Abstract
AcrD, a transporter belonging to the resistance-nodulation-division family, was shown to participate in the efflux of aminoglycosides. Deletion of the acrD gene decreased the MICs of amikacin, gentamicin, neomycin, kanamycin, and tobramycin by a factor of two to eight, and ΔacrD cells accumulated higher levels of [3H]dihydrostreptomycin and [3H]gentamicin than did the parent strain.
Multidrug efflux pumps play an important role in establishing the intrinsic level of resistance of gram-negative bacteria to a number of agents (16, 17). Many of these pumps belong to the resistance-nodulation-division (RND) family, whose members have been known to pump out mostly lipophilic or amphiphilic molecules or toxic divalent cations (16, 19). The Escherichia coli genome contains several genes coding for RND transporters. Among these, acrB is constitutively expressed and is largely responsible for the intrinsic resistance of E. coli to a very wide range of compounds, including lipophilic drugs, detergents (including bile salts), and dyes (8, 10, 18, 20). The gene acrF has a high degree of similarity to acrB (77% identity at the amino acid level, with no gaps) and is also expected to pump out a wide variety of lipophilic and amphiphilic agents. Indeed, AcrF overexpression strains can be isolated as suppressors of acrAB mutants (J. Xu, M. L. Nilles, and K. P. Bertrand, Abstr. 93rd Gen. Meet. Am. Soc. Microbiol. 1993, abstr. K-169, p. 290, 1993) and seem to have a resistance phenotype similar to that of the acrAB+ wild-type strain. However, disruption of the acrF gene in the wild-type strain does not produce a drug hypersusceptibility phenotype (8), suggesting that acrF is weakly expressed in wild-type E. coli.
Disruption of the acrD gene also did not result in hypersusceptibility to lipophilic and amphiphilic drugs (8). However, recently RND-type transporters that pump out very hydrophilic compounds, aminoglycosides, have been observed in Burkholderia pseudomallei (13) and in Pseudomonas aeruginosa (1). Furthermore, comparison of the P. aeruginosa aminoglycoside efflux pump MexY sequence with open reading frames in the E. coli genome sequence using the gapped BLAST program (2) shows that AcrD has the highest similarity score at the amino acid level. In light of these findings, we have reexamined the drug susceptibility of an acrD disruption mutant and found it to be hypersusceptible to a variety of aminoglycosides.
Deletion-insertion of the acrD gene was done as follows. Plasmid pBW7, which corresponds to vector pEMBL8(+) containing a 5.5-kb fragment of E. coli K-12 chromosome with the dapE (msgB) gene and the upstream acrD gene (21), was obtained from D. Ang. This plasmid was digested with ClaI (which cuts at position 710 of the acrD gene) and EcoRV (which cuts at position 2370), creating a deletion within acrD of 1,660 bp, and the AvaI-EcoRI fragment of pBR322 containing the tet gene was inserted in this interval. The plasmid was linearized and used to transform JC7623 [K-12 argE3 hisG4 leuB6 Δ(gpt-proA) thr-1 thi-1 ara-14 galK2 lacY1 mtl-1 xyl-1 kdgK51 tsx-33 recB21 recC22 sbcB15 supE44 rpsL31 rac] as previously described (9), and a tetracycline-resistant clone was isolated as JZM320. The disruption of the acrD gene was confirmed by PCR amplification using genomic DNA of both the parent JC7623 and JZM320.
The antibiotic susceptibility of JZM320 was determined by the serial twofold broth dilution method, first in Luria-Bertani (LB) medium (10 g of tryptone, 10 g of Bacto yeast extract, and 5 g of NaCl per liter). The inoculum was 104 cells per ml, and the results were read after overnight incubation at 37°C. Table 1 shows that MICs of all the aminoglycosides tested were significantly decreased in the case of JZM320. MICs of streptomycin were not determined because of the presence of a ribosomal mutation, rpsL31, which makes the JC7623 strain highly resistant to this drug. MICs of erythromycin and polymyxin B were slightly but reproducibly decreased in JZM320. MICs of crystal violet, novobiocin, rifampin, chloramphenicol, carbenicillin, cefoxitin, nalidixic acid, norfloxacin, and ampicillin were not altered in a reproducible manner in JZM320, although in some experiments twofold differences were observed with some of the drugs. When the intact acrD+ allele was introduced by transforming JZM320 with plasmid pBW7, the aminoglycoside MICs returned to those for the parent strain, JC7623 (Table 1).
TABLE 1.
