Abstract
Ex vivo confocal microscopy (EVCM) generates digitally colored purple-pink images similar to H&E, without time-consuming tissue processing. It can be used during Mohs surgery for rapid detection of basal cell carcinoma (BCC); however, reading EVCM images requires specialized training. An automated approach using a Deep Learning algorithm to BCC detection in EVCM images can aid in diagnosis. 40 BCCs and 28 negative (“not-BCC”) samples were collected at Memorial Sloan Kettering Cancer Center to create three training datasets: 1) EVCM image dataset (663 images), 2) H&E image dataset (516 images), and 3) a combination of the two datasets. 7 BCCs and 4 negative samples were collected to create a EVCM test dataset (107 images). The model trained with the EVCM dataset achieved 92% diagnostic accuracy, similar to the H&E model (93%). The area under ROC was 0.94, 0.95, and 0.94 for EVCM, H&E, and combination trained models, respectively. We developed an algorithm for automatic BCC detection in EVCM images (comparable accuracy to dermatologists). This approach could be used to assist with BCC detection during Mohs surgery. Furthermore, we found that a model trained with only H&E images (which are more available than EVCM images) can accurately detect BCC in EVCM images.
Keywords: ex vivo confocal microscopy, digitally stained purple and pink images, basal cell carcinoma, convolutional neural network, deep learning
INTRODUCTION
Basal cell carcinoma (BCC) is the most common skin cancer accounting for ~2 millions of cases annually in the United States alone [Rogers et al. 2015]. Biopsy, followed by histopathology, is the gold standard for diagnosis and subtyping of BCCs (as aggressive or non-aggressive) for appropriate management — surgical treatment for aggressive tumors versus topical treatment (non-surgical) for less aggressive BCCs. For aggressive or recurrent tumors, especially those located in cosmetically sensitive sites such as the face, Mohs micrographic surgery (MMS) is the treatment of choice with high cure rates [Van Loo et al. 2014]. However, MMS is a tedious and time-consuming procedure as it involves careful removal of skin cancer layer-by-layer. Each excised layer undergoes frozen sectioning and microscopic evaluation, requiring up to 20-45 minutes. Often times multiple layers are removed to achieve complete tumor clearance; thus, the entire surgical procedure may last for several hours [Keena and Que 2016], increasing patient’s waiting time, complications, and cost of the procedure. Additionally, frozen sectioning can cause tissue destruction and create artefacts that may hinder the final diagnosis.
To expedite the surgical procedure, various ex vivo optical imaging devices have been developed [Bennàssar et al. 2013; Dalimier and Salomon 2012; Gareau et al. 2009; Karen et al. 2009]. These devices can rapidly image freshly excised tissues at “near-histopathological” resolution, obviating the need for destructive and time-consuming tissue processing.
Ex vivo confocal microscopy (EVCM) is an emerging imaging technique that can evaluate freshly (un-processed) excised whole-tissue samples without the need for tissue processing (frozen sectioning). As there is no tissue processing involved, EVCM can image tissues rapidly (less than a minute for a tissue measuring up to 2 cm), reducing the time for the Mohs surgery and enabling real-time imaging in the surgical suite [Keena and Que 2016]. Furthermore, EVCM creates digitally colored purple and pink images (similar to H&E images) by converting fluorescence signal originating from the nucleus into purple color and reflectance signal from the cytoplasm into pink color. These digitally colored images can be read by Mohs surgeons trained in pathology [Mu et al. 2016]. Although EVCM has demonstrated an overall high sensitivity and specificity (~ 90%) for detection of BCC [Gareau et al. 2009; Karen et al. 2009] during MMS [Bennàssar et al. 2013], it is only been utilized in very few academic centers. We believe that the integration of an automatic algorithm for the detection of BCC in EVCM images could immensely aid Mohs surgeons, increasing adoption of this technology. Moreover, EVCM technology may be useful for surgical pathologists and dermatologists to obtain faster results from standard excisions [Bennàssar A et al. 2012; Debarbieux et al. 2015] or even for biopsies of inflammatory skin lesions [Bağcı et al. 2019; Bağcı et al. 2021; Bertoni et al. 2018]. This approach can achieve the goal of a real “bedside” pathology, similar to the ongoing integration of this technique for assessment of non-dermatology specimens in surgical pathology [Panarello et al. 2020].
Artificial intelligence (AI) is currently transforming healthcare [Hinton 2018]. A popular AI technique for image classification is Convolutional Neural Networks (CNNs), a deep-learning approach inspired from the human brain. In CNNs, inputs such as images go through several layers of artificial “neurons” before an output is finally rendered such as the diagnoses of those images. CNN algorithms are being used in radiology and pathology [Topol 2019] to classify images as neoplastic or non-neoplastic and have shown a proficiency at par or even exceeding human performance [Campanella et al. 2019]. Likewise, in dermatology, CNNs have reported comparable performance to an expert dermatologist in skin cancer diagnosis using clinical [Esteva et al. 2017; Fujisawa et al. 2019, Han et al. 2018] and dermoscopy images [Brinker et al. 2019; Codella et al. 2016; Haenssle et al. 2018; Haenssle et al. 2020]. Recently, CNN has also been applied successfully to reflectance confocal microscopy (RCM) images to classify skin lesions [Kose et al. 2020, Wodzkinski et al. 2019; Wodzkinski et al. 2020, Campanella et al. 2021]. However, to the best of our knowledge, CNNs have not been developed and tested to diagnose skin cancers in digitally colored EVCM images.
