo in patient-derived xenograft models.39 Fulvestrant and GDC-0927 displayed superior anti-proliferative activity compared with GDC-0810 and AZD9496. Fulvestrant, GDC-0927, GDC-0810 and AZD9496 all induced similar strong ER degradation activity in the MCF7 breast cancer cell line, but GDC-0810 and AZD9496 did not perform as well in other cell lines and exhibited weak ER transcriptional agonism.39
The mechanism of action of fulvestrant and GDC-0927 compared with GDC-0810 and AZD9496 was further interrogated. ER protein expression was not affected after 10–45 minute exposure of fulvestrant or GDC-0927 in nuclear soluble protein lysate. At 4 hours, the nuclear ER was slightly reduced and this was reversed when treated with proteasome inhibitor MG132, indicating that the reduction was due to proteasome degradation.39 In contrast, reduction of cytoplasmic ER may not be due to protein degradation, but rather be due to ER translocation to the nucleus.39 Fulvestrant and GDC-0927 also reduced ER mobility in the nucleus and limited ER accessibility to chromatin, prompting increased ER turnover.39 The basic side chain SERD GDC-0927 was superior to the acrylic acid side chain SERDs GDC-0810 and AZD9496 in achieving full ER antagonism.34
GDC-0810, AZD9496, GDC-0927 and LSZ102 have all discontinued further development. Lessons from the shortcomings of the initial oral SERD candidates led to the development of AZD9833 (camizestrant),40 GDC-9545 (giredestrant),41 SAR439859 (amcenestrant)42 and LY3484356 (imlunestrant)43 which all have basic side chains. Along with RAD1901 (elacestrant),44 these compounds have demonstrated activity against wild-type and mutant ERα breast cancer cell lines and patient-derived xenograft models, and have reached phase III trials. The acrylic acid side chain SERDs G1T48 (rintodestrant)45 and ZN-c5,46 and other oral SERDs D-050247 and ZB716,48 also have demonstrated encouraging preclinical activity, and are currently in the early phase trial stage.
Oral SERD Monotherapy Early Phase Trials
Table 1 summarizes the anti-tumor activity and side effects observed in early phase oral SERD trials. Responses to oral SERDs have occurred in heavily pretreated ER positive, HER2 negative metastatic breast cancer, including patients with ESR1 mutant tumors, and patients who had progressed on CDK4/6 inhibitor and/or fulvestrant therapy. The oral SERDs have been generally well tolerated with the most frequent class-related side effects being grade 1–2 nausea and fatigue. Arthralgia and hot flushes which are common with AIs were also seen with some SERDs. Elacestrant has predominantly gastrointestinal side effects including nausea, vomiting and dyspepsia but these were half as prevalent with the tablet formulation compared with the capsule form.49
Table 1.
Results from early phase SERD trials
| Trial | SERD and Doses Assessed | Phase | RP2D for Monotherapy | N (Total) | N (at RP2D) | % ESR1mut | % Prior CDK4/6i | % Prior Fulvestrant | Lines of Prior ET Median (Range) | DLTs | Adverse Events in ≥10% Patients (Any Grade) | Adverse Events Grade ≥3 | ORR at RP2D | CBR at RP2D | Median PFS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMEERA-1(Parts A/B)86 | Amcenestrant 20-600mg | I/II | 400mg | 65 | 49 | 43% (21/49) | 61% (30/49) | 45% (22/49) | 2 (1-4) | Nil | Hot flush (10%) | Nil | 11% (5/46)– 1 CR, 4 PR | 28% (13/46) | NR |
| SERENA-1(Parts A/B)50 | Camizestrant 25-450mg | I | NR | 98 | NR | 43% (42/98)a | 62% (61/98)a | 53% (52/98)a | 2 (0-6)a | 3 DLTsb | Fatigue (11%), nausea (6%), bradycardia (4%), visual disturbance (3%)c | Dizziness (1%), bradycardia (1%), atrial fibrillation (1%), visual disturbance (1%), and as per DLTs | 10% (7/70)– all PRa | 35% (24/68)a | 5.4 months all doses |
| RAD1901–00549 | Elacestrant 200-600mg | I | 400mg | 57 | 50 | 50% (25/50) | 52% (26/50) | 52% (26/50) | 2.5 (1-7) | Nil | Nausea (33%), hypertriglyceridemia (25%), hypophosphatemia (25%) dyspepsia (21%), fatigue (21%), constipation (20%) | Hyperglycemia (4%) hypophosphatemia (8%) | 19% (6/31)– all PR | 43% (20/47) | 4.5 months 400mg |
| GO3993251 | Giredestrant 10-250mg | I | 30mg | 111 | 41 | 51% (21/41) | 66% (27/41) | 20% (8/41) | 1 (0-2)d | Nil | Nausea (20%), arthralgia (20%), back pain (17%), fatigue (17%), diarrhea (12%), constipation (12%) | NR | 15% (6/40)– all PR | 55% (22/40) | 7.2 months 30mg |
| EMBER90 | Imlunestrant 200-1200mg | I | 400mg | 114 | 51 | 49% (53/108)e | 92% (105/114)e | 51% (58/114)e | 2 (0-8)d | Nil | Nausea (35%), fatigue (28%), diarrhea (25%), arthralgia (15%), cough (13%), headache (10%), urinary infection (10%), hot flush (10%)f | Nausea (3%), fatigue (3%), diarrhea (1%) | 12% (4/34)– all PR | 55% (28/51) | 4.3 months all doses |
| G1T48-0184 | Rintodestrant 200-1000mg | I | 800mg | 67 | 7 | 45% (29/64)g | 86% (6/7) | 86% (6/7) | 4 (2-5)d | Nil | Hot flush (43%), fatigue (29%), diarrhea (14%) | Nil | 0% (0/7) | 14% (1/7) | 2.6 months 600mg, 3.6 months 1000mg |
| ZN-c5-00191 | ZN-c5 50–300mg | I | 50mg | 56 | 16 | 64% (9/14) | 44% (7/16) | 19% (3/16) | 1 (0-3) | Nil | Nil ≥10% | Nil | 0% (0/14) | 44% (7/16) | 3.9 months all doses |
Notes: Unless otherwise annotated, all percentages are relating to RP2D cohort only. aData only available for pooled population of 98 patients receiving doses 25–450 mg camizestrant. bDLTs observed were G3 QTc prolongation at 300 mg, G3 vomiting at 450 mg, G2 visual disturbance, headache and imbalance at 450 mg. cData on 98 patients enrolled only reports adverse events grade ≥ 2. dData only available for prior lines of therapy inclusive of chemotherapy. eData only available for the pooled population receiving imlunestrant across all dose levels. fData only available for pooled advanced breast cancer and endometrioid carcinoma patients receiving 400 mg imlunestrant. gData only available for the pooled population of 64 evaluable patients receiving rintodestrant across all dose levels.
Abbreviations: CBR, clinical benefit rate; CR, complete response; DLT, dose limiting toxicity; ET, endocrine therapy; N, patient enrolment; NR, not reported; ORR, objective response rate; PFS, progression free survival; PR, partial response; RP2D, recommended phase 2 dose; SERD, selective estrogen receptor degrader.
Toxicities of special interest with SERDs are bradycardia and visual disturbance, which are dose dependent. Bradycardia was observed with camizestrant and giredestrant. In the phase I camizestrant trial, most cases of bradycardia were grade 1 (asymptomatic), and there were a few cases of grade 2 bradycardia at doses of 150–450 mg. QTcF prolongation was a dose-limiting toxicity (DLT) for 1 patient at 300 mg.50 In the phase I giredestrant trial, bradycardia was observed in 7% patients receiving giredestrant monotherapy. The majority were grade 1 except for a single case of grade 2 toxicity at the highest dose assessed of 250 mg.51
To further assess the cardiac effects of giredestrant, an open-label study was performed of giredestrant 100 mg daily, with or without palbociclib.52 Patients underwent echocardiograms, Holter monitors and treadmill testing. For the 20 patients enrolled, the mean heart rate was lower for patients while on treatment compared with baseline, though this was not clinically significant. There were no episodes of heart rate <50 beats per minute, nor second and third degree heart block. Four patients had paroxysmal supraventricular tachycardia (SVT), though 2 patients had paroxysmal SVT at baseline. Importantly, none of these events required intervention or dose changes, and there was no change in the exercise intensity achieved on treadmill exercise testing after giredestrant treatment.52 Of note the lower dose of 30 mg giredestrant has been selected for monotherapy and combination with CDK4/6 inhibitor therapy.