MICs of various antibiotics
| Medium and strain | MIC (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
| Amikacin | Gentamicin | Tobramycin | Kanamycin | Neomycin | Erythromycin | Polymyxin B | |
| LB medium | |||||||
| JC7623 (acrD+) | 3 | 6 | 6 | 12 | 25 | 200 | 0.05 |
| JZM320 (ΔacrD) | 0.8 | 1.5 | 3 | 3 | 12 | 100 | 0.025 |
| JZM320/pBW7 | 3 | 6 | 3 | 12 | 25 | 200 | 0.05 |
| Nutrient broth | |||||||
| JC7623 | 0.006 | 0.006 | 0.006 | 0.05 | 0.006 | 12 | 0.025 |
| JZM320 | 0.0008 | 0.0015 | 0.0015 | 0.006 | 0.0015 | 6 | 0.012 |
| MOPS medium | |||||||
| JC7623 | 0.2 | 0.1 | 0.05 | 0.4 | 0.1 | NDa | ND |
| JZM320 | 0.1 | 0.025 | 0.013 | 0.2 | 0.05 | ND | ND |
ND, not determined.
Aminoglycoside MICs are known to be influenced strongly by the nature of the medium used (12). When the same batch of LB medium was used, the MICs were reproducible, except that there were occasional twofold differences that are expected in twofold dilution assays. In contrast, when three lots each of Difco tryptone and Difco yeast extract were used to make LB broth, the absolute values of the gentamicin MIC for JC7623 varied up to sixfold, although the difference in MIC between JC7623 and JZM320 remained similar, four- to eightfold. (However, with one lot each of more recent Becton Dickinson-Difco ingredients, the difference between the gentamicin MICs for these two strains decreased to only twofold.) Since salts present in the medium are known to have a large influence on aminoglycoside susceptibility (3, 12), we also tested MICs in Difco nutrient broth, which contains very low concentrations of salts (only 11 mM Na+, 0.3 mM Mg2+, and 0.03 mM Ca2+, according to reference 12). In nutrient broth, both the parent and the mutant strains became much more susceptible to aminoglycosides, as reported earlier (3, 12). The absence of AcrD nevertheless produced similar changes in aminoglycoside susceptibility, making the ΔacrD mutant up to eightfold more susceptible to both amikacin and kanamycin. The addition of 5 mM MgCl2 to the nutrient broth made both strains much more resistant to aminoglycosides (data not shown), as reported earlier for E. coli and P. aeruginosa (12). Because the constituents of complex media had a not-totally-predictable influence on aminoglycoside susceptibility, we also determined the MIC in a synthetic medium, MOPS (morpholinepropanesulfonic acid) medium (15), supplemented with required amino acids at 40 μg/ml. We confirmed that the ΔacrD mutant was more susceptible to aminoglycosides in this medium also (Table 1).
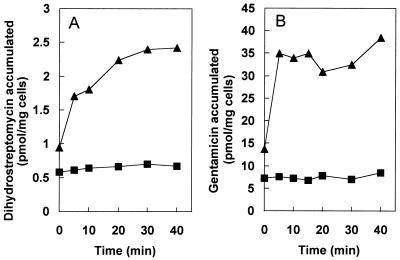
The accumulation kinetics of antibiotics, especially the steady-state level of accumulation, is a sensitive indicator of an active efflux process. Cells of JC7623 and JZM320 were grown in LB broth up to a density of about 109 cells/ml with aeration by shaking and were harvested and washed once at room temperature with a 50 mM phosphate buffer, pH 7.0, containing 1 mM MgSO4 and 0.2% (wt/vol) glucose. The washed cells were resuspended in the same buffer at a density of 2 mg (dry weight) per ml. Aminoglycoside uptake was measured by preequilibrating 0.5 ml of the cell suspension for 10 min at 30°C with shaking and then removing 50-μl samples at various times after the addition of 3H-labeled aminoglycosides. The samples were immediately diluted into 1 ml of ice-cold 0.1 M LiCl–50 mM KPO4, pH 7.0, and the mixture was filtered on a 0.45-μm-pore-size Gelman GN6 Metricel membrane filter. The filter was quickly washed with 5 ml of the ice-cold LiCl-KPO4 solution, dried, and used for determination of radioactivity in a Beckman liquid scintillation counter. The final concentrations of aminoglycosides used were 0.8 μM [3H]dihydrostreptomycin (20 Ci/mmol; American Radiolabeled Chemical, Inc., St. Louis, Mo.) and 10 μM for [3H]gentamicin (specific radioactivity, 200 mCi/g; American Radiolabeled Chemical). As shown in Fig. 1A, at 20 min the accumulation level of dihydrostreptomycin in the ΔacrD mutant strain was about four times higher than that in the isogenic parent strain. Similar differences were also observed reproducibly for gentamicin (Fig. 1B). The accumulation levels of dihydrostreptomycin and gentamicin in the ΔacrD strain (2.4 and 34 nmol/mg [dry weight], respectively) correspond to intracellular concentrations of about 0.8 and 11 μM, respectively, if we assume that 1 mg (dry weight) of E. coli cells has a volume of 3 μl (14). Interestingly, this is very close to the external concentrations of aminoglycosides used, i.e., 0.8 and 10 μM, respectively, although it does not allow much room for accumulation against a concentration gradient or for drug binding to ribosomes.