The goal of this study was to develop and test the performance of CNN algorithms for detecting BCC in digitally colored EVCM images obtained from freshly excised tissues from Mohs surgery. For this study, 40 BCCs and 28 negative (“not-BCC”) skin tissue samples were collected from 42 patients to create three different image datasets to train CNN models: 1) an EVCM image dataset with 663 images, 2) an H&E image dataset with 516 images, and 3) a combination of the two datasets (EVCM and H&E image datasets) with 1179 images. The performance of these 3 trained models were evaluated and compared on a separate test set (not used in training), which comprised of 97 EVCM images created using 7 BCCs and 4 negative (“not-BCC”) skin tissue samples were from 11 patients.
RESULTS
Patient demographics and lesion characteristics:
A total of 53 patients were enrolled in the study. Mean age was 61 years (± 13, range 36-95 years); 64% (34/53) were males and 36% (19/53) females. Majority of the lesions, 63.3% (50/79) were located on the head and neck. A total of 47 BCCs were imaged including 18 nodular BCCs (nBCCs), 11 superficial BCCs (sBCCs), 10 infiltrative BCCs (iBCCs), 8 mix-subtype BCCs and 32 skin samples did not present BCC. Patient demographics and lesion characteristics are detailed in the Supplementary Table S1.
Model performance:
The main outcome measures were sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) (Table 1 and Supplementary Figure S1).
Table 1.
Comparison of the performance of trained models for diagnosis of BCC in the EVCM images
| Training Dataset |
Results of BCC detection in EVCM images- Metrics (CI 95%) | ||||
|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
| EVCM TRAINING | 0.96 (0.88-1) | 0.89 (0.80-0.97) | 0.86 (0.75-0.97) | 0.96 (0.91-1) | 0.92 (0.86-0.98) |
| H&E TRAINING | 0.93 (0.85-1) | 0.92 (0.84-1) | 0.89 (0.8-0.99) | 0.95 (0.87-1) | 0.93 (0.87-0.98) |
| EVCM + H&E TRAINING | 0.78 (0.65-0.91) | 0.92 (0.84-1) | 0.87 (0.76-0.99) | 0.85 (0.76-0.94) | 0.86 (0.79-0.93) |
Abbreviations: CI= confidence interval, PPV= positive predictive value, NPV =negative predictive value. Bold fonts indicate highest accuracy results.
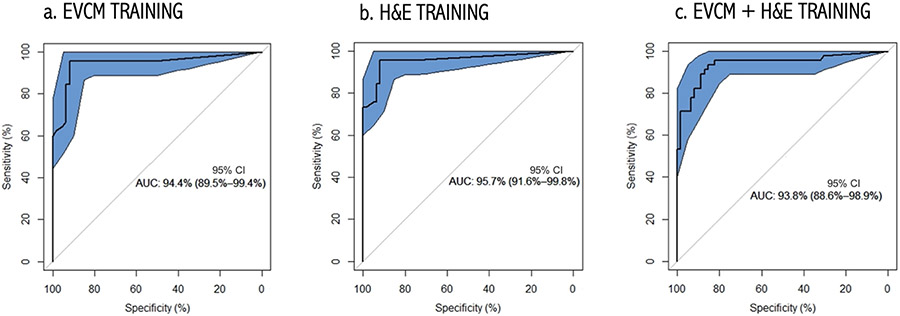
The model trained with the EVCM dataset (EVCM model) achieved 92% diagnostic accuracy, similar to the H&E model (93%). Compared to the H&E model, the EVCM model had a higher sensitivity (96% vs. 93%) but lower specificity (89% vs. 92%). The combined model had the lowest diagnostic accuracy (86%) with a high specificity (92%; similar to H&E model) but the lowest sensitivity (78%). Area under the curve (AUC) of receiver operating characteristics (ROC) for diagnostic dichotomous classification was calculated for each the three training datasets (Figure 1). The AUC was 0.94, 0.95, and 0.94 for EVCM, H&E and combination trained models, respectively.
Figure 1. Receiver Operating Characteristic (ROC) Curves obtained using:
(a) EVCM-trained model, (b) H&E-trained model, and (c) combined (EVCM+H&E) trained model. The 95% CI bounds of the ROC curve were calculated via bootstrapping. The proposed algorithm achieves an AUC of 94.4%, 95.7% and 93.8%, respectively. The shaded ellipse represents the 95% CI area for the estimate of the sensitivity and specificity of the algorithm calculated via bootstrapping.
Gradient maps:
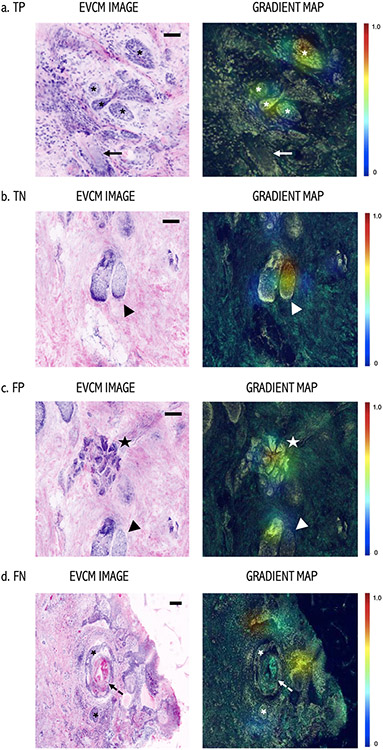
Gradient maps created with Grad-CAM [Selvaraju RR, 2016] highlighted important regions in red color in the images for predicting the presence/absence of tumor after all the three trainings. Figure 2 shows gradient map examples of a true positive (TP), a true negative (TN), a false positive (FP), and a false negative (FN) tissue sample. For the TP example, the algorithm identified even small BCC nodules (asterisks) as important areas for BCC prediction, while not taking the hair follicle (arrow) as an important region. Likewise, for the TN example, sebaceous gland was correctly identified as an important region within the image for the negative prediction for tumor. We had a very few images with false positive and false negative results. For FP example, sebaceous glands (arrowhead) and eccrine ducts (star) were detected as important for BCC detection. On the other hand, a FN example where BCC nodules (asterisks) were not considered important and the algorithm prediction was no-BCC.