Visual toxicities have been only observed with camizestrant. Most cases were grade 1, but grade 2 visual disturbance was a DLT for camizestrant monotherapy and for camizestrant in combination with palbociclib.50,53 Ophthalmological reviews of affected patients were all unremarkable, and no patients discontinued camizestrant due to visual disturbance.
Oral SERD with CDK4/6 Inhibitor Early Phase Trials
The use of CDK4/6 inhibitors have been a major advance in the ER positive, HER2 negative breast cancer treatment landscape. AI or fulvestrant in combination with a CDK4/6 inhibitor is now the standard first-line therapy for advanced disease. CDK4/6 inhibitors block the phosphorylation of the retinoblastoma protein by the CDK4/6-Cyclin D complex and thereby halt progression through the cell cycle and tumor growth. Compared with an AI alone, the addition of a CDK4/6 inhibitor in the first-line setting prolongs PFS by 9–13 months.54–56 OS results have been mixed, with significant OS benefit demonstrated for the addition of ribociclib to letrozole,57 but not for the addition of palbociclib to letrozole.58 Compared with fulvestrant alone, the addition of the CDK4/6 inhibitor ribociclib in the first- or second-line setting prolongs PFS by 8 months,59 and OS by 12 months.60 Up to 15–20% patients though fail to have clinical benefit from this standard first-line therapy,55,56,59 and all patients eventually develop treatment resistance and disease progression.
Oral SERDs have demonstrated promising activity in combination with the CDK4/6 inhibitor palbociclib in early phase trials, which are summarized in Table 2. The objective response rates (ORRs) and clinical benefit rates (CBRs) reported for combination therapy have been higher than for oral SERD monotherapy, and even complete responses have been seen with the combination of high-dose giredestrant and palbociclib,61 and with camizestrant plus palbociclib.53 Anti-tumor activity has also been evident as reduction in the ESR1 mutation allele frequency or ctDNA levels with treatment,50,61–64 and as downregulation of ER, PR and Ki67 in paired pre- and on-treatment tumor biopsies in early stage breast cancer.61,64 The combinations have been well tolerated and like AI plus CDK4/6 inhibitor therapy, the most frequent side effect of oral SERD and CDK4/6 inhibitor therapy has been neutropenia.
Table 2.
Results from early phase trials of oral SERDs in combination with CDK4/6 inhibitors
| Trial | SERD and CDK4/6 Inhibitor Combination | Phase | N | % ESR1 mut | %Prior CDK4/6i | % Prior Fulvestrant | Lines of Prior ET Median (Range) | Adverse Events in ≥10% Patients (Any Grade) | Adverse Events Grade ≥ 3 | ORR | CBR | Median PFS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMEERA-1 (Parts C/D)62,65 | Amcenestrant 200mg + Palbociclib | I/II | 39 | NR | 5% (2/39) | 8% (3/39) | 1 (0–4) | Neutropenia (95%), nausea (18%), fatigue (15%), arthralgia (10%), asthenia (10%), dry skin (10%), hot flushes (10%) | Neutropenia (56%), deep vein thrombosis (3%) | 32% (11/34) – all PR | 74% (25/34) | 14.7 months |
| SERENA-1 (Parts C/D)53 | Camizestrant 75mg + Palbociclib | I | 25 | 44% (11/25) | 80% (20/25) | 68% (17/25) | 2 (1–4) | Neutropenia (80%), visual disturbance (44%), bradycardia (16%), fatigue (16%), nausea (16%), vomiting (12%), diarrhea (12%) | Neutropenia (68%), infections (8%), anemia (4%) | 12% (3/25) – 2 CR, 1 PR | 28% (7/25) | NR |
| GO3993261,64 | Giredestrant 100mg + Palbociclib +LHRH agonist | I | 48 | 29% (14/48) | 0% (0/48) | 7% (3/48) | 1 (0–2) | Neutropenia (77%), bradycardia (31%), diarrhea (33%), fatigue (29%), nausea (21%), constipation (21%), cough (21%), dizziness (19%), anemia (17%), thrombocytopenia (17%) | Neutropenia (60%), thrombocytopenia (6%), diarrhea (2%) | 48% (21/44) – 5 CR, 16 PR | 81% (39/48) | 18.2 months |
| G1T48-0163 | Rintodestrant 800mg + Palbociclib | I | 40 | 41% (16/39) | 0% (0/40) | 15% (6/40) | 1 (0–1) | Neutropenia (90%), leukopenia (45%), anemia (15%), asymptomatic bacteriuria (10%), thrombocytopenia (10%) | Neutropenia (53%), leukopenia (18%), anemia (5), lymphopenia (5%) | 5% (2/40)– all PR | 60% (24/40) | 7.4 months |
Abbreviations: CBR, clinical benefit rate; CR, complete response; DLT, dose-limiting toxicity; ET, endocrine therapy; LHRH, luteinizing hormone releasing hormone; N, patient enrollment; NR, not reported; ORR, objective response rate; PFS, progression-free survival; PR, partial response; SERD, selective estrogen receptor degrader.
Pharmacokinetic studies found no interaction between palbociclib and camizestrant,50,53 giredestrant64 or rintodestrant.63 An interaction between amcenestrant and palbociclib is possible, with amcenestrant having a moderate cytochrome P450 3A4 induction effect and reducing palbociclib exposure in a dose-dependent manner, hence the recommended dose for amcenestrant is 200 mg in combination with palbociclib, compared with 400 mg as monotherapy.65 Several oral SERDs are undergoing evaluation in combination with abemaciclib, but no results are reported yet. These include trials of amcenestrant (NCT03284957), camizestrant (NCT03616587 and NCT04964934), imlunestrant (NCT04188548 and NCT04975308) and ZN-c5 (NCT04514159). There are no trials currently combining oral SERDs and ribociclib in view of both agents potentially causing bradycardia and QTc prolongation.
Randomized Trials of Oral SERDs in ER Positive, HER2 Negative Advanced Breast Cancer
The randomized phase II and III trials of oral SERDs in ER positive, HER2 negative advanced breast cancer are listed in Table 3. The phase III EMERALD trial is currently the only positive trial to date. The EMERALD trial enrolled patients with advanced ER positive, HER2 negative breast cancer who progressed after 1–2 lines of ET for advanced or metastatic disease, including a line in combination with a CDK4/6 inhibitor.66 477 patients, 48% of whom had ESR1 mutant tumors, and 78% of whom were naïve to chemotherapy in the metastatic breast cancer setting, were randomized 1:1 to elacestrant 400 mg daily or investigator’s choice of fulvestrant or AI. The co-primary endpoints of PFS in all patients and in those with ESR1 mutations were both met. The PFS in all patients was 2.79 months for elacestrant versus 1.91 months for standard of care (HR 0.70 [95% CI 0.55–0.88], p = 0.002). Patients with ESR1 mutations had increased benefit from elacestrant with PFS of 3.78 months for elacestrant versus 1.87 months for standard of care (HR 0.55 [95% CI 0.39–0.77], p = 0.0005).66 The PFS benefit of elacestrant was seen across all prespecified subgroups including patients who received prior fulvestrant, even when the 6 patients who received fulvestrant on trial and as a prior line of therapy were excluded.66 OS data are expected to be mature for analysis by early 2023.67 Elacestrant was well tolerated with most side effects being grade 1–2. The most common elacestrant side effects were nausea, vomiting, fatigue and arthralgia. No cardiac or visual toxicities were observed.66
Table 3.