FIG. 1.
Accumulation of [3H]dihydrostreptomycin (A) and [3H]gentamicin (B) by E. coli cells resuspended in buffer containing glucose. Cells were incubated with 0.8 μM dihydrostreptomycin or 10 μM gentamicin, and the uptake of drugs by cells of JC7263 (parent) (■) and JZM 320 (ΔacrD mutant) (▴) was examined as described in the text.
With other multidrug efflux pumps, protonophores such as carbonyl cyanide m-chlorophenylhydrazone have been used to abolish the energy supply for pumps and to show an increase in the level of accumulation (reviewed in reference 16). However, when this protonophore was added to the parent strain, only a minor increase in the aminoglycoside accumulation was seen (data not shown). Similarly, a protonophore-induced increase in aminoglycoside accumulation has not been reported in previous publications dealing with aminoglycoside efflux pumps (1, 13). This is most likely due to the fact that aminoglycoside entry into the cytoplasm requires membrane potential (4, 11), which is abolished by protonophores. Nevertheless, our data suggest that the difference in aminoglycoside accumulation between the acrD+ and ΔacrD strains (Fig. 1) is due to the presence and absence of efflux rather than to differences in the drug permeability of the cytoplasmic membrane, because a slow, steadily increasing intracellular accumulation of aminoglycosides is predicted in the latter model (see Fig. 2A of reference 16), whereas low, steady-state levels of accumulation were clearly attained in acrD+ cells in our experiment (Fig. 1), as anticipated by the former model.
Aminoglycosides are usually thought to be taken up by bacterial cells in a two-phase process, with the slow, initial phase I uptake followed by a massive, rapid influx of drugs in phase II (4). Phase II uptake requires the aminoglycoside-induced inhibition of translation (and most likely misreading) (4). Since JC7263 and its acrD derivative were resistant to the action of streptomycin and dihydrostreptomycin thanks to the rpsL mutation present, the absence of phase II uptake in the Fig. 1A experiment was expected. However, because the gentamicin concentration used in the Fig. 1B experiment was similar to its MIC (in the LB medium) for the parent strain, it was unexpected that the phase II uptake was not seen. This is most probably due to the inhibition of aminoglycoside uptake by the salts present in our assay media, which contained 1 mM MgSO4 and 50 mM phosphate buffer; Mg2+ at 1 mM is known to decrease streptomycin uptake by E. coli cells by more than 95%, and even 5.7 mM phosphate buffer is sufficient to decrease the uptake by 50% (3). In fact, many of the “two-phase” uptake curves were generated by using resuspension media with exceptionally low ionic strengths (3).
Other RND-type pump genes in E. coli usually occur together with the genes for membrane fusion protein (MFP) family members, such as acrA and acrE, and this fact suggests that the transporter forms a multisubunit assembly with a member of MFP and an outer membrane channel (presumably TolC, as shown in reference 6), so that the pumped-out drug molecules do not accumulate in the periplasm (16). In contrast, the acrD gene is not associated with any gene coding for an MFP. There are at least two ways in which this genetic organization can be rationalized. (i) AcrD may become associated with a member of the MFP family and an outer membrane channel, both encoded elsewhere on the chromosome, to function as a part of the multisubunit transporter complex. (Indeed, the homologous MexY pump in P. aeruginosa is encoded by a gene located next to the gene mexX, which codes for an MFP, and these two proteins were shown to work in association with an outer membrane channel, OprM [1].) Disruption of the tolC gene, coding for an important outer membrane channel (6), or disruption of acrA did not, however, make K-12 more susceptible to the aminoglycosides listed in Table 1 (data not shown). (ii) Most lipophilic and amphiphilic drugs can cross the cytoplasmic membrane barrier spontaneously. Thus, their accumulation in the periplasm accelerates the spontaneous reentry of the drugs into the cytoplasm. This is why it is critical for their efflux systems, such as AcrAB-TolC of E. coli, to have a multisubunit construction in order to pump out the drug molecules all the way into the medium. In contrast, aminoglycosides, owing to their hydrophilic structure and the presence of multiple positive charges, do not easily cross the cytoplasmic membrane. Indeed, their uptake usually requires high levels of membrane potential (5, 11) and has been proposed by some to utilize specific transport pathways (7). Thus, accumulation in the periplasm will not be as problematic for aminoglycosides as for lipophilic agents, and the AcrD protein perhaps can function without the participation of MFP and the outer membrane channels.