Figure 2. Gradient Map: Left: EVCM images and Right: Gradient maps.
(a) True positive (TP) example of a BCC with small tumor nodules (asterisks). High prediction attributes over the tumor nodules in the Gradient map. Note, that a hair follicle (arrow) in the same field was not considered “important” for the prediction by the algorithm. (b) True negative example of an area with no-BCC, where a sebaceous gland (arrowhead) was identified “important” region for negative prediction. (c) False positive (FP) example where eccrine glands (star) and sebaceous gland (arrowhead) were detected as important for the prediction of tumor. (d) False Negative (FN) example, where BCC tumor nodules (asterisks) were not considered important by the algorithm for tumor detection. Note, this BCC tumor nodule has an extensive cystic degeneration in the center (dashed arrow), which could have resulted in the false prediction in this case. Color scalebar: Red color, high attribution and blue color low attribution for a given prediction by the algorithm. Scale bars: a, b, c, d) 200 μm.
DISCUSSION
BCC is the most common skin cancer worldwide [Leiter et al. 2014]. Although, BCC has a low metastatic potential it can be locally invasive causing extensive tissue damage and loss of regional function [Nehal and Bichakjian 2018]. MMS is a specialized surgical procedure capable of achieving complete clearance of BCC, while maximizing normal tissue preservation, making it a preferred treatment for recurrent BCCs and BCCs located on cosmetically sensitive and functionally challenging sites, such as the face [Jain et al. 2017]. However, MMS is not only a time-consuming surgery (due to frozen section analysis) but it is also an expensive procedure that requires an extensive laboratory set-up and specialized surgeons and technicians. Ex vivo confocal microscopy (EVCM) is an emerging imaging technique that generates digitally colored purple and pink images similar to H&E, without any time-consuming tissue processing [Mu et al. 2016; Schüürmann et al. 2019]. It can be used during Mohs surgery (MMS) for rapid detection of residual basal cell carcinoma (BCC); however, reading EVCM images requires specialized training. An automated approach to BCC detection in EVCM images can aid in diagnosis.
Currently, AI is being implemented extensively in the field of dermatology and pathology for the automated diagnosis of skin cancers and non-neoplastic lesions (psoriasis, atopic dermatitis and onychomycosis) [Han et al. 2018] in clinical and dermoscopy images using CNN, a deeplearning algorithm [Esteva et al. 2017, Schüürmann et al. 2019]. In our study we used CNN for detection of BCC in EVCM images. CNN was first used in skin cancer detection by Nasr-Esfahani et al. (2016) for the diagnosis of melanoma. They trained the algorithm with a small dataset of 170 clinical images from melanocytic lesions. Similar to our study, the authors used augmentation methods such as random rotation, and resizing of the images to increase the number of images (from 170 original images to 6120 images) in the training dataset, which yielded a sensitivity of 81% and a specificity of 80% in the diagnosis of melanoma.
CNNs has been applied to non-invasive in vivo imaging technique such as reflectance confocal microscopy (RCM). Wodzinski et al. (2019) reported an accuracy of 91% in the diagnosis of BCC in RCM images. Recently, Campanella et al. (2021) developed a deep learning model to automatically detect BCC in RCM images acquired from lesions clinically equivocal for BCC and compared the results with the RCM expert readers. The proposed model achieved an area under the curve (AUC) for the receiver operator characteristic (ROC) curve of 89.7%, which was on par with the expert readers. We achieved similar results with 92% diagnostic accuracy for detection of BCC in EVCM images using EVCM training dataset. The use of H&E images to train deep-learning algorithms has been extensively used in pathology. Towards this end, Campanella et al. (2019) reported an accuracy above 98% in the diagnosis of BCC in conventional H&E stained images. On the contrary, we achieved a diagnostic accuracy of 93% with H&E-trained model for the detection of BCC in EVCM images. This difference in diagnostic accuracy could be attributed to the differences in the type of images used for the training and testing datasets in our study i.e. H&E images trained model was tested on EVCM image dataset compared to the use of only H&E images in the training and testing datasets in their study. Another reason could be the use of a relatively larger number of images (9,962) used to train their model.
Our study demonstrates CNN’s high-level performance in classifying BCC in EVCM images. Even with the use of freshly discarded tumor margins in this study, which typically has less tumor burden than the central tumor de-bulk tissue, we achieved a high sensitivity and specificity in the diagnosis of BCC in EVCM images, which is at par with dermatologists’ reported level in the literature [Gareau et al. 2009; Karen et al. 2009; Mu et al. 2016]. The highest sensitivity value of 96% was obtained when the CNN was trained with EVCM images, whereas the specificity decreased to 89%. Our results showed the best accuracy with H&E-trained model, which could be attributed to the better and sharper image quality of H&E images compared to digitally-colored EVCM images. Because we demonstrated that H&E images can successfully train CNNs to diagnose BCC in EVCM images, one can collect a large dataset comprising of only H&E images to train such a model (as H&E images are more readily available than EVCM images). Due to large number of images used in the H&E training dataset, less time-consuming weakly supervised CNN training could be used [Campanella et al. 2019] instead of the completely supervised training used in our study.