Phase II and III oral SERD trials in metastatic breast cancer
| Trial | Oral SERD | Phase | N | ET line | Investigational Arm | Comparator Arm | Randomization | Primary Endpoint | Design | ClinicalTrials.gov |
|---|---|---|---|---|---|---|---|---|---|---|
| AMEERA-3 | Amcenestrant | II | 367b | 1–2 | Amcenestrant 400mg | Physician’s choice ET (Fulvestrant, AI, Tamoxifen) | 1:1 | PFS | Open-label | NCT04059484 |
| AMEERA-5 | Amcenestrant | III | 1068b | 1 | Amcenestrant 200mg + Palbocicliba | Letrozole + Palbocicliba | 1:1 | PFS | Double-blind, placebo controlled | NCT04478266 |
| persevERA | Giredestrant | III | 978 | 1 | Giredestrant 30mg + Palbocicliba | Letrozole + Palbocicliba | 1:1 | PFS | Double-blind, placebo controlled | NCT04546009 |
| SERENA-4 | Camizestrant | III | 1342 | 1 | Camizestrant 75mg + Palbocicliba | Anastrozole + Palbocicliba | 1:1 | PFS | Double-blind, placebo controlled | NCT04711252 |
| SERENA-6 | Camizestrant | III | 302 | 1 (ESR1mut ctDNA) | Camizestrant 75mg + Palbociclib/Abemacicliba | AI (letrozole/ anastrozole) + Palbociclib/Abemacicliba | 1:1 | PFS | Double-blind, placebo controlled | NCT04964934 |
| EMBER-3 | Imlunestrant | III | 800 | 2 (prior AI alone or with CDK4/6i) | Imlunestrant 400mg vs Imlunestrant + Abemacicliba | Physician’s choice ET (fulvestrant/ exemestane)a | 1:1:1 | PFS | Open-label | NCT04975308 |
| SERENA-2 | Camizestrant | II | 240b | 2 | Camizestrant75/150/300mg | Fulvestrant | 1:1:1:1 | PFS | Open-label | NCT04214288 |
| EMERALD | Elacestrant | III | 477b | 2–3, post CDK4/6i | Elacestrant 400mg | Physician’s choice ET (Fulvestrant/AI) | 1:1 | PFS in all patients and in ESR1mut | Open-label | NCT03778931 |
| acelERA | Giredestrant | II | 303 b | 2-3 | Giredestrant 30mg | Physician’s choice ET (Fulvestrant/AI) | 1:1 | PFS | Open-label | NCT04576455 |
Notes: aAlso with luteinizing hormone releasing hormone agonist if premenopausal (or male in EMBER-3). bRecruitment completed.
Abbreviations: AI, aromatase inhibitor; ET, endocrine therapy; N, target enrolment; PFS, progression free survival; SERD, selective estrogen receptor degrader.
The phase II AMEERA-3 and acelERA trials are negative trials for amcenestrant monotherapy and giredestrant monotherapy respectively in advanced ER positive breast cancer. Both trials have announced that the primary endpoint of improved PFS compared with physician’s choice ET was not reached.68,69 The full results of these trials are awaited with interest, to understand the characteristics of the trial participants and their tumors, particularly the prevalence of ESR1 mutations. The other trials in Table 3, some of which are of oral SERD in combination with CDK4/6 inhibitor therapy, are ongoing.
AI to SERD Stable Switch Strategy
The phase III PADA-1 trial assessed the novel strategy of switching from AI to the SERD fulvestrant at time of rising ESR1 mutations in ctDNA.70 1017 patients already receiving first-line AI with palbociclib for ER positive, HER2 negative metastatic breast cancer were enrolled into the first step of the trial and were screened for ESR1 mutations in ctDNA every 2 months. After a median of 15.6 months, 172 patients in step one had detectable or rising ESR1 mutations in the absence of clinical or radiological disease progression. These patients proceeded to be randomized 1:1 open-label to continue their AI with palbociclib, or to substitute the AI with fulvestrant. The co-primary endpoints were PFS from time of randomization and safety. The median PFS was 11.9 months for those who switched to fulvestrant with palbociclib, versus 5.7 months for those who stayed on AI with palbociclib (HR 0.63 [95% CI 0.45–0.88], p = 0.007).71
The PADA-1 trial had an optional third step, where patients in the standard of care arm could cross-over to fulvestrant with palbociclib upon disease progression. Forty-seven out of 70 eligible patients crossed-over and the median second PFS for these patients was 3.5 months (95% CI 2.7–5.1 months),71 which when combined with the median first PFS for these patients was still less than median first PFS for patients who switched in step two. Detecting and addressing endocrine resistance early therefore was advantageous, and OS results are awaited.
The stable switch strategy is the basis of design of the ongoing international SERENA-6 trial.72 In this double-blind phase III randomized controlled trial, patients who have been on first-line non-steroidal AI with CDK4/6 inhibitor (palbociclib or abemaciclib) for > 6 months are centrally screened for ESR1 mutations in ctDNA every 2–3 months. Upon detection of ESR1 mutations, provided there is no clinical or radiological progression, patients are randomized 1:1 to continue their same AI and CDK4/6 inhibitor combination, or to continue the same CDK4/6 inhibitor with switch of the ET backbone to the oral SERD camizestrant. The primary endpoint is PFS and the results will further our understanding of the place of liquid biopsy and the stable switch strategy in the ET treatment paradigm.
Oral SERD Adjuvant Therapy Trials
In ER positive early-stage breast cancer, an AI or tamoxifen is used as adjuvant ET, with choice of agent and use of concurrent ovarian function suppression guided by patient menopausal status, comorbidities, and risk stratification. Some patients have poor tolerance of AIs and tamoxifen, so oral SERDs have the potential to be another option for these patients. There are currently 2 phase III trials evaluating oral SERDs in the adjuvant setting, AMEERA-6 (NCT05128773) evaluating amcenestrant versus tamoxifen in patients who have not tolerated AIs, and lidERA (NCT04961996) evaluating giredestrant versus standard of care ET in medium-high risk stage I–III ER positive breast cancer.
The prevalence of ESR1 mutations is estimated at only 1% in early ER positive breast cancer patients prior to any ET,73 and 6% in patients with advanced breast cancer who received AI in the adjuvant setting.28 Therefore, the activity of oral SERDs against ESR1 mutations may have less significance in the early breast cancer setting. Additional studies showed a higher rate of ESR1 mutations in recurrent disease and further studies will be needed to determine the prevalence of these mutations after 5–10 years of adjuvant AI therapy.74,75 The results of current and future trials will inform if oral SERDs have a role in the adjuvant setting, and if so whether their use would be general or restricted to extended ET and certain higher risk subpopulations.
Oral SERD Window Trials
Most oral SERDS under clinical evaluation have a neoadjuvant window trial in their trial portfolio, and these are summarized in Table 4. The neoadjuvant window setting provides an opportunity to assess the biological activity of ET, and to potentially identify biomarkers predictive of benefit to an ET. CoopERA and AMEERA-4 are the oral SERD window trials with reported results to date.
Table 4.
Phase II and III oral SERD trials in early ER positive breast cancer
| Trial | SERD | Phase | N | Investigational Arm | Comparator Arm | Population | Randomization | Duration of Intervention | Primary Endpoint | Design | ClinicalTrials.gov |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjuvant | |||||||||||
| AMEERA-692 | Amcenestrant | III | 3738 | Amcenestrant 200mgb | Tamoxifenb | Stage IIB/III, toxicity after≥6 months of (neo)adjuvant AI; includes HER2+ patients | 1:1 | 5 years | Invasive breast cancer free survival | Double-blind, placebo controlled | NCT05128773 |
| lidERA | Giredestrant | III | 4100 | Giredestrant 30mgb | Physician’s choice ET (Tamoxifen/ anastrozole/ letrozole/ exemestane)b | Med-High risk stage I-IIIc | 1:1 | 5 years | Invasive disease free survival | Open-label | NCT04961996 |
| Window/ Neoadjuvant | |||||||||||
| AMEERA-478 | Amcenestrant | II | 105a | Amcenestrant 200mg/400mg | Letrozole | Stage I-III post-menopausal | 1:1:1 | 2 weeks | ΔKi67 | Open-label | NCT04191382 |
| Giredestrant | I | 75 a | Giredestrant 3 dose levels | - | Stage I-III post-menopausal | - | 2 weeks | ΔKi67 | Open-label, single-arm | NCT03916744 | |
| coopERA76 | Giredestrant | II | 221 a | Giredestrant 30mg + Palbociclib (window giredestrant only) | Anastrozole + Palbociclib (window anastrozole only) | Stage I-III post-menopausal | 1:1 | 2 weeks (window) 16 weeks (neoadjuvant) | ΔKi67 | Open-label | NCT04436744 |
| SERENA-3 | Camizestrant | II | 92 | Camizestrant 75/150mg | - | Stage I-III post-menopausal | 1:1 | 5–7 days | ΔER expression | Open-label | NCT04588298 |
| EMBER-2 | Imlunestrant | I | 90 | Imlunestrant 3 dose levels | - | Stage I-III post-menopausal | - | NR | ΔER expression | Open-label | NCT04647487 |
| I-SPY2 EOP93 | Amcenestrant | II | 120 | Amcenestrant 200mg vs amcenestrant 200mg + abemaciclib vs amcenestrant 200mg + letrozoleb | - | Stage II/III, ≥2.5cm primary tumour; MammaPrint low-risk, or high-risk and cN0 | 1:1:1 | 6 months | Feasibility:≥75% patients completing ≥75% study therapy | Open-label | NCT01042379 |
| ELIPSE | Elacestrant | 0 | 24 | Elacestrant 400mg | - | Tumor ≥1.5cm, cN0, and Ki67 ≥10% | - | 4 weeks | Rate of complete cell cycle arrest (Ki67≤ 2.7%) | Open-label, single-arm | NCT04797728 |
Notes: aRecruitment completed. bAlso with luteinizing hormone releasing hormone agonist if male/premenopausal/peri-menopausal. cMedium-High risk defined as T4 and any pN stage; any T stage with ≥ pN1; pN0 with primary tumor >1cm and at least one of the following: grade 3, Ki67 ≥ 20%, Oncotype Dx® ≥ 26, High-Risk MammaPrint; patients who have had neoadjuvant chemotherapy must have residual disease in lymph nodes to be eligible.
Abbreviations: AI, aromatase inhibitor; ET, endocrine therapy; N, target enrolment; NR, not reported; SERD, selective estrogen receptor degrader.
In the coopERA trial, 211 patients with ER positive, HER2 negative early breast cancer with a primary tumor ≥ 1.5 cm and Ki67 ≥ 5% were randomized 1:1 to giredestrant 30 mg daily or anastrozole 1 mg daily for 2 weeks (window of opportunity phase).76 Palbociclib 125 mg was then added to the treatment regimen of each arm for 16 weeks before surgery (neoadjuvant treatment phase).76 The primary endpoint was change in the Ki67 score in the window of opportunity phase, and giredestrant was superior with geometric mean relative reduction in Ki67 of −75% compared with −67% for anastrozole (p = 0.0433).76 At the end of the neoadjuvant combination treatment phase, geometric mean relative reduction in Ki67 was again superior in the giredestrant arm at −81% compared with −74%, but there was no difference in the ORR nor pathological complete response rate between the trial arms.77
In the AMEERA-4 trial, 105 patients with ER positive, HER2 negative early breast cancer with a primary tumor ≥ 1.0 cm and Ki67 ≥ 15% were randomized 1:1:1 to amcenestrant 400 mg daily, amcenestrant 200 mg daily, or letrozole 2.5 mg daily for 2 weeks before surgery.78 No formal statistical comparisons were performed as the study had closed early due to slow trial enrollment. Similar reductions in Ki67 were observed in all 3 arms, but the amcenestrant arms did demonstrate large decreases in ER H-score that were not seen with letrozole, reflective of the action of amcenestrant on the ER.78
Future Perspectives
While oral SERDs have the potential to alter the natural history of ER positive breast cancer and provide an additional effective ET, the clinical benefit of SERDs will be limited by eventual acquired drug resistance. Resistance to SERDs may develop from activation of alternative growth factor and signaling pathways, such as the PI3K/AKT/mTOR pathway. In a study of 156 patients with metastatic ER positive breast cancer following AI therapy, PIK3CA mutations were seen in 40% of patients, and 44% of these patients also had ESR1 mutations.22 Mutations in PIK3CA have been demonstrated in vitro to confer resistance to fulvestrant and contribute to cell migration.79 The combination of the PIK3CA inhibitor alpelisib with fulvestrant has superior PFS compared with fulvestrant alone in PIK3CA mutant ER positive, HER2 negative advanced breast cancer.80 The challenge of the combination therapy however is toxicity, with alpelisib discontinuation rates of 21–25% observed in trials.80,81 Imlunestrant is being evaluated in combination with alpelisib in the phase I EMBER trial (NCT04188548). Amcenestrant is being evaluated in combination with alpelisib or the mTOR inhibitor everolimus in parts F-I of the phase I/II AMEERA-1 trial (NCT03284957). Camizestrant is being evaluated in combination with everolimus or the AKT inhibitor capivasertib in the phase I SERENA-1 trial (NCT03616587).
The plasmaMATCH study has provided further insights into mechanisms of fulvestrant resistance.82 Cohort A of this study enrolled patients with ER positive advanced breast cancer with baseline ESR1 mutations. ctDNA samples at baseline and at end-of-treatment with fulvestrant were analyzed and 51% of evaluable patients had acquired mutations at end of treatment. These included mutations in PIK3CA, HER2, BRAF, PTEN, BRCA1/2 but interestingly also in L536 and F404 of the ligand-binding domain of ESR1. F404 mutations are rare in patients who are fulvestrant naïve. In vitro, double-mutant D538-F404L and E380Q-F404L cells display fulvestrant resistance, but sensitivity to tamoxifen and novel oral SERDs.82
While the clinical development of oral SERDs has been impressively rapid, there are some groups of patients with ER positive breast cancer which have been excluded so far. ER positive, HER2 positive patients are excluded from all the current trials except for AMEERA-6 (NCT05128773) and the phase I EMBER trial (NCT04188548). Cross-talk between the ER and HER2 pathways can facilitate tumor escape and resistance, which can be reduced by targeting both pathways in ER positive, HER2 positive breast cancer. Patients with brain metastases are another subset of patients that have mostly been excluded, and there are limited data of the efficacy of ET in the treatment of intracranial disease. In an intracranial ER positive tumor mouse xenograft model, elacestrant demonstrated activity against brain metastases whereas fulvestrant did not.44 There is a phase II trial (NCT04791384) in progress specifically assessing the efficacy of elacestrant and abemaciclib together in patients with brain metastases.
Advances in the technologies of liquid biopsy and in functional imaging have occurred in parallel with oral SERD development. These technologies have the potential to help guide ET treatment decisions. The feasibility and application of ESR1 mutation screening in ctDNA was illustrated in the PADA-1 trial.83 Over 12,000 blood samples from 83 centers across France were processed with a median turnaround time for centralized ESR1 mutation assessment of 13 days.83 The mutant allele frequency limit of detection was 0.1% and there was strong correlation of droplet digital PCR results with those from next-generation sequencing.83
16α-18F-fluoro-17β-estradiol (FES) positron emission tomography (PET) provides a non-invasive method to quantify change in ER expression in response to ET. A prospective study was performed in 16 patients receiving fulvestrant, and ≥ 75% relative decrease in FES uptake was predictive of clinical benefit.29 Nine patients achieved a median reduction in FES tumor uptake of ≥ 75% and for these patients the CBR was 8/9 (89%) and the median PFS was 11.7 months. This was compared with a CBR of 1/6 (17%) and median PFS of 3.3 months among the 6 patients who had a median reduction in FES tumor uptake of < 75%.29 FES-PET has already been incorporated into oral SERD trials as a pharmacodynamic marker together with or instead of paired tumor biopsy.84–86
Oral SERDs do not represent the only developments in ER positive breast cancer, and there are other novel classes of oral ETs in early development including Selective Estrogen Receptor Covalent Antagonists (SERCAs), Complete Estrogen Receptor Antagonists (CERANs) and PROteolysis Targeting Chimera (PROTAC) protein degraders. H3B-6545 is a first-in-class SERCA and binds at cysteine 530 on wild-type and mutant ERα, thereby inactivating the receptor.87 OP-1250 is a CERAN which degrades and inactivates ER, and blocks the ER transcript at the activation function domains (AF-1 and AF-2).88 The PROTAC ARV-471 is an ER antagonist with the ubiquitin E3 ligase attached, capable of wild-type and mutant ER degradation.89 These have displayed signals of activity in phase I/II trials.87–89 Interestingly off-target cardiac side effects have been observed with H3B-6545 and OP-1250, and the visual side effect photopsia has occurred with OP-1250. A second ER-targeting PROTAC AC682 has entered a phase I trial (NCT05080842).
Conclusions
ET remains an effective therapy for most patients with ER positive breast cancer and has altered the natural history of this disease through its widespread use in the adjuvant setting, and continuing use as a backbone in combination with targeted therapies in advanced breast cancer, where patients are treated with multiple lines of ET during the course of their disease, as the ER signaling pathway continues to drive cancer growth. There is a need for new ET agents with strong and durable activity in heavily treated patients, including after standard of care first-line therapy with CDK4/6 inhibitors, and in those with ESR1 mutations. Oral SERDs are showing great promise in meeting this need. The outcomes of ongoing trials will determine the ideal place of oral SERDs in the ET landscape, including if oral SERDs have a role in the early ER positive breast cancer setting. In addition, determining toxicities that are class effects and unique to each compound would also have a significant impact on clinical uptake and influence patient and clinician preference for individual agents.
Funding Statement
EL is supported by the National Breast Cancer Foundation Endowed Chair (EC17-02) and Love Your Sister foundation. RJ receives funding from the National Institute of Health/ National Cancer Institute (R01CA237414-01) and the Claudia Adams Barr Program.
Disclosure
R.J. received research funding from Pfizer and Lilly and is a consultant for Carrick Therapeutics and Luminex. E.L. is on the advisory board for AstraZeneca, Lilly, Pfizer, Roche, Novartis and Gilead Australia, and has received research funding from Pfizer, Novartis and Bayer. The authors report no other conflicts of interest in this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5). doi: 10.1093/jnci/dju055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51(15):3867–3873. [PubMed] [Google Scholar]
- 4.Wakeling AE, Bowler J. Steroidal pure antioestrogens. J Endocrinol. 1987;112(3):R7–R10. doi: 10.1677/joe.0.112r007 [DOI] [PubMed] [Google Scholar]
- 5.Pike ACW, Brzozowski AM, Walton J, Hubbard RF. Structural insights into the mode of action of a pure antiestrogen. Structure. 2001;9:145–153. doi: 10.1016/S0969-2126(01)00568-8 [DOI] [PubMed] [Google Scholar]
- 6.Bowler J, Lilley TJ, Pittam JD, Wakeling AE. Novel steroidal pure antiestrogens. Steroids. 1989;54(1):71–99. doi: 10.1016/0039-128x(89)90076-7 [DOI] [PubMed] [Google Scholar]
- 7.Parker MG, Arbuckle N, Dauvois S, Danielian P, White R. Structure and function of the estrogen receptor. Ann N Y Acad Sci. 1993;684:119–126. doi: 10.1111/j.1749-6632.1993.tb32276.x [DOI] [PubMed] [Google Scholar]
- 8.Dauvois S, Danielian PS, White R, Parker MG. Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proc Natl Acad Sci U S A. 1992;89(9):4037–4041. doi: 10.1073/pnas.89.9.4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howell A, Osborne CK, Morris C, Wakeling AE. ICI 182,780 (Faslodex): development of a novel, “pure” antiestrogen. Cancer. 2000;89(4):817–825. doi: [DOI] [PubMed] [Google Scholar]
- 10.Wakeling AE, Bowler J. Novel antioestrogens without partial agonist activity. J Steroid Biochem. 1988;31(4B):645–653. doi: 10.1016/0022-4731(88)90014-3 [DOI] [PubMed] [Google Scholar]
- 11.Wakeling AE, Bowler J. Biology and mode of action of pure antioestrogens. Drugs Exp Clin Res. 1988;14(12):729–734. [PubMed] [Google Scholar]
- 12.Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20(16):3386–3395. doi: 10.1200/JCO.2002.10.058 [DOI] [PubMed] [Google Scholar]
- 13.Howell A, Robertson JF, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396–3403. doi: 10.1200/JCO.2002.10.057 [DOI] [PubMed] [Google Scholar]
- 14.McCormack P, Sapunar F. Pharmacokinetic profile of the fulvestrant loading dose regimen in postmenopausal women with hormone receptor-positive advanced breast cancer. Clin Breast Cancer. 2008;8(4):347–351. doi: 10.3816/CBC.2008.n.040 [DOI] [PubMed] [Google Scholar]
- 15.Robertson JF, Nicholson RI, Bundred NJ, et al. Comparison of the short-term biological effects of 7alpha-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)-nonyl]estra-1,3,5, (10)-triene-3,17beta-diol (Faslodex) versus tamoxifen in postmenopausal women with primary breast cancer. Cancer Res. 2001;61(18):6739–6746. [PubMed] [Google Scholar]
- 16.Kuter I, Gee JM, Hegg R, et al. Dose-dependent change in biomarkers during neoadjuvant endocrine therapy with fulvestrant: results from NEWEST, a randomized Phase II study. Breast Cancer Res Treat. 2012;133(1):237–246. doi: 10.1007/s10549-011-1947-7 [DOI] [PubMed] [Google Scholar]