Acknowledgments
D. Ma thanks J. E. Hearst for support and encouragement and M. Alberti for help in DNA sequencing.
This study was supported in part by U.S. Public Health Service grant AI-09644.
REFERENCES
- 1.Aires J R, Köhler T, Nikaido H, Plésiat P. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother. 1999;43:2624–2628. doi: 10.1128/aac.43.11.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell B D, Kadner R J. Relation of aerobiosis and ionic strength to the uptake of dihydrostreptomycin in Escherichia coli. Biochim Biophys Acta. 1980;593:1–10. doi: 10.1016/0005-2728(80)90002-x. [DOI] [PubMed] [Google Scholar]
- 4.Davis B D. Mechanism of bactericidal action of aminoglycosides. Microbiol Rev. 1987;51:341–350. doi: 10.1128/mr.51.3.341-350.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraimow H S, Greenman J B, Leviton I M, Dougherty T J, Miller M H. Tobramycin uptake in Escherichia coli is driven by either electrical potential or ATP. J Bacteriol. 1991;173:2800–2808. doi: 10.1128/jb.173.9.2800-2808.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fralick J A. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J Bacteriol. 1996;178:5803–5805. doi: 10.1128/jb.178.19.5803-5805.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashiwagi K, Tsuhako M H, Sakata K, Saisho T, Igarashi A, Pinto da Costa S O, Igarashi K. Relationship between spontaneous aminoglycoside resistance in Escherichia coli and a decrease in oligopeptide binding protein. J Bacteriol. 1998;180:5484–5488. doi: 10.1128/jb.180.20.5484-5488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma D, Cook D N, Alberti M, Hearst J E, Nikaido H. Efflux pumps and drug resistance in gram-negative bacteria. Trends Microbiol. 1994;2:489–493. doi: 10.1016/0966-842x(94)90654-8. [DOI] [PubMed] [Google Scholar]
- 9.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J Bacteriol. 1993;175:6299–6313. doi: 10.1128/jb.175.19.6299-6313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 11.Mates S M, Eisenberg E S, Mandel L J, Patel L, Kaback H R, Miller M H. Membrane potential and gentamicin uptake in Staphylococcus aureus. Proc Natl Acad Sci USA. 1982;79:6693–6697. doi: 10.1073/pnas.79.21.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medeiros A A, O'Brien T F, Wacker W E C, Yulug N F. Effect of salt concentration on the apparent in-vitro susceptibility of Pseudomonas and other Gram-negative bacilli to gentamicin. J Infect Dis. 1971;124:S59–S64. doi: 10.1093/infdis/124.supplement_1.s59. [DOI] [PubMed] [Google Scholar]
- 13.Moore R A, DeShazer D, Reckseidler S, Weissman A, Woods D E. Efflux-mediated aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob Agents Chemother. 1999;43:465–470. doi: 10.1128/aac.43.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mühlradt P F, Menzel J, Golecki J R, Speth V. Lateral mobility and surface density of lipopolysaccharide in the outer membrane of Salmonella typhimurium. Eur J Biochem. 1974;43:533–539. doi: 10.1111/j.1432-1033.1974.tb03440.x. [DOI] [PubMed] [Google Scholar]
- 15.Neidhardt F C, Bloch P L, Smith D F. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikaido H. Multiple antibiotic resistance and efflux. Curr Opin Microbiol. 1998;1:516–523. doi: 10.1016/s1369-5274(98)80083-0. [DOI] [PubMed] [Google Scholar]
- 18.Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol. 1996;178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saier M H, Jr, Tam R, Reizer A, Reizer J. Two novel families of bacterial membrane proteins concerned with nodulation, cell division and transport. Mol Microbiol. 1994;11:841–847. doi: 10.1111/j.1365-2958.1994.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 20.Thanassi D G, Cheng L W, Nikaido H. Active efflux of bile salts by Escherichia coli. J Bacteriol. 1997;179:2512–2518. doi: 10.1128/jb.179.8.2512-2518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu B, Georgopoulos C, Ang D. The essential Escherichia coli msgB gene, a multicopy suppressor of a temperature-sensitive allele of the heat shock gene grpE, is identical to dapE. J Bacteriol. 1992;174:5258–5264. doi: 10.1128/jb.174.16.5258-5264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]