Although the combined (H&E and EVCM training dataset) model improved the specificity of BCC detection in EVCM images, it had the lowest diagnostic accuracy and sensitivity compared to the other two models (EVCM and H&E models). It is possible that the model trained with the combined dataset may not have had sufficient training time (i.e., number of epochs), which can be explored in future work.
Furthermore, the gradient map created in this study could be combined with the model prediction to aid surgeons in the real-time diagnosis and also as a teaching-training tool for novices [Campanella et al. 2021].
Our study had some limitations. First, while our dataset covered all the common subtypes of BCCs, it had an overall small size samples of each subtype, which could not represent all the morphological appearance of BCCs encountered in clinical practice. Also, this study did not include analysis on pigmented BCCs, which may be important to be tested in future studies. Secondly, the algorithm has yet to be tested in actual clinical practice. Also, for imaging with the EVCM device, we used freshly discarded surgical margins from Mohs surgery, which often has less tumor burden than the central de-bulk tissue. Even with the smaller tumor burden in these samples our algorithm achieved high diagnostic values; thus, we anticipate better results using de-bulk specimens with higher tumor burden. Lastly, all the images were acquired at a single institution, which does not account for the variability in staining protocols and tissue processing. Thus, our results should be further validated on EVCM images acquired from various centers (multi-centric study).
In conclusion, we present the results of a deep-learning algorithm in classifying BCC in EVCM images. The various models developed could diagnose BCC in digitally colored purple and pink EVCM images which was at par with reported dermatologists’ accuracy in the literature. Furthermore, we found that a model trained with only H&E images (which are more available than EVCM images) can accurately detect BCC in EVCM images. Training deep-learning technology with H&E images to diagnose EVCM images expands the possibility our approach to be generalized to diagnose a variety of skin lesions (neoplastic and non-neoplastic) in excised tissues. Ultimately, deep-learning models could be integrated in existing EVCM devices to aid Mohs surgeons in identifying BCCs automatically. Prospective and larger scale studies are needed to validate this technology in real clinical practice.
MATERIAL AND METHODS:
Tissue sample collection and image acquisition:
All the tissues used for creating the training and test datasets were collected at Memorial Sloan Kettering Cancer Center (NY, USA) under institutional review protocols (IRBs #17-078 and # 08-066) approved by Memorial Sloan Kettering Cancer Center Ethics Committee and after written, informed consent from patients.
a). Tissue sample collection and image acquisition for EVCM images:
Freshly discarded whole tissues samples (excised en face tumor margins) were collected consecutively from BCC cases undergoing Mohs surgery under a prospective IRB protocol (# 08-066). These samples were dipped in 0.6 mM acridine orange dye (a fluorescent dye) for 20 seconds and immediately placed on a commercial EVCM device (Vivascope 2500; Caliber ID, Rochester, NY, USA) for imaging [Jain et al. 2017]. The tissue was similarly oriented as for the frozen sectioning, which enabled an en face view of the entire tissue. Digitally-colored purple and pink images were acquired by combining signals from the fluorescent and reflectance channels, respectively (Supplementary Figure S2). First, we acquired an overview image, covering the entire tissue (measuring up to 2 cm in maximum diameter) section. Then we acquired multiple smaller sized zoomed-in images (within the overview image) of BCC tumors and normal surrounding skin structures such as sebaceous glands, hair follicles, epidermis, and eccrine ducts.
b). Tissue sample collection and image acquisition for H&E images:
Under another IRB approved protocol (#17-078), a Dataline search was performed to identify lesions with a histopathology confirmed diagnosis of BCC. We retrieved routine histopathology H&E stained slides from these lesions from the Department of Dermatopathology. The glass slides were then digitized using Aperio AT2 slide Scanner (Leica Biosystems, Nussloch, Germany) in the Dermatology research lab. Similar to EVCM image acquisition, we acquired multiple H&E images at various magnifications (2x, 8x, 10x) from BCC tumors and normal surrounding skin structures (sebaceous glands, hair follicles, epidermis, and eccrine ducts). On average, we acquired 24 images (ranging from 1 to 49 images) of varied sizes measuring ~1200x600 pixels to ~12000x12000 pixels per tissue sample using EVCM and H&E images.
Image Labelling:
The H&E and EVCM images were analyzed for the presence or absence of BCC by a dermatologist (MSM) and a pathologist specialized in optical imaging techniques (MJ). Each image was labelled as “BCC” and “not-BCC” and used to create training and test datasets (see below).
Dataset Creation:
Training Datasets:
EVCM training dataset: A total of 663 digitally stained purple and pink EVCM images (190 “BCC” images and 473 “not-BCC” images) were obtained from 14 fresh BCC tissues (5 nodular BCC, 4 infiltrative BCC, 3 superficial BCC, and 2 infiltrative-nodular BCCs) and 15 negative (“not-BCC”) controls normal skin tissues.
H&E training dataset: A total of 516 H&E images (170 “BCC” images and 346 “not-BCC”) were obtained from 26 H&E stained slides of BCC (11 nodular BCC, 4 infiltrative BCC, 6 superficial BCC, 3 infiltrative-nodular and 2 superficial-nodular BCC), and 13 negative (“not-BCC”) controls normal skin tissues.
Combined EVCM and H&E training dataset: A total of 1,179 images, which was created by combining all 516 images from the EVCM database and all 663 images from the H&E dataset.
Test Dataset:
The CNN models built using the above three training datasets were tested on a new set of 107 EVCM images (45 BCC and 62 normal images) obtained from 7 BCCs and 4 normal skin tissue samples that were not previously shown to the algorithm. Images artifacts were introduced for both training and testing sets to simulate real conditions. Composition of the training and test datasets are detailed in Table 2. Example images from the training and test datasets can be seen in Figure 3.
Table 2.
Samples and images used for creating training and test datasets.
| Training datasets | N° of Tissue Samples and their diagnosis |
Total N° of images/dataset |
N° of BCC positive and negative images |
||
|---|---|---|---|---|---|
| 1. EVCM TRAINING DATASET | 14 BCCs | 5 nBCC (35.7%) 4 iBCC (28.6%) 3 sBCC (21.4%) 2 inBCC (14.3%) |
663 | “BCC” 190 (28,7%) | |
| “not-BCC” 473 (71,3%) | |||||
| 15 normal (“not-BCC”) skin samples | |||||
| 2. H&E TRAINING DATASET | 26 BCCs | 11 nBC (42.3%) 4 iBCC (15.4%) 6 sBCC (23.1%) 3 inBCC(l 1.5%) 2 snBCC (7.7%) |
516 | “BCC” 170 (32,9%) | |
| “not BCC” 346 (67,1%) | |||||
| 13 normal (“not-BCC”) skin samples | |||||
| 3. COMBINED EVCM + H&E TRAINING DATASET (Datasets 1 and 2) | 40 BCC and 28 normal (“not-BCC”) skin samples 1,179 images: 360 “BCC” images and 820 “not-BCC” images |
||||
| Test-set | N° of Tissue Samples and their diagnosis |
N° of images |
N° of BCC positive and negative images |
||
| EVCM TEST SET | 7 BCCs | 2 sBCCs (28.6%) 2 nBCCs(28.6%) 2 iBCCs (28.6%) 1 snBCC(14.3%) |
107 | 45 “BCC” (44,4%) | |
| 4 normal (“not-BCC”) skin samples | 62 “not-BCC”(53,6%) | ||||
Abbreviations: BCC= basal cell carcinoma, nBCC=nodular BCC, sBCC= superficial BCC, iBCC= infiltrative BCC, inBCC= infundibular BCC, snBCC= superficial and nodular BCC.
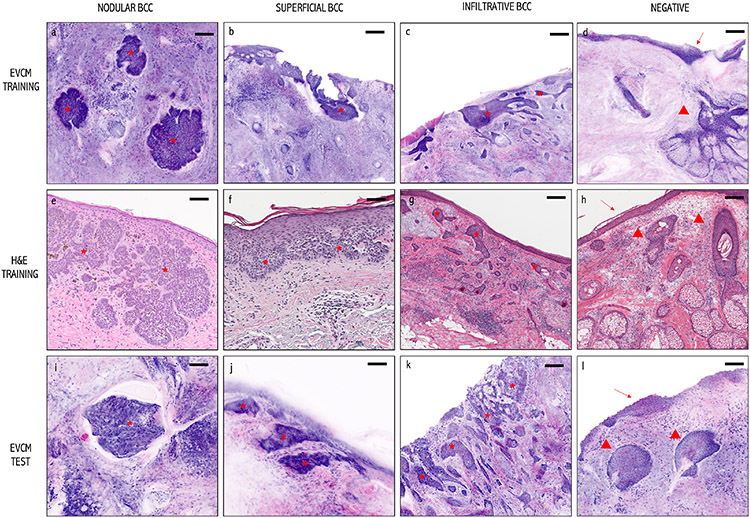
Figure 3. Images used to create training and test datasets:
EVCM images (upper and lower panel: Training and Test datasets): Purple and pink digitally colored EVCM images of: (a, i) nodular BCCs, (b, j) superficial BCC, (c, k) infiltrative BCC, and (d, l) normal (“not-BCC”) skin tissue with epidermis (arrow) and pilosebaceous gland (arrowhead). Conventional H&E-stained images (middle panel) of a: (e) a nodular BCC, (f) a superficial BCC, (g) an infiltrative BCC, and (h) normal (“not-BCC”) skin tissue with epidermis (arrow) and hair follicles (arrowheads). BCCs are shown with an asterisk. Scale bars: a, b, c, e, i, j) 100 μm and d, f, g, h, k) 200 μm.
CNN Architecture:
In this study, we trained and evaluated ResNet50 (Residual Neural Network) [He et al. 2016], a type of CNN, using both EVCM and H&E images. Unlike a standard CNN architecture, a ResNet architecture can handle greater number of hidden layers (higher model complexity), allowing for the extraction of more complex patterns and features. We used 181 hidden layers in our ResNet. We integrated Transfer Learning in our CNN to improve the efficacy of feature extraction. Transfer Learning stores knowledge gained from another problem and applies it to a different problem [Pan and Yang, 2010]. In this case, we used a CNN model pre-trained with the popular ImageNet dataset (which includes images from a large number of categories, including animal, plant, and objects but not medical images) and applied its knowledge to train our BCC-detection models.
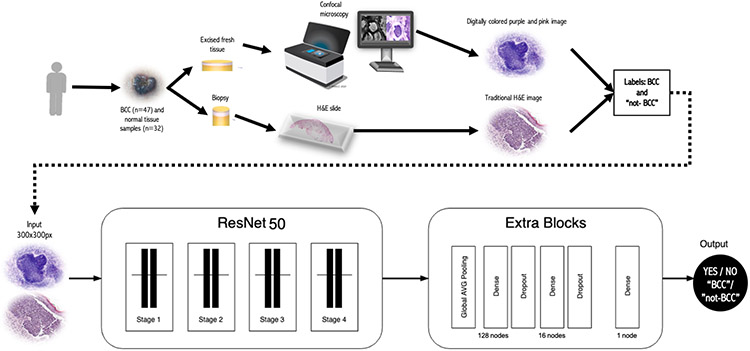
The distribution of images labelled as “BCC” and “not-BCC” were imbalanced because we used different number of images per class within each training dataset (Table 2). Images in each class were augmented, increasing the total number of images to 10000 per class. To perform data-augmentation, synthetic copies were created by applying image transformation methods such as random rotation, shifting, and resizing [Wodzinski et al. 2020]. To speed up model training time and reduce model complexity, all input images were resized to 300x300 pixels [Wodzinski et al. 2019]. A total of 20 epochs (number of complete passes through a training dataset) were used to train each of the models. A schematic of the methodology is shown in Figure 4.
Figure 4. Experimental workflow:
a) Tissue acquisition, imaging and datasets generation, and b) CNN model used in this study. A ResNet50 of 181 hidden layers and pretrained with ImageNet was used on “BCC” and “not-BCC” labelled images. Abbreviations: BCC= Basal Cell Carcinoma.
The dataset was divided in three different subsets (training, validation and testing) in order to avoid the overfitting. The training and validation subsets were used to train the CNN and the testing subset was only used to see how well the model performs on unseen data. Besides, we also used Dropout Regularization [Liang et al. 2021] to reduce overfitting. Dropout works by probabilistically removing, or “dropping out,” inputs to a layer, which may be input variables in the data sample or activations from a previous layer. It has the effect of simulating a large number of networks with very different network structure and, in turn, making nodes in the network generally more robust to the inputs.
Gradient maps:
Gradient maps were created with Grad-CAM [Selvaraju RR et al. 2016]. Grad-CAM is a technique for visual explanation of CNNs that highlights the regions of the input that are “important” for the prediction in a particular CNN model. Grad-CAM determines the weight for each of CNN feature maps to compute the weighted sum of the activations and then up sampling the result to the image size to plot the original image with the heatmap, highlighting the important regions (red color) for the model prediction. A subset of gradient maps is shown in Figure 2.
Statistical analysis:
All statistical analyses was performed in R (v4.03) [R Core Team, 2020]. The ability to discriminate the classes (“BCC” or “not-BCC”) inferred by the model was used to generate ROC curves with 95% intervals, using the package “pROC” (version 1.17.0.1) [Robin et al. 2011]. ROC curves and 95% confidence intervals for sensitivity, specificity, positive predictive and negative predictive values, and accuracy measures were generated.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr. Kivanc. Kose, PhD at Memorial Sloan Kettering Center for his critical review of the manuscript.
Funding sources:
This research is funded by a grant from the National Cancer Institute/National Institutes of Health (P30-CA008748) made to the Memorial Sloan Kettering Cancer Center.
This research was funded, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748. The funder had no role in the design and conduct of the study: collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
The remaining authors declare none.
Abbreviations Used:
- BCC
Basal Cell carcinoma
- MMS
Mohs Micrographic Surgery
- AI
Artificial Intelligence
- EVCM
Ex Vivo Confocal Microscopy
- ROC
Receiver Operator Characteristic
- AUC
Area Under the Curve
- CNN
Convolutional Neural Network
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERESTS
AR: Almirall: Consulatant; Mavig: Travel; Merz: Consultant; Dynamed: Consultant; Canfield Scientific: Consultant; Allergan Inc: Advisory Board; Evolus: Consultant; Biofrontera: Consultant; Quantia MD: Consultant; Lam Therapeutics; Consultant; Regeneron; consultant; Cutera, consultant; Skinfix, advisor; L’oreal, travel, DAR companies: Founder
ASLMS: A Ward Memorial Research Grant
Skin Cancer Foundation: Research Grant
Regen: Research / Study Funding
LeoPharma: Research / Study Funding
Biofrontera: Research Study Funding
Editorial Board: Lasers in Surgery and Medicine; CUTIS
Editorial Board: Journal of the American Academy of Dermatology (JAAD); Dermatologic Surgery
Board Member: ASDS
Committee Member and / or Chair: AAD; ASDS; ASLMS
DATA AVAILABILITY
The deep learning model that was trained to generate the presented results and the code is available at https://github.com/boxyware/confocal-ex-vivo.
REFERENCES
- Bağcı IS, Aoki R, Krammer S, Ruzicka T, Sárdy M, French LE, Hartmann D. Ex vivo confocal laser scanning microscopy for bullous pemphigoid diagnostics: new era in direct immunofluorescence? J Eur Acad Dermatol Venereol. 2019. Nov;33(11):2123–2130. doi: 10.1111/jdv.15767. [DOI] [PubMed] [Google Scholar]
- Bağcı IS, Aoki R, Vladimirova G, Ergün E, Ruzicka T, Sárdy M, et al. New-generation diagnostics in inflammatory skin diseases: Immunofluorescence and histopathological assessment using ex vivo confocal laser scanning microscopy in cutaneous lupus erythematosus. Exp Dermatol. 2021. May;30(5):684–690. doi: 10.1111/exd.14265. [DOI] [PubMed] [Google Scholar]
- Bennàssar A, Vilalta A, Carrera C, Puig S, Malvehy J. Rapid diagnosis of two facial papules using ex vivo fluorescence confocal microscopy: toward a rapid bedside pathology. Dermatol Surg. 2012. Sep;38(9):1548–51. doi: 10.1111/j.1524-4725.2012.02467.x. [DOI] [PubMed] [Google Scholar]
- Bennàssar A, Carrera C, Puig S, Vilalta A, Malvehy J. Fast evaluation of 69 basal cell carcinomas with ex vivo fluorescence confocal microscopy: criteria description, histopathological correlation, and interobserver agreement. JAMA Dermatol. 2013. Jul;149(7):839–47. doi: 10.1001/jamadermatol.2013.459. [DOI] [PubMed] [Google Scholar]
- Bertoni L, Azzoni P, Reggiani C, Pisciotta A, Carnevale G, Chester J, et al. Ex vivo fluorescence confocal microscopy for intraoperative, real-time diagnosis of cutaneous inflammatory diseases: A preliminary study. Exp Dermatol 2018;27:1152–9. 10.1111/exd.13754. [DOI] [PubMed] [Google Scholar]
- Brinker TJ, Hekler A, Hauschild A, Berking C, Schilling B, Enk AH, et al. Comparing artificial intelligence algorithms to 157 German dermatologists: the melanoma classification benchmark. Eur J Cancer 2019;111:30–7. 10.1016/j.ejca.2018.12.016. [DOI] [PubMed] [Google Scholar]
- Campanella G, Hanna MG, Geneslaw L, Miraflor A, Werneck Krauss Silva V, Busam KJ, et al. Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nat Med 2019;25:1301–9. 10.1038/s41591-019-0508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella G, Navarrete-Dechent C, Liopyris K, Monnier J, Aleissa S, Minas B, et al. Deep Learning for Basal Cell Carcinoma Detection for Reflectance Confocal Microscopy. J Invest Dermatol. 2021. Jul 12:S0022-202X(21)01437-8. doi: 10.1016/j.jid.2021.06.015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codella N, Nguyen Q-B, Pankanti S, Gutman D, Helba B, Halpern A, et al. Deep Learning Ensembles for Melanoma Recognition in Dermoscopy Images. IBM J Res Dev 2016;61. [Google Scholar]
- Dalimier E, Salomon D. Full-field optical coherence tomography: A new technology for 3D high-resolution skin imaging. Dermatology 2012;224:84–92. 10.1159/000337423. [DOI] [PubMed] [Google Scholar]
- Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017;542:115–8. 10.1038/nature21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debarbieux S, Gaspar R, Depaepe L, Dalle S, Balme B, Thomas L. Intraoperative diagnosis of nonpigmented nail tumours with ex vivo fluorescence confocal microscopy: 10 cases. Br J Dermatol. 2015. Apr;172(4):1037–44. doi: 10.1111/bjd.13384. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Otomo Y, Ogata Y, Nakamura Y, Fujita R, Ishitsuka Y, et al. Deep-learning-based, computer-aided classifier developed with a small dataset of clinical images surpasses board-certified dermatologists in skin tumour diagnosis. Br J Dermatol 2019;180:373–81. 10.1111/bjd.16924. [DOI] [PubMed] [Google Scholar]
- Gareau DS, Karen JK, Dusza SW, Tudisco M, Nehal KS, Rajadhyaksha M. Sensitivity and specificity for detecting basal cell carcinomas in Mohs excisions with confocal fluorescence mosaicing microscopy. J Biomed Opt 2009;14:034012. 10.1117/1.3130331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenssle HA, Fink C, Schneiderbauer R, Toberer F, Buhl T, Blum A, et al. Man against Machine: Diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists. Ann Oncol 2018;29:1836–42. 10.1093/annonc/mdv166. [DOI] [PubMed] [Google Scholar]
- Haenssle HA, Fink C, Toberer F, Winkler J, Stolz W, Deinlein T, et al. Man against machine reloaded: performance of a market-approved convolutional neural network in classifying a broad spectrum of skin lesions in comparison with 96 dermatologists working under less artificial conditions. Ann Oncol 2020;31:137–43. 10.1016/j.annonc.2019.10.013. [DOI] [PubMed] [Google Scholar]
- Han SS, Kim MS, Lim W, Park GH, Park I, Chang SE. Classification of the Clinical Images for Benign and Malignant Cutaneous Tumors Using a Deep Learning Algorithm. J Invest Dermatol 2018;138:1529–38. 10.1016/j.jid.2018.01.028. [DOI] [PubMed] [Google Scholar]
- Han SS, Park GH, Lim W, Kim MS, Na JI, Park I, et al. Deep neural networks show an equivalent and often superior performance to dermatologists in onychomycosis diagnosis: Automatic construction of onychomycosis datasets by region-based convolutional deep neural network. PLoS One 2018;13. 10.1371/journal.pone.0191493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. Proc. IEEE Comput. Soc. Conf. Comput. Vis. Pattern Recognit, vol. 2016- December, IEEE Computer Society; 2016, p. 770–8. 10.1109/CVPR.2016.90. [DOI] [Google Scholar]
- Hinton G. Deep learning-a technology with the potential to transform health care. JAMA - J Am Med Assoc 2018;320:1101–2. 10.1001/jama.2018.11100. [DOI] [PubMed] [Google Scholar]
- Jain M, Rajadhyaksha M, Nehal K. Implementation of fluorescence confocal mosaicking microscopy by “early adopter” Mohs surgeons and dermatologists: recent progress. J Biomed Opt 2017;22:24002. 10.1117/1.JBO.22.2.024002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karen JK, Gareau DS, Dusza SW, Tudisco M, Rajadhyaksha M, Nehal KS. Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal mosaicing microscopy. Br J Dermatol 2009;160:1242–50. 10.1111/j.1365-2133.2009.09141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keena S, Que T. Research Techniques Made Simple: Noninvasive Imaging Technologies for the Delineation of Basal Cell Carcinomas. J Invest Dermatol 2016;136:e33–8. 10.1016/j.jid.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Kose K, Bozkurt A, Alessi-Fox C, Brooks DH, Dy JG, Rajadhyaksha M, et al. Utilizing Machine Learning for Image Quality Assessment for Reflectance Confocal Microscopy. J Invest Dermatol 2020;140:1214–22. 10.1016/j.jid.2019.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Wu L, Li J, Wang Y, Meng Q, Qin T, et al. R-Drop: Regularized Dropout for Neural Networks 2021. arXiv:2106.14448 [Google Scholar]
- Mu EW, Lewin JM, Stevenson ML, Meehan SA, Carucci JA, Gareau DS. Use of digitally stained multimodal confocal mosaic images to screen for nonmelanoma skin cancer. JAMA Dermatology 2016;152:1335–41. 10.1001/jamadermatol.2016.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr-Esfahani E, Samavi S, Karimi N, Soroushmehr SMR, Jafari MH, Ward K, et al. Melanoma detection by analysis of clinical images using convolutional neural network. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS, vol. 2016-October, Institute of Electrical and Electronics Engineers Inc.; 2016, p. 1373–6. 10.1109/EMBC.2016.7590963. [DOI] [PubMed] [Google Scholar]
- Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med 2018;379:363–74. 10.1056/NEJMra1708701. [DOI] [PubMed] [Google Scholar]
- Leiter U, Eigentler T, Garbe C. Epidemiology of Skin Cancer. Sunlight, Vitam. D Ski. Cancer, vol. 810, New York, NY: Springer New York; 2014, p. 120–40. 10.1007/978-1-4939-0437-2_7. [DOI] [PubMed] [Google Scholar]
- Pan SJ, Yang Q. A survey on transfer learning. IEEE Trans Knowl Data Eng 2010;22:1345–59. 10.1109/TKDE.2009.191. [DOI] [Google Scholar]
- Panarello D, Compérat E, Seyde O, Colau A, Terrone C, Guillonneau B. Atlas of Ex Vivo Prostate Tissue and Cancer Images Using Confocal Laser Endomicroscopy: A Project for Intraoperative Positive Surgical Margin Detection During Radical Prostatectomy. Eur Urol Focus. 2020. Sep 15;6(5):941–958. doi: 10.1016/j.euf.2019.01.004 [DOI] [PubMed] [Google Scholar]
- R Core Team (2020). A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. n.d. [Google Scholar]
- Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12. 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the us population, 2012. JAMA Dermatology 2015;151:1081–6. 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- Selvaraju RR, Cogswell M, Das A, Vedantam R, Parikh D, Batra D. Grad-CAM: Visual Explanations from Deep Networks via Gradient-based Localization. Int J Comput Vis 2016;128:336–59. 10.1007/s11263-019-01228-7. [DOI] [Google Scholar]
- Schüürmann M*, Stecher MM, Paasch U, Simon JC, Grunewald S. Evaluation of digital staining for ex vivo confocal laser scanning microscopy. J Eur Acad Dermatology Venereol 2019:jdv.16085. 10.1111/jdv.16085. [DOI] [PubMed] [Google Scholar]
- Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med 2019;25:44–56. 10.1038/s41591-018-0300-7. [DOI] [PubMed] [Google Scholar]
- Van Loo E, Mosterd K, Krekels GAM, Roozeboom MH, Ostertag JU, Dirksen CD, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: A randomised clinical trial with 10 year follow-up. Eur J Cancer 2014;50:3011–20. 10.1016/j.ejca.2014.08.018 [DOI] [PubMed] [Google Scholar]
- Wodzinski M, Skalski A, Witkowski A, Pellacani G, Ludzik J. Convolutional Neural Network Approach to Classify Skin Lesions Using Reflectance Confocal Microscopy. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS, vol. 2019, Institute of Electrical and Electronics Engineers Inc.; 2019, p. 4754–7. 10.1109/EMBC.2019.8856731. [DOI] [PubMed] [Google Scholar]
- Wodzinski M, Pajak M, Skalski A, Witkowski A, Pellacani G, Ludzik J. Automatic Quality Assessment of Reflectance Confocal Microscopy Mosaics using Attention-Based Deep Neural Network. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS, vol. 2020- July, Institute of Electrical and Electronics Engineers Inc.; 2020, p. 1824–7. 10.1109/EMBC44109.2020.9176557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The deep learning model that was trained to generate the presented results and the code is available at https://github.com/boxyware/confocal-ex-vivo.