- 17.Robertson JF, Semiglazov V, Nemsadze G, et al. Effects of fulvestrant 250mg in premenopausal women with oestrogen receptor-positive primary breast cancer. Eur J Cancer. 2007;43(1):64–70. doi: 10.1016/j.ejca.2006.08.019 [DOI] [PubMed] [Google Scholar]
- 18.Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28(30):4594–4600. doi: 10.1200/JCO.2010.28.8415 [DOI] [PubMed] [Google Scholar]
- 19.Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106(1):djt337. doi: 10.1093/jnci/djt337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, Phase 3 trial. Lancet. 2016;388(10063):2997–3005. doi: 10.1016/S0140-6736(16)32389-3 [DOI] [PubMed] [Google Scholar]
- 21.Robertson JF, Lindemann JP, Llombart-Cussac A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136(2):503–511. doi: 10.1007/s10549-012-2192-4 [DOI] [PubMed] [Google Scholar]
- 22.Spoerke JM, Gendreau S, Walter K, et al. Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun. 2016;7:11579. doi: 10.1038/ncomms11579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45(12):1439–1445. doi: 10.1038/ng.2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nettles KW, Bruning JB, Gil G, et al. NFkappaB selectivity of estrogen receptor ligands revealed by comparative crystallographic analyses. Nat Chem Biol. 2008;4(4):241–247. doi: 10.1038/nchembio.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fanning SW, Jeselsohn R, Dharmarajan V, et al. The SERM/SERD bazedoxifene disrupts ESR1 helix 12 to overcome acquired hormone resistance in breast cancer cells. Elife. 2018;7. doi: 10.7554/eLife.37161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fanning SW, Mayne CG, Dharmarajan V, et al. Estrogen receptor alpha somatic mutations Y537S and D538G confer breast cancer endocrine resistance by stabilizing the activating function-2 binding conformation. Elife. 2016;5. doi: 10.7554/eLife.12792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fribbens C, O’Leary B, Kilburn L, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2016;34(25):2961–2968. doi: 10.1200/JCO.2016.67.3061 [DOI] [PubMed] [Google Scholar]
- 28.Schiavon G, Hrebien S, Garcia-Murillas I, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med. 2015;7(313):313ra182. doi: 10.1126/scitranslmed.aac7551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Kruchten M, de Vries EG, Glaudemans AW, et al. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov. 2015;5(1):72–81. doi: 10.1158/2159-8290.CD-14-0697 [DOI] [PubMed] [Google Scholar]
- 30.O’Leary B, Hrebien S, Morden JP, et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun. 2018;9(1):896. doi: 10.1038/s41467-018-03215-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willson TM, Henke BR, Momtahen TM, et al. 3-[4-(1,2-Diphenylbut-1-enyl)phenyl]acrylic acid: a non-steroidal estrogen with functional selectivity for bone over uterus in rats. J Med Chem. 1994;37(11):1550–1552. doi: 10.1021/jm00037a002 [DOI] [PubMed] [Google Scholar]
- 32.Wu YL, Yang X, Ren Z, et al. Structural basis for an unexpected mode of SERM-mediated ER antagonism. Mol Cell. 2005;18(4):413–424. doi: 10.1016/j.molcel.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 33.Connor CE, Norris JD, Broadwater G, et al. Circumventing tamoxifen resistance in breast cancers using antiestrogens that induce unique conformational changes in the estrogen receptor. Cancer Res. 2001;61(7):2917–2922. [PubMed] [Google Scholar]
- 34.Mottamal M, Kang B, Peng X, Wang G. From pure antagonists to pure degraders of the estrogen receptor: evolving strategies for the same target. ACS Omega. 2021;6(14):9334–9343. doi: 10.1021/acsomega.0c06362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Zheng S, Akerstrom VL, et al. Fulvestrant-3 boronic acid (ZB716): an orally bioavailable Selective Estrogen Receptor Downregulator (SERD). J Med Chem. 2016;59(17):8134–8140. doi: 10.1021/acs.jmedchem.6b00753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahraman M, Govek SP, Nagasawa JY, et al. Maximizing ER-alpha degradation maximizes activity in a tamoxifen-resistant breast cancer model: identification of GDC-0927. ACS Med Chem Lett. 2019;10(1):50–55. doi: 10.1021/acsmedchemlett.8b00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Savi C, Bradbury RH, Rabow AA, et al. Optimization of a novel binding motif to (E)-3-(3,5-difluoro-4-((1R,3R)-2-(2-fluoro-2-methylpropyl)-3-methyl-2,3,4,9-tetra hydro-1H-pyrido[3,4-b]indol-1-yl)phenyl)acrylic acid (AZD9496), a potent and orally bioavailable selective estrogen receptor downregulator and antagonist. J Med Chem. 2015;58(20):8128–8140. doi: 10.1021/acs.jmedchem.5b00984 [DOI] [PubMed] [Google Scholar]
- 38.Tria GS, Abrams T, Baird J, et al. Discovery of LSZ102, a potent, orally bioavailable selective estrogen receptor degrader (SERD) for the treatment of estrogen receptor positive breast cancer. J Med Chem. 2018;61(7):2837–2864. doi: 10.1021/acs.jmedchem.7b01682 [DOI] [PubMed] [Google Scholar]
- 39.Guan J, Zhou W, Hafner M, et al. Therapeutic ligands antagonize estrogen receptor function by impairing its mobility. Cell. 2019;178(4):949–963 e18. doi: 10.1016/j.cell.2019.06.026 [DOI] [PubMed] [Google Scholar]
- 40.Scott JS, Moss TA, Balazs A, et al. Discovery of AZD9833, a potent and orally bioavailable selective estrogen receptor degrader and antagonist. J Med Chem. 2020;63(23):14530–14559. doi: 10.1021/acs.jmedchem.0c01163 [DOI] [PubMed] [Google Scholar]
- 41.Liang J, Zbieg JR, Blake RA, et al. GDC-9545 (Giredestrant): a potent and orally bioavailable selective estrogen receptor antagonist and degrader with an exceptional preclinical profile for ER+ breast cancer. J Med Chem. 2021;64(16):11841–11856. doi: 10.1021/acs.jmedchem.1c00847 [DOI] [PubMed] [Google Scholar]
- 42.El-Ahmad Y, Tabart M, Halley F, et al. Discovery of 6-(2,4-Dichlorophenyl)-5-[4-[(3S)-1-(3-fluoropropyl)pyrrolidin-3-yl]oxyphenyl]-8, 9-dihydro-7H-benzo[7]annulene-2-carboxylic acid (SAR439859), a Potent and Selective Estrogen Receptor Degrader (SERD) for the treatment of estrogen-receptor-positive breast cancer. J Med Chem. 2020;63(2):512–528. doi: 10.1021/acs.jmedchem.9b01293 [DOI] [PubMed] [Google Scholar]
- 43.Bhagwat SV, Zhao B, Shen W, et al. Abstract 1236: preclinical characterization of LY3484356, a novel, potent and orally bioavailable selective estrogen receptor degrader (SERD). Cancer Res. 2021;81(13_Supplement):1236. doi: 10.1158/1538-7445.Am2021-1236 [DOI] [Google Scholar]
- 44.Garner F, Shomali M, Paquin D, Lyttle CR, Hattersley G. RAD1901: a novel, orally bioavailable selective estrogen receptor degrader that demonstrates antitumor activity in breast cancer xenograft models. Anticancer Drugs. 2015;26(9):948–956. doi: 10.1097/CAD.0000000000000271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andreano KJ, Wardell SE, Baker JG, et al. G1T48, an oral selective estrogen receptor degrader, and the CDK4/6 inhibitor lerociclib inhibit tumor growth in animal models of endocrine-resistant breast cancer. Breast Cancer Res Treat. 2020;180(3):635–646. doi: 10.1007/s10549-020-05575-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samatar AA, Li J, Hegde S, et al. Abstract 4373: discovery of ZN-c5, a novel potent and oral selective estrogen receptor degrader. Cancer Res. 2020;80(16_Supplement):4373. doi: 10.1158/1538-7445.Am2020-4373 [DOI] [Google Scholar]
- 47.Osborne C, Richards DA, Wilks ST, et al. Abstract PS11-26: a phase 1 study of D-0502, an orally bioavailable SERD, for advanced or metastatic HR-positive and HER2-negative breast cancer. Cancer Res. 2021;81(4_Supplement):PS11-26-PS11-26. doi: 10.1158/1538-7445.Sabcs20-ps11-26 [DOI] [Google Scholar]
- 48.Guo S, Zhang C, Bratton M, et al. ZB716, a steroidal selective estrogen receptor degrader (SERD), is orally efficacious in blocking tumor growth in mouse xenograft models. Oncotarget. 2018;9(6):6924–6937. doi: 10.18632/oncotarget.24023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bardia A, Kaklamani V, Wilks S, et al. Phase I study of elacestrant (RAD1901), a novel selective estrogen receptor degrader, in ER-positive, HER2-negative advanced breast cancer. J Clin Oncol. 2021;39(12):1360–1370. doi: 10.1200/JCO.20.02272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baird R, Oliveira M, Gil EMC, et al. Abstract PS11-05: updated data from SERENA-1: a phase 1 dose escalation and expansion study of the next generation oral SERD AZD9833 as a monotherapy and in combination with palbociclib, in women with ER-positive, HER2-negative advanced breast cancer. Cancer Res. 2021;81(4_Supplement):PS11-05-PS11-05. doi: 10.1158/1538-7445.Sabcs20-ps11-05 [DOI] [Google Scholar]
- 51.Jhaveri KL, Boni V, Sohn J, et al. Safety and activity of single-agent giredestrant (GDC-9545) from a phase Ia/b study in patients (pts) with estrogen receptor-positive (ER+), HER2-negative locally advanced/metastatic breast cancer (LA/mBC). J Clin Oncol. 2021;39(15_suppl):1017. doi: 10.1200/JCO.2021.39.15_suppl.1017 [DOI] [Google Scholar]
- 52.Neilan TG, Villanueva-Vázquez R, Bellet M, et al. Abstract P5-18-07: heart rate changes, cardiac safety, and exercise tolerance from a phase Ia/b study of giredestrant (GDC-9545) ± palbociclib in patients with estrogen receptor-positive, HER2-negative locally advanced/metastatic breast cancer. Cancer Res. 2022;82(4_Supplement):P5-18-07-P5-18-07. doi: 10.1158/1538-7445.Sabcs21-p5-18-07 [DOI] [Google Scholar]
- 53.Oliveira M, Hamilton EP, Incorvati J, et al. Serena-1: updated analyses from a phase 1 study (parts C/D) of the next-generation oral SERD camizestrant (AZD9833) in combination with palbociclib, in women with ER-positive, HER2-negative advanced breast cancer. J Clin Oncol. 2022;40(16_suppl):1032. doi: 10.1200/JCO.2022.40.16_suppl.1032 [DOI] [Google Scholar]
- 54.Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5. doi: 10.1038/s41523-018-0097-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–1547. doi: 10.1093/annonc/mdy155 [DOI] [PubMed] [Google Scholar]
- 56.Rugo HS, Finn RS, Dieras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–729. doi: 10.1007/s10549-018-05125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386(10):942–950. doi: 10.1056/NEJMoa2114663 [DOI] [PubMed] [Google Scholar]
- 58.Finn RS, Rugo HS, Dieras VC, et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): analyses from PALOMA-2. J Clin Oncol. 2022;40(17_suppl):LBA1003–LBA1003. doi: 10.1200/JCO.2022.40.17_suppl.LBA1003 [DOI] [Google Scholar]
- 59.Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–2472. doi: 10.1200/JCO.2018.78.9909 [DOI] [PubMed] [Google Scholar]
- 60.Slamon DJ, Neven P, Chia S, et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol. 2021;32(8):1015–1024. doi: 10.1016/j.annonc.2021.05.353 [DOI] [PubMed] [Google Scholar]
- 61.Turner NC, Loi S, Moore HM, et al. Abstract PD13-07: activity and biomarker analyses from a phase Ia/b study of giredestrant (GDC-9545; G) with or without palbociclib (palbo) in patients with estrogen receptor-positive, HER2-negative locally advanced/metastatic breast cancer (ER+/HER2- LA/mBC). Cancer Res. 2022;82(4_Supplement):PD13-07-PD13-07. doi: 10.1158/1538-7445.Sabcs21-pd13-07 [DOI] [Google Scholar]
- 62.Chandarlapaty S, Linden HM, Neven P, et al. Abstract P1-17-11: updated data from AMEERA-1: phase 1/2 study of amcenestrant (SAR439859), an oral selective estrogen receptor (ER) degrader (SERD), combined with palbociclib in postmenopausal women with ER+/HER2- advanced breast cancer. Cancer Res. 2022;82(4_Supplement):P1-17-11-P1-17-11. doi: 10.1158/1538-7445.Sabcs21-p1-17-11 [DOI] [Google Scholar]
- 63.Maglakelidze M, Bulat I, Ryspayeva D, et al. Rintodestrant (G1T48), an oral selective estrogen receptor degrader, in combination with palbociclib for ER+/HER2– advanced breast cancer: phase 1 results. J Clin Oncol. 2021;39(15_suppl):1063. doi: 10.1200/JCO.2021.39.15_suppl.1063 [DOI] [Google Scholar]
- 64.Lim E, Jhaveri KL, Perez-Fidalgo JA, et al. A phase Ib study to evaluate the oral selective estrogen receptor degrader GDC-9545 alone or combined with palbociclib in metastatic ER-positive HER2-negative breast cancer. J Clin Oncol. 2020;38(15_suppl):1023. doi: 10.1200/JCO.2020.38.15_suppl.1023 [DOI] [Google Scholar]
- 65.Chandarlapaty S, Linden HM, Neven P, et al. AMEERA-1: phase 1/2 study of amcenestrant (SAR439859), an oral selective estrogen receptor (ER) degrader (SERD), with palbociclib (palbo) in postmenopausal women with ER+/ human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer (mBC). J Clin Oncol. 2021;39(15_suppl):1058. doi: 10.1200/JCO.2021.39.15_suppl.1058 [DOI] [Google Scholar]
- 66.Bidard FC, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022;JCO2200338. doi: 10.1200/JCO.22.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaklamani VG, Bardia A, Aftimos PG, et al. Subgroup analysis of patients with no prior chemotherapy in EMERALD: a phase 3 trial evaluating elacestrant, an oral selective estrogen receptor degrader (SERD), versus investigator’s choice of endocrine monotherapy for ER+/HER2-advanced/metastatic breast cancer (mBC). J Clin Oncol. 2022;40(16_suppl):1100. doi: 10.1200/JCO.2022.40.16_suppl.1100 [DOI] [Google Scholar]
- 68.Sanofi. Sanofi provides update on phase 2 study evaluating amcenestrant in ER+/HER2- advanced or metastatic breast cancer; 2022. Available from: https://www.sanofi.com/en/media-room/press-releases/2022/2022-03-14-06-00-00-2402216. Accessed August 5, 2022.
- 69.Taylor P. Roche’s oral SERD giredestrant fails breast cancer trial. Available from: https://pharmaphorum.com/news/roches-oral-serd-giredestrant-fails-breast-cancer-trial/. Accessed August 5, 2022.
- 70.Berger F, Marce M, Delaloge S, et al. Randomised, open-label, multicentric phase III trial to evaluate the safety and efficacy of palbociclib in combination with endocrine therapy, guided by ESR1 mutation monitoring in oestrogen receptor-positive, HER2-negative metastatic breast cancer patients: study design of PADA-1. BMJ Open. 2022;12(3):e055821. doi: 10.1136/bmjopen-2021-055821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bidard F-C, Hardy-Bessard A-C, Bachelot T, et al. Abstract GS3-05: fulvestrant-palbociclib vs continuing aromatase inhibitor-palbociclib upon detection of circulating ESR1 mutation in HR+ HER2- metastatic breast cancer patients: results of PADA-1, a UCBG-GINECO randomized phase 3 trial. Cancer Res. 2022;82(4_Supplement):GS3-05-GS3-05. doi: 10.1158/1538-7445.Sabcs21-gs3-05 [DOI] [Google Scholar]
- 72.Bidard F-C, Kalinsky K, Cristofanilli M, et al. Abstract OT2-11-05: SERENA-6: a Phase III study to assess the efficacy and safety of AZD9833 (camizestrant) compared with aromatase inhibitors when given in combination with palbociclib or abemaciclib in patients with HR+/HER2- metastatic breast cancer with detectable ESR1m who have not experienced disease progression on first-line therapy. Cancer Res. 2022;82(4_Supplement):OT2-11-05-OT2-11-05. doi: 10.1158/1538-7445.Sabcs21-ot2-11-05 [DOI] [Google Scholar]
- 73.Dahlgren M, George AM, Brueffer C, et al. Preexisting somatic mutations of estrogen receptor alpha (ESR1) in early-stage primary breast cancer. JNCI Cancer Spectr. 2021;5(2). doi: 10.1093/jncics/pkab028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zundelevich A, Dadiani M, Kahana-Edwin S, et al. ESR1 mutations are frequent in newly diagnosed metastatic and loco-regional recurrence of endocrine-treated breast cancer and carry worse prognosis. Breast Cancer Res. 2020;22(1):16. doi: 10.1186/s13058-020-1246-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuang Y, Siddiqui B, Hu J, et al. Unraveling the clinicopathological features driving the emergence of ESR1 mutations in metastatic breast cancer. NPJ Breast Cancer. 2018;4:22. doi: 10.1038/s41523-018-0075-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hurvitz SA, Quiroga V, Park YH, et al. Abstract PD13-06: neoadjuvant giredestrant (GDC-9545) + palbociclib versus anastrozole + palbociclib in postmenopausal women with estrogen receptor-positive, HER2-negative, untreated early breast cancer: primary analysis of the randomized, open-label, phase II coopERA breast cancer study. Cancer Res. 2022;82(4_Supplement):PD13-06-PD13-06. doi: 10.1158/1538-7445.Sabcs21-pd13-06 [DOI] [Google Scholar]
- 77.Fasching PA, Bardia A, Quiroga V, et al. Neoadjuvant giredestrant (GDC-9545) plus palbociclib (P) versus anastrozole (A) plus P in postmenopausal women with estrogen receptor–positive, HER2-negative, untreated early breast cancer (ER+/HER2– eBC): final analysis of the randomized, open-label, international phase 2 coopERA BC study. J Clin Oncol. 2022;40(16_suppl):589. doi: 10.1200/JCO.2022.40.16_suppl.589 [DOI] [Google Scholar]
- 78.Campone M, Dong Y, Ling B, Wang L, Herold CI. AMEERA-4: a preoperative window-of-opportunity (WOO) study to assess the pharmacodynamic (PD) activity of amcenestrant or letrozole in postmenopausal patients with ER+/HER2− primary breast cancer. J Clin Oncol. 2022;40(16_suppl):528. doi: 10.1200/JCO.2022.40.16_suppl.528 [DOI] [Google Scholar]
- 79.Huang D, Tang L, Yang F, Jin J, Guan X. PIK3CA mutations contribute to fulvestrant resistance in ER-positive breast cancer. Am J Transl Res. 2019;11(9):6055–6065. [PMC free article] [PubMed] [Google Scholar]
- 80.Andre F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–1940. doi: 10.1056/NEJMoa1813904 [DOI] [PubMed] [Google Scholar]
- 81.Rugo HS, Lerebours F, Ciruelos E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22(4):489–498. doi: 10.1016/S1470-2045(21)00034-6 [DOI] [PubMed] [Google Scholar]
- 82.Kingston B, Pearson A, Herrera-Abreu MT, et al. ESR1 F404 mutations and acquired resistance to fulvestrant in the plasmaMATCH study. J Clin Oncol. 2022;40(16_suppl):1009. doi: 10.1200/JCO.2022.40.16_suppl.100935077194 [DOI] [Google Scholar]
- 83.Callens C, Bidard F-C, Curto-Taribo A, et al. Abstract P1-07-01: real time detection of ESR1 mutation in blood by droplet digital PCR in the PADA-1 trial: feasibility and cross-validation with NGS. Cancer Res. 2022;82(4_Supplement):P1-07-01-P1-07-01. doi: 10.1158/1538-7445.Sabcs21-p1-07-01 [DOI] [PubMed] [Google Scholar]
- 84.Aftimos P, Neven P, Pegram M, et al. Abstract PS12-04: rintodestrant (G1T48), an oral selective estrogen receptor degrader in ER+/HER2- locally advanced or metastatic breast cancer: updated phase 1 results and dose selection. Cancer Res. 2021;81(4_Supplement):PS12-04-PS12-04. doi: 10.1158/1538-7445.Sabcs20-ps12-04 [DOI] [Google Scholar]
- 85.Jager A, de Vries EGE, der Houven van Oordt CWM, et al. A phase 1b study evaluating the effect of elacestrant treatment on estrogen receptor availability and estradiol binding to the estrogen receptor in metastatic breast cancer lesions using (18) F-FESPET/CT imaging. Breast Cancer Res. 2020;22(1):97. doi: 10.1186/s13058-020-01333-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bardia A, Chandarlapaty S, Linden HM, et al. AMEERA-1 phase 1/2 study of amcenestrant, SAR439859, in postmenopausal women with ER-positive/HER2-negative advanced breast cancer. Nat Commun. 2022;13(1):4116. doi: 10.1038/s41467-022-31668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamilton EP, Wang JS, Pluard T, et al. Abstract P1-17-10: H3B-6545, a novel selective estrogen receptor covalent antagonist (SERCA), in estrogen receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2-) advanced breast cancer - A phase II study. Cancer Res. 2022;82(4_Supplement):P1-17-10-P1-17-10. doi: 10.1158/1538-7445.Sabcs21-p1-17-10 [DOI] [Google Scholar]
- 88.Patel M, Alemany C, Mitri Z, et al. Abstract P1-17-12: preliminary data from a phase I/II, multicenter, dose escalation study of OP-1250, an oral CERAN/SERD, in subjects with advanced and/or metastatic estrogen receptor (ER)-positive, HER2-negative breast cancer. Cancer Res. 2022;82(4_Supplement):P1-17-12-P1-17-12. doi: 10.1158/1538-7445.Sabcs21-p1-17-12 [DOI] [Google Scholar]
- 89.Hamilton E, Vahdat L, Han HS, et al. Abstract PD13-08: first-in-human safety and activity of ARV-471, a novel PROTAC® estrogen receptor degrader, in ER+/HER2- locally advanced or metastatic breast cancer. Cancer Res. 2022;82(4_Supplement):PD13-08-PD13-08. doi: 10.1158/1538-7445.Sabcs21-pd13-08 [DOI] [Google Scholar]
- 90.Jhaveri KL, Jeselsohn R, Lim E, et al. A phase 1a/b trial of imlunestrant (LY3484356), an oral selective estrogen receptor degrader (SERD) in ER-positive (ER+) advanced breast cancer (aBC) and endometrial endometrioid cancer (EEC): monotherapy results from EMBER. J Clin Oncol. 2022;40(16_suppl):1021. doi: 10.1200/JCO.2022.40.16_suppl.1021 [DOI] [Google Scholar]
- 91.Kalinksy K, Abramson V, Chalasani P, et al. Abstract P1-17-02: ZN-c5, an oral selective estrogen receptor degrader (SERD), in women with advanced estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2 negative (HER2-) breast cancer. Cancer Res. 2022;82(4_Supplement):P1-17-02-P1-17-02. doi: 10.1158/1538-7445.Sabcs21-p1-17-02 [DOI] [Google Scholar]
- 92.Meyskens T, Metzger O, Poncet C, et al. Adjuvant study of amcenestrant (SAR439859) versus tamoxifen for patients with hormone receptor-positive (HR+) early breast cancer (EBC), who have discontinued adjuvant aromatase inhibitor therapy due to treatment-related toxicity (AMEERA-6). J Clin Oncol. 2022;40(16_suppl):TPS607–TPS607. doi: 10.1200/JCO.2022.40.16_suppl.TPS607 [DOI] [Google Scholar]
- 93.Chien AJ, Kalinsky KM, Molina-Vega J, et al. Abstract OT1-10-02: i-SPY2 endocrine optimization protocol (EOP): a pilot neoadjuvant endocrine therapy study with amcenestrant as monotherapy or in combination with abemacicilib or letrozole in molecularly selected HR+/HER2- clinical stage 2/3 breast cancer. Cancer Res. 2022;82(4_Supplement):OT1-10-02-OT1-10-02. doi: 10.1158/1538-7445.Sabcs21-ot1-10-02 [DOI] [Google Scholar]