Abstract
The treatment goal for patients with early stage lung cancer is cure. Multidisciplinary discussions of surgical resectability and medical operability determine the modality of definitive local treatment (surgery or radiotherapy) and associated systemic therapies to further improve the likelihood of cure. Trial evidence supports cisplatin-based adjuvant therapy either after surgical resection or concurrently with radiotherapy. Consensus guidelines support neoadjuvant chemotherapy in lieu of adjuvant chemotherapy and carboplatin-based regimens for patients who are ineligible for cisplatin. The incorporation of newer agents, now standard for patients with stage IV lung cancer, into the curative therapy paradigm has lagged owing to inefficient trial designs, the lengthy follow-up needed to assess survival end points, and a developmental focus on the advanced-stage disease setting. Surrogate end points such as pathologic response are being studied and might shorten trial durations. In 2018, the anti-PD-L1 antibody durvalumab was approved for patients with stage III lung cancer after concurrent chemoradiotherapy. Since then, the study of targeted and immunotherapies in patients with early stage lung cancer has rapidly expanded. In this Review, we present current considerations for treating patients with early stage lung cancer, and explore the current and future state of clinical research to develop systemic therapies for non-metastatic lung cancer.
TOC blurb
The authors of this Review present current considerations for treating patients with early stage lung cancer, discussing the critical determination of resectability by thoracic surgical oncologists and the management of both resectable and unresectable disease with a focus on systemic therapy selection. They also address innovations in drug development, trial design and efforts to identify early stage cancers.
Introduction
Over the past 60 years, the development of systemic therapies for non-metastatic lung cancer has been hampered by disease heterogeneity, patient comorbidities, and a lack of safe, tolerable and effective drug therapies. Compared to patients with breast or colon cancer, individuals with lung cancer tend to be older on average, and are predominantly cigarette smokers with high rates of emphysema and heart disease leading to higher all-cause mortality and debility caused by surgery (especially pneumonectomy)1. Combined, these factors reduce the tolerability of cytotoxic drugs, which have been the best option for these patients until the past decade. Further challenges in the development of systemic therapies for non-metastatic lung cancer include the fact that one-third of patients are diagnosed at stages in which multimodality therapy is indicated2, and that studies requiring an overall survival (OS) end point take decades to complete.
The demographics of patients with lung cancer have changed in the past decade3–5, with decreases in smoking prevalence, a lower stage at diagnosis, and improved pre-operative staging (using 18F-fluorodeoxyglucose (FDG)-PET and MRI of the brain). With further implementation of lung cancer screening, we hope that a stage migration to earlier stages of disease at diagnosis will also be seen. Refinements of surgical techniques have advanced such that, stage for stage, the survival of patients treated with surgery alone is steadily improving6,7, and morbidity following surgery has been reduced8,9. Similarly, radiotherapy techniques have improved, resulting in higher conformality of the radiation fields targeting the tumour and markedly decreased toxicities with intensity-modulated radiation therapy (IMRT) compared with 2D or 3D techniques10. Proton therapy and MRI-guided radiotherapy have the potential to further improve dose delivery. Systemically, the arsenal of agents used to treat metastatic NSCLC has also expanded dramatically, with the targeting of mutant oncogenes by tyrosine-kinase inhibitors (TKIs) and the use of monoclonal antibodies to block immune checkpoints11. A multitude of agents are now approved by the FDA for the treatment of patients with advanced-stage lung cancers (Fig. 1). This large number of options contrasts starkly with the limited number of drug indications in the guidelines for non-metastatic NSCLC. The successes of newer therapies in the advanced-stage setting, including patients without disease progression ≥5 years after diagnosis12, presents the extraordinary opportunity to expand the use of these agents to personalize care for individuals with non-metastatic cancers to enhance their chances of remaining relapse free from the lung cancer being treated and, ultimately, cured.
Fig. 1 |. Treatment of metastatic and non-metastatic NSCLC.
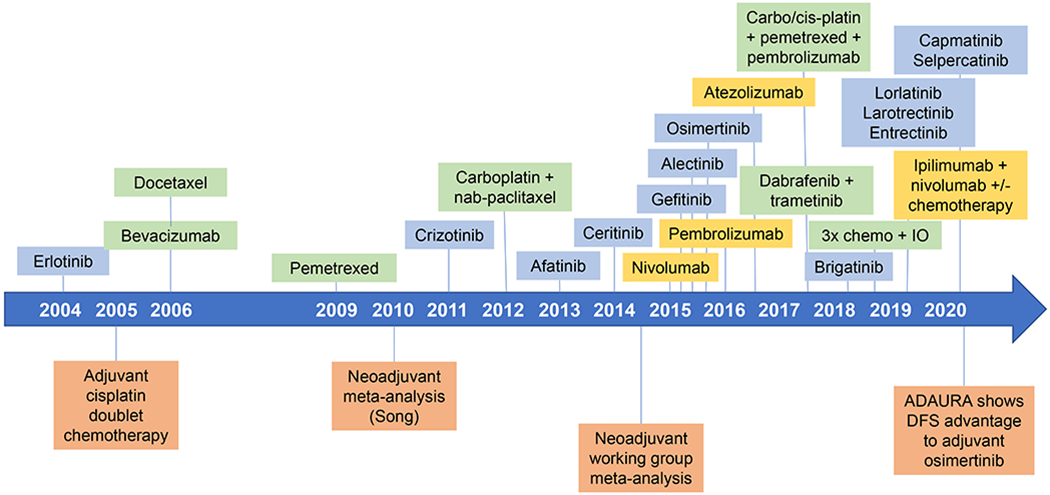
Timeline showing drugs approved or indicated for the treatment of metastatic and non-metastatic non-small cell lung cancer (NSCLC) as of December 2020. When several approvals were made in a year, they are arranged chronologically from top to bottom.
This Review explores the essential considerations for the management of patients with non-metastatic NSCLC. We discuss the critical determination of resectability by thoracic surgical oncologists and the management of both resectable and unresectable disease with a focus on how systemic therapy selection has changed. Finally, we present how innovations in drug development, trial design and efforts to identify early stage cancers through lung cancer screening have reinvigorated a historically barren research landscape and prompted clinicians to re-imagine the care of patients with potentially curable lung cancer.
Surgical considerations
The thoracic surgeon’s decision to offer surgery in the management of non-metastatic NSCLC depends on both the tumour extent and its relationship to surrounding structures (technical resectability) and the extent of the surgery needed to completely remove the tumour in the context of patient fitness (medical operability). If the tumour is not completely resectable or the patient is not medically operable, the patient is referred for definitive non-surgical management with radiotherapy, as discussed in the next section.
Technical resectability
Technical resectability is assessed by the thoracic surgical oncologist who evaluates imaging to determine whether complete resection of the tumour and regional lymph nodes (R0 resection) is possible. Resectability of stage I and II tumours is usually defined by review of preoperative imaging. By contrast, the criteria for resectability of stage III tumours are more complex, defined by tumour characteristics (size or invasion) or the extent of N2 nodal involvement and relationship of the tumour to the airways and vasculature. Tumour characteristics, for example invasion of the vertebral bodies or brachial plexus, sometimes require involvement of a neurosurgeon as part of the multidisciplinary team. Nodal involvement can be estimated through the use of non-invasive imaging such as CT and PET, but is more accurately determined by invasive mediastinal staging using endobronchial ultrasound13 and mediastinoscopy. The number and location of lymph nodes that enter into the resectability decision varies by region, centre and surgeon. General agreement exists that confluent bulky lymph nodes should not be considered as resectable, whereas a single station involvement is accepted to be resectable in all guidelines11,14,15. No consensus exists on the number of involved mediastinal lymph node stations that make an R0 resection unlikely. In some centres, studies have shown promise using neoadjuvant cytotoxic chemotherapy to induce conversion of borderline resectable NSCLC to resectable NSCLC; however, this approach is not considered standard16,17. We anticipate that such an approach will gain momentum as regimens with improved efficacy are moved into the non-metastatic setting.
Medical operability
Medical operability is generally determined by cardiac and pulmonary fitness. Cardiac fitness is required for general anaesthesia and the stresses of surgery and postoperative recovery. Pulmonary fitness is defined by pulmonary function testing18, including diffusion capacity19,20, ventilation–perfusion imaging, and, at times, cardiopulmonary exercise testing21,22. Poor pulmonary function can prohibit a surgery, although occasionally, borderline pulmonary function can remain stable or improve postoperatively if a volume reduction following surgery positively affects respiratory mechanics.
Both technical resectability and medical operability should be determined by a multidisciplinary team at the time of diagnosis and staging to assign a treatment plan. If the tumour grows through neoadjuvant therapy or the patient has interval medical events or complications of therapy, both resectability and operability might need to be redetermined after completion of neoadjuvant therapy.
Unresectable or inoperable disease
For most patients deemed to have medically inoperable stage I and II, node-negative NSCLC, standard therapy is definitive stereotactic body radiation therapy (SBRT; also known as stereotactic ablative body radiation) to a biologically effective dose ≥100 Gy11. Rarely, SBRT is not possible in patients with large primary tumours, central tumours and some other conditions, such as interstitial lung disease, for which the risks of SBRT are unknown. With this approach, long-term local control rates of the treated primary tumour are 90–95%, with nodal and systemic progression-free survival (PFS) of 70–80%23–26. Systemic therapy can be difficult to administer to these patients owing to frailty, age, and the medical comorbidities that rendered them inoperable. Although patients with larger or more FDG-avid tumours are at increased risk of intrathoracic, nodal and systemic disease progression, the role of systemic anticancer therapy in these patients has not been defined27–29.
Concurrent chemotherapy and radiation therapy
Definitive concurrent chemotherapy and radiation therapy (cCRT; typically to a total dose of 60–66 Gy in 30–33 fractions) followed by 1 year of durvalumab is the standard of care for patients with unresectable, locally advanced, node-positive (stage IIB–IIIC) NSCLC11,30,31. Prior to 2018, the standard of care for patients with unresectable locally advanced-stage NSCLC was treatment with cCRT to a total radiation dose of 60 Gy in 30 fractions32, although these patients had poor outcomes largely driven by poor distant control32. Multiple failed attempts were made to improve upon this regimen with intensification of systemic therapy and escalation of the radiation dose33–35. RTOG 0617 explored whether 74 Gy in 37 fractions was superior to the standard dose of 60 Gy in 30 fractions, but treatment with 74 Gy was associated with reduced OS and poorer local control36,37. The addition of cetuximab to weekly carboplatin and paclitaxel only added toxic effects. Cisplatin and pemetrexed were also tested. In a study published in 201638, cisplatin and pemetrexed did not improve OS, however this regimen is often adopted in clinical practice owing to the milder adverse effects of this combination relative to cisplatin and etoposide11,38.
Immunotherapy
The PACIFIC trial established a new standard of care by investigating the addition of durvalumab (an anti-PD-L1 monoclonal antibody) following cCRT30, consisting of at least two cycles of platinum-based doublet chemotherapy delivered concurrently with definitive radiotherapy to a total dose of 54–66 Gy. Patients without disease progression after cCRT were randomly assigned 2:1 to receive either durvalumab or placebo. Durvalumab was initiated within 42 days from the end of cCRT. Durvalumab improved median PFS by 11 months31 and median OS by 18.4 months39. The study was stratified by patient age, sex, and smoking history, but not tumour PD-L1 expression. Preplanned subset analyses did include PD-L1 expression at a 25% threshold, with benefit seen in both subsets. However, an unplanned retrospective analysis found that durvalumab did not improve OS in a subgroup with PD-L1 expression of <1%40. Grade 3 or 4 pneumonitis rates were comparable between the study arms31. A broad exploration of intrathoracic versus extrathoracic failures demonstrated that consolidative durvalumab reduced the sites of first progression in both intrathoracic and extrathoracic sites compared with placebo41. Interestingly, first progression most commonly occurred in intrathoracic sites; historically, the greatest risk in patients treated for locoregional disease has been distant metastatic failure. The observation of common intrathoracic progression in the setting of improved systemic control begs the question of whether optimized intrathoracic tumour control by optimized radiation plans or surgery in resectable cases might be able to further improve outcomes.
Building on the success of immunotherapy following cCRT, multiple efforts are taking place to expand the role of immunotherapy in unresectable NSCLC. Even patients with stage I NSCLC remain at a 20–30% risk of progression and death8. Studies have been initiated to add adjuvant immunotherapy after definitive SBRT for node-negative stage I NSCLC. Some studies are focusing on patients with larger tumours (≥2 cm diameter) or higher standardized uptake values (≥6.2)42; others include patients with broader criteria43. Trials are also examining the duration of adjuvant immunotherapy (up to 6 months in SWOG S191444 versus 24 months in PACIFIC-443), initiation of immunotherapy relative to SBRT (two cycles before SBRT in SWOG S191444 versus after SBRT in PACIFIC-443), placebo control arm (only PACIFIC-4), as well as type and dosing of immunotherapy agents.
PACIFIC-2 (REF.45) is a randomized, double-blind, phase III trial investigating the use of concurrent durvalumab (1,500 mg) every 4 weeks with cCRT compared to placebo and cCRT. This study is enrolling patients prior to cCRT and will thus provide much needed detail on the chemotherapy and radiotherapy parts absent from the PACIFIC trial. The comparator arm of this trial is cCRT alone, rather than the current standard of care of cCRT followed by consolidative durvalumab. Dual immune-checkpoint inhibition with ipilimumab plus nivolumab with cCRT is being explored and compared to nivolumab with cCRT or durvalumab with cCRT in the phase III trial CheckMate73L (REF.46).
The search continues to develop therapies for patients who are not candidates for cCRT because of comorbidities precluding them from undergoing cCRT, including frailty or tumour extent. These patients are typically treated with sequential CRT or RT alone. PACIFIC-647 is a phase II study investigating the addition of durvalumab after sequential CRT in this patient subgroup.
Although the studies so far have focused on immunotherapy, efforts are being made to add targeted therapy after definitive cCRT. The LAURA48 study is the first to prescribe adjuvant osimertinib or placebo to patients with EGFR-mutated NSCLC following cCRT.
Lastly, improvements in radiotherapy techniques will likely further decrease radiation-related toxicities, a concern when radiation is combined with novel systemic therapies. Radiation doses to the lungs49,50, heart37,51, great vessels52, oesophagus50,53,54, and circulating lymphocytes55–58 have been correlated with the risks of pneumonitis, esophagitis and decreased survival. Proton therapy offers considerable potential in minimizing doses to at-risk organs, especially in patients with extensive disease involvement (for example, of the bilateral mediastinum, supraclavicular fossae or lower lobes of the lungs (Fig. 2)). RTOG 1308 (REF.59) is a phase III trial comparing photon and proton radiotherapy as part of cCRT with durvalumab.
Fig. 2 |. Graphic depiction of a definitive proton radiotherapy dose distribution in a patient with stage IIIC non-small cell lung cancer (NSCLC).
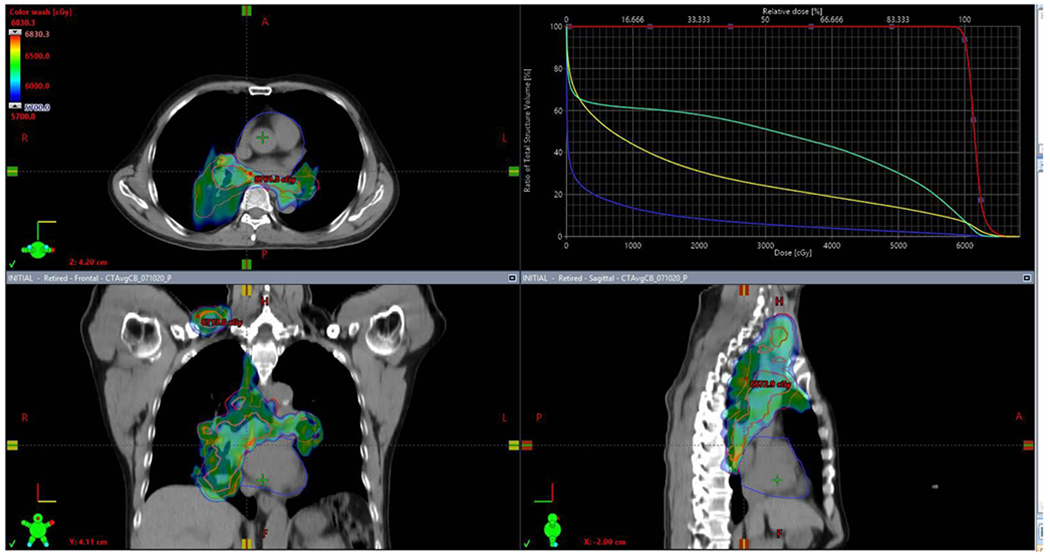
On the axial (upper left), coronal (lower left) and sagittal (lower right) views of the radiotherapy plan, the red target delineation represents the gross tumour volume extending from the primary tumour in the medial right lower lobe to the bilateral hilar areas, mediastinum and right supraclavicular fossa. The dose–volume histogram (upper right) represents the radiation dose delivered to each structure of interest; it demonstrates that the gross tumour volume (red) receives close to 100% of the intended radiation dose with a steep dose fall-off to the oesophagus (green), bilateral lungs combined (yellow) and the heart (blue). Courtesy of Dr A.F. Shepherd, Memorial Sloan Kettering Cancer Center, New York, USA.
Adjuvant therapy for resected NSCLC
More than 50% of patients experience recurrence after surgery alone8. In patients with resected disease, the most accurate prognostic factor is pathologic stage8. The best way to risk-stratify a lung cancer population prior to enrollment in an adjuvant drug trial is pathologic assessment of the resection specimen and pathologic stage, the gold standard. Using surgical pathology and pathologic stage, an accurate assessment of the risk of recurrence is possible60,61. Adjuvant therapy is prescribed based on stage and patient fitness. The use of additional predictive biomarker testing for the precise assignment of adjuvant therapy has yet to make its way into routine clinical practice, although we anticipate routine testing for EGFR mutations based on the recently reported results of a trial of adjuvant osimertinib62.
Chemotherapy
Following successful surgery, randomized controlled trials of patients with stages I–III NSCLC have demonstrated that postoperative, cisplatin-based therapy significantly reduces the risk of death, especially in stage II and III disease63. The efficacy of cytotoxic therapy has been established by two fundamental approaches: 1) intense, toxicity-limited, two-drug, cisplatin-based combinations delivered intravenously over a few months to eliminate micro-metastatic disease64; or 2) prolonged 1–2 year delivery of oral antimetabolites (uracil–tegafur) to suppress cancer growth65. Dose-intense cytotoxic approaches are limited by issues of tolerance and safety. Clear evidence exists that benefits are often outweighed by harms, or inability to deliver the planned therapy. For example, clinical trials using cytotoxic three-drug regimens or in which high numbers of patients also received postoperative radiotherapy (PORT) were unable to demonstrate benefit, whereas studies that used lower-dose cisplatin-based doublet regimens in patients without N2 disease were successful66,67. Although PORT does not interfere with the benefit of cytotoxic chemotherapy68, data from 2020 raise concerns that PORT can increase the risk of non-cancer death69. Of note, highly toxic adjuvant chemotherapies also increase the risk of non-cancer death, and are clearly more harmful than helpful for patients with low-risk (stage IA) cancers64,70. By contrast, prolonged treatment with antimetabolites is well-tolerated and can be effective, even for patients with stage I disease71,72. However, clinical trials demonstrating the benefit of prolonged therapy with uracil–tegafur have not been replicated outside of Japan owing to issues of drug availability and practice preferences in the rest of the world.
The current approach to adjuvant cytotoxic chemotherapy is to take into account a combination of pathologic stage and a medical assessment of the patient, balance the benefits and harms, and proceed with treatment as a shared decision with individuals who are willing to take on additional adverse effects and risk. For example, meta-analyses have shown that four cycles of cisplatin-based, two-drug combinations lower the risk of death by 20% (relative risk reduction) compared with surgery alone in patients with pathologic stage II and III NSCLC64. Given that the risk of cancer-related death in this population is high with surgery alone (50–80%), the absolute risk reduction is also high (10–16%)64. This absolute risk reduction is balanced against the risks associated with cytotoxic chemotherapy as adjuvant therapy after complete surgical resection: death (1%, typically related to neutropenic sepsis)74, permanent hearing loss or kidney damage (3–5%), nausea (common) and fatigue (nearly universal)73–75. Advanced patient age does not predict benefit from cisplatin but does predict increased risk of treatment-related toxicity76. As the age of patients with newly diagnosed NSCLC is increasing, cisplatin-based therapy is often not an acceptable option.
The benefit of adjuvant cisplatin doublets with etoposide or vinorelbine took decades to establish34,64,67. To avoid multi-decade trials in the United States, expert consensus alone has been sufficient to establish newer regimens combining cisplatin with pemetrexed, gemcitabine or docetaxel.11 In Japan, a clinical trial of cisplatin with pemetrexed versus vinorelbine in patients with resected lung adenocarcinoma was done for regulatory approval of pemetrexed in this setting. The study failed to show superiority of pemetrexed but confirmed a reduced toxicity profile77. US guidelines recommend pemetrexed in this setting11. Also backed by expert consensus is the common practice to use carboplatin instead of cisplatin in patients with medical contraindications to cisplatin, such as hearing loss or renal insufficiency78. Adding drugs with different mechanisms of action, such as angiogenesis inhibitors and cancer antigen-targeted vaccines, to cisplatin-based adjuvant treatments has not improved outcomes in prospective studies73,79. However, numerous new drugs with activity in the treatment of advanced-stage NSCLC are worthy of study (Fig. 1).
Targeted or immunotherapy
In metastatic NSCLC, cytotoxic chemotherapy has been replaced with targeted therapy or immunotherapy in biomarker-selected populations80,81. However, evidence is currently lacking to replace chemotherapy in the curative adjuvant setting82. Incorporation of platinum-based therapy has been either a mandate or an option in most trials looking to register new drugs in the adjuvant setting in non-metastatic NSCLC (Table 1). None of these adjuvant studies yet has mature data. However, the ADAURA study, in which patients with completely resected NSCLC received adjuvant osimertinib or placebo for 3 years after completion of standard of care adjuvant chemotherapy, reported a striking improvement in disease-free survival (DFS) (hazard ratio (HR) 0.17; 95% CI 0.12–0.23) in patients with resected stage II–III EGFR-mutated NSCLC62. ADAURA is the first biomarker-selected adjuvant study anticipated to change the standard of care in resected lung cancer. The results of the other listed adjuvant TKI and immunotherapy studies are eagerly anticipated.
Table 1 |.
Ongoing phase III studies of adjuvant therapy in non-metastatic non-small cell lung cancer.
| Drug | Comparator group | n | Biomarker tested | Biomarker selected | Chemotherapy | End point | Refs |
|---|---|---|---|---|---|---|---|
| Crizotinib | Observation | 168 | ALK fusion | Yes | SOC | OS | NCT02201992 (REF.132) |
| Erlotinib | Observation | 450 | EGFR mutation | Yes | SOC | OS | NCT02193282 (REF.133) |
| Osimertinib | Placebo | 688 | EGFR mutation | Yes | SOC | DFS | NCT02511106 (REF.62) |
| Nivolumab | Observation | 905 | PD-L1 positivity | No | SOC | DFS and OS in all patients DFS in patients with high PD-L1 (≥50% staining) |
NCT02595944 (REF.134) |
| Pembrolizumab | Placebo | 1,177 | PD-L1 positivity | No | SOC | DFS | NCT02504372 (REF.135) |
| Atezolizumab | Observation | 1,280 | PD-L1 positivity | No | Cisplatin doublet | DFS in all patients (including PD-L1 subgroup)a | NCT02486718 (REF.136) |
| Durvalumab | Placebo | 1,360 | PD-L1 positivity | No | SOC | DFS in patients with PD-L1 ≥25% in tumour cells | NCT02273375 (REF.137) |
| Durvalumab | Placebo | 332 | ctDNA | Yes | Platinum doublet | DFS | NCT04385368 (REF.129) |
| Canakinumab | Placebo | 1,500 | None | No | SOC | DFS | NCT03447769 (REF.138) |
Further details not provided.
ctDNA, circulating tumour DNA; DFS, disease-free survival; OS, overall survival; PD-L1, programmed death ligand 1; SOC, standard of care.
Neoadjuvant therapy
Chemotherapy
In 2005, the survival results of the decade-long adjuvant studies presented above67,83 led to early closure of concurrently running neoadjuvant trials because these trials did not include an adjuvant component and adjuvant chemotherapy was the new standard of care. Therefore, few completed phase III studies are available to guide neoadjuvant therapy. Results from meta-analyses evaluating the use of neoadjuvant70 or adjuvant64 platinum doublet chemotherapy in patients with resectable stage IB–IIIA NSCLC concluded that both approaches yield an absolute benefit in 5-year OS of approximately 5% (HR 0.87 for pooled neoadjuvant studies; HR 0.89 for the adjuvant studies; both hazard ratios are for therapy versus surgery alone). As a result, the guidelines support the use of neoadjuvant platinum-based chemotherapy in patients with clinical stages that would merit adjuvant chemotherapy11. Neoadjuvant treatment offers several advantages over adjuvant therapy, including improved patient tolerance prior to surgery84, tumour downstaging85, an earlier opportunity to eradicate micrometastases, and more rapid assessment of therapeutic efficacy either before surgery with scans or at the time of resection86. Neoadjuvant approaches have the additional advantage of permitting a change in systemic treatment either preoperatively based on imaging results87 or postoperatively, based on pathologic assessment of the resection specimen. Neoadjuvant therapy also provides an opportunity to evaluate surrogate markers of clinical efficacy that might correlate with improved survival88.
Immunotherapy
Immune-checkpoint inhibitors targeting CTLA4, PD-1 and its ligand PD-L1 have changed the treatment landscape for patients with advanced-stage NSCLC89. Immunotherapy in the neoadjuvant setting has the hypothesized advantage of priming an anti-tumour response and imparting immunological memory early in the disease process90. This strategy provides unprecedented opportunities to advance care and for translational work. Elevated neoantigen burden and reduced neoantigen heterogeneity are associated with longer survival in patients with early stage NSCLC91, suggesting that the neoadjuvant setting might represent the optimal time point to achieve the maximal clinical benefit of immunotherapy. Two doses of neoadjuvant nivolumab (anti-PD-1) produced a 45% complete or major (≤10% viable tumour cells) pathologic response (MPR) rate in 20 resected tumours92. In the multicenter Lung Cancer Consortium 3 (LCMC3) study93, neoadjuvant atezolizumab (anti-PD-L1) produced a 19% MPR rate94, comparable to cisplatin-based neoadjuvant chemotherapy in earlier studies. In 2020, a neoadjuvant study evaluating the PD-1 inhibitor, sintilimab, in 37 Chinese patients with resectable NSCLC reported an MPR rate of 41%95. While the results of all of the aforemented neoadjuvant immunotherapy trials are intriguing, few pathologic complete responses (pCRs) were seen and no robust predictive biomarker for response was identified. Reported results of the phase II randomized NEOSTAR study evaluating neoadjuvant nivolumab or nivolumab plus ipilimumab in patients with resectable NSCLC reported a 38% MPR rate with the combination regimen in 21 treated patients, most of these pCRs96.
Combination immunotherapy and chemotherapy
Combining immunotherapy with chemotherapy in the perioperative setting might further increase clinical efficacy. Indeed, chemotherapy might synergize with immunotherapy by killing tumour cells, improving the T cell:cancer cell ratio, reducing immunosuppressive substances released by the tumour, and releasing antigens for presentation, thereby expanding the anti-tumour response97. Chemotherapy also stimulates PD-L1 expression in NSCLC98. The addition of immunotherapy to chemotherapy improved outcomes of patients with metastatic NSCLC89,99–101, encouraging the investigation of the combination strategy in the preoperative setting. In resectable NSCLC, atezolizumab plus carboplatin and nab-paclitaxel produced an MPR in 57% (95% CI 37–75%), including pCRs in 33% (95% CI 19–51%) of 30 patients102. In the phase II NADIM study, neoadjuvant nivolumab plus carboplatin and paclitaxel induced MPR in 34 (83%; 95% CI 68–93%) of 41 patients with resected stage IIIA (N2) NSCLC, of whom 26 (63%; 95% CI 62–91%) had a pCR103. The 18-month OS in NADIM was 93.5%103. The SAKK single-arm study of durvalumab and chemotherapy in patients with stage IIIA (N2) NSCLC showed comparable pathologic remission rate results104. The results of these studies have prompted multiple randomized phase III studies of neoadjuvant chemotherapy with or without immunotherapy (Table 2). We anticipate the readout of pathologic end points to some of these studies within the next year105.
Table 2 |.
Ongoing phase III studies of neoadjuvant therapy in non-metastatic non-small cell lung cancer.
| Neoadjuvant | Control | n | Adjuvant | End points | Biomarker | Refs |
|---|---|---|---|---|---|---|
| Platinum doublet + durvalumab | Platinum doublet + placebo | 800 | Durvalumab versus placebo | MPR EFS |
PD-L1 positivity | NCT03800134 (REF.139) |
| Platinum doublet + atezolizumab | Platinum doublet + placebo | 450 | Atezolizumab versus placebo | EFS MPR |
PD-L1 positivity | NCT03456063 (REF.140) |
| Cisplatin doublet + pembrolizumab | Cisplatin doublet + placebo | 786 | Pembrolizumab versus placebo | EFS OS |
PD-L1 positivity | NCT03425643 (REF.141) |
| Ipilimumab + nivolumab or chemotherapy + nivolumab | Chemotherapy | 350 | None | EFS pCR |
PD-L1 positivity | NCT02998528 (REF.142) |
| Osimertinib or osimertinib + chemotherapy | Chemotherapy | 351 | Not specified | MPR | EGFR mutation | NCT04351555 (REF.108) |
EFS, event-free survival; MPR, major pathologic response; pCR, pathologic complete response; OS, overall survival.
In advanced-stage disease, biomarker-matched molecular therapies are better tolerated than chemotherapy and can produce response rates that exceed 50%106. Initial studies of neoadjuvant targeted therapies are promising. The EMERGING/CTONG1103 trial demonstrated that neoadjuvant erlotinib produced an MPR in 10% of patients with stage IIIA-N2 EGFR-mutated NSCLC; this result compares with a 0% MPR in the neoadjuvant chemotherapy arm107. The NeoADAURA phase III trial comparing neoadjuvant osimertinib versus chemotherapy versus the combination is planned for patients with EGFR-mutated lung cancer108. Finally, the Lung Cancer Mutation Consortium has proposed an umbrella trial for resectable (stages IB–IIIB) NSCLC that will identify 10 actionable oncogenic drivers at diagnosis and match patients with corresponding neoadjuvant targeted therapies86.
Clinically, neoadjuvant therapy has many advantages over adjuvant therapy to both the patient and the treating clinician. The results of the neoadjuvant therapy studies suggest that their promise will be realized for correlative science as well. We are hopeful that the results of the phase III neoadjuvant studies in progress will demonstrate both the theoretical advantage of improved efficacy to immunotherapy with the tumour in situ and the prospect of earlier trial readouts based on pathologic end points that serve as surrogates for clinical outcomes.
Trimodality therapy
Trimodality therapy in resectable NSCLC refers to the use of systemic therapy, radiotherapy and surgery. To date, no level 1 evidence exists to support trimodality therapy in most patients with resectable NSCLC. Three scenarios remain in which trimodality therapy is considered: induction chemoradiotherapy for superior sulcus tumours, PORT for resected N2 disease, and induction chemoradiotherapy for resectable N2 disease11.
Induction chemoradiotherapy
Induction chemoradiotherapy followed by surgery and adjuvant therapy for tumours of the superior sulcus (Pancoast tumours) remains a standard of care based on the findings of the Intergroup Trial 0160, a single-arm study that showed superior outcomes in patients who received induction cisplatin plus etoposide plus radiotherapy to 45 Gy compared to historic controls of surgery or radiotherapy alone109,110. With the identification of more effective and better tolerated systemic therapies, some now advocate for induction chemotherapy only in patients with Pancoast tumours, reserving radiotherapy for the postoperative setting where persistent or bulky disease can be more precisely targeted111.
Induction chemoradiotherapy for patients with resectable N2 disease without Pancoast tumours remains a therapeutic option in the NCCN guidelines and is considered a standard of care by many of the NCCN institutions11. The accumulated data shows that preoperative chemoradiotherapy is associated with more toxicity and no improvement in resection rates or survival over induction chemotherapy alone112,113.
Postoperative radiotherapy (PORT)
PORT to the mediastinum for patients with resected NSCLC involving the N2 lymph nodes has been considered a therapeutic option based on findings from multiple database studies and subgroup analyses of adjuvant chemotherapy treatment trials114–117. These studies show a clear improvement in local control and a 5% improvement in OS with PORT to 50–54 Gy in patients with completely resected N2 disease. The results of the phase III Lung ART study, a prospective, randomized trial of PORT were presented at the 2020 ESMO Congress69. The study was designed to show a 10% improvement in DFS at 3 years. This study failed to meet its prespecified primary end points, which was not surprising as retrospective studies have shown only a 5% survival advantage at 5 years. An increase in non-cancer-related deaths in the radiation arm of the Lung ART study again raise doubts about the overall benefit of trimodality therapy69.
Trial design
The pace of progress in early stage resectable NSCLC has been slowed by the long time required for data maturity when drugs are studied in the adjuvant setting. Although surgical pathology and surgical staging enables accurate study inclusion and stratification, the duration of follow-up necessary for clinical outcomes is nearly a decade long, as illustrated by the National Clinical Trial Network’s ALCHEMIST study platform evaluating adjuvant erlotinib for patients with resected EGFR-mutated NSCLC and adjuvant crizotinib for patients with resected ALK-rearranged NSCLC118. These trials of TKIs opened in 2014 and neither is fully enrolled, likely owing to the rarity of patient populations in the United States, a lack of routine clinical biomarker testing, and the toxicity profiles of the drugs. Both drugs are no longer the agents of choice for their respective diseases and, already, another placebo-controlled trial of adjuvant osimertinib has demonstrated a substantial benefit in DFS with very few treatment discontinuations as a result of toxicity62, making the ALCHEMIST outcomes related to targeted therapy, once available, of questionable relevance. The rapid pace of advancements in systemic therapy in metastatic NSCLC (Fig. 1) cannot be reasonably replicated in the adjuvant space. Two trial paradigms might accelerate progress. The first is neoadjuvant investigation with evaluation of pathologic end points as surrogates for later clinical end points. The second is utilization of predictive biomarkers for risk enrichment to select the adjuvant population at highest risk — the group most in need of better adjuvant therapies and most likely to provide a demonstration of benefit with the fewest patients in the shortest time.
Surrogate end points
Surrogate end points are objective and reproducible measures that result from study intervention. As per regulatory authorities in the US, accelerated approval can be considered based on a surrogate end point that is reasonably likely to predict the traditional clinical end point119. DFS has been statistically validated as a surrogate end point for OS in a meta-analysis of trials of surgery or radiotherapy and chemotherapy in patients with non-metastatic NSCLC120. Whether this association is valid when evaluating mechanistically distinct drugs such as immunotherapies or targeted therapies is unclear. The controversy of using DFS as an appropriate end point has been reignited by the ADAURA data, which showed that patients treated with osimertinib had marked improvement in DFS compared with patients receiving placebo62; however, a co-primary OS end point was not included in the trial. Thus, whether TKIs will improve cure rate or just delay recurrence is unknown. The lack of data on whether the DSF advantage with adjuvant TKIs will translate into an OS advantage has sparked sharp debate over the use of osimertinib in the adjuvant setting, and many skeptic investigators await longer follow-up and the secondary OS end point results.
Pathologic response can also be used as a surrogate end point with neoadjuvant systemic therapy. Owing to its rarity after treatment with chemotherapy or single-agent immunotherapy, the use of pCR as a surrogate end point after induction cisplatin-doublet chemotherapy followed by surgery in patients with non-metastatic NSCLC was not useful121. A more clinically relevant frequency of events was seen when the potential surrogate event was defined as MPR (≤10% viable tumour cells). MPR to neoadjuvant chemotherapy occurred in 19% of patients, and OS and DFS were prolonged in patients who achieved MPR compared with patients who had >10% viable tumour cells after neoadjuvant chemotherapy (5-year OS 85% versus 40%, P <0.0001; 5-year DFS 78% versus 35%, P <0.001)121. The positive association between MPR and clinical outcomes in patients with resectable NSCLC has been reproduced in phase II studies of neoadjuvant chemotherapy122 and chemotherapy plus anti-angiogenic therapies123,124, which illustrates how MPR facilitates the rapid evaluation of neoadjuvant therapies88. Consequently, MPR has been proposed and adopted as an end point of interest in many neoadjuvant clinical trials in patients with resectable NSCLC, including those evaluating immunotherapies125. Furthermore, a standardized approach for MPR determination by pathologists across studies has been described86,126. However, prospective validation of pCR or MPR as a surrogate end point in lung cancer is pending completion of several studies (Table 2).
Most immunotherapy adjuvant studies in completely resected NSCLC (Tables 1 and 2) have completed enrollment, and the current trial landscape in early stage NSCLC has moved towards neoadjuvant investigation. However, many patients are pathologically upstaged at the time of surgery and adjuvant investigation remains important. Studies have demonstrated that the presence of circulating tumour DNA (ctDNA) following definitive treatment is associated with a high risk of disease recurrence127. Next-generation study proposals have been made to use this prognostic factor to select a high-risk patient population for adjuvant investigation128. NCT04385368 (Table 1, REF.129) is the first phase III study to select a high-risk population of patients with completely resected non-metastatic NSCLC based on the persistence of ctDNA after surgery. This strategy offers escalation of care in patients at increased risk of recurrence and can enable clinical end points to be reached more quickly.
Conclusions
Systemic therapy for non-metastatic lung cancer continues to evolve. The durvalumab trial following cCRT in patients with unresectable lung cancer30,40 was the first of many studies expected to alter routine care and improve the prognosis of individuals with local but still potentially lethal disease. We are now seeing biomarker testing of resected specimens and adjuvant osimertinib enter the clinic for patients with resected EGFR-mutated tumours62. However, the attitudes of the thoracic oncology community must evolve as have our therapies. We must revisit our opinions that the current survival rates for any patients with early stage lung cancer are acceptable8. Innovative clinical trials are needed to bring the personalized advances (Figure 1), which are now routinely applied to patients with stage IV disease, to patients with curable lung cancers.
We know that the earliest targeted therapy approvals for NSCLC have been associated with improvement in survival rates for patients with stage IV lung cancer diagnosed between 2013 and 2016130. Immunotherapy is poised to further alter these survival expectations. The important questions about the incorporation of immunotherapy and targeted therapy into the non-metastatic setting will focus on whether drugs should be given in sequence or combination, whether neoadjuvant or adjuvant therapy is preferable, and the optimal duration of therapy. Over time, the survival curves for all patients should continue to shift upwards. Our experiences with new therapies for individuals with metastatic lung cancers assure us that it will.
Key points.
Cisplatin-based adjuvant chemotherapy remains the standard of care for patients with resected high-risk non-metastatic non-small cell lung cancer (NSCLC).
Anti-PD-L1 therapy with durvalumab after concurrent chemotherapy and radiotherapy for unresectable or inoperable non-metastatic NSCLC improves overall survival.
Osimertinib for 3 years after standard adjuvant therapy improves disease-free survival in patients with NSCLC harbouring EGFR mutations.
Immunotherapy is being extensively studied in the preoperative and postoperative settings.
Novel clinical trial designs are needed to accelerate advances in the treatment of patients with curable NSCLC.
Acknowledgements
We would like to thank Clare Wilhelm for critically reading the manuscript and for editorial contributions, and Annemarie F. Shepherd for contributions to Figure 2. The work of the authors is supported in part by an NIH grant P30 CA008748 to Memorial Sloan Kettering Cancer Center. T.C. is the recipient of an ASCO Career Development Award and the MDACC P30 CA016672 award.
Competing interests
J.E.C. reports consulting fees from AstraZeneca, Bristol–Myers Squibb, Flame Biosciences, Genentech, Merck and Novartis; and clinical research funding to Memorial Sloan Kettering Cancer Center from AstraZeneca, Bristol–Myers Squibb, Genentech and Merck. A.R. reports grants from Boehringer Ingelheim, Pfizer and Varian Medical Systems; grants and personal fees from AstraZeneca and Merck; personal fees from Cybrexa, More Health and Research to Practice; and non-financial support from Philips–Elekta, outside the submitted work. W.W. reports advisory board and speaker fees from AstraZeneca, and teaching grant and speaker fees from Covidien–Medtronic. M.G.K. reports personal fees from AstraZeneca, Daiichi–Sankyo, Pfizer and Regeneron, from outside the submitted work; and honoraria for participation in educational programmes from AstraZeneca, AXIS, Carvive Systems, Creative Educational Concepts, i3 Health, Intellisphere, OncLive, Paradigm Medical Communications, Peerview, Physicians Education Resources, Prime Oncology, Research to Practice and WebMD. Funds for travel and lodging, and food and beverage have been provided by AstraZeneca, Genentech, Pfizer and Regeneron. M.G.K. is an employee of Memorial Sloan Kettering. Memorial Sloan Kettering has received research funding from Genentech Roche, the Lung Cancer Research Foundation, the National Cancer Institute (USA) and PUMA Biotechnology for research conducted by M.G.K. Memorial Sloan Kettering has licensed testing for EGFR T790M to MolecularMD. T.C. reports consulting fees from Bristol–Myers Squibb and MedImmune–AstraZeneca, advisory role fees from Bristol–Myers Squibb and EMD Serono, and clinical research funding to MD Anderson Cancer Center from Boehringer Ingelheim, Bristol–Myers Squibb, EMD Serono and MedImmune–AstraZeneca. C.G.A. declares no competing interests.
References
- 1.Shapiro M et al. Predictors of major morbidity and mortality after pneumonectomy utilizing the Society for Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 90, 927–934; discussion 934–925, doi: 10.1016/j.athoracsur.2010.05.041 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD & Jemal A Cancer statistics, 2020. CA: a cancer journal for clinicians 70, 7–30, doi: 10.3322/caac.21590 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Bade BC & Dela Cruz CS Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clinics in chest medicine 41, 1–24, doi: 10.1016/j.ccm.2019.10.001 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Fischer B et al. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med 361, 32–39, doi: 10.1056/NEJMoa0900043 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Fuchs HE & Jemal A Cancer Statistics, 2021. CA: a cancer journal for clinicians 71, 7–33, doi: 10.3322/caac.21654 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Pagès PB et al. Impact of video-assisted thoracic surgery approach on postoperative mortality after lobectomy in octogenarians. The Journal of thoracic and cardiovascular surgery 157, 1660–1667, doi: 10.1016/j.jtcvs.2018.11.098 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Hristov B et al. Minimally Invasive Lobectomy Is Associated With Lower Noncancer-specific Mortality in Elderly Patients: A Propensity Score Matched Competing Risks Analysis. Annals of surgery 270, 1161–1169, doi: 10.1097/sla.0000000000002772 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstraw P et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 11, 39–51, doi: 10.1016/j.jtho.2015.09.009 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Yang CF et al. Use and Outcomes of Minimally Invasive Lobectomy for Stage I Non-Small Cell Lung Cancer in the National Cancer Data Base. Ann Thorac Surg 101, 1037–1042, doi: 10.1016/j.athoracsur.2015.11.018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun SG et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 35, 56–62, doi: 10.1200/jco.2016.69.1378 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines):Non-Small Cell Lung Cancer, https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (2020).
- 12.Garon EB et al. Five-Year Overall Survival for Patients With Advanced NonSmall-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 37, 2518–2527, doi: 10.1200/JCO.19.00934 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Um SW et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 10, 331–337, doi: 10.1097/JTO.0000000000000388 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Vansteenkiste J et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology 24, vi89–vi98 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Planchard D et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 29, iv192–iv237 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Curioni-Fontecedro A et al. Preoperative chemotherapy and radiotherapy concomitant to cetuximab in resectable stage IIIB NSCLC: a multicentre phase 2 trial (SAKK 16/08). Br J Cancer 120, 968–974, doi: 10.1038/s41416-019-0447-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kocher F et al. Multicenter phase II study evaluating docetaxel and cisplatin as neoadjuvant induction regimen prior to surgery or radiochemotherapy with docetaxel, followed by adjuvant docetaxel therapy in chemonaive patients with NSCLC stage II, IIIA and IIIB (TAX-AT 1.203 Trial). Lung Cancer 85, 395–400, doi: 10.1016/j.lungcan.2014.06.019 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Kearney DJ, Lee TH, Reilly JJ, DeCamp MM & Sugarbaker DJ Assessment of operative risk in patients undergoing lung resection. Importance of predicted pulmonary function. Chest 105, 753–759, doi: 10.1378/chest.105.3.753 (1994). [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Olak J & Ferguson MK Diffusing capacity predicts operative mortality but not long-term survival after resection for lung cancer. The Journal of thoracic and cardiovascular surgery 117, 581–586; discussion 586–587, doi: 10.1016/s0022-5223(99)70338-7 (1999). [DOI] [PubMed] [Google Scholar]
- 20.Bousamra M, 2nd et al. Early and late morbidity in patients undergoing pulmonary resection with low diffusion capacity. Ann Thorac Surg 62, 968–974; discussion 974–965, doi: 10.1016/0003-4975(96)00476-6 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Datta D & Lahiri B Preoperative evaluation of patients undergoing lung resection surgery. Chest 123, 2096–2103, doi: 10.1378/chest.123.6.2096 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Burke JR, Duarte IG, Thourani VH & Miller JI Preoperative risk assessment for marginal patients requiring pulmonary resection. The Annals of Thoracic Surgery 76, 1767–1773, doi: 10.1016/S0003-4975(03)00650-7 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Timmerman R et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 303, 1070–1076, doi: 10.1001/jama.2010.261 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Videtic GM et al. A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer: NRG Oncology RTOG 0915 (NCCTG N0927). Int J Radiat Oncol Biol Phys 93, 757–764, doi: 10.1016/j.ijrobp.2015.07.2260 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ball D et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol 20, 494–503, doi: 10.1016/S1470-2045(18)30896–9 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ & Senan S Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 13, 802–809, doi: 10.1016/S1470-2045(12)70242-5 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Leeman JE et al. Histologic Subtype in Core Lung Biopsies of Early-Stage Lung Adenocarcinoma is a Prognostic Factor for Treatment Response and Failure Patterns After Stereotactic Body Radiation Therapy. International Journal of Radiation Oncology, Biology, Physics 97, 138–145, doi: 10.1016/j.ijrobp.2016.09.037 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohutek ZA et al. FDG-PET maximum standardized uptake value is prognostic for recurrence and survival after stereotactic body radiotherapy for non-small cell lung cancer. Lung Cancer 89, 115–120, doi: 10.1016/j.lungcan.2015.05.019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuaron JJ et al. Stereotactic Body Radiation Therapy for Primary Lung Cancers >3 Centimeters. Journal of Thoracic Oncology 8, 1396–1401, doi: 10.1097/JTO.0b013e3182a47181 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Antonia SJ et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 377, 1919–1929, doi: 10.1056/NEJMoa1709937 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Antonia SJ et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 379, 2342–2350, doi: 10.1056/NEJMoa1809697 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Bradley JD et al. Long-Term Results of NRG Oncology RTOG 0617: Standard-Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 38, 706–714, doi: 10.1200/JCO.19.01162 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vokes EE et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 25, 1698–1704, doi: 10.1200/JCO.2006.07.3569 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Hanna N et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 26, 5755–5760, doi: 10.1200/JCO.2008.17.7840 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Tsujino K et al. Is consolidation chemotherapy after concurrent chemo-radiotherapy beneficial for patients with locally advanced non-small-cell lung cancer? A pooled analysis of the literature. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 8, 1181–1189, doi: 10.1097/JTO.0b013e3182988348 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Bradley JD et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 16, 187–199, doi: 10.1016/S1470-2045(14)71207-0 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thor M et al. Modeling the Impact of Cardiopulmonary Irradiation on Overall Survival in NRG Oncology Trial RTOG 0617. Clin Cancer Res, doi: 10.1158/1078-0432.CCR-19-2627 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senan S et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 34, 953–962, doi: 10.1200/JCO.2015.64.8824 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Faivre-Finn C et al. LBA49 Durvalumab after chemoradiotherapy in stage III NSCLC: 4-year survival update from the phase III PACIFIC trial. Annals of Oncology 31, S1178–S1179, doi: 10.1016/j.annonc.2020.08.2281 (2020). [DOI] [Google Scholar]
- 40.Paz-Ares L et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Annals of Oncology 31, 798–806, doi: 10.1016/j.annonc.2020.03.287 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raben D et al. Patterns of disease progression with durvalumab in stage III non-small cell lung cancer (PACIFIC). Int J Radiat Oncol 105, 683, doi:DOI 10.1016/j.ijrobp.2019.08.034 (2019). [DOI] [Google Scholar]
- 42.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04214262. (2020). [DOI] [PubMed]
- 43.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03833154. (2020). [DOI] [PubMed]
- 44.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00686166. (2018). [DOI] [PubMed]
- 45.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03519971. (2020). [DOI] [PubMed]
- 46.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04026412. (2020). [DOI] [PubMed]
- 47.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03693300. (2020). [DOI] [PubMed]
- 48.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03521154. (2020). [DOI] [PubMed]
- 49.Marks LB et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 76, S70–76, doi: 10.1016/j.ijrobp.2009.06.091 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thor M et al. Toward personalized dose-prescription in locally advanced non-small cell lung cancer: Validation of published normal tissue complication probability models. Radiother Oncol 138, 45–51, doi: 10.1016/j.radonc.2019.05.011 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Speirs CK et al. Heart Dose Is an Independent Dosimetric Predictor of Overall Survival in Locally Advanced Non-Small Cell Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 12, 293–301, doi: 10.1016/j.jtho.2016.09.134 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Xue J et al. Validity of Current Stereotactic Body Radiation Therapy Dose Constraints for Aorta and Major Vessels. Semin Radiat Oncol 26, 135–139, doi: 10.1016/j.semradonc.2015.11.001 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Dehing-Oberije C et al. Development, external validation and clinical usefulness of a practical prediction model for radiation-induced dysphagia in lung cancer patients. Radiother Oncol 97, 455–461, doi: 10.1016/j.radonc.2010.09.028 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Werner-Wasik M, Yorke E, Deasy J, Nam J & Marks LB Radiation dose-volume effects in the esophagus. Int J Radiat Oncol Biol Phys 76, S86–93, doi: 10.1016/j.ijrobp.2009.05.070 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sung S, Son SH, Park EY & Kay CS Prognosis of locally advanced rectal cancer can be predicted more accurately using pre-and post-chemoradiotherapy neutrophil-lymphocyte ratios in patients who received preoperative chemoradiotherapy. PLoS One 12, e0173955, doi: 10.1371/journal.pone.0173955 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scilla KA et al. Neutrophil-Lymphocyte Ratio Is a Prognostic Marker in Patients with Locally Advanced (Stage IIIA and IIIB) Non-Small Cell Lung Cancer Treated with Combined Modality Therapy. Oncologist 22, 737–742, doi: 10.1634/theoncologist.2016-0443 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thor M et al. Are unsatisfactory outcomes after concurrent chemoradiotherapy for locally advanced non-small cell lung cancer due to treatment-related immunosuppression? Radiother Oncol 143, 51–57, doi: 10.1016/j.radonc.2019.07.016 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang C et al. Lymphopenia Association With Gross Tumor Volume and Lung V5 and Its Effects on Non-Small Cell Lung Cancer Patient Outcomes. International Journal of Radiation Oncology*Biology*Physics 89, 1084–1091, doi: 10.1016/j.ijrobp.2014.04.025 (2014). [DOI] [PubMed] [Google Scholar]
- 59.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01993810. (2018). [DOI] [PubMed]
- 60.Travis WD et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 11, 1204–1223, doi: 10.1016/j.jtho.2016.03.025 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Nicholson AG et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 11, 300–311, doi: 10.1016/j.jtho.2015.10.008 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Wu YL et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med, doi: 10.1056/NEJMoa2027071 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Bradbury P et al. Postoperative Adjuvant Systemic Therapy in Completely Resected Non-Small-Cell Lung Cancer: A Systematic Review. Clinical lung cancer 18, 259–273.e258, doi: 10.1016/j.cllc.2016.07.002 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Pignon JP et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 26, 3552–3559, doi: 10.1200/JCO.2007.13.9030 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Hamada C et al. Meta-analysis of postoperative adjuvant chemotherapy with tegafur-uracil in non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 23, 4999–5006, doi: 10.1200/JCO.2005.09.017 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Scagliotti GV et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst 95, 1453–1461, doi: 10.1093/jnci/djg059 (2003). [DOI] [PubMed] [Google Scholar]
- 67.Winton T et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 352, 2589–2597, doi: 10.1056/NEJMoa043623 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Arriagada R et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet (London, England) 375, 1267–1277, doi: 10.1016/s0140-6736(10)60059-1 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Le Pechoux C et al. LBA3_PR An international randomized trial, comparing post-operative conformal radiotherapy (PORT) to no PORT, in patients with completely resected non-small cell lung cancer (NSCLC) and mediastinal N2 involvement: Primary end-point analysis of LungART (IFCT-0503, UK NCRI, SAKK) NCT00410683. Annals of Oncology 31, S1178, doi: 10.1016/j.annonc.2020.08.2280 (2020). [DOI] [Google Scholar]
- 70.Nsclc Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet (London, England) 383, 1561–1571, doi: 10.1016/S0140-6736(13)62159-5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kato H et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 350, 1713–1721, doi: 10.1056/NEJMoa032792 (2004). [DOI] [PubMed] [Google Scholar]
- 72.Hamada C et al. Effect of postoperative adjuvant chemotherapy with tegafur-uracil on survival in patients with stage IA non-small cell lung cancer: an exploratory analysis from a meta-analysis of six randomized controlled trials. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 4, 1511–1516, doi: 10.1097/JTO.0b013e3181bbf1f2 (2009). [DOI] [PubMed] [Google Scholar]
- 73.Wakelee HA et al. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 18, 1610–1623, doi: 10.1016/S1470-2045(17)30691-5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petrelli F & Barni S Non-cancer-related mortality after cisplatin-based adjuvant chemotherapy for non-small cell lung cancer: a study-level meta-analysis of 16 randomized trials. Medical oncology (Northwood, London, England) 30, 641, doi: 10.1007/s12032-013-0641-5 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Douillard JY et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 5, 220–228, doi: 10.1097/JTO.0b013e3181c814e7 (2010). [DOI] [PubMed] [Google Scholar]
- 76.Pepe C et al. Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 25, 1553–1561, doi: 10.1200/JCO.2006.09.5570 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Kenmotsu H et al. Randomized Phase III Study of Pemetrexed Plus Cisplatin Versus Vinorelbine Plus Cisplatin for Completely Resected Stage II to IIIA Nonsquamous Non-Small-Cell Lung Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 38, 2187–2196, doi: 10.1200/jco.19.02674 (2020). [DOI] [PubMed] [Google Scholar]
- 78.Kris MG et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non-Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 35, 2960–2974, doi: 10.1200/JCO.2017.72.4401 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Vansteenkiste JF et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 17, 822–835, doi: 10.1016/S1470-2045(16)00099-1 (2016). [DOI] [PubMed] [Google Scholar]
- 80.Chan BA & Hughes BGM Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res 4, 36–54, doi: 10.3978/j.issn.2218-6751.2014.05.01 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reck M et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 375, 1823–1833, doi: 10.1056/NEJMoa1606774 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Zhong WZ et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 19, 139–148, doi: 10.1016/S1470-2045(17)30729-5 (2018). [DOI] [PubMed] [Google Scholar]
- 83.Arriagada R et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350, 351–360, doi: 10.1056/NEJMoa031644 (2004). [DOI] [PubMed] [Google Scholar]
- 84.Felip E et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 28, 3138–3145, doi: 10.1200/JCO.2009.27.6204 (2010). [DOI] [PubMed] [Google Scholar]
- 85.Betticher DC et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 21, 1752–1759, doi: 10.1200/jco.2003.11.040 (2003). [DOI] [PubMed] [Google Scholar]
- 86.Blumenthal GM et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 13, 1818–1831, doi: 10.1016/j.jtho.2018.09.017 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Chaft JE et al. Adaptive Neoadjuvant Chemotherapy Guided by (18)F-FDG PET in Resectable Non-Small Cell Lung Cancers: The NEOSCAN Trial. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 11, 537–544, doi: 10.1016/j.jtho.2015.12.104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blakely CM & McCoach CE Role of MPR as an Early Signal for Efficacy in Neoadjuvant Studies. Clin Cancer Res 26, 3499–3500, doi: 10.1158/1078-0432.CCR-20-1129 (2020). [DOI] [PubMed] [Google Scholar]
- 89.Chiang AC & Herbst RS Frontline immunotherapy for NSCLC - the tale of the tail. Nat Rev Clin Oncol 17, 73–74, doi: 10.1038/s41571-019-0317-y (2020). [DOI] [PubMed] [Google Scholar]
- 90.Topalian SL, Taube JM & Pardoll DM Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 367, eaax0182, doi: 10.1126/science.aax0182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McGranahan N et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469, doi: 10.1126/science.aaf1490 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forde PM et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 378, 1976–1986, doi: 10.1056/NEJMoa1716078 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02927301. (2020). [DOI] [PubMed]
- 94.Kwiatkowski DJ et al. Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): Interim analysis and biomarker data from a multicenter study (LCMC3). Journal of Clinical Oncology 37, doi: 10.1200/JCO.2019.37.15_suppl.8503 (2019). [DOI] [Google Scholar]
- 95.Gao S et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 15, 816–826, doi: 10.1016/j.jtho.2020.01.017 (2020). [DOI] [PubMed] [Google Scholar]
- 96.Cascone T et al. Neoadjuvant nivolumab (N) or nivolumab plus ipilimumab (NI) for resectable non-small cell lung cancer (NSCLC): Clinical and correlative results from the NEOSTAR study. Journal of Clinical Oncology 37, 8504–8504, doi: 10.1200/JCO.2019.37.15_suppl.8504 (2019). [DOI] [Google Scholar]
- 97.Herbst RS & Sznol M Diminished but not dead: chemotherapy for the treatment of NSCLC. Lancet Oncol 17, 1464–1465, doi: 10.1016/S1470-2045(16)30524-1 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Parra ER et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J Immunother Cancer 6, 48, doi: 10.1186/s40425-018-0368-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gadgeel S et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non–Small-Cell Lung Cancer. Journal of Clinical Oncology 38, 1505–1517, doi: 10.1200/JCO.19.03136 (2020). [DOI] [PubMed] [Google Scholar]
- 100.Paz-Ares L et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. Journal of Thoracic Oncology 15, 1657–1669, doi: 10.1016/j.jtho.2020.06.015 (2020). [DOI] [PubMed] [Google Scholar]
- 101.Reck M et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. The Lancet Respiratory Medicine 7, 387–401, doi: 10.1016/S2213-2600(19)30084-0 (2019). [DOI] [PubMed] [Google Scholar]
- 102.Shu CA et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 21, 786–795, doi: 10.1016/S1470-2045(20)30140-6 (2020). [DOI] [PubMed] [Google Scholar]
- 103.Provencio M et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 21, 1413–1422, doi: 10.1016/s1470-2045(20)30453-8 (2020). [DOI] [PubMed] [Google Scholar]
- 104.Rothschild S et al. SAKK 16/14: Anti-PD-L1 antibody durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small cell lung cancer (NSCLC)—A multicenter single-arm phase II trial. Journal of Clinical Oncology 38, 9016–9016, doi: 10.1200/JCO.2020.38.15_suppl.9016 (2020). [DOI] [PubMed] [Google Scholar]
- 105.Bristol Myers Squibb. Opdivo (nivolumab) Plus Chemotherapy Shows Statistically Significant Improvement in Pathologic Complete Response as Neoadjuvant Treatment of Resectable Non-Small Cell Lung Cancer in Phase 3 CheckMate −816 Trial, https://www.businesswire.com/news/home/20201007005273/en/ (2020).
- 106.Mayekar MK & Bivona TG Current Landscape of Targeted Therapy in Lung Cancer. Clin Pharmacol Ther 102, 757–764, doi: 10.1002/cpt.810 (2017). [DOI] [PubMed] [Google Scholar]
- 107.Zhong WZ et al. Erlotinib Versus Gemcitabine Plus Cisplatin as Neoadjuvant Treatment of Stage IIIA-N2 EGFR-Mutant Non-Small-Cell Lung Cancer (EMERGING-CTONG 1103): A Randomized Phase II Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 37, 2235–2245, doi: 10.1200/JCO.19.00075 (2019). [DOI] [PubMed] [Google Scholar]
- 108.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04351555. (2020). [DOI] [PubMed]
- 109.Rusch VW et al. Induction chemoradiation and surgical resection for non-small cell lung carcinomas of the superior sulcus: Initial results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). The Journal of thoracic and cardiovascular surgery 121, 472–483, doi: 10.1067/mtc.2001.112465 (2001). [DOI] [PubMed] [Google Scholar]
- 110.Rusch VW et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). Journal of clinical oncology : official journal of the American Society of Clinical Oncology 25, 313–318, doi: 10.1200/JCO.2006.08.2826 (2007). [DOI] [PubMed] [Google Scholar]
- 111.Robinson LA et al. Induction chemoradiotherapy versus chemotherapy alone for superior sulcus lung cancer. Lung Cancer 122, 206–213, doi: 10.1016/j.lungcan.2018.06.021 (2018). [DOI] [PubMed] [Google Scholar]
- 112.Thomas M et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol 9, 636–648, doi: 10.1016/s1470-2045(08)70156-6 (2008). [DOI] [PubMed] [Google Scholar]
- 113.Pless M et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet (London, England) 386, 1049–1056, doi: 10.1016/S0140-6736(15)60294-X (2015). [DOI] [PubMed] [Google Scholar]
- 114.Billiet C et al. Modern post-operative radiotherapy for stage III non-small cell lung cancer may improve local control and survival: a meta-analysis. Radiother Oncol 110, 3–8, doi: 10.1016/j.radonc.2013.08.011 (2014). [DOI] [PubMed] [Google Scholar]
- 115.Mikell JL et al. Postoperative radiotherapy is associated with better survival in non-small cell lung cancer with involved N2 lymph nodes: results of an analysis of the National Cancer Data Base. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 10, 462–471, doi: 10.1097/JTO.0000000000000411 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Corso CD et al. Re-evaluation of the role of postoperative radiotherapy and the impact of radiation dose for non-small-cell lung cancer using the National Cancer Database. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 10, 148–155, doi: 10.1097/jto.0000000000000406 (2015). [DOI] [PubMed] [Google Scholar]
- 117.Robinson CG et al. Postoperative radiotherapy for pathologic N2 non-small-cell lung cancer treated with adjuvant chemotherapy: a review of the National Cancer Data Base. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 33, 870–876, doi: 10.1200/jco.2014.58.5380 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Govindan R et al. ALCHEMIST Trials: A Golden Opportunity to Transform Outcomes in Early-Stage Non-Small Cell Lung Cancer. Clin Cancer Res 21, 5439–5444, doi: 10.1158/1078-0432.CCR-15-0354 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Johnson JR, Williams G & Pazdur R End points and United States Food and Drug Administration approval of oncology drugs. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 21, 1404–1411, doi: 10.1200/JCO.2003.08.072 (2003). [DOI] [PubMed] [Google Scholar]
- 120.Mauguen A et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: a re-analysis of meta-analyses of individual patients’ data. Lancet Oncol 14, 619–626, doi: 10.1016/S1470-2045(13)70158-X (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pataer A et al. Histopathologic Response Criteria Predict Survival of Patients with Resected Lung Cancer After Neoadjuvant Chemotherapy. Journal of Thoracic Oncology 7, 825–832, doi: 10.1097/JTO.0b013e318247504a (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cascone T et al. Induction Cisplatin Docetaxel Followed by Surgery and Erlotinib in Non-Small Cell Lung Cancer. Ann Thorac Surg 105, 418–424, doi: 10.1016/j.athoracsur.2017.08.052 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chaft JE et al. Phase II trial of neoadjuvant bevacizumab plus chemotherapy and adjuvant bevacizumab in patients with resectable nonsquamous non-small-cell lung cancers. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 8, 1084–1090, doi: 10.1097/JTO.0b013e31829923ec (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cascone T et al. A Phase I/II Study of Neoadjuvant Cisplatin, Docetaxel, and Nintedanib for Resectable Non-Small Cell Lung Cancer. Clin Cancer Res 26, 3525–3536, doi: 10.1158/1078-0432.CCR-19-4180 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hellmann MD et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 15, e42–50, doi: 10.1016/S1470-2045(13)70334-6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Travis WD et al. IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 15, 709–740, doi: 10.1016/j.jtho.2020.01.005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chaudhuri AA et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer Discov 7, 1394–1403, doi: 10.1158/2159-8290.CD-17-0716 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li BT et al. Liquid biopsy for ctDNA to revolutionize the care of patients with early stage lung cancers. Ann Transl Med 5, 479, doi: 10.21037/atm.2017.09.02 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04385368. (2020). [DOI] [PubMed]
- 130.Howlader N et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med 383, 640–649, doi: 10.1056/NEJMoa1916623 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Song W-A. et al. Survival benefit of neoadjuvant chemotherapy in non-small cell lung cancer: an updated meta-analysis of 13 randomized control trials. J. Thorac. Oncol 5, 510–516 (2010) [DOI] [PubMed] [Google Scholar]
- 132.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02201992. (2020). [DOI] [PubMed]
- 133.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02193282. (2020). [DOI] [PubMed]
- 134.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02595944. (2020). [DOI] [PubMed]
- 135.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02504372. (2020). [DOI] [PubMed]
- 136.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02486718. (2020). [DOI] [PubMed]
- 137.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02273375. (2020). [DOI] [PubMed]
- 138.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03447769. (2020). [DOI] [PubMed]
- 139.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03800134. (2020). [DOI] [PubMed]
- 140.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03456063. (2020). [DOI] [PubMed]
- 141.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03425643. (2020). [DOI] [PubMed]
- 142.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02998528. (2020). [DOI] [PubMed